Abstract
Background
Traumatic hyphema is the entry of blood into the anterior chamber (the space between the cornea and iris) subsequent to a blow or a projectile striking the eye. Hyphema uncommonly causes permanent loss of vision. Associated trauma (e.g. corneal staining, traumatic cataract, angle recession glaucoma, optic atrophy, etc.) may seriously affect vision. Such complications can lead to permanent impairment of vision. People with sickle cell trait/disease may be particularly susceptible to increases of elevated intraocular pressure. If rebleeding occurs, the rates and severity of complications increase.
Objectives
To assess the effectiveness of various medical interventions in the management of traumatic hyphema.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) (2018, Issue 6); MEDLINE Ovid; Embase.com; PubMed (1948 to June 2018); the ISRCTN registry; ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP). The date of the search was 28 June 2018.
Selection criteria
Two review authors independently assessed the titles and abstracts of all reports identified by the electronic and manual searches. In this review, we included randomized and quasi‐randomized trials that compared various medical (non‐surgical) interventions versus other medical intervention or control groups for the treatment of traumatic hyphema following closed‐globe trauma. We applied no restrictions regarding age, gender, severity of the closed‐globe trauma, or level of visual acuity at the time of enrollment.
Data collection and analysis
Two review authors independently extracted the data for the primary outcomes, visual acuity and time to resolution of primary hemorrhage, and secondary outcomes including: secondary hemorrhage and time to rebleed; risk of corneal blood staining, glaucoma or elevated intraocular pressure, optic atrophy, or peripheral anterior synechiae; adverse events; and duration of hospitalization. We entered and analyzed data using Review Manager 5. We performed meta‐analyses using a fixed‐effect model and reported dichotomous outcomes as risk ratios (RR) and continuous outcomes as mean differences (MD).
Main results
We included 20 randomized and seven quasi‐randomized studies with a total of 2643 participants. Interventions included antifibrinolytic agents (systemic and topical aminocaproic acid, tranexamic acid, and aminomethylbenzoic acid), corticosteroids (systemic and topical), cycloplegics, miotics, aspirin, conjugated estrogens, traditional Chinese medicine, monocular versus bilateral patching, elevation of the head, and bed rest.
We found no evidence of an effect on visual acuity for any intervention, whether measured within two weeks (short term) or for longer periods. In a meta‐analysis of two trials, we found no evidence of an effect of aminocaproic acid on long‐term visual acuity (RR 1.03, 95% confidence interval (CI) 0.82 to 1.29) or final visual acuity measured up to three years after the hyphema (RR 1.05, 95% CI 0.93 to 1.18). Eight trials evaluated the effects of various interventions on short‐term visual acuity; none of these interventions was measured in more than one trial. No intervention showed a statistically significant effect (RRs ranged from 0.75 to 1.10). Similarly, visual acuity measured for longer periods in four trials evaluating different interventions was also not statistically significant (RRs ranged from 0.82 to 1.02). The evidence supporting these findings was of low or very low certainty.
Systemic aminocaproic acid reduced the rate of recurrent hemorrhage (RR 0.28, 95% CI 0.13 to 0.60) as assessed in six trials with 330 participants. A sensitivity analysis omitting two studies not using an intention‐to‐treat analysis reduced the strength of the evidence (RR 0.43, 95% CI 0.17 to 1.08). We obtained similar results for topical aminocaproic acid (RR 0.48, 95% CI 0.20 to 1.10) in two studies with 121 participants. We assessed the certainty of these findings as low and very low, respectively. Systemic tranexamic acid had a significant effect in reducing the rate of secondary hemorrhage (RR 0.31, 95% CI 0.17 to 0.55) in five trials with 578 participants, as did aminomethylbenzoic acid as reported in one study (RR 0.10, 95% CI 0.02 to 0.41). The evidence to support an associated reduction in the risk of complications from secondary hemorrhage (i.e. corneal blood staining, peripheral anterior synechiae, elevated intraocular pressure, and development of optic atrophy) by antifibrinolytics was limited by the small number of these events. Use of aminocaproic acid was associated with increased nausea, vomiting, and other adverse events compared with placebo. We found no evidence of an effect in the number of adverse events with the use of systemic versus topical aminocaproic acid or with standard versus lower drug dose.
The number of days for the primary hyphema to resolve appeared to be longer with the use of systemic aminocaproic acid compared with no use, but this outcome was not altered by any other intervention.
The available evidence on usage of systemic or topical corticosteroids, cycloplegics, or aspirin in traumatic hyphema was limited due to the small numbers of participants and events in the trials.
We found no evidence of an effect between a single versus binocular patch or ambulation versus complete bed rest on the risk of secondary hemorrhage or time to rebleed.
Authors' conclusions
We found no evidence of an effect on visual acuity by any of the interventions evaluated in this review. Although evidence was limited, it appears that people with traumatic hyphema who receive aminocaproic acid or tranexamic acid are less likely to experience secondary hemorrhaging. However, hyphema took longer clear in people treated with systemic aminocaproic acid.
There is no good evidence to support the use of antifibrinolytic agents in the management of traumatic hyphema other than possibly to reduce the rate of secondary hemorrhage. Similarly, there is no evidence to support the use of corticosteroids, cycloplegics, or non‐drug interventions (such as binocular patching, bed rest, or head elevation) in the management of traumatic hyphema. As these multiple interventions are rarely used in isolation, further research to assess the additive effect of these interventions might be of value.
Plain language summary
Medical interventions for traumatic hyphema
What is the aim of this review? The aim of this Cochrane Review was to find out what medical treatments are effective for traumatic hyphema, a condition in which blood collects in the eye following trauma, usually a blow to the eye. We collected and analyzed all relevant studies to answer this question.
Key messages We found no evidence that any medical intervention affected vision, whether measured within a few weeks or longer. We also found that no medical intervention resulted in fewer complications from the hyphema itself, although this evidence is weak because few events occurred. We found limited evidence that antifibrinolytics, drugs that affect how blood is clotted, reduced the risk of new bleeding in the eye.
What was studied in the review? It was important to evaluate current medication interventions for traumatic hyphema because complications from the condition can affect final vision. We found 27 studies with a total of 2643 participants addressing this question. Studies were from the USA, Canada, Sweden, Denmark, China, South Africa, Malysia, Iran, and Israel. The studies included more males than females, and participants tended to be children or young adults. Interventions included antifibrinolytic agents taken orally or applied directly to the eye (aminocaproic acid, tranexamic acid, and aminomethylbenzoic acid), oral or topical corticosteroids, other kinds of eyedrops, aspirin, estrogens, traditional Chinese medicine, patching, elevation of the head, and bed rest. Most studies looked at how often fresh bleeding occurred, because this secondary bleeding is often associated with more complications. Other outcomes examined included visual acuity and the length of time it took for the blood in the eye to be absorbed.
What are the main results of the review? We found no evidence that any medical intervention affected final vision, but we graded the evidence as generally of low certainty. Antifibrinolytic agents did appear to reduce the risk of new bleeding in the eye, but participants taking oral aminocaproic acid (an antifibrinolytic agent) appeared to have more nausea and vomiting compared with participants in the control group. It was unclear whether antifibrinolytics reduced complications of secondary bleeding, because these events were infrequent in the studies. We found no evidence for effectiveness of any other medical intervention in reducing the rate of fresh bleeding or the number of complications, but the evidence for a beneficial effect of any of these interventions was uncertain because the numbers of participants and events were small.
How up‐to‐date is this review? We reviewed studies published up to 28 June 2018.
Summary of findings
Background
Description of the condition
Introduction
Traumatic hyphema is the entry of blood into the anterior chamber (the space between the cornea and iris) subsequent to a blow or a projectile striking the eye. Apart from the direct consequences of the initial trauma, traumatic hyphema is usually a self limiting condition that rarely causes permanent loss of vision in the absence of associated damage to the cornea, lens, or optic nerve. Traumatic hyphema is an important clinical entity because of the risks associated with significant initial reduction in vision and because of associated injuries to the tissues of the eye. In young children, it can lead to the development of irreversible amblyopia. Complications resulting from secondary hemorrhage, such as glaucoma, corneal blood staining, or optic atrophy, can lead to permanent impairment of vision, especially if the hyphema is prolonged in association with elevated intraocular pressure (IOP).
Epidemiology
Traumatic hyphema is usually seen in children or young adults, with an incidence of approximately 2 per 10,000 children per year (Wright 2003). Males predominate, with a male‐to‐female ratio of 3:1 (Crouch 1993). Sports injuries account for 60% of traumatic hyphemas (Crouch 1999).
Presentation and diagnosis
Patients usually present with a sudden decrease or loss of vision following an injury to the eye. The loss of vision depends on the level of hyphema: a patient with a microhyphema occasionally may present with normal vision or with somewhat blurred vision, whereas a patient with a full hyphema may present with almost complete loss of vision. With time, blood in the anterior chamber is forced by gravity to the bottom of the anterior chamber. Subsequently, vision clears gradually unless associated injuries, traumatic uveitis, glaucoma, optic atrophy, or corneal blood staining contributes to further losses of vision.
The severity of traumatic hyphema varies from microhyphema, where red blood cells are suspended in the anterior chamber, to a layered hyphema, where fresh or clotted blood may be observed grossly in the lower anterior chamber. In a full or total hyphema, the entire anterior chamber is filled with blood.
Recurrent hemorrhage, occurring at a rate of 2% to 38% (Walton 2002), increases the time to visual recovery and is associated with poorer visual outcomes. Secondary hemorrhage typically occurs three to five days after the incident hyphema and may occur due to clot lysis and retraction within the traumatized vessels.
Hyphema in the setting of sickle cell trait/disease appears to be particularly dangerous because the naturally hypoxic and relatively acidotic anterior chamber induces sickling of red blood cells. Sickling in turn prevents normal egress of those blood cells through the trabecular meshwork. Hyphema patients with sickle cell trait/disease may be at a higher risk for elevated IOP (Lai 2001).
The most important sign for diagnosing hyphema is the presence of blood in the anterior chamber assessed by a slit‐lamp exam. Various grading schemes for hyphema have been proposed. Objective quantification of the level of hyphema is critical, because a sudden increase in the height of a layered hyphema is indicative of 'rebleed.' Immediate measurement of IOP and a dilated ophthalmoscopic exam (to rule out traumatic retinal tears, dialyses, and detachment) are also indicated at a relatively early time after clearance of hyphema.
Description of the intervention
Management of traumatic hyphema focuses on preventing repeated eye trauma and rebleed, promoting the settling of blood away from the visual axis, controlling traumatic anterior uveitis, and monitoring in order to initiate early prophylaxis or treatment for both secondary glaucoma and corneal blood staining. Methods employed to prevent recurrent or iatrogenic trauma include shielding the eye, bed rest, and avoidance of diagnostic interventions such as scleral depression or gonioscopy that could deform the globe. Elevation of the head while sleeping, topical corticosteroids, and cycloplegic medications are mainstays in the management of traumatic hyphema. Hospitalization, once considered essential in order to enforce bed rest, has been questioned and is currently advocated only for patients perceived to be at high risk of rebleed, at risk of noncompliance with bed rest at home, or possibly with sickle cell trait/disease.
The use of antifibrinolytic agents such as epsilon‐aminocaproic acid and tranexamic acid in traumatic hyphema is controversial. These agents are reported to potentially reduce the rate of recurrent hemorrhage, but are known to have several possible side effects, such as nausea, vomiting, muscle cramps, conjunctival suffusion, headache, rash, pruritis, dyspnea, toxic confusional states, arrhythmias, and systemic hypotension. Epsilon‐aminocaproic acid is contraindicated in women who are pregnant and in people with coagulopathies or renal diseases, and should be used cautiously in people with hepatic, cardiovascular, or cerebrovascular diseases. A topical gel form of epsilon‐aminocaproic acid has not yet received US Food and Drug Administration (FDA) approval; it appears to have comparable effectiveness, with fewer side effects, as compared with the oral form, and thus might be used on an outpatient basis. Tranexamic acid (Cyklokapron) is reported to be more potent than epsilon‐aminocaproic acid and has similar side effects, but with fewer gastric side effects (Rahmani 1999).
Corticosteroids have also been used to treat hyphema and are reported to be effective (Walton 2002). Investigators have studied both topical and systemic corticosteroids, applying these agents for varying lengths of time with or without other interventions, such as bed rest or cycloplegics. Topical administration of corticosteroids avoids the side effects of systemic corticosteroid use, but it is not known whether topically applied corticosteroids are as effective as systemic corticosteroids in reducing the rate of rebleed. The mechanism of action of corticosteroids is thought to be due to stabilization of the blood‐ocular barrier, direct inhibition of fibrinolysis, or reduced inflammation (Walton 2002).
Surgical evacuation of hyphema is generally not needed. In the past, surgical evacuation was often contraindicated due to the possibility of sudden decreases in IOP and increased risk of recurrent hemorrhage (due to decompression of the damaged iris and ciliary body). However, surgical 'washout' is advocated in patients with non‐clearing hyphema, in whom secondary glaucoma threatens to cause permanent visual loss due to glaucomatous optic neuropathy or to corneal blood staining. Surgical washout is often performed (via simple paracentesis) in patients with sickle cell trait because of the increased risk of elevated IOP.
How the intervention might work
The mode of action of medications used to treat traumatic hyphema, especially the antifibrinolytics, is through slowing or inhibiting the resorption of the blood clot within traumatized blood vessels. Aminocaproic acid slows the dissolution of the fibrin blood clot by competing at sites that bind lysine, including lysine sites on tissue plasminogen activator, inhibiting the conversion of plasminogen to plasmin, the enzyme involved in the breakdown of the fibrin clot (Sheppard 2009; Walton 2002). Aminocaproic acid also competitively inhibits the binding of plasmin to the fibrin clot itself. Both of these mechanisms result in a slowing of the breakdown of the fibrin clot, thus stabilizing it and reducing the risk of secondary hemorrhage. Tranexamic acid also binds to fibrin and is believed to act through a similar mechanism. The action of aminobenzoic acid involves inhibition of fibrinolysis, and estrogens decrease antithrombin activity, both of which result in delays of clot resorption (Westlund 1982). In addition to inhibition of fibrinolysis, corticosteroids are also believed to stabilize the blood‐ocular barrier and reduce inflammation.
The goal of most of the other interventions used in the management of traumatic hyphema is to prevent complications from the trauma or from a rebleed, including further trauma, anterior uveitis, secondary glaucoma, optic atrophy, or corneal blood staining. These interventions include bed rest and eye patching to prevent further trauma; use of mydriatic or miotic agents to prevent motion of the iris, increased IOP, or uveitis; corticosteroids to prevent inflammation; and elevation of the head to facilitate settling of the blood in the anterior chamber. Hospitalization facilitates close monitoring of more severe cases of trauma or rebleeding (or both), allowing more timely medical or surgical intervention, if warranted.
Why it is important to do this review
Despite the existence of guidelines for the management of traumatic hyphema (Crouch 1999; Rhee 1999; Sheppard 2009), the safety and effectiveness of various therapeutic modalities such as use of antifibrinolytic agents, their routes of administration, use of corticosteroids, and hospitalization are controversial. The evidence for the impact of rebleed on visual outcomes, glaucoma, optic atrophy, and blood staining is limited. Furthermore, rebleed, which is a surrogate outcome (rather than visual outcome), dominates the published literature on management of traumatic hyphema. It is important to examine the impact of the various antifibrinolytic medications, routes of administration, and dosages used across various populations.
Objectives
To assess the effectiveness of various medical interventions in the management of traumatic hyphema.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized and quasi‐randomized trials.
Types of participants
We included trials in which the study population consisted of people with traumatic hyphema following closed‐globe trauma. We applied no restrictions regarding age, gender, or severity of the closed‐globe trauma or level of visual acuity (VA) at the time of enrollment.
Types of interventions
We considered trials in which:
antifibrinolytic agents (e.g. epsilon‐aminocaproic acid, tranexamic acid) or corticosteroids in any form or dosage, with the intention to treat or reduce the signs or symptoms of traumatic hyphema, were compared with other treatments, placebo, or no treatment. There was no time limit on the duration of treatment;
bed rest was compared with ambulatory management;
bilateral patching was compared with unilateral or no patching;
outpatient management was compared with inpatient management; or
any other medical (non‐surgical) intervention was compared with another medical intervention or no intervention.
Types of outcome measures
Primary outcomes
Proportion of participants with best‐corrected visual acuity (BCVA) of 20/40 or better assessed at short‐, medium‐, and long‐term follow‐up, defined respectively as two weeks or less; more than two weeks but within two months; and more than two months from the traumatic event. We also assessed VA at resolution of hyphema.
Time to resolution of primary hemorrhage (hyphema) defined as the length of time from onset to resolution of hyphema.
Secondary outcomes
Secondary outcomes for this review were sequelae of traumatic hyphema assessed at the time of last study follow‐up.
Proportion of participants with rebleed (i.e. secondary hemorrhage), defined as (a) an increase in height of layered hyphema using a biomicroscopic caliper or by any other method; or (b) the occurrence of fresh (red) blood in the eye with the existing clot. We also reported the average time to rebleed among participants with rebleed when this information was available.
Proportion of participants with corneal blood staining.
Proportion of participants with peripheral anterior synechiae (PAS) formation.
Proportion of participants with pathologic increase in IOP or glaucoma development, as defined by trial investigators.
Proportion of participants with optic atrophy development.
Adverse effects
We summarized the reported adverse effects related to treatment.
Quality of life outcomes
We described available data on indicators of quality of life.
Economic outcomes
We assessed the need for bed rest or hospitalization versus outpatient care. We also compared length of hospital stay as described in the primary reports. No other economic outcomes were reported.
Follow‐up
There were no restrictions based on length of follow‐up.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist conducted systematic searches in the following electronic databases for RCTs and controlled clinical trials. There were no language or publication year restrictions. The date of the search was 28 June 2018.
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 6) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 28 June 2018) (Appendix 1).
MEDLINE Ovid (1946 to 28 June 2018) (Appendix 2).
Embase.com (1980 to 28 June 2018) (Appendix 3).
PubMed (1948 to 28 June 2018) (Appendix 4).
ISRCTN registry (www.isrctn.com/editAdvancedSearch; searched 28 June 2018) (Appendix 6).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 28 June 2018) (Appendix 6).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched 28 June 2018) (Appendix 7).
Searching other resources
We searched the reference lists of included trial reports to find additional trials. We also searched the ISI Web of Science Social Sciences Citation Index (SSCI) to find studies that have cited the included trials. We planned to contact the primary investigators of included trials for details of additional trials, but were unable to do so because most trials were published more than 10 years ago. We did not conduct manual searches of conference proceedings or abstracts specifically for this review.
Data collection and analysis
Selection of studies
Two review authors independently assessed the titles and abstracts of all reports identified by the electronic and manual searches as per the Criteria for considering studies for this review. We classified the abstracts as (a) definitely include, (b) unsure, or (c) definitely exclude. We obtained full copies of those abstracts classified as (a) or (b) and reassessed them as per the Criteria for considering studies for this review. We assessed the studies as (1) include, (2) awaiting assessment, or (3) exclude. We documented the concordance between review authors and resolved any disagreements by consensus or by consulting a third review author. We planned to contact authors of studies classified as (2) for clarification of unclear inclusion and exclusion criteria, but were unable to do so. We excluded from the review studies identified by both review authors as (3) and documented our reasons for exclusion in the Characteristics of excluded studies table. We included studies identified as (1) in the review and described them in the Characteristics of included studies table. The review authors were unmasked to the reports' authors, institutions, and trial results during this assessment.
Data extraction and management
Two review authors independently extracted the data for the primary and secondary outcomes onto data collection forms developed by the Cochrane Eyes and Vision Group. Any discrepancies were resolved by discussion. We attempted to contact primary investigators for missing data, but were unable to do so. One review author entered all data into Review Manager 5 (RevMan 5) (Review Manager 2014), and a second review author verified all values.
Assessment of risk of bias in included studies
Two review authors assessed the sources of systematic bias in trials according to methods described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We considered the following parameters: adequate sequence generation and allocation concealment (selection bias), masking of participants and researchers (performance bias), masking of outcome assessors (detection bias), adequate handling of incomplete data by reporting rates of follow‐up and using intention‐to‐treat analysis (attrition bias), and complete reporting of outcomes (reporting bias). We assessed each of the parameters as low, unclear, or high risk of bias. We documented agreement between authors and resolved any disagreements by consensus or by involving a third review author. We used masking of participants and care providers as a quality criterion only in interventions where masking was feasible. We contacted the authors of trials categorized as at unclear risk of bias for additional information when contact information for the trial authors was available. In cases where we were unable to contact the study authors or the study authors did not respond to our request, we assigned a grade based on the available information.
Measures of treatment effect
Dichotomous data
For dichotomous outcomes, we calculated summary risk ratios (RR) with 95% confidence intervals (CIs). We analyzed VA outcomes as dichotomous variables. For each follow‐up period with sufficient data, we compared the proportion of participants with VA 20/40 or better between the treatment and control groups. We analyzed data on the proportion of participants with secondary hemorrhage, corneal blood stain, PAS formation, glaucoma development, and optic atrophy development as dichotomous data.
Continuous data
We calculated mean differences (MD) for continuous outcomes. We analyzed the time to resolution of primary hemorrhage (hyphema), defined as the length of time from onset to resolution, as a continuous variable. We also analyzed the length of time to rebleed, the duration of hospitalization, and other quality of life and economic outcomes as continuous data.
Ordinal data
We summarized ordinal data qualitatively.
Counts and rate data
We summarized counts and rate data in rate ratios when the event was rare, and as continuous outcome data when the event was more common. We analyzed adverse events data as counts and rates.
Unit of analysis issues
The unit of analysis for this review was the affected eye or eyes of the individual participant.
Dealing with missing data
We contacted the authors of included studies to obtain additional data when contact information for the trial authors was available. When we were unable to retrieve additional data because we were unable to contact the authors or received no response, we imputed data with the information that was available in the study report. We reported loss to follow‐up for each study when this information was available. We also noted when intention‐to‐treat analyses were performed.
Assessment of heterogeneity
We used the I2 statistic to assess for statistical heterogeneity and examined clinical heterogeneity using forest plots. We considered I2 values greater than 40% to represent statistical heterogeneity between studies.
Assessment of reporting biases
We did not use funnel plots to assess the possibility of small‐study effects or reporting biases because we included no more than 10 studies in a meta‐analysis.
Data synthesis
We analyzed data according to the guidelines in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017). We tested for statistical heterogeneity. When we detected no statistical heterogeneity and there was no clinical heterogeneity among the trials, we combined the results in a meta‐analysis using a fixed‐effect model. In cases of statistical or clinical heterogeneity, we did not combine study results but presented a tabulated summary.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses according to age, race, presence of sickle cell trait/disease, presenting IOP, and severity of hyphema, but we did not perform these because sufficient numbers of trials were not available. We planned to present results by subgroup as an Additional table.
Sensitivity analysis
We conducted sensitivity analyses to determine the impact of excluding studies of lower methodologic quality. We had planned to conduct sensitivity analyses to determine the impact of excluding unpublished studies or industry‐funded studies, but did not because we included no studies with these characteristics.
Summary of findings
We assessed each outcome using the GRADE approach, which judges the certainty of the evidence based on risk of bias, inconsistency, indirectness, imprecision, and publication bias in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2017). We prepared a 'Summary of findings' table for each main comparison and included the following outcomes.
Proportion of participants with BCVA of 20/40 or better assessed at short‐term follow‐up, defined as two weeks or less from the traumatic event.
Proportion of participants with BCVA of 20/40 or better assessed at medium‐term follow‐up, defined as more than two weeks but within two months of the traumatic event.
Proportion of participants with BCVA of 20/40 or better assessed at long‐term follow‐up, defined as more than two months from the traumatic event.
Proportion of participants with BCVA of 20/40 or better assessed at resolution of hyphema.
Time to resolution of primary hemorrhage (hyphema), defined as the length of time from onset to resolution of hyphema.
Proportion of participants with rebleed (i.e. secondary hemorrhage).
Proportion of participants with adverse effects.
Results
Description of studies
Results of the search
The original electronic literature searches conducted in June 2010 identified 836 potentially relevant references for this review. After duplicate review of the titles and abstracts, we classified 748 references as 'definitely exclude,' 23 as 'definitely include,' and 65 as unsure. Seventeen of the 65 references assessed as unsure were letters or editorials that did not report original data and were excluded. We obtained full‐text copies of the 48 remaining references classified as unsure and reviewed them in duplicate. Of these, we excluded 40 and included eight. A manual search of other resources, including reference lists of included studies and citation index databases, yielded four additional potentially relevant full‐text references for this review, of which we included two and excluded two. In the 2011 publication of this review (Gharaibeh 2011), we included 26 studies as reported in 33 publications and excluded 41 studies in 42 publications.
After revising and updating the electronic searches as of August 2013, we identified 460 additional references for review. After duplicate review of the titles and abstracts, we classified 438 references as definitely exclude and 22 as unsure. We obtained full‐text copies of the references classified as unsure and reviewed them in duplicate. Seventeen of the references were not in the English language, and we identified colleagues who read the relevant languages to assist with assessing the articles in duplicate. Of the 22 references reviewed in full, we excluded 20; one was a reference for a study already included in the review; and one was included as a new study in the review. A manual search of other resources, including reference lists of included studies and citation index databases, yielded four additional potentially relevant full‐text references for this review, of which one was excluded and the remaining three were from studies already included in this review. In the 2013 publication of this review (Gharaibeh 2013), we included 27 studies reported by 38 publications, and excluded 62 studies reported by 63 publications.
We updated the searches for this review in June 2018 (Figure 1). Of 479 records identified by the searches, we examined the full‐text reports of two studies and excluded both (Zhang 2013; Zhang 2014). We identified no new eligible trials since the 2013 version of this review, thus the review includes 27 studies.
1.
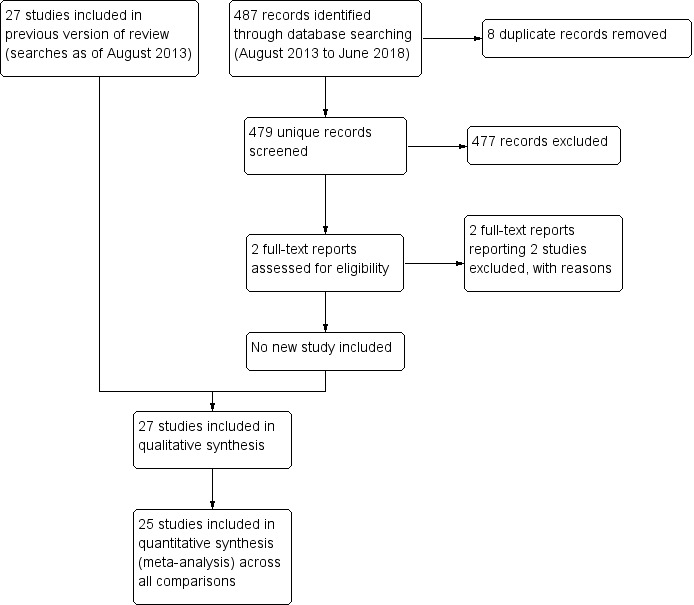
8 Study flow diagram.
Included studies
The 27 studies included in this review are described in the Characteristics of included studies table. Twenty of the included studies were randomized controlled trials (RCTs), and seven used a quasi‐randomized method to assign participants to treatment groups. The review outcomes reported by the included studies are listed in Table 7.
1. Summary of outcomes* reported by intervention.
| Interventions | Primary outcomes | Secondary outcomes | Adverse effects | Duration of hospitalization or quality of life outcomes | ||||||
| VA | Time to resolution of primary hemorrhage | Secondary hemorrhage | Risk of corneal blood staining | Risk of PAS formation | Risk of pathologic increase in IOP or glaucoma | Risk of optic atrophy | ||||
| Risk of rebleed | Time to rebleed | |||||||||
| Aminocaproic acid vs placebo | ||||||||||
| Oral aminocaproic acid | ||||||||||
| Christianson 1979 | Not reported | Partially reported** | Risk of rebleed reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Crouch 1976 | Long‐term VA reported | Days to resolution reported | Risk of rebleed reported | Time to rebleed reported | Risk of corneal blood staining reported | Partially reported** | Not reported | Risk of optic atrophy reported | Not reported | Not reported |
| Kraft 1987 | Long‐term VA reported | Days to resolution reported | Risk of rebleed reported | Time to rebleed reported | Not reported | Not reported | Persistent increases in IOP reported | Not reported | Adverse effects reported | Not reported |
| Kutner 1987 | Short‐term VA reported | Days to resolution reported | Risk of rebleed reported | Time to rebleed reported | Not reported | Not reported | Persistent increases in IOP reported | Not reported | Adverse effects reported | Not reported |
| McGetrick 1983 | Final VA reported | Days to resolution reported | Risk of rebleed reported | Time to rebleed reported | Not reported | Not reported | Not reported | Not reported | Adverse effects reported | Partially reported** |
| Teboul 1995 | Final VA reported | Days to resolution reported | Risk of rebleed reported | Time to rebleed reported | Not reported | Not reported | Transient increases in IOP reported | Not reported | Not reported | Duration of hospitalization reported |
| Topical aminocaproic acid | ||||||||||
| Karkhaneh 2003 | Reported as NS | Days to resolution reported | Risk of rebleed reported | Time to rebleed reported | Not reported | Not reported | Reported as NS | Not reported | Not reported | Not reported |
| Pieramici 2003 | Short‐term VA reported | Reported as NS | Risk of rebleed reported | Time to rebleed reported | Not reported | Not reported | Transient increases in IOP reported | Not reported | Adverse effects reported | Not reported |
| Low‐dose vs standard‐dose aminocaproic acid | ||||||||||
| Palmer 1986 | Final VA reported | Days to resolution reported | Risk of rebleed reported | Time to rebleed reported | Not reported | Not reported | Transient increases in IOP reported | Not reported | Adverse effects reported | Duration of hospitalization reported |
| Oral vs topical aminocaproic acid | ||||||||||
| Crouch 1997 | Final VA reported | Not reported | Risk of rebleed reported | Time to rebleed reported | Risk of corneal blood staining reported | Partially reported** | Not reported | Risk of optic atrophy reported | Adverse effects reported | Not reported |
| Tranexamic acid vs control | ||||||||||
| Rahmani 1999 | Short‐term VA reported | Days to resolution reported | Risk of rebleed reported | Time to rebleed reported | Not reported | Not reported | Transient increases in IOP reported | Not reported | Adverse effects reported | Duration of hospitalization reported |
| Sukumaran 1988 | Short‐term VA reported | Days to resolution reported | Risk of rebleed reported | Time to rebleed reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Vangsted 1983 | Short‐term VA reported | Partially reported** | Risk of rebleed reported | No rebleeds occurred | Risk of corneal blood staining reported | Not reported | Transient increases in IOP reported | Not reported | Not reported | Duration of hospitalization and days off work reported |
| Varnek 1980 | Partially reported** | Not reported | Risk of rebleed reported | Time to rebleed reported | Risk of corneal blood staining reported | Not reported | Transient increases in IOP reported | Risk of optic atrophy reported | Not reported | Duration of hospitalization reported |
| Welsh 1983 | Not reported | Partially reported** | Risk of rebleed reported | Not reported | Not reported | Not reported | Transient increases in IOP reported | Not reported | Adverse effects reported | Not reported |
| Aminomethylbenzoic acid vs placebo | ||||||||||
| Liu 2002 | Not reported | Not reported | Risk of rebleed reported | Not reported | Not reported | Not reported | Not reported | Not reported | Adverse effects reported | Not reported |
| Corticosteroids vs control | ||||||||||
| Oral corticosteroids | ||||||||||
| Rahmani 1999 | Short‐term VA reported | Days to resolution reported | Risk of rebleed reported | Time to rebleed reported | Not reported | Not reported | Transient increases in IOP reported | Not reported | Adverse effects reported | Duration of hospitalization reported |
| Spoor 1980 | Final VA reported | Days to resolution reported | Risk of rebleed reported | Time to rebleed reported | Risk of corneal blood staining reported | Risk of PAS formation reported | Transient increases in IOP reported | Not reported | Not reported | Not reported |
| Topical corticosteroids | ||||||||||
| Rakusin 1972 | Short‐term VA reported | Partially reported** | Risk of rebleed reported | Not reported | Partially reported** | Partially reported** | Not reported | Not reported | Not reported | Not reported |
| Zetterstrom 1969 | Short‐term VA reported | Not reported | Risk of rebleed reported | Not reported | Risk of corneal blood staining reported | Not reported | Transient increases in IOP reported | Risk of optic atrophy reported | Not reported | Duration of hospitalization reported |
| Oral aminocaproic acid vs oral prednisone | ||||||||||
| Farber 1991 | Short‐term VA reported | Partially reported** | Risk of rebleed reported | Not reported | Not reported | Not reported | Reported as NS | Not reported | Not reported | Not reported |
| Conjugated estrogen vs placebo | ||||||||||
| Spaeth 1966 | Partially reported** | Not reported | Risk of rebleed reported | Partially reported** | Risk of corneal blood staining reported | Partially reported** | Partially reported** | Not reported | Not reported | Not reported |
| Cycloplegics vs miotics | ||||||||||
| Bedrossian 1974 | Not reported | Days to resolution reported | Risk of rebleed reported | Time to rebleed reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Rakusin 1972 | Short‐term VA reported | Partially reported** | Risk of rebleed reported | Not reported | Reported as NS | Reported as NS | Not reported | Not reported | Not reported | Not reported |
| Aspirin vs observation | ||||||||||
| Marcus 1988 | Not reported | Not reported | Risk of rebleed reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Traditional Chinese medicine vs control treatment | ||||||||||
| Wang 1994 | Partially reported** | Partially reported** | Partially reported** | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Monocular vs binocular patching | ||||||||||
| Edwards 1973 | Final VA reported | Not reported | Risk of rebleed reported | Time to rebleed reported | Risk of corneal blood staining reported | Not reported | Risk of secondary glaucoma reported | Not reported | Not reported | Quality of life outcomes reported |
| Rakusin 1972 | Short‐term VA reported | Partially reported** | Risk of rebleed reported | Not reported | Reported as NS | Reported as NS | Not reported | Not reported | Not reported | Not reported |
| Ambulatory vs conservative treatment | ||||||||||
| Rakusin 1972 | Short‐term VA reported | Partially reported** | Risk of rebleed reported | Not reported | Reported as NS | Reported as NS | Not reported | Not reported | Not reported | Not reported |
| Read 1974 | Partially reported** | Days to resolution reported | Risk of rebleed reported | Partially reported** | Risk of corneal blood staining reported | Not reported | Transient increases in IOP reported | Not reported | Not reported | Not reported |
| Elevation of the head vs control | ||||||||||
| Zi 1999 | Not reported | Days to resolution reported | Not reported | Not reported | Not reported | Not reported | Risk of secondary glaucoma reported | Not reported | Not reported | Not reported |
*See Types of outcome measures for detailed descriptions of outcomes. **Noted as 'partially reported' if some information was reported, but it was insufficient for quantitative data analyses.
Abbreviations: IOP: intraocular pressure; NS: not significant; PAS: peripheral anterior synechiae; VA: visual acuity.
All but two of the studies restricted entry to people with primary traumatic hyphema; Welsh 1971 also included people with perforated globes that had been sutured and were treated as closed‐globe injuries, and Palmer 1986 also included some people with secondary hemorrhage. Most studies included all age groups, although some studies excluded very young children (e.g. less than four or seven years) (Farber 1991; Kutner 1987; Marcus 1988; Pieramici 2003; Vangsted 1983; Welsh 1983), and one study included children only (Kraft 1987). Of studies reporting demographic data, the mean age of participants ranged from 10 to 32 years, and the proportion of male participants ranged from 67% to 100%. Studies took place in a number of different countries: three in China; two each in Iran, Sweden, and South Africa; one each in Denmark, Israel, and Malaysia; and the remainder in Canada and the USA. The race of participants varied by country, and nine studies reported 50% or more black participants.
