Abstract
Background
The World Health Organization (WHO) recommends artemisinin‐based combination therapies (ACTs) to treat uncomplicated Plasmodium falciparum (P falciparum) malaria. Concerns about artemisinin resistance have led to global initiatives to develop new partner drugs to protect artemisinin derivatives in ACT. Pyronaridine‐artesunate is a novel ACT.
Objectives
To evaluate the efficacy of pyronaridine‐artesunate compared to alternative ACTs for treating people with uncomplicated P falciparum malaria, and to evaluate the safety of pyronaridine‐artesunate and other pyronaridine treatments compared to alternative treatments.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL), published in the Cochrane Library; MEDLINE; Embase; and LILACS. We also searched ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform Search Portal, and the International Standard Randomized Controlled Trial Number (ISRCTN) registry for ongoing or recently completed trials. The date of the last search was 8 May 2018.
Selection criteria
Efficacy analysis: randomized controlled trials (RCTs) of pyronaridine‐artesunate for treating uncomplicated P falciparum malaria.
Safety analysis: RCTs of pyronaridine‐artesunate or pyronaridine for treating P falciparum or P vivax malaria.
Data collection and analysis
For this update, two review authors independently re‐extracted all data and assessed certainty of evidence. We meta‐analysed data to calculate risk ratios (RRs) for treatment failures between comparisons, and for safety outcomes between and across comparisons.
Main results
We included 10 relevant studies. Seven studies were co‐funded by Shin Poong Pharmaceuticals which manufactures the drug. Three studies were funded by government agencies.
For efficacy analysis we identified five RCTs with 5711 participants. This included 4465 participants from 13 sites in Africa, and 1246 participants from five sites in Asia. It included 541 children aged less than five years.
For polymerase chain reaction (PCR)‐adjusted failures at day 28, pyronaridine‐artesunate may have fewer failures compared to artemether‐lumefantrine (RR 0.59, 95% confidence interval (CI) 0.26 to 1.31; 4 RCTs, 3068 participants, low‐certainty evidence), artesunate‐amodiaquine (RR 0.55, 95% CI 0.11 to 2.77; 1 RCT, 1245 participants, low‐certainty evidence), and mefloquine plus artesunate (RR 0.37, 95% CI 0.13 to 1.05; 1 RCT, 1117 participants, low‐certainty evidence).
For unadjusted failures at day 28, pyronaridine‐artesunate may have fewer failures compared to artemether‐lumefantrine (RR 0.27, 95% CI 0.13 to 0.58; 4 RCTs, 3149 participants, low‐certainty evidence), and probably has fewer failures compared to artesunate‐amodiaquine (RR 0.49, 95% CI 0.30 to 0.81; 1 RCT, 1257 participants, moderate‐certainty evidence) and mefloquine plus artesunate (RR 0.36, 95% CI 0.17 to 0.78; 1 RCT, 1120 participants, moderate‐certainty evidence).
For PCR‐adjusted failures at day 42, pyronaridine‐artesunate may make little or no difference compared to artemether‐lumefantrine (RR 0.86, 95% CI 0.49 to 1.51; 4 RCTs, 2575 participants, low‐certainty evidence) and artesunate‐amodiaquine (RR 0.98, 95% CI 0.20 to 4.83; 1 RCT, 1091 participants, low‐certainty evidence), but may have higher failures than mefloquine plus artesunate (RR 1.80, 95% CI 0.90 to 3.57; 1 RCT, 1037 participants, low‐certainty evidence). Overall, pyronaridine‐artesunate had a PCR‐adjusted treatment failure rate of less than 5%.
For unadjusted failures at day 42, pyronaridine‐artesunate may have fewer failures compared to artemether‐lumefantrine (RR 0.61, 95% CI 0.46 to 0.82; 4 RCTs, 3080 participants, low‐certainty evidence), may make little or no difference compared to mefloquine plus artesunate (RR 0.84, 95% CI 0.54 to 1.31; 1 RCT, 1059 participants, low‐certainty evidence), and probably makes little or no difference compared to artesunate‐amodiaquine (RR 0.98, 95% CI 0.78 to 1.23; 1 RCT, 1235 participants, moderate‐certainty evidence).
For the safety analysis of severe adverse events and liver function, we identified eight RCTs with 6614 participants comparing pyronaridine‐artesunate to other antimalarials, four of which were not in the previous version of this review. A further two RCTs, comparing pyronaridine alone to other treatments, contributed to the synthesis of all adverse events.
Raised alanine aminotransferase (ALT) greater than five times the upper limit of normal (> 5 x ULN) is more frequent with pyronaridine‐artesunate compared to other antimalarials (RR 3.34, 95% CI 1.63 to 6.84; 8 RCTS, 6581 participants, high‐certainty evidence). There is probably little or no difference for raised bilirubin > 2.5 x ULN between pyronaridine‐artesunate and other antimalarials (RR 1.03, 95% CI 0.49 to 2.18; 7 RCTs, 6384 participants, moderate‐certainty evidence). There was one reported case in which raised ALT occurred with raised bilirubin, meeting criteria for moderate drug‐induced liver injury. No study reported severe drug‐induced liver injury. Electrocardiograph (ECG) abnormalities were less common with pyronaridine‐artesunate compared to other antimalarials. We identified no other safety concerns.
