Abstract
Background
Asthma is a common respiratory condition in children that is characterised by symptoms including wheeze, shortness of breath, chest tightness, and cough. Children with asthma may be able to manage their condition more effectively by improving inhaler technique, and by recognising and responding to symptoms. Schools offer a potentially supportive environment for delivering interventions aimed at improving self‐management skills among children. The educational ethos aligns with skill and knowledge acquisition and makes it easier to reach children with asthma who do not regularly engage with primary care. Given the multi‐faceted nature of self‐management interventions, there is a need to understand the combination of intervention features that are associated with successful delivery of asthma self‐management programmes.
Objectives
This review has two primary objectives.
• To identify the intervention features that are aligned with successful intervention implementation.
• To assess effectiveness of school‐based interventions provided to improve asthma self‐management among children.
We addressed the first objective by performing qualitative comparative analysis (QCA), a synthesis method described in depth later, of process evaluation studies to identify the combination of intervention components and processes that are aligned with successful intervention implementation.
We pursued the second objective by undertaking meta‐analyses of outcomes reported by outcome evaluation studies. We explored the link between how well an intervention is implemented and its effectiveness by using separate models, as well as by undertaking additional subgroup analyses.
Search methods
We searched the Cochrane Airways Trials Register for randomised studies. To identify eligible process evaluation studies, we searched MEDLINE, Embase, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycINFO, the Cochrane Database of Systematic Reviews (CDSR), Web of Knowledge, the Database of Promoting Health Effectiveness Reviews (DoPHER), the Database of Abstracts of Reviews of Effects (DARE), the International Biography of Social Science (IBSS), Bibliomap, Health Technology Assessment (HTA), Applied Social Sciences Index and Abstracts (ASSIA), and Sociological Abstracts (SocAbs). We conducted the latest search on 28 August 2017.
Selection criteria
Participants were school‐aged children with asthma who received the intervention in school. Interventions were eligible if their purpose was to help children improve management of their asthma by increasing knowledge, enhancing skills, or changing behaviour. Studies relevant to our first objective could be based on an experimental or quasi‐experimental design and could use qualitative or quantitative methods of data collection. For the second objective we included randomised controlled trials (RCTs) where children were allocated individually or in clusters (e.g. classrooms or schools) to self‐management interventions or no intervention control.
Data collection and analysis
We used qualitative comparative analysis (QCA) to identify intervention features that lead to successful implementation of asthma self‐management interventions. We measured implementation success by reviewing reports of attrition, intervention dosage, and treatment adherence, irrespective of effects of the interventions.
To measure the effects of interventions, we combined data from eligible studies for our primary outcomes: admission to hospital, emergency department (ED) visits, absence from school, and days of restricted activity due to asthma symptoms. Secondary outcomes included unplanned visits to healthcare providers, daytime and night‐time symptoms, use of reliever therapies, and health‐related quality of life as measured by the Asthma Quality of Life Questionnaire (AQLQ).
Main results
We included 55 studies in the review. Thirty‐three studies in 14,174 children provided information for the QCA, and 33 RCTs in 12,623 children measured the effects of interventions. Eleven studies contributed to both the QCA and the analysis of effectiveness. Most studies were conducted in North America in socially disadvantaged populations. High school students were better represented among studies contributing to the QCA than in studies contributing to effectiveness evaluations, which more commonly included younger elementary and junior high school students. The interventions all attempted to improve knowledge of asthma, its triggers, and stressed the importance of regular practitioner review, although there was variation in how they were delivered.
QCA results highlighted the importance of an intervention being theory driven, along with the importance of factors such as parent involvement, child satisfaction, and running the intervention outside the child's own time as drivers of successful implementation.
Compared with no intervention, school‐based self‐management interventions probably reduce mean hospitalisations by an average of about 0.16 admissions per child over 12 months (SMD –0.19, 95% CI ‐0.35 to ‐0.04; 1873 participants; 6 studies, moderate certainty evidence). They may reduce the number of children who visit EDs from 7.5% to 5.4% over 12 months (OR 0.70, 95% CI 0.53 to 0.92; 3883 participants; 13 studies, low certainty evidence), and probably reduce unplanned visits to hospitals or primary care from 26% to 21% at 6 to 9 months (OR 0.74, 95% CI 0.60 to 0.90; 3490 participants; 5 studies, moderate certainty evidence). Self‐management interventions probably reduce the number of days of restricted activity by just under half a day over a two‐week period (MD 0.38 days 95% CI ‐0.41 to ‐0.18; 1852 participants; 3 studies, moderate certainty evidence). Effects of interventions on school absence are uncertain due to the variation between the results of the studies (MD 0.4 fewer school days missed per year with self‐management (‐1.25 to 0.45; 4609 participants; 10 studies, low certainty evidence). Evidence is insufficient to show whether the requirement for reliever medications is affected by these interventions (OR 0.52, 95% CI 0.15 to 1.81; 437 participants; 2 studies; very low‐certainty evidence). Self‐management interventions probably improve children's asthma‐related quality of life by a small amount (MD 0.36 units higher on the Paediatric AQLQ(95% CI 0.06 to 0.64; 2587 participants; 7 studies, moderate certainty evidence).
Authors' conclusions
School‐based asthma self‐management interventions probably reduce hospital admission and may slightly reduce ED attendance, although their impact on school attendance could not be measured reliably. They may also reduce the number of days where children experience asthma symptoms, and probably lead to small improvements in asthma‐related quality of life. Many of the studies tested the intervention in younger children from socially disadvantaged populations. Interventions that had a theoretical framework, engaged parents and were run outside of children's free time were associated with successful implementation.
Plain language summary
Are asthma self‐management interventions effective when delivered in schools for children, and how should they be delivered?
Background to the question
Asthma is a common condition among children. Schools are potential sites for developing self‐management skills, but evidence that school‐based interventions improve asthma control has not been reviewed systematically.
Review question
We sought to address two questions.
• Which parts of school‐based asthma self‐management interventions are more likely to make these interventions successful?
• What effect do interventions have on children's asthma control, school attendance, and attendance at GP and hospital settings?
Study characteristics
We included 55 studies. Thirty three of these studies helped us to gain a better understanding of the best way to deliver an asthma self‐management intervention. Thirty three studies helped us to determine whether these interventions are successful in improving children's health and well‐being. Eleven studies contributed to both.
Key results
We included 23 studies in quantitative models measuring children's asthma outcomes (an outcome is something you can measure to find out if an intervention worked). Results show that school‐based self‐management interventions could improve outcomes such as hospitalisations, emergency department visits, and health‐related quality of life. Fewer studies reported improved unplanned medical visits or reduced numbers of days on which children could not do their normal activities. Interventions did not reduce school absences, symptoms, or reliever medication use. The more effective interventions were based on theories about how the intervention might work. Researchers found that including parents in the intervention, making sure children were happy with the intervention, and running the intervention during school hours helped increase fidelity.
Certainty of the evidence
Studies that measured whether an intervention worked were usually well designed; however sometimes they were difficult to carry out, and some may not have measured outcomes accurately. Reviewers found that some of the studies conducted to understand how an intervention should be delivered were at risk of bias, and certainty of the evidence was generally lower for these studies.
Take‐home message
Evidence suggests that school‐based self‐management interventions can help children with asthma and can reduce hospital admissions and trips to the emergency department. Study findings suggest that interventions that were based on a theory about how an intervention can be planned and delivered could prove useful in improving children's outcomes, reaching large numbers of children, and keeping dropout rates low, and indicate that those designing interventions should consider factors such as including parents.
This review is current to August 2017.
Summary of findings
Summary of findings for the main comparison. Effects of school‐based asthma interventions compared to usual care for asthma among children and adolescents.
| Effects of school‐based asthma interventions compared to usual care for asthma among children and adolescents | ||||||
| Patient or population: asthma among children and adolescents Setting: primary/elementary schools through to high/senior schools Intervention: effects of school‐based asthma interventions Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with effect of school‐based asthma interventions | |||||
| Exacerbations leading to hospitalisation (hospitalisations) assessed with RCT Follow‐up: range 1 week to 12 months | Mean level of hospitalisation at post‐treatment in the intervention group was 0.19 standard deviations lower than in the control group (0.35 to 0.04 lower) |
‐ | 1873 (6 RCTs) | ⊕⊕⊕⊝ MODERATEa | Meta‐analysis based on SMD including data transformed from OR (data on median level from Gerald 2006 not included) | |
| Asthma symptoms leading to emergency hospital visits (ED visits) Follow‐up: range 1 week to 12 months | Less than 10% experience ED visit annually | OR 0.70 (0.53 to 0.92) | 3883 (13 RCTs) | ⊕⊕⊝⊝ LOWb | Data from Gerald 2006 on median visits not combined Assumed risk based on rates over 12 months < 10% based on Horner 2008, McGhan 2010, Velsor‐Friedrich 2005 ≥ 10% based on Cicutto 2013, McGhan 2003 |
|
| 75 per 1000 | 54 per 1000 (41 to 69) | |||||
| Over 10% experience ED visit annually | ||||||
| 281 per 1000 | 215 per 1000 (172 to 264) | |||||
| Unplanned visit to hospital or GP due to asthma symptoms (unplanned medical visits) Follow‐up: range 1 week to 12 months | Unplanned visits over 6 to 9 months | OR 0.74 (0.60 to 0.90) | 3283 (5 RCTs) | ⊕⊕⊕⊝ MODERATEc | Unplanned visits over 6 to 9 months based on McGhan 2003, Splett 2006; unplanned visits over 12 months based on Cicutto 2013, McGhan 2010 | |
| 264 per 1000 | 210 per 1000 (177 to 244) | |||||
| Unplanned visits over 12 months | ||||||
| 318 per 1000 | 257 per 1000 (219 to 296) | |||||
| Absence from school Follow‐up: range 1 week to 15 months | Mean absence from school was 4.3 school days missed annually | MD 0.399 school days missed annually lower (1.254 lower to 0.456 higher) | ‐ | 4609 (10 RCTs) | ⊕⊕⊝⊝ LOWd | Meta‐analysis based on SMD including data transformed from OR; transformation to mean difference undertaken based on data from Cicutto 2005 |
| Experience of daytime and night‐time symptoms ‐ daytime symptoms (daytime symptoms) Follow‐up: range 2 months to 12 months | Mean experience of daytime and night‐time symptoms ‐ daytime symptoms was 3.3 days experienced in past 2 weeks | MD 0.377 days experienced in past 2 weeks lower (0.828 lower to 0.05 higher) | ‐ | 1065 (5 RCTs) | ⊕⊕⊕⊝ MODERATEe | CI for this pooled estimate crossed the line of no effect by a small margin. Original meta‐analysis based on SMDs, including transformations from ORs. SMD to MD based on Bruzzese 2011 |
| Use of reliever therapies, e.g. beta₂‐agonists (reliever therapies) Follow‐up: range 1 week to 15 months | Study population | OR 0.52 (0.15 to 1.81) | 437 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWf | ||
| 228 per 1000 | 133 per 1000 (42 to 349) | |||||
| Health‐related quality of life Follow‐up: range 1 week to 12 months | Mean health‐related quality of life was 4.96 Paediatric Asthma Quality of Life Questionnaire points | MD 0.36 Paediatric Asthma Quality of Life Questionnaire points higher (0.06 higher to 0.64 higher) | ‐ | 2587 (7 RCTs) | ⊕⊕⊕⊝ MODERATEg | Two studies provided information on change in QoL. Both showed positive intervention effects. Risk with usual care based on follow‐up scores |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ED: emergency department; GP: general practitioner; MD: mean difference; OR: odds ratio; QoL: quality of life; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aStudies with high or unclear risk of bias contribute the least to the overall effect size. Hospitalisations may be due to reasons other than asthma (‐1 for indirectness).
bFour studies had high risk of bias around allocation concealment; four also had high risk of bias around attrition; many other studies had unclear risks of bias. However, these risks did not appear to inflate the effect size nor systematically influence the effect. A high degree of inconsistency was evident, as measured by heterogeneity statistics in the meta‐analysis, which was partially explained by subgroup analyses. A large degree of variation was evident in measurement of the outcome, prompting concerns about indirectness; similarly, wide confidence intervals were detected (0.53 to 0.95). Study results led to concerns that not all ED visits may be due to asthma (‐1 for inconsistency; ‐1 for indirectness).
cNo guarantee that unplanned medical visits were due to asthma (‐1 for indirectness).
dSchool absences could be due to causes other than asthma; heterogeneity statistics suggested a large degree of statistical inconsistency (‐1 for indirectness; ‐1 for inconsistency).
eHigh risk of bias detected in at least one domain for two out of five studies, which accounted for around a third of the pooled effect size. This included high risk of bias suspected for attrition bias in one study (‐1 for risk of bias).
fRisk of bias deemed high for attrition and reporting bias for one of the two studies included in the meta‐analysis; very wide confidence interval; although both studies were consistent in the direction of effect, they showed large differences in the magnitude of effect (‐1 for risk of bias; ‐1 for inconsistency; ‐1 for imprecision).
gImprecision was deemed to be serious based on the nature of the outcome; five of the seven studies were deemed to have high risk of bias in at least one domain. This included three studies deemed to have high risk of bias for allocation concealment. However, these did not appear to differentially influence the effect size (‐1 for imprecision).
Background
Description of the condition
Asthma is a chronic respiratory condition characterised by bronchoconstriction, airway inflammation, and mucus hypersecretion leading to variable airflow limitation. Resulting symptoms include wheeze, dyspnoea, cough, and tightness in the chest. No single definitive diagnostic 'test' for asthma is available; instead asthma is diagnosed clinically upon assessment of respiratory symptoms and clinical response to inhaled therapy, and review of evidence of reversible airflow limitation or airway hyper‐responsiveness ‐ as in BTS 2016 and Levy 2014 ‐ and elevated exhaled breath nitric oxide ‐ as in NICE 2017. Asthma is the most common chronic disease among children (Neuzil 2000; To 2012), with more than a million children in the UK living with this chronic condition (Asthma UK 2013). Many countries report high prevalence rates of childhood asthma. The International Study of Asthma and Allergy in Children (ISAAC) study, for example, found high prevalence in Australasia and the United Kingdom (Asher 2006). Much of the evidence on non‐pharmacological interventions derives from North America, where prevalence is among the highest globally, at 21.5% and 16.7% for six‐ to seven‐year‐old boys and girls, respectively, and 19.8% and 23.3% among children 13 to 14 years of age (Mallol 2013).
In the UK, children from black and white ethnic backgrounds have higher levels of asthma symptoms compared with children from South Asian backgrounds (Netuveli 2005), although substantial variation in the risk of developing asthma has been found within these broad ethnic groups (Kneale 2010). Successful management of asthma among UK children is associated, in part, with social position and socio‐economic status. For example, although South Asian children are at lower risk of asthma, they, along with black children, are at higher risk than white children of admission following asthma complications (Netuveli 2005). Indeed a systematic review of socio‐economic status and health outcomes found evidence to suggest that the risk of developing asthma is highest among children in the UK from lower‐income families (Spencer 2012). Overall, the UK government estimates that a billion pounds is spent annually through the National Health Service (NHS) on treatment and prevention of asthma among adults and children (Department of Health 2012). Thus population‐based interventions that improve asthma control have the potential to generate significant savings for the UK NHS.
Description of the intervention
Globally, a large proportion of people with asthma do not receive adequate self‐management education and training in primary care, and in England in 2014, more than a quarter of people (adults and children) living with asthma had not undergone an asthma review in the previous 15 months (HSCIC 2014). Moreover, inadequate knowledge of the condition and patient non‐adherence with clinician recommendations for asthma treatment (e.g. overuse of long‐acting beta₂‐agonists, under‐use of inhaled corticosteroids) may contribute towards poor asthma management among children (Piecoro 2001; Walsh 1999).
Children who experience an asthma exacerbation are at risk of hospitalisation and death (Bush 2017). Of the 65,000 hospitalisations for asthma occurring in 2011‐2012 in the UK, more than one‐third (38%) occurred in children (aged birth to 14 years); moreover, in an in‐depth study of asthma deaths, 14% (28 of 195) of confirmed deaths from asthma in the UK over a year occurred among children and young people 20 years of age and younger (Levy 2014). Effective self‐management of asthma could reduce levels of hospitalisation, which may reduce the financial implications of asthma and improve outcomes for children and adults with asthma, while reducing asthma‐related deaths in children.
Living with asthma can impact many other child health and social outcomes, and asthma, particularly severe asthma, is associated with a range of developmental, emotional, and behavioural problems (Blackman 2007). Some studies suggest that children with asthma are disadvantaged in terms of their peer relationships, and other studies report that some children with asthma are bullied (Harris 2017; Wildhaber 2012). Moreover, children with asthma are more likely to limit participation in activities as the result of dyspnoea and other asthma‐related symptoms (Van Den Bemt 2011).
Children with asthma tend to have poorer school attendance rates than their peers (Rodriguez 2013). For example, one US study reported that children living with asthma miss an average of 1.5 additional days of school annually compared with their peers, and that increased asthma severity was associated with an increase in the number of days absent from school (Moonie 2006). Furthermore, average school days missed masks large heterogeneity in experience, with some children missing many school days as a result of asthma. A school‐based survey, conducted by two members of the review team (KH, JG), assessed current levels of asthma control and school attendance in a sample of 766 children with asthma attending London secondary schools (Harris 2017). Overall, 20.9% of London school children self‐reported at least one school absence due to asthma over a four‐week period. Moreover, children with poor asthma control (n = 350) had greater rates of school absence compared to their peers with good asthma control (32.7% vs 10.9%) (Harris 2017). Fowler 1992 found that grade failure is more frequent among children with asthma.
Self‐management consists of educating and enabling children to achieve good control over their own asthma symptoms, thereby preventing future exacerbations (Kotses 2010);self‐management is viewed as a cornerstone of asthma treatment and care (Bateman 2008; BTS 2016; GINA 2018). Asthma control refers to the degree to which asthma symptoms can be observed and subsequently improved with treatment (GINA 2018). Well‐controlled asthma is associated with reduced daytime and night‐time symptoms, decreased long‐term morbidity, and diminished risk of life‐threatening asthma attacks (Juniper 2006). Asthma control tends to improve with age among children; one study reported excellent or satisfactory control in 38% of children four to six years of age and in 66% of children 13 to 16 years of age (Kuehni 2002).
For chronic respiratory diseases, self‐management is defined by the British Thoracic Society (BTS) as "the tasks that individuals must undertake to live with chronic conditions, including have the confidence to deal with medical management, role management and emotional management of their conditions" (BTS 2016). For asthma, successful self‐management skills include good inhaler technique and ability to recognise and respond to asthma symptoms. Self‐management also encourages an alliance between the physician or healthcare professional and the patient for the purpose of managing asthma (Kotses 2010). For the purposes of the present review, we have included only self‐management studies that provided education on asthma symptoms and their avoidance and management, omitting studies that provided education solely on the nature of asthma.
One main indirect cost of childhood asthma is absence from school, and costs of hospitalisation and of asthma medication drive most of the direct costs of this condition (Bahadori 2009). Although delivery of an asthma self‐management intervention in schools has the potential to reduce asthma burden, the effectiveness of this approach across various "proximal" (e.g. improvement in asthma symptoms), "intermediate" (e.g. healthcare usage), and "distal" outcomes (e.g. school achievement) remains unclear (Figure 1). Even when interventions are delivered in similar school settings, several factors can influence success, including variation in treatment settings, study populations, and ways in which school‐based asthma self‐management interventions and intervention components are delivered, in addition to the role of intervention mediators such as changes in school‐level policies around asthma or asthma medication (Al Aloola 2014).
1.
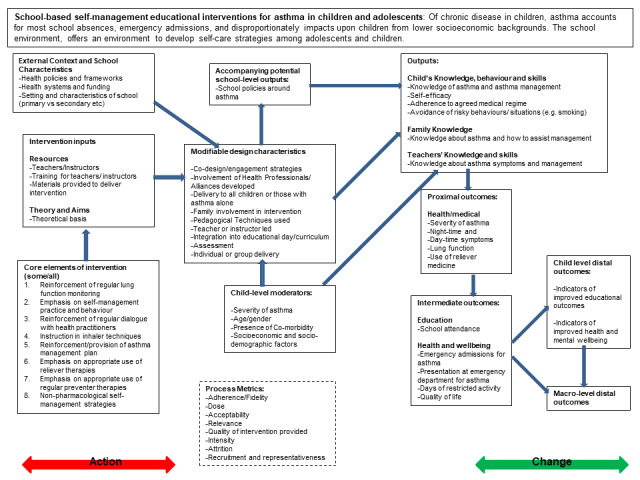
Logic model of school‐based asthma interventions.
How the intervention might work
Self‐managment works by enabling patients to control their asthma symptoms, thereby preventing future exacerbations and improving their quality of life. Schools are a familiar environment for children's learning, and interventions provided at school have the potential to include large numbers of children with asthma at a single location (Ahmad 2011; Bruzzese 2009; Coffman 2009).
A previous systematic review of self‐management interventions delivered in clinic, home, and school environments for children with asthma found that these were positively associated with moderate improvements in lung function, school absenteeism, emergency visits to hospital, and self‐efficacy (Guevara 2003). A separate Cochrane Review reported that targeted self‐management interventions can lead to reduced hospital admissions among those at risk of hospitalisation (Boyd 2009). Participants included in both reviews were children from birth to 18 years of age with a diagnosis of asthma. Guevara 2003 excluded children with a pulmonary diagnosis other than asthma. Neither review noted participant comorbidities. Other reviews of self‐management interventions for children with asthma suggest that educational interventions delivered to children with asthma can be effective; however, these reviews have considered interventions delivered within schools alongside those delivered in other settings, including the clinic and the home (e.g. Smith 2005; Wolf 2002). Indeed Welsh 2011 points to lack of consensus around the optimal setting for asthma interventions. To date, only two systematic reviews have evaluated the evidence for interventions delivered exclusively within schools. These reviews reported a positive impact on school absenteeism but provided less conclusive evidence on the impact on health outcomes such as hospitalisations (Ahmad 2011; Coffman 2009). Notably, both reviews used a narrative approach to synthesis (Ahmad 2011; Coffman 2009). Another review examined outcomes for primary school age children only (Al Aloola 2014).
To date, few reviews have included analyses of accompanying "process‐level" measures, such as changes in school policy. Pinnock 2015 is one exception. These review authors explored how asthma self‐management interventions should be implemented, although they did not focus on school interventions alone. Nevertheless, based on analysis of two studies conducted in schools, they identified high school turnover and lack of parental involvement as challenges to implementation. Analysis of such process factors would further illuminate the modifiable components of interventions that may be most critical in determining the success (or failure) of interventions, and in mapping out the diverse processes undertaken as part of the intervention.
Systematic reviews of self‐management interventions in adults with asthma highlight the importance of gaining a deeper understanding of intervention characteristics and implementation processes. For example, Denford 2013 found that active involvement was associated with greater effect size, but that focus on stress management techniques was potentially counterproductive. Previous studies of self‐management in children have focused on child‐level moderators. Consequently, the effectiveness of different aspects of school‐based interventions for children with asthma is currently unclear.
Background to the methods used in this review
In this review, we aim to synthesise the evidence for school‐based interventions by addressing asthma self‐management, for the first time, using a mixed methods approach. Mixed methods involves synthesising qualitative and process evaluation evidence, as well as quantitative evidence, in an integrated way. Process evaluation studies explore the implementation, receipt, and setting of an intervention. Although "process" and "qualitative" are often mistakenly used interchangeably, data for process evaluation can be both quantitative and qualitative (Oakley 2006). Process evaluations can be used to develop mechanistic theories around how interventions work, although no universally agreed definition is available for what a process evaluation is and which core components it should include.
Investigators in one study defined a process evaluation as evaluating the quality of the intervention and measuring the disparity between the way in which an intervention was intended to be implemented and the way in which it is actually implemented (Shepherd 2010). This focus on evaluating the processes of delivery and the factors "responsible for successful outcomes, implementation of the intervention, and intervention integrity" is also shared elsewhere (Waters 2006). Meanwhile, UK Medical Research Council guidance on how to conduct process evaluations states that core components of process evaluations include (I) clear description (and evaluation) of implementation and processes of implementation; (ii) clear analysis of the mechanism of impact (participant responses to and interactions with the intervention); and (iii) clear description of context and analysis of how contextual factors affect mechanisms and implementation (Moore 2015).
Qualitative comparative analysis (QCA)
Although other reviews have set out to apply a mixed methods approach (albeit applied to other health topics) (Hurley 2013; Husk 2016), we sought to review the literature using both meta‐analyses of quantitative studies to assess the effectiveness of interventions and qualitative comparative analysis (QCA) to discern the importance of different configurations of intervention features. QCA has its basis in set‐theoretic logic, and is a well‐placed method for synthesising data from a small number of studies with complex characteristics. This approach aims to uncover the degree of overlap between a set of studies that are successful in their implementation and sets of studies that share different configurations of intervention characteristics. In pursuing the aim described above, we used a logic model to help structure and synthesise review findings (Figure 1), in accordance with the practices described in previous reviews (Glenton 2013).
Logic models
Logic models are tools that can be used to evaluate the effectiveness of a programme and/or to guide programme planning and implementation (NHS Scotland 2014). The protocol authors developed a logic model to outline some school‐based asthma self‐management intervention components that may be influential (Figure 1). We developed the logic model from the outcomes to be included in this review, and we worked backwards, theorising the causal chain necessary to lead to these outcomes. We developed the logic model using published literature and systematic reviews, including existing logic models used in studies and policy documents. Use of a logic model assisted us in identifying the types of data that may need to be captured if we are to gain an understanding of intervention components and implementation processes (Kneale 2015). The underlying idea behind a logic model is that a target or final goal is identified, and the pre‐conditions needed to reach this goal are hypothesised as different steps, building up a theorised chain of intervention actions and how they may impact outcomes. The logic model in Figure 1 shows the steps needed to reach the distal (long‐term) outcome of improvement in general health, well‐being, and educational outcomes among children with asthma; to achieve this long‐term outcome, we hypothesise that improvement in more intermediate outcomes such as episodes of healthcare usage and school absences is needed; to achieve improvement in these outcomes, we would expect improvement in asthma symptoms and lung function to be a necessary pre‐condition, and, in turn, to improve these, we theorise that children need better knowledge about asthma and improved skill in using inhalers. Changes in children's knowledge and skills follow from exposure to the intervention, although several modifiable intervention design characteristics may cause the intervention to have a differential impact, and may influence the characteristics of children themselves and the context in which the intervention takes place. Each intervention however includes various core elements (reflecting our definition of self‐management), as well as a set of resources and theories underlying its delivery. In addition, the logic model recognises that interventions can fail to effect change in children's outcomes because of issues of design or implementation, and a box on 'process metrics' incorporates ways of understanding the success of intervention implementation.
Why it is important to do this review
Educational impacts attributable to asthma are larger among children from lower socio‐economic groups and/or ethnic minority groups (Milton 2004), with children from ethnic minorities more likely than others to report asthma‐related hospitalisations (Netuveli 2005). Such differentials may, in part, reflect the failure of existing intervention models to deliver asthma self‐management training equitably to children across socio‐demographic groups. Given that the school environment offers a platform by which children from all socio‐economic backgrounds can receive the same asthma self‐management interventions, delivery of asthma self‐management interventions at this level could reduce inequalities in self‐management. Indeed, schools were previously identified as effective sites for the delivery of asthma self‐management interventions because the school environment is commonly associated with learning of new skills. Schools also provide access to large numbers of children with asthma, including those who do not have a general practitioner (GP) and those who do not regularly attend GP appointments. However, 'school age' (usually five to 18 years old) spans a wide spectrum of child development stages and consequently represents different teaching needs and various responses to self‐management interventions. Therefore, an understanding of the processes of implementation (and their success) is essential for the development of mechanistic theories of how and why interventions work that can be understood in the context of the child's characteristics.
In planning the current review, we placed strong emphasis on documenting and understanding the different processes that occur during school‐based asthma self‐management interventions. We envisaged that this approach would help us to understand the different mechanisms involved and would allow future trialists to evaluate the generalisability of processes and outcomes described and measured. The focus on delivery of interventions to help children self‐manage their own chronic condition is encouraged by advisory groups to UK policy‐makers. They view the integration of health and educational (and social care) services as critical in improving the quality of life of children with chronic conditions such as asthma, and in reducing differentials in outcomes such as school attendance (Lewis 2012). This systematic review draws on a mixed methods approach, looking at different sets of literature that evaluate intervention implementation and effectiveness, and using different methods to combine this literature. This approach will provide a rich account of school‐based asthma interventions by examining whether these interventions are effective in changing children's outcomes and by discerning how they effect change.
Objectives
This review has two primary objectives.
To identify the intervention features that are aligned with successful intervention implementation.
To assess effectiveness of school‐based interventions provided to improve asthma self‐management among children.
We addressed the first objective by performing qualitative comparative analysis (QCA), a synthesis method described in depth later, of process evaluation studies to identify the combination of intervention components and processes that are aligned with successful intervention implementation.
We pursued the second objective by undertaking meta‐analyses of outcomes reported by outcome evaluation studies. We explored the link between how well an intervention is implemented and its effectiveness by using separate models, as well as by undertaking additional subgroup analyses.
Methods
Criteria for considering studies for this review
Types of studies
We addressed our first objective (to identify intervention components and processes that are aligned with successful intervention implementation) by exploring process evaluation reports. We pursued the second objective (to assess the effectiveness of school‐based interventions for improvement of asthma self‐management) by examining outcome evaluation reports (i.e. randomised parallel‐group design involving individual or cluster randomisation).
Identifying the intervention components and processes aligned with intervention success in process evaluation studies
In this review, we identified process evaluations as involving systematic measurements to determine the extent to which a particular programme was implemented, in keeping with the guidance described above. Measures of implementation were focused on fidelity and specifically on attrition, adherence, and dosage. To capture the breadth of evidence about implementation, we identified a process evaluation study as (I) a study that was a self‐defined "process evaluation"; or (ii) a study that included the elements of a process evaluation as defined in a section of an outcome evaluation; or (iii) a study in which researchers integrated process evaluation data within an outcome evaluation but provided within the results measures around processes that were detailed and extractable. Studies not self‐identified as process evaluation studies must have contained (I) an assessment of core components (implementation, mechanisms, context); (ii) clear research questions guiding the process evaluation; and (iii) use of recognised evaluation methods (described by Moore 2015). We also included studies with a focus on the presence/development of school asthma policies (as represented in the logic model (Figure 1)); we expanded this to include studies measuring broader school‐level commitment. In this way, use of a logic model explicitly impacted study selection decisions (Kneale 2015).
Previous systematic reviews of process evaluation studies have tended to include only process evaluation studies linked to an outcome evaluation (e.g. Murta 2007). In this review, we have linked included process evaluation studies to randomised controlled trials (RCTs) assessing the effectiveness of the intervention; we have also included trials evaluating the implementation of a variety of study designs, provided they met our other inclusion criteria. This allowed us to use process evaluation data for theory development and testing within a mixed method framework.
Publication date and language
We imposed criteria around the date on which studies were published to help ensure that the content of self‐management interventions was broadly reflective of today's recommendations. Recommendations around the management of asthma in the UK were first developed in 1990 on the basis of articles that had appeared in British Medical Journal and Archives of Diseases in Childhood, from 1989 onwards (British Asthma Guidelines 1997); recommendations were developed in the USA around the same time (National Institute of Health 1997). Therefore, we excluded studies that pre‐dated the impetus around development of guidelines for the management of asthma, and we included only studies published from 1995 onwards (corresponding with publication of the first Global Initiative for Asthma (GINA) guidelines, which provided a foundation for asthma guidelines globally). We included only studies published in English.
Types of participants
We included school‐aged children and young people (five to 18 years old) with asthma. When the intervention included young people and adults (e.g. when provided in colleges with students 16 to 24 years of age), we intended to include these studies only if most participants were 18 years of age or younger (although we observed no such instance). We also included interventions if they incorporated some components that were delivered to peers, teachers, and/or parents and families, although only when they involved at least partial delivery of the intervention to school‐aged participants with asthma within school environments. We included studies reporting on interventions among children and young people with intermittent or mild to severe or persistent asthma.
We did not impose criteria regarding the types of schools that we included in our scope, as long as schools represented the physical location where intervention participants usually received most of their education.
Types of interventions
We included asthma self‐management interventions delivered at school. Eligible interventions aimed to develop and enhance self‐management of asthma among children by achieving the following.
Increasing knowledge of asthma self‐management.
Enhancing self‐management skills.
Improving self‐management behaviours and practice.
Eligible interventions must have included the active transfer of information around at least one of the aspects of asthma self‐management outlined below. However, we recognise that for asthma self‐management to be effective, a combination of these must be incorporated into the interventions.
Reinforcement of regular monitoring of lung function.
Emphasis on the importance of self‐management practice and behaviour.
Development of a partnership/alliance between patient and primary care/healthcare practitioners (including school nursing staff) for the management of asthma.
Instruction on inhaler techniques.
Reinforcement/provision of an individualised written asthma management plan.
Emphasis on the importance and appropriate use of reliever therapies such as beta₂‐agonists (BTS 2016).
Emphasis on the importance and appropriate use of regular preventer therapies such as inhaled corticosteroids and combination inhaled corticosteroid and long‐acting beta₂‐agonist therapies (BTS 2016).
Non‐pharmacological self‐management strategies focused on avoiding or reducing the risk of experiencing asthma or asthma attacks, including lifestyle and behavioural modifications (as set out in BTS 2016).
Interventions that focused only on treating children's asthma in schools, and not on enhancing self‐management skills, were not eligible. For example, interventions that provided directly observed therapy but did not seek to actively improve children's self‐management skills inside and outside school were not eligible for inclusion. This included studies in which we determined that most of the self‐management component of the intervention had not occurred in the school environment. This led to the omission of some studies that otherwise met the inclusion criteria and have been included in previous reviews (e.g. Halterman 2011; Halterman 2012).
Interventions may focus on improving the climate for asthma self‐management within schools, for example, by changing school policies around the way that teaching staff may assist in asthma self‐management. However, studies that did not also include the development and evaluation of asthma self‐management skills and behaviours among children were not eligible. We included self‐management interventions if they fit the definition given in the guidelines produced by the British Thoracic Society/Scottish Intercollegiate Guidelines Network, or in the GINA guidelines (BTS 2016; GINA 2018), as described in the Background section. We excluded studies that concentrated on breathing exercise methods (including yoga interventions) if they did not directly focus on the other aspects of self‐management listed above.
The intervention could be provided by a trained educator, nurse (including school, practice, or community nurse), doctor or physician, peer, or social worker, and most delivery or access must have been provided on the premises of the school attended by the children. Interventions for which the school setting was not involved in delivery were not eligible for inclusion.
Comparisons
For outcome evaluation studies, comparison groups were restricted to usual care or to a self‐management or health intervention with a focus other than asthma (placebo).
For process evaluation studies, a comparison group could have received another asthma intervention, or the study may not have included a comparison group at all; all process evaluation studies must have included other parameters as described above in terms of study population, study setting, and contents of the asthma intervention.
Types of outcome measures
Outcomes for meta‐analyses
Our primary outcomes were based on those identified as indicators of good asthma control (BTS 2016), represented as intermediate outcomes in Figure 1. We were also interested in several secondary outcomes (represented as proximal and intermediate outcomes in Figure 1, as well as a measure of acceptability/implementation in withdrawal from the intervention).
Primary outcomes
Asthma symptoms or exacerbations leading to admission to hospital (children with one or more admissions or high admission rates)
Asthma symptoms or exacerbations leading to emergency department visits
Parent‐reported absence from school
Days of restricted activity
Secondary outcomes
Unplanned visit to hospital or GP due to asthma symptoms
Experience of daytime and night‐time symptoms (*these were differentiated from 'any' symptomatology by stating that symptoms occurred either in the daytime or at night‐time)
Lung function (e.g. forced expiratory volume in one second (FEV₁) in clinic, peak flow at home)
Use of reliever therapies such as beta₂‐agonists
Corticosteroid dosage and/or use of add‐on therapies (e.g. long‐acting beta₂‐agonists (LABAs), leukotriene receptor antagonists (LTRAs))
Health‐related quality of life (HRQoL) as measured by a validated questionnaire
Withdrawal from the study
We extracted data for all points at which the outcomes above were measured and pooled data as appropriate.
Outcomes for qualitative comparative analysis (QCA): defining a successful intervention
Qualitative comparative analysis (QCA) as used in this review and described in further detail below, is a method of evidence synthesis that enables understanding of which configurations of intervention components and processes trigger successful outcomes. QCA is predicated upon set theory, and in this context essentially involves exploring the degree of overlap between a set of successfully implemented studies and a set of studies with a particular range of intervention components and processes.
A first step in our use of QCA was deciding how 'successful' implementation could be identified. Currently, no approach has been established for categorising the implementation of an intervention as 'successful' or 'not successful' (Schellenberg 2012). We began by examining aspects of intervention implementation that were related to intervention fidelity as well as evidence around attrition, dosage, and adherence. A literature review of implementation scoring methods for public health interventions ‐ Schellenberg 2012 ‐ included one study that examined the implementation of a complex intervention that included a school component (Rosecrans 2008). Study authors used the following criteria: "process indictors for which standards were set, such as fidelity (e.g. % of minimum foods stocked) or dose received (e.g. % of family pack cards completed and returned), were assigned to categories of implementation as follows: low (0–49%), moderate (50–74%) or high (75–100%)" (Rosecrans 2008; p75). This 75% threshold also corresponds with the 25% attrition rate that is often incorporated within study sample size calculations for public health trials involving children (Berry 2013; Bruzzese 2011; Clark 1986).
A 75% threshold formed the basis of our coding scheme for the outcome, by which 75% was used as a cross‐over point for a 'high' or 'successful' implementation score. Implementation reflected reports of attrition, dosage, and adherence. For each of these indicators, we set values by using a blend of direct and transformational assignment (see Table 2), whereby we assigned values to qualitative data and then calibrated all data using transformational assignment. This blended approach was necessary to combine qualitative and quantitative data. To derive an outcome variable that reflected intervention implementation more holistically, we aggregated the three separate indicators into a single outcome variable by adding each separate value and calibrating the summed score. This outcome value reflected the mainstay of the analyses and distinguished our successfully implemented intervention set.
1. Detailed coding framework for conditions and outcomes.
| Field | Instructions for extractors | Coding values and method | |
| Setting and participants | |||
| 1 | Number of children | Recorded total number of children involved in intervention | Transformational assignment implemented to condition, reflecting whether it was a ‘large intervention’. Interventions with 15 or fewer children = 0; interventions with 90 children = 0.5; interventions with 300 or more children = 1. Other values fell between 0 and 1 |
| 2 | Multiple settings | Evidence if delivered at more than 1 school | Direct assignment: yes (mentioned) = 1; no evidence = 0 |
| 3 | Single sex school | Evidence if delivered at a single sex school | Direct assignment: yes (mentioned) = 1; no evidence = 0 |
| 4 | Type of school | High school; primary/elementary; junior/middle; other Variable transformed to reflect whether the intervention took place at a high school |
Direct assignment: high school = 1; middle/junior = 0.66; elementary/primary = 0.33; missing = 0.5; mixture of high schools and middle schools = 0.75 |
| 5 | Ethnicity of children | Whether minority ethnic children were targeted/represented. Actual proportions recorded where possible | Transformational assignment Interventions with 25% or fewer children from ethnic minority = 0; interventions with 33.3% of children from ethnic minority = 0.5; interventions with 50% or more children from ethnic minority = 1 When value is missing (and no qualitative statement supports assumption of targeting), assume that this is ‘probably not’ – i.e. probably not targeted – input value of 0.25 |
| 6 | Socio‐economic status of children | Whether children from lower socio‐economic groups were targeted/represented Actual proportions recorded where possible Indicators included parents with low levels of education; low household income; receipt of free school meals |
Transformational assignment Interventions with 25% or fewer children from low socio‐economic groups = 0; interventions with 33.3% of children from low socio‐economic groups = 0.5; interventions with 50% or more children from low socio‐economic groups = 1 Where value is missing (and no qualitative statement supports assumption of targeting), assume that this is ‘probably not’ – i.e. probably not targeted – input value of 0.25 |
| 7 | Child age | Age groups/classes targeted: ages 5 to 10 | Direct assignment: yes (mentioned) = 1; no evidence = 0 |
| 8 | Age groups/classes targeted: ages 11 to 14 | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 9 | Age groups/classes targeted: ages 15 to 18 | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 10 | Direct recipients | Children directed recipients | Direct assignment: yes (mentioned) = 1; no evidence = 0 |
| 11 | Teachers directed recipients | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 12 | Parents directed recipients | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 13 | School nurses directed recipients | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| Programme design | |||
| 14 | Theory driven | Did the study name a theoretical framework that underpins the intervention design or delivery style? | Direct assignment: yes (mentioned) = 1; no evidence = 0 |
| 15 | Intensity of the programme | Coded initially as follows: high intensity = 6+ sessions (group and individual); medium intensity = 3 to 5 sessions; low intensity/no evidence of med/high = 1 to 2 sessions; unclear. Variable transformed to reflect whether the intervention was of high intensity | Direct assignment: high intensity = 1, medium intensity = 0.66; low intensity = 0.33. When no evidence on intensity of intervention was included (1 study = (Richmond 2011)), this was coded as 0.33 (no evidence of high intensity) – interpreted as no evidence of high intensity; for Splett (Splett 2006), such is the degree of personalisation/tailoring that 0.5 was selected as the intensity – each individual session was personalised and lengthy |
| 16 | Personalisation/tailoring | Did the programme include individual sessions or use personalisation in any way to alter curriculum to individual students’ needs? | Direct assignment: yes, all sessions implemented were personalised/tailored = 1; some sessions were personalised/tailored = 0.66; personalisation/tailored sessions account for only a minor component = 0.5; no evidence, only generic group sessions implemented = 0 Note that this was personalised by or individual sessions were held with an instructor (included guided online sessions); self‐study components including homework were not included here |
| 17 | Timing of the intervention | Did the intervention interfere with the child’s own time (during lunch or after school)? | Direct assignment: yes, all sessions did = 1; yes, but not all sessions = 0.75; missing data = 0.5; described as not interfering with child’s own time = 0 |
| 18 | Did the intervention interfere with the child’s lessons/other education? | Direct assignment: yes, all sessions did = 1; yes, but not all sessions = 0.75; missing data = 0.5; described as not interfering with child’s lessons/other education = 0 | |
| 19 | Information about control condition | Described whether trialists were also providing a control for the main intervention (intended to capture complexity of running an intervention and a control) | Direct assignment: yes, an equivalent control = 1; yes, but not an equivalent = 0.66; no control described = 0 |
| 20 | Instructor or facilitator | Teacher | Direct assignment: yes, main instructor = 1; secondary instructor or facilitator = 0.66; not mentioned as an instructor/facilitator = 0 |
| 21 | Peer | Direct assignment: yes, main instructor = 1; secondary instructor or facilitator = 0.66; not mentioned as an instructor/facilitator = 0 | |
| 22 | School nurse | Direct assignment: yes, main instructor = 1; secondary instructor or facilitator = 0.66; not mentioned as an instructor/facilitator = 0 | |
| 23 | Self‐directed/child‐directed | Direct assignment: yes, main instructor = 1; secondary instructor or facilitator = 0.66; not mentioned as an instructor/facilitator = 0 | |
| 24 | Parent | Direct assignment: yes, main instructor = 1; secondary instructor or facilitator = 0.66; not mentioned as an instructor/facilitator = 0 | |
| 25 | Other | Direct assignment: yes, main instructor = 1; secondary instructor or facilitator = 0.66; not mentioned as an instructor/facilitator = 0 | |
| Programme content | |||
| 26 | Curriculum | Lung physiology/asthma biology | Direct assignment: yes (mentioned) = 1; no evidence = 0 |
| 27 | Asthma acceptance/asthma into identity | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 28 | Symptom monitoring and correct medication use | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 30 | Avoiding triggers | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 31 | General health including exercise | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 32 | Strengthening alliances including asthma action plans with primary care providers | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 33 | Specific focus on smoking | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 34 | Personalised/tailored (individualised) | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 35 | School performance | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 36 | Emergencies | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 37 | Unknown | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 38 | Specific focus on breathing/relaxation techniques | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 39 | Learning styles | Problem‐solving component | Direct assignment: yes (mentioned) = 1; no evidence = 0 |
| 40 | Self‐directed (including homework) component | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 41 | Peer delivery component | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 42 | Interactive (non‐didactic) components | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 43 | Didactic components | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 44 | Other style/unclear | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 45 | Programme ethos/aims | Emphasis on social benefit | Direct assignment: yes (mentioned) = 1; no evidence = 0 |
| 46 | Emphasis on improving well‐being | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 47 | Emphasis on having fun | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 48 | Emphasis on fostering independence/personal responsibility | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 49 | Emphasis on developing children's knowledge | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 50 | Emphasis on collaboration | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 51 | Emphasis on tailoring for specific group needs | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 52 | Emphasis on breathing technique | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 53 | Unclear | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 54 | Additional components – school asthma policy | Additional support provided for developing school policy | Direct assignment: yes (mentioned) = 1; no evidence = 0 |
| 55 | School asthma policy developed organically | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| Additional processes undertaken – planned and unplanned | |||
| 56 | Recruitment methods ‐ school | Ad hoc/convenience sample of schools | Direct assignment: yes (mentioned) = 1; no evidence = 0 |
| 57 | Census of school district (all schools invited and potentially eligible) | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 58 | Unspecified methods of school recruitment | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 59 | Additional processes to improve/attenuate attrition/enrolment | Marketing materials sent to parents | Direct assignment: yes (mentioned) = 1; no evidence = 0 |
| 60 | Low motivation of students acknowledged and addressed | Direct assignment: yes (mentioned) = 1; no evidence = 0 Note that 1 study received a value of 0.75, as low motivation was acknowledged but was not explicitly described as being addressed (Magzamen 2008) |
|
| 61 | Incentives used (child or parent) | Direct assignment: yes (mentioned) = 1; no evidence = 0 Incentives for teachers and no evidence for children/teachers coded as 0.5 |
|
| 62 | Make‐up/catch‐up sessions provided | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 63 | Reminders sent to parents/children | Direct assignment: yes (mentioned) = 1; no evidence = 0 | |
| 64 | Relationships/engagement | Did teachers engage or participate in the way they were expected to? | Direct assignment: yes, good reported throughout = 1; yes, some weaker evidence of good relationships or evidence that relationships improved during the course of the intervention = 0.75; missing, not applicable, or undetermined = 0.5; no, some weaker evidence of poorer relationships or evidence that relationships deteriorated during the course of the intervention = 0.25; evidence of poor relationships throughout = 0 |
| 65 | Did parents engage or participate in the way they were expected to? | Direct assignment: yes, good reported throughout = 1; yes, some weaker evidence of good relationships or evidence that relationships improved during the course of the intervention = 0.75; missing, not applicable, or undetermined = 0.5; no, some weaker evidence of poorer relationships or evidence that relationships deteriorated during the course of the intervention = 0.25; evidence of poor relationships throughout = 0 One study described good levels of engagement, but review authors assigned value of 0.25 as a third of parents did not engage as expected (Kintner 2012); similar rationale for Mujuru 2011 |
|
| 66 | Did school nurses engage or participate in the way they were expected to? | Direct assignment: yes, good reported throughout = 1; yes, some weaker evidence of good relationships or evidence that relationships improved during the course of the intervention = 0.75; missing, not applicable, or undetermined = 0.5; no, some weaker evidence of poorer relationships or evidence that relationships deteriorated during the course of the intervention = 0.25; evidence of poor relationships throughout = 0 | |
| 67 | Did other relevant stakeholders engage or participate in the way they were expected to? | Direct assignment: yes, good reported throughout = 1; yes, some weaker evidence of good relationships or evidence that relationships improved during the course of the intervention = 0.75; missing, not applicable, or undetermined = 0.5; no, some weaker evidence of poorer relationships or evidence that relationships deteriorated during the course of the intervention = 0.25; evidence of poor relationships throughout = 0 | |
| Process outcomes | |||
| 68 | Child satisfaction | Put in level of satisfaction (%) or record qualitative statement on child satisfaction with the intervention experience. Indicators of satisfaction include children reporting that they enjoyed the intervention; whether the children would recommend the intervention to others; whether children found the intervention helpful. Knowledge development should not be included here | Elements of direct and transformational assignment included here [First] Direct assignment: where there is a qualitative statement indicating positive agreement, assign value of 0.66; where a qualitative statement indicating negative agreement, assign value of 0.33; where no child satisfaction data were collected or data were missing, assign value of 0.5 [Second; including of direct above] Transformational assignment implemented to condition reflecting whether children were satisfied. Interventions with 25% or fewer children satisfied = 0; interventions with 50% of children satisfied = 0.5; missing data coded as 0.5; interventions with 75% or more children satisfied See text for further justification on use of the 75% threshold |
| 69 | Child attrition (overall level) | Put in level of completion (%) or record qualitative statement on child completion rate | Elements of direct and transformational assignment here. Note thresholds were higher than for satisfaction, as fewer data were missing [First] Direct assignment: where there is a qualitative statement indicating high level of completion, assign value of 0.83; where a qualitative statement indicating problematic completion, assign value of 0.66. Where data are missing, assign value of 0.75 [Second; including of direct above] Transformational assignment implemented to condition reflecting level of completion. Interventions with 66% or fewer children completing the intervention = 0; interventions with 75% of children completing the intervention = 0.5; interventions with 83% or more children completing the intervention = 1. Missing data coded as 0.5 See text for further justification on the use of thresholds |
| 70 | Child dosage level | Did the children receive the intended dosage of the intervention? Put in level of dosage (%) or record qualitative statement on child dosage. | Elements of direct and transformational assignment here. Note thresholds are higher than for satisfaction, as fewer data are missing [First] Direct assignment: where there is a qualitative statement indicating high level of dosage, assign value of 0.83; where a qualitative statement indicating problematic dosage, assign value of 0.66. Where data are missing, assign value of 0.75 [Second; including of direct above] Transformational assignment implemented to condition reflecting level of dosage. Interventions with 66% or fewer children receiving the full dosage = 0; interventions with 75% of children receiving the full dosage = 0.5; interventions with 83% or more of children receiving the full dosage = 1. Missing data coded as 0.5 See text for further justification on the use of thresholds |
| 71 | Child adherence | Did the children adhere to the intervention instructions, e.g. students being compliant with paperwork; completing homework; going to visit PCPs as instructed, etc. Put in level of adherence (%) or record qualitative statement on child dosage | Elements of direct and transformational assignment here. Note thresholds are higher than for satisfaction as fewer data are missing [First] Direct assignment: where there is a qualitative statement indicating high level of adherence, assign value of 0.83; where a qualitative statement indicating problematic adherence, assign value of 0.66. Where data are missing, assign value of 0.75 [Second; including of direct above] Transformational assignment implemented to condition reflecting level of adherence. Interventions with 66% or fewer children adherent = 0; interventions with 75% of children adherent = 0.5; interventions with 83% or more children adherent = 1. Missing data coded as 0.5 See text for further justification on the use of thresholds |
| 72 | Consolidated process variable | Summation of attrition, adherence, and dosage scores as a marker of implementation success | Transformational assignment Score of 0 = 0 implementation not successful; score of 1.5 = mid point between successful and unsuccessful implementation; score of 3 = full implementation success |
Search methods for identification of studies
Electronic searches
We searched the Cochrane Airways Group Specialised Register (see Appendix 1) for trials, using the strategy presented in Appendix 2, which was developed by the Cochrane Airways Information Specialist (Liz Stovold). We conducted searches in April 2015 and updated them in April 2016. We conducted further searches on 25 August 2017.
We searched the databases below for process evaluations for our qualitative comparative analyses, using the search criteria identified in Appendix 1, although we modified these criteria to account for the different search syntax/parameters used in additional databases (see Appendix 3,Appendix 4,Appendix 5,Appendix 6, and Appendix 7 for example search strategies).
Database of Promoting Health Effectiveness Reviews (DoPHER).
Cochrane Database of Systematic Reviews (CDSR).
Database of Abstracts of Reviews of Effects (DARE).
The Campbell Library.
National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Programme website/journals library.
Health Technology Assessment (HTA) database.
We applied search strategies to a comprehensive search of the following clinical, public health, psychology, and social care databases from 1995 to the present*.
Allied and Complementary Medicine Database (AMED)
Applied Social Sciences Index and Abstracts (ASSIA).
Bibliomap (EPPI‐Centre Database of Health Promotion Research).
ClinicalTrials.gov
Cochrane Database of Systematic Reviews (CDSR).
Cochrane Central Register of Controlled Trials (CENTRAL).
Cumulative Index to Nursing and Allied Health Literature (CINAHL).
Excerpta Medica dataBASE (EMBASE)
Health Management Information Consortium (HMIC).
International Bibliography of the Social Sciences (IBSS).
National Health Service Economic Evaluation Database (NHS EED).
PsychInfo.
PubMed.
Sociological Abstracts (SocAbs).
Social Policy and Practice (SPP).
Social Services Abstracts
Web of Knowledge.
*MEDLINE, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), Embase, the Allied and Complementary Medicine Database (AMED), and PsycINFO are included within the Cochrane Airways Group Specialised Register search.
Searching other resources
We handsearched Google Scholar, Social Policy Digest (for content up to 2014), and other sources such as the British Thoracic Society and Asthma UK for further studies.
We initially identified integral process evaluations (sibling studies) through backwards and forwards citation searches. As expected, we identified multiple process evaluations for some intervention studies; our strategy also allowed for inclusion of process evaluations without linkage to a trial included for quantitative analyses.
Data collection and analysis
Selection of studies
We piloted criteria for title and abstract screening on a random subset of studies for which the review authors who were involved in screening (DK, KH) took part in moderation exercises; we resolved disagreements by discussion and developed a shared understanding of the inclusion criteria. We achieved an agreement rate exceeding 90% in three consecutive samples before we proceeded to independent screening (DK, KH). We also employed priority screening (text mining) for independent title and abstract screening (Thomas 2011), after achieving a sufficiently high agreement rate, to locate likely included studies more quickly. However, both review authors (DK, KH) screened all abstracts.
We applied inclusion criteria successively to titles and abstracts, and to full reports. We obtained full‐text reports when studies appeared to meet the criteria for title and abstract, or when information was insufficient for a decision. For outcome evaluation studies, screening criteria covered populations (children five to 18 years of age), disease status (asthma), interventions (school‐based and focused on self‐management), comparators (usual care or placebo), study design (randomised controlled trials or cluster randomised controlled trials), date (publication year after 1995), and language (English language). We entered full‐text reports into EPPI‐Reviewer and reapplied the inclusion criteria (Thomas 2010); we included studies that met these study design criteria (irrespective of the actual outcomes collected). We developed a similar set of inclusion criteria for process evaluation studies covering populations, disease status, interventions, date, and language; additional criteria stipulated that studies must include the core components expected within a process evaluation and must use structured or recognised tools to collect data.
Data extraction and management
Data management
We uploaded records identified by searches to the specialist systematic review software EPPI‐Reviewer 4 for duplicate stripping and screening (Thomas 2010). This software recorded the bibliographic details of each study considered in the review, the origins of all studies (including search strings), and reasons for their inclusion or exclusion. We first extracted all data into EPPI‐Reviewer 4 and later exported them, as appropriate, into other software for synthesis (RevMan 2014; StataCorp 2013; Thiem 2013).
Extraction and management of data from process evaluation studies
Process evaluation measures ‐ data selection
Overall approach
The primary aim of exploring process evaluations using QCA was to identify the combinations of components and processes undertaken for interventions that were associated with successful intervention implementation. QCA is based on set theory, and, in this review, we explored the extent of overlap between a set of studies with successful implementation (our process outcome) and sets of studies that share combinations of different intervention components and processes. We presented extracted intervention components and processes (equivalent to antecedents and referred to as conditions from hereon in, in line with QCA terminology) as modifiable design characteristics in the logic model (Figure 1).
Extracting data and building the data table: initial data reduction and assignment of values
Two review authors (DK, KH) independently extracted the conditions (process evaluation measures) of interest from eligible studies. We developed an extensive data table of information supporting over 90 conditions for each study. These data represented quantitative indicators (showing the level of presence of a condition (e.g. the proportion of children from an ethnic minority recruited into an intervention)); binary indicators (representing whether or not a condition was present (e.g. study authors reported that the asthma curriculum contained information on lung physiology)); or qualitative statements (e.g. when study authors published quotes illustrative of child satisfaction with the intervention). In accordance with guidance provided by Rihoux and De Meur (Rihoux 2009), we developed a set of rules for assigning values to conditions (Table 2); these rules reflect a mixture of direct and transformational assignment (we have provided further explanation and an example in Appendix 8).
Reduction of data on conditions
We extracted more data than any QCA model could support ‐ a problem referred to as 'limited diversity in QCA terminology'. Recognising that many of the conditions extracted were binary indicators of constructs related to the same underlying condition, we implemented cluster analyses of linked items (e.g. elements of the curriculum) to create natural groupings and to reduce the number of conditions included in some models (Thomas 2014). We have displayed original and reduced data for these conditions in Table 3. In addition, we used the logic model presented in Figure 1 to guide our analysis, to rationalise and prioritise the conditions entered into models, and to limit the number of conceptually similar conditions that were entered into models.
2. Original and reduced conditions for curriculum content, delivery style, and programme emphasis.
| Curriculum – original conditions | Curriculum – reduced conditionsa |
| I. Lung physiology ii. Asthma acceptance iii. Symptom monitoring and treatment iv. Trigger avoidance v. General health vi. Forming alliances vii. Smoking viii. Tailored/personalised ix. School performance x. Emergencies xi. Unknown content |
I. Symptom monitoring and alliances ii. Lung physiology and general health iii. Symptom monitoring and trigger avoidance iv. Other various foci v. Unknown |
| Pedagogical delivery style – original conditions | Pedagogical delivery style – reduced conditionsb |
| I. Problem‐solving ii. Self‐direct iii. Peer delivery iv. Interactive v. Didactic vi. No information/other focus |
I. Interactive focused style ii. Diverse style iii. Unknown style |
| Intervention emphasis – original conditions | Intervention emphasis – reduced conditionsc |
| I. Emphasis on social benefit ii. Emphasis on well‐being iii. Emphasis on having fun iv. Emphasis on personal responsibility v. Emphasis on children’s knowledge vi. Emphasis on collaboration vii. Emphasis on tailoring/personalisation viii. Emphasis unclear |
I. Emphasis on tailoring/personalisation ii. Emphasis on personal responsibility iii. Diffuse emphasis/other |
aPseudo‐F index = 5.66. bPseudo‐F index = 8.36. cPseudo‐F index = 6.50.
Reduction of cases
Although cluster analysis reduced the number of conditions examined, we made the decision to focus on cases (studies) that were coded as providing high‐ or medium‐intensity interventions. We did not explicitly mention this in the protocol (therefore it is reported as a deviation), although this approach is congruent with indicators such as attrition and dosage.
Extraction and management of data from outcome evaluation studies (RCTs)
Outcome measures ‐ data extraction
Two review authors (DK, KH) independently extracted study characteristics and numerical outcome data from studies meeting the eligibility criteria of the review. In agreement meetings, review authors resolved discrepancies by discussion; we encountered no disagreements that needed resolution through arbitration by senior members of the review team. When we encountered missing data, we recorded these instances and contacted study authors for further information.
Assessment of risk of bias in included studies
Assessment of risk of bias in included RCTs
We assessed how the following sources of bias may affect the results of an individual study.
Sequence generation: we deemed that studies that used a computer‐generated allocation procedure, a random number table, or other recognised low‐risk means were at low risk of bias (as advised by the Cochrane tool for assessing risk of bias). We deemed that studies that used items such as clinic visit date or date of birth when the order of treatment group assignment was predictable or open to external influence were at high risk of bias. We described studies for which we were unable to ascertain methods of randomisation and allocation as having unclear risk of bias. Given the potential impact of socio‐economic imbalance between cluster sites within the same study, we also considered whether study authors had stratified socio‐economic variables.
Allocation concealment: we deemed that studies for which researchers took measures to prevent disclosure of treatment group assignment, such as off‐site allocation or allocation by a third party not involved in the study, were at low risk of bias. For cluster randomised studies, an additional consideration was timing of recruitment into the study in relation to assignment.
Blinding (performance bias and detection bias): we deemed that studies for which investigators took measures to ensure that personnel collecting data were unaware of participants' treatment group assignment were at low risk of bias. However, given the nature of the intervention and the difficulty involved in blinding recipients, a degree of performance bias may have impacted some outcomes, particularly patient‐reported outcomes, and this was unavoidable.
Handling of missing data and attrition: we deemed that studies for which data sets were complete, or for which reasons for missing data were not related to treatment, were at low risk of bias. When attrition rates were particularly high or imbalanced and unexplained, and only an available case set was presented, we deemed that the study was at high risk of bias. We deemed that studies for which study authors did not report the attrition rate separately for treatment and control groups, and for which we were unable to determine satisfactorily the reasons for withdrawal, were at high risk of bias.
Selective reporting: we restricted assessments of selective reporting to examination of available data related to outcomes included in the 'Summary of findings' table.
Other bias: we examined baseline imbalances in the characteristics of participants (see also the first point around stratification) for potential bias. We also looked for evidence of contamination between intervention and control groups. We restricted sensitivity analysis to primary outcomes of the review, and we derived overall judgements for each study at the outcome level.
Assessment of risk of bias in included process evaluation studies
We assessed the quality of process evaluation studies using elements of two tools. The first tool was developed at the EPPI‐Centre to assess the methodological rigour of 'views' studies that aimed to collect information on people's experiences during trials (Harden 2004). This tool considers seven criteria, including (I) whether the study includes an explicit theoretical framework and/or literature review; (ii) clearly stated aims and objectives; (iii) a clear description of context; (iv) a clear description of the sample and how it was recruited; (v) a clear description of methods used to collect and analyse data; (vi) attempts made to establish the reliability or validity of data analysis; and (vii) inclusion of sufficient original data to mediate between evidence and interpretation. The second tool, which was developed by the EPPI‐Centre to assess the quality of process evaluation data (O'Mara‐Eves 2013), assesses (I) methods of data collection; (ii) process evaluation participants as described; (iii) timing of the process evaluation with respect to the intervention; (iv) process evaluation data collection methods; (v) process evaluation data analysis methods; (vi) whether findings were supported by data; (vii) breadth and depth of findings; (viii) the extent to which the process evaluation gave privilege to the views of participants; (ix) reliability of findings; and (x) usefulness of process evaluation. As some of these domains overlap, we combined elements from both tools to assess the quality of process measures. This strategy also covers the main domains that had been set out in the Cochrane Qualitative Methods Group guidance that was current at the time (Hannes 2011).
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol (Harris 2015), and we reported deviations from it under Differences between protocol and review.
Measures of treatment effect
Continuous data
We planned to calculate mean differences (MDs) when continuous data were measured by the same scale or unit; however, this did not occur for most outcomes (one MD model had been constructed to explore quality of life as an outcome). Instead, when similar outcomes were measured by different scales or units, we used standardised mean differences (SMDs) (Hedges' (adjusted) g).
Dichotomous data
For dichotomous data, we calculated odds ratios (ORs), and, when appropriate, we combined results from different trials.
Ordinal data
We planned to analyse ordinal outcomes (such as quality of life scales) as continuous variables; when appropriate thresholds were identified, we analysed these as dichotomous variables.
Count data
We planned to calculate rate ratios for any count data that we encountered that represented the ratio of events experienced between two groups, such as episodes of hospitalisation or absences from school.
Unit of analysis issues
Cluster randomised studies
We included cluster randomised controlled trials in which schools or classes within schools rather than individuals with asthma were the unit of allocation. As variation in response to treatment between clusters may also be influenced by cluster membership, meaning that cluster members' data can no longer be considered independent of one another, we extracted data when study authors had undertaken analysis that properly adjusted for a clustered design. When study authors provided no intracluster correlation coefficient (ICC), we intended to estimate the ICC and the design effect according to methods recommended in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). No study provided a direct estimate of ICC; however, we selected 0.05 based on the ICC estimate used in one of the included papers to calculate the sample size (McCann 2006). We adjusted effect estimates using methods described in Higgins 2011.
Choice of measurement point
For trials that reported outcomes at multiple time points, such as at post‐test with longer follow‐up, we extracted all data and combined in meta‐analyses the follow‐up points most consistently reported among trials.
Dealing with missing data
When study characteristics and numerical outcome data were missing from studies, we contacted study authors to request missing information. For quantitative aspects of process evaluations, such as satisfaction or participation data, we applied the same procedure. Recording of the 'missingness' of qualitative data in the process evaluations that we include is more oblique, although we recorded instances in which investigators indicated that the data collected were not reported upon as part of the quality assessment.
Assessment of heterogeneity
We assessed statistical heterogeneity by using the I² measure (Higgins 2003). We explored possible sources of variation by conducting prespecified sensitivity and subgroup analyses and performing meta‐regression analyses. These included those set out in the protocol (Harris 2015), as well those that we developed from QCAs.
We intended to construct random‐effects multi‐variate meta‐regression models using STATA, which would allow us to model the impact of different covariates simultaneously after first exploring the impact of these potential effect size study‐level moderators in bivariate models. However, a relatively small number of studies (our largest meta‐analysis model included 13 studies) meant that we were unable to extend the modelling in this way without compromising the underlying assumptions.
Assessment of reporting biases
We recorded the number of studies for which we were not able to ascertain the analysis of data related to our primary outcomes. We also recorded the number of studies for which we were not able to extract process measures, and we assessed the breadth and depth of those studies in terms of information on processes. We selected all process evaluation studies conditionally on addressing process‐related research questions, although the core process outcomes included within these did not always match our own selected process outcomes (e.g. some studies addressed different recruitment techniques as a central process of interest, although this focus did not match our own focus).
We plotted the distribution of effect sizes for each (outcome) study against study standard errors as a funnel plot for primary outcomes and based our assessment of publication bias on visual inspection (if 10 or more studies contributed to the outcome); we also undertook formal tests for small‐study publication bias using Egger's test (Harbord 2009).
Data synthesis
Data synthesis ‐ adopting a mixed methods approach
In the first strand of analyses, we explored which intervention features (components and processes) are associated with successful implementation of an intervention. This first strand involved undertaking qualitative comparative analysis (QCA) to uncover which configurations of these features (known as 'conditions' in QCA terminology) are aligned with successful intervention implementation. The QCA served to generate hypotheses about the importance of different intervention components and processes that were tested in meta‐analyses (below). Conditions identified through QCA helped us to identify which conditions matter for implementing an intervention, and structuring the meta‐analysis helped us to identify their potential impact on the overall effectiveness of interventions. The possibility that hypotheses were generated and tested on the same dataset was avoided due to very little overlap between studies included in the QCA synthesis and studies included in the meta‐analyses.
To examine the effectiveness of school‐based asthma self‐management interventions in improving children's outcomes, we undertook meta‐analyses. We performed subgroup analyses based upon results of the QCA described above.
We undertook the synthesis of process evaluations performed before the RCTs were conducted to remain blinded to the possible impact of specific measures. We further examined the link between implementation and effectiveness by estimating whether interventions defined as 'successful' in terms of their implementation were those with greater effect sizes. These analyses focused on a subgroup of studies adopting diverse designs (as outlined below).
Data synthesis part 1 ‐ using process evaluation studies for qualitative comparative analysis (QCA) of determinant conditions for successful intervention implementation
Qualitative comparative analysis (QCA) is used to identify configurations of conditions associated with successful intervention implementation. QCA takes a study‐based approach (accounting for several of the study's observed characteristics simultaneously) rather than a variable‐based approach, so that the focus is on different configurations of conditions (Thomas 2014). As this approach is relatively novel to systematic reviews, we have provided further information on the underlying principles and operationalisation of the approach in Appendix 8. The QCA approach used here aimed to generate theories about components 'sufficient' for triggering successful implementation; 'sufficient' relationships signify that an outcome is triggered in the presence of a sufficient condition or a sufficient condition set, but that other pathways to triggering the outcome may also exist. Here the outcome is successful implementation, and conditions are intervention characteristics and processes. In analysing our data, we followed the steps laid out by others (Ragin 2009; Thomas 2014).
We began by operationalising our data and creating a set of rules on how data should be coded for creating a data table of intervention characteristics (known as 'conditions' in QCA terminology) and the extent to which an intervention was successfully implemented (the outcome in this case). In the section titled Secondary outcomes, we have described the way in which we derived our outcome variable, and in the section titled Data extraction and management, we have described our coding framework for other intervention characteristics of interest. Two review authors (DK, KH) coded data for each study and grouped the information into separate data tables reflecting different domains of an intervention (i.e. conditions): setting and participants (Table 4); recruitment and retention processes (Table 5); curriculum and pedagogical factors (Table 6); modifiable intervention design features (Table 6); and stakeholder involvement (Table 7). We adopted this strategy to avoid 'limited diversity', whereby too many possible combinations of intervention characteristics are unsupported by observed studies.
We constructed truth tables that move beyond examining individual studies (i.e. one row per study) to examining configurations of conditions. Configurations could be supported by no studies, one study, or multiple studies. Truth tables also show the extent to which a 'set' of studies belonging to a configuration overlap with the outcome set.
We checked the quality of the truth tables. For each truth table, we considered whether a spread of positive and negative outcomes was triggered; whether configurations were supported by (multiple) cases (especially for configurations triggering a successful outcome); whether some configurations were counterintuitive and whether some conditions showed identical patterns; and whether some conditions occurred too infrequently. Our most important check involved whether we observed contradictory configurations when evidence suggested that configurations triggered positive and negative outcomes. When we were unable to resolve these issues according to guidance provided in Thomas 2014, the analysis progressed no further (see Appendix 8).
We then implemented Boolean minimisation to identify the most logically simple expression of a 'pathway' to a successful outcome. A pathway in this case represents a configuration of conditions that is observed to sufficiently trigger an outcome. This solution is based on observed configurations of conditions only and is known as a 'complex solution'.
When we detected logical remainders, we incorporated these into further models as 'intermediate solutions' to simplify the solution and maintain its theoretical coherence (see Appendix 8). For intermediate solutions, review authors (DK, KH) set expectations on whether the conditions entered were likely to lead to success.
A sixth stage involved interpretation, when review authors considered the plausibility of the solution and determined whether conclusions were consistent with evidence obtained from individual cases. We constructed a consolidated model, using evidence from preceding models. We checked the quality of the overall solution to ensure that it did not trigger negation of the outcome; we also assessed the parameters of fit and the validity of simplifying assumptions.
3. Data table for QCA model 1 ‐ setting and participants.
| Successful intervention | School‐based health centre | High school | Parents directly involved | Teachers received training | School nurses or other stakeholders received training | |
| Joseph 2010 | 0.52 | 0.55 | 1 | 0 | 0 | 0 |
| Kouba 2012 | 0.33 | 0.33 | 1 | 1 | 0 | 0 |
| Dore‐Stites 2007 | 0.67 | 0.66 | 0 | 1 | 0 | 0 |
| Joseph 2013 | 1.00 | 0.55 | 1 | 0 | 0 | 0 |
| Mujuru 2011 | 0.67 | 0.66 | 0 | 0 | 1 | 0 |
| Henry 2004 | 0.83 | 0.33 | 1 | 0 | 1 | 0 |
| Pike 2011 | 0.67 | 0.33 | 0 | 0 | 1 | 0 |
| Spencer 2000 | 0.33 | 0.66 | 0 | 1 | 0 | 0 |
| Engelke 2013 | 0.50 | 0.66 | 0.5 | 1 | 1 | 1 |
| Splett 2006 | 0.50 | 1.00 | 0.5 | 0 | 1 | 1 |
| Kintner 2012 | 0.83 | 0.66 | 1 | 1 | 0 | 1 |
| Berg 2004 | 0.83 | 0.66 | 1 | 0 | 0 | 0 |
| Howell 2005 | 0.33 | 0.75 | 0 | 1 | 0 | 0 |
| Gerald 2006 | 0.33 | 0.55 | 0 | 0 | 0 | 0 |
| Langenfeld 2010 | 0.33 | 0.66 | 0 | 0 | 1 | 0 |
| Al‐Sheyab 2012 | 0.83 | 0.33 | 1 | 0 | 0 | 0 |
| Levy 2006 | 0.52 | 0.33 | 0 | 0 | 1 | 0 |
| Terpstra 2012 | 1.00 | 0.66 | 0.66 | 1 | 0 | 0 |
| Horner 2015 | 0.67 | 0.66 | 0 | 0 | 0 | 0 |
| Bruzzese 2008 | 0.94 | 0.66 | 0.66 | 1 | 0 | 0 |
| Lee 2011 | 0.50 | 0.66 | 0 | 0 | 0 | 0 |
| Bruzzese 2004 | 0.33 | 0.55 | 1 | 0 | 0 | 1 |
| Cicutto 2013 | 0.67 | 0.33 | 0 | 0 | 0 | 1 |
| Brasler 2006 | 0.00 | 0.66 | 0.66 | 1 | 0 | 0 |
| Crane 2014 | 0.50 | 0.33 | 0 | 0 | 0 | 0 |
| Bruzzese 2011 | 0.88 | 0.55 | 1 | 0 | 0 | 1 |
| Magzamen 2008 | 0.19 | 0.55 | 0.75 | 0 | 1 | 0 |
QCA: qualitative comparative analysis.
4. Data table for QCA model 2 ‐ recruitment and retention processes.
| Successful intervention | Provision of additional marketing materials | Provision of incentives | Make‐up sessions provided | Reminders provided for attendance at activity | |
| Joseph 2010 | 0.52 | 1 | 1 | 0 | 0 |
| Kouba 2012 | 0.33 | 1 | 0 | 1 | 0 |
| Dore‐Stites 2007 | 0.67 | 1 | 1 | 0 | 0 |
| Joseph 2013 | 1.00 | 1 | 1 | 0 | 0 |
| Mujuru 2011 | 0.67 | 0 | 0 | 0 | 1 |
| Henry 2004 | 0.83 | 0 | 0 | 0 | 0 |
| Pike 2011 | 0.67 | 0 | 0.5 | 0 | 0 |
| Spencer 2000 | 0.33 | 1 | 0 | 0 | 0 |
| Engelke 2013 | 0.50 | 0 | 0 | 0 | 0 |
| Splett 2006 | 0.50 | 0 | 0 | 0 | 0 |
| Kintner 2012 | 0.83 | 1 | 1 | 1 | 0 |
| Berg 2004 | 0.83 | 0 | 1 | 0 | 0 |
| Howell 2005 | 0.33 | 0 | 1 | 1 | 1 |
| Gerald 2006 | 0.33 | 0 | 0 | 0 | 0 |
| Langenfeld 2010 | 0.33 | 0 | 1 | 0 | 0 |
| Al‐Sheyab 2012 | 0.83 | 0 | 0 | 0 | 0 |
| Levy 2006 | 0.52 | 0 | 0 | 0 | 0 |
| Terpstra 2012 | 1.00 | 1 | 1 | 1 | 1 |
| Horner 2015 | 0.67 | 0 | 0 | 0 | 0 |
| Bruzzese 2008 | 0.94 | 0 | 0 | 1 | 0 |
| Lee 2011 | 0.50 | 0 | 0.75 | 0 | 0 |
| Bruzzese 2004 | 0.33 | 0 | 1 | 0 | 1 |
| Cicutto 2013 | 0.67 | 0 | 0 | 1 | 0 |
| Brasler 2006 | 0.00 | 1 | 1 | 1 | 1 |
| Crane 2014 | 0.50 | 0 | 0 | 0 | 0 |
| Bruzzese 2011 | 0.88 | 0 | 0 | 1 | 0 |
| Magzamen 2008 | 0.19 | 1 | 1 | 0 | 1 |
QCA: qualitative comparative analysis.
5. Data table for QCA model 4 ‐ modifiable design features.
| Successful intervention | Theory driven | Personalised or individual sessions | Intervention takes place during lesson time | Intervention takes place during students’ own free time | School nurse involved in delivery of the intervention | |
| Joseph 2010 | 0.52 | 1 | 1 | 1 | 0.33 | 0 |
| Kouba 2012 | 0.33 | 1 | 1 | 0 | 1 | 0 |
| Dore‐Stites 2007 | 0.67 | 1 | 0 | 0.33 | 0.33 | 0.66 |
| Joseph 2013 | 1.00 | 1 | 1 | 0.75 | 0.75 | 0 |
| Mujuru 2011 | 0.67 | 0 | 0 | 1 | 0 | 0 |
| Henry 2004 | 0.83 | 0 | 0 | 1 | 0 | 0 |
| Pike 2011 | 0.67 | 0 | 0 | 1 | 0 | 0 |
| Spencer 2000 | 0.33 | 0 | 1 | 0.33 | 0.33 | 0.66 |
| Engelke 2013 | 0.50 | 0 | 0.66 | 0.33 | 0.33 | 1 |
| Splett 2006 | 0.50 | 0 | 1 | 0.33 | 0.33 | 1 |
| Kintner 2012 | 0.83 | 1 | 0 | 1 | 1 | 0.66 |
| Berg 2004 | 0.83 | 1 | 0.66 | 0.33 | 0.33 | 0.66 |
| Howell 2005 | 0.33 | 1 | 1 | 0.33 | 0.33 | 0.66 |
| Gerald 2006 | 0.33 | 0 | 0 | 1 | 0.33 | 0 |
| Langenfeld 2010 | 0.33 | 0 | 1 | 0.33 | 0.33 | 1 |
| Al‐Sheyab 2012 | 0.83 | 1 | 0 | 0.33 | 0.33 | 0 |
| Levy 2006 | 0.52 | 0 | 0.66 | 0.33 | 0.33 | 1 |
| Terpstra 2012 | 1.00 | 1 | 0 | 0 | 1 | 0.66 |
| Horner 2015 | 0.67 | 1 | 0 | 0 | 1 | 0 |
| Bruzzese 2008 | 0.94 | 1 | 0 | 0.33 | 0.33 | 0.66 |
| Lee 2011 | 0.50 | 1 | 0 | 1 | 0 | 0.66 |
| Bruzzese 2004 | 0.33 | 1 | 1 | 0.75 | 0.75 | 0 |
| Cicutto 2013 | 0.67 | 1 | 0 | 0 | 1 | 0 |
| Brasler 2006 | 0.00 | 0 | 0 | 0.75 | 0.75 | 0.66 |
| Crane 2014 | 0.50 | 1 | 0 | 0 | 1 | 0.66 |
| Bruzzese 2011 | 0.88 | 1 | 1 | 0.33 | 0.33 | 0 |
| Magzamen 2008 | 0.19 | 0 | 0 | 0 | 1 | 1 |
QCA: qualitative comparative analysis.
6. Data table for QCA model 5 ‐ stakeholder involvement and engagement.
| Successful intervention | School asthma policy | Good relationships/engagement with parents | Good relationships/engagement with school nurses | Child Satisfaction | School asthma policy | |
| Joseph 2010 | 0.52 | 0 | 0 | 0 | 0 | 0 |
| Kouba 2012 | 0.33 | 0 | 0 | 0 | 0 | 0 |
| Dore‐Stites 2007 | 0.67 | 0 | 0.75 | 1 | 1 | 0 |
| Joseph 2013 | 1.00 | 0 | 1 | 0 | 0 | 0 |
| Mujuru 2011 | 0.67 | 0 | 0.25 | 0 | 0 | 0 |
| Henry 2004 | 0.83 | 1 | 0 | 0 | 0 | 1 |
| Pike 2011 | 0.67 | 0 | 0 | 0 | 0 | 0 |
| Spencer 2000 | 0.33 | 0 | 1 | 1 | 0 | 0 |
| Engelke 2013 | 0.50 | 1 | 1 | 0 | 0 | 1 |
| Splett 2006 | 0.50 | 1 | 0 | 1 | 0 | 1 |
| Kintner 2012 | 0.83 | 0 | 0.25 | 0 | 1 | 0 |
| Berg 2004 | 0.83 | 0 | 0 | 0 | 1 | 0 |
| Howell 2005 | 0.33 | 0 | 0.75 | 0.75 | 0.633333 | 0 |
| Gerald 2006 | 0.33 | 0 | 0 | 0 | 0 | 0 |
| Langenfeld 2010 | 0.33 | 1 | 0 | 1 | 0 | 1 |
| Al‐Sheyab 2012 | 0.83 | 0 | 0 | 0 | 0.633333 | 0 |
| Levy 2006 | 0.52 | 1 | 0 | 0 | 0 | 1 |
| Terpstra 2012 | 1.00 | 0 | 0.25 | 0 | 0 | 0 |
| Horner 2015 | 0.67 | 0 | 0 | 0 | 0 | 0 |
| Bruzzese 2008 | 0.94 | 0 | 1 | 0 | 1 | 0 |
| Lee 2011 | 0.50 | 0 | 0 | 0 | 0 | 0 |
| Bruzzese 2004 | 0.33 | 0 | 0 | 0 | 0.633333 | 0 |
| Cicutto 2013 | 0.67 | 1 | 0 | 0 | 0 | 1 |
| Brasler 2006 | 0.00 | 1 | 0 | 1 | 0.633333 | 1 |
| Crane 2014 | 0.50 | 0 | 0 | 1 | 0 | 0 |
| Bruzzese 2011 | 0.88 | 0 | 0 | 0 | 0 | 0 |
| Magzamen 2008 | 0.19 | 0 | 0 | 0 | 0 | 0 |
QCA: qualitative comparative analysis.
We constructed all QCA models using R and a package developed by Thiem and Dusa (Thiem 2013). We have outlined further details of all steps, as well as the background to the method, in Appendix 8.
Data synthesis part 2 ‐ using RCTs for meta‐analyses of effectiveness
We combined data in Review Manager 5.3 (RevMan 2014), and we conducted some analyses and data transformations in STATA (when we encountered cluster randomised trials, we converted our standard errors using EPPI‐Reviewer functions (Thomas 2010)). We expected outcomes to be reported as similar units of analysis, although we encountered several variations and used Chinn's formulae for converting effect sizes and standard errors between SMDs and ORs (Chinn 2000), according to direction provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In addition, although we had originally specified daytime and night‐time symptoms as a single outcome, we split this into two separate outcomes to maintain conceptual coherence.
Occasionally, we could not incorporate some data into the meta‐analyses because of methodological difficulties in combining these data (including data based on rank (e.g. median)). Other changes and forms of imputation for missingness included the following: (I) basing the effect size for quality of life from Al‐Sheyab 2012 on the P value because of uncertainty regarding the effect size derived from point estimates and the precision provided; (ii) basing effect sizes for Cicutto 2013 on approximations of the numbers of participants in control and treatment groups; and (iii) estimating the numbers in treatment and control arms for Clark 2005 (assuming equal distribution of the overall sample size); we also imputed an OR of 0.996 for a value reported as 1.00 for Clark 2005 for ED visits, so we could combine the information from different models.
Data synthesis part 3: adjunct meta‐analyses exploring the link between implementation and effectiveness of school‐based asthma self‐management interventions
Methods used by review authors for the adjunct meta‐analyses followed the same processes as were used for the main meta‐analysis (part 2) in terms of the approaches taken in extracting effect sizes and combining data. The difference between analyses is that results of part 3 are based both on RCTs included in the main analyses (part 2) and on studies included in part 1 that allow for calculation of an effect size for school absences and/or emergency department visits. All studies included here must have included a control group and must have allowed for calculation of successful implementation, which we defined in the same way as our QCA analysis (part 2), and represented a combined indicator around attrition, adherence, and dosage.
Rating the certainty of the evidence
The certainty of evidence rating reflects the extent to which we can be confident that results for review outcomes reflect the true effect (Guyatt 2008). We rated the certainty of evidence for our main outcomes using methods developed by the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) Working Group (http://www.gradeworkinggroup.org/publications/JCE_series.htm). We considered the possible impact of each of the following factors on our outcomes of interest.
Risk of bias.
Imprecision.
Inconsistency.
Indirectness.
Publication bias.
We attempted to identify a representative control group risk to illustrate the effects of our meta‐analysis results in absolute terms. We tabulated GRADE ratings alongside absolute and relative effects in a 'Summary of findings' (SoF) table for the following outcomes.
Asthma symptoms or exacerbations leading to admission to hospital.
Asthma symptoms or exacerbations leading to emergency department (ED) visits.
Unplanned visit to hospital or GP due to asthma symptoms.
School absence.
Experience of daytime symptoms.
Use of reliever therapies such as beta₂‐agonists.
(Health‐related) quality of life.
We generated the SoF table using the GRADE Guideline Development tool (GDT). We have described elsewhere further analyses undertaken to explore heterogeneity in effect size.
Subgroup analysis and investigation of heterogeneity
We conducted a statistical test for heterogeneity across subgroups using an I² statistic. We planned to construct a multi‐variate meta‐regression model based on our results for different outcomes. However, the small number of included studies precluded this possibility. We undertook prespecified subgroup analyses to investigate heterogeneity on the basis of the following characteristics, which are represented in our logic model as child‐level, school‐level, and contextual moderators, as well as modifiable design characteristics of the intervention itself, which we identified on the basis of QCA.