The included studies investigated three types of antifibrinolytic agents (epsilon‐aminocaproic acid (aminocaproic acid), tranexamic acid, and aminomethylbenzoic acid). Other types of pharmaceuticals investigated were corticosteroids, including prednisone, prednisolone, hydrocortisone, and cortisone; conjugated estrogen; aspirin; and topical mydriatics and miotics. One study compared traditional Chinese medicine (Yunnan Baiyao) versus antihemorrhagic agents. Non‐pharmaceutical interventions included the use of monocular or binocular patching, eye shields, bed rest, and elevation of the head. The primary outcome for all but three studies was the risk of a secondary hemorrhage.
Aminocaproic acid
Eight studies investigated the use of aminocaproic acid compared with placebo in treating traumatic hyphema: six studies prescribed oral aminocaproic acid (Christianson 1979; Crouch 1976; Kraft 1987; Kutner 1987; McGetrick 1983; Teboul 1995), and two studies prescribed topical aminocaproic acid (Karkhaneh 2003; Pieramici 2003). The dosage of oral aminocaproic acid used in five studies was 100 mg/kg of body weight every four hours for five days (Crouch 1976; Kraft 1987; Kutner 1987; McGetrick 1983; Teboul 1995); the remaining study used a loading dose of 75 mg/kg of body weight, then doses of 60 mg/kg of body weight every four hours, although the length of treatment was not reported (Christianson 1979). The six studies included a total of 331 participants (34 to 94 participants per study); 175 participants were randomized to receive oral aminocaproic acid, and 156 participants were randomized to receive placebo pills. The follow‐up periods ranged from the length of hospitalization (typically about one to two weeks) to 3.4 years after discharge.
Two studies evaluated topical aminocaproic acid and included 206 participants. Karkhaneh 2003 had three treatment groups: 45 participants were randomized to receive aminocaproic acid (two drops of 25% aminocaproic acid in 2% carboxymethylene gel applied to the inferior fornix of the affected eye every six hours for five days) plus homatropine eyedrops three times per day; 44 participants were randomized to receive placebo gel plus homatropine eyedrops; and 66 participants were randomized to receive homatropine eyedrops only. Homatropine is a cycloplegic agent used to prevent eye muscles from moving temporarily and to enlarge the pupil. The follow‐up period for this study was 14 days. In Pieramici 2003, 24 participants were randomized to receive aminocaproic acid (30% aminocaproic acid in 2% gel instilled in the inferior fornix following one drop of 0.05% proparacaine hydrochloride every six hours for five days), and 27 participants were randomized to receive placebo gel applied in the same manner as in the intervention group. Participants in this study were managed on an outpatient or inpatient basis and followed for seven days.
One included study compared oral aminocaproic acid versus topical aminocaproic acid for the treatment of traumatic hyphema (Crouch 1997). Of the 118 participants eligible for inclusion in the study, 64 participants agreed to be randomized to receive either topical aminocaproic acid (0.2 mL of 30% aminocaproic acid in 2% carboxymethylene gel applied to the inferior fornix every six hours plus oral placebo solution every four hours for five days) or oral aminocaproic acid (50 mg/kg of body weight of oral aminocaproic acid, up to 30 g per day, plus placebo gel every four hours for five days). The 54 participants who declined study entry were followed as an untreated control group. The participants in this study were hospitalized and followed for five days.
The last study investigating the use of aminocaproic acid compared low‐dose oral aminocaproic acid (50 mg/kg, up to 5 g per dose or 30 g per day every four hours for five days) versus the standard‐dose oral aminocaproic acid (100 mg/kg, up to 5 g per dose or 30 g per day every four hours for five days) for the treatment of traumatic hyphema (Palmer 1986). The participants in this study, 26 in the low‐dose group and 33 in the standard‐dose group, were followed for the duration of hospitalization.
Tranexamic acid
Five studies investigated the use of oral tranexamic acid compared with a control in treating traumatic hyphema (Rahmani 1999; Sukumaran 1988; Vangsted 1983; Varnek 1980; Welsh 1983). The studies included a total of 581 participants: 279 were assigned to tranexamic acid and 302 to a control intervention. The doses of tranexamic acid administered in these studies varied from 1.75 mg/kg per day for five days to 1.5 g per day for seven days. Participants were followed for five to 12 days. The study using the lowest dose of tranexamic acid assigned 82 participants to oral tranexamic acid 1.75 mg/kg daily for five days; 81 to prednisone 0.75 mg/kg daily for five days; and 81 to daily placebo for five days. All participants were followed for five days (Rahmani 1999). In two studies, participants were assigned to tranexamic acid 25 mg/kg per day for seven days (Sukumaran 1988; Vangsted 1983). In Sukumaran 1988, both the group receiving tranexamic acid (n = 17) and the control group (n = 18) received bilateral patching, bed rest, sedation, analgesics, and topical corticosteroid drops from day three through day seven. Both groups were followed for one week. In Vangsted 1983, 59 participants were randomized to receive tranexamic acid and 53 participants were randomized to receive complete bed rest for six days; participants were followed for seven days. Varnek 1980 compared the same dose of tranexamic acid, 25 mg/kg daily for seven days, along with hospitalization and bed rest (n = 102), versus hospitalization and bed rest alone in the control group (n = 130). Participants were followed for 12 days. In Welsh 1983, 19 participants were randomized to receive the largest dose of tranexamic acid, three 500 mg tablets of oral tranexamic acid three times a day for seven days (for an overall total dose of 31.5 g tranexamic acid), and 20 participants were randomized to receive three tablets of placebo three times a day for seven days.
Aminomethylbenzoic acid
One included study compared oral aminomethylbenzoic acid versus placebo for the treatment of traumatic hyphema (Liu 2002). The study, published in Chinese, randomized 60 participants to the intervention group and 32 participants to the placebo group. Participants in the intervention group received oral aminomethylbenzoic acid 0.5 g plus oral vitamin B1 20 mg three times a day for six days. The dosage of aminomethylbenzoic acid was modified for children to "follow age‐recommended dose"; the vitamin B1 dosage remained the same. Participants in the control group received oral vitamin B1 (20 mg) three times a day for six days. The follow‐up period for the study was one week post‐blood resolution.
Corticosteroids
Four studies examined the use of corticosteroids, two using an oral preparation (Rahmani 1999; Spoor 1980), and two using a topical preparation (Rakusin 1972; Zetterstrom 1969). Spoor 1980 compared oral prednisone versus placebo for the treatment of traumatic hyphema. Twenty‐three participants were randomized to the treatment group: oral prednisone, 40 mg/day for adults and children over 10 years old; 15 mg/day for children between four and 10 years; and 10 mg/day for children between 18 months and four years, for seven days; and 20 participants were randomized to the control group: lactose placebo capsules administered daily for seven days. All participants were followed for seven days and some for up to six months. The second study consisted of three intervention arms with 244 participants (Rahmani 1999). One arm of the study included 82 participants who received oral tranexamic acid 75 mg/kg per day, divided into three doses per day, for five days. The second arm included 81 participants who received oral prednisolone 0.75 mg/kg per day, divided into two doses per day, for five days. The third group included 81 participants who received placebo administered three times per day. The follow‐up period for this study was five days or until discharge. The remaining two studies administered topical corticosteroids. In Zetterstrom 1969, atropine plus corticosteroid eyedrops (Decadron) was administered five times daily in 58 participants, while the control group of 59 participants simply received bed rest. The fourth study, Rakusin 1972, compared the use of 0.5% hydrocortisone acetate in 13 participants versus topical 0.5% chloramphenicol in 21 participants.
Antifibrinolytic agents versus corticosteroids
Two studies compared the use of antifibrinolytic agents versus corticosteroids for the treatment of traumatic hyphema. The first study included 122 participants: 64 were allocated to receive oral aminocaproic acid and 58 to receive oral prednisone. All participants were followed through the treatment period (Farber 1991). Participants in the aminocaproic acid group received 50 mg/kg oral aminocaproic acid (up to 30 g per day) every four hours plus two doses of placebo for five days. Participants in the prednisone group received 40 mg/day of oral prednisone in two doses plus six doses of placebo; children and adults weighing less than 60 kg were given 0.6 mg/kg/day of prednisone for five days. The second study, described above, divided participants into three groups: oral prednisolone, tranexamic acid, and placebo (Rahmani 1999).
Conjugated estrogen
One included study compared the use of conjugated estrogen versus placebo to treat traumatic hyphema (Spaeth 1966). Participants randomized to receive conjugated estrogen were given 5 mg intramuscularly (children less than five years of age); 10 mg intramuscularly (children five years of age but less than 10 years of age); and 20 mg intravenously (children 10 years of age or older and adults), for five days. The 85 participants included in the study were followed for five days or until discharge.
Cycloplegics versus miotics
Two studies compared the use of cycloplegics (agents that enlarge the pupil) versus miotics (agents that constrict the pupil). Bedrossian 1974 evaluated 1% atropine ointment in 28 participants versus 2% pilocarpine (or eserine) ointment in 30 participants, who were followed until the hyphema cleared (one to seven days). Rakusin 1972 examined the effects of 1% homatropine eyedrops in 17 participants; 4% pilocarpine in 17 participants; homatropine plus pilocarpine in 17 participants; and neither agent in 19 participants over a period of one to two weeks.
Aspirin
One included study compared aspirin (500 mg three times a day for five days) versus observation for the treatment of traumatic hyphema (Marcus 1988). Of the 51 included participants, 23 were randomized to the aspirin group and 28 to the observation group. All participants were followed for seven days.
Traditional Chinese medicine
One included study compared Yunnan Baiyao, a traditional Chinese medicine formula, versus control treatment for traumatic hyphema (Wang 1994). Yunnan Baiyao is an herbal supplement with hemostatic and anti‐inflammatory properties. The 45 participants in the Yunnan Baiyao group received 0.5 g of the medicine four times a day orally in addition to oral antibiotics and topical 0.5% vinegar eye drops. The 38 participants in the control group received antihemorrhagic agents such as carbazochrome and etamsylate. Participants were treated for up to five days (until complete resolution of the hyphema), and follow‐up was one week.
Monocular versus binocular patching
Two studies compared monocular versus binocular patching. Edwards 1973 compared monocular patching in 35 participants versus binocular patching in 29 participants. Follow‐up was one to seven days. In one of the comparisons conducted by Rakusin 1972, 27 participants wore binocular patches; 26 wore monocular patches; and 10 wore no patch. Participants were followed up for one to two weeks.
Ambulatory versus conservative treatment
In two studies, the test and control interventions consisted of multiple components but could be assessed as treatments allowing moderate activity compared with bed rest. Read 1974 evaluated an intervention that included bed rest with elevation of the head, bilateral patches, an eye shield over the injured eye, and sedation in 66 participants compared with an intervention comprised of moderate ambulatory activity, patching, shielding of the injured eye, and no sedation in 71 participants. The second study, Rakusin 1972, compared bed rest versus ambulation in 26 participants each.
Combination and other interventions
In one study (Rakusin 1972), various components of a multiple‐component intervention were tested sequentially and separately. Four of these comparisons are described above (i.e. 0.5% hydrocortisone eyedrops versus 0.5% chloramphenicol eyedrops, monocular versus binocular patching, cycloplegics versus miotics, and ambulation versus bed rest). In addition, Rakusin 1972 also presented results on the following comparisons: 1) oral trypsin in 15 participants compared with oral papase in 18 participants or no treatment in 10 participants; and 2) acetazolamide 250 mg in 18 participants compared with oral glycerol 1 mL/kg in 18 participants and no treatment in 10 participants.
The remaining study compared the time to resolution for participants lying flat either on the right or left side versus remaining in a semi‐reclined position (i.e. with the head elevated) (Zi 1999).
Excluded studies
We excluded 64 studies. The reasons for exclusion are provided in the Characteristics of excluded studies table. We excluded 45 studies because the study design was not a randomized or controlled clinical trial; nine studies because they included non‐traumatic hyphema cases and did not report outcomes for traumatic hyphema cases separately; seven studies because no original data were presented; and three studies because they investigated interventions outside the scope this review (e.g. surgical interventions and patient education interventions).
Risk of bias in included studies
Allocation
Twenty of the 27 studies included in the review were RCTs. Seven studies specified using computerized randomization to generate the allocation sequence, and one study used a randomization list; we judged these eight studies as having a low risk of sequence generation bias (Figure 2). Twelve of the 20 RCTs did not report methods of allocation, therefore we assessed these studies as having an unclear risk of sequence generation bias. Of the 20 included RCTs, eight reported the implementation of allocation concealment: one study used sealed, numbered envelopes; two studies used coded bottles; and five studies maintained the randomization code at a pharmacy or other central study center. The remaining 12 RCTs did not report methods of allocation concealment. The seven studies that were not RCTs were controlled clinical trials but did not use randomization to assign participants to treatment. Participants were allocated by alternation in four studies, and by date of admission in one study. The method of allocation was not reported in the remaining two controlled clinical trials.
2.
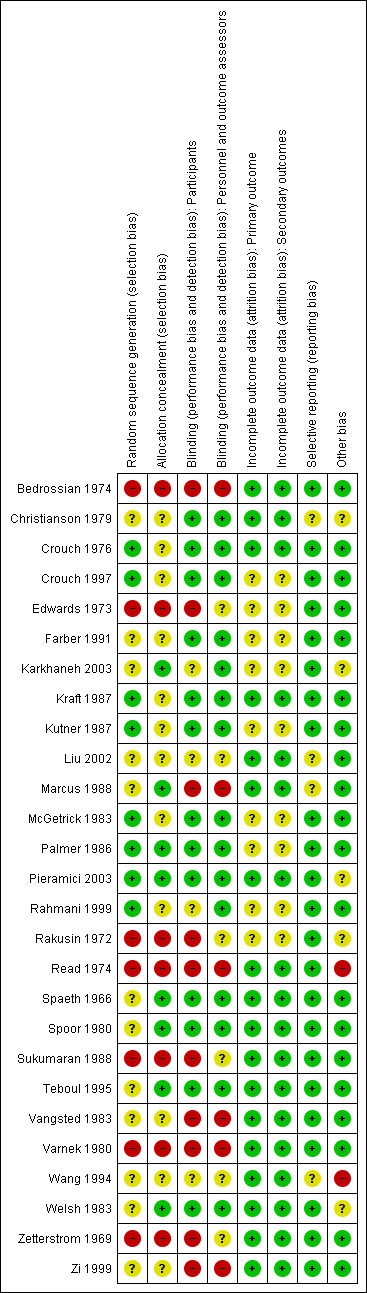
Methodologic quality summary: review authors' judgments about each methodologic quality item for each included study. Green: low risk of bias; red: high risk of bias; yellow: unclear risk of bias.
Blinding
Twelve of the 20 included RCTs were double‐masked (participants and investigators), placebo‐controlled trials. One study investigating two doses of oral aminocaproic acid was also double‐masked (Palmer 1986). Participants and treating physicians were partially masked in two studies in which there was only one placebo‐controlled group for two intervention groups that had different treatment regimens (Karkhaneh 2003; Rahmani 1999). In both of these studies, it was noted that the ophthalmologists and outcome assessors were not involved in participant treatment and were masked to the treatment groups. The interventions of interest in two studies precluded masking: the first study compared aspirin three times daily versus observation only (Marcus 1988), and the second study compared bed confinement versus walking and oral tranexamic acid three times daily (Vangsted 1983). Two studies did not mention whether or not masking occurred (Liu 2002; Wang 1994), and the authors of one study reported that no masking was done (Zi 1999).
Masking of participants was not possible because of the type of interventions in four of the seven quasi‐randomized studies included in this review (Edwards 1973; Rakusin 1972; Read 1974; Zetterstrom 1969), and was not reported in one (Bedrossian 1974). Masking of participants with the use of placebo pills could have been implemented, but was not achieved in the remaining two quasi‐randomized studies (Sukumaran 1988; Varnek 1980). Masking of outcome assessors was not reported or unclear in all seven quasi‐randomized studies.
Incomplete outcome data
Attrition rates for the included studies were minimal due to the nature of the condition and treatment regimens. Typically, treatment duration for traumatic hyphema at the time the studies were completed was one week or less, and hospitalization was frequently implemented. Eighteen of the 27 included studies reported no exclusions or losses to follow‐up, and thus used intention‐to‐treat analyses. Of the nine studies that excluded participants from the analysis, four studies excluded only one or two participants due to an adverse effect of treatment (Crouch 1997; Kutner 1987; Palmer 1986), treatment failure (Palmer 1986), or loss of a participant's medical record (McGetrick 1983). The remaining five studies did not conduct intention‐to‐treat analyses, although all reported the number of exclusions and losses to follow‐up.
Selective reporting
All but five of the included studies reported risk of a secondary hemorrhage as a primary outcome: in two studies, time to resolution of the hyphema was reported as the primary outcome (Bedrossian 1974; Zi 1999); in another two studies, secondary hemorrhage was reported as a secondary outcome with no primary outcome identified (Edwards 1973; Read 1974); and in the fifth study, absence of secondary hemorrhage was part of the composite outcome of being "cured" (Wang 1994). All investigators except Zi and colleagues and Wang and colleagues reported results for secondary hemorrhage. In four included studies the risk of reporting bias was unclear: due to the lack of study details available in the abstract, and no full version being published (Christianson 1979); because study outcomes were not clearly stated in the publication (Liu 2002; Wang 1994); and because only results for secondary hemorrhage were reported, although VA and IOP were measured throughout the duration of the study (Marcus 1988).
Other potential sources of bias
We detected no other potential sources of bias in 18 of the included studies. We classified four studies as having an unclear risk of other bias because the publications had poor descriptions of study methods and results (Christianson 1979; Liu 2002; Marcus 1988; Wang 1994). In two studies, some participants were selected to receive surgery either at recruitment (Rakusin 1972), or after having been assigned to a treatment group (Read 1974). We classified three studies as having an unclear risk of other bias because they were funded by pharmaceutical companies that either manufactured the drug being investigated in the study or that supplied study drug (Karkhaneh 2003; Pieramici 2003; Welsh 1983).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Summary of findings for the main comparison. Systemic aminocaproic acid compared with placebo for traumatic hyphema.
| Systemic aminocaproic acid compared with placebo for traumatic hyphema | ||||||
|
Patient or population: people with traumatic hyphema Settings: hospital Intervention: 100 mg aminocaproic acid every 4 hours Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Systemic aminocaproic acid | |||||
| Short‐term visual acuity 20/40 or better ≤ 2 weeks after treatment |
769 per 1000 |
699 per 1000 (438 to 1015) |
RR 0.87 (0.57 to 1.32) | 34 (1) | ⊕⊕⊝⊝ low1,2 | Outcome not reported by 5 other studies. |
| Medium‐term visual acuity 20/40 or better > 2 weeks and ≤ 2 months after treatment | See comment | ‐ | ‐ | ‐ | ‐ | Outcome not reported. |
| Long‐term visual acuity 20/40 or better > 2 months after treatment | 731 per 1000 | 752 per 1000 (599 to 942) | RR 1.03 (0.82 to 1.29) | 108 (2) | ⊕⊕⊝⊝ low1,3 | Outcome not reported by 4 other studies. |
| Final visual acuity 20/40 or better at resolution of hyphema | 866 per 1000 | 908 per 1000 (805 to 1021) | RR 1.05 (0.93 to 1.18) | 143 (2) | ⊕⊕⊝⊝ low1,2 | Outcome not reported by 4 other studies. |
| Time to resolution of primary hemorrhage | See comment | ‐ | ‐ | 330 (6) | ⊕⊕⊝⊝ low1,2 | Average time to resolution of the hemorrhage ranged from 4.1 to 6.7 days in participants receiving oral aminocaproic acid and from 2.4 to 6.3 days in participants receiving placebo (data not meta‐analyzable). |
| Secondary hemorrhage at any time point | 148 per 1000 | 42 per 1000 (19 to 89) | RR 0.28 (0.13 to 0.60) | 330 (6) | ⊕⊕⊝⊝ low2,3 | |
| Adverse effects: nausea or vomiting | 17 per 1000 | 148 per 1000 (36 to 612) | RR 8.60 (2.09 to 35.50) | 131 (3) | ⊕⊕⊕⊝ moderate1 | Outcome not reported by 3 other studies. |
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1Downgraded for imprecision (‐1). 2Downgraded for indirectness (outcome unrelated to visual acuity or possible complications) (‐1). 3Downgraded for inconsistency (large variation in effect estimate across trials) (‐1).
Summary of findings 2. Topical aminocaproic acid compared with placebo for traumatic hyphema.
| Topical aminocaproic acid compared with placebo for traumatic hyphema | ||||||
|
Patient or population: people with traumatic hyphema Settings: hospital Intervention: 25% to 30% aminocaproic acid in gel every 6 hours Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Topical aminocaproic acid | |||||
| Short‐term visual acuity 20/40 or better ≤ 2 weeks after treatment | 481 per 1000 | 419 per 1000 (226 to 770) | RR 0.87 (0.47 to 1.60) | 51 (1) | ⊕⊕⊝⊝ low1 | Outcome not reported by 1 other study. |
| Medium‐term visual acuity 20/40 or better > 2 weeks and ≤ 2 months after treatment | See comment | ‐ | ‐ | ‐ | ‐ | 1 study reported no difference between groups after 2 weeks of follow‐up. |
| Long‐term visual acuity 20/40 or better > 2 months after treatment | See comment | ‐ | ‐ | ‐ | ‐ | Outcome not reported. |
| Final visual acuity 20/40 or better at resolution of hyphema | See comment | ‐ | ‐ | ‐ | ‐ | Outcome not reported. |
| Time to resolution of primary hemorrhage | See comment | ‐ | ‐ | 142 (2) | ⊕⊕⊝⊝ low2,3 | In 1 study, average time to resolution of the hemorrhage was 11.1 days in participants receiving topical aminocaproic acid and 9.3 and 9.5 days in those receiving placebo; in the second study, the authors reported "no difference" in time to resolution between study groups. |
| Secondary hemorrhage at any time point | 227 per 1000 | 109 per 1000 (45 to 250) | RR 0.48 (0.20 to 1.10) | 131 (2) | ⊕⊕⊝⊝ low3,4 | |
| Adverse effects: systemic hypotension | See comment | ‐ | ‐ | ‐ | ‐ | 1 study reported that 13% of participants in the topical aminocaproic acid group versus 11% of participants in the placebo group had systemic hypotension. |
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1Downgraded for imprecision (‐2). 2Downgraded for inconsistency (large variation in effect estimate across trials) (‐1). 3Downgraded for indirectness (outcome unrelated to visual acuity or possible complications) (‐1). 4Downgraded for imprecision (‐1).
Summary of findings 3. Systemic tranexamic acid compared with control for traumatic hyphema.
| Systemic tranexamic acid compared with control for traumatic hyphema | ||||||
|
Patient or population: people with traumatic hyphema Settings: hospital Intervention: 25 to 75 mg/kg tranexamic acid per day Comparison: control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Tranexamic acid | |||||
| Short‐term visual acuity 20/40 or better ≤ 2 weeks after treatment | 680 per 1000 | 754 per 1000 (666 to 850) | RR 1.11 (0.98 to 1.25) | 303 (3) | ⊕⊕⊝⊝ low1,2 | Outcome not reported by 2 other studies. |
| Medium‐term visual acuity 20/40 or better > 2 weeks and ≤ 2 months after treatment | See comment | ‐ | ‐ | ‐ | ‐ | Outcome not reported. |
| Long‐term visual acuity 20/40 or better > 2 months after treatment | See comment | ‐ | ‐ | ‐ | ‐ | Outcome not reported. |
| Final visual acuity 20/40 or better at resolution of hyphema | See comment | ‐ | ‐ | ‐ | ‐ | Outcome not reported. |
| Time to resolution of primary hemorrhage | See comment | ‐ | ‐ | 549 (5) | ⊕⊝⊝⊝ very low1,3,4 | In 1 study, average time to resolution of the hemorrhage was 4.0 days in 72 participants receiving tranexamic acid and 3.7 days in 59 participants recieving placebo. In another study, average time to resolution was 4.6 days in 17 participants receiving tranexamic acid and 3.9 days in 18 participants not receiving drug. A third study reported that resolution was delayed in the tranexamic acid group, and a fourth study reported faster resolution in the tranexamic acid group. |
| Secondary hemorrhage at any time point | 150 per 1000 | 46 per 1000 (25 to 82) | RR 0.31 (0.17 to 0.55) | 578 (5) | ⊕⊕⊝⊝ low1,4 | |
| Adverse effects: nausea | See comment | ‐ | ‐ | ‐ | ‐ | 1 study reported that 1 of 19 participants receiving tranexamic acid complained of nausea; another study reported that no adverse events were observed in either the drug‐treated or the control group. |
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1Downgraded for risk of bias (‐1). 2Downgraded for imprecision (‐1). 3Downgraded for inconsistency (large variation in effect estimate across trials) (‐1). 4Downgraded for indirectness (outcome unrelated to visual acuity or possible complications) (‐1).
Summary of findings 4. Systemic or topical corticosteroids compared with usual treatment for traumatic hyphema.
| Systemic or topical corticosteroids compared with usual treatment for traumatic hyphema | ||||||
|
Patient or population: people with traumatic hyphema Settings: hospital Intervention: oral or topical corticosteroids Comparison: usual treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Corticosteroids | Usual treatment | |||||
| Short‐term visual acuity between 20/20 and 20/40 ≤ 2 weeks after treatment; oral corticosteroids | 438 per 1000 | 534 per 1000 (385 to 739) | RR 1.22 (0.88 to 1.99) | 155 (1) | ⊕⊕⊝⊝ low1,2 | |
|
Final visual acuity between 20/20 and 20/40 at end of treatment; oral corticosteroids |
900 per 1000 |
909 per 1000 (756 to 1107) |
RR 1.01 (0.84 to 1.23) | 41 (1) | ⊕⊕⊝⊝ low1,2 | |
|
Short‐term visual acuity between 20/20 and 20/40 ≤ 2 weeks after treatment; topical corticosteroids |
619 per 1000 | 464 per 1000 (235 to 910) | RR 0.75 (0.38 to 1.47) | 34 (1) | ⊕⊝⊝⊝ very low1,2,3 | |
|
Final visual acuity between 20/20 and 20/25 at end of treatment; topical corticosteroids |
943 per 1000 |
962 per 1000 (887 to 1047) |
RR 1.02 (0.94 to 1.11) | 111 (1) | ⊕⊕⊝⊝ low1,3 | |
|
Time to resolution of primary hemorrhage; oral corticosteroids |
See comment | See comment | ‐ | 166 (2) | ⊕⊕⊝⊝ low1,2 | First study reported that average resolution of primary hemorrhage was 3.5 days in the oral corticosteroid group and 3.7 days in the control group. The second study reported that average resolution of primary hemorrhage was 4.45 days in the oral corticosteroid group and 4.48 days in the control group. |
|
Time to resolution of primary hemorrhage; topical corticosteroids |
See comment | See comment | ‐ | 34 (1) | ⊕⊝⊝ very low1,2,3 | A single study reported that hyphema had cleared in 10/13 participants in the corticosteroid group and 16/21 participants in control group. |
| Risk of secondary hemorrhage; oral corticosteroids | 250 per 1000 | 170 per 1000 (98 to 295) | RR 0.68 (0.39 to 1.18) | 201 (2) | ⊕⊕⊝⊝ low1,2 | |
| Risk of secondary hemorrhage; topical corticosteroids | 75 per 1000 | 22 per 1000 (4 to 115) | RR 0.29 (0.05 to 1.53) | 151 (2) | ⊕⊝⊝⊝ very low1,2,3 | |
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1Downgraded for imprecision (‐1). 2Downgraded for indirectness (outcome unrelated to visual acuity or possible complications) (‐1). 3Downgraded for risk of bias (‐1).
Summary of findings 5. Other pharmaceutical agents compared with placebo or other control interventions for traumatic hyphema.
| Other pharmaceutical agents compared with placebo or other control interventions for traumatic hyphema | ||||||
|
Patient or population: people with traumatic hyphema Settings: hospital Intervention: other pharmaceutical agent Comparison: placebo or usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Pharmaceutical agent | |||||
| Short‐term visual acuity between 20/20 and 20/60 at end of treatment; cycloplegics versus miotics | 529 per 1000 | 434 per 1000 (211 to 768) | RR 0.82 (0.46 to 1.45) | 34 (1) | ⊕⊕⊝⊝ low1,2 | 1% homatropine versus 4% pilocarpine Visual acuity measured at end of treatment, typically within 2 weeks of occurence of hyphema. |
| Risk of secondary hemorrhage; conjugated estrogen | 217 per 1000 | 257 per 1000 (120 to 552) | RR 1.18 (0.55 to 2.54) | 85 (1) | ⊕⊕⊕⊝ moderate1 | Conjugated estrogen, 5 to 10 mg intramuscularly for children under 10 years of age and 20 mg intravenously for children 10 years of age or older and adults, versus placebo |
| Risk of secondary hemorrhage; cycloplegics | 22 per 1000 | 22 per 1000 (3 to 149) | RR 1.03 (0.15 to 6.99) | 92 (2) | ⊕⊝⊝⊝ very low1,2,3 | 1% homatropine versus 4% pilocarpine in first study; 1% atropine versus 2% pilocarpine in second study |
| Risk of secondary hemorrhage; aspirin | 71 per 1000 | 130 per 1000 (24 to 716) | RR 1.83 (0.33 to 10.02) | 51 (1) | ⊕⊕⊝⊝ low1,2 | 500 mg aspirin 3 times/day for 5 days versus observation |
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1Downgraded for imprecision (‐1). 2Downgraded for risk of bias (‐1). 3Downgraded for inconsistency (large variation in effect estimate across trials) (‐1).
Summary of findings 6. Non‐pharmaceutical interventions compared with usual care for traumatic hyphema.
| Non‐pharmaceutical interventions compared with usual care for traumatic hyphema | ||||||
|
Patient or population: people with traumatic hyphema Settings: hospital Intervention: non‐pharmaceutical interventions Comparison: control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Non‐pharmacuetical agent | |||||
|
Short‐term visual acuity between 20/20 and 20/60 at end of treatment; monocular versus binocular eye patching |
808 per 1000 | 662 per 1000 (541 to 808) | RR 0.82 (0.67 to 1.00) | 46 (1) | ⊕⊕⊝⊝ low1,2 | Visual acuity measured at end of treatment, typically within 2 weeks of occurrence of hyphema. |
| Final visual acuity between 20/20 and 20/50; monocular versus binocular eye patching | 846 per 1000 | 803 per 1000 (652 to 998) | RR 0.95 (0.77 to 1.18) | 53 (1) | ⊕⊕⊝⊝ low1,2 | Time frame when visual acuity measured not reported. |
|
Short‐term visual acuity between 20/20 and 20/50 at end of treatment; ambulatory versus bed rest |
846 per 1000 | 931 per 1000 (710 to 1218) | RR 1.10 (0.84 to 1.44) | 52 (1) | ⊕⊕⊝⊝ low1,2 | Visual acuity measured at end of treatment, typically within 2 weeks of occurrence of hyphema. |
|
Time to resolution of primary hemorrhage; ambulatory versus bed rest |
See comment | See comment | ‐ | 137 (1) | ⊕⊕⊝⊝ low1,2 | A single study reported that mean resolution of primary hemorrhage was 5.8 days in ambulatory group and 5.6 days in bed rest group. |
| Risk of secondary hemorrhage; monocular versus binocular eye patching | 148 per 1000 | 114 per 1000 (51 to 254) | RR 0.77 (0.35 to 1.72) | 117 (2) | ⊕⊕⊝⊝ low1,2 | |
| Risk of secondary hemorrhage; ambulatory treatment versus bed rest | 185 per 1000 | 238 per 1000 (108 to 445) | RR 1.28 (0.68 to 2.40) | 189 (2) | ⊕⊕⊝⊝ low1,3 | |
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1Downgraded for imprecision (‐1). 2Downgraded for risk of bias (‐1). 3Downgraded for inconsistency (large variation in effect estimate across trials) (‐1).
Systemic antifibrinolytics versus control
Six studies evaluated oral aminocaproic acid versus placebo (Christianson 1979; Crouch 1976; Kraft 1987; Kutner 1987; McGetrick 1983; Teboul 1995). We assessed all six studies as at overall low risk of bias.
Visual acuity
No study observed a difference in VA measured at two weeks or less after the hospital admission. At the time of discharge, Kutner 1987 observed VA of 20/40 or better in 14 of 21 (67%) participants in the systemic aminocaproic acid group and in 10 of 13 (77%) participants in the placebo group (risk ratio (RR) 0.87, 95% confidence interval (CI) 0.57 to 1.32; Analysis 1.1). We graded the certainty of evidence for short‐term VA outcomes as low, downgrading for imprecision (wide confidence interval) and indirectness (lack of standard follow‐up time point) (Table 1)
1.1. Analysis.
Comparison 1 Systemic aminocaproic acid versus placebo, Outcome 1 Short‐term visual acuity from 20/20 to 20/40.
No study of systemic aminocaproic acid reported VA outcomes at medium‐term follow‐up (more than two weeks but within two months).
Two studies evaluating systemic aminocaproic acid measured long‐term VA at nine months or from six months to 2.5 years after discharge (Crouch 1976; Kraft 1987). Neither study found a difference in the proportion of participants who achieved useful final VA, defined as VA between 20/20 and 20/40 (Analysis 1.2). Kraft 1987 reported that 17 of 24 (70.8%) participants who had been assigned to aminocaproic acid had VA between 20/20 and 20/40, compared with 20 of 25 (80%) participants assigned to placebo. Crouch 1976 reported similar results, with 25 of 32 (79%) participants assigned to drug versus 18 of 27 (67%) participants assigned to placebo achieving useful VA. The summary RR for these two studies was 1.03 (95% CI 0.82 to 1.29). We graded the certainty of evidence for long‐term VA outcomes as low, downgrading for imprecision and inconsistency (Table 1)
1.2. Analysis.
Comparison 1 Systemic aminocaproic acid versus placebo, Outcome 2 Long‐term visual acuity between 20/20 and 20/40.
Two additional studies evaluated final VA with the time of measurement including both short‐ and long‐term time points ranging from five days to 3.4 years, in Teboul 1995, or from zero to nine months, in McGetrick 1983. Forty‐six of 48 (95.8%) children in the aminocaproic acid group and 44 of 46 (95.6%) children in the placebo group had good final VA in Teboul 1995. McGetrick 1983 reported that the number of participants with final VA of 20/40 or better was 22 of 28 (78.6%) in the aminocaproic acid group and 14 of 21 (66.6%) in the placebo group. The summary RR for final VA of 20/40 or better for these two studies was 1.05 (95% CI 0.93 to 1.18; Analysis 1.3). We graded the certainty of evidence for final VA outcomes as low, downgrading for imprecision and indirectness (Table 1).
1.3. Analysis.
Comparison 1 Systemic aminocaproic acid versus placebo, Outcome 3 Final visual acuity between 20/20 and 20/40.
Time to resolution of primary hemorrhage
In general, the hyphemas in participants assigned to systemic aminocaproic acid took longer to clear than those in participants assigned to placebo or control groups (Analysis 1.4). Christianson 1979 noted that drug‐treated hyphemas tended to take longer to clear compared with controls but reported that this was significant only among hyphemas filling more than half of the anterior chamber. Of the five remaining studies using systemic aminocaproic acid, the mean time to resolution of the primary hemorrhage ranged from 4.1 to 6.7 days in the aminocaproic acid group and 2.4 to 6.3 days in the placebo group among all participants. Two studies evaluated time to clear the initial hyphema after excluding participants who rebled (Crouch 1976; Kraft 1987). In both studies, the group receiving aminocaproic acid took longer to clear the initial hyphema than the group receiving placebo (4.0 days versus 2.8 days in Crouch 1976, and 5.3 days versus 2.6 days in Kraft 1987). In Kraft 1987, the time to resolution appeared to be associated with initial hyphema severity, with larger initial hyphemas taking longer to resolve. The longer resolution times for drug‐treated groups were statistically significant as reported in the Kraft and Teboul studies individually; however, there were insufficient data to perform a meta‐analysis. In contrast, in McGetrick 1983, the mean time to resolution was longer in the placebo than in the aminocaproic acid group. We graded the certainty of evidence for time to resolution of primary hemorrhage as low, downgrading for imprecision and indirectness (Table 1).