Authors' conclusions
Pyronaridine‐artesunate was efficacious against uncomplicated P falciparum malaria, achieved a PCR‐adjusted treatment failure rate of less than 5% at days 28 and 42, and may be at least as good as, or better than other marketed ACTs.
Pyronaridine‐artesunate increases the risk of episodes of raised ALT > 5 x ULN. This meets criteria for mild drug‐induced liver injury. On one instance this was linked to raised bilirubin, indicating moderate drug‐induced liver injury. No episodes of severe drug‐induced liver injury were reported. The findings of this review cannot fully inform a risk‐benefit assessment for an unselected population. Readers should remain aware of this uncertainty when considering use of pyronaridine‐artesunate in patients with known or suspected pre‐existing liver dysfunction, and when co‐administering with other medications which may cause liver dysfunction.
12 April 2019
Up to date
All studies incorporated from most recent search
All eligible published studies found in the last search (8 May, 2018) were included
Plain language summary
Pyronaridine‐artesunate for treating uncomplicated Plasmodium falciparum (P falciparum) malaria
What is the aim of this review?
The aim of this Cochrane Review was to find out if the antimalarial drug pyronaridine‐artesunate is effective and safe to treat uncomplicated cases of an important type of malaria (P falciparum). We collected and analysed all relevant studies to answer this question and found 10 studies.
Key messages
Pyronaridine‐artesunate is effective in treating uncomplicated P falciparum malaria. Pyronaridine‐artesunate is generally safe, but some people who receive it have blood tests suggesting liver damage. This appears to neither be long‐lasting nor make people ill.
What was studied in the review?
The World Health Organization (WHO) recommends that malaria is treated with combinations of drugs called artemisinin‐based combination therapies (ACTs). Pyronaridine‐artesunate is a new ACT. New ACTs are needed to treat malaria that has become resistant to currently available ACTs, and to help prevent malaria becoming more resistant to treatment.
We compared pyronaridine‐artesunate to other ACTs to evaluate its efficacy against P falciparum malaria, and compared pyronaridine‐artesunate and pyronaridine alone to other drugs to evaluate its safety.
What are the main results of the review?
We included 10 relevant studies. Seven studies were co‐funded by Shin Poong Pharmaceuticals which manufactures the drug. Three studies were funded by government agencies.
Three studies compared pyronaridine‐artesunate to artemether‐lumefantrine in adults and children of all ages in Africa and Asia. One study compared pyronaridine‐artesunate to artesunate‐amodiaquine in adults and older children in Africa. One study compared pyronaridine‐artesunate to mefloquine plus artesunate in adults and older children in Africa and Asia. We included another five studies when we looked at the safety of the drug.
Pyronaridine‐artesunate effectively treated uncomplicated P falciparum malaria, and may be at least as good as or better than existing ACTs (low‐ to moderate‐certainty evidence).
Pyronaridine‐artesunate increases the risk of having blood tests which suggest mild liver injury (moderate‐ to high‐certainty evidence). We did not find evidence that any such liver injury was severe or irreversible. We do not know how pyronaridine‐artesunate might affect people who already have liver damage.
We found two trials that exclusively recruited children under 12, with a total of 732 participants. Using the data from these trials, we did not find differences in treatment efficacy or safety between pyronaridine‐artesunate and artemether‐lumefantrine.
How up‐to‐date is the review?
We searched for studies that had been published up to 8 May 2018.
Summary of findings
Background
Description of the condition
Malaria poses a global health challenge, with an estimated 216 million cases and 445,000 deaths in 2016. Plasmodium falciparum (P falciparum) is the most important species of malaria, causing 99% of malaria cases in the World Health Organization (WHO) Africa region, and 66% in the South‐East Asia region (WHO 2017).
The WHO defines uncomplicated malaria by the absence of clinical features of severe malaria, in the presence of an asexual P falciparum parasitaemia (WHO 2015). Severe malaria is P falciparum parasitaemia with one or more of: impaired consciousness, prostration, multiple convulsions, acidosis, hypoglycaemia, severe malarial anaemia, renal impairment, jaundice, pulmonary oedema, significant bleeding, shock, raised lactate, or a parasitaemia of greater than 10%. If untreated, uncomplicated malaria can develop into severe malaria.
The WHO has recommended artemisinin‐based combination therapies (ACTs) as first‐line treatment of uncomplicated P falciparum malaria since 2006, recognising the risk of resistance with monotherapy (WHO 2006). Artemisinin resistance has emerged in South‐East Asia, initially from the Thai‐Cambodian border, and has since become prevalent in Laos, Myanmar, Thailand, and Vietnam (Dondorp 2009; Noedl 2008). This resistance remains a key concern, as further spread of artemisinin resistance could lead to high mortality (Lubell 2014). These concerns have led to global initiatives to contain the spread of artemisinin resistance, which includes the development of new drugs to partner and protect the artemisinin derivatives in ACT (WHO 2011).
Description of the intervention
The WHO currently recommends the following five ACTs for first‐line treatment of malaria.
