Abstract
Background
Procedural sedation and analgesia (PSA) is used frequently in the emergency department (ED) to facilitate painful procedures and interventions. Capnography, a monitoring modality widely used in operating room and endoscopy suite settings, is being used more frequently in the ED setting with the goal of reducing cardiopulmonary adverse events. As opposed to settings outside the ED, there is currently no consensus on whether the addition of capnography to standard monitoring modalities reduces adverse events in the ED setting.
Objectives
To assess whether capnography in addition to standard monitoring (pulse oximetry, blood pressure and cardiac monitoring) is more effective than standard monitoring alone to prevent cardiorespiratory adverse events (e.g. oxygen desaturation, hypotension, emesis, and pulmonary aspiration) in ED patients undergoing PSA.
Search methods
We searched the Cochrane Central Register of Controlled Trials (2016, Issue 8), and MEDLINE, Embase, and CINAHL to 9 August 2016 for randomized controlled trials (RCTs) and quasi‐randomized trials of ED patients requiring PSA with no language restrictions. We searched meta‐registries (www.controlled‐trials.com, www.clinicalstudyresults.org, and clinicaltrials.gov) for ongoing trials (February 2016). We contacted the primary authors of included studies as well as scientific advisors of capnography device manufacturers to identify unpublished studies (February 2016). We handsearched conference abstracts of four organizations from 2010 to 2015.
Selection criteria
We included any RCT or quasi‐randomized trial comparing capnography and standard monitoring to standard monitoring alone for ED patients requiring PSA.
Data collection and analysis
Two authors independently performed study selection, data extraction, and assessment of methodological quality for the 'Risk of bias' tables. An independent researcher extracted data for any included studies that our authors were involved in. We contacted authors of included studies for incomplete data when applicable. We used Review Manager 5 to combine data and calculate risk ratios (RR) and 95% confidence intervals (CI) using both random‐effects and fixed‐effect models.
Main results
We identified three trials (κ = 1.00) involving 1272 participants. Comparing the capnography group to the standard monitoring group, there were no differences in the rates of oxygen desaturation (RR 0.89, 95% CI 0.48 to 1.63; n = 1272, 3 trials; moderate quality evidence) and hypotension (RR 2.36, 95% CI 0.98 to 5.69; n = 986, 1 trial; moderate quality evidence). There was only one episode of emesis recorded without significant difference between the groups (RR 3.10, 95% CI 0.13 to 75.88, n = 986, 1 trial; moderate quality evidence). The quality of evidence for the primary outcomes was moderate with downgrades primarily due to heterogeneity and reporting bias.
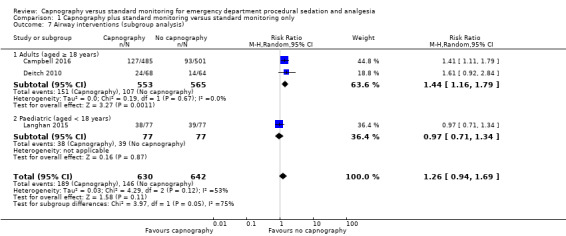
There were no differences in the rate of airway interventions performed (RR 1.26, 95% CI 0.94 to 1.69; n = 1272, 3 trials; moderate quality evidence). In the subgroup analysis, we found a higher rate of airway interventions for adults in the capnography group (RR 1.44, 95% CI 1.16 to 1.79; n = 1118, 2 trials; moderate quality evidence) with a number needed to treat for an additional harmful outcome of 12. Although statistical heterogeneity was reduced, there was moderate quality of evidence due to outcome definition heterogeneity and limited reporting bias. None of the studies reported recovery time.
Authors' conclusions
There is a lack of convincing evidence that the addition of capnography to standard monitoring in ED PSA reduces the rate of clinically significant adverse events. Evidence was deemed to be of moderate quality due to population and outcome definition heterogeneity and limited reporting bias. Our review was limited by the small number of clinical trials in this setting.
Plain language summary
Use of capnography in emergency department patients being sedated for procedures
Review question
Will carbon dioxide detection monitoring help reduce heart, lung, and airway complications for emergency department patients being sedated for painful procedures?
Background
Medications are often used in order to reduce pain or awareness (or both) for patients having painful procedures. Sometimes, complications involving a patient's heart, lungs, or airway (breathing tubes) can occur due to these medicines (e.g. vomit inhaled by the lungs). Healthcare workers monitor heart rate, blood pressure, breathing rate, and blood oxygen content to help prevent complications.
Capnography (measuring carbon dioxide gas as a patient breathes out) use has been proposed to further increase the safety of sedating patients in the emergency department. This study was performed to determine if capnography makes a difference when added to standard monitoring.
Study characteristics
We searched for studies using multiple research databases, conference research abstracts, and by contacting experts in the field. The evidence is current to August 2016. We only considered studies with participants being sedated for procedures in the emergency department. We only included studies that compared capnography and standard monitoring to standard monitoring only.
The main outcomes involved events of low blood oxygen content, low blood pressure, and vomiting. We also recorded how many times the healthcare providers had to help the patient breathe easier. This could mean simple actions such as opening of the mouth to more serious manoeuvres such as mechanically breathing for the patient.
Three studies with 1272 people, containing moderate evidence, were included in our study.
Key results
There was no difference in heart, lung, or airway complications with the addition of capnography. When only adults were studied, healthcare providers performed more manoeuvres to help the patient breathe when capnography was used. This could be due to false alarms.
Quality of evidence
The level of evidence was determined to be moderate.
Summary of findings
Summary of findings for the main comparison. Capnography and standard monitoring compared with standard monitoring for emergency department patients undergoing procedural sedation and analgesia.
| Capnography and standard monitoring compared with standard monitoring for emergency department patients undergoing procedural sedation and analgesia | ||||||
|
Patient or population: patients undergoing PSA Settings: emergency departments in North America Intervention: capnography and standard monitoring Comparison: standard monitoring | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard monitoring | Capnography and standard monitoring | |||||
| Oxygen desaturation | Medium risk population | RR 0.89 (0.48 to 1.63) | 1272 participants (3 studies) | ⊕⊕⊕⊝ Moderateb | ‐ | |
| 8 per 1000a | 7 per 1000 (4 to 13) | |||||
| Hypotension | Medium risk population | RR 2.36 (0.98 to 5.69) | 986 participants (1 study) | ⊕⊕⊕⊝ Moderated | ‐ | |
| 6 per 1000c | 14 per 1000 (6 to 34) | |||||
| Emesis, pulmonary aspiration | Medium risk population | RR 3.10 (0.13 to 75.88) | 986 participants (1 study) | ⊕⊕⊕⊝ Moderated | None of the studies recorded pulmonary aspiration events. | |
| 4 per 1000e |
4 per 1000 (1 to 304) |
|||||
| Airway interventions | Medium risk population | RR 1.26 (0.94 to 1.69) | 1272 participants (3 studies) | ⊕⊕⊕⊝ Moderateg | 2 studies included verbal/physical stimulation and supplemental oxygen as airway interventions (not consistent with our definition) but only reported total airway interventions (as dichotomous outcomes). | |
| 150 per 1000f | 189 per 1000 (141 to 254) | |||||
| Airway interventions adult subgroup analysis (aged ≥ 18 years)h | Medium risk population | RR 1.44 (1.16 to 1.79) | 1118 participants (2 studies) | ⊕⊕⊕⊝ Moderatej | ‐ | |
| 190 per 1000i | 274 per 1000 (220 to 340) | |||||
| Recovery time | None of the studies reported recovery time. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PSA: procedural sedation and analgesia; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aCampbell 2006; Cudny 2013. No study found to determine assumed risk for all‐age population, combined incidence of these studies used as a surrogate. Hypoxia defined as oxygen saturation < 90% at any time with baseline oxygen saturation ≥ 95% for Campbell 2006. Unknown definition of hypoxia for Cudny 2013.
b Although statistics show low to moderate heterogeneity (I2 = 42%, P = 0.18), quality downgraded due to heterogeneity in study designs.
cCampbell 2006. Hypotension defined as systolic blood pressure < 85 mmHg at any time with baseline systolic blood pressure ≥ 100 mmHg.
d Downgraded for reporting bias in one study.
eLanghan 2012. Used this paediatric study as surrogate for all ages population.
g Downgraded due to significant heterogeneity (I2 = 53%).
h The study by Campbell 2016 reported adults aged 16 years or greater whereas the study by Deitch 2010 reported adults aged greater than 18 years.
j Downgraded due to heterogeneity in outcome definitions as well as small number of studies.
Background
Description of the condition
Procedural sedation and analgesia (PSA) is used very frequently in the modern emergency department (ED) under the auspices of emergency physicians and allied healthcare professionals. PSA is defined as the use of medications to induce an altered state of consciousness to safely facilitate painful procedures and interventions such as fracture reduction, cardioversion, and incision and drainage of abscesses without loss of spontaneous cardiopulmonary function (Godwin 2005). Despite widespread use, PSA is not without risk. Overshoot in the intended depth of sedation potentially resulting in respiratory compromise, loss of airway protection, and cardiovascular depression occurs in 1.3% to 10% of cases (Campbell 2006; Miner 2003; Swanson 1996). In one study, respiratory depression occurred in up to 39% of patients undergoing PSA with propofol (Deitch 2010). Emesis with or without pulmonary aspiration is of particular concern in patients with full stomachs who may be intoxicated and undergoing PSA in the ED. Safeguards to reduce complications in ED PSA include monitoring heart rate, respiratory rate, blood pressure, and oxygen saturation.
Description of the intervention
Capnography, the continuous measurement of exhaled carbon dioxide (CO2) partial pressures (PCO2), can be used in conjunction with other monitoring variables to determine the effects of sedation medications on ventilation and potentially prevent hypoxic events. This is accomplished by placement of an infrared sensor at the facemask or nasal cannula. The data output can be interpreted in the form of capnograms (CO2 waveforms versus time or expired volume) or end‐tidal CO2 partial pressure (an estimation of alveolar PCO2 (Kodali 2013). It is a widely accepted modality and used extensively in the operating room and the endoscopy suite in addition to standard PSA monitoring (Swanson 1996). While capnography use in the ED was historically limited to confirmation of endotracheal tube placement and for cardiac arrest, it is being used with increasing frequency for PSA (Krauss 2007).