1.4. Analysis.
Comparison 1 Systemic aminocaproic acid versus placebo, Outcome 4 Time to resolution of primary hemorrhage (days).
| Time to resolution of primary hemorrhage (days) | ||||
|---|---|---|---|---|
| Study | Mean (SD) time to resolution in drug treated group | Number of participants in drug treated group | Mean (SD) time to resolution in control group | Number of participants in control group |
| Christianson 1979 | NR | 22 | NR | 23 |
| Crouch 1976 | 4.1 days (4.0 days in study participants without secondary hemorrhage) | 32 (31 without a secondary hemorrhage) | 3.8 days (2.8 days in study participants without secondary hemorrhage) | 27 (18 without a secondary hemorrhage) |
| Kraft 1987 | 8 days (5.3 days in study participants without secondary hemorrhage) | 24 (22 without a secondary hemorrhage) | 5 days (2.6 days in study participants without a secondary hemorrhage) | 25 (24 without a secondary hemorrhage) |
| Kutner 1987 | 4.8 days in all study participants | 21 (no participant had a secondary hemorrhage | 2.4 days in all study participants | 10 study participants without a secondary hemorrhage |
| McGetrick 1983 | 4.5 days in all study participants | 28 (1 study participant had a secondary hemorrhage) | 6.3 days in all study participants | 21 (7 study participants had a secondary hemorrhage) |
| Teboul 1995 | 6.7 days in all study participants | 48 (1 study participant had a secondary hemorrhage) | 2.6 days in all study participants | 46 (2 study participants had a secondary hemorrhage) |
Risk of secondary hemorrhage
All RCTs comparing systemic aminocaproic acid versus placebo reported results on the risk of secondary hemorrhage. Participants assigned to the systemic aminocaproic acid group experienced a secondary hemorrhage less often than participants in the placebo group (RR 0.28, 95% CI 0.13 to 0.60; Analysis 1.5). Because an intention‐to‐treat analysis was not performed in two studies of systemic aminocaproic acid, each of which excluded one participant from analysis (Kutner 1987; McGetrick 1983), we performed a sensitivity analysis to assess the effect of excluding these studies. This resulted in an inconclusive effect of aminocaproic acid (RR 0.43, 95% CI 0.17 to 1.08). We graded the certainty of evidence for risk of secondary hemorrhage as low, downgrading for inconsistency and indirectness (Table 1).
1.5. Analysis.
Comparison 1 Systemic aminocaproic acid versus placebo, Outcome 5 Risk of secondary hemorrhage.
Of the six studies comparing systemic aminocaproic acid versus placebo, four excluded people with sickle cell trait (Kraft 1987; Kutner 1987; McGetrick 1983; Teboul 1995). Crouch 1976 reported that eight participants had sickle cell trait, although the trial investigators do not say to which group these participants were assigned. The one participant who had a secondary hemorrhage in the aminocaproic acid group and two of the nine participants who had a secondary hemorrhage in the placebo group also had sickle cell trait. Of the eight participants with sickle cell trait, five rebled.
Almost all studies reported initial hyphema severity, mostly by the proportion of anterior chamber filled with blood or by the height of the hyphema in millimeters. There did not appear to be any overall pattern in the proportion of participants who had a secondary hemorrhage within groups defined by initial hyphema severity (Table 8). Two studies reported that all secondary hemorrhages occurred in initially less severe hyphemas (Kutner 1987; Teboul 1995), while one study found evidence of a higher proportion of secondary hemorrhages when the initial hyphema was more severe (Kraft 1987).
2. Outcomes by initial hyphema severity.
| Study | Severity scale | Reported severity | Secondary hemorrhage | Other outcomes |
| Systemic aminocaproic acid vs control | ||||
| Christianson 1979 | NR | NR | NR | Time to resolution of the primary hyphema was significantly longer (P < 0.05) for participants receiving drug for whom the hyphema filled more than ½ of the anterior chamber. |
| Crouch 1976 | Blood filling < ⅓ of anterior chamber | Reported no statistically significant differences across groups | NR | NR |
| Blood filling ⅓ to ½ of anterior chamber | ||||
| Blood filling > ½ to ¾ of anterior chamber | ||||
| Blood filling > ¾ to total of anterior chamber, but excluded total hyphema | ||||
| Kraft 1987 | Blood filling < ⅓ of anterior chamber | 30/49 (61%) participants: 13/24 (54%) in drug group; 17/25 (68%) in placebo group | 1/3 (33%) secondary hemorrhage (in placebo group) | Excluding secondary hemorrhages, mean time to resolution of 3.4 days in drug group (range 1 to 11 days); mean time to resolution of 2.2 days in placebo group (range 1 to 4 days) |
| Blood filling ⅓ to ½ of anterior chamber | 14/49 (29%) participants: 9/24 (37.5%) in drug group; 5/25 (20%) in placebo group | 1/3 (33%) secondary hemorrhage (in drug group) | Excluding secondary hemorrhages, mean time to resolution of 7.1 days in drug group (range 6 to 9 days); mean time to resolution of 4.0 days in placebo group (range 3 to 4 days) | |
| Blood filling ½ or more of anterior chamber | 5/49 (10%) participants: 2/24 (8.3%) in drug group; 3/25 (12%) in placebo group | 1/3 (33%) secondary hemorrhage (in drug group) | Excluding secondary hemorrhages, time to resolution of 10 days in drug group; mean of placebo 4.3 days (range 3 to 5 days) | |
| Kutner 1987 | Mean hyphema height | 2.2 mm (SD 1.7, n = 21) in drug group; 1.7 mm (SD 1.0, n = 13) in placebo group | "All who rebled had initial hyphemas of 15% or less" | NR |
| McGetrick 1983; | Mean hyphema height | 100% (28/28) hyphemas in drug group were < 25% of anterior chamber; 86% (18/21) hyphemas in placebo group were < 25% of anterior chamber. | 1 secondary hemorrhage in drug group; 6 secondary hemorrhages in placebo group | NR |
| Teboul 1995 | Blood filling < ⅓ of anterior chamber | 88/94 (94%) participants: 44/48 (92%) in drug group; 44/46 (96%) in placebo group | 1 secondary hemorrhage in drug group and 2 secondary hemorrhages in placebo group | NR |
| Blood filling ⅓ to ½ of anterior chamber | 6/94 (6%) participants: 4/48 (8%) in aminocaproic acid group; 2/46 (4%) in placebo group | No rebleeds | NR | |
| Topical aminocaproic acid vs control | ||||
| Karkhaneh 2003 | Blood filling < ¼ of anterior chamber; excluded microscopic hyphemas | 65/80 (81%) participants: 34/41 (83%) in drug group; 31/39 (79.5%) in placebo group | Reported no effect of hyphema size on secondary hyphema (RR 0.7, 95% CI 0.2 to 2.5) | NR |
| Blood filled ¼ to ½ of anterior chamber | 14/80 (18%) participants: 7/41 (17%) in drug group; 7/39 (18%) in placebo group | |||
| Blood filling > ½ of anterior chamber; excluded total or blackball hyphemas | 1/80 (1%) participants: 0/41 in drug group; 1/39 (2.5%) in placebo group | |||
| Pieramici 2003 | Mean hyphema height in millimeters | 1 mm (SE 0) in drug group (range 0 to 4 mm); 2 mm (SE 0) in placebo group (range 0 to 8 mm) | Size of primary hyphema in 2 participants with secondary hemorrhages in drug group: 0.3 and 1 mm; in 8 participants in the placebo group: 0.8, 0.9, 1, 1.4, 1.8, 2, 2, and 4.5 mm | NR |
| Low‐dose vs standard‐dose aminocaproic acid | ||||
| Palmer 1986 | Mean hyphema height in millimeters | 1.7 mm (SD 2.0, range 0.1 to 9.9) in low‐dose group (n = 25); 1.5 mm (SD 2.2, range 0.1 to 9.9) in standard‐dose group (n = 33) | 1 secondary hemorrhage in low‐dose group; 5 secondary hemorrhages in standard‐dose group | NR |
| Systemic vs topical aminocaproic acid | ||||
| Crouch 1997 | Blood filling < ⅓ of anterior chamber | 44/64 (69%) participants | NR | NR |
| Blood filling ⅓ to ½ of anterior chamber | 6/64 (9%) participants | |||
| Blood filling > ½ to ¾ of anterior chamber | 8/64 (13%) participants | |||
| Blood filling > ¾ to total of anterior chamber | 6/64 (9%) participants | |||
| Tranexamic acid vs control | ||||
| Rahmani 1999 | Microscopic, but excluding individuals with unlayered microscopic hyphemas | 17/238 (7%) participants: 6/80 (7%) in aminocaproic acid group; 4/78 (5%) in prednisolone group; 7/80 (9%) in placebo group | 2/43 (5%) secondary hemorrhages | NR |
| Blood filling < ¼ of anterior chamber | 173/238 (72%) participants: 56/80 (70%) in aminocaproic acid group; 61/78 (78%) in prednisolone group; 56/80 (70%) in placebo group | 30/43 (70%) secondary hemorrhages | ||
| Blood filling ¼ to ½ of anterior chamber | 36/238 (15%) participants: 13/80 (16%) in aminocaproic acid group; 10/78 (13%) in prednisolone group; 13/80 (16%) in placebo group | 7/43 (16%) secondary hemorrhages | ||
| Blood filling > ½ of anterior chamber; excluded total hyphemas | 12/238 (5%) participants: 5/80 (6%) in aminocaproic acid group; 3/78 (4%) in prednisolone group; 4/80 (5%) in placebo group | 4/43 (9%) secondary hemorrhages | ||
| Sukumaran 1988 | Hyphema height of 0 to 1 mm | 8/35 (23%) participants: 4/17 (24%) in drug group; 4/18 (22%) in control group | NR | NR |
| Hyphema height of 2 to 3 mm | 12/35 (34%) participants: 6/17 (35%) in drug group; 6/18 (33%) in control group | |||
| Hyphema height of 4 to 5 mm | 10/35 (29%) participants: 5/17 (29%) in drug group; 5/18 (28%) in control group | |||
| Hyphema height of 6 to 7 mm | 5/35 (14%) participants: 2/17 (12%) in drug group; 3/18 (17%) in control group | |||
| Vangsted 1983 | Hyphema height of 1 mm | 10/112 (9%) participants: 8/59 (14%) in drug group; 2/53 (4%) in control group | NR | NR |
| Hyphema height of 2 mm | 33/112 (29%) participants: 15/59 (25%) in drug group; 18/53 (34%) in control group | |||
| Hyphema height of 3 mm | 37/112 (33%) participants: 18/59 (31%) in drug group; 19/53 (36%) in control group | |||
| Hyphema height of 4 mm | 18/112 (16%) participants: 9/59 (15%) in drug group; 9/53 (17%) in control group | |||
| Hyphema height of 5 mm | 9/112 (8%) participants: 6/59 (10%) in drug group; 3/53 (6%) in control group | |||
| Hyphema height of 6 mm | 4/112 (4%) participants: 3/59 (5%) in drug group; 1/53 (2%) in control group | |||
| Hyphema height of 7 mm | None in either group | |||
| Hyphema height of 8 mm | 1/112 (1%) participants: 0/59 (0%) in drug group; 1/53 (2%) in control group | |||
| Varnek 1980 | Mean hyphema height in millimeters | 2.0 mm in drug group (n = 102); 2.1 mm in control group (n = 130) | 1.0 mm in 2 participants in drug group with a secondary hemorrhage; 2.2 mm in 12 participants in control group with a secondary hemorrhage | NR |
| Welsh 1983 | Mean of proportion of anterior chamber area filled with blood | 68% in drug group (n = 19); 63% in placebo group (n = 20) | NR | NR |
| Aminomethylbenzoic acid vs control | ||||
| Liu 2002 | Blood filling < ⅓ of anterior chamber and level is lower than the inferior border of pupil | 47/92 (51%) participants: 31/60 (52%) in drug group; 16/32 (50%) in control group | NR | NR |
| Blood filling ½ of anterior chamber and level is higher than the inferior border of the pupil, but not exceeding the median line | 30/92 (33%) participants: 19/60 (32%) in drug group; 11/32 (34%) in control group | |||
| Blood filling > ½ of anterior chamber or filling the entire anterior chamber | 15/92 (16%) participants: 10/60 (17%) in drug group; 5/32 (16%) in control group | |||
| Systemic corticosteroids vs control | ||||
| Spoor 1980 | 0% to 33% of anterior chamber area filled with blood | 38/43 (88%) participants: 21/23 (91%) in prednisone group; 17/20 (85%) in placebo group | 2/4 (50%) secondary hemorrhages | 1. 30 hyphemas resolved in 5 days or less; 8 hyphemas resolved in more than 5 days 2. 34 participants with final visual acuity between 20/20 and 20/50 |
| > 33% to 75% of anterior chamber filled with blood | 5/43 (12%) participants: 2/23 (9%) in prednisone group; 3/20 (15%) in placebo group | 2/4 (50%) secondary hemorrhages | 1. 1 hyphema resolved in 5 days or less; 4 hyphemas resolved in more than 5 days 2. 5 participants with final visual acuity between 20/20 and 20/50 |
|
| Rahmani 1999 | See above under "Tranexamic acid vs control" | |||
| Topical corticosteroids vs control | ||||
| Zetterstrom 1969 | Mean hyphema height in millimeters | 2.5 mm in topical corticosteroid group (n = 58); 3.5 mm in control group (n = 59) | No participant with secondary hemorrhage in topical corticosteroid group; 4 participants with secondary hemorrhage in control group | NR |
| Antifibrinolytics vs systemic corticosteroids | ||||
| Farber 1991 | Microscopic | 24/112 (21%) participants: 11/56 (20%) in aminocaproic acid group; 13/56 (23%) in prednisone group | 3/8 (38%) secondary hemorrhages: 2 in aminocaproic acid group; 1 in prednisone group | NR |
| Hyphema height 0.1 to 3.9 mm | 80/112 (71%) participants: 41/56 (73%) in aminocaproic acid group; 39/56 (70%) in prednisone group | 4/8 (50%) secondary hemorrhages: 1 in aminocaproic acid group; 3 in prednisone group | ||
| Hyphema height 4.0 to 5.9 mm | 4/112 (4%) participants: 3/56 (6%) in aminocaproic acid group; 1/56 (2%) in prednisone group | No secondary hemorrhages in either group | ||
| Hyphema height 6.0 to 11 mm | 2/112 (2%) participants: 0/56 (0%) in aminocaproic acid group; 2/56 (4%) in prednisone group | No secondary hemorrhages in either group | ||
| Total hyphema | 2/112 (2%) participants: 1/56 (2%) in aminocaproic acid group; 1/56 (2%) in prednisone group | 1/8 (12%) secondary hemorrhage: 1 in aminocaproic acid group; none in prednisone group | ||
| Rahmani 1999 | See above under "Tranexamic acid vs control" | |||
| Conjugated estrogens vs control | ||||
| Spaeth 1966 | Blood filling < 20% of anterior chamber | 55/85 (65%) participants: 28/39 (72%) in estrogen group; 27/46 (59%) in control group | 13/20 (65%) secondary hemorrhages: 8 in estrogen group; 5 in control group | NR |
| Blood filling 20% to 40% of anterior chamber | 17/85 (20%) participants: 5/39 (13%) in estrogen group; 12/46 (26%) in control group | 4/20 (20%) secondary hemorrhages: 1 in estrogen group; 3 in control group | ||
| Blood filling 40% to 60% of anterior chamber | 5/85 (6%) participants: 2/39 (5%) in estrogen group; 3/46 (7%) in control group | 1/20 (5%) secondary hemorrhage: none in estrogen group; 1 in control group | ||
| Blood filling 60% to 80% of anterior chamber | 2/85 (2%) participants: 1/39 (3%) in estrogen group; 1/46 (2%) in control group | No secondary hemorrhages in either group | ||
| Blood filling > 80% of anterior chamber | 6/85 (7%) participants: 3/39 (8%) in estrogen group; 3/46 (7%) in control group | 2/20 (10%) secondary hemorrhages: 1 in estrogen group; 1 in control group | ||
| Cycloplegics vs miotics | ||||
| Bedrossian 1974 | Hyphema height of 1 mm | 20/58 (34%) participants: 10/28 (36%) in the cycloplegic group; 10/30 (33%) in the miotic group | 1/1 (100%) secondary hemorrhage (in cycloplegic group) | Mean time to resolution in cycloplegic group of 1.9 days (SD 1.4); mean time to resolution in miotic group of 2.5 days (SD 1) |
| Hyphema height of 2 mm | 22/58 (38%) participants: 10/28 (36%) in the cycloplegic group; 12/30 (40%) in the miotic group | No secondary hemorrhages in either group | Mean time to resolution in cycloplegic group of 3.3 days (SD 1.8); mean time to resolution in miotic group of 4.2 days (SD 1.3) | |
| Hyphema height of 3 mm | 12/58 (21%) participants: 6/28 (21%) in the cycloplegic group; 6/30 (20%) in the miotic group | No secondary hemorrhages in either group | Mean time to resolution in cycloplegic group of 3.2 days (SD 1.9); mean time to resolution in miotic group of 4.0 days (SD 1.1) | |
| Hyphema height of 4 mm | 4/58 (7%) participants: 2/28 (7%) in the cycloplegic group; 2/30 (7%) in the miotic group | No secondary hemorrhages in either group | Mean time to resolution in cycloplegic group of 2.5 days (1 resolved on day 2 and 1 on day 3); mean time to resolution in miotic group of 4.0 days (1 resolved on day 3 and 1 on day 5) | |
| Aspirin vs no aspirin | ||||
| Marcus 1988 | Reported that "the two groups were comparable with respect to age, cause, and extent of hyphema" and that 2 of 3 eyes with a secondary hemorrhage in the aspirin group (n = 23) had an initial total hyphema, while of the 2 eyes with a secondary hemorrhage in the control group (n = 28), 1 had 30% and 1 had almost total hyphema | NR | ||
| Traditional Chinese medicine vs control treatment | ||||
| Wang 1994 | Any level | No significant differences between groups | NR | Proportion of participants who were "cured" (defined as the resolution of the primary hemorrhage after 5 days of treatment, visual acuity of 0.7 or better after resolution of the primary hemorrhage, and no recurrence of bleeding for 1 week following resolution of the primary hemorrhage) was 29/45 (64%) in the traditional Chinese medicine group and 10/38 (26%) in the control group. |
| Monocular vs binocular patching | ||||
| Edwards 1973 | Blood filling < ⅓ of anterior chamber | 42/64 (66%) participants: 21/35 (60%) in the monocular patching group; 21/29 (72%) in the binocular patching group | 7/14 (50%) secondary hemorrhages: 4 in the monocular group; 3 in the binocular group | 62% (13/21) of participants with final visual acuity of 20/50 or better in the monocular group; 71% (15/21) of participants with final visual acuity of 20/50 or better in the binocular group |
| Blood filling ⅓ to ½ of anterior chamber | 14/64 (22%) participants: 9/35 (26%) in the monocular patching group; 5/29 (17%) in the binocular patching group | 7/14 (50%) secondary hemorrhages: 4 in the monocular group; 3 in the binocular group | 57% (8/14) of participants with final visual acuity of 20/50 or better in the monocular group; 62% (5/8) of participants with final visual acuity of 20/50 or better in the binocular group | |
| Blood filling ½ or more of anterior chamber | 8/64 (12%) participants: 5/35 (14%) in the monocular patching group; 3/29 (10%) in the binocular patching group | |||
| Ambulatory vs conservative treatment | ||||
| Read 1974 | Blood filling < ⅓ of anterior chamber | 79/137 (58%) participants: 47/71 (66%) in the ambulatory group; 32/66 (48%) in the conservative group | 16/30 (53%) secondary hemorrhages: 9 in the ambulatory group; 7 in the conservative group | NR |
| Blood filling ⅓ to ½ of anterior chamber | 28/137 (20%) participants: 11/71 (16%) in the ambulatory group; 17/66 (26%) in the conservative group | 5/30 (17%) secondary hemorrhages: 4 in the ambulatory group; 1 in the conservative group | ||
| Blood filling ½ but not total anterior chamber | 19/137 (14%) participants: 8/71 (11%) in the ambulatory group; 11/66 (17%) in the conservative group | 6/30 (20%) secondary hemorrhages: 3 in the ambulatory group; 3 in the conservative group | ||
| Total hyphema | 11/137 (8%) participants: 5/71 (7%) in the ambulatory group; 6/66 (9%) in the conservative group | 3/30 (10%) secondary hemorrhages: 2 in the ambulatory group; 1 in the conservative group | ||
| Elevation of head vs lying flat | ||||
| Zi 1999 | Blood filling < ½ of anterior chamber, and level was lower than the inferior border of pupil | 36/74 (49%) participants: 18/35 (51%) with elevation of the head; 18/39 (46%) lying flat | NR | NR |
| Blood filling ½ of anterior chamber, and level was higher than the inferior border of the pupil | 19/74 (26%) participants: 6/35 (17%) with elevation of the head; 13/39 (33%) lying flat | NR | NR | |
| Blood filling > ½ of anterior chamber or filling the entire anterior chamber | 19/74 (26%) participants: 11/35 (31%) with elevation of the head; 8/39 (21%) lying flat | NR | NR | |
| Other | ||||
| Rakusin 1972 * | Blood filling < ½ of anterior chamber | 213 participants | NR | 1. 4% (8/213) of participants with elevated intraocular pressure across all participants 2. 22% (47/213) of participants with complications 3. 78% (166/213) of participants with final visual acuity better than 20/60 |
| Blood filling > ½ of anterior chamber | 157 participants | NR | 1. 85% (133/157) of participants with elevated intraocular pressure across all participants 2. 78% (123/157) of participants with complications 3. 28% (44/157) of participants with final visual acuity better than 20/60 |
|
*Rakusin 1972 reported severity for entire study population rather than by trials of topical corticosteroids, cycloplegics vs miotics, monocular vs binocular patching, and ambulatory vs conservative treatment. See under "Other."
Abbreviations: CI: confidence interval; n: number of participants; NR: not reported; RR: risk ratio; SD: standard deviation; SE: standard error.
Time to rebleed
Five of the six studies that studied systemic aminocaproic acid reported data on the time between the initial injury and a secondary hemorrhage (Analysis 1.6). Of the 10 participants who had a secondary hemorrhage in Crouch 1976, the one participant in the aminocaproic acid group rebled on day one, and the nine placebo‐treated participants rebled between days two and seven. Of the three participants in Kraft 1987 who experienced a secondary hemorrhage, the two treated with aminocaproic acid had a rebleed on days three and four, and the placebo‐treated participant rebled on day four. All three participants who rebled in Kutner 1987 were in the placebo group and rebled on day two. In the one aminocaproic acid‐treated participant who rebled in McGetrick 1983, the secondary hemorrhage occurred on day four, and three of the five participants in the placebo group rebled on day three, one on day five, and one on day six. Of the three participants who rebled in Teboul 1995, one rebled on day two (placebo); one rebled on day six (aminocaproic acid); and one rebled on day seven (placebo).
1.6. Analysis.
Comparison 1 Systemic aminocaproic acid versus placebo, Outcome 6 Time to rebleed (days).
| Time to rebleed (days) | ||||
|---|---|---|---|---|
| Study | Number of rebleeds in drug treated group | Time to rebleed in drug treated group | Number of rebleeds in control group | Time to rebleed in control group |
| Christianson 1979 | 2 of 22 | NR | 1 of 23 | NR |
| Crouch 1976 | 1 of 32 | Day 1 | 9 of 27 | Days 2 to 7: 2 on day 2; 2 on day 3; 4 on day 4; and 1 on day 7 |
| Kraft 1987 | 2 of 24 | Days 3 and 4 | 1 of 25 | Day 4 |
| Kutner 1987 | 0 of 21 | NA | 3 of 13 | All rebled on Day 2 |
| McGetrick 1983 | 1 of 28 | Day 4 | 7 of 21 | Days 3 to 6: 5 on day 3; 1 on day 5; and 1 on day 6 |
| Teboul 1995 | 1 of 48 | Day 6 | 2 of 46 | Days 2 and 7 |
Overall, there appeared to be little difference in the time for a secondary hemorrhage to occur, although the small numbers of events make statistical testing unreliable. We graded the certainty of evidence for time to secondary hemorrhage as low, downgrading for imprecision and indirectness.
Risk of corneal blood stain
One study examining oral aminocaproic acid reported outcomes for corneal blood stain (Crouch 1976). Two participants in the placebo group who also had secondary hemorrhages required surgery "due to increased intraocular pressure and early corneal bloodstaining" (RR 0.17, 95% CI 0.01 to 3.39; Analysis 1.7; Table 9). We graded the certainty of evidence for corneal blood stain as low, downgrading for imprecision and indirectness.
1.7. Analysis.
Comparison 1 Systemic aminocaproic acid versus placebo, Outcome 7 Risk of corneal blood stain.
3. Risk of corneal blood staining.
| Study | Test intervention | No. with outcome/no. in group | Control intervention | No. with outcome/no. in group | Total no. with outcome/total no. |
| Aminocaproic acid | |||||
| Crouch 1976 | Sytemic aminocaproic acid | 0/32 | Placebo | 2/27 | 2/59 |
| Crouch 1997 | Systemic aminocaproic acid | 0/29 | Topical aminocaproic acid | 0/35 | 0/64 |
| Tranexamic acid | |||||
| Vangsted 1983 | Tranexamic acid | 0/59 | Bed rest only | 0/53 | 0/112 |
| Varnek 1980 | Tranexamic acid | 1/102 | Conservative treatment | 0/130 | 1/232 |
| Prednisone/cortisone | |||||
| Spoor 1980 | Systemic prednisone | NR | Placebo | NR | 1/43 |
| Zetterstrom 1969 | Atropine plus cortisone eyedrops | 0/58 | Conservative treatment | 1/59 | 1/117 |
| Estrogen | |||||
| Spaeth 1966 | Estrogen | 2/39 | Placebo | 2/46 | 4/85 |
| Non‐drug medical interventions | |||||
| Edwards 1973 | Monocular patching | 1/35 | Binocular patching | 1/29 | 2/64 |
| Read 1974 | Moderate ambulatory activity, patching and shielding of injured eye | 5/71 | Bed rest with elevation of the head, bilateral patches and eye shield | 4/66 | 9/137 |
NR: not reported.
Risk of peripheral anterior synechiae formation
Crouch 1976 reported that 14 participants in the study cohort experienced PAS formation. The difference between groups was reported to be non‐significant, although the number of participants for each group was not reported (Table 10).
4. Risk of peripheral anterior synechiae.
| Study | Test intervention | No. with outcome/no. in group | Control intervention | No. with outcome/no. in group | Total no. with outcome/total no. |
| Aminocaproic acid | |||||
| Crouch 1997 | Systemic aminocaproic acid | NR | Topical aminocaproic acid | NR | 4/64 |
| Prednisone | |||||
| Spoor 1980 | Systemic prednisone | 0/23 | Placebo | 0/20 | 0/43 |
| Conjugated estrogen | |||||
| Spaeth 1966 | Conjugated estrogens | NR | Placebo | NR | 15/85 |
| Non‐drug medical interventions | |||||
| Read 1974 | Moderate ambulatory activity, patching and shielding of injured eye | NR | Bed rest with elevation of the head, bilateral patches and eye shield | NR | 9/137 |
NR: not reported
Risk of glaucoma or elevated intraocular pressure
Three studies reported the number of participants with elevated IOP in the systemic aminocaproic acid and placebo groups (Kraft 1987; Kutner 1987; Teboul 1995). None of the studies included participants with sickle cell disease/trait (Analysis 1.8). Of these three studies, one (Teboul 1995) reported that six participants (three in each group) developed transient increases in IOP that did not persist following discharge (RR 0.96, 95% CI 0.18 to 5.00). In the remaining two studies, the investigators reported that participants had IOP greater than 25 mmHg at follow‐up, with Kraft 1987 reporting that two participants (one in each group) had elevated IOP, and Kutner 1987 reporting that four participants (one in the aminocaproic acid group and three in the control group) had elevated IOP at time of discharge (summary RR 0.38, 95% CI 0.08 to 1.82; Analysis 1.9; Table 11). We graded the certainty of evidence for IOP as low, downgrading for imprecision and indirectness.
1.8. Analysis.
Comparison 1 Systemic aminocaproic acid versus placebo, Outcome 8 Risk of glaucoma or increases in IOP.
| Risk of glaucoma or increases in IOP | ||||
|---|---|---|---|---|
| Study | Odds Ratio [95% CI] | Total patients (N) | Definition of outcome | Patients with sickle cell/trait |
| Transient increase in IOP | ||||
| Teboul 1995 | 0.96 [0.18, 5.00] | 94 | Transient IOP greater than 25 mmHg, all patients had normal IOP at discharge (5 days) | None (excluded) |
| Persistent increase in IOP | ||||
| Kraft 1987 | 1.04 [0.06, 17.69] | 49 | IOP greater than 25 mmHg at follow‐up (6 weeks to 18 months) | None (excluded) |
| Kutner 1987 | 0.17 [0.02, 1.81] | 34 | Elevated IOP at time of discharge (6 days) | None (excluded) |
1.9. Analysis.
Comparison 1 Systemic aminocaproic acid versus placebo, Outcome 9 Risk of glaucoma or elevated IOP.
5. Risk of elevated intraocular pressure.
| Study | Test intervention | No. with outcome/no. in group | Control intervention | No. with outcome/no. in group | Total no. with outcome/total no. |
| Aminocaproic acid | |||||
| Kraft 1987 | Systemic aminocaproic acid | 1/24 | Placebo | 1/25 | 2/49 |
| Kutner 1987 | Systemic aminocaproic acid | 1/21 | Placebo | 3/13 | 4/34 |
| Teboul 1995 | Systemic aminocaproic acid | 3/48 | Placebo | 3/46 | 6/94 |
| Pieramici 2003 | Topical aminocaproic acid | 2/24 | Placebo | 1/27 | 3/51 |
| Palmer 1986 | Standard‐dose systemic aminocaproic acid | 2/33 | Low‐dose oral aminocaproic acid | 0/26 | 2/59 |
| Tranexamic acid | |||||
| Vangsted 1983 | Tranexamic acid | 8/59 | Bed rest only | 6/53 | 14/112 |
| Varnek 1980 | Tranexamic acid | 8/102 | Conservative treatment | 7/130 | 15/232 |
| Rahmani 1999 | Tranexamic acid | 12/80 | Placebo | 12/80 | 24/160 |
| Welsh 1983 | Tranexamic acid | 1/19 | Placebo | 2/20 | 3/39 |
| Prednisone/cortisone | |||||
| Spoor 1980 | Systemic prednisone | 0/23 | Placebo | 0/20 | 0/43 |
| Rahmani 1999 | Systemic prednisone | 9/78 | Placebo | 12/80 | 21/158 |
| Zetterstrom 1969 | Atropine plus cortisone eyedrops | 3/58 | Conservative treatment | 2/59 | 5/117 |
| Non‐drug medical interventions | |||||
| Edwards 1973 | Monocular patching | 3/35 | Binocular patching | 0/29 | 3/64 |
| Read 1974 | Ambulation | 17/71 | Bed rest | 19/66 | 36/137 |
| Zi 1999 | Lying in right and left lateral position | 7/39 | Lying in semi‐reclining position | 8/35 | 15/74 |
Risk of optic atrophy
Crouch 1976 reported that two participants (7.4%) in the placebo group and no participants in the aminocaproic acid group developed optic atrophy (RR 0.17, 95% CI 0.01 to 3.39; Analysis 1.10; Table 12). We graded the certainty of evidence for optic atrophy as low, downgrading for imprecision and indirectness.
1.10. Analysis.
Comparison 1 Systemic aminocaproic acid versus placebo, Outcome 10 Risk of optic atrophy.
6. Risk of optic atrophy.
| Study | Test intervention | No. with outcome/no. in group | Control intervention | No. with outcome/no. in group | Total no. with outcome/total no. |
| Aminocaproic acid | |||||
| Crouch 1976 | Systemic aminocaproic acid | 0/32 | Placebo | 2/27 | 2/59 |
| Crouch 1997 | Systemic aminocaproic acid | 0/29 | Topical aminocaproic acid | 0/35 | 0/64 |
| Tranexamic acid | |||||
| Varnek 1980 | Tranexamic acid | 1/102 | Conservative treatment | 0/130 | 1/232 |
| Cortisone | |||||
| Zetterstrom 1969 | Atropine plus cortisone eyedrops | 0/58 | Conservative treatment | 1/59 | 1/117 |
| Non‐drug medical interventions | |||||
| Read 1974 | Moderate ambulatory activity, patching and shielding of injured eye | NR | Bed rest with elevation of the head, bilateral patches and eye shield | NR | 8/137 |
NR: not reported.
Adverse effects
Adverse events occurred significantly more often in participants treated with oral aminocaproic acid than in participants who received placebo (RR 8.60, 95% CI 2.09 to 35.50; Analysis 1.11). We graded the certainty of evidence as moderate, downgrading for imprecision (Table 1). In addition to increased nausea and vomiting in the aminocaproic acid group, McGetrick 1983 reported that two participants experienced diarrhea and one participant had muscle cramps (Table 13). No participants in Kutner 1987 had diarrhea or muscle cramps, but 10 (45%) of the participants in the aminocaproic acid group had at least one complication compared with only one participant (8%) in the placebo group. Complications other than nausea and vomiting reported in Kutner 1987 included lightheadedness and systemic hypotension.
1.11. Analysis.
Comparison 1 Systemic aminocaproic acid versus placebo, Outcome 11 Adverse effects: nausea or vomiting.
7. Risk of non‐ocular adverse effects.
| Study ID | Comparison | Type of complication | Results |
| Aminocaproic acid | |||
| Kraft 1987 | Systemic aminocaproic acid vs placebo | Nausea | Drug group: 8 of 24; placebo group 1 of 25 |
| Kutner 1987 | Systemic aminocaproic acid vs placebo | Nausea or vomiting | Drug group: 6 of 21; placebo group: 0 of 13 |
| Lightheadedness | Drug group: 7 of 21; placebo group: 1 of 13 | ||
| Systemic hypotension | Drug group: 4 of 21; placebo group: 1 of 13 | ||
| Total complications | Drug group: 10 of 21; placebo group: 1 of 13 | ||
| McGetrick 1983 | Systemic aminocaproic acid vs placebo | Nausea or vomiting | Drug group: 6 of 28; placebo group: 0 of 20 |
| Diarrhea | Drug group: 2 of 28; placebo group: 0 of 20 | ||
| Muscle cramps | Drug group: 1 of 28; placebo group: 0 of 20 | ||
| Pieramici 2003 | Topical aminocaproic acid vs placebo | Systemic hypotension | Drug group: 3 of 24; placebo group: 3 of 27 |
| Crouch 1997 | Systemic vs topical aminocaproic acid | Dizziness, nausea, vomiting | Oral group: 5 of 29; topical group: 1 of 35 |
| Palmer 1986 | Low‐dose vs standard‐dose systemic aminocaproic acid | Nausea or vomiting | Low‐dose group: 5 of 25; standard‐dose group: 9 of 33 |
| Dizziness and hypotension | Low‐dose group: 0 of 25; standard‐dose group: 5 of 33 | ||
| Syncope | Low‐dose group: 0 of 25; standard‐dose group: 2 of 33 | ||
| Diarrhea | Low‐dose group: 1 of 25; standard‐dose group: 0 of 33 | ||
| Rash or pruritis | Low‐dose group: 1 of 25; standard‐dose group: 2 of 33 | ||
| Hot flashes | Low‐dose group: 1 of 25; standard‐dose group: 0 of 33 | ||
| Dry mouth or nose | Low‐dose group: 1 of 25; standard‐dose group: 0 of 33 | ||
| Farber 1991 | Systemic aminocaproic acid vs oral prednisone | Any adverse event | Aminocaproic acid group: 0 of 56; prednisone group: 0 of 56 |
| Tranexamic acid | |||
| Welsh 1983 | Tranexamic acid vs placebo | Nausea | Drug group: 1 of 19; placebo group: 0 of 20 |
| Rahmani 1999 | Tranexamic acid vs placebo | Nausea | Drug group: 0 of 80; placebo group: 0 of 80 |
| Aminomethylbenzoic acid | |||
| Liu 2002 | Systemic aminomethylbenzoic acid vs placebo | Nausea and vomiting | Drug group: 7 of 60; placebo group: NR |
NR: not reported.