Setting: elementary/primary school versus secondary/high school.
Age: five to 10 years; 11 to 15 years; 16 years and older.
Socio‐economic level: low or mixed/high/unclear.
Delivery of intervention: healthcare provider (e.g. health educator, school nurse, other healthcare professional) versus other professional (e.g. teacher, mixture) versus other model of delivery (e.g. peer led).
Other (prespecified): intervention moderators developed from hypotheses generated through syntheses of process evaluation data including whether the intervention was theory driven, whether parents were actively involved, and the timing of the intervention during the school day. We entered these as single conditions and as groups reflecting configurations.
We measured some indicators, such as socio‐economic status, very differently, and we used broad groupings based on income, social class, or other indicators of social position, such as having received means tested benefits.
Sensitivity analysis
We undertook sensitivity analyses on the basis of the following.
Risk of bias assessment: we included all studies in the primary analysis and restricted included studies to those that were not classed as having high risk of bias for any single domain.
Fixed‐effect modelling.
Exclusion of cluster study data from outcomes (originally intended when external or imputed ICCs had been used, although this applied to most included cluster RCTs).
We did not plan to apply an equivalent for QCA modelling, although we did conduct robustness checks, including whether solutions predicted negation of the outcome.
We had intended to run sensitivity analyses based on the severity of children's asthma; however, no intervention specifically targeted children at particular levels of asthma severity, and inconsistent and low levels of reporting of asthma severity meant that we did not conduct these analyses. We have reported elsewhere other deviations from the protocol.
Results
Description of studies
We have reported the characteristics of all included studies in the Characteristics of included studies section; Table 8 presents an additional summary of how process evaluations met the review inclusion criteria.
7. Included process evaluation studies: methodological characteristics and processes described.
| Study | Type of study | Approach | Process evaluation elements |
| Al‐Sheyab 2012a | Feasibility study | Qualitative | Thematic analyses of student perceptions |
| Berg 2004 | Outcome and process evaluation | Qualitative and quantitative | Thematic analyses of student perceptions |
| Bignall 2015 | Feasibility study | Qualitative and quantitative | Thematic analyses of student perceptions |
| Brasler 2006 | Feasibility/case study of implementation | Quantitative data and trialist reports | Implementation challenges and facilitators identified |
| Bruzzese 2004 | Feasibility study | Qualitative and quantitative | Section evaluating intervention reach, dosage, and student satisfaction |
| Bruzzese 2011 | Outcome evaluation with section on process evaluation | Quantitative | Section evaluating intervention reach (dosage) |
| Bruzzese 2008 | Feasibility study | Qualitative and quantitative | Stand‐alone section on process evaluation results assessing implementation and student perceptions |
| Carpenter 2016 | Outcome and process evaluation | Qualitative and quantitative | Thematic analyses of student perceptions |
| Cicutto 2013 | Outcome and process evaluation | (Mainly) Quantitative | In addition to information on other processes of interest, provided a description of wider school support through policy changes (process of interest included in the logic model) |
| Crane 2014 | Feasibility study | Quantitative | Study was included as it represented an implementation study (through focus on the impact of changing dosage schedule) |
| Dore‐Stites 2007 | Feasibility study | Quantitative | In addition to information on other processes of interest, provided information on student satisfaction |
| Engelke 2013 | Feasibility study | Quantitative | Detailed process/implementation information was provided |
| Gerald 2006 | Outcome and process evaluation | (Mainly) Quantitative | In addition to information on other processes of interest, provided a description of implementation challenges |
| Henry 2004 | Outcome and process evaluation | (Mainly) Quantitative | In addition to information on other processes of interest, provided a description of wider school support through policy changes (process of interest in the logic model) and assessment of sustainability |
| Horner 2015 | Outcome evaluation with process evaluation information | Quantitative | Included detailed information on attrition and cost‐effectiveness |
| Howell 2005 | Outcome and process evaluation | Quantitative | In addition to information on other processes of interest, provided information on student satisfaction |
| Jackson 2006 | Outcome evaluation with process evaluation information | Quantitative | In addition to information on other processes of interest, provided information on student satisfaction |
| Joseph 2010 | Outcome and process evaluation | Quantitative | In addition to information on other processes of interest, provided detailed information on non‐adherence |
| Joseph 2013 | Outcome and process evaluation | Quantitative | Included detailed studies of non‐adherence and relationship with student characteristics |
| Kintner 2012 | Feasibility study | Quantitative | In addition to information on other processes of interest, provided information on student satisfaction |
| Kouba 2012 | Outcome evaluation with process evaluation information | Quantitative | In addition to information on other processes of interest, provided detailed information on dosage (and dose‐response) |
| Langenfeld 2010 | Implementation study | Quantitative | In addition to information on other processes of interest, provided detailed information on dosage (and dose‐response) |
| Lee 2011 | Implementation study | Qualitative | In addition to information on other processes of interest, provided detailed information on instructor experiences |
| Levy 2006 | Outcome evaluation with process evaluation information | Quantitative | In addition to information on other processes of interest, provided information on parental adherence to intervention protocol |
| Magzamen 2008 | Outcome evaluation with process evaluation information | Quantitative | In addition to information on other processes of interest, provided information on attrition |
| McCann 2006 | Outcome evaluation with process evaluation information | Quantitative | In addition to information on other processes of interest, provided information on teacher adherence/school level commitment |
| Mickel 2016 | Outcome and process evaluation | Qualitative and quantitative | Thematic analyses of student perceptions |
| Mujuru 2011 | Outcome and process evaluation | (Mainly) Quantitative | In addition to information on other processes of interest, provided a description of parental satisfaction |
| Pike 2011 | Outcome and process evaluation | (Mainly) Quantitative | In addition to information on other processes of interest, provided information on teacher adherence/school level commitment |
| Richmond 2011 | Outcome and process evaluation | (Mainly) Quantitative | Included detailed information on adherence and awareness |
| Spencer 2000 | Outcome and process evaluation | Quantitative | In addition to information on other processes of interest, provided information on instructor satisfaction and school level commitment |
| Splett 2006 | Outcome and process evaluation | Quantitative | In addition to information on other processes of interest, provided information on adherence and school level commitment |
| Terpstra 2012 | Outcome and process evaluation | Quantitative | In addition to information on other processes of interest, represented an implementation study by including a focus on the impact of parental involvement/increasing parental awareness |
Results of the search
We performed the first search in April 2015, and an updated search in April 2016. We conducted further searches on 25 August 2017. Two members of the review team (KH, DK) conducted the searches for process evaluation studies (see Figure 2). The Cochrane Airways Information Specialist, Liz Stovold, conducted the searches for outcome evaluation studies (see Figure 3). Review team members (KH, DK) performed initial automated checks for duplication using EPPI‐Reviewer software during the data screening and extraction process. After de‐duplication, we (KH, DK) screened 29,384 titles and abstracts of potential process evaluation studies, facilitated by text mining, as well as 350 title and abstracts for eligibility as outcome evaluations. Following application of inclusion criteria to review of titles and abstracts, KH and DK independently assessed the remaining 1066 full‐text process evaluation records and 105 full‐text outcome evaluation records for eligibility for inclusion. We included 54 papers, from 33 different studies, for further analysis as process evaluation studies, and 44 papers, from 33 different studies, for further analysis as outcome evaluation studies.
2.
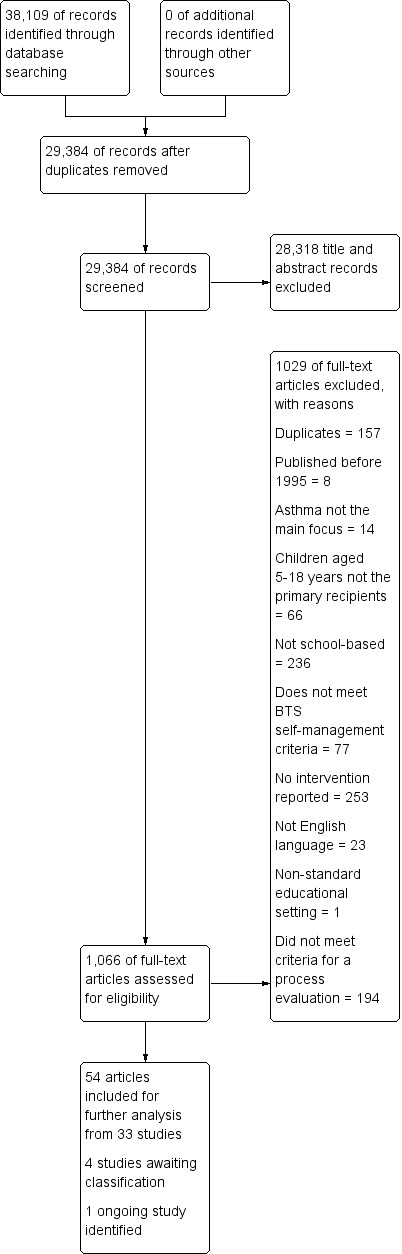
Process evaluation study flow diagram.
3.
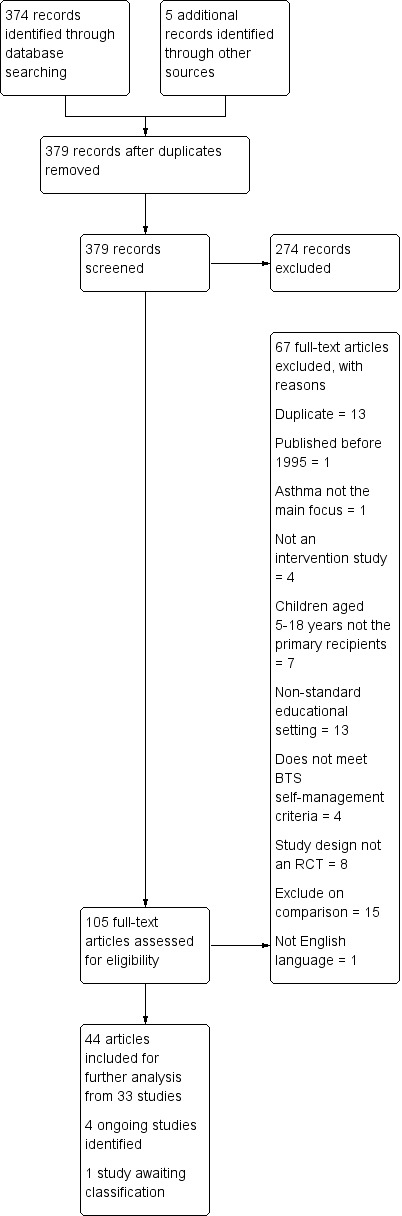
Outcome evaluation study flow diagram.
We identified several potential additional sources as ongoing studies (n = 4; see Characteristics of ongoing studies) and other studies as awaiting classification (n = 5; see Characteristics of studies awaiting classification).
Included studies
We included in the review 33 process evaluation studies and 33 outcome evaluation studies that met the inclusion criteria. We have described the characteristics of process and outcome evaluation studies separately below. We noted little overlap between the 33 studies included in both sets of studies, with Bruzzese 2004,Bruzzese 2008,Bruzzese 2011,Cicutto 2013,Gerald 2006,Henry 2004,Horner 2015,Howell 2005,Levy 2006,McCann 2006, and Splett 2006 (11/33) common to both sets of studies, although Bruzzese 2004 and McCann 2006 did not contribute data to the meta‐analyses.
Characteristics of process evaluation studies
Study population and intervention characteristics
Process evaluations of asthma self‐management interventions in schools reported on a diversity of intervention models. Nine studies included evaluations of the effectiveness of Open Airways for Schools (OAS) (American Lung Association 2018), or modifications to this programme (see Table 9). OAS consists of six 40‐minute sessions, aimed at groups of children aged eight to 11 who learn about different topics including general information about asthma, how to recognise and manage asthma symptoms, and problem‐solving and decision‐making about asthma medication. Authors of process evaluation studies described other intervention models (e.g. PowerBreathing (Berg 2004); Staying Healthy‐Asthma Responsible and Prepared (SHARP; Kintner 2012); Asthma Self‐Management for Adolescents (ASMA; Bruzzese 2004; Bruzzese 2008)), although these were diffuse across studies and were common to no more than two included studies.
8. Process evaluation studies ‐ summary of intervention characteristics.
| Named theoretical framework | Aim | Intervention type | Control | Intensity | Included in QCA | |
| Al‐Sheyab 2012a | Developmental stages (not named) | To assess feasibility in the Jordanian context of a peer‐led, school‐based asthma education programme | Triple A. Children received education through interactive teaching and learning activities | N/A | 14 hours over 6 days | Setting and participants; further modifiable design features; stakeholder involvement and engagement |
| Berg 2004 | Social learning theory | To evaluate effects of the Power Breathing programme and individual coaching sessions on asthma knowledge and functional health status | Power Breathing. Children received education in a group session on asthma management | N/A | 2 weeks | Stakeholder involvement and engagement |
| Bignall 2015 | None | To test the feasibility and preliminary efficacy of a school‐based RCT on breathing retraining for asthma outcomes and anxiety symptoms | Single workshop for children. Children received information on relaxation/breathing techniques | 30 minutes of standard asthma education | 2 face‐to‐face visits 1 month apart | None |
| Brasler 2006 | None | To provide adolescents with knowledge and skills to take control of their asthma; to enhance knowledge and skills of school staff, health professionals, and parents | Power Breathing. Children received basic asthma education and addressed social/lifestyle concerns | N/A | 3× 90‐minute or 6× 45‐minute sessions | None |
| Bruzzese 2004 | Self‐regulation theory | To help students weave asthma and management strategies into their self‐identity | ASMA. Students were taught how to manage their asthma to prevent symptoms and reduced quality of life. Continued medical education was also offered to medical providers | Usual care | 3 workshops 2 or 3 weeks apart for 8 weeks | Stakeholder involvement and engagement |
| Bruzzese 2011 | Social cognitive theory | To test the efficacy of ASMA | ASMA; academic detailing. Students attended workshops to empower them to manage their asthma. Parents received training on how to support their child's need to manage his or her asthma | Usual care | 8‐week programme/3× 45‐minute sessions and individual coaching sessions once a week for 5 weeks | Further modifiable design features |
| Bruzzese 2008 | Social cognitive theory; cognitive‐behavioural therapy | To test the feasibility and short‐term outcomes of asthma: it’s a family affair! | OAS and ASMA; caregiver education. Intervention students received education about asthma, based on existing materials, from coping with asthma at home and at school; OAS and ASMA | Usual care | 6× 75‐minute group sessions once a week for 6 weeks; caregiver 5× 90‐minute sessions once a week | Setting and participants; further modifiable design features; stakeholder involvement and engagement |
| Carpenter 2016 | None | To test whether a tailored inhaler technique video intervention could be feasibly implemented by school nurses; to improve the inhaler technique of children with asthma | Multiple sessions for children. Children watched a tailored video and demonstrated their inhaler technique before and after | N/A | 6 weeks or less | None |
| Cicutto 2013 | Social cognitive theory | To prepare and support children with asthma to be successful managers of their asthma, thereby reducing school absenteeism, interrupted activity, and health service use | Roaring Adventures of Puff. Workshops included goal‐setting and self‐monitoring, trigger identification, control and avoidance, basic pathophysiology, medication use, symptom recognition, and the asthma action plan, using interactive techniques | Usual care | Unclear | Setting and participants |
| Crane 2014 | Educational theory of Jean Piaget | To pilot a shorter, condensed OAS education programme as an alternative, yet still effective, delivery approach compared to the lengthier original programme | OAS. Children received education from OAS | Non‐equivalent intervention | 10 weeks | Setting and participants; further modifiable design features |
| Dore‐Stites 2007 | None | Unclear | OAS; Quest for the Code. Children received a computer game, home activities, and caregiver information | N/A | 20 minutes a week for 8 to 9 weeks | Further modifiable design features |
| Engelke 2013 | Case management theory | To identify the process of case management used by school nurses, and when they provide case management to students with asthma. The second aim was to identify the impact of case management on parent perception of how well the child manages illness; parent perception of how well the child keeps up with school work; quality of life and academic achievement of children | Case management; nurse meetings; multiple sessions for children; multiple sessions for staff. Children received education and counselling, and parent/family education was delivered, as well as education and healthcare co‐ordination for teachers/staff | N/A | Unclear | None |
| Gerald 2006 | None | To evaluate a comprehensive school‐based asthma management programme in an inner city, largely African American school system | OAS. The intervention included 3 educational programmes and medical management for children, as well as education for school staff | Usual care | Unclear | None |
| Henry 2004 | Unclear | To determine whether an asthma education programme in schools would have a direct impact on student knowledge and attitudes toward asthma and quality of life of students with asthma; an indirect impact on teacher knowledge and attitudes on asthma and on school policies about asthma; and a sustainable programme after resources were withdrawn | Asthma education. A package about asthma was taught within the PD/H/PE (Personal Development, Health and Physical Education) strand of the school curriculum | Usual care | Unclear | Setting and participants |
| Horner 2015 | Bruhn’s theoretical model of asthma self‐management | To test effects of 2 modes of delivering an asthma educational intervention on health outcomes and asthma management | 7‐topic curriculum. The intervention was designed for children in rural areas and included asthma information | In‐school asthma classes | 16× 15‐minute sessions for 5 weeks | None |
| Howell 2005 | Learning theory and behaviour modification | To examine whether it was feasible to implement an interactive computer game at school health centres. Second, to examine whether exposure to the game was effective in increasing asthma knowledge, reducing asthma symptoms, and reducing unnecessary healthcare use compared with no exposure to the game | Quest for the Code. Computer game | Usual care | 4× 30‐minute sessions | None |
| Jackson 2006 | None | To evaluate knowledge and attitude outcomes of an educational asthma programme for third grade children with and without asthma | Single sessions for children. Children completed an educational programme. Teachers were also encouraged to attend | N/A | 3 classes per session for 11 sessions | None |
| Joseph 2010 | None | To develop and evaluate a multi‐media, web‐based asthma management programme | Puff City. A web‐based programme was delivered to children to focus on adherence, inhaler availability, and smoking cessation/reduction | Generic asthma websites | Unclear | Further modifiable design features; stakeholder involvement and engagement |
| Joseph 2013 | Behavioural theory | To evaluate a school‐based RCT to evaluate Puff City | Adapted version of the Puff City computer programme | Generic asthma education | 4× 15‐minute sessions | None |
| Kintner 2012 | Lifespan development perspective | To evaluate the feasibility of the SHARP programme for students, their family, school personnel, and community partners | SHARP; Community Coalition component | N/A | Once a week for 10 weeks plus a 3‐hour community component | Setting and participants; further modifiable design features; stakeholder involvement and engagement |
| Kouba 2012 | Orem’s self‐care deficit theory | To determine the effectiveness of the ICAN programme for nutrition knowledge and dietary behaviours | Single workshop for staff; multiple sessions for children; Quest for the Code; Fight Asthma Now; additional nurse meetings; combined education | N/A | 8 weeks | None |
| Langenfeld 2010 | None | Unclear | OAS; case management; stand‐alone respiratory therapy. Children received the OAS curriculum and case management asthma strategies developed with teachers | N/A | 6× 40‐minute sessions for 1 school year | None |
| Lee 2011 | The functional context approach | To evaluate the effectiveness and feasibility of using undergraduate nursing students as facilitators to deliver an asthma management programme | OAS. Children received the OAS curriculum | N/A | Unclear | Further modifiable design features |
| Levy 2006 | None | To evaluate the effectiveness of a school‐based nurse case management approach to asthma in students with poor control | OAS; monitoring of students; health status. Students received OAS education and weekly monitoring of their health status | Usual care | 1 school term | None |
| Magzamen 2008 | None | To evaluate the implementation of Kickin’ Asthma | Multiple sessions for children; Kickin’ Asthma. Educational sessions, similar to the OAS curriculum. Customised letters were also sent home to describe health needs and goals for each child | N/A | 3 months | None |
| McCann 2006 | None | To assess whether a school‐based intervention would produce clinical and psychological benefits for children with asthma | Education; role‐play. The intervention focused on describing the respiratory condition through a role‐play | Respiratory education | 45‐minute session | None |
| Mickel 2016 | None | To provide Iggy education to more than 75% of children with asthma; To increase asthma knowledge; increase families’ awareness of asthma; and cultivate collaboration between school nurses and asthma providers | Iggy and the Inhalers intervention. Children received an asthma education video, poster, comic book, sticker, and trading card programme | N/A | Unclear | None |
| Mujuru 2011 | None | To demonstrate the feasibility of a school‐based asthma education programme for students and to evaluate parents’ perspectives on the intervention | OAS. Children received the OAS curriculum | N/A | 40‐minute session once a week for 2 months | None |
| Pike 2011 | None | To assess student asthma knowledge gain, teacher acceptance, and grade appropriateness after an intervention | Multiple sessions for children; integrated into the curriculum. Teachers taught lessons with information about asthma | Usual care | 7 lesson plans | Setting and participants |
| Richmond 2011 | None | To increase the number of current provider‐written asthma action plans submitted to the school nurse at the beginning of the school year | Breathe Your Best. Students were encouraged to receive an asthma action plan from their doctor and to collect their prescriptions | N/A | Unclear | None |
| Spencer 2000 | None | To evaluate the OAS programme for children | OAS. Children received the OAS curriculum | N/A | 6× 40‐minute sessions | None |
| Splett 2006 | None | To evaluate the effectiveness and sustainability of the Healthy Learners Asthma Initiative | Children received training on asthma self‐management. Licensed nurses and healthcare assistants received coaching and reinforcement from asthma resource nurses | Usual care | Varied according to asthma severity and need | None |
| Terpstra 2012 | Social cognitive theory | To test a version of an intervention with a caregiver newsletter vs no newsletter | Multiple sessions for children; materials for parents. Children received skills training on how to use a peak flow meter. Parents received a newsletter about an important theme from the research | Intervention or intervention with a newsletter | 6‐week training | Setting and participants; further modifiable design features |
ASMA: Asthma Self‐Management for Adolescents.
ICAN: I Can Control Asthma and Nutrition Now.
N/A: not applicable.
OAS: Open Airways for Schools.
RCT: randomised controlled trial.
SHARP: Staying Healthy–Asthma Responsible & Prepared.
Triple A: Adolescent Asthma Action.
Across all studies, investigators taught a diverse curriculum. Although most studies mentioned that the intervention involved developing knowledge and skills around asthma physiology and monitoring and treatment of symptoms, fewer included studies explicitly mentioned that investigators aimed to develop alliances between children/parents and their care provider(s) (Dore‐Stites 2007; Gerald 2006; Richmond 2011; Terpstra 2012), and a greater number did involve parents in the intervention in other ways. Most interventions were reliant on trialists, research staff, and others from outside schools to deliver the intervention, although some interventions were primarily delivered, or supported pivotally, by school nurses (Engelke 2013; Langenfeld 2010; Levy 2006; Magzamen 2008; Splett 2006), teachers (Henry 2004; Mujuru 2011; Pike 2011), or children's peers (Magzamen 2008).
Several studies explicitly drew on social cognitive theory (Bruzzese 2008; Bruzzese 2011; Cicutto 2013; Terpstra 2012). Two studies from the same research team drew upon the Health Belief Model (Joseph 2010; Joseph 2013). Other theoretical models featured in only a single study included self‐regulation theory (Bruzzese 2004), learning or social learning theory (Berg 2004; Howell 2005), Piaget's pedagogical theory (Crane 2014), Orem's self‐care deficit theory (Kouba 2012), attribution theory (Joseph 2013), miscellaneous theoretical concepts that contributed to a theoretical framework (Al‐Sheyab 2012a), biopsychosocial theory (Dore‐Stites 2007), a transtheoretical model (Joseph 2010), and a functional context model (Lee 2011). A small minority of studies named a theoretical framework that was specific to asthma, with Horner 2015 employing Bruhn's theoretical model of asthma self‐management to underpin an intervention (Bruhn 1983), and Kintner 2012 drawing upon an asthma acceptance model (alongside a life course development perspective). These theoretical frameworks also differed in their use and in whether they supported the premise and emphasis of the intervention in a holistic manner, or whether they supported a particular pedagogical technique that was favoured in delivery of the intervention; this distinction was not clear in some studies. Few studies presented a clear logic model or theory of change to describe the underlying conceptual framework (Kneale 2015).
Five studies evaluated implementation of interventions involving delivery of self‐management education in part or mainly through electronic games or training provided through computers (Dore‐Stites 2007; Howell 2005; Joseph 2010; Joseph 2013; Kouba 2012). In two of these interventions (Joseph 2010; Joseph 2013), the information provided was tailored to children based on their input. In total, nine interventions had components that tailored content towards the needs of an individual child through delivery on a one‐to‐one basis or through delivery of personalised content (Bruzzese 2004; Bruzzese 2008; Howell 2005; Joseph 2010; Joseph 2013; Langenfeld 2010; Spencer 2000; Splett 2006).
Most studies took place in the USA (29/33 studies); several of these US‐based studies explicitly mentioned that the intervention took place in an urban or inner city area, or explicitly made reference to the diverse socio‐economic or ethnic background of participants (Berg 2004; Bignall 2015; Brasler 2006; Bruzzese 2004; Bruzzese 2010; Bruzzese 2011; Gerald 2006; Joseph 2010; Joseph 2013; Kouba 2012; Levy 2006; Magzamen 2008; Mickel 2016; Pike 2011; Richmond 2011; Splett 2006); in contrast, just two studies specifically explored implementation in rural areas (Horner 2015; Mujuru 2011). Fewer studies took place in high schools (14 studies) than in junior, middle, or elementary/primary schools (see Table 10).
9. Process evaluation studies ‐ summary of study design, setting, and population.
| Study design | Number of children | Country | Type of School | Recipients | Age of children (years) | Representation of children from BME backgrounds | Representation of children from low SES backgrounds | |
| Al‐Sheyab 2012a | Case study | 31 | Jordan | High | Children | 11 to 18 | Unclear | Unclear |
| Berg 2004 | Quasi‐experimental | 13 | USA | High | Children | 15 to 18 | 46.2% African American | Unclear |
| Bignall 2015 | Parallel‐group RCT | 33 | USA | High | Children | 11 to 18 | 100% Black or African American | Unclear |
| Brasler 2006 | Case study | 342 | USA | Junior/middle | Children; teachers; parents | 11 to 14 | Unclear | Unclear |
| Bruzzese 2004 | Parallel‐group RCT | 45 | USA | High | Children | 11 to 18 | Unclear | Unclear |
| Bruzzese 2011 | Parallel‐group RCT | 345 | USA | High | Children | 11 to 18 | 45.5% Hispanic; 37.7% African American; 11.6% mixed; 5.2% other | 75% free school meals |
| Bruzzese 2008 | Parallel‐group RCT | 24 | USA | Junior/middle | Children; parents | 11 to 14 | 41% Hispanic; 17% White; 8% African American; 34% other | 8% unemployed; 21% part‐time employment; 71% full‐time employment |
| Carpenter 2016 | Quasi‐experimental | 25 | USA | All school types | Children; nurses | Unclear | 72% White; 12% Hispanic; 8% African American; 8% Black | Unclear |
| Cicutto 2013 | Cluster RCT | 1316 | Canada | Primary/elementary | Children; school board; head teacher; teachers; peers | 5 to 10 | Unclear | 25% to 50% deprived |
| Crane 2014 | Quasi‐experimental | 45 | USA | Primary/elementary | Children | 5 to 10 | Unclear | Unclear |
| Dore‐Stites 2007 | Quasi‐experimental | 32 | USA | Primary/elementary | Children; parents | 5 to 10 | 39% African American; 28.6% Caucasian; 14.3% Hispanic; 18% biracial | 34.6% < $20,000; 53.8% $21,000 to $40,000 |
| Engelke 2013 | Quasi‐experimental | 143 | USA | All school types | Children; teachers; parents; nurses | Unclear | 40.6% Caucasian; 37.8% African American; 7% Latino; 14% other | 63.6% on Medicaid |
| Gerald 2006 | Cluster RCT | 736 | USA | Primary/elementary | Children; teachers | 5 to 10 | 97% African American | Unclear |
| Henry 2004 | Cluster RCT | 4161 | Australia | High | Children; teachers | 11 to 14 | Predominantly Caucasian | Unclear |
| Horner 2015 | Cluster RCT | 292 | USA | Primary/elementary | Children | 5 to 10 | 21.2% African American; 25% Spanish speaking | 30.7% low SES |
| Howell 2005 | Cluster RCT | 24 | USA | Primary/elementary | Children; parents | 5 to 10 | 75% African American | Unclear |
| Jackson 2006 | Quasi‐experimental | 943 | USA | Primary/elementary | Children | 5 to 10 | Unclear | Unclear |
| Joseph 2010 | Parallel‐group RCT | 314 | USA | High | Children | 11 to 18 | Unclear | 52% eligible for free school meals |
| Joseph 2013 | Parallel‐group RCT | 422 | USA | High | Children | 11 to 18 | 98% African American | 73% on Medicaid |
| Kintner 2012 | Quasi‐experimental | 28 | USA | High | Children; peers; families; teachers | 11 to 14 | 53.6% African American; 32.1% White; 3.6% American; 10.7% biracial | 35.7% low SES; 42.9% low middle SES; 17.8% upper middle SES; 3.6% high SES |
| Kouba 2012 | Quasi‐experimental | 25 | USA | High | Children | 11 to 18 | 92% African American; 4% Hispanic; 4% mixed | 25% to 50% deprived |
| Langenfeld 2010 | Quasi‐experimental | 286 | USA | Primary/elementary | Children; teachers | 5 to 10 | 63% African American; 23.9% Hispanic; 6.4% White; 2.6% Asian | High percentage on free school meals |
| Lee 2011 | Quasi‐experimental | 827 | USA | Primary/elementary | Children | 5 to 10 | Unclear | Unclear |
| Levy 2006 | Cluster RCT | 243 | USA | Primary/elementary | Children; teachers | 5 to 10 | 97% African American | 80% on Medicaid |
| Magzamen 2008 | Quasi‐experimental | 845 | USA | High; junior/middle | Children | 11 to 18 | Unclear | Unclear |
| McCann 2006 | Parallel‐group RCT | 219 | UK | Primary/elementary | Children; teachers | 5 to 10 | Unclear | < 25% deprived |
| Mickel 2016 | Quasi‐experimental | 173 | USA | Primary/elementary | Children | 5 to 10 | 63.6% African American; 13.3% Hispanic; 20.2% White | > 50% deprived |
| Mujuru 2011 | Quasi‐experimental | 18 | USA | Primary/elementary | Children; parents | 5 to 10 | Unclear | 39% Medicaid |
| Pike 2011 | Quasi‐experimental | 236 | USA | Primary/elementary | Children; teachers | 5 to 10 | 75% African American (approx.) | 80% free school meals (approx.) |
| Richmond 2011 | Narrative | Unclear | USA | Primary/elementary | Children | 5 to 10 | 100% African American | 80% free school meals |
| Spencer 2000 | Quasi‐experimental | 369 | USA | Primary/elementary | Children; parents | 5 to 14 | Unclear | 34% free school meals |
| Splett 2006 | Cluster RCT | 1561 | USA | All school types | Children; school staff | Unclear | 66% African American; 6% Hispanic; 5% American Indian; 3% Asian; 20% White | 73% free school meals |
| Terpstra 2012 | Quasi‐experimental | 58 | USA | Junior/middle | Children; parents | 11 to 14 | > 50% BME | > 50% deprived |
BME: black and minority ethnicity.
RCT: randomised controlled trial.
Time of assessment of process outcome measurements
Twenty‐one process evaluation studies collected pre‐ and post‐hoc data. Four studies collected post‐hoc data only (Al‐Sheyab 2012a; Berg 2004; Bruzzese 2004; Richmond 2011). Several studies collected data immediately after the intervention or within three months of cessation of the intervention (Bignall 2015; Bruzzese 2004; Bruzzese 2008; Carpenter 2016; Crane 2014; Gerald 2006; Howell 2005; Jackson 2006; Kintner 2012; Kouba 2012; Magzamen 2008; Mickel 2016; Mujuru 2011; Pike 2011; Spencer 2000; Splett 2006). The longest follow‐up data collection period lasted for 12 months post testing (Bruzzese 2011; Cicutto 2013; Horner 2015; Joseph 2010; Joseph 2013; McCann 2006). In a small number of studies, the follow‐up duration was unclear (Al‐Sheyab 2012a; Dore‐Stites 2007; Engelke 2013; Langenfeld 2010; Levy 2006; Richmond 2011; Terpstra 2012).
Measurement of process outcomes
We included 33 process evaluation studies, most of which adopted a quantitative approach to analyses. Process evaluation elements across these studies included thematic analysis of student perceptions, identification of implementation challenges and facilitators, reach of the intervention, and student satisfaction. We have provided further details of inclusion criteria and process evaluation elements for all process evaluation studies in Table 8 The descriptions below refer to all studies included as process evaluation studies, although we included in QCAs only those that we deemed to be of moderate or high intensity (see section on reduction of cases). Similarly, we transformed the data and ratings described below using direct and indirect transformations (see earlier methods).
Attrition
A total of 18 studies provided evidence that attrition was low. Five studies showed substantial attrition (Bruzzese 2004; Gerald 2006; Levy 2006; Magzamen 2008; Richmond 2011), with levels of attrition exceeding 20% and/or reported by trial authors as a substantial challenge.
Adherence to the intervention
A total of 21 studies reported child adherence. 'Child adherence' broadly referred to the extent to which children followed directions of the intervention, for example, in completing homework assignments, undertaking and completing intervention modules, or completing evaluation instruments. Fourteen studies presented evidence that child adherence with the intervention was good. Six studies highlighted evidence that adherence was not problematic among other stakeholders (Bruzzese 2011; Cicutto 2013; Jackson 2006; Joseph 2013; Kintner 2012; Splett 2006). Child adherence was problematic in eight studies (Brasler 2006; Gerald 2006; Howell 2005; Joseph 2010; Kouba 2012; Magzamen 2008; Richmond 2011; Spencer 2000); these judgements were based on reports from trialists and on reports of completion rates of intervention modules and/or completion of evaluation instruments.
Dosage of intervention received
'Dosage' broadly referred to the extent to which children received the intervention as intended, for example, in attending the expected number of sessions. This differed from attrition, in that children could have received a low dosage but may have not permanently dropped out; this also differed from adherence, in that children could have received a low dosage but were otherwise adherent. Participants received the intended dose of the intervention in nine studies (Bignall 2015; Bruzzese 2011; Jackson 2006; Joseph 2013; Kintner 2012; McCann 2006; Mickel 2016; Pike 2011; Terpstra 2012). In one study, researchers noted a dose‐response relationship (Kouba 2012). Seven studies reported that the intended dose was not achieved (Brasler 2006; Bruzzese 2008; Gerald 2006; Howell 2005; Joseph 2010; Langenfeld 2010; Magzamen 2008), with substantial numbers not receiving the intended intervention. In one study (Gerald 2006), this finding was based on reports of shortening of sessions. In another study, in which parental involvement was an integral component, study authors reported additional problems with dosage received (Bruzzese 2008). One study comparing an individualised intervention model versus a generic intervention model reported that the individualised model had higher levels of dosage, although both models showed relatively low levels of completion of all modules (Joseph 2010).
Combined indicator of 'successful' implementation
We combined data from process evaluation studies on attrition, adherence, and dosage into a single indicator. We summed scores across the three indicators and calibrated them to fall between zero and one, with 0.5 the point of maximum ambiguity and values over 0.5 indicating partial membership of the successful implementation set, up to a maximum possible value of one, which indicated full membership of the successful implementation set, values under 0.5 indicating more out of than in the set, and a value of 0 indicating full non‐membership of the successful implementation set. Eight studies were either fully or strongly within the successful implementation set (Al‐Sheyab 2012a; Berg 2004; Bruzzese 2008; Bruzzese 2011; Henry 2004; Joseph 2013; Kintner 2012; Terpstra 2012), and another five studies had scores that were mainly within the successful implementation set (Cicutto 2013; Dore‐Stites 2007; Horner 2015; Mujuru 2011; Pike 2011). A further 14 studies provided scores that were ambiguous or low implementation scores (Brasler 2006; Bruzzese 2004; Crane 2014; Engelke 2013; Howell 2005; Gerald 2006; Joseph 2010; Kouba 2012; Langenfeld 2010; Lee 2011; Levy 2006; Magzamen 2008; Spencer 2000; Splett 2006).
Characteristics of outcome evaluation studies (RCTs)
We have included in Table 11 further details of studies that met the criteria for study design, but from which we did not include data in the meta‐analysis.
10. Outcome evaluation studies not included in the analyses.
| Study included as outcome | Reason data not included in quantitative analysis |
| Bruzzese 2004 | Feasibility study uses randomised controlled trial (RCT) design with no quantitative data presented |
| Bruzzese 2010 | Abstract only located and outcomes were not presented in an extractable format |
| Clark 2004 | Published effect sizes that were extractable but of a different effect size from other studies |
| Clark 2010 | No outcome measured in the study matched the review protocol |
| McCann 2006 | Outcomes were not presented in an extractable format (disaggregated data for asthmatic children unavailable) |
| Monforte 2012 | Abstract only located and outcomes were not presented in an extractable format |
| Mosnaim 2011 | No outcome measured in the study matched the review protocol |
| Praena‐Crespo 2010 | Abstract only located and outcomes were not presented in an extractable format |
| Pulcini 2007 | No outcome measured in the study matched the review protocol |
| Srof 2012 | Outcomes were not presented in an extractable format (data on overall quality of life were not presented in full; only subdomains of quality of life are available) |
Study population and intervention characteristics
Most studies took place in the USA (22/33 studies), with fewer taking place in high schools (eight studies) than in junior, middle, or elementary/primary schools (see Table 12). Study reports showed substantial variation in the types of interventions that were trialled, although nine studies included evaluations of the effectiveness of Open Airways for School, or modifications to this programme (see Table 13). Study reports also showed substantial variety in the ways in which asthma self‐management interventions were delivered. Children received long programmes of sessions in some interventions, with 16 sessions delivered in two studies (Horner 2008; Horner 2015), and 10 sessions and eight sessions delivered in others (Kintner 2009; Patterson 2005, respectively). In contrast, researchers delivered three interventions in a single group session to children (Gerald 2006; Howell 2005; McCann 2006), although these interventions were supported by other activities including nurse visits or staff training. The number of sessions was not always commensurate with the quantity of content delivered however; for example, the intervention delivered in Atherly 2009 amounted to 4.5 hours of instruction over three sessions, and Horner 2015 delivered 4 hours of content over 16 sessions.
11. Outcome evaluation studies ‐ summary of study design, setting, and population.
| Study design | Number of children | Country | Type of school | Recipients |
Age of children (years) |
Representation of children from BME backgrounds | Representation of children from low SES backgrounds | |
| Al‐Sheyab 2012 | Clustered parallel RCT | 261 | Jordan | 4 public high schools | Children | 11 to 15 | Unclear | Unclear |
| Atherly 2009 | Clustered parallel RCT | 524 | USA | Junior and high schools | Children | 11 to 15 | Unclear | Unclear |
| Bartholomew 2006 | Clustered parallel RCT | 948 | USA | Elementary schools | Children; care providers; parents/carers | 5 to 10 | 45% African American; 51% Hispanic; 3% Caucasian | Deprived individuals > 50% |
| Bruzzese 2004 | RCT | 45 | USA | 2 public high schools | Children | Unclear | Unclear | Unclear |
| Bruzzese 2008 | Clustered parallel RCT | 24 | USA | 1 middle school | Children; caregivers | 11 to 15 | 41% Hispanic; 17% African American | 71% parents full‐time employment |
| Bruzzese 2010 | Clustered parallel RCT | Unclear | USA | 25 public schools | Children; caregivers | Mean age, 12.8 | Unclear | Unclear |
| Bruzzese 2011 | Clustered parallel RCT | 340 | USA | 5 high schools | Children | Mean age, 15 | > 80% BME | Unclear |
| Cicutto 2005 | Clustered parallel RCT | 256 | Canada | 26 elementary schools | Children | 5 to 10 | Unclear | Average household income $53,000 |
| Cicutto 2013 | Clustered RCT | 1316 | Canada | 170 primary/elementary schools | Children; families | 5 to 10 | Unclear | Deprived individuals 25% to 50% |
| Clark 2004 | Clustered parallel RCT | 835 | USA | 14 public high schools | Children; parents/carers; classmates; school personnel | 5 to 10 | 98% African American | 45% annual income < $15,000 |
| Clark 2005 | Clustered parallel RCT | 639 | China | 21 elementary schools | Children | 7 to 11 | Unclear | Unclear |
| Clark 2010 | Clustered parallel RCT | 1292 | USA | 19 middle schools | Children | Mean age, 11.6 | 93% African American | 48% annual income < $15,000 |
| Gerald 2006 | Parallel‐group RCT | 736 | USA | 54 elementary schools | Children | Mean age, 11 | 97% Black | Unclear |
| Gerald 2009 | Parallel‐group RCT | 290 | USA | Unclear | Children | 5 to 10 | 91% Black | Unclear |
| Henry 2004 | Clustered parallel RCT | Unclear | Australia | Secondary schools | Children | 11 to 15 | < 50% BME | Unclear |
| Horner 2008 | Clustered parallel RCT | 183 | USA | 18 elementary schools | Children | 5 to 10 | 47% Hispanic; 30% White; 22% African American | Unclear |
| Horner 2015 | Clustered parallel RCT | 196 | USA | 3 elementary schools | Children | 5 to 10 | > 50% BME | Deprived individuals 25% to 50% |
| Howell 2005 | Clustered parallel RCT | 25 | USA | 4 elementary schools | Children; families | 5 to 10 | 75% African American | Unclear |
| Kintner 2009 | Clustered parallel RCT | 59 | USA | 5 schools | Children | 9 to 12 | 30% Black; 36% White; 18% biracial | Deprived individuals 25% to 50% |
| Levy 2006 | Clustered parallel RCT | 243 | USA | 14 elementary schools | Children | 5 to 10 | 98% African American | 85% TennCare |
| McCann 2006 | Clustered parallel RCT | 229 | England | 24 primary/junior schools | Children; parents | 5 to 10 | Unclear | Deprived individuals < 25% |
| McGhan 2003 | Clustered parallel RCT | 162 | Canada | 18 elementary schools | Children | 5 to 10 | < 50% BME | Deprived individuals 25% to 50% |
| McGhan 2010 | Clustered parallel RCT | 206 | Canada | Elementary schools | Children; parents/carers; teachers | Mean age, 8.6 | Unclear | Unclear |
| Monforte 2012 | Clustered parallel RCT | Unclear | USA | 8 elementary schools | Children | 5 to 10 | Unclear | Unclear |
| Mosnaim 2011 | Clustered parallel RCT | 344 youth; 192 teens | USA | Elementary schools | Children | Median age 10 | > 50% BME | Deprived individuals > 50% |
| Patterson 2005 | Clustered parallel RCT | 175 | Ireland | Primary schools | Children | 7 to 11 | Unclear | Deprived individuals 25% to 50% |
| Persaud 1996 | Parallel‐group RCT | 36 | USA | 10 schools | Children | Mean age, 10.2 | 69% African American | 69% received Medicaid |
| Praena‐Crespo 2010 | Clustered parallel RCT | 279 | Spain | 16 high schools | Children | 11 to 15 | Unclear | Unclear |
| Pulcini 2007 | Clustered parallel RCT | 40 | USA | Middle schools | Children | 11 to 15 | Unclear | Unclear |
| Shah 2001 | Clustered parallel RCT | 272 | Australia | High schools | Children | 11 to 15 | Unclear | Unclear |
| Splett 2006 | Clustered parallel RCT | 1561 | USA | K‐8 schools | Children | 5 to 15 | 66% African American | 73% free school meals |
| Srof 2012 | Parallel group RCT | 39 | USA | High schools | Children | 14 to 18 | Unclear | Unclear |
| Velsor‐Friedrich 2005 | Clustered parallel RCT | 52 | USA | 4 elementary schools | Children | Mean age, 10.1 | 100% African American | Unclear |
BME: black and minority ethnicity.
RCT: randomised controlled trial.
12. Outcome evaluation studies ‐ summary of intervention characteristics.
| Named theoretical framework | Aim | Intervention type | Control | Intensity | Outcomes Included in meta‐analysis | |
| Al‐Sheyab 2012 | Self‐efficacy | To test the impact of the Triple A programme on health‐related outcomes in high school students | Triple A. Bilingual health workers trained peer leaders from year 11 to deliver 3 Triple A lessons | Unclear | 3× lessons | HRQoL |
| Atherly 2009 | None | To describe an analysis and results of the cost‐effectiveness of the Power Breathing programme | Power Breathing. This intervention focussed on education about asthma, asthma control strategies, and psychosocial concerns | Unclear | 3× 90‐minute lessons | Hospitalisations; ED visits; Experience of daytime and night‐time symptoms |
| Bartholomew 2006 | Social cognitive theory | To describe the evaluation of a school‐based intervention to improve asthma self‐management, medical care, the school environment, symptoms, and the functional status of children | Multi‐component intervention involving direct delivery to children, care providers, and parents/guardians. Children received education through the Watch, Discover, Think and Act interactive computer programme | Unclear | Unclear | Withdrawal |
| Bruzzese 2004 | None | Unclear | ASMA. Continued medical education was also offered to medical providers | Usual care | 3× lessons | None |
| Bruzzese 2008 | Social cognitive theory; cognitive‐behavioural theory | To describe asthma: it’s a family affair; to present feasibility and preliminary outcome data from a pilot RCT | Elements of OAS and ASMA were provided to students; caregivers also received education | Usual care | 6× lessons | Experience of daytime and night‐time symptoms; Withdrawal |
| Bruzzese 2010 | None | To test the efficacy of an RCT: it’s a family affair, a school‐based, family‐focussed intervention to improve asthma outcomes in pre‐adolescents | ASMA and academic detailing. Students received workshops to empower them to manage their asthma. Parents received training to support their child’s need to manage their asthma | Unclear | Children: 6× lessons; caregivers: 5× lessons | Withdrawal |
| Bruzzese 2011 | Social cognitive theory | Unclear | ASMA. Students received group sessions and individual tailored coaching sessions, delivered by trained health educators | Wait‐list control | 3× group sessions; individual coaching sessions | Hospitalisations; ED visits; School absence; Restricted activity days; Unplanned GP or hospital visits; Experience of daytime and night‐time symptoms; Use of corticosteroids; Withdrawal |
| Cicutto 2005 | Social cognitive theory; self‐regulation theory | To evaluate an asthma education programme for children with asthma | Roaring Adventures of Puff. Children received group sessions on asthma and goal‐setting | Usual care | 6× lessons | Hospitalisations; ED visits; School absence; Restricted activity days |
| Cicutto 2013 | Social cognitive theory | To implement an elementary school‐based asthma self‐management education programme for children with asthma; to work with schools to create an asthma‐friendly supportive school environment; to evaluate the programme | Roaring Adventures of Puff. Children received group sessions on asthma and goal‐setting | Usual care | 6× lessons | ED visits; School absence; Restricted activity days; Unplanned GP or hospital visit; HRQoL; Withdrawal |
| Clark 2004 | None | To assess the impact of a comprehensive school‐based asthma programme | OAS; control strategies for schools | Wait‐list control | 6× lessons and 2× classroom sessions | School absence |
| Clark 2005 | Social cognitive theory | To assess effectiveness in children in China of an asthma education programme adapted from a model developed in the USA | OAS; intervention directed at children only | Unclear | 5× lessons | Hospitalisations; ED visits |
| Clark 2010 | None | To assess self‐management and self‐management plus peer involvement | OAS; peer component. In the first treatment arm, an adapted form of OAS was delivered to children. In the second treatment arm, a peer education component was added | Usual care | 6× lessons | Experience of daytime and night‐time symptoms |
| Gerald 2006 | None | Unclear | OAS. The intervention included educational programmes and medical management for children, as well as education for school staff | Usual care | 6× lessons | Hospitalisations; ED visits; School absence |
| Gerald 2009 | None | To determine the effectiveness of school‐based supervised asthma therapy in improving asthma control | Children received asthma education, including a discussion of trigger avoidance (not manualised) | Usual care | 1× lesson; multiple supervisions | School absence; Lung function; Use of reliever therapies; Withdrawal |
| Henry 2004 | None | To determine whether an asthma education programme in schools would have a direct impact on student knowledge and attitudes on asthma and an indirect impact on teacher knowledge and attitudes | Asthma education. A package about asthma was taught within the PD/H/PE strand of the school curriculum | Usual care | 3× lessons | HRQoL |
| Horner 2008 | Asthma health education model | To examine changes in rural children’s asthma self‐management after they received classes, but before they received the family education session | Asthma self‐management. The curriculum included a 7‐step asthma self‐management plan | Health promotion education | 16× lessons | Hospitalisations; Withdrawal |
| Horner 2015 | Bruhn’s theoretical model of asthma self‐management | To test effects of 2 modes of delivering an asthma educational intervention on health outcomes and asthma self‐management in school‐aged children living in rural areas | 7‐topic curriculum. The intervention was designed for children in rural areas and included asthma information | Health promotion education | 16× lessons | Hospitalisations; ED visits; Withdrawal |
| Howell 2005 | Social learning theory | To examine the feasibility of an interactive computer game in school‐based health centres; to test whether exposure to the game was effective in improving knowledge and reducing symptoms and healthcare use | Quest for the Code computer game. The caregiver also participated in medication interviews and received a home visit | Usual care | 30‐minute session | ED visits; Experience of daytime and night‐time symptoms; HRQoL; School absence; Corticosteroid dosage |
| Kintner 2009 | Lifespan development perspective | To evaluate the preliminary efficacy of SHARP | SHARP. Students worked through the SHARP curriculum. Caregivers also received a 3‐hour information sharing programme | Usual care | 10× lessons | HRQoL; Withdrawal |
| Levy 2006 | None | To evaluate the effectiveness of a school‐based nurse case management approach to asthma in students with poor control | OAS; monitoring of students; health status. Students received OAS education and weekly monitoring of their health status | Usual care | Weekly group sessions and weekly individual sessions | Hospitalisations; ED visits; Withdrawal |
| McCann 2006 | None | To assess whether schools are an appropriate context for an intervention designed to produce clinical and psychological benefits for children with asthma | Education; role‐play. The intervention focussed on describing the respiratory condition through a role‐play | Education about the respiratory system | 1× workshop | None |
| McGhan 2003 | Social cognitive theory | To determine whether an interactive childhood asthma education programme improved asthma management behaviours, health status, and quality of life in elementary school children | Roaring Adventures of Puff. Children received education on asthma in a group setting. Parents and teachers were invited to participate in a school‐based asthma awareness event | Usual care | 6× lessons | ED visits; School absence; Unplanned GP or hospital visit; Experience of daytime and night‐time symptoms; Withdrawal |
| McGhan 2010 | Social cognitive theory; self‐regulation theory | To assess the feasibility and impact of the Roaring Adventures of Puff programme | Roaring Adventures of Puff delivered to children. Parents and teachers participated in an asthma awareness event. | Usual care | 6× lessons | ED visits; School absence; Unplanned GP or hospital visit; Experience of daytime and night‐time symptoms; Withdrawal |
| Monforte 2012 | None | To evaluate the implementation of OAS | OAS. No further information was given | Unclear | Unclear | HRQoL |
| Mosnaim 2011 | None | To assess the impact of the Fight Asthma Now educational programme among 2 populations of predominantly low‐income minority students | One‐to‐one training on spacer technique, peak flow meter use, and use of an asthma action plan. Teens also received education on tobacco avoidance and peer pressure | Usual care | 4× sessions | None |
| Patterson 2005 | PRECEDE model | To evaluate the effectiveness of a programme of asthma clubs in improving quality of life for primary school children with asthma | SCAMP. Children used a workbook during sessions to learn about asthma | Wait‐list control | 8× sessions | Restricted activity days; Lung function; HRQoL; Withdrawal |
| Persaud 1996 | None | To assess the effectiveness of an intervention on knowledge, locus of control, attitudes towards asthma, functional status, school attendance, and ED visits | Individualised education sessions. Children had a personal peak flow meter in the school health office. The school nurse also reviewed the student asthma diary and discussed this with them | Usual care | 3× lessons and weekly education sessions | ED visits; School absence |
| Praena‐Crespo 2010 | None | To verify whether an asthma education program in schools would have direct benefit for student knowledge and attitudes towards asthma and quality of life for students with asthma | Asthma programme. No further information was given (abstract only) | Unclear | 3× lessons | None |
| Pulcini 2007 | None | To determine the effectiveness of an intervention to increase the number of AAPs in schools | Peak flow education. Children were given a peak flow meter and were educated on the correct technique to measure lung function | Unclear | Daily for 2 weeks | None |
| Shah 2001 | None | To determine the effects of a peer‐led programme for asthma education on quality of life and related morbidity in adolescents with asthma | Triple‐A: asthma education and empowerment. Students learnt how to educate their peers about asthma. Peers also led 3 health lessons for classes in school | Wait‐list control | 3× sessions | Experience of daytime and night‐time symptoms; Lung function; HRQoL; Withdrawal |
| Splett 2006 | None | To improve asthma management among school children and reduce asthma‐related school absences, hospitalisations, and ED visits | Children received training on managing their asthma. Licensed nurses and healthcare assistants received coaching and reinforcement from asthma resource nurses | Usual care | Unclear | School absence; Unplanned GP or hospital visit |
| Srof 2012 | Health promotion model | To determine effects of coping skills on asthma self‐efficacy, social support, quality of life, and peak flow among adolescents | Asthma diary; 5× coping skills sessions. Students received coping skills training and completed diary entries | Usual care | Sessions over 5 weeks | None |
| Velsor‐Friedrich 2005 | Self‐care deficit theory | To test a 2‐part intervention on selected psychosocial and health outcomes for children with asthma | OAS; nurse practitioner visits. Children received the OAS education curriculum and nurse practitioner visits to assess asthma health and further education | Usual care | 6× group sessions; individual nurse sessions | ED visits; Experience of daytime and night‐time symptoms; Lung function |
AAP: XXX.
ASMA: Asthma Self‐Management for Adolescents.
ED: emergency department.
GP: general practitioner.
HRQoL: health‐related quality of life.
ICAN: I Can Control Asthma and Nutrition Now.
OAS: Open Airways for Schools.
PD/H/PE: personal development/health/physical education.
PRECEDE: Predisposing, Reinforcing, and Enabling Causes in Educational Diagnosis and Evaluation.
RCT: randomised controlled trial.
SCAMP: School Care and Asthma Management Project.
SHARP: Staying Healthy–Asthma Responsible & Prepared.
Triple A: Adolescent Asthma Action.
Several studies collected outcome data immediately after the intervention or within three months (Atherly 2009; Bruzzese 2004; Bruzzese 2008; Gerald 2006; Horner 2008; Howell 2005; Kintner 2009; Mosnaim 2011; Patterson 2005; Persaud 1996; Shah 2001; Srof 2012), or they appeared to collect data concurrently with intervention delivery (Splett 2006). The longest period between the end of the intervention and data collection was 36 months in Bartholomew 2006, and 24 months in Clark 2004 and Clark 2010, although for a minority of studies, the length of follow‐up was not clear (Levy 2006; Monforte 2012; Pulcini 2007). We included many studies on the basis of study design, although these studies did not contribute to the meta‐analyses, as they did not collect data on the outcomes of interest or did not collect these data in an extractable format (see Table 11).
Primary outcomes
Asthma symptoms or exacerbations leading to admission to hospital
Six outcome studies provided data on asthma exacerbations leading to admission to hospital that were combined in meta‐analyses (Atherly 2009; Bruzzese 2011; Clark 2005; Horner 2008; Horner 2015; Levy 2006). One study collected information on hospitalisations but did not disaggregate the information by treatment status (Bartholomew 2006), and another study provided disaggregated information on median hospitalisations that could not be combined in meta‐analyses (Gerald 2006). Two studies assessed hospitalisations using hospital or school medical records (Gerald 2006; Levy 2006); three studies assessed hospitalisations using parent reports (Clark 2005; Horner 2015; Horner 2008); and two studies used child reports (Atherly 2009; Bruzzese 2011). Of the six studies included in the meta‐analyses, most collected outcome data on hospitalisations after a substantial period between receipt of the intervention and assessment of the outcome had elapsed (12 months in the case of Bruzzese 2011; Clark 2005; and Horner 2015; and seven months in the case of Horner 2008); less time had elapsed in the case of Atherly 2009 and Levy 2006, in which assessment took place within three months of receipt of the intervention. Studies in which a longer time had elapsed between intervention and assessment tended to be those with a longer exposure time over which the outcome was measured.
Asthma symptoms or exacerbations leading to emergency department visits
Fifteen outcome evaluation studies collected data on asthma symptoms or exacerbations leading to an emergency department (ED) visit (Atherly 2009; Bartholomew 2006; Bruzzese 2011; Cicutto 2005; Cicutto 2013; Clark 2005; Gerald 2006; Horner 2008; Horner 2015; Howell 2005; Levy 2006; McGhan 2003; McGhan 2010; Persaud 1996; Velsor‐Friedrich 2005). However, we did not use data from Bartholomew 2006 because study authors did not disaggregate the data by treatment status, and we could not combine data from Gerald 2006 because of incompatibility in the unit of assessment. Three studies used school or hospital administrative records to assess ED visits, with records provided by the medical hospital (Gerald 2006; Levy 2006; Persaud 1996). Parents were frequently the sources of ED data: one study collected these data using tracking sheets of ED attendance provided by parents (Cicutto 2013); another study collected data through parent interviews (Cicutto 2005); six studies used various parent self‐completion questionnaires (Clark 2005; Horner 2015; Horner 2008; Howell 2005; McGhan 2003; McGhan 2010), and one specifically used the Usherwood symptom questionnaire (Bartholomew 2006). One study collected data from children's asthma diaries (Velsor‐Friedrich 2005), and others collected data from children's reports (Atherly 2009; Bruzzese 2011).
Of the 13 studies included in the meta‐analyses, most collected outcome data on ED visits after a substantial period had elapsed between receipt of the intervention and assessment of the outcome (12 months in the case of Bruzzese 2011,Cicutto 2005,Cicutto 2013,Clark 2005,Horner 2015,McGhan 2003,McGhan 2010; seven months in the case of Horner 2008; and 20 weeks in the case of Persaud 1996); less time had elapsed in the case of Atherly 2009,Howell 2005, and Levy 2006, which performed assessment within three months of receipt of the intervention. As was the case above, studies in which a longer time had elapsed between intervention and assessment were those with a longer exposure time over which the outcome was measured (see Table 12 for full details).
Absence from school
Twelve outcome evaluation studies assessed school absence or attendance (Bartholomew 2006; Bruzzese 2011; Cicutto 2005; Cicutto 2013; Clark 2004; Gerald 2006; Gerald 2009; Howell 2005; McGhan 2003; McGhan 2010; Persaud 1996; Splett 2006).
Four studies used administrative school records (Bartholomew 2006; Gerald 2006; Persaud 1996; Splett 2006). One study collected school absenteeism data from parents/guardians using tracking sheets (Cicutto 2013), and five studies used parental interviews or questionnaires (Cicutto 2013; Clark 2004; Howell 2005; McGhan 2003; McGhan 2010). In another study, school staff entered absence data into an intervention tracking system (Gerald 2009). Bruzzese 2011 was the only study that collected self‐reported absence data directly from children.
Bartholomew 2006 did not present disaggregated information, and we will not consider this study further here. Clark 2004 presented information on effectiveness of the intervention in terms of school absence in the form of a risk difference, which was not combined in the meta‐analyses, although researchers showed a significant intervention effect in reducing absences at three months and 12 months.
We included data from 10 studies in meta‐analysis models. Six of these studies considered long‐term impact of the intervention, with follow‐up data from nine months or longer collected and included in the meta‐analysis (Bruzzese 2011; Cicutto 2005; Cicutto 2013; Gerald 2009; McGhan 2003; McGhan 2010). However, three studies collected follow‐up data after three months or sooner (Persaud 1996; Howell 2005; Splett 2006), and one study provided unclear information on this (Gerald 2006). Differences in the exposure period over which absences were considered ranged from a year in three studies ‐ as in Cicutto 2005,Cicutto 2013, and McGhan 2010 ‐ to two weeks in one study ‐ as in Bruzzese 2011. Three studies considered any instance of recorded absence from school (Cicutto 2013; McGhan 2003; McGhan 2010), and the remaining seven studies measured mean number of days of absence or attendance at school. Most studies included in the meta‐analysis collected data on any form of absence, with only Gerald 2009 collecting data on absence related to asthma/respiratory illness.
Days of restricted activity
Three outcome evaluation studies reported days of restricted activity (Bruzzese 2011; Cicutto 2005; Cicutto 2013). One study used parent tracking sheets/diaries to record days of interrupted activity due to asthma (Cicutto 2013), another study used data from parent interviews (Cicutto 2005), and another study collected information directly from children (Bruzzese 2011). We included data from all three studies in the meta‐analyses, and all three studies collected data at 12 months' follow‐up. Two studies collected data on the mean number of days of restricted activity (Bruzzese 2011; Cicutto 2005), and Cicutto 2013 collected data on any instance of a day of restricted activity.
Secondary outcomes
Unplanned visit to a hospital or GP due to asthma symptoms
Five outcome evaluation studies reported on unplanned visits to a hospital or GP due to asthma symptoms (Bruzzese 2011; Cicutto 2013; McGhan 2003; McGhan 2010; Splett 2006). One study recorded unplanned visits using tracking sheets provided to parents (Cicutto 2013); two studies used a parental questionnaire (McGhan 2003; McGhan 2010); one study collected data directly from children (Bruzzese 2011); and a final study collected information on episodic asthma‐related visits to a school‐based health facility from administrative data (Splett 2006).
We included data from all five studies in the meta‐analyses. One study originally collected information on the mean number of unscheduled visits (Bruzzese 2011), and the remaining studies collected information on any instances of unscheduled visits to a medical provider (not captured in hospitalisation or ED utilisation data (above)). All studies collected data after substantial time had elapsed since the intervention began; this extended to nine to 12 months in four studies (Bruzzese 2011; Cicutto 2013; McGhan 2003; McGhan 2010), and in Splett 2006, longitudinal data collection occurred concurrently alongside delivery of the intervention over a period of six months.
Experience of daytime and night‐time symptoms
Nine outcome evaluation studies assessed children's experiences of daytime and night‐time symptoms (Atherly 2009; Bruzzese 2008; Bruzzese 2011; Clark 2004; Clark 2010; Howell 2005; McGhan 2003; Shah 2001; Velsor‐Friedrich 2005). These studies specifically reported on symptoms occurring during the day or during the night. Data were not combined in meta‐analyses for either Clark 2004 or Clark 2010. Clark 2004 collected data on daytime and night‐time symptoms as a risk difference, which indicated that the intervention had a positive effect in reducing daytime symptoms for all children but reduced the incidence of night‐time symptoms only for children with severe or persistent asthma (yielding a negative effect on night‐time symptoms for children with mild asthma). We did not include this in the meta‐analyses as it was incompatible with other units of analysis. Meanwhile, Clark 2010 collected information on a change in daytime symptoms, which indicated that the intervention had a positive, but non‐statistically significant, impact in terms of a drop in daytime symptoms (an effect size was extractable for one of the treatment arms only, although it was not used in meta‐analyses because of statistical and conceptual differences between post‐test data and changes in post‐test outcome data).
Among the seven studies included in the meta‐analysis, five studies reported on the incidence of daytime symptoms (Atherly 2009; Bruzzese 2008; Bruzzese 2011; Shah 2001; Velsor‐Friedrich 2005), and in the case of Shah 2001, researchers reported the incidence of daytime symptoms specifically occurring within school; four studies reported on night‐time awakenings (Bruzzese 2008; Bruzzese 2011; Howell 2005; McGhan 2003), with two studies reporting on both daytime and night‐time symptoms (Bruzzese 2008; Bruzzese 2011). Four studies reported on intervention effects six to 12 months after the intervention (Bruzzese 2011; McGhan 2003; Shah 2001; Velsor‐Friedrich 2005), and the remaining three studies included in the meta‐analyses information collected from children or parents two to three months post intervention. Similarly, data show a relatively even split between studies reporting on the mean level of asthma symptoms occurring in the daytime/at night‐time ‐ Atherly 2009,Bruzzese 2008,Bruzzese 2011,Howell 2005 ‐ and those focused on measuring any reported incidence of daytime/night‐time symptoms ‐ McGhan 2003, Shah 2001, and Velsor‐Friedrich 2005.
Lung function
Five outcome evaluation studies assessed lung function (Gerald 2009; Horner 2015; Patterson 2005; Shah 2001; Velsor‐Friedrich 2005), although studies measured this in different ways. One study assessed lung function using the peak expiratory flow rate (PEFR) and specifically focused on the occurrence of poor readings (red and yellow readings defined as less than 80% of best value) (Gerald 2009). A second study measured spirometry by measuring the percentage predicted change in forced expiratory volume in one second (FEV₁) (Patterson 2005). Shah 2001 reported forced vital capacity (FVC) before use of a bronchodilator. Velsor‐Friedrich 2005 measured peak flow increases as a percentage of pretest peak (i.e. change in peak flow); Horner 2015 measured airway inflammation by measuring exhaled nitric oxide as a biomarker of airway inflammation.
Because of conceptual differences in the outcomes collected, we did not combine these in meta‐analyses. Table 14 shows that the individual effects extracted exhibited considerable heterogeneity in the direction and magnitude of effect, confirming that meta‐analysis was not desirable due to statistical heterogeneity.
13. Details of data transformations and adjustments made for meta‐analyses.
| Study | Indicator | Collection/reporting point | Mean cluster size (if applicable) | Intracluster correlation coefficient applied (if applicable) | Data transformation | Original effect size and standard error (with adjustment for clustering if applicable) | Final or transformed effect size and standard error (with adjustment for clustering if applicable) |
| Hospitalisations | |||||||
| Atherly 2009 | Instances of hospitalisation in previous 4 weeks | Post intervention (3‐month follow‐up) | 45.8 | 0.05 | Yes – transformed from odds ratio to SMD | OR (0.7736); SE (lnOR) (1.385) | SMD (‐0.141); SE (0.764) |
| Bruzzese 2011 | Hospitalisations in the past 2 months | Post intervention (12‐month follow‐up) | N/A | N/A | No | N/A | SMD (‐0.219); SE (0.120) |
| Clark 2005 | Hospitalisations | Post intervention (12‐month follow‐up) | Deemed that analysis methods accounted for clustering | Deemed that analysis methods accounted for clustering | Yes – transformed from odds ratio to SMD | OR (1.43); SE (estimated from P value (lnOR)) 0.39 | SMD (‐0.197); SE (0.215) |
| Gerald 2006 | Median hospitalisations (not combined) | N/A | N/A | N/A | N/A | N/A | N/A |
| Horner 2008 | Any hospital stays in the past 12 months (based on parents reporting any stay) | Post intervention (7‐month follow‐up) | 10.1 (reported by study authors) | 0.05 | Yes – transformed from odds ratio to SMD | OR (0.882); SE (lnOR) (0.791) | SMD (‐0.069); SE (0.436) |
| Horner 2015 | Mean number of hospitalisations since the previous data collection (at 8 months) | Post intervention (12‐month follow‐up) | 8.9 (approx.) | 0.05 | No | N/A | SMD (‐0.057); SE (0.169) |
| Levy 2006 | Mean hospital days | Post test (at intervention end) | 17.36 | 0.05 | No | N/A | SMD (‐0.293); SE (0.174) |
| Emergency department visits | |||||||
| Atherly 2009 | Instances of ED visits in previous 4 weeks | Post intervention (3‐month follow‐up) | 45.8 | 0.05 | No | N/A | OR (1.036); SE (lnOR) (0.916) |
| Bruzzese 2011 | Mean ED visits in the past 2 months | Post intervention (12‐month follow‐up) | N/A | N/A | Yes ‐ transformed from SMD to OR | SMD (‐0.289); SE (0.120) | OR (0.592); SE (lnOR) (0.218) |
| Cicutto 2005 | ED visits in the past year | Post intervention (12‐month follow‐up) | 9.85 | 0.05 | No | N/A | OR (0.697); SE (lnOR) (0.407) |
| Cicutto 2013 | ED visits in the past year (reports of) | Post intervention (12‐month follow‐up) | 7.7 | 0.05 | No | N/A | OR (0.318); SE (lnOR) (0.317) |
| Clark 2005 | ED visits | Post intervention (12‐month follow‐up) | Deemed that analysis methods accounted for clustering | Deemed that analysis methods accounted for clustering | No, but see notes | N/A | OR (1.002)*; SE (estimated from P value (lnOR)) 0.072 *Note that the OR was reported as 1.00 in the paper with a P value of 0.98. So information could be used and an SE extracted, a small correction to an OR of 1.002 was applied |
| Gerald 2006 | Median ED visits (not combined) | N/A | N/A | N/A | N/A | N/A | N/A |
| Horner 2008 | Any ED visits in the past 12 months (based on parents reporting any stay) | Post intervention (7‐month follow‐up) | 10.1 (reported by study authors) | 0.05 | No | N/A | OR (0.857); SE (lnOR) (0.461) |
| Horner 2015 | Mean number of ED visits since the previous data collection (8 months) | Post intervention (12‐month follow‐up) | 8.9 (approx.) | 0.05 | Yes ‐ transformed from SMD to OR | SMD (0); SE (0.169) | OR (1.00); SE (0.306) |
| Howell 2005 | Mean number of ED visits in the past 6 weeks | Post intervention (3‐month follow‐up) | 4.25 | 0.05 | Yes ‐ transformed from SMD to OR | SMD (‐0.331); SE (0.578) | OR (0.549); SE (1.049) |
| Levy 2006 | Mean urgent care or emergency visits | Post test (at intervention end) | 17.36 | 0.05 | Yes ‐ transformed from SMD to OR | SMD (‐0.286); SE (0.174) | OR (0.595); SE (0.318) |
| McGhan 2003 | ED visits in the past year (any) | Post intervention (9‐month follow‐up) | 9 | 0.05 | No | N/A | OR (1.283); SE (lnOR) (0.649) |
| McGhan 2010 | ED visits in the past year (any) | Post intervention (12‐month follow‐up) | 8.3 | 0.05 | No | N/A | OR (2.64); SE (lnOR) (0.707) |
| Persaud 1996 | Children with ED Visits (20‐week period post intervention) | Post intervention (events in 20‐week period post intervention) | N/A | N/A | No | N/A | OR (0.286); SE (lnOR) (0.737) |
| Velsor‐Friedrich 2005 | Urgent doctor visits (any in the past 12 months) | Post intervention (12‐month follow‐up) | 13 | 0.05 | No | N/A | OR (0.683); SE (lnOR) (0.933) |
| Absence from school | |||||||
| Bruzzese 2011 | Mean self‐reported absence in past 2 weeks (any absence) | Post intervention (12‐month follow‐up) | N/A | N/A | No | N/A | SMD (‐0.382); SE (0.121) |
| Cicutto 2005 | Parent‐reported absence (any absence) over a year | Post intervention (12‐month follow‐up) | 9.85 | 0.05 | No | N/A | SMD (‐0.256); SE (0.151) |
| Cicutto 2013 | Parent‐reported absence (any absence) over a year | Post intervention (12‐month follow‐up) | 7.7 | 0.05 | Yes – transformed from odds ratio to SMD | OR (0.660); SE (lnOR) (0.129) | SMD (‐0.229); SE (0.071) |
| Gerald 2006 | Absences recorded on school records | Post test (unclear duration) | Clustering accounted for in analytical strategy | Clustering accounted for in analytical strategy | No | N/A | SMD (‐0.199); SE (0.084) |
| Gerald 2009 | Absence from school due to respiratory illness/asthma *December measure used |
Post intervention (15‐month follow‐up) | N/A | N/A | Yes – transformed from odds ratio to SMD | OR (1.1667); SE (lnOR) (0.364) | SMD (0.085); SE (0.227) |
| Howell 2005 | School days missed in past 6 weeks | Post intervention (3‐month follow‐up) | 3.25 | 0.05 | No | N/A | SMD (0.152); SE (0.635) |
| McGhan 2003 | Any missed school days | Post intervention (9‐month follow‐up) | 9 | 0.05 | Yes – transformed from odds ratio to SMD | OR (0.720); SE (lnOR) (0.413) | SMD (‐0.181); SE (0.227) |
| McGhan 2010 | (No) Missed school days (any) over past 12 months | Post intervention (12‐month follow‐up) | 8.3 | 0.05 | Yes – transformed from odds ratio to SMD | OR (0.640); SE (lnOR) (0.353) | SMD (0.246); SE (0.195) (note: inverse taken as the intervention favours control) |
| Persaud 1996 | Mean school days of absence based on school records | Post intervention (immediately afterwards) | N/A | N/A | No | N/A | SMD (‐0.236); SE (0.335) |
| Splett 2006 | Mean percentage of days attended | Post intervention (12‐month follow‐up) | Deemed that analysis methods accounted for clustering | Deemed that analysis methods accounted for clustering | No | N/A | SMD (0.019); SE (0.051) |
| Days of restricted activity | |||||||
| Bruzzese 2011 | Mean self‐reported days of restricted activity in past 2 weeks | Post intervention (12‐month follow‐up) | N/A | N/A | No | N/A | SMD (‐0.349); SE (0.120) |
| Cicutto 2005 | Days of limited activity due to asthma | Post intervention (12‐month follow‐up) | 9.85 | 0.05 | No | N/A | SMD (‐0.318); SE (0.151) |
| Cicutto 2013 | Percentage of students reporting days of restricted activity | Post intervention (12‐month follow‐up) | 7.7 | 0.05 | Yes – transformed from odds ratio to SMD | OR (0.612); SE (lnOR) (0.130) | SMD (‐0.271); SE (0.072) |
| Unplanned visits to medical providers | |||||||
| Bruzzese 2011 | Mean acute care visits in the past 2 months | Post intervention (12‐month follow‐up) | N/A | N/A | Yes – transformed from SMD to OR | SMD (‐0.283); SE (0.120) | OR (0.598); SE (0.217) |
| Cicutto 2013 | Unscheduled care in the past year (reports of) | Post intervention (12‐month follow‐up) | 7.7 | 0.05 | No | OR (0.703); SE (lnOR) (0.143) | SMD (‐0.194); SE (0.079) |
| McGhan 2003 | Any unscheduled doctor visits | Post intervention (9‐month follow‐up) | 9 | 0.05 | No | OR (0.886); SE (lnOR) (0.426) | SMD (‐0.067); SE (0.235) |
| McGhan 2010 | Unscheduled GP visits (any) over past 12 months | Post intervention (12‐month follow‐up) | 8.3 | 0.05 | No | OR (1.169); SE (lnOR) (0.397) | SMD (0.086); SE (0.219) |
| Splett 2006 | Episodic asthma visits to school health office (over 6 months following start of intervention) | Over 6 months following start of intervention | 97.6 | 0.05 | No | OR (0.913); SE (lnOR) (0.282) | SMD (‐0.046); SE (0.156) |
| Daytime symptoms | |||||||
| Atherly 2009 | Mean number of days with asthma symptoms | Post intervention (3‐month follow‐up) | 45.8 | 0.05 | No | N/A | SMD (‐0.026); SE (0.168) |
| Bruzzese 2008 | Mean days last 2 weeks with asthma symptoms | Post intervention (2‐month follow‐up) | N/A | N/A | No | N/A | SMD (‐0.151); SE (0.418) |
| Bruzzese 2011 | Mean days last 2 weeks with asthma symptoms | Post intervention (12‐month follow‐up) | N/A | N/A | No | N/A | SMD (‐0.210); SE (0.120) |
| Shah 2001 | Number of students reporting attacks in school at follow‐up | Post intervention (6‐month follow‐up) | 41.8 | 0.05 | Yes – transformed from odds ratio to SMD | OR (0.647); SE (lnOR) (0.488) | SMD (‐0.240); SE (0.269) |
| Velsor‐Friedrich 2005 | Symptom days in past 2 weeks | Post intervention (12‐month follow‐up) | 13 | 0.05 | Yes – transformed from odds ratio to SMD | OR (0.846); SE (lnOR) (0.705) | SMD (‐0.030); SE (0.413) |
| Night‐time symptoms | |||||||
| Bruzzese 2008 | Mean nights woken last 2 weeks with asthma symptoms | Post intervention (2‐month follow‐up) | N/A | N/A | No | N/A | SMD (‐0.433); SE (0.423) |
| Bruzzese 2011 | Mean self‐reported night‐time awakenings in past 2 weeks | Post intervention (12‐month follow‐up) | N/A | N/A | No | N/A | SMD (‐0.388); SE (0.121) |
| Howell 2005 | Mean number of night‐time awakenings in past 6 weeks | Post intervention (3‐month follow‐up) | 4.25 | 0.05 | No | N/A | SMD (0.253); SE (0.478) |
| McGhan 2003 | Waking up in past 2 weeks twice or more | Post intervention (9‐month follow‐up) | 9 | 0.05 | Yes – transformed from odds ratio to SMD | OR (1.237); SE (lnOR) (0.412) | SMD (0.117); SE (0.227) |
| Use of reliever therapies | |||||||
| Gerald 2009 | Rescue medication use over twice per week *November measure used |
Post intervention (15‐month follow‐up) | N/A | N/A | N/A | OR (0.228); SE (lnOR) (0.582) | N/A |
| McGhan 2003 | Number of students with appropriate use of reliever medication | Post intervention (9‐month follow‐up) | 9 | 0.05 | N/A | OR (3.48); SE (lnOR) (0.565) | N/A |
| McGhan 2010 | Used short‐acting bronchodilators in past 2 weeks | Post intervention (12‐month follow‐up) | 8.3 | 0.05 | N/A | OR (0.878); SE (lnOR) (0.356) | N/A |
| Splett 2006 | Students with access to reliever medication visiting health office (over 6 months following start of intervention) *Note low levels of children with reliever medication |
Over 6 months following start of intervention | 97.6 | 0.05 | N/A | OR (1.28); SE (lnOR) (0.282) | N/A |
| Use of corticosteroids and/or use of add‐on therapies | |||||||
| Bruzzese 2011 | Use of controller medication | Post intervention (12‐month follow‐up) | N/A | N/A | No | N/A | OR (1.451); SE (lnOR) (0.240) |
| Horner 2015 | Inhaled corticosteroid adherence | Post intervention (5‐month follow‐up) | 8.9 | 0.05 | No | N/A | SMD (‐0.605); SE (0.173) |
| Howell 2005 | Inhaled corticosteroid adherence as prescribed (during past week) | Post intervention (3‐month follow‐up) | 4.25 | 0.05 | No | N/A | SMD (0.953); SE (0.546) |
| McGhan 2003 | Currently using inhaled steroids | Post intervention (9‐month follow‐up) | 9 | 0.05 | No | N/A | OR (1.112); SE (lnOR) (0.418) |
| McGhan 2010 | Currently using inhaled steroids | Post intervention (12‐month follow‐up) | 8.3 | 0.05 | No | N/A | OR (0.962); SE (lnOR) (0.376) |
| Splett 2006 | Students with access to controller medication visiting health office (over 6 months following start of intervention) *Note low levels of children with controller medication |
Over 6 months following start of intervention | 97.6 | 0.05 | N/A | OR (1.703); SE (lnOR) (0.806) | SMD (0.293); SE (0.445) |
| Lung function | |||||||
| Gerald 2009 | Poor peak flow measures (red/amber readings) | Post‐intervention (15‐month follow‐up) | N/A | N/A | No | OR (0.94); SE (lnOR) (0.334) | |
| Horner 2015 | Airway inflammation (exhaled nitric oxide, collected using the single‐use RTube collection device, was the biomarker of airway inflammation) |
Post intervention (12‐month follow‐up) | 8.9 | 0.05 | No | N/A | SMD (‐0.011); SE (0.169) |
| Shah 2001 | Forced expiratory volume in 1 second: forced vital capacity before bronchodilator |
Post intervention (3‐month follow‐up) | Deemed that analysis methods accounted for clustering | Deemed that analysis methods accounted for clustering | No | N/A | SMD (0.074); SE (0.127) |
| Patterson 2005 | Forced expiratory volume in 1 second (% predicted change) |
Post intervention (2‐month follow‐up) | Deemed that analysis methods accounted for clustering | Deemed that analysis methods accounted for clustering | No | N/A | SMD (‐0.05); SE (0.177) |
| Velsor‐Friedrich 2005 | Peak flow increases as a percentage of pretest peak flow (change) |
Post intervention (12‐month follow‐up) | 13 | 0.05 | No | N/A | SMD (‐5.905); SE (0.839) |
| Quality of life | Mean difference (QoL only) | Standardised mean difference (QoL only) | |||||
| Al‐Sheyab 2012 | Arabic version of the Pediatric Asthma Quality of Life Questionnaire (PAQLQ) *because of uncertainty about SD values, derived from t/P value of difference between means |
Post intervention (3‐month follow‐up) | Deemed that analysis methods accounted for clustering | Deemed that analysis methods accounted for clustering | No | MD 1.35 (CI 0.96 to 1.74) | SMD (0.299); SE (0.129) |
| Cicutto 2005 | Juniper Pediatric Asthma Quality of Life Questionnaire overall quality of life | Post intervention (2‐month follow‐up) | 9.85 | 0.05 | No | MD 0.50 (CI 0.00 to 1.00) | SMD (0.356); SE (0.151) |
| Cicutto 2013 | Juniper Pediatric Asthma Quality of Life Questionnaire overall quality of life | Post intervention (12‐month follow‐up) | 7.7 | 0.05 | No | MD 0.40 (CI 0.21 to 0.59) | SMD (0.308); SE (0.064) |
| Henry 2004 | Juniper Pediatric Asthma Quality of Life Questionnaire overall quality of life | Post intervention (6‐month follow‐up) | 15.2 | 0.05 | No | MD 0.16 (CI ‐0.22 to 0.54) | SMD (0.128); SE (0.114) |
| Horner 2008 | Juniper Pediatric Asthma Quality of Life Questionnaire overall quality of life | Post intervention (7‐month follow‐up) | 10.2 | 0.05 | No | MD 0.05 (CI ‐0.21 to 0.31) | SMD (0.083); SE (0.196) |
| Howell 2005 | Juniper Pediatric Asthma Quality of Life Questionnaire overall quality of life | Post intervention (3‐month follow‐up) | 6 | 0.05 | No | MD 0.03 (CI ‐1.71 to 1.77) | SMD (0.020); SE (0.484) |
| Kintner 2009 | Quality of life is defined through the Participation in Life Activities Scale |
Immediately post intervention | Deemed that analysis methods accounted for clustering | Deemed that analysis methods accounted for clustering | No | N/A | SMD (0.583); SE (0.263) |
| Patterson 2005 | Change in Juniper Pediatric Asthma Quality of Life Questionnaire overall quality of life | Change in quality of life between baseline and 4 months post intervention | Deemed that analysis methods accounted for clustering | Deemed that analysis methods accounted for clustering | No | MD 0.07 (CI ‐0.26 to 0.40) | N/A |
| Shah 2001 | Juniper Pediatric Asthma Quality of Life Questionnaire overall quality of life; percentage of students with clinically significant improvement | Post intervention (3‐month follow‐up) | Deemed that analysis methods accounted for clustering | Deemed that analysis methods accounted for clustering | No | MD 0.09 (CI ‐0.23 to 0.41) | N/A |
| Withdrawal | |||||||
| Al‐Sheyab 2012 | Withdrew between baseline and outcome collection | Post intervention (3‐month follow‐up) | 65.25 | 0.05 | No | N/A | OR (0.511); SE (lnOR) (1.074) |
| Bartholomew 2006 | Lost to follow‐up at post‐test measure | Post intervention (duration unclear) | 11.2 | 0.05 | No | N/A | OR (0.237); SE (lnOR) (0.145) |
| Bruzzese 2008 | Withdrew between baseline and outcome collection | Immediate post intervention | N/A | N/A | No | N/A | OR (0.307); SE (lnOR) (1.683) |
| Bruzzese 2011 | Withdrew between baseline and outcome collection | Post intervention (12‐month follow‐up) | N/A | N/A | No | N/A | OR (1.313); SE (lnOR) (0.279) |
| Cicutto 2005 | Withdrew between baseline and outcome collection | Post intervention (6‐month follow‐up) | 9.85 | 0.05 | No | N/A | OR (1.788); SE (lnOR) (0.629) |
| Gerald 2009 | Withdrew between baseline and outcome collection | Post intervention (6‐month follow‐up) | N/A | N/A | No | N/A | OR (1.788); SE (lnOR) (0.613) |
| Horner 2008 | Withdrew between baseline and outcome collection | Post intervention (7‐month follow‐up) | 10.2 | 0.05 | No | N/A | OR (1.333); SE (lnOR) (0.531) |
| Horner 2015 | Failed to complete final data collection | Post intervention (12‐month follow‐up) | 8.9 | 0.05 | No | N/A | OR (0.75); SE (lnOR) (0.486) |
| Kintner 2009 | Withdrew during intervention and between end of intervention and follow‐up | Post intervention (12‐month follow‐up) | 13.2 | 0.05 | No | N/A | OR (30.176); SE (lnOR) (1.860) |
| Levy 2006 | Failure to complete outcome evaluation | Post intervention (12‐month follow‐up) | 17.36 | 0.05 | No | N/A | OR (0.357); SE (lnOR) (0.3881) |
| McGhan 2003 | Withdrew between baseline and outcome collection | Post intervention (9‐month follow‐up) | 9 | 0.05 | No | N/A | OR (1.147); SE (lnOR) (0.5381) |
| McGhan 2010 | Withdrew between baseline and interim outcome collection | Post intervention (6‐month follow‐up) | 8.3 | 0.05 | No | N/A | OR (1.007); SE (lnOR) (0.387) |
| Patterson 2005 | Withdrew during intervention | Post intervention – immediately following intervention | 7.95 | 0.05 | No | N/A | OR (5.675); SE (lnOR) (1.087) |
| Shah 2001 | Withdrew between baseline and outcome collection | Post intervention (3‐month follow‐up) | 45.3 | 0.05 | No | N/A | OR (1.343); SE (lnOR) (0.475) |
CI: confidence interval.