Artemether‐lumefantrine
Artesunate‐amodiaquine
Artesunate‐mefloquine
Artesunate‐sulphadoxine‐pyrimethamine
Dihydroartemisinin‐piperaquine
The artemisinin in ACTs rapidly clears parasites from the blood. It also kills some sexual forms of the parasite, and may reduce onward transmission to mosquitoes. The longer‐acting partner drug clears residual infections, and protects against resistance to artemisinin (WHO 2015). Drug combinations with long half‐lives (artesunate‐mefloquine and dihydroartemisinin‐piperaquine) can provide a period of post‐treatment prophylaxis which may last for up to six weeks (Sinclair 2009).
Pyronaridine is a potential partner drug for artesunate. Researchers in China developed pyronaridine during the mid‐1970s, using the nucleus of an earlier antimalarial compound (mepacrine) with an added amodiaquine side‐chain (Fu 1991). Clinicians thereafter used pyronaridine extensively as monotherapy for P falciparum and P vivax infections in China (Chen 1992). Concerns about observed in vitro resistance to pyronaridine lead Chinese researchers to use pyronaridine in combinations with sulphadoxine and pyrimethamine, and primaquine (Fu 1991).
A public‐private partnership including the Medicines for Malaria Venture (MMV) and Shin Poong Pharmaceuticals Incorporated developed pyronaridine‐artesunate in combination from 2002 onwards (MMV 2002), with its first national registration in 2011 (with the Korean Food and Drug Administration). For uncomplicated malaria, the treatment is taken once‐daily for three days. Treatment is provided as tablets for adults and children over 20 kg, or in granules for children and infants between 5 kg and 20 kg.
How the intervention might work
The mode of action of pyronaridine is unclear, with several possible mechanisms (Croft 2012). Pyronaridine has been shown to have potent in vitro activity versus P falciparum (Basco 1992; Chen 1992; Childs 1988; Pradines 1998; Ringwald 1999), even in strains with resistance to other antimalarials, including chloroquine, cycloguanil, amodiaquine, and sulfadoxine‐pyrimethamine (Chavalitshewinkoon‐Petmitr 2000; Kurth 2009; Price 2010). In vitro studies also indicate synergy between pyronaridine and artesunate versus parasites which are resistant to either agent (Peters 1997; Vivas 2008).
Why it is important to do this review
In the absence of resistance, ACTs are effective drugs. However, with emerging resistance to the above currently recommended ACTs, it is necessary to identify new drug combinations with equivalent efficacy. This review is an update of a Cochrane Review first published in 2007 (Unnikrishnan 2007), and previously updated in 2014 (Bukirwa 2014). The latest update of this review concluded that pyronaridine‐artesunate performed well in these trials compared to artemether‐lumefantrine and mefloquine plus artesunate. At day 28, polymerase chain reaction (PCR)‐adjusted treatment failure (where PCR is used to confirm recrudescence rather than reinfection) was below the 5% standard set by the WHO. However, the review recommended further efficacy and safety studies in African and Asian children to clarify whether the combination is an option for first‐line treatment of uncomplicated P falciparum malaria.
Following this review, the latest edition of the WHO guidelines for the treatment of malaria did not recommend pyronaridine‐artesunate for general use (WHO 2015). The Guideline Development Group recommended further data were required for efficacy in children less than five years of age, and safety, including safety of repeat dosing. They noted the undesirable effects of elevated liver function tests.
Since the previous update and WHO Guidelines, the West African Network for Clinical Trials of Antimalarial Drugs has published a new study of pyronaridine‐artesunate (Sagara 2018), and the European Medicines Agency (EMA) has adopted a positive scientific opinion of pyronaridine‐artesunate (EMA 2015). In view of this, we have updated the review to inform future guideline development.
Objectives
To evaluate the efficacy of pyronaridine‐artesunate compared to alternative ACTs for treating people with uncomplicated P falciparum malaria, and to evaluate the safety of pyronaridine‐artesunate and other pyronaridine treatments compared to alternative treatments.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs).
Types of participants
Adults and children with uncomplicated Plasmodium falciparum (P falciparum) malaria, as confirmed by either microscopy or rapid diagnostic tests.
For an additional safety analysis we extended the inclusion criteria to adults and children with P vivax malaria.
Types of interventions
Intervention
Pyronaridine‐artesunate
Control
World Health Organization (WHO)‐recommended artemisinin‐based combination therapies (ACTs) for treating malaria
For the analysis of adverse events, we extended the inclusion criteria to all RCTs comparing pyronaridine alone or in combination with any other antimalarial.