Quality of life outcomes
No study reported any quality of life outcomes.
Economic outcomes
Two studies reported duration of hospitalization, although insufficient details were provided to perform a meta‐analysis (Analysis 1.12). McGetrick 1983 reported that the mean duration of hospitalization was 5.7 days for the aminocaproic acid group and 7.3 days for the placebo group; the difference was not statistically significant. This trend was the reverse in Teboul 1995, in which the aminocaproic acid group had a longer hospital stay (7.3 days) compared with the placebo group (5.4 days) (P < 0.001).
1.12. Analysis.
Comparison 1 Systemic aminocaproic acid versus placebo, Outcome 12 Duration of hospitalization (days).
| Duration of hospitalization (days) | ||||
|---|---|---|---|---|
| Study | Mean (SD) duration of hospitalization for drug treated group | Number of participants in drug treated group | Mean (SD) duration of hospitalization in control group | Number of participants in control group |
| McGetrick 1983 | 5.7 days | 28 | 7.3 days | 20 |
| Teboul 1995 | 7.3 days | 48 | 5.4 days | 46 |
Topical antifibrinolytics versus control
Two studies evaluated topical aminocaproic acid versus placebo (Karkhaneh 2003; Pieramici 2003). We assessed both studies as at overall low risk of bias.
Visual acuity
Pieramici 2003 reported that 10 of 24 (42%) participants in the topical aminocaproic acid group and 13 of 27 (48%) participants in the placebo group had VAs of 20/40 or better seven days after study enrollment (RR 0.87, 95% CI 0.47 to 1.60; Analysis 2.1). We graded the certainty of evidence for short‐term visual acuity outcomes as low, downgrading two levels for imprecision (Table 2).
2.1. Analysis.
Comparison 2 Topical aminocaproic acid versus placebo, Outcome 1 Short‐term visual acuity from 20/20 to 20/40.
Karkhaneh 2003 did not report on the proportion of participants with good VA, but did report that there was no significant difference in VA between topical aminocaproic acid‐treated participants and placebo‐treated participants after two weeks of follow‐up.
Neither study reported long‐term VA or final VA outcomes.
Time to resolution of primary hemorrhage
The mean time to resolution of primary hemorrhage in participants receiving topical aminocaproic acid in Karkhaneh 2003 was 11.1 days (standard deviation (SD) 4.7) versus 9.3 days (SD 4.2) in participants in the placebo group receiving gel and 9.5 days (SD 3.9) in the placebo group not receiving get. Pieramici 2003 reported no significant difference in time to clearance of the primary hyphema between topical aminocaproic acid‐treated participants and placebo‐treated participants. However, these studies included all participants, including those with a secondary hemorrhage (Analysis 2.2). We graded the certainty of evidence for resolution of primary hemorrhage as low, downgrading for inconsistency and indirectness (Table 2).
2.2. Analysis.
Comparison 2 Topical aminocaproic acid versus placebo, Outcome 2 Time to resolution of primary hemorrhage (days).
| Time to resolution of primary hemorrhage (days) | ||||
|---|---|---|---|---|
| Study | Mean (SD) time to resolution in drug treated group | Number of participants in drug treated group | Mean (SD) time to resolution in control group | Number of participants in control group |
| Karkhaneh 2003 | 11.1 (4.7) days | 41 | + Placebo gel: 9.3 (4.2) days No placebo gel: 9.5 (3.9) days |
+ Placebo gel: 39 No placebo gel: 52 |
| Pieramici 2003 | Reported as "no difference between treatment groups" | 24 | Reported as "no difference between treatment groups" | 27 |
Risk of secondary hemorrhage
Participants receiving topical aminocaproic acid experienced a secondary hemorrhage less often than participants receiving placebo (RR 0.48, 95% CI 0.20 to 1.10; Analysis 2.3). Pieramici 2003 reported that two participants in the aminocaproic acid group and one in the placebo group had sickle cell trait, but they did not report on the rebleed rate for participants with sickle cell trait/disease. Karkhaneh 2003 reported no effect of initial hyphema size on secondary hemorrhages. We graded the certainty of evidence for secondary hemorrhage as low, downgrading for imprecision and indirectness (Table 2).
2.3. Analysis.
Comparison 2 Topical aminocaproic acid versus placebo, Outcome 3 Risk of secondary hemorrhage.
Time to rebleed
The mean time to rebleed in the five participants receiving topical aminocaproic acid who rebled in Karkhaneh 2003 was 3.2 days (SD 0.5) versus 3.0 days (SD 0.8) in the seven participants who rebled in the placebo group (P = 0.18). Pieramici 2003 reported that of the participants in their study who rebled, those receiving topical aminocaproic acid took longer to rebleed (one participant on day six) compared with those receiving placebo (eight participants; range in days two to six). However, this result was observed after the exclusion of one participant in the aminocaproic acid group who had taken aspirin and rebled on day three. Overall, there appeared to be little difference in the time for a secondary hemorrhage to occur, although the small numbers of events make statistical testing unreliable (Analysis 2.4).
2.4. Analysis.
Comparison 2 Topical aminocaproic acid versus placebo, Outcome 4 Time to rebleed (days).
| Time to rebleed (days) | ||||
|---|---|---|---|---|
| Study | Number of rebleeds in drug treated group | Time to rebleed in drug treated group | Number of rebleeds in control group | Time to rebleed in control group |
| Karkhaneh 2003 | 5 of 41 | Days 2 to 4: Mean = 3.2 days; SD = 0.5 | + Placebo gel: 7 of 39 No placebo gel: 8 of 52 |
+ Placebo gel: Mean = 3 days; SD = 0.8 No placebo gel: Mean = 3 days; SD = 0.8 |
| Pieramici 2003 | 2 of 24 | Days 3 and 6 | 8 of 27 | Days 2 to 6: 3 on day 2; 1 on day 3; 2 on day 4; and 2 on day 6 |
Risk of corneal blood stain
Neither study reported outcomes for corneal blood stain.
Risk of peripheral anterior synechiae formation
Neither study reported outcomes for PAS formation.
Risk of glaucoma or elevated intraocular pressure
Pieramici 2003 reported the number of participants receiving aminocaproic acid who had elevated IOP during the seven‐day trial compared with participants receiving placebo (RR 2.25, 95% CI 0.22 to 23.28; Analysis 2.5). This study enrolled three participants (6%) with sickle cell disease/trait, but it was not clear if any of these participants developed elevated IOP. Karkhaneh 2003 reported no significant differences in initial or final IOP between treatment groups (Table 11). We graded the certainty of evidence for IOP outcomes as low, downgrading two levels for imprecision.
2.5. Analysis.
Comparison 2 Topical aminocaproic acid versus placebo, Outcome 5 Risk of glaucoma or elevated IOP.
Risk of optic atrophy
Neither study reported outcomes for optic atrophy.
Adverse effects
Systemic hypotension was observed in 13% of participants in the topical aminocaproic acid group versus 11% of participants in the placebo group in Pieramici 2003 (Table 13). Karkhaneh 2003 did not report adverse events.
Quality of life outcomes
Neither study reported any quality of life outcome.
Economic outcomes
Neither study reported any economic outcome.
Low‐ versus standard‐dose aminocaproic acid
Only one study compared low‐dose (50 mg/kg) versus standard dose (100 mg/kg) of oral aminocaproic acid (Palmer 1986), therefore we did not perform meta‐analyses for any outcome.
Visual acuity
Although "final" VA was measured, the time from injury to final VA was not reported. Final VAs of 20/40 or better were attained by 16 of 25 (64.0%) participants receiving low‐dose aminocaproic acid and by 25 of 32 (78.1%) participants receiving standard‐dose aminocaproic acid (RR 0.82, 95% CI 0.58 to 1.16; Analysis 3.1). We graded the certainty of evidence for final VA as low, downgrading for imprecision and indirectness.
3.1. Analysis.
Comparison 3 Low‐dose versus standard‐dose aminocaproic acid, Outcome 1 Unspecified time for visual acuity between 20/20 and 20/40.
Time to resolution of primary hemorrhage
No significant difference was reported between groups regarding time to resolution of the primary hemorrhage (mean difference (MD) ‐0.14 days, 95% CI ‐1.24 to 0.96). The mean time for resolution of the primary hemorrhage was 3.1 days (SD 2.3) in the low‐dose group and 3.3 days (SD 1.8) in the standard‐dose group (Analysis 3.2). We graded the certainty of evidence as moderate, downgrading for imprecision.
3.2. Analysis.
Comparison 3 Low‐dose versus standard‐dose aminocaproic acid, Outcome 2 Time to resolution of primary hemorrhage (days).
Risk of secondary hemorrhage
The investigators reported that one of 25 (4.0%) eyes receiving low‐dose aminocaproic acid rebled, and five of 33 (15.2%) eyes receiving standard‐dose aminocaproic acid rebled (RR 0.26, 95% CI 0.03 to 2.12; Analysis 3.3). Participants with sickle cell trait were excluded from this study, and there did not appear to be an effect of initial hyphema severity on the rate of secondary hemorrhage (Table 8). We graded the certainty of evidence for secondary hemorrhage as low, downgrading two levels for imprecision.
3.3. Analysis.
Comparison 3 Low‐dose versus standard‐dose aminocaproic acid, Outcome 3 Risk of secondary hemorrhage.
Time to rebleed
The one participant who rebled in the low‐dose group rebled on day four. Of the five participants who rebled in the standard‐dose group, one did so on day two, two on day three, and two on day six (Analysis 3.4).
3.4. Analysis.
Comparison 3 Low‐dose versus standard‐dose aminocaproic acid, Outcome 4 Time to rebleed (days).
| Time to rebleed (days) | ||||
|---|---|---|---|---|
| Study | Number of rebleeds in the low dose group | Time to rebleed in the low dose group | Number of rebleeds in the standard dose group | Time to rebleed in the standard dose group |
| Palmer 1986 | 1 of 25 | Day 4 | 5 of 32 | Days 2 to 6: 1 on day 2; 2 on day 3; and 2 on day 6 |
Risk of corneal blood stain
Palmer 1986 did not report this outcome.
Risk of peripheral anterior synechiae formation
Palmer 1986 did not report this outcome.
Risk of glaucoma or elevated intraocular pressure
No participant in the low‐dose group and two participants in the standard‐dose group experienced elevated IOP requiring surgical intervention (RR 0.25, 95% CI 0.01 to 5.06; Analysis 3.5; Table 11). We graded the certainty of evidence for IOP outcomes as low, downgrading two levels for imprecision.
3.5. Analysis.
Comparison 3 Low‐dose versus standard‐dose aminocaproic acid, Outcome 5 Risk of glaucoma or elevated IOP.
Risk of optic atrophy
Palmer 1986 did not report this outcome.
Adverse effects
Adverse events reported between groups are summarized in Analysis 3.6 and Table 13. Nausea or vomiting was reported in five participants in the low‐dose group and nine participants in the standard‐dose group. Dizziness and hypotension were reported in five participants and syncope in two participants in the standard‐dose group. Other adverse events in the low‐dose group included diarrhea and dry mouth or nose, each with one participant. Rash or pruritis was reported in one participant in the low‐dose group and two participants in the standard‐dose group. We graded the certainty of evidence for adverse events as low, downgrading two levels for imprecision.
3.6. Analysis.
Comparison 3 Low‐dose versus standard‐dose aminocaproic acid, Outcome 6 Adverse effects.
Quality of life outcomes
No study reported any quality of life outcome.
Economic outcomes
The mean hospital stay was 5.4 days (SD 1.1) in the low‐dose group and 5.5 days (SD 1.4) in the standard‐dose group (MD ‐0.10, 95% CI ‐0.75 to 0.55; Analysis 3.7). We graded the certainty of evidence as moderate, downgrading for imprecision.
3.7. Analysis.
Comparison 3 Low‐dose versus standard‐dose aminocaproic acid, Outcome 7 Duration of hospitalization (days).
Systemic versus topical aminocaproic acid
One study compared systemic versus topical aminocaproic acid (Crouch 1997).
Visual acuity
Final VAs of 20/40 or better were attained by 20 of 29 (85.7%) participants receiving systemic aminocaproic acid and 30 of 35 (69.0%) participants receiving topical aminocaproic acid (RR 0.80, 95% CI 0.61 to 1.06; Analysis 4.1). We graded the certainty of evidence for final VA as low, downgrading for imprecision and indirectness.
4.1. Analysis.
Comparison 4 Systemic versus topical aminocaproic acid, Outcome 1 Short‐term visual acuity from 20/20 to 20/40.
Time to resolution of primary hemorrhage
Crouch 1997 did not report this outcome.
Risk of secondary hemorrhage
One of 29 (3%) eyes in the oral group versus one of 35 (3%) eyes in the topical group had a secondary hemorrhage (RR 1.21, 95% CI 0.08 to 18.46; Analysis 4.2). Two participants in each of the treatment groups had sickle cell trait, but there was no report on the rate of secondary hemorrhage by this condition or by initial hyphema severity. We graded the certainty of evidence for secondary hemorrhage as low, downgrading two levels for imprecision.
4.2. Analysis.
Comparison 4 Systemic versus topical aminocaproic acid, Outcome 2 Risk of secondary hemorrhage.
Time to rebleed
Crouch 1997 reported that the secondary hemorrhage in the participant in the systemic aminocaproic acid group occurred on day three, and the secondary hemorrhage in the participant in the topical aminocaproic acid group occurred on day five.
Risk of corneal blood stain
No incident of corneal blood staining was reported in either treatment group (Table 9).
Risk of peripheral anterior synechiae formation
Crouch 1997 reported that four participants experienced PAS formation, but the number of participants for each group was not reported.
Risk of glaucoma or elevated intraocular pressure
Crouch 1997 did not report this outcome.
Risk of optic atrophy
No incident of optic atrophy was reported in either treatment group (Table 12).
Adverse effects
There were no significant differences between groups in adverse events reported (Analysis 4.3; Table 13; Table 14). Of the 35 participants in the topical aminocaproic acid group, four reported feeling a conjunctival or corneal foreign body sensation; three experienced transient punctate corneal staining; and one had dizziness, nausea, and vomiting on two occasions. Five of the 29 participants in the systemic aminocaproic acid group had dizziness, nausea, and vomiting. We graded the certainty of evidence for adverse events as low, downgrading two levels for imprecision.
4.3. Analysis.
Comparison 4 Systemic versus topical aminocaproic acid, Outcome 3 Adverse effects.
8. Risk of other ocular events.
| Study | Outcome | Test intervention | No. with outcome/no. in group | Control intervention | No. with outcome/no. in group | Total no. with outcome/total no. |
| Aminocaproic acid | ||||||
| Crouch 1997 | Conjunctival/corneal foreign body sensation | Topical aminocaproic acid | 4/35 | Systemic aminocaproic acid | 0/29 | 4/64 |
| Transient punctate corneal staining | Topical aminocaproic acid | 3/35 | Systemic aminocaproic acid | 0/29 | 3/64 | |
| Tranexamic acid | ||||||
| Varnek 1980 | Vitreous and retinal hemorrhage | Tranexamic acid | 5/102 | Conservative treatment | 5/130 | 10/232 |
| Traumatic cataract | Tranexamic acid | 2/102 | Conservative treatment | 0/130 | 2/232 | |
| Non‐drug medical intervention | ||||||
| Read 1974 | Traumatic cataract | Moderate ambulatory activity, patching and shielding of injured eye | NR | Bed rest with elevation of the head, bilateral patches and eye shield | NR | 8/137 |
| Vitreous hemorrhage | Moderate ambulatory activity, patching and shielding of injured eye | NR | Bed rest with elevation of the head, bilateral patches and eye shield | NR | 11/137 | |
| Commotio retinae | Moderate ambulatory activity, patching and shielding of injured eye | NR | Bed rest with elevation of the head, bilateral patches and eye shield | NR | 4/137 | |
| Occluded pupil | Moderate ambulatory activity, patching and shielding of injured eye | NR | Bed rest with elevation of the head, bilateral patches and eye shield | NR | 2/137 | |
| Optic atrophy with nasalization of optic cup | Moderate ambulatory activity, patching and shielding of injured eye | NR | Bed rest with elevation of the head, bilateral patches and eye shield | NR | 4/137 | |
| Optic atrophy without nasalization of optic cup | Moderate ambulatory activity, patching and shielding of injured eye | NR | Bed rest with elevation of the head, bilateral patches and eye shield | NR | 8/137 | |
NR: not reported.
Quality of life outcomes
Crouch 1997 did not report any quality of life outcome.
Economic outcomes
Crouch 1997 did not report any economic outcome.
Tranexamic acid versus control
We analyzed data from five studies reporting results comparing tranexamic acid versus control (Rahmani 1999; Sukumaran 1988; Vangsted 1983; Varnek 1980; Welsh 1983). Three studies were RCTs, and two were quasi‐randomized controlled clinical trials.
Visual acuity
Four studies reported short‐term VA. Rahmani 1999 measured VA at the time of discharge (range five to 15 days); 41 of 77 (57%) participants in the tranexamic acid group had VA of 20/40 or better compared with 35 of 79 (44%) participants in the placebo group. Sukumaran 1988 reported that all participants had a final VA of 20/30 or better with the exception of one participant in the control group. The time of measurement for final VA was not reported, but participants were followed up for only one week. Vangsted 1983 reported that all 59 participants in the tranexamic acid group had VA between 20/20 and 20/40 two weeks after the initial trauma. All 53 participants in the control group had VA between 20/20 and 20/50 two weeks after the initial trauma. A meta‐analysis of these three studies showed no effect to a slightly beneficial effect of tranexamic acid (RR 1.11, 95% CI 0.98 to 1.25; Analysis 5.1). In addition, Varnek 1980 reported mean VAs of 0.9 in both the tranexamic acid and control groups at day five after the trauma. Welsh 1983 did not report VA. We graded the certainty of evidence for short‐term VA as low, downgrading for risk of bias and imprecision (Table 3).
5.1. Analysis.
Comparison 5 Tranexamic acid versus control, Outcome 1 Short‐term visual acuity from 20/20 to 20/40.
Time to resolution of primary hemorrhage
Five studies reported time to resolution of primary hemorrhage (Analysis 5.2). Rahmani 1999 found no significant difference for time to primary resolution between participants receiving tranexamic acid (mean 4.0 days, SD 2.2) and those receiving placebo (mean 3.7 days, SD 1.6), after excluding participants who had secondary hemorrhages. Sukumaran 1988 also found no difference in time to resolution between groups, but included participants with and without secondary hemorrhages in the analysis (tranexamic group: mean 4.6, SD 2.4; control group: mean 3.9, SD 2.4). Vangsted 1983 reported a delay in resolution in the tranexamic acid group. Although Welsh 1983 did not report time to resolution of the primary hyphema directly, the investigators estimated the daily rate of improvement in the hyphema by calculating the geometric mean of the per cent area of the hyphema remaining at each day following injury; these calculations indicated that tranexamic acid‐treated hyphemas cleared faster than those treated with placebo. We graded the certainty of evidence for time to resolution as very low, downgrading for risk of bias, inconsistency, and indirectness (Table 3).
5.2. Analysis.
Comparison 5 Tranexamic acid versus control, Outcome 2 Time to resolution of primary hemorrhage (days).
| Time to resolution of primary hemorrhage (days) | ||||
|---|---|---|---|---|
| Study | Mean (SD) time to resolution in drug treated group | Number of participants in drug treated group | Mean (SD) time to resolution in control group | Number of participants in control group |
| Rahmani 1999 | 4.0 (2.2) days in study participants without secondary hemorrhage | 72 | 3.7 (1.6) days in study participants without secondary hemorrhage | 59 |
| Sukumaran 1988 | 4.6 (2.4) days in all study participants | 17 (2 study participants had a secondary hemorrhage) | 3.9 (2.4) days in all study participants | 18 (6 study participants had a secondary hemorrhage) |
| Vangsted 1983 | Reported as delayed | 59 | NR | 53 |
| Varnek 1980 | NR | 102 | NR | 130 |
| Welsh 1983 | NR | 19 | NR | 20 |
Risk of secondary hemorrhage
All five studies reported the risk of a secondary hemorrhage (Analysis 5.3). Using a fixed‐effect model, the summary RR comparing oral tranexamic acid to placebo or control was 0.31 (95% CI 0.17 to 0.55). We graded the certainty of evidence for secondary hemorrhage as low, downgrading for risk of bias and indirectness (Table 3).
5.3. Analysis.
Comparison 5 Tranexamic acid versus control, Outcome 3 Risk of secondary hemorrhage.
No study evaluating tranexamic acid reported on the presence of sickle cell trait. Two of the studies had all‐white populations, thus it is unlikely any participant would have had this condition (Rahmani 1999; Varnek 1980). Although all investigators reported initial hyphema severity, only Rahmani 1999 reported the proportion of secondary hemorrhages in groups defined by the severity of the initial hyphema, finding no effect of severity on rebleed rate (Table 8). Varnek 1980 reported that the initial size of the hyphemas that underwent secondary hemorrhage was 1.0 mm (one secondary hemorrhage) in the study group and 2.2 mm (12 secondary hemorrhages) in the control group.
Time to rebleed
Three studies reported the time interval between the initial injury and the time of the secondary hemorrhage (Analysis 5.4). In Rahmani 1999, the mean time to rebleed in eight participants who experienced a secondary hemorrhage in the tranexamic acid group was 3.4 days (SD 0.7) compared with 3.8 days (SD 1.0) in the 21 participants who rebled in the placebo group. This difference was reported as not significant. In Sukumaran 1988, rebleeding occurred between days two and three in the participants who rebled in both groups, and Varnek 1980 reported that the secondary hemorrhage took place at day three in the two participants in the tranexamic group who experienced this event, and that time to rebleed ranged from day two to day seven in the 12 participants who rebled in the control group. We graded the certainty of evidence as low, downgrading for risk of bias and imprecision.
5.4. Analysis.
Comparison 5 Tranexamic acid versus control, Outcome 4 Time to rebleed (days).
| Time to rebleed (days) | ||||
|---|---|---|---|---|
| Study | Number of rebleeds in drug treated group | Time to rebleed in drug treated group | Number of rebleeds in control group | Time to rebleed in control group |
| Rahmani 1999 | 8 of 80 | Days 2 to 4: Mean = 3.4 days; SD = 0.7 | 21 of 80 | Days 2 to 6: Mean = 3.8 days; SD = 1.0 |
| Sukumaran 1988 | 2 of 17 | Days 2 to 3 | 6 of 18 | Days 2 to 3 |
| Vangsted 1983 | 0 of 59 | NA | 0 of 53 | NA |
| Varnek 1980 | 2 of 102 | Day 3 | 12 of 130 | Days 2 to 7: 5 occurred on Day 4 |
| Welsh 1983 | 1 of 19 | NR | 6 of 20 | NR |
Risk of corneal blood stain
Two studies reported corneal blood staining as an outcome (Analysis 5.5; Table 9). Vangsted 1983 observed corneal blood staining in one participant of 53 in the control group, and Varnek 1980 reported observing no corneal bleeding in either the tranexamic acid group or the placebo group.
5.5. Analysis.
Comparison 5 Tranexamic acid versus control, Outcome 5 Risk of corneal blood stain.
Risk of peripheral anterior synechiae formation
No study comparing tranexamic acid with control reported this outcome.
Risk of glaucoma or elevated intraocular pressure
Four of the five studies for this comparison reported the number of participants with transient increases in IOP in each group following the treatment period (Rahmani 1999; Vangsted 1983; Varnek 1980; Welsh 1983). None of the studies reported including participants with sickle cell disease/trait. Rahmani 1999 defined elevated IOP as greater than 21 mmHg during the hospital stay and requiring medical or surgical treatment, or both. Vangsted 1983 and Varnek 1980 defined transient elevated IOP as 25 mmHg or greater. Welsh 1983 did not define IOP by a pressure level but stated that three participants required surgery for elevated IOP. The summary RR was 1.20 (95% CI 0.73 to 1.98) when comparing tranexamic acid versus control (Analysis 5.6; Table 11). In addition, Vangsted 1983 reported no instances of secondary glaucoma. We graded the certainty of evidence for elevated IOP as low, downgrading for risk of bias and imprecision.
5.6. Analysis.
Comparison 5 Tranexamic acid versus control, Outcome 6 Risk of glaucoma or elevated IOP.
Risk of optic atrophy
Varnek 1980 reported one incident of optic atrophy in the tranexamic acid group and no incidents in the placebo group (Table 12).
Adverse effects
Two studies reported adverse effects (Analysis 5.7; Table 13). Welsh 1983 reported that one of 19 participants receiving tranexamic acid complained of nausea. Rahmani 1999 reported that medical staff observed no adverse events in either the drug‐treated or the control group.
5.7. Analysis.
Comparison 5 Tranexamic acid versus control, Outcome 7 Adverse effects: nausea or vomiting.
Quality of life outcomes
No study reported any quality of life outcome.
Economic outcomes
Three studies reported on length of hospitalization (Analysis 5.8). Rahmani 1999 reported that the mean hospital stay was six days (SD 1.6) for participants in the tranexamic acid group and 6.3 days (SD 1.8) for participants in the control group. This difference was not significant. In Vangsted 1983, the mean length of hospitalization for the tranexamic acid group was six days compared with seven days for the control group. Varnek 1980 reported that the length of hospitalization for the tranexamic acid group was 6.8 days compared with 6.5 days for the control group.
5.8. Analysis.
Comparison 5 Tranexamic acid versus control, Outcome 8 Duration of hospitalization (days).
| Duration of hospitalization (days) | ||||
|---|---|---|---|---|
| Study | Mean (SD) duration of hospitalization for drug treated group | Number of participants in drug treated group | Mean (SD) duration of hospitalization in control group | Number of participants in control group |
| Rahmani 1999 | 6.0 (1.6) days | 80 | 6.3 (1.8) days | 80 |
| Vangsted 1983 | 6 days | 59 | 7 days | 53 |
| Varnek 1980 | 6.8 days | 102 | 6.5 days | 130 |
One study reported the mean number of days off work (Vangsted 1983). The mean period off work for the tranexamic acid group was 17 days compared with 20 days for the control group. We graded the certainty of evidence for duration of hospitalization as low, downgrading for risk of bias and inconsistency.
Aminomethylbenzoic acid versus placebo
We did not perform meta‐analysis because only one study compared aminomethylbenzoic acid with placebo (Liu 2002).
Visual acuity
Liu 2002 did not report this outcome.
Time to resolution of primary hemorrhage
Liu 2002 did not report this outcome.
Risk of secondary hemorrhage
Liu 2002 reported that participants treated with oral aminomethylbenzoic acid were less likely to rebleed compared with participants treated with placebo (RR 0.10, 95% CI 0.02 to 0.41; Analysis 6.1). We graded the certainty of evidence for secondary hemorrhage as low, downgrading for risk of bias and indirectness.
6.1. Analysis.
Comparison 6 Aminomethylbenzoic acid versus placebo, Outcome 1 Risk of secondary hemorrhage.
Time to rebleed
Liu 2002 did not report this outcome.
Risk of corneal blood stain
Liu 2002 did not report this outcome.
Risk of peripheral anterior synechiae formation
Liu 2002 did not report this outcome.
Risk of glaucoma or elevated intraocular pressure
Liu 2002 did not report this outcome.
Risk of optic atrophy
Liu 2002 did not report this outcome.
Adverse events
Of the 60 participants who received oral aminomethylbenzoic acid, seven reported nausea and vomiting; adverse events for the placebo group were not reported (Table 13).
Quality of life outcomes
Liu 2002 did not report any quality of life outcome.
Economic outcomes
Liu 2002 did not report any economic outcome.
Corticosteroids versus control
Visual acuity
Two studies compared systemic corticosteroids versus placebo. Visual acuity outcomes between studies could not be combined because they were assessed at different follow‐up times, and participants were divided by cut points into different levels of VA. In Rahmani 1999, short‐term VA was compared for participants in each treatment group. At time of discharge (range five to 12 days), 40 of 75 (53%) participants in the corticosteroid group had VA of 20/40 or better compared with 35 of 80 (44%) participants in the placebo group. These results were not statistically different (RR 1.22, 95% CI 0.88 to 1.99; Analysis 7.1) Spoor 1980 reported that 21 of 23 (91%) participants in the prednisone group achieved final VA between 20/20 and 20/50 compared with 18 of 20 (90%) participants in the placebo group (RR 1.01, 95% CI 0.84 to 1.23; Analysis 7.2). We graded the certainty of evidence for variable‐length VA as low, downgrading for indirectness and imprecision. We graded the certainty of evidence for short‐term VA as low, downgrading for indirectness and imprecision (Table 4).
7.1. Analysis.
Comparison 7 Corticosteroids versus control, Outcome 1 Short‐term (5 to 14 days) visual acuity from 20/20 to 20/40, systemic corticosteroids.
7.2. Analysis.
Comparison 7 Corticosteroids versus control, Outcome 2 Visual acuity between 20/20 and 20/50 at resolution of hyphema, systemic corticosteroids.
Two studies administering topical corticosteroids reported short‐term VA. Again, the VA outcomes could not be combined because different cut points were used across studies (Rakusin 1972; Zetterstrom 1969). Rakusin 1972 reported that six of 13 (46%) participants assigned to corticosteroid eyedrops and 13 of 21 (62%) participants assigned to control eyedrops achieved short‐term VA better than 20/60 (RR 0.75, 95% CI 0.38 to 1.47; Analysis 7.3). We graded the certainty of the evidence for short‐term visual acuity as very low, downgraded for imprecision, indirectness, and risk of bias. Zetterstrom 1969 reported that 56 of 58 (97%) participants in the corticosteroid group had final VA of 0.9 (between 20/20 and 20/25), and 53 of 59 (90%) in the control group achieved VA better than 0.7 (about 20/30). At discharge, mean VA in the group assigned to corticosteroids was 0.96, compared with 0.91 in the control group (RR 1.02, 95% CI 0.94 to 1.11; Analysis 7.4). We graded the certainty of the evidence for VA at discharge as low, downgrading for imprecision and risk of bias (Table 4).
7.3. Analysis.
Comparison 7 Corticosteroids versus control, Outcome 3 Short‐term (5 to 14 days) visual acuity from 20/20 to 20/40, topical corticosteroids.
7.4. Analysis.
Comparison 7 Corticosteroids versus control, Outcome 4 Final visual acuity between 20/20 and 20/25, topical corticosteroids.
Time to resolution of primary hemorrhage
Two studies evaluated the effect of systemic corticosteroids on time to resolution of the hyphema (Analysis 7.5). In Spoor 1980, the authors reported means of 4.4 days and 4.5 days for the resolution of primary hemorrhage in groups receiving prednisone and placebo, respectively. This result remained non‐significant when participants who rebled were excluded from the analysis. Spoor 1980 reported that time to resolution was shorter in hyphemas that were initially less severe (Table 8). Rahmani 1999 also found no significant difference for time to resolution of primary hemorrhage in participants without a secondary hemorrhage between the prednisolone group (mean 3.5 days, SD 1.8) and the placebo group (mean 3.7 days, SD 1.6). We graded the certainty of evidence for time to resolution of primary hemorrhage as low, downgrading for indirectness and imprecision. In the one study evaluating topical corticosteroids that measured time to resolution of primary hemorrhage (Table 4). Rakusin 1972 reported that the primary hyphema was resolved within one week in 10 of 13 (77%) participants assigned to corticosteroid eyedrops and in 16 of 21 (76%) participants assigned to the control group (Analysis 7.6). We graded the certainty of evidence as very low, downgrading for indirectness, imprecision, and risk of bias (Table 4).
7.5. Analysis.
Comparison 7 Corticosteroids versus control, Outcome 5 Time to resolution of primary hemorrhage (days), systemic corticosteroids.
| Time to resolution of primary hemorrhage (days), systemic corticosteroids | ||||
|---|---|---|---|---|
| Study | Time to resolution in drug group | Number of participants in drug group | Time to resolution in control group | Number of participants in control group |
| Rahmani 1999 | 3.5 days (SD = 1.8) in study participants without a secondary hemorrhage | 64 | 3.7 days (SD = 1.6) in study participants without a secondary hemorrhage | 59 |
| Spoor 1980 | 4.45 days (4.01 days in study participants without a secondary hemorrhage) | 23 (20 without a secondary hemorrhage) | 4.48 days (3.60 days in study participants without a secondary hemorrhage) | 20 (16 without a secondary hemorrhage) |
7.6. Analysis.
Comparison 7 Corticosteroids versus control, Outcome 6 Time to resolution of primary hemorrhage (days), topical corticostroids.
| Time to resolution of primary hemorrhage (days), topical corticostroids | ||||
|---|---|---|---|---|
| Study | Time to resolution in drug group | Number of participants in drug group | Time to resolution in control group | Number of participants in control group |
| Rakusin 1972 | 10 resolved within 7 days | 13 (1 study participant had a secondary hemorrhage) | 16 resolved within 7 days | 21 (2 study participants had a secondary hemorrhage) |
Risk of secondary hemorrhage
We analyzed data from two studies evaluating systemic corticosteroids and reporting results for the risk of secondary hemorrhage (Rahmani 1999; Spoor 1980). Using a fixed‐effect model, the summary RR comparing oral corticosteroids to placebo was 0.68 (95% CI 0.39 to 1.18; Analysis 7.7); however, this was not an intention‐to‐treat analysis due to missing data from the exclusion of four participants by Rahmani 1999. We graded the certainty of evidence for risk of secondary hemorrhage as low, downgrading for indirectness and imprecision (Table 4). A meta‐analysis of secondary hemorrhage including data from Rakusin 1972 (topical corticosteroids versus placebo eyedrops) and Zetterstrom 1969 (topical corticosteroids versus complete bed rest with no simultaneous local therapy) did not show a statistically significant difference (RR 0.29, 95% CI 0.05 to 1.53; Analysis 7.8). We graded the certainty of evidence as very low, downgrading for indirectness, imprecision, and risk of bias (Table 4).
7.7. Analysis.
Comparison 7 Corticosteroids versus control, Outcome 7 Risk of secondary hemorrhage, systemic corticosteorids.
7.8. Analysis.
Comparison 7 Corticosteroids versus control, Outcome 8 Risk of secondary hemorrhage, topical corticosteroids.
None of the four studies reported on the presence of sickle cell trait.
Rahmani 1999 observed no effect of initial hyphema severity on the proportion of participants with a secondary hemorrhage, but Spoor 1980 found that there was a lower proportion of secondary hemorrhages in participants with hyphemas that were initially less severe (2/38 (13%) versus 2/5 (40%), where severity was defined as blood filling one‐third versus more than one‐third of the anterior chamber) (Table 8).
Time to rebleed
In Rahmani 1999, rebleeding occurred a mean of 3.2 days (SD 0.8) from the time of trauma in the 14 participants who rebled in the prednisolone group and 3.8 days (SD 1.0) in the 21 participants who rebled in the placebo group. This difference was reported as not significant. In Spoor 1980, the mean time to rebleed in three participants who experienced a secondary hemorrhage in the prednisone group was 2.3 days compared with 2.6 days in the four participants who rebled in the placebo group. As in Rahmani 1999, this difference was not significant (Analysis 7.9).