How the intervention might work
Sedation medications act to depress minute ventilation by decreasing respiration rate or tidal volume, or both. On a physiological level, alterations in PCO2 are used to estimate changing pulmonary CO2 levels, a surrogate of changes in minute ventilation. Capnography can be used to detect decreasing trends of minute ventilation (i.e. ventilatory depression) earlier and steps can be taken (airway intervention or medication or dose change) to correct the undesired physiological disturbance (Burton 2006). Specifically, capnography data can be interpreted by monitoring for changes in end‐tidal PCO2 (PETCO2), the shape of the capnogram, or arterial (a) and end‐tidal PaCO2 ‐ PETCO2 gradients (Kodali 2013). It is theorized that by using capnography in addition to other physiological variables to detect ventilatory depression, cardiorespiratory complications such as hypoxia can be detected and treated earlier.
Why it is important to do this review
There has been a trend towards increased use of capnography in ED PSA despite limited evidence demonstrating its efficacy in this environment. The controlled environments of the operating room and endoscopy suite are in contrast to the ED with respect to both patient population and procedures performed. ED visits are unplanned and patients often present in significant distress with full stomachs and they may have recently consumed alcohol or recreational drugs. Considering this, conclusions derived from studies in operating room and endoscopy settings do not necessarily apply to ED PSA. Only one published randomized controlled trial (RCT) had been performed at the protocol stage studying capnography as a primary intervention in ED patients undergoing PSA (Deitch 2010). This study found that capnography both reduced and provided earlier detection of hypoxic events. The authors used definitions inconsistent with other literature, notably defining hypoxia as a blood oxygen saturation (SpO2) less than 93% for 15 seconds or greater, complicating the interpretation of their conclusions. A complete review of relevant studies is needed to improve generalizability and ultimately to provide a more powerful consensus on the utility of capnography in the ED.
Objectives
To assess whether capnography in addition to standard monitoring (pulse oximetry, blood pressure and cardiac monitoring) is more effective than standard monitoring alone to prevent cardiorespiratory adverse events (e.g. oxygen desaturation, hypotension, emesis, and pulmonary aspiration) in ED patients undergoing procedural sedation and analgesia.
Methods
Criteria for considering studies for this review
Types of studies
We only considered RCTs and quasi‐RCTs for inclusion. We imposed no language restrictions. We included unpublished data if the trial met the inclusion criteria.
Types of participants
We included all ED patients requiring PSA were included, regardless of age. Procedural sedation refers to "the technique of administering sedatives or dissociative agents with or without analgesics to induce an altered state of consciousness that allows the patient to tolerate unpleasant procedures while preserving cardiorespiratory function" (Godwin 2005). We excluded participants receiving PSA in non‐ED environments such as endoscopy suites or operating rooms.
Types of interventions
All participants had to be randomized to either capnography in addition to standard monitoring (pulse oximetry, blood pressure and cardiac monitoring) or standard monitoring alone.
Types of outcome measures
Primary outcomes
Oxygen desaturation (percentage of oxygen saturation in arterial blood (SaO2) less than 90% for 30 seconds).
Hypotension (systolic blood pressure less than 90 mmHg).
Emesis, pulmonary aspiration.
Secondary outcomes
Airway interventions performed (airway repositioning manoeuvres, positive pressure ventilation (PPV), oral pharyngeal or nasal pharyngeal airway placement, endotracheal intubation (ETI)).
Recovery time (time from end of procedure to cessation of monitoring).
Note: this definition of oxygen desaturation was most consistent with other relevant studies in the literature (Anderson 2007; Campbell 2006; Deitch 2007; Hart 1997; Langhan 2012; Mallory 2011; Miner 2002; Wright 1992). We would argue that this is clinically significant due to the fact that as oxygen saturation falls below 90%, the steep area of the oxyhaemoglobin dissociation curve is entered mandating medical intervention.
Outcomes did not form part of the study eligibility assessment so that studies that met the design, participant, intervention, and comparison criteria could be included in the review even if they reported no relevant outcomes.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, 2016, Issue 8); MEDLINE (1980 to 9 August 2016); Embase (1980 to 9 August 2016); and CINAHL (1982 to 9 August 2016) to identify all clinical trials relating to our objectives. We imposed no language or publication restrictions, or publication status restrictions. We reviewed the reference lists of all available primary studies and review articles to identify potential relevant citations.
See Appendix 1 (CENTRAL), Appendix 2 (MEDLINE), Appendix 3 (Embase), and Appendix 4 (CINAHL) for our search strategies.
Searching other resources
We contacted the authors of the primary studies and scientific advisors of the various capnography device manufacturers to enquire about additional published or unpublished studies (February 2016). We searched for trials in progress using: www.controlled‐trials.com, www.clinicalstudyresults.org, and clinicaltrials.gov (February 2016).
We handsearched Canadian Association of Emergency Physicians (CAEP); American College of Emergency Physicians (ACEP); Society for Academic Emergency Medicine (SAEM); and International Conference on Emergency Medicine (ICEM) conference abstract submissions from 2010 to 2015.
Finally, we personally contacted colleagues, collaborators, and other trialists working in the field of ED PSA to identify potentially relevant studies.
Data collection and analysis
Selection of studies
We combined results from the search strategies into a reference manager program (EndNote X7.4) with duplicates excluded. Two authors (BFW, KDM) selected all trials which appeared relevant on the basis of title, abstract, and MeSH headings for full review.
From the potentially relevant articles identified, two authors (BFW, KDM) independently selected trials (based on the full‐text format) for inclusion in this review (see Appendix 5 for the article inclusion form). Agreement was measured using simple agreement and kappa statistics. We resolved disagreements by discussion to reach consensus or third party adjudication (SGC). Authors were not blinded to information about the articles such as publication names, institutions involved, or conclusions made. A list of all included trials was completed (Appendix 6). Adjudication of the final list was performed by a separate author (BFW) and an independent third party (Anna MacDonald) for inclusion or exclusion from the selection of studies conducted by authors of this review.
Data extraction and management
Two authors (BFW, KDM) independently extracted data from the trials using an electronic data collection form (see Appendix 7). Disagreement not resolved by consensus was adjudicated by a third author (SGC). Any additional trial data required was obtained by contacting the first trial author (BFW). Data for trials conducted by authors of this review were extracted by a separate author (BFW) and an independent third party (Anna MacDonald). Data extraction included the following items.
General information: title, authors, contact address, publication source, publication year, country.
Methodological characteristics and study design.
Population characteristics: age, sedation agent used, procedure performed.
Intervention: capnography, definition of; capnography alert level.
Control: blood pressure, heart rate, oxygen saturation.
Outcome measures as noted above.
Assessment of risk of bias in included studies
We performed a methodological quality assessment using the 'Risk of bias' tool detailed in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The quality assessment form is attached as Appendix 8. Two authors (BFW, KDM) independently performed this assessment with disagreement not resolved by consensus arbitrated by a third author (SGC). Assessors were not blinded to the study authors or the results of the studies.
We assessed the following domains: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective reporting; and all other biases.
Measures of treatment effect
For dichotomous variables, we calculated individual and pooled statistics as risk ratios (RR) with 95% confidence intervals (CI). For continuous outcomes, we calculated individual and pooled statistics as mean differences (MD) or standardized mean differences (SMD) and 95% CIs.
Unit of analysis issues
We did not include cluster‐randomized trials and cross‐over trials in the review, and therefore, there were no unit of analysis issues.
Dealing with missing data
An intention‐to‐treat (ITT) analysis, which deals with 'missing data' from the individual trials, was completed.
Assessment of heterogeneity
We assessed heterogeneity by analysing the methodological diversity (risk of bias assessment), clinical diversity (participant age, procedure performed, sedation agent used, definition of hypotension, definition of oxygen desaturation), and trial size (publication bias). We visually inspected Forest plots for evidence of heterogeneity and calculated both Chi2 and I2 statistics. Given the small number of included studies, we classified significant heterogeneity as a measured I2 statistic greater than 50% (Higgins 2011). We performed subgroup and sensitivity analyses to address clinical heterogeneity.
Assessment of reporting biases
We addressed publication bias, when found, by adjusting the results using the Egger approach (Egger 1997), and the 'trim and fill' method. In the event that 10 or more studies were included in the review, we planned to create a funnel plot. In addition, we planned to use quality weighting to test the robustness of the results.
Data synthesis
In the absence of significant heterogeneity, we performed a meta‐analysis using Review Manager 5 (RevMan 2014). To ensure the robustness of the results, we used both random‐effects and fixed‐effect modelling. The criteria for statistical significance was a P value less than 0.05 and 95% CIs that did not intersect.
Subgroup analysis and investigation of heterogeneity
As the utility of capnography in ED PSA may be dependent on the age of the participant, the sedation agent used, and the procedure performed, subgroup analysis was planned a priori in the event significant heterogeneity was detected (determined by the forest plot and I2 statistic).
Adults (aged over 18 years) versus paediatrics.
Sedation agent used (propofol, ketamine, midazolam, lorazepam, fentanyl, etomidate, pentobarbital, methohexital).
Procedure performed (fracture reduction, joint reduction, incision and drainage, suturing, cardioversion, lumbar puncture, diagnostic imaging, endoscopy, Foley catheter insertion, central line placement, dressing changes, burn debridement, ophthalmic procedure, dental procedure, temporomandibular joint reduction, chest tube insertion, other).
Sensitivity analysis
In order to test the robustness of our findings, we planned to perform a sensitivity analysis for trials with low risk versus high risk of bias. We planned to use random‐effects and fixed‐effect model estimates for each outcome variable. In the event of any missing data, we planned to use best‐case and worst‐case scenarios for imputation of missing data.
'Summary of findings' table and GRADE
We used the GRADE system to assess the quality of the evidence associated with specific outcomes (oxygen desaturation, hypotension, emesis, pulmonary aspiration, airway interventions performed, recovery time) in our review and to construct a 'Summary of findings' table using the GRADE software (Guyatt 2008). The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of effect estimates, and risk of publication bias.