ED: emergency department.
lnOR: log odds ratio.
MD: mean difference.
N/A: not applicable.
OR: odds ratio.
PAQLQ: Pediatric Asthma Quality of Life Questionnaire.
QoL: quality of life.
SD: standard deviation.
SE: standard error.
SMD: standardised mean difference.
Use of reliever therapies such as beta₂‐agonists
Four outcome evaluation studies assessed use of reliever therapies (Gerald 2009; McGhan 2003; McGhan 2010; Splett 2006). We combined in meta‐analyses two studies that reported on the use of rescue medication and short‐acting bronchodilators (SABAs), respectively (Gerald 2009; McGhan 2010). The former captured information on instances when rescue medication was used more than twice a week, and the latter measured any instance in which rescue medication was used; these studies sought to measure long‐term intervention effects at 12 months ‐ as in McGhan 2010 ‐ and at 15 months ‐ as in Gerald 2009. The remaining two studies measured appropriate use of reliever medication and access to reliever medication, respectively (McGhan 2003; Splett 2006). Because of conceptual differences in the way in which researchers measured use of reliever therapies, we chose not to meta‐analyse this information. We have presented information provided by all four studies in Table 14.
Corticosteroid dosage and/or use of add‐on therapies
Six studies measured corticosteroid usage and dosage (Bruzzese 2011; Horner 2015; Howell 2005; McGhan 2003; McGhan 2010; Splett 2006). One study measured whether children had access to controller medication while visiting the school health office (Splett 2006). Two studies measured whether children were adhering to guidance provided around the correct use of corticosteroid (Horner 2015; Howell 2005), and three studies measured any reported usage of corticosteroid or controller medication (Bruzzese 2011; McGhan 2003; McGhan 2010). We meta‐analysed data from these five studies separately, as adherence was deemed to conceptually differ from reports of usage. Horner 2015 and Howell 2005 included information from children at five months and three months, respectively, in meta‐analyses of corticosteroid adherence. All three studies in the second meta‐analysis on reported instances of corticosteroid or controller medication usage collected information at nine months or 12 months post intervention. We have presented data from all six studies in Table 14.
Health‐related quality of life (HRQoL)
Twelve outcome evaluation studies measured quality of life (Al‐Sheyab 2012; Cicutto 2005; Cicutto 2013; Clark 2010; Henry 2004; Horner 2008; Howell 2005; Kintner 2009; McCann 2006; McGhan 2010; Patterson 2005; Shah 2001). McCann 2006,McGhan 2010, and Clark 2010 did not present data in an extractable format (i.e. described data narratively, did not disaggregate data, or did not include the necessary information to extract an effect size); Patterson 2005 measured change in quality of life; and Shah 2001 measured clinically significant improvements (see Table 14). Among the nine studies that calculated an effect size, eight were based on the Juniper Pediatric Asthma Quality of Life Questionnaire overall quality of life (see Juniper 1996); Al‐Sheyab 2012 used an Arabic version of this questionnaire. Kintner 2009 measured quality of life by reviewing responses to the Participation in Life Activities Scale.
We constructed two sets of meta‐analyses for a model measuring changes in quality of life. One of these used SMD to calculate effect sizes; this allowed us to incorporate data from Kintner 2009. We meta‐analysed change scores to obtain an MD from the data reported in Patterson 2005 and Shah 2001. Therefore data from six studies were common to both models. Several studies measured quality of life within four months of the intervention (Al‐Sheyab 2012; Cicutto 2005; Howell 2005; Kintner 2009; Patterson 2005; Shah 2001), two studies collected data at six to seven months after the intervention (Henry 2004; Horner 2008), and one study collected data 12 months after the intervention (Cicutto 2013).
Withdrawal from the study
Researchers frequently presented withdrawal data, although not always in a format that allowed extraction of data to form an effect size. This often occurred because studies reported overall numbers lost during the study without disaggregating by treatment arm (Cicutto 2013; Velsor‐Friedrich 2005), or because studies reported no losses (Persaud 1996). Fourteen studies provided enough data to allow calculation of an effect size (OR) (Al‐Sheyab 2012; Bartholomew 2006; Bruzzese 2008; Bruzzese 2011; Cicutto 2005; Gerald 2009; Horner 2008; Horner 2015; Kintner 2009; Levy 2006; McGhan 2003; McGhan 2010; Patterson 2005; Shah 2001). Few studies reported on active withdrawal processes occurring during the intervention; instead investigators reported on failure to collect children's data at follow‐up (collected from children and parents). Researchers collected data at different points between intervention and follow‐up, including at four months or less (Al‐Sheyab 2012; Bruzzese 2008; Patterson 2005; Shah 2001), at six to seven months (Cicutto 2005; Gerald 2009; Horner 2008; McGhan 2010), and at nine to 12 months (Bruzzese 2011; Horner 2015; Kintner 2009; Levy 2006; McGhan 2003). Duration was unclear in one study (Bartholomew 2006).
Excluded studies
From the title and abstract screening, we excluded 28,318 records because they were clearly outside the remit of the review of process evaluations. Following full‐text screening, we excluded another 1029 records, for reasons detailed in the PRISMA diagram (Figure 2).
Based on title and abstract screening, we excluded 274 records as they were outside the remit of the review of outcome evaluation studies. Following full‐text screening, we excluded 67 additional records, for reasons detailed in the PRISMA diagram (Figure 3).
Risk of bias in included studies
We have displayed results of the risk of bias assessment for process and outcome evaluation studies in the risk of bias table and graph. We have presented the agreed judgement of two review authors (DK, KH) regarding the risk of bias for each included study as percentages for each bias item in the risk of bias graph (Figure 4; Figure 5).
4.
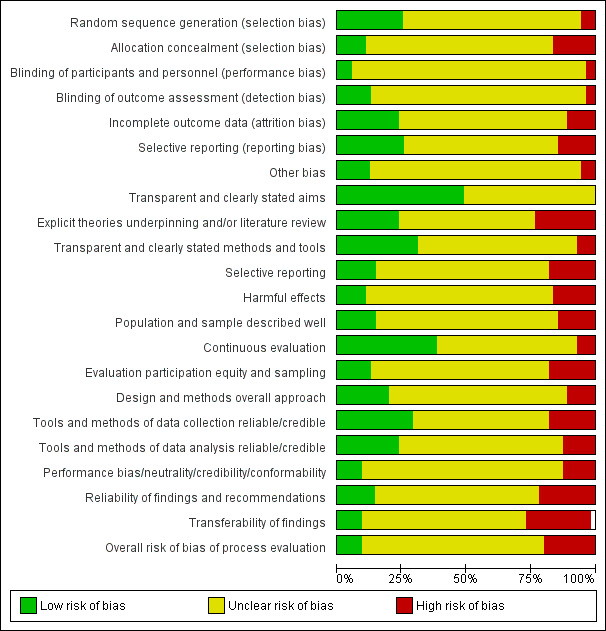
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
5.
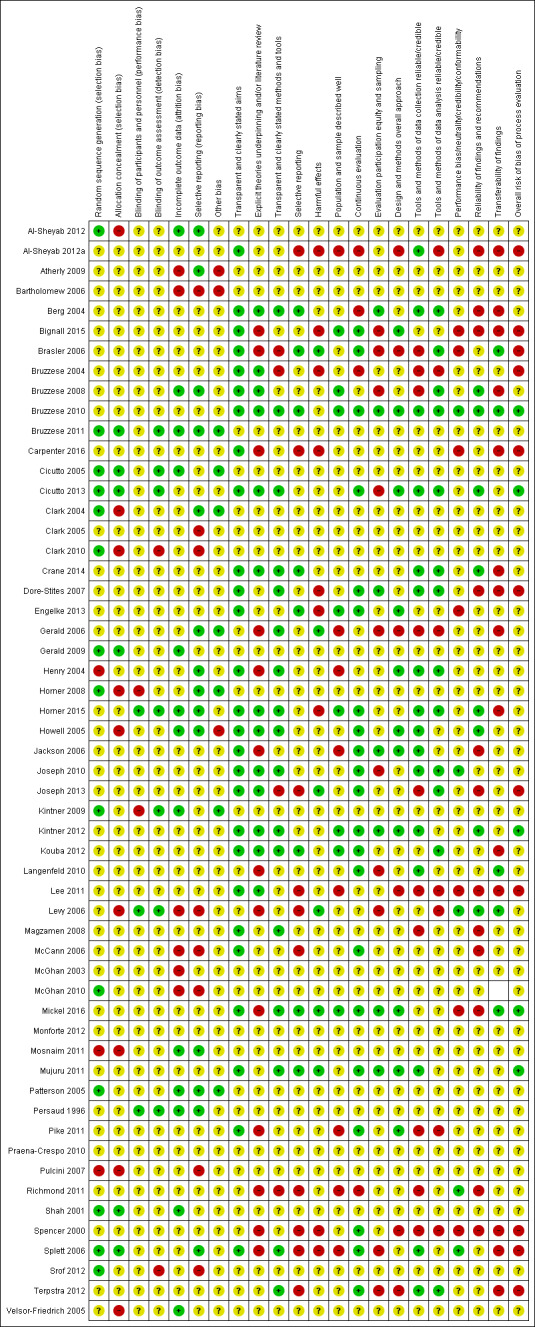
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Risk of bias ‐ process evaluation studies
For process evaluation studies, we assessed risk of bias using a combination of two tools. The first tool was developed at the EPPI‐Centre (Harden 2004) to assess the methodological rigour of 'views' studies; the second tool, which was developed by the EPPI‐Centre to assess the quality of process evaluation data (O'Mara‐Eves 2013),
We assessed reporting quality across five indicators.
Transparent and clearly stated aims (0 high risk of bias, 27 low risk of bias, 6 unclear risk).
Explicit theories underpinning the intervention (10 high risk of bias, 14 low risk of bias, 9 unclear risk).
Transparent and clearly stated methods and tools (4 high risk of bias, 17 low risk of bias, 12 unclear risk).
Selective reporting (10 high risk of bias, 8 low risk of bias, 15 unclear risk).
Harmful effects (8 high risk of bias, 5 low risk of bias, 20 unclear risk).
We assessed population and selection factors using four indicators.
Population and sample described well (8 high risk of bias, 8 low risk of bias, 17 unclear risk).
Continuous evaluation (3 high risk of bias, 8 low risk of bias, 22 unclear risk).
Evaluation participation equity and sampling (9 high risk of bias, 7 low risk of bias, 17 unclear risk).
Design and methods overall approach (6 high risk of bias, 10 low risk of bias, 16 unclear risk).
We assessed reliability and transferability of findings using two indicators.
Reliability of findings and recommendations (11 high risk of bias, 8 low risk of bias, 14 unclear risk).
Transferability of findings (13 high risk of bias, 5 low risk of bias, 15 unclear risk).
Overall, process evaluation studies consisted of 10 high‐risk studies, five low‐risk studies, and 18 studies at unclear risk.
Risk of bias ‐ outcome evaluation (RCT) studies
Allocation
We judged 14 outcome evaluation studies to be at low risk of bias for random sequence generation (Al‐Sheyab 2012; Bruzzese 2011; Cicutto 2005; Cicutto 2013; Clark 2004; Clark 2010; Gerald 2009; Horner 2008; Kintner 2009; McGhan 2010; Patterson 2005; Shah 2001; Splett 2006; Srof 2012). We judged three to be at high risk (Henry 2004; Mosnaim 2011; Pulcini 2007). We judged the remainder to be at unclear risk. We judged six of these studies to be at low risk of allocation concealment bias (Bruzzese 2011; Cicutto 2005; Cicutto 2013; Gerald 2009; Shah 2001; Splett 2006). We judged nine studies to be at high risk of allocation concealment bias (Clark 2010; Horner 2008; Howell 2005; Kintner 2009; Levy 2006; McGhan 2010; Mosnaim 2011; Pulcini 2007; Velsor‐Friedrich 2005).
Blinding
We judged three outcome evaluation studies to be at low risk of bias for blinding of participants and personnel (Cicutto 2013; Horner 2015; Levy 2006). We judged two studies to be at high risk of bias for this component (Horner 2008; Kintner 2009). We judged seven outcome evaluation studies to be at low risk for blinding of outcome assessment (Bruzzese 2011; Cicutto 2005; Cicutto 2013; Horner 2015; Kintner 2009; Levy 2006; Persaud 1996). For two outcome evaluation studies, we determined that risk of bias for blinding of outcome assessment was high (Clark 2010; Srof 2012).
Incomplete outcome data
We judged 13 outcome evaluation studies to be at low risk of bias for incomplete outcome data (Al‐Sheyab 2012; Bruzzese 2011; Bruzzese 2008; Cicutto 2005; Gerald 2009; Horner 2015; Howell 2005; Kintner 2009; Mosnaim 2011; Patterson 2005; Persaud 1996; Shah 2001; Velsor‐Friedrich 2005). We judged six outcome evaluation studies to be at high risk of bias for incomplete outcome data (Atherly 2009; Bartholomew 2006; Levy 2006; McCann 2006; McGhan 2010; McGhan 2003).
Selective reporting
We judged 14 outcome evaluation studies to be at low risk of bias for selective reporting (Al‐Sheyab 2012; Atherly 2009; Bruzzese 2011; Bruzzese 2008; Clark 2004; Gerald 2006; Henry 2004; Horner 2015; Horner 2008; Howell 2005; Mosnaim 2011; Patterson 2005; Persaud 1996; Splett 2006). We judged eight studies to be at high risk of bias for selective reporting (Bartholomew 2006; Clark 2005; Clark 2010; Levy 2006; McCann 2006; McGhan 2010; Pulcini 2007; Srof 2012).
Other potential sources of bias
We judged 13 outcome evaluation studies to be at low risk of bias (Al‐Sheyab 2012; Atherly 2009; Bruzzese 2008; Bruzzese 2011; Gerald 2009; Gregory 2000; Horner 2008; Kintner 2009; Patterson 2005; Persaud 1996; Shah 2001; Splett 2006; Velsor‐Friedrich 2005), along with seven studies at high risk of bias, for missingness (Bartholomew 2006; Bruzzese 2004; Cicutto 2005; Howell 2005; Levy 2006; McGhan 2010; Praena‐Crespo 2010).
We judged 15 outcome evaluation studies to be at low risk of bias for baseline imbalance (Bruzzese 2008; Bruzzese 2011; Cicutto 2005; Cicutto 2013; Clark 2004; Gerald 2006; Gerald 2009; Gregory 2000; Horner 2008; Kintner 2009; Levy 2006; McGhan 2010; Splett 2006; Srof 2012; Velsor‐Friedrich 2005). We judged six studies to be at high risk for baseline imbalance (Al‐Sheyab 2012; Atherly 2009; Clark 2010; Howell 2005; McCann 2006; McGhan 2003).
We judged 27 outcome evaluation studies to be at low risk for contamination (Al‐Sheyab 2012; Atherly 2009; Bartholomew 2006; Bruzzese 2011; Cicutto 2005; Cicutto 2013; Clark 2004; Clark 2005; Clark 2010; Gerald 2006; ; Henry 2004; Horner 2008; Horner 2015; Howell 2005; Kintner 2009; Levy 2006; McCann 2006; McGhan 2003; McGhan 2010; Monforte 2012; Mosnaim 2011; Patterson 2005; Praena‐Crespo 2010; Pulcini 2007; Shah 2001; Splett 2006; Velsor‐Friedrich 2005), and we determined that five outcome evaluation studies were at high risk (Bruzzese 2004; Bruzzese 2008; Gerald 2009; Persaud 1996; Srof 2012).
Effects of interventions
See: Table 1
Results of synthesis ‐ part 1: qualitative comparative analysis of determinant conditions for successful intervention implementation
Descriptive results from process evaluation studies on implementation success
Across the 27 included studies, review authors identified eight studies as having high implementation scores for our combined outcome (attrition, adherence, dosage) and classified these studies as mainly or fully included in a set of studies marked as successfully implemented (Al‐Sheyab 2012a; Berg 2004; Bruzzese 2008; Bruzzese 2011; Henry 2004; Joseph 2010; Kintner 2012; Terpstra 2012). In contrast, we identified eight studies as having low implementation success scores and as mainly or entirely outside the successfully implemented set of studies (Brasler 2006; Bruzzese 2004; Gerald 2006; Howell 2005; Kouba 2012; Langenfeld 2010; Magzamen 2008; Spencer 2000). Other studies were more ambiguous regarding their implementation success and had high levels of missing data or conflicting results across indicators.
For many studies reporting lower implementation success, we viewed the difficulty of incorporating an intervention into the busy school curriculum and into children's busy schedules as undermining the intervention (Brasler 2006; Bruzzese 2004; Gerald 2006; Howell 2005; Kouba 2012). Additional factors included difficulties in terms of high staff turnover (Gerald 2006); high child turnover and/or chaotic families (Brasler 2006; Howell 2005); and low motivation among children, particularly in the absence of incentives (Magzamen 2008). Similarly, researchers provided a diverse set of explanations for successful implementation, including high levels of school‐level commitment (Henry 2004; Kintner 2012); high levels of child and teacher motivation (Al‐Sheyab 2012a; Berg 2004); and development of group cohesion (Bruzzese 2008), as well as specific intervention design features, including tailoring of messages to children, as in Bruzzese 2011 and Joseph 2010, and additional communications with parents, as in Terpstra 2012.
In the QCA analyses below, we examine factors that could further explain successful implementation by examining which characteristics are shared among studies that were successfully implemented, and whether these differ from studies that were not successfully implemented.
Summary of results from qualitative comparative analysis
We first explored different domains of implementation separately, before bringing this evidence together in a final model (Table 15). We used this strategy mainly because of the problem of limited diversity, by which observed studies did not support too many possible combinations of intervention characteristics. We found no configurations of characteristics that consistently triggered successful implementation with respect to recruitment and retention, as well as pedagogical factors, although these may be important in other ways for children's outcomes.
14. Summary of interventions, conditions entered, and model results.
| Domain (model) | Conditions entered | Sufficient configurations identified that trigger successful implementation |
| 1. Setting and participant features | School health centre; high school; parents direct intervention recipients; teachers direct intervention recipients; school nurses/others direct intervention recipients | Yes |
| 2. Recruitment and retention processes | Additional marketing materials; provision of incentives; provision of catch‐up sessions; provision of reminders | No |
| 3. Curriculum, pedagogy, and intervention emphasis | Focus on establishing alliances with care providers; focus on asthma symptom recognition and management; tailored content; emphasis on personal responsibility; interactive pedagogical style; diverse pedagogical style | No |
| 4. Modifiable intervention processes | Theory driven; run in class time; run in students' free time; school nurse key role in delivery or teaching; personalised or individual 1‐to‐1 instruction | Yes |
| 5. Stakeholder engagement | School asthma policy; child satisfaction; teachers engaged/relationships developed; parents engaged/relationships developed; school nurses engaged/relationships developed | Yes |
| 6. Consolidated model | Theory driven; run in students' free time; child satisfaction; parents engaged/relationships developed; high school | Yes |
In our consolidated model, we prioritised conditions that were included in configurations with high consistency and coverage scores. To facilitate interpretation in the consolidated model, we focused on conditions with a consistent direction. Working from the raw data (Table 16), we created a truth table (Table 17), which showed the extent to which sets of studies with particular configurations of conditions overlapped with a set of studies included in our successful intervention set. Boolean minimisation helped to simplify the solution (Table 18), and we inserted assumptions about logical remainders (configurations with no observed cases) to further simplify the solution (Table 19). After doing this, we observed that four pathways (or configurations of conditions) triggered the outcome, thereby forming our 'solution' (summarised in Table 20).
15. Data table for QCA model 6 ‐ consolidated model.
| Successful intervention | High school | Child satisfaction | Theory driven | Intervention takes place during students' own free time | Good relationships/engagement with parents | |
| Joseph 2010 | 0.52 | 1 | 0 | 1 | 0.33 | 0 |
| Kouba 2012 | 0.33 | 1 | 0 | 1 | 1 | 0 |
| Dore‐Stites 2007 | 0.67 | 0 | 1 | 1 | 0.33 | 0.75 |
| Joseph 2013 | 1.00 | 1 | 0 | 1 | 0.75 | 1 |
| Mujuru 2011 | 0.67 | 0 | 0 | 0 | 0 | 0.25 |
| Henry 2004 | 0.83 | 1 | 0 | 0 | 0 | 0 |
| Pike 2011 | 0.67 | 0 | 0 | 0 | 0 | 0 |
| Spencer 2000 | 0.33 | 0 | 0 | 0 | 0.33 | 1 |
| Engelke 2013 | 0.50 | 0.5 | 0 | 0 | 0.33 | 1 |
| Splett 2006 | 0.50 | 0.5 | 0 | 0 | 0.33 | 0 |
| Kintner 2012 | 0.83 | 1 | 1 | 1 | 1 | 0.25 |
| Berg 2004 | 0.83 | 1 | 1 | 1 | 0.33 | 0 |
| Howell 2005 | 0.33 | 0 | 0.633333 | 1 | 0.33 | 0.75 |
| Gerald 2006 | 0.33 | 0 | 0 | 0 | 0.33 | 0 |
| Langenfeld 2010 | 0.33 | 0 | 0 | 0 | 0.33 | 0 |
| Al‐Sheyab 2012 | 0.83 | 1 | 0.633333 | 1 | 0.33 | 0 |
| Levy 2006 | 0.52 | 0 | 0 | 0 | 0.33 | 0 |
| Terpstra 2012 | 1.00 | 0.66 | 0 | 1 | 1 | 0.25 |
| Horner 2015 | 0.67 | 0 | 0 | 1 | 1 | 0 |
| Bruzzese 2008 | 0.94 | 0.66 | 1 | 1 | 0.33 | 1 |
| Lee 2011 | 0.50 | 0 | 0 | 1 | 0 | 0 |
| Bruzzese 2004 | 0.33 | 1 | 0.633333 | 1 | 0.75 | 0 |
| Cicutto 2013 | 0.67 | 0 | 0 | 1 | 1 | 0 |
| Brasler 2006 | 0.00 | 0.66 | 0.633333 | 0 | 0.75 | 0 |
| Crane 2014 | 0.50 | 0 | 0 | 1 | 1 | 0 |
| Bruzzese 2011 | 0.88 | 1 | 0 | 1 | 0.33 | 0 |
| Magzamen 2008 | 0.19 | 0.75 | 0 | 0 | 1 | 0 |
QCA: qualitative comparative analysis.
16. Truth table for QCA model 6 ‐ consolidated model.
| High school | Child satisfaction | Theory driven | Intervention takes place during students' own free time | Good relationships/ engagement with parents | Outcome code (based on consistency score) | Number of studies with membership in causal combination > 0.5 | Consistency score with subset relationship (n = 27 in each assessment) | Proportional reduction in inconsistency | Cases |
| 1 | 1 | 1 | 0 | 0 | 1 | 2 | 1 | 1 | Al‐Sheyab 2012; Berg 2004 |
| 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Joseph 2013 |
| 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | Bruzzese 2008 |
| 1 | 0 | 1 | 0 | 0 | 1 | 2 | 0.924 | 0.841 | Bruzzese 2011; Joseph 2010 |
| 1 | 1 | 1 | 1 | 0 | 1 | 2 | 0.853 | 0.752 | Bruzzese 2004; Kintner 2012 |
| 0 | 1 | 1 | 0 | 1 | 1 | 2 | 0.815 | 0.668 | Dore‐Stites 2007; Howell 2005 |
| 1 | 0 | 1 | 1 | 0 | 0 | 2 | 0.768 | 0.595 | Kouba 2012; Terpstra 2012 |
| 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0.763 | 0 | Engelke 2013; Spencer 2000 |
| 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0.762 | 0.615 | Henry 2004 |
| 0 | 0 | 1 | 1 | 0 | 0 | 3 | 0.675 | 0.463 | Cicutto 2013; Crane 2014; Horner 2015 |
| 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0.67 | 0.322 | Gerald 2006; Langenfeld 2010; Levy 2006; Mujuru 2011; Pike 2011; Splett 2006 |
| 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0.6 | 0 | Lee 2011 |
| 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0.358 | 0 | Magzamen 2008 |
| 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | Brasler 2006 |
QCA: qualitative comparative analysis.
17. Complex solution for QCA model 6 ‐ consolidated model.
| Consistency score with subset relationship (n = 27 in each assessment) | Proportional reduction in inconsistency | Raw coverage | Unique coverage | Cases | ||
| 1 | CHILDSAT*THEORYDRIVEN*runinstudenttime*GOODRELPAR | 0.846 | 0.756 | 0.106 | 0.106 | Bruzzese 2008; Dore‐Stites 2007; Howell 2005 |
| 2 | HIGHSCHOOL*CHILDSAT*THEORYDRIVEN*goodrelpar | 0.845 | 0.786 | 0.162 | 0.063 | Al‐Sheyab 2012; Berg 2004; Bruzzese 2004; Kintner 2012 |
| 3 | HIGHSCHOOL*THEORYDRIVEN*runinstudenttime*goodrelpar | 0.949 | 0.914 | 0.177 | 0.078 | Al‐Sheyab 2012; Berg 2004; Bruzzese 2011; Joseph 2010 |
| 4 | HIGHSCHOOL*childsat*THEORYDRIVEN*RUNINSTUDENTTIME*GOODRELPAR | 1 | 1 | 0.064 | 0.064 | Joseph 2013 |
| M1 | 0.875 | 0.823 | 0.41 |
QCA: qualitative comparative analysis.
[Notation: Upper case = condition is present; Lower case = condition is absent; * = logical and; + logical or; Key: HIGHSCHOOL = High School (lower case not in high school); THEORYDRIVEN = Authors explicitly named theory or presented conceptual model for intervention; RUNINSTUDENTTIME = Substantial component run in students' own time (e.g. lunchtime); GOODRELPAR = Good level of reported in engagement and/or developing relationships with parents; CHILDSAT = Children reported as satisfied; SUCCESSFULIMPLEMENTATION = Implementation of intervention successful]
18. Intermediate solution for QCA model 6 ‐ consolidated model.
| Consistency score with subset relationship (n = 27 in each assessment) | Proportional reduction in inconsistency | Raw coverage | Unique coverage | Cases | ||
| 1 | HIGHSCHOOL*CHILDSAT*THEORYDRIVEN | 0.839 | 0.791 | 0.21 | 0.053 | Al‐Sheyab 2012; Berg 2004; Bruzzese 2004; Bruzzese 2008; Kintner 2012 |
| 2 | HIGHSCHOOL*THEORYDRIVEN*GOODRELPAR | 1 | 1 | 0.138 | 0.064 | Bruzzese 2008; Joseph 2013 |
| 3 | HIGHSCHOOL*THEORYDRIVEN*runinstudenttime | 0.961 | 0.942 | 0.235 | 0.078 | Al‐Sheyab 2012; Berg 2004; Bruzzese 2008; Bruzzese 2011; Joseph 2013 |
| 4 | CHILDSAT*THEORYDRIVEN*runinstudenttime*GOODRELGPAR | 0.846 | 0.756 | 0.106 | 0.064 | Bruzzese 2008; Dore‐Stites 2007; Howell 2005 |
| M1 | 0.862 | 0.81 | 0.432 |
QCA: qualitative comparative analysis.
[Notation: Upper case = condition is present; Lower case = condition is absent; * = logical and; + logical or; Key: HIGHSCHOOL = High School (lower case not in high school); THEORYDRIVEN = Authors explicitly named theory or presented conceptual model for intervention; RUNINSTUDENTTIME = Substantial component run in students' own time (e.g. lunchtime); GOODRELPAR = Good level of reported in engagement and/or developing relationships with parents; CHILDSATB = Children reported as satisfied; SUCCESSFULIMPLEMENTATION = Implementation of intervention successful]
19. Summary of results from consolidated model.
| Consolidated model | Theory driven | Run in children's free time | Child satisfaction | Parents engaged/relationships developed | High school | Successful intervention |
| Pathway 1 | Present | ‐ | Present | ‐ | Present | Yes |
| Pathway 2 | Present | ‐ | ‐ | Present | Present | Yes |
| Pathway 3 | Present | Absent | ‐ | ‐ | Present | Yes |
| Pathway 4 | Present | Absent | Present | Present | ‐ | Yes |
Absent: absence of condition is essential in triggering success.
Present: presence of condition is essential in triggering success.
‐ (symbol): presence or absence of condition is not essential in triggering success.
This solution emphasises the importance of a theory‐driven intervention across all settings for successful implementation. Three of these pathways are specific to high schools. Here, the evidence suggests that in addition to the importance of a theory‐based intervention, good levels of engagement with parents, high levels of child satisfaction, or running the intervention outside the child's own time can lead to a successfully implemented intervention. A pathway that is not specific to high schools reinforces these findings by showing that being theory‐based, fostering high levels of child satisfaction, reporting good levels of parental engagement, and running an intervention outside the child's own time are sufficient conditions for triggering a positive outcome.
As a whole solution, these pathways had a consistency score of 0.862, suggesting that they were sufficient in triggering the outcome. Interventions that are designed with these sets of characteristics are therefore highly likely to be successfully implemented. We also checked whether any of the configurations described also predicted negation of the outcome, but we found no such evidence. Our coverage score of 0.432, which is modest, suggests that other pathways can also trigger successful implementation, which may be explained by factors not explored in these models. We were not able to incorporate risk of bias judgements directly into the QCA solution.
Based on results of QCAs, we intended to include the following conditions in meta‐analyses, either in the form of subgroup analyses or as covariates in meta‐regression. We planned to examine these as binary or ordinal variables in meta‐analyses; they reflect the single conditions thought to most commonly trigger a successful outcome.
Type of school: high school; primary/elementary school; junior/middle school; other.
Theory driven: does the study name a theoretical framework that underpins the intervention design or delivery style?
Parental engagement: did parents engage or participate in the ways they were expected to?
Child satisfaction: did at least 75% of children report satisfaction with the intervention, or did study authors report high levels of satisfaction?
Timing of the intervention: does the intervention interfere with the child's own time (during lunch or after school)?
Due to data constraints, we were not able to explore child satisfaction in meta‐analyses, as very few studies captured this information, and we operationalised parental engagement as 'parental involvement' ‐ whether or not parents were actively included in the intervention ‐ for similar reasons. We entered the factors beginning "Theory driven", "Parental engagement", and "Timing of the intervention" into subgroup analyses as configurations of conditions in an attempt to replicate the results of the QCA (above). We further explored the link between implementation and outcomes in the next section.
Results of synthesis ‐ part 2: meta‐analyses of effectiveness
Primary outcome: asthma symptoms or exacerbations leading to hospitalisation
We extracted effect sizes from seven studies (Atherly 2009; Bruzzese 2011; Clark 2005; Gerald 2006; Horner 2008; Horner 2015; Levy 2006), and we analysed the data from six. Evidence showed that school‐based asthma self‐management interventions were effective in reducing numbers of hospitalisations among children (standardised mean difference (SMD) ‐0.19, 95% confidence interval (CI) ‐0.35 to ‐0.04; participants = 1873; Figure 6Analysis 1.1). Effect sizes from all six studies were in the same direction, and I² and Q statistic values provided no evidence of statistical heterogeneity. Gerald 2006 presented data on the median number of hospitalisations, which were not compatible with other extracted data, although it is worth noting that the median level of hospitalisation appeared higher for the intervention group than for the control group post intervention.
6.
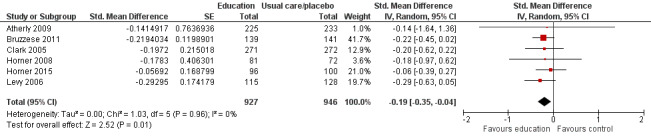
Forest plot of comparison: 1 School‐based asthma interventions vs usual care: outcome: 1.1. Exacerbations leading to hospitalisation.
1.1. Analysis.
Comparison 1 Effects of school‐based asthma interventions vs usual care, Outcome 1 Exacerbations leading to hospitalisation.
Given that we found no indication of heterogeneity in these models and the likelihood that these analyses would be underpowered, we did not conduct further subgroup analyses. We considered sensitivity analyses, although the small number of studies included in the models precluded a full analysis. All but one of the studies ‐ Bruzzese 2011 ‐ reported on cluster randomised trials, and half of the studies originally reported on binary outcomes (Atherly 2009; Clark 2010; Horner 2008), although sensitivity analyses on these factors revealed no significant differences in effect size. Egger's test for publication bias suggested no evidence of publication bias (the P value for the bias coefficient stood at 0.626), although the small number of studies meant that the test and observations of the funnel plot (not displayed) were ultimately underpowered.
The small number of included studies precluded a detailed investigation of the way in which risk of bias influenced the effect size for this outcome. However, two of the largest studies, which contributed three‐fifths of weighting to the pooled effect size, had low or unclear risk of bias across all domains (Bruzzese 2011; Horner 2015), and in the case of Horner 2015, low risk of bias was seen for each domain, apart from blinding of participants and personnel (unclear risk of bias).
Evidence therefore suggests that school‐based asthma self‐management interventions do reduce the frequency of asthma symptoms and exacerbations requiring hospitalisation among children, with a high level of consistency in the direction and magnitude of effect.
Primary outcome: asthma symptoms or exacerbations leading to emergency department visits
We meta‐analysed effect sizes from 13 studies and found clear evidence that school‐based asthma self‐management interventions were effective in reducing the frequency of ED visits (odds ratio (OR) 0.70, 95% CI 0.53 to 0.92; participants = 3883). Gerald 2006 presented data on the median number of hospitalisations, which were not compatible with other extracted data (full details in Table 14), although the median level of ED visits was observed to be slightly lower for the intervention group than for the control group post intervention.
Heterogeneity in the effects of studies was evident, in terms of both magnitude and direction of effect, with three studies having negligible effect sizes (close to one ‐ Atherly 2009; Clark 2005; Horner 2015) and two studies suggesting a negative intervention effect (McGhan 2003; McGhan 2010); this resulted in an I² value of 26%. The number of studies and the level of heterogeneity allowed us to explore potential study characteristics that could help to explain the observed variation.
Subgroup analyses: exacerbations leading to emergency department visits
Subgroup analyses suggested that the heterogeneity shown in Figure 7 ‐ Analysis 1.2 ‐ was not explained by school type (Figure 8), age (Analysis 3.1), or socio‐economic status of children and intervention deliverers involved in the intervention (Analysis 4.1; Analysis 5.1).
7.
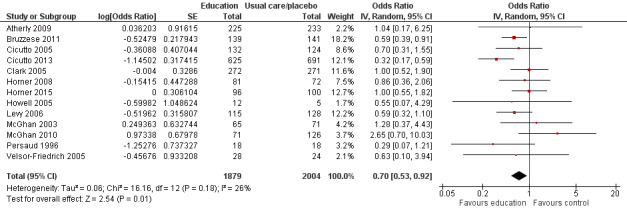
Forest plot of comparison: 1. Effect of school‐based asthma interventions vs usual care, outcome: 1.2. Exacerbations leading to emergency department (ED) visits.
1.2. Analysis.
Comparison 1 Effects of school‐based asthma interventions vs usual care, Outcome 2 Exacerbations leading to emergency department (ED) visits.
8.
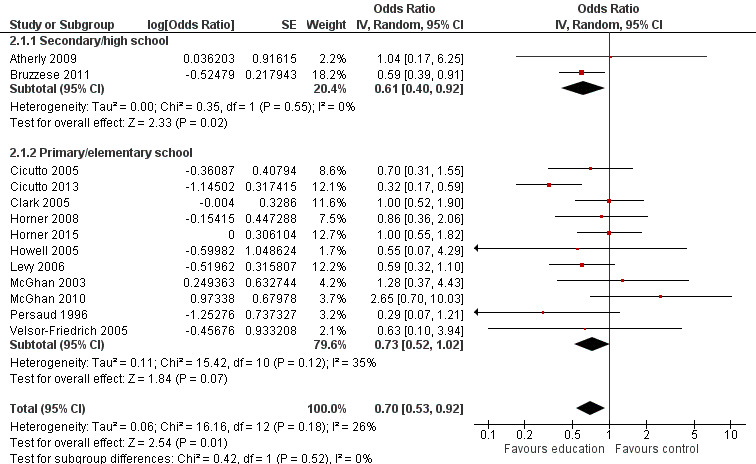
Forest plot of comparison: 2. Effect of school‐based asthma interventions vs usual care subgrouped by school type, outcome: 2.1. Exacerbations leading to emergency department (ED) visits.
3.1. Analysis.
Comparison 3 Effects of school‐based asthma interventions vs usual care subgrouped by age of children, Outcome 1 Exacerbations leading to emergency department (ED) visits.
4.1. Analysis.
Comparison 4 Effects of school‐based asthma interventions vs usual care subgrouped by child socio‐economic status (SES), Outcome 1 Exacerbations leading to emergency department (ED) visits.
5.1. Analysis.
Comparison 5 Effects of school‐based asthma interventions vs usual care subgrouped by involvement of school staff in direct delivery of self‐management skills to children, Outcome 1 Exacerbations leading to emergency department (ED) visits.
We employed subgroup analyses to examine whether any of the intervention conditions that consistently predicted successful implementation in earlier QCAs, namely, explicit use of theory (Analysis 6.1), inclusion of parents (Analysis 7.1), or timing of the intervention (Analysis 8.1), also helped to explain any of the observed heterogeneity in effect sizes. However, we found no evidence that these factors helped to explain heterogeneity.
6.1. Analysis.
Comparison 6 Effects of school‐based asthma interventions vs usual care subgrouped by explicit use of theory, Outcome 1 Exacerbations leading to emergency department (ED) visits.
7.1. Analysis.
Comparison 7 Effects of school‐based asthma interventions vs usual care subgrouped by whether design included active inclusion or participation of parents, Outcome 1 Exacerbations leading to emergency department (ED) visits.
8.1. Analysis.
Comparison 8 Effects of school‐based asthma interventions vs usual care subgrouped by timing of intervention, Outcome 1 Exacerbations leading to emergency department (ED) visits.
We also constructed a variable that attempted to replicate some of the implicants (combinations of intervention characteristics) identified in QCAs that trigger successful implementation; however, results appeared to contradict the findings of earlier analyses. A subgroup of studies that replicated one of the configurations theorised to trigger successful intervention implementation (five studies that were theory driven, did not take place in children's own time, and did not involve school nurses) had inconclusive effect sizes as a group (OR 0.85, 95% CI 0.47 to 1.52); in contrast, a subgroup of studies that did not replicate a configuration were found to trigger successful intervention implementation in the QCAs (OR 0.67, 95% CI 0.47 to 0.94). We also created a variable based on a count of intervention characteristics found to trigger successful implementation in our earlier QCAs, and we tested these in subgroup analyses. We constructed a variable reflecting a count of three of the conditions generally found to trigger successful implementation (theory driven, not run in children's own time, and parental engagement (assessed by active involvement of parents)), whereby studies could include zero to three of these 'ingredients'. All studies included in the meta‐analyses had incorporated at least one of these conditions, and subgroup analyses suggested that the number of components was inversely related to effect size, with studies with one component (three studies; OR 0.56, 95% CI 0.33 to 0.97) or two components (seven studies; OR 0.67, 95% CI 0.49 to 0.94) having lower effect sizes than the three studies that included all three components (OR 1.48, 95% CI 0.65 to 3.40); however, the test for differences between subgroups did not suggest that these differences were significant, and moderate heterogeneity remained within one of the subgroups. Among the latter group of studies, two of the three studies evaluated the effectiveness of the RAP (Roaring Adventures of Puff) intervention (McGhan 2003; McGhan 2010). One of these studies provided evidence of a baseline imbalance that could influence the outcome (McGhan 2003), whereby the proportion of intervention group children who had been admitted to an ED was almost ten percentage points higher in the intervention group (23.7%) than in the control group (14%). The second study provided evidence that the mean number of ED visits was higher post intervention in the control group (McGhan 2010), although study authors did not present full data allowing for extraction of the mean number of visits, and the measure used reflected the odds of reporting ED visits.
Sensitivity analyses: exacerbations leading to emergency department visits
We conducted sensitivity analyses to explore the impact of decisions to transform or combine the data. We detected no differences between effect sizes that were originally measured through binary effect sizes (ORs) and those that were originally measured through continuous measures (SMDs). We detected no differences in whether studies assessed intervention effects at 12 months, four to seven months, or within three months (intervals reflecting the spread of studies). All but two studies ‐ Bruzzese 2011 and Persaud 1996 ‐ had randomised children at the school level (cluster RCTs); little evidence suggested that this distinction explained heterogeneity in effect sizes.
In assessing the impact of study quality on effect sizes, we undertook supplementary analyses using meta‐regression in STATA, and, due to the limited number of studies, we combined categories of high and unclear risk when assessing the impact of study quality. We classified none of the studies included in the meta‐analysis for ED visits as having high risk of bias for random sequence generation, although we deemed that eight studies were at unclear risk. Results of sensitivity analyses provided moderate evidence that studies had high or unclear risk of selection bias with respect to breaches in allocation concealment with significantly different effect sizes (OR 0.86, 95% CI 0.64 to 1.16), compared to the three studies that we deemed to have low risk of bias (OR 0.51, 95% CI 0.33 to 0.78). Finally, evidence showed that studies with low risk of bias with respect to collection of outcome data and blinding of collectors were significantly more effective (OR 0.58, 95% CI 0.41 to 0.81) than the seven studies with unclear or high risk of bias (OR 1.04, 95% CI 0.69 to 1.58). Differences in the risk of bias classification for other domains did not significantly explain heterogeneity in effect sizes between studies. We conducted sensitivity analyses to explore the impact of a random‐effects specification on pooled effect size, noting only moderate differences in point estimates between fixed‐effect (OR 0.68, 95% CI 0.55 to 0.85) and random‐effects models (OR 0.70, 95% CI 0.53 to 0.92); however, the level of heterogeneity (I² = 26%) suggested that studies were not measuring a single common effect size, thereby undermining the fixed‐effect assumption (and model results).
Our investigations into the potential impact of publication bias revealed that neither the funnel plot nor Egger's test was indicative of publication bias (the bias coefficient provided weak evidence that smaller studies differed systematically from studies with larger sample sizes).
Evidence therefore suggests that school‐based asthma self‐management interventions do reduce the frequency of asthma symptoms and exacerbations requiring emergency care among children, although variation in the magnitude and direction of effect was not explained coherently by planned subgroup analyses.
Primary outcome: absences from school
Ten studies contributed to our meta‐analyses of effects of interventions on school absences, although there was uncertainty as to whether school‐based self‐management interventions had an impact on reducing absences from school (SMD ‐0.07, 95% CI ‐0.22 to 0.08; participants = 4609; Analysis 1.3; Figure 9). These studies showed substantial heterogeneity between effect size estimates, with I² estimated at 70%. Effect sizes from half of the studies included in the meta‐analysis indicated that the intervention had a negative impact in slightly or significantly increasing the number of school absences in the intervention group relative to the control group (Gerald 2006; Gerald 2009; Howell 2005; McGhan 2010; Splett 2006).
1.3. Analysis.
Comparison 1 Effects of school‐based asthma interventions vs usual care, Outcome 3 Absence from school.
9.
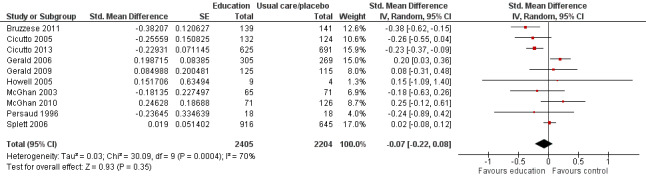
Forest plot of comparison: 1. Effect of school‐based asthma interventions vs usual care, outcome: 1.3. Absence from school.
Subgroup analyses: absences from school
We undertook subgroup analyses to explore study‐level characteristics that could explain this between‐study heterogeneity, although it is worth noting that these analyses were likely to be underpowered and to represent indicative factors that could explain observed differences in the direction and magnitude of effect sizes across studies. The only study included in the meta‐analyses that focused on high schools (and consequently older children) was highly effective in reducing school absences (SMD ‐0.38, 95% CI ‐0.62 to ‐0.15) (Bruzzese 2011); this study appeared to drive much of the heterogeneity explained by subgroup analyses examining school type and child age (Figure 10; Analysis 2.2; Analysis 3.2). Studies that included 25% to 50% children from lower socio‐economic backgrounds were significantly more effective in reducing levels of school absence (SMD ‐0.23, 95% CI ‐0.36 to ‐0.09; studies = 2) than studies with greater numbers of children from deprived backgrounds (over 50%) for whom the effect was negligible (SMD 0.01, 95% CI ‐0.09 to 0.11; 2 studies) and studies in which less than 25% of children were from deprived backgrounds or in which this was unclear, where the pooled effect size indicated negligible effect (SMD ‐0.02, 95% CI ‐0.29 to 0.24; 6 studies).
10.
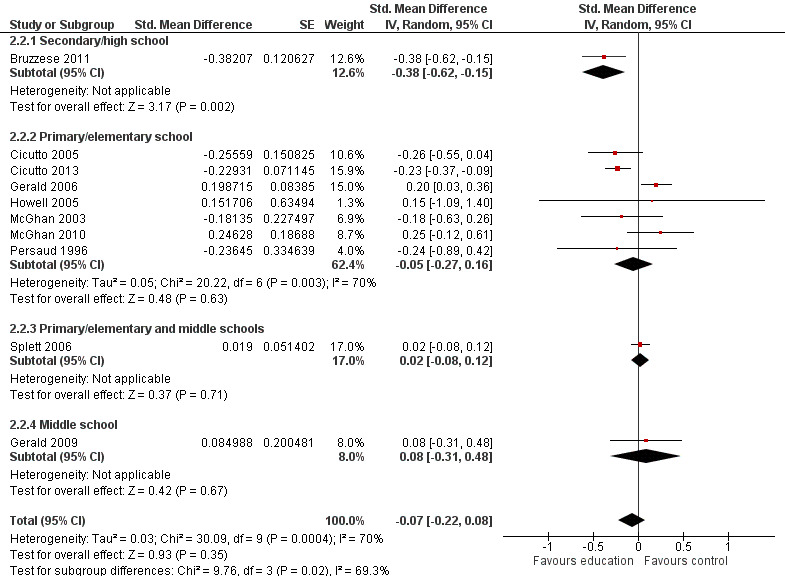
Forest plot of comparison: 2. Effect of school‐based asthma interventions vs usual care subgrouped by school type, outcome: 2.2. Absence from school.
2.2. Analysis.
Comparison 2 Effects of school‐based asthma interventions vs usual care subgrouped by school type, Outcome 2 Absence from school.
3.2. Analysis.
Comparison 3 Effects of school‐based asthma interventions vs usual care subgrouped by age of children, Outcome 2 Absence from school.
Studies that involved existing school staff (teachers or school nurses) in delivery of the intervention were significantly less effective (SMD 0.08, 95% CI ‐0.08 to 0.24; 3 studies) than studies in which the intervention was mainly delivered and facilitated by stakeholders who were external to the school (SMD ‐0.17, 95% CI ‐0.32 to ‐0.02; 7 studies; Analysis 5.2;Figure 11). Findings of the earlier QCA show that involvement of internal stakeholders within the school in delivery of the intervention did not always lead to successful intervention implementation, but they also show that involving school staff in intervention delivery may be one of a configuration of conditions that trigger successful implementation, none of which are sufficient alone. Similar processes may occur around their role in reducing the level of school absence.
5.2. Analysis.
Comparison 5 Effects of school‐based asthma interventions vs usual care subgrouped by involvement of school staff in direct delivery of self‐management skills to children, Outcome 2 Absence from school.
11.
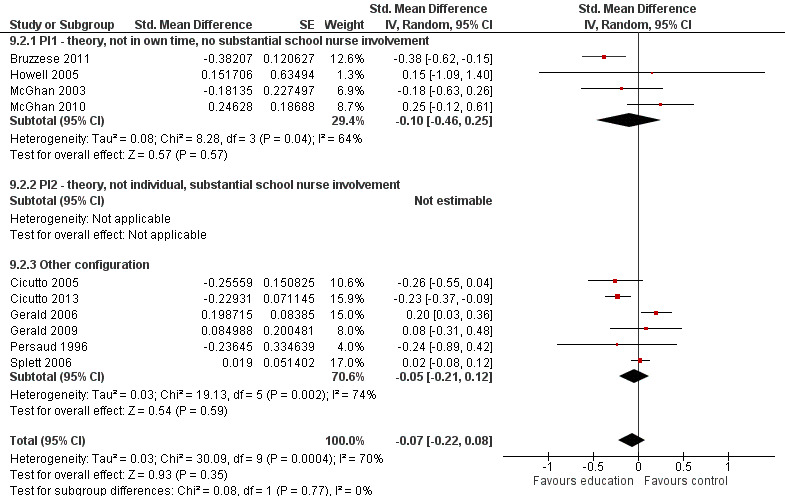
Forest plot of comparison: 10. Effect of school‐based asthma interventions vs usual care subgrouped by configuration of conditions (iI), outcome: 10.3. Absence from school.
We conducted subgroup analyses involving the conditions and configurations found to be sufficient in earlier QCAs to trigger successful implementation. But these findings did not significantly explain the heterogeneity in effect sizes, with two exceptions. Analysis 8.2 provided evidence that interventions that took place during the child's own time had significantly greater impacts in reducing school absence (SMD ‐0.23, 95% CI ‐0.36 to ‐0.11; 2 studies) than those that took place at another point in the school day (SMD ‐0.01, 95% CI ‐0.18 to 0.16; 8 studies), although a substantial level of heterogeneity remained among this latter group of studies (I² = 62%). We noted strong evidence around the role of theory (Analysis 6.2), whereby studies that reported drawing upon a defined theoretical framework had a significantly more impactful pooled effect size (SMD ‐0.20, 95% CI ‐0.36 to ‐0.04; 6 studies) than studies that did not (SMD 0.08, 95% CI ‐0.05 to 0.20; 4 studies). Although moderate levels of heterogeneity remained (I² = 41% for studies that explicitly drew upon theory and I² = 28% for those that did not), and even though interpretation of these results is not straightforward (see discussion), this result indicates that theory‐driven studies may achieve better outcomes with respect to this domain.
8.2. Analysis.
Comparison 8 Effects of school‐based asthma interventions vs usual care subgrouped by timing of intervention, Outcome 2 Absence from school.
6.2. Analysis.
Comparison 6 Effects of school‐based asthma interventions vs usual care subgrouped by explicit use of theory, Outcome 2 Absence from school.
Sensitivity analyses: absences from school
We conducted sensitivity analyses to explore whether the following factors, reflecting study design or analytical decisions made during the review process, helped to explain heterogeneity in effect size: (I) transformations were made to the original effect size (conversions between ORs and SMDs; Chinn 2000); (ii) cluster RCT or not; (iii) the data collection period; and (iv) the study's risk of bias . We found no evidence to suggest that transformations in effect sizes explained heterogeneity, and no evidence indicated that the unit of randomisation (school vs child) explained variation in effect size. The three studies that collected absence data within three months post intervention (or for which the collection date was unclear) did exhibit a weaker effect in reducing school absences (Gerald 2006; Howell 2005; Persaud 1996), with Gerald 2006 and Howell 2005 showing a negative intervention impact, although this was not significantly different from studies that assessed absences over the 12 months post intervention. Little evidence suggests that risk of bias influenced the effect size obtained; however, studies that had taken steps to blind assessment of outcomes and to avoid detection bias had a greater impact in reducing school absences (SMD ‐0.27, 95% CI ‐0.38 to ‐0.17; 3 studies) than studies that did not take these steps (SMD ‐0.07, 95% CI ‐0.02 to 0.16; 7 studies).
We investigated the potential impact of publication bias by examining a funnel plot and the results of Egger's test. These tests did not provide strong evidence that data were impacted by publication bias (the bias coefficient provided weak evidence that smaller studies differed systematically from studies with larger sample sizes). We examined differences between the fixed‐effect model and the random‐effects model reported above. The fixed‐effect model showed that the pooled point estimate remained similar, but with a less conservative confidence interval (SMD ‐0.05, 95% CI ‐0.11 to 0.02). However the level of heterogeneity was substantial (I² = 70%), which suggested that these studies were not measuring a single common effect size and thereby undermined the fixed‐effect assumption (and model results).
Evidence from the overall pool of studies therefore suggests that school‐based asthma self‐management interventions did not have an impact in reducing absence from school, although variation in direction and magnitude was substantial. Planned subgroup analyses assisted in identifying particular groups of studies and did, or did not, have a beneficial effect.
Primary outcome: days of restricted activity
Three studies contributed data to our meta‐analysis of the impact of school‐based asthma self‐management interventions in reducing the number of days of restricted activity that children experienced (Bruzzese 2011; Cicutto 2005; Cicutto 2013). These studies provided evidence that the intervention mode could reduce the number of days of restricted activity experienced (SMD ‐0.30, 95% CI ‐0.41 to ‐0.18; 1852 participants; 3 studies; Analysis 1.4), albeit based on a limited number of studies, two of which evaluated the same intervention design (the Roaring Adventures of Puff) (Cicutto 2005; Cicutto 2013). All three studies provided relatively consistent evidence around the direction and magnitude of effect (I² = 0%). Reporting on the results of subgroup analyses is not meaningful in the presence of low heterogeneity and small numbers of studies, and many sensitivity analyses could not be conducted for the same reason, although it is worth noting that we rated none of the included studies as having high risk of bias for any domain assessed for the outcome evaluation risk of bias tool (Figure 12).
1.4. Analysis.
Comparison 1 Effects of school‐based asthma interventions vs usual care, Outcome 4 Days of restricted activity.
12.
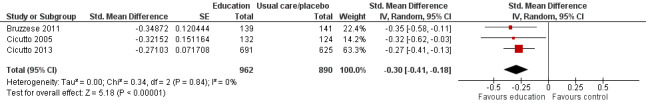
Forest plot of comparison: 1. Effect of school‐based asthma interventions vs usual care, outcome: 1.4. Days of restricted activity.
Secondary outcome: unplanned visits to a medical provider
From a meta‐analysis of five studies (Analysis 1.5), evidence shows that school‐based asthma self‐management interventions did reduce the number of unplanned or unscheduled visits to a medical provider (OR 0.74, 95% CI 0.60 to 0.90; 3490 participants; 5 studies). Despite inconsistency in the magnitude (and direction) of effect in the case of McGhan 2003, which indicated a small negative intervention impact, the meta‐analysis provided little evidence of statistical heterogeneity (I² = 0%). As was the case above, the small number of studies and the absence of heterogeneity did not support meaningful investigation of subgroup analyses, nor the opportunity to undertake a full assessment of some of the assumptions made in pooling the data (see Table 14 for further details on the derivation of effect sizes). Similarly, we were not able to assess the potential impact of publication bias. Two studies contributed almost 75% towards the pooled effect size (Bruzzese 2011; Cicutto 2013), and we rated neither study as having high risk of bias in any domain (Figure 13).
1.5. Analysis.
Comparison 1 Effects of school‐based asthma interventions vs usual care, Outcome 5 Unplanned visit to hospital or GP due to asthma symptoms.
13.
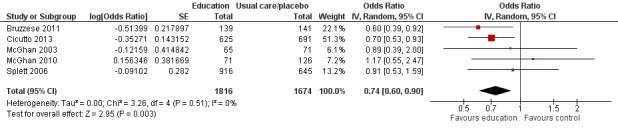
Forest plot of comparison: 1. Effect of school‐based asthma interventions vs usual care, outcome: 1.5. Unplanned visit to hospital or GP due to asthma symptoms.
Secondary outcome: experience of daytime and night‐time symptoms
As described in the section on Included studies, trialists adopted different strategies in measuring the impact of interventions on children's daytime and night‐time symptoms. We constructed separate models of meta‐analysis for studies reporting on daytime symptoms (Analysis 1.6) and night‐time symptoms (Analysis 1.7), although some variation remained in the way in which symptom data were collected (Table 14).
1.6. Analysis.
Comparison 1 Effects of school‐based asthma interventions vs usual care, Outcome 6 Experience of daytime and night‐time symptoms ‐ daytime symptoms.
1.7. Analysis.
Comparison 1 Effects of school‐based asthma interventions vs usual care, Outcome 7 Experience of daytime and night‐time symptoms ‐ night‐time symptoms.
Uncertainty surrounded the question of whether school‐based self‐management interventions reduced the level of daytime symptoms that children experienced (SMD ‐0.15, 95% CI ‐0.32 to 0.02; I² = 0%; 1065 participants; 5 studies), with the confidence interval just crossing the line of no effect (zero). However, study reports show consistency in the direction of effects (Figure 14). Even greater uncertainty surrounded whether self‐management interventions in schools reduced the level of night‐time symptoms reported by children in random effects meta‐analysis (SMD ‐0.18, 95% CI ‐0.52 to 0.15; I² = 40%; 459 participants; 4 studies), with two studies providing weak evidence that night‐time symptoms actually increased among children receiving school‐based asthma self‐management interventions. We performed sensitivity analyses using a fixed‐effect model, with the pooled effect size across the four studies indicating that night‐time symptoms decreased (SMD ‐0.26, 95% CI ‐0.46 to ‐0.06; 4 studies), although given the inconsistency in the direction of effect, the underlying assumptions of the fixed‐effect model cannot be substantiated, and the random‐effects model may provide a more realistic estimate of intervention effects on night‐time symptoms.
14.
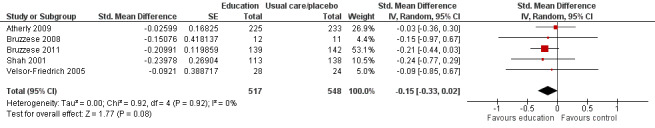
Forest plot of comparison: 1. Effect of school‐based asthma interventions vs usual care, outcome: 1.6. Experience of daytime and night‐time symptoms ‐ daytime symptoms.
Reporting on the results of subgroup analyses was not meaningful with the few included studies; other sensitivity analyses could not be conducted for the same reason. One study that measured change in daytime symptoms showed a weak effect of the intervention in lowering the level of daytime symptoms (see Table 14) (Clark 2010).
Secondary outcome: lung function
We extracted outcomes measuring trial impacts on lung function from five studies, although we did not combine these data in meta‐analyses due to conceptual (and statistical) heterogeneity. We have presented these outcomes in full in Table 14.
Secondary outcome: use of reliever therapies
Four studies reported on the use of reliever therapies among children who had received self‐management interventions in school (Table 14), and we included effect sizes from two studies with clinical and conceptual equivalence in a random‐effects meta‐analysis (Figure 15; Analysis 1.8). The pooled result provided uncertain evidence on the impact of the intervention on children's use of reliever therapies (OR 0.52, 95% CI 0.15 to 1.81; 437 participants; 2 studies). The level of heterogeneity between studies was substantial (I² = 68%), although both were somewhat consistent in the direction of effect, indicating lower odds of (frequent) reliever therapy usage. One study had low or unclear risk of bias across all domains considered (Gerald 2009), and we judged McGhan 2010 to have high risk of bias in terms of attrition bias and selective reporting.
15.
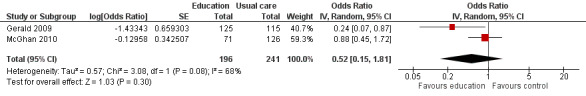
Forest plot of comparison: 1. Effect of school‐based asthma interventions vs usual care, outcome: 1.8. Use of reliever therapies, e.g. beta₂‐agonists.
1.8. Analysis.
Comparison 1 Effects of school‐based asthma interventions vs usual care, Outcome 8 Use of reliever therapies, e.g. beta₂‐agonists.
Secondary outcome: corticosteroid dosage and use of add‐on therapies
We found unclear evidence on the impact of interventions on children's use of corticosteroids and add‐on therapies (OR 1.25, 95% CI 0.88 to 1.77; 614 participants; 3 studies; Figure 16; Analysis 1.9). We noted no evidence of statistical heterogeneity between these study impacts on corticosteroid usage (I² = 0%), and as reporting on the results of subgroup analyses is not meaningful with low heterogeneity and few studies, we could not conduct other sensitivity analyses for the same reason. We deemed one study included in the model to have low risk of bias for all domains except blinding of participants and personnel, for which we deemed the risk to be unclear (Bruzzese 2011); we deemed the other two studies to have high risk of bias in one and two domains (McGhan 2003; McGhan 2010), respectively, with both deemed to have high risk of attrition bias from incomplete and unexplained dropouts at outcome data collection.
16.
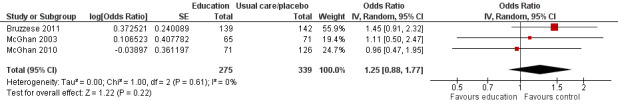
Forest plot of comparison: 1. Effect of school‐based asthma interventions vs usual care, outcome: 1.9. Corticosteroid dosage and/or use of add‐on therapies (usage of).
1.9. Analysis.
Comparison 1 Effects of school‐based asthma interventions vs usual care, Outcome 9 Corticosteroid dosage and/or use of add‐on therapies (usage of).
We included two studies reporting appropriate usage of corticosteroids and add‐on therapies. Although the direction of findings differed substantially between studies, resulting in considerably high levels of heterogeneity (I² = 87%), we did not estimate a pooled effect size (Analysis 1.10).
1.10. Analysis.
Comparison 1 Effects of school‐based asthma interventions vs usual care, Outcome 10 Corticosteroid dosage and/or use of add‐on therapies (appropriate usage of).
Secondary outcome: health‐related quality of life
Nine studies provided data on the effectiveness of school‐based self‐management interventions in improving children's quality of life. Because of conceptual differences in the way in which the outcome was measured, one meta‐analysis of seven studies explored intervention impacts on quality of life measures assessed through standardised mean differences using mainly the Paediatric Asthma Quality of Life Questionnaire (PAQLQ) (Figure 17; Analysis 1.11), and provided evidence of effectiveness (SMD 0.27, 95% CI 0.18 to 0.36; 2587 participants; 7 studies). This model provided no evidence of statistical heterogeneity in effectiveness (I² = 0%), with all studies providing estimates of positive improvements, although these were not all statistically significant in all studies. The low level of heterogeneity and the few included studies meant that conducting subgroup analyses was not appropriate. We deemed that five of the seven studies included in the meta‐analysis were at high risk of bias in at least one domain (Al‐Sheyab 2012; Henry 2004; Horner 2008; Howell 2005; Kintner 2009), although the two studies with low or unclear risk of bias in all domains contributed over 60% of the weighted effect size (Cicutto 2005; Cicutto 2013). Explorations of the funnel plot and Egger's test were underpowered, and publication bias could not be adequately tested.
17.
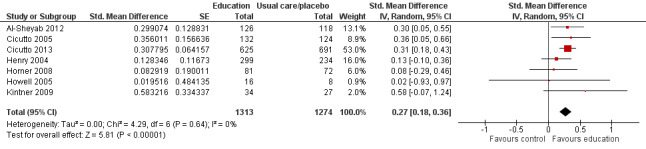
Forest plot of comparison: 1. Effect of school‐based asthma interventions vs usual care, outcome: 1.11. Health‐related quality of life (SMD).
1.11. Analysis.
Comparison 1 Effects of school‐based asthma interventions vs usual care, Outcome 11 Health‐related quality of life (SMD).
A second meta‐analysis involving eight studies also provided evidence that children in intervention groups had higher HRQoL than children in control groups (MD 0.35, 95% CI 0.06 to 0.64; 2950 participants; 8 studies) based on PAQLQ results at follow‐up (Analysis 1.12). The mean difference, while again indicating that the impact did not cross the threshold of no effect, fell below 0.5 ‐ the threshold considered to indicate a clinically significant change in HRQoL on this scale. Heterogeneity among studies was considerably high (I² = 81%). One study in particular had relatively high levels of baseline imbalance, and a sensitivity analysis removing this value resulted in a lower point estimate but much lower levels of heterogeneity (MD 0.21, 95% CI 0.07 to 0.36; I² = 24%) (Al‐Sheyab 2012). We included this same study in Analysis 1.11, although we used different data to obtain an effect size (P value and sample size). We did not further explore heterogeneity because included studies were few and, similarly, explorations of the funnel plot and Egger's test were underpowered; therefore, we could not adequately assess publication bias. We deemed that four of the studies included in Analysis 1.12 were at high risk of bias in at least one domain. A further sensitivity analysis involving constructing a fixed‐effect model yielded a similar point estimate (MD 0.32, 95% CI 0.21 to 0.43; I² = 81%; 8 studies), although the considerably high level of heterogeneity indicates that this is not a suitable analytical framework.
1.12. Analysis.
Comparison 1 Effects of school‐based asthma interventions vs usual care, Outcome 12 Health‐related quality of life (MD).
Despite the additional study included in Analysis 1.12, we consider the results from Analysis 1.11 to be more reliable because of the considerably high heterogeneity observed in the model for MD and the insufficient number of studies to fully explore drivers of this heterogeneity.
Therefore, evidence suggests that school‐based asthma self‐management interventions do improve children's quality of life, although this finding may not reach a point of clinically significant improvement. Although all studies provided an indication of a positive beneficial effect, variation in the size of the effect was substantial.
Secondary outcome: withdrawal from the study
Meta‐analysis provided no evidence that participation in the intervention was linked to withdrawal from the study (OR 1.14, 95% CI 0.92 to 1.43; 3442 participants; 13 studies; Figure 18; Analysis 1.13). We detected no substantial statistical heterogeneity (I² = 0%), although some qualitative differences were apparent between studies that reported very low levels of withdrawal among those receiving treatment relative to those in control groups (Bruzzese 2008), and relative to those with very high levels of withdrawal (Kintner 2009; Patterson 2005); in neither case would the level of withdrawal be described as problematic (not exceeding 25% of participants), and the stark relative effect was driven by very small sample sizes in some studies (Bruzzese 2008; Kintner 2009).
18.
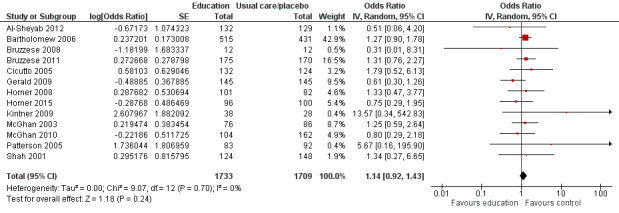
Forest plot of comparison: 1. Effect of school‐based asthma interventions vs usual care, outcome: 1.13. Withdrawal from the study.
1.13. Analysis.
Comparison 1 Effects of school‐based asthma interventions vs usual care, Outcome 13 Withdrawal from the study.
Despite the low level of heterogeneity, we have presented subgroup analyses because of the link between this outcome and the QCAs presented earlier. When we replicated one set of configurations in the subgroup analysis to mirror QCA findings (Analysis 9.3), we found weak/uncertain evidence to suggest that studies that used theory, while avoiding running the intervention in children's own time and having no substantial school staff involvement, were less likely to have children drop out before outcomes were assessed (OR 0.88, 95% CI 0.55 to 1.40; 4 studies) when compared with studies with other configurations of conditions (OR 1.23, 95% CI 0.95 to 1.58; 8 studies). We also found no evidence that withdrawal from the intervention was associated with school type (Figure 19).
9.3. Analysis.
Comparison 9 Effects of school‐based asthma interventions vs usual care subgrouped by configuration of conditions, Outcome 3 Withdrawal from the study.
19.
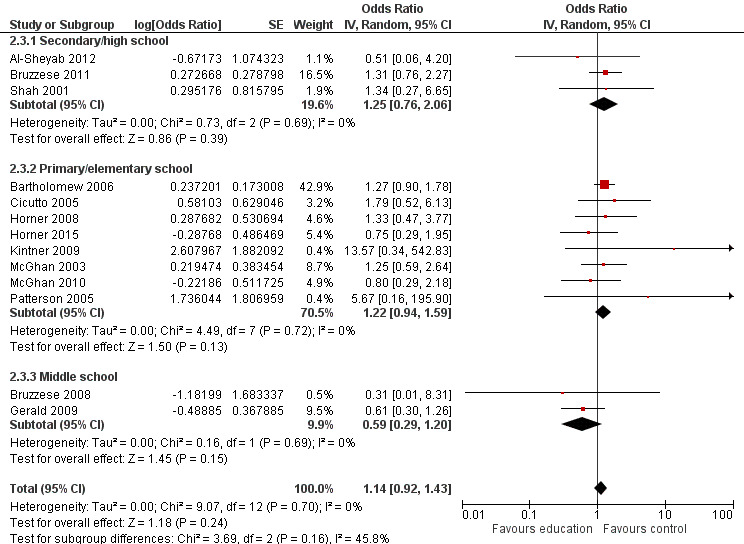
Forest plot of comparison: 2. Effect of school‐based asthma interventions vs usual care subgrouped by school type, outcome: 2.3. Withdrawal from the study.
Subgroup analyses seeking to reveal patterns of heterogeneity in the odds of withdrawal did not show that timing of assessment, unit of randomisation (cluster vs individually randomised trials), or risk of bias explained patterns of withdrawal. This included risk of attrition bias assessments, although the meta‐analysis explored differential patterns of attrition and did not account for instances in which both intervention and control groups had high levels of attrition (as was the case when risk of attrition bias was assessed). We found no evidence to show that publication bias was an issue in terms of withdrawal data. Because of the low level of statistical heterogeneity, fixed‐effect and random‐effects specifications for the meta‐analyses were equivalent, with one study accounting for 46% of the weighting; we classified this study as having high risk of bias in three domains and unclear risk of bias in the remaining four domains (Bartholomew 2006).
Results of synthesis ‐ part 3: adjunct meta‐analyses exploring the link between implementation and effectiveness of school‐based asthma self‐management interventions
We conducted adjunct meta‐analyses to explore whether interventions that were deemed successful in terms of implementation were also deemed successful in terms of their effectiveness (see Figure 2), using a subset of studies contained within the process evaluations. For inclusion in these analyses, we considered studies that included a control group; however studies could have employed randomisation or quasi‐experimental methods, and control group children could have received an alternative intervention that might have included an asthma component.
Because of conceptual and methodological differences in study design, these studies provide indicative evidence only pertaining to the impact of self‐management interventions on children's asthma outcomes, but they help us to establish links between implementation factors and asthma outcomes. Researchers defined successful implementation the same way it was defined in our QCA, and this represented a combined indicator around attrition, adherence, and dosage. We considered two outcomes ‐ ED visits and school absences ‐ when we found sufficient studies to form a meta‐analysis. Both models included effect sizes from seven studies, with five studies in each appearing in earlier meta‐analyses (with studies considered as process and outcome evaluation studies (Bruzzese 2011; Cicutto 2013; Horner 2015; Howell 2005; Levy 2006)), and two studies in each meta‐analysis included as process evaluation studies only (Joseph 2010; Joseph 2013).
Meta‐analysis of ED visits shows that the included interventions were successful in reducing the number of ED visits (Figure 20; Analysis 11.1), but with a high I² value (52%) signalling substantial levels of heterogeneity. Subgroup analyses, based on implementation scores, indicated that studies classified as successfully implemented had a greater impact in reducing ED visits (SMD ‐0.26, 95% CI ‐0.48 to ‐0.04; 4 studies) than studies that were not as successful (SMD ‐0.09, 95% CI ‐0.28 to 0.10; 3 studies), although this difference was not statistically significant (P value for subgroup differences = 0.26). Meta‐analysis of the impact of self‐management interventions provided uncertain evidence that these interventions were successful in reducing children's absences from school (SMD ‐0.12, 95% CI ‐0.28 to 0.04; 7 studies). However, subgroup analyses based on the combined implementation score indicate that studies that were successfully implemented had significantly higher effect sizes (SMD ‐0.28, 95% CI ‐0.39 to ‐0.18; 3 studies) than those that were not successfully implemented (SMD 0.04, 95% CI ‐0.09 to 0.18; Figure 21).
20.
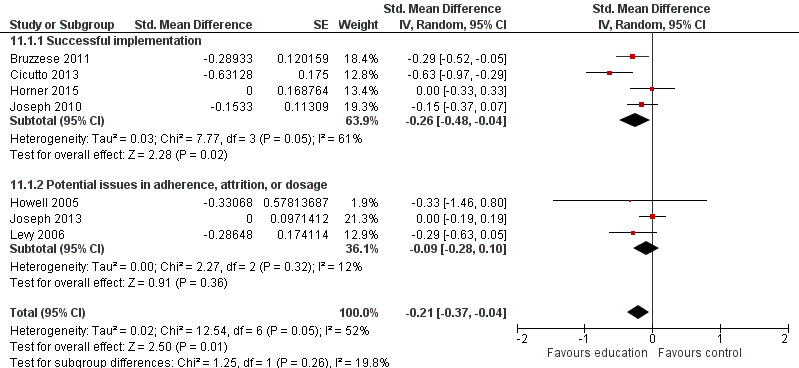
Forest plot of comparison: 11. Adjunct analyses ‐ impact of Implementation on selected outcomes, outcome: 11.1. Exacerbations leading to emergency department (ED) visits.
11.1. Analysis.
Comparison 11 Adjunct analyses ‐ impact of Implementation on selected outcomes, Outcome 1 Exacerbations leading to emergency department (ED) visits.
21.
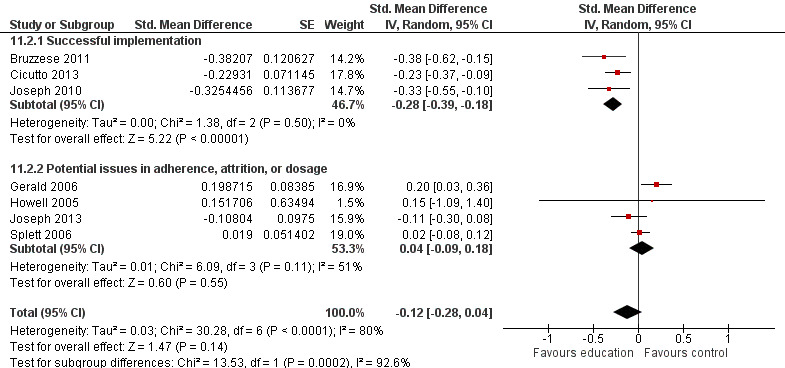
Forest plot of comparison: 11. Adjunct analyses ‐ impact of Implementation on selected outcomes, outcome: 11.2. Absence from school.
In both models, had the focus been restricted to well‐implemented studies only, the conclusions would have changed, and these studies would have provided evidence that school‐based asthma self‐management interventions were effective in reducing these outcomes. Although restricted to selected outcomes and a subset of studies, these models help to illuminate the links between successful implementation and intervention effectiveness, and provide justification for meta‐analyses based on earlier QCAs to test emerging hypotheses.
Part 4: update of the logic model
Figure 22 presents an updated logic model. This is a graphical depiction of synthesised evidence showing that school‐based asthma interventions have a positive impact in reducing healthcare usage, improving quality of life (albeit not at a clinically meaningful level), and reducing days of restricted activity (shaded green). These were termed 'intermediate outcomes' in our original model (Figure 1), although some of the pathways through which these improvements are achieved remain poorly understood, particularly around proximal outcomes including lung function and daytime/night‐time symptoms (shaded blue and grey). We found evidence of a link between successful implementation (through results presented in part 3) and improved outcomes, although Figure 22 shows that other factors around the intervention design may directly lead to improvement in 'intermediate' outcomes. Of these, being theory driven is likely to be the most important element leading to successful implementation, and later, successfully improving children's outcomes, although the logic model shows that other conditions are likely to be important in certain circumstances.
22.
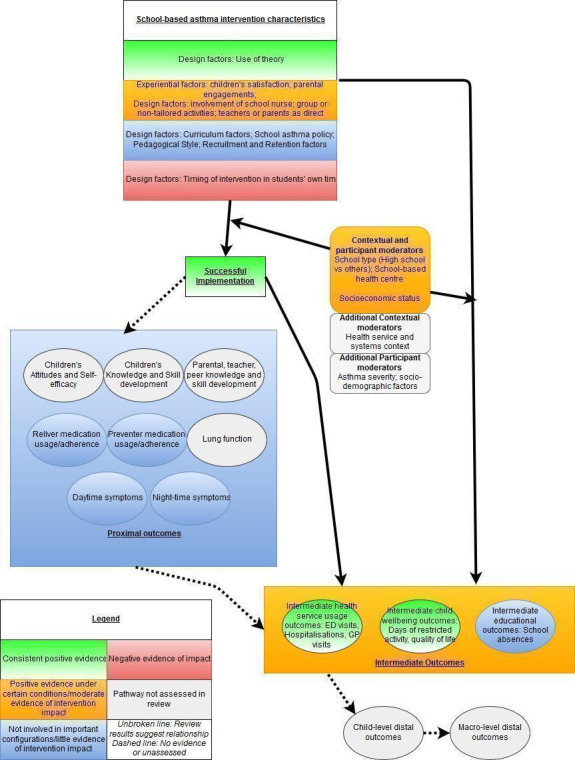
Use of QCA alongside meta‐analysis has helped to disentangle the ways that school‐based asthma interventions 'work' to a certain extent. The logic model helps to show the strength of evidence for many parts of the causal chain but also shows gaps in evidence on which future reviewers may focus their efforts (boxes shaded grey in Figure 22), including (I) establishing which proximal outcomes are important elements of the causal chain between intervention and intermediate outcomes; (ii) improving understanding of the role of contextual and participant characteristics; and (iii) examining distal characteristics and stability of improved outcomes.
Discussion
Summary of main results
Summary and further description of qualitative comparative analysis (QCA) results
One of the most consistently positive conditions that appeared in configurations triggering a successfully run intervention was a named theoretical framework described as underpinning the intervention (Table 21). However, a diverse set of theoretical standpoints were represented (see Description of studies), and we are unable to attribute a successful intervention to a single conceptual or theoretical framework. Merely the use of named or explicitly expressed theory, in conjunction with other conditions, led to better implementation. These configurations also included interventions not run in children's own time, good levels of engagement from parents and satisfaction from children reported, and some configurations specific to high schools. It is not clear whether the theories used to underpin an intervention were equally suitable, and we were not able to ascertain how the theoretical framework was used to shape or inform different stages of the intervention.
20. Summary of QCA results based on intermediate solutions.
| Model 1. Setting and participant features | School health centre | High school | Parents direct intervention recipients | Teachers direct intervention recipients | School nurses/others direct intervention recipients | Successful intervention | |
| Pathway 1 | Present | Present | Present | Absent | ‐ | Yes | |
| Pathway 2 | Absent | Present | Absent | ‐ | ‐ | Yes | |
| Pathway 3 | Absent | ‐ | Absent | Absent | Absent | Yes | |
| Pathway 4 | Present | Present | Present | ‐ | Present | Yes | |
| Model 2. Recruitment and retention processes | Additional marketing materials | Provision of incentives | Provision of catch‐up sessions | Provision of reminders | Successful intervention | ||
| ‐ | ‐ | ‐ | ‐ | ‐ | No solution found | ||
| Model 3. Curriculum, pedagogy, and intervention emphasis | Focus on establishing alliances with care providers | Focus on asthma symptom recognition and management | Tailored content | Emphasis on personal responsibility | Interactive pedagogical style | Diverse pedagogical style | Successful intervention |
| ‐ | ‐ | ‐ | ‐ | ‐ | No solution found | ||
| Model 4. Modifiable intervention processes | Theory driven | Run in class time | Run in students’ free time | School nurse key role in delivery or teaching | Personalised or individual 1‐to‐1 instruction | Successful intervention | |
| Pathway 1 | Present | ‐ | Absent | Absent | ‐ | Yes | |
| Pathway 2 | Present | ‐ | ‐ | Present | Absent | Yes | |
| Model 5. Stakeholder engagement | School asthma policy | Child satisfaction | Teachers engaged/ relationships developed | Parents engaged/ relationships developed | School nurses engaged/ relationships developed | Successful intervention | |
| Pathway 1 | Absent | ‐ | ‐ | Present | Absent | Yes | |
| Pathway 2 | ‐ | Present | ‐ | ‐ | Absent | Yes | |
| Model 6. Consolidated model | Theory driven | Run in students’ free time | Child satisfaction | Parents engaged/ relationships developed | High school | Successful intervention | |
| Pathway 1 | Present | ‐ | Present | ‐ | Present | Yes | |
| Pathway 2 | Present | ‐ | ‐ | Present | Present | Yes | |
| Pathway 3 | Present | Absent | ‐ | ‐ | Present | Yes | |
| Pathway 4 | Present | Absent | Present | Present | ‐ | Yes |
Absent: absence of condition is essential in triggering success.
Present: presence of condition is essential in triggering success.
QCA: qualitative comparative analysis.
‐ (symbol): presence or absence of condition is not essential in triggering success.
We found that good levels of engagement from parents and positive experiences among children, in combination with other conditions, were sufficient conditions for a successful intervention. Positive parental engagement reflected high levels of co‐operation in providing information to trialists, as noted by study authors (Dore‐Stites 2007; Joseph 2013), or more active forms of engagement, including co‐operation with home or school visits (Engelke 2013; Howell 2005), attendance at seminars (Bruzzese 2008), or telephone appointments received from the trialists (Engelke 2013). In contrast, a different set of studies reported difficulties in engaging parents to provide consent (when consent was actively sought) or to assist with data collection (Berg 2004; Gerald 2006; Terpstra 2012); difficulties in participation (Brasler 2006; Cicutto 2013; Kintner 2012; Kouba 2012; Levy 2006); or problems with adherence or behaviour change (Mujuru 2011). Children's satisfaction was found to be a sufficient condition for successful implementation (in combination with other conditions) and was collected in eight included studies, with four studies providing evidence that most children were satisfied through qualitative statements based on children's other stakeholders' perceptions (Al‐Sheyab 2012a; Brasler 2006; Bruzzese 2004; Howell 2005), and four studies providing evidence based on quantitative data (Berg 2004; Bruzzese 2008; Dore‐Stites 2007; Kintner 2012). None of the studies included in the QCAs reported low levels of child satisfaction, although one study (not included in the QCAs), which provided a low‐intensity intervention, did report low levels, with low levels of satisfaction (64% and 67%) for some indicators (Jackson 2006).
With respect to school nurse involvement, the presence and involvement of school nurses in interventions appear to be instrumental in triggering a successful intervention under certain conditions when children are not engaged in personalised or tailored interventions. Finally, the timing of interventions was important in triggering successful interventions, with interventions that did not interrupt children's own time triggering successful implementation in two different configurations.
No single condition appeared in isolation as a trigger for successful implementation. This highlights the complexity of triggering a successful intervention, as well as the utility of the QCA approach in capturing complex causal recipes. This finding is further supported by modest levels of coverage of any pathway.
Summary of outcome evaluation results
Results from meta‐analyses show that school‐based self‐management interventions led to small average improvements in several important outcomes, including hospitalisations (six studies), emergency department (ED) visits (13 studies), and health‐related quality of life (seven studies). A smaller number of studies contributed to meta‐analyses suggesting positive results for unplanned medical visits (five studies) and days of restricted activity (three studies). Effects on school absences, symptoms, and use of medication were also small, although our certainty for these outcomes was low or very low and confidence intervals included small or no effect. The effect on withdrawals suggested similar levels of attrition between intervention and control conditions.
The original logic model and the updated logic model show that evidence for effectiveness of the intervention was stronger than for urgent care contact and quality of life than for symptoms (Figure 1; Figure 22, respectively). We did not measure distal outcomes (e.g. academic achievement). This is likely a partial reflection of heterogeneity in measurement approach in terms of lung function and daytime and night‐time symptoms.