Types of outcome measures
Primary outcomes
Total treatment failure at day 28 (PCR‐adjusted and unadjusted)
Total treatment failure at day 42 (PCR‐adjusted and unadjusted)
Secondary outcomes
-
Early treatment failure (WHO 2009):
danger signs or severe malaria on day 1, 2 or 3, in the presence of parasitaemia
parasitaemia on day 2 higher than on day 0, irrespective of axillary temperature
parasitaemia on day 3 with axillary temperature ≥ 37.5 °C
parasitaemia on day 3 ≥ 25% of count on day 0
Adverse events (safety analysis)
Serious adverse events (leading to death, requiring hospitalization or prolongation of existing hospitalization, are life threatening, or result in persistent or significant disability or incapacity)
Adverse events leading to withdrawal from treatment (discontinuation of trial drug or withdrawal from trial)
Elevated liver function tests
Other adverse events
Comment on outcome measures
We base our primary outcome measures on WHO recommendations (WHO 2003; WHO 2009), which advise a 28‐day follow‐up to capture most failures, and 42‐day follow‐up to capture failures for drugs with a longer elimination half‐life (mefloquine and piperaquine). This is also consistent with previous Cochrane Reviews. We do not report ‘adequate clinical and parasitological response' as this is defined in terms of absence of failure and therefore represents duplication.
The previous published protocol for this review listed a priori secondary outcomes to include parasite clearance, fever clearance, and gametocyte carriage (Bukirwa 2014). The protocol did not clearly define these outcomes, including whether they refer to durations, rates, or proportions of patients at given time points. We encountered considerable heterogeneity in these measures between studies, and therefore present a narrative synthesis.
We encountered heterogeneity in the threshold at which elevated liver function tests were deemed by study authors to be significant, which we have detailed in Table 5. The reader should note that these thresholds do not necessarily correspond with internationally accepted definitions of drug‐induced liver injury (Aithal 2011, summarized in Appendix 1).
1. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin increase grading.
| Trial | ALT increased (grade 3 and above) | AST increased (grade 3 and above) | Blood bilirubin increased |
| Kayentao 2012 | 10 × ULN | 10 × ULN | 3 × ULN |
|
Poravuth 2011 Rueangweerayut 2012 Sagara 2018 Tshefu 2010 |
5 × ULN | 5 × ULN | 2.5 × ULN |
|
Roth 2018 Shin 2011 |
3 × ULN | 3 × ULN | ‐ |
| Nelwan 2015 | 3 × ULN if associated with bilirubin > 2 × ULN | ‐ | ‐ |
Abbreviations: ALT: alanine aminotransferase; AST: aspartate aminotransferase; ULN: upper limit of normal.
Search methods for identification of studies
We sought to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress).
Electronic searches
The review authors and the Cochrane Infectious Diseases Group (CIDG) Information Specialist, Vittoria Lutje (VL), attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress). The date of the last search was 8 May 2018.
VL searched the following databases using the search terms and strategy described in Appendix 2: the Cochrane Infectious Diseases Group Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL), published in the Cochrane Library; MEDLINE (PubMed, from 1966); Embase (OVID; from 1947); and LILACS (BIREME; from 1982). We also searched ClinicalTrials.gov (clinicaltrials.gov), the WHO International Clinical Trial Registry Platform (ICTRP; www.who.int/ictrp/search/en), and the International Standard Randomized Controlled Trial Number (ISRCTN) registry (www.isrctn.com/) for ongoing or recently completed trials using 'pyronaridine', 'pyramax', and 'malaria' as search terms.
Searching other resources
Conference proceedings
The previous authors of this review searched conference proceedings for relevant abstracts (Bukirwa 2014). We did not handsearch conference proceedings for this update as relevant abstracts are likely to be indexed and detected by the electronic search.
Reference lists
We checked the reference lists of all trials identified by the above methods.
Contacting organizations and experts
We did not formally contact experts for this update.
Data collection and analysis
Selection of studies
For this review update, Joseph Pryce (JP) and Paul Hine (PH) independently screened the results of the search update to identify potentially relevant trials and obtain the full‐text reports of these trials. JP and PH used a standard eligibility form to assess newly identified studies. There were no disagreements. Due to the change in authorship, and to ensure validity, we rescreened all the results of the search, and verified the eligibility of previously included studies. We documented the reason for excluding trials in the ‘Characteristics of excluded studies' table. We prepared a PRISMA diagram to summarise the identification, screening, and inclusion of studies in this review (Moher 2009).
Data extraction and management
For this update, to ensure accuracy and consistency, the new authors (JP and PH) independently re‐extracted all data using a new data extraction form.
Unadjusted total failure rate: day 28, day 42
We extracted the following data, and summed it, to form the numerator.
Early treatment failure.
Late clinical failure.
Late parasitological failure.
We aimed to extract the following data, and subtract it from the number of participants randomized, to form the denominator.
Those found not to be fulfilling inclusion criteria after randomization.
Those voluntarily withdrawing consent.
Those lost to follow‐up.
Those violating protocol, including (but not limited to) missed or vomited doses, those failing to complete treatment, and those taking additional antimalarials.
PCR‐adjusted total failure rate: day 28, day 42
We aimed to extract the following data, and sum it, to form the numerator.
Early treatment failure due to PCR‐confirmed recrudescence.
Late clinical failure due to PCR‐confirmed recrudescence.
Late parasitological failure due to PCR‐confirmed recrudescence.
We aimed to extract the following data, and subtract it from the number of participants randomized, to form the denominator.
Those with indeterminate PCR results.
Those with missing PCR results.
Those with PCR‐confirmed new infections.
Those found not to be fulfilling inclusion criteria after randomization.