Results
Description of studies
Results of the search
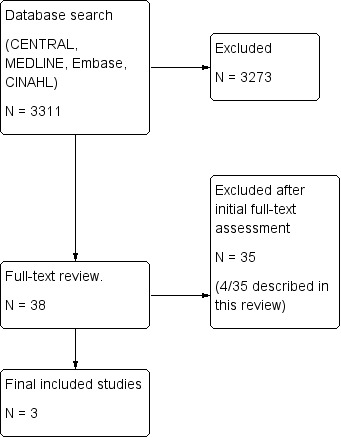
The electronic search resulted in 3311 studies after removal of duplicates using bibliographic software (Figure 1). We identified 38 studies for full paper review by screening titles and abstracts. Of these 38 references, we included three in our review (Characteristics of included studies table) and excluded 35 due to the following:
1.
Search flow diagram.
10 were review articles;
eight were non‐randomized trials;
eight involved other interventions;
eight were letters or questionnaires;
one was a systematic review.
Of these 35 excluded reports, four were identified as potentially relevant but were then excluded (Characteristics of excluded studies table).
Included studies
We included three articles comparing 1272 participants in our review (Characteristics of included studies table).
Setting
All three studies took place in single‐centred academic EDs in the US or Canada. Two studies involved physicians performing the sedation (Deitch 2010; Langhan 2015), and one study involved advanced level paramedics performing the sedation, an evidence‐based standard at that institution (Campbell 2016). All providers involved in procedural sedation were trained in interpreting capnography.
Population
Two of the included studies enrolled adults only with Campbell 2016 enrolling 986 participants (aged greater than 16 years) and Deitch 2010 enrolling 132 participants (aged greater than 18 years). The other study enrolled 154 paediatric participants (aged less than 20 years) (Langhan 2015). All studies included patients undergoing PSA but generally excluded critically ill patients. The paediatric study (Langhan 2015), excluded patients who had supplemental oxygen at baseline whereas the adult studies administered baseline supplemental oxygen as standard of care (Campbell 2016; Deitch 2010).
Interventions
The control groups differed in one study (Campbell 2016), using only standard monitoring without capnography whereas two studies used standard monitoring and blinded capnography (Deitch 2010; Langhan 2015). Standard monitoring was similar in all studies, consisting of cardiac monitoring, blood pressure monitoring, and pulse oximetry. Capnography alert levels were specified and similar in two of the studies (Deitch 2010; Langhan 2015), whereas one study did not specify alert levels (Campbell 2016).
Outcomes
All three studies recorded the primary outcome of oxygen desaturation but there was some variance in the definition.
One study defined hypoxia as less than 93% for greater than 15 seconds (Deitch 2010). The author was unable to be reached for unpublished data.
One study defined hypoxia as less than 90% for greater than 30 seconds (Campbell 2016).
One study defined hypoxia as less than 95% (Langhan 2015). We were able to contact the authors and collect unpublished data for episodes of hypoxia less than 90%.
Campbell 2016 recorded vomiting and hypotensive events, with hypotension defined as systolic blood pressure (SBP) less than 100 mmHg or less than 85 mmHg if baseline less than 100 mmHg. Langhan 2015 did not measure vomiting and hypotension. Deitch 2010 did not report vomiting and hypotension despite the methods section stating the recording of such events. None of the studies recorded pulmonary aspiration events.
All three studies recorded the secondary outcome of airway interventions but there was significant variance in classification of such interventions. Only Campbell 2016 and Langhan 2015 provided data detailing the number of specific interventions performed. Deitch 2010 and Langhan 2015 published dichotomous data, whereas we contacted the authors of Campbell 2016 to obtain dichotomous data (only continuous outcome data was published).
None of the studies reported recovery time (excluding procedure time).
Funding
The Campbell 2016 and Langhan 2015 studies were supported by independent government grants. Deitch 2010 had capnography equipment donated by a private medical company.
Excluded studies
We excluded four studies due to retrospective analysis of capnography data in RCTs comparing other interventions (Deitch 2007;Deitch 2008; Hart 1997; Sivilotti 2010) (see Characteristics of excluded studies table).
Studies awaiting classification
There are no studies awaiting classification.
Ongoing studies
We identified no ongoing studies.
Risk of bias in included studies
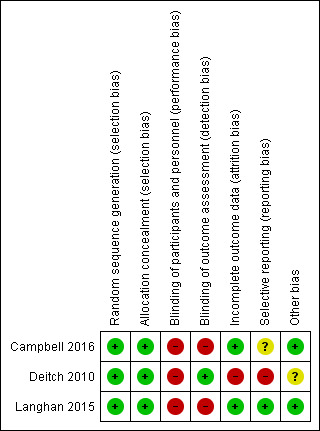
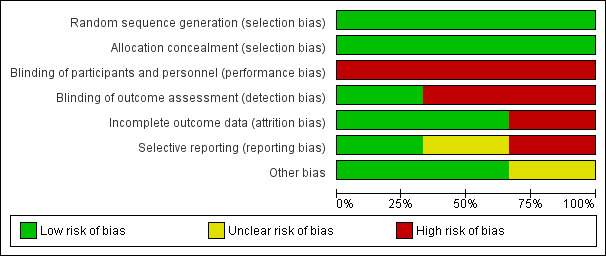
We have included the risk of bias in Figure 2 and Figure 3. Overall quality of evidence was moderate with downgrades resulting from outcome definition heterogeneity and limited reporting bias in one study.
2.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
All three studies used random sequence generation using a computer (Campbell 2016; Deitch 2010; Langhan 2015). Allocation concealment was appropriate in all studies. Although none of the studies mentioned use of an ITT protocol, there were no participants given an intervention other than their assigned group intervention. We found that there was low risk of allocation bias.
Blinding
None of the studies blinded either treating personnel or participants to the intervention. We deemed this an inherent bias due to the monitoring nature of the intervention; treating personnel are required to see the monitoring technique to perform the procedure. Likewise, the logistics of blinding a patient to bedside monitoring while maintaining safety is understandably difficult. We deemed this to be low risk of bias because future studies would likely not be able to improve upon this.
Incomplete outcome data
Two studies accounts for all data, with low risk for attrition bias (Campbell 2016; Langhan 2015). One study excluded 12% of participants enrolled in the study from analysis based on a priori exclusion criteria of greater than 35% data loss (Deitch 2010). This was deemed a high source of attrition bias.
Selective reporting
Given the small number of studies included in the meta‐analysis, it was difficult to assess for publication bias. The review did not include the minimum number of studies stipulated to produce a funnel plot. However, we are confident that our robust search strategy identified all relevant published studies. With respect to intra‐study reporting bias, one study collected data on adverse events (hypotension, emesis) but did not report them in the results section (Deitch 2010). This study reported only absolute numbers of airway interventions and not specific types although these data were also reported to be collected. We found this study to be at a high risk of reporting bias due to these discrepancies between the methods and results reported. One study did not enrol in a trial registry and thus reporting bias was unknown in this study (Campbell 2016).
Other potential sources of bias
All three included studies defined oxygen desaturation differently. A priori, we defined oxygen desaturation as an oxygen saturation of less than 90% for 30 seconds, the same definition used by Campbell 2016. This definition was most consistent with other relevant studies in the literature (Anderson 2007; Campbell 2006; Deitch 2007; Hart 1997; Langhan 2012; Mallory 2011; Miner 2002; Wright 1992), and we would argue, makes physiological sense based on the oxyhaemoglobin dissociation curve and is clinically significant mandating medical intervention. Although Langhan 2015 defined hypoxia as an oxygen saturation of less than 95%, the authors provided unpublished individual participant data such that rates of oxygen desaturation, as defined above, could be calculated. Deitch 2010 defined hypoxia as an oxygen saturation of less than 93% for greater than 15 seconds. Attempts to contact the authors were unsuccessful and we were required to include these participants in the analysis despite an obvious difference in outcome definition that would introduce more oxygen desaturation events. We performed a sensitivity analysis to explore the effect of this heterogeneity on the overall results.
Two studies were supported by independent government grants (Campbell 2016; Langhan 2015). One study had a capnography device donated by a private medical manufacturing company (Deitch 2010).
Effects of interventions
See: Table 1
Primary outcomes
1. Oxygen desaturation
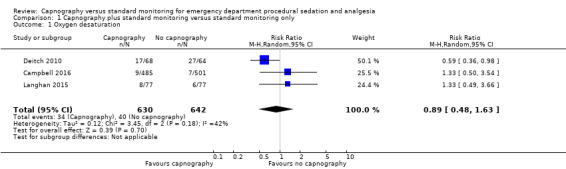
A total of 74 hypoxic events occurred in 1272 participants with an incidence of 33.3% in Deitch 2010, 9.1% in Langhan 2015, and 1.6% in Campbell 2016. The significantly higher incidence in Deitch 2010 likely represents the much more conservative definition of hypoxia used in this study. There was no significant difference in the rate of hypoxia between the intervention and control groups (RR 0.89, 95% CI 0.48 to 1.63; I2 = 42%) (Analysis 1.1; Figure 4). A sensitivity analysis to address heterogeneity was performed excluding one study with a different hypoxia definition (Deitch 2010). This proved to reduce heterogeneity but did not show a significant difference in the rate of hypoxia with the use of capnography (RR 1.33, 95% CI 0.66 to 2.69; I2 = 0%) (Figure 5). The quality of the evidence was downgraded to moderate quality due to heterogeneity between outcome definitions.
1.1. Analysis.
Comparison 1 Capnography plus standard monitoring versus standard monitoring only, Outcome 1 Oxygen desaturation.
4.
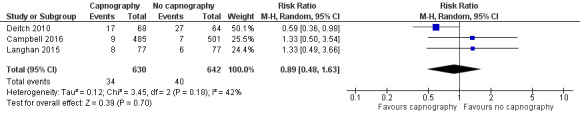
Forest plot of comparison: capnography plus standard monitoring versus standard monitoring, outcome: 1.1 oxygen desaturation.
5.