Researchers observed the most prominent intervention impacts for outcomes involving healthcare usage. Although conceptually relatively homogeneous, they were measured in several different ways, prompting us to undertake several transformations to facilitate meta‐analysis. The magnitude of effect sizes for hospitalisations, ED visits, and other instances of unplanned healthcare usage was similarly small across all three outcomes when considered in absolute terms. However, this indicates that intervention effect can also reach across children's healthcare pathways to include both primary and secondary episodes of care. In contrast, it was anticipated that a greater effect would be evident for school absences than was apparent in our review. However, heterogeneity was substantial in meta‐analyses of school absence (I² = 70%), and additional subgroup analyses suggest that the way in which the intervention was implemented may have had a substantial impact on this outcome.
Effects of the intervention were relatively consistent across outcomes, with the exception of school absences and ED visits. Most planned investigations of heterogeneity were generally uninformative or inconclusive in explaining variation in our results. Indications suggest that both school type and age of the child may help to explain some between‐study heterogeneity in models for school absence, with the intervention exerting a greater impact on older children in high schools, although this result was primarily driven by a single study (Bruzzese 2011). Two studies suggested that interventions with moderate to high numbers of children from lower socio‐economic backgrounds (between 25% and 50% of children) resulted in fewer school absences for intervention children (Cicutto 2013; McGhan 2003), although the relationship between the proportion of children from a lower socio‐economic background and effect size was not linear. We found generally mixed evidence around the impact of including parents. Based on subgroup analyses, interventions that did include parents appeared to confer no additional benefit compared with those that did not. Similarly, meta‐analyses provided contrasting evidence as to whether involvement of school nurses had a positive impact on children's outcomes.
Contribution of a mixed methods approach
The mixed methods approach adopted here allowed us to (I) understand design and implementation processes associated with more successful implementation of school‐based self‐management interventions; (ii) develop judicious and theory‐driven hypotheses for testing in subsequent meta‐analyses with covariates that reflected configurations of study conditions as well as single conditions; and (ii) explore the links between successful implementation and intervention outcomes.
Adjunct analyses showed links between intervention implementation and more impactful interventions, although the strength of these relationships differed for Analysis 11.1 and Analysis 11.2. Analysis of ED visits did not rule out differential effects between subgroups. We classified implementation of the intervention as successful in four studies. Study authors reported lower levels of ED visits with the intervention, and this finding was consistent with results for subgroups of studies that classified interventions as not successful. However, the result was inconclusive for studies that did not implement the intervention successfully. In the case of school absence, evidence shows greater impact of studies that were well implemented versus those that were not successfully implemented. This held when we restricted our focus to direct comparison of interventions (five studies) versus usual care.
11.2. Analysis.
Comparison 11 Adjunct analyses ‐ impact of Implementation on selected outcomes, Outcome 2 Absence from school.
Meta‐analyses based on the findings of earlier QCAs, which assessed the impact of school‐based asthma self‐management interventions in lowering levels of school absence, also show that individual conditions that were frequently part of configurations that triggered successful intervention implementation explained some of the between‐study heterogeneity. Notably, studies that were theory driven had greater impact on reducing school absences than those that were not, with the confidence interval for the subgroup of studies that explicitly used theory clearly within the boundaries of an effective intervention.
Further meta‐analyses suggesting that interventions that did not involve existing school staff in a substantial delivery or facilitating role were those that achieved greater levels of impact in lowering school absence. This corresponds with QCA findings that involvement of school staff could be counterproductive in certain configurations. Well‐implemented interventions that are supported by theory and can be implemented independently of existing school staff appear to be sufficient for lowering levels of school absence in these analyses.
Translating evidence into practice
The financial implications of asthma treatment and care for healthcare systems are significant; costs up to £1 billion per year are reported in the UK. A formal economic evaluation would be needed to determine how the reduction in healthcare use observed in this review impacts the financial burden on healthcare systems incurred by managing asthma. Although a similar reduction in school absence has not been established in this review, subgroup analyses developed on the basis of earlier QCAs identified study‐level characteristics associated with substantial reductions in absence, most notably interventions explicitly using theory.
In terms of the design of interventions, the importance of theory was emphasised in QCA results and was given further limited support by some of the subgroup analyses conducted as part of the meta‐analyses. However, it is not clear if the use of theory in interventions is a marker of the quality of the interventions and the experience of researchers, or is more integral to intervention success and provides an anchor for trialists to return to and actively draw upon. Based on QCA results, when trialists take steps to measure levels of child satisfaction (including levels of enjoyment and fulfilment from activities), this is reflected in delivery of a successful intervention. The presence and involvement of school nurses appear to be instrumental in successful implementation of the intervention under certain conditions, particularly when children are not engaged in personalised or tailored interventions.
Overall completeness and applicability of evidence
Most of the included studies were conducted in the USA, specifically in inner city areas with large numbers of children from ethnic minority backgrounds and/or lower‐income households; very few of the included studies came from the UK or Europe. Although we anticipated that broader contextual factors around health policy and access to health care are likely to shape the design and implementation of the intervention (see logic model in Figure 22), we have not synthesised the impact of these contextual factors.
The US focus of studies may have differing implications for the transferability of interventions. The nature of healthcare delivery and the large number of people without adequate healthcare coverage could mean that the intervention has a greater impact in US settings, particularly among lower‐income populations with substantial levels of underdiagnosis and low levels of access to appropriate medication plans. Several interventions (e.g. those of Bruzzese 2011 and Gerald 2009) were developed precisely on the basis of this rationale, focusing on low‐income groups or ethnic minority groups with inadequate access to health care, and selected schools as the delivery site because of the universality of education (as opposed to health care) in these settings. The implications for transferability could mean that weaker effect sizes are achieved in settings with better healthcare coverage, higher rates of diagnosis, and greater equality in access to medication (e.g. in settings such as the UK, where health care is universally free at the point of delivery). In contrast, many of the findings around intervention implementation are likely to be universal across several settings because of the relative universality of the way in which children attend schools, for example, better implementation when the intervention takes place outside children's own time.
Many outcomes with stronger evidence of an intervention effect were those commonly experienced by children with relatively severe asthma. For example, in Atherly 2009, when an intervention was implemented in high schools among children with mild to severe asthma, around 3% of children had been hospitalised for asthma at baseline, and less than 10% had visited an ED. Values suggesting that unplanned secondary healthcare utilisation is relatively rare among children with asthma are also observed in prevalence studies, for example, in Harris 2017, which examined asthma patterns in London high schools.
Many of the studies included in the QCA and in the meta‐analyses were conducted as cluster randomised controlled trials (RCTs); however few of these studies described the impact of this clustering effect either quantitatively or qualitatively. It is likely that conducting school‐level randomisation is an important consideration in terms of the feasibility of the study and serves as a step toward prevention of contamination of treatment impact, although the opportunity to explore implementation and impact of school‐level designs is not taken up by many trialists. This means that we are unable to comment on the generalisability of study findings with regards to school cultures.
High schools were better represented among studies included as process evaluations than among those included as outcome evaluations. Whether this is a reflection of the challenge of implementing RCT designs in high schools compared to primary schools was not directly addressed by the studies included in this review, although distinct configurations of conditions that triggered successful interventions were identified in QCAs for high schools and/or older children. Meta‐analyses revealed little 'qualitative' impact of conducting interventions in high schools rather than in other types of schools for most outcomes except school absences, although this assertion is based on inclusion of few high schools in subgroup analyses and low heterogeneity for many healthcare usage outcomes.
Many studies did not report on the outcomes specified in the protocol for this review and encountered further issues with the incompatibility of some reported effect sizes. In fact, any of the meta‐analyses performed (the largest including 13 studies) provided only a partial account of the total number of studies included. Some models, especially subgroup analyses, may have been effectively underpowered. Future systematic reviewers exploring public health interventions may wish to explicitly include a narrative synthesis of all studies in terms of study design, which may examine both the nature of the intervention, the types of outcomes collected, and the impact of interventions on these outcomes, including graphical representations (Thomson 2013), for a more complete account and understanding of the impact and feasibility of the model.
Finally, because we excluded studies that delivered similar interventions in different settings, we do not know the added value of running an intervention in a school compared with running an intervention in a hospital or community setting. What is clear, however, is that schools provide access to large numbers of children with asthma, including those who do not regularly attend appointments with their medical provider; therefore the school environment can be considered an important third space for delivery of interventions that can improve both children's outcomes and healthcare usage. This review has shown that school‐based self‐management interventions are effective in improving several outcomes for children with asthma, and that those who design future interventions should consider a number of configurations, including instructor, theory, and time of day, in their design. The outcomes of this review will directly inform the development of a school‐based self‐management intervention for children with asthma in London secondary schools.
Certainty of the evidence
The 'Summary of findings' table highlights our reasons for downgrading the certainty of evidence for the main outcomes of interest in this review, with process evaluations considered separately below. We noted issues in the execution of all studies, although the impact of risk of bias differed across outcomes. We deemed that several studies had high or unclear risk of bias, although these results did not appear to inflate the effect size relative to that provided by low‐risk studies, and in most cases they did not influence the direction of effect. Studies that we deemed to have unclear or high risk of bias may nevertheless have contributed to decisions to downgrade the certainty of evidence through other factors, including directness of outcome measurements. For example, school absences were measured in a variety of ways, and not all approaches were specific to asthma‐related school absences.
We deemed the certainty of evidence to be moderate for four outcomes delineated in the 'Summary of findings' table: hospitalisation, unplanned medical visits, quality of life, and symptoms. Each of these outcomes showed positive intervention effects (or effects that were very close to being classed as positive effects in the case of daytime symptoms). For two of the outcomes reported in the 'Summary of findings' table, we deemed that the certainty of evidence was low (school absences and ED visits), and we found evidence certainty to be very low for a further outcome on medication usage. Again, indirectness and unexplained heterogeneity were the main drivers for downgrading of evidence.
Additional considerations not necessarily captured in the 'Summary of findings' table should be considered when quality of the evidence is examined. First, we decided to include in our analyses some cluster RCTs with relatively low numbers of clusters. Although these studies tended to be comparatively small by their nature and therefore did not contribute greatly to pooled effect sizes, there remains the possibility that the intervention effects are slightly exaggerated compared to those of individually randomised trials or large cluster RCTs (see also the section on bias below). Nevertheless, this risk should be balanced against the potential bias introduced by overlooking information from such (smaller) trials. Similarly, effect sizes were harmonised for most outcomes, with the most substantial transformations involving conversion between standardised mean differences (SMDs) and odds ratios (ORs) to develop a common metric; although this appeared to have minimal impact, and different effect sizes tended to be consistent in direction/impact regardless of original measurement (see Analysis 12.1 through to Analysis 12.27), this is further evidence of indirectness in outcome measures, which is an indicator of lower‐certainty evidence.
12.1. Analysis.
Comparison 12 Effects of school‐based asthma interventions vs usual care, including disaggregated effect sizes, Outcome 1 Exacerbations leading to hospitalisation ‐ standardised mean difference.
12.27. Analysis.
Comparison 12 Effects of school‐based asthma interventions vs usual care, including disaggregated effect sizes, Outcome 27 Withdrawal from the study.
In contrast, we judged the quality of the process evaluation literature to be almost uniformly poor, with many studies having high or unclear risk of bias across several domains. This is likely due to various factors but most plausibly is a reflection of previous lack of guidance around the conduct of process evaluations, as well as difficulty in identifying process evaluations in the literature; there remains a methodological gap in terms of tools to report on and help in identifying process evaluations (as opposed to guidance on conducting process evaluations (Moore 2015)). This review included process evaluation studies that were integrated with outcome evaluation studies, that were presented as separate sections, or that could be considered stand‐alone evaluations. The tool used to measure risk of bias in process evaluation studies was an amalgamation of two tools used in reviews of process evaluation studies and resulted in a comprehensive assessment (O'Mara‐Eves 2013; Shepherd 2010). We deemed only four studies to have low risk of bias in most domains (Bruzzese 2011; Kintner 2012; Kouba 2012; Mujuru 2011). Of these, only Kintner 2012 could be considered a stand‐alone evaluation, with Mujuru 2011 including defined sections evaluating processes, and Bruzzese 2011 and Kouba 2012 presenting process evaluation data that were more integrated. We classified the latter two studies as process evaluations due to their exploration of process‐related questions using recognised tools and exploration of context and potential mechanisms. The main weakness of the process evaluation studies included is that they lacked breadth and had considered only a single process of importance in‐depth. The impact of these poor quality studies on the QCA is difficult to ascertain, although absence of richer and broader process data may have been a factor as to why we were able to explain only a relatively modest amount of successful implementation via QCA models. A commonly occurring risk of bias among the included process evaluation studies is that the tools and methods of collecting and analysing data were not always deemed to be reliable or credible.
Potential biases in the review process
Current evidence around the introduction of potential bias through restrictions on publication language is mixed, with some recent studies finding no systematic bias in effect size estimates when languages other than English were excluded (Morrison 2012), although many remain concerned that the results of ineffective trials will be submitted to local (non‐English language) journals, leading to the potential for language restrictions and systematic bias (Guyatt 2011). We assessed a potential impact of this restriction by conducting explorations of the impact of publication bias. Imposing a language restriction may also have influenced results of the synthesis of process evaluation data, and may impede the generalisability of results to individuals of non‐English speaking cultures, although we were not able to explore the impact of this decision in this review.
We encountered the following limitations in the review process.
Potential measurement error: we noted variation in the way in which many outcomes were measured, for example, lung function and school absences. Although no 'gold standard' is available for measuring school absences, lack of continuity across studies may reduce the validity of findings. Further, data for both school attendances and healthcare use may be subject to substantial measurement error, for example, we cannot say for certain that all school absences and healthcare visits that were recorded were specifically due to asthma, or were authorised by either the school or the medical centre. Similarly, measurement error may be a factor with some of the covariates used in subgroup analyses, for example, socio‐economic status (SES) can be measured in different ways ‐ through stated household income or evidence of free school meals ‐ although it was not possible to further explore these differences in measurement in the present review.
Effect size transformations: this review sought to include comprehensive trial data within meta‐analytical models, while maintaining construct validity across effect sizes. This often necessitated transforming the data to ensure statistical compatibility, following recommendations within the Cochrane Handbook for Systematic Reviews of Interventions, and undertaking Chinn's transformation (Chinn 2000). Although we have attempted to ensure transparency in fully presenting disaggregated effect sizes alongside those that have been consolidated, and despite sensitivity analyses conducted to ensure the validity of findings, there is potential for these analyses themselves to be confounded, and underlying assumptions around the transformation of effect sizes may not hold with further interrogation. For example, to facilitate transformations, we combined data on SMDs and ORs, although the skewness usually associated with data such as hospitalisations, for example, may not have been fully accounted for in the transformation. This is an important limitation, but it needs to be balanced against research wastage and information lost by excluding studies that use different approaches in measuring outcomes. Encountering such diverse data reinforces our recommendation below for development of a core outcome set.
Potentially underpowered analyses and treatment of heterogeneity: we included few studies in many of the meta‐analysis models, and for random‐effects models, the models themselves may have been underpowered (Jackson 2017). In addition, when heterogeneity was encountered, the low number of studies meant either that subgroup analyses were unsuitable, or that the subgroups themselves included few studies. We deemed that planned meta‐regression analyses were not suitable for any of the outcomes. Furthermore, unlike many other systematic reviews, we did not present all planned subgroup analyses when we encountered a low number of studies (under 10) and/or a low level of heterogeneity; in this respect, several deviations from the protocol occurred. However, we have greater confidence in the results of subgroup analyses because of our judicious use of these methods.
Identification of process evaluation studies: identification of process evaluations was a challenge in this review. Although guidance is available to assist trialists in conducting a process evaluation (Moore 2015), this did not necessarily aid in the identification of process evaluation studies from a systematic review perspective. All process evaluation studies included an examination of a given process (or processes) and implementation outcome(s) of interest, as well as their relationship to context (in this case, the immediate context of the schools). However, this group spanned a range of studies ‐ from those that were self‐described process evaluations, to those with defined process evaluation sections, to those that included process evaluation data embedded within other evaluation data. Although we developed an inclusive strategy around identification of process evaluation studies, there remains the possibility that some trialists may not have considered their own study as fulfilling the remit of a process evaluation. In addition, although guidance for process evaluations states that they can adopt a range of methods for data collection (Moore 2015), unlike other recent reviews (Dickson 2016), many of the studies that we included did not draw upon robust qualitative methods of data collection, which in turn may have limited our understanding of some of the issues surrounding implementation. Consequently, we deemed there remained greater scope within several of the included studies to explore the way in which the school context, and particularly the broader health service context, influenced delivery of the intervention, and we graded much of the process evaluation information as having high risk of bias because of this weakness. This review highlights the need for greater support for review authors in identifying process evaluation studies. In the current review, our original logic model was instrumental in helping to identify the processes and process metrics of interest and informed the selection of studies (Figure 1); in the absence of clearer guidance in this area, the use of logic models may represent an important step in helping review authors to draw criteria around which processes should be considered in a process evaluation.
Harmful effects: some studies reported negative intervention impacts among children, such as increased levels of ED visits. Such negative effects may reflect the content of self‐management information delivered to children, which may, for example, have recommended greater contact with healthcare providers when experiencing exacerbations (although such detail was not reported in studies), in which case an increase in ED visits could be viewed as a positive. A narrative approach to synthesis of outcome evaluations data could lead to a more nuanced understanding.
Alternative explanations: many other factors might also have influenced review results. For example, although these are school‐based asthma self‐management interventions, few, if any, of the studies considered seasonality of asthma exacerbations and their relationship within the school year. Another Cochrane Review considered the issue of seasonality and showed that seasonal omalizumab treatment between four and six weeks before children return to school might reduce the number of asthma exacerbations seen in autumn (Pike 2018); however the effect of this on outcomes such as asthma control remains unclear.
Low number of clusters: some of the cluster RCTs included in this review randomised only a small number of schools. Although it is universally agreed that randomising one cluster per arm would entirely conflate the randomisation/intervention and clustering effect, there is less agreement on the minimum number of clusters needed for a study to qualify as a cluster RCT (one source recommends four clusters per arm). Studies involving a low number of clusters are generally indicative of a small trial and often contribute only sparse data to any one model. Sensitivity analyses for studies with a low number of clusters per arm were conducted (two or three clusters per arm: Al‐Sheyab 2012; Howell 2005; Kintner 2012; Shah 2001; Velsor‐Friedrich 2005). Results were generally inconclusive and inclusion/exclusion of these studies in models did not qualitatively change results of meta‐analyses, with the exception of Al‐Sheyab 2012 in one quality of life model. These studies may be particularly prone to baseline imbalances, as well as to issues involving introduction of bias, and their inclusion does represent a potential source of bias.
Agreements and disagreements with other studies or reviews
This review is one of the first of its kind to employ a mixed study and mixed methods approach to understanding how school‐based asthma self‐management interventions work, and whether they are effective. It is also the first to undertake quantitative synthesis of studies seeking to develop children's asthma self‐management skills in the school environment. Direct comparisons are challenging, but a number of similar reviews have focused on different settings, different study designs, or use of different synthesis methods, which allows us to understand results in the context of other evidence.
Pinnock 2015 is one of few reviews that have explored how asthma self‐management interventions should be implemented. Review authors focused on a range of settings and age groups and addressed a targeted question around whether interventions primarily targeted at patients, professionals, or the organisation, or explicitly targeting all three levels simultaneously, were differentially effective in changing outcomes, or in changing process measures. They found that complex interventions that explicitly address patient education, professional training, and organisational commitment were associated with improvements in process measures, markers of asthma control, and reduced use of unscheduled health care. Their conclusions that 'individually, the separate components (professional, patient, organisation) of comprehensive self‐management support do not appear to be sufficient consistently to improve outcomes in asthma' (p14) are congruent with our own findings from QCA synthesis, which emphasised that no single condition was necessary and sufficient to trigger successful implementation outside a configuration of conditions.
An earlier Cochrane Review explored the effectiveness of self‐management education interventions for children aged two to 18 with asthma across a range of settings between 1980 and 2002 (Wolf 2002). Review findings were similar to the findings of this review, with data suggestive of moderate reductions in ED visits and in days of restricted activity. This earlier review also found evidence that self‐management education led to a small reduction in school absences, and review authors were able to ascertain a small impact on lung function. It is unclear to what extent the discrepancy in settings, age groups, or inclusion criteria for studies on date would drive the discrepancy in school absence, or another factor. In contrast to the promising results observed for night‐time symptoms in the previous review (Wolf 2002), our review did not find evidence that the intervention made a positive impact, although this was consistent with the findings of a later review that narratively summarised study results (Coffman 2009).
Subsequent reviews include Al Aloola 2014, which focused on primary schools and used a narrative approach to synthesise data. Review authors concluded that most studies were suggestive of positive effects, but as was the case in the present review, they were critical of the measurement of outcomes, which varied greatly among included studies. They also highlighted lack of detail in the descriptions provided for intervention content and processes, which is consistent with the outcome evaluations included here. Ahmad 2011 also took a narrative approach to synthesising outcome data from studies that involved school nurses, but nevertheless concluded that results indicated that a decrease in school absences could be expected, but that results for reductions in ED visits and hospital admissions were less certain, in contrast to the results provided here. Coffman 2009 also undertook a narrative descriptive synthesis of the effectiveness of a school‐based approach, although review authors concluded that there was heterogeneity in the direction and/or magnitude of effect on quality of life, symptom days, night‐time symptoms, and school absences, which largely corroborates the findings of the present review. Finally, a more recent review included school‐based self‐management interventions provided across a diffuse set of studies with regards to design (Carvalho 2016). These review authors also took a narrative approach when synthesising study results, and again showed an overall trend suggestive of heterogeneity in magnitude and direction of effect across a range of outcomes.
This systematic review makes a contribution to the literature by providing the first meta‐analyses of asthma self‐management interventions focused in schools, and it provides evidence of the effectiveness of this approach in reducing healthcare usage. Methodologically, this is also one of the first Cochrane Reviews to employ a mixed methods approach in synthesising evidence. This mixed methods approach helped to show that although intervention as a whole did not appear to be effective in reducing school absences, interventions that were drawing upon theory were effective in improving school absences.
Authors' conclusions
Implications for practice.
School‐based asthma self‐management interventions probably reduce hospitalisations and improve symptoms (moderate‐certainty evidence), may lower emergency department (ED) attendance (low‐certainty evidence), and may decrease children's unplanned and urgent healthcare visits (low‐certainty evidence). Their impact on school absence varied between studies (low‐certainty evidence), and probably lead to small improvements in quality of life (moderate‐certainty evidence). The effects of these interventions on the requirement for reliever medication are uncertain.
Hospitalisation was reduced by an average of about 0.16 admissions per child over a 12‐month period. The proportion of children attending the ED was reduced from 75 per 1000 children to 54 per 1000 children over the course of a year. Similar results were observed for unplanned medical visits. For health policy‐makers, the results highlight that schools may be an effective location for delivering asthma self‐management interventions to potentially large numbers of children, although formal cost‐effectiveness analysis is needed to determine how reductions in healthcare usage affect financial burden on health systems. Many of the included studies tested the intervention among financially deprived populations, and judging the applicability of the results to more socially diverse populations is difficult.
The mixed methods design of this review has revealed important features of interventions that are of particular interest to educational practitioners and teachers. Variation in school absences may be driven by the results from a subset of explicitly theory‐driven interventions that achieved modest decreases. Trialists may wish to take account of this when designing interventions that they intend to evaluate. Our process evaluation shows that when trialists are concerned about the level of child satisfaction (including levels of enjoyment and fulfilment from activities), and when they take steps to measure levels of satisfaction, this is reflected in the delivery of a successfully implemented intervention.
Implications for research.
The evidence presented in this review for school‐based asthma self‐management interventions varies in degrees of certainty across the outcomes of interest. The updated logic model summarises where evidence has been identified but also highlights where uncertainties remain (Figure 22). In particular, the mechanisms that link participation in a school‐based asthma intervention with achievement of these relatively distal outcomes remain undefined. Many analyses of intermediary outcomes provided inconclusive evidence (e.g. analyses reported asthma symptoms (Analysis 1.6; Analysis 1.7); data were insufficient for inclusion in meta‐analyses (e.g. lung function data (see Table 14)). In other cases, these outcomes were not included in our protocol. For example, although knowledge was not explicitly measured, we can hypothesise that knowledge and skill development are essential components for changes in self‐management and therefore changes in healthcare usage. The current review also did not assess these, and overall, many of the intermediary stages and accompanying changes in healthcare service usage between receipt of the intervention and behaviour change remain unidentified, signalling some of the pathways for future research.
Evaluation of healthcare usage in future studies would help to establish whether the intervention effect transfers to other settings. Researchers providing data on ED visits observed heterogeneity in the magnitude and direction of effect across studies. Research conducted specifically to determine when and how the intervention might increase attendance as observed for a subset of studies would help to explain the variation in direction of effect. For example, although baseline imbalances may be a contributory factor in explaining negative or negligible impacts for some studies implementing the "Roaring Adventures of Puff" manualised intervention in certain settings (McGhan 2003; McGhan 2010), further targeted analyses may reveal the context and mechanisms that explain its effectiveness in others (Cicutto 2005; Cicutto 2013).
This review identified a heterogeneous group of process evaluation studies that were often of low quality and did not present a broad or deep understanding of the processes undertaken and the mechanisms of action reflective of the complexity of the intervention. The quality of the process evaluation literature has been criticised previously (Oakley 2006), and this is relevant when one seeks to understand the causal chains of actions occurring within public health interventions such as school‐based asthma self‐management interventions. Although guidance on the conduct of process evaluation studies is available (Moore 2015), this review highlights that many trialists do not adequately assess the implementation and context of their interventions. It is notable that only a third of included studies contributed to both sets of syntheses conducted in our review. Enhancing understanding of the barriers preventing conduct and publication of process evaluations is a priority for future research. Systematic reviews would benefit from the development of a tool or checklist that can be used to help identify process evaluation studies during screening and/or to better design searches for relevant studies.
The largest meta‐analysis includes 13 of the 33 RCTs identified in this review. The need for a more standardised approach to evaluating key asthma outcomes is clear based on this finding. Approaches to developing core outcome sets for clinical trials are increasingly common (Williamson 2012). Some work has been undertaken to consider which domains should be captured in trials involving children with asthma (Sinha 2012). Our review shows that many studies, including those recently published, continue to capture diffuse outcomes that may have little clinical value and/or policy resonance. Further development, refinement, and implementation of a core outcome set for this intervention model would be welcome and would facilitate future reviews, which could include information not only on which domains should be captured, but also on how this information should be measured.
Subgroup analyses suggest that intervention effects were generally consistent across different types of schools (high/senior vs primary/elementary schools) for outcomes for which we were able to explore differences in effect size. However, further studies within high/senior schools are needed to extend the applicability of the evidence base to children older than those recruited to many of the studies to date. These results should also be considered in light of results from process evaluations, which suggest that the distinction between high/senior school and other types of schools may be important from an implementation perspective, necessitating a modified approach to the design and running of school‐based asthma self‐management interventions.
Although this review has shown that schools can provide an effective setting for self‐management interventions that reduce healthcare usage, we have not been able to explore the optimal setting. This would be a natural direction for future primary research studies and systematic reviews. In addition, although the intervention aim and the setting were the same in all studies included here, interventions have differed substantially. Future reviews should explore whether differences in outcomes are observed across different modes of asthma intervention, and should examine the comparative effectiveness of different programmes (e.g. Open Airways for Schools). Review authors could provide a better understanding of the links between intervention input and more distal outcomes, and this may prove valuable for public health decision‐makers. The feasibility of such research is contingent on emergence of a more mature evidence base for this type of intervention in terms of the number of available studies, as well as improvements in collection of standardised outcomes and reporting of processes undertaken and implemented.
What's new
| Date | Event | Description |
|---|---|---|
| 28 January 2019 | Amended | Mistake in the plain language summary corrected. The total number of included studies was 55, not 66. |
Acknowledgements
Thanks to Elizabeth Stovold for helping to design the search strategy. Thanks also to Emma Dennett for comments and ongoing assistance. Thanks to the NIHR Collaborative Leadership in Applied Health Research and Care (CLAHRC) North Thames for continued support.
Chris Cates was the Editor for this review and commented critically on the review.
The Background and Methods sections of this protocol are based on a standard template used by the Cochrane Airways Group.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL | Monthly |
| MEDLINE (Ovid) | Weekly |
| Embase (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 2. Search strategy to identify relevant trials from the CAGR (via the Cochrane Register of Studies – CRS)
#1 AST:MISC1
#2 MeSH DESCRIPTOR Asthma Explode All
#3 asthma*:ti,ab
#4 #1 or #2 or #3
#5 MeSH DESCRIPTOR Schools Explode All
#6 MeSH DESCRIPTOR School Health Services
#7 MeSH DESCRIPTOR School Nursing
#8 school*:ti,ab,kw
#9 academ*:ti,ab,kw
#10 colleg*:ti,ab,kw
#11 lesson*:ti,ab,kw
#12 pupil*:ti,ab,kw
#13 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12
#14 MeSH DESCRIPTOR Self Care Explode All
#15 MeSH DESCRIPTOR Health Education Explode All
#16 MeSH DESCRIPTOR Case Management
#17 MeSH DESCRIPTOR Patient Education as Topic
#18 educat*:ti,ab,kw
#19 manag*:ti,ab,kw
#20 self‐car*:ti,ab,kw
#21 self NEXT car*:ti,ab,kw
#22 train*:ti,ab,kw
#23 instruct*:ti,ab,kw
#24 teach*:ti,ab,kw
#25 patient‐cent*:ti,ab,kw
#26 patient NEXT cent*:ti,ab,kw
#27 MeSH DESCRIPTOR Patient‐Centered Care
#28 patient‐focus*:ti,ab,kw
#29 patient NEXT focus*:ti,ab,kw
#30 coach*:ti,ab,kw
#31 skill*:ti,ab,kw
#32 knowledge NEXT develop*:ti,ab,kw
#33 tutor*:ti,ab,kw
#34 #14 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33
#35 #4 AND #13 AND #34
[Note: in search line #1, MISC1 denotes the field in the record in which the reference has been coded for condition, in this case, asthma]
Appendix 3. CENTRAL search strategy
Ti ab kw combined rather than ti; ab for asthma
| #1 | MESH DESCRIPTOR asthma EXPLODE ALL TREES |
| #2 | asthma*:TI,AB,KY |
| #3 | #1 OR #2 |
| #4 | MESH DESCRIPTOR Schools EXPLODE ALL TREES |
| #5 | MESH DESCRIPTOR School Health Services EXPLODE ALL TREES |
| #6 | MESH DESCRIPTOR School Nursing EXPLODE ALL TREES |
| #7 | school*:TI,AB,KY OR academ*:TI,AB,KY OR colleg*:TI,AB,KY OR lesson*:TI,AB,KY OR pupil*:TI,AB,KY |
| #8 | #4 OR #5 OR #6 OR #7 |
| #9 | educat*:TI,AB,KY OR manag*:TI,AB,KY OR self‐car*:TI,AB,KY OR self NEXT car*:TI,AB,KY OR train*:TI,AB,KY OR instruct*:TI,AB,KY OR teach*:TI,AB,KY OR patient‐cent*:TI,AB,KY OR patient NEXT cent*:TI,AB,KY OR patient‐focus*:TI,AB,KY OR patient NEXT focus*:TI,AB,KY OR coach*:TI,AB,KY OR skill*:TI,AB,KY OR knowledge NEXT develop*:TI,AB,KY OR tutor*:TI,AB,KY |
| #10 | MESH DESCRIPTOR Self Care EXPLODE ALL TREES |
| #11 | MESH DESCRIPTOR Health Education EXPLODE ALL TREES |
| #12 | MESH DESCRIPTOR Case Management EXPLODE ALL TREES |
| #13 | MESH DESCRIPTOR Patient Education EXPLODE ALL TREES |
| #14 | MESH DESCRIPTOR Patient‐Centred Care EXPLODE ALL TREES |
| #15 | #9 OR #10 OR #11 OR #12 OR #13 OR #14 |
| #16 | #3 AND #8 AND #15 |
Appendix 4. CT.gov search strategy
| #1 AST:MISC1 |
| #2 MeSH DESCRIPTOR Asthma Explode All |
| #3 asthma*:ti,ab |
| #4 #1 or #2 or #3 |
| #5 MeSH DESCRIPTOR Schools Explode All |
| #6 MeSH DESCRIPTOR School Health Services |
| #7 MeSH DESCRIPTOR School Nursing |
| #8 school*:ti,ab,kw |
| #9 academ*:ti,ab,kw |
| #10 colleg*:ti,ab,kw |
| #11 lesson*:ti,ab,kw |
| #12 pupil*:ti,ab,kw |
| #13 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 |
| #14 MeSH DESCRIPTOR Self Care Explode All |
| #15 MeSH DESCRIPTOR Health Education Explode All |
| #16 MeSH DESCRIPTOR Case Management |
| #17 MeSH DESCRIPTOR Patient Education as Topic |
| #18 educat*:ti,ab,kw |
| #19 manag*:ti,ab,kw |
| #20 self‐car*:ti,ab,kw |
| #21 self NEXT car*:ti,ab,kw |
| #22 train*:ti,ab,kw |
| #23 instruct*:ti,ab,kw |
| #24 teach*:ti,ab,kw |
| #25 patient‐cent*:ti,ab,kw |
| #26 patient NEXT cent*:ti,ab,kw |
| #27 MeSH DESCRIPTOR Patient‐Centered Care |
| #28 patient‐focus*:ti,ab,kw |
| #29 patient NEXT focus*:ti,ab,kw |
| #30 coach*:ti,ab,kw |
| #31 skill*:ti,ab,kw |
| #32 knowledge NEXT develop*:ti,ab,kw |
| #33 tutor*:ti,ab,kw |
| #34 #14 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 |
| #35 #4 AND #13 AND #34 |
Appendix 5. CINAHL search strategy
| #1 | asthma* |
| #2 | (MH "Asthma+") |
| #3 | (MH "Schools+") OR (MH "School Health Services+") OR (MH "School Nursing+") OR school* OR academ* OR colleg* OR lesson* OR pupil* |
| #4 | (MH "Self Care+") OR (MH "Health Education+") OR (MH "Case Management+") OR (MH "Patient Education+") OR educat* OR manag* OR self‐car* OR self n1 car* OR train* OR instruct* OR teach* OR patient‐cent* |
| #5 | patient n1 cent* OR (MH "Patient‐Centred Care+") OR patient‐focus* OR patient N1 focus* OR coach* OR skill* OR knowledge n1 develop* OR tutor* |
| #6 | S4 OR S5 |
| #7 | S1 OR S2 |
| #8 | S3 AND S6 AND S7 |
Appendix 6. AMED search strategy
| #1 | exp Asthma/ |
| #2 | exp Schools/ |
| #3 | asthma*.mp. [mp=title, other title, abstract, heading words] |
| #4 | 1 or 3 |
| #5 | exp School health services/ |
| #6 | exp School nursing/ |
| #7 | (school* or academ* or colleg* or lesson* or pupil*).mp. [mp=title, other title, abstract, heading words] |
| #8 | 2 or 5 or 6 or 7 |
| #9 | (educat* or manag* or self‐car* or train* or instruct* or teach* or patient‐cent* or coach* or skill* or tutor*).mp. [mp=title, other title, abstract, heading words] |
| #10 | ((self adj1 car*) or (patient adj1 cent*) or (patient adj1 focus*) or (knowledge adj1 develop*)).mp. [mp=title, other title, abstract, heading words] |
| #11 | exp Self care/ |
| #12 | exp Health education/ |
| #13 | exp Case management/ |
| #14 | exp Patient education/ |
| #15 | exp patient centred care/ |
| #16 | 9 or 10 or 11 or 12 or 13 or 14 or 15 |
| #17 | 4 and 8 and 16 |
| #18 | from 17 keep 1‐100 |
| #19 | limit 18 to yr="1995 ‐Current" |
Appendix 7. Embase search strategy
| #1 | 'Asthma' |
| #2 | 'Schools' |
| #3 | 'School Health Services' |
| #4 | 'School Nursing' |
| #5 | 'School' |
| #6 | Academy' |
| #7 | 'Academic' |
| #8 | 'Academies' |
| #9 | 'college' |
| #10 | 'Colleges' |
| #11 | 'lesson' |
| #12 | 'Lessons' |
| #13 | 'pupil' |
| #14 | 'Pupils' |
| #15 | #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 |
| #16 | 'Self Care' |
| #17 | 'Health Education' |
| #18 | 'Case Management' |
| #19 | 'Patient Education' |
| #20 | 'Educate' |
| #21 | 'Education' |
| #22 | 'Educator' |
| #23 | 'Manage' |
| #24 | 'Management' |
| #25 | 'self‐care' |
| #26 | 'train' |
| #27 | 'Training' |
| #28 | 'trainer' |
| #29 | 'Instruct' |
| #30 | 'instructor' |
| #31 | 'Instruction' |
| #32 | 'teach' |
| #33 | 'Teacher' |
| #34 | 'patient‐center' |
| #35 | 'patient center' |
| #36 | 'Patient‐Centered Care' |
| #37 | 'patient‐focus' |
| #38 | 'patient focus' |
| #39 | 'Coach' |
| #40 | 'skill' |
| #41 | 'Skills' |
| #42 | 'knowledge develop' |
| #43 | 'Tutor' |
| #44 | #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 |
| #45 | #1 AND #15 AND #44 |
Appendix 8. Additional information on the synthesis of process evaluation data and qualitative comparative analysis
Background and theoretical basis for qualitative comparative analysis (QCA)
The QCA approach was developed in the political sciences during the 1980s by Charles Ragin (Ragin 2008), and in turn was based on mathematical developments in electrical engineering and analytical philosophy (Miech 2015; Thiem 2015). In its application within political sciences, QCA was utilised in comparing the characteristics of nations to enhance understanding of the conditions associated with different forms of governance and rule (Thomas 2014). Since then, its use has broadened, and it has been applied in as fields as diverse as ecology (e.g. Hellström 2001), education (Cooper 2005), and dentistry (Singh 2012). In each case, QCA was employed as a solution to the challenge of analysing data containing a small number of cases, each with an extensive array of conditions that may be necessary to trigger a given outcome. This 'small N‐many variables' challenge is similar to that often faced by systematic review authors, and we followed the approach developed by Thomas and colleagues in employing QCA to understand conditions associated with our outcome of interest based on published data within primary studies (Thomas 2014). In this review, however, rather than attempting to understand how different configurations of conditions are associated with differentials in effect size (see further examples in Brunton 2014 and Brunton 2014a), we explored their association with different levels of implementation success.
QCA has its basis in set‐theoretic logic, and it can be conceptualised as bridging the qualitative and quantitative divide, not only in terms of the types of data employed to undertake QCA but also in the research process and theoretical standpoints taken during stages of the QCA. Aspects of QCA that are aligned with a qualitative standpoint include the iterative process of case selection, reconceptualisation of conditions (or variables), and reconceptualisation of the outcome of interest that takes place during the model specification (Schneider 2010); in this review, the approach adopted very much mirrors the hypothesis‐generating role of qualitative research. In contrast, exploration of patterns in their alignment of conditions with the outcome of interest mirrors the quantitative practice of testing variables simultaneously in a regression framework (Schneider 2010). Unlike most quantitative research, QCA is based on set‐theoretic principles, where the focus is on sets of conditions as units, rather than on the individual constituent components. This is aligned closely with the statements that social scientists routinely make about the nature of social phenomena that involve descriptions of groups as subsets of larger groups (Ragin 2008). Furthermore, the nature of these relationships is asymmetrical, unlike the symmetrical principles of statistical correlational research.
QCAs allow us to consider two aspects of set relationships. First, the number of those with an outcome who share a given condition, and second, the number with a given condition who share an outcome. The first aspect allows us to consider the degree to which a condition is a 'necessary' component of triggering the outcome (necessity); the second allows us to consider the extent to which a given condition is a sufficient condition for triggering the outcome (sufficiency), with particular application to exploring combinations of cases (Ragin 2008). It It is this second application that is of greatest interest, as it allows us to consider more complex configurations that may trigger an outcome. QCA allows for quantification of these relationships through exploration of different combinations of conditions that achieve an outcome. QCA was developed first by exploring conditions in binary form, although later extensions have allowed for fuzzy‐set QCA that allows for ambiguity in both outcome and condition sets (Ragin 2009).
A set membership score for each case based on its characteristics is calculated from the data table, and these are analysed against outcome membership scores. Subset relationships are identified by observing when the membership scores in one set (i.e. a combination of conditions) are consistently less than those in another set (i.e. outcome) (see Ragin 2009). In line with the guidance set out by Ragin (Ragin 2009), and reflective of the size of the data set, we set a frequency threshold of one around the number of cases with a membership score greater than 0.5 in each combination (i.e. configuration of conditions and outcomes). QCA is reliant on Boolean algebra to reduce multiple configurations of conditions that lead to outcomes to their instrumental parts, to form a parsimonious solution. Although QCA is analogous in some ways to data reduction techniques employed in statistical analyses, the conditions tested in QCA analyses are included only on the basis of pre‐existing theoretical knowledge of the analyst. In this case, our logic model, presented in Figure 1, helped to guide much of our thinking.
Explanation and example of coding strategy
We developed a strategy involving direct and indirect transformation in assembling our data for the QCA. For example, we developed a single variable to reflect whether an intervention took place within a high school. Those interventions that took place exclusively within high schools were assigned a value of 1 (fully within the set), and those that took place exclusively within elementary/primary schools were assigned a value of 0. Further details of this example are found below in Table 1.
Appendix Table 1. Example coding of direct assignment of values – whether the intervention took place in a high school
| Condition = high school | Directly assigned value |
| High school(s) | 1 |
| High schools and junior/middle schools | 0.75 |
| Junior/middle school(s) | 0.66 |
| Missing information on age | 0.5 |
| Primary/elementary school(s) | 0 |
When values were directly assigned in this way, no further calibration was required. In other cases, the assignment followed a combination of direct and transformational assignment. Direct assignment involves a researcher directly assigning values (usually based on categorical or binary source indicators); transformational assignment involves developing rules for how values that are more continuous in nature (and not necessarily bounded by 0 and 1) are coded between zero and one. Transformational assignment was conducted using R, as was most of the QCA synthesis, and a full explanation of transformational assignment and the underlying theoretical principles is provided in Thiem and Dusa (Thiem 2012). In all cases, transformational assignment was based on positive endpoint values, and involved setting thresholds indicating full exclusion from a set, a cross‐over point (maximum ambiguity (0.5); which was also used in the case of missing data), and a threshold for full inclusion. An example is provided in Appendix Table 2 below for identification of whether an intervention could be considered a 'large' intervention based on the total number of students involved.
Appendix Table 2. Example coding of transformational assignment of values – whether the intervention was a large intervention
| Condition = large intervention | Threshold values |
| Large interventions (whole school interventions taking place in large schools) | 300 |
| Moderately large interventions (approximately 3 classes in the intervention) (maximum ambiguity) |
90 |
| Small interventions (less than a single class) | 15 |
We extracted information supporting several conditions (over 90) for studies. We identified five key domains in which these belonged.
Outcome: process outcomes.
Condition group 1: setting and participants.
Condition group 2: programme design.
Condition group 3: programme content and style.
Condition group 4: additional processes undertaken to facilitate implementation (planned and unplanned).
To limit the problem of limited diversity, when a large number of possible logical combinations are not supported by cases, we tested the relationship between each condition group and the outcome separately to identify the individual pathway recipes to successful implementation. We then consolidated the information to understand the instrumental components across all four groups and their membership in the outcome set. This approach is analogous to stepwise entry of antecedent variables into a regression model.
Initial results from QCA modelling: a single domain
Model 1. Setting and participant characteristics
We initially considered constructing sets based on a number of conditions (size of intervention, presence of existing health facilities in schools, high schools, black and minority ethnic students, low socio‐economic status, whether teachers received additional training, whether school nurses or others received additional training, and whether parents received an intervention). However, given that we were working with 27 studies, we were concerned that limited diversity would be an issue and re‐examined the theoretical justification for inclusion of each condition. In the case of the condition measuring the size of the intervention, we were concerned that this would reflect only a distinction between whether the intervention was a pilot/feasibility study or a full intervention and would not reflect modifiable 'process' and interaction with context per se. With regards to ethnicity and socio‐economic status of children, we were concerned that this would be uninformative with regards to modifiable 'processes' and their interaction with context, and we did not include these conditions in the model. A data table and a truth table were constructed for the five remaining conditions (see Table 4 and Table 34).
21. Truth table for QCA model 1 ‐ setting and participants.
| School‐based health centre | High school | Parents directly involved | Teachers received training | School nurses or other stakeholders received training | Outcome code (based on consistency score) | Number of studies with membership in causal combination > 0.5 | Consistency score with subset relationship (n = 27 in each assessment) | Proportional reduction in inconsistency | Cases |
| 1 | 1 | 0 | 1 | 0 | 1 | 2 | 1 | 1 | Bruzzese 2008; Terpstra 2012 |
| 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | Henry 2004 |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Kintner 2012 |
| 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0.995 | 0.99 | Cicutto 2013 |
| 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0.918 | 0.588 | Crane 2014; Pike 2011 |
| 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0.889 | 0.811 | Al‐Sheyab 2012 |
| 1 | 1 | 0 | 0 | 1 | 0 | 2 | 0.865 | 0.662 | Bruzzese 2004; Bruzzese 2011 |
| 1 | 1 | 0 | 0 | 0 | 0 | 4 | 0.852 | 0.761 | Berg 2004; Joseph 2010; Joseph 2013; Magzamen 2008 |
| 0 | 1 | 0 | 0 | 0 | 0 | 4 | 0.845 | 0.543 | Horner 2015; Langenfeld 2010; Lee 2011; Mujuru 2011 |
| 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0.763 | 0.136 | Levy 2006 |
| 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0.754 | 0 | Gerald 2006 |
| 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0.751 | 0.647 | Kouba 2012 |
| 0 | 1 | 0 | 1 | 0 | 0 | 3 | 0.73 | 0.56 | Dore‐Stites 2007; Howell 2005; Spencer 2000 |
| 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | Brasler 2006 |
QCA: qualitative comparative analysis.
The truth table showed six configurations that were indicative of a subset relationship with the outcome set and showed good distribution of configurations associated with the outcome and its negation. Several studies formed sets with high levels of consistency, and five showed proportional reduction in inconsistency (PRI) scores above 0.6. PRI is indicative of how distinct a subset configuration is of the outcome compared to negation of the outcome. No suggestions were provided as to an adequate threshold for PRI scores, although 0.6 falls between high and low values suggested elsewhere (Schwellnus 2013). We then proceeded to explore whether the truth table contained contradictory configurations. As we were conducting fsQCA, identification of contradictory configurations was less straightforward than would be the case for crisp‐set QCA, and we explored the stability of rows supported by multiple cases for potential contradictory configurations, primarily by examining the original data in Table 4, although we found no evidence.
A complex solution was generated through Boolean minimisation (Table 35), suggesting five pathways towards generating the outcome of interest. We then incorporated information from logical remainders making explicit hypotheses that the presence of school‐based health services (including school nurses), the involvement of parents, and the provision of additional training for teachers and other stakeholders would be beneficial to a successful intervention, but making no specific hypotheses about the impact of the intervention conducted in a high school. The intermediate solution gave four potential minimal sums (Table 36), each of which contained three essential prime implicants (three essential routes to the outcome) and two inessential prime implicants (interchangeable routes to the outcome needed to complete the minimal sum). Selection of the minimal solution was based on the most theoretically plausible; we also confirmed that no contradictory simplifying assumptions were made on the outcome and its negation. We explored whether any of the prime implicants selected were associated with negation of the outcome, finding little evidence based on consistency scores achieved for 'unsuccessful' interventions. The solution is displayed below (where upper case notation represents that the condition is present, and lower case represents that the condition is absent).
22. Complex solution for QCA model 1 ‐ setting and participants.
| Consistency score with subset relationship (n = 27 in each assessment) | Proportional reduction in inconsistency | Raw coverage | Unique coverage | Cases | ||
| 1 | HIGHSCHOOL*schoolbasedhealth*parentdirect*anyothdir | 0.913 | 0.861 | 0.176 | 0.043 | Al‐Sheyab 2012; Henry 2004 |
| 2 | schoolbasedhealth*teacherdirect*parentdirect*anyothdir | 0.913 | 0.769 | 0.294 | 0.16 | Al‐Sheyab 2012; Crane 2014; Pike 2011 |
| 3 | highschool*schoolbasedhealth*TEACHERDIRECT*parentdirect*ANYOTHDIR | 0.995 | 0.99 | 0.042 | 0.042 | Cicutto 2013 |
| 4 | HIGHSCHOOL*SCHOOLBASEDHEALTH*teacherdirect*PARENTDIRECT*anyothdir | 1 | 1 | 0.105 | 0.105 | Bruzzese 2008; Terpstra 2012 |
| 5 | HIGHSCHOOL*SCHOOLBASEDHEALTH*TEACHERDIRECT*PARENTDIRECT*ANYOTHDIR | 1 | 1 | 0.074 | 0.074 | Kintner 2012 |
| M1 | 0.952 | 0.901 | 0.558 |
QCA: qualitative comparative analysis.
[Notation: Upper case = condition is present; Lower case = condition is absent; * = logical and; + logical or; Key: HIGHSCHOOL = High School (lower case not in high school); SCHOOLBASEDHEALTH = School Based Health Centre; TEACHERDIRECT = Teachers received directly received component of intervention; PARENTDIRECT = Parents directly received component of intervention; ANYOTHDIR = School nurses or other stakeholders (apart from children) directly received component of intervention; SUCCESSFULIMPLEMENTATION = Implementation of intervention successful]
23. Intermediate solution for QCA model 1 ‐ setting and participants.
| Consistency score with subset relationship (n = 27 in each assessment) | Proportional reduction in inconsistency | Raw coverage | Unique coverage | Cases | ||
| 1 | HIGHSCHOOL*schoolbasedhealth*parentdirect | 0.904 | 0.838 | 0.226 | 0.093 | Al‐Sheyab 2012; Henry 2004 |
| 2 | HIGHSCHOOL*SCHOOLBASEDHEALTH*teacherdirect*PARENTDIRECT | 1 | 1 | 0.105 | 0.105 | Bruzzese 2008; Terpstra 2012 |
| 3 | schoolbasedhealth*teacherdirect*parentdirect*anyothdir | 0.913 | 0.769 | 0.294 | 0.16 | Crane 2014; Pike 2011 |
| 4 | highschool*TEACHERDIRECT*ANYOTHDIR | 0.778 | 0.5 | 0.074 | 0.042 | Cicutto 2013 |
| 5 | HIGHSCHOOL*SCHOOLBASEDHEALTH*PARENTDIRECT*ANYOTHDIR | 1 | 1 | 0.074 | 0.042 | Kintner 2012 |
| Solution | 0.915 | 0.831 | 0.608 |
QCA: qualitative comparative analysis.
Overall solution
HIGHSCHOOL*schoolbasedhealth*parentdirect +
schoolbasedhealth*teacherdirect*parentdirect*anyothdir +
HIGHSCHOOL*SCHOOLBASEDHEALTH*teacherdirect*PARENTDIRECT +
(highschool*TEACHERDIRECT*ANYOTHDIR + HIGHSCHOOL*SCHOOLBASEDHEALTH*PARENTDIRECT*ANYOTHDIR)
=> SUCCESSFULIMPLEMENTATION
[Notation: Upper case = condition is present; Lower case = condition is absent; * = logical and; + logical or; Key: HIGHSCHOOL = High School (lower case not in high school); SCHOOLBASEDHEALTH = School Based Health Centre; TEACHERDIRECT = Teachers received directly received component of intervention; PARENTDIRECT = Parents directly received component of intervention; ANYOTHDIR = School nurses or other stakeholders (apart from children) directly received component of intervention; SUCCESSFULIMPLEMENTATION = Implementation of intervention successful]
Overall solution
HIGHSCHOOL*schoolbasedhealth*parentdirect +
schoolbasedhealth*teacherdirect*parentdirect*anyothdir +
HIGHSCHOOL*SCHOOLBASEDHEALTH*teacherdirect*PARENTDIRECT +
(highschool*TEACHERDIRECT*ANYOTHDIR + HIGHSCHOOL*SCHOOLBASEDHEALTH*PARENTDIRECT*ANYOTHDIR)
=> SUCCESSFULIMPLEMENTATION
[Notation: Upper case = condition is present; Lower case = condition is absent; * = logical and; + logical or; => leads to; Key: HIGHSCHOOL = High School (lower case not in high school); SCHOOLBASEDHEALTH = School Based Health Centre; TEACHERDIRECT = Teachers received directly received component of intervention; PARENTDIRECT = Parents directly received component of intervention; ANYOTHDIR = School nurses or other stakeholders (apart from children) directly received component of intervention; SUCCESSFULIMPLEMENTATION = Implementation of intervention successful]
Our intermediate model achieved an overall coverage score of 0.61 and a consistency score of 0.951, indicating that the solution accounted for most instances of the outcome and was highly sufficient in triggering the outcome (Table 36). The three essential prime implicants were identified, two of which suggested very different pathways to running a successful intervention in a high school. In the first pathway, supported by two studies (Al‐Sheyab 2012a; Henry 2004), successful interventions were observed with no school‐based health centre and no direct parental involvement. In contrast, evidence from Bruzzese 2008 and Terpstra 2012 suggested that successful interventions were observed with a school‐based health centre and direct parental involvement (but no additional training for teachers). When we explored further contextual characteristics (not included within the model due to issues around limited diversity and difficulties in convergence with inclusion of these conditions), both studies indicating that support from a school‐based health facility and direct involvement of parents were not essential for running a successful intervention took place in schools with low numbers of children from ethnic minorities or low socio‐economic status backgrounds. In contrast, both studies suggesting that school‐based health centres and involvement of parents were necessary conditions for a successful intervention took place in locations with large numbers of children from lower socio‐economic status backgrounds and large numbers from an ethnic minority background (i.e. not the majority ethnic group in the country). This suggests that interventions taking place among larger numbers of marginalised children with asthma are successful support is received from existing school medical facilities or personnel, or when parents are directly involved; this support is not necessary when interventions take place among children who are not predominantly disadvantaged. This is reconfirmed by inclusion of a further (inessential) pathway suggesting that successful interventions in high schools are accompanied by direct parental involvement, additional training for school nurses and other stakeholders (not teachers), and current school‐based health facilities (row 5; Table 36). Here the supporting case, represented by Kintner 2012, also took place within a location with a large number of children from low socio‐economic status backgrounds and large numbers of children from ethnic minority backgrounds.
Two further prime implicants were identified. The first (inessential) implicant suggested that when interventions were implemented outside high schools, additional teacher training and training of other stakeholders were conditions that were sufficient to generate a successful outcome. A second (essential) primary implicant suggested that not having school‐based health facilities, not having additional training for teachers or other stakeholders, and not having additional parental involvement were sufficient conditions to generate an outcome, regardless of whether the intervention took place in a high school. Both of these prime implicants were supported by studies that took place in primary/elementary schools, although additional systematic differences in context were not identifiable.
Such complexity in causal pathways is perhaps an artefact of the fuzzy QCA implemented here, in which we have focused on the setting and on actors involved in the intervention. Results suggest that even among this limited set of conditions, successful interventions with regards to implementation are triggered through a variety of seemingly contradictory pathways that may also reflect non‐modifiable contextual characteristics. The evidence presented here suggests that when interventions take place in high schools (or junior schools) with large numbers of marginalised children with asthma, additional components involving parents or support from school‐based health facilities are important conditions for ensuring successful implementation. These additional conditions are not necessary found in high schools with a less marginalised student body and may be detrimental to successful implementation. A similar difference was not immediately apparent for interventions taking place outside high schools.
Model 2. Recruitment and retention processes
We attempted to construct a truth table to explore a number of conditions based on recruitment and retention processes, eventually focusing on the use of incentives, marketing materials, reminders, and provision of make‐up sessions. Nevertheless, we were unable to detect configurations that were potential subsets of the outcome based on the truth table output (not shown; see Table 5 for data). No configuration was identified as stable enough to support classification as a subset of a positive outcome value. We also tested these conditions against the negation of the outcome but again detected high levels of inconsistency. As such, we decided that this group of conditions did not form configurations that were subsets of successful interventions, and we did not consider them further in our consolidated model.
Model 3. Curriculum, pedagogy, and intervention emphasis
We constructed a model to explore the impact of conditions reflecting curriculum content, pedagogical style, and authors' descriptions of the emphasis of the intervention (see Table 37 for data). After several iterations, six conditions were entered into a model reflecting (I) whether the curriculum reflected forming alliances and monitoring symptoms, (ii) whether the curriculum reflected learning about asthma triggers and monitoring symptoms, (iii) whether study authors emphasised the intervention as tailored or personalised, (iv) whether study authors emphasised developing personal responsibility as an aim of the intervention, (v) whether the pedagogical style focused on interactive methods, and (vi) whether a diverse pedagogical style was employed. These conditions were selected on the basis of being the most theoretically informative (i.e. not simply a reflection of diffuse styles, e.g. other curriculum foci). A truth table was constructed but showed just two configurations that were sufficient to trigger a successful intervention and were supported by a single study each (Table 38). This low coverage of the outcome precluded further analysis. The same conditions were tested against the negation of the outcome, but no configuration displayed adequate levels of consistency.
24. Data table for QCA model 3 ‐ curriculum, pedagogy, and intervention emphasis.
| Successful intervention | Curriculum reflected forming alliances and monitoring symptoms | Curriculum reflected learning about asthma triggers and monitoring symptoms | Emphasised the intervention as being tailored or personalised | Emphasised developing personal responsibility as aim of the intervention | Pedagogical style focused on interactive methods | Diverse pedagogical style employed | |
| Joseph 2010 | 0.52 | 0 | 1 | 1 | 0 | 0 | 0 |
| Kouba 2012 | 0.33 | 0 | 0 | 0 | 1 | 0 | 0 |
| Dore‐Stites 2007 | 0.67 | 1 | 0 | 0 | 1 | 0 | 0 |
| Joseph 2013 | 1.00 | 0 | 1 | 1 | 0 | 0 | 1 |
| Mujuru 2011 | 0.67 | 0 | 1 | 0 | 0 | 0 | 0 |
| Henry 2004 | 0.83 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pike 2011 | 0.67 | 0 | 1 | 0 | 0 | 0 | 0 |
| Spencer 2000 | 0.33 | 0 | 0 | 0 | 0 | 1 | 0 |
| Engelke 2013 | 0.50 | 0 | 0 | 0 | 0 | 0 | 1 |
| Splett 2006 | 0.50 | 0 | 0 | 0 | 0 | 1 | 0 |
| Kintner 2012 | 0.83 | 0 | 1 | 0 | 0 | 0 | 0 |
| Berg 2004 | 0.83 | 0 | 1 | 1 | 0 | 0 | 0 |
| Howell 2005 | 0.33 | 0 | 1 | 0 | 0 | 0 | 0 |
| Gerald 2006 | 0.33 | 1 | 0 | 0 | 0 | 0 | 0 |
| Cheung 2015 | 0.33 | 0 | 0 | 0 | 1 | 0 | 0 |
| Al‐Sheyab 2012 | 0.83 | 0 | 1 | 0 | 1 | 0 | 1 |
| Levy 2006 | 0.52 | 0 | 0 | 0 | 0 | 0 | 1 |
| Terpstra 2012 | 1.00 | 1 | 0 | 0 | 1 | 0 | 0 |
| Horner 2015 | 0.67 | 1 | 0 | 0 | 1 | 0 | 0 |
| Bruzzese 2008 | 0.94 | 0 | 1 | 0 | 1 | 0 | 0 |
| Lee 2011 | 0.50 | 0 | 0 | 0 | 0 | 0 | 1 |
| Bruzzese 2004 | 0.33 | 0 | 0 | 1 | 0 | 0 | 0 |
| Cicutto 2013 | 0.67 | 1 | 0 | 0 | 0 | 0 | 0 |
| Brasler 2006 | 0.00 | 0 | 1 | 0 | 0 | 0 | 0 |
| Crane 2014 | 0.50 | 0 | 0 | 0 | 1 | 0 | 0 |
| Bruzzese 2011 | 0.88 | 0 | 0 | 1 | 0 | 0 | 0 |
| Magzamen 2008 | 0.19 | 0 | 1 | 0 | 0 | 0 | 0 |
QCA: qualitative comparative analysis.
25. Truth table for QCA model 3 ‐ curriculum, pedagogy, and intervention emphasis.
| Curriculum reflected forming alliances and monitoring symptoms | Curriculum reflected learning about asthma triggers and monitoring symptoms | Emphasised the intervention as being tailored or personalised | Emphasised developing personal responsibility as aim of the intervention | Pedagogical style focused on interactive methods | Diverse pedagogical style employed | Outcome code (based on consistency score) | Number of studies with membership in causal combination > 0.5 | Consistency score with subset relationship (n=27 in each assessment); [proportional reduction in inconsistency] | Cases |
| 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 [1] | Joseph 2013 |
| 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0.938 [0.9333] | Bruzzese 2008 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.833 [0.8] | Henry 2004 |
| 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0.833 [0.8] | Al‐Sheyab 2012 |
| 1 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 0.778 [0.714] | Dore‐Stites 2007; Horner 2015; Terpstra 2012 |
| 0 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 0.677 [0.523] | Berg 2004; Joseph 2010 |
| 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0.604 [0.486] | Bruzzese 2004; Bruzzese 2011 |
| 0 | 0 | 0 | 0 | 0 | 1 | 0 | 3 | 0.507 [0.027] | Engelke 2013; Lee 2011; Levy 2006 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.5 [0.25] | Cicutto 2013; Gerald 2006 |
| 0 | 1 | 0 | 0 | 0 | 0 | 0 | 6 | 0.448 [0.287] | Brasler 2006; Howell 2005; Kintner 2012; Magzamen 2008; Mujuru 2011; Pike 2011 |
| 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0.417 [0] | Spencer 2000; Splett 2006 |
| 0 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 0.389 [0] | Crane 2014; Kouba 2012; Langenfeld 2010 |
QCA: qualitative comparative analysis.
Model 4. Further modifiable intervention design features
Conditions included in this model reflected modifiable design features of the intervention that were reported by study authors. The first condition reflected the extent to which study authors reported that their interventions were grounded in a named theoretical framework underpinning the intervention. Although it is likely that all interventions were grounded in theory to some extent, and a vast majority of studies presented supporting literature to justify delivery of the intervention, reporting a named theoretical framework in the process evaluation may signal that a theoretical framework continued to shape the study and was used as a reference point throughout the design of all stages of the trial. Two conditions reflected whether students' own time was interrupted (e.g. lunchtime, free periods), or whether their normal educational programme was interrupted by delivery of the intervention. We included a condition reflecting the extent to which the intervention was delivered or facilitated by a school nurse to capture the importance (or not) of having known medical personnel involved in the intervention as a condition for successful implementation. Finally, it was hypothesised that running personalised or individual sessions may impact the ability of trialists to deliver a successful intervention; negatively, this may impact on trialists' ability to balance individualised sessions across a larger cohort of students.
From the raw data (Table 6), configurations were created and were examined for their sufficiency in generating a successful intervention initially through construction of a truth table (Table 39). This initially showed six configurations that were associated with generating a successful intervention, all of which had been theory driven and included as a condition (although just one combination was supported by multiple cases). This table was then minimised, and a complex solution was generated (Table 40). However, a number of logical remainders were omitted from the derivation of this solution (17) and were used in developing a parsimonious solution (not shown) and an intermediate solution (Table 41). In developing the intermediate solutions, we hypothesised that conditions that reflected the presence of personalised and individual sessions, or that suggested that the intervention interrupted students' free time, would be negatively associated with successful implementation, and the presence of other conditions entered would impact positively.
26. Truth table for QCA model 4 ‐ modifiable design features.
| Theory driven | Personalised or individual sessions | Intervention takes place during lesson time | Intervention takes place during students’ own free time | School nurse involved in delivery of the intervention | Outcome code (based on consistency score) | Number of studies with membership in causal combination > 0.5 | Consistency score with subset relationship (n = 27 in each assessment) | Proportional reduction in inconsistency | Cases |
| 1 | 0 | 0 | 0 | 1 | 1 | 2 | 1 | 1 | Bruzzese 2008; Dore‐Stites 2007 |
| 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | Al‐Sheyab 2012 |
| 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Kintner 2012 |
| 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0.996 | 0.993 | Bruzzese 2011 |
| 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0.931 | 0.816 | Joseph 2010 |
| 1 | 1 | 1 | 0 | 0 | 1 | 2 | 0.931 | 0.872 | Crane 2014; Terpstra 2012 |
| 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0.903 | 0.729 | Lee 2011 |
| 1 | 1 | 1 | 1 | 0 | 0 | 2 | 0.852 | 0.729 | Bruzzese 2004; Joseph 2013 |
| 1 | 0 | 0 | 1 | 0 | 0 | 2 | 0.833 | 0.706 | Cicutto 2013; Horner 2015 |
| 1 | 1 | 0 | 0 | 1 | 0 | 2 | 0.753 | 0.602 | Berg 2004; Howell 2005 |
| 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0.732 | 0.481 | Kouba 2012 |
| 0 | 0 | 1 | 0 | 0 | 0 | 5 | 0.659 | 0.035 | Engelke 2013; Langenfeld 2010; Levy 2006; Spencer 2000; Splett 2006 |
| 0 | 1 | 0 | 0 | 1 | 0 | 0 | 4 | 0.638 | 0.484 |
| 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0.5 | 0 |
| 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0.444 | 0 |
QCA: qualitative comparative analysis.
27. Complex solution for QCA model 4 ‐ modifiable design features.
| Consistency score with subset relationship (n = 27 in each assessment) | Proportional reduction in inconsistency | Raw coverage | Unique coverage | Cases | ||
| 1 | THEORYDRIVEN*personalorindividual*SCHOOLNURSEINSTRUCT | 0.926 | 0.876 | 0.253 | 0.148 | Bruzzese 2008; Crane 2014; Dore‐Stites 2007; Kintner 2012; Lee 2011; Terpstra 2012 |
| 2 | THEORYDRIVEN*PERSONALORINDIVIDUAL*runinstudenttime*schoolnurseinstruct | 0.938 | 0.866 | 0.151 | 0.033 | Bruzzese 2011; Joseph 2013 |
| 3 | THEORYDRIVEN*personalorindividual*runinlessons*runinstudenttime | 0.999 | 0.998 | 0.149 | 0.001 | Al‐Sheyab 2012a; Bruzzese 2008; Dore‐Stites 2007 |
| M1 | 0.933 | 0.883 | 0.426 |
QCA: qualitative comparative analysis.
[Notation: Upper case = condition is present; Lower case = condition is absent; * = logical and; + logical or; Key: THEORYDRIVEN = Authors explicitly named theory or presented conceptual model for intervention; SCHOOLNURSEINSTRUCT = Substantial component delivered by schools' nurse; PERSONALORINDIVIDUAL = Substantial components delivered that were individually personalised or delivered to individuals; RUNINSTUDENTTIME = Substantial component run in students' own time (e.g. lunchtime); RUNINLESSONS = Substantial component run during lesson time; SUCCESSFULIMPLEMENTATION = Implementation of intervention successful]
28. Intermediate solution for QCA model 4 ‐ further modifiable intervention design features.
| Consistency score with subset relationship (n = 27 in each assessment) | Proportional reduction in inconsistency | Raw coverage | Unique coverage | Cases | ||
| 1 | THEORYDRIVEN*personalorindividual*SCHOOLNURSEINSTRUCT | 0.926 | 0.876 | 0.253 | 0.167 | Bruzzese 2008; Crane 2014; Dore‐Stites 2007; Kintner 2012; Lee 2011; Terpstra 2012 |
| 2 | THEORYDRIVEN*runinstudenttime*schoolnurseinstruct | 0.963 | 0.92 | 0.258 | 0.172 | Al‐Sheyab 2012; Bruzzese 2011; Joseph 2010 |
| M1 | 0.933 | 0.883 | 0.425 |
QCA: qualitative comparative analysis.
[Notation: Upper case = condition is present; Lower case = condition is absent; * = logical and; + logical or; Key: THEORYDRIVEN = Authors explicitly named theory or presented conceptual model for intervention; SCHOOLNURSEINSTRUCT = Substantial component delivered by schools' nurse; PERSONALORINDIVIDUAL = Substantial components delivered that were individually personalised or delivered to individuals; RUNINSTUDENTTIME = Substantial component run in students' own time (e.g. lunchtime); RUNINLESSONS = Substantial component run during lesson time; SUCCESSFULIMPLEMENTATION = Implementation of intervention successful]
Overall solution
THEORYDRIVEN*runinstudenttime*schoolnurseinstruct +
THEORYDRIVEN*personalorindividual*SCHOOLNURSEINSTRUCT => PROCOUTSUM
Overall solution
THEORYDRIVEN*runinstudenttime*schoolnurseinstruct +
THEORYDRIVEN*personalorindividual*SCHOOLNURSEINSTRUCT => SUCCESSFULIMPLEMENTATION
[Notation: Upper case = condition is present; Lower case = condition is absent; * = logical and; + logical or; => leads to; Key: THEORYDRIVEN = Authors explicitly named theory or presented conceptual model for intervention; SCHOOLNURSEINSTRUCT = Substantial component delivered by schools' nurse; PERSONALORINDIVIDUAL = Substantial components delivered that were individually personalised or delivered to individuals; RUNINSTUDENTTIME = Substantial component run in students' own time (e.g. lunchtime); RUNINLESSONS = Substantial component run during lesson time; SUCCESSFULIMPLEMENTATION = Implementation of intervention successful]
The intermediate solution above was checked for the presence of contradictory simplifying assumptions (none were detected), and the sufficiency of the configurations was checked for negation of the outcome (no sufficient configurations were detected). The solution confirms the importance of being theory driven as a sufficient condition in generating the outcome and identifies two configurations that include this condition. Two pathways were identified: the first pathway (row 1) suggests that school nurse involvement is needed if the intervention does not involve personalised or individualised sessions. This suggests that when an intervention provides only group‐based or generic content, school nurse input is needed to ensure successful implementation. However, the second pathway suggests that when interventions are not provided during students' free time (and therefore implicitly take place during students' learning time), successful implementation is achieved when involvement of a school nurse is not observed. A factorised (simplified) version of the solution is displayed below:
THEORYDRIVEN*(personalorindividual*SCHOOLNURSEINSTRUCT + runinstudenttime*schoolnurseinstruct) => SUCCESSFULIMPLEMENTATION
The factorised solution emphasised that being theory driven is a common condition for triggering a successful outcome within specific configurations. The presence of a school nurse in facilitating successful implementation is important in group or non‐tailored interventions, although absence of the school nurse is important for interventions that may take place within lesson or assembly time. Although the model captures two configurations with high levels of sufficiency (both consistency scores and overall model score are over 0.9), modest coverage scores (0.43 for the model) suggest that there remain several other pathways in which the outcome is triggered that are not identified in this set of conditions (Table 41).
Model 5. Stakeholder involvement and engagement
We explored levels of stakeholder involvement and engagement (I) across the school level (through development of school policies for asthma), (ii) at the child level (by measuring child satisfaction), and (iii) at the level of other stakeholders by exploring teacher, parent, and school nurse engagement. These latter conditions reflected whether study authors reported instances of problematic or enthusiastic engagement. For the latter four conditions, a missing code was initially entered into models to reflect where the condition was irrelevant (the intervention did not include involvement of a given stakeholder) or where the information was not collected. High levels of missing data precluded construction of appropriate configurations of conditions, and missing data were later coded as 'zero', indicating that the condition was absent or unreported (this is reflected in the raw data table (Table 7); this also meant that negative instances of a condition were coded in the same way as missing data or 'not applicable', although no instances of negative child satisfaction were identified). We lowered the consistency threshold to 0.8 (which remained relatively high) to reflect the low frequency of occurrence of these conditions.
The initial truth table revealed substantial amounts of limited diversity, and no configurations predicting the outcome were supported by multiple cases (Table 42); therefore we removed teacher engagement due to its infrequent occurrence (low coverage as a condition). We then explored the revised truth table, identifying three configurations sufficient for the outcome and supported by five studies. The truth table also revealed that five configurations remained as logical remainders that were not supported by observations. We then implemented Boolean minimisation to generate a complex solution (Table 43), and we imposed specific directional hypotheses on child satisfaction and development of the school asthma policy (both expected to lead to positive outcomes) to create an intermediate solution (Table 44).
29. Truth table for QCA model 5 ‐ stakeholder involvement and engagement.
| School asthma policy | Good relationships/ engagement with parents | Good relationships/ engagement with school nurses | Child satisfaction | Outcome code (based on consistency score) | Number of studies with membership in causal combination > 0.5 | Consistency score with subset relationship (n = 27 in each assessment) | Proportional reduction in inconsistency | Cases | |
| 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | Joseph 2013 |
| 2 | 0 | 1 | 0 | 1 | 1 | 1 | 0.958 | 0.939 | Bruzzese 2008 |
| 3 | 0 | 0 | 0 | 1 | 1 | 4 | 0.857 | 0.786 | Al‐Sheyab 2012; Berg 2004; Bruzzese 2004; Kintner 2012 |
| 4 | 0 | 1 | 1 | 1 | 0 | 2 | 0.723 | 0.465 | Dore‐Stites 2007; Howell 2005 |
| 5 | 1 | 0 | 0 | 0 | 0 | 3 | 0.674 | 0.515 | Cicutto 2013; Henry 2004; Levy 2006 |
| 6 | 0 | 0 | 0 | 0 | 0 | 10 | 0.615 | 0.405 | Bruzzese 2011; Gerald 2006; Horner 2015; Joseph 2010; Kouba 2012; Lee 2011; Magzamen 2008; Mujuru 2011; Pike 2011; Terpstra 2012 |
| 7 | 0 | 0 | 1 | 0 | 0 | 1 | 0.6 | 0 | Crane 2014 |
| 8 | 1 | 1 | 0 | 0 | 0 | 1 | 0.5 | 0 | Engelke 2013 |
| 9 | 0 | 1 | 1 | 0 | 0 | 1 | 0.488 | 0 | Spencer 2000 |
| 10 | 1 | 0 | 1 | 0 | 0 | 2 | 0.352 | 0 | Langenfeld 2010; Splett 2006 |
| 11 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | Brasler 2006 |
QCA: qualitative comparative analysis.
30. Complex solution for QCA model 5 ‐ stakeholder involvement and engagement.
| Consistency score with subset relationship (n = 27 in each assessment) | Proportional reduction in inconsistency | Raw coverage | Unique coverage | Cases | ||
| 1 | anyschpol*goodrelnur*CHILDSAT | 0.846 | 0.794 | 0.243 | 0.152 | Al‐Sheyab 2012; Berg 2004; Bruzzese 2004; Bruzzese 2008; Kintner 2012 |
| 2 | anyschpol*GOODRELPAR*goodrelnur | 0.979 | 0.972 | 0.187 | 0.095 | Bruzzese 2008; Joseph 2013 |
| M1 | 0.884 | 0.849 | 0.339 |
QCA: qualitative comparative analysis.
[Notation: Upper case = condition is present; Lower case = condition is absent; * = logical and; + logical or; Key: ANYSCHPOL = School asthma policy; GOODRELNUR = Good level of engagement and/or developing relationships with school nurses; GOODRELPAR = Good level of reported in engagement and/or developing relationships with parents; CHILDSAT = Children reported as satisfied; SUCCESSFULIMPLEMENTATION = Implementation of intervention successful]
31. Intermediate solution for QCA model 5 ‐ stakeholder involvement and engagement.
| Consistency score with subset relationship (n = 27 in each assessment) | Proportional reduction in inconsistency | Raw coverage | Unique coverage | Cases | ||
| 1 | goodrelnur*CHILDSAT | 0.846 | 0.794 | 0.243 | 0.152 | Al‐Sheyab 2012; Berg 2004; Bruzzese 2004; Bruzzese 2008; Kintner 2012 |
| 2 | anyschpol*GOODRELGPAR*goodrelnur | 0.979 | 0.972 | 0.187 | 0.095 | Bruzzese 2008; Joseph 2010 |
| M1 | 0.884 | 0.849 | 0.339 |
QCA: qualitative comparative analysis.
[Notation: Upper case = condition is present; Lower case = condition is absent; * = logical and; + logical or; Key: ANYSCHPOL = School asthma policy; GOODRELNUR = Good level of engagement and/or developing relationships with school nurses; GOODRELGPAR = Good level of reported in engagement and/or developing relationships with parents; CHILDSAT = Children reported as satisfied; SUCCESSFULIMPLEMENTATION = Implementation of intervention successful]
Overall solution
goodrelnur*CHILDSAT +
anyschpol*GOODRELPAR*goodrelnur => SUCCESSFULIMPLEMENTATION
[Notation: Upper case = condition is present; Lower case = condition is absent; * = logical and; + logical or; Key: ANYSCHPOL = School asthma policy; GOODRELNUR = Good level of engagement and/or developing relationships with school nurses; GOODRELPAR = Good level of reported in engagement and/or developing relationships with parents; CHILDSAT = Children reported as satisfied; SUCCESSFULIMPLEMENTATION = Implementation of intervention successful]
The intermediate solution indicated two essential prime implicants (pathways) sufficient to generate a positive outcome. One included child satisfaction as a sufficient condition, and one included reporting good levels of engagement with parents. Student satisfaction, although rarely measured, is a sufficient condition for generating a successful intervention. Similarly, parental engagement, even when parents were not necessarily directly involved in the intervention beyond providing consent for student participation, appeared to be a sufficient condition for successful implementation later. Both were sufficient only in the presence (or absence) of other conditions. Each implicant had high levels of consistency and high PRI levels, suggestive of sufficient configurations, although individual implicants exhibited low levels of coverage, and overall the solution had low levels of raw coverage (0.339, lying beneath thresholds suggested elsewhere (e.g. Ho et al., 2016)). This low coverage reflects the low frequency of reporting of included conditions by study authors. Although such low coverage would usually indicate that conditions lacked empirical importance, it is difficult to make this link with these data. What the evidence in model 5 does reinforce is the importance of measuring child satisfaction and parental engagement, as higher levels of both are found to trigger successful implementation. Despite this link, these conditions go unreported by trialists in almost half of studies, even within process evaluations (see data in Table 7).
Model 6. Consolidated model
We developed a consolidated model using evidence from models 1, 4, and 5 to understand some of the most important conditions to consider in designing an intervention. As we wanted to identify conditions that were empirically meaningful as well as sufficient, we selected conditions that were included in configurations with high consistency and coverage scores. To aid interpretability of the solutions, we focused on conditions with a consistent direction in a solution; this also reflected the type of data that we were working with that are dependent on trialists' reports, as opposed to standardised inventories of activities from across interventions.
We selected four conditions from model 4 (modifiable intervention design features) that reflected whether interventions were theory‐driven, were delivered as personalised or individual sessions, or took place during students' own free time, and whether a school nurse was involved in delivery of the intervention. In addition, we included whether high levels of child satisfaction were reported (from model 5) and whether the intervention took place in a high school (model 1). The data table for this model is found in Table 16. The initial truth table showed six configurations of conditions spread across 10 studies that were classified as triggering a successful intervention (Table 17). Each of these configurations was theory‐driven, and a complex solution was generated based on this initial truth table identifying four essential prime implicants sufficient for triggering successful implementation of an intervention (see Table 18). Logical remainders were an issue, with 18 configurations unsupported by cases, and parsimonious and intermediate solutions were generated using simplifying assumptions from these configurations. The intermediate solution, presented in Table 19, makes directional assumptions on four of the five conditions, with child satisfaction, parental engagement, and interventions being theory driven expected to lead to a positive outcome, while running an intervention during students' own time was expected to lead to a negative outcome.
Overall solution
HIGHSCHOOL*CHILDSAT*THEORYDRIVEN +
HIGHSCHOOL*THEORYDRIVEN* GOODRELGPAR +
HIGHSCHOOL*THEORYDRIVEN*runinstudenttime +
CHILDSAT*THEORYDRIVEN*runinstudenttime*GOODRELENGPAR => SUCCESSFULIMPLEMENTATION
[Notation: Upper case = condition is present; Lower case = condition is absent; * = logical and; + logical or; Key: HIGHSCHOOL = High School (lower case not in high school); THEORYDRIVEN = Authors explicitly named theory or presented conceptual model for intervention; RUNINSTUDENTTIME = Substantial component run in students' own time (e.g. lunchtime); GOODRELPAR = Good level of reported in engagement and/or developing relationships with parents; CHILDSAT = Children reported as satisfied; SUCCESSFULIMPLEMENTATION = Implementation of intervention successful]
The solution emphasises the importance of being theory‐driven across all settings. Three of the essential prime implicants were restricted in coverage to high schools. Here the evidence suggests that in addition to being theory‐based, having good levels of engagement with parents, or having high levels of child satisfaction, or running the intervention outside the students' own time leads to a successfully implemented intervention. An essential prime implicant that is not restricted to high schools also reinforces these findings by showing that being theory‐based, fostering high levels of student satisfaction, reporting good levels of parental engagement, and running an intervention outside students' own time are sufficient conditions for triggering a positive outcome. As with a large portion of the data included here, this solution has modest levels of coverage, accounting for almost half of the instances of the outcome, and indicating that there remain diverse pathways to running a successful intervention that have not been included within the QCA solution. A factorised representation of the minimal sum, as presented below, helps to simplify the solution.
CHILDSATB*THEORYDRIVEN*runinstudenttime*GOODRELPAR + HIGHSCHOOL*THEORYDRIVEN*(CHILDSAT + runinstudenttime + GOODRELPAR) => SUCCESSFULIMPLEMENTATION
[Notation: Upper case = condition is present; Lower case = condition is absent; * = logical and; + logical or; Key: HIGHSCHOOL = High School (lower case not in high school); THEORYDRIVEN = Authors explicitly named theory or presented conceptual model for intervention; RUNINSTUDENTTIME = Substantial component run in students' own time (e.g. lunchtime); GOODRELPAR = Good level of reported in engagement and/or developing relationships with parents; CHILDSATB = Children reported as satisfied; SUCCESSFULIMPLEMENTATION = Implementation of intervention successful]
We checked for the presence of contradictory configurations in developing the solution and examined whether any of the configurations described above also predicted negation of the outcome, but we found no evidence in either case. This confirms that these combinations did consistently lead to successful implementation of school‐based asthma interventions, and were not associated with poor levels of implementation, but that they were not necessarily observed with a high degree of frequency.
Data and analyses
Comparison 1. Effects of school‐based asthma interventions vs usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exacerbations leading to hospitalisation | 6 | 1873 | Std. Mean Difference (Random, 95% CI) | ‐0.19 [‐0.35, ‐0.04] |
| 2 Exacerbations leading to emergency department (ED) visits | 13 | 3883 | Odds Ratio (Random, 95% CI) | 0.70 [0.53, 0.92] |
| 3 Absence from school | 10 | 4609 | Std. Mean Difference (Random, 95% CI) | ‐0.07 [‐0.22, 0.08] |
| 4 Days of restricted activity | 3 | 1852 | Std. Mean Difference (Random, 95% CI) | ‐0.30 [‐0.41, ‐0.18] |
| 5 Unplanned visit to hospital or GP due to asthma symptoms | 5 | 3490 | Odds Ratio (Random, 95% CI) | 0.74 [0.60, 0.90] |
| 6 Experience of daytime and night‐time symptoms ‐ daytime symptoms | 5 | 1065 | Std. Mean Difference (Random, 95% CI) | ‐0.15 [‐0.33, 0.02] |
| 7 Experience of daytime and night‐time symptoms ‐ night‐time symptoms | 4 | 459 | Std. Mean Difference (Random, 95% CI) | ‐0.18 [‐0.52, 0.15] |
| 8 Use of reliever therapies, e.g. beta₂‐agonists | 2 | 437 | Odds Ratio (Random, 95% CI) | 0.52 [0.15, 1.81] |
| 9 Corticosteroid dosage and/or use of add‐on therapies (usage of) | 3 | 614 | Odds Ratio (Random, 95% CI) | 1.25 [0.88, 1.77] |
| 10 Corticosteroid dosage and/or use of add‐on therapies (appropriate usage of) | 2 | Std. Mean Difference (Random, 95% CI) | Totals not selected | |
| 11 Health‐related quality of life (SMD) | 7 | 2587 | Std. Mean Difference (Random, 95% CI) | 0.27 [0.18, 0.36] |
| 12 Health‐related quality of life (MD) | 8 | 2950 | Mean Difference (IV, Random, 95% CI) | 0.35 [0.06, 0.64] |
| 13 Withdrawal from the study | 13 | 3442 | Odds Ratio (Random, 95% CI) | 1.14 [0.92, 1.43] |
Comparison 2. Effects of school‐based asthma interventions vs usual care subgrouped by school type.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exacerbations leading to emergency department (ED) visits | 13 | Odds Ratio (Random, 95% CI) | 0.70 [0.53, 0.92] | |
| 1.1 Secondary/high school | 2 | Odds Ratio (Random, 95% CI) | 0.61 [0.40, 0.92] | |
| 1.2 Primary/elementary school | 11 | Odds Ratio (Random, 95% CI) | 0.73 [0.52, 1.02] | |
| 2 Absence from school | 10 | Std. Mean Difference (Random, 95% CI) | ‐0.07 [‐0.22, 0.08] | |
| 2.1 Secondary/high school | 1 | Std. Mean Difference (Random, 95% CI) | ‐0.38 [‐0.62, ‐0.15] | |
| 2.2 Primary/elementary school | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.05 [‐0.27, 0.16] | |
| 2.3 Primary/elementary and middle schools | 1 | Std. Mean Difference (Random, 95% CI) | 0.02 [‐0.08, 0.12] | |
| 2.4 Middle school | 1 | Std. Mean Difference (Random, 95% CI) | 0.08 [‐0.31, 0.48] | |
| 3 Withdrawal from the study | 13 | Odds Ratio (Random, 95% CI) | 1.14 [0.92, 1.43] | |
| 3.1 Secondary/high school | 3 | Odds Ratio (Random, 95% CI) | 1.25 [0.76, 2.06] | |
| 3.2 Primary/elementary school | 8 | Odds Ratio (Random, 95% CI) | 1.22 [0.94, 1.59] | |
| 3.3 Middle school | 2 | Odds Ratio (Random, 95% CI) | 0.59 [0.29, 1.20] |
2.1. Analysis.
Comparison 2 Effects of school‐based asthma interventions vs usual care subgrouped by school type, Outcome 1 Exacerbations leading to emergency department (ED) visits.