7.9. Analysis.
Comparison 7 Corticosteroids versus control, Outcome 9 Time to rebleed (days), systemic corticosteroids.
| Time to rebleed (days), systemic corticosteroids | ||||
|---|---|---|---|---|
| Study | Number of rebleeds in the drug group | Mean time to rebleed in the drug group | Number of rebleeds in the control group | Mean time to rebleed in the control group |
| Rahmani 1999 | 14 of 78 | 3.2 days (SD = 0.8) | 21 of 80 | 3.8 days (SD = 1.0) |
| Spoor 1980 | 3 of 23 | 2.3 days | 4 of 20 | 2.6 days |
Risk of corneal blood stain
One of 43 participants included in Spoor 1980 experienced corneal blood staining. The study group in which the blood stain occurred was not reported (Analysis 7.10). In Zetterstrom 1969, one participant in the control group experienced corneal blood staining compared with no participants in the group receiving corticosteroid eyedrops (RR 0.34, 95% CI 0.01 to 8.15; Analysis 7.11).
7.10. Analysis.
Comparison 7 Corticosteroids versus control, Outcome 10 Risk of corneal blood stain, systemic cortiocsteroids.
7.11. Analysis.
Comparison 7 Corticosteroids versus control, Outcome 11 Risk of corneal blood stain, topical corticosteroids.
Complications of hyphema, including corneal blood staining; pigment on endothelium, anterior lens capsule, or vitreous; posterior synechiae; PAS; anterior chamber blood clots; and fibrous membrane formation, were documented among participants in Rakusin 1972. The study reported that 54% of participants in the corticosteroid group had complications compared with 70% of participants in the control group, although this difference was not significant, and the risk of corneal blood staining was not reported separately (Table 9).
Risk of peripheral anterior synechiae formation
Spoor 1980 reported that there was no instance of PAS formation in either group (Analysis 7.12; Table 10).
7.12. Analysis.
Comparison 7 Corticosteroids versus control, Outcome 12 Risk of peripheral anterior synechiae, systemic corticosteroids.
Risk of glaucoma or elevated intraocular pressure
Rahmani 1999 reported that nine (11.5%) of 78 participants in the prednisolone group and 12 (15%) of 80 participants in the placebo group had an IOP greater than 21 mmHg during hospitalization that required medical treatment, surgical treatment, or both. Two participants in Spoor 1980 had elevated IOP that was controlled by acetazolamide therapy alone, one each in the prednisolone and the control group. No participant in this cohort had IOP greater than 35 mmHg (RR 0.78, 95% CI 0.36 to 1.68; Analysis 7.13). Five participants in Zetterstrom 1969 developed "elevated" IOP (undefined), three of 58 in the topical corticosteroids group and two of 59 in the control group (RR 1.53, 95% CI 0.26 to 8.80; Analysis 7.14). This information is included in Table 11.
7.13. Analysis.
Comparison 7 Corticosteroids versus control, Outcome 13 Risk of glaucoma or elevated IOP, systemic corticosteroids.
7.14. Analysis.
Comparison 7 Corticosteroids versus control, Outcome 14 Risk of glaucoma or elevated IOP, topical corticosteroids.
Risk of optic atrophy
Zetterstrom 1969 reported one incident of optic atrophy in the 58 participants assigned to topical corticosteroid eyedrops (RR 3.05, 95% CI 0.13 to 73.39; Analysis 7.15).
7.15. Analysis.
Comparison 7 Corticosteroids versus control, Outcome 15 Risk of optic atrophy, topical corticosteroids.
Adverse effects
Rahmani 1999 reported that medical staff observed no adverse events in either the drug‐treated or control group.
Quality of life outcomes
No study reported any quality of life outcome.
Economic outcomes
In Rahmani 1999, participants treated with prednisolone were hospitalized a mean of 5.9 days (SD 1.4); those treated with placebo were hospitalized a mean of 6.3 days (SD 1.8). The mean difference between groups was ‐0.40 days (95% CI ‐0.90 to 0.10) (Analysis 7.16).
7.16. Analysis.
Comparison 7 Corticosteroids versus control, Outcome 16 Duration of hospitalization (days), systemic corticosteroids.
Zetterstrom 1969 reported the duration of hospitalization: the mean length of stay for participants assigned to corticosteroid drops was 5.9 days compared with 8.9 days for participants assigned to the control group (Analysis 7.17).
7.17. Analysis.
Comparison 7 Corticosteroids versus control, Outcome 17 Duration of hospitalization (days), topical corticosteroids.
| Duration of hospitalization (days), topical corticosteroids | ||||
|---|---|---|---|---|
| Study | Mean (SD) duration of hospitalization for drug treated group | Number of participants in drug treated group | Mean (SD) duration of hospitalization in control group | Number of participants in control group |
| Zetterstrom 1969 | 5.9 days (SD not reported) | 58 | 8.9 days (SD not reported) | 59 |
Systemic aminocaproic acid versus systemic prednisone
Visual acuity
We performed no meta‐analysis because only one study compared systemic aminocaproic acid versus systemic prednisone (Farber 1991). After five days of hospitalization, 10 of 56 (18%) participants in the aminocaproic acid group had short‐term VA of 20/200 or worse compared with seven of 56 (12.5%) participants in the prednisone group. These results were not statistically different (RR 0.84, 95% CI 0.58 to 1.2; Analysis 8.1). Likewise, there was no difference in final VA of 20/40 or better between groups (26 of 56 (46%) participants in the aminocaproic acid group and 31 of 56 (55%) participants in the prednisone group).
8.1. Analysis.
Comparison 8 Aminocaproic acid versus prednisone, Outcome 1 Short‐term (5 to 14 days) visual acuity from 20/20 to 20/40.
Time to resolution of primary hemorrhage
Farber 1991 did not follow participants past discharge and so did not report on time to resolution of the primary hyphema. However, they did report that "at discharge" (mean time to discharge = five days) 43% of participants in the aminocaproic acid group compared with 75% of participants in the prednisone group had complete resolution of their hyphema. This difference was statistically significant (P = 0.001).
Risk of secondary hemorrhage
The risk of secondary hemorrhage was equal for both groups, four eyes out of 56 eyes per group (RR 1.00, 95% CI 0.26 to 3.80; Analysis 8.2). Participants with sickle cell trait/disease were excluded from this study. Initial hyphema severity did not appear to have an influence on rate of secondary hemorrhage (Table 8).
8.2. Analysis.
Comparison 8 Aminocaproic acid versus prednisone, Outcome 2 Risk of secondary hemorrhage.
Time to rebleed
Farber 1991 did not report this outcome.
Risk of corneal blood stain
Farber 1991 did not report this outcome.
Risk of peripheral anterior synechiae formation
Farber 1991 did not report this outcome.
Risk of glaucoma or elevated intraocular pressure
No significant differences were reported for mean IOPs at time of discharge between groups.
Risk of optic atrophy
Farber 1991 did not report this outcome.
Adverse events
Farber 1991 reported that no participant experienced any adverse event (Analysis 8.3).
8.3. Analysis.
Comparison 8 Aminocaproic acid versus prednisone, Outcome 3 Adverse effect: any adverse event.
Quality of life outcomes
Farber 1991 did not report this outcome.
Economic outcomes
Farber 1991 reported an overall mean duration of hospitalization of five days across both treatment groups.
Conjugated estrogen versus placebo
Visual acuity
Visual acuity at time of discharge was partially reported by the one study that compared conjugated estrogen versus placebo (Spaeth 1966). Among all participants, 61% had VA better than 6/12; 30% had VA better than 6/60; and 9% had VA of 6/60 or worse at time of discharge. These results were not reported by treatment group. We graded the certainty of evidence for visual acuity as moderate, downgrading for imprecision.
Time to resolution of primary hemorrhage
Spaeth 1966 did not report this outcome.
Risk of secondary hemorrhage
Spaeth 1966 reported that 10 of 39 estrogen‐treated participants rebled (25.6%), and 10 of 46 placebo‐treated participants rebled (21.7%). These results were not statistically different (RR 1.18, 95% CI 0.55 to 2.54; Analysis 9.1).
9.1. Analysis.
Comparison 9 Conjugated estrogen versus placebo, Outcome 1 Risk of secondary hemorrhage.
Spaeth 1966 did not report on the presence of sickle cell trait/disease. The risk of secondary hemorrhage by initial hyphema severity did not appear to differ across severity ratings (Table 8).
Time to rebleed
The time to rebleed among all participants was a mean of 3.5 days after injury, with a range of one to eight days. These results were not reported by treatment group.
Risk of corneal blood stain
In the estrogen group, two of 39 (5%) participants had corneal blood staining compared with two of 46 (4%) participants in the placebo group (RR 1.18, 95% CI 0.55 to 7.99; Analysis 9.2).
9.2. Analysis.
Comparison 9 Conjugated estrogen versus placebo, Outcome 2 Risk of corneal blood stain.
Risk of peripheral anterior synechiae formation
Fifteen cases of PAS were reported among all participants. These results were not reported by treatment group (Table 10).
Risk of glaucoma or elevated intraocular pressure
Thirteen cases of secondary glaucoma were reported among all participants, four of which occurred prior to secondary hemorrhage (Table 11). These results were not reported by treatment group.
Risk of optic atrophy
Spaeth 1966 did not report this outcome.
Adverse events
Spaeth 1966 did not report this outcome.
Quality of life outcomes
Spaeth 1966 did not report any quality of life outcome.
Economic outcomes
Spaeth 1966 did not report this outcome.
Cycloplegics versus miotics
Two studies evaluated the effect of cycloplegics compared with miotics (Bedrossian 1974; Rakusin 1972).
Visual acuity
Rakusin 1972 reported that nine of 17 (53%) participants in the homatropine group and 11 of 17 (65%) participants in the pilocarpine group had short‐term VA better than 20/60 (RR 0.82, 95% CI 0.46 to 1.45; Analysis 10.1). We graded the certainty of evidence for short‐term VA as low, downgrading for imprecision and risk of bias (Table 5). Bedrossian 1974 did not report on VA.
10.1. Analysis.
Comparison 10 Cycloplegics versus miotics, Outcome 1 Short‐term visual acuity.
Time to resolution of primary hemorrhage
Bedrossian 1974 reported a longer time to resolution with the pilocarpine group (mean 3.6 days, SD 1.3) compared with the atropine group (mean 2.7 days, SD 1.7), an MD of ‐0.82 days (95% CI ‐1.68 to ‐0.12; Analysis 10.2). The time to resolution showed a slight increase with larger size of initial hyphema (Table 8). In Rakusin 1972, there was no significant difference in the proportion of participants with absorption within one week between cycloplegic (12/17) and miotic (13/17) groups.
10.2. Analysis.
Comparison 10 Cycloplegics versus miotics, Outcome 2 Time to resolution of primary hemorrhage (days).
Risk of secondary hemorrhage
In Bedrossian 1974, only one participant experienced a secondary hemorrhage, who was in the cycloplegic group and had an initial hyphema height of 1 mm. The one participant with a secondary hemorrhage in Rakusin 1972 was in the group receiving homatropine. The meta‐analysis combining results from these two studies was not significant (RR 1.03, 95% CI 0.15 to 6.99; Analysis 10.3; Table 8). We graded the certainty of the evidence as very low, downgrading for imprecision, risk of bias, and inconsistency (Table 5).
10.3. Analysis.
Comparison 10 Cycloplegics versus miotics, Outcome 3 Risk of secondary hemorrhage.
Time to rebleed
Bedrossian 1974 reported that the time to rebleed in the one participant with a secondary hyphema was two days (Analysis 10.4).
10.4. Analysis.
Comparison 10 Cycloplegics versus miotics, Outcome 4 Time to rebleed (days).
| Time to rebleed (days) | ||||
|---|---|---|---|---|
| Study | Number of rebleeds in the cycloplegic group | Mean time to rebleed in the cycloplegic group | Number of rebleeds in the miotic group | Mean time to rebleed in the miotic group |
| Bedrossian 1974 | 1 of 28 | 2 days | 0 of 30 | NA |
Risk of corneal blood stain
Rakusin 1972 reported that the number of complications of hyphema, including corneal blood staining; pigment on endothelium, anterior lens capsule, or vitreous; posterior synechiae; PAS; anterior chamber blood clots; and fibrous membrane formation, were similar in all groups.
Risk of peripheral anterior synechiae formation
Rakusin 1972 reported that the number of complications of hyphema, including corneal blood staining; pigment on endothelium, anterior lens capsule, or vitreous; posterior synechiae; PAS; anterior chamber blood clots; and fibrous membrane formation, were similar in all groups.
Risk of glaucoma or elevated intraocular pressure
Neither Bedrossian 1974 nor Rakusin 1972 reported this outcome.
Risk of optic atrophy
Neither Bedrossian 1974 nor Rakusin 1972 reported this outcome.
Adverse events
Neither Bedrossian 1974 nor Rakusin 1972 reported this outcome.
Quality of life outcomes
Neither Bedrossian 1974 nor Rakusin 1972 reported any quality of life outcome.
Economic outcomes
Neither Bedrossian 1974 nor Rakusin 1972 reported this outcome.
Aspirin versus observation
Only one study compared aspirin versus observation (Marcus 1988), therefore we did not perform a meta‐analysis.
Visual acuity
Marcus 1988 did not report this outcome.
Time to resolution of primary hemorrhage
Marcus 1988 did not report this outcome.
Risk of secondary hemorrhage
Marcus 1988 reported that three of 23 (13%) eyes in the aspirin group rebled, and two of 28 (7%) eyes in the observation group rebled. These results were not statistically different (RR 1.83, 95% CI 0.33 to 10.02; Analysis 11.1). The study investigators reported that two of the three eyes that rebled in the aspirin group initially had a total hyphema, while of the two eyes that rebled in the control group, one had an initial hyphema of 30% and one an "almost total" hyphema (Table 8).
11.1. Analysis.
Comparison 11 Aspirin versus observation, Outcome 1 Risk of secondary hemorrhage.
Time to rebleed
Marcus 1988 did not report this outcome.
Risk of corneal blood stain
Marcus 1988 did not report this outcome.
Risk of peripheral anterior synechiae formation
Marcus 1988 did not report this outcome.
Risk of glaucoma or elevated intraocular pressure
Marcus 1988 did not report this outcome.
Risk of optic atrophy
Marcus 1988 did not report this outcome.
Adverse events
Marcus 1988 did not report this outcome.
Quality of life outcomes
Marcus 1988 did not report this outcome.
Economic outcomes
Marcus 1988 did not report this outcome.
Traditional Chinese medicine versus control
We did not perform a meta‐analysis for traditional Chinese medicine versus control treatment since only one study evaluated these interventions (Wang 1994). The authors of Wang 1994 reported only one outcome: the proportion of participants who were "cured." The outcome of being cured was a composite outcome defined as the resolution of the primary hemorrhage after five days of treatment; VA of 0.7 or better after resolution of the primary hemorrhage; and no recurrence of bleeding for one week following resolution of the primary hemorrhage. One week after completing treatment, 29 of 45 (64%) participants in the traditional Chinese medicine group and 10 of 38 (26%) participants in the control group met these criteria for being "cured."
Visual acuity
Wang 1994 did not report this outcome.
Time to resolution of primary hemorrhage
Wang 1994 did not report this outcome.
Risk of secondary hemorrhage
Wang 1994 did not report this outcome.
Time to rebleed
Wang 1994 did not report this outcome.
Risk of corneal blood stain
Wang 1994 did not report this outcome.
Risk of peripheral anterior synechiae formation
Wang 1994 did not report this outcome.
Risk of glaucoma or elevated intraocular pressure
Wang 1994 did not report this outcome.
Risk of optic atrophy
Wang 1994 did not report this outcome.
Adverse events
Wang 1994 did not report this outcome.
Quality of life outcomes
Wang 1994 did not report this outcome.
Economic outcomes
Wang 1994 did not report this outcome.
Monocular versus binocular patching
We identified two studies that compared the use of monocular versus binocular patches (Edwards 1973; Rakusin 1972).
Visual acuity
Rakusin 1972 reported that 22 of 26 (85%) participants in the monocular group compared with 24 of 27 (89%) participants in the binocular group had short‐term VA better than 20/60 (RR 0.82, 95% CI 0.67 to 1.00; Analysis 12.1). Edwards 1973 reported that 21 of 26 (81%) participants in the monocular group compared with 20 of 20 (100%) participants in the binocular group had VA better than 20/50, although the time at which VA was measured was not specified (RR 0.95, 95% CI 0.77 to 1.18; Analysis 12.2). We graded the certainty of the evidence for both of these analyses of VA as low, downgrading for imprecision and risk of bias (Table 6). Edwards 1973 also reported that of participants with an initial hyphema filling less than one‐third of the anterior chamber, 67% (28/42) had VA of 20/50 or better compared with 59% (13/22) of those with more severe hyphemas (Table 8).
12.1. Analysis.
Comparison 12 Monocular versus binocular patching, Outcome 1 Short‐term visual acuity.
12.2. Analysis.
Comparison 12 Monocular versus binocular patching, Outcome 2 Variable time length 'final' visual acuity.
Time to resolution of primary hemorrhage
Rakusin 1972 reported that the primary hyphema was resolved within one week in 22 of 26 (85%) participants with monocular patching and in 24 of 27 (89%) participants with binocular patching.
Risk of secondary hemorrhage
In Edwards 1973, there were eight participants each with a secondary hemorrhage from the group with a patch on both eyes (n = 35; 23%) and the group with a patch only on the injured eye (n = 29; 28%). The results from Rakusin 1972 also showed no difference between groups for risk of secondary hemorrhage (one of 26 (3.8%) in the group with a monocular patch and two of 27 (7.4%) in the group with binocular patches). Meta‐analyzing these two studies produced an RR of 0.77 (95% CI 0.35 to 1.72; Analysis 12.3). The proportion of secondary hyphemas in Edwards 1973 was greater in participants with initially more severe hyphemas (32% (seven of 22)) than in those with an initial hyphema filling less than one‐third of the anterior chamber (17% (seven of 42)) (Table 8).
12.3. Analysis.
Comparison 12 Monocular versus binocular patching, Outcome 3 Risk of secondary hemorrhage.
Time to rebleed
A mean of three days between injury and secondary hemorrhage was reported for eight participants in the group with a monocular patch as well as for eight participants in the group with binocular patches (Analysis 12.4) (Edwards 1973).
12.4. Analysis.
Comparison 12 Monocular versus binocular patching, Outcome 4 Time to rebleed (days).
| Time to rebleed (days) | ||||
|---|---|---|---|---|
| Study | Number of rebleeds in monocular patching group | Time to rebleed in monocular patching group | Number of rebleeds in binocular patching group | Time to rebleed in binocular patching group |
| Edwards 1973 | 8 of 35 | Mean 3 days | 8 of 29 | Mean 3 days |
Risk of corneal blood stain
One participant in each of the two treatment groups experienced corneal blood staining in Edwards 1973 (RR 0.83, 95% CI 0.95 to 12.68; Analysis 12.5).
12.5. Analysis.
Comparison 12 Monocular versus binocular patching, Outcome 5 Risk of corneal blood stain.
Rakusin 1972 reported that the risk of complications of hyphema, including corneal blood staining; pigment on endothelium, anterior lens capsule, or vitreous; posterior synechiae; PAS; anterior chamber blood clots; and fibrous membrane formation, were similar in both groups.
Risk of peripheral anterior synechiae formation
Rakusin 1972 reported that the risk of complications of hyphema, including corneal blood staining; pigment on endothelium, anterior lens capsule, or vitreous; posterior synechiae; PAS; anterior chamber blood clots; and fibrous membrane formation, were similar in both groups.
Risk of glaucoma or elevated intraocular pressure
In Edwards 1973, three participants in the monocular patching group developed secondary glaucoma, while no participants in the binocular patching group developed secondary glaucoma (RR 5.83, 95% CI 0.31 to 108.52; Analysis 12.6; Table 11).
12.6. Analysis.
Comparison 12 Monocular versus binocular patching, Outcome 6 Risk of glaucoma or elevated IOP.
Risk of optic atrophy
Neither Edwards 1973 nor Rakusin 1972 reported this outcome.
Adverse events
Neither Edwards 1973 nor Rakusin 1972 reported this outcome.
Quality of life outcomes
Edwards 1973 noted no difference between groups on the "cooperation index," which included a number of outcomes including those associated with quality of life (pain, restlessness, activity, and emotional state while in the hospital).
Economic outcomes
Neither Edwards 1973 nor Rakusin 1972 reported this outcome.
Ambulatory versus conservative treatment
Visual acuity
Two studies compared ambulatory (i.e. moderate activity allowed) versus conservative treatment, which comprised bed rest alone (Rakusin 1972), or bed rest with elevation of the head, bilateral ocular patches, and a shield over the injured eye (Read 1974). In Read 1974, VA was not reported by treatment group, but the authors distinguished between poor VA due to the initial trauma and that due to secondary effects of the hyphema. They stated that poor VA due to hyphema occurred in nine of 71 (13%) participants in the ambulatory group compared with four of 66 (6%) participants in the conservative group. Overall, the proportion of participants with good VA was 104 of 137 (76%) with more participants in the ambulatory group having good VA. In Rakusin 1972, 22 of 26 (85%) participants in the ambulatory group had short‐term VA better than 20/60 compared with 20 of 26 (77%) participants in the conservative group (RR 1.10, 95% CI 0.84 to 1.44; Analysis 13.1). We graded the certainty of the evidence as low, downgrading for imprecision and risk of bias (Table 6).
13.1. Analysis.
Comparison 13 Ambulatory versus conservative treatment, Outcome 1 Short‐term visual acuity.
Time to resolution of primary hemorrhage
Read 1974 reported a mean of 5.8 days between the initial injury and resolution of the hyphema in the ambulatory group compared with 5.6 days in the group receiving bed rest. However, Rakusin 1972 observed a significant difference in the speed of reabsorption. The primary hyphema was resolved within one week in 13 of 26 (50%) participants in the ambulatory group compared with 22 of 26 (85%) participants in the conservative group (Analysis 13.2). We graded the certainty of the evidence as low, downgrading for imprecision and risk of bias (Table 6).
13.2. Analysis.
Comparison 13 Ambulatory versus conservative treatment, Outcome 2 Time to resolution of primary hemorrhage.
| Time to resolution of primary hemorrhage | ||||
|---|---|---|---|---|
| Study | Time to resolution in ambulatory group | Number of participants in ambulatory group | Time to resolution in control group | Number of participants in control group |
| Read 1974 | 5.8 days | 5.6 days | ||
Risk of secondary hemorrhage
Eighteen of 71 (25%) participants in the ambulatory group and 12 of 66 (18%) participants in the group receiving bed rest developed a secondary hemorrhage in Read 1974. Rakusin 1972 reported only one secondary hemorrhage, which occurred in the conservatively treated group. Combining the results of the two trials showed no evidence of a treatment effect (RR 1.28, 95% CI 0.68 to 2.40; Analysis 13.3). We graded the certainty of this evidence as low, downgrading for imprecision and inconsistency (Table 6). In Read 1974, the proportion of participants with a secondary hemorrhage appeared to be smaller with more severe initial hyphemas (16 of 30 (53%) versus 14 of 90 (16%) for those with an initial hyphema filling less than one‐third compared with one‐third or more of the anterior chamber) (Table 8).
13.3. Analysis.
Comparison 13 Ambulatory versus conservative treatment, Outcome 3 Risk of secondary hemorrhage.
Time to rebleed
Read 1974 reported that the majority of secondary hemorrhages occurred between days two and five following injury, although two secondary hemorrhages took place on day seven following the initial injury.
Risk of corneal blood stain
Nine participants in Read 1974 developed corneal blood staining: five of 71 (7%) participants in the ambulatory group and four of 66 (6%) participants in the group receiving bed rest (RR 0.83, 95% CI 0.47 to 1.46; Analysis 13.4; Table 9).
13.4. Analysis.
Comparison 13 Ambulatory versus conservative treatment, Outcome 4 Risk of corneal blood stain.
Rakusin 1972 reported that the risk of complications of hyphema, including corneal blood staining; pigment on endothelium, anterior lens capsule, or vitreous; posterior synechiae; PAS; anterior chamber blood clots; and fibrous membrane formation, were similar in both groups.
Risk of peripheral anterior synechiae formation
Read 1974 did not report on this outcome. Rakusin 1972 reported that the risk of complications of hyphema, including corneal blood staining; pigment on endothelium, anterior lens capsule, or vitreous; posterior synechiae; PAS; anterior chamber blood clots; and fibrous membrane formation, were similar in both groups .
Risk of glaucoma or elevated intraocular pressure
In Read 1974, 17 of the 71 (23.9%) participants in the group that was allowed moderate activity developed IOP of 25 mmHg or greater, while 19 of the 66 (28.8%) participants in the group assigned to bed rest developed an elevated IOP (RR 0.83, 95% CI 0.47 to 1.46; Analysis 13.5; Table 11).
13.5. Analysis.
Comparison 13 Ambulatory versus conservative treatment, Outcome 5 Risk of glaucoma or elevated IOP.
Risk of optic atrophy
Neither Rakusin 1972 nor Read 1974 reported this outcome.
Adverse events
Neither Rakusin 1972 nor Read 1974 reported this outcome.
Quality of life outcomes
Neither Rakusin 1972 nor Read 1974 reported this outcome.
Economic outcomes
Neither Rakusin 1972 nor Read 1974 reported this outcome.
Elevation of the head versus control
One study compared elevation of the head by assigning participants to a semi‐reclined body position or to lying on their right or left side (Zi 1999).
Visual acuity
Zi 1999 did not report this outcome.
Time to resolution of primary hemorrhage
Time to resolution was compared by level of hyphema. The time to resolution was somewhat shorter for participants with their head elevated compared with those lying flat if the initial hyphema filled up to half of the anterior chamber, but longer if the blood filled more than half (level of blood < one‐half of the anterior chamber: 1.7 days (n = 18) versus 2.8 days (n = 18); level of blood = one‐half of the anterior chamber: 2.2 days (n = 6) versus 3.1 days (n = 13); level of blood > one‐half of anterior chamber: 9.0 days (n = 11) versus 8.0 days (n = 8)) (Table 8).
Risk of secondary hemorrhage
Zi 1999 did not report this outcome.
Time to rebleed
Zi 1999 did not report this outcome.
Risk of corneal blood stain
Zi 1999 did not report this outcome.
Risk of peripheral anterior synechiae formation
Zi 1999 did not report this outcome.
Risk of glaucoma or elevated intraocular pressure
Fifteen participants developed secondary glaucoma, eight of 35 (23%) in the group in the semi‐reclined position and seven of 39 (18%) in the group lying flat (Table 11) (Zi 1999).
Risk of optic atrophy
Zi 1999 did not report this outcome.
Adverse events
Zi 1999 did not report this outcome.
Quality of life outcomes
Zi 1999 did not report this outcome.
Economic outcomes
Zi 1999 did not report this outcome.
Discussion
Summary of main results
We included 27 studies in this review, of which 20 were RCTs and seven used a quasi‐randomized method to assign participants to treatment groups. The primary outcome for all but three studies was the risk of a secondary hemorrhage. The primary outcomes for this review were VA and time to resolution of primary hemorrhage. Secondary outcomes for this review were sequelae of the traumatic hyphema, including risk of and time to rebleed, risk of corneal blood staining, risk of PAS formation, risk of pathologic increase in IOP or glaucoma development, and risk of optic atrophy development.
Antifibrinolytic agents
The use of antifibrinolytic agents such as aminocaproic acid and tranexamic acid in traumatic hyphema is controversial because while they are reported to reduce the rate of recurrent hemorrhage, this is at the cost of gastric and other adverse events. We found no effect of any antifibrinolytic agent on VA measured at any time point. Neither systemic nor topical aminocaproic acid had an effect on final VA, nor did tranexamic acid. Hyphemas in participants administered systemic aminocaproic acid appeared to take a somewhat longer time to clear than those in participants not receiving systemic aminocaproic acid, although the numbers were small and the conclusions unreliable. As expected, it took less time for hyphemas to clear in participants who did not have a secondary hemorrhage than in those who experienced a secondary hemorrhage. Antifibrinolytics appeared to prolong the time to resolution in both groups, that is those who had a rebleed and those who did not, but the available evidence was insufficient to make any firm conclusions about the time for a hyphema to clear in those treated with an antifibrinolytic.
Oral aminocaproic acid appeared to reduce the risk of a secondary hemorrhage, but in a sensitivity analysis excluding studies that did not adhere to an intention‐to‐treat analysis, we found a non‐significant effect of this drug on the rate of rebleeds. Likewise, evidence showing an effect of topical aminocaproic acid on the rate of rebleeds was equivocal: although appearing to reduce the rate of secondary hemorrhage, the number of events was small, thus although there was some evidence supporting an effect of aminocaproic acid in reducing the risk of secondary hemorrhage, it appears to be less convincing than previously reported (Walton 2002). There appeared to be little difference in the time for a secondary hemorrhage to occur between participants receiving aminocaproic acid (systemic or topical) and controls, but again the evidence is weak due to a small number of incidents. In addition, there appears to be no effect of either systemic or topically applied aminocaproic acid on the timing of the rebleed or on the number of events related to the traumatic hyphema itself (i.e. corneal blood staining, PAS formation, elevated IOP, or development of optic atrophy). However, the small number of events renders significance testing unreliable. Unfortunately, there was insufficient evidence to conclude whether aminocaproic acid would be beneficial specifically for individuals with sickle cell trait/disease. Whether aminocaproic acid is useful for people with sickle cell trait/disease is of extreme importance because such individuals are at higher risk for elevated IOP (Lai 2001).
Aminocaproic acid is reported to have several side effects, including nausea, vomiting, muscle cramps, conjunctival suffusion, headache, rash, pruritis, dyspnea, toxic confusional states, arrhythmias, and systemic hypotension. Its use is contraindicated in pregnant women and in people with coagulopathies or with renal diseases, and it should be used cautiously in people with hepatic, cardiovascular, or cerebrovascular diseases. There were no statistically significant differences in adverse events reported between systemic and topical aminocaproic acid or between standard versus low doses of aminocaproic acid.
Tranexamic acid was not statistically different from controls in terms of final VA, time to resolution of primary hemorrhage, time to rebleed, or duration of hospitalization. Tranexamic acid is reported to have fewer gastric side effects than aminocaproic acid. The results of one study comparing aminomethylbenzoic acid versus placebo suggested that people treated with oral aminomethylbenzoic acid were less likely to rebleed compared with those given placebo.
Corticosteroids
Corticosteroids have also been used to treat hyphema; the mechanism of action of corticosteroids is believed to be due to reduced inflammation, stabilization of the blood‐ocular barrier, or direct inhibition of fibrinolysis, thus preventing secondary rebleeds. Two studies evaluated the effect of systemic corticosteroids (Rahmani 1999; Spoor 1980), and two studies evaluated the effect of topical corticosteroids (Rakusin 1972; Zetterstrom 1969). No significant differences in terms of time to resolution of primary hemorrhage, time to rebleed, or increased IOP were found.
One study compared systemic aminocaproic acid versus prednisolone (Farber 1991). This study concluded that more hyphemas had resolved at discharge in participants in the prednisolone group than in participants in the systemic aminocaproic acid group. No other differences between agents were noted in this study, although the investigators did not follow participants after discharge.
Other pharmaceutical interventions
Two studies compared homatropine as a cycloplegic (agent that enlarges the pupil) to pilocarpine as a miotic (agent that constricts the pupil) (Bedrossian 1974; Rakusin 1972). A secondary hemorrhage occurred in only one participant in each study. Such small numbers of events makes significance testing unreliable. The traumatic hyphemas took a longer time to resolve in participants receiving pilocarpine. No other outcomes or other miotics or cycloplegics were studied.
No effect was seen with the use of conjugated estrogens in one study (Spaeth 1966).
No statistically significant difference was reported for risk of rebleed in participants who were given aspirin in comparison with those who were not (Marcus 1988).
One study compared traditional Chinese medicine versus antihemorrhagics (Wang 1994), but this study only measured a composite outcome of "cure" that was defined as complete resolution within five days, VA of 0.7 or better, and no rebleed within one week of resolution of the primary hemorrhage. No single outcome was reported separately and so could not be compared. Although the authors reported a positive effect of the intervention, the findings should be interpreted with caution due to the biases present in the study and the use of a composite outcome measure.
Non‐pharmaceutical interventions
No differences in VA, risk of secondary hemorrhage, or time to rebleed were reported in participants receiving a single versus binocular patch (Edwards 1973; Rakusin 1972).
One study evaluated the effect of raising the head (semi‐reclined position) compared with alternatively right and left lateral positions on time to resolution of primary hemorrhage (Zi 1999). The results were inconsistent: small hyphemas resolved sooner but larger hyphemas took longer when the head was raised. The time of follow‐up was not mentioned, and participants were not masked to treatment assignment.
Studies comparing moderate activity versus complete bed rest did not show any statistically significant difference in risk of secondary hemorrhage, final VA, time to rebleed, or time to resolution of the primary hemorrhage (Rakusin 1972; Read 1974). Occurrences of complications (elevated IOP or corneal blood staining) were also comparable.
Overall completeness and applicability of evidence
Our search strategy was comprehensive. We believe that we identified all or a high proportion of published trials of interventions for hyphema and that our review is reasonably complete.
Only a few studies or a single study evaluated a particular intervention. For example, only one study compared a low dose (50 mg/kg) versus the standard dose (100 mg/kg) of oral aminocaproic acid, and one study compared aminomethylbenzoic acid versus placebo (Liu 2002). Only two studies compared topical corticosteroids versus control (Rakusin 1972; Zetterstrom 1969), and only two studies compared systemic corticosteroids versus control (Rahmani 1999; Spoor 1980). One study compared aminocaproic acid versus prednisolone (Farber 1991), and just one study compared conjugated estrogen versus placebo (Spaeth 1966). Only two studies compared cycloplegic versus miotic usage, both of which evaluated homatropine versus pilocarpine (Bedrossian 1974; Rakusin 1972). One study compared aspirin versus control (Marcus 1988). One study compared traditional Chinese medicine versus antihemorrhagic agents as the control (Wang 1994). Only two studies evaluated monocular versus binocular patching (Edwards 1973; Rakusin 1972), and no studies compared binocular or monocular patching versus no patching. Only one study compared the effect of elevation of the head versus control (Zi 1999). The limited number of studies evaluating a particular intervention made the application of meta‐analytic methods unreliable or impossible for many outcomes.
Another limitation of the validity of some results was the lack of information on people with sickle cell disease/trait. Two studies included in this review reported on the occurrence of secondary hemorrhage in participants with sickle cell trait/disease. Crouch 1976 mentioned that the one participant who had a secondary hemorrhage in the aminocaproic acid group and two of the nine participants who had a secondary hemorrhage in the placebo group also had sickle cell trait, but they did not state to which group the eight sickle cell trait participants were originally assigned. Pieramici 2003 reported that two participants in the aminocaproic acid group and one in the placebo group had sickle cell trait, but did not comment on the rebleed rate of these participants. The subgroup of patients with sickle cell trait/disease is especially important because this group has been shown to be at higher risk for elevated IOP (Lai 2001). It has been shown that even modest elevations in IOP are potentially deleterious in sickle cell disease/trait (Goldberg 1979a; Goldberg 1979b; Goldberg 1979c), and specifically that permanent infarction of the optic nerve with substantial loss of vision can occur in such individuals. Careful monitoring of IOP is indicated, and early surgery to decompress the eye is often required.
Quality of the evidence
We included 27 studies in this review, of which 20 were RCTs and seven were quasi‐randomized studies. Overall, the risk of bias was higher in the non‐randomized studies in that the sequence generation and allocation concealment were inadequate. In many cases, the studies were not reported clearly, and participants were inappropriately excluded from the analyses in some studies.
Potential biases in the review process
Many of the studies were published more than 20 years ago, and it was not possible to contact the investigators to obtain missing information. One review author abstracted data from some of the foreign language articles.
Agreements and disagreements with other studies or reviews
We found some evidence for an effect of aminocaproic acid and tranexamic acid on the risk of secondary hemorrhage. The evidence for a preventive effect of antifibrinolytics on rebleeds was not nearly as strong as that reported in the reviews by Walton 2002 and Sheppard 2009. However, Walton 2002 included RCTs, controlled clinical trials, as well as observational studies, but did not take into account any biases in the individual studies. Sheppard 2009 cited only some of the trials and also included observational studies. In these two reviews, no effect of either aminocaproic acid or tranexamic acid was found on VA. Walton 2002 presented a stronger case for the use of corticosteroids to prevent secondary hemorrhage than we report here or than is reported by Sheppard 2009. Our review agrees with most of the existing literature in that there is little evidence for the use of bilateral patching, topical cycloplegics, sedation, or bed rest, although these interventions are often recommended (Sheppard 2009; Walton 2002).