Those voluntarily withdrawing consent.
Those lost to follow‐up.
Those violating protocol, including (but not limited to) missed or vomited doses, those failing to complete treatment, and those taking additional antimalarials.
Adverse events data
For adverse events, we extracted the number of people experiencing the events in each study as the numerator. In contrast to the efficacy analysis, we extracted the number of people who received at least one dose of the study drug as the denominator. Recognising that studies often use different terminology to describe adverse events, we referenced the Medical Dictionary for Regulatory Activities to find the preferred term (MedDRA 2018), and grouped adverse events according to MedDRA's "High Level Term" descriptors.
Comment on data extraction
This approach is based on standard WHO definitions (WHO 2003; WHO 2009). The WHO protocol has a primary goal to "provide guidance in obtaining the minimum essential information about the clinical and parasitological response to antimalarial drugs among populations at greatest risk of severe morbidity or mortality due to malaria" (WHO 2003). Many antimalarial efficacy studies have used adapted versions of this protocol since its publication. Within this protocol, a high number of randomized participants are excluded from the final efficacy outcome as losses to follow‐up or voluntary or involuntary withdrawals.
In some instances, we could not extract the individual components required to form the denominator. We discuss this issue further in ‘Dealing with missing data' below.
The primary outcomes relate to failure due to P falciparum. The denominators for each include participants developing P vivax parasitaemia that continued to be followed up within the trial.
Assessment of risk of bias in included studies
For this review update, JP and PH assessed the risk of bias for the new trial inclusion using the Cochrane tool for assessing risk of bias (Higgins 2011). JP and PH assigned a judgement of ‘high risk', ‘low risk', and ‘unclear risk' to each domain recording these in ‘Risk of bias' tables, and a summary ‘Risk of bias' graph.
For efficacy, we assessed the following domains.
Sequence generation.
Allocation concealment.
Blinding of participants, trial personnel and outcome assessors.
Incomplete outcome data.
Selective reporting.
Other sources of bias.
For adverse events, we assessed the two following domains, selected based on Cochrane and PRISMA recommendations (Loke 2007; Zorzela 2016).
Adverse event detection.
Incomplete reporting of adverse events.
Appendix 3 gives examples of ‘Risk of bias' assessment decisions.
Measures of treatment effect
We extracted data from each included trial to calculate risk ratios (RRs) for dichotomous data, and mean differences (MDs) for continuous data. We present all measures with the corresponding 95% confidence interval (CI).
Unit of analysis issues
We did not encounter any unit of analysis issues.
Dealing with missing data
In the event of missing or unclear data, we contacted trial authors for clarifications or to provide further information. It was not always possible to extract each data item required to itemise the denominator for treatment failures, particularly where study authors reported amalgamations of the denominator component. Where this was the case, we kept clear records of inferences made to inform the denominator data.
Assessment of heterogeneity
We visually inspected the forest plots for overlapping CIs as an indicator of clinical heterogeneity. We also took into account Chi2 and I2 tests of heterogeneity. We considered a Chi2 test P < 0.1 and/or an I2 statistic > 75% as indicating substantial heterogeneity. If we judged there to be substantial heterogeneity we did not pool the results in a meta‐analysis, and instead presented a narrative synthesis of the findings.
Assessment of reporting biases
There were too few trials to examine funnel plot asymmetry for evidence of small trial effects or publication bias.
Data synthesis
We analysed data using Review Manager 2014. For the primary analysis we stratified by comparator ACT. We performed meta‐analysis where appropriate after assessment and investigation of heterogeneity. In the first instance, we used a fixed‐effect model. Where there was evidence of heterogeneity, we used a random‐effects model, and applied this consistently across similar outcomes.
We deemed it inappropriate to combine continuous data for the outcomes of parasite clearance, fever clearance and gametocyte carriage, due to heterogeneity in the measurements of these outcomes.
Subgroup analysis and investigation of heterogeneity
We intended to explore causes of heterogeneity using subgroup analysis of age, country and geographic region. We deemed that there were too few trials to use these subgroup analyses. However, to explore the applicability of the evidence to child populations, we presented the findings from a subset of trials that exclusively recruited paediatric participants.
Sensitivity analysis
We planned to conduct a series of sensitivity analyses as detailed in Appendix 4. The aim of this was to restore the integrity of the randomizations process by adding excluded groups back into the analysis in a stepwise fashion. However, as we were unable to reliably extract data pertinent to the missing or indeterminate PCR values, we did not conduct the sensitivity analysis.
Certainty of the evidence
We assessed the certainty of the evidence using the GRADE approach (Schünemann 2013). We appraised the certainty of the evidence in relation to the following criteria.
Study design
Risk of bias
Inconsistency
Indirectness
Imprecision
Other considerations (including publication bias)
We used GRADEpro GDT 2015 to create ‘Summary of findings' tables for each comparison included in the review. We included our primary outcomes and adverse event outcomes, and used the tables to guide our conclusions.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies sections.
Results of the search
The search (dated 8 May 2018) identified 40 database records and 12 trials registry records. After contacting authors of a relevant registered trial, we obtained one additional published record. JP and PH independently screened all titles. This process replaced that of the previous version of this review (Bukirwa 2014), as we are a new authorship team.