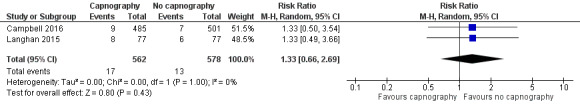
Forest plot of comparison: capnography plus standard monitoring) versus standard monitoring, outcome: 1.6 oxygen desaturation (sensitivity analysis based on definition of oxygen desaturation. Deitch 2010 excluded).
2. Hypotension
Only one study reported hypotension with a total of 23 events in 986 participants, an incidence of 2.3% (Campbell 2016). There was a trend of more hypotensive events in the capnography group compared to the control group that was not statistically significant (RR 2.36, 95% CI 0.98 to 5.69) (Analysis 1.2). One study stated in the methods section it would record hypotensive events but did not report on these findings in the results (Deitch 2010). Due to this reporting bias, the quality of evidence was downgraded to moderate.
1.2. Analysis.
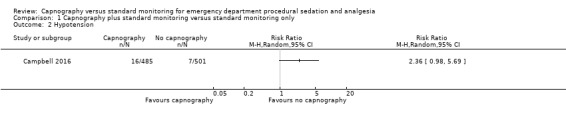
Comparison 1 Capnography plus standard monitoring versus standard monitoring only, Outcome 2 Hypotension.
3. Emesis, pulmonary aspiration
Only one study reported emesis outcomes with one event occurring in 986 participants, an incidence of 0.1% (Campbell 2016). There was no significant difference between the intervention and control groups (RR 3.10, 95% CI 0.13 to 75.88) (Analysis 1.3). Although one study stated in their methods that episodes of vomiting would be recorded, no such events were reported in the results section (Deitch 2010). Attempts to contact the authors to clarify this were unsuccessful. Due to this reporting bias, the quality of evidence was downgraded to moderate. None of the studies recorded pulmonary aspiration events.
1.3. Analysis.
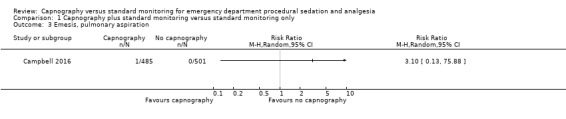
Comparison 1 Capnography plus standard monitoring versus standard monitoring only, Outcome 3 Emesis, pulmonary aspiration.
Secondary outcomes
1. Airway interventions
A total of 335 airway interventions were performed in 1272 participants, an incidence of 26.3%. In two studies, there were no interventions recorded involving ETI and only two interventions of PPV (Campbell 2016; Langhan 2015). One study did not report the specific interventions (Deitch 2010). There was no significant difference between the capnography and standard monitoring groups (RR 1.26, 95% CI 0.94 to 1.69) (Analysis 1.4). There was significant heterogeneity (I2 = 53%) and thus the quality of evidence was downgraded to moderate. A sensitivity analysis was performed using a fixed‐effect model resulting in a significant difference favouring the standard monitoring group (RR 1.31, 95% CI 1.09 to 1.58; I2 = 53%) with a number needed to treat for an additional harmful outcome (NNTH) of 14. We believe that due to the heterogeneity in outcome definitions between the studies, along with a high I2 value, that the random‐effects model was more appropriate for this outcome.
1.4. Analysis.
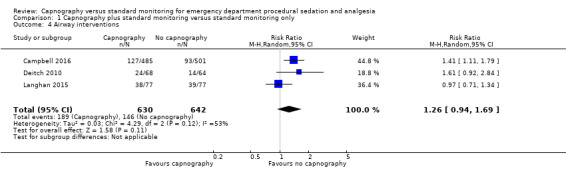
Comparison 1 Capnography plus standard monitoring versus standard monitoring only, Outcome 4 Airway interventions.
2. Recovery time
None of the included studies reported recovery time.
Subgroup analysis for oxygen desaturation
Age subgroup analysis did not reveal any significant differences between the control and intervention groups (Analysis 1.5).
1.5. Analysis.
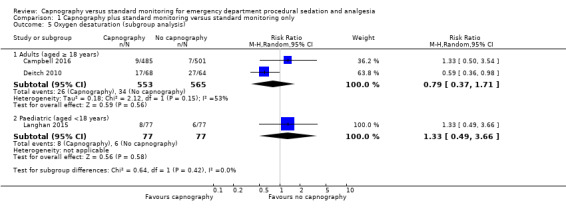
Comparison 1 Capnography plus standard monitoring versus standard monitoring only, Outcome 5 Oxygen desaturation (subgroup analysis).
Subgroup analysis for airway interventions
Analysis of the adults‐only group reduced heterogeneity and revealed a significant difference with respect to the use of airway interventions favouring the control group over the capnography group (RR 1.44, 95% CI 1.16 to 1.79; I2 = 0) with an NNTH of 12 (Figure 6). The paediatrics‐only group showed no significant difference between the intervention and control group (RR 0.97, 95% CI 0.71 to 1.34). The quality of the evidence was downgraded to moderate quality due to variance in the definition of airway interventions.
6.
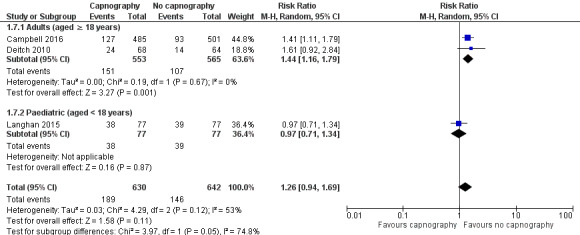
Forest plot of comparison: capnography plus standard monitoring versus standard monitoring, outcome: 1.7 airway interventions (subgroup analysis based on participant age).
Subgroup analysis of sedation agent used
None of the included studies recorded data for analysis.
Subgroup analysis of procedure performed
None of the included studies recorded data for analysis.
Inter‐observer agreement
There was complete agreement between both evaluators regarding article selection (Kappa statistic 1.0).
Discussion
Summary of main results
Primary and secondary outcomes
This review summarized the results of three studies comprising 1272 participants, demonstrating a lack of convincing evidence that the addition of capnography to standard monitoring for PSA in the ED affects the rate of oxygen desaturation or hypotension. Similarly, there was no significant difference in the rate of airway interventions across the three studies based on moderate quality evidence. There was only one recorded event of emesis, and no significant difference, in the one study that reported this outcome.
Capnography is an instrument, and like any tool, its effectiveness is heavily dependent upon the skill of the user. The reality of emergency medicine is that the variety of practitioners using capnography, from physicians to allied health providers, introduces inherent heterogeneity for any review in this subject area. Even if one were to only include studies where ED PSA was performed by physicians, the skill set and training of emergency physicians is highly variable not only from country to country but also within countries. Likewise, the variety of patient presentations and procedures performed in the ED also has associated heterogeneity. While some might argue that this heterogeneity precludes the conduction of a meta‐analysis, this is the very nature of emergency medicine and such reviews are important for both clinicians and policy decision‐makers. Although this review found no evidence supporting the use of capnography for ED PSA, these results should be viewed with caution; they may not be applicable to certain practitioners, for patients in an unstable condition, or for procedures known to have higher risk of adverse events. Considering this, we do not condemn the wholesale use of capnography, but rather find no evidence supporting the mandatory use of capnography for routine ED PSA at this point in time.
Varying definitions of oxygen desaturation accounted for one source of heterogeneity in this meta‐analysis. A sensitivity analysis excluding the one study that defined oxygen desaturation as less than 93% for 15 seconds eliminated heterogeneity but failed to produce a different conclusion than what is reported above (Deitch 2010). When the definition of an oxygen saturation of less than 90% was used, the event incidence was dramatically reduced from 33.3% (Deitch 2010), to 2.6% (Campbell 2016; Langhan 2015). The trend favouring the use of capnography was reversed to favour standard monitoring with this sensitivity analysis but did not reach significance (RR 1.33, 95% CI 0.66 to 2.69). The quality of the evidence was downgraded due to the varying outcome definitions.
The question of what constitutes a clinically significant level of oxygen saturation is difficult to answer. The partial pressure of oxygen in the blood decreases exponentially below 90% in accordance with the standard oxygen dissociation curve, the primary physiological reason we used this definition of hypoxia. This definition has been cited in other relevant studies in the literature (Anderson 2007; Campbell 2006; Deitch 2007; Hart 1997; Langhan 2012; Mallory 2011; Miner 2002; Wright 1992).
In the paediatric setting, there is no consensus on providing baseline supplemental oxygen for PSA. In fact, studies generally refer to supplemental oxygen as an adverse event. Despite this, the paediatric study (Langhan 2015) found no significant heterogeneity in the oxygen desaturation outcome compared to the two adult studies (Campbell 2016; Deitch 2010). There was heterogeneity shown in the secondary outcome of airway interventions. This result may be partially explained by pulse oximetry detecting respiratory depression earlier than capnography in patients breathing room air, a conclusion reached in multiple studies (Deitch 2008; Sivilotti 2010). A lower rate of respiratory depression events detected by capnography than the standard monitoring may have necessitated fewer airway interventions in these paediatric participants breathing room air.
As determined in one large case study of 979 adults, PSA in the ED setting without the use of capnography is a safe procedure with clinically significant complications of hypoxia (1%) and hypotension (0.8%) occurring very rarely (Campbell 2006). Using data from this meta‐analysis and assuming an adverse event rate ranging from 3% to 23%, anywhere from 1600 to nearly 10,000 participants would need to be enrolled in each arm of the study to show a 25% decrease in the rate of complications (Sealed 2012). The magnitude and costs of such a trial would likely be unfeasible and, as such, future studies showing a difference in complication rate is improbable. Likewise, in the paediatric ED population, one case study of 204 sedations showed incidences of hypoxia of 0.5% and airway interventions of 2.0% (Cudny 2013). There were no reports of PPV, ETI, hypotension, or aspiration.
This type of monitoring intervention study has inherent bias because of the inability to blind providers to the group assignment (after randomization). This exposes results to the John Henry Effect (Saretsky 1972); theoretically, providers may alter and improve their standard monitoring practice because they know they do not have the additional capnography tool. This may have biased results in favour of the control group. Likewise, providers may have acted according to the Hawthorne Effect (McCarney 2007), increasing quality of the standard monitoring secondary to observation only. Theoretically, this would affect both intervention groups and be unlikely to bias the results in a particular direction. It is impossible to design a study without these sources of bias and therefore the evidence is still deemed high quality.