2.3. Analysis.
Comparison 2 Effects of school‐based asthma interventions vs usual care subgrouped by school type, Outcome 3 Withdrawal from the study.
Comparison 3. Effects of school‐based asthma interventions vs usual care subgrouped by age of children.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exacerbations leading to emergency department (ED) visits | 13 | Odds Ratio (Random, 95% CI) | 0.70 [0.53, 0.92] | |
| 1.1 Aged 11 to 15, 16 to 18 | 1 | Odds Ratio (Random, 95% CI) | 0.59 [0.39, 0.91] | |
| 1.2 Aged 11 to 15 | 1 | Odds Ratio (Random, 95% CI) | 1.04 [0.17, 6.25] | |
| 1.3 Aged 5 to 10, 11 to 15 | 2 | Odds Ratio (Random, 95% CI) | 0.64 [0.15, 2.76] | |
| 1.4 Aged 5 to 10 | 9 | Odds Ratio (Random, 95% CI) | 0.74 [0.51, 1.06] | |
| 2 Absence from school | 10 | Std. Mean Difference (Random, 95% CI) | ‐0.07 [‐0.22, 0.08] | |
| 2.1 Aged 11 to 15, 16 to 18 | 1 | Std. Mean Difference (Random, 95% CI) | ‐0.38 [‐0.62, ‐0.15] | |
| 2.2 Aged 5 to 10, 11 to 15 | 4 | Std. Mean Difference (Random, 95% CI) | 0.03 [‐0.06, 0.13] | |
| 2.3 Aged 5 to 10 | 5 | Std. Mean Difference (Random, 95% CI) | ‐0.09 [‐0.34, 0.16] | |
| 3 Withdrawal from the study | 13 | Odds Ratio (Random, 95% CI) | 1.14 [0.92, 1.43] | |
| 3.1 Aged 11 to 15, 16 to 18 | 1 | Odds Ratio (Random, 95% CI) | 1.31 [0.76, 2.27] | |
| 3.2 Aged 11 to 15 | 3 | Odds Ratio (Random, 95% CI) | 0.82 [0.25, 2.67] | |
| 3.3 Aged 5 to 10, 11 to 15 | 4 | Odds Ratio (Random, 95% CI) | 1.08 [0.48, 2.43] | |
| 3.4 Aged 5 to 10 | 5 | Odds Ratio (Random, 95% CI) | 1.19 [0.90, 1.58] |
3.3. Analysis.
Comparison 3 Effects of school‐based asthma interventions vs usual care subgrouped by age of children, Outcome 3 Withdrawal from the study.
Comparison 4. Effects of school‐based asthma interventions vs usual care subgrouped by child socio‐economic status (SES).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exacerbations leading to emergency department (ED) visits | 13 | Odds Ratio (Random, 95% CI) | 0.70 [0.53, 0.92] | |
| 1.1 Low SES over 50% | 2 | Odds Ratio (Random, 95% CI) | 0.53 [0.30, 0.94] | |
| 1.2 Low SES over 25% | 3 | Odds Ratio (Random, 95% CI) | 0.69 [0.28, 1.69] | |
| 1.3 Unclear or not low SES | 8 | Odds Ratio (Random, 95% CI) | 0.76 [0.57, 1.01] | |
| 2 Absence from school | 10 | Std. Mean Difference (Random, 95% CI) | ‐0.07 [‐0.22, 0.08] | |
| 2.1 Low SES over 50% | 2 | Std. Mean Difference (Random, 95% CI) | 0.01 [‐0.09, 0.11] | |
| 2.2 Low SES over 25% | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.36, ‐0.09] | |
| 2.3 Unclear or not low SES | 6 | Std. Mean Difference (Random, 95% CI) | ‐0.02 [‐0.28, 0.24] | |
| 3 Withdrawal from the study | 13 | Odds Ratio (Random, 95% CI) | 1.14 [0.92, 1.43] | |
| 3.1 Low SES over 50% | 1 | Odds Ratio (Random, 95% CI) | 1.27 [0.90, 1.78] | |
| 3.2 Low SES over 25% | 4 | Odds Ratio (Random, 95% CI) | 1.16 [0.61, 2.23] | |
| 3.3 Unclear or not low SES | 8 | Odds Ratio (Random, 95% CI) | 1.03 [0.73, 1.45] |
4.2. Analysis.
Comparison 4 Effects of school‐based asthma interventions vs usual care subgrouped by child socio‐economic status (SES), Outcome 2 Absence from school.
4.3. Analysis.
Comparison 4 Effects of school‐based asthma interventions vs usual care subgrouped by child socio‐economic status (SES), Outcome 3 Withdrawal from the study.
Comparison 5. Effects of school‐based asthma interventions vs usual care subgrouped by involvement of school staff in direct delivery of self‐management skills to children.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exacerbations leading to emergency department (ED) visits | 13 | Odds Ratio (Random, 95% CI) | 0.70 [0.53, 0.92] | |
| 1.1 Teachers involved in delivery (with or without school nurses) | 2 | Odds Ratio (Random, 95% CI) | 1.00 [0.55, 1.83] | |
| 1.2 School nurses alone involved in delivery | 1 | Odds Ratio (Random, 95% CI) | 0.29 [0.07, 1.21] | |
| 1.3 Existing school staff not involved in delivery | 10 | Odds Ratio (Random, 95% CI) | 0.69 [0.51, 0.94] | |
| 2 Absence from school | 10 | Std. Mean Difference (Random, 95% CI) | ‐0.07 [‐0.22, 0.08] | |
| 2.1 School nurses or teachers involved in delivery | 3 | Std. Mean Difference (Random, 95% CI) | 0.08 [‐0.08, 0.24] | |
| 2.2 Existing school staff not involved in delivery | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.16 [‐0.32, ‐0.00] | |
| 3 Withdrawal from the study | 13 | Odds Ratio (Random, 95% CI) | 1.14 [0.92, 1.43] | |
| 3.1 School nurses involved in delivery | 1 | Odds Ratio (Random, 95% CI) | 5.67 [0.16, 195.90] | |
| 3.2 Existing school staff not involved in delivery | 12 | Odds Ratio (Random, 95% CI) | 1.14 [0.91, 1.42] |
5.3. Analysis.
Comparison 5 Effects of school‐based asthma interventions vs usual care subgrouped by involvement of school staff in direct delivery of self‐management skills to children, Outcome 3 Withdrawal from the study.
Comparison 6. Effects of school‐based asthma interventions vs usual care subgrouped by explicit use of theory.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exacerbations leading to emergency department (ED) visits | 13 | Odds Ratio (Random, 95% CI) | 0.70 [0.53, 0.92] | |
| 1.1 Theoretical framework utilised explicitly | 10 | Odds Ratio (Random, 95% CI) | 0.75 [0.54, 1.04] | |
| 1.2 Use of theory not explicit | 3 | Odds Ratio (Random, 95% CI) | 0.56 [0.33, 0.97] | |
| 2 Absence from school | 10 | Std. Mean Difference (Random, 95% CI) | ‐0.07 [‐0.22, 0.08] | |
| 2.1 Theoretical framework utilised explicitly | 6 | Std. Mean Difference (Random, 95% CI) | ‐0.19 [‐0.35, ‐0.03] | |
| 2.2 Use of theory not explicit | 4 | Std. Mean Difference (Random, 95% CI) | 0.08 [‐0.05, 0.20] | |
| 3 Withdrawal from the study | 13 | Odds Ratio (Random, 95% CI) | 1.14 [0.92, 1.43] | |
| 3.1 Theoretical framework utilised explicitly | 12 | Odds Ratio (Random, 95% CI) | 1.22 [0.97, 1.54] | |
| 3.2 Use of theory not explicit | 1 | Odds Ratio (Random, 95% CI) | 0.61 [0.30, 1.26] |
6.3. Analysis.
Comparison 6 Effects of school‐based asthma interventions vs usual care subgrouped by explicit use of theory, Outcome 3 Withdrawal from the study.
Comparison 7. Effects of school‐based asthma interventions vs usual care subgrouped by whether design included active inclusion or participation of parents.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exacerbations leading to emergency department (ED) visits | 13 | Odds Ratio (Random, 95% CI) | 0.70 [0.53, 0.92] | |
| 1.1 Parents actively included | 8 | Odds Ratio (Random, 95% CI) | 0.82 [0.53, 1.25] | |
| 1.2 Not included/unclear | 5 | Odds Ratio (Random, 95% CI) | 0.58 [0.42, 0.81] | |
| 2 Absence from school | 10 | Std. Mean Difference (Random, 95% CI) | ‐0.07 [‐0.22, 0.08] | |
| 2.1 Parents actively included | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.02 [‐0.23, 0.18] | |
| 2.2 Not included/unclear | 3 | Std. Mean Difference (Random, 95% CI) | ‐0.18 [‐0.50, 0.15] | |
| 3 Withdrawal from the study | 13 | Odds Ratio (Random, 95% CI) | 1.14 [0.92, 1.43] | |
| 3.1 Parents actively included | 9 | Odds Ratio (Random, 95% CI) | 1.21 [0.93, 1.58] | |
| 3.2 Not included/unclear | 4 | Odds Ratio (Random, 95% CI) | 0.97 [0.62, 1.53] |
7.2. Analysis.
Comparison 7 Effects of school‐based asthma interventions vs usual care subgrouped by whether design included active inclusion or participation of parents, Outcome 2 Absence from school.
7.3. Analysis.
Comparison 7 Effects of school‐based asthma interventions vs usual care subgrouped by whether design included active inclusion or participation of parents, Outcome 3 Withdrawal from the study.
Comparison 8. Effects of school‐based asthma interventions vs usual care subgrouped by timing of intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exacerbations leading to emergency department (ED) visits | 13 | Odds Ratio (Random, 95% CI) | 0.70 [0.53, 0.92] | |
| 1.1 Intervention mainly delivered during students' free time | 5 | Odds Ratio (Random, 95% CI) | 0.71 [0.45, 1.13] | |
| 1.2 Intervention took place during school day (exact time unclear or variable) | 8 | Odds Ratio (Random, 95% CI) | 0.67 [0.48, 0.92] | |
| 2 Absence from school | 10 | Std. Mean Difference (Random, 95% CI) | ‐0.07 [‐0.22, 0.08] | |
| 2.1 Intervention mainly delivered during students' free time | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.36, ‐0.11] | |
| 2.2 Intervention took place during school day (exact time unclear or variable) | 8 | Std. Mean Difference (Random, 95% CI) | ‐0.01 [‐0.17, 0.16] | |
| 3 Withdrawal from the study | 13 | Odds Ratio (Random, 95% CI) | 1.14 [0.92, 1.43] | |
| 3.1 Intervention took place during class time | 1 | Odds Ratio (Random, 95% CI) | 13.57 [0.34, 542.83] | |
| 3.2 Intervention mainly delivered during students' free time | 4 | Odds Ratio (Random, 95% CI) | 1.19 [0.65, 2.16] | |
| 3.3 Intervention took place during school day (exact time unclear or variable) | 8 | Odds Ratio (Random, 95% CI) | 1.13 [0.89, 1.43] |
8.3. Analysis.
Comparison 8 Effects of school‐based asthma interventions vs usual care subgrouped by timing of intervention, Outcome 3 Withdrawal from the study.
Comparison 9. Effects of school‐based asthma interventions vs usual care subgrouped by configuration of conditions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exacerbations leading to emergency department (ED) visits | 13 | Odds Ratio (Random, 95% CI) | 0.70 [0.53, 0.92] | |
| 1.1 PI1 ‐ theory, not in own time, no substantial school nurse involvement | 5 | Odds Ratio (Random, 95% CI) | 0.85 [0.47, 1.52] | |
| 1.2 PI2 ‐ theory, not individual, substantial school nurse involvement | 0 | Odds Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Other configuration | 8 | Odds Ratio (Random, 95% CI) | 0.67 [0.47, 0.94] | |
| 2 Absence from school | 10 | Std. Mean Difference (Random, 95% CI) | ‐0.07 [‐0.22, 0.08] | |
| 2.1 PI1 ‐ theory, not in own time, no substantial school nurse involvement | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.10 [‐0.46, 0.25] | |
| 2.2 PI2 ‐ theory, not individual, substantial school nurse involvement | 0 | Std. Mean Difference (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Other configuration | 6 | Std. Mean Difference (Random, 95% CI) | ‐0.05 [‐0.21, 0.12] | |
| 3 Withdrawal from the study | 13 | Odds Ratio (Random, 95% CI) | 1.14 [0.92, 1.43] | |
| 3.1 PI1 ‐ theory, not in own time, no substantial school nurse involvement | 4 | Odds Ratio (Random, 95% CI) | 0.88 [0.55, 1.40] | |
| 3.2 PI2 ‐ theory, not individual, substantial school nurse involvement | 1 | Odds Ratio (Random, 95% CI) | 5.67 [0.16, 195.90] | |
| 3.3 Other configuration | 8 | Odds Ratio (Random, 95% CI) | 1.23 [0.95, 1.58] |
9.1. Analysis.
Comparison 9 Effects of school‐based asthma interventions vs usual care subgrouped by configuration of conditions, Outcome 1 Exacerbations leading to emergency department (ED) visits.
9.2. Analysis.
Comparison 9 Effects of school‐based asthma interventions vs usual care subgrouped by configuration of conditions, Outcome 2 Absence from school.
Comparison 10. Effects of school‐based asthma interventions vs usual care subgrouped by number of consistent conditions (use of theory, parental involvement, not in own time).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exacerbations leading to emergency department (ED) visits | 13 | Odds Ratio (Random, 95% CI) | 0.70 [0.53, 0.92] | |
| 1.1 No conditions | 0 | Odds Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 One condition | 3 | Odds Ratio (Random, 95% CI) | 0.56 [0.33, 0.97] | |
| 1.3 Two conditions | 7 | Odds Ratio (Random, 95% CI) | 0.67 [0.49, 0.94] | |
| 1.4 Three conditions | 3 | Odds Ratio (Random, 95% CI) | 1.48 [0.65, 3.40] | |
| 2 Absence from school | 10 | Std. Mean Difference (Random, 95% CI) | ‐0.07 [‐0.22, 0.08] | |
| 2.1 No conditions | 0 | Std. Mean Difference (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 One condition | 3 | Std. Mean Difference (Random, 95% CI) | 0.02 [‐0.08, 0.11] | |
| 2.3 Two conditions | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.16 [‐0.43, 0.11] | |
| 2.4 Three conditions | 3 | Std. Mean Difference (Random, 95% CI) | 0.07 [‐0.22, 0.37] | |
| 3 Withdrawal from the study | 13 | Odds Ratio (Random, 95% CI) | 1.14 [0.92, 1.43] | |
| 3.1 No conditions | 0 | Odds Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 One condition | 1 | Odds Ratio (Random, 95% CI) | 0.61 [0.30, 1.26] | |
| 3.3 Two conditions | 7 | Odds Ratio (Random, 95% CI) | 1.22 [0.83, 1.80] | |
| 3.4 Three conditions | 5 | Odds Ratio (Random, 95% CI) | 1.22 [0.91, 1.64] |
10.1. Analysis.
Comparison 10 Effects of school‐based asthma interventions vs usual care subgrouped by number of consistent conditions (use of theory, parental involvement, not in own time), Outcome 1 Exacerbations leading to emergency department (ED) visits.
10.2. Analysis.
Comparison 10 Effects of school‐based asthma interventions vs usual care subgrouped by number of consistent conditions (use of theory, parental involvement, not in own time), Outcome 2 Absence from school.
10.3. Analysis.
Comparison 10 Effects of school‐based asthma interventions vs usual care subgrouped by number of consistent conditions (use of theory, parental involvement, not in own time), Outcome 3 Withdrawal from the study.
Comparison 11. Adjunct analyses ‐ impact of Implementation on selected outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exacerbations leading to emergency department (ED) visits | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.21 [‐0.37, ‐0.04] | |
| 1.1 Successful implementation | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.26 [‐0.48, ‐0.04] | |
| 1.2 Potential issues in adherence, attrition, or dosage | 3 | Std. Mean Difference (Random, 95% CI) | ‐0.09 [‐0.28, 0.10] | |
| 2 Absence from school | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.12 [‐0.28, 0.04] | |
| 2.1 Successful implementation | 3 | Std. Mean Difference (Random, 95% CI) | ‐0.28 [‐0.39, ‐0.18] | |
| 2.2 Potential issues in adherence, attrition, or dosage | 4 | Std. Mean Difference (Random, 95% CI) | 0.04 [‐0.09, 0.18] |
Comparison 12. Effects of school‐based asthma interventions vs usual care, including disaggregated effect sizes.
12.2. Analysis.
Comparison 12 Effects of school‐based asthma interventions vs usual care, including disaggregated effect sizes, Outcome 2 Exacerbations leading to hospitalisation ‐ odds ratio.
12.3. Analysis.
Comparison 12 Effects of school‐based asthma interventions vs usual care, including disaggregated effect sizes, Outcome 3 Exacerbations leading to hospitalisation ‐ harmonised effect sizes.
12.4. Analysis.
Comparison 12 Effects of school‐based asthma interventions vs usual care, including disaggregated effect sizes, Outcome 4 Exacerbations leading to emergency department (ED) visits ‐ standardised mean difference.
12.5. Analysis.
Comparison 12 Effects of school‐based asthma interventions vs usual care, including disaggregated effect sizes, Outcome 5 Exacerbations leading to emergency department (ED) visits ‐ odds ratio.
12.6. Analysis.
Comparison 12 Effects of school‐based asthma interventions vs usual care, including disaggregated effect sizes, Outcome 6 Exacerbations leading to emergency department (ED) visits ‐ harmonised effect sizes.
12.7. Analysis.
Comparison 12 Effects of school‐based asthma interventions vs usual care, including disaggregated effect sizes, Outcome 7 Absence from school ‐ standardised mean difference.
12.8. Analysis.
Comparison 12 Effects of school‐based asthma interventions vs usual care, including disaggregated effect sizes, Outcome 8 Absence from school ‐ odds ratio.
12.9. Analysis.
Comparison 12 Effects of school‐based asthma interventions vs usual care, including disaggregated effect sizes, Outcome 9 Absence from school ‐ harmonised effect sizes.
12.10. Analysis.
Comparison 12 Effects of school‐based asthma interventions vs usual care, including disaggregated effect sizes, Outcome 10 Days of restricted activity ‐ standardised mean difference.
12.11. Analysis.
Comparison 12 Effects of school‐based asthma interventions vs usual care, including disaggregated effect sizes, Outcome 11 Days of restricted activity ‐ odds ratio.
12.12. Analysis.
Comparison 12 Effects of school‐based asthma interventions vs usual care, including disaggregated effect sizes, Outcome 12 Days of restricted activity ‐ harmonised effect sizes.
12.13. Analysis.
Comparison 12 Effects of school‐based asthma interventions vs usual care, including disaggregated effect sizes, Outcome 13 Experience of daytime and night‐time symptoms ‐ daytime symptoms ‐ standardised mean difference.
12.14. Analysis.
Comparison 12 Effects of school‐based asthma interventions vs usual care, including disaggregated effect sizes, Outcome 14 Experience of daytime and night‐time symptoms ‐ daytime symptoms ‐ odds ratio.
12.15. Analysis.
Comparison 12 Effects of school‐based asthma interventions vs usual care, including disaggregated effect sizes, Outcome 15 Experience of daytime and night‐time symptoms ‐ daytime symptoms ‐ harmonised effect sizes.
12.16. Analysis.
Comparison 12 Effects of school‐based asthma interventions vs usual care, including disaggregated effect sizes, Outcome 16 Experience of daytime and night‐time symptoms ‐ night‐time symptoms ‐ standardised mean difference.
12.17. Analysis.
Comparison 12 Effects of school‐based asthma interventions vs usual care, including disaggregated effect sizes, Outcome 17 Experience of daytime and night‐time symptoms ‐ night‐time symptoms ‐ odds ratio.
12.18. Analysis.
Comparison 12 Effects of school‐based asthma interventions vs usual care, including disaggregated effect sizes, Outcome 18 Experience of daytime and night‐time symptoms ‐ night‐time symptoms ‐ harmonised effect sizes.
12.19. Analysis.
Comparison 12 Effects of school‐based asthma interventions vs usual care, including disaggregated effect sizes, Outcome 19 Use of reliever therapies, e.g. beta₂‐agonists ‐ odds ratio.
12.20. Analysis.
Comparison 12 Effects of school‐based asthma interventions vs usual care, including disaggregated effect sizes, Outcome 20 Corticosteroid dosage and/or use of add‐on therapies (usage of).
12.21. Analysis.
Comparison 12 Effects of school‐based asthma interventions vs usual care, including disaggregated effect sizes, Outcome 21 Corticosteroid dosage and/or use of add‐on therapies (appropriate usage of).
12.22. Analysis.
Comparison 12 Effects of school‐based asthma interventions vs usual care, including disaggregated effect sizes, Outcome 22 Health‐related quality of life ‐ standardised mean difference.
12.23. Analysis.
Comparison 12 Effects of school‐based asthma interventions vs usual care, including disaggregated effect sizes, Outcome 23 Health‐related quality of life (MD).
12.24. Analysis.
Comparison 12 Effects of school‐based asthma interventions vs usual care, including disaggregated effect sizes, Outcome 24 Unplanned visit to hospital or GP due to asthma symptoms ‐ standardised mean difference.
12.25. Analysis.
Comparison 12 Effects of school‐based asthma interventions vs usual care, including disaggregated effect sizes, Outcome 25 Unplanned visit to hospital or GP due to asthma symptoms ‐ odds ratio.
12.26. Analysis.
Comparison 12 Effects of school‐based asthma interventions vs usual care, including disaggregated effect sizes, Outcome 26 Unplanned visit to hospital or GP due to asthma symptoms ‐ harmonised effect sizes.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al‐Sheyab 2012.
| Methods |
Included as outcome evaluation Study design: clustered parallel‐group design with schools selected as the unit of randomisation Setting: study was conducted in 4 public high schools in the Irbid region of northern Jordan ‐ 2 schools randomised to intervention arm and 2 to control arm Period: dates in which study was conducted ‐ intervention and subsequent data collection ‐ not clear |
|
| Participants |
Eligible sample frame: 261 pupils found to be eligible Randomised: 261 pupils randomised at the school level: 132 to the treatment group and 129 to the control group Completed (intervention): 244 pupils completed the trial Inclusion criteria: students were eligible for participation in the study if they had reported wheezing in the last 12 months as identified by the Arabic version of the ISAAC written questionnaire; were physically and cognitively capable of completing the survey; were able to read and converse in both Arabic and English; and regularly attended school classes Exclusion criteria: students with other diseases that could affect quality of life measures or who were concurrently involved in another health‐related study were not eligible Baseline characteristics Age of children: exact age not given; all children in years 8, 9, and 10 (usually 12 to 15 years old) Ethnicity: not reported Socio‐economic status: not reported Gender: 113 female participants (43.3%); note intervention and control took place in single‐sex schools (1 of each in treatment and control arms) Asthma status: 184 students (70.5%) had a formal asthma diagnosis; 87 students (33.3%) reported use of reliever medication; and 57 students (21.8%) reported use of preventer medication |
|
| Interventions |
Intervention: bilingual health workers trained peer leaders from year 11 to deliver 3 Triple‐A lessons. The content of Triple‐A is not described here, but typical topics in Triple‐A include basic information on asthma, its triggers, and management; and barriers to optimal asthma management, including risk‐taking behaviours such as smoking (see earlier description of Triple A provided in Gibson 1998) Control: not clearly stated (usual care) Intensity: target asthmatic students received 3 lessons from peer leaders (year 11 students) Instructor: peers Theoretical framework: theories involving self‐efficacy underpinned the intervention Parental engagement: not reported Child satisfaction: not reported Timing of intervention in school day: not reported |
|
| Outcomes |
Extractable outcomes were collected for: Health‐related quality of life (measured through the Paediatric Asthma Quality of Life Questionnaire) Withdrawal |
|
| Notes | This intervention tests a model of asthma self‐management education developed elsewhere, although modifications to the intervention are not fully described Considered as a process evaluation but excluded as did not seek to address process evaluation research questions Funding source: Jordan University of Science and Technology (Irbid, Jordon) and Nursing Council |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Low risk ‐ closed‐envelope technique used to select initial schools that were stratified by gender |
| Allocation concealment (selection bias) | High risk | Closed envelope, although no further details were provided and only a small number of schools (4) were involved, potentially compromising the concealment of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No further details were given around blinding of personnel and participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No further details were given around blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low levels of attrition, roughly spread across intervention and control arms |
| Selective reporting (reporting bias) | Low risk | All stated outcomes were reported upon |
| Other bias | Unclear risk | Missingness ‐ low risk ‐ low levels of missingness Baseline imbalance ‐ high risk ‐ differences in asthma reliever therapies at baseline between groups Risk of contamination ‐ low ‐ school‐based randomisation minimises the potential for contamination between intervention and control groups |
| Transparent and clearly stated aims | Unclear risk | N/A |
| Explicit theories underpinning and/or literature review | Unclear risk | N/A |
| Transparent and clearly stated methods and tools | Unclear risk | N/A |
| Selective reporting | Unclear risk | N/A |
| Harmful effects | Unclear risk | N/A |
| Population and sample described well | Unclear risk | N/A |
| Continuous evaluation | Unclear risk | N/A |
| Evaluation participation equity and sampling | Unclear risk | N/A |
| Design and methods overall approach | Unclear risk | N/A |
| Tools and methods of data collection reliable/credible | Unclear risk | N/A |
| Tools and methods of data analysis reliable/credible | Unclear risk | N/A |
| Performance bias/neutrality/credibility/conformability | Unclear risk | N/A |
| Reliability of findings and recommendations | Unclear risk | N/A |
| Transferability of findings | Unclear risk | N/A |
| Overall risk of bias of process evaluation | Unclear risk | N/A |
Al‐Sheyab 2012a.
| Methods |
Included as process evaluation Intervention study design: non‐experimental design with post‐test only evaluation of feasibility among intervention groups Unit of allocation: N/A Process evaluation methods: surveys and focus groups among key stakeholders |
|
| Participants |
Setting: a private girl's high school in Jordan Age of children: students in years 7 to 11 received the intervention delivered by children in years 10 and 11 Child characteristics (BME/SES): no information Asthma status: no information Intervention recipients: children only |
|
| Interventions |
School type: high school (private girl's high school) Intervention description: study authors report that the Adolescent Asthma Action (Triple A) programme uses a 3‐step cascade process from senior to junior students to deliver asthma education and has well‐developed resources, including standardised training manuals, educational videos, asthma‐related models and devices, and first aid kits. Trained health workers provide initial training for peer leaders and facilitate the steps of the programme. Programme content covers management of asthma exacerbations, resisting pressure to smoke, and asthma medication and triggers. Programme delivery occurs through interactive teaching and learning activities, including role‐play and group discussion, all of which are said to be more effective than traditional didactic education for adolescents Control description: N/A ‐ feasibility study with no control group Theoretical framework: study authors report that Triple A is grounded in universally applicable theoretical concepts including peer leadership, self‐efficacy, and empowerment, suggesting its potential for use in different cultural contexts |
|
| Outcomes | Core processes evaluated (child level): the intervention explores child satisfaction in‐depth, with the intervention ostensibly implemented as intended | |
| Notes |
Process evaluation category: stand‐alone; core process questions were central Breadth and depth: neither broad nor deep Voice of children given prominence: featured but not sufficiently; unclear about the extent to which children were able to express negative views of the intervention Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Unclear risk | N/A |
| Transparent and clearly stated aims | Low risk | Study aims were clearly reported |
| Explicit theories underpinning and/or literature review | Unclear risk | Literature was sufficient to support the direction of the intervention, but a specific theory was not named to provide evidence of a sound theoretical basis |
| Transparent and clearly stated methods and tools | Unclear risk | Data collection methods and tools were reported; however data analysis methods are unclear |
| Selective reporting | High risk | Absence of outcome data (e.g. asthma‐related emergencies) directly related to the aims of the programme |
| Harmful effects | High risk | No evidence that any harmful effects were considered |
| Population and sample described well | High risk | Difficulty in distinguishing between numbers involved in the intervention and numbers involved in the process evaluation |
| Continuous evaluation | High risk | Data collected only post intervention |
| Evaluation participation equity and sampling | Unclear risk | Although the voice of young people was given prominence, it is unclear whether intervention sessions required school lessons to be moved, and how teachers felt about this |
| Design and methods overall approach | High risk | Description of research design and methods was limited, particularly with regards to the analysis, as study authors stated that this was beyond the scope of the study |
| Tools and methods of data collection reliable/credible | Low risk | Instruments used were suitable for the study and have been implemented elsewhere |
| Tools and methods of data analysis reliable/credible | High risk | Study authors did not report on an analytical framework and did not describe the methods used for data analysis |
| Performance bias/neutrality/credibility/conformability | Unclear risk | Unclear whether this was addressed during the study |
| Reliability of findings and recommendations | High risk | Study authors considered this to be a feasibility study, and the paper suggests that it was conducted successfully. However, the data presented do not support this in all instances |
| Transferability of findings | High risk | Study authors acknowledged that findings were limited in transferability, as the sample was derived from a single private girl's school, where English was not studied extensively |
| Overall risk of bias of process evaluation | High risk | This study had many unclear or high risk of bias classifications |
Atherly 2009.
| Methods |
Included as outcome evaluation Study design: clustered parallel‐group RCT Setting: junior high and high schools Period: 2003‐2004 school year |
|
| Participants |
Eligible sample frame: not reported Randomised: numbers for these data are disaggregated. The study included 524 children: 458 children randomised at the school level: 225 to the intervention group; and 233 to the control group Completed (intervention): 458 Inclusion criteria: not reported Exclusion criteria: not reported Baseline characteristics Age of children: mean age, 13.9 in the intervention group; 13.4 in the control group Ethnicity: not reported Socio‐economic status: not reported Gender: 46.6% female in the intervention group; 50.7% female in the control group Asthma status: asthmatic children only |
|
| Interventions |
Intervention: the Power Breathing programme focused on education about asthma, asthma control strategies, and psychosocial concerns Control: not reported Intensity: three 90‐minute educational sessions Instructor: teachers and school nurses were involved in the intervention; however their role is unclear Theoretical framework: not reported Parental engagement: not reported Child satisfaction: not reported Timing of intervention in school day: not reported |
|
| Outcomes |
Extractable outcomes were collected for: Exacerbations leading to admission to hospital Asthma symptoms leading to an emergency hospital visit Experience of daytime and night‐time symptoms |
|
| Notes | Study presented an economic evaluation of the intervention Considered for inclusion as a process evaluation but not deemed to fulfil the criteria of a process evaluation Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detail provided on random assignment procedures "The schools were then randomly assigned to the intervention or control group" |
| Allocation concealment (selection bias) | Unclear risk | Not addressed by study authors |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not addressed by study authors |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not addressed by study authors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Although attrition was relatively low overall, the study did not provide details of the spread of attrition across arms and was deemed at high risk of bias "The study included 524 adolescents in grades 6–12 from middle and high schools. Surveys were administered at baseline, immediately postintervention and three months post‐intervention. A total of 458 children completed all surveys, including 225 in the intervention group and 233 in the control group" |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | High risk | Missingness ‐ low risk ‐ besides attrition, no additional missing data were reported Baseline imbalance ‐ high risk ‐ indications showed poorer asthma control at baseline in the control group (e.g. higher level of ED visits) Risk of contamination ‐ low ‐ randomisation occurred at a school level |
| Transparent and clearly stated aims | Unclear risk | N/A |
| Explicit theories underpinning and/or literature review | Unclear risk | N/A |
| Transparent and clearly stated methods and tools | Unclear risk | N/A |
| Selective reporting | Unclear risk | N/A |
| Harmful effects | Unclear risk | N/A |
| Population and sample described well | Unclear risk | N/A |
| Continuous evaluation | Unclear risk | N/A |
| Evaluation participation equity and sampling | Unclear risk | N/A |
| Design and methods overall approach | Unclear risk | N/A |
| Tools and methods of data collection reliable/credible | Unclear risk | N/A |
| Tools and methods of data analysis reliable/credible | Unclear risk | N/A |
| Performance bias/neutrality/credibility/conformability | Unclear risk | N/A |
| Reliability of findings and recommendations | Unclear risk | N/A |
| Transferability of findings | Unclear risk | N/A |
| Overall risk of bias of process evaluation | Unclear risk | N/A |
Bartholomew 2006.
| Methods |
Included as outcome evaluation Study design: clustered parallel‐group design with schools selected as the unit of randomisation Setting: study was conducted across inner city elementary schools in Texas, USA. All schools from a single district were invited to participate, with 84 agreeing to participate. 60 schools were selected because two‐thirds of students were in receipt of free school meals Period: study conducted and data collected from 1997 to 2000 |
|
| Participants |
Eligible sample frame: 982 pupils eligible and consented Randomised: 946 pupils randomised at school level: 515 into treatment group and 431 into control group Completed (intervention): 503 pupils were available at follow‐up; 16 had actively withdrawn, 325 were lost to follow‐up, and 102 had graduated Inclusion criteria: not clearly stated ‐ case detection procedure implemented to discover asthmatic students Exclusion criteria: not clearly stated at child level (school‐level criteria included that schools would have two‐thirds of pupils in receipt of free school meals) Baseline characteristics Age of children: based on reports from 88.3% of pupils: mean age 7.7 years Ethnicity: based on reports from 88.3% of pupils: 45% of children were African American, 51% Hispanic, 3% Caucasian, and 1% from other ethnic groups Socio‐economic status: most students were from households with incomes < $20,000 per year; 28% reported incomes < $9999 Gender: based on reports from 88.3% of pupils: 400 girls (47.9%) and 435 boys (52.1%) Asthma status: indicators of asthma severity not provided |
|
| Interventions |
Intervention: multi‐component intervention involving direct delivery to children, care providers, and parents/guardians. Children received self‐management education through the "Watch, Discover, Think, and Act" interactive computer programme, which was based on National Institutes of Health guidelines and pedagogical and self‐management theories. Children participated in the intervention for approximately 1 year, during which time they played the computer game and their parents received training materials on managing asthma. School nurses received training to improve communication with community primary care providers and to encourage greater monitoring of children's asthma status. Children with persistent asthma in 15 schools received an enhanced intervention, which involved meetings with a project physician to develop an Asthma Action Plan and receipt of a month's supply of medication, with the plan sent to the child's community primary care provider. Researchers also assessed the quality of the school environment with regards to asthma triggers in intervention schools and communicated these findings with recommendations to schools Control: not clearly stated (usual care) Intensity: not clearly described: children were involved in the intervention over the course of a year, and all children completed all levels of the intervention computer programme, although patterns and intensity of usage were not described Instructor: main standardised instruction provided through a computer programme; other components involved school nurses Theoretical framework: a logic model was provided and the computer programme was reported as being based on social cognitive theory Parental engagement: not reported directly. Problems reported in gaining consent: "only about 64% of parents returned case detection surveys, and about half of the families of children with probable asthma agreed to participate in the study" Child satisfaction: not reported Timing of intervention in school day: not reported |
|
| Outcomes | Withdrawal from study | |
| Notes | Several primary outcomes of interest were collected but were not disaggregated by treatment status (exacerbations leading to admission to hospital and absence from school) or were not presented in the results (asthma symptoms leading to emergency department visits) Also considered for possible inclusion as a process evaluation ‐ while the study purports to include a process evaluation section, this was not deemed to include the core components of a process evaluation (i.e. at least a partial focus on implementation outcomes and the relationship with context) Funding source: National Heart, Lung, and Blood Institute, National Institutes of Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was provided on how randomisation occurred, except schools were randomly assigned to treatment and control groups |
| Allocation concealment (selection bias) | Unclear risk | No information was provided on how allocation of schools occurred, except that schools were randomly assigned to treatment and control groups Also little detail on how schools were additionally allocated to enhanced intervention: in 15 schools, an enhanced intervention allowed children and their parents to meet with a project physician, develop an asthma action plan, and receive a 1‐month supply of medication; the project physician then followed up with the child's community physician |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No measures to limit performance and detection biases were described (not considered by trial authors) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No measures to blind outcome assessment were described (not considered by trial authors) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Very high levels of attrition were noted in both outcome and control groups: 43% in control and 49.5% in treatment at post test. Some evidence provided by study authors indicated that "attrition did not create any significant group differences in the variables measured in the study sample" |
| Selective reporting (reporting bias) | High risk | Some outcomes were not reported or results were not disaggregated by intervention and control (e.g. levels of hospitalisation) |
| Other bias | High risk | Missingness ‐ children were not tracked from school to school because of problems with treatment and control group migration. Around 10% of children had missing data at baseline or were not considered to have "usable data", and no imputation strategies were described Baseline imbalance ‐ no data on baseline demographic characteristics were given to illuminate the split between control and intervention groups. No differences between groups were found in health status variables, school performance, attendance, or levels of environmental allergens in schools Risk of contamination ‐ low ‐ randomisation occurred at the school level |
| Transparent and clearly stated aims | Unclear risk | N/A |
| Explicit theories underpinning and/or literature review | Unclear risk | N/A |
| Transparent and clearly stated methods and tools | Unclear risk | N/A |
| Selective reporting | Unclear risk | N/A |
| Harmful effects | Unclear risk | N/A |
| Population and sample described well | Unclear risk | N/A |
| Continuous evaluation | Unclear risk | N/A |
| Evaluation participation equity and sampling | Unclear risk | N/A |
| Design and methods overall approach | Unclear risk | N/A |
| Tools and methods of data collection reliable/credible | Unclear risk | N/A |
| Tools and methods of data analysis reliable/credible | Unclear risk | N/A |
| Performance bias/neutrality/credibility/conformability | Unclear risk | N/A |
| Reliability of findings and recommendations | Unclear risk | N/A |
| Transferability of findings | Unclear risk | N/A |
| Overall risk of bias of process evaluation | Unclear risk | N/A |
Berg 2004.
| Methods |
Included as process evaluation Intervention study design: quasi‐experimental, post test Unit of allocation: not reported Process evaluation methods: descriptive/bivariate (surveys), thematic/grounded theory |
|
| Participants |
Country: USA Age of children: 15 to 18 years Child characteristics (BME/SES): 46.2% were of African American ethnicity. The sample was 69.2% female; SES information was not reported Asthma status: asthmatic only Intervention recipients: children only |
|
| Interventions |
School type: intervention was implemented in 1 high school Intervention description: the Power Breathing programme consisted of educational sessions in which the children met as a group and were instructed on aspects of asthma management, including triggers, symptoms, and causes Control description: not reported Theoretical framework: intervention was grounded in social learning theory |
|
| Outcomes | Core processes evaluated (child level): attrition and adherence were not problematic; information on dosage was not reported | |
| Notes |
Process evaluation category: integrated Breadth and depth: neither broad nor deep Voice of children given prominence: sufficient coverage Funding source: intramural grant from the University of California, Los Angeles, School of Nursing |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Unclear risk | N/A |
| Transparent and clearly stated aims | Low risk | Study aims were clearly reported |
| Explicit theories underpinning and/or literature review | Low risk | Theoretical framework guiding the intervention was described as social learning theory |
| Transparent and clearly stated methods and tools | Low risk | Process of collecting data and the tools used were well described |
| Selective reporting | Low risk | All results of the intervention appear to be documented |
| Harmful effects | Unclear risk | Unclear how much difficulty was involved in engaging with parents |
| Population and sample described well | Unclear risk | Population was generally described well; however the severity of asthma among participants was not reported |
| Continuous evaluation | High risk | Data were collected only post intervention |
| Evaluation participation equity and sampling | Low risk | All students who participated in the pilot were also included in the process evaluation |
| Design and methods overall approach | Unclear risk | Some of the indicators in Table 3 are unconvincing |
| Tools and methods of data collection reliable/credible | Low risk | Tools and methods used for data collection were reported on |
| Tools and methods of data analysis reliable/credible | Low risk | Methods of data analysis were appropriate for the data |
| Performance bias/neutrality/credibility/conformability | Unclear risk | Unclear what effect negative cases had on outcomes. Analysis of those who consented vs those who did not would have been helpful |
| Reliability of findings and recommendations | High risk | Sample size was too small to allow for too much endorsement of study findings |
| Transferability of findings | High risk | Small sample size limits the transferability of findings. The profile of participating students is noticeably different from the profile of the school overall |
| Overall risk of bias of process evaluation | Unclear risk | Not much evidence to determine low risk; however evidence is sufficient for a process evaluation of a feasibility study |
Bignall 2015.
| Methods |
Included as process evaluation Intervention study design: randomised controlled trial Unit of allocation: child Process evaluation methods: descriptive/bivariate (quantitative), descriptive (qualitative) |
|
| Participants |
Setting: single high school in a midwestern city in the USA Age of children: 12 to 17 years (mean age, 15.47 years) Child characteristics (BME/SES): African American Asthma status: asthmatic only Intervention recipients: children |
|
| Interventions |
School type: high school Intervention description: 2 short instructional sessions for children on relaxation/breathing retraining techniques. Participants completed 2 in‐person visits spaced 1 month apart and were given a copy of the script and a CD with breathing retraining techniques to help them practise at home Control description: participants in the control group received 30 minutes of standard asthma education Theoretical framework: no specific framework was named (although supporting literature around breathing exercises was provided) |
|
| Outcomes | Core processes evaluated (child level): attrition, dosage, adherence | |
| Notes |
Process evaluation category: named section(s) on processes included Breadth and depth: neither broad nor deep Voice of children given prominence: featured but not sufficiently Note: not included as outcome evaluation because control received asthma education Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Unclear risk | N/A |
| Transparent and clearly stated aims | Low risk | Study aims were clearly stated |
| Explicit theories underpinning and/or literature review | High risk | No named theoretical framework was presented |
| Transparent and clearly stated methods and tools | Unclear risk | Although methods and tools were clearly described, it is unclear who delivered the intervention. However, tools used and the content of interviews were well described |
| Selective reporting | Unclear risk | Interviews may have been underreported |
| Harmful effects | High risk | Not much scope for harmful effects, such as impact of disruption of the intervention, to be studied |
| Population and sample described well | Low risk | The most relevant characteristics of the sample were captured |
| Continuous evaluation | Low risk | Data were collected before and after the intervention |
| Evaluation participation equity and sampling | High risk | Only child‐level data were collected |
| Design and methods overall approach | Low risk | Two sets of data were provided |
| Tools and methods of data collection reliable/credible | Unclear risk | Difficult to establish whether these were reliable, as they were interviews |
| Tools and methods of data analysis reliable/credible | Unclear risk | Analysis of quantitative data was comprehensive. However, treatment/analysis of qualitative data was unclear |
| Performance bias/neutrality/credibility/conformability | High risk | Because of the way in which qualitative interviews were conducted, risk of performance bias was increased |
| Reliability of findings and recommendations | High risk | Study included a small sample, and target numbers for the study were not achieved. Presentation of qualitative data was limited |
| Transferability of findings | High risk | Study authors did consider transferability of findings; however analysis of qualitative data was absent |
| Overall risk of bias of process evaluation | High risk | Some data were collected well; however treatment of qualitative data reveals high risk of bias |
Brasler 2006.
| Methods |
Included as process evaluation Intervention study design: single‐group pre‐post design Unit of allocation: N/A Process evaluation methods: descriptive analysis of outcome and process factors |
|
| Participants |
Setting: conducted in 12 culturally and socio‐economically diverse junior and middle schools in school districts in Anchorage, Alaska (AK), and Kansas City (KC) suburbs Age of children: 11 to 13 years Child characteristics (BME/SES): 29% of children in Anchorage and 48% in Kansas City were African American, Asian, or Native American. An average of 27% of children across both sites were eligible for free school meals Asthma status: asthmatic only Intervention recipients: children only |
|
| Interventions |
School type: junior/middle Intervention description: PBP: "the program, designed for adolescents aged 11‐19, covers asthma basics and management, addresses adolescents' social and lifestyle concerns, and encourages them to take control of their asthma". It is a manualised program developed by the Asthma and Allergy Foundation of America Control description: N/A Theoretical framework: no single theoretical framework was named |
|
| Outcomes | Core processes evaluated (child level): mainly attrition, dosage, adherence | |
| Notes |
Process evaluation category: named section Breadth and depth: breadth not depth ‐ a broad range of processes explored on a superficial level Voice of children given prominence: not featured ‐ data collected from children did not allow the voice of children to be heard Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Unclear risk | N/A |
| Transparent and clearly stated aims | Low risk | Study aims were clearly stated |
| Explicit theories underpinning and/or literature review | High risk | No evidence of a named theoretical framework |
| Transparent and clearly stated methods and tools | High risk | Most of the content was based on reflective note taking |
| Selective reporting | Low risk | Notes reflected low levels of initial co‐operation |
| Harmful effects | Low risk | Although elements of the intervention did not match the plan, study authors have discussed these |
| Population and sample described well | Unclear risk | Some detail was absent, for example, ethnicity and SES data were not well described |
| Continuous evaluation | Low risk | Data were collected before and after the intervention |
| Evaluation participation equity and sampling | High risk | This was a multi‐component intervention, but the 'voices' of teachers, nurses, and parents were absent |
| Design and methods overall approach | High risk | Tools that were used were unstructured |
| Tools and methods of data collection reliable/credible | High risk | Tools used were unstructured, thus reducing reliability |
| Tools and methods of data analysis reliable/credible | Low risk | Analysis of the data was fair |
| Performance bias/neutrality/credibility/conformability | High risk | Study authors did not consider performance bias |
| Reliability of findings and recommendations | Unclear risk | Tools used to collect these data were not clearly described, although the conclusions drawn appear to match the data presented |
| Transferability of findings | Low risk | Process evaluation findings were regarded as transferable; the extent to which evidence of effectiveness was transferable across sites is unclear |
| Overall risk of bias of process evaluation | High risk | Unstructured data collection methods were used for process evaluation |
Bruzzese 2004.
| Methods |
Included as process evaluation and outcome evaluation Intervention study design: randomised controlled trial with the child selected as the unit of allocation Setting: children were recruited from 2 inner city public high schools Period: not reported |
|
| Participants |
Eligible sample frame: 65 students were eligible; 45 were randomised to intervention or control group Randomised: 45 students were randomised: 23 to the intervention group and 22 to the delayed‐treatment control group across the 2 schools Completed (intervention): 100% of children in the intervention group received workshop 1; 91% received workshop 2; 61% of children in the intervention group received workshop 3, as time did not permit a make‐up session Inclusion criteria: students with persistent asthma symptoms, at least 3 days a week or 3 nights a month Exclusion criteria: not reported Baseline characteristics Age of children: students in 9th and 10th grades Ethnicity: not reported Socio‐economic status: not reported Gender: not reported Asthma status: asthmatic students only |
|
| Interventions |
School type: high school Intervention description: Open Airways for Schools, academic detailing Control description: usual care/nothing Theoretical framework: self‐regulation theory Intervention: students received the ASMA programme, in which students were taught how to manage their asthma to prevent symptoms and improve quality of life. One goal of ASMA is to help students incorporate asthma management strategies into their self‐identity. Continued medical education was also offered to students' medical providers Intensity: intervention was delivered over an 8‐week period, comprising 3 workshops spaced 2 to 3 weeks apart Instructor: intervention was delivered by a trained health educator Parental engagement: not reported Child satisfaction: most students found the sessions helpful; however a third confirmed that they may or may not participate again Timing of intervention in school day: every attempt was made to meet with students during their free time |
|
| Outcomes |
Extractable outcomes were collected for: none Core processes/outcomes evaluated (child level): attrition, adherence |
|
| Notes |
Process evaluation category: stand‐alone Breadth and depth: neither broad nor deep Voice of children given prominence: sufficient coverage Funding source: Speakers' fund for public health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only detail provided was that students were randomly assigned to treatment or control groups |
| Allocation concealment (selection bias) | Unclear risk | No details were provided except that students were randomly assigned to treatment or control groups |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Methods used for blinding were not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Methods used for blinding were not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Low levels of post‐test outcomes collected: 14 students who attended group workshop #3 ‐ 64% of all treatment students ‐ completed an evaluation of the programme |
| Selective reporting (reporting bias) | Unclear risk | No data from the control group were presented. The procedure followed for control group students is unclear |
| Other bias | Unclear risk | Baseline data were not reported, so we are unable to determine if any imbalances existed at baseline |
| Transparent and clearly stated aims | Low risk | Study aims were clearly stated |
| Explicit theories underpinning and/or literature review | Low risk | Theory guiding the intervention was provided as self‐regulation theory |
| Transparent and clearly stated methods and tools | High risk | Tools used for data collection were inferred, as opposed to being stated |
| Selective reporting | Unclear risk | Outcomes that study authors intended to measure are unclear; therefore we are unable to say whether evidence shows selective reporting |
| Harmful effects | High risk | No provision was made for harmful effects |
| Population and sample described well | Unclear risk | Some aspects of the sample were described in sufficient detail; however no information was provided on ethnicity, SES, or asthma severity among participants |
| Continuous evaluation | High risk | Study authors did not capture the people who dropped out |
| Evaluation participation equity and sampling | Unclear risk | Not reported by study authors |
| Design and methods overall approach | Unclear risk | Little information was given about the methods used |
| Tools and methods of data collection reliable/credible | High risk | Small sample size suggests that different approaches to analysis and collection should have been used |
| Tools and methods of data analysis reliable/credible | High risk | Small sample size suggests that different approaches to analysis and collection should have been used |
| Performance bias/neutrality/credibility/conformability | Unclear risk | Not reported by study authors |
| Reliability of findings and recommendations | Unclear risk | Not reported by study authors |
| Transferability of findings | Unclear risk | Some of the lessons learnt around time‐tabling were transferable; however study authors did not assess transferability |
| Overall risk of bias of process evaluation | High risk | Points around sampling and absence of continuous evaluation contribute to high risk of bias |
Bruzzese 2008.
| Methods |
Included as outcome evaluation and process evaluation Study design: parallel‐group design with families selected as the unit of randomisation Setting: study recruited children and their families from one middle school in New York City, New York, USA Period: dates on which study was conducted ‐ intervention and subsequent data collection ‐ not clear; follow‐up data collected 2 months post intervention |
|
| Participants |
Eligible sample frame: 78 pupils found to be eligible; 24 agreed to participate Randomised: 24 students randomised (at the student/family level): 12 each in control and intervention groups Completed (intervention): 12 students and 10 caregivers in the treatment group, and 11 students and 8 caregivers in the control group, completed immediate follow‐up Inclusion criteria: after completing a case detection survey, eligible students were identified as having had "an asthma diagnosis from a medical provider, and over the past 12 months exhibited asthma symptoms an average of three times per month and used asthma medication" Exclusion criteria: families were excluded if "(1) the child had a co‐morbid disease that affects lung functioning or highly specialized developmental or learning needs, (2) the child and/or the caregiver did not speak English, or (3) the caregiver and child did not live together" Baseline characteristics Age of children: mean age, 12.8 years (grades 6 to 8) Ethnicity: child ethnicity described as Hispanic (41%); White, not of Hispanic origin (17%); African American, not of Hispanic origin (8%); and other (34%) Socio‐economic status: employment status of participating caregivers (but socio‐economic circumstances of family) presented. Unemployed (8%); employed part‐time (21%); employed full‐time (71%). Data on highest educational level completed by caregiver were also presented, with 66% having post‐compulsory education Gender: males accounted for 13 of the child participants (54%), and females for 11 of the participants (46%) Asthma status: direct information on severity not presented |
|
| Interventions |
School type: junior/middle Intervention description: OAS, ASMA, caregiver education Control description: usual care/nothing Theoretical framework: social cognitive theory and cognitive‐behavioural therapy Intervention: implemented "Asthma: It's a Family Affair!" intervention. "Intervention students received six group sessions on prevention and management of asthma. Lesson topics included: (1) information and feelings about asthma; (2) asthma medication; (3) prevention and management of asthma symptoms; (4) problem‐solving and coping with negative feelings about asthma; and (5) communicating about asthma to peers and teachers, relaxation exercises, and healthy behaviours, including smoking refusal skills and avoiding exposure to secondhand smoke". The sixth session included a comprehensive review designed to reinforce key messages and to enhance students' confidence in managing their asthma. Curriculum was based on existing materials from Coping with Asthma at Home and at School, OAS, and ASMA. Caregivers participated in five 90‐minute group sessions held once per week intended to support the child's autonomy and development of asthma self‐management skills Control: no treatment (usual care) Intensity: children received 6 lessons on a weekly basis Instructor: children's sessions were delivered by a developmental psychologist Theoretical framework: integration of 4 psychological theories: social cognition theory, cognitive‐behavioural theory, and 2 forms of family systems theory (parenting styles and behavioural family systems theory) Parental engagement: low attrition among caregivers and high levels of satisfaction Child satisfaction: high levels of satisfaction: "All students reported that the intervention gave them a better understanding of asthma, and 91% reported that the handouts helped them understand the topics discussed. Many reported that the best aspect of the program was 'talking about my asthma'" Timing of intervention in school day: not reported |
|
| Outcomes |
Extractable outcomes were collected for: Experience of daytime and night‐time symptoms Withdrawal from the study Core processes/outcomes evaluated (child level): attrition, dosage, adherence |
|
| Notes |
Process evaluation category: named section Breadth and depth: neither broad nor deep Voice of children given prominence: featured but not sufficiently Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on how randomisation occurred |
| Allocation concealment (selection bias) | Unclear risk | No information on how allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information; blinding was not assessed by study authors |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information; blinding was not assessed by study authors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few participants dropped out (1 out of 12 students in the control group) |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | Missingness ‐ low risk ‐ no evidence of missing indicators Baseline imbalance ‐ low risk ‐ no evidence of systematic differences in baseline characteristics Contamination ‐ high ‐ randomisation was by family |
| Transparent and clearly stated aims | Low risk | Study aims were clearly stated |
| Explicit theories underpinning and/or literature review | Low risk | Intervention development was guided by social cognitive theory and cognitive‐behavioural therapy |
| Transparent and clearly stated methods and tools | Unclear risk | Medium bias ‐ tools were poorly described |
| Selective reporting | Unclear risk | As data collection tools were not that well stated, it is difficult to assess whether results show any evidence of selective reporting |
| Harmful effects | Unclear risk | Not considered by trial authors |
| Population and sample described well | Low risk | Population and sample were well described |
| Continuous evaluation | Unclear risk | Data were collected before and after the intervention; however the level of participation was low |
| Evaluation participation equity and sampling | High risk | Little evidence of evaluation from children |
| Design and methods overall approach | Unclear risk | Unclear how data were collected |
| Tools and methods of data collection reliable/credible | High risk | Unclear whether tools used had been validated |
| Tools and methods of data analysis reliable/credible | Low risk | Analysis appears to reflect the data |
| Performance bias/neutrality/credibility/conformability | Unclear risk | Unclear whether this was given consideration |
| Reliability of findings and recommendations | Low risk | Reliability of findings clear; conclusions justified |
| Transferability of findings | High risk | No consideration given to transferability of findings |
| Overall risk of bias of process evaluation | Unclear risk | Medium bias |
Bruzzese 2010.
| Methods |
Included as outcome evaluation and process evaluation Intervention study design: RCT, parallel group, randomised at the child level Setting: 25 public schools in New York City Period: intervention was conducted over 4 years |
|
| Participants |
Eligible sample frame: 393 students were eligible; 288 completed the 12‐month follow‐up Randomised: 393 students were randomised; numbers of students in intervention and control groups not reported Completed (intervention): 288 students completed the 12‐month follow‐up Inclusion criteria: students with persistent asthma and their caregivers Exclusion criteria: not reported Baseline characteristics Age of children: 14 to 16 Child characteristics (BME/SES): 45.51% Hispanic; 75% of students on free school meals Asthma status: asthmatic only Intervention recipients: children only |
|
| Interventions |
School type: high school Intervention description: ASMA, academic detailing Control description: usual care/nothing Theoretical framework: social cognitive theory Intervention: a family affair programme. Students in the intervention group attended 6 workshops to empower them to manage their asthma; their parents attended training group workshops to teach childrearing skills that support their child's growing autonomy and need to manage their asthma Intensity: children attended 6 workshops, and parents/caregivers attended 5 Instructor: not reported Parental engagement: not reported Child satisfaction: not reported Timing of intervention in school day: not reported |
|
| Outcomes |
Extractable outcomes were collected for: Withdrawal Core processes evaluated (child level): attrition, dosage, adherence |
|
| Notes |
Process evaluation category: integrated Breadth and depth: neither broad nor deep Voice of children given prominence: featured but not sufficiently Funding source: National Heart, Lung, and Blood Institute; NYC Speakers' Fund |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported by study authors |
| Allocation concealment (selection bias) | Unclear risk | Not reported by study authors |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Abstract only; no information on blinding presented |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Abstract only; no information on blinding presented |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract only; no information on blinding presented |
| Selective reporting (reporting bias) | Unclear risk | Abstract only; no information on blinding presented |
| Other bias | Unclear risk | Abstract only; no information on blinding presented |
| Transparent and clearly stated aims | Low risk | Study aims were clearly stated |
| Explicit theories underpinning and/or literature review | Low risk | Social cognitive theory guided development of the intervention |
| Transparent and clearly stated methods and tools | Low risk | Methods and tools used were clearly described |
| Selective reporting | Low risk | All collected data were reported on |
| Harmful effects | Unclear risk | Medium bias ‐ some harmful effects around differential reach can be inferred |
| Population and sample described well | Low risk | Sufficient data were included to provide a depiction of context |
| Continuous evaluation | Low risk | Two rounds of follow‐up were described for outcomes |
| Evaluation participation equity and sampling | Low risk | Available data with adjustments for main baseline imbalances could serve as confounders |
| Design and methods overall approach | Low risk | Administrative and survey data were collected at multiple points |
| Tools and methods of data collection reliable/credible | Low risk | Validated tools were used |
| Tools and methods of data analysis reliable/credible | Low risk | Credible analysis ‐ zip regression modelling was employed to address skewness |
| Performance bias/neutrality/credibility/conformability | Low risk | Blinding was explicitly mentioned |
| Reliability of findings and recommendations | Low risk | No issues were reported |
| Transferability of findings | Low risk | Study authors have considered this; however their findings have comparatively low transferability "We are also unable to extrapolate study results to other populations of high school students with asthma (e.g. white suburban adolescents with mild asthma) because we limited enrolment to minority youth with moderate to severe persistent asthma" |
| Overall risk of bias of process evaluation | Low risk | Within the narrow confines of the data, this is a well‐conducted study |
Bruzzese 2011.
| Methods |
Included as outcome evaluation Intervention study design: randomised controlled trial parallel group Setting: conducted at 5 participating high schools in New York, USA Period: study enrolment took place over 4 consecutive school years from 2001 to 2004 |
|
| Participants |
Eligible sample frame: 261 pupils found to be eligible Randomised: 345 students randomised: 175 to intervention group and 170 to control group Completed (intervention): 139 (79.4%) in the intervention group completed follow‐up, as did 142 (83.5%) in the control group Inclusion criteria: 9th and 10th graders with moderate to severe persistent asthma who were taking medication prescribed by a medical provider in the last 12 months Exclusion criteria: none stated Baseline characteristics Age of children: mean age, 15.10 years Ethnicity: 45.5% Hispanic/Latino/a or Hispanic American; 37.7% African American/African or Caribbean American/Caribbean; 11.6% mixed ethnicity; 5.2% other ethnicity Socio‐economic status: not reported Gender: 29.6% male; 70.4% female Asthma status: 68.70% moderate persistent asthma, 31.30% severe persistent asthma. No information on SES |
|
| Interventions |
Intervention: ASMA consisted of 2 components: (I) an 8‐week intensive programme for students, and (ii) academic detailing for adolescents’ medical providers. Student intervention consisted of three 45‐ to 60‐minute group sessions, and individual tailored coaching sessions held at least once per week for 5 weeks. Sessions were delivered by trained health educators during the school day. Students were taught asthma management skills and ways to cope with asthma, and were encouraged to see their medical provider for clinical evaluation and treatment (see Bruzzese 2004 for a full outline of ASMA content) Control: wait‐list control (usual care) Intensity: three 45‐ to 60‐minute group sessions for children over 8 weeks and individual tailored coaching sessions once a week for 5 weeks Instructor: health educators Theoretical framework: ASMA described as grounded in social cognitive theory Parental engagement: not reported Child satisfaction: not reported Timing: unclear but at some point during the school day |
|
| Outcomes |
Extractable outcomes were collected for: Exacerbations leading to hospital admission Asthma symptoms leading to emergency hospital visits Absence from school Days of restricted activity Unplanned GP or hospital visit due to asthma Experience of daytime and night‐time symptoms Corticosteroid dosage Withdrawal |
|
| Notes | Funding source: National Heart, Lung, and Blood Institute; NYC Speakers' Fund | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study authors reported: "Within each stratum, we randomised students to control or intervention using computerized randomisation lists generated in advance by the data manager who concealed them until randomisation" |
| Allocation concealment (selection bias) | Low risk | Randomisation lists were generated in advance by the data manager, who concealed them until randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Interviewers were blind to group assignment. Whether participants were blinded is unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Interviewers were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No differences were noted between the 2 groups ‐ incomplete data were unlikely to affect outcomes |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | Missingness ‐ low risk ‐ appears that more participants who did not drop out submitted their data Baseline imbalance ‐ low risk ‐ intervention and control groups were relatively evenly matched in characteristics Risk of contamination ‐ low ‐ informal interviews with control participants regarding their contact with other students in the programme suggest that contamination did not occur |
| Transparent and clearly stated aims | Unclear risk | N/A |
| Explicit theories underpinning and/or literature review | Unclear risk | N/A |
| Transparent and clearly stated methods and tools | Unclear risk | N/A |
| Selective reporting | Unclear risk | N/A |
| Harmful effects | Unclear risk | N/A |
| Population and sample described well | Unclear risk | N/A |
| Continuous evaluation | Unclear risk | N/A |
| Evaluation participation equity and sampling | Unclear risk | N/A |
| Design and methods overall approach | Unclear risk | N/A |
| Tools and methods of data collection reliable/credible | Unclear risk | N/A |
| Tools and methods of data analysis reliable/credible | Unclear risk | N/A |
| Performance bias/neutrality/credibility/conformability | Unclear risk | N/A |
| Reliability of findings and recommendations | Unclear risk | N/A |
| Transferability of findings | Unclear risk | N/A |
| Overall risk of bias of process evaluation | Unclear risk | N/A |
Carpenter 2016.
| Methods |
Included as process evaluation Intervention study design: study design was quasi‐experimental, with pre‐post follow‐up and no control Unit of allocation: N/A Process evaluation methods: descriptive/bivariate |
|
| Participants |
Country: USA Age of children: 25 children 7 to 17 years old were recruited Child characteristics (BME/SES): sample comprised 72% non‐Hispanic white children Asthma status: asthmatic children only Intervention recipients: children and nurses |
|
| Interventions |
School type: intervention was delivered at 7 different schools, consisting of high schools, junior/middle schools, and primary/elementary schools Intervention description: the intervention consisted of 2 sessions for children. Children were asked to watch a tailored video and to demonstrate their inhaler technique before and after the video. One month later, at the second session, children demonstrated their inhaler technique again to the school nurse and were allowed to watch the video again Control description: N/A Theoretical framework: not reported |
|
| Outcomes | Core processes evaluated (child level): adherence | |
| Notes |
Process evaluation category: named section Breadth and depth: neither broad nor deep Voice of children given prominence: sufficient coverage Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Unclear risk | N/A |
| Transparent and clearly stated aims | Low risk | Study aims were clearly stated |
| Explicit theories underpinning and/or literature review | High risk | No theoretical framework was presented to inform the intervention |
| Transparent and clearly stated methods and tools | Unclear risk | Medium bias ‐ content and conduct of the focus group were not presented |
| Selective reporting | High risk | Study authors provided some example quotations; however it is unclear how themes were derived |
| Harmful effects | High risk | The premise of the intervention could be considered harmful, as the information that was collected should be provided only by demographically equivalent people. However, this was not explored in depth |
| Population and sample described well | Unclear risk | The reach of this study is unclear; very little information on the sample was presented |
| Continuous evaluation | Unclear risk | Not reported by study authors |
| Evaluation participation equity and sampling | Unclear risk | Some information was collected from different actors; however information was not collected to the same degree of robustness as for other participants |
| Design and methods overall approach | Unclear risk | Medium bias ‐ premise of the intervention is suspect, and approach needs to be grounded in providing information around a need ‐ but this info is lacking |
| Tools and methods of data collection reliable/credible | Unclear risk | The focus of the intervention is not reflected strongly enough in the data collected |
| Tools and methods of data analysis reliable/credible | Unclear risk | No information was provided on how data were collected in the focus group, so whether data analysis methods used were appropriate is not clear |
| Performance bias/neutrality/credibility/conformability | High risk | No evidence indicates whether concerns were addressed during the data collection process |
| Reliability of findings and recommendations | Unclear risk | Although data were presented, how data were collected remains unclear |
| Transferability of findings | High risk | No evidence indicates this; the premise of the intervention makes a large focus on transferability very important |
| Overall risk of bias of process evaluation | High risk | The premise of the intervention was not explored |
Cicutto 2005.
| Methods |
Included as outcome evaluation Study design: clustered parallel‐group design with schools selected as the unit of randomisation Setting: study was conducted across 26 elementary schools in a suburb of Toronto (Canada) Period: dates on which the study was conducted ‐ including intervention and subsequent data collection ‐ not clear; intervention and data collection was conducted over the period of a year |
|
| Participants |
Eligible sample frame: 40 elementary schools were randomly selected from a pool of 147 potential elementary schools for inclusion in the Toronto area. Parents and children were invited to participate, and eligibility was assessed (see below). Based on the information returned, 26 schools had sufficient numbers of pupils (more than 7 pupils per school) to allow the trial to go ahead. In total, 297 eligible pupils were identified across the 26 schools Randomised: 256 pupils randomised at the school level: 132 to the treatment group and 124 to the control group Completed (intervention): 248 pupils remained at the 6‐month data collection point: 130 treatment group children and 118 control group children. 239 children completed the intervention: 121 in the treatment and 118 in the control group. Inclusion criteria: students were eligible for participation if they were reported as (I) having physician‐diagnosed asthma, (ii) having used an asthma medication (i.e. bronchodilator and/or anti‐inflammatory agents) for breathing difficulties, and (iii) having experienced asthma symptoms 3 or more times in the past year. Students had to meet all 3 criteria to be eligible Exclusion criteria: children were excluded from the study if they had a second (major) chronic illness with a pulmonary component (e.g. cystic fibrosis) Baseline characteristics Age of children: mean age, 8.6 years across treatment and control groups Ethnicity: not reported Socio‐economic status: indicative evidence that none of the children were from low‐income families: "The average income of the parent/guardian who participated in the study was approximately $53,000.00 (Canadian dollars) with a range of $20,500.00 to $200,000.00. In Canada, low‐income families earn $19,000.00 per year" Gender: treatment group: 58.3% male, 41.7% female; control group: 59.6% male, 40.4% female Asthma status: At baseline, according to parental report: Treatment group: 68.2% of children had mild asthma, 20.4% had moderate asthma, 5.4% had severe asthma; Control group: 69.5% of children had mild asthma; 23.4% had moderate asthma; 7.3% had severe asthma |
|
| Interventions |
Intervention: children in treatment group received "Roaring Adventures of Puff" (RAP) intervention. This consisted of 6 sessions that included: "(1) getting to know each other, goal setting, use of a peak flowmeter, and diary monitoring; (2) trigger identification, control, and avoidance, and basic pathophysiology; (3) medications and the proper use of inhalers; (4) symptom recognition and action plan use; (5) lifestyle, exercise, and managing an asthma episode; and (6) sharing asthma information with teachers and parents". Parents were invited to attend the final session and were encouraged to take part in assisting with children's homework Control: usual care Intensity: children attended 6 sessions 50 to 60 minutes in length that were held once a week over 6 consecutive weeks Instructor: asthma educator Theoretical framework: theories involving social cognitive theory and self‐regulation underpinned the intervention Parental engagement: reported as low ‐ < 20% of children had a parent who attended the final session Child satisfaction: not reported Timing of intervention in school day: sessions took place over the lunch period |
|
| Outcomes |
Extractable outcomes were collected for: Exacerbations leading to hospital admission Asthma symptoms leading to emergency hospital visits Absence from school Days of restricted activity |
|
| Notes | Considered for process evaluation but not deemed to address implementation questions nor to provide any in‐depth study of implementation processes Funding source: Change Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was centrally controlled by a computerised randomisation programme |
| Allocation concealment (selection bias) | Low risk | Randomisation was centrally controlled by a computerised randomisation programme |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not addressed by study authors |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors and participants were blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low levels of attrition/dropout with outcome data collected from 93% of students, who were randomised |
| Selective reporting (reporting bias) | Unclear risk | Not all outcomes were fully reported, but they were described narratively in some places |
| Other bias | Low risk | Missingness ‐ unclear risk ‐ intention‐to‐treat analyses were implemented, although the extent of missingness (as opposed to attrition) is unclear Baseline imbalance ‐ low risk ‐ demographic and asthma‐related characteristics were similar between the 2 study groups and did not demonstrate statistically significant differences Risk of contamination ‐ low ‐ allocation was provided at the school level |
| Transparent and clearly stated aims | Unclear risk | N/A |
| Explicit theories underpinning and/or literature review | Unclear risk | N/A |
| Transparent and clearly stated methods and tools | Unclear risk | N/A |
| Selective reporting | Unclear risk | N/A |
| Harmful effects | Unclear risk | N/A |
| Population and sample described well | Unclear risk | N/A |
| Continuous evaluation | Unclear risk | N/A |
| Evaluation participation equity and sampling | Unclear risk | N/A |
| Design and methods overall approach | Unclear risk | N/A |
| Tools and methods of data collection reliable/credible | Unclear risk | N/A |
| Tools and methods of data analysis reliable/credible | Unclear risk | N/A |
| Performance bias/neutrality/credibility/conformability | Unclear risk | N/A |
| Reliability of findings and recommendations | Unclear risk | N/A |
| Transferability of findings | Unclear risk | N/A |
| Overall risk of bias of process evaluation | Unclear risk | N/A |
Cicutto 2013.
| Methods |
Included as process evaluation and outcome evaluation Intervention study design: cluster RCT Setting: 170 primary/elementary schools from 5 public health units across Ontario, Canada Period: not reported |
|
| Participants |
Eligible sample frame: 180 schools were eligible and 170 schools were randomised (85 in each arm). 2502 families were eligible and 1316 were enrolled Randomised: 1316 participants were enrolled Completed (intervention): 1172 completed the study; 144 children withdrew, representing an 11% withdrawal rate Inclusion criteria: parental report of physician‐diagnosed asthma, use of asthma medications, asthma symptoms at least 3 times in the past year, enrolment in grades 1 through 5 at school, ability to speak English, no other chronic conditions that could mimic asthma Exclusion criteria: not reported Baseline characteristics Age of children: 8 years old Ethnicity: not reported Socio‐economic status: 26.4% of children had a mother who did not have a high school diploma Gender: the sample comprised 57.4% males; 58.4% of control children and 56.6% of intervention children were male Asthma status: asthmatic only |
|
| Interventions |
School type: primary/elementary Intervention description: Roaring Adventures of Puff Control description: usual care/nothing Theoretical framework: social cognitive theory Intervention: workshops included goal setting and self‐monitoring, trigger identification, control and avoidance, basic pathophysiology, medication and proper inhaler use, symptom recognition and the asthma action plan, lifestyle and exercise, managing an asthma exacerbation, and showcasing learning with teachers and parents. The intervention used interactive techniques to educate the children. Make‐up sessions were available for those who missed a class Intensity: children attended six 45‐minute sessions Instructor: public health nurses Parental engagement: difficulty in getting parents/guardians involved Child satisfaction: not reported Timing of intervention in school day: lunchtime |
|
| Outcomes |
Extractable outcomes collected for: Asthma symptoms leading to emergency hospital visits Parent‐reported absence from school Days of restricted activity Unplanned visit to hospital or GP due to asthma symptoms Health‐related quality of life Withdrawal Core processes evaluated (child level): attrition |
|
| Notes |
Process evaluation category: stand‐alone and Integrated (2 papers) Breadth and depth: breadth ‐ not depth Voice of children given prominence: not featured Funding source: Ontario Ministry of Health and Long Term Care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence: of the 180 schools, 170 schools with the largest numbers of students with asthma were randomised to intervention or control groups through a computer‐generated table of random numbers |
| Allocation concealment (selection bias) | Low risk | Central allocation indicated: of the 180 schools, 170 schools with the largest numbers of students with asthma were randomised to intervention or control groups through a computer‐generated table of random numbers |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not clear whether measures were taken and whether this would have influenced the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study authors reported: "Data collectors were blinded to group assignment. A post survey of data collectors revealed that blinding was successful. Data collectors became unblinded to group assignment for 9% of participating families, which occurred during the data collection interviews with parents/guardians" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition data were presented, although data were not disaggregated by treatment status |
| Selective reporting (reporting bias) | Unclear risk | Absence of clear numbers hinders interpretation of some data |
| Other bias | Unclear risk | Missingness ‐ high risk ‐ evidence of unexplained missing data in Table 2 Baseline imbalance ‐ low risk ‐ study authors reported: "Randomisation was successful in that comparison of baseline variables for the control and experimental groups suggested they were similar or balanced before the intervention" Risk of contamination ‐ low ‐ unit of randomisation was at the school level, reducing the threat of contamination |
| Transparent and clearly stated aims | Low risk | Study aims were clearly stated |
| Explicit theories underpinning and/or literature review | Low risk | Intervention development was informed by social cognitive theory |
| Transparent and clearly stated methods and tools | Low risk | Tools and methods were clearly reported |
| Selective reporting | Unclear risk | Unclear how this was addressed |
| Harmful effects | Unclear risk | Study authors did report on low parental engagement; however they offered no explanation as to why all treatment schools did not adopt the policy |
| Population and sample described well | Unclear risk | Individual schools were not described sufficiently |
| Continuous evaluation | Low risk | Policy development was monitored early on; 2 sets of data were collected after baseline |
| Evaluation participation equity and sampling | High risk | Stakeholders were not directly involved |
| Design and methods overall approach | Low risk | Pre‐post assessment data were collected |
| Tools and methods of data collection reliable/credible | Low risk | Study authors used validated data collection tools |
| Tools and methods of data analysis reliable/credible | Low risk | Data analysis methods used were suitable for the data |
| Performance bias/neutrality/credibility/conformability | Unclear risk | Blinding was attempted but was not always successful |
| Reliability of findings and recommendations | Low risk | Clearly reported by study authors. Findings appear to be reliable |
| Transferability of findings | Unclear risk | Not explored |
| Overall risk of bias of process evaluation | Low risk | Important indicators, for example, design methods and continuous evaluation, were at low risk |
Clark 2004.
| Methods |
Included as outcome evaluation Study design: clustered parallel‐group design with schools selected as the unit of randomisation Setting: study was conducted at 14 public high schools in Detroit, Michigan, USA. Seven schools (416 children) were assigned to the treatment arm, and 7 schools (419 children) to the control group Period: dates on which study was conducted ‐ intervention and subsequent data collection ‐ were not clear; follow‐up data were collected 2 years after baseline |
|
| Participants |
Eligible sample frame: following a case detection survey, 1217 children were identified as eligible and 835 parents provided consent for their child to participate Randomised: 835 pupils randomised at the school level: 416 to the treatment group and 419 to the control group Completed (intervention): unclear; 674 parents participated in follow‐up survey 2 years later, but this was not disaggregated and completion figures were not provided Inclusion criteria: students were eligible for participation in the study if they had: "(1) a physician’s diagnosis of asthma and active symptoms, or a diagnosis and received a prescription for asthma medications in the previous year; or (2) no physician’s diagnosis, but reported presence of three or more of seven asthma symptoms in the past year, or reported either of two exercise‐related asthma symptoms with frequency of three times or more, in the past year" Exclusion criteria: no additional exclusion criteria reported Baseline characteristics Age of children: all children were in grades 2 through 5; 93% were between 7 years and 10 years of age Ethnicity: 98% of children were African American Socio‐economic status: schools were located in areas of high poverty. Almost half of students (45%) were from families with annual income under $15,000 Gender: no gender breakdown was provided Asthma status: 236 students had mild persistent asthma (28.3%), 128 had moderate asthma (15.3%), and 40 had severe asthma (4.7%) |
|
| Interventions |
Intervention This was a comprehensive programme of asthma self‐management targeted at children, caregivers, and the wider school: "The program elements were as follows: (1) OAS disease management training for children adapted to local needs (for example, related to smoking among elementary school‐aged children), which included handouts and homework assignments involving parents; (2) 'Environmental Detective', two classroom sessions for classmates to enhance their understanding of factors that may influence respiratory health in general, and to help them develop empathy for children with asthma in particular; (3) orientation to asthma and control strategies for school principals and counsellors; (4) briefings and building walk‐throughs for custodial personnel regarding potential environmental triggers to asthma symptoms and practical means of remediation; (5) school fairs for children and their caretakers, including asthma care question‐and‐answer sessions for the adults; (6) written communication on behalf of the family with the child’s clinician providing information about the school program, encouraging completion of an asthma action plan for the child, and requesting provision of a copy to the school" Control: wait‐list control (usual care) Intensity: target students received OAS training (which usually consists of 6*60‐minute sessions) as well as 2 additional classroom sessions through the 'environmental detective' component Instructor: not reported Theoretical framework: not explicitly described Parental engagement: not reported in detail, although elements of the programme reported as having been "completed with reasonable success", except the element that involved written communication with the child's physician Child satisfaction: not reported Timing of intervention in school day: not reported |
|
| Outcomes | Selected outcomes were extracted but were not combined in meta‐analyses due to incompatibility of unit of analysis (risk difference) between this study and others | |
| Notes | Absence from school and experience of daytime and night‐time symptoms were collected in the study, but sample sizes disaggregated by study arm that could allow for extraction and inclusion in meta‐analysis were not included Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Schools were randomly assigned via a random numbers table to receive the programme (7 schools and 416 children) or to be assigned to a wait‐list control group (7 schools and 419 children) |
| Allocation concealment (selection bias) | High risk | Use of pre‐defined random number table potentially compromised allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information was provided on blinding; this was not addressed by study authors |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was provided on blinding; this was not addressed by study authors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear ‐ full information on attrition was not presented |
| Selective reporting (reporting bias) | Low risk | No evidence indicated selective reporting (although the unit of analysis was incompatible for the meta‐analytical framework implemented) |
| Other bias | Low risk | Missingness ‐ unclear ‐ information on missing data was not provided Baseline imbalance ‐ low risk ‐ no differences in characteristics of intervention and control groups were described Risk of contamination ‐ low ‐ schools were the unit of randomisation, providing low risk of contamination |
| Transparent and clearly stated aims | Unclear risk | N/A |
| Explicit theories underpinning and/or literature review | Unclear risk | N/A |
| Transparent and clearly stated methods and tools | Unclear risk | N/A |
| Selective reporting | Unclear risk | N/A |
| Harmful effects | Unclear risk | N/A |
| Population and sample described well | Unclear risk | N/A |
| Continuous evaluation | Unclear risk | N/A |
| Evaluation participation equity and sampling | Unclear risk | N/A |
| Design and methods overall approach | Unclear risk | N/A |
| Tools and methods of data collection reliable/credible | Unclear risk | N/A |
| Tools and methods of data analysis reliable/credible | Unclear risk | N/A |
| Performance bias/neutrality/credibility/conformability | Unclear risk | N/A |
| Reliability of findings and recommendations | Unclear risk | N/A |
| Transferability of findings | Unclear risk | N/A |
| Overall risk of bias of process evaluation | Unclear risk | N/A |
Clark 2005.
| Methods |
Included as outcome evaluation Intervention study design: clustered parallel‐group randomised controlled trial with schools selected as the unit of randomisation; "schools similar in size and student body within the same district were randomly assigned to either the intervention or control group" Setting: study was conducted at 21 elementary schools in 1 agricultural area and 1 industrial area of Beijing, China Period: not clear Length: dates on which study was conducted ‐ intervention and subsequent data collection ‐ not clear |
|
| Participants |
Eligible sample frame: 9040 parents of children surveyed in case detection survey, with 8724 returning a questionnaire, revealing 639 children with a diagnosis of asthma Randomised: 639 children randomised according to their school (note numbers of children in intervention and control groups were not provided) Completed (intervention): unclear; 543 parents of children returned follow‐up questionnaire a year after start (note numbers of children in intervention and control groups were not provided) Inclusion criteria: children eligible if they had "(1) three or more of seven asthma symptoms reported in the past year; (2) one or more of two exercise symptoms reported three times or more in the past year; and (3) a physician's diagnosis of asthma, with any symptoms reported or medication prescribed in the past year" Exclusion criteria: not directly reported Baseline characteristics Age of children: all 7 to 11 years of age Ethnicity: not reported Socio‐economic status: not reported Gender: not reported Asthma status: mixed severity: "29% of the children were classified as having mild (20%) or moderate persistent asthma (9%) and 71% as having mild intermittent asthma. No severe persistent asthma was noted" |
|
| Interventions |
Intervention: based on Open Airways for Schools (OAS) model. The OAS programme manual and teaching materials were translated into Chinese and adapted for specifics related to the Beijing idiom. Some changes were made, but researchers reported that the substantial focus of the programme was not modified. Topics covered included "basic information/feelings about asthma, recognising and managing asthma symptoms, solving problems with medicines, deciding severity of symptoms, finding and controlling asthma triggers, staying healthy and doing well at school" Control: not clearly stated (usual care) Intensity: 5 sessions over a 5‐week period, each lasting approximately 25 minutes. Sessions were split according to children's age/grade Instructor: teachers provided the programme in schools and were trained in advance Theoretical Framework: social cognitive theory, especially principles of self‐regulation Parental engagement: no info Child satisfaction: no info Timing: children met for the programme at the end of the class day |
|
| Outcomes |
Extractable outcome data collected for: Exacerbations leading to hospital admission Asthma symptoms leading to emergency hospital visits |
|
| Notes | Unclear how many participants were included in intervention and control groups Quality of life and withdrawal data collected but not extractable from the information presented Funding source: Thrasher Fund Award |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No additional information was provided to support a judgement |
| Allocation concealment (selection bias) | Unclear risk | No additional information was provided to support a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No additional information was provided to support a judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No additional information was provided to support a judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information was provided to support a judgement. Around 100 children dropped out of the intervention (although the spread of these children across treatment arms remains unclear) |
| Selective reporting (reporting bias) | High risk | Many outcomes could not be extracted in full for meta‐analyses |
| Other bias | Unclear risk | Missingness ‐ unclear risk ‐ additional reports of missingness were not described by study authors Baseline imbalance ‐ unclear risk ‐ this was not addressed by study authors Risk of contamination ‐ low ‐ schools were the unit of randomisation, lowering the risk of contamination |
| Transparent and clearly stated aims | Unclear risk | N/A |
| Explicit theories underpinning and/or literature review | Unclear risk | N/A |
| Transparent and clearly stated methods and tools | Unclear risk | N/A |
| Selective reporting | Unclear risk | N/A |
| Harmful effects | Unclear risk | N/A |
| Population and sample described well | Unclear risk | N/A |
| Continuous evaluation | Unclear risk | N/A |
| Evaluation participation equity and sampling | Unclear risk | N/A |
| Design and methods overall approach | Unclear risk | N/A |
| Tools and methods of data collection reliable/credible | Unclear risk | N/A |
| Tools and methods of data analysis reliable/credible | Unclear risk | N/A |
| Performance bias/neutrality/credibility/conformability | Unclear risk | N/A |
| Reliability of findings and recommendations | Unclear risk | N/A |
| Transferability of findings | Unclear risk | N/A |
| Overall risk of bias of process evaluation | Unclear risk | N/A |
Clark 2010.