Authors' conclusions
Implications for practice.
Limited evidence suggests that people with traumatic hyphema who receive aminocaproic acid are less likely to experience secondary hemorrhage than those who do not. Complications resulting from secondary hemorrhage, such as glaucoma, corneal blood staining, or optic atrophy, can lead to permanent impairment of vision. We did not identify a significant effect on or final visual acuity (VA) following hyphema. Moreover, oral aminocaproic acid was shown to yield significant side effects including gastrointestinal upset and systemic hypotension, and participants without secondary hyphemas who were treated with aminocaproic acid showed slower clearing of hyphema than participants in control groups.
Tranexamic acid seems to be as effective as aminocaproic acid in terms of effect on secondary hemorrhage but with fewer gastric side effects. Data from the few studies that evaluated the effect of corticosteroids on final VA and risk of secondary hemorrhage do not support the presumed benefits, though corticosteroid usage may aid in relieving the associated inflammation in such cases.
Given the risk of side effects for various potential medical treatments for traumatic hyphema (antifibrinolytic agents, corticosteroids, and cycloplegics) without the presence of solid scientific evidence to support their benefit, it might be reasonable to recommend their usage only in those individuals at high risk of complications (such as those with sickle cell trait/disease).
Controlled clinical trials comparing non‐drug treatment modalities versus placebo did not show a protective effect. We found no convincing evidence of benefit of binocular patching over monocular patching, bed rest over moderate activity, or elevation of the head in a semi‐reclined position in the treatment of traumatic hyphema. Given that most of these interventions were used collectively in many of the studies presented, it was not possible to assess the extent to which any of these interventions may have contributed to any reported positive results.
Implications for research.
There is insufficient high‐quality evidence from large randomized controlled trials to support the use of corticosteroids or cycloplegics, and limited evidence for the use of antifibrinolytics in the treatment of traumatic hyphema. It is possible that topical aminocaproic acid or a lower dose of systemic aminocaproic acid (50 mg/kg instead of 100 mg/kg) may be efficacious in reducing secondary hemorrhage with a potential reduction in the risk of side effects. Future research with such agents aimed at assessing impact on final VA after the resolution of the hyphema, time to achieve final VA, cost, and quality of life (side effects and time lost from school and employment) would be most helpful to guide treatment recommendations. Ongoing or future studies on the medical treatment of hyphema should study individuals with sickle cell disease/trait in particular. Studies with direct comparisons of aminocaproic acid versus tranexamic acid do not yet exist, and only one study compared aminocaproic acid versus prednisolone. Further research to study the additive effect of non‐medical interventions in hyphema management might be of value, as they are not usually used independently of one another.
What's new
| Date | Event | Description |
|---|---|---|
| 18 December 2018 | New search has been performed | Issue 1, 2019: Searches updated, no new studies identified for inclusion. |
| 18 December 2018 | New citation required but conclusions have not changed | Issue 1, 2019: Summary of findings tables have been included. |
History
Protocol first published: Issue 3, 2005 Review first published: Issue 1, 2011
| Date | Event | Description |
|---|---|---|
| 22 November 2013 | New search has been performed | Issue 12, 2013: Searches updated. |
| 22 November 2013 | New citation required but conclusions have not changed | Issue 12, 2013: One new study added. |
| 7 August 2008 | New search has been performed | Converted to new review format |
Acknowledgements
We acknowledge the peer reviewers who made valuable comments on the protocol and the manuscript of the review. We thank Dr Milan Mathew for his contribution to the development of the protocol. We also thank Dr Tianjing Li for her comments on the protocol and translation of a Chinese language trial report. We acknowledge Xue Wang and Tsung Yu for assessing and translating Chinese language trial reports. In addition, we thank Kinnar Merchant and Aliaksei Pustavoitau for translating Russian trial reports. Lori Rosman, Information Specialist for Cochrane Eyes and Vision (CEV), executed the search strategies for this review.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Hyphema explode all trees #2 hyphem* or hyphaem* #3 MeSH descriptor Anterior Chamber explode all trees with qualifier: IN #4 MeSH descriptor Eye Hemorrhage, this term only #5 MeSH descriptor Hemorrhage, this term only #6 MeSH descriptor Eye Injuries explode all trees #7 (#5 AND #6) #8 (#1 OR #2 OR #3 OR #4 OR #7)
Appendix 2. MEDLINE Ovid search strategy
1. Randomized Controlled Trial.pt. 2. Controlled Clinical Trial.pt. 3. (randomized or randomised).ab,ti. 4. placebo.ab,ti. 5. drug therapy.fs. 6. randomly.ab,ti. 7. trial.ab,ti. 8. groups.ab,ti. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp animals/ not humans.sh. 11. 9 not 10 12. exp Hyphema/ 13. (hyphem* or hyphaem*).tw. 14. exp *Anterior Chamber/in [Injuries] 15. *Eye Hemorrhage/ 16. Hemorrhage/ 17. exp Eye Injuries/ 18. 16 and 17 19. 12 or 13 or 14 or 15 or 18 20. 11 and 19
Appendix 3. Embase.com search strategy
1. 'randomized controlled trial'/exp 2. 'randomization'/exp 3. 'double blind procedure'/exp 4. 'single blind procedure'/exp 5. random*:ab,ti 6. 1 OR 2 OR 3 OR 4 OR 5 7. 'animal'/exp OR 'animal experiment'/exp 8. 'human'/exp 9. 7 AND 8 10. 7 NOT 9 11. 6 NOT 10 12. 'clinical trial'/exp 13. (clin* NEAR/3 trial*):ab,ti 14. ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti 15. 'placebo'/exp 16. placebo*:ab,ti 17. random*:ab,ti 18. 'experimental design'/exp 19. 'crossover procedure'/exp 20. 'control group'/exp 21. 'latin square design'/exp 22. 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19 OR 20 OR 21 23. 22 NOT 10 24. 23 NOT 11 25. 'comparative study'/exp 26. 'evaluation'/exp 27. 'prospective study'/exp 28. control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti 29. 25 OR 26 OR 27 OR 28 30. 29 NOT 10 31. 30 NOT (11 OR 23) 32. 11 OR 24 OR 31 33. 'hyphema'/exp 34. hyphem*:ab,ti OR hyphaem*:ab,ti 35. 'anterior eye chamber'/mj AND 'injury'/exp 36. 'bleeding'/de AND 'eye injury'/exp 37. 33 OR 34 OR 35 OR 36 38. 32 AND 37
Appendix 4. PubMed search strategy
1. ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomised[tiab] OR randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh]) 2. (hyphem*[tiab] or hyphaem*[tiab]) NOT Medline[sb] 3. #1 AND #2
Appendix 5. ISRCTN search strategy
hyphema or hyphaema
Appendix 6. ClinicalTrials.gov search strategy
hyphema or hyphaema
Appendix 7. WHO ICTRP search strategy
hyphema or hyphaema
Data and analyses
Comparison 1. Systemic aminocaproic acid versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term visual acuity from 20/20 to 20/40 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Long‐term visual acuity between 20/20 and 20/40 | 2 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.82, 1.29] |
| 3 Final visual acuity between 20/20 and 20/40 | 2 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.93, 1.18] |
| 4 Time to resolution of primary hemorrhage (days) | Other data | No numeric data | ||
| 5 Risk of secondary hemorrhage | 6 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.13, 0.60] |
| 6 Time to rebleed (days) | Other data | No numeric data | ||
| 7 Risk of corneal blood stain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Risk of glaucoma or increases in IOP | Other data | No numeric data | ||
| 8.1 Transient increase in IOP | Other data | No numeric data | ||
| 8.2 Persistent increase in IOP | Other data | No numeric data | ||
| 9 Risk of glaucoma or elevated IOP | 2 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.08, 1.82] |
| 10 Risk of optic atrophy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 Adverse effects: nausea or vomiting | 3 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.60 [2.09, 35.50] |
| 12 Duration of hospitalization (days) | Other data | No numeric data |
Comparison 2. Topical aminocaproic acid versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term visual acuity from 20/20 to 20/40 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Time to resolution of primary hemorrhage (days) | Other data | No numeric data | ||
| 3 Risk of secondary hemorrhage | 2 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.20, 1.10] |
| 4 Time to rebleed (days) | Other data | No numeric data | ||
| 5 Risk of glaucoma or elevated IOP | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 3. Low‐dose versus standard‐dose aminocaproic acid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Unspecified time for visual acuity between 20/20 and 20/40 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Time to resolution of primary hemorrhage (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Risk of secondary hemorrhage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Time to rebleed (days) | Other data | No numeric data | ||
| 5 Risk of glaucoma or elevated IOP | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Nausea or vomiting | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Dizziness or hypotension | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Syncope | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Diarrhea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.5 Rash or pruritis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.6 Hot flashes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.7 Dry mouth or nose | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Duration of hospitalization (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 4. Systemic versus topical aminocaproic acid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term visual acuity from 20/20 to 20/40 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Risk of secondary hemorrhage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Conjunctival corneal foreign body sensation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Transient punctate corneal staining | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Dizziness, nausea, vomiting | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Tranexamic acid versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term visual acuity from 20/20 to 20/40 | 3 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.98, 1.25] |
| 2 Time to resolution of primary hemorrhage (days) | Other data | No numeric data | ||
| 3 Risk of secondary hemorrhage | 5 | 578 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.17, 0.55] |
| 4 Time to rebleed (days) | Other data | No numeric data | ||
| 5 Risk of corneal blood stain | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Risk of glaucoma or elevated IOP | 4 | 543 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.73, 1.98] |
| 7 Adverse effects: nausea or vomiting | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Duration of hospitalization (days) | Other data | No numeric data |
Comparison 6. Aminomethylbenzoic acid versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Risk of secondary hemorrhage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 7. Corticosteroids versus control.
Comparison 8. Aminocaproic acid versus prednisone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term (5 to 14 days) visual acuity from 20/20 to 20/40 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Risk of secondary hemorrhage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Adverse effect: any adverse event | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. Conjugated estrogen versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Risk of secondary hemorrhage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Risk of corneal blood stain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 10. Cycloplegics versus miotics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term visual acuity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Time to resolution of primary hemorrhage (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Risk of secondary hemorrhage | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.15, 6.99] |
| 4 Time to rebleed (days) | Other data | No numeric data |
Comparison 11. Aspirin versus observation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Risk of secondary hemorrhage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 12. Monocular versus binocular patching.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term visual acuity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Variable time length 'final' visual acuity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Risk of secondary hemorrhage | 2 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.35, 1.72] |
| 4 Time to rebleed (days) | Other data | No numeric data | ||
| 5 Risk of corneal blood stain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Risk of glaucoma or elevated IOP | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 13. Ambulatory versus conservative treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term visual acuity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Time to resolution of primary hemorrhage | Other data | No numeric data | ||
| 3 Risk of secondary hemorrhage | 2 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.68, 2.40] |
| 4 Risk of corneal blood stain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Risk of glaucoma or elevated IOP | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bedrossian 1974.
| Methods | Study design: Quasi‐randomized controlled series. Exclusions after allocation: None. Losses to follow‐up: None. Intention‐to‐treat: All participants were analyzed in the group to which they were assigned. Sample size calculations: Not reported. |
|
| Participants | Country: USA. Dates: Not reported. Number allocated: 58 consecutive patients alternately assigned to treatment group after classification based on the size of initial hyphema. Age: Not reported. Sex: Not reported. Race: Not reported. Sickle cell disease: Not reported. Participants appeared to be balanced with respect to baseline characteristics. Inclusion criteria: Non‐total traumatic hyphema. |
|
| Interventions | Cycloplegics (n = 28): 1% atropine ointment. Miotics (n = 30): 2% pilocarpine ointment (or eserine ointment). Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Time to resolution of primary hemorrhage. Secondary outcomes:
Follow‐up: Days 1 to 7. |
|
| Notes | Funding source not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation was not randomized: participants alternately assigned to treatment groups based on the blood level in the anterior chamber. |
| Allocation concealment (selection bias) | High risk | Allocation was assigned on an alternate basis. |
| Blinding (performance bias and detection bias) Participants | High risk | Masking was not reported. |
| Blinding (performance bias and detection bias) Personnel and outcome assessors | High risk | Masking was not reported. |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | All participants were analyzed in the group to which they had been assigned. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | All participants were analyzed in the group to which they had been assigned. |
| Selective reporting (reporting bias) | Low risk | Reported results for primary and secondary outcomes |
| Other bias | Low risk | No other sources of potential bias were identified. |
Christianson 1979.
| Methods | Study design: Randomized, double‐masked, placebo‐controlled clinical trial. Exclusions after randomization: None reported. Losses to follow‐up: None reported. Intention‐to‐treat: All participants were analyzed in the group to which they had been randomly assigned. Sample size calculations: Not reported. |
|
| Participants | Country: USA. Dates: Not reported. Number randomized: 45. Age: Not reported. Sex: Not reported. Race: Not reported. Sickle cell disease: Not reported. Inclusion criteria: Traumatic hyphema. Exclusion criteria: Not reported. |
|
| Interventions | Treatment (n = 22): Oral aminocaproic acid, loading dose 75 mg/kg, followed by 60 mg/kg every 4 hours; length of treatment not reported. Control (n = 23): Placebo, presumably every 4 hours. |
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, details not reported. Secondary outcomes: Time to resolution of primary hyphema, details not reported. |
|
| Notes | Abstract of unpublished study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) Participants | Low risk | Authors used a placebo control and stated that the study was double‐masked. |
| Blinding (performance bias and detection bias) Personnel and outcome assessors | Low risk | Authors used a placebo control and stated that the study was double‐masked. |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | Unclear if number randomized equaled the number reported and analyzed in the abstract, but no exclusions or losses to follow‐up were reported. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | Unclear if number randomized equaled the number reported and analyzed in the abstract, but no exclusions or losses to follow‐up were reported. |
| Selective reporting (reporting bias) | Unclear risk | Few study details available in the abstract, and no full version was published. |
| Other bias | Unclear risk | Few study details available in the abstract, and no full version was published. |
Crouch 1976.
| Methods | Study design: Randomized, double‐masked, placebo‐controlled clinical trial. Exclusions after randomization: None. Losses to follow‐up: None. Intention‐to‐treat: All participants were analyzed in the group to which they had been randomly assigned. Sample size calculations: Not reported. |
|
| Participants | Country: USA. Dates: September 1972 to October 1974. Number randomized: 59. Age: 83% aged 6 to 30 years. Sex: 83% male. Race: 65% black, 35% white. Sickle cell disease: 8/59 (14%) had sickle cell trait. Participants appeared to be balanced with respect to baseline characteristics. Inclusion criteria: Traumatic hyphema. Exclusion criteria:
|
|
| Interventions | Treatment (n = 32): Oral aminocaproic acid 100 mg/kg every 4 hours for 5 days. Control (n = 27): Placebo (200 mL of aromatic elixir (5% glucose, water, and ethanol) in 1000 mL sterile water) every 4 hours for 5 days. Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, assessed by daily slit‐lamp exam, and documented by 3 observers. Secondary outcomes:
Follow‐up: 1 week, 1, 2, 3, 6, 12, 18, and 24 months. |
|
| Notes | Funded by the National Eye Institute, US National Institutes of Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants assigned to treatment groups using computerized randomization. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) Participants | Low risk | Authors used a placebo control and stated that the study was double‐masked. |
| Blinding (performance bias and detection bias) Personnel and outcome assessors | Low risk | Authors used a placebo control and stated that the study was double‐masked. |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | There were no exclusions or losses to follow‐up. All participants were analyzed in the group to which they had been randomly assigned. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | There were no exclusions or losses to follow‐up. All participants were analyzed in the group to which they had been randomly assigned. |
| Selective reporting (reporting bias) | Low risk | Reported results for primary and secondary outcomes |
| Other bias | Low risk | No other sources of potential bias were identified. |
Crouch 1997.
| Methods | Study design: Randomized, double‐masked clinical trial. Exclusions after randomization: 1 individual assigned to oral aminocaproic acid and topical placebo excluded due to side effect of drug (vomiting). Losses to follow‐up: None. Intention‐to‐treat: All participants were analyzed in the group to which they had been randomly assigned. Sample size calculations: Sample size was determined to be 25 to 30 participants in each of the 3 groups based on alpha of 0.05 and power of 80%. Additional comments: The investigators also studied a control group that did not receive either topical or systemic aminocaproic acid and had refused randomization. We did not include these individuals in our analyses. |
|
| Participants | Country: USA. Dates: March 1990 to May 1996. Number randomized: 64: 29 to oral aminocaproic acid plus topical placebo, 35 to oral placebo plus topical aminocaproic acid. Additional 54 participants included as control group. Age: 72% younger than 21 years. Sex: 67% male. Race: 50% black, 49% white, and 1% (1 participant) Asian. Sickle cell disease: 2/35 (6%) of participants assigned to topical aminocaproic acid, and 2/29 (7%) of participants assigned to oral aminocaproic acid had sickle cell trait. Participants appeared to be balanced with respect to baseline characteristics. Inclusion criteria: Traumatic hyphema. Exclusion criteria:
|
|
| Interventions | Treatment: 0.2 mL of 30% aminocaproic acid in 2% carboxymethylene gel applied to inferior fornix every 6 hours plus oral placebo solution every 4 hours for 5 days. Control: Oral aminocaproic acid 50 mg/kg (up to 30 g/day) plus placebo gel every 4 hours for 5 days. Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, assessed by daily slit‐lamp exam, and documented by a sketch each day. Secondary outcomes:
|
|
| Notes | Funded in part by the Lions Medical Eye Bank and Research Center of Eastern Virginia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to treatment groups using computerized randomization. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) Participants | Low risk | Authors used a placebo control and stated that the study was double‐masked. Placebo pills were given to the topical group and placebo gel administered to the systemic group to make both regimens similar. |
| Blinding (performance bias and detection bias) Personnel and outcome assessors | Low risk | Authors used a placebo control and stated that the study was double‐masked. "Data were compiled by observers who did not know what patients were in the treated and untreated control groups." |
| Incomplete outcome data (attrition bias) Primary outcome | Unclear risk | 1 participant who was assigned to oral aminocaproic acid and topical placebo was excluded due to side effect of drug (vomiting). The remaining participants were analyzed in the group to which they had been randomly assigned. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | 1 participant who was assigned to oral aminocaproic acid and topical placebo was excluded due to side effect of drug (vomiting). The remaining participants were analyzed in the group to which they had been randomly assigned. |
| Selective reporting (reporting bias) | Low risk | Reported results for primary and secondary outcomes |
| Other bias | Low risk | No other sources of potential bias were identified. |
Edwards 1973.
| Methods | Study design: Quasi‐randomized controlled series. Exclusions after allocation: Individuals over 20 years old were excluded from the study because of the small number enrolled. Losses to follow‐up: None. Intention‐to‐treat: Participants aged 20 years and younger were analyzed in the group to which they had been assigned. Sample size calculations: Not reported. |
|
| Participants | Country: USA. Dates: 1969 to 1971. Number allocated: 64 consecutive patients alternately assigned to treatment group. Age: Mean 10 years (up to 20 years). Sex: 61 (95%) men and 3 (5%) women. Race: Not reported. Sickle cell disease: Not reported. Participants appeared to be balanced with respect to baseline characteristics. Inclusion criteria: Traumatic hyphema. Exclusion criteria: Individuals over 20 years of age. |
|
| Interventions | Treatment: Monocular patching (n = 35) Control: Binocular patching (n = 29) Treatment for both groups included:
|
|
| Outcomes | Primary and secondary outcomes not specified. Measured outcomes:
Follow‐up: Days 1 to 7. |
|
| Notes | Funded by Research to Prevent Blindness Inc, Public Health Service Training Grant, and the National Institutes of Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation was not randomized: an independent study director assigned participants to treatment groups on an alternate basis by turning a card. Occasionally the card was not turned each time, which led to an uneven number of participants in each group. |
| Allocation concealment (selection bias) | High risk | Allocation was assigned on an alternate basis. |
| Blinding (performance bias and detection bias) Participants | High risk | Masking of participants was not possible given the interventions being studied. |
| Blinding (performance bias and detection bias) Personnel and outcome assessors | Unclear risk | The authors reported that the study was double‐masked, although this statement was not clear. The study investigators seldom participated in participant care to allow other examiners with less experience in monocular patching to collect data in hopes of minimizing observation bias. |
| Incomplete outcome data (attrition bias) Primary outcome | Unclear risk | Individuals over 20 years of age were excluded after allocation to treatment group. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | Individuals over 20 years of age were excluded after allocation to treatment group. |
| Selective reporting (reporting bias) | Low risk | Reported results for all outcomes |
| Other bias | Low risk | No other sources of potential bias were identified. |
Farber 1991.
| Methods | Study design: Randomized, double‐masked clinical trial. Exclusions after randomization: 6 participants in the aminocaproic acid group were excluded: 4 were administered prednisone instead of aminocaproic acid (treatment cross‐over); 1 participant had an unrelated seizure; and 1 participant developed thrombocytopenia. 1 participant in the prednisone group was administered aminocaproic acid instead of prednisone (treatment cross‐over) and was excluded. Losses to follow‐up: 2 participants in the aminocaproic acid group and 1 participant in the prednisone group withdrew from the study. Intention‐to‐treat: The participants lost to follow‐up or excluded were not included in the analyses, and the intention‐to‐treat principle was not followed in the analyses. Sample size calculations: Not reported. Additional comments: The authors noted that there were no secondary hemorrhages in the individuals who were excluded or who withdrew from the study. |
|
| Participants | Country: USA. Dates: July 1985 to March 1990. Number randomized: 122: 64 to aminocaproic acid, 58 to prednisone. Age: Mean age in aminocaproic acid group: 23.8 ± 13.8 years (range 4 to 64 years); prednisone group: 23.3 ± 13.4 years (range 1.5 to 62 years). Sex: 79% male. Race: 53% black, 22% white, 22% Hispanic, and 3% of other ethnic or racial group. Study groups were not balanced by race: 57% black and 20% white in aminocaproic acid group vs 48% black and 25% white in prednisone group. Sickle cell disease: None; excluded. Inclusion criteria: Traumatic hyphema. Exclusion criteria:
|
|
| Interventions | Treatment: Oral aminocaproic acid 50 mg/kg (up to 30 g/day) every 4 hours plus 2 doses placebo for 5 days. Control: Oral prednisone 40 mg/day in 2 doses plus 6 doses placebo; children and adults weighing less than 60 kg were given 0.6 mg/kg/day prednisone, for 5 days. Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, recorded daily by slit‐lamp exam, documented by measuring height in millimeters and defined as a definite increase in level of presence of "fresh" blood visible over darker clotted blood. Secondary outcomes:
|
|
| Notes | Funded by the National Eye Institute of the National Institutes of Health, Bethesda, MD, and Research to Prevent Blindness | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, but method of allocation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) Participants | Low risk | Authors used a double‐dummy placebo design and stated that the study was double‐masked. |
| Blinding (performance bias and detection bias) Personnel and outcome assessors | Low risk | Authors used a double‐dummy placebo design and stated that the study was double‐masked. "All of the treating physicians and nurses were masked to the identity of the treatment." |
| Incomplete outcome data (attrition bias) Primary outcome | Unclear risk | The participants lost to follow‐up or excluded were not included in the analyses, and the intention‐to‐treat principle was not followed in the analyses. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | The participants lost to follow‐up or excluded were not included in the analyses, and the intention‐to‐treat principle was not followed in the analyses. |
| Selective reporting (reporting bias) | Low risk | Reported results for primary and secondary outcomes |
| Other bias | Low risk | No other sources of potential bias were identified. |
Karkhaneh 2003.
| Methods | Study design: Randomized, double‐masked clinical trial. Exclusions after randomization: None. Losses to follow‐up: 23: 4 to homatropine drops plus topical aminocaproic acid gel group, 5 to homatropine drops plus topical placebo gel group, 14 to homatropine drops‐only group. Intention‐to‐treat: The participants lost to follow‐up were not included in the analyses, and the intention‐to‐treat principle was not followed in the analyses. Sample size calculations: Not reported. |
|
| Participants | Country: Iran. Dates: 1998‐99. Number randomized: 155: 45 to homatropine drops plus topical aminocaproic acid gel group, 44 to homatropine drops plus placebo gel group, 66 to homatropine drops‐only group. Age: 4 to 30 years. Sex: 87% (not including those lost to follow‐up) male. Race: Not reported. Sickle cell disease: Not reported. Participants appeared to be balanced with respect to baseline characteristics. Inclusion criteria: Non‐penetrating traumatic hyphema and emergency room outpatient of Farabi Eye Hospital. Exclusion criteria:
|
|
| Interventions | Treatment 1: 2 drops of 25% aminocaproic acid in 2% carboxymethylene gel applied to inferior fornix of affected eye every 6 hours plus homatropine eyedrops 3 times/day, for 5 days. Control 1: 2 drops 2% carboxymethylene (placebo) gel applied to inferior fornix of affected eye every 6 hours plus homatropine eyedrops 3 times/day, for 5 days. Control 2: Homatropine eyedrops 3 times/day, for 5 days. Treatment for all groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, assessed daily by slit‐lamp exam for 7 days, and then at day 14. Method for documentation and definition not reported. Secondary outcomes: All measured daily for 7 days and at day 14:
|
|
| Notes | Conducted with support from Sina Darou (an ophthalmic pharmaceutical company in Iran), who provided the aminocaproic acid preparation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, but method of allocation was not reported. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed from investigators by use of coded bottles. |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Authors used coded bottles to mask participants to the topical medication, but the group assigned to homatropine drops and no topical medication was not masked. |
| Blinding (performance bias and detection bias) Personnel and outcome assessors | Low risk | Authors used coded bottles to mask healthcare providers and outcomes assessors. "The ophthalmologist who examined the patients did not know if they were treated or not." |
| Incomplete outcome data (attrition bias) Primary outcome | Unclear risk | The participants lost to follow‐up were not included in the analyses, and the intention‐to‐treat principle was not followed in the analyses. 23 participants were lost to follow‐up: 4 to homatropine drops plus topical aminocaproic acid gel, 5 to homatropine drops plus topical placebo gel, 14 to homatropine drops only. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | The participants lost to follow‐up were not included in the analyses, and the intention‐to‐treat principle was not followed in the analyses. 23 participants were lost to follow‐up: 4 to homatropine drops plus topical aminocaproic acid gel, 5 to homatropine drops plus topical placebo gel, 14 to homatropine drops only. |
| Selective reporting (reporting bias) | Low risk | Reported results for primary and secondary outcomes |
| Other bias | Unclear risk | Conducted with support from Sina Darou (an ophthalmic pharmaceutical company in Iran), who provided the aminocaproic acid preparation |
Kraft 1987.
| Methods | Study design: Randomized, double‐masked clinical trial. Exclusions after randomization: None. Losses to follow‐up: None. Intention‐to‐treat: All participants were analyzed in the group to which they had been randomly assigned. Sample size calculations: Not reported. |
|
| Participants | Country: Canada Dates: May 1978 to December 1984 Number randomized: 49: 24 to oral aminocaproic acid; 25 to placebo. Age: 3 to 18 years. Mean age: aminocaproic acid group 10.6 years, placebo group 11.2 years. Sex: 73% male. Race: 3 black participants in the aminocaproic acid group; 1 in the placebo group. The ethnicity or race of the other participants was not reported. Sickle cell disease: None; excluded. Participants appeared to be balanced with respect to baseline characteristics. Inclusion criteria: Children with non‐penetrating traumatic hyphema treated at the Hospital for Sick Children in Toronto, Canada. Exclusion criteria:
|
|
| Interventions | Treatment: Oral aminocaproic acid 100 mg/kg every 4 hours, for 5 days. Control: Placebo every 4 hours for 5 days. Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, assessed daily by slit‐lamp exam; documented by 2 observers and defined as definite increase in amount of blood compared with amount at admission or fresh red blood over darker clotted blood. Secondary outcomes: Outcomes measured daily during hospitalization (up to 5 days), then at 6 weeks, and 3, 6, 12, and 18 months after discharge.
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants assigned to treatment groups using computerized randomization. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) Participants | Low risk | Authors used a placebo control and stated that the study was double‐masked. |
| Blinding (performance bias and detection bias) Personnel and outcome assessors | Low risk | Authors used a placebo control and stated that the study was double‐masked. |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | There was no loss to follow‐up, and all participants were analyzed in the group to which they had been randomly assigned. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | There was no loss to follow‐up, and all participants were analyzed in the group to which they had been randomly assigned. |
| Selective reporting (reporting bias) | Low risk | Reported results for primary and secondary outcomes |
| Other bias | Low risk | No other sources of potential bias were identified. |
Kutner 1987.
| Methods | Study design: Randomized, double‐masked clinical trial. Exclusions after randomization: 1 participant was excluded from the aminocaproic acid group due to systemic hypotension attributable to the study drug. Losses to follow‐up: None. Intention‐to‐treat: The participant excluded from the study was not included in the analyses, and the intention‐to‐treat principle was not followed in the analyses. Sample size calculations: Not reported. |
|
| Participants | Country: USA. Dates: November 1983 to January 1986. Number randomized: 34: 21 to aminocaproic acid, 13 to placebo. Age: Mean age: aminocaproic acid 18.9 ± 7.7 years, placebo 22.8 ± 7.6 years. Sex: 88% male. Race: 85% white. Sickle cell disease: None; excluded. Participants appeared to be balanced with respect to baseline characteristics. Inclusion criteria: Non‐penetrating traumatic hyphema. Exclusion criteria:
|
|
| Interventions | Treatment: Oral aminocaproic acid 100 mg/kg every 4 hours (up to 5 g/dose and 30 g/day), for 5 days. Control: Placebo every 4 hours for 5 days. Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, assessed daily by slit‐lamp exam, for 6 days and 1 week after discharge. Defined as a definite increase in the amount of blood in the anterior chamber compared with the amount noted on the previous day's exam. Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to treatment groups using computerized randomization. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) Participants | Low risk | Authors used a placebo control and stated that the study was double‐masked. |
| Blinding (performance bias and detection bias) Personnel and outcome assessors | Low risk | Authors used a placebo control and stated that the study was double‐masked. Assignment codes were maintained by a central data evaluator who had no clinical contact with any participant. "Physicians caring for study patients did not have access to the cumulative data until the code was broken." |
| Incomplete outcome data (attrition bias) Primary outcome | Unclear risk | 1 participant was excluded from the aminocaproic acid group due to systemic hypotension attributable to the study drug. This participant reportedly did not rebleed. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | 1 participant was excluded from the aminocaproic acid group due to systemic hypotension attributable to the study drug. Data for this participant were analyzed until time of study withdrawal. |
| Selective reporting (reporting bias) | Low risk | Reported results for primary and secondary outcomes |
| Other bias | Low risk | No other sources of potential bias were identified. |
Liu 2002.
| Methods | Study design: Randomized clinical trial. Exclusions after randomization: None. Losses to follow‐up: None. Intention‐to‐treat: All participants were analyzed in the group to which they had been randomly assigned. Sample size calculations: Not reported. |
|
| Participants | Country: China. Dates: December 1997 to December 2000. Number randomized: 92: 60 to aminomethylbenzoic acid, 32 to control. Age: Mean age: aminomethylbenzoic acid 32.7 ± 11.25 years, control 33.4 ± 10.75 years. Sex: 75% male. Race: Not reported. Sickle cell disease: Not reported. Participants appeared to be balanced with respect to baseline characteristics. Inclusion criteria: Traumatic hyphema. Exclusion criteria:
|
|
| Interventions | Treatment: Oral aminomethylbenzoic acid 0.5 g plus oral vitamin B1 20 mg 3 times/day, for 6 days. For children, the dosage of aminomethylbenzoic acid was modified to "follow age‐recommended dose"; the vitamin B1 dosage remained the same. Control: Oral vitamin B1 20 mg 3 times/day, for 6 days. Treatment for both groups included 0.3% ofloxacin eyedrops 4 times/day, for 6 days. |
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, details not reported. Secondary outcomes: Risk of complications and adverse events. |
|
| Notes | Poor description of study methods in publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, but method of allocation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) Participants | Unclear risk | The authors do not state whether masking was used. |
| Blinding (performance bias and detection bias) Personnel and outcome assessors | Unclear risk | The authors do not state whether masking was used. |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | No exclusions or loss to follow‐up. All participants were analyzed in the group to which they had been randomly assigned. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | No exclusions or loss to follow‐up. All participants were analyzed in the group to which they had been randomly assigned. |
| Selective reporting (reporting bias) | Unclear risk | Study outcomes of interest not clearly stated. |
| Other bias | Low risk | No other sources of potential bias were identified. |
Marcus 1988.
| Methods | Study design: Randomized clinical trial. Exclusions after randomization: None. Losses to follow‐up: None. Intention‐to‐treat: All participants were analyzed in the group to which they had been randomly assigned. Sample size calculations: Not reported. |
|
| Participants | Country: Israel. Dates: Not reported. Number randomized: 51: 23 to aspirin, 28 to observation. Age: Mean age: 20 years. Sex: Not reported. Race: Not reported. Sickle cell disease: Not reported. Author stated that participants were balanced with respect to baseline characteristics. Inclusion criteria: Traumatic hyphema. Exclusion criteria:
|
|
| Interventions | Treatment: Aspirin 500 mg 3 times/day for 5 days. Control: Observation. Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, assessed daily. Documented by estimating percentage involvement and plotting diagrammatically; definition not reported. Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, but method of allocation not reported. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed from investigators by use of sequentially numbered envelopes. |
| Blinding (performance bias and detection bias) Participants | High risk | The participants were not masked to treatment. No placebo was given to the control group. |
| Blinding (performance bias and detection bias) Personnel and outcome assessors | High risk | The healthcare providers were not masked to treatment. No placebo was given to the control group. |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | No exclusions or loss to follow‐up. All participants were analyzed in the group to which they had been randomly assigned. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | No exclusions or loss to follow‐up. All participants were analyzed in the group to which they had been randomly assigned. |
| Selective reporting (reporting bias) | Unclear risk | Results are only reported for secondary hemorrhage. |
| Other bias | Low risk | Poor description of study methods and results in publication |
McGetrick 1983.
| Methods | Study design: Randomized, double‐masked clinical trial. Exclusions after randomization: The chart of 1 participant in the placebo group was "lost," and this participant was excluded. Losses to follow‐up: None. Intention‐to‐treat: The excluded participant was not included in the analyses, and the intention‐to‐treat principle was not followed in the analyses. Sample size calculations: Not reported. |
|
| Participants | Country: USA. Dates: August 1980 to February 1982. Number randomized: 50: 28 to aminocaproic acid, 22 to placebo. Age: 86% aged 6 to 40 years. Sex: 81% male. Race: 69% black, 21% Hispanic, and 10% white. Sickle cell disease: None; excluded. Participants appeared to be balanced with respect to baseline characteristics. Inclusion criteria: Non‐penetrating traumatic hyphema. Exclusion criteria:
|
|
| Interventions | Treatment: Oral aminocaproic acid 100 mg/kg (up to 5 g/dose and 30 g/day) every 4 hours, for 5 days. Control: Placebo every 4 hours for 5 days. Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, assessed daily by slit‐lamp exam. Defined as a definite increase in the amount of blood in the anterior chamber following admission. Secondary outcomes:
|
|
| Notes | Funded by the National Eye Institute, National Institutes of Health, Bethesda, MD and Research to Prevent Blindness, Inc | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to treatment groups using computerized randomization. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) Participants | Low risk | Authors used a placebo control and stated that the study was double‐masked. |
| Blinding (performance bias and detection bias) Personnel and outcome assessors | Low risk | Authors used a placebo control and stated that the study was double‐masked. Assignment codes were not broken until the study was terminated. |
| Incomplete outcome data (attrition bias) Primary outcome | Unclear risk | The chart of 1 participant in the placebo group was "lost," and this participant was excluded. The excluded participant was not included in the analyses, and the intention‐to‐treat principle was not followed in the analyses. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | The chart of 1 participant in the placebo group was "lost," and this participant was excluded. The excluded participant was not included in the analyses, and the intention‐to‐treat principle was not followed in the analyses. |
| Selective reporting (reporting bias) | Low risk | Reported results for primary and secondary outcomes |
| Other bias | Low risk | No other sources of potential bias were identified. |
Palmer 1986.