We encountered some reporting bias from one study that resulted in downgrading the quality of the evidence in multiple outcomes (Deitch 2010). Data were stated to have been collected in the methods section (hypotension, emesis) but then not reported in the results section. With such a small meta‐analysis, this impacted our outcomes significantly. In future updates, we hope to continue to make attempts to contact the authors for unpublished data.
Subgroup analysis
The subgroup analysis based on participant age revealed a significant increase in the rate of airway interventions for adults in the capnography group, with an NNTH of 12. This significant difference was not shown in the paediatrics subgroup. One explanation accounting for this would be that the highly sensitive capnography detects respiratory depression early in patients on supplemental oxygen without an increase in detection of clinically significant oxygen desaturation, causing an increase in unnecessary interventions. A second theory would be that the use of capnography increased clinician confidence resulting in higher rate or dose (or both) of sedative administration.
It should be noted that the airway interventions recorded were largely not serious in nature, mostly comprising airway repositioning with no ETIs reported in any study. So while there was a statistically significant increase in the rate of airway interventions in the capnography group, the clinical significance of this is uncertain.
Overall completeness and applicability of evidence
This study identified and analysed three trials to address our review question. Despite the small number of studies, the number of participants was significant and reflected the population seen in modern western academic EDs. Significant sources of heterogeneity were largely explained by differences in outcome definitions and study populations (adults versus paediatric). Sensitivity analyses did not change the conclusion that there is a lack of evidence that capnography decreases the rate of major adverse events in all age groups, and in fact, it may increase unnecessary airway interventions in adult populations. Airway interventions were noted to be minor in nature but may lead to waking the patient and theoretically result in additional sedative administration in order to successfully finish the procedure.
The three studies included in this meta‐analysis were performed in busy North American academic centres by skilled, experienced clinicians. The applicability of the evidence to settings that PSA is infrequently performed or where training differs is uncertain and could be the subject of future trials.
Quality of the evidence
Overall quality of the three studies was moderate after accounting for inherent bias associated with interventional studies comparing monitoring modalities (performance bias). Variance in outcome definition contributed to qualitative heterogeneity and resulted in moderate quality evidence. There were too few studies to properly assess for publication bias. There may have been some degree of selective reporting bias in one of the studies when comparing methods to reported results for the secondary outcomes (Deitch 2010). One of the studies was not enrolled in a clinical trials registry and thus may have also been affected by selective reporting bias (Campbell 2016).
We assessed heterogeneity through sensitivity and subgroup analyses, with the major source deemed to be due to slight variations in outcome definitions. In future updates, we will make further attempts to contact authors for unpublished data.
Potential biases in the review process
Three co‐authors of this study (KDM, SGC, PJZ) were also authors of one of the studies included in this meta‐analysis (Campbell 2016). Another author (BFW) and an independent researcher (Anna MacDonald) extracted data from this study since neither were involved in the included study. This method was stated a priori (Wall 2013).
A decision was made to perform a sensitivity analysis to address heterogeneity in the oxygen desaturation outcome after it was discovered there was variance in the outcome definition. This possibility was outlined a priori in the previously published protocol to address sources of heterogeneity (Wall 2013).
The safety of using paramedics as sedation providers under the supervision of emergency physicians may be contested by some. The Campbell 2006 study, the largest observational trial studying complications in ED PSA, showed that outcomes were similar to when the providers were emergency physicians.
As mentioned in the 'Discussion', an inability to reach one of the author of an included study to collect unpublished data resulted in significant downgrades to the evidence quality.
Agreements and disagreements with other studies or reviews
We found no other systematic reviews studying capnography. Burton 2012, a short cut review, stated that "capnography may provide early warning of ventilatory changes that could result in hypoxia." This did not necessarily disagree with our findings but the conclusion was primarily based on one well‐designed RCT (Deitch 2010).
One clinical policy guideline stated Level B recommendation ("based on evidence from one or more class of evidence II studies or strong consensus of class of evidence III studies") that capnography may be used to detect hypoventilation and apnoea earlier than standard monitoring in ED patients undergoing PSA (Godwin 2014). The authors noted that serious adverse events were quite rare in this setting and that evidence was lacking showing that capnography reduced these complications. Conclusions were also based heavily on the findings of Deitch 2010 in addition to multiple non‐RCT reviews and RCTs performed in settings other than the ED.
These reviews were written before two of the studies included in this review were published. Their conclusions disagreed with our results and promoted the routine use of capnography for ED PSA but were based only on one RCT rather than the three included in this review.
Authors' conclusions
Implications for practice.
We have found no convincing evidence that capnography in addition to standard monitoring for patients undergoing procedural sedation and analgesia (PSA) in the emergency department (ED) decreases the rate of oxygen desaturation, hypotension, or vomiting, and may actually increase the rate of airway interventions in adults. These conclusions are based on moderate quality evidence.
Conclusions reached in this review are contrary to expert opinions in the emergency medicine field (Burton 2012; Godwin 2014; Mohr 2013). The impact of this review may prevent the additional costs of expensive equipment and training involved in capnography, and further reinforces evidence that PSA performed by skilled clinicians in the ED with standard monitoring is a safe procedure with rare adverse events.
Implications for research.
The incidence of serious adverse events in ED PSA is so rare that a single study powered to detect a significant difference would have to be quite large. Even if capnography was successfully shown to decrease rates of oxygen desaturation, we wonder as to the clinical significance of this theoretical result given the relative simple and safe solutions to correct hypoxia secondary to oversedation. When hypoxic episodes do occur, there are no corresponding increases in clinically relevant outcomes such as anoxic brain injury, aspiration, death, hospital admission, or unplanned endotracheal intubation (Green 2010).
There was a trend towards increased hypotensive events in the capnography groups. Further research to investigate the relationship between hypotensive episodes, airway interventions, and dose of drug given could be beneficial. For particularly high‐risk procedures or patients in an unstable condition undergoing PSA (or both), it is possible that capnography may be of more utility given the presumably higher incidence of adverse events. Studies in these populations would help delineate any benefits over standard monitoring alone.
Given that the included studies were performed in busy academic EDs by skilled PSA practitioners, further research involving smaller ED sites would be useful to apply conclusions more broadly.
In future updates of this review, we would like to address any reporting bias by continuing attempts to contact authors. For any forthcoming trials, we hope that more consensus and uniformity can be found among authors in defining outcomes such as oxygen desaturation. This would allow for a stronger meta‐analysis without having to seek unpublished data.
What's new
| Date | Event | Description |
|---|---|---|
| 3 January 2019 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Acknowledgements
We would like to thank Jane Cracknell (managing editor), Harald Herkner (content editor), Vibeke E Horstmann (statistical editor), Baruch Krauss, Teresa A Williams, Cheuk San Law (peer reviewers), and Janet Wale (consumer editor) for their help and editorial advice during the preparation of this systematic review.
Anna MacDonald helped with the task of data extraction for one study as an independent researcher (Campbell 2016).
We would also like to thank Melissa Langhan (included study author) for responding to our requests for unpublished data. Finally, we thank the Canadian Association of Emergency Physicians for the resident research grant that helped fund this study (see Sources of support).
Appendices
Appendix 1. CENTRAL (2016, Issue 8)
#1 MeSH descriptor: [Anesthesia, Intravenous] explode all trees
#2 MeSH descriptor: [Conscious Sedation] explode all trees
#3 MeSH descriptor: [Hypnotics and Sedatives] explode all trees
#4 anesthes* or ANAESTHES* or SEDATE* or SEDATION* or SEDATIVE* or HYPNOTIC*
#5 MeSH descriptor: [Capnography] explode all trees
#6 MeSH descriptor: [Respiratory Insufficiency] explode all trees
#7 MeSH descriptor: [Monitoring, Physiologic] explode all trees
#8 MeSH descriptor: [Anoxia] explode all trees
#9 MeSH descriptor: [Carbon Dioxide] explode all trees
#10 CAPNOGRAPH* or "Respiratory Insufficiency" or "respiratory failure" or ANOXIA* or HYPOXI* or "CARBON DIOXIDE"
#11 MeSH descriptor: [Emergency Medical Services] explode all trees
#12 MeSH descriptor: [Emergency Service, Hospital] explode all trees
#13 EMERGENC* or casualt*
#14 (#1 or #2 or #3 or #4)
#15 (#5 or #6 or #7 or #8 or #9 or #10)
#16 (#11 or #12 or #13)
#17 (#14 and #15 and #16)
Appendix 2. MEDLINE (via PubMed) (1980 to 9 August 2016)
1. "Anesthesia, Intravenous"[Mesh]
2. "Conscious Sedation"[Mesh]
3. "Hypnotics and Sedatives"[Mesh]
4. (anesthes*[TIAB] OR ANAESTHES*[TIAB] OR SEDATE*[TIAB] OR SEDATION*[TIAB] OR SEDATIVE*[TIAB] OR HYPNOTIC*[TIAB])
5. "Capnography"[Mesh]
6. "Respiratory Insufficiency"[Mesh]
7. "Monitoring, Physiologic"[Mesh]
8. "Anoxia"[Mesh]
9. "Carbon Dioxide"[Mesh])
10. (CAPNOGRAPH*[TIAB] OR "Respiratory Insufficiency"[TIAB] OR "respiratory failure"[tiab] OR ANOXIA*[TIAB] OR HYPOXI*[TIAB] OR "CARBON DIOXIDE"[TIAB])
11. "Emergency Medical Services"[Mesh]
12. "Emergency Service, Hospital"[Mesh]
13. (EMERGENC*[TIAB]) OR (casualt*[TIAB])
14. 1 or 2 or 3 or 4
15. 5 or 6 or 7 or 8 or 9 or 10
16. 11 or 12 or 13
17. 14 and 15 and 16
Appendix 3. Embase (via Ovid) (1980 to 9 August 2016)
1. exp intravenous anesthesia/
2. exp conscious sedation/
3. exp hypnotic sedative agent/
4. (anesthes* or anaesthes* or sedate* or sedation* or sedative* or hypnotic*).ab,ti.