| Methods |
Included as outcome evaluation Study design: clustered parallel‐group design with schools selected as the unit of randomisation Setting: study was conducted at 19 middle schools in Detroit, Michigan, USA. Seven schools were randomised to one of the treatment arms, 6 schools to another treatment arm, and 6 schools to a control arm Period: this was a 5‐year study with a 2‐year enrolment period starting in 2003 |
|
| Participants |
Eligible sample frame: through a case detection survey, 1292 students were identified as probably having asthma (and eligible for the intervention) Randomised: students randomised at the school level ‐ 468 pupils in one treatment arm, 416 in a second treatment arm, and 408 in a control arm Completed (intervention): data from 921 pupils were collected at 12‐month follow‐up; disaggregated numbers by trial arm were not reported Inclusion criteria: students were eligible for participation in the study if they met the "definition of probable asthma as well as levels of severity based on NAEPP guidelines" Exclusion criteria: no further criteria reported Baseline characteristics Age of children: mean age, 11.6 years across all 3 trial arms Ethnicity: 93% of children involved in the study were African American; this varied between 90% and 98% across trial arms Socio‐economic status: a large proportion of children were from low‐income households ‐ 48% of all children (44% to 50% across different arms) were from households with annual income under $15,000 per annum Gender: 48% of children were female (ranging from 46% to 50% across different trial arms) Asthma status: indicators of asthma severity at baseline included 58% of children (caregivers) reporting obtaining prescriptions for asthma medications in the past year and 52% reporting persistent night‐time asthma |
|
| Interventions |
Intervention: trial included 2 treatment arms and 1 control arm. Both treatment arms tested the effectiveness of different forms of Open Airways for Schools (OAS): Treatment arm 1: adapted form of OAS delivered as a 6‐ to 7‐lesson curriculum including interactive problem‐solving activities appropriate for groups of pre‐teens. Topics included (I) basic information and feelings about asthma; (ii) facts about asthma medicines and their use (emphasising partnership with the physician); (iii) how to make good decisions about activities; (iv) how to manage an asthma attack at home or school, deciding when to go to the doctor, and making the doctor visit more effective; (v) how to keep yourself healthy, including smoking avoidance; and (vi) personal characteristics, actions, and environmental factors that lead to successful asthma control. Groups of pre‐teens met during school hours for approximately 1½ hours each week for 6 weeks. Take‐home assignments and handout materials for parents were provided at each session Treatment arm 2: included the adapted OAS above and a peer education component. Peer leaders were sought from the general population of eighth grade students and were trained by project staff to provide 3 asthma awareness lessons to seventh grade students. Two to three peer leaders were trained as a team. Peer leaders developed skits and game shows as part of training to impart an important message about asthma. Teams of peer leaders taught the 3 asthma awareness lessons to seventh grade students. Participants discussed a video, played games demonstrating and testing asthma knowledge, and discussed barriers to self‐management. Finally, younger students voted on key messages to communicate to sixth grade schoolmates. In step 3, with help from peer leaders, project staff, and a teacher, seventh grade students translated asthma messages into skits, songs, and dramas, and performed these for an assembly of sixth grade students. All 3 steps focused on enabling students to understand and support their classmates with asthma Control: treatment not described (usual care) Intensity: students in treatment group 1 received 6 lessons; students in treatment group 2 received the same 6 lessons and additional input that differed depending on school grade Instructor: OAS sessions were led by graduate students and community leaders who were trained in programme methods and approach; in treatment arm 2, some components may have been delivered directly by peers Theoretical framework: not explicitly discussed Parental engagement: not reported Child satisfaction: not reported Timing of intervention in school day: described as during school day (not after school), but exact timing not reported |
|
| Outcomes | Data on daytime and night‐time symptoms were extracted | |
| Notes | Health‐related quality of life and experience of daytime and night‐time symptoms were collected, but data needed for extraction and inclusion in meta‐analysis were not presented Considered as a process evaluation: did not include implementation research questions nor in‐depth process or contextual information (did not meet the criteria for a process evaluation) Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Schools were stratified by geographical location and disease prevalence to ensure homogeneity across groups. Within each of the resulting 4 strata, schools were randomised via a table of random numbers |
| Allocation concealment (selection bias) | High risk | Potential for concealment to be breached through open random numbers table |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear which measures were taken to ensure blinding of participants |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Likely any blinding of interviewers would have been breached through data collection methods: data were collected from school records and through face‐to‐face interviews with students at each school |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data and attrition did occur ‐ but impacts were said to have been attenuated through multiple imputations |
| Selective reporting (reporting bias) | High risk | Risk of selective reporting was high, as outcomes were not reported in full and we were not able to extract several outcomes of interest |
| Other bias | Unclear risk | Missingness ‐ unclear risk ‐ missingness was present, but multiple imputations were implemented and impact is unknown Baseline imbalance ‐ high risk ‐ at baseline, despite randomisation, one intervention arm had lower grades than controls. This may impact responsiveness to the intervention Risk of contamination ‐ low ‐ schools were the unit of analysis |
| Transparent and clearly stated aims | Unclear risk | N/A |
| Explicit theories underpinning and/or literature review | Unclear risk | N/A |
| Transparent and clearly stated methods and tools | Unclear risk | N/A |
| Selective reporting | Unclear risk | N/A |
| Harmful effects | Unclear risk | N/A |
| Population and sample described well | Unclear risk | N/A |
| Continuous evaluation | Unclear risk | N/A |
| Evaluation participation equity and sampling | Unclear risk | N/A |
| Design and methods overall approach | Unclear risk | N/A |
| Tools and methods of data collection reliable/credible | Unclear risk | N/A |
| Tools and methods of data analysis reliable/credible | Unclear risk | N/A |
| Performance bias/neutrality/credibility/conformability | Unclear risk | N/A |
| Reliability of findings and recommendations | Unclear risk | N/A |
| Transferability of findings | Unclear risk | N/A |
| Overall risk of bias of process evaluation | Unclear risk | N/A |
Crane 2014.
| Methods |
Included as process evaluation Intervention study design: study design was quasi‐experimental. Pre‐post follow‐up was provided Unit of allocation: school Process evaluation methods: quantitative: survey/questionnaire |
|
| Participants |
Country: USA Age of children: 8 to 12 years old Child characteristics (BME/SES): not reported Asthma status: asthmatic only Intervention recipients: 45 children; 49% male |
|
| Interventions |
School type: 1 Tulsa‐area elementary school Intervention description: modification of Open Airways for Schools (OAS). Children received 10 sessions lasting 20 minutes over a lunch period. Six education topics from the original OAS programme were taught, and students received handouts from the original programme Control description: Open Airways for Schools (OAS) (standard) Theoretical framework: based on Piaget's educational theory |
|
| Outcomes | Core processes evaluated (child level): attrition, dosage | |
| Notes |
Process evaluation category: stand‐alone Breadth and depth: depth ‐ not breadth Voice of children given prominence: featured but not sufficiently Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Unclear risk | N/A |
| Transparent and clearly stated aims | Low risk | Study aims were clearly stated |
| Explicit theories underpinning and/or literature review | Low risk | Based on the educational theory of Jean Piaget |
| Transparent and clearly stated methods and tools | Low risk | Methods and tools were well described |
| Selective reporting | Low risk | What was purported to be measured was included in the report |
| Harmful effects | Unclear risk | Harmful effects were discussed, for example, time‐tabling issues and conflicts. However, harmful effects were not collected by structured means |
| Population and sample described well | Unclear risk | Information around asthma burden and ethnicity was not collected |
| Continuous evaluation | Unclear risk | Only 1 drop out was reported; however relevant data were not collected |
| Evaluation participation equity and sampling | Unclear risk | Not all stakeholders were included in the evaluation |
| Design and methods overall approach | Unclear risk | This was reported, but not a lot of information was provided |
| Tools and methods of data collection reliable/credible | Low risk | All data collection methods and tools were reliable |
| Tools and methods of data analysis reliable/credible | Low risk | Data analysis methods were credible for the data |
| Performance bias/neutrality/credibility/conformability | Unclear risk | Unclear how confidentiality was maintained |
| Reliability of findings and recommendations | Low risk | Findings of the process evaluation were sufficiently supported by the data |
| Transferability of findings | High risk | Small sample size makes it difficult for findings to be transferable |
| Overall risk of bias of process evaluation | Unclear risk | The narrow confines of the focus probably account for medium risk when viewed as a 'process evaluation' |
Dore‐Stites 2007.
| Methods |
Included as process evaluation Intervention study design: quasi‐experimental single‐group intervention examining change pre‐post intervention Unit of allocation: N/A Process evaluation methods: descriptive/bivariate (hypothesis testing) |
|
| Participants |
Setting: 5 elementary schools in the Detroit area, USA Age of children: 5 to 10 years (mean age, 9.1 years) Child characteristics (BME/SES): 39% African American, 14% Hispanic, and 18% mixed ethnicity children; 34.6% were from low‐income families (< USD20,000 per annum) Asthma status: asthmatic only Intervention recipients: children and parents |
|
| Interventions |
School type: primary/elementary Intervention description: treatment consisted of 3 components: a computer‐based educational game (Asthma: Quest for the Code), home activities, and caregiver information. The computer game included modules on: Lung Physiology; Symptom Recognition; Trigger Recognition; Peak Flow Meter Usage; Appropriate Use of Long‐Term and Reliever Medication; Correct Usage of Common Asthma Medication; and Effect of Asthma Medications on Lungs. The study author reports: "The self‐directed activities spanned from 10 to 20 minutes per session and were presented to participants individually during their school day. Modules were embedded within a larger, multiphase game and advancement to the next level occurred contingent upon answering quiz questions correctly. Children completed one or two modules per session dependent upon progression through each section and academic schedule. At a minimum, an individual participant could complete all activities in Asthma: Quest for the Code in approximately 1.25 hours although typically children utilised the CD‐ROM for approximately 20 minutes once per week over the course of eight to nine weeks for an approximate total time of 2.5 hours in instruction" Control description: N/A Theoretical framework: not 1 overarching theory utilised, but the study draws upon several theoretical standpoints |
|
| Outcomes | Core processes evaluated (child level): attrition | |
| Notes |
Process evaluation category: integrated Breadth and depth: neither broad nor deep Voice of children given prominence: sufficient coverage Funding source: Blue Cross Blue Shield Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Unclear risk | N/A |
| Transparent and clearly stated aims | Low risk | Study aims were clearly stated and were related to exploring feasibility |
| Explicit theories underpinning and/or literature review | Unclear risk | Sufficent literature was provided to support the study, drawing upon several theoretical standpoints |
| Transparent and clearly stated methods and tools | Low risk | Methods and tools were clearly stated |
| Selective reporting | Unclear risk | Parental engagement was a key component of the study but was deemed to have not been reported in full |
| Harmful effects | High risk | Not fully addressed ‐ implementation data on the support needed to get children to play the game were lacking |
| Population and sample described well | Unclear risk | Caregiver demographics were not reported in full |
| Continuous evaluation | Low risk | A pre‐post design was utilised |
| Evaluation participation equity and sampling | Low risk | All participants involved in the programme had the opportunity to participate in the evaluation |
| Design and methods overall approach | Unclear risk | This was reported; however not much information was provided |
| Tools and methods of data collection reliable/credible | Low risk | Tools that were used were well described and were recognised tools |
| Tools and methods of data analysis reliable/credible | Low risk | Analytical plan seems to be appropriate for the data; however it is unclear how (or if) the hierarchical nature of the data was accounted for |
| Performance bias/neutrality/credibility/conformability | Unclear risk | Not addressed by the study author |
| Reliability of findings and recommendations | High risk | The study sample was very small, reducing the reliability of study findings |
| Transferability of findings | High risk | Transferability of findings is unclear; however given the small sample size, transferability of study findings should be limited |
| Overall risk of bias of process evaluation | High risk | Data regarding dosage and adherence are limited. Moreover, the small sample size compromised the study as a whole |
Engelke 2013.
| Methods |
Included as process evaluation Intervention study design: quasi‐experimental, pre‐post, no control Unit of allocation: N/A Process evaluation methods: bivariate analysis |
|
| Participants |
Country: USA Age of children: 143 children in grades 1 to 12 Child characteristics (BME/SES): 40.6% of children were Caucasian; 50.3% were male; 63.6% received Medicaid Asthma status: asthmatic only Intervention recipients: children, teachers, parents, nurses |
|
| Interventions |
School type: 303 schools, including junior/middle, primary/elementary, and high schools, from 24 school districts participated Intervention description: interventions were divided into 5 categories, including direct care, student education/counselling, parent/family education, teacher/staff education, and healthcare co‐ordination. After initial assessment, the nurse chose an individual goal for each student Control description: N/A Theoretical framework: case management theory |
|
| Outcomes | Core processes evaluated (child level): not reported | |
| Notes |
Process evaluation category: named section Breadth and depth: depth ‐ not breadth Voice of children given prominence: not featured Funding source: Kate B. Reynolds Charitable Trust |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Unclear risk | N/A |
| Transparent and clearly stated aims | Low risk | Study aims were clearly stated |
| Explicit theories underpinning and/or literature review | Unclear risk | Literature around case management was provided; however the actual theory remains unclear |
| Transparent and clearly stated methods and tools | Unclear risk | Instruments used are not entirely clear, for example, scoring and analysis tools |
| Selective reporting | Low risk | All outcomes were reported on satisfactorily |
| Harmful effects | High risk | Any negative effects of the intervention on the nurse workload were not addressed |
| Population and sample described well | Low risk | Study population was adequately described |
| Continuous evaluation | Low risk | Pre‐post assessment findings were reported |
| Evaluation participation equity and sampling | Unclear risk | Voice of the children was not included in the evaluation |
| Design and methods overall approach | Low risk | Parent and nurse reports were collected twice |
| Tools and methods of data collection reliable/credible | Unclear risk | It is unclear if the analytical method or the tool itself was reliable |
| Tools and methods of data analysis reliable/credible | Unclear risk | It is unclear if the analytical method or the tool itself was reliable |
| Performance bias/neutrality/credibility/conformability | High risk | No steps were taken to reduce this |
| Reliability of findings and recommendations | Unclear risk | Multi‐variate analysis was not used |
| Transferability of findings | Unclear risk | School factors are unclear, for example, what kinds of schools were used and what types of findings were obtained |
| Overall risk of bias of process evaluation | Unclear risk | Because many classifications were unclear, it is difficult to categorise this trial; however as a process evaluation, this is not a bad study |
Gerald 2006.
| Methods |
Included as outcome evaluation and process evaluation Study design: parallel‐group design. The study was split across cohorts, and schools within cohorts were randomised Setting: the study was conducted at 54 elementary schools in Birmingham, Alabama, USA Period: the intervention was implemented over 3 years, with 1 cohort receiving the intervention each year |
|
| Participants |
Eligible sample frame: 736 were enrolled into the study Randomised: 736 pupils enrolled, but unclear how many were included in each arm Completed (intervention): not reported Inclusion criteria: children identified by the case detection procedure as having previously diagnosed asthma or suspected asthma Exclusion criteria: not reported Baseline characteristics Age of children: elementary school age ‐ grades 1 to 4 (even split) Ethnicity: 97% black ethnicity Socio‐economic status: not reported. Gender: 56% and 52% of participants were male in intervention and control groups, respectively Asthma status: not reported |
|
| Interventions |
Intervention: the intervention consisted of 3 separate educational programmes and medical management for children with asthma: asthma education for school faculty and staff (Managing Asthma: A Guide for Schools), for the general student body (Asthma Awareness: A Curriculum for the Elementary School Classroom), and for students with asthma (Open Airways for Schools). Education was provided for school faculty and staff at an in‐service meeting. Asthma awareness classes were provided to all children at each elementary school. Content was modified slightly for children younger than age 8 Control: delayed intervention, but usual care at the time of data collection Intensity: the Open Airways for Schools programme consists of six 40‐minute sessions Instructor: in the first cohort, teachers were trained to deliver sessions. In the second cohort, classes were taught by study personnel Theoretical framework: not reported Parental engagement: parental satisfaction was low. Parents either did not attend scheduled visits or did not return completed questionnaires Child satisfaction: not reported Timing of intervention in school day: during physical education periods |
|
| Outcomes |
Core processes evaluated: attrition, dosage, adherence Extractable outcomes were collected for: Exacerbations leading to admission to hospital Asthma symptoms leading to an emergency hospital visit Parent‐reported absence from school |
|
| Notes |
Process evaluation category: named section(s)/integrated within the study Breadth and depth: neither broad nor deep Voice of children given prominence: not featured directly Funding source: National Heart, Lung, and Blood Institute |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | How schools were randomised was not described |
| Allocation concealment (selection bias) | Unclear risk | Not addressed by study authors |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not addressed by study authors |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not addressed by study authors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition was evident but was not fully disaggregated |
| Selective reporting (reporting bias) | Low risk | No evidence shows selective reporting |
| Other bias | Low risk | Misssingness ‐ unclear risk ‐ unclear the extent to which missing data were due to attrition or to additional survey non‐response Baseline imbalance ‐ low risk ‐ both arms were balanced on characteristics of importance Risk of contamination ‐ low ‐ schools were the unit of randomisation |
| Transparent and clearly stated aims | Unclear risk | Medium bias ‐ study aims are not explicitly clear |
| Explicit theories underpinning and/or literature review | High risk | No theoretical framework was presented |
| Transparent and clearly stated methods and tools | Low risk | Methods were clearly reported |
| Selective reporting | Unclear risk | Many aspects did not match the plan, although the tools used to collect this information were not entirely structured |
| Harmful effects | Low risk | Difficulty in maintaining fidelity was discussed |
| Population and sample described well | High risk | Unclear how many dropped out of intervention and treatment groups |
| Continuous evaluation | Unclear risk | Medium bias ‐ use of administrative records lowered the risk of bias |
| Evaluation participation equity and sampling | High risk | The voices of children, parents, and teachers were not included in the evaluation |
| Design and methods overall approach | High risk | Many of the methods used did not capture key process data |
| Tools and methods of data collection reliable/credible | High risk | Study authors reported on the high level of measurement error |
| Tools and methods of data analysis reliable/credible | High risk | No adjustment was made for missing data |
| Performance bias/neutrality/credibility/conformability | Unclear risk | The degree of neutrality is not clear |
| Reliability of findings and recommendations | Unclear risk | A lot of variation in implementation is evident |
| Transferability of findings | High risk | The quality of the study is not high enough to support the claim that this style of intervention does work in inner city schools |
| Overall risk of bias of process evaluation | Unclear risk | Use of unstructured tools to collect process data means that the risk of bias is not entirely clear |
Gerald 2009.
| Methods |
Included as outcome evaluation Study design: parallel‐group design, with a 2‐group randomised longitudinal design, randomised by the child Setting: USA Period: baseline data collection occurred from October 2005 to December 2006. Children were randomised in January 2006. The study comprised a longitudinal design with 15‐month follow‐up. Follow‐up data were collected from January 2006 to December 2006 |
|
| Participants |
Eligible sample frame: 290 children were randomised Randomised: 290 children were randomised ‐ 145 in each arm Completed (intervention): 240 (83%) Inclusion criteria: children were eligible if they had physician‐diagnosed persistent asthma requiring daily controller medication, they attended one of the 36 participating schools, and they were able to use a dry powder inhaler and a peak flow meter Exclusion criteria: not reported Baseline characteristics Age of children: mean age, 11.0 years Ethnicity: 91% black ethnicity Socio‐economic status: not reported. Gender: 57% male; 43% female in total Asthma status: mixed levels of severity ‐ 15% mild persistent asthma, 79% moderate persistent asthma, 6% severe persistent asthma |
|
| Interventions |
Intervention: children were given 20 minutes of asthma education, including discussion about avoidance of triggers. Children in the supervised therapy group also received supervision from study staff each day on the use of inhaled corticosteroids. If a child was observed using the inhaler incorrectly, staff provided education with the aid of a placebo inhaler Control: usual care Intensity: a single education session for 20 minutes; multiple supervisions for the intervention group Instructor: study personnel Theoretical framework: not reported Parental engagement: not reported Child satisfaction: not reported Timing of intervention in school day: not reported |
|
| Outcomes |
Extractable outcomes were collected for: Parent‐reported absence from school Lung function Use of reliever therapies Withdrawal |
|
| Notes | This paper did not provide much detail; however the study was previously reported in a separate paper (Gerald 2009) Considered for process evaluation: implementation data were not considered to have been collected via structured or recognised tools Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study authors reported: "a random sequence of treatment codes, stratified by school system, was generated using the SAS System (Version 9.1, Cary, North Carolina) by the statistician" |
| Allocation concealment (selection bias) | Low risk | Centrally generated: study authors reported: "a random sequence of treatment codes, stratified by school system, was generated using the SAS System (Version 9.1, Cary, North Carolina) by the statistician" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No measures were described as implemented around blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No measures were described as implemented around blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors described: "79.3% completion rate in the control group and 86% completion rate in the intervention group. Reasons and details provided" |
| Selective reporting (reporting bias) | Unclear risk | No evidence shows selective reporting |
| Other bias | Unclear risk | Missingness ‐ low risk ‐ all those who were followed up submitted information Baseline imbalance ‐ low risk ‐ no significant differences between groups were found in baseline demographic characteristics or asthma symptom Risk of contamination ‐ high ‐ children were the unit of randomisation; children with different treatment allocations were present in the same school |
| Transparent and clearly stated aims | Unclear risk | N/A |
| Explicit theories underpinning and/or literature review | Unclear risk | N/A |
| Transparent and clearly stated methods and tools | Unclear risk | N/A |
| Selective reporting | Unclear risk | N/A |
| Harmful effects | Unclear risk | N/A |
| Population and sample described well | Unclear risk | N/A |
| Continuous evaluation | Unclear risk | N/A |
| Evaluation participation equity and sampling | Unclear risk | N/A |
| Design and methods overall approach | Unclear risk | N/A |
| Tools and methods of data collection reliable/credible | Unclear risk | N/A |
| Tools and methods of data analysis reliable/credible | Unclear risk | N/A |
| Performance bias/neutrality/credibility/conformability | Unclear risk | N/A |
| Reliability of findings and recommendations | Unclear risk | N/A |
| Transferability of findings | Unclear risk | N/A |
| Overall risk of bias of process evaluation | Unclear risk | N/A |
Henry 2004.
| Methods |
Included as outcome evaluation and process evaluation Study design: clustered parallel‐group design Setting: secondary schools in Newcastle, New South Wales, Australia Period: baseline data were collected between February and March 1993. Follow‐up data were collected between August and October 1993. Follow‐up questionnaires were sent to the heads of participating schools in 1999 Process evaluation methods: descriptive/bivariate |
|
| Participants |
Eligible sample frame: 33 schools were eligible for participation, with a total of 4475 year 8 students, 23% of whom had current asthma. In total, 76.7% of all eligible students and 82.7% of students recruited into th initial phase contributed to data analysis Randomised: not reported Completed (intervention): not reported Inclusion criteria: not reported Exclusion criteria: not reported Baseline characteristics Age of children: year 8 students were eligible; adolescents aged 13 to 14 years were targeted Ethnicity: numbers were not reported; however schools included a predominantly Caucasian population Socio‐economic status: not reported. Gender: males represented 52.4% of intervention students with matched data and 52.9% of control adolescents Asthma status: not reported |
|
| Interventions |
Intervention: intervention schools received a 3‐lesson package about asthma designed to be taught within the Personal Development/Health/Physical Education (PD/ H/PE) strand of the school curriculum. Each school was invited to send a delegate to learn the curriculum and was provided with the Living With Asthma teaching kit Control: usual care Intensity: 3 lessons; however the duration of these lessons was not reported Instructor: in some schools, teachers who attended the training seminar delivered the lessons; in other schools, teachers trained their colleagues Theoretical framework: not reported Parental engagement: not reported Child satisfaction: not reported Timing of intervention in school day: taught within the Personal Development/Health/Physical Education strand of the school curriculum |
|
| Outcomes |
Core processes evaluated (child level): adherence (long‐term) Extractable outcomes were collected for: Health‐related quality of life (HRQoL) |
|
| Notes |
Process evaluation category: integrated Breadth and depth: depth ‐ not breadth Voice of children given prominence: featured but not sufficiently Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Method of stratification is unclear: schools were randomised to control or intervention, with an attempt to obtain similar demographic mixes in the 2 groups |
| Allocation concealment (selection bias) | Unclear risk | Not addressed by study authors |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not addressed by study authors |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not addressed by study authors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition data for asthmatic children were not provided in full |
| Selective reporting (reporting bias) | Low risk | All data were reported by study authors |
| Other bias | Unclear risk | Missingness ‐ unclear risk ‐ missing data are apparent with no explanation provided Baseline imbalance ‐ unclear risk ‐ this was not addressed by study authors Risk of contamination ‐ low ‐ risk of contamination was low due to the study design |
| Transparent and clearly stated aims | Low risk | Study aims were clearly stated |
| Explicit theories underpinning and/or literature review | High risk | No theoretical framework and very little supporting literature were provided |
| Transparent and clearly stated methods and tools | Low risk | Study aims were clearly stated |
| Selective reporting | Unclear risk | Medium bias ‐ problems with linking surveys were experienced (pre‐post intervention) |
| Harmful effects | Unclear risk | How this was addressed is unclear. In particular, this might not have been beneficial for many children |
| Population and sample described well | High risk | Level of baseline imbalance was not reported |
| Continuous evaluation | Unclear risk | Pre‐post assessment data were used for the majority, but an element of continuous evaluation was included in the school policy analysis |
| Evaluation participation equity and sampling | Unclear risk | Moderate evidence shows that the voice of children was reflected adequately |
| Design and methods overall approach | Low risk | Design and methods were appropriate for this study |
| Tools and methods of data collection reliable/credible | Low risk | Data collection tools used were credible and reliable |
| Tools and methods of data analysis reliable/credible | Low risk | Analysis of quantitative data was reliable |
| Performance bias/neutrality/credibility/conformability | Unclear risk | No evidence shows how confidentiality was maintained |
| Reliability of findings and recommendations | Unclear risk | Level of baseline imbalance is unclear |
| Transferability of findings | Unclear risk | Transferability was not assessed by study authors |
| Overall risk of bias of process evaluation | Unclear risk | Some aspects of study design and study characteristics were not explained fully |
Horner 2008.
| Methods |
Included as outcome evaluation Study design: clustered parallel‐group design with schools selected as the unit of randomisation Setting: elementary schools in the USA; 10 treatment and 8 attention control schools Period: each of the participating school districts participated for 1 academic year, and the whole project spanned 2003 to 2006 academic years. Analysis occurred over 12 weeks of the academic year from study enrolment to 6 weeks post intervention |
|
| Participants |
Eligible sample frame: 541 families of children were invited to participate Randomised: 183 pupils were randomised: 101 into treatment group and 82 into control group Completed (intervention): 163 pupils completed the trial Inclusion criteria: students were eligible for participation in the study if they had doctor‐diagnosed asthma, had experienced asthma symptoms in the previous 12 months, had no other significant comorbidity that would preclude participation in classes, spoke either English or Spanish, and were enrolled in grades 2 to 5 Exclusion criteria: not reported Baseline characteristics Age of children: mean age, 8.78 years Ethnicity: within the sample, 47% were Mexican American, 30% white, 22% African American, and 1% other Socio‐economic status: not reported Gender: 108 male; 75 female Asthma status: not reported |
|
| Interventions |
Intervention: the curriculum presented a 7‐step asthma self‐management plan Control: the attention‐control group mirrored the treatment group and received education on health promotion topics appropriate for children Intensity: 16 sessions, each for 15 minutes Instructor: 18 lay health educators Theoretical framework: the asthma health education model that informed the study was adapted from Bruhn's theoretical model of asthma management Parental engagement: not reported Child satisfaction: not reported Timing of intervention in school day: lunch breaks |
|
| Outcomes |
Extractable outcomes were collected for: Hospitalisation Withdrawal |
|
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study authors reported: "schools were randomised through a simple coin toss at a summer meeting held with elementary school principals" |
| Allocation concealment (selection bias) | High risk | Risk that concealment of allocation was breached |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors reported: "Because the participants could not be blinded to their treatment condition, this information was disclosed to parents during this first telephone call" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not assessed by study authors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing outcome data roughly balanced across intervention and control groups, with similar reasons for missingness and study authors reporting: "Comparing baseline scores of those who dropped out of the study with those who were retained (i.e. completers) showed no significant differences in terms of demographic or study variables" |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | Missingness ‐ low risk ‐ strategies implemented for missingness (which accounted for less than 10% of the sample) described as follows: "Missing items were handled by substituting the participant's mean score for the missed item in those cases where fewer than 10% of the items were missing for a scale. In this study, there were no instances of more than 10% of missed items for a scale" Baseline imbalance ‐ low risk ‐ comparing groups at baseline on study measures revealed no significant differences between groups except for asthma severity, which was greater in the treatment group Risk of contamination ‐ low ‐ schools were the unit of randomisation, lowering the potential for contamination |
| Transparent and clearly stated aims | Unclear risk | N/A |
| Explicit theories underpinning and/or literature review | Unclear risk | N/A |
| Transparent and clearly stated methods and tools | Unclear risk | N/A |
| Selective reporting | Unclear risk | N/A |
| Harmful effects | Unclear risk | N/A |
| Population and sample described well | Unclear risk | N/A |
| Continuous evaluation | Unclear risk | N/A |
| Evaluation participation equity and sampling | Unclear risk | N/A |
| Design and methods overall approach | Unclear risk | N/A |
| Tools and methods of data collection reliable/credible | Unclear risk | N/A |
| Tools and methods of data analysis reliable/credible | Unclear risk | N/A |
| Performance bias/neutrality/credibility/conformability | Unclear risk | N/A |
| Reliability of findings and recommendations | Unclear risk | N/A |
| Transferability of findings | Unclear risk | N/A |
| Overall risk of bias of process evaluation | Unclear risk | N/A |
Horner 2015.
| Methods |
Included as outcome evaluation and process evaluation Intervention study design: cluster parallel‐group design, with schools selected as unit of randomisation, using a stratified design according to school characteristics Setting: 33 elementary schools in 5 rural Texas, USA, districts participated in the study Period: dates on which study was conducted ‐ intervention and subsequent data collection ‐ not clear; data were collected over a 12‐month period |
|
| Participants |
Eligible sample frame: a total of 292 children were enrolled Randomised: a total of 292 children were enrolled and were randomised to 1 of 3 groups; information from 2 groups of interest provided here. 96 children were randomised to the school‐based intervention group and 100 children to the attention control group Completed (intervention): 84 children completed the intervention and attention control Inclusion criteria: children were eligible if they had doctor‐diagnosed asthma, had experienced asthma symptoms in the previous 12 months, and could speak either English or Spanish Exclusion criteria: no significant comorbidity that would preclude participation in classes Baseline characteristics Age of children (based on completers): mean age, 8.8 years Ethnicity (based on completers): treatment: 22.9% white, 55.2% Hispanic, 21.9% African American; control: 11.3% white, 60.8% Hispanic, 27.8% African American Socio‐economic status (based on completers): 31.2% defined as lower SES group in intervention group and 29.8% in control group Gender (based on completers): treatment: 55.2% male, 44.6% female; control: 76.0% male, 24.0% female Asthma status: mixed levels of asthma severity; similar levels between treatment and control groups when measured on the Severity of Chronic Asthma Scale |
|
| Interventions |
Intervention: the "Asthma Plan for Kids" curriculum, specifically designed for children in rural areas, was provided to the intervention group as group instruction. Topics within the curriculum included: "(1) identifying lung function, asthma warning signs, symptoms, and triggers; (2) learning skills to manage symptoms, including peak expiratory flow score interpretation, communication with adults, medication use, and inhaler technique; (3) evaluating asthma symptoms and the effectiveness of management; and (4) discussing how to safely keep active during physical activity and sports" Control: an equivalent attention control was provided. This mirrored the structure of the school‐based intervention but differed in providing a non‐asthma‐based curriculum. The content consisted of topics on general health information identified by school nurses as useful, including handwashing, nutrition, brushing teeth, and exercising Intensity: 15‐minute lessons spread over 16 sessions that took place within a 5‐week period Instructor: trained lay health educators Theoretical framework: Bruhn's theoretical model of asthma self‐management Parental engagement: not reported Child satisfaction: not reported Timing: sessions held during lunchtime (15‐minute blocks) |
|
| Outcomes |
Quantitative outcomes Extractable outcomes were collected for: Exacerbations leading to hospital admission Asthma symptoms leading to emergency hospital visits Withdrawal Core processes evaluated (child level): attrition, adherence |
|
| Notes | This study compared the treatment (a school‐based intervention) group vs an attention control group; also compared the school‐based intervention vs the same intervention provided at an asthma day camp (although these data are not extracted here) Data were collected on office visits, but unclear whether office visits were restricted to community primary care providers or included specialist consultations Additional measures of process evaluation quality Process evaluation category: integrated within outcome evaluation Breadth and depth: neither broad nor deep Voice of children given prominence: featured but not sufficiently Funding source: National Institutes of Health; Nursing Research; National Heart, Lung, and Blood Institute |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not addressed in full by study authors: actual method of randomisation unclear |
| Allocation concealment (selection bias) | Unclear risk | Not addressed by study authors |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Evidence shows that steps were taken to ensure blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study authors reported: "Data were collected 4 times over the 12‐month study during home visits, scheduled at times convenient to the families, by RAs who were blind to group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors reported: "A total of 292 children were enrolled and 257 completed the 12‐month study (87.7% retention). There were no significant baseline differences in age, gender, race/ethnicity, SES, language spoken by parents, or asthma severity between the children who completed the study and those who dropped out" |
| Selective reporting (reporting bias) | Low risk | All collected outcomes apparently reported in full |
| Other bias | Unclear risk | Missingness ‐ unclear risk ‐ no evidence of additional missing data Baseline imbalance ‐ unclear risk ‐ children in the control group were more likely to be male; the impact that this could have on study outcomes is unclear Risk of contamination ‐ low ‐ schools were the unit of randomisation, lowering the risk of contamination |
| Transparent and clearly stated aims | Low risk | Study aims were clearly stated |
| Explicit theories underpinning and/or literature review | Low risk | Intervention was guided by Bruhn's theoretical model of asthma self‐management |
| Transparent and clearly stated methods and tools | Low risk | Study methods were clearly stated |
| Selective reporting | Unclear risk | A limited set of data was presented for the process evaluation |
| Harmful effects | High risk | Only a few negative outcomes were considered |
| Population and sample described well | Low risk | Study sample was well described |
| Continuous evaluation | Low risk | Data were collected at several time points |
| Evaluation participation equity and sampling | Unclear risk | Not many stakeholders were involved in the evaluation |
| Design and methods overall approach | Unclear risk | This is unclear, as risk of bias for outcome evaluation was low; however risk of bias for process evaluation was high |
| Tools and methods of data collection reliable/credible | Low risk | Tools used for data collection were credible |
| Tools and methods of data analysis reliable/credible | Low risk | Data analysis methods were appropriate for the data |
| Performance bias/neutrality/credibility/conformability | Unclear risk | Credible findings focused mainly on outcomes |
| Reliability of findings and recommendations | Low risk | Findings are transparent |
| Transferability of findings | High risk | Some factors around transferability were assessed, for example, reach and attrition; however no other factors were assessed |
| Overall risk of bias of process evaluation | Unclear risk | As an outcome evaluation, few concerns are evident; as a process evaluation, this study is questionable |
Howell 2005.
| Methods |
Included as outcome evaluation and process evaluation Study design: clustered parallel‐group design with schools selected as the unit of randomisation Setting: 4 elementary schools in Syracuse City, New York, USA Period: conducted December 2003 to January 2004; subsequent data collected through September 2004 Process evaluation methods: descriptive/bivariate |
|
| Participants |
Eligible sample frame: 40 families found to be eligible and 30 consented, with 5 others unable to participate before randomisation Randomised: 25 families (children and caregiver), with 16 selected into the intervention group and 9 into the control group Completed (intervention): no reports of permanent attrition Inclusion criteria: target child was between 8 and 11 years of age and was in third to fifth grade with a diagnosis of asthma and prescribed daily medications for asthma as reported by nurse and/or parent Exclusion criteria: coexisting chronic illness that required daily medication (e.g. insulin‐dependent diabetes) Baseline characteristics Age of children: mean age, 9 years 7 months; range, 8 to 11 years Ethnicity: 75% of children were African American Socio‐economic status: unclear Gender: 63% male Asthma status: all children had a record of asthma, although study authors did not describe asthma severity |
|
| Interventions |
School type: primary/elementary Intervention description: children in the intervention condition received four 30‐minute sessions on the "Quest for the Code" computer game at their school. The computer game included modules on Lung Physiology; Symptom Recognition; Trigger Recognition; Peak Flow Meter Usage; Appropriate Use of Long‐Term and Reliever Medication; Correct Usage of Common Asthma Medication; and Effect of Asthma Medications on Lungs. The child's primary caregiver participated in a medication routine interview in the home and received a 1‐time home visit at which a medication routine plan was developed Control description: usual care/no additional intervention Theoretical framework: learning theory principles and behaviour modification |
|
| Outcomes | Core processes evaluated (child level): attrition, dosage, adherence | |
| Notes |
Process evaluation category: named section Breadth and depth: neither broad nor deep Voice of children given prominence: the voice of children was featured but not sufficiently Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not given: this study was a pretest, intervention, post‐test, follow‐up (PPF) RCT with random assignment based on school site |
| Allocation concealment (selection bias) | High risk | Information not given; few students were randomised and concealment may have been breached |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not addressed in the study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not addressed in the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low risk ‐ low levels of attrition are reported in the study |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | High risk | Missingness ‐ high risk ‐ small sample and evidence of missingness for some indicators
Baseline imbalance ‐ high risk ‐ described in the introduction as imbalanced Risk of contamination ‐ low ‐ randomisation occurred at a school level |
| Transparent and clearly stated aims | Low risk | Study aims were clearly stated |
| Explicit theories underpinning and/or literature review | Low risk | Intervention was informed by learning theory principles and behaviour modification |
| Transparent and clearly stated methods and tools | Low risk | Study included a broad‐ranging description of tools implemented at different time points |
| Selective reporting | Unclear risk | Some statements from parents are impenetrable; other statements describe kids enjoying the intervention but are not entirely clear on how this feedback was obtained |
| Harmful effects | Unclear risk | Study methods do not highlight the possibility of negative effects |
| Population and sample described well | Unclear risk | Only basic demographic information was included |
| Continuous evaluation | Low risk | Not continuous; however 3 time points were considered |
| Evaluation participation equity and sampling | Unclear risk | Information was collected from parents and children only |
| Design and methods overall approach | Low risk | This was an RCT but with dedicated attention to feasibility |
| Tools and methods of data collection reliable/credible | Low risk | Validated measures were used |
| Tools and methods of data analysis reliable/credible | Unclear risk | Small study sample precludes usefulness of confounding data |
| Performance bias/neutrality/credibility/conformability | Unclear risk | Process was limited |
| Reliability of findings and recommendations | Low risk | How the data relate to study findings is clear |
| Transferability of findings | Unclear risk | Study sample was too small, so transferability is limited |
| Overall risk of bias of process evaluation | Unclear risk | Small sample compromises much of the information provided in this study |
Jackson 2006.
| Methods |
Included as process evaluation Intervention study design: quasi‐experimental design with pre‐post follow‐up and no control group Unit of allocation: N/A Process evaluation methods: descriptive/bivariate analysis methods |
|
| Participants |
Country: USA Age of children: this study recruited 943 third grade students, aged 8 to 9 years Child characteristics (BME/SES): not reported Asthma status: asthmatic and non‐asthmatic children Intervention recipients: children only |
|
| Interventions |
School type: public and private elementary schools in Chicago Intervention description: children completed a 1‐hour asthma education programme entitled "The lion who couldn't roar". Teachers were encouraged to attend the workshop Control description: N/A Theoretical framework: not reported |
|
| Outcomes | Core processes evaluated (child level): attrition, dosage, adherence | |
| Notes |
Process evaluation category: integrated Breadth and depth: breadth ‐ not depth Voice of children given prominence: sufficient coverage Funding source: Midwestern University and Majestic Steel Erections |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Unclear risk | N/A |
| Transparent and clearly stated aims | Low risk | Study aims were clearly stated |
| Explicit theories underpinning and/or literature review | High risk | No evidence of a theoretical framework guiding intervention development |
| Transparent and clearly stated methods and tools | Unclear risk | Some detail provided; however information is limited |
| Selective reporting | Unclear risk | Unclear whether selective reporting was considered, but most aspects of the data collected were presented; they are not entirely interpretable |
| Harmful effects | Unclear risk | Most aspects of the data collected were presented; they are not entirely interpretable |
| Population and sample described well | High risk | No demographic information about the children was provided |
| Continuous evaluation | Low risk | Appropriate for intensity of the intervention |
| Evaluation participation equity and sampling | Low risk | Relevant stakeholders were included in the evaluation |
| Design and methods overall approach | Low risk | Appropriate for intensity of the intervention |
| Tools and methods of data collection reliable/credible | Low risk | Tools used were appropriate and well described |
| Tools and methods of data analysis reliable/credible | Unclear risk | Presentation of data collection methods and analysis is difficult to follow |
| Performance bias/neutrality/credibility/conformability | Unclear risk | Data are unclear |
| Reliability of findings and recommendations | High risk | No evidence from the intervention data that findings are reliable |
| Transferability of findings | Unclear risk | No exploration of how the impact of study findings differed by school |
| Overall risk of bias of process evaluation | Unclear risk | Data are unclear |
Joseph 2010.
| Methods |
Included as process evaluation Intervention study design: parallel‐group randomised controlled trial Unit of allocation: child Process evaluation methods: survey based, including multi‐variate analyses of outcomes |
|
| Participants |
Setting: 6 high schools in Detroit, Michigan, USA Age of children: mean age, 15.3 years Child characteristics (BME/SES): 97% of students were African American; 52% were eligible for federal school lunch programmes Asthma status: asthmatic only; severity unclear Intervention recipients: children only |
|
| Interventions |
School type: high schools Intervention description: tailored computer programme (Puff City): the web‐based programme focuses on 3 core behaviours, namely, controller medication adherence, rescue inhaler availability, and smoking cessation/reduction, and consists of 4 consecutive educational computer sessions that make use of both normative ("compared with other students") and ipsative ("compared with your last session") feedback. Messages are voiced over to accommodate low literacy. Participant‐specific information necessary for tailoring is obtained at baseline and during the 4 sessions. Control description: students randomised to the control group were directed to existing generic asthma websites Theoretical framework: not 1 single framework was named, but theories around content were mentioned |
|
| Outcomes | Core processes evaluated (child level): attrition, dosage, adherence | |
| Notes |
Process evaluation category: stand‐alone and integrated (2 papers) Breadth and depth: breadth and depth Voice of children given prominence: featured but not sufficiently Note: study is not included as an outcome evaluation because the comparison group received asthma education (this study evaluated the added impact of providing tailored messaging) Funding source: National Heart, Lung, and Blood Institute, National Institutes of Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Unclear risk | N/A |
| Transparent and clearly stated aims | Low risk | Each paper includes clearly stated aims |
| Explicit theories underpinning and/or literature review | Low risk | Not a single theory, but some aspects of learning are grounded in pedagogical techniques |
| Transparent and clearly stated methods and tools | Low risk | All methods were clearly stated |
| Selective reporting | Unclear risk | Data were apparently collected at different time points, but only follow‐up data were presented. Otherwise no evidence indicates that data collected were not presented |
| Harmful effects | Unclear risk | Some consideration of negative factors in the design ‐ e.g. access to the referral co‐ordinator. However, whether the analysis fully accounts for this is unclear |
| Population and sample described well | Unclear risk | Characteristics of the population were generally described well ‐ but ambiguities between papers that ostensibly describe the same study population surround the numbers involved |
| Continuous evaluation | Low risk | Data were collected at different time points, but only follow‐up data were presented |
| Evaluation participation equity and sampling | High risk | High level of non‐participation is a matter of concern |
| Design and methods overall approach | Unclear risk | Evaluation took into account multiple time points (all data were not necessarily presented): however data from multiple stakeholders were not collected |
| Tools and methods of data collection reliable/credible | Low risk | Tools and methods used were reliable |
| Tools and methods of data analysis reliable/credible | Low risk | Analysis was straightforward, in part because of the relatively straightforward research design |
| Performance bias/neutrality/credibility/conformability | Low risk | One of the papers explicitly addressed negative cases |
| Reliability of findings and recommendations | Unclear risk | Taken together, the 3 papers associated with this study present an accurate description of the processes undertaken, but numerous children did not participate and no clear explanation for this was provided |
| Transferability of findings | Unclear risk | Study authors did not address transferability, but the data are rich enough for exploration of contextual factors, etc. No explanation is provided as to why so many children did not participate |
| Overall risk of bias of process evaluation | Unclear risk | This study was well conducted in most respects, but it is unclear why so many children failed to participate |
Joseph 2013.
| Methods |
Included as process evaluation Intervention study design: parallel‐group randomised controlled trial Unit of allocation: child Process evaluation methods: survey based, including multi‐variate analyses of outcomes |
|
| Participants |
Setting: 6 high schools in Detroit, Michigan, USA Age of children: mean age, 15.6 years Child characteristics (BME/SES): 98% African American; 74% of children were in receipt of free or reduced price school meals Asthma status: asthmatic only; severity unclear Intervention recipients: children only |
|
| Interventions |
School type: high school Intervention description: this is an adapted version of a tailored computer programme (Puff City) that was tested in Joseph 2010. Puff City focusses on 3 behaviours: controller medication adherence, keeping an inhaler nearby, and smoking reduction or cessation. This new intervention included new submodules designed to target teens with characteristics shown to be associated with lack of behaviour change in the previous trial, and who exhibited no change after 1 or more sessions. Students were provided 4 sessions in total Control description: controls received 4 sessions of generic asthma education to match the experience of students in the treatment group Theoretical framework: Puff City uses tailoring to apply behavioural theory. Also includes Health Belief Model, Attribution Theory, and motivational interviewing |
|
| Outcomes | Core processes evaluated (child level): attrition, dosage, adherence | |
| Notes |
Process evaluation category: stand‐alone and integrated (2 papers) Breadth and depth: breadth and depth Voice of children given prominence: featured but not sufficiently Note: study is not included as an outcome evaluation because the comparison group received asthma education (this study evaluated the added impact of providing tailored messaging) Funding source: National Institutes of Health; National Heart, Lung, and Blood Institute |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Unclear risk | N/A |
| Transparent and clearly stated aims | Low risk | Study aims were clearly stated |
| Explicit theories underpinning and/or literature review | Low risk | Behavioural theory informed the intervention |
| Transparent and clearly stated methods and tools | High risk | Information collected from caregivers is unclear |
| Selective reporting | High risk | Data were collected from caregivers, but study authors did not state what these data included |
| Harmful effects | Low risk | Subgroup analyses of the impact of potential risk group were undertaken |
| Population and sample described well | Unclear risk | More could have been done to describe caregivers |
| Continuous evaluation | Low risk | Pre‐post assignment data were collected |
| Evaluation participation equity and sampling | Unclear risk | Data collected are unclear |
| Design and methods overall approach | Unclear risk | Data were collected but were not presented; a clear outline of the research design was not presented |
| Tools and methods of data collection reliable/credible | High risk | Information collected from caregivers is unclear |
| Tools and methods of data analysis reliable/credible | Low risk | Data presented were analysed in a straightforward way |
| Performance bias/neutrality/credibility/conformability | Unclear risk | Steps taken to address this are unclear |
| Reliability of findings and recommendations | High risk | Very low proportion of eligible students took part, so reliability of study findings was compromised |
| Transferability of findings | Unclear risk | Subgroup analyses were conducted; however study authors did not address transferability of findings, and the high level of non‐response does impede data transferability |
| Overall risk of bias of process evaluation | High risk | Data collected were not presented clearly; high level of non‐participation impinges on ability to generalise, even to the population in question |
Kintner 2009.
| Methods |
Included as outcome evaluation Study design: clustered parallel‐group design, with schools selected as the unit of randomisation Setting: 5 schools in a south‐central Michigan school district Period: dates from recruitment to data collection spanned from September 2006 to June 2007 |
|
| Participants |
Eligible sample frame: 85 pupils found to be eligible Randomised: 66 pupils randomised at the school level: 38 to treatment group and 28 to control group Completed (intervention): 59 pupils completed the trial (7 dropped out of the intervention group) Inclusion criteria: student eligibility criteria included (a) diagnosis of asthma, (b) availability to participate in scheduled classes or make‐up sessions, and (c) verbal and written assent to participate Exclusion criteria: student's expressed unwillingness to participate, lack of consent from parent or legal guardian Baseline characteristics Age of children: 9 to 12 years of age, with mean age, approximately 10.5 years Ethnicity: 32% African American; 15% mixed race; 11% other; 3% Hispanic Socio‐economic status: unclear: mean score > 50 on Nam‐Powers Socioeconomic Status Scale Gender: overall, 52% of participants were male Asthma status: unclear: mean score on the Severity of Illness Scale was > 5.8 for intervention and control groups |
|
| Interventions |
Intervention: study evaluated the SHARP programme (Staying Healthy ‐ Asthma Responsible and Prepared). Study authors describe that 'the SHARP programme was integrated into the schools as a teaching module. Students met for 50‐minute sessions delivered once a week for 10 weeks from January through March. Students worked through the 100‐page SHARP workbook, which was designed to be colourful, entertaining, educational, and developmentally appropriate, as well as diverse with regards to gender, race, and culture. The programme was incorporated into the existing curriculum as an elective course through inclusion of spelling words, math problems, reading and writing assignments, discussions, demonstrations, and hands‐on learning activities from biology, psychology, and sociology. To support SHARP students, caregivers and others participated in a 3‐hour information sharing programme Control: not clearly stated (usual care) Intensity: targeted asthmatic students received 10 sessions in total Instructor: unclear (potentially teachers, although not clear) Theoretical framework: a lifespan development perspective guided this study and served as the framework for development of the Acceptance of Asthma Model. Cognitive, behavioural, and psychosocial needs of students with asthma were addressed to foster acceptance of asthma by increasing long‐term responsibility for maintaining and promoting health, and for preventing complications Parental engagement: caregivers were involved, but level of engagement is unclear Child satisfaction: not reported Timing of intervention in school day: integrated into class time |
|
| Outcomes |
Extractable outcomes were collected for: Health‐related quality of life (study authors described participation in activities as a measure of quality of life) Withdrawal |
|
| Notes | Considered as a process evaluation but did not include the core components of a process evaluation, with process data collected via use of structured tools Funding source: National Institutes of Health and Staying Healthy – Asthma Responsible and Prepared, National Institute of Nursing Research (Primary); National Heart, Lung, and Blood Institute; National Institute of Allergy & Infectious Diseases; National Institute of Child Health & Human Development |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer‐generated following Time 1 data collection |
| Allocation concealment (selection bias) | Unclear risk | Unclear; few schools were allocated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interveners and participants were not blinded to randomisation after schools were designated to treatment and control groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Evaluators were instructed to not assume or ask the randomisation status of participants. Participants were requested to not disclose randomisation status to evaluators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Relatively low levels of attrition; baseline characteristics of those who dropped out of the intervention group did not differ from characteristics of those who completed post‐intervention assessment |
| Selective reporting (reporting bias) | Unclear risk | A large body of data was collected; data were aggregated into various scores, hindering their interpretation and use within meta‐analyses |
| Other bias | Low risk | Missingness ‐ low risk ‐ baseline characteristics of those who dropped out of the intervention group did not differ from characteristics of those who completed post‐intervention assessment
Baseline imbalance ‐ low risk ‐ baseline group imbalance was addressed by adjusting for baseline values of outcomes in the analyses Risk of contamination ‐ low ‐ schools were the unit of randomisation |
| Transparent and clearly stated aims | Unclear risk | N/A |
| Explicit theories underpinning and/or literature review | Unclear risk | N/A |
| Transparent and clearly stated methods and tools | Unclear risk | N/A |
| Selective reporting | Unclear risk | N/A |
| Harmful effects | Unclear risk | N/A |
| Population and sample described well | Unclear risk | N/A |
| Continuous evaluation | Unclear risk | N/A |
| Evaluation participation equity and sampling | Unclear risk | N/A |
| Design and methods overall approach | Unclear risk | N/A |
| Tools and methods of data collection reliable/credible | Unclear risk | N/A |
| Tools and methods of data analysis reliable/credible | Unclear risk | N/A |
| Performance bias/neutrality/credibility/conformability | Unclear risk | N/A |
| Reliability of findings and recommendations | Unclear risk | N/A |
| Transferability of findings | Unclear risk | N/A |
| Overall risk of bias of process evaluation | Unclear risk | N/A |
Kintner 2012.
| Methods |
Included as process evaluation Intervention study design: quasi‐experimental, pre‐post, no control Unit of allocation: N/A Process evaluation methods: descriptive/bivariate |
|
| Participants |
Country: USA Age of children: sixth and seventh grades Child characteristics (BME/SES): 53.6% African American; 35.7% lower SES Asthma status: asthmatic only Intervention recipients: students, members of their social network |
|
| Interventions |
School type: high school Intervention description: SHARP, community coalition component Control description: N/A Theoretical framework: asthma model and lifespan development perspective |
|
| Outcomes | Core processes evaluated (child level): dosage, adherence | |
| Notes |
Process evaluation category: stand‐alone Breadth and depth: breadth and depth Voice of children given prominence: sufficient coverage Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Unclear risk | N/A |
| Transparent and clearly stated aims | Low risk | Study aims were clearly stated |
| Explicit theories underpinning and/or literature review | Low risk | A theoretical framework was clearly presented |
| Transparent and clearly stated methods and tools | Low risk | Methods were well described |
| Selective reporting | Unclear risk | Not all indicators were fully reported, for example, asthma severity |
| Harmful effects | Unclear risk | These effects were not presented by study authors but can be inferred |
| Population and sample described well | Low risk | Participant demographics were clearly described |
| Continuous evaluation | Low risk | Pre‐post assignment assessment of data was provided |
| Evaluation participation equity and sampling | Low risk | All relevant stakeholders were apparently involved in the evaluation |
| Design and methods overall approach | Low risk | Data were collected from multiple sources and at multiple time points |
| Tools and methods of data collection reliable/credible | Low risk | Tools and methods were well described |
| Tools and methods of data analysis reliable/credible | Unclear risk | Not everything was presented in full |
| Performance bias/neutrality/credibility/conformability | Unclear risk | These were not fully addressed; only a few steps appear to have been taken to minimise the possibility of performance bias |
| Reliability of findings and recommendations | Low risk | How findings came about is clearly shown by study data |
| Transferability of findings | Unclear risk | Study authors did not focus on what might need to be changed to scale up. Some measures (e.g. individual interviews with dyads) are not transferable, although it is not clear if study authors shared this view |
| Overall risk of bias of process evaluation | Low risk | This is a good example of a process evaluation study |
Kouba 2012.
| Methods |
Included as process evaluation Intervention study design: quasi‐experimental single‐group study examining change pre‐post intervention Unit of allocation: N/A Process evaluation methods: descriptive and bivariate methods of analyses of survey‐based data (as well as administrative records) |
|
| Participants |
Setting: 2 urban high schools in the USA Age of children: ninth through 12th grade; average age was 15.9 years Child characteristics (BME/SES): 92% African American, 4% Hispanic, 4% mixed ethnicity. Combined median family income ranged from USD30,000 to USD39,000 Asthma status: asthmatic only; 66% of children were deemed to have control of their asthma at baseline according to ACT tests Intervention recipients: children only (targeted overweight/obese children) |
|
| Interventions |
School type: 2 high schools Intervention description: I Can Control Asthma and Nutrition Now (ICAN): the ICAN programme was developed as an adaptation of an existing intervention and is composed of 3 elements: (I) asthma education; (ii) coping skills training; and (iii) nurse practitioner‐reinforcement visits. In this study, 60% of students were overweight or obese. Because of concerns about the increasing prevalence of both youth asthma and obesity, study authors added a nutrition component to the intervention, so that the intervention could address these comorbidities. The ICAN programme is thus composed of 4 elements: (I) asthma education, (ii) nutrition education synthesised with CST, targeting obesity prevention and management, (iii) reinforcement visits with a registered nurse (RN) and a dietetic intern, and (iv) a family information meeting Control description: N/A Theoretical framework: Orem's self‐care deficit theory (SCDT) |
|
| Outcomes | Core processes evaluated (child level): attrition, dosage | |
| Notes |
Process evaluation category: integrated within outcome evaluation Breadth and depth: depth ‐ not breadth Voice of children given prominence: voice and views of children not featured Funding source: Loyola University Chicago Niehoff School of Nursing |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Unclear risk | N/A |
| Transparent and clearly stated aims | Low risk | Study aims were clearly stated |
| Explicit theories underpinning and/or literature review | Low risk | Orem's self‐care deficit theory guided the intervention |
| Transparent and clearly stated methods and tools | Low risk | Methods and tools were fully described |
| Selective reporting | Low risk | All planned outcomes were reported on |
| Harmful effects | Unclear risk | Generalisability was not considered. Some of the challenges of working with obese kids who are not adherent were not reported |
| Population and sample described well | Low risk | Population demographics were clearly described |
| Continuous evaluation | Low risk | Two follow‐ups post intervention |
| Evaluation participation equity and sampling | Unclear risk | No evidence of satisfaction evaluation and no child perspectives given |
| Design and methods overall approach | Unclear risk | Everything required for a good process evaluation was not captured |
| Tools and methods of data collection reliable/credible | Unclear risk | Nothing suggests that this study was at high risk of bias; however no study steps were described |
| Tools and methods of data analysis reliable/credible | Low risk | All methods of data analysis are appropriate for the study |
| Performance bias/neutrality/credibility/conformability | Unclear risk | Not much evidence of this; however analysis was carried out by research associates under the supervision of the statistician |
| Reliability of findings and recommendations | Unclear risk | This included a small sample, and the voice of children did not feature prominently |
| Transferability of findings | High risk | Small sample size limits the transferability of findings |
| Overall risk of bias of process evaluation | Unclear risk | As a process evaluation, this study was limited, and the breadth of the study in including nutrition alongside asthma education was not evaluated |
Langenfeld 2010.
| Methods |
Included as process evaluation Intervention study design: quasi‐experimental, pre‐post, with no control Unit of allocation: N/A Process evaluation methods: bivariate analysis methods |
|
| Participants |
Country: USA Age of children: students from grades 3, 4, and 5 were enrolled. All students were between 5 and 10 years old Child characteristics (BME/SES): 63% African American; large number of students were eligible for a free, or reduced price, lunch Asthma status: asthmatic only Intervention recipients: children and teachers |
|
| Interventions |
School type: 286 students from 12 elementary schools Intervention description: children participated in the OAS programme, consisting of six 40‐minute education sessions on asthma and asthma management. Children and staff also participated in the asthma programme, developed through case management strategies. This included 5 core components, including case management for specific children selected by the school nurse, and staff development and education about asthma Control description: N/A Theoretical framework: not reported |
|
| Outcomes | Core processes evaluated (child level): dosage | |
| Notes |
Process evaluation category: stand‐alone Breadth and depth: depth ‐ not breadth Voice of children given prominence: not featured Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Unclear risk | N/A |
| Transparent and clearly stated aims | Unclear risk | Study aims can be inferred by the reader but were not clearly stated |
| Explicit theories underpinning and/or literature review | High risk | No theoretical framework was provided, and very little supporting literature is available |
| Transparent and clearly stated methods and tools | Unclear risk | Methods described are relatively transparent, but some ambiguity remains |
| Selective reporting | Unclear risk | This paper did not claim to present outcomes but focused on whether the right people received the right intervention |
| Harmful effects | Unclear risk | Harmful effects in terms of recruitment were definitely addressed, but other harmful effects were not considered |
| Population and sample described well | Unclear risk | Study population and sample were generally well described, but school demographics were not reported |
| Continuous evaluation | Low risk | Pre‐post assessment was conducted |
| Evaluation participation equity and sampling | High risk | Nurse voice and student voices were obfuscated |
| Design and methods overall approach | Unclear risk | Not clearly described by study authors |
| Tools and methods of data collection reliable/credible | Low risk | Tools used for data collection were appropriate for the data |
| Tools and methods of data analysis reliable/credible | Unclear risk | No in‐depth analysis was provided; insights gained from nurse interviews are unclear |
| Performance bias/neutrality/credibility/conformability | Unclear risk | Premise of the paper involves thinking about the robustness of certain processes, but it is unclear who conducted the analysis |
| Reliability of findings and recommendations | Unclear risk | This paper clearly presented one point, but obscurity surrounds nurses' ratings |
| Transferability of findings | Low risk | Evidence is sufficient that the main thrust of the paper is transferable |
| Overall risk of bias of process evaluation | Unclear risk | As a process evaluation, its low breadth introduces high risk of bias to this trial, but in evaluating a single process, this is a really good trial |
Lee 2011.
| Methods |
Included as process evaluation Intervention study design: quasi‐experimental single‐group intervention examining change pre‐post intervention Unit of allocation: N/A Process evaluation methods: qualitative and quantitative data collection; descriptive/bivariate, thematic/grounded theory, narrative data analysis |
|
| Participants |
Setting: selected schools (67 schools) in Cleveland Metropolitan School District Age of children: 8 to 11 years old Child characteristics (BME/SES): no information Asthma status: asthmatic only Intervention recipients: children only |
|
| Interventions |
School type: primary/elementary Intervention description: Open Airways for Schools (also testing the feasibility of undergraduate nursing students as instructors). The study author described that "the curriculum consists of six 40‐minute group lessons held during the school day. These lessons use group discussion, stories, role‐playing, and games to help the children understand more about asthma and to engage them more in the empowerment of managing their disease. The topics that are included in the program are basic information about asthma, recognizing and managing asthma symptoms, using medication, avoiding asthma triggers, getting enough exercise, and doing well in school. Each lesson focuses on one of the above topics with a review of previous information for enforcement of the skills and knowledge learned. The overall goals of the program are to (a) improve asthma self‐management skills, (b) decrease asthma emergencies, (c) raise awareness among parents/guardians, and (d) promote broader asthma management coordination among physicians, parents, and schools" Control description: N/A Theoretical framework: functional context approach |
|
| Outcomes | Core processes evaluated (child level): explored some indicators of adherence | |
| Notes |
Process evaluation category: integrated within outcome evaluation Breadth and depth: neither broad nor deep Voice of children given prominence: featured but not sufficiently Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Unclear risk | N/A |
| Transparent and clearly stated aims | Low risk | Study aims were clearly stated |
| Explicit theories underpinning and/or literature review | Low risk | Functional context approach guided development of the intervention |
| Transparent and clearly stated methods and tools | Unclear risk | In terms of the outcome evaluation and information collected from children, lack of transparency surrounds the sample frame and tools |
| Selective reporting | High risk | Many instruments were not presented |
| Harmful effects | Unclear risk | Some elements that could be negative were included, but nothing from the children is included |
| Population and sample described well | High risk | Not much information was included on participants, and some details of the children were not described. More information was provided about the schools themselves |
| Continuous evaluation | Unclear risk | Pre‐post assessment, but post hoc only for nurses |
| Evaluation participation equity and sampling | Unclear risk | Nothing from the teachers was reported |
| Design and methods overall approach | High risk | Structured data about implementation ‐ e.g. attrition, adherence ‐ are insufficient |
| Tools and methods of data collection reliable/credible | High risk | Lack of transparency is evident among the methods used to assess child outcomes |
| Tools and methods of data analysis reliable/credible | High risk | Quantitative data were not analysed fully ‐ e.g. lack of subgroup analyses |
| Performance bias/neutrality/credibility/conformability | High risk | How aspects around neutrality were addressed is unclear |
| Reliability of findings and recommendations | High risk | Study design did not support the research question ‐ results from different instructors were not compared |
| Transferability of findings | High risk | Generalisability was not explicitly considered in enough detail |
| Overall risk of bias of process evaluation | High risk | Study has several limitations, including reporting bias and lack of transparency |
Levy 2006.
| Methods |
Included as outcome evaluation and process evaluation Intervention study design: cluster parallel‐group RCT with schools selected as unit of randomisation, based on a stratified design according to school characteristics Setting: 14 elementary schools in Memphis, Tennessee, USA, school district participated in the study Period: study was conducted over 2 school years between 1999 and 2001 |
|
| Participants |
Eligible sample frame: see below Randomised (based on year 1): 14 schools randomised. In 8 treatment group schools, 115 students participated, and in 6 usual care schools, 128 students participated. This represented a consent rate of 48% for both groups of students, whose parents reported asthma on the student's registration form Completed (intervention) (based on year 1): in the treatment group, 90 (78.3%) parents completed both pre‐ and post‐test surveys; in the usual care group, 72 (56.3%) parents participated in post‐test surveys Inclusion criteria: children 6 to 10 years of age with a diagnosis of asthma reported on school health forms and whose parents provided consent (see above) Exclusion criteria: not reported Baseline characteristics Age of children: 6 to 10 years of age; further breakdown not provided Ethnicity (based on year 1): 97% of children in the treatment group and 99% of children in the control group were African American Socio‐economic status: (based on year 1): 81% of students in the treatment group and 85% in the control group were in receipt of TennCare health insurance (a state‐specific version of Medicare) Gender (based on year 1): treatment: 58% male, 42% female; control: 57% male, 53% female Asthma status: not reported directly |
|
| Interventions |
Intervention: the intervention consisted of the following: "(1) education (delivery of the Open Airways curriculum to students in a weekly group setting at school), (2) weekly monitoring of students' health status (following up on absences and symptoms with students, families, and teachers), and (3) coordination of care (contacting students, family members, school personnel, and medical providers to facilitate disease management and mitigate environmental triggers at school and at home)" The intervention aimed to introduce the following principles of self‐management: "(1) periodic physiologic assessment and monitoring of asthma symptoms, (2) appropriate use of medications, (3) patient education, and (4) control of factors contributing to asthma severity" Control: usual care Intensity: weekly group sessions and weekly individual sessions; "nurse case managers met with students weekly from October through May to teach and coach students on asthma knowledge and treatment techniques" Instructor: school‐based nurses Theoretical framework: not directly reported Parental engagement: difficulties reported; low levels of consent and high levels of attrition at post‐test survey Child satisfaction: not reported Timing of intervention: during school day; exact time unclear |
|
| Outcomes |
Extractable outcomes were collected for: Exacerbations leading to hospital admission Asthma symptoms leading to emergency hospital visits Withdrawal Core processes outcomes evaluated (child level): attrition |
|
| Notes |
Notes for outcome evaluation: only data for year 1 used, as year 2 data not collected through randomised study design Additional measures of process evaluation quality Process evaluation category: integrated Breadth and depth: breadth ‐ not depth Voice of children given prominence: not featured Funding source: Tennessee Department of Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on method of randomisation was provided |
| Allocation concealment (selection bias) | High risk | Study author description suggests that allocation concealment was broken: "although schools were matched on demographic variables, a greater number of schools were randomised to intervention status than usual care because of staffing considerations and the school district's request for intervention in as many schools as possible" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Some measures were taken to ensure blinding of personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clear that blinding was implemented for several outcomes based on study author's description of staff being blinded to students' experimental condition in dealing with abstracted medical data from computerised hospital records |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study authors reported a differential loss to follow‐up: "there was a 24% loss in follow‐up with intervention parents and a 44% dropout rate with usual care parents" |
| Selective reporting (reporting bias) | High risk | High risk, as study authors tended to report in passing or incompletely outcomes that were not significantly different |
| Other bias | Unclear risk | Missingness ‐ high risk ‐ evidence shows substantial missing data, as study authors described that "in the usual care parent group, only 72 of the possible 128 parents participated in the posttest surveys, and considerable data were missing". How this affected outcomes is unclear Baseline imbalance ‐ low risk ‐ no evidence shows a baseline imbalance in the characteristics of children Risk of contamination ‐ low ‐ randomisation took place by school, thereby lowering the risk of contamination |
| Transparent and clearly stated aims | Unclear risk | Medium bias ‐ aims were inferred |
| Explicit theories underpinning and/or literature review | High risk | No theory and not much literature were provided |
| Transparent and clearly stated methods and tools | Unclear risk | These data were not clearly reported by study authors |
| Selective reporting | High risk | Some outcomes were not fully reported (outcomes as opposed to processes) |
| Harmful effects | Low risk | Study authors did consider harmful effects and paid attention to harmful processes or implementation problems |
| Population and sample described well | Unclear risk | Medium bias ‐ some conflation between year 1 and year 2 populations is evident |
| Continuous evaluation | Unclear risk | Medium bias ‐ pre‐post for most of the process evaluation |
| Evaluation participation equity and sampling | High risk | No input from children or teachers and no satisfaction data were reported |
| Design and methods overall approach | Unclear risk | Data were collected at multiple time points and from different sources; but they were not very well reported |
| Tools and methods of data collection reliable/credible | Unclear risk | Lack of transparency surrounds some aspects, so it is difficult to categorise this risk of bias appropriately |
| Tools and methods of data analysis reliable/credible | High risk | Lack of disaggregated data and the bivariate nature of the data mean that this study is likely at high risk of bias |
| Performance bias/neutrality/credibility/conformability | Low risk | Study authors attempted to introduce rigour and provided a full outline of the caveats. Staff who were blinded to students' experimental condition abstracted medical data from computerised hospital records |
| Reliability of findings and recommendations | Low risk | How conclusions were reached based on the data is clear |
| Transferability of findings | Low risk | Study findings are transferable to the wider population |
| Overall risk of bias of process evaluation | Unclear risk | Bivariate nature of this study makes it have high risk of bias |
Magzamen 2008.
| Methods |
Included as process evaluation Intervention study design: quasi‐experimental single‐group intervention examining change pre‐post intervention Unit of allocation: N/A Process evaluation methods: survey based using descriptive/bivariate analyses. Some regression analyses reported controlling for previous baseline observations |
|
| Participants |
Setting: 15 schools in Oakland, California, USA Age of children: 11 to 16 years of age (although more than 80% were 11 to 12 years old) Child characteristics (BME/SES): ethnicity of children is unclear (although intervention took place in a diverse catchment area). Socio‐economic status of children unclear, although intervention took place within a deprived school system Asthma status: asthmatic only (diagnosed asthma) Intervention recipients: children only |
|
| Interventions |
School type: junior/middle schools and high schools Intervention description: study authors presented results from Kickin' Asthma, described as "a 4‐session curriculum developed jointly by experts, nurses and peer educators and delivered over a 3‐year period. Kickin' Asthma is similar structurally to Open Airways for Schools, a curriculum designed for children at the elementary school level but with more advanced topics and learning modalities more suitable for adolescents' level of cognition and awareness. The 4 Kickin' Asthma sessions were each taught by a specialist nurse, about 50 minutes in length, and were spaced 1 week apart. The 4 sessions covered (1) lung physiology and asthma basics; (2) triggers, symptoms, and warning signs; (3) medication; and (4) emergencies, problem solving, and review. Each session has optional modules for skits, games, videos, and role‐playing scenarios and allowed certain modules to be taught by either the health educator or peer educators. Customized letters were sent home to the parents or guardians of all Kickin' Asthma participants that described the curriculum along with the specific health needs and goals of each student as assessed by the nurse educator" Control description: N/A (no control) Theoretical framework: no information |
|
| Outcomes | Core processes evaluated (child level): attrition, dosage, adherence | |
| Notes |
Process evaluation category: stand‐alone and named section (2 papers) Breadth and depth: depth ‐ not breadth Voice of children given prominence: not featured Funding source: Centers for Disease Control and Prevention Controlling Asthma in American Cities Project |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Unclear risk | N/A |
| Transparent and clearly stated aims | Low risk | Study aims were clearly stated |
| Explicit theories underpinning and/or literature review | Unclear risk | Some supporting literature was provided, but no theoretical framework was stated |
| Transparent and clearly stated methods and tools | Low risk | Lots of details about the tools used and how the study was conducted were provided |
| Selective reporting | Unclear risk | Data were collected individually and were presented ecologically |
| Harmful effects | Unclear risk | Harmful impacts ‐ particularly around equity and gathering a range of stakeholder views ‐ were not considered |
| Population and sample described well | Unclear risk | Data on SES and ethnicity are missing |
| Continuous evaluation | Unclear risk | Data that support different cohorts were provided, but whether they were analysed continuously (i.e. whether data were analysed and used to make any necessary changes to the programme) remains unclear |
| Evaluation participation equity and sampling | Unclear risk | Equitable sampling was described, along with a low response rate; data were not collected from all stakeholders involved |
| Design and methods overall approach | Unclear risk | Only one source of evidence was provided; however this is disaggregated across different cohorts |
| Tools and methods of data collection reliable/credible | High risk | Very low response rates suggest that methods of data collection used are unreliable |
| Tools and methods of data analysis reliable/credible | Unclear risk | Two sets of analyses were conducted ‐ ecological and individual. Insights gained by using both sets remain unclear |
| Performance bias/neutrality/credibility/conformability | Unclear risk | Study authors did not describe steps taken to minimise performance bias. Negative outcomes are discussed, as is delivery of the intervention by nurses vs researchers. Confidentiality/anonymity is not discussed |
| Reliability of findings and recommendations | High risk | Study authors reported low rates of response to the surveys used |
| Transferability of findings | Unclear risk | This was not assessed by study authors, but the information provided is rich enough to assess transferability |
| Overall risk of bias of process evaluation | Unclear risk | As an impact study, issues are evident, but as a process evaluation, data provided are useful |
McCann 2006.
| Methods |
Included as outcome evaluation Study design: clustered parallel‐group RCT with schools selected as the unit of randomisation Setting: primary/junior schools in the south of England; 24 schools randomised (with one dropout); 12 treatment schools and 11 control schools included Period: 2000 to 2001. Outcomes were collected 6 months before and after the intervention |
|
| Participants |
Eligible sample frame: 361 children and their parents were invited to participate Randomised: 219 children and their parents agreed to participate. 113 pupils at 12 schools were in the control group; 106 pupils at 12 schools were in the intervention group Completed (intervention): 1 school withdrew, resulting in 6 children withdrawing. 20 children withdrew because they moved out the area. Unclear whether these children came from the intervention group or the control group Inclusion criteria: not reported Exclusion criteria: not reported Baseline characteristics Age of children: 7 to 9 years of age Ethnicity: not reported Socio‐economic status: 25% of pupils in the control group and 15% of those in the intervention group were socially deprived Gender: 97 females and 122 males: 50 females and 56 males in the intervention group, 47 females and 66 males in the control group Asthma status: asthmatic and non‐asthmatic children were included, and selected outcomes for asthmatic children were presented separately |
|
| Interventions |
School type: 24 primary schools Intervention description: intervention workshops focused on a description of the respiratory condition, consistent with the national science curriculum. Role‐play between a teacher and a school nurse demonstrated what it was like to have asthma, and how one can help a friend who is coughing and struggling to breathe Control description: children in the control group took part in a workshop about the respiratory system and how the body defends itself against infection; however asthma was not mentioned during the workshop Theoretical framework: not reported Intensity: one 45‐minute workshop Instructor: school nurse Parental engagement: not reported Child satisfaction: not reported Timing of the intervention in the school day: not reported |
|
| Outcomes |
Extractable outcomes were not collected Core processes evaluated (child level): attrition, dosage, adherence |
|
| Notes |
Process evaluation category: integrated Breadth and depth: depth ‐ not breadth Voice of children given prominence: featured but not sufficiently Note: extractable outcomes were not collected for meta‐analysis; this study described a low‐intensity single‐session intervention and was not included in QCAs Funding source: National Health Service Research and Development Grant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This was not addressed by study authors, who described that "Pairs of schools were matched on demographic characteristics and randomly assigned within pairs" |
| Allocation concealment (selection bias) | Unclear risk | This was not addressed by study authors, who described that "Pairs of schools were matched on demographic characteristics and randomly assigned within pairs" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was not addressed by study authors |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This was not addressed by study authors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition was not disaggregated; 219 parents of children with asthma agreed their children could participate, of whom 193 (88.1%) completed the study |
| Selective reporting (reporting bias) | High risk | Full attendance data were not presented; full data for other outcomes also were not presented |
| Other bias | Unclear risk | Missingness ‐ unclear risk ‐ this is not fully described by study authors Baseline imbalance ‐ high risk ‐ differences were apparent (e.g. the control group was twice as likely to report both parents smoking); these were accounted for only in some analyses Risk of contamination ‐ unclear ‐ schools were the unit of randomisation |
| Transparent and clearly stated aims | Low risk | Study aims were clearly stated |
| Explicit theories underpinning and/or literature review | Unclear risk | Some literature was provided to support a whole school approach, but no theory was named |
| Transparent and clearly stated methods and tools | Unclear risk | Tools used are not clear, and it is not clear whether they were validated |
| Selective reporting | High risk | Although the direction of the result was presented in some cases, actual data were not presented, meaning that they are not extractable |
| Harmful effects | Unclear risk | Some subgroup analyses were undertaken, but not always with justification |
| Population and sample described well | Unclear risk | Whether the population consists of all children or only asthmatic children is not always clear |
| Continuous evaluation | Low risk | Pre‐post assessment was conducted |
| Evaluation participation equity and sampling | Unclear risk | This was not clearly reported by study authors |
| Design and methods overall approach | Unclear risk | Data were collected from multiple stakeholders, but they were not collected continuously |
| Tools and methods of data collection reliable/credible | Unclear risk | Not much information about the questionnaires used was presented |
| Tools and methods of data analysis reliable/credible | Unclear risk | It appears that analysis of the data was conducted sensibly, but it is unclear what happened in the light of results presented |
| Performance bias/neutrality/credibility/conformability | Unclear risk | This was not addressed by study authors |
| Reliability of findings and recommendations | High risk | Data were not presented in full, and study authors reported statistical significance, rather than presenting effect sizes |
| Transferability of findings | Unclear risk | This was not explicitly considered, but processes of implementation were described in full (simple intervention in some ways) |
| Overall risk of bias of process evaluation | Unclear risk | Adherence and attrition data are not fully applicable in a single‐session intervention, and issues surround the disaggregation of outcome data between asthmatic and non‐asthmatic children |
McGhan 2003.
| Methods |
Included as outcome evaluation Study design: clustered parallel‐group RCT, with schools selected as the unit of randomisation Setting: 18 elementary schools in Edmonton, Canada Period: dates on which study was conducted ‐ intervention and subsequent data collection ‐ are not clear (post‐test data collection took place 9 months after the intervention) |
|
| Participants |
Eligible sample frame: eligibility based on school and parent assent and asthma status ‐ 162 children found to be eligible across 18 schools Randomised: 162 pupils were randomised at the school level: 76 to the treatment group and 86 to the control group Completed (intervention): 136 pupils completed the trial Inclusion criteria: Study authors stated: "the target population was children with asthma ages 7–12 years; however, other ages were included if the parent and child were interested in participating. Criteria for selection included: (1) a diagnosis of asthma by a physician, (2) informed consent from the parent/guardian, (3) ability to speak English, and (4) no previous participation in RAP" Exclusion criteria: no additional exclusion criteria described Baseline characteristics Age of children: wide age range, with children 5 to 13 years old participating (most were 8 to 10 years of age) Ethnicity: approximately 77.8% of children were white Socio‐economic status: unclear Gender: approximately 59.2% of participants were male Asthma status: approximately 66.6% of children were deemed to have mild asthma, and 6.3% were deemed to have severe asthma |
|
| Interventions |
Intervention: involved testing the effectiveness of "Roaring Adventures of Puff" (RAP). Study authors describe that "using the 30 page manual, the instructors taught six 60‐minute sessions: (1) getting to know each other, goal setting, use of a peak flow meter, diary monitoring; (2) trigger identification, control and avoidance, basic pathophysiology; (3) medications and proper use of inhalers; (4) symptom recognition and action plan; (5) lifestyle, exercise, managing an asthma episode; and (6) sharing this information with teachers and parents. Parents and teachers in the intervention schools were invited to participate in a RAP parent/teacher asthma awareness event at the school" Control: not clearly stated (usual care) Intensity: asthmatic students received 6*60‐minute lessons Instructor: nursing and pharmacy students were asked to be RAP instructors Theoretical framework: social cognitive theory Parental engagement: it was intended that parents would be involved, although parent and teacher attendance ranged from 10% to 80% Child satisfaction: not reported Timing of intervention in school day: not reported |
|
| Outcomes |
Outcomes were extracted for: Asthma symptoms leading to emergency hospital visits Parent‐reported absence from school Unplanned GP or hospital visit due to asthma Experience of daytime and night‐time symptoms Withdrawal |
|
| Notes |
Considered for process evaluation: did not meet the definition of a process evaluation Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This is not addressed by study authors, who describe: "the study compared children with asthma in randomly assigned intervention schools with those in control schools" |
| Allocation concealment (selection bias) | Unclear risk | This is not addressed by study authors, who describe: "the study compared children with asthma in randomly assigned intervention schools with those in control schools" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not addressed by study authors |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not addressed by study authors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Twenty‐six families (16%) dropped out of the study; indications show that children who dropped out had substantially different characteristics from children who remained engaged: "the dropouts were significantly less likely to have reported seasonal asthma and unscheduled doctor visits in the last year" |
| Selective reporting (reporting bias) | Unclear risk | No evidence shows selective reporting |
| Other bias | Unclear risk | Missingness ‐ unclear risk ‐ information on missing data was not provided in detail Baseline imbalance ‐ high risk ‐ the intervention group was more likely to have received previous education on asthma, which is likely to have influenced response to the intervention Risk of contamination ‐ low ‐ unit of randomisation was schools |
| Transparent and clearly stated aims | Unclear risk | N/A |
| Explicit theories underpinning and/or literature review | Unclear risk | N/A |
| Transparent and clearly stated methods and tools | Unclear risk | N/A |
| Selective reporting | Unclear risk | N/A |
| Harmful effects | Unclear risk | N/A |
| Population and sample described well | Unclear risk | N/A |
| Continuous evaluation | Unclear risk | N/A |
| Evaluation participation equity and sampling | Unclear risk | N/A |
| Design and methods overall approach | Unclear risk | N/A |
| Tools and methods of data collection reliable/credible | Unclear risk | N/A |
| Tools and methods of data analysis reliable/credible | Unclear risk | N/A |
| Performance bias/neutrality/credibility/conformability | Unclear risk | N/A |
| Reliability of findings and recommendations | Unclear risk | N/A |
| Transferability of findings | Unclear risk | N/A |
| Overall risk of bias of process evaluation | Unclear risk | N/A |
McGhan 2010.
| Methods |
Included as outcome evaluation Study design: clustered parallel‐group design Setting: elementary schools in Edmonton, Canada Period: not reported |
|
| Participants |
Eligible sample frame: all schools in Edmonton were eligible; an estimated 646 students from the 18 participating schools were eligible Randomised: 162 pupils from 18 schools: 76 pupils in the intervention group and 86 in the control group Completed (intervention): 136 pupils: 65 in the intervention group and 71 in the control group Inclusion criteria: the target population was children 7 to 12 years of age with asthma. Pupils were eligible if they had received a diagnosis of asthma from their doctor and informed consent from their parent/guardian, were able to speak Eglish, and had not previously participated in Roaring Adventures of Puff (RAP) Exclusion criteria: not reported Baseline characteristics Age of children: 5 to 10 years of age; 27.6% in the intervention group and 23.3% in the control group were 5 to 7 years old, and 61.8% in the intervention group and 57% in the control group were 8 to 10 years old Ethnicity: 81.6% of children in the intervention group and 74.4% of children in the control group were Caucasian Socio‐economic status: not reported; however 26.8% of children in the intervention group and 36.4% in the control group had a father who achieved education grade 12 or less Gender: males represented 55.3% of intervention students and 62.8% of control students Asthma status: mixed levels of severity: 62.7% of intervention children and 66.7% of control children had mild asthma; 29.3% of intervention children and 28.6% of control children had moderate asthma; 8% of intervention children and 4.8% of control children had severe asthma |
|
| Interventions |
Intervention: parents and teachers in intervention schools were invited to participate in a RAP asthma awareness event at school. Parents and children in the intervention schools received information letters to share with their doctors, including suggested guidelines for a written action plan to be used at home and school. Doctors also received a summary letter from the RAP instructor at the end of the programme Control: usual care Intensity: six 60‐minute sessions Instructor: third year nursing and pharmacy students were RAP instructors under the guidance of their supervisor Theoretical framework: social cognitive theory informed the intervention Parental engagement: not reported Child satisfaction: not reported Timing of intervention in school day: not reported |
|
| Outcomes |
Extractable outcomes were collected for: Asthma symptoms leading to emergency hospital visit Parent‐reported absence from school Unplanned visit to hospital or GP due to asthma symptoms Experience of daytime and night‐time symptoms Withdrawal |
|
| Notes |
Considered for process evaluation: did not meet the definition of a process evaluation Funding source: Alberta Heritage Foundation for Medical Research ‐ Health Research Fund |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Schools were randomly assigned to RAP educational intervention or usual care (control group) via a random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Selection of schools is unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not addressed by study authors |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not addressed by study authors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Over one‐quarter of children dropped out; no reasons were provided |
| Selective reporting (reporting bias) | High risk | Measures of variance were not presented, hindering extraction of some outcomes |
| Other bias | Unclear risk | Missingness ‐ unclear risk ‐ no descriptions of missingness were provided Baseline imbalance ‐ low risk ‐ no differences between intervention and control arms are apparent Risk of contamination ‐ low ‐ randomisation at the school level |
| Transparent and clearly stated aims | Unclear risk | N/A |
| Explicit theories underpinning and/or literature review | Unclear risk | N/A |
| Transparent and clearly stated methods and tools | Unclear risk | N/A |
| Selective reporting | Unclear risk | N/A |
| Harmful effects | Unclear risk | N/A |
| Population and sample described well | Unclear risk | N/A |
| Continuous evaluation | Unclear risk | N/A |
| Evaluation participation equity and sampling | Unclear risk | N/A |
| Design and methods overall approach | Unclear risk | N/A |
| Tools and methods of data collection reliable/credible | Unclear risk | N/A |
| Tools and methods of data analysis reliable/credible | Unclear risk | N/A |
| Performance bias/neutrality/credibility/conformability | Unclear risk | N/A |
| Reliability of findings and recommendations | Unclear risk | N/A |
| Overall risk of bias of process evaluation | Unclear risk | N/A |
Mickel 2016.