| Methods | Study design: Randomized, double‐masked clinical trial. Exclusions after randomization: 2 participants were excluded: 1 from the low‐dose aminocaproic acid group due to need for surgery and 1 from the standard‐dose aminocaproic acid group due to severe hypotension. Losses to follow‐up: None. Intention‐to‐treat: The intention‐to‐treat principle was followed only for analyses of adverse events. The 2 excluded participants were not included in the analyses, and the intention‐to‐treat principle was not followed in the analyses. Sample size calculations: Not reported. |
|
| Participants | Country: USA. Dates: July 1982 to December 1983. Number randomized: 59: 26 to low‐dose aminocaproic acid, 33 to standard‐dose aminocaproic acid. Age: Mean age: low‐dose aminocaproic acid 20 years (range 4 to 46 years), standard‐dose aminocaproic acid 22.8 years (range 3 to 50 years). Sex: 23 participants (88%) in low‐dose aminocaproic acid group and 27 participants (82%) in standard‐dose aminocaproic acid group were male. Race: 13 (50%) black, 7 (27%) white, and 5 (19%) Hispanic in the low‐dose aminocaproic acid group (the race of the excluded participant was not reported); and 17 (52%) black, 7 (27%) white, and 9 (21%) Hispanic in the standard‐dose aminocaproic acid group. Sickle cell disease: None; excluded. Participants appeared to be balanced with respect to baseline characteristics. Inclusion criteria: Traumatic hyphema, including both primary and secondary hemorrhages. Exclusion criteria:
|
|
| Interventions | Treatment: Low‐dose (50 mg/kg) oral aminocaproic acid (up to 5 g/dose or 30 g/day) every 4 hours, for 5 days. Control: Standard‐dose (100 mg/kg) oral aminocaproic acid (up to 5 g/dose or 30 g/day) every 4 hours, for 5 days. Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Incidence of secondary hyphema, assessed daily by slit‐lamp exam. Documented by level in millimeters and percentage of anterior chamber filled with blood. Defined as a definite increase in the amount of fresh blood in the anterior chamber over level at admission. Secondary outcomes:
|
|
| Notes | Funded by the National Eye Institute, National Institutes of Health, Bethesda, MD, Research to Prevent Blindness, Inc, and Lederle‐Cyanamid Laboratories for serum assays | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assignments determined by computerized randomization in the pharmacy. |
| Allocation concealment (selection bias) | Low risk | Allocation was possibly concealed from investigators by pharmacy preparation of drugs. |
| Blinding (performance bias and detection bias) Participants | Low risk | Participants were masked by preparation of drugs by pharmacy. "The treating physicians and the patients were not told of the admission dose in order to maintain the double‐masked status." |
| Blinding (performance bias and detection bias) Personnel and outcome assessors | Low risk | Healthcare providers and outcomes assessors were masked by preparation of drugs by pharmacy. "The treating physicians and the patients were not told of the admission dose in order to maintain the double‐masked status." |
| Incomplete outcome data (attrition bias) Primary outcome | Unclear risk | 2 participants were excluded: 1 from the low‐dose aminocaproic acid group due to need for surgery and 1 from the standard‐dose aminocaproic acid group due to severe hypotension. It was noted that excluding the participant from the standard‐dose group did not affect the statistical results. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | 2 participants were excluded: 1 from the low‐dose aminocaproic acid group due to need for surgery and 1 from the standard‐dose aminocaproic acid group due to severe hypotension. The intention‐to‐treat principle was followed only for analyses of adverse events. |
| Selective reporting (reporting bias) | Low risk | Reported results for primary and secondary outcomes |
| Other bias | Low risk | No other sources of potential bias were identified. |
Pieramici 2003.
| Methods | Study design: Randomized, double‐masked, placebo‐controlled clinical trial. Exclusions after randomization: None. Losses to follow‐up: None. Intention‐to‐treat: All participants were analyzed in the group to which they had been randomly assigned. Sample size calculations: 124 participants based on secondary hemorrhage rate of 15% and 3% in placebo‐ and aminocaproic acid‐treated participants, respectively, with alpha = 0.05, power = 80%, and one‐tailed test of significance; study terminated due to slow enrollment. Notes: Multicenter study with 8 centers. |
|
| Participants | Country: USA. Dates: Not reported, although study was conducted over 14 months. Number randomized: 51: 24 to aminocaproic acid, 27 to placebo. Age: Mean age: aminocaproic acid group 24 ± 4 years (range 4 to 73 years), placebo group 23 ± 3 years (range 6 to 48 years). Sex: 21 (88%) of aminocaproic acid group and 23 (85%) of placebo group were male. Race: 15 (63%) white, 8 (33%) black, and 1 (1%) other in aminocaproic acid group and 13 (48%) white, 11 (41%) black, and 3 (11%) other in placebo group. Sickle cell disease: 2/24 (8%) of participants in aminocaproic acid group and 1/27 (4%) of participants in placebo group had sickle cell trait. Participants appeared to be balanced with respect to baseline characteristics except for race and size of primary hyphema, with larger hyphemas found in the placebo group. Inclusion criteria: Traumatic hyphema. Exclusion criteria:
|
|
| Interventions | Treatment: Following 1 drop of 0.05% proparacaine hydrochloride, 30% aminocaproic acid in 2% gel instilled in inferior fornix every 6 hours, for 5 days. Control: Following 1 drop of 0.05% proparacaine hydrochloride, placebo gel instilled in inferior fornix every 6 hours, for 5 days. Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, assessed daily by slit‐lamp exam for 7 days; defined as increase in height of hyphema of at least 0.5 mm above darker blood, color change of blood of at least 0.5 mm, obvious new "trickle" of blood on iris, or reappearance of blood after resolution. Secondary outcomes:
|
|
| Notes | Funded by Orphan Medical Inc; Covance Inc; National Eye Institute, National Institutes of Health, Bethesda, MD; and Research to Prevent Blinding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants assigned to treatment groups using computerized randomization. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed from investigators, as treatment assignments were based on a trial number obtained from a contract research organization. |
| Blinding (performance bias and detection bias) Participants | Low risk | Authors used a placebo control and stated that the study was double‐masked. "The investigators and patients were masked to the treatment arm." |
| Blinding (performance bias and detection bias) Personnel and outcome assessors | Low risk | Authors used a placebo control and stated that the study was double‐masked. "The investigators and patients were masked to the treatment arm." |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | No exclusions or loss to follow‐up. All participants were analyzed in the group to which they had been randomly assigned. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | No exclusions or loss to follow‐up. All participants were analyzed in the group to which they had been randomly assigned. |
| Selective reporting (reporting bias) | Low risk | Reported results for primary and secondary outcomes |
| Other bias | Unclear risk | "There were a number of protocol violations noted in both study groups." "During the course of the study, only 8 of the original 13 sites enrolled patients, and at 14 months a total of 51 patients were enrolled overall. The study was terminated at this point by Orphan Medical, the manufacturer, against the advice of the principal investigators, because of slow enrollment." |
Rahmani 1999.
| Methods | Study design: Randomized, placebo‐controlled clinical trial. Exclusions after randomization: 6: 2 participants in the tranexamic acid group, 3 in the prednisone group, and 1 in the placebo group left the hospital before the end of the study and were excluded. Losses to follow‐up: None. Intention‐to‐treat: The excluded participants were not included in the analyses, and the intention‐to‐treat principle was not followed in the analyses. Sample size calculations: Not reported. |
|
| Participants | Country: Iran. Dates: January 1991 to May 1992. Number randomized: 244: 82 to tranexamic acid, 81 to prednisone, 81 to placebo. Age: Median age: tranexamic acid 11 years (range 1 to 65 years); prednisone 11.5 years (range 1 to 50 years); placebo 12 years (range 1 to 58 years). Sex: 63 participants (79%) in tranexamic acid group, 58 participants (73%) in prednisone group, and 66 participants (82%) in placebo group were male. Race: 100% white. Sickle cell disease: Not reported, but all‐white study population. Participants appeared to be balanced with respect to baseline characteristics. Inclusion criteria: Traumatic hyphema. Exclusion criteria:
|
|
| Interventions | Treatment 1: Oral tranexamic acid 75 mg/kg per day, divided into 3 doses/day, for 5 days. Treatment 2: Oral prednisolone 0.75 mg/kg per day, divided into 2 doses/day, for 5 days. Control: Placebo administered 3 times/day. Treatment for all groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, assessed daily by slit‐lamp exam for 5 days. Defined as definite increase in size of level of blood or appearance of fresh blood over darker clotted blood in the anterior chamber. Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was based on a randomization list. |
| Allocation concealment (selection bias) | Unclear risk | Participants were assigned to treatment groups using a randomization list, but it was not clear whether list was revealed before allocation to individuals enrolling participants. |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Participants partially masked as authors used a placebo control for the tranexamic acid, but not for prednisone. |
| Blinding (performance bias and detection bias) Personnel and outcome assessors | Low risk | Healthcare providers partially masked as authors used a placebo control for the tranexamic acid, but not for prednisone; however, ophthalmologists and outcome assessors were masked. |
| Incomplete outcome data (attrition bias) Primary outcome | Unclear risk | 6 participants were excluded from the study: 2 in the tranexamic acid group, 3 in the prednisone group, and 1 in the placebo group left the hospital before the end of the study and were excluded. The excluded participants were not included in the analyses, and the intention‐to‐treat principle was not followed in the analyses. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | 6 participants were excluded from the study: 2 in the tranexamic acid group, 3 in the prednisone group, and 1 in the placebo group left the hospital before the end of the study and were excluded. The excluded participants were not included in the analyses, and the intention‐to‐treat principle was not followed in the analyses. |
| Selective reporting (reporting bias) | Low risk | Reported results for primary and secondary outcomes |
| Other bias | Low risk | No other sources of potential bias were identified. |
Rakusin 1972.
| Methods | Study design: Quasi‐randomized controlled series. Exclusions after allocation: 59 patients in the series with large hyphemas underwent surgery and were not included in the analysis. Losses to follow‐up: 20. Intention‐to‐treat: Not all participants were accounted for in the final analyses, thus intention‐to‐treat analysis was not performed. Sample size calculations: Not reported. |
|
| Participants | Country: South Africa. Dates: 1966‐69. Number allocated: 390 consecutive patients. Age: Not reported. Sex: Not reported. Sickle cell disease: Not reported. Race: 90% African origin, 10% Asiatic origin. Inclusion criteria: Traumatic hyphema. Exclusion criteria: Surgical treatment indicated. |
|
| Interventions | Series of comparisons based on 6 variable factors:
Treatment and control groups followed the same regimen, except even‐numbered participants received the variable factor, and odd‐numbered participants did not. Excluding the variable factor for each series, all participants received bed rest, single pad over the injured eye, and topical chloramphenicol. |
|
| Outcomes | Primary outcomes:
Follow‐up: Range 1 to 2 weeks to 3 years. |
|
| Notes | Funded by the University of Witwatersrand, the South African Medical Research Council, Leo Laboratories, Mer‐National, and Warner Pharmaceutical Co. In the third comparison group, antibiotics versus corticosteroids, 3 participants were assigned to receive neither treatment, but this group was discontinued after all 3 participants developed a mucous conjunctival discharge. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Method of allocation unclear, not all patients in the series were allocated to the 6 comparisons under study; 59 patients were selected for surgery. Also, even and odd patient number allocation is not applicable to comparison with 3 treatment groups. |
| Allocation concealment (selection bias) | High risk | Method of allocation concealment not reported, not randomized. |
| Blinding (performance bias and detection bias) Participants | High risk | Masking of participants was not possible for some variables (i.e. bed rest and eye patching). Use of placebo for other variables was not mentioned. |
| Blinding (performance bias and detection bias) Personnel and outcome assessors | Unclear risk | Masking was not reported. |
| Incomplete outcome data (attrition bias) Primary outcome | Unclear risk | 79 participants were not included in the analyses, and the intention‐to‐treat principle was not followed. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Unclear risk | 79 participants were not included in the analyses, and the intention‐to‐treat principle was not followed. |
| Selective reporting (reporting bias) | Low risk | Reported results for primary and secondary outcomes |
| Other bias | Unclear risk | The primary interventions of interest for this study were not clear. Although the majority of the patients in the series were assigned to 1 of 6 conservative‐treatment comparison groups, 59 recruited patients were selected for surgery. |
Read 1974.
| Methods | Study design: Quasi‐randomized controlled series. Exclusions after allocation: None. Losses to follow‐up: None. Intention‐to‐treat: All participants were analyzed in the groups to which they had been assigned. Sample size calculations: Not reported. |
|
| Participants | Country: USA. Dates: February 1970 to July 1972. Number allocated: 137 consecutive patients. Age: Mean 15.9 years. Sex: 108 men and 29 women; 79% male. Race: 101 (74%) African‐American. Sickle cell disease: Not reported. Participants were similar with respect to baseline characteristics. Inclusion criteria: Traumatic hyphema. Exclusion criteria:
|
|
| Interventions | Medical treatment #1 (n = 66): Bed rest with elevation of head to 30°, bilateral ocular patches and shield over injured eye, and sedation. Medical treatment #2 (n = 71): Moderate ambulatory activity in the hospital, patching and shielding of the traumatized eye only, and no sedation. Eyedrops were not administered in either medical treatment regimen. On day 5, participants with remaining major primary or secondary hyphemas (n = 16) were alternately assigned to continue with medical treatment or to receive surgical intervention (ab externo corneal section with clot expression). |
|
| Outcomes | Primary and secondary outcomes not specified. Measured outcomes:
Follow‐up: 1 week, 1, 3, and 6 months (range 3 months to 2.5 years; mean 16.5 months). |
|
| Notes | Funded by a grant from Research to Prevent Blindness, Inc | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation was not randomized; alternately assigned patients to treatment groups at time of admission. Imbalance in number assigned to each group (66 vs 71) suggests alternation was not systematic. |
| Allocation concealment (selection bias) | High risk | Allocation was assigned on an alternate basis. |
| Blinding (performance bias and detection bias) Participants | High risk | Masking of participants was not possible given the interventions under study. |
| Blinding (performance bias and detection bias) Personnel and outcome assessors | High risk | All participants were treated by the primary investigator in order to standardize therapy and to record results as accurately as possible. |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | All participants were analyzed in the group to which they had been assigned. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | All participants were analyzed in the group to which they had been assigned. |
| Selective reporting (reporting bias) | Low risk | Reported results for all outcomes |
| Other bias | High risk | A subset of participants with major hyphema on day 5 were alternately allocated to either continue with medical treatment as originally assigned or to undergo surgical intervention. The participants that had surgery were thus censored on day 5 from their medical treatment outcomes. |
Spaeth 1966.
| Methods | Study design: Randomized, double‐masked, placebo‐controlled clinical trial. Exclusions after randomization: None. Losses to follow‐up: None. Intention‐to‐treat: All participants were analyzed in the group to which they had been randomly assigned. Sample size calculations: Not reported. |
|
| Participants | Country: USA. Dates: 1963‐64. Number randomized: 85: 39 to estrogen, 46 to placebo. Age: Mean age: estrogen 16.2 years (range 2 to 62 years), placebo 18.9 years (range 0.5 to 65 years). Sex: 80% of estrogen group, 85% of placebo group were male. Race: 72% of estrogen group, 70% of placebo group were black; remaining participants were white. Sickle cell disease: Not reported. Participants appeared to be balanced with respect to baseline characteristics. Inclusion criteria: Traumatic hyphema. Exclusion criteria:
|
|
| Interventions | Treatment: Conjugated estrogen, 5 mg intramuscularly for children < 5 years; 10 mg intramuscularly for children 5 years or older but < 10 years; and 20 mg intravenously for children 10 years or older and adults, for 5 days. Control: Placebo, for 5 days. Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, assessed daily by "complete ocular examination" for 5 days. Documentation and definition not reported. Secondary outcomes:
|
|
| Notes | Placebo and conjugated estrogen supplied by Ayerst Laboratories. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, but method of allocation not reported. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed from investigators by use of coded bottles. |
| Blinding (performance bias and detection bias) Participants | Low risk | Authors used coded bottles to mask participants. "Neither the person administering nor the patient receiving the medications knew whether estrogen or placebo was being given." |
| Blinding (performance bias and detection bias) Personnel and outcome assessors | Low risk | Authors used coded bottles to mask healthcare providers and outcomes assessors. "Neither the person administering nor the patient receiving the medications knew whether estrogen or placebo was being given." |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | There were no exclusions and no loss to follow‐up. All participants were analyzed in the group to which they had been randomly assigned. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | There were no exclusions and no loss to follow‐up. All participants were analyzed in the group to which they had been randomly assigned. |
| Selective reporting (reporting bias) | Low risk | Reported results for primary and secondary outcomes |
| Other bias | Low risk | No other sources of potential bias were identified. |
Spoor 1980.
| Methods | Study design: Randomized, double‐masked, placebo‐controlled clinical trial. Exclusions after randomization: None. Losses to follow‐up: None. Intention‐to‐treat: All participants were analyzed in the group to which they had been randomly assigned. Sample size calculations: Not reported. |
|
| Participants | Country: USA. Dates: September 1975 to December 1977. Number randomized: 43: 23 to prednisone, 20 to placebo. Age: Mean age: prednisone group 20.1 years (range 5 to 61 years), placebo group 21.2 years (range 9 to 51 years). Sex: 16 (70%) of prednisone group, 16 (80%) of placebo group were male. Race: 14 (61%) white participants, 6 (26%) Hispanic participants, and 3 (13%) black participants in prednisone group; 11 (55%) white participants, 7 (35%) Hispanic participants, and 2 (10%) black participants in placebo group. Sickle cell disease: Not reported. Participants appeared to be balanced with respect to baseline characteristics. Inclusion criteria: Traumatic hyphema. Exclusion criteria:
|
|
| Interventions | Treatment: Oral prednisone 40 mg/day for adults and children > 10 years; 15 mg/day for children ages 4 to 10 years; and 10 mg/day for children ages 18 months to 4 years, for 7 days. Control: Lactose placebo capsules administered daily for 7 days. Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, assessed daily for 7 days, using slit‐lamp exam, documented by drawings or photography. Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, but method of allocation not reported. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed from investigators by use of encoded capsules prepared by pharmacy. |
| Blinding (performance bias and detection bias) Participants | Low risk | Participants masked to treatment assignment by use of encoded capsules prepared by pharmacy. "Neither the doctor nor the patient knew which capsule the patient was receiving until the conclusion of the course of treatment and follow‐up." |
| Blinding (performance bias and detection bias) Personnel and outcome assessors | Low risk | Healthcare providers and outcomes assessors masked to treatment assignment by use of encoded capsules prepared by pharmacy. "Neither the doctor nor the patient knew which capsule the patient was receiving until the conclusion of the course of treatment and follow‐up." |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | There were no exclusions and no loss to follow‐up. All participants were analyzed in the group to which they had been randomly assigned. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | There were no exclusions and no loss to follow‐up. All participants were analyzed in the group to which they had been randomly assigned. |
| Selective reporting (reporting bias) | Low risk | Reported results for primary and secondary outcomes |
| Other bias | Low risk | No other sources of potential bias were identified. |
Sukumaran 1988.
| Methods | Study design: Quasi‐randomized controlled series. Exclusions after allocation: None. Losses to follow‐up: None. Intention‐to‐treat: All participants were analyzed in the group to which they had been assigned. Sample size calculations: Not reported. |
|
| Participants | Country: Malaysia. Dates: Not reported. Number allocated: 35 consecutive patients. Age: 80% below the age of 30 years. Sex: 35 men. Race: Not reported. Sickle cell disease: Not reported. Inclusion criteria: Traumatic hyphema. Exclusion criteria:
|
|
| Interventions | Treatment (n = 17): oral tranexamic acid (Cyklokapron) 25 mg/kg divided into 3 doses for 7 days in addition to routine treatment. Control (n = 18): Routine treatment. Routine treatment for both groups included:
|
|
| Outcomes | Primary outcomes:
Follow‐up: At least 1 week. |
|
| Notes | Funding source not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Method of allocation unclear, not randomized. |
| Allocation concealment (selection bias) | High risk | Method of allocation concealment not reported, not randomized. |
| Blinding (performance bias and detection bias) Participants | High risk | No placebo was used for the control group. |
| Blinding (performance bias and detection bias) Personnel and outcome assessors | Unclear risk | Masking was not reported. |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | All participants were analyzed in the group to which they had been assigned. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | All participants were analyzed in the group to which they had been assigned. |
| Selective reporting (reporting bias) | Low risk | Reported results for primary and secondary outcomes |
| Other bias | Low risk | No other sources of potential bias were identified. |
Teboul 1995.
| Methods | Study design: Randomized, double‐masked, placebo‐controlled clinical trial. Exclusions after randomization: None. Losses to follow‐up: None. Intention‐to‐treat: All participants were analyzed in the group to which they had been randomly assigned. Sample size calculations: The authors reported that sample sizes were not calculated because the rate of secondary hemorrhage in children was unknown and that of other populations was too variable to estimate. |
|
| Participants | Country: Canada. Dates: November 1987 to February 1994. Number randomized: 94: 48 to aminocaproic acid, 46 to placebo. Age: Mean age: aminocaproic acid group 8.2 years, placebo group 10.6 years. Sex: 42 (88%) of aminocaproic acid group, 39 (85%) of placebo group were male. Race: 43 (90%) of aminocaproic acid group, 42 (91%) of placebo group were white. Sickle cell disease: None; excluded. Participants appeared to be balanced with respect to baseline characteristics, except for mean age where the aminocaproic acid group was younger (8.2 to 10.6 years). Inclusion criteria: Traumatic hyphema. Exclusion criteria:
|
|
| Interventions | Treatment: Oral aminocaproic acid 100 mg/kg every 4 hours (up to 30 g/day), for 5 days. Control: Placebo every 4 hours for 5 days. Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, assessed by daily slit‐lamp exam for 5 days; documented by drawing of hyphema with distinction between fresh and clotted blood. Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, but method of allocation not reported. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed from investigators by preparation of drugs by pharmacy; it was stated that investigators were unaware of next treatment assignment. |
| Blinding (performance bias and detection bias) Participants | Low risk | Participants masked to treatment assignment by use of medications prepared by pharmacy. |
| Blinding (performance bias and detection bias) Personnel and outcome assessors | Low risk | Healthcare providers and outcomes assessors masked to treatment assignment by use of medications prepared by pharmacy. "The double‐blind code was not broken until completion of the study." |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | There were no exclusions and no loss to follow‐up. All participants were analyzed in the group to which they had been randomly assigned. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | There were no exclusions and no loss to follow‐up. All participants were analyzed in the group to which they had been randomly assigned. |
| Selective reporting (reporting bias) | Low risk | Reported results for primary and secondary outcomes |
| Other bias | Low risk | "The authors have no proprietary interest in aminocaproic acid or any competing drug." |
Vangsted 1983.
| Methods | Study design: Randomized clinical trial. Exclusions after randomization: None. Losses to follow‐up: None. Intention‐to‐treat: All participants were analyzed in the group to which they had been randomly assigned. Sample size calculations: Not reported. |
|
| Participants | Country: Sweden. Dates: November 1978 to May 1981. Number randomized: 112: 59 to tranexamic acid, 53 to bed rest. Age: Mean age: tranexamic acid group 23.5 years (range 9 to 60 years), bed rest group 23.5 years (range 9 to 67 years). Sex: Ratio of male:female 4:1. Race: Not reported. Sickle cell disease: Not reported. Participants appeared to be balanced with respect to baseline characteristics. Inclusion criteria: Traumatic hyphema. Exclusion criteria:
|
|
| Interventions | Treatment: Oral tranexamic acid 25 mg/kg 3 times/day, for 7 days. Control: Complete bed rest for 6 days. Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, assessed daily by slit‐lamp exam at days 2 and 7. Documentation and definition not reported. Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, but method of allocation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) Participants | High risk | Participants were not masked to treatment assignment (bed rest vs tranexamic acid). |
| Blinding (performance bias and detection bias) Personnel and outcome assessors | High risk | Healthcare providers and outcome assessors were not masked to treatment assignment (bed rest vs tranexamic acid). |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | There were no exclusions and no loss to follow‐up. All participants were analyzed in the group to which they had been randomly assigned. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | There were no exclusions and no loss to follow‐up. All participants were analyzed in the group to which they had been randomly assigned. |
| Selective reporting (reporting bias) | Low risk | Reported results for primary and secondary outcomes |
| Other bias | Low risk | No other sources of potential bias were identified. |
Varnek 1980.
| Methods | Study design: Quasi‐randomized controlled series. Exclusions after allocation: None. Losses to follow‐up: None. Intention‐to‐treat: All participants were analyzed in the group to which they had been assigned. Sample size calculations: Not reported. |
|
| Participants | Country: Denmark. Dates: March 1978 to November 1979. Number allocated: 232 consecutive patients from 4 study centers. Age: Mean 24.4 years. Sex: 188 men, 44 women; 81% male. Race: 100% white. Sickle cell disease: Not reported, but all‐white study population. Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Treatment (n = 102): Oral tranexamic acid 25 mg/kg divided into 3 doses for 6 days. Control (n = 130): Conservative treatment. Treatment for both groups included:
|
|
| Outcomes | Primary outcomes:
Follow‐up: Days 5 and 12. |
|
| Notes | Funding source not reported. Method used to calculate mean VA not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation was not randomized; patients were assigned to treatment groups based on date of admission. |
| Allocation concealment (selection bias) | High risk | Method of allocation based on even versus odd admission dates. |
| Blinding (performance bias and detection bias) Participants | High risk | No placebo was used for the control group. |
| Blinding (performance bias and detection bias) Personnel and outcome assessors | High risk | Masking was not done because of the noticeable delay in resolution time between treatment groups. Tranexamic acid was considered to induce persistence of the primary clot a priori. |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | All participants were analyzed in the group to which they had been assigned. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | All participants were analyzed in the group to which they had been assigned. |
| Selective reporting (reporting bias) | Low risk | Reported results for primary and secondary outcomes |
| Other bias | Low risk | No other sources of potential bias were identified. |
Wang 1994.
| Methods | Study design: Randomized clinical trial. Exclusions after randomization: None. Losses to follow‐up: None. Intention‐to‐treat: All participants were analyzed in the group to which they had been randomly assigned. Sample size calculations: Not reported. |
|
| Participants | Country: China. Dates: Not reported. Number randomized: 83: 45 in treatment group, 38 in control group. Age: Range 4 to 49 years. Sex: 56 (67%) men, 27 (33%) women. Race: Not reported. Sickle cell disease: Not reported. Participants appeared to be balanced with respect to baseline characteristics (P > 0.05 for between‐group comparisons for anterior chamber blood volume, IOP, gender, and age). Severity of hyphema not reported; however, in the treatment group, 29 (64%) participants received medicine within 24 hours after the trauma; 13 (29%) participants received medicine within 3 days after the trauma; and 3 (7%) participants received medicine at day 5 after the trauma, while in the control group, 31 (82%) participants received medicine (carbazochrome or etamsylate) within 24 hours after the trauma, and 7 (18%) participants received medicine within 3 days after the trauma. Inclusion criteria: Any degree of traumatic hyphema. Exclusion criteria: Not reported. |
|
| Interventions | Treatment (n = 45): Yunnan Baiyao (a traditional Chinese medicine) was given to participants in the treatment group. The participants were assigned to take 0.5 g of the medicine 4 times/day orally, accompanied by vitamin C and vitamin K also taken orally, and with 0.5% vinegar eyedrops. The length of treatment was up to 5 days (until complete resolution). Control (n = 38): participants in the control group were given medicines such as carbazochrome or etamsylate to help stop bleeding. Follow‐up: 1 week. |
|
| Outcomes | Primary outcome: Number of participants "cured," defined as complete resolution within 5 days, VA of 0.7 or better, and no rebleed within 1 week. Secondary outcomes: None reported. |
|
| Notes | Funding source not reported. Poor description of study methods and outcomes in publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, but method of allocation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Masking of participants was not reported. |
| Blinding (performance bias and detection bias) Personnel and outcome assessors | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | There were no exclusions and no loss to follow‐up. All participants were analyzed in the group to which they had been randomly assigned. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | There were no exclusions and no loss to follow‐up. All participants were analyzed in the group to which they had been randomly assigned. |
| Selective reporting (reporting bias) | Unclear risk | Study outcomes of interest not clearly stated. |
| Other bias | High risk | 2 different control interventions were described, but method used to decide which participants received which control intervention was not stated. Why "vinegar eyedrops" were used in the experimental group was not described. Length of time between onset of hyphema and initiation of treatment differed between treatment groups. |
Welsh 1983.
| Methods | Study design: Randomized, double‐masked, placebo‐controlled clinical trial. Exclusions after randomization: None. Losses to follow‐up: None. Intention‐to‐treat: All participants were analyzed in the group to which they had been randomly assigned. Sample size calculations: Not reported. |
|
| Participants | Country: South Africa. Dates: Not reported. Number randomized: 39: 19 to tranexamic acid, 20 to placebo. Age: Mean age: tranexamic acid group 25.2 years (range 15 to 38 years), placebo group 25.2 years (range 14 to 52 years). Sex: 15 (79%) of tranexamic acid group, 17 (85%) of placebo group were male. Race: 100% black. Sickle cell disease: Not reported. Participants appeared to be balanced with respect to baseline characteristics. 3 of 39 participants had a hyphema due to cataract surgery: 2 in the tranexamic group and 1 in the control group. Inclusion criteria: Hyphema; either non‐perforated or perforated, if the latter then the wound was sutured and treated as closed injury. Exclusion criteria:
|
|
| Interventions | Treatment: 3 x 500 mg tablets of oral tranexamic acid 3 times/day for 7 days, for an overall total of 31.5 g of tranexamic acid. Control: 3 tablets of placebo 3 times/day for 7 days. Treatment for both groups included:
|
|
| Outcomes | Primary outcome: Risk of secondary hemorrhage, assessed daily by visual exam. Documentation and definition not reported. Secondary outcomes:
|
|
| Notes | Tranexamic acid and placebo supplied by Adcock Ingram Laboratories. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, but method of allocation not reported. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed from investigators by preparation of drugs by pharmacy; it was stated that investigators were unaware of next treatment assignment. |
| Blinding (performance bias and detection bias) Participants | Low risk | Participants were masked to treatment assignment by use of medications prepared by pharmacy. "Neither patient nor staff knew which tablet the patient was receiving and the code was broken by the pharmaceutical firm at the end of the trial." |
| Blinding (performance bias and detection bias) Personnel and outcome assessors | Low risk | Healthcare providers and outcomes assessors were masked to treatment assignment by use of medications prepared by pharmacy. "Neither patient nor staff knew which tablet the patient was receiving and the code was broken by the pharmaceutical firm at the end of the trial." |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | There were no exclusions and no loss to follow‐up. All participants were analyzed in the group to which they had been randomly assigned. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | There were no exclusions and no loss to follow‐up. All participants were analyzed in the group to which they had been randomly assigned. |
| Selective reporting (reporting bias) | Low risk | Reported results for primary and secondary outcomes |
| Other bias | Unclear risk | Tranexamic acid (Cyklokapron) and placebo tablets were supplied by Adcock Ingram Laboratories. |
Zetterstrom 1969.
| Methods | Study design: Quasi‐randomized controlled series. Exclusions after allocation: None. Losses to follow‐up: None. Intention‐to‐treat: All participants were analyzed in the group to which they had been assigned. Sample size calculations: Not reported. |
|
| Participants | Country: Sweden. Dates: September 1967 to September 1968. Number allocated: 117 consecutive patients. Age: Mean: 22.0 years (range 5 to 57 years). Sex: 102 men and 17 women (as reported); 86% male. Race: Not reported. Sickle cell disease: Not reported. Inclusion criteria: Traumatic hyphema. Exclusion criteria: Perforation of the eyeball. |
|
| Interventions | Treatment (n = 58): Topical atropine with dexamethasone (Decadron) eyedrops 5 times/day and moderate ambulatory activity within hospital. Control (n = 59): Conservative treatment consisting of complete bed rest without pinhole glasses or simultaneous local therapy. Treatment for both groups included inpatient care until VA in the injured eye was satisfactory, the hyphema was absorbed, and IOP did not deviate from normal. |
|
| Outcomes | Primary outcomes:
Follow‐up: Followed until discharge; some participants with iritis were seen as outpatients after discharge. |
|
| Notes | Funding source not reported. Method used to calculate mean VA not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation was not randomized; patients alternately assigned to treatment groups based on order of admission. |
| Allocation concealment (selection bias) | High risk | Method of allocation based on order of admission. |
| Blinding (performance bias and detection bias) Participants | High risk | Masking of participants was not possible given the interventions under study. |
| Blinding (performance bias and detection bias) Personnel and outcome assessors | Unclear risk | Masking was not reported, but unlikely due to the types of interventions being studied. |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | All participants were analyzed in the group to which they had been assigned. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | All participants were analyzed in the group to which they had been assigned. |
| Selective reporting (reporting bias) | Low risk | Reported results for primary and secondary outcomes |
| Other bias | Low risk | No other sources of potential bias were identified. |
Zi 1999.
| Methods | Study design: Randomized controlled series. Exclusions after allocation: None. Losses to follow‐up: None. Intention‐to‐treat: All participants were analyzed in the group to which they had been assigned. Sample size calculations: Not reported. |
|
| Participants | Country: China. Dates: September 1990 to 1997. Number randomized: 79. Age: Mean: 24.5 years (range 7 to 43 years). Sex: 70 men and 4 women (as reported); 95% male. Race: Not reported. Sickle cell disease: Not reported. Inclusion criteria: Hyphema. Exclusion criteria: Not reported. |
|
| Interventions | Treatment (n = 39): Alternatively right and left lateral position. Control (n = 35): Semi‐reclined position. |
|
| Outcomes | Primary outcomes: Time to resolution by severity. Secondary outcomes:
Follow‐up: Not reported. |
|
| Notes | Funding source not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, but method of allocation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) Participants | High risk | Participants were not masked to treatment assignment (lying either semi‐reclining or on side). |
| Blinding (performance bias and detection bias) Personnel and outcome assessors | High risk | Healthcare providers and outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) Primary outcome | Low risk | All participants were analyzed in the group to which they had been assigned. |
| Incomplete outcome data (attrition bias) Secondary outcomes | Low risk | All participants were analyzed in the group to which they had been assigned. |
| Selective reporting (reporting bias) | Low risk | Reported results for primary and secondary outcomes |
| Other bias | Low risk | No other sources of potential bias were identified. |
IOP: intraocular pressure; n: number of participants; VA: visual acuity.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amirova 1991 | Included non‐traumatic hyphema cases in trial and could not separate outcomes in traumatic hyphema cases; the method of choosing the control group was not mentioned. |
| Anderson 1971 | Not a clinical trial, case reports |
| Berrios 1995 | Review of traumatic hyphema, no original data |
| Bramsen 1977 | Not a clinical trial, used historical controls |
| Bramsen 1980 | Review of previously published studies, no original data |
| Campana 1969 | Not a clinical trial, case reports and experimental studies in rabbits |
| Cherkasov 1989 | Did not include traumatic hyphema cases, all had vitreous hemorrhage. |
| Crawford 1976 | Not a clinical trial, retrospective cohort study |
| Dralands 1981 | Not a clinical trial, used historical controls |
| Dumitrache 2011 | Not a clinical trial, case reports |
| Gabler 2002 | Review of treatment strategies for ocular emergencies, no original data |
| Gastaldi 1970 | Review of treatments for traumatic hyphema, no original data |
| Ghisolfi 1972 | Included non‐traumatic hyphema cases in trial and could not separate outcomes in traumatic hyphema cases |
| Gilbert 1973 | Not a clinical trial, used historical controls |
| Gillan 1961 | Not a clinical trial, used historical controls |
| Goldberg 1960 | Not a clinical trial, cohort study using chart review |
| Gundorova 1985 | Not a clinical trial. There were only 3 participants with post‐traumatic hyphema and no obvious control group was defined. |
| Guseva 2010 | Included non‐traumatic hyphema cases and could not separate outcomes in traumatic hyphema cases; the method of choosing treatment groups was not mentioned. |
| Heath 1966 | Not a clinical trial, case reports |
| Jrasnov 1986 | Not a clinical trial, all participants on same drug therapy, compared those who eventually had surgery vs those who did not. |
| Kirschner 2012 | Summary of review, no original data |
| Kotas 1990 | Not a clinical trial, case report |
| Krasnov 1971a | There were only 6 participants with post‐traumatic hyphema without surgery or penetrating injuries; participants with different types of glaucoma were classified and treated with glycerin alone or with glycerin and thromboplastin accordingly. |
| Krasnov 1971b | Not a clinical trial, 2 case series and 1 report of an animal study |
| Latinovic 1981 | Interventional case series, no control group |
| Li 1997 | Included non‐traumatic hyphema cases in trial and could not separate outcomes in traumatic hyphema cases |
| Li 2009 | Not a clinical trial, cohort study |
| Logai 1974 | Not a clinical trial, case series of 74 eyes with hyphema, 28 had non‐penetrating traumatic hyphema |
| Mathis 1987 | Not a clinical trial, case reports |
| Missotten 1977 | Not a clinical trial, used historical controls |
| Mortensen 1978 | Not a clinical trial, used historical controls |
| Munoz Negrete 1989 | Interventional case series, no control group |
| Murzin 1966 | Not a clinical trial, appears to be without a control group, and the author tested 2 different drugs in various combinations for various types of bleeds in the eye, which occurred at various times before the onset of treatment. |
| Ohrstrom 1972 | Not a clinical trial, cohort study |
| Oksala 1967 | Not a clinical trial, cohort study |
| Pierse 1964 | Not a clinical trial, case reports |
| Pogorel'skii 1966 | Not a clinical trial, cohort study comparing people with hemophthalmos treated with chymotrypsin vs people with hemorrhage into the eye cavity treated with resorption therapy |
| Polychronakos 1967 | Not a clinical trial, case reports |
| Rakusin 1971 | Not eligible, surgical interventions |
| Roberts 2006 | Editorial calling for trial of traumatic hyphema to be done, no original data |
| Romano 1986 | Review of corticosteroids for the treatment of traumatic hyphema, no original data |
| Romashchenko 1985 | Groups of patients with bleeds in the eye: group 1 was a mix of post‐traumatic and postoperative hyphemas (no clear group with post‐traumatic hyphemas); the control group was taken from a retrospective study of case notes from 1979 to 1981 and had received an entirely different set of drugs as treatment for their bleeds in the eye. |
| Rouher 1966 | Not a clinical trial, report of 10 cases, only some patients had hyphema |
| Spoor 1990 | Not a clinical trial, cohort study |
| Stepanov 2002 | Not a clinical trial, no control group |
| Surel 1987 | Not a clinical trial, used historical controls |
| Tartakovskaia 1972 | Not a clinical trial, no control group |
| Travkin 1997 | Included non‐traumatic hyphema cases in trial and could not separate outcomes in traumatic hyphema cases |
| Uusitalo 1988 | Not a clinical trial, used historical controls |
| Volpe 1991 | Combined randomized and non‐randomized participants into 1 cohort |
| Wang 2010 | Not related to medical treatments for hyphema, compared satisfaction in 2 groups based on whether or not they had received education on having glaucoma secondary to traumatic hyphema. |
| Watkins 1974 | Not a clinical trial, animal study and case reports |
| Welsh 1971 | Not a clinical trial, case reports |
| Williams 1993 | Not a clinical trial, interventional case series |
| Williamson 1973 | Not a clinical trial, report of 4 cases |
| Wilson 1990 | Not a clinical trial, cohort study |
| Wright 1964 | Included non‐traumatic hyphema cases in trial and could not separate outcomes in traumatic hyphema cases |
| Yan 2012 | Included participants who may have been treated surgically prior to study enrollment |
| Yasuna 1974 | Not a clinical trial, used historical controls |
| Zhang 2013 | Retrospective study comparing routine treatment with or without chymotrypsin for grade III contusive hyphema |
| Zhang 2014 | Compared routine treatment with or without Hexue mingmu tablets for hyphema; method of randomization not reported. |
| Zhou 1982 | Not a clinical trial, groups were selected based on severity of injury |
| Zobina 1987 | Not a clinical trial, case series, no control group |
| Zobina 1996 | Not a clinical trial, description of therapy with observational findings |
Differences between protocol and review
We have updated the Assessment of risk of bias in included studies section of the Methods to reflect modified Cochrane methodology regarding assessments of the risk of bias in included studies.