5. exp capnography/
6. exp respiratory failure/
7. exp patient monitoring/
8. exp anoxia/
9. exp carbon dioxide/
10. (capnograph* or 'respiratory insufficiency' or anoxia* or hypoxi* or 'carbon dioxide' or 'respiratory failure').ab,ti.
11. exp emergency health service/ or (emergenc* or casualt*).ab,ti.
12. 1 or 2 or 3 or 4
13. 5 or 6 or 7 or 8 or 9 or 10
14. 11 and 12 and 13
Appendix 4. CINAHL (via EBSCOhost) (1980 to August 2016)
S1. (MH "Anesthesia, Intravenous")
S2. (MH "Hypnotics and Sedatives+")
S3. (MH "Conscious Sedation")
S4. (AB anesthes* OR TI anesthes* OR AB ANAESTHES* OR TI ANAESTHES* OR AB SEDATE* OR TI SEDATE* OR AB SEDATION* OR TI SEDATION* OR AB SEDATIVE* OR TI SEDATIVE* OR AB HYPNOTIC* OR TI HYPNOTIC*)
S5. (MH "Capnography")
S6. (MH "Respiratory Failure+")
S7. (MH "Monitoring, Physiologic+")
S8. (MH "Anoxia+")
S9. (MH "Carbon Dioxide")
S10. (TI CAPNOGRAPH* OR AB CAPNOGRAPH* OR TI "Respiratory Insufficiency" OR AB "Respiratory Insufficiency" OR TI "respiratory failure" OR AB "respiratory failure" OR TI ANOXIA* OR AB ANOXIA* OR TI HYPOXI* OR AB HYPOXI* OR TI "CARBON DIOXIDE" OR AB "CARBON DIOXIDE")
S11. (MH "Emergency Medical Services+")
S12. (MH "Emergency Service+")
S13. (TI casualt* OR AB casualt* OR TI EMERGENC* OR AB EMERGENC*)
S14. (S1 or S2 or S3 or S4)
S15. (S5 or S6 or S7 or S8 or S9 or S10)
S16. (S11 or S12 or S13)
S17. (S14 and S15 and S16)
Appendix 5. Article inclusion criteria form
THE USE OF CAPNOGRAPHY IN EMERGENCY DEPARTMENT PROCEDURAL SEDATION AND ANALGESIA: A SYSTEMATIC REVIEW CRITERIA FOR INCLUSION
Citation # ____________
Reviewer: BFW KDM
Please assess the following questions for each paper. WHEN YOU OBTAIN ONE X (NOT INCLUDED) STOP. The inclusion criteria are:
[1] DESIGN
[ ] Randomized controlled clinical trial OR Quazi randomized controlled clinical trial
[ ] Exclude all studies which are non‐experimental (cohort study, case‐control study, before‐after studies, case series, letters, reviews, etc.).
[2] POPULATIONS
[ ] Include if patients were selected due to undergoing PSA in an Emergency Department.
[ ] Exclude papers where the patients were classified as: inpatients, day surgery patients, or endoscopy suite patients.
[3] INTERVENTIONS
[ ] Include all primary research in which patients were monitored with capnography and standard monitoring (BP cuff, oxygen saturation, cardiac monitoring) versus standard monitoring only.
[ ] Exclude if capnography was not the primary research question.
[4] OUTCOMES
[ ] Must have clinically relative outcomes (i.e. airway intervention required, hypotension, oxygen desaturation, CO2 levels).
[ ] Exclude all studies that do not report clinically relevant outcomes.
[5] FINAL DECISION
[ ] INCLUDED (meets inclusion criteria above)
[ ] NOT INCLUDED
[ ] CAN'T TELL (need more information from authors to make decision
Appendix 6. Studies meeting inclusion criteria
| Unique ID | Study ID (Lead Author, year) | PMID | Source (Journal, conference, etc) |
| 1 | |||
| 2 | |||
| 3 |
Appendix 7. Data extraction form
| Review title or ID |
| Study ID(surname of first author and year first full report of study was published e.g. Smith 2001) |
| Report IDs of other reports of this study(e.g. duplicate publications, follow‐up studies) |
|
Notes: |
1. General Information
| Date form completed(dd/mm/yyyy) | |
| Name/ID of person extracting data | |
|
Report title (title of paper/ abstract/ report that data are extracted from) |
|
|
Report ID (ID for this paper/ abstract/ report) |
|
| Reference details | |
| Report author contact details | |
|
Publication type (e.g. full report, abstract, letter) |
|
|
Study funding sources (including role of funders) |
|
|
Possible conflicts of interest (for study authors) |
|
| Notes: | |
3. Population and setting
|
Description Include comparative information for each group (i.e. intervention and controls) if available |
Location in text (pg/fig/table) |
||
|
Population description (from which study participants are drawn) |
|||
|
Setting (including location and social context) |
|||
| Inclusion criteria | |||
| Exclusion criteria | |||
| Method of recruitment of participants | |||
| Informed consent obtained | Yes No Unclear | ||
| Notes: | |||
4. Methods
|
Descriptions as stated in report/paper |
Location in text (pg/fig/table) |
||
| Aim of study | |||
| Design(e.g. parallel, crossover, cluster) | |||
|
Unit of allocation (by individuals, cluster/ groups or body parts) |
|||
| Start date | |||
| End date | |||
| Total study duration | |||
| Ethical approval needed/ obtained for study | Yes No Unclear | ||
| Notes: | |||
6. Participants
Provide overall data and, if available, comparative data for each intervention or comparison group.
|
Description as stated in report/paper |
Location in text (pg/fig/table) |
|
|
Total no. randomized (or total pop. at start of study for NRCTs) |
||
|
Clusters (if applicable, no., type, no. people per cluster) |
||
| Baseline imbalances | ||
|
Withdrawals and exclusions (if not provided below by outcome) |
||
| Age | ||
| Sex | ||
| Race/Ethnicity | ||
| Severity of illness | ||
| Co‐morbidities | ||
| Other treatment received(additional to study intervention) | ||
| Other relevant sociodemographics | ||
| Subgroups measured | ||
| Subgroups reported | ||
| Notes: | ||
7. Intervention groups
Copy and paste table for each intervention and comparison group
Intervention Group 1
|
Description as stated in report/paper |
Location in text (pg/fig/table) |
|
| Group name | ||
|
No. randomized to group (specify whether no. people or clusters) |
||
| Theoretical basis(include key references) | ||
| Description(include sufficient detail for replication, e.g. content, dose, components) | ||
| Duration of treatment period | ||
| Timing(e.g. frequency, duration of each episode) | ||
| Delivery(e.g. mechanism, medium, intensity, fidelity) | ||
|
Providers (e.g. no., profession, training, ethnicity etc. if relevant) |
||
| Co‐interventions | ||
| Economic variables (i.e. intervention cost, changes in other costs as result of intervention) | ||
|
Resource requirements to replicate intervention (e.g. staff numbers, cold chain, equipment) |
||
| Notes: | ||
8. Outcomes
Oxygen Desaturation
|
Description as stated in report/paper |
Location in text (pg/fig/table) |
||
| Outcome name | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear | ||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
| Notes: | |||
Hypotension
|
Description as stated in report/paper |
Location in text (pg/fig/table) |
||
| Outcome name | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear | ||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
| Notes: | |||
Airway Intervention Performed
|
Description as stated in report/paper |
Location in text (pg/fig/table) |
||
| Outcome name | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear | ||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
| Notes: | |||
Emesis/Aspiration
|
Description as stated in report/paper |
Location in text (pg/fig/table) |
||
| Outcome name | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear | ||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
| Notes: | |||
Recovery Time
|
Description as stated in report/paper |
Location in text (pg/fig/table) |
||
| Outcome name | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear | ||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
| Notes: | |||
9. Results
Copy and paste the appropriate table for each outcome, including additional tables for each time point and subgroup as required.
Dichotomous outcome
|
Description as stated in report/paper |
Location in text (pg/fig/table) |
|||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Timepoint (specify whether from start or end of intervention) | ||||||
| Results | Intervention | Comparison | ||||
| No. events | No. participants | No. events | No. participants | |||
| No. missing participants and reasons | ||||||
| No. participants moved from other group and reasons | ||||||
| Any other results reported | ||||||
| Unit of analysis(by individuals, cluster/groups or body parts) | ||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||
| Reanalysis required?(specify) | Yes No Unclear | |||||
| Reanalysis possible? | Yes No Unclear | |||||
| Reanalysed results | ||||||
| Notes: | ||||||
Continuous outcome
|
Description as stated in report/paper |
Location in text (pg/fig/table) |
|||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Timepoint (specify whether from start or end of intervention) | ||||||||||
| Post‐intervention or change from baseline? | ||||||||||
| Result | Intervention | Comparison | ||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||
| No. missing participants and reasons | ||||||||||
| No. participants moved from other group and reasons | ||||||||||
| Any other results reported | ||||||||||
|
Unit of analysis (individuals, cluster/ groups or body parts) |
||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Reanalysis required?(specify) | Yes No Unclear | |||||||||
| Reanalysis possible? | Yes No Unclear | |||||||||
| Reanalysed results | ||||||||||
| Notes: | ||||||||||
Other outcome
|
Description as stated in report/paper |
Location in text (pg/fig/table) |
|||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Timepoint (specify whether from start or end of intervention) | ||||||
| Results | Intervention result | SD (or other variance) | Control result | SD (or other variance) | ||
| Overall results | SE (or other variance) | |||||
| No. participants | Intervention | Control | ||||
| No. missing participants and reasons | ||||||
| No. participants moved from other group and reasons | ||||||
| Any other results reported | ||||||
| Unit of analysis(by individuals, cluster/groups or body parts) | ||||||
| Statistical methods used and appropriateness of these methods | ||||||
| Reanalysis required?(specify) | Yes No Unclear | |||||
| Reanalysis possible? | Yes No Unclear | |||||
| Reanalysed results | ||||||
| Notes: | ||||||
10. Applicability
| Have important populations been excluded from the study?(consider disadvantaged populations, and possible differences in the intervention effect) | Yes No Unclear |
|
| Is the intervention likely to be aimed at disadvantaged groups?(e.g. lower socioeconomic groups) | Yes No Unclear |
|
|
Does the study directly address the review question? (any issues of partial or indirect applicability) |
Yes No Unclear |
|
| Notes: | ||
11. Other information
|
Description as stated in report/paper |
Location in text (pg/fig/table) |
|
| Key conclusions of study authors | ||
| References to other relevant studies | ||
| Correspondence required for further study information(from whom, what and when) | ||
| Notes: | ||
Appendix 8. Risk of bias assessment
| Domain | Risk of bias |
Support for judgement |
Location in text (pg/fig/table) |
||
| Low risk | High risk | Unclear | |||
|
Random sequence generation (selection bias) |
|||||
|
Allocation concealment (selection bias) |
|||||
|
Blinding of participants and personnel (performance bias) |
Outcome group: All/ |
||||
|
Blinding of outcome assessment (detection bias) |
Outcome group: All/ |
||||
|
Incomplete outcome data (attrition bias) |
|||||
|
Selective outcome reporting? (reporting bias) |
|||||
| Other bias | |||||
| Notes: | |||||
Data and analyses
Comparison 1. Capnography plus standard monitoring versus standard monitoring only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oxygen desaturation | 3 | 1272 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.48, 1.63] |
| 2 Hypotension | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Emesis, pulmonary aspiration | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Airway interventions | 3 | 1272 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.94, 1.69] |
| 5 Oxygen desaturation (subgroup analysis) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Adults (aged ≥ 18 years) | 2 | 1118 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.37, 1.71] |
| 5.2 Paediatric (aged <18 years) | 1 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.49, 3.66] |
| 6 Oxygen desaturation (sensitivity analysis), Deitch 2010 excluded | 2 | 1140 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.66, 2.69] |
| 7 Airway interventions (subgroup analysis) | 3 | 1272 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.94, 1.69] |
| 7.1 Adults (aged ≥ 18 years) | 2 | 1118 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.16, 1.79] |
| 7.2 Paediatric (aged < 18 years) | 1 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.71, 1.34] |