| Methods |
Included as process evaluation Intervention study design: quasi‐experimental single‐group intervention with change pre‐post intervention examined Unit of allocation: N/A Process evaluation methods: descriptive/bivariate, descriptive qualitative analysis |
|
| Participants |
Setting: 7 schools in an urban Midwest school district Age of children: mean age, 9.3 years Child characteristics (BME/SES): 63.6% of children were African American; 13.3% Hispanic; 20.2% white. Backgrounds of individual children were not presented, although schools were recruited from districts in low‐income areas Asthma status: asthmatic and non‐asthmatic children were included, with asthma diagnosed in approximately half of the 348 children Intervention recipients: children only |
|
| Interventions |
School type: primary/elementary Intervention description: study authors described an intervention (Iggy and the Inhalers (Iggy)). Iggy is an asthma education video, poster, comic book, sticker, and trading card programme for children between the ages of 7 and 12 years. Iggy education takes place over 1 session lasting approximately 30 minutes. Sessions include a brief welcome (2 minutes), the Iggy video (11 minutes), and interactive discussion based on posters (7 minutes). Each pre‐ and post‐test worksheet takes approximately 5 minutes to complete. Children then take home trading cards, stickers, and comic books to share with their parents Control description: N/A Theoretical framework: no information |
|
| Outcomes | Core processes evaluated (child level): attrition (to post‐test) | |
| Notes |
Process evaluation category: named section Breadth and depth: breadth and depth Voice of children given prominence: sufficient coverage Funding source: Wisconsin State Asthma Coalition mini‐grant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Unclear risk | N/A |
| Transparent and clearly stated aims | Low risk | Study aims were clearly stated |
| Explicit theories underpinning and/or literature review | High risk | Some literature was provided, but no theoretical framework was presented |
| Transparent and clearly stated methods and tools | Low risk | Study approach was well described |
| Selective reporting | Low risk | No evidence shows selective reporting, as negative comments were presented |
| Harmful effects | Low risk | Negative responses from children were presented and discussed |
| Population and sample described well | Low risk | Demographic information was provided |
| Continuous evaluation | Low risk | Pre‐post assessment was conducted |
| Evaluation participation equity and sampling | Low risk | Parent, nurse, and child responses were collected |
| Design and methods overall approach | Low risk | Short intervention ‐ data were collected at multiple time points |
| Tools and methods of data collection reliable/credible | Unclear risk | Description of qualitative data is limited |
| Tools and methods of data analysis reliable/credible | Unclear risk | How themes were developed remains unclear |
| Performance bias/neutrality/credibility/conformability | High risk | These aspects were not considered, and programme staff collected qualitative data |
| Reliability of findings and recommendations | High risk | Some of the claims around sustained impact were based on a 1‐month post‐test |
| Transferability of findings | Low risk | Enough data were provided for assessment of the relevance of challenges |
| Overall risk of bias of process evaluation | Low risk | Overall low risk, although some reservations around reporting of study design and analysis of qualitative aspects remain |
Monforte 2012.
| Methods |
Included as outcome evaluation Study design: clustered parallel‐group RCT Setting: 8 urban public elementary schools Period: not reported |
|
| Participants |
Eligible sample frame: 22 schools were eligible and 4 participated; unclear how many students were eligible Randomised: 49 intervention students and 41 control students ‐ between 32% and 64% of eligible students across schools Completed (intervention): 49 students in the intervention group and 41 students in the control group Inclusion criteria: not reported Exclusion criteria: not reported Baseline characteristics Age of children: 90 children in grades 3 to 6 Ethnicity: not reported Socio‐economic status: not reported Gender: not reported Asthma status: asthma only |
|
| Interventions |
Intervention: children were enrolled in the OAS programme; however no further information was provided Control: not reported Intensity: not reported Instructor: not reported Theoretical framework: not reported Parental engagement: not reported Child satisfaction: not reported Timing of intervention in school day: not reported |
|
| Outcomes |
Extractable outcomes were collected for: Health‐related quality of life |
|
| Notes | This study was presented as an abstract only Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Unclear risk | N/A |
| Transparent and clearly stated aims | Unclear risk | N/A |
| Explicit theories underpinning and/or literature review | Unclear risk | N/A |
| Transparent and clearly stated methods and tools | Unclear risk | N/A |
| Selective reporting | Unclear risk | N/A |
| Harmful effects | Unclear risk | N/A |
| Population and sample described well | Unclear risk | N/A |
| Continuous evaluation | Unclear risk | N/A |
| Evaluation participation equity and sampling | Unclear risk | N/A |
| Design and methods overall approach | Unclear risk | N/A |
| Tools and methods of data collection reliable/credible | Unclear risk | N/A |
| Tools and methods of data analysis reliable/credible | Unclear risk | N/A |
| Performance bias/neutrality/credibility/conformability | Unclear risk | N/A |
| Reliability of findings and recommendations | Unclear risk | N/A |
| Transferability of findings | Unclear risk | N/A |
| Overall risk of bias of process evaluation | Unclear risk | N/A |
Mosnaim 2011.
| Methods |
Included as outcome evaluation Study design: clustered parallel‐group RCT Setting: elementary schools in Chicago Period: not reported |
|
| Participants |
Eligible sample frame: not reported Randomised: 344 pupils within the youth group (275 in the intervention group and 69 in the control group) and 192 within the teen group (141 in the intervention group and 51 in the control group) participated. 25 youth classes (19 intervention groups and 6 control groups) and 16 teen classes (11 intervention group and 5 control groups) from 26 schools participated Completed (intervention): not reported Inclusion criteria: not reported Exclusion criteria: not reported Baseline characteristics Age of children: youths and teens 5 to 15 years old. Median age was 10 for the youth group and 13 for the treatment group Ethnicity: 65.5% in the youth intervention group were African American; 11.6% were Hispanic; and 22.3% were other. In the teen intervention group, 85.1% were African American, 7.1% were Hispanic, and 7.1% were other Socio‐economic status: not reported, but study author described participants as predominantly low income Gender: females represented 43% of participants. In the youth intervention group, 41.5% were female; in the teen intervention group, 48.2% were female Asthma status: not reported |
|
| Interventions |
Intervention: certified asthma educators provided 1‐to‐1 training on spacer technique, peak flow meter use, and use of an asthma action plan. The teen programme also addressed tobacco avoidance, asthma‐related peer pressure, and asthma self‐management skills Control: usual care Intensity: four 45‐minute sessions conducted in school on 4 consecutive days Instructor: certified asthma educators Theoretical framework: not reported Parental engagement: not reported Child satisfaction: not reported Timing of intervention in school day: not explicitly reported, but sessions were scheduled at times with the least impact on instruction, as determined by each school |
|
| Outcomes | Extractable outcomes were collected for: None | |
| Notes |
Considered for process evaluation: study did not include the core components of a process evaluation Funding source: Abbott Laboratories Unrestricted Grant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Potential breach of randomisation schedule through systematic bias in selection of schools for the control group: "The allocation scheme first determined whether an eligible school could accommodate the intervention schedule. Those that could not were automatically assigned to the control group, whereas those that could were subject to the 3:1 randomisation scheme" |
| Allocation concealment (selection bias) | High risk | Potential breach of randomisation schedule through systematic bias in selection of schools for the control group: "The allocation scheme first determined whether an eligible school could accommodate the intervention schedule. Those that could not were automatically assigned to the control group, whereas those that could were subject to the 3:1 randomisation scheme" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not addressed by study authors |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not addressed by study authors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Relatively low levels of missing data; study authors state that this had no impact on the outcome: "Approximately 15% of the participants overall were missing posttest scores (39 youth and 44 teen participants). Based on feedback from our trained educators as to the source of the absenteeism, we concluded that these missing data were missing at random and did not merit use of data imputation methods" |
| Selective reporting (reporting bias) | Low risk | No evidence of indicators measured but not reported. However, indicators that were not included in our protocol were collected |
| Other bias | Unclear risk | Missingness ‐ unclear risk ‐ missing data but no imputation strategy ‐ study authors stated that it was not necessary to implement imputation strategies Baseline imbalance ‐ unclear risk ‐ some ethnic and gender differences at baseline but impact on outcomes unclear; no differences in asthma knowledge at baseline Risk of contamination ‐ low ‐ unit of randomisation was the school, lowering the risk of contamination |
| Transparent and clearly stated aims | Unclear risk | N/A |
| Explicit theories underpinning and/or literature review | Unclear risk | N/A |
| Transparent and clearly stated methods and tools | Unclear risk | N/A |
| Selective reporting | Unclear risk | N/A |
| Harmful effects | Unclear risk | N/A |
| Population and sample described well | Unclear risk | N/A |
| Continuous evaluation | Unclear risk | N/A |
| Evaluation participation equity and sampling | Unclear risk | N/A |
| Design and methods overall approach | Unclear risk | N/A |
| Tools and methods of data collection reliable/credible | Unclear risk | N/A |
| Tools and methods of data analysis reliable/credible | Unclear risk | N/A |
| Performance bias/neutrality/credibility/conformability | Unclear risk | N/A |
| Reliability of findings and recommendations | Unclear risk | N/A |
| Transferability of findings | Unclear risk | N/A |
| Overall risk of bias of process evaluation | Unclear risk | N/A |
Mujuru 2011.
| Methods |
Included as process evaluation Intervention study design: quasi‐experimental design, pre‐post follow‐up, no control Unit of allocation: N/A Process evaluation methods: descriptive/bivariate analysis methods |
|
| Participants |
Country: USA Age of children: 18 students in grades 3 to 5 Child characteristics (BME/SES): 39% of students were in receipt of Medicaid. Ethnicity data were not reported Asthma status: asthmatic only Intervention recipients: children and parents |
|
| Interventions |
School type: 1 elementary school Intervention description: study used the OAS programme to provide educational workshops in schools Control description: N/A Theoretical framework: not reported |
|
| Outcomes | Core processes evaluated (child level): attrition | |
| Notes |
Process evaluation category: integrated Breadth and depth: breadth and depth Voice of children given prominence: featured but not sufficiently Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Unclear risk | N/A |
| Transparent and clearly stated aims | Low risk | Study aims were clearly stated |
| Explicit theories underpinning and/or literature review | Unclear risk | A named theory is not present, but supporting literature was presented |
| Transparent and clearly stated methods and tools | Low risk | Data collection tools were reasonably well described |
| Selective reporting | Low risk | Negative aspects of the intervention were reported |
| Harmful effects | Low risk | Low parental engagement and compliance were reported |
| Population and sample described well | Unclear risk | Some expected fields, for example, ethnicity, were not reported |
| Continuous evaluation | Low risk | Pre‐post assessment was conducted; however post follow‐up engagement was low |
| Evaluation participation equity and sampling | Low risk | Parents were involved, but little information was received from teachers or instructors |
| Design and methods overall approach | Low risk | The overall design and methods were well described and suitable for the study |
| Tools and methods of data collection reliable/credible | Low risk | Tools used for data collection were reported fully |
| Tools and methods of data analysis reliable/credible | Unclear risk | The validity of the parental survey is unclear. This survey contained a 32‐item questionnaire designed by investigators as based on a review of published medical literature |
| Performance bias/neutrality/credibility/conformability | Unclear risk | Some aspects were covered, but not all aspects were reported on |
| Reliability of findings and recommendations | Unclear risk | Some process outcomes might be generalisable, but study authors themselves suggest that the "sample size was too small to generalise the results to a larger population" |
| Transferability of findings | Unclear risk | Some process outcomes might be generalisable, but study authors themselves suggest that the "sample size was too small to generalise the results to a larger population" |
| Overall risk of bias of process evaluation | Low risk | No factors were considered high risk |
Patterson 2005.
| Methods |
Included as outcome evaluation Study design: clustered parallel‐group design, with schools as the unit of randomisation Setting: primary schools in Belfast, Northern Ireland Period: participating schools entered the trial between September 2002 and September 2003. Preliminary assessment through to follow‐up assessment took 31 weeks |
|
| Participants |
Eligible sample frame: 102 eligible children in intervention schools and 126 eligible children in control schools Randomised: 84 eligible children in intervention schools and 92 eligible children in control schools Completed (intervention): 99 children in intervention schools and 92 children in control schools Inclusion criteria: children were eligible if they were between 7 and 11 years of age and had received a diagnosis of asthma Exclusion criteria: not reported Baseline characteristics Age of children: mean age, 9.01 years in the intervention group and 8.99 years in the control group Ethnicity: not reported Socio‐economic status: 32% of children in the intervention group and 22% of children in the control group were eligible for free school meals Gender: males represented 45% of the intervention group and 58% of the control group Asthma status: not reported |
|
| Interventions |
Intervention: each session began with brief reinforcement of previous training and ended with session feedback. The SCAMP club workbook used during sessions was given to children at prize giving, along with the child‐held asthma care pathway record and action plan Control: control group received the same intervention after a 16‐week interval Intensity: weekly sessions for 8 weeks Instructor: school nurse and a health visitor Theoretical framework: study was informed by the Predisposing, Reinforcing, and Enabling Causes in Educational Diagnosis and Evaluation (PRECEDE) model Parental engagement: not reported Child satisfaction: not reported Timing of intervention in school day: intervention was designed to be delivered at lunchtime |
|
| Outcomes |
Extractable outcomes were collected for: Days of restricted activity Lung function Health‐related quality of life Withdrawal |
|
| Notes |
Considered for process evaluation: study did not contain core components of a process evaluation Funding source: South and East Belfast Health and Social Services Trust, Primary Care and Development Fund, Eastern Health and Social Services Board, Department of Child Health, Queen's University Belfast |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | In each pair, the toss of a coin was used to randomise schools to immediate or delayed intervention |
| Allocation concealment (selection bias) | Unclear risk | Given that allocation was done within pairs of schools, allocation concealment might have been breached |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not addressed by study authors |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not addressed by study authors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Very low levels of attrition (2/83 in the intervention group) |
| Selective reporting (reporting bias) | Low risk | No indication that outcomes were collected and not reported upon |
| Other bias | Low risk | Missingness ‐ low risk ‐ no missing data among those who did not drop out Baseline imbalance ‐ unclear risk ‐ some differences are apparent; not clear if these differences would significantly alter response to the intervention Risk of contamination ‐ low ‐ schools were the unit of randomisation |
| Transparent and clearly stated aims | Unclear risk | N/A |
| Explicit theories underpinning and/or literature review | Unclear risk | N/A |
| Transparent and clearly stated methods and tools | Unclear risk | N/A |
| Selective reporting | Unclear risk | N/A |
| Harmful effects | Unclear risk | N/A |
| Population and sample described well | Unclear risk | N/A |
| Continuous evaluation | Unclear risk | N/A |
| Evaluation participation equity and sampling | Unclear risk | N/A |
| Design and methods overall approach | Unclear risk | N/A |
| Tools and methods of data collection reliable/credible | Unclear risk | N/A |
| Tools and methods of data analysis reliable/credible | Unclear risk | N/A |
| Performance bias/neutrality/credibility/conformability | Unclear risk | N/A |
| Reliability of findings and recommendations | Unclear risk | N/A |
| Transferability of findings | Unclear risk | N/A |
| Overall risk of bias of process evaluation | Unclear risk | N/A |
Persaud 1996.
| Methods |
Included as outcome evaluation Study design: parallel‐group design with children selected as the unit of randomisation Setting: study was conducted in Galveston, Texas, USA, across children in 10 schools Period: the intervention was conducted between September and December 1992 |
|
| Participants |
Eligible sample frame: 60 pupils found to be eligible; 43 were contacted and 36 agreed to participate Randomised: 36 children were randomised, with 18 each selected into intervention and control arms Completed (intervention): no students were described as having dropped out Inclusion criteria: children 8 to 12 years of age with a diagnosis of asthma (several prior episodes of airway obstruction, clinical response to bronchodilator, and absence of other pulmonary disease) Exclusion criteria: no additional exclusion criteria provided Baseline characteristics Age of children: mean age, 10.2 years Ethnicity: 69% of children were African American Socio‐economic status: 69% of children were from families in receipt of Medicaid Gender: 64% of children were male Asthma status: 44% of children had mild asthma, 50% had moderately severe asthma, and 6% had severe asthma |
|
| Interventions |
Intervention: study authors described that "intervention subjects received individualized, weekly, 20‐min education sessions with the school nurse for 8 weeks. Each child had a personal peak flow meter in the school health office to use during teaching sessions. At each visit, the school nurse reviewed the asthma diary with the student and discussed progress, symptoms, and ability to take appropriate measures to control asthma. At each visit, the child demonstrated proper use of inhaled medications and the peak flow meter. The school nurses recorded each student's weekly progress on a checklist in the teaching manual" Control: usual care Intensity: target asthmatic students received 3 lessons from peer leaders (year 11 students) Instructor: school nurses Theoretical framework: no overarching theory named Parental engagement: not reported Child satisfaction: not reported Timing of intervention in school day: not reported |
|
| Outcomes |
Extractable outcomes were collected for: Emergency department visits Absences from school |
|
| Notes |
Considered for process evaluation: study did not include the core components of a process evaluation, and process data were collected with the use of structured tools Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No further information beyond this: students within each school were randomly assigned to intervention or control groups |
| Allocation concealment (selection bias) | Unclear risk | No further information beyond this: students within each school were randomly assigned to intervention or control groups |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All primary care providers were blinded as to assignment to treatment or control groups; primary care providers collected most of these data |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All primary care providers were blinded as to assignment to treatment or control groups; primary care providers collected most of these data |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition was reported among the 36 children |
| Selective reporting (reporting bias) | Low risk | Selective reporting was evident and outcomes were extracted |
| Other bias | Unclear risk | Missingness ‐ low risk ‐ no apparent missing data Baseline imbalance ‐ unclear risk ‐ control group had lived with asthma for longer, which could inflate the impact of the intervention Risk of contamination ‐ high ‐ students were randomised within schools; this could lead to contamination across groups |
| Transparent and clearly stated aims | Unclear risk | N/A |
| Explicit theories underpinning and/or literature review | Unclear risk | N/A |
| Transparent and clearly stated methods and tools | Unclear risk | N/A |
| Selective reporting | Unclear risk | N/A |
| Harmful effects | Unclear risk | N/A |
| Population and sample described well | Unclear risk | N/A |
| Continuous evaluation | Unclear risk | N/A |
| Evaluation participation equity and sampling | Unclear risk | N/A |
| Design and methods overall approach | Unclear risk | N/A |
| Tools and methods of data collection reliable/credible | Unclear risk | N/A |
| Tools and methods of data analysis reliable/credible | Unclear risk | N/A |
| Performance bias/neutrality/credibility/conformability | Unclear risk | N/A |
| Reliability of findings and recommendations | Unclear risk | N/A |
| Transferability of findings | Unclear risk | N/A |
| Overall risk of bias of process evaluation | Unclear risk | N/A |
Pike 2011.
| Methods |
Included as process evaluation Intervention study design: quasi‐experimental, pre‐post, control groups Unit of allocation: children in 15 classes were provided with the intervention (data available for 10), and 4 additional classrooms served as controls; 167 children were in the intervention group and 69 were in the control group Process evaluation methods: survey data were collected with descriptive/bivariate analyses of data |
|
| Participants |
Setting: elementary schools in a district in St Louis, Missouri, USA Age of children: 9 to 11 years of age (based on grade) Child characteristics (BME/SES): 81% of control group and 69% of intervention group were African American; 78% of intervention group and 86% of control group were receiving free school meals Asthma status: asthmatic and non‐asthmatic (mixed class; this is a core feature of the intervention so as not to disrupt normal school functioning) Intervention recipients: children and teachers |
|
| Interventions |
School type: primary/elementary Intervention description: a curriculum was developed for teachers that contained 15 lesson plans created or adapted from various existing sources and aligned with existing standards for communication arts, science, mathematics, and health. Intervention classroom teachers were asked to teach 7 of the 15 lesson plans, including 3 specific lesson plans chosen by the investigators (which included information on asthma basics, signs and symptoms, triggers, and use of a peak flow metre); the remaining 4 lesson plans were self‐selected by the teacher Control description: usual care/no additional asthma education Theoretical framework: no information |
|
| Outcomes | Core processes evaluated (child level): dosage | |
| Notes |
Process evaluation category: stand‐alone Breadth and depth: depth ‐ not breadth Voice of children given prominence: not featured Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Unclear risk | N/A |
| Transparent and clearly stated aims | Low risk | Study aims were clearly stated |
| Explicit theories underpinning and/or literature review | High risk | No theory was named and little literature was presented |
| Transparent and clearly stated methods and tools | Unclear risk | Some tools or aspects of tools were not clearly explained, for example, asthma knowledge |
| Selective reporting | Unclear risk | A full account of what was collected for assessment was not presented; some aspects were not reported ‐ e.g. the teacher focus group |
| Harmful effects | Unclear risk | How study authors accounted for this remains unclear |
| Population and sample described well | High risk | Some demographic characteristics, particularly the asthma status of children, were not explained well |
| Continuous evaluation | Low risk | Pre‐post assessment was included |
| Evaluation participation equity and sampling | Unclear risk | Sample information was collected from several stakeholders |
| Design and methods overall approach | Low risk | Multiple sources of evidence were used |
| Tools and methods of data collection reliable/credible | High risk | How asthma knowledge was measured remains unclear |
| Tools and methods of data analysis reliable/credible | High risk | No way to assess this without seeing a full output ‐ e.g. of the focus group |
| Performance bias/neutrality/credibility/conformability | Unclear risk | Some blinding was undertaken |
| Reliability of findings and recommendations | Unclear risk | Whether this was an effective intervention is not clear, as the information was not presented fully |
| Transferability of findings | Unclear risk | Study authors explained how the curriculum was developed, so transferability is low for part of this study ‐ but not enough information was provided in other sections |
| Overall risk of bias of process evaluation | Unclear risk | This is a good study of teachers, but study authors did not provide a lot of other information |
Praena‐Crespo 2010.
| Methods |
Included as outcome evaluation Study design: clustered parallel‐group RCT Setting: 16 high schools Period: baseline data were collected between November and December 2008 |
|
| Participants |
Eligible sample frame: 16 high schools (4090 children) Randomised: 15 high schools (3827 children) Completed (intervention): 15 high schools (3550 children) Inclusion criteria: not reported Exclusion criteria: not reported Baseline characteristics Age of children: 13 and 14 years of age Ethnicity: not reported Socio‐economic status: not reported Gender: mixed Asthma status: asthmatic and non‐asthmatic children |
|
| Interventions |
Intervention: children received an asthma programme, but no details were provided Control: not reported Intensity: 3 lessons Instructor: teachers Theoretical framework: not reported Parental engagement: not reported Child satisfaction: not reported Timing of intervention in school day: during personal development/health/physical education |
|
| Outcomes |
Extractable outcomes were collected for: None |
|
| Notes | This study was reported as an abstract only Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This study was presented as an abstract only. The abstract states that schools were randomised but does not describe how this was done |
| Allocation concealment (selection bias) | Unclear risk | This study was presented as an abstract only. The abstract states that schools were randomised but does not describe how this was done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not addressed in the abstract |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not addressed in the abstract |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Questionnaires were returned by 3827/4090 students (279 with asthma) at baseline and by 3550 at follow‐up (261 with asthma) |
| Selective reporting (reporting bias) | Unclear risk | Not addressed in the abstract |
| Other bias | Unclear risk | Missing data ‐ high risk ‐ many forms were not returned and data were not disaggregated Baseline imbalances ‐ not addressed in the abstract Risk of contamination ‐ low ‐ schools were the unit of allocation |
| Transparent and clearly stated aims | Unclear risk | N/A |
| Explicit theories underpinning and/or literature review | Unclear risk | N/A |
| Transparent and clearly stated methods and tools | Unclear risk | N/A |
| Selective reporting | Unclear risk | N/A |
| Harmful effects | Unclear risk | N/A |
| Population and sample described well | Unclear risk | N/A |
| Continuous evaluation | Unclear risk | N/A |
| Evaluation participation equity and sampling | Unclear risk | N/A |
| Design and methods overall approach | Unclear risk | N/A |
| Tools and methods of data collection reliable/credible | Unclear risk | N/A |
| Tools and methods of data analysis reliable/credible | Unclear risk | N/A |
| Performance bias/neutrality/credibility/conformability | Unclear risk | N/A |
| Reliability of findings and recommendations | Unclear risk | N/A |
| Transferability of findings | Unclear risk | N/A |
| Overall risk of bias of process evaluation | Unclear risk | N/A |
Pulcini 2007.
| Methods |
Included as outcome evaluation Study design: clustered parallel‐group design, with schools as the unit of randomisation Setting: middle schools in Massachusetts, USA Period: number of AAPs received was recorded by the school nurse in the fall of 2005 and was reported as a total number in early 2006 at the end of data collection |
|
| Participants |
Eligible sample frame: not reported Randomised: 40 students from 4 school districts ‐ 20 students in each group Completed (intervention): not reported Inclusion criteria: children were eligible if they had received a diagnosis of asthma with medications ordered at school, had no current asthma action plan on file, were from English‐speaking families, did not have any developmental disorders, and had a regular primary care provider or asthma specialist Exclusion criteria: not reported Baseline characteristics Age of children: not reported, but children in grades 6 to 8 were recruited Ethnicity: not reported Socioeconomic status: not reported Gender: not reported Asthma status: not reported |
|
| Interventions |
Intervention: each student was given a peak flow meter and was educated on the correct technique for measuring lung function. Peak flow was measured for 2 weeks and scores were recorded. All scores were sent to the physician along with a request for an asthma action plan Control: school nurses in the control group continued to follow their standard procedure of requesting an AAP via the student's parents Intensity: peak flow measured and recorded on a daily basis for 2 weeks Instructor: school nurse Theoretical framework: not reported Parental engagement: not reported Child satisfaction: not reported Timing of intervention in school day: not reported |
|
| Outcomes |
Extractable outcomes were collected for: None |
|
| Notes | AAPs are important but are not a part of the outcomes in this review, so they cannot be extracted Funding source: National Association of School Nurses Research Grant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not specified, and low numbers randomised: each school district participating in the study was required to have at least 2 middle schools, which were randomly assigned to experimental or control groups |
| Allocation concealment (selection bias) | High risk | Not specified, and low numbers randomised: each school district participating in the study was required to have at least 2 middle schools, which were randomly assigned to experimental or control groups |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not addressed by study authors |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not addressed by study authors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed by study authors |
| Selective reporting (reporting bias) | High risk | Peak flow data were collected but were not published in full |
| Other bias | Unclear risk | Missingness ‐ unclear risk ‐ not all data were published Baseline imbalance ‐ unclear risk ‐ not addressed in the study Risk of contamination ‐ low ‐ allocation was done on a school basis |
| Transparent and clearly stated aims | Unclear risk | N/A |
| Explicit theories underpinning and/or literature review | Unclear risk | N/A |
| Transparent and clearly stated methods and tools | Unclear risk | N/A |
| Selective reporting | Unclear risk | N/A |
| Harmful effects | Unclear risk | N/A |
| Population and sample described well | Unclear risk | N/A |
| Continuous evaluation | Unclear risk | N/A |
| Evaluation participation equity and sampling | Unclear risk | N/A |
| Design and methods overall approach | Unclear risk | N/A |
| Tools and methods of data collection reliable/credible | Unclear risk | N/A |
| Tools and methods of data analysis reliable/credible | Unclear risk | N/A |
| Performance bias/neutrality/credibility/conformability | Unclear risk | N/A |
| Reliability of findings and recommendations | Unclear risk | N/A |
| Transferability of findings | Unclear risk | N/A |
| Overall risk of bias of process evaluation | Unclear risk | N/A |
Richmond 2011.
| Methods |
Included as process evaluation Intervention study design: quasi‐experimental, post follow‐up only, no control Unit of allocation: N/A Process evaluation methods: descriptive/bivariate analysis methods |
|
| Participants |
Country: USA Age of children: elementary school age was reported, but no further details were given Child characteristics (BME/SES): almost 100% of students were African American; approximately 80% received free or reduced price lunch Asthma status: asthmatic only Intervention recipients: children only |
|
| Interventions |
School type: 14 elementary schools across 3 school districts Intervention description: breathe your best was implemented in the schools. Students were encouraged to received an asthma action plan from their healthcare provider and to collect prescriptions from the pharmacy. Students were also advised to give their action plans and medications to their school nurse at the beginning of the school year Control description: N/A Theoretical framework: not reported |
|
| Outcomes | Core processes evaluated (child level): attrition, adherence | |
| Notes |
Process evaluation category: stand‐alone Breadth and depth: neither broad nor deep Voice of children given prominence: not featured Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Unclear risk | N/A |
| Transparent and clearly stated aims | Unclear risk | Study report shows some confusion over the purpose of the study |
| Explicit theories underpinning and/or literature review | High risk | No literature was provided; no named theory guided the intervention |
| Transparent and clearly stated methods and tools | High risk | How the random sample was selected is not clear |
| Selective reporting | High risk | Information discussed in the interviews is not clear ‐ no schedule |
| Harmful effects | Unclear risk | This is inferred by the reader ‐ impact on nurses if implemented across the board |
| Population and sample described well | High risk | Age and gender were missing, as were the demographic characteristics of asthmatic kids |
| Continuous evaluation | High risk | Post hoc evaluation only ‐ particularly worrisome, as the intervention failed in the first year but continued into the second year |
| Evaluation participation equity and sampling | Unclear risk | Parents were interviewed and some information was collected from school nurses |
| Design and methods overall approach | Unclear risk | Interviews with parents seem to be the most important element; these were covered adequately |
| Tools and methods of data collection reliable/credible | High risk | Tools used were not presented (i.e. interview schedule) |
| Tools and methods of data analysis reliable/credible | Unclear risk | Only descriptive/narrative analysis reported |
| Performance bias/neutrality/credibility/conformability | Low risk | Attention was given to negative cases and non‐participation was investigated |
| Reliability of findings and recommendations | High risk | Quantity of data collected was unclear; not easy to ascertain whether there was more to be understood here |
| Transferability of findings | Unclear risk | Results show lack of understanding of the degree to which age and school factors played a part ‐ much more remained to be said about this |
| Overall risk of bias of process evaluation | Unclear risk | Risk of bias not very high because of the nature of the messages and the simple methods employed; however reporting errors occurred |
Shah 2001.
| Methods |
Included as outcome evaluation Study design: clustered parallel‐group design Setting: high schools in Tamworth, rural New South Wales, Australia Period: pupils were recruited in February 1998 and completed the study in October 1998 ‐ 3 months after the intervention was completed |
|
| Participants |
Eligible sample frame: 325 pupils Randomised: 272 pupils: 148 in the control group and 124 in the intervention group Completed (intervention): 251 pupils; 138 in the control group and 113 in the intervention group Inclusion criteria: not reported Exclusion criteria: not reported Baseline characteristics Age of children: 118 pupils in year 7; 133 pupils in year 10 Ethnicity: not reported Socio‐economic status: not reported Gender: 62% to 68% female in the intervention group; 44% to 48% female in the control group Asthma status: 69% to 80% had received an asthma diagnosis |
|
| Interventions |
Intervention: the intervention involved a 3‐step approach to educating and empowering students with asthma. In step 1, students learnt how to educate their peers about asthma and its management using games, videos, worksheets, and discussions as teaching tools. In step 2, peer leaders conducted three 45‐mnute health lessons for year 10 classes at school. In step 3, year 10 students developed and presented to year 7 students key messages learnt in the lessons Control: wait‐list control group received usual care during data collection Intensity: in step 1, volunteers from year 11 were trained as asthma peers during a 6‐hour workshop. In step 2, three 45‐minute sessions were taught. No information was recorded on length and intensity in step 3 Instructor: peers Theoretical framework: not reported Parental engagement: not reported Child satisfaction: not reported Timing of intervention in school day: not reported |
|
| Outcomes |
Extractable outcomes were collected for: Experience of daytime and night‐time symptoms Lung function Health‐related quality of life Withdrawal |
|
| Notes | School absence data were collected as median values but were not reported in full Funding source: Commonwealth Department of Health and Aged Care and Asthma NSW |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Concealed random allocation was performed by study author (who was not involved in administration of the study), using a random number generator and the closed envelope technique |
| Allocation concealment (selection bias) | Low risk | Concealed random allocation was performed by PGG (who was not involved in administration of the study), using a random number generator and the closed envelope technique |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not addressed by study authors |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not addressed by study authors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low levels of attrition: 272 participated in baseline testing; matched data at baseline and after the intervention were available for 251 students |
| Selective reporting (reporting bias) | Unclear risk | No direct evidence, although median number of days missed was collected and could not be combined in the meta‐analysis |
| Other bias | Unclear risk | Missingness ‐ low risk ‐ missing data described as uncommon and occurred owing to misclassification, students changing schools or being absent on the day of testing, or failure to complete the questionnaire Baseline imbalance ‐ unclear risk ‐ differences between groups, although it is unclear if these are significant for the outcome Risk of contamination ‐ low ‐ schools were the unit of randomisation, thereby lowering risk of contamination |
| Transparent and clearly stated aims | Unclear risk | N/A |
| Explicit theories underpinning and/or literature review | Unclear risk | N/A |
| Transparent and clearly stated methods and tools | Unclear risk | N/A |
| Selective reporting | Unclear risk | N/A |
| Harmful effects | Unclear risk | N/A |
| Population and sample described well | Unclear risk | N/A |
| Continuous evaluation | Unclear risk | N/A |
| Evaluation participation equity and sampling | Unclear risk | N/A |
| Design and methods overall approach | Unclear risk | N/A |
| Tools and methods of data collection reliable/credible | Unclear risk | N/A |
| Tools and methods of data analysis reliable/credible | Unclear risk | N/A |
| Performance bias/neutrality/credibility/conformability | Unclear risk | N/A |
| Reliability of findings and recommendations | Unclear risk | N/A |
| Transferability of findings | Unclear risk | N/A |
| Overall risk of bias of process evaluation | Unclear risk | N/A |
Spencer 2000.
| Methods |
Included as process evaluation Intervention study design: quasi‐experimental single‐group intervention examining change pre‐post intervention Unit of allocation: N/A Process evaluation methods: survey‐based methods with descriptive/bivariate analyses of results |
|
| Participants |
Setting: the study included 40 schools from 8 school districts throughout New York State, USA Age of children: wide range of ages, with children 6 to 13 years old Child characteristics (BME/SES): 36% of children were receiving free or reduced price lunch Asthma status: asthmatic only Intervention recipients: children and parents |
|
| Interventions |
School type: primary/elementary Intervention description: Open Airways for Schools (OAS) described by study authors as consisting of "six weekly (40‐minute) hands‐on sessions for the children, one or two sessions for the children's parents, and a graduation ceremony for both parents and children. The children's portion of the program covered such areas as: (I) basic information and feelings about asthma; (ii) recognizing and managing asthma symptoms; (iii) solving problems with medicines and deciding how bad symptoms are; (iv) finding and controlling asthma triggers; (v) getting enough exercise; and (vi) doing well at school. The parents' program briefly covered content similar to the children's sessions. Parents also received letters that familiarized them with the children's classroom content" Control description: N/A Theoretical framework: no information |
|
| Outcomes | Core processes evaluated (child level): adherence | |
| Notes |
Process evaluation category: integrated Breadth and depth: neither broad nor deep Voice of children given prominence: not featured Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Unclear risk | N/A |
| Transparent and clearly stated aims | Unclear risk | Aims of OAS were clearly stated, but aims of the study were not explained |
| Explicit theories underpinning and/or literature review | High risk | No literature or theoretical framework was provided |
| Transparent and clearly stated methods and tools | Unclear risk | Not much detail provided on tools for parents and nurses |
| Selective reporting | High risk | Not everything was reported; instruments were poorly reported |
| Harmful effects | High risk | No evidence of provision for measuring harmful effects |
| Population and sample described well | Unclear risk | No information about asthma severity |
| Continuous evaluation | Low risk | Pre‐post assessment was implemented |
| Evaluation participation equity and sampling | Unclear risk | How almost half the sample of kids dropped out remains unclear |
| Design and methods overall approach | High risk | Limited detail on design and methods was provided; problems with internal validity were noted |
| Tools and methods of data collection reliable/credible | High risk | Not all tools were clearly described |
| Tools and methods of data analysis reliable/credible | High risk | No indication that clustering was accounted for; not enough information (mean cluster size) was provided to estimate this |
| Performance bias/neutrality/credibility/conformability | High risk | No attempt at blinding was presented |
| Reliability of findings and recommendations | High risk | This study was not reported well enough to warrant that findings were reliable |
| Transferability of findings | High risk | Details about context were lacking, making the findings difficult to transfer |
| Overall risk of bias of process evaluation | High risk | Issues surround the tools and methods used to collect study data |
Splett 2006.
| Methods |
Included as outcome evaluation and process evaluation Study design: clustered parallel‐group design, randomised at the school level Setting: K‐8 schools in Minneapolis, Minnesota, USA Period: in 2000 and 2001, the HLAI was implemented in schools and was tested for effectiveness; in 2001 and 2002, the HLAI was expanded to all K‐8 schools |
|
| Participants |
Eligible sample frame: not reported; however 700 students with asthma were required in each group to detect a positive change in attendance Randomised: 916 in intervention schools and 645 in control schools Completed (intervention): not reported Inclusion criteria: not reported Exclusion criteria: not reported Baseline characteristics Age of children: not reported Ethnicity: 66% were African American, 6% were Hispanic, 5% were American‐Indian, and 20% were white Socio‐economic status: 73% of participants were eligible for free or reduced price lunches Gender: males represented 58% of participants Asthma status: not reported |
|
| Interventions |
Intervention: in participating schools, licensed school nurses, licensed practical nurses, and health service assistants received coaching and reinforcement by asthma resource nurses. Clinics also received training on NIH guidelines and guidance on implementing standards of care for asthma. Study authors reported: "staff followed 'Core Components of Asthma Management in the School Health Office' (Core Components), including case identification, nursing care procedures, care coordination, emergency care, and student education, to provide more systematic and consistent care to students with asthma and improve communication with school staff, parents, and health care providers", although further details of the student education component were not provided Control: usual care Intensity: not reported Instructor: school nurse Theoretical framework: not reported Parental engagement: not reported Child satisfaction: not reported Timing of intervention in school day: not reported |
|
| Outcomes |
Extractable outcomes were collected for: Absence from school Unplanned visit to hospital or GP due to asthma symptoms Core processes evaluated (child level): no information (other outcomes considered around sustainability) |
|
| Notes | This study conducted an ecological analysis Process evaluation category: stand‐alone, named section (2 papers) Breadth and depth: neither broad nor deep Voice of children given prominence: not featured Funding source: Member Organisations of the Healthy Learners Board, Controlling Asthma in American Cities Grant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study authors reported: "A random sequence of treatment codes, stratified by school system, was generated using the SAS System (Version 9.1, Cary, North Carolina) by the statistician" |
| Allocation concealment (selection bias) | Low risk | Centrally allocated: study authors reported: "A random sequence of treatment codes, stratified by school system, was generated using the SAS System (Version 9.1, Cary, North Carolina) by the statistician" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No measures were described as implemented around blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No measures were described as implemented around blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No direct reports describe attrition |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting was found |
| Other bias | Unclear risk | Missingness ‐ low risk ‐ all those who were followed up submitted information Baseline imbalance ‐ low risk ‐ no evidence of baseline imbalances between intervention and control groups Risk of contamination ‐ high ‐ children were the unit of randomisation; potential was present for children with different treatment allocations to share materials/information, etc |
| Transparent and clearly stated aims | Low risk | Study aims were clearly stated |
| Explicit theories underpinning and/or literature review | High risk | No theoretical framework or supporting literature was provided |
| Transparent and clearly stated methods and tools | Low risk | Methods and tools were clearly stated |
| Selective reporting | High risk | A focus group is mentioned in the DuPlessis paper, but whether this occurred before or after or during the intervention is not clear. In addition, relevant results were not presented |
| Harmful effects | High risk | Very broad study; harmful effects were not directly considered |
| Population and sample described well | High risk | Some information is missing, including age of participants |
| Continuous evaluation | Low risk | Process evaluation data were apparently collected throughout |
| Evaluation participation equity and sampling | High risk | No information was collected from nurses |
| Design and methods overall approach | Unclear risk | Some core elements are missing |
| Tools and methods of data collection reliable/credible | Low risk | Based on administrative records ‐ straightforward constructs |
| Tools and methods of data analysis reliable/credible | Unclear risk | Lack of age data makes it difficult to interpret some outcomes, although the models include controls for age |
| Performance bias/neutrality/credibility/conformability | Low risk | As based on administrative records, little reason was provided to assign anything but low risk of bias |
| Reliability of findings and recommendations | Unclear risk | Reliability of findings was compromised by the K‐12 age group |
| Transferability of findings | High risk | Details are lacking, and standardisation introduced difficulties related to transferability ‐ particularly the wide age range |
| Overall risk of bias of process evaluation | High risk | Details around the ages of children and other key factors that could influence outcomes are lacking |
Srof 2012.
| Methods |
Included as outcome evaluation Study design: parallel‐group design, randomised at the child level Setting: high schools in midwestern America Period: not reported, but post‐test data were collected 6 weeks after the intervention was completed |
|
| Participants |
Eligible sample frame: 299 students with asthma across the 3 participating high schools Randomised: 39 students: 21 in the intervention group and 18 in the control group Completed (intervention): 39 students Inclusion criteria: not reported Exclusion criteria: not reported Baseline characteristics Age of children: 14 to 18 years of age; average age was 15.67 years Ethnicity: not reported Socio‐economic status: not reported Gender: the intervention group comprised 10 males and 11 females Asthma status: not reported |
|
| Interventions |
Intervention: each morning for 2 weeks, the PI and the CNS assisted students to complete asthma diary entries. Students in the intervention group also received 5 coping skills training sessions Control: usual care Intensity: sessions ranged from 40 minutes to 60 minutes in duration and were conducted over a period of 5 weeks Instructor: principal investigator (PI) Theoretical framework: Health Promotion Model (HPM) informed development of the intervention Parental engagement: not reported Child satisfaction: not reported Timing of intervention in school day: sessions took place during the reserved study or resource period time block of the school day |
|
| Outcomes |
Extractable outcomes were collected for: None |
|
| Notes | Only some of the data in this study were reported, indicating evidence of possible selective reporting Considered for process evaluation but did not include expected required components Funding source: American Lung Association of Metropolitan Chicago, Nu Omicron Chapter of Sigma Theta Tau International, Pedipress Fulfillment Center, Respironics: HealthScan and Allergy Products |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Names of participating students were placed in an envelope and were drawn for random assignment to treatment and control groups within each school |
| Allocation concealment (selection bias) | Unclear risk | Unclear whether closed envelope technique was used and whether allocation could be concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not addressed by study authors |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Lack of blinding was cited as a disadvantage of the study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed in full by study authors |
| Selective reporting (reporting bias) | High risk | Evidence of some selective reporting; outcomes were not reported consistently throughout |
| Other bias | Unclear risk | Missingness ‐ unclear risk ‐ not addressed in full by study authors Baseline imbalance ‐ low risk ‐ no differences noted between groups on any of the baseline variables, as would be expected following random assignment Risk of contamination ‐ high ‐ children were randomised within schools, raising the risk of contamination |
| Transparent and clearly stated aims | Unclear risk | N/A |
| Explicit theories underpinning and/or literature review | Unclear risk | N/A |
| Transparent and clearly stated methods and tools | Unclear risk | N/A |
| Selective reporting | Unclear risk | N/A |
| Harmful effects | Unclear risk | N/A |
| Population and sample described well | Unclear risk | N/A |
| Continuous evaluation | Unclear risk | N/A |
| Evaluation participation equity and sampling | Unclear risk | N/A |
| Design and methods overall approach | Unclear risk | N/A |
| Tools and methods of data collection reliable/credible | Unclear risk | N/A |
| Tools and methods of data analysis reliable/credible | Unclear risk | N/A |
| Performance bias/neutrality/credibility/conformability | Unclear risk | N/A |
| Reliability of findings and recommendations | Unclear risk | N/A |
| Transferability of findings | Unclear risk | N/A |
| Overall risk of bias of process evaluation | Unclear risk | N/A |
Terpstra 2012.
| Methods |
Included as process evaluation Intervention study design: quasi‐experimental design, pre‐post follow‐up, control group Unit of allocation: school Process evaluation methods: multi‐variate analysis |
|
| Participants |
Country: USA Age of children: mean age, 12.52 in the intervention group and 12.10 in the control group Child characteristics (BME/SES): 44% of intervention children and 56% of control group children were Latino; average annual income was less than $20,000 Asthma status: asthmatic only Intervention recipients: children and parents |
|
| Interventions |
School type: 2 middle schools Intervention description: children received skills training addressing topics such as how to use a peak flow meter. These sessions took place over 6 weeks. Parents received a newsletter that was centred on an important theme identified during the research Control description: equivalent intervention in which children received the intervention but parents did not receive the newsletter Theoretical framework: social cognitive theory |
|
| Outcomes | Core processes evaluated (child level): attrition, dosage, adherence | |
| Notes |
Process evaluation category: integrated Breadth and depth: neither broad nor deep Voice of children given prominence: not featured Funding source: American Lung Association |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Unclear risk | N/A |
| Transparent and clearly stated aims | Unclear risk | Study aims were not clearly stated |
| Explicit theories underpinning and/or literature review | Unclear risk | Some theory and supporting literature were provided, but why a newsletter was used was not explained |
| Transparent and clearly stated methods and tools | Low risk | All tools were clearly described |
| Selective reporting | High risk | This was not considered ‐ particularly the absence of measures showing whether people did receive the newsletter Also, as acknowledged by study authors, not only caregivers received the newsletter |
| Harmful effects | Unclear risk | This was not recorded, and the paper included no information on child outcomes |
| Population and sample described well | Unclear risk | School characteristics were poorly described |
| Continuous evaluation | Low risk | Pre‐post assessment was carried out |
| Evaluation participation equity and sampling | High risk | No child input was included |
| Design and methods overall approach | High risk | Low breadth of scope was evident, for example, not all stakeholders and not all outcomes were reported |
| Tools and methods of data collection reliable/credible | Low risk | Tools used for data collection were appropriate for the data |
| Tools and methods of data analysis reliable/credible | Low risk | How conclusions were reached is clearly explained |
| Performance bias/neutrality/credibility/conformability | Unclear risk | Absence of 'other' stakeholders who received the newsletter might reduce credibility; absence of any child outcomes certainly does reduce credibility |
| Reliability of findings and recommendations | Unclear risk | Intervention did not work |
| Transferability of findings | High risk | Information provided is not rich enough to support a similar study |
| Overall risk of bias of process evaluation | High risk | Intervention was unsuccessful |
Velsor‐Friedrich 2005.
| Methods |
Included as outcome evaluation Study design: clustered parallel‐group design with schools selected as the unit of randomisation Setting: 4 inner city elementary schools in the USA Period: dates on which study was conducted ‐ intervention and subsequent data collection ‐ are not clear. Outcomes were collected from children immediately after they participated in the school‐based asthma intervention programme (2 weeks) and at 5 and 12 months |
|
| Participants |
Eligible sample frame: 73 met inclusion criteria guidelines and were enrolled in the study Randomised: 73 pupils were randomised at the school level, although distribution between groups is unclear Completed (intervention): a total of 52 students were included in the final analysis: 28 students in the treatment group and 24 in the control group Inclusion criteria: children for whom a physician had diagnosed asthma, or who had demonstrated asthma‐related symptoms and frequent asthma‐related emergency department visits or hospital admissions Exclusion criteria: no additional exclusion criteria Baseline characteristics Age of children: mean age of children in the treatment group was 10.2 years; mean age of children in the control group was 9.9 years Ethnicity: all children were African American Socio‐economic status: unclear Gender: the sample of completers was evenly split in terms of sex: 26 females and 26 males Asthma status: children who received a diagnosis were 8 to 13 years old (mean age, 10 years of age) |
|
| Interventions |
Intervention: 2‐part intervention First part consisted of Open Airways for School (OAS) as described by study authors: "the purposes of the Open Airways Program are to: (a) empower children with asthma by teaching them how to prevent asthma episodes and emergencies; and (b) to help schools control asthma by creating partnerships in asthma care with school personnel, school nurses, physicians, and families. The program consists of six 45‐minute sessions offered once per week in which small groups of children learn new asthma management skills. The session topics include: (a) basic information about asthma; (b) how to recognize and respond to asthma symptoms; (c) using asthma medication and deciding when to seek help; (d) how to keep physically active; (e) identifying and controlling triggers to minimize asthma symptoms; and (f) handling problems related to asthma and school. The curriculum incorporates an interactive teaching approach utilizing group discussion, stories, games, and role‐play to promote children's active involvement in the learning process" Second part of the intervention consisted of nurse practitioner visits, which consisted of the following: "5 monthly visits with the NP at the school‐based health clinic. These follow‐up visits were initiated after the students completed the asthma educational program. During the visits, the nurse assessed the student's asthma health, including auscultation of breath sounds, assessment of current medication use and availability, and history of symptoms, visits to the emergency department, and hospitalizations. Students were asked to demonstrate skills such as medication administration and peak flow meter techniques. The nurse included age‐appropriate asthma education information (from the Open Airways curriculum) as deemed necessary to reinforce and/or increase asthma knowledge" Control: wait‐list control; the control group received the intervention after evaluation Intensity: 6 group‐based lessons plus individual nurse practitioner session Instructor: principal Investigator for the first part, and nurse practitioner for the second part Theoretical framework: Orem's Self‐Care Deficit Theory of Nursing served as the guiding framework for this study Parental engagement: not reported Child satisfaction: not reported Timing of intervention in school day: unclear |
|
| Outcomes |
Extractable outcomes were collected for: Emergency department visits Daytime and night‐time symptoms Lung function |
|
| Notes | Considered as a process evaluation, but study did not seek to address process evaluation/implementation research questions and did not include sufficient process data Funding source: National Institute of Nursing Research, Loyola University Research Award, Respironics Corporation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear ‐ not described in the study: the 4 schools were randomly assigned to treatment or comparison groups |
| Allocation concealment (selection bias) | High risk | Not described in the study but potentially high, given the low number of randomised schools and study authors' description of study design |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not addressed by study authors |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not addressed by study authors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low risk posed by attrition ‐ only 3 students dropped out |
| Selective reporting (reporting bias) | Unclear risk | Unclear ‐ some data have high levels of variance |
| Other bias | Unclear risk | Missingness ‐ unclear risk ‐ not addressed by study authors Baseline imbalance ‐ low risk ‐ not described by study authors as problematic Risk of contamination ‐ low ‐ schools (low number) were the unit of randomisation |
| Transparent and clearly stated aims | Unclear risk | N/A |
| Explicit theories underpinning and/or literature review | Unclear risk | N/A |
| Transparent and clearly stated methods and tools | Unclear risk | N/A |
| Selective reporting | Unclear risk | N/A |
| Harmful effects | Unclear risk | N/A |
| Population and sample described well | Unclear risk | N/A |
| Continuous evaluation | Unclear risk | N/A |
| Evaluation participation equity and sampling | Unclear risk | N/A |
| Design and methods overall approach | Unclear risk | N/A |
| Tools and methods of data collection reliable/credible | Unclear risk | N/A |
| Tools and methods of data analysis reliable/credible | Unclear risk | N/A |
| Performance bias/neutrality/credibility/conformability | Unclear risk | N/A |
| Reliability of findings and recommendations | Unclear risk | N/A |
| Transferability of findings | Unclear risk | N/A |
| Overall risk of bias of process evaluation | Unclear risk | N/A |
AAP: Asthma Action Plan.
ACT: Asthma Control Test.
ASMA: Asthma Self‐Management for Adolescents.
BME: black and minority ethnicity.
CNS: clinical nurse specialist.
CST: Corticosteroids.
ED: emergency department.
GP: general practitioner.
HLAI: Health Learners Asthma Initiative.
HPM: Health Promotion Model.
HRQoL: health‐related quality of life.
ICAN: I Can Control Asthma and Nutrition Now.
ISACC: International Study of Asthma and Allergies in Childhood.
N/A: not applicable.
NAEPP: National Asthma Education and Prevention Program.
NHLBI: National Heart, Lung, and Blood Institute.
OAS: Open Airways for Schools.
PBP: Power Breathing Program.
PD/H/PE: personal development/health/physical education.
PI: principal investigator.
PPF: pre‐test/post‐test/follow‐up.
PRECEDE: Predisposing, Reinforcing, and Enabling Causes in Educational Diagnosis and Evaluation.
RAP: Roaring Adventures of Puff.
RCT: randomised controlled trial.
SCDT: self‐care deficit theory.
SES: socio‐economic status.
SHARP: Staying Healthy ‐ Asthma Responsible & Prepared.
Triple A: Adolescent Asthma Action.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akasawa 2016 | Considered for process evaluation: not published in the English language |
| Al Aloola 2017 | Considered for process evaluation: core processes not available; educational programme for teachers ‐ child data not collected |
| Al‐Sheyab 2015 | Considered for outcome evaluation: excluded on comparison (tested effects of TAJ‐Plus vs TAJ) |
| Alreshidi 2015 | Considered for process evaluation: excluded as did not include implementation research questions. |
| Anderson 2004 | Considered for process evaluation: excluded as did not reflect a school setting ‐ school specifically designed for children with chronic disease |
| Ando 2016 | Considered for process evaluation: core processes not evaluated |
| Arnold 2012 | Considered for process evaluation: did not include implementation research questions nor in‐depth process or contextual information (did not meet the criteria for a process evaluation) |
| Arıkan‐Ayyıldız 2016 | Considered for process evaluation: not school based; clinical settings |
| Augustin 2003 | Considered for outcome evaluation: excluded on comparison: intervention group received weekly workshops for 6 weeks, control group was given standard educational materials on asthma management Considered for process evaluation: did not include implementation research questions (did not meet the criteria for a process evaluation) |
| Becker 2003 | Considered for outcome evaluation: not school based |
| Bignall 2015a | Considered for outcome evaluation: excluded, as comparison received an intervention (intervention group (20 minutes breathing retraining plus education) or control group (20 minutes standard education)) |
| Bollinger 2010 | Considered for process evaluation: included only information on cost‐effectiveness, not information on implementation |
| Bowen 2013 | Considered for outcome evaluation: not school based Considered for process evaluation: did not contain core components of a process evaluation; did not include implementation research questions nor in‐depth process or contextual information (did not meet the criteria for a process evaluation) |
| Brooten 2008 | Considered for process evaluation: did not include implementation research questions nor in‐depth process or contextual information (did not meet the criteria for a process evaluation) |
| Bruzzese 2001 | Considered for process evaluation: did not include implementation research questions nor in‐depth process or contextual information (did not meet the criteria for a process evaluation) |
| Bruzzese 2006 | Considered for outcome evaluation: excluded, as focussed on family‐level self‐management, rather than child‐level self‐management |
| Bruzzese 2011a | Considered for outcome evaluation: unclear whether asthmatic students (with diagnosed asthma) were included. Considered for process evaluation: in addition to the above, did not represent a study of implementation using recognised tools |
| Burgess 2017 | Considered for process evaluation: did not address process questions |
| Burkhart 2003 | Considered for outcome evaluation: large number of children under 5 were included (mean age, < 5) |
| Bush 2014 | Considered for outcome evaluation: not an intervention study (observational design) Considered for process evaluation: no information to suggest that evaluating processes of implementation formed a key part of the intervention |
| Butz 2005 | Considered for outcome evaluation: excluded on comparison: usual care not provided to comparison group Considered for process evaluation: did not include implementation research questions nor in‐depth process or contextual information (did not meet the criteria for a process evaluation) |
| Carpenter 2016b | Considered for process evaluation: not school‐based; school not instrumental for delivery |
| Cheung 2015 | Considered for process evaluation: excluded, as provided a detailed description of planned intervention but not of implementation |
| Chini 2011 | Considered for process evaluation: did not contain core components of a process evaluation |
| Christiansen 1997 | Considered for process evaluation: did not include implementation research questions nor in‐depth process or contextual information (did not meet the criteria for a process evaluation) |
| Clark 1986 | Considered for outcome evaluation: published before cutoff point |
| Clark 2003 | Considered for outcome evaluation: duplicate (on manual screening) |
| Coté 1997 | Considered for outcome evaluation: not school based |
| De Godoi 2016 | Considered for process evaluation: not solely about asthma |
| de Greef, 2017 | Considered for process evaluation: not an intervention study |
| DePue 2007 | Considered for process evaluation: limited process data were presented, although they were not deemed to be collected via recognised tools nor reported by standardised means |
| Eakin 2012 | Considered for outcome evaluation: large number of children under 5 years of age (mean age, < 5) |
| Evans 2001 | Considered as a process evaluation study and an outcome evaluation study |
| Fernandes 2006 | Considered for outcome evaluation: large number of participants outside the 5‐ to 18‐year‐old target age range |
| Francis 2001 | Considered for process evaluation: not deemed to have included the core components of a process evaluation via structured tools |
| Gardida 2002 | Considered for outcome evaluation: not published in the English language |
| Gerald 2016 | Considered for process evaluation: not an intervention study |
| Gibson 1998 | Considered for outcome evaluation: schools were not randomised, and inclusion of only 2 schools means that intervention and randomisation effects would conflate if schools were randomised Considered for process evaluation: did not contain the core components of a process evaluation |
| Grad 2009 | Considered for process evaluation: not deemed to have included the core components of a process evaluation via structured tools |
| Greenberg 2010 | Considered for process evaluation: focus of the study was long‐term impact on student health, not implementation. Focus group data were collected, although these data were not presented |
| Greer 2009 | Considered for process evaluation and outcome evaluation: focus on improving knowledge about asthma among children without asthma |
| Gregory 2000 | Considered for outcome evaluation: excluded on the basis of study design. Only 2 sites randomised ‐ 1 school in each arm. Any intervention effect was conflated with school effect Considered for process evaluation: was deemed to not address implementation research questions |
| Halterman 2004 | Considered for outcome evaluation: excluded ‐ deemed to not include a sufficient component of self‐management |
| Halterman 2011 | Considered for outcome evaluation: excluded – delivered in part at school and in part in the home – included a substantial home component; not possible to disentangle which part may be driving any change |
| Halterman 2011a | Considered for outcome evaluation: excluded, as comparison received asthma care Considered for process evaluation: stand‐alone process evaluation identified but focused on an allied part of the trial that was not school based |
| Halterman 2012 | Considered for outcome evaluation: excluded, as comparison received asthma care Considered for process evaluation: deemed to not include the core components of a process evaluation using structured tools |
| Hemate 2012 | Considered for process evaluation: did not contain the core components of a process evaluation |
| Hill 1991 | Considered for outcome evaluation: excluded, as the intervention did not foster self‐management skills |
| Horner 1998 | Considered for outcome evaluation: excluded ‐ study not designed as an RCT Considered for process evaluation: deemed to not include the core components of a process evaluation using structured tools |
| Horner 2003 | Considered for outcome evaluation: study design was non‐experimental |
| Hughes | Considered for process evaluation: although some satisfaction data were collected, the study did not include the core components of a process evaluation; process data were collected using structured tools |
| Johnson 2016 | Considered for process evaluation: not school based; clinical settings only |
| Jones 2005 | Considered for process evaluation: school site was not judged to be instrumental for delivery of the intervention; sites external to school were also used for intervention delivery |
| Joseph 2004 | Considered for outcome evaluation: not school based |
| Joseph 2007 | Considered for outcome evaluation (along with linked papers): excluded, as comparison included asthma education Note: included in process evaluation |
| Joseph 2013a | Considered for outcome evaluation (along with linked papers): excluded, as comparison included asthma education Note: included in process evaluation |
| Kaufman 2011 | Considered for process evaluation: study did not include the core components of a process evaluation; process data were collected using structured tools |
| Kenny 2016 | Considered for process evaluation: not school based |
| Khan 2014 | Considered for outcome evaluation: excluded, as not school based |
| Khoshnavay 2013 | Considered for process evaluation: received from study author; did not include core components of a process evaluation; process data were collected using structured tools |
| Kintner 2015 | Considered for outcome evaluation: excluded on comparison as the control group received alternative asthma education Considered for process evaluation: did not contain the core components of a process evaluation |
| Knight 2005 | Considered for process evaluation: study did not include the core components of a process evaluation; process data were collected using structured tools |
| Krishna 2006 | Considered for outcome evaluation: deemed as not school based |
| Lakupoch 2017 | Considered for process evaluation: not school based |
| Lewis 2005 | Considered for outcome evaluation: study as designed included no randomisation |
| Li 2017 | Considered for process evaluation: did not address process evaluation research questions |
| Liao 2006 | Considered for process evaluation: study did not include the core components of a process evaluation; process data were collected using structured tools; included home visit components |
| Lin 2017 | Considered for process evaluation: school setting not central for delivery |
| Lipman 2017 | Considered for process evaluation: did not address process evaluation research questions |
| Loman 2017 | Considered for process evaluation: did not address process evaluation research questions centrally |
| Louisias 2016 | Considered for process evaluation: did not address process questions |
| Lu 2017 | Considered for process evaluation: did not include core components of process evaluation |
| Lurie 2001 | Considered for process evaluation: study did not include the core components of a process evaluation; process data were collected using structured tools; some data on stakeholder perceptions were collected, but study did not address implementation research questions |
| Lwebuga‐Mukasa 2002 | Considered for process evaluation: study did not include the core components of a process evaluation |
| Maa 2010 | Considered for process evaluation: did not contain core components of a process evaluation |
| MacPherson 2011 | Considered for process evaluation: did not contain core components of a process evaluation |
| Mangan 2006 | Considered for process evaluation: did not contain core components of a process evaluation |
| Marabini 2002 | Considered for outcome evaluation: not focussed on children (mean age, approximately 50) |
| McClure 2008 | Considered for process evaluation: did not fall into the category of self‐management (supported management through observation) |
| McElmurry 1999 | Considered for process evaluation: study did not include the core components of a process evaluation; process data were collected using structured tools; included home visit components |
| McEwen 1998 | Considered for process evaluation: study did not include the core components of a process evaluation; process data were collected using structured tools; included home visit components |
| McLaughlin 2006 | Considered for process evaluation: study did not include the core components of a process evaluation; process data were collected using structured tools |
| Meng 2000 | Considered for process evaluation: study did not include the core components of a process evaluation; process data were collected using structured tools |
| Meurer 1999 | Considered for process evaluation: study did not include the core components of a process evaluation; process data were collected using structured tools |
| Millard 2003 | Considered for outcome and process evaluation: not focussed on self‐management; educational activities were aimed at parents; study did not contain the core components of a process evaluation |
| Mitchell 2017 | Considered for process evaluation: core processes not available |
| Morphew 2013 | Considered for process evaluation: presented an economic evaluation ‐ not a process evaluation |
| Morphew 2017 | Considered for process evaluation: core processes not available |
| Morton 2017 | Considered for process evaluation: not reliant on schools for delivery |
| Mosnaim 2017 | Considered for process evaluation: not an intervention study |
| NCT00217776 | Considered for outcome evaluation: not an intervention study (trial protocol) |
| Neuharth‐Pritchett 2016 | Considered for process evaluation: not focussed on children; focussed exclusively on training educators |
| Nuss 2016 | Considered for process evaluation: study did not include the core components of a process evaluation; process data were collected using structured tools |
| Otim 2015 | Considered for process evaluation: presented an economic evaluation ‐ not a process evaluation |
| Patel 2007 | Considered for process evaluation: presented an outcome and economic evaluation ‐ not a process evaluation |
| Peers 2017 | Considered for process evaluation: did not include core processes |
| Pender‐Phaneuf 2016 | Considered for process evaluation: study did not include the core components of a process evaluation; process data were collected using structured tools |
| Perry 2000 | Considered for outcome evaluation: study not considered to be an RCT |
| Petrie 2010 | Considered for process evaluation: study did not evaluate processes |
| Quaranta 2012 | Considered for process evaluation: study did not report on implementation processes |
| Quaranta 2015 | Considered for process evaluation: study did not involve an intervention |
| Rasberry 2014 | Considered for process evaluation: study did not report on implementation processes |
| Raun 2017 | Considered for process evaluation: correlational analysis |
| Rhee 2012 | Considered for process evaluation: presented an outcome and economic evaluation ‐ not a process evaluation |
| Richterová 2016 | Considered for process evaluation: not published in the English language |
| Rodriguez‐Martinez 2017 | Considered for process evaluation: focussed on an economic evaluation |
| Sabla 2017 | Considered for process evaluation: did not contain core components of a process evaluation ‐ focused on evaluating the validity of teaching materials |
| Salisbury 2002 | Considered for outcome evaluation: excluded, as comparison group received additional intervention beyond usual care Considered for process evaluation: study did not include the core components of a process evaluation; process data were collected using structured tools |
| Scherer 2016 | Considered for process evaluation: not focussed on self‐management among children |
| Schlueter 2011 | Considered for process evaluation: study implementation focussed on parental smoke reduction |
| Schneider 1997 | Considered for process evaluation: study did not include the core components of a process evaluation; process data were collected using structured tools; some processes and context were described but were not evaluated |
| Schuller 2015 | Considered for process evaluation: study did not include the core components of a process evaluation; process data were collected using structured tools; some processes and context were described but were not evaluated |
| Scott 2006 | Considered for process evaluation: did not allow for implementation processes to be evaluated; only 6 students were included, precluding assessment of core components of a school‐based asthma intervention |
| Scott 2008 | Considered for process evaluation: study did not include the core components of a process evaluation; process data were collected using structured tools; unclear if school was not instrumental in delivery of the intervention |
| Scott 2011 | Considered for process evaluation: study did not include the core components of a process evaluation; process data were collected using structured tools; unclear if school was not instrumental in delivery of the intervention |
| Shanovich 2009 | Considered for outcome evaluation: study was not judged to be an RCT |
| Sharek 2002 | Considered for outcome evaluation: study was not school based |
| Shaw 2005 | Considered for process evaluation: study did not include the core components of a process evaluation (reported that process evaluation was conducted but did not report the findings) |
| Shaw 2016 | Considered for process evaluation: not school based |
| Shegog 2001 | Considered for outcome evaluation: delivery of the intervention not contingent on schools (not school based) |
| Shelef 2016 | Considered for process evaluation: described development of study protocol, not implementation |
| Staudt 2015 | Considered for process evaluation: study did not include the core components of a process evaluation |
| Suwannakeeree 2016 | Considered for process evaluation: study did not include the core components of a process evaluation; included diaries for symptom monitoring alone |
| Szefler 2016 | Considered for process evaluation: not an intervention study |
| Szefler 2017 | Considered for process evaluation: core aspects of the process evaluation were not addressed |
| Tate 2009 | Considered for process evaluation: study did not seek to address process evaluation/implementation research questions and did not include process data |
| Terpstra 2012a | Considered for outcome evaluation: excluded on comparison, as trial tested added impact on additional engagement with caregivers in an established intervention Note: included as a process evaluation |
| Thornton 2016 | Considered for process evaluation: school not instrumental for delivery; main components delivered at home |
| Urrutia‐Pereira 2017 | Considered for process evaluation: core aspects of process evaluation not addressed |
| Valery 2007 | Considered for outcome evaluation: intervention not school based |
| Velsor‐Friedrich 2004 | Considered for outcome evaluation: no randomisation described (not an RCT) Considered for process evaluation: study did not seek to address process evaluation/implementation research questions and did not include process data |
| Velsor‐Friedrich 2012 | Considered for outcome evaluation: excluded on comparison (study compared alternative asthma interventions) Considered for process evaluation: study did not seek to address process evaluation/implementation research questions and did not include process data |
| Volerman 2017 | Considered for process evaluation: core aspects of process evaluation not addressed |
| Walter 2016 | Considered for process evaluation: review in progress; not an intervention study |
| Walton 2004 | Considered for process evaluation: study did not seek to address process evaluation/implementation research questions and did not include sufficient process data |
| Webber 2005 | Considered for process evaluation: study did not seek to address process evaluation/implementation research questions and did not include sufficient process data |
| Weng 2007 | Considered for outcome evaluation: study not deemed to be an RCT |
| Wensley 2004 | Considered for outcome evaluation: not a school‐based intervention |
| Whitman 1985 | Considered for outcome evaluation: published before cutoff date |
| Willeboordse 2016 | Considered for process evaluation: school not instrumental in delivery |
| Wilson 2008 | Considered for process evaluation: did not contain the core components expected in a process evaluation; focussed on implementation at a school district level rather than among students and within schools |
| Wyatt 2008 | Considered for process evaluation: study did not seek to address process evaluation/implementation research questions and did not include sufficient process data |
| Wyatt 2013 | Considered for process evaluation: study provided in‐depth description of the process of developing content but not implementation; study therefore did not seek to address process evaluation/implementation research questions |
| Yawn 2000 | Considered for outcome evaluation: not focussed on asthmatic children; study did not report on outcomes for asthmatic children separately from non‐asthmatic children Considered for process evaluation: study did not seek to address process evaluation/implementation research questions and did not include sufficient process data |
| Yoshida 2011 | Considered for process evaluation: study was not an intervention study |
| Young 2001 | Considered for process evaluation: some implementation notes included, but study did not seek to address process evaluation/implementation research questions using structured tools |
| Zografos 2007 | Considered for process evaluation: study did not seek to address process evaluation/implementation research questions using structured tools |
RCT: randomised controlled trial.
TAJ: XXX.
Characteristics of studies awaiting assessment [ordered by study ID]
Liptzin 2016a.
| Methods | Pre‐post study |
| Participants | Children with asthma across a wide age range |
| Interventions | Step‐Up Asthma Program, applying National Asthma Education and Prevention Program‐National Heart, Lung, and Blood Institute guidelines for evidence‐based programmes for children with asthma |
| Outcomes | Number of asthma action plans; access to medication; asthma knowledge; asthma exacerbations |
| Notes | Status as process evaluation to be classified |
McCallum 2017.
| Methods | Pre‐post study |
| Participants | Participants in high schools with large numbers of Indigenous Australian children; not all children were known to be asthmatic |
| Interventions | Peer‐led, school‐based educational programme called the Asthma and Smoking Prevention Project (ASPP); split focus between asthma and smoking prevention |
| Outcomes | Lung function and wheezing; smoking status |
| Notes | Status as process evaluation to be classified |
Praena‐Crespo 2017.
| Methods | Cluster randomised parallel‐group trial |
| Participants | Students engaging in physical activity lessons, including approximately 10% with asthma |
| Interventions | Asthma, Sport, and Health (ASAH) programme taught by physical activity teachers |
| Outcomes | Quality of life and asthma knowledge |
| Notes | Status as outcome evaluation to be classified; results for quality of life not expected to change conclusions |
Reznik 2016.
| Methods | Cluster randomised parallel‐group trial |
| Participants | Children with asthma in primary schools |
| Interventions | School‐wide asthma awareness event; facilitated collaboration with child's primary care provider, classroom‐based physical activity, and asthma education for families and school personnel |
| Outcomes | Symptom‐free days, medication adherence, and physical activity levels |
| Notes | Status as outcome evaluation to be classified |
Warren 2016.
| Methods | Pre‐post evaluation design |
| Participants | Children with asthma in high schools |
| Interventions | Student Asthma Research Team (START), engaged high school youth in a Photovoice investigation of factors impacting asthma |
| Outcomes | Asthma knowledge and lung function |
| Notes | Status as process evaluation to be classified |
ASAH: Asthma, Sport, and Health programme.
ASPP: Asthma and Smoking Prevention Project.
START: Student Asthma Research Team.
Characteristics of ongoing studies [ordered by study ID]
Halterman 2017.
| Trial name or title | Development of School‐Based Asthma Management Programs in Rochester, New York: Presented in Honor of Dr Robert Haggerty |
| Methods | Randomised trial |
| Participants | Children with asthma |
| Interventions | Telemedicine intervention |
| Outcomes | Symptom days |
| Starting date | Unclear |
| Contact information | Jill Halterman; jill_halterman@urmc.rochester.edu |
| Notes |
Lemanske 2016.
| Trial name or title | School‐Based Asthma Management Program (SAMPRO) |
| Methods | Study design unclear |
| Participants | Children with asthma and numerous stakeholders |
| Interventions | Multi‐component intervention |
| Outcomes | Unclear |
| Starting date | Unclear |
| Contact information | Robert F. Lemanske, Jr, MD; rfl@medicine.wisc.edu |
| Notes | Description of establishment published in cited reference |
NCT03032744.
| Trial name or title | Project IMPACT in Schools to Prevent Asthma Symptoms |
| Methods | Randomised trial |
| Participants | 60 students 6 to 16 years of age |
| Interventions | Project IMPACT is a school‐based health centre intervention programme that institutes guideline‐based long‐term asthma care and provides supervised administration with daily preventive asthma medications to improve asthma symptoms and lung function, reduce emergency visits, and decrease missed days of school among children from communities with health disparities |
| Outcomes | Asthma Symptoms; ACT score; Lung function; Missed days of school; Decrease in ED/urgent care visits; Hospitalisations; Oral/parenteral steroid use |
| Starting date | January 2017 |
| Contact information | Lucy C Holmes, MD; lholmes@upa.chob.edu |
| Notes |
Perry 2015.
| Trial name or title | Breath Connection |
| Methods | Clustered parallel‐group RCT |
| Participants | Children 7 to 14 years of age, with a median age of 9.6 years |
| Interventions | Study used the Breath Connection programme to provide asthma education via telemedicine to rural children with asthma, their caregivers, and school nurses |
| Outcomes | Lung function; Use of reliever therapies |
| Starting date | Unclear |
| Contact information | |
| Notes | This study is available as an abstract only and describes ongoing recruitment; study author was contacted for further information |
Phipatanakul 2017.
| Trial name or title | The School Inner‐City Asthma Intervention Study |
| Methods | Randomised, blinded, sham‐controlled intervention trial |
| Participants | Plan to enrol 300 students with asthma from multiple classrooms in 40 northeastern inner city elementary schools. |
| Interventions | School environmental intervention |
| Outcomes | Asthma symptoms |
| Starting date | Unclear |
| Contact information | Boston Children's Hospital |
| Notes |
ACT: XXX.
ED: emergency department.
RCT: randomised controlled trial.
SAMPRO: School‐Based Asthma Management Program.
Differences between protocol and review
Our original protocol specified that we would locate process evaluation studies, although our inclusion criteria were expanded to include studies that evaluated the process of implementing interventions, regardless of whether they were stand‐alone process evaluations. Although our original protocol did not specify that we would include only stand‐alone process evaluations, this clarification does represent a difference between the language of protocol and the studies included in the review.
We extracted all data on school absences, and they were eligible for analysis, regardless of collection method, including those collected through administrative records. This is a deviation from the published protocol, which specified that parent‐reported absences alone would be our outcome of interest.
We intended to conduct a sensitivity analysis of alternative estimated ICCs to studies for which these values are missing; however a suitable alternative ICC apart from the estimate used in analyses was not identified; thus we did not conduct additional sensitivity analyses.
Sensitivity analyses were planned on the basis of country (OECD country vs other), although we did not carry out these analyses due to lack of variation according to this characteristic.
We had originally specified daytime and night‐time symptoms as a single outcome, but we split this into two separate outcomes to maintain conceptual coherence. Similarly, we developed two models for corticosteroid usage in an attempt to ensure conceptual coherence.
Our protocol originally specified that thorough investigation of heterogeneity would take place only when heterogeneity as measured as I² exceeding 25%. This was the case for ED visits and school absences; no other outcome met this threshold. However, we also conducted subgroup analyses of withdrawals, despite low heterogeneity, because of the conceptual similarity of this outcome and the implementation success that was the focus of the QCA.
Our protocol reported that we would include all outcomes in the 'Summary of findings' table (except withdrawals). In practice, we did not include lung function because of insufficient evidence to develop a meta‐analysis. In addition, we did not include evidence of corticosteroid dosage because it is unclear whether the outcome reflected dosage as part of step‐up or step‐down therapy (see discussion).
Because our QCA involved examining dosage, attrition, and adherence as process outcomes, we deemed that these were not suitable for examining effects among studies of short duration. This meant we excluded six reports of interventions that involved one or two face‐to‐face sessions (Bignall 2015; Carpenter 2016; Jackson 2006; McCann 2006; Mickel 2016; Richmond 2011), which we did not explicitly describe in the protocol.
Contributions of authors
| Role | Author |
| Drafting the protocol | KH, DK, TL, JG, VM, JT |
| Developing a search strategy | KH, DK, TL, JG, VM, JT |
| Searching for trials | KH, DK |
| Obtaining copies of trials | KH, DK |
| Providing subject expertise | JG |
| Providing methodological expertise | JT, TL |
| Selecting which trials to include | KH, DK, JG, JT |
| Extracting data from trials | KH, DK |
| Entering data into RevMan | KH, DK |
| Carrying out the analysis | KH, DK |
| Interpreting the analysis | KH, DK, TL, JG, JT |
| Drafting the final review | KH, DK, TL, JG, JT |
| Updating the review | KH, DK |
Dylan Kneale and Katherine Harris are the joint lead authors of this review.
Sources of support
Internal sources
The review authors declare that no internal source of funding was received for this systematic review, UK.
External sources
-
NIHR CLAHRC North Thames, UK.
Katherine Harris is in receipt of funding from the NIHR CLAHRC North Thames for her PhD.
Declarations of interest
A grant from NIHR North Thames CLAHRC is supporting some of this work, although it is not contributing to any individual time spent on the review (James Thomas).
This paper presents independent research and was funded by the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care (NIHR CLAHRC) North Thames at Bart's Health NHS Trust.
The views expressed are those of the review author(s) and are not necessarily those of the NHS, the NIHR, or the Department of Health.
Edited (no change to conclusions)
References
References to studies included in this review
Al‐Sheyab 2012 {published data only}
- Al‐sheyab N, Gallagher R, Crisp J, Shah S. Peer‐led education for adolescents with asthma in Jordan: a cluster‐randomized controlled trial. Pediatrics 2012;129(1):e106‐12. [DOI] [PubMed] [Google Scholar]
- Al‐sheyab NA, Shah S, Gallagher R, Crisp J. Effectiveness of a peer‐led education program for adolescents with asthma in Jordanian schools. American Journal of Respiratory and Critical Care Medicine 2010;181:A2253. [Google Scholar]
Al‐Sheyab 2012a {published data only}
- Al‐Sheyab NA, Gallagher R, Roydhouse JK, Crisp J, Shah S. Feasibility of a peer‐led, school‐based asthma education programme for adolescents in Jordan. Eastern Mediterranean Health Journal 2012;18(5):468‐73. [DOI] [PubMed] [Google Scholar]
Atherly 2009 {published data only}
- Atherly A, Nurmagambetov T, Williams S, Griffith M. An economic evaluation of the school‐based “power breathing” asthma program. Journal of Asthma 2009;46(6):596‐9. [DOI] [PubMed] [Google Scholar]
Bartholomew 2006 {published data only}
- Bartholomew KL, Sockrider M, Abramson SL, Swank PR, Czyzewski DI, Tortolero SR, et al. Partners in school asthma management: evaluation of a self‐management program for children with asthma. Journal of School Health 2006;76(6):283‐90. [DOI] [PubMed] [Google Scholar]
Berg 2004 {published data only}
- Berg J, Tichacek MJJ, Theodorakis R. Evaluation of an educational program for adolescents with asthma. Journal of School Nursing 2004;20(1):29‐35. [DOI] [PubMed] [Google Scholar]
Bignall 2015 {published data only}
- Bignall WJR, Luberto CM, Cornette AF, Haj‐Hamed M, Cotton S. Breathing retraining for African‐American adolescents with asthma: a pilot study of a school‐based randomized controlled trial. Journal of Asthma 2015;52(9):889‐96. [DOI] [PubMed] [Google Scholar]
Brasler 2006 {published data only}
- Brasler M, Lewis M. Teens: taking control of asthma. Journal of School Health 2006;76(6):269‐72. [DOI] [PubMed] [Google Scholar]
Bruzzese 2004 {published data only}
- Bruzzese JM, Bonner S, Vincent EJ, Sheares BJ, Mellins RB, Levison MJ, et al. Asthma education: the adolescent experience. Special Issue: Educating and Counseling Children about Physical Health 2004;55(3):396‐406. [DOI] [PubMed] [Google Scholar]
Bruzzese 2008 {published data only}
- Bruzzese JM, Unikel L, Gallagher R, Evans D, Colland V. Feasibility and impact of a school‐based intervention for families of urban adolescents with asthma: results from a randomized pilot trial. Family Process 2008;47(1):95‐113. [DOI] [PubMed] [Google Scholar]
Bruzzese 2010 {published data only}
- Bruzzese JM, Stepney C, Gallagher R, Wang J, Petkova E, Evans D. Reducing morbidity and urgent healthcare utilization in urban pre‐adolescents with asthma: results of a randomized control trial of asthma: it's a family affair. American Journal of Respiratory and Critical Care Medicine 2010;181:A2250. [Google Scholar]
Bruzzese 2011 {published data only}
- Bruzzese JM, Sheares BJ, Vincent EJ, Du Y, Sadeghi H, Levison MJ, et al. Effects of a school‐based intervention for urban adolescents with asthma. A controlled trial. American Journal of Respiratory and Critical Care Medicine 2011;183(8):998‐1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carpenter 2016 {published data only}
- Carpenter DM, Alexander DS, Elio A, DeWalt D, Lee C, Sleath BL. Using tailored videos to teach inhaler technique to children with asthma: results from a school nurse‐led pilot study. Journal of Pediatric Nursing 2016;31(4):380‐9. [DOI] [PubMed] [Google Scholar]
Cicutto 2005 {published data only}
- Cicutto L, Murphy S, Coutts D, O'Rourke J, Lang G, Chapman C, et al. Breaking the access barrier: evaluating an asthma center's efforts to provide education to children with asthma in schools. Chest 2005;128(4):1928‐35. [DOI] [PubMed] [Google Scholar]
- Cicutto L, Murphy S, Coutts D, O'Rourke J, Lang G, Chapman C, et al. Evaluating an elementary school based asthma education program: effects on quality of life and self efficacy [abstract]. American Thoracic Society 99th International Conference; 2003 May 16‐21; Seattle. 2003.
Cicutto 2013 {published data only}
- Cicutto L, Conti E, Evans H, Lewis R. Creating asthma‐friendly schools: a public health approach. Journal of School Health 2006;76(6):255. [DOI] [PubMed] [Google Scholar]
- Cicutto L, To T, Murphy S. A randomized controlled trial of a public health nurse‐delivered asthma program to elementary schools. Journal of School Health 2013;83(12):876‐84. [DOI] [PubMed] [Google Scholar]
Clark 2004 {published data only}
- Clark NM, Brown R, Joseph CL, Anderson EW, Liu M, Valerio MA. Effects of a comprehensive school‐based asthma program on symptoms, parent management, grades, and absenteeism. Chest 2004;125(5):1674‐9. [DOI] [PubMed] [Google Scholar]
- Petteway RJ, Valerio MA, Patel MR. What about your friends? Exploring asthma‐related peer interactions. Journal of Asthma 2011;48(4):393‐9. [DOI] [PubMed] [Google Scholar]
Clark 2005 {published data only}
- Clark NM, Gong M, Kaciroti N, Yu J, Wu G, Zeng Z, et al. A trial of asthma self‐management in Beijing schools. Chronic Illness 2005;1(1):31‐8. [DOI] [PubMed] [Google Scholar]
- Clark NM, Gong MZ, Kaciroti N, Yu J, Zeng Z, Wu G, et al. Effect of self management education on school children with asthma in Beijing, China [abstract]. European Respiratory Society 13thAnnual Congress; 2003 Sep 28‐29; Vienna. 2003; Vol. 22, issue Suppl 45:410s.