The primary outcome of this review was previously defined as duration of visual impairment (length of time from onset to resolution of hyphema) in the protocol; we have redefined it as time to resolution of primary hemorrhage (length of time from onset to resolution of hyphema) in the review.
We revised the searches of electronic databases from the original 2011 publication of this review (Gharaibeh 2011). We updated the search to incorporate new MeSH terms in the MEDLINE search; we also searched PubMed and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP), which we did not originally search.
In this version of the review, we re‐analyzed all meta‐analyses using risk ratio instead of odds ratio because the number of events was not rare, and therefore the use of odds ratios was inappropriate. We also added methods for producing 'Summary of findings' tables and incorporating GRADE assessments.
Contributions of authors
Conceiving the review: HS, AG Designing the review: RS, HS Co‐ordinating the review: RS Undertaking manual searches: RS Screening search results: RS, KL, AG Organizing retrieval of papers: KL Screening retrieved papers against inclusion criteria: RS, KL, AG Appraising quality of papers: RS, KL Abstracting data from papers: RS, KL,AG Writing to authors of papers for additional information: RS, KL Providing additional data about papers: HS, MG Data management for the review: RS, KL Entering data into Review Manager 5: RS, KL Analysis of data: RS, KL Interpretation of data: AG, HS, MG, RS Writing the review: RS, KL, AG, HS, MG Performing previous work that was the foundation of current study: AG, HS, RS, MG Updating the review: RS, KL Providing substantial feedback and final approval: AG, HS, MG
Sources of support
Internal sources
No sources of support supplied
External sources
Contract N‐01‐EY‐2‐1003 and Grant 1 U01 EY020522‐01, National Eye Institute, National Institutes of Health, USA.
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the NIHR to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- This review was supported by the NIHR, via Cochrane Infrastructure funding to the CEV UK editorial base.
The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service, or the Department of Health.
Declarations of interest
AG HS, RS, and KL: none known.
MG was an investigator for some of the trials included in this review and is a paid consultant for Panoptic Inc (not related to hyphema) and a non‐paid board member for Eyetech Inc (not related to hyphema).
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bedrossian 1974 {published data only}
- Bedrossian RH. The management of traumatic hyphema. Annals of Ophthalmology 1974;6(10):1016‐8. [PubMed] [Google Scholar]
Christianson 1979 {published data only}
- Christianson MD, Crawford JS. Epsilon aminocaproic acid in the treatment of traumatic hyphema. American Journal of Ophthalmology 1979;88(4):782. [Google Scholar]
Crouch 1976 {published data only}
- Crouch ER Jr, Frenkel M. Aminocaproic acid in the treatment of traumatic hyphema. American Journal of Ophthalmology 1976;81(3):355‐60. [DOI] [PubMed] [Google Scholar]
Crouch 1997 {published data only}
- Crouch ER Jr, Williams PB, Gray MK, Crouch ER, Chames M. Topical aminocaproic acid in the treatment of traumatic hyphema. Archives of Ophthalmology 1997;115(9):1106‐12. [DOI] [PubMed] [Google Scholar]
- Crouch ER, Williams PB. Topical aminocaproic acid in the treatment of patients with traumatic hyphema—reply. Archives of Ophthalmology 1998;116(3):395‐6. [DOI] [PubMed] [Google Scholar]
Edwards 1973 {published data only}
- Edwards WC, Layden WE. Monocular versus binocular patching in traumatic hyphema. American Journal of Ophthalmology 1973;76(3):359‐62. [DOI] [PubMed] [Google Scholar]
Farber 1991 {published data only}
- Farber MD, Fiscella R, Goldberg MF. A randomized clinical trial of Amicar vs. prednisone for the treatment of traumatic hyphema. American Academy of Ophthalmology; 1990 10‐11; Atlanta, Georgia. 1990:119. [DOI] [PubMed]
- Farber MD, Fiscella R, Goldberg MF. Aminocaproic acid versus prednisone for the treatment of traumatic hyphema. A randomized clinical trial. Ophthalmology 1991;98(3):279‐86. [DOI] [PubMed] [Google Scholar]
Karkhaneh 2003 {published data only}
- Karkhaneh R, Naeeni M, Chams H, Abdollahi M, Mansouri MR. Topical aminocaproic acid to prevent rebleeding in cases of traumatic hyphema. European Journal of Ophthalmology 2003;13(1):57‐61. [DOI] [PubMed] [Google Scholar]
Kraft 1987 {published data only}
- Kraft SP, Christianson MD, Crawford JS, Wagman RD, Antoszyk JH. Traumatic hyphema in children. Treatment with epsilon‐aminocaproic acid. Ophthalmology 1987;94(10):1232‐7. [DOI] [PubMed] [Google Scholar]
Kutner 1987 {published data only}
- Anonymous. Traumatic hyphema therapy. Ophthalmology 1988;95(5):707‐9. [DOI] [PubMed] [Google Scholar]
- Kutner B, Fourman S, Brein K, Hobson S, Mrvos D, Sheppard J, et al. Aminocaproic acid reduces the risk of secondary hemorrhage in patients with traumatic hyphema. Archives of Ophthalmology 1987;105(2):206‐8. [DOI] [PubMed] [Google Scholar]
Liu 2002 {published data only}
- Liu SC, Den JS. The effect of aminomethylbenzoic acid on preventing secondary hyphema. Journal of Eye Injuries and Ophthalmic Occupational Diseases 2002;24(2):127‐8. [Google Scholar]
Marcus 1988 {published data only}
- Marcus M, Biedner B, Lifshitz T, Yassur Y. Aspirin and secondary bleeding after traumatic hyphema. Annals of Ophthalmology 1988;20(4):157‐8. [PubMed] [Google Scholar]
McGetrick 1983 {published data only}
- Anonymous. Aminocaproic acid. Archives of Ophthalmology 1984;102(6):818, 820‐1. [DOI] [PubMed] [Google Scholar]
- McGetrick JJ, Jampol LM, Goldberg MF, Frenkel M, Fiscella RG. Aminocaproic acid decreases secondary hemorrhage after traumatic hyphema. Archives of Ophthalmology 1983;101(7):1031‐3. [DOI] [PubMed] [Google Scholar]
Palmer 1986 {published data only}
- Palmer DJ, Goldberg MF, Frenkel M, Fiscella R, Anderson RJ. A comparison of two dose regimens of epsilon aminocaproic acid in the prevention and management of secondary traumatic hyphemas. Ophthalmology 1986;93(1):102‐8. [DOI] [PubMed] [Google Scholar]
Pieramici 2003 {published data only}
- Pieramici DJ, Goldberg MF, Melia BM. Topical aminocaproic acid (Caprogel) in the treatment of traumatic hyphema: results of a phase III multicenter placebo controlled trial. Investigative Ophthalmology & Visual Science 2000;41:ARVO Abstract 1616. [Google Scholar]
- Pieramici DJ, Goldberg MF, Melia M, Fekrat S, Bradford CA, Faulkner A, et al. A phase III, multicenter, randomized, placebo‐controlled clinical trial of topical aminocaproic acid (Caprogel) in the management of traumatic hyphema. Ophthalmology 2003;110(11):2106‐12. [DOI] [PubMed] [Google Scholar]
Rahmani 1999 {published data only}
- Rahmani B, Jahadi HR. Comparison of tranexamic acid and prednisolone in the treatment of traumatic hyphema. A randomized clinical trial. Ophthalmology 1999;106(2):375‐9. [DOI] [PubMed] [Google Scholar]
- Rahmani B, Jahadi HR, Rajaeefard A. An analysis of risk for secondary hemorrhage in traumatic hyphema. Ophthalmology 1999;106(2):380‐5. [DOI] [PubMed] [Google Scholar]
- Rahmani B, Jahadi HR, Salour H. Comparison of tranexamic acid and prednisolone in treatment of traumatic hyphema. American Academy of Ophthalmology; 1997 10‐11; San Francisco, California. 1997:210. [DOI] [PubMed]
Rakusin 1972 {published data only}
- Rakusin W. Emergencies in ophthalmology. South African Medical Journal 1973;47(30):1338. [PubMed] [Google Scholar]
- Rakusin W. Traumatic hyphema. American Journal of Ophthalmology 1972;74(2):284‐92. [DOI] [PubMed] [Google Scholar]
Read 1974 {published data only}
- Read J. Traumatic hyphema: surgical vs medical management. Annals of Ophthalmology 1975;7(5):659‐62, 664‐6, 668‐70. [PubMed] [Google Scholar]
- Read J, Goldberg MF. Comparison of medical treatment for traumatic hyphema. Transactions of the American Academy of Ophthalmology and Otolaryngology 1974;78(5):OP799‐OP815. [Google Scholar]
Spaeth 1966 {published data only}
- Spaeth GL, Levy PM. Traumatic hyphema: its clinical characteristics and failure of estrogens to alter its course. A double‐blind study. American Journal of Ophthalmology 1966;62(6):1098‐106. [DOI] [PubMed] [Google Scholar]
Spoor 1980 {published data only}
- Spoor TC, Hammer M, Belloso H. Traumatic hyphema. Failure of steroids to alter its course: a double‐blind prospective study. Archives of Ophthalmology 1980;98(1):116‐9. [DOI] [PubMed] [Google Scholar]
Sukumaran 1988 {published data only}
- Sukumaran K. The role of tranexamic acid (Cyklokapron) in the treatment of traumatic hyphaema. Medical Journal of Malaysia 1988;43(2):155‐8. [PubMed] [Google Scholar]
Teboul 1995 {published data only}
- Teboul BK, Jacob JL, Barsoum‐Homsy M, Brunette I, Chevrette L, Milot J, et al. Clinical evaluation of aminocaproic acid for managing traumatic hyphema in children. Ophthalmology 1995;102(11):1646‐53. [DOI] [PubMed] [Google Scholar]
- Teboul BK, Jacob JL, Barsoum‐Homsy M, Brunette I, Chevrette L, Milot J, et al. Clinical evaluation of aminocaproic acid for the management of traumatic hyphema in children. American Academy of Ophthalmology; 1995 10‐12; Atlanta Georgia. 1995:175. [DOI] [PubMed]
Vangsted 1983 {published data only}
- Vangsted P, Nielsen PJ. Tranexamic acid and traumatic hyphaema. A prospective study. Acta Ophthalmologica 1983;61(3):447‐53. [DOI] [PubMed] [Google Scholar]
Varnek 1980 {published data only}
- Varnek L, Dalsgaard C, Hansen A, Klie F. The effect of tranexamic acid on secondary haemorrhage after traumatic hyphaema. Acta Ophthalmologica 1980;58(5):787‐93. [DOI] [PubMed] [Google Scholar]
Wang 1994 {published data only}
- Wang XL. Yunnan Baiyao for contusion hyphema in 45 cases. Jiangsu Journal of Traditional Chinese Medicine 1994;15(7):21. [Google Scholar]
Welsh 1983 {published data only}
- Welsh N, Schoeman H, Blignaut P. The effect of Cyklokapron in traumatic hyphema. A double masked study. South African Archives of Ophthalmology 1983;10(3‐4):67‐74. [Google Scholar]
Zetterstrom 1969 {published data only}
- Zetterstrom B. The treatment of contusion of the eye. Acta Ophthalmologica 1969;47(2):784‐91. [DOI] [PubMed] [Google Scholar]
Zi 1999 {published data only}
- Zi QL. The clinical observation of alternatively right and left lateral position in the cases of hyphema. Journal of Nursing Science 1999;14(5):263‐4. [Google Scholar]
References to studies excluded from this review
Amirova 1991 {published data only}
- Amirova EKh, Dubilei OV. Experience in the use of streptodecase in the treatment of intraocular hemorrhage. Vestnik Oftalmologii 1991;107(6):34‐6. [PubMed] [Google Scholar]
Anderson 1971 {published data only}
- Anderson TW. Treatment of hyphema with atropinization of contralateral eye. Eye, Ear, Nose & Throat Monthly 1971;50(7):266‐7. [PubMed] [Google Scholar]
Berrios 1995 {published data only}
- Berrios RR, Dreyer EB. Traumatic hyphema. International Ophthalmology Clinics 1995;35(1):93‐103. [DOI] [PubMed] [Google Scholar]
Bramsen 1977 {published data only}
- Bramsen T. Traumatic eye hemorrhage (hyphema) treated with the antifibrinolytic preparation tranexamic acid. Ugeskrift for Laeger 1977;139(24):1422‐4. [PubMed] [Google Scholar]
Bramsen 1980 {published data only}
- Bramsen T. The influence of antifibrinolytica on traumatic hyphaema and corneal oedema. Acta Ophthalmologica ‐ Supplementum 1980, (145):1‐53. [PubMed] [Google Scholar]
Campana 1969 {published data only}
- Campana G, Boschi MC. Clinical and experimental results of the treatment of some ocular diseases with desferrioxamine B. Annali di Ottalmologia e Clinica Oculistica 1969;95(3):200‐24. [PubMed] [Google Scholar]
Cherkasov 1989 {published data only}
- Cherkasov IS, Pashkovskaia VA, Novik AI, Shekhab M. Collalysine treatment of intraocular hemorrhages. Oftalmologicheskii Zhurnal 1989, (5):289‐90. [PubMed] [Google Scholar]
Crawford 1976 {published data only}
- Crawford JS. The effect of aspirin on rebleeding in traumatic hyphema. Transactions of the American Ophthalmological Society 1976;74:357‐62. [PMC free article] [PubMed] [Google Scholar]
Dralands 1981 {published data only}
- Dralands L, Clippeleir L, Missotten L. The prevention of secondary traumatic hyphema. Bulletin de la Société Belge d'Ophtalmologie 1981;193:77‐8. [PubMed] [Google Scholar]
Dumitrache 2011 {published data only}
- Dumitrache M, Ciocâlteu AM, Cioboată M, Potop V. Posttraumatic hyphema with secondary glaucoma. Oftalmologia 2011;55(2):50‐3. [PubMed] [Google Scholar]
Gabler 2002 {published data only}
- Gabler B. Ophthalmological emergencies. 2. Trauma and acute loss of vision. MMW Fortschritte der Medizin 2002;144(45):44‐6. [PubMed] [Google Scholar]
Gastaldi 1970 {published data only}
- Gastaldi TS, Grassi MS. Treatment of the complications of traumatic hyphema [Tratamiento de las complicaciones del hipema traumatico]. Archivos de Oftalmologia de Buenos Aires 1970;45(11):477‐83. [PubMed] [Google Scholar]
Ghisolfi 1972 {published data only}
- Ghisolfi A, Manfredini U. Clinical study on a polyenzymic antiinflammatory preparation in some ocular inflammatory conditions [Ricerche cliniche su un antiflogistico polienzimatico in alcune flogosi oculari]. Annali di Ottalmologia e Clinica Oculistica 1972;98(2):69‐77. [Google Scholar]
Gilbert 1973 {published data only}
- Gilbert HD, Jensen AD. Atropine in the treatment of traumatic hyphema. Annals of Ophthalmology 1973;5(12):1297‐300. [PubMed] [Google Scholar]
Gillan 1961 {published data only}
- Gillan JG. Treatment and prophyaxis of hyphaema by conjugated estrogens. Transactions of the Canadian Ophthalmological Society 1961;24:217‐22. [PubMed] [Google Scholar]
Goldberg 1960 {published data only}
- Goldberg JL. Conjugated estrogens in the prevention of secondary hyphema after ocular trauma. Archives of Ophthalmology 1960;63(6):127‐30. [DOI] [PubMed] [Google Scholar]
Gundorova 1985 {published data only}
- Gundorova RA, Romashchenko AD. Immobilized streptokinase (streptodecase) in the treatment of traumatic intra‐ocular hemorrhage. Voprosy Meditsinskoi Khimii 1985;31(4):47‐51. [PubMed] [Google Scholar]
Guseva 2010 {published data only}
- Guseva MR, Beslaneeva MB. Clinical rationale for the efficiency of using the domestic antioxidant agent Histochrome. Vestnik Oftalmologii 2010;126(3):37‐40. [PubMed] [Google Scholar]
Heath 1966 {published data only}
- Heath W. Experiences with urokinase in secondary traumatic hyphaema. Transactions of the Ophthalmological Societies of the United Kingdom 1966;86:843‐5. [PubMed] [Google Scholar]
Jrasnov 1986 {published data only}
- Jrasnov MM, Lariukhina GM, Ronzina IA. Intravitreal use of enzymes in the sequelae of eye injury. Vestnik Oftalmologii 1986;102(1):25‐30. [PubMed] [Google Scholar]
Kirschner 2012 {published data only}
- Kirschner J, Seupaul RA. Do medical interventions for traumatic hyphema reduce the risk of vision loss?. Annals of Emergency Medicine 2012;60(2):197‐8. [DOI] [PubMed] [Google Scholar]
Kotas 1990 {published data only}
- Kotas R, Neumann D, Ashkenazi I. Epsilon aminocaproic acid for management of traumatic hyphema with large blood clot in the anterior chamber. Harefuah 1990;119(9):269‐70. [PubMed] [Google Scholar]
Krasnov 1971a {published data only}
- Krasnov AM. Beneficial action of glycerine in traumatic hyphema. Vestnik Oftalmologii 1971;6:43‐6. [PubMed] [Google Scholar]
Krasnov 1971b {published data only}
- Krasnov AM. Washing the anterior chamber in expressed traumatic hyphema [O promyvanii perednei kamery pri vyrazhennoi travmaticheskoi gifeme]. Oftalmologicheskii Zhurnal 1971;26(2):135‐8. [PubMed] [Google Scholar]
Latinovic 1981 {published data only}
- Latinovic S, Pellegrino N. Glycerin‐vitamin C in the treatment of traumatic hyphema [Glicerolo‐vitamina C nel trattamento degli ipoemi traumatici]. Bollettino Chimico Farmaceutico 1981;120(3):156‐8. [PubMed] [Google Scholar]
Li 1997 {published data only}
- Li SW. Integrated traditional Chinese and western medicine for contusive intraocular hemorrhage in 52 cases. Jiangsu Journal of Traditional Chinese Medicine 1997;18(3):18‐9. [Google Scholar]
Li 2009 {published data only}
- Li Y, Zhu Y. Clinical study of medication for the treatment of traumatic hyphema. International Journal of Ophthalmology 2009;9(10):2025‐6. [Google Scholar]
Logai 1974 {published data only}
- Logai IM. Treatment of traumatic hemorrhages into the vitreous body with fibrinolysin in combination with other methods. Oftalmologicheskii Zhurnal 1974;29(4):260‐5. [PubMed] [Google Scholar]
Mathis 1987 {published data only}
- Mathis A, Malecaze F, Vigne J, Bec P. Treatment of traumatic recurrent hyphema with sodium hyaluronate [Traitement des hyphemas traumatiques recidivants par le hyaluronate de sodium]. Bulletin des Sociétés d'Ophtalmologie de France 1987;87(4):503‐4. [PubMed] [Google Scholar]
Missotten 1977 {published data only}
- Missotten L, Clippeleir L, Tornout I, Beenders P. The value of tranexamic acid (Cyklokapron) in the prevention of secondary bleeding, a complication of traumatic hyphaemia. Bulletin de la Société Belge d'Ophtalmologie 1977;179:47‐52. [PubMed] [Google Scholar]
Mortensen 1978 {published data only}
- Mortensen KK, Sjolie AK. Secondary hemorrhage following traumatic hyphema. A comparative study of conservative and tranexamic acid treatment. Acta Ophthalmologica 1978;56(5):763‐8. [DOI] [PubMed] [Google Scholar]
Munoz Negrete 1989 {published data only}
- Munoz Negrete FJ, Elosua De Juan I. Prospective study of the efficacy of low dose epsilon aminocaproic acid for the prevention of secondary bleeding after traumatic hyphema. Archivos de la Sociedad Espanola de Oftalmologia 1989;57(2):105‐10. [Google Scholar]
Murzin 1966 {published data only}
- Murzin AA. Experiences with the use of fibrinolysin and trypsin in intraocular hemorrhages [Opyt primeneniia fibrinolizina i tripsina pri vnutriglaznykh krovoizliianiiakh]. Vestnik Oftalmologii 1966;79(3):19‐21. [PubMed] [Google Scholar]
Ohrstrom 1972 {published data only}
- Ohrstrom A. Treatment of traumatic hyphaema with corticosteroids and mydriatics. Acta Ophthalmologica 1972;50(4):549‐55. [DOI] [PubMed] [Google Scholar]
Oksala 1967 {published data only}
- Oksala A. Treatment of traumatic hyphaema. British Journal of Ophthalmology 1967;51(5):315‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pierse 1964 {published data only}
- Pierse D. The use of urokinase in the anterior chamber. Transactions of the Ophthalmological Societies of the United Kingdom 1964;84:271‐4. [PubMed] [Google Scholar]
Pogorel'skii 1966 {published data only}
- Pogorel'skii AI. The use of chemotrypsin in hemophthalmia. Vestnik Oftalmologii 1966;79(3):21‐3. [PubMed] [Google Scholar]
Polychronakos 1967 {published data only}
- Polychronakos D, Razoglou C. Treatment of total hyphema with fibrinolysin. Ophthalmologica 1967;154(1):31‐8. [DOI] [PubMed] [Google Scholar]
Rakusin 1971 {published data only}
- Rakusin W. The role of urokinase in the management of traumatic hyphaema. Ophthalmologica 1973;167(5):373‐82. [DOI] [PubMed] [Google Scholar]
- Rakusin W. Urokinase in the management of traumatic hyphaema. British Journal of Ophthalmology 1971;55(12):826‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 2006 {published data only}
- Roberts I. Antifibrinolytic agents in traumatic hemorrhage. Annals of Epidemiology 2006;16(2):113‐4. [DOI] [PubMed] [Google Scholar]
Romano 1986 {published data only}
- Romano PE. Pro steroids for systemic antifibrinolytic treatment for traumatic hyphema. Journal of Pediatric Ophthalmology and Strabismus 1986;23(2):92‐5. [DOI] [PubMed] [Google Scholar]
Romashchenko 1985 {published data only}
- Romashchenko AD, Makarova VP. Experience in treating intraocular hemorrhage using immobilized streptokinase [Opyt lecheniia vnutrioglaznogo krovoizliianiia s pomoshch'iu immobilizovannoi streptokinazy]. Oftalmologicheskii Zhurnal 1985;3:148‐51. [PubMed] [Google Scholar]
Rouher 1966 {published data only}
- Rouher F, Sole P, Serpin G. Treatment of traumatic intraocular hemorrhages by mannitol. Bulletin des Societes d'Ophtalmologie de France 1966;66(4):455‐64. [PubMed] [Google Scholar]
Spoor 1990 {published data only}
- Spoor TC, Kwitko GM, O'Grady JM, Ramocki JM. Traumatic hyphema in an urban population. American Journal of Ophthalmology 1990;109(1):23‐7. [DOI] [PubMed] [Google Scholar]
Stepanov 2002 {published data only}
- Stepanov AV, Bolkvadze ER, Belogurov AA, Tovarova II. Prospects for treating intraocular traumatic hemorrhage using a new fibrinolytic hemase [Vozmozhnosti terapii vnutriglaznykh travmaticheskikh krovoizliianii s pomoshch'iu novogo fibrinolitika gemaza]. Vestnik Oftalmologii 2002;118(5):25‐7. [PubMed] [Google Scholar]
Surel 1987 {published data only}
- Surel Z, Cicekdag E. Tranexamic acid treatment in secondary hemorrhage after traumatic hyphema. Turk Oftalmologji Gazetesi 1987;17(1):49‐56. [Google Scholar]
Tartakovskaia 1972 {published data only}
- Tartakovskaia AI, Mikhailova NA. Pathogenetic role of fibrinolysis in recurring intra‐ocular hemorrhages and their treatment with sigma‐aminocapronic acid [Patogeneticheskaia rol' fibrinoliza pri retsidiviruiushchikh vnutriglaznykh krovoizliianiikah i ikh terapiia sigma‐aminokapronovoi kislotoi]. Vestnik Oftalmologii 1972;1:41‐5. [PubMed] [Google Scholar]
Travkin 1997 {published data only}
- Travkin AG, Romashchenko AD. New class of drugs for treatment of eye vascular diseases. Vestnik Oftalmologii 1997;113(6):35‐6. [PubMed] [Google Scholar]
Uusitalo 1988 {published data only}
- Uusitalo RJ, Ranta‐Kemppainen L, Tarkkanen A. Management of traumatic hyphema in children. An analysis of 340 cases. Archives of Ophthalmology 1988;106(9):1207‐9. [DOI] [PubMed] [Google Scholar]
Volpe 1991 {published data only}
- Volpe NJ, Larrison WI, Hersh PS, Kim T, Shingleton BJ. Secondary hemorrhage in traumatic hyphema. American Journal of Ophthalmology 1991;112(5):507‐13. [DOI] [PubMed] [Google Scholar]
Wang 2010 {published data only}
- Wang Y‐M. Effects of health education on patients with glaucoma secondary to traumatic hyphema. International Journal of Ophthalmology 2010;10(7):1415‐6. [Google Scholar]
Watkins 1974 {published data only}
- Watkins G, Venable HP. E‐Aminocaproic acid in traumatic hyphema. Journal of the National Medical Association 1974;66(6):484‐6. [PMC free article] [PubMed] [Google Scholar]
Welsh 1971 {published data only}
- Welsh N. The management of traumatic glaucoma. South African Medical Journal 1971;45(44):1250‐2. [PubMed] [Google Scholar]
Williams 1993 {published data only}
- Williams C, Laidlaw A, Diamond J, Pollock W, Bloom P. Outpatient management of small traumatic hyphaemas: is it safe?. Eye 1993;7(1):155‐7. [DOI] [PubMed] [Google Scholar]
Williamson 1973 {published data only}
- Williamson J, Forrester JV. Treatment of vitreous haemorrhage with urokinase. Lancet 1973;1(7808):888. [DOI] [PubMed] [Google Scholar]
Wilson 1990 {published data only}
- Wilson TW, Nelson LB, Jeffers JB, Manley DR. Outpatient management of traumatic microhyphemas. Annals of Ophthalmology 1990;22(10):366‐8. [PubMed] [Google Scholar]
Wright 1964 {published data only}
- Wright JC. Hyphema treated by buccal varidase: a double‐blind study. American Journal of Ophthalmology 1964;58(3):479‐82. [PubMed] [Google Scholar]
Yan 2012 {published data only}
- Yan XY, He P, Liu J, Xia T. Safety discussion of pilocarpine for the treatment of hyphema. International Eye Science 2012;12(9):1797‐8. [Google Scholar]
Yasuna 1974 {published data only}
- Yasuna E. Management of traumatic hyphemas. Archives of Ophthalmology 1974;91(3):190‐1. [DOI] [PubMed] [Google Scholar]
Zhang 2013 {published data only}
- Zhang H, Sun HQ, Chen R. Clinical analysis on the chymotrypsin treatment of 42 patients with contusive hyphema at grade III. International Eye Science 2013;13(12):2517‐8. [Google Scholar]
Zhang 2014 {published data only}
- Zhang HF, Kang J, Ma QM, Zhao ZH, Jia ZY. Effect of Hexue mingmu tablets in the treatment of hyphema. International Eye Science 2014;14(9):1710‐2. [Google Scholar]
Zhou 1982 {published data only}
- Zhou FU. Direct current iontherapy with radix pseudoginseng solution for hyphema. Chinese Journal of Ophthalmology 1982;18(2):83‐4. [PubMed] [Google Scholar]
Zobina 1987 {published data only}
- Zobina LV, Romashchenko AD. Streptodecase magnetophoresis in the treatment of posttraumatic intraocular hemorrhage. Vestnik Oftalmologii 1987;103(1):51‐4. [PubMed] [Google Scholar]
Zobina 1996 {published data only}
- Zobina LV, Malkov IuV. Streptodecase magnetophoresis in the Polius‐3 treatment of post‐traumatic intraocular bleeding. Meditsinskaia Tekhnika 1996, (6):41‐2. [PubMed] [Google Scholar]
Additional references
Crouch 1993
- Crouch ER Jr, Williams PB. Trauma: ruptures and bleeding. In: Tasman W, Jaeger EM editor(s). Duane's Clinical Ophthalmology. Vol. 4, Philadelphia: JB Lippincott, 1993:1‐18. [Google Scholar]
Crouch 1999
- Crouch ER Jr, Crouch ER. Management of traumatic hyphema: therapeutic options. Journal of Pediatric Ophthalmology and Strabismus 1999;36(5):238. [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JPT, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Goldberg 1979a
- Goldberg MF. Sickled erythrocytes, hyphema, and secondary glaucoma: I. The diagnosis and treatment of sickled erythrocytes in human hyphemas. Ophthalmic Surgery 1979;10(4):17‐31. [PubMed] [Google Scholar]
Goldberg 1979b
- Goldberg MF, Dizon R, Moses VK. Sickled erythrocytes, hyphema, and secondary glaucoma: VI. The relationship between intracameral blood cells and aqueous humor pH, PO2, and PCO2. Ophthalmic Surgery 1979;10(4):78‐88. [PubMed] [Google Scholar]
Goldberg 1979c
- Goldberg MF, Tso MO. Sickled erythrocytes, hyphema, and secondary glaucoma: VII. The passage of sickled erythrocytes out of the anterior chamber of the human and monkey eye: light and electron microscopic studies. Ophthalmic Surgery 1979;10(4):89‐123. [PubMed] [Google Scholar]
Higgins 2017
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Lai 2001
- Lai JC, Fekrat S, Barrron Y, Goldberg MF. Traumatic hyphema in children: risk factors for complications. Archives of Ophthalmology 2001;119(1):64‐70. [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rhee 1999
- Rhee D, Pyfer M. The Wills Eye Manual. Lippincott Williams and Wilkins, 1999. [Google Scholar]
Schünemann 2017
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Akl E, Guyatt GH on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Sheppard 2009
- Sheppard JD, Crouch ER, Williams PB, Crouch ER Jr, Rastogi S, Garcia‐Valenzuela E. Hyphema. Updated: 22 December 2009. emedicine.medscape.com/1190165.overview (accessed 8 January 2010).
Walton 2002
- Walton W, Hagen S, Grigorian R, Zarbin M. Management of traumatic hyphema. Survey of Ophthalmology 2002;47(4):297‐334. [DOI] [PubMed] [Google Scholar]
Westlund 1982
- Westlund LE, Lundén R, Wallén P. Effect of EACA, PAMBA, AMCA and AMBOCA on fibrinolysis induced by streptokinase, urokinase and tissue activator. Haemostasis 1982;11(4):235‐41. [DOI] [PubMed] [Google Scholar]
Wright 2003
- Wright KW. Pediatric ocular trauma: pediatric hyphema. In: Wright KW, Spiegel PH editor(s). Pediatric Ophthalmology and Strabismus. Vol. 6, Springer, 2003:79‐82. [Google Scholar]
References to other published versions of this review
Gharaibeh 2011
- Gharaibeh A, Savage HI, Scherer RW, Goldberg MF, Lindsley K. Medical interventions for traumatic hyphema. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD005431.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gharaibeh 2013
- Gharaibeh A, Savage HI, Scherer RW, Goldberg MF, Lindsley K. Medical interventions for traumatic hyphema. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD005431.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Savage 2005
- Savage HI, Gharaibeh A‐M, Mathew MC, Scherer R. Medical interventions for traumatic hyphema. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD005431] [DOI] [Google Scholar]