1.6. Analysis.
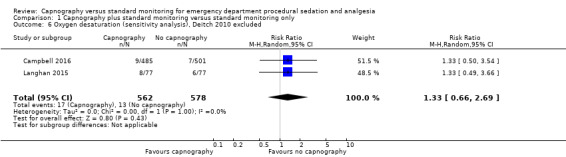
Comparison 1 Capnography plus standard monitoring versus standard monitoring only, Outcome 6 Oxygen desaturation (sensitivity analysis), Deitch 2010 excluded.
1.7. Analysis.
Comparison 1 Capnography plus standard monitoring versus standard monitoring only, Outcome 7 Airway interventions (subgroup analysis).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Campbell 2016.
| Methods |
Design: prospective, RCT Blinding: research associates blinded, participants and personnel unblinded, allocation concealed Dates: April 2006 to April 2010 |
|
| Participants |
n = 986 Included: adults (aged > 16 years) requiring PSA Excluded: critically ill people or people unable to give consent Location: emergency department, tertiary care university hospital, Canada |
|
| Interventions |
Control (n = 501) Standard monitoring (cardiac monitoring, blood pressure monitoring, pulse oximetry) Intervention (n = 485) Standard monitoring (as above) and end‐tidal capnography (measured every 5 seconds or absence of waveform at any time) |
|
| Outcomes |
Primary Oxygen desaturation (< 90% SpO2 for > 30 seconds) Airway interventions (airway repositioning manoeuvre, positive pressure ventilation, oral/nasal airway placement, endotracheal intubation)* Secondary Hypotension (SBP < 100 mmHg or < 85 mmHg if baseline < 100 mmHg) Sedation time (time from first dose of drug administration to commencement of procedure Recovery time (time from end of procedure to cessation of monitoring) Vomiting |
|
| Funding sources | Research grant from government organization (Capital Health Research Fund, Halifax, Nova Scotia, Canada) | |
| Declarations of interest | No authors declared any actual or potential conflicts of interest. | |
| Notes | * Published data for airway interventions was continuous. Dichotomous data obtained by contacting authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned to each group by research associates (blinded) using computer‐generated randomization. |
| Allocation concealment (selection bias) | Low risk | Allocation concealed using opaque white envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No data excluded, all data included in analysis. |
| Selective reporting (reporting bias) | Unclear risk | All participants accounted for. Study was not enrolled in a trial registry and thus modifications to the study methods could not be assessed. |
| Other bias | Low risk | Funding: supported by an independent government grant. |
Deitch 2010.
| Methods |
Design: prospective RCT Blinding: research associates blinded, participants and personnel unblinded, allocation concealed Dates: November 2006 to February 2008 |
|
| Participants |
n = 132 Included: adults (aged > 18 years) requiring PSA Excluded: severe chronic obstructive pulmonary disease; chronic oxygen requirements; haemodynamic instability; respiratory distress; pregnancy; inability to provide informed consent; allergy to propofol, morphine, or fentanyl (or other components of its formulation); or if, in the judgement of the attending emergency physician, procedural sedation could compromise participant safety; critically ill patients or patients unable to give consent Location: emergency department, tertiary care university hospital, US |
|
| Interventions |
Control (n = 64) Standard monitoring (cardiac monitoring, pulse oximetry, and blood pressure monitoring) and blinded capnography Intervention (n = 68) Standard monitoring and capnography (measured every 5 seconds with waveform, respiratory depression defined as > 50 mmHg, absolute increase or decrease from baseline of > 10%, or loss of waveform for > 15 seconds)* |
|
| Outcomes |
Primary Hypoxia defined as < 93% SpO2 for > 15 seconds Secondary Airway Interventions (verbal or physical stimulation; airway realignment; use of additional oxygen; and use of airway adjuncts, assisted ventilation, or intubation) Hypotension (undefined, not reported in results) Vomiting (not reported in results) |
|
| Funding sources | Capnography equipment donated by private company (Oridian Medical, Needham, MA) | |
| Declarations of interest | No authors declared any actual or potential conflicts of interest | |
| Notes | * Disqualified graphs if > 35% data loss | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned to each group by research associates (blinded) using a computer‐generated randomization list. |
| Allocation concealment (selection bias) | Low risk | Research associates and treating physicians were blinded to the randomization choice until after enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although all participants had capnography, only the participants/personnel in the capnography group could see the capnography monitor, whereas the standard group knew that they could not see the capnography monitor. Therefore, this was not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators evaluating graphs for outcome measures were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Participant data excluded if 35% data loss. This resulted in 18/150 (12%) participants excluded. |
| Selective reporting (reporting bias) | High risk | Primary outcome data all accounted for outside the above exclusions. Secondary outcomes listed including vomiting and hypotension were mentioned in methods section but not reported on in results. The specific airway interventions were not reported (only total number of interventions) despite the methods section stating these data were collected. |
| Other bias | Unclear risk | Oxygen desaturation outcome defined as SpO2 < 93%. We were unable to reach author for unpublished data regarding this outcome. Funding: a device performing capnography was donated by a private medical manufacturing company. |
Langhan 2015.
| Methods |
Design: prospective RCT Blinding: research associates unblinded, participants and personnel unblinded, allocation concealed Dates: September 2011 to January 2013. |
|
| Participants |
n = 154 Included: paediatric participants (aged 1 to 20 years) requiring PSA Excluded: intubation, administration of baseline supplemental oxygen without preceding hypoxaemia, and conditions associated with abnormal ETCO2 values such as lower airway disease (e.g. asthma), diabetic ketoacidosis, moderate to severe dehydration, and major trauma. Participants were excluded if they did not tolerate the capnography cannula or if the participant cried for > 20% of the sedation Location: paediatric emergency department, tertiary care university hospital, US |
|
| Interventions |
Control (n = 77) Standard monitoring (cardiac monitoring, pulse oximetry, and blood pressure monitoring) and blinded capnography Intervention (n = 77) Standard monitoring and capnography (alerts at ETCO2 levels of < 30 mmHg and > 50 mmHg) |
|
| Outcomes |
Primary Oxygen desaturation (SpO2 < 95%) Airway interventions (verbal or physical stimulation, bag‐valve mask ventilation, jaw thrust, head tilt, use of a shoulder roll, supplemental oxygen, or reversal agents)* Secondary Oxygen desaturation (SpO2 < 90%)** |
|
| Funding sources | Research grant from government organization (National Center for Advancing Translational Science, components of the National Institutes of Health) | |
| Declarations of interest | Not stated in publication | |
| Notes | *Airway interventions: verbal or physical stimulation, bag‐valve mask ventilation, airway repositioning (jaw thrust or head tilt), use of a shoulder roll, supplemental oxygen or reversal agents **Primary outcome was SpO2 < 95%. Secondary outcome SpO2 < 90% obtained by contacting author directly. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A statistician provided blocked randomization using Proc Plan (Sas 9.2, Cary, NC), with a 7‐digit random seed; group assignments were allocated to participants in a random sequence within blocks of 6. |
| Allocation concealment (selection bias) | Low risk | Allocation concealed with sealed, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel not blinded to capnography screens. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Research associates during procedures not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outside of a priori defined exclusion criteria, all data included. |
| Selective reporting (reporting bias) | Low risk | All participant outcomes accounted for upon contacting author directly. |
| Other bias | Low risk | Unpublished data for the oxygen desaturation outcome was obtained from author. Funding: supported by an independent government grant. |
ETCO2: end‐tidal carbon dioxide partial pressure; n: number of participants; PSA: procedural sedation and analgesia; RCT: randomized controlled trial; SBP: systolic blood pressure; SpO2: blood oxygen saturation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Deitch 2007 | Not relevant interventions: supplemental oxygen vs compressed air for midazolam and fentanyl PSA. Retrospectively analysed blinded capnography data. |
| Deitch 2008 | Not relevant interventions: supplemental oxygen vs compressed air for propofol PSA. Retrospectively analysed blinded capnography data. |
| Hart 1997 | Non‐relevant interventions: RCT comparing fentanyl vs fentanyl‐midazolam vs meperidine‐promethazine‐chlorpromethazine compound as sedation drugs for PSA. |
| Sivilotti 2010 | Non‐relevant interventions: RCT comparing sedation drugs for PSA then retrospectively analysed for adverse events and utility of capnography. |
PSA: procedural sedation and analgesia; RCT: randomized controlled trial.