Clark 2010 {published data only}
- Clark NM, Shah S, Dodge JA, Thomas LJ, Andridge RR, Little RJ, et al. An evaluation of asthma interventions for preteen students. Journal of School Health 2010;80(2):80‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crane 2014 {published data only}
- Crane LMM, O'Neal KSS, Honey BLL, Kirkpatrick A. Effectiveness of a modified open airways curriculum. Journal of Asthma 2014;52(5):1‐9. [DOI] [PubMed] [Google Scholar]
Dore‐Stites 2007 {published data only}
- Dore‐Stites DJ. Evaluation of a School‐based Program Targeting Pediatric Asthma Self‐Management Skills in an Urban Population [thesis]. Kalamazoo, Michigan: Western Michigan University, 2007. [Google Scholar]
Engelke 2013 {published data only}
- Engelke MK, Swanson M, Guttu M. Process and outcomes of school nurse case management for students with asthma. Journal of School Nursing 2013;30(3):196‐205. [DOI] [PubMed] [Google Scholar]
Gerald 2006 {published data only}
- Gerald LB, Wittich AR, Erwin S, Hains C, Hemstreet MP, Redden D, et al. Outcomes for a comprehensive school‐based asthma management program. Journal of School Health 2006;76(6):291‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gerald 2009 {published data only}
- Gerald LB, Gerald JK, Gibson L, Patel K, Zhang S, McClure LA. Changes in environmental tobacco smoke exposure and asthma morbidity among urban school children. Chest 2009;135(4):911‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerald LB, McClure LA, Harrington KF, Mangan JM, Gibson L, Atchison J, et al. Design of the supervised asthma therapy study: implementing an adherence intervention in urban elementary schools. Contemporary Clinical Trials 2008;29(2):304‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerald LB, McClure LA, Mangan JM, Harrington KF, Gibson L, Erwin S, et al. Increasing adherence to inhaled steroid therapy among schoolchildren: randomized, controlled trial of school‐based supervised asthma therapy. Pediatrics 2009;123(2):466‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henry 2004 {published data only}
- Henry RL, Gibson PG, Vimpani GV, Francis JL, Hazell J. Randomized controlled trial of a teacher‐led asthma education program. Pediatric Pulmonology 2004;38(6):434‐42. [DOI] [PubMed] [Google Scholar]
Horner 2008 {published data only}
- Horner SD, Brown A. Evaluating the effect of an asthma self‐management intervention for rural families. Journal of Asthma 2014;51(2):168‐77. [DOI] [PubMed] [Google Scholar]
- Horner SD, Fouladi RT. Improvement of rural children's asthma self‐management by lay health educators. Journal of School Health 2008;78(9):506‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner SD, Rew DL, Brown SA. Enhancing asthma management among rural Mexican American, white and African American school‐aged children and their parents [Abstract]. American Thoracic Society International Conference; 2007 May 18‐23; San Francisco. 2007.
Horner 2015 {published data only}
- Horner SD, Brown A, Brown SA, Rew DL. Enhancing asthma self‐management in rural school‐aged children: a randomized controlled trial. Journal of Rural Health 2015;32(3):260‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Howell 2005 {published data only}
- Howell KJ. "Quest for the Code": A Study of a Computer Based Education Program for Children With Asthma [dissertation]. Syracuse University (Thesis). Syracuse: Syracuse University Libraries, 2005. [Google Scholar]
Jackson 2006 {published data only}
- Jackson TL, Stensland SL, Todd TJ, Lullo A, Mazan J, Masood AM. Evaluation of a pediatric asthma awareness program. Journal of Asthma 2006;43(4):311‐7. [DOI] [PubMed] [Google Scholar]
Joseph 2010 {published data only}
- Joseph CLM, Baptist AP, Stringer S, Havstad S, Ownby DR, Johnson CC, et al. Identifying students with self‐report of asthma and respiratory symptoms in an urban high school setting. Journal of Urban Health 2007;84(1):60‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph CLM, Havstad SL, Johnson D, Saltzgaber J, Peterson EL, Resnicow K, et al. Factors associated with nonresponse to a computer‐tailored asthma management program for urban adolescents with asthma. Journal of Asthma 2010;47(6):667‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph CLM, Stringer S, Ownby DR, Peterson E, Hoerauf S, Gibson‐Scipio W, et al. Preliminary results of the Puff City program for urban teens with asthma. Journal of Allergy and Clinical Immunology 2005;115(2):S63. [Google Scholar]
- Joseph ChLM, Peterson E, Havstad S, Johnson CC, Hoerauf S, Stringer S, et al. A web‐based, tailored asthma management program for urban African‐American high school students. American Journal of Respiratory and Critical Care Medicine 2007;175(9):888‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joseph 2013 {published data only}
- Guglani L, Havstad SL, Johnson CC, Ownby DR, Joseph CLM. Effect of depressive symptoms on asthma intervention in urban teens. Annals of Allergy, Asthma and Immunology 2012;109(4):237‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglani L, Havstad SL, Ownby DR, Saltzgaber J, Johnson DA, Johnson Christine C, et al. Exploring the impact of elevated depressive symptoms on the ability of a tailored asthma intervention to improve medication adherence among urban adolescents with asthma. Allergy, Asthma and Clinical Immunology 2013;9(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglani LMD, Havstad SL. Usefulness of a home affluence scale administered to urban adolescents with asthma to estimate the family's socioeconomic status. Annals of Epidemiology 2015;30:1e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph CLM, Ownby DR, Havstad SL, Saltzgaber J, Considine S, Johnson D, et al. Evaluation of a web‐based asthma management intervention program for urban teenagers: reaching the hard to reach. Journal of Adolescent Health 2013;52(4):419‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph CLM, Saltzgaber J, Havstad SL, Johnson CC, Johnson D, Peterson EL, et al. Comparison of early‐, late‐, and non‐participants in a school‐based asthma management program for urban high school students. Trials 2011;12(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio MA, Peterson EL, Wittich AR, Joseph CL. Examining health literacy among urban African‐American adolescents with asthma. Journal of Asthma 2016;53(10):1041‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kintner 2009 {published data only}
- Kintner EK, Sikorskii A. Randomized clinical trial of a school‐based academic and counseling program for older school‐age students. Nursing Research 2009;58(5):321‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kintner 2012 {published data only}
- Kintner E, Cook G, Allen A, Meeder L, Bumpus J, Lewis K. Feasibility and benefits of a school‐based academic and counseling program for older school‐age students with asthma. Research in Nursing and Health 2012;35(5):507‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintner EK, Clary M, Cook GD. Curriculum development for the theory‐driven, research guided, school‐based Staying Healthy‐Asthma Responsible and Prepared Education and Counseling Program. Journal of Allergy and Clinical Immunology 2007;119(1):S285. [Google Scholar]
Kouba 2012 {published data only}
- Kouba J, Velsor‐Friedrich B, Militello L, Harrison PR, Becklenberg A, White B, et al. Efficacy of the I Can Control Asthma and Nutrition Now (ICAN) pilot program on health outcomes in high school students with asthma. Journal of School Nursing 2012;29(3):235‐47. [DOI] [PubMed] [Google Scholar]
Langenfeld 2010 {published data only}
- Langenfeld NA, Mast DK, Rasberry CN, Cheung K, Luna P, Buckley R, et al. Strategies for identifying students in need of school‐based asthma services: challenges and questions that emerged from a rapid evaluation of a school‐based asthma program. Journal of Asthma and Allergy Educators 2010;1(3):109‐16. [Google Scholar]
Lee 2011 {published data only}
- Lee E. The Effectiveness of a School‐Based Asthma Management Program on Children With Asthma [Thesis]. Minneapolis, Minnesota, USA: Walden University, 2011. [Google Scholar]
Levy 2006 {published data only}
- Levy M, Heffner B, Stewart T, Beeman G. The efficacy of asthma case management in an urban school district in reducing school absences and hospitalizations for asthma. Journal of School Health 2006;76(6):320‐4. [DOI] [PubMed] [Google Scholar]
Magzamen 2008 {published data only}
- Davis A, Brown AS, Edelstein J, Tager IB. Identification and education of adolescents with asthma in an urban school district: results from a large‐scale asthma intervention. Journal of Urban Health 2008;85(3):361‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magzamen S, Mortimer KM, Davis A, Tager IB. School‐based asthma surveillance: a comparison of student and parental report. Pediatric Allergy and Immunology 2005;16(8):669‐78. [DOI] [PubMed] [Google Scholar]
- Magzamen S, Patel B, Davis A, Edelstein J, Tager IB. Kickin' Asthma: school‐based asthma education in an urban community. Journal of School Health 2008;78(12):655‐65. [DOI] [PubMed] [Google Scholar]
- Patel SB, Hasenbush A, Davis A, Tager I, Magzamen S. Medication use patterns among urban youth participating in school‐based asthma education. Journal of Urban Health 2011;88(Suppl 1):73‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCann 2006 {published data only}
- McCann DC, McWhirter J, Coleman H, Calvert M, Warner JO. A controlled trial of a school‐based intervention to improve asthma management. European Respiratory Journal 2006;27(5):921‐8. [DOI] [PubMed] [Google Scholar]
- McWhirter J, McCann D, Coleman H, Calvert M, Warner J. Can schools promote the health of children with asthma?. Health Education Research 2008;23(6):917‐30. [DOI] [PubMed] [Google Scholar]
McGhan 2003 {published data only}
- McGhan S, Jhangri G, Wells H, Wong E, Boechler V, Befus D, et al. Results of a controlled study of a school based asthma education program [abstract]. American Journal of Respiratory and Critical Care Medicine 2000;161:A903. [Google Scholar]
- McGhan SL, Wells HM, Befus AD. The "Roaring Adventures of Puff": a childhood asthma education program. Journal of Pediatric Health Care 1998;12(4):191‐5. [DOI] [PubMed] [Google Scholar]
- McGhan SL, Wong E, Jhangri GS, Wells HM, Michaelchuk DR, Boechler VL, et al. Evaluation of an education program for elementary school children with asthma. Journal of Asthma 2003;40(5):523‐33. [DOI] [PubMed] [Google Scholar]
McGhan 2010 {published data only}
- Mandhane PJ, McGhan SL, Sharpe HM, Wong E, Hessel PA, Befus AD, et al. A child's asthma quality of life rating does not significantly influence management of their asthma. Pediatric Pulmonology 2010;45(2):141‐8. [DOI] [PubMed] [Google Scholar]
- McGhan SL, Wong E, Sharpe HM, Hessel PA, Mandhane P, Boechler VL, et al. A children's asthma education program: Roaring Adventures of Puff (RAP) improves quality of life. Canadian Respiratory Journal 2010;17(2):67‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mickel 2016 {published data only}
- Mickel CF, Shanovich KK, Evans MD, Jackson DJ. Evaluation of a school‐based asthma education protocol: Iggy and the inhalers. Journal of School Nursing 2016;1:1‐9. [DOI] [PubMed] [Google Scholar]
- Mickel CF, Shanovich KK, Evans MD, Jackson DJ. Evaluation of a school‐based asthma education protocol: Iggy and the inhalers. Journal of School Nursing 2017;33(3):189‐97. [DOI] [PubMed] [Google Scholar]
Monforte 2012 {published data only}
- Monforte SE, Gleason M, Covar R, Cicutto L, Szelfer SJ. Reducing health disparities for asthma with a school based asthma education program [Abstract]. Journal of Allergy and Clinical Immunology 2012;129:AB41 [156]. [Google Scholar]
Mosnaim 2011 {published data only}
- Mosnaim GS, Li H, Damitz M, Sharp LK, Li Z, Talati A, et al. Evaluation of the Fight Asthma Now (FAN) program to improve asthma knowledge in urban youth and teenagers. Annals of Allergy, Asthma and Immunology 2011;107(4):310‐6. [DOI] [PubMed] [Google Scholar]
Mujuru 2011 {published data only}
- Mujuru P, Salana H, Kellam N, Howell C. Challenges to childhood asthma intervention delivery in hard‐to‐reach small rural communities: a school‐based approach. Journal of Asthma and Allergy Educators 2011;2(5):225‐32. [Google Scholar]
Patterson 2005 {published data only}
- Patterson EE, Brennan MP, Linskey KM, Webb DC, Shields MD, Patterson CC, et al. A cluster randomised intervention trial of asthma clubs to improve quality of life in primary school children: the School Care and Asthma Management Project (SCAMP). Archives of Disease in Childhood 2005;90(8):786‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields MD, Patterson EE, Brennan MP, Linskey K, Webb D, Patterson CC, et al. A cluster randomised intervention trial of asthma clubs to improve quality of life in primary school children ‐ the school care and asthma management project (SCAMP) [Abstract]. Thorax 2004;59(Suppl II):ii21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Persaud 1996 {published data only}
- Persaud DI, Barnett SE, Weller SC, Baldwin CD, Niebuhr V, McCormick DP. An asthma self‐management program for children, including instruction in peak flow monitoring by school nurses. Journal of Asthma 1996;33(1):37‐43. [DOI] [PubMed] [Google Scholar]
Pike 2011 {published data only}
- Pike EV, Richmond CM, Hobson A, Kleiss J, Wottowa J, Sterling DA. Development and evaluation of an integrated asthma awareness curriculum for the elementary school classroom. Journal of Urban Health 2011;88(Suppl 1):61‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Praena‐Crespo 2010 {published data only}
- Praena‐Crespo M, Fernandez‐Truan J, Gálvez‐González J, Murillo‐Fuentes A, Castro‐Gómez L, Cenizo‐Benjumea J. Randomised controlled trial of educational intervention directed by physical education teachers in high schools. European Journal of Allergy and Clinical Immunology 2010;65:190‐1. [Google Scholar]
Pulcini 2007 {published data only}
- Pulcini J, DeSisto MC, McIntyre CL. An intervention to increase the use of Asthma Action Plans in schools: a MASNRN study. Journal of School Nursing 2007;23(3):170‐6. [DOI] [PubMed] [Google Scholar]
Richmond 2011 {published data only}
- Richmond CM, Hobson A, Pike E, Kleiss J, Wottowa J, Sterling DA. Breathe Your Best for School Success: evaluation of an initiative to enhance asthma action plans in the school setting. Journal of Urban Health 2011;88(Suppl 1):68‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2001 {published data only}
- Shah S, Peat JK, Mazurski EJ, Wang H, Sindhusake D, Bruce C, et al. Effect of peer led programme for asthma education in adolescents: cluster randomised controlled trial. BMJ 2001;322(7286):583‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spencer 2000 {published data only}
- Spencer GA, Atav S, Johnston Y, Harrigan JF. Managing childhood asthma: the effectiveness of the Open Airways for Schools program. Family and Community Health 2000;23(2):20‐30. [Google Scholar]
Splett 2006 {published data only}
- Erickson CD, Splett PL, Mullett SS, Jensen C, Belseth SB. The healthy learner model for student chronic condition management ‐ part II: the asthma initiative. Journal of School Nursing 2006;22(6):319‐29. [DOI] [PubMed] [Google Scholar]
- Splett PL, Erickson CD, Belseth SB, Jensen C. Evaluation and sustainability of the Healthy Learners Asthma Initiative. Journal of School Health 2006;76(6):276‐82. [DOI] [PubMed] [Google Scholar]
Srof 2012 {published data only}
- Srof BJ, Velsor‐Friedrich B, Penckofer S. The effects of coping skills training among teens with asthma. Western Journal of Nursing Research 2012;34(8):1043‐61. [DOI] [PubMed] [Google Scholar]
Terpstra 2012 {published data only}
- Terpstra JL, Chavez LJ, Ayala GX. An intervention to increase caregiver support for asthma management in middle school‐aged youth. Journal of Asthma 2012;49(3):267‐74. [DOI] [PubMed] [Google Scholar]
Velsor‐Friedrich 2005 {published data only}
- Velsor‐Friedrich B, Pigott T, Srof B. A practitioner‐based asthma intervention program with African American inner‐city school children. Journal of Pediatric Health Care 2005;19(3):163‐71. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Akasawa 2016 {published data only}
- Akasawa A. Asthma management in school. Japanese Journal of Allergology 2016;65(7):901‐6. [DOI] [PubMed] [Google Scholar]
Al Aloola 2017 {published data only}
- Al Aloola NA, Saba M, Nissen L, Alewairdhi HA, Alaloola A, et al. Development and evaluation of a school‐based asthma educational program. Journal of Asthma 2017;54(4):419‐29. [DOI] [PubMed] [Google Scholar]
Alreshidi 2015 {published data only}
- Alreshidi N. The impact of a school‐based asthma health education programme on quality of life, knowledge and attitudes of Saudi children with asthma. Clinical and Experimental Allergy 2015;45(2):514. [Google Scholar]
Al‐Sheyab 2015 {published data only}
- Al‐Sheyab N, Alomari M, Shah S, Gallagher R. 'Class smoke‐free' pledge impacts on nicotine dependence in male adolescents: a cluster randomized controlled trial. Tropical Medicine and International Health 2015;20:255‐6. [Google Scholar]
Anderson 2004 {published data only}
- Anderson ME, Freas MR, Wallace AS, Kempe A, Gelfand EW, Liu AH. Successful school‐based intervention for inner‐city children with persistent asthma. Journal of Asthma 2004;41(4):445‐53. [DOI] [PubMed] [Google Scholar]
Ando 2016 {published data only}
- Ando T, Yamamoto‐Hanada K, Nagao M, Fujisawa T, Ohya Y. Combined program with computer‐based learning and peer education in early adolescents with asthma: a pilot study. Journal of Allergy and Clinical Immunology 2016;137(2):AB157. [Google Scholar]
Arnold 2012 {published data only}
- Arnold RJ, Stingone JA, Claudio L. Computer‐assisted school‐based asthma management: a pilot study. JMIR Research Protocols 2012;1(2):e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arıkan‐Ayyıldız 2016 {published data only}
- Arıkan‐Ayyıldız Z, Işık S, Çağlayan‐Sözmen Ş, Ana Ö, Karaman Ö, Uzuner N. Efficacy of asthma education program on asthma control in children with uncontrolled asthma. Turkish Journal of Pediatrics 2016;58(4):383‐8. [DOI] [PubMed] [Google Scholar]
Augustin 2003 {published data only}
- Augustin J. An Intensive Asthma Intervention in a School‐Based Clinic [Dissertation]. Chicago, IL: Rush University, 2003:14p. [Google Scholar]
Becker 2003 {published data only}
- Becker AB, Whitters D, Gillespie CA, Filuk SE, McColm JE, Thomas NJ, et al. Impact of a randomized asthma education program on asthma control in children [Abstract]. Journal of Asthma and Clinical Immunology 2003;111:S212. [Google Scholar]
Bignall 2015a {published data only}
- Bignall WJ, Luberto CM, Cornette AF, Haj‐Hamed M, Cotton S. Breathing retraining for African‐American adolescents with asthma: a pilot study of a school‐based randomized controlled trial. Journal of Asthma 2015;52(9):889‐96. [DOI] [PubMed] [Google Scholar]
Bollinger 2010 {published data only}
- Bollinger ME, Morphew T, Mullins CD. The Breathmobile program: a good investment for underserved children with asthma. Annals of Allergy, Asthma and Immunology 2010;105(4):274‐81.e1. [DOI] [PubMed] [Google Scholar]
Bowen 2013 {published data only}
- Bowen F. Asthma education and health outcomes of children aged 8 to 12 years. Clinical Nursing Research 2013;22(2):172‐85. [DOI] [PubMed] [Google Scholar]
Brooten 2008 {published data only}
- Brooten D, Youngblut JM, Royal S, Cohn S, Lobar SL, Hernandez L. Outcomes of an asthma program: Healthy Children, Healthy Homes. Pediatric Nursing 2008;34(6):448‐55. [PubMed] [Google Scholar]
Bruzzese 2001 {published data only}
- Bruzzese JM, Markman LB, Appel D, Webber M. An evaluation of Open Airways for Schools: using college students as instructors. Journal of Asthma 2001;38(4):337‐42. [DOI] [PubMed] [Google Scholar]
Bruzzese 2006 {published data only}
- Bruzzese JM, Evans D, Wiesemann S, Pinkett‐Heller M, Levison MJ, Du Y, et al. Using school staff to establish a preventive network of care to improve elementary school students' control of asthma. Journal of School Health 2006;76(6):307‐12. [DOI] [PubMed] [Google Scholar]
Bruzzese 2011a {published data only}
- Bruzzese JM, Cespedes A, Sheares BJ, Kingston S, Evans D, Sheares BJ. Feasibility and preliminary outcomes of a school‐based approach to helping urban ethnic minority adolescents with undiagnosed asthma. Patient Education and Counseling 2011;85(2):290‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burgess 2017 {published data only}
- Burgess K, Smith B. Efficacy of telemedicine in delivering pediatric asthma education in a rural West Alabama elementary school. Journal of Investigative Medicine 2017;65(2):412‐3. [Google Scholar]
Burkhart 2003 {published data only}
- Burkhart PV, Ward HJ. Children's self‐reports of characteristics of their asthma episodes. Journal of Asthma 2003;40(8):909‐16. [DOI] [PubMed] [Google Scholar]
Bush 2014 {published data only}
- Bush JS, Waller JL, Ownby DR, Tingen MS. Do parents influence health literacy and impact asthma self‐management in rural Georgia high school students?. Annals of Allergy, Asthma and Immunology 2014;113(5 Suppl 1):A20. [Google Scholar]
Butz 2005 {published data only}
- Butz A, Pham L, Lewis L, Lewis C, Hill K, Walker J, et al. Rural children with asthma: impact of a parent and child asthma education program. Journal of Asthma 2005;42(10):813‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J, Winkelstein M, Land C, Lewis‐Boyer L, Quartey R, Pham L, et al. Factors that influence quality of life in rural children with asthma and their parents. Journal of Pediatric Health Care 2008;22(6):343‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carpenter 2016b {published data only}
- Carpenter DM, Geryk LL, Sage A, Arrindell C, Sleath BL. Exploring the theoretical pathways through which asthma app features can promote adolescent self‐management. Translational Behavioral Medicine 2016;6(4):509‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheung 2015 {published data only}
- Cheung K, Rasberry CN, Dunville RL, Buckley R, Cook D, Daniels B, et al. A multicomponent school‐based asthma management program: enhancing connections to clinical care. Journal of School Health 2015;85(2):135‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chini 2011 {published data only}
- Chini L, Iannini R, Chianca M, Corrente S, Graziani S, Rocca M, et al. Happy Air®, a successful school‐based asthma educational and interventional program for primary school children. Journal of Asthma 2011;48(4):419‐26. [DOI] [PubMed] [Google Scholar]
Christiansen 1997 {published data only}
- Christiansen SC, Martin SB, Schleicher NC, Koziol JA, Mathews KP, Zuraw BL. Evaluation of a school‐based asthma education program for inner‐city children. Journal of Allergy and Clinical Immunology 1997;100(5):613‐7. [DOI] [PubMed] [Google Scholar]
Clark 1986 {published data only}
- Clark NM, Feldman CH, Evans D, Duzey O, Levison MJ, Wasilewski Y, et al. Managing better: children, parents, and asthma. Patient Education and Counseling 1986;8(1):27‐38. [DOI] [PubMed] [Google Scholar]
Clark 2003 {published data only}
- Clark NM. School‐based approaches to help pre‐teens manage asthma. CRISP (Computer Retrieval of Information on Scientific Projects) 2003;31/01/2008 End date:1‐2. [Google Scholar]
Coté 1997 {published data only}
- Coté J, Cartier A, Robichaud P, Boutin H, Malo JL, Rouleau M, et al. Influence on asthma morbidity of asthma education programs based on self‐management plans following treatment optimization. American Journal of Respiratory and Critical Care Medicine 1997;155(5):1509‐14. [DOI] [PubMed] [Google Scholar]
De Godoi 2016 {published data only}
- Molino CG, Romano‐Lieber NS, Ribeiro E, Melo DO. Non‐communicable disease clinical practice guidelines in Brazil: a systematic assessment of methodological quality and transparency. PLoS ONE 2016;11(11):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Greef, 2017 {published data only}
- de GreefM, Pijnenburg HM, Hattum MJ, McLeod BD, et al. Parent‐professional alliance and outcomes of child, parent, and family treatment: a systematic review. Journal of Child and Family Studies 2017;26(4):961‐76. [Google Scholar]
DePue 2007 {published data only}
- DePue JD, McQuaid EL, Koinis‐Mitchell D, Camillo C, Alario A, Klein RB. Providence school asthma partnership: school‐based asthma program for inner‐city families. Journal of Asthma 2007;44(6):449‐53. [DOI] [PubMed] [Google Scholar]
Eakin 2012 {published data only}
- Eakin MN, Rand CS, Bilderback A, Bollinger ME, Butz A, Kandasamy V, et al. Asthma in Head Start children: effects of the Breathmobile program and family communication on asthma outcomes. Journal of Allergy and Clinical Immunology 2012;129(3):664‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 2001 {published data only}
- Evans D, Clark NM, Levison MJ, Levin B, Mellins RB. Can children teach their parents about asthma?. Health Education and Behavior 2001;28(4):500‐11. [DOI] [PubMed] [Google Scholar]
Fernandes 2006 {published data only}
- Fernandes L, Fonseca JA, Costa‐pereira A, Delgado L, Martins S, Moreira A, et al. Effect on quality of life of multidisciplinary psycho‐educational group interventions: a randomized controlled trial [Abstract]. Journal of Allergy and Clinical Immunology 2006;117:S139. [Google Scholar]
Francis 2001 {published data only}
- Francis C. Setting up a school‐based clinic to improve adolescent asthma. Community Nurse 2001;7(6):19. [Google Scholar]
Gardida 2002 {published data only}
- Gardida A, Rojas M, Tavera C, Catalan M. Evaluation of an educational program to control asthma in school age children in the Morelos state, Mexico. [Spanish]. Revista del Instituto Nacional de Enfermedades Respiratorias 2002;15(1):27‐30. [Google Scholar]
Gerald 2016 {published data only}
- Gerald JK, Gerald LB. The unfulfilled promise of school‐centered asthma care. Journal of Allergy and Clinical Immunology 2016;4(5):980‐1. [DOI] [PubMed] [Google Scholar]
Gibson 1998 {published data only}
- Gibson PG, Shah S, Mamoon HA. Peer‐led asthma education for adolescents: impact evaluation. Journal of Adolescent Health 1998;22(1):66‐72. [DOI] [PubMed] [Google Scholar]
Grad 2009 {published data only}
- Grad R, McClure L, Zhang S, Mangan J, Gibson L, Gerald L. Peak flow measurements in children with asthma: what happens at school?. Journal of Asthma 2009;46(6):535‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenberg 2010 {published data only}
- Greenberg C, Luna P, Simmons G, Huhman M, Merkle S, Robin L, et al. Follow‐up of an elementary school intervention for asthma management: do gains last into middle school?. Journal of Asthma 2010;47(5):587‐93. [DOI] [PubMed] [Google Scholar]
Greer 2009 {published data only}
- Greer M, Lin L, Atkinson RK. Using a computer game to teach school‐aged children about asthma. Interactive Learning Environments 2017;25(4):431‐8. [Google Scholar]
- Greer MZ. Effect of a CD‐ROM Game to Teach School‐Age Children About Asthma [doctoral thesis]. Tempe, AZ: Arizona State University, 2009. [Google Scholar]
Gregory 2000 {published data only}
- Gregory EK. Empowering students on medication for asthma to be active participants in their care: an exploratory study. Journal of School Nursing 2000;16(1):20‐7. [DOI] [PubMed] [Google Scholar]
Halterman 2004 {published data only}
- Halterman JS, Szilagyi PG, Yoos HL, Conn KM, Kaczorowski JM, Holzhauer RJ, et al. Benefits of a school‐based asthma treatment program in the absence of secondhand smoke exposure: results of a randomized clinical trial. Archives of Pediatrics and Adolescent Medicine 2004;158(5):460‐7. [DOI] [PubMed] [Google Scholar]
Halterman 2011 {published data only}
- Halterman JS, Riekert K, Bayer A, Fagnano M, Tremblay P, Blaakman S, et al. A pilot study to enhance preventive asthma care among urban adolescents with asthma. Journal of Asthma 2011;48(5):523‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Halterman 2011a {published data only}
- Blaakman S, Tremblay PJ, Halterman JS, Fagnano M, Borrelli B. Implementation of a community‐based secondhand smoke reduction intervention for caregivers of urban children with asthma: process evaluation, successes and challenges. Health Education Research 2012;28(1):141‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halterman JS, Szilagyi PG, Fisher SG, Fagnano M, Tremblay P, Conn KM, et al. Randomized controlled trial to improve care for urban children with asthma: results of the school‐based asthma therapy trial. Archives of Pediatrics and Adolescent Medicine 2011;165(3):262‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes K, Bajorska A, Fisher S, Sauer J, Fagnano M, Halterman JS. Cost‐effectiveness of the School‐Based Asthma Therapy (SBAT) program. Pediatrics 2013;131(3):e709‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Halterman 2012 {published data only}
- Halterman JS, Fagnano M, Montes G, Fisher S, Tremblay P, Tajon R, et al. The school‐based preventive asthma care trial: results of a pilot study. Journal of Pediatrics 2012;161(6):1109‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halterman JSS, Sauer J, Fagnano M, Montes G, Fisher S, Tremblay P, et al. Working toward a sustainable system of asthma care: development of the School‐Based Preventive Asthma Care Technology (SB‐PACT) trial. Journal of Asthma 2012;49(4):395‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hemate 2012 {published data only}
- Hemate Z, Ghazavi Z, Hasanpor M, Iranpor R, Alidosti M. An examination of the effect of health promotion plan in high school students' on knowledge and performance on peers suffering from asthma in high schools of district 3 in Esfahan, 2010. Journal of Education and Health Promotion 2012;1:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hill 1991 {published data only}
- Hill R, Williams J, Britton J, Tattersfield A. Can morbidity associated with untreated asthma in primary school children be reduced? A controlled intervention study. BMJ 1991;303(6811):1169‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Horner 1998 {published data only}
- Horner SD. Using the Open Airways curriculum to improve self‐care for third grade children with asthma. Journal of School Health 1998;68(8):329‐33. [DOI] [PubMed] [Google Scholar]
Horner 2003 {published data only}
- Horner SD. Enhancing children's and parents' asthma management. CRISP (Computer Retrieval of Information on Scientific Projects) 2003;30/04/2007 end date:1. [Google Scholar]
Hughes {published data only}
- Hughes M, Murphy M. Evaluation of a pilot national online asthma E‐learning program for secondary school students*. Issues in Comprehensive Pediatric Nursing 2013;37(2):136‐46. [DOI] [PubMed] [Google Scholar]
Johnson 2016 {published data only}
- Johnson KB, Patterson BL, Ho YX, Chen QX, Nian H, Davison CL, et al. The feasibility of text reminders to improve medication adherence in adolescents with asthma. Journal of the American Medical Informatics Association 2016;23(3):449‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2005 {published data only}
- Jones CA, Clement LT, Hanley‐Lopez J, Morphew T, Kwong KYC, Lifson F, et al. The Breathmobile(trademark) Program: structure, implementation, and evolution of a large‐scale, urban, pediatric asthma disease management program. Disease Management 2005;8(4):205‐22. [DOI] [PubMed] [Google Scholar]
- Jones CA, Clement LT, Morphew T, Kwong KY, Hanley‐Lopez J, Lifson F, et al. Achieving and maintaining asthma control in an urban pediatric disease management program: the Breathmobile Program. Journal of Allergy and Clinical Immunology 2007;119(6):1445‐53. [DOI] [PubMed] [Google Scholar]
Joseph 2004 {published data only}
- Joseph V. A study compliance to two alternative drug regimens and the effect of health education on drug compliance in school age children with bronchial asthma. Nursing Journal of India 2004;95(7):153‐4. [PubMed] [Google Scholar]
Joseph 2007 {published data only}
- Joseph CL, Peterson E, Havstad S, Johnson CC, Hoerauf S, Stringer S, et al. A web‐based, tailored asthma management program for urban African‐American high school students. American Journal of Respiratory and Critical Care Medicine 2007;175(9):888‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joseph 2013a {published data only}
- Joseph CL, Ownby DR, Havstad SL, Saltzgaber J, Considine S, Johnson D, et al. Evaluation of a web‐based asthma management intervention program for urban teenagers: reaching the hard to reach. Journal of Adolescent Health 2013;52(4):419‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaufman 2011 {published data only}
- Kaufman D, Sauve L, Renaud L. Enhancing learning through an online secondary school educational game. Journal of Educational Computing Research 2011;44(4):409‐28. [Google Scholar]
- Kaufman D, Sauve L, Renaud L. The impact of an online educational game on knowledge and attitudes. Proceedings of the 6th International Conference on E‐Learning 2011;44:173‐82. [Google Scholar]
Kenny 2016 {published data only}
- Kenny P, Dunne M. Asthma Friendly School Award ‐ a new way of engaging schools, students and families in asthma management education. Irish Journal of Medical Science 2016;185:S483. [Google Scholar]
Khan 2014 {published data only}
- Khan R, Maharaj R, Seerattan N, Babwah F. Effectiveness of personalized written asthma action plans in the management of children with partly controlled asthma in Trinidad: a randomized controlled trial. Journal of Tropical Pediatrics 2014;60(1):17‐26. [DOI] [PubMed] [Google Scholar]
Khoshnavay 2013 {published data only}
- Khoshnavay F, Kharazmi RA. The effectiveness of rational emotive behavior therapy (REBT) on the quality of life in asthmatic children. Iranian Journal of Allergy, Asthma and Immunology 2013;12(1):S130. [Google Scholar]
Kintner 2015 {published data only}
- Kintner E, Cook G, Marti CN, Stoddard D, Gomes M, Harmon P, et al. Comparative effectiveness on cognitive asthma outcomes of the SHARP Academic Asthma Health Education and Counseling Program and a non‐academic program. Research in Nursing and Health 2015;38(6):423‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintner EK, Cook G, Marti CN, Allen A, Stoddard D, Harmon P, et al. Effectiveness of a school‐ and community‐based academic asthma health education program on use of effective asthma self‐care behaviors in older school‐age students. Journal for Specialists in Pediatric Nursing 2015;20(1):62‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knight 2005 {published data only}
- Knight DD. Expanding Asthma Awareness in Adolescents: A Pilot Investigation [dissertation]. Honolulu, HI: University of Hawaii at Manoa, 2005. [Google Scholar]
Krishna 2006 {published data only}
- Krishna S, Balas EA, Francisco BD, Konig P. Effective and sustainable multimedia education for children with asthma: a randomized controlled trial. Children's Health Care 2006;35(1):75‐90. [Google Scholar]
Lakupoch 2017 {published data only}
- Lakupoch K, Manuyakorn W, Preutthipan A, Kamalaporn H. The effectiveness of newly developed written asthma action plan in improvement of asthma outcome in children. Asian Pacific Journal of Allergy and Immunology 2017;1:1‐5. [DOI] [PubMed] [Google Scholar]
Lewis 2005 {published data only}
- Lewis CJ, Thompson RE, Butz AM, Hill KL, Huss K, Lewis‐Bowyer LL, et al. Asthma education increases knowledge of rural parents and children with asthma and affects parents' reports of their child's asthma symptoms [Abstract]. Journal of Allergy and Clinical Immunology 2005;115:S132. [Google Scholar]
Li 2017 {published data only}
- Li Z, Leite WL, Thompson LA, Gross HE, Shenkman EA, Reeve BB, et al. Determinants of longitudinal health‐related quality‐of‐life change in children with asthma from low‐income families: a report from the PROMIS® Pediatric Asthma Study. Clinical and Experimental Allergy 2017;47(3):383‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liao 2006 {published data only}
- Liao O, Morphew T, Amaro S, Galant SP. The Breathmobile: a novel comprehensive school‐based mobile asthma care clinic for urban underprivileged children. Journal of School Health 2006;76(6):313‐9. [DOI] [PubMed] [Google Scholar]
Lin 2017 {published data only}
- Lin CH, Zedeck SS, Garcia Lloret MI. UCLA (Long Beach) Breathmobile: improving asthma outcomes in low‐income pediatric patients. Journal of Allergy and Clinical Immunology 2017;139(2):AB99. [Google Scholar]
Lipman 2017 {published data only}
- Lipman TH. Using tailored videos to teach inhaler technique to children with asthma: results from a school nurse‐led pilot study. MCN. The American Journal of Maternal Child Nursing 2017;42(3):185. [DOI] [PubMed] [Google Scholar]
Loman 2017 {published data only}
- Loman DG, Kwong CG, Henry LD, Mahl C, Meadows L, Ellis AG. Asthma control and obesity in urban African American children. Journal of Asthma 2017;54(6):578‐83. [DOI] [PubMed] [Google Scholar]
Louisias 2016 {published data only}
- Louisias M, Goldmann D, Phipatanakul W. School nurses' perspectives on barriers to implementing school‐based asthma management plans. Journal of Allergy and Clinical Immunology 2016;137(2):AB100. [Google Scholar]
Lu 2017 {published data only}
- Lu KD, Cooper D, Haddad F, Lakes KD, Radom‐Aizik S. Four months of a school based exercise intervention improved fitness in normal weight and overweight/obese children with asthma in a minority, low SES population ‐ a pilot study. American Journal of Respiratory and Critical Care Medicine 2017;195:1. [Google Scholar]
Lurie 2001 {published data only}
- Lurie N, Bauer EJ, Brady C. Asthma outcomes at an inner‐city school‐based health center. Journal of School Health 2001;71(1):9‐16. [DOI] [PubMed] [Google Scholar]
Lwebuga‐Mukasa 2002 {published data only}
- Lwebuga‐Mukasa J, Dunn‐Georgiou E. A school‐based asthma intervention program in the Buffalo, New York, schools. Journal of School Health 2002;72(1):27‐32. [DOI] [PubMed] [Google Scholar]
Maa 2010 {published data only}
- Maa SH, Chang YC, Chou CL, Ho SC, Sheng TF, MacDonald K, et al. Evaluation of the feasibility of a school‐based asthma management programme in Taiwan. Journal of Clinical Nursing 2010;19(17‐18):2415‐23. [DOI] [PubMed] [Google Scholar]
MacPherson 2011 {published data only}
- MacPherson A, Snider C, Kakepetum‐Schults T, McGhan S. Growing healthy children and youth: an asthma education program. Canadian Respiratory Journal 2011;18:26A. [Google Scholar]
Mangan 2006 {published data only}
- Mangan JMM, Gerald LBB. Asthma agents: monitoring asthma in school. Journal of School Health 2006;76(6):300‐2. [DOI] [PubMed] [Google Scholar]
Marabini 2002 {published data only}
- Marabini A, Brugnami G, Curradi F, Casciola G, Stopponi R, Pettinari L, et al. Short‐term effectiveness of an asthma educational program: results of a randomized controlled trial. Respiratory Medicine 2002;96(12):993‐8. [DOI] [PubMed] [Google Scholar]
McClure 2008 {published data only}
- McClure LA, Harrington KF, Graham H, Gerald LB. Internet‐based monitoring of asthma symptoms, peak flow meter readings, and absence data in a school‐based clinical trial. Clinical Trials 2008;5(1):31‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
McElmurry 1999 {published data only}
- McElmurry BJ, Buseh AG, Dublin M. Health education program to control asthma in multiethnic, low‐income urban communities: the Chicago Health Corps Asthma Program. Chest 1999;116(4 Suppl 1):198S‐9S. [PubMed] [Google Scholar]
McEwen 1998 {published data only}
- McEwen M, Johnson P, Neatherlin J, Millard MW, Lawrence G. School‐based management of chronic asthma among inner‐city African‐American schoolchildren in Dallas, Texas. Journal of School Health 1998;68(5):196‐201. [DOI] [PubMed] [Google Scholar]
McLaughlin 2006 {published data only}
- McLaughlin T, Maljanian R, Kornblum R, Clark P, Simpson J, McCormack K. Evaluating the availability and use of asthma action plans for school‐based asthma care: a case study in Hartford, Connecticut. Journal of School Health 2006;76(6):325‐8. [DOI] [PubMed] [Google Scholar]
Meng 2000 {published data only}
- Meng A. A school‐based asthma clinic: a partnership model for managing childhood asthma. Nurse Practitioner Forum ‐ Current Topics and Communications 2000;11(1):38‐47. [PubMed] [Google Scholar]
Meurer 1999 {published data only}
- Meurer JR, McKenzie S, Mischler E, Subichin S, Malloy M, George V. The Awesome Asthma School Days program: educating children, inspiring a community. Journal of School Health 1999;69(2):63‐8. [DOI] [PubMed] [Google Scholar]
Millard 2003 {published data only}
- Millard MW, Johnson PT, McEwen M, Neatherlin J, Lawrence G, Kennerly DK, et al. A randomized controlled trial using the school for anti‐inflammatory therapy in asthma. Journal of Asthma 2003;40(7):769‐76. [DOI] [PubMed] [Google Scholar]
Mitchell 2017 {published data only}
- Mitchell DK, McQuaid EL, Fritz GK, Kopel SJ, Seifer R, Esteban CA, et al. Culturally and contextually tailored asthma self‐management for urban, Latino middle school students: the Rhode Island‐Puerto Rico Asmas program. American Journal of Respiratory and Critical Care Medicine 2017;195:1. [Google Scholar]
Morphew 2013 {published data only}
- Morphew T, Scott L, Li M, Galant SP, Wong W, Lloret Maria IG, et al. Mobile health care operations and return on investment in predominantly underserved children with asthma: the Breathmobile Program. Population Health Management 2013;16(4):261‐9. [DOI] [PubMed] [Google Scholar]
- Xi SC, Morphew T, Kwong K, Li M, Thobani S, Nichols B, et al. Association between obesity and asthma control in children: the Breathmobile Program. Annals of Allergy, Asthma and Immunology 2015;115(5):A23. [Google Scholar]
Morphew 2017 {published data only}
- Morphew T, Altamirano W, Bassin SL, Galant SP. The Breathmobile improves the asthma medication ratio and decreases emergency department utilization. American Journal of Managed Care 2017;23(4):120‐6. [PubMed] [Google Scholar]
Morton 2017 {published data only}
- Morton RW, Elphick HE, Rigby AS, Daw WJ, King DA, Smith LJ, et al. STAAR: a randomised controlled trial of electronic adherence monitoring with reminder alarms and feedback to improve clinical outcomes for children with asthma. Thorax 2017;72(4):347‐54. [DOI] [PubMed] [Google Scholar]
Mosnaim 2017 {published data only}
- Mosnaim GS, Akkoyun E, Eng J, Shalowitz MU. Behavioral interventions to improve asthma outcomes: a systematic review of recent publications. Current Opinion in Allergy and Clinical Immunology 2017;17(3):194‐200. [DOI] [PubMed] [Google Scholar]
NCT00217776 {published data only}
- NCT00217776. School‐based approaches to help pre‐teens manage asthma. clinicaltrials.gov/ct2/show/NCT00217776 (first received 22 September 2005).
Neuharth‐Pritchett 2016 {published data only}
- Neuharth‐Pritchett S, Getch YQ. The effectiveness of a brief asthma education intervention for child care providers and primary school teachers. Early Childhood Education Journal 2016;44(6):555‐61. [Google Scholar]
Nuss 2016 {published data only}
- Nuss HJ, Hester LL, Perry MA, Stewart‐Briley C, Reagon VM, Collins P. Applying the social ecological model to creating asthma‐friendly schools in Louisiana. Journal of School Health 2016;86(3):225‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Otim 2015 {published data only}
- Otim ME, Jayasinha R, Forbes H, Smita S. Building evidence for peer‐led interventions: assessing the cost of the Adolescent Asthma Action program in Australia. Australian Journal of Primary Health 2015;21(4):438‐43. [DOI] [PubMed] [Google Scholar]
Patel 2007 {published data only}
- Patel B, Sheridan P, Detjen P, Donnersberger D, Gluck E, Malamut K, et al. Success of a comprehensive school‐based asthma intervention on clinical markers and resource utilization for inner‐city children with asthma in Chicago: the Mobile C.A.R.E. Foundation's asthma management program. Journal of Asthma 2007;44(2):113‐8. [DOI] [PubMed] [Google Scholar]
Peers 2017 {published data only}
- Peers CB, Mullan A, Chodhari R. Asthma innovation research ‐ air. Archives of Disease in Childhood 2017;102:A168. [Google Scholar]
Pender‐Phaneuf 2016 {published data only}
- Pender‐Phaneuf K. Pediatric asthma management in Massachusetts schools: facilitators and barriers. Nursing Research 2016;65(2):E31‐2. [Google Scholar]
Perry 2000 {published data only}
- Perry CS, Toole KA. Impact of school nurse case management on asthma control in school‐aged children. Journal of School Health 2000;70(7):303‐4. [DOI] [PubMed] [Google Scholar]
Petrie 2010 {published data only}
- Petrie JL, Segal AR. Clinical pharmacy services provided to asthma patients in a school‐based clinic. American Journal of Health‐System Pharmacy 2010;67(3):185, 188‐9. [DOI] [PubMed] [Google Scholar]
Quaranta 2012 {published data only}
- Quaranta J, Brown K, Logvis K, Ponticiello D. Using nursing students as open airways facilitators through a community partnership to influence asthma outcomes. Journal of Asthma and Allergy Educators 2012;3(2):56‐63. [Google Scholar]
Quaranta 2015 {published data only}
- Quaranta JE, Spencer GA. Using the health belief model to understand school nurse asthma management. Journal of School Nursing 2015;31(6):430‐40. [DOI] [PubMed] [Google Scholar]
Rasberry 2014 {published data only}
- Rasberry CN, Cheung K, Buckley R, Dunville R, Daniels B, Cook D, et al. Indicators of asthma control among students in a rural, school‐based asthma management program. Journal of Asthma 2014;51(8):876‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raun 2017 {published data only}
- Raun LH, Campos LA, Stevenson E, Ensor KB, Johnson G, Persse D. Analyzing who, when, and where: data for better targeting of resources for school‐based asthma interventions. Journal of School Health 2017;87(4):253‐61. [DOI] [PubMed] [Google Scholar]
Rhee 2012 {published data only}
- Rhee H, Pesis‐Katz I, Xing JP. Cost benefits of a peer‐led asthma self‐management program for adolescents. Journal of Asthma 2012;49(6):606‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Richterová 2016 {published data only}
- Richterová J, Richter J. Allergy and asthma at school. Alergie 2016;2016(2):102‐8. [Google Scholar]
Rodriguez‐Martinez 2017 {published data only}
- Rodriguez‐Martinez CE, Sossa‐Briceno MP, Castro‐Rodriguez JA. A cost‐effectiveness threshold analysis of a multidisciplinary structured educational intervention in pediatric asthma. Journal of Asthma 2017;1:1‐10. [DOI] [PubMed] [Google Scholar]
Sabla 2017 {published data only}
- Sabla GE, McDowell KM, Kercsmar CM, Braun WE. Creating a collaborative and sustainable partnership with a public school to promote peer to peer asthma education. American Thoracic Society International Conference; 2017 May 19‐24; Washington. 2017:A4831.
Salisbury 2002 {published data only}
- Salisbury C, Francis C, Rogers C, Parry K, Thomas H, Chadwick S, et al. A randomised controlled trial of clinics in secondary schools for adolescents with asthma. British Journal of General Practice 2002;52:988‐96. [PMC free article] [PubMed] [Google Scholar]
Scherer 2016 {published data only}
- Scherer YK, Foltz‐Ramos K, Fabry D, Chao YY. Evaluating simulation methodologies to determine best strategies to maximize student learning. Journal of Professional Nursing 2016;32(5):349‐57. [DOI] [PubMed] [Google Scholar]
Schlueter 2011 {published data only}
- Schlueter DF, Rasberry CN, Buckley R, Mast DK, Cheung K, Luna PJ, et al. Secondhand tobacco smoke exposure among school‐aged youth enrolled in school‐based asthma management programs: a mixed methods analysis. Journal of Asthma and Allergy Educators 2011;2(4):173‐80. [Google Scholar]
Schneider 1997 {published data only}
- Schneider SL, Richard M, Huss K, Huss RW, Thompson LC, Butz AM, et al. Moving health care education into the community. Nursing Management 1997;28(9):40‐3. [PubMed] [Google Scholar]
Schuller 2015 {published data only}
- Schuller L, Faulkner G. Providing better asthma care for children in school. Nursing Times 2015;111(40):12‐4. [PubMed] [Google Scholar]
Scott 2006 {published data only}
- Scott VL. Self‐Management Skills for School‐Age Youth With Asthma [PhD thesis]. New York: Hofstra University, 2006. [Google Scholar]
Scott 2008 {published data only}
- Scott L, Nichols B, Choi Kwong KY, Morphew T, Jones CA. Longitudinal patterns of predominant asthma disease activity in pediatric patients enrolled in an asthma‐specific disease management program. Journal of Asthma 2008;45(6):501‐5. [DOI] [PubMed] [Google Scholar]
Scott 2011 {published data only}
- Scott L, Morphew T, Bollinger ME, Samuelson S, Galant S, Clement L, et al. Achieving and maintaining asthma control in inner‐city children. Journal of Allergy and Clinical Immunology 2011;128(1):56‐63. [DOI] [PubMed] [Google Scholar]
Shanovich 2009 {published data only}
- Shanovich KK, Sorkness CA, Wise ME, Pulvermacher AD, Bhattacharya A, Gustafson DH. Internet telehealth for pediatric nurse case management improves asthma control [Abstract]. Journal of Allergy and Clinical Immunology 2009;123(2 Suppl 1):S43. [Google Scholar]
Sharek 2002 {published data only}
- Sharek PJ, Mayer ML, Loewy L, Robinson TN, Shames RS, Umetsu DT, et al. Agreement among measures of asthma status: a prospective study of low‐income children with moderate to severe asthma. Pediatrics 2002;110(4):797‐804. [DOI] [PubMed] [Google Scholar]
Shaw 2005 {published data only}
- Shaw SF, Marshak HH, Dyjack DT, Neish CM. Effects of a classroom‐based asthma education curriculum on asthma knowledge, attitudes, self‐efficacy, quality of life, and self‐management behaviors among adolescents. American Journal of Health Education 2005;36(3):140‐7. [Google Scholar]
Shaw 2016 {published data only}
- Shaw N, Souëf P, Turkovic L, McCahon L, Kicic A, Sly PD. Pressurised metered dose inhaler‐spacer technique in young children improves with video instruction. European Journal of Pediatrics 2016;175(7):1007‐12. [DOI] [PubMed] [Google Scholar]
Shegog 2001 {published data only}
- Shegog R, Bartholomew LK, Parcel GS, Sockrider MM, Mâsse L, Abramson SL. Impact of a computer‐assisted education program on factors related to asthma self‐management behavior. Journal of the American Medical Informatics Association 2001;8(1):49‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shelef 2016 {published data only}
- Shelef DQ, Rand C, Streisand R, Horn IB, Yadav K, Stewart L, et al. Using stakeholder engagement to develop a patient‐centered pediatric asthma intervention. Journal of Allergy and Clinical Immunology 2016;138(6):1512‐7. [DOI] [PubMed] [Google Scholar]
Staudt 2015 {published data only}
- Staudt AM, Alamgir H, Long DL, Inscore SC, Wood PR. Developing and implementing a citywide asthma action plan: a community collaborative partnership. Southern Medical Journal 2015;108(12):710‐4. [DOI] [PubMed] [Google Scholar]
Suwannakeeree 2016 {published data only}
- Suwannakeeree P, Deerojanawong J, Prapphal N. School‐based educational interventions can significantly improve health outcomes in children with asthma. Journal of the Medical Association of Thailand 2016;99(2):166‐74. [PubMed] [Google Scholar]
Szefler 2016 {published data only}
- Szefler SJ. Examining causes of the urban (inner city) asthma epidemic: implementing new management strategies. Allergy and Proceedings 2016;37(1):4‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Szefler 2017 {published data only}
- Szefler SJ, Cloutier M, Villarreal M, Hollenbach J, Gleason M, Howard CH, et al. Building bridges for asthma care: reducing school absence for children with health disparities. American Journal of Respiratory and Critical Care Medicine 2017;195:A5092. [Google Scholar]
Tate 2009 {published data only}
- Tate ED. Asthma in the Community: Designing Instruction to Help Students Explore Scientific Dilemmas That Impact Their Lives [thesis]. Berkeley, CA: University of California, 2009. [Google Scholar]
Terpstra 2012a {published data only}
- Terpstra JL, Chavez LJ, Ayala GX. An intervention to increase caregiver support for asthma management in middle school‐aged youth. Journal of Asthma 2012;49(3):267‐74. [DOI] [PubMed] [Google Scholar]
Thornton 2016 {published data only}
- Thornton E, Kennedy S, Hayes‐Watson C, Krouse RZ, Mitchell H, Cohn RD, et al. Adapting and implementing an evidence‐based asthma counseling intervention for resource‐poor populations. Journal of Asthma 2016;53(8):825‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Urrutia‐Pereira 2017 {published data only}
- Urrutia‐Pereira M, To T, Cruz Á, Solé D. The school as a health promoter for children with asthma: the purpose of an education programme. Allergologia et Immunopathologia 2017;45(1):93‐8. [DOI] [PubMed] [Google Scholar]
Valery 2007 {published data only}
- Valery PC, Masters IB, Clements V, Taylor B, Laifoo Y, Chang AB. A randomised controlled study on education intervention for childhood asthma by the Aboriginal and Torres Strait Islander health workers in Torres Strait region [Abstract]. Medical Journal of Australia 2007;12:A193. [DOI] [PubMed] [Google Scholar]
Velsor‐Friedrich 2004 {published data only}
- Velsor‐Friedrich B, Pigott TD, Louloudes A, Velsor‐Freidrich B. The effects of a school‐based intervention on the self‐care and health of African‐American inner‐city children with asthma. Journal of Pediatric Nursing 2004;19(4):247‐56. [DOI] [PubMed] [Google Scholar]
Velsor‐Friedrich 2012 {published data only}
- Velsor‐Friedrich B, Militello LK, Richards MH, Harrison PR, Gross IM, Romero E, et al. Effects of coping‐skills training in low‐income urban African‐American adolescents with asthma. Journal of Asthma 2012;49:372‐9. [DOI] [PubMed] [Google Scholar]
Volerman 2017 {published data only}
- Volerman A, Hull A, Ignoffo S, Press VG. Overcoming inhaler misuse: looking outside the healthcare setting for assessment and education. American Journal of Respiratory and Critical Care Medicine 2017;195:A3329. [Google Scholar]
Walter 2016 {published data only}
- Walter H, Sadeque‐IF, Ulysse R, Castillo D, Fitzpatrick A, Singleton J. Effectiveness of school‐based family asthma educational programs on quality of life and asthma exacerbations in asthmatic children aged five to 18: a systematic review. JBI Database of Systematic Reviews and Implementation Reports 2016;14(11):113‐38. [DOI] [PubMed] [Google Scholar]
Walton 2004 {published data only}
- Walton I, Harding J, Stewart A, Tunna K. Project for the evaluation of asthma in Tipton schools (PEATS). Quality in Primary Care 2004;12(1):53‐8. [Google Scholar]
Webber 2005 {published data only}
- Webber MP, Hoxie AM, Odlum M, Oruwariye T, Lo Y, Appel D. Impact of asthma intervention in two elementary school‐based health centers in the Bronx, New York City. Pediatric Pulmonology 2005;40(6):487‐93. [DOI] [PubMed] [Google Scholar]
Weng 2007 {published data only}
- Weng HC, Yuan BC, Su YT, Perng DS, Chen WH, Lin LJ, et al. Effectiveness of a nurse‐led management programme for paediatric asthma in Taiwan. Journal of Paediatrics and Child Health 2007;43(3):134‐8. [DOI] [PubMed] [Google Scholar]
Wensley 2004 {published data only}
- Wensley D, Silverman M. Peak flow monitoring for guided self‐management in childhood asthma: a randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2004;170(6):606‐12. [DOI] [PubMed] [Google Scholar]
Whitman 1985 {published data only}
- Whitman N, West D, Brough FK, Welch M. A study of a self‐care rehabilitation program in pediatric asthma. Health Education Quarterly 1985;12(4):333‐42. [DOI] [PubMed] [Google Scholar]
Willeboordse 2016 {published data only}
- Willeboordse M, Kant KDG, Tan FE, Mulkens S, Schellings J, et al. A multifactorial weight reduction programme for children with overweight and asthma: a randomized controlled trial. PLoS One 2016;11(6):e0157158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2008 {published data only}
- Wilson KD, Kurz RS. Bridging implementation and institutionalization within organizations: proposed employment of continuous quality improvement to further dissemination. Journal of Public Health Management and Practice 2008;14(2):109‐16. [DOI] [PubMed] [Google Scholar]
Wyatt 2008 {published data only}
- Wyatt TH, Hauenstein EJ. Pilot testing OKAY WITH ASTHMA: an online asthma intervention for school‐age children. Journal of School Nursing 2008;24(3):145‐50. [DOI] [PubMed] [Google Scholar]
Wyatt 2013 {published data only}
- Wyatt TH, Li XP, Huang Y, Farmer R, Reed D, Burkhart PV. Developing an interactive story for children with asthma. Nursing Clinics of North America 2013;48(2):271‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yawn 2000 {published data only}
- Yawn BP, Algatt‐Bergstrom PJ, Yawn RA, Wollan P, Greco M, Gleason M, et al. An in‐school CD‐ROM asthma education program. Journal of School Health 2000;70(4):153‐9. [DOI] [PubMed] [Google Scholar]
Yoshida 2011 {published data only}
- Yoshida K, Masuko I, Akada T, Itazawa T, Adachi Y, Aka‐Sawa A, et al. The association between asthma symptoms and obesity in adolescents. Journal of Allergy and Clinical Immunology 2011;127(2):AB153. [Google Scholar]
Young 2001 {published data only}
- Young NL, Foster AM, Parkin PC, Reisman J, MacLusky I, Gold M, et al. Assessing the efficacy of a school‐based asthma education program for children: a pilot study. Canadian Journal of Public Health‐Revue Canadienne De Sante Publique 2001;92(1):30‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zografos 2007 {published data only}
- Zografos K, Marshak HH, Dyjack DT, Neis C. The effects of an adolescent asthma education intervention on knowledge, intention, behavior, self‐efficacy and self‐consciousness. Californian Journal of Health Promotion 2007;8(1):60‐71. [Google Scholar]
References to studies awaiting assessment
Liptzin 2016a {published data only}
- Liptzin DR, Gleason MC, Cicutto LC, Cleveland CL, Shocks DJ, White MK, et al. Developing, implementing, and evaluating a school‐centered asthma program: step‐up asthma program. Journal of Allergy and Clinical Immunology 2016;4(5):972‐99.e1. [DOI] [PubMed] [Google Scholar]
McCallum 2017 {published data only}
- McCallum GB, Chang AB, Wilson CA, Petsky HL, Saunders J, Pizzutto SJ, et al. Feasibility of a peer‐led asthma and smoking prevention project in Australian schools with high indigenous youth. Frontiers in Pediatrics 2017;5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Praena‐Crespo 2017 {published data only}
- Praena‐Crespo M, Aquino‐Llinares N, Fernández‐Truan JC, Castro‐Gómez L, Segovia‐Ferrera C, GESA network. Asthma education taught by physical education teachers at grade schools: a randomised cluster trial. Allergologia et Immunopathologia 2017;45(4):375‐86. [DOI] [PubMed] [Google Scholar]
Reznik 2016 {published data only}
- Reznik M, Ozuah PO. Addressing barriers to physical activity in schoolchildren with asthma: results of a pilot cluster randomized controlled trial. European Journal of Pediatrics 2016;175(11):1604‐5. [Google Scholar]
Warren 2016 {published data only}
- Warren CM, Dyer A, Blumenstock J, Gupta RS. Leveraging mobile technology in a school‐based participatory asthma intervention: findings from the student media‐based asthma research team (SMART) Study. American Journal of Health Education 2016;47(2):59‐70. [Google Scholar]
- Yarbrough M, Blumenstock J, Warren C, Dyer A, Wilson J, Smith B, et al. SMART (student media‐based asthma research team): engaging adolescents to understand asthma in their communities. Progress in Community Health Partnerships‐Research Education and Action 2016;10(4):523‐32. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Halterman 2017 {published data only}
- Halterman JS, Tajon R, Tremblay P, Fagnano M, Butz A, Perry TT, et al. Development of school‐based asthma management programs in Rochester, New York: presented in honor of Dr Robert Haggerty. Academic Pediatrics 2017;17(6):595‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lemanske 2016 {published data only}
- Lemanske RF, Kakumanu S, Shanovich K, Antos N, Cloutier MM, Mazyck D, et al. Creation and implementation of SAMPRO (TM): a school‐based asthma management program. Journal of Allergy and Clinical Immunology 2016;138(3):711‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT03032744 {published data only}
- NCT03032744. Project IMPACT in schools to prevent asthma symptoms [Intervention and management program for asthma control and treatment in schools]. clinicaltrials.gov/show/NCT03032744 (first received 26 January 2017).
Perry 2015 {published data only}
- Perry TT, Halterman JS, Brown RH, Hunter CR, Randle SM, Tilford JM, et al. Breath Connection: a school‐based telemedicine program for rural children with asthma. Journal of Allergy and Clinical Immunology 2015;135(2 Suppl 1):Abs169. [Google Scholar]
Phipatanakul 2017 {published data only}
- Phipatanakul W, Koutrakis P, Coull BA, Kang CM, Wolfson JM, Ferguson ST, et al. The school inner‐city asthma intervention study: design, rationale, methods, and lessons learned. Contemporary Clinical Trials 2017;60:14‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Ahmad 2011
- Ahmad E, Grimes D. The effects of self‐management education for school‐age children on asthma morbidity: a systematic review. Journal of School Nursing 2011;27(4):282‐92. [DOI] [PubMed] [Google Scholar]
Al Aloola 2014
- Al Aloola NA, Naik‐Panvelkar P, Nissen L, Saini B. Asthma interventions in primary schools ‐ a review. Journal of Asthma 2014;51(8):779‐98. [DOI] [PubMed] [Google Scholar]
American Lung Association 2018
- American Lung Association. About Open Airways for Schools. http://www.lung.org/lung‐health‐and‐diseases/lung‐disease‐lookup/asthma/asthma‐education‐advocacy/open‐airways‐for‐schools/about‐open‐airways.html (accessed prior to 12 November 2018).
Asher 2006
- Asher M, Montefort S, Bjorksten B, Lai C, Strachan D, Weiland K, et al. Worldwide trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross‐sectional surveys. Lancet 2006;368:733‐43. [DOI] [PubMed] [Google Scholar]
Asthma UK 2013
- Asthma UK. 2 million people unaware they are at risk of an asthma attack. asthma.org.uk/News/2‐million‐people‐unaware‐they‐are‐at‐risk‐of‐an‐asthma‐attack (accessed 4 February 2015).
Bahadori 2009
- Bahadori K, Doyle‐Waters MM, Marra C, Lynd L, Alasaly K, Swiston J, et al. Economic burden of asthma: a systematic review. BMC Pulmonary Medicine 2009;9(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bateman 2008
- Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, et al. Global strategy for asthma management and prevention: GINA executive summary. European Respiratory Journal 2008;31(1):143‐78. [DOI] [PubMed] [Google Scholar]
Berry 2013
- Berry DC, Neal M, Hall EG, McMurray RG, Schwartz TA, Skelly AH, et al. Recruitment and retention strategies for a community‐based weight management study for multi‐ethnic elementary school children and their parents. Public Health Nursing 2013;30(1):80‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blackman 2007
- Blackman JA, Gurka MJ. Developmental and behavioral comorbidities of asthma in children. Journal of Developmental and Behavioral Pediatrics 2007;28(2):92‐9. [DOI] [PubMed] [Google Scholar]
Boyd 2009
- Boyd M, Lasserson TJ, McKean MC, Gibson PG, Ducharme FM, Haby M. Interventions for educating children who are at risk of asthma‐related emergency department attendance. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD001290.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
British Asthma Guidelines 1997
- British Asthma Guidelines Coordinating Committee. British guidelines on asthma management: 1995 review and position statement. Thorax 1997;52(Suppl 1):S1‐20. [Google Scholar]
Bruhn 1983
- Bruhn JG. The application of theory in childhood asthma self‐help programs. Journal of Allergy and Clinical Immunology 1983;72(5):561‐77. [DOI] [PubMed] [Google Scholar]
Brunton 2014
- Brunton G, Caird J, Stokes G, Stansfield C, Kneale D, Richardson M, et al. Community engagement for health via coalitions, collaborations and partnerships: a systematic review and meta‐analysis. London EPPI‐Centre 2014;1:1‐548. [Google Scholar]
Brunton 2014a
- Brunton G, O'Mara‐Eves A, Thomas J. The 'active ingredients' for successful community engagement with disadvantaged expectant and new mothers: a qualitative comparative analysis. Journal of Advanced Nursing 2014;70(12):2847‐60. [DOI] [PubMed] [Google Scholar]
Bruzzese 2009
- Bruzzese JM, Evans D, Kattan M. School‐based asthma programs. Journal of Allergy and Clinical Immunology 2009;124(2):195‐200. [DOI] [PubMed] [Google Scholar]
BTS 2016
- British Thoracic Society. BTS/SIGN Asthma Guideline 2016. brit‐thoracic.org.uk/document‐library/clinical‐information/asthma/btssign‐asthma‐guideline‐2016/ (accessed before 20 June 2018).
Bush 2017
- Bush A, Griffiths C. Improving treatment of asthma attacks in children. BMJ: British Medical Journal (Online) 2017;359:j5763. [DOI] [PubMed] [Google Scholar]
Carvalho 2016
- Carvalho C, Ana C, Barretto C, Laís S, Souza‐Machado C de, et al. The impacts of educational asthma interventions in schools: a systematic review of the literature. Canadian Respiratory Journal 2016 Aug 30 [Epub ahead of print]:8476206. [DOI: 10.1155/2016/8476206] [DOI] [PMC free article] [PubMed]
Chinn 2000
- Chinn S. A simple method for converting an odds ratio to effect size for use in meta‐analysis. Statistics in Medicine 2000;19(22):3127‐31. [DOI] [PubMed] [Google Scholar]
Coffman 2009
- Coffman JM, Cabana MD, Yelin MH. Do school‐based asthma education programs improve self‐management and health outcomes?. Pediatrics 2009;124(2):729‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2005
- Cooper B. Applying Ragin's crisp and fuzzy set QCA to large datasets: social class and educational achievement in the National Child Development Study. Sociological Research Online 2005;10(2):1‐20. [Google Scholar]
Denford 2013
- Denford S, Taylor RD, Campbell JL, Greaves CJ. Effective behavior change techniques in asthma self‐care interventions: systematic review and meta‐regression. Health Psychology 33;7:577‐87. [DOI] [PubMed] [Google Scholar]
Department of Health 2012
- Department of Health. NHS atlas of variation in healthcare for people with respiratory disease. Reducing unwarranted variation to increase value and improve quality. September 2012. http://www.rightcare.nhs.uk/index.php/atlas/respiratorydisease/ (accessed 5 February 2015).
Dickson 2016
- Dickson K, Melendez‐Torres GJ, Fletcher A, Hinds K, Thomas J, Stansfield C, et al. How do contextual factors influence implementation and receipt of positive youth development programs addressing substance use and violence? A qualitative meta‐synthesis of process evaluations. American Journal of Health Promotion 2016:1‐12. [DOI: 10.1177/0890117116670302] [DOI] [PubMed] [Google Scholar]
Fowler 1992
- Fowler M, Davenport M, Garg R. School functioning of US children with asthma. Pediatrics 1992;90(6):939‐44. [PubMed] [Google Scholar]
GINA 2018
- Global Initiative for Asthma. 2018 GINA Report, Global Strategy for Asthma Management and Prevention. https://ginasthma.org/2018‐gina‐report‐global‐strategy‐for‐asthma‐management‐and‐prevention/ (accessed before 20 June 2018).
Glenton 2013
- Glenton C, Colvin CJ, Carlsen B, Swartz A, Lewin S, Noyes J, et al. Barriers and facilitators to the implementation of lay health worker programmes to improve access to maternal and child health: qualitative evidence synthesis. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD010414.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guevara 2003
- Guevara JP, Wolf FM, Grum CM, Clark NM. Effects of educational interventions for self management of asthma in children and adolescents: systematic review and meta‐analysis. BMJ 2003;326(7402):1308‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Gunn E, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: what is “quality of evidence” and why is it important to clinicians?. British Medical Journal 2008;336(7650):924‐6. [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence: publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [DOI] [PubMed] [Google Scholar]
Hannes 2011
- Hannes K. Chapter 4. Critical appraisal of qualitative research. In: Noyes J, Booth A, Hannes K, Harden A, Harris J, Lewin S, et al (editors). Supplementary Guidance for Inclusion of Qualitative Research in Cochrane Systematic Reviews of Interventions. Version 1 [updated August 2011]. Cochrane Collaboration Qualitative Methods Group, 2011. Critical appraisal of qualitative research. http://cqim.cochrane.org/supplemental‐handbook‐guidance (accessed 5 February 2015).
Harbord 2009
- Harbord RM, Harris RJ, Sterne JAC. Updated tests for small‐study effects in meta‐analyses. Stata Journal 2009;9(2):197. [Google Scholar]
Harden 2004
- Harden AJ, Garcia S, Oliver R, Rees M, Shepherd J, Brunton G, et al. Applying systematic review methods to studies of people's views: an example from public health research. Journal of Epidemiology and Community Health 59;9:794‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 2015
- Harris KM, Kneale D, Lasserson TJ, McDonald VM, Grigg J, Thomas J. School‐based self management interventions for asthma in children and adolescents: a mixed methods systematic review. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.CD011651] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 2017
- Harris K, Mosler G, Williams SA, Whitehouse A, Raine R, Grigg J. Asthma control in London secondary school children. Journal of Asthma 2017;54:1‐8. [DOI] [PubMed] [Google Scholar]
Hellström 2001
- Hellström E. Conflict Cultures: Qualitative Comparative Analysis of Environmental Conflicts in Forestry. 2nd Edition. Helsinki, Finland: Finnish Society of Forest Science [and] Finnish Forest Research Institute, 2001. [Google Scholar]
Higgins 2003
- Higgins J, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 (updated March 2011). The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
HSCIC 2014
- Health and Social Care Information Centre. Compendium of population health indicators (CCG Outcome Indicators Dataset). england.nhs.uk/wp‐content/uploads/2013/12/ccg‐ois‐1415‐tech‐guid.pdf (accessed 5 February 2015).
Hurley 2013
- Hurley M, Dickson K, Walsh N, Hauari H, Grant R, Cumming J, et al. Exercise interventions and patient beliefs for people with chronic hip and knee pain: a mixed methods review. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD010842] [DOI] [PMC free article] [PubMed] [Google Scholar]
Husk 2016
- Husk KR, Lovell C, Cooper C, Stahl‐Timmins W, Garside R. Participation in environmental enhancement and conservation activities for health and well‐being in adults: a review of quantitative and qualitative evidence. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD010351.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jackson 2017
- Jackson D, Turner R. Power analysis for random‐effects meta‐analysis. Research Synthesis Methods 2017;8:290‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Juniper 1996
- Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in children with asthma. Quality of Life Research 1996;5(1):35‐46. [DOI] [PubMed] [Google Scholar]
Juniper 2006
- Juniper EF, Bousquet J, Abetz L, Bateman ED. Identifying 'well‐controlled' and 'not well‐controlled' asthma using the Asthma Control Questionnaire. Respiratory Medicine 2006;100(4):616‐21. [DOI] [PubMed] [Google Scholar]
Kneale 2010
- Kneale D. Child health. In: Hansen K, Jones E, Joshi H, Budge D editor(s). Millennium Cohort Study: Fourth Survey: A User's Guide to Initial Findings. London: Centre for Longitudinal Studies, Institute of Education, 2010:177‐210. [Google Scholar]
Kneale 2015
- Kneale D, Thomas J, Harris K. Developing and optimising the use of logic models in systematic reviews: exploring practice and good practice in the use of programme theory in reviews. PloS one 2015;10:1‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kotses 2010
- Kotses H, Creer TL. Asthma self‐management. Asthma, Health and Society. New York City: Springer, 2010:117‐39. [Google Scholar]
Kuehni 2002
- Kuehni CE, Frey U. Age‐related differences in perceived asthma control in childhood: guidelines and reality. European Respiratory Journal 2002;20(4):880‐9. [DOI] [PubMed] [Google Scholar]
Levy 2014
- Levy M, Andrews R, Buckingham R, Evans H, Francis C, Houston R, et al. Why asthma still kills: the National Review of Asthma Deaths (NRAD). Clinical Effectiveness and Evaluation Unit. London: Royal College of Physicians, 2014. [Google Scholar]
Lewis 2012
- Lewis I, Lenehan C. Report of the children and young people's health outcomes forum. Report of the Long‐term Conditions, Disabilities and Palliative Care Subgroup. London: Department of Health, 2012. [Google Scholar]
Mallol 2013
- Mallol J, Crane J, Mutius E, Odhiambo J, Keil U, Stewart A, et al. The International study of asthma and allergies in childhood (ISAAC) phase three: a global synthesis. Allergy and Immunopathology 2013;41(2):73‐85. [DOI] [PubMed] [Google Scholar]
Miech 2015
- Miech E, Bravata DM, Woodward‐Hagg H. Evaluating lean implementation: challenges in developing a research agenda for lean enterprise transformation in healthcare. General Internal Medicine and Geriatrics 2015;1:2172. [Google Scholar]
Milton 2004
- Milton B, Whitehead M, Holland P, Hamilton V. The social and economic consequences of childhood asthma across the lifecourse: a systematic review. Child 2004;30(6):711‐28. [DOI] [PubMed] [Google Scholar]
Moonie 2006
- Moonie SA, Sterling DA, Figgs L, Castro M. Asthma status and severity affects missed school days. Journal of School Health 2006;76(1):18‐24. [DOI] [PubMed] [Google Scholar]
Moore 2015
- Moore GF, Audrey S, Barker M, Bond L, Bonnell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350:h1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morrison 2012
- Morrison A, Polisena J, Husereau D, Moulton K, Clark M, Fiander M, et al. The effect of English‐language restriction on systematic review‐based meta‐analyses: a systematic review of empirical studies. International Journal of Technology Assessment in Health Care 2012;28(2):138‐44. [DOI] [PubMed] [Google Scholar]
Murta 2007
- Murta SG, Sanderson K, Oldenburg B. Process evaluation in occupational stress management programs: a systematic review. American Journal of Health Promotion 2007;21(4):248‐54. [DOI] [PubMed] [Google Scholar]
National Institute of Health 1997
- National Institute of Health. Guidelines for the Diagnosis and Management of Asthma (EPR‐3). http://www.nhlbi.nih.gov/health‐pro/guidelines/current/asthma‐guidelines (accessed 6 February 2015).
Netuveli 2005
- Netuveli G, Hurwitz B, Levy M, Fletcher M, Barnes G, Durham SR, et al. Ethnic variations in UK asthma frequency, morbidity, and health‐service use: a systematic review and meta‐analysis. Lancet 2005;365(9456):312‐7. [DOI] [PubMed] [Google Scholar]
Neuzil 2000
- Neuzil, KM, Wright PF, Mitchel EF Jr, Griffin MR. The burden of influenza illness in children with asthma and other chronic medical conditions. Journal of Pediatrics 2000;137(6):856‐64. [DOI] [PubMed] [Google Scholar]
NHS Scotland 2014
- NHS Scotland. Logic Models. http://www.healthscotland.com/scotlands‐health/planning/logic‐models.aspx 2014.
NICE 2017
- NICE. Asthma: diagnosis, monitoring and chronic asthma management: NICE guideline [NG80]. https://www.nice.org.uk/guidance/NG80 (accessed prior to 12 November 2018).
O'Mara‐Eves 2013
- O'Mara‐Eves A, Brunton G, McDaid G, Oliver S, Kavanagh J, Jamal F, et al. Community Engagement to Reduce Inequalities in Health: A Systematic Review, Meta‐analysis and Economic Analysis. Southampton (UK): NIHR, 2013. [PubMed] [Google Scholar]
Oakley 2006
- Oakley A, Strange V, Bonell C, Allen E, Stephenson J. Process evaluation in randomised controlled trials of complex interventions. BMJ (Clinical research ed.) 2006;332(7538):413‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Piecoro 2001
- Piecoro LT, Potoski M, Talbert JC, Doherty DE. Asthma prevalence, cost, and adherence with expert guidelines on the utilization of health care services and costs in a state Medicaid population. Health Services Research 2001;36(2):357. [PMC free article] [PubMed] [Google Scholar]
Pike 2018
- Pike KC, Harris KM, Kneale D. Interventions for autumn exacerbations of asthma in children. Cochrane Database of Systematic Reviews 2018, Issue 3. [DOI: 10.1002/14651858.CD012393] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pinnock 2015
- Pinnock H, Epiphaniou E, Pearce G, Parke H, Greenhalgh T, Sheikh A, et al. Implementing supported self‐management for asthma: a systematic review and suggested hierarchy of evidence of implementation studies. BMC Medicine 2015;13:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ragin 2008
- Ragin CC. Redesigning Social Inquiry: Fuzzy Sets and Beyond. 240th. London, UK: Wiley Online Library, 2008. [Google Scholar]
Ragin 2009
- Ragin CC. Qualitative comparative analysis using fuzzy sets (fsQCA). In: Rihoux B, Ragin CC editor(s). Configurational Comparative Methods: Qualitative Comparative Analysis (QCA) and Related Techniques. Thousand Oaks, CA: Sage, 2009. [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rihoux 2009
- Rihoux BE, Meur G. Crisp‐set qualitative comparative analysis (csQCA). In: Rihoux B, Ragin CC editor(s). Configurational Comparative Methods: Qualitative Comparative Analysis (QCA) and Related Techniques. Thousand Oaks, CA: Sage, 2009. [Google Scholar]
Rodriguez 2013
- Rodriguez E, Rivera DA, Perlroth D, Becker E, Wang NE, Landau M. School nurses' role in asthma management, school absenteeism, and cost savings: a demonstration project. Journal of School Health 2013;83(12):842‐50. [DOI] [PubMed] [Google Scholar]
Rosecrans 2008
- Rosecrans AM, Gittelsohn J, Ho LS, Harris SB, Naqshbandi M, Sharma S. Process evaluation of a multi‐institutional community‐based program for diabetes prevention among First Nations. Health Education Research 2008;23(2):272‐86. [DOI] [PubMed] [Google Scholar]
Schellenberg 2012
- Schellenberg JA, Bobrova N, Avan BI. Measuring implementation strength: literature review draft report. ideas.lshtm.ac.uk/wp‐content/uploads/2017/08/IDEAS‐Measuring‐implementation‐strength‐report.pdf (accessed prior to 19 January 2018).
Schneider 2010
- Schneider CQ, Wagemann C. Standards of good practice in qualitative comparative analysis (QCA) and fuzzy‐sets. Comparative Sociology 2010;9(3):397‐418. [Google Scholar]
Schwellnus 2013
- Schwellnus G. Eliminating the influence of irrelevant cases on the consistency and coverage of necessary and sufficient conditions in Fuzzy‐Set QCA. ecpr.eu/Filestore/PaperProposal/99d7fbea‐b604‐4251‐847c‐f8e33c82cb41.pdf (accessed prior to 19 January 2018).
Shepherd 2010
- Shepherd J, Kavanagh J, Picot J, Cooper K, Harden A, Barnett‐Page E, et al. The effectiveness and cost‐effectiveness of behavioural interventions for the prevention of sexually transmitted infections in young people aged 13‐19: a systematic review and economic evaluation. Health Technology Assessment 2010;14(7):1‐206. [DOI] [PubMed] [Google Scholar]
Singh 2012
- Singh HP, Shetty DC, Wadhwan V, Aggarwal P. A quantitative and qualitative comparative analysis of collagen fibers to determine the role of connective tissue stroma on biological behavior of odontogenic cysts: a histochemical study. National Journal of Maxillofacial Surgery 2012;3(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sinha 2012
- Sinha IP, Gallagher R, Williamson PR, Smyth RL. Development of a core outcome set for clinical trials in childhood asthma: a survey of clinicians, parents, and young people. Trials 2012;13(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2005
- Smith JR, Mugford M, Holland R, Candy B, Noble MJ, Harrison BDW, et al. A systematic review to examine the impact of psycho‐educational interventions on health outcomes and costs in adults and children with difficult asthma. Health Technology Assessment 2005;9(23):iii‐iv, 1‐167. [DOI] [PubMed] [Google Scholar]
Spencer 2012
- Spencer N, Thanh TM, Louise S. Low income/socioeconomic status in early childhood and physical health in later childhood/adolescence: a systematic review. Maternal and Child Health 2012;17(3):424‐31. [DOI] [PubMed] [Google Scholar]
StataCorp 2013 [Computer program]
- StataCorp. Stata. Version 15. College Station, TX, USA: StataCorp, 2017.
Thiem 2012
- Thiem A, Dusa A. Qualitative Comparative Analysis With R: A User's Guide. 5th Edition. Berlin, Germany: Springer Science & Business Media, 2012. [Google Scholar]
Thiem 2013
- Thiem A, Dusa A. QCA: a package for qualitative comparative analysis. The R Journal 2013;5(1):87‐97. [Google Scholar]
Thiem 2015
- Thiem A. Standards of good practice and the methodology of necessary conditions in qualitative comparative analysis: a critical view on Schneider and Wagemann's Theory‐Guided/Enhanced Standard Analysis, COMPASSS Working Paper Series 2015‐83. www.researchgate.net/publication/283794175_Standards_of_Good_Practice_and_the_Methodology_of_Necessary_Conditions_in_Qualitative_Comparative_Analysis_A_Critical_View_on_Schneider_and_Wagemann%27s_Theory‐GuidedEnhanced_Standard_Analysis (accessed before 19 January 2018).
Thomas 2010 [Computer program]
- In: Thomas J, Brunton J, Graziosi S (eds). EPPI‐Reviewer 4: Software for Research Synthesis. EPPI‐Centre Software. Version 4.0. London: Social Science Research Unit, Institute of Education, 2010.
Thomas 2011
- Thomas J, McNaught J, Ananiadou S. Applications of text mining within systematic reviews. Research Synthesis Methods 2011;2(1):1‐14. [DOI] [PubMed] [Google Scholar]
Thomas 2014
- Thomas J, O'Mara‐Eves A, Brunton G. Using qualitative comparative analysis (QCA) in systematic reviews of complex interventions: a worked example. Systematic Reviews 2014;3(1):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomson 2013
- Thomson HJ, Thomas S. The effect direction plot: visual display of non‐standardised effects across multiple outcome domains. Research Synthesis Methods 2013;4(1):95‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
To 2012
- To T, Stanojevic S, Moores G, Gershon AS, Bateman ED, Cruz AA, et al. Global asthma prevalence in adults: findings from the cross‐sectional world health survey. BMC Public Health 2012;12(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Den Bemt 2011
- Bemt L, Kooijman S, Linssen V, Lucassen P, Muris J, Slabbers G, et al. How does asthma influence the daily life of children? Results of focus group interviews. Health and Quality of Life Outcomes 2011;8(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 1999
- Walsh LJ, Wong CA, Cooper S, Guhan AR, Pringle M, Tattersfield AE. Morbidity from asthma in relation to regular treatment: a community based study. Thorax 1999;54(4):296‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Waters 2006
- Waters E, Doyle J, Jackson N, Howes F, Brunton G, Oakley A, et al. Evaluating the effectiveness of public health interventions: the role and activities of the Cochrane Collaboration. Journal of Epidemiology and Community Health 2006; Vol. 60, issue 4:285‐9. [DOI] [PMC free article] [PubMed]
Welsh 2011
- Welsh E, Hasan M, Li P. Home‐based educational interventions for children with asthma. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD008469] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wildhaber 2012
- Wildhaber J, Carroll WD, Brand PLP. Global impact of asthma on children and adolescents' daily lives: the room to breathe survey. Pediatric Pulmonology 2012;47(4):346‐57. [DOI] [PubMed] [Google Scholar]
Williamson 2012
- Williamson PR, Altman DG, Blazeby JM, Clarke M, Devane D, Gargon E, et al. Developing core outcome sets for clinical trials: issues to consider. Trials 2012;13(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolf 2002
- Wolf FM, Guevara JP, Grum CM, Clark NM, Cates CJ. Educational interventions for asthma in children. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD000326] [DOI] [PubMed] [Google Scholar]