We identified 31 potentially relevant full‐text records through title and abstract screening. We excluded 10 records after further assessment (see ‘Characteristics of excluded studies' table).
This process identified 21 records, relating to 10 studies, which we included in qualitative and quantitative synthesis (see Characteristics of included studies). We identified four studies that were not included in the previous version of this review (Nelwan 2015; Roth 2018; Sagara 2018; Shin 2011).
The Sagara 2018 trial compared different drug combinations at different sites, but presented the results in an aggregated analysis, in which the numbers of outcome events were summarized across sites. We were concerned that this presented an unpredictable bias and so contacted the trial authors to obtain data disaggregated to site level for the efficacy and raised liver enzyme outcomes. We were therefore able to present the data from individual sites separately in the meta‐analysis. However, we did not have disaggregated data for the outcomes of serious adverse events or adverse events leading to withdrawal of treatment. We therefore present these outcomes in narrative form. Further details of the comparisons examined and the number of participants are provided in the ‘Characteristics of included studies' tables for each site.
Figure 1 illustrates the search results in a flow diagram (PRISMA).
1.
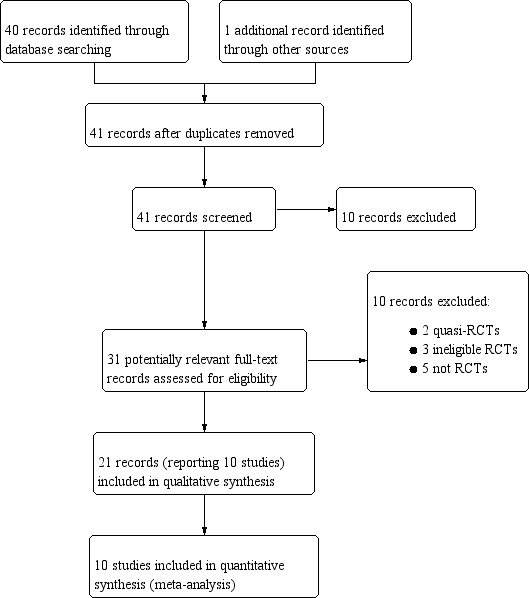
Study flow diagram.
Included studies
Studies meeting the inclusion criteria for efficacy outcomes
Five studies met the inclusion criteria for efficacy outcomes (Kayentao 2012; Roth 2018; Rueangweerayut 2012; Sagara 2018; Tshefu 2010; see Characteristics of included studies). Each of the studies had a length of follow‐up of 42 days.
Comparison 1: Pyronaridine‐artesunate versus artemether‐lumefantrine
Four randomized controlled trials (RCTs) evaluated this comparison (Kayentao 2012; Roth 2018; Sagara 2018; Tshefu 2010).
Sample sizes ranged from 197 participants in Roth 2018 to 1323 participants in Sagara 2018, yielding a total number of 3327 for inclusion in quantitative synthesis. Two studies were multicentred in Africa and Asia (Kayentao 2012; Tshefu 2010), one was multicentred in Africa (Sagara 2018), and one was a single‐centre in Africa (Roth 2018). None of the studies described the P falciparum resistance profile to currently available antimalarials. In total, 3128 (94%) participants were recruited in Africa, and 213 (6%) participants were recruited in Asia.
Two studies included adults and children (Sagara 2018; Tshefu 2010), and two studies included children only (Kayentao 2012; Roth 2018). In total, 541 (16%) participants were aged less than five years. All studies included both male and female participants. In total 1568 (47%) participants were female.
All studies used three‐day regimens of pyronaridine‐artesunate with dose adjusted according to weight. There were minimal differences in dose by weight. The two paediatric trials used granule formulation.
All studies reported "adequate clinical and parasitological response" rate) at day 28 and day 42, PCR‐adjusted and unadjusted. All studies also reported parasite clearance time (defined as first dose to aparasitaemia), and fever clearance time (defined as first dose to apyrexia).
Comparison 2: Pyronaridine‐artesunate versus artesunate‐amodiaquine
One RCT evaluated this comparison, taking place in multiple centres in West Africa (Sagara 2018). The study did not describe the P falciparum resistance profile to currently available antimalarials. In total, 1317 participants randomized to this comparison received at least one study treatment. Of these, 477 (36%) participants were aged less than five years, and 658 (50%) participants were female.
Both pyronaridine‐artesunate and artesunate‐amodiaquine were administered once‐daily for three days at doses according to bodyweight.
Comparison 3: Pyronaridine‐artesunate versus mefloquine plus artesunate
A single trial evaluated this comparison (Rueangweerayut 2012).
The sample size was 1271 participants. Most participants (1033, 81.3%) were from Asia (Cambodia, India, Thailand, and Vietnam), with a smaller number (238, 18.7%) from Africa (Burkina Faso, Ivory Coast, and Tanzania). Malaria endemicity was described by the trial authors as high in most sites. In Cambodia, significantly extended parasite clearance times (for both treatment arms) were suggestive of in vivo resistance to artemisinin. The resistance in the other sites or to other antimalarials was not described. The trial planned to recruit participants aged between three to 60 years, the youngest participant was five years old.