Differences between protocol and review
We made the following changes to the published protocol (Wall 2013).
An explanation for the definition of the primary outcome oxygen desaturation was added to the 'Types of outcome measures' section.
The description in the 'Objectives' section was simplified to conform to Cochrane standards but no prespecified primary or secondary objectives were changed.
Contributions of authors
Brian F Wall (BFW), Kirk Magee (KDM), Samuel G Campbell (SGC), Peter J Zed (PJZ)
Conceiving the review: BFW, KDM, PJZ.
Co‐ordinating the review: BFW, KDM.
Undertaking manual searches: BFW, KDM.
Screening search results: BFW, KDM.
Organizing retrieval of papers: BFW.
Screening retrieved papers against inclusion criteria: BFW, KDM, SGC.
Appraising quality of papers: BFW, KDM.
Abstracting data from papers: BFW, KDM.
Writing to authors of papers for additional information: BFW.
Providing additional data about papers: BFW.
Obtaining and screening data on unpublished studies: BFW.
Data management for the review: BFW, KDM.
Entering data into Review Manager 5 (RevMan 2014): BFW, KDM.
RevMan statistical data: BFW, KDM.
Other statistical analysis not using RevMan: BFW, KDM.
Interpretation of data: BFW, KDM, PJZ.
Statistical inferences: BFW, KDM, PJZ.
Writing the review: BFW, KDM, PJZ.
Securing funding for the review: BFW, KDM.
Performing previous work that was the foundation of the present study: SGC, PJZ.
Guarantor for the review: BFW.
Person responsible for reading and checking review before submission: KDM.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Canadian Association of Emergency Physicians (CAEP) Research Grant, Canada.
A national grant competition was won providing financial support to pay for statistician services and reference software.
Declarations of interest
Brian F Wall: none known.
Kirk Magee: was a listed author of one of the included studies in this review (Campbell 2016).
Samuel G Campbell: was a listed author of one of the included studies in this review (Campbell 2016).
Peter J Zed: was a listed author of one of the included studies in this review (Campbell 2016).
Edited (no change to conclusions)
References
References to studies included in this review
Campbell 2016 {published and unpublished data}
- Campbell SG, Magee KD, Zed PJ, Froese P, Etsell G, LaPierre A, et al. End‐tidal capnometry during emergency department procedural sedation and analgesia: a randomized trial. World Journal of Emergency Medicine 2016;7(1):13‐8. [PUBMED: 27006732] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deitch 2010 {published data only}
- Deitch K, Miner J, Chudnofsky CR, Dominici P, Latta D. Does end tidal CO2 monitoring during emergency department procedural sedation and analgesia with propofol decrease the incidence of hypoxic events? A randomized, controlled trial. Annals of Emergency Medicine 2010;5(3):258‐64. [DOI: 10.1016/j.annemergmed.2009.07.030; PUBMED: 19783324] [DOI] [PubMed] [Google Scholar]
Langhan 2015 {published data only}
- Langhan ML, Shabanova V, Li FY, Bernstein SL, Shapiro ED. A randomized controlled trial of capnography during sedation in a pediatric emergency setting. American Journal of Emergency Medicine 2015;33(1):25‐30. [PUBMED: 25445871] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Deitch 2007 {published data only}
- Deitch K, Chudnofsky CR, Dominici P. The utility of supplemental oxygen during emergency department procedural sedation and analgesia with midazolam and fentanyl: a randomized, controlled trial. Annals of Emergency Medicine 2007;49(1):1‐8. [PUBMED: 16978741] [DOI] [PubMed] [Google Scholar]
Deitch 2008 {published data only}
- Deitch K, Chudnofsky CR, Dominici P. The utility of supplemental oxygen during emergency department procedural sedation with propofol: a randomized, controlled trial. Annals of Emergency Medicine 2008;52(1):1‐8. [PUBMED: 18294729] [DOI] [PubMed] [Google Scholar]
Hart 1997 {published data only}
- Hart LS, Berns SD, Houck CS, Boenning DA. The value of end‐tidal CO2 monitoring when comparing three methods of conscious sedation for children undergoing painful procedures in the emergency department. Pediatric Emergency Care 1997;13(3):189‐93. [PUBMED: 9220504] [DOI] [PubMed] [Google Scholar]
Sivilotti 2010 {published data only}
- Sivilotti MLA, Messenger DW, Vlymen J, Dungey PE, Murray HE. A comparative evaluation of capnometry versus pulse oximetry during procedural sedation and analgesia on room air. Canadian Journal of Emergency Medicine 2010;12(5):397‐404. [PUBMED: 20880431] [DOI] [PubMed] [Google Scholar]
Additional references
Anderson 2007
- Anderson JL, Junkins E, Pribble C, Guenther E. Capnography and depth of sedation during propofol sedation in children. Annals of Emergency Medicine 2007;49:9‐13. [DOI] [PubMed] [Google Scholar]
Burton 2006
- Burton JH, Harrah JD, Germann CA, Dillon DC. Does end‐tidal carbon dioxide monitoring detect respiratory events prior to current sedation monitoring practices?. Academic Emergency Medicine 2006;13(5):500‐4. [DOI] [PubMed] [Google Scholar]
Burton 2012
- Burton F. Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. BET 2: should capnography be routinely used during procedural sedation in the emergency department?. Emergency Medicine Journal 2012;29:164‐6. [DOI] [PubMed] [Google Scholar]
Campbell 2006
- Campbell SG, Magee KD, Kovacs GJ, Petrie DA, Tallon JM, McKinley R, et al. Procedural sedation and analgesia in a Canadian adult tertiary care emergency department: a case series. Canadian Journal of Emergency Medicine 2006;8(2):85‐93. [DOI] [PubMed] [Google Scholar]
Cudny 2013
- Cudny M, Wang N, Bardas S, Nguyen C. Adverse events associated with procedural sedation in pediatric patients in the emergency department. Hospital Pharmacy 2013;48(2):134‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Phillips AN. Meta‐analysis: principles and procedures. BMJ 1997;315:1533‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
EndNote X7.4 [Computer program]
- Thomson Reuters. EndNote X7.4. Thomson Reuters, 2015.
Godwin 2005
- Godwin SA, Caro DA, Wolf SJ, Jagoda AS, Charles R, Marett BE, et al. American College of Emergency Physicians. Clinical policy: procedural sedation and analgesia in the emergency department. Annals of Emergency Medicine 2005;45:177‐96. [DOI] [PubMed] [Google Scholar]
Godwin 2014
- Godwin S, Burton J, Gerardo C, Hatten B, Mace S, Silvers S, et al. Clinical Policy: Procedural Sedation and Analgesia in the Emergency Department. Annals of Emergency Medicine 2014;63:247‐58. [DOI] [PubMed] [Google Scholar]
Green 2010
- Green SM, Pershad J. Should capnographic monitoring be standard practice during emergency department procedural sedation and analgesia? Pro and con. Annals of Emergency Medicine 2010;55:265‐7. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians. BMJ 2008;336:995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kodali 2013
- Kodali BS. Capnography outside the operating rooms. Anesthesiology 2013;118:192‐201. [DOI] [PubMed] [Google Scholar]
Krauss 2007
- Krauss B, Hess DR. Capnography for procedural sedation and analgesia in the emergency department. Annals of Emergency Medicine 2007;50:172‐81. [PUBMED: 17222941 ] [DOI] [PubMed] [Google Scholar]
Langhan 2012
- Langhan ML, Mallory M, Hertzog J, Lowrie L, Cravero J. Physiologic monitoring practices during pediatric procedural sedation. A report from the Pediatric Sedation Research Consortium. Archives of Pediatrics & Adolescent Medicine 2012;166:990‐8. [DOI] [PubMed] [Google Scholar]
Mallory 2011
- Mallory MD, Baxter AL, Yanosky DJ, Cravero JP. Emergency physician administered propofol sedation: a report on 25,433 sedations from the Pediatric Sedation Research Consortium. Annals of Emergency Medicine 2011;57:462‐8. [DOI] [PubMed] [Google Scholar]
McCarney 2007
- McCarney R, Warner J, Iliffe S, Haselen R, Griffin M, Fisher P. The Hawthorne Effect: a randomised, controlled trial. BMC Medical Research Methodology 2007;7(30):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miner 2002
- Miner JR, Heegaard W, Plummer D. End‐tidal carbon dioxide monitoring during procedural sedation. Academic Emergency Medicine 2002;9:275‐80. [DOI] [PubMed] [Google Scholar]
Miner 2003
- Miner JR, Biros M, Krieg S, Johnson C, Heegaard W, Plummer D. Randomized clinical trial of propofol versus methohexital for procedural sedation in the emergency department. Academic Emergency Medicine 2003;10:931‐7. [DOI] [PubMed] [Google Scholar]
Mohr 2013
- Mohr NM, Wessman B. Routine capnography in procedural sedation. Annals of Emergency Medicine 2013;61:697‐8. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Saretsky 1972
- Saretsky G. The OEO P.C. Experiment and the John Henry Effect. The Phi Delta Kappan 1972;53(9):579‐81. [Google Scholar]
Sealed 2012
- Sealed Envelope Ltd. 2012. Power calculator for binary outcome superiority trial. [Online]. www.sealedenvelope.com/power/binary‐superiority/ (last accessed 15 June 2016).
Swanson 1996
- Swanson E, Seaberg DC, Mathias S. The use of propofol for sedation in the emergency department. Academic Emergency Medicine 1996;3:234‐8. [DOI] [PubMed] [Google Scholar]
Wright 1992
- Wright SW. Conscious sedation in the emergency department: the value of capnography and pulse oximetry. Annals of Emergency Medicine 1992;21:551‐5. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Wall 2013
- Wall BF, Magee K, Campbell SG, Zed PJ. Capnography versus standard monitoring for emergency department procedural sedation and analgesia. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD010698] [DOI] [PMC free article] [PubMed] [Google Scholar]