Both pyronaridine‐artesunate and mefloquine plus artesunate were administered once‐daily for three days. The trial did not use a fixed dose combination of mefloquine and artesunate. The mefloquine dose ranged from 6.2 mg/kg to 12.5 mg/kg and the artemether dose ranged from 2.2 mg/kg to 5.0 mg/kg.
Studies meeting the inclusion criteria for safety outcomes
In addition to the five studies meeting the inclusion criteria for efficacy, we included five further studies which met the inclusion criteria for safety outcomes. Two studies had a follow‐up period of 14 days (Ringwald 1996; Ringwald 1998), two had a follow‐up of 42 days (Poravuth 2011; Shin 2011), and one study followed up for one year (Nelwan 2015).
Of these, we included three in a meta‐analysis pertaining to serious adverse events and liver function tests (Nelwan 2015; Poravuth 2011; Shin 2011), in addition to the studies included in the efficacy analysis. These three studies contributed a further 666 participants to the meta‐analysis, and included participants with P vivax malaria recruited from sites in Asia. One study included only adult male soldiers (Nelwan 2015). No participants were aged less than five years. Two studies excluded participants with existing hepatic impairment (Nelwan 2015; Poravuth 2011). Further details of the inclusion and exclusion criteria are provided in the ‘Characteristics of included studies' tables.
The Nelwan 2015 study compared pyronaridine‐artesunate versus artesunate alone or dihydroartemisinin‐piperaquine. The other two studies were based on the same protocol (Poravuth 2011; Shin 2011), and compared pyronaridine‐artesunate versus chloroquine.
We included two further studies (Ringwald 1996; Ringwald 1998), contributing a further 184 participants, in the analysis of other adverse events; these studies compared pyronaridine monotherapy to chloroquine.
As is common to clinical trials, patients with known or suspected pre‐existing liver dysfunction were excluded. Concomitant paracetamol (acetaminophen) administration was allowed in at least two of the trial protocols (Poravuth 2011, Sagara 2018), but the remaining trials do not record whether concomitant paracetamol was allowed or to the extent that it was used.
Excluded studies
We excluded 10 records after further assessment (see Characteristics of excluded studies).
Two were quasi‐RCTs, three were RCTs that were not relevant to this review, and five were not RCTs.
Risk of bias in included studies
See Figure 2.
2.
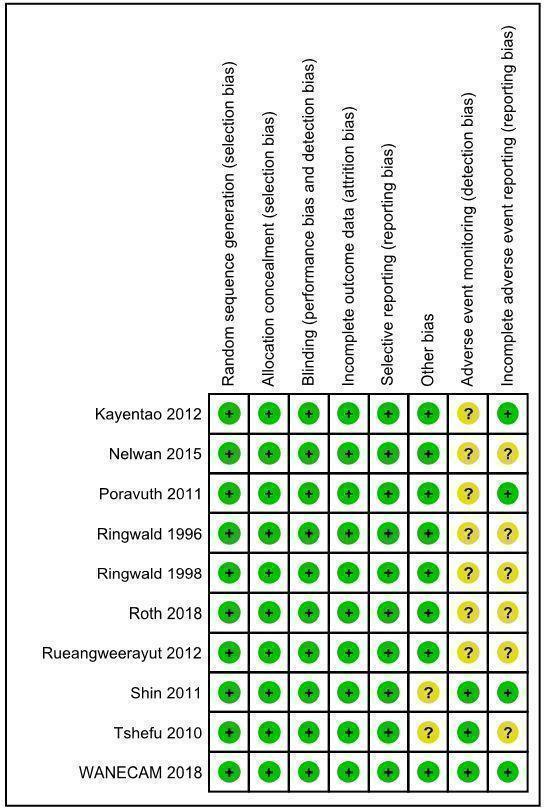
‘Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Seven studies reported the use of computer generated allocation sequences (Kayentao 2012; Poravuth 2011; Roth 2018; Rueangweerayut 2012; Sagara 2018; Shin 2011; Tshefu 2010). The Nelwan 2015 study reported "statistician block‐allocated treatment". Two studies reported block randomization, but it is unclear how blocks were generated (Ringwald 1996; Ringwald 1998). We judged random sequence generation to present a low risk of bias across studies.
Five studies concealed allocation using sealed opaque envelopes (Nelwan 2015; Poravuth 2011; Roth 2018; Sagara 2018; Shin 2011). Three studies concealed allocation using individually numbered treatment packs (Kayentao 2012; Rueangweerayut 2012; Tshefu 2010). Two studies reported central randomization in correspondence with the previous authors of this review (Ringwald 1996; Ringwald 1998). We judged allocation concealment to present a low risk of bias across studies.
Blinding
Five studies reported that the participants were blinded to treatment allocation (Poravuth 2011; Ringwald 1996; Ringwald 1998; Roth 2018; Tshefu 2010). Six studies reported that the investigators performing clinical assessments were blinded to treatment allocation (Kayentao 2012; Poravuth 2011; Roth 2018; Rueangweerayut 2012; Shin 2011; Tshefu 2010). Eight studies reported that the investigators performing parasitological assessments were blinded to treatment allocation (Kayentao 2012; Nelwan 2015; Poravuth 2011; Roth 2018; Rueangweerayut 2012; Sagara 2018; Shin 2011; Tshefu 2010).
Notwithstanding the different degrees to which studies were blinded, we judged there to be a low risk of performance bias and detection bias in relation to the outcomes assessed.
Incomplete outcome data
All of the included trials reported attrition with details of all randomized participants. Our analysis focused on evaluable participants. We did not have concerns that there was differential loss to follow‐up between interventions.
Selective reporting
We located trial registration documents for eight studies (Kayentao 2012; Nelwan 2015; Poravuth 2011; Roth 2018; Rueangweerayut 2012; Sagara 2018; Shin 2011; Tshefu 2010). These appeared to be free of selective reporting based on comparison of registration documents and trial protocols, where available. Though trial registration documents were not available for the remaining two studies (Ringwald 1996; Ringwald 1998), we also considered them to be at low risk of reporting bias, as all the expected outcomes were reported.
Other potential sources of bias
Seven of the 10 included studies were funded by the public‐private partnership of Medicines for Malaria Venture and Shin Poong Pharmaceuticals (Kayentao 2012; Nelwan 2015; Poravuth 2011; Rueangweerayut 2012; Sagara 2018; Shin 2011; Tshefu 2010). The Medicines for Malaria Venture and/or Shin Poong Pharmaceuticals employed study authors in six of these studies (Kayentao 2012; Poravuth 2011; Rueangweerayut 2012; Sagara 2018; Shin 2011; Tshefu 2010). We considered this to pose low risk of bias as all authors took responsibility for reporting accuracy, apart from in one study (Shin 2011), where the lead authors were Shin Poong Pharmaceuticals employees.
Of the remaining three studies not funded by Medicines for Malaria Venture and Shin Poong Pharmaceuticals, one assessed pyronaridine‐artesunate (Roth 2018); the other two assessed pyronaridine monotherapy (Ringwald 1996; Ringwald 1998), and did not contribute to the main analyses.
We considered one study to have unclear risk of other bias in relation to bioavailability of lumefantrine (Tshefu 2010).
Adverse event monitoring (detection bias)
Seven studies provided unclear descriptions, definitions, or schedules for adverse advent monitoring, and therefore we deemed them to have unclear risk of detection bias for adverse events (Kayentao 2012; Nelwan 2015; Poravuth 2011; Ringwald 1996; Ringwald 1998; Roth 2018; Rueangweerayut 2012). We deemed the remaining studies to be at low risk of detection bias for adverse events (Sagara 2018; Shin 2011; Tshefu 2010).
Incomplete adverse event reporting (reporting bias)
In five studies we identified unclear reporting of adverse events, with differences in reporting numbers or thresholds, and deemed these to have unclear risk of reporting bias for adverse events (Nelwan 2015; Ringwald 1996; Ringwald 1998; Roth 2018; Rueangweerayut 2012). We judged the remaining studies to be at low risk of reporting bias for adverse events (Kayentao 2012; Poravuth 2011; Sagara 2018; Shin 2011; Tshefu 2010).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Pyronaridine‐artesunate (PY‐AS) compared to artemether‐lumefantrine (AL) for adults and children with uncomplicated Plasmodium falciparum malaria.
| Pyronaridine‐artesunate (PY‐AS) compared to artemether‐lumefantrine (AL) for adults and children with uncomplicated Plasmodium falciparum malaria | ||||||
| Patient or population: adults and children with uncomplicated P falciparum malaria Setting: malaria transmission settings Intervention: pyronaridine‐artesunate (PY‐AS) Comparison: artemether‐lumefantrine (AL) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with AL | Risk with PY‐AS | |||||
| Total failure: day 28 (PCR‐adjusted) | 15 per 1000 | 9 per 1000 (4 to 19) | RR 0.59 (0.26 to 1.31) | 3068 (4 RCTs) | ⊕⊕⊝⊝
LOWa,b,c Due to indirectness and imprecision |
Compared to AL, PY‐AS may have fewer PCR‐adjusted failures at day 28. |
| Total failure: day 42 (PCR‐adjusted) | 23 per 1000 | 20 per 1000 (12 to 35) | RR 0.86 (0.49 to 1.51) | 2575 (4 RCTs) | ⊕⊕⊝⊝
LOWa,b Due to indirectness and imprecision |
There may be little or no difference in PCR‐adjusted failures at day 42 between PY‐AS and AL. |
| Total failure: day 28 (unadjusted) | 126 per 1000 | 34 per 1000 (16 to 73) | RR 0.27 (0.13 to 0.58) | 3149 (4 RCTs) | ⊕⊕⊝⊝
LOWa,d,e Due to indirectness and inconsistency |
Compared to AL, PY‐AS may have fewer unadjusted failures at day 28. |
| Total failure: day 42 (unadjusted) | 254 per 1000 | 155 per 1000 (117 to 208) | RR 0.61 (0.46 to 0.82) | 3080 (4 RCTs) | ⊕⊕⊝⊝
LOWa,d,e Due to indirectness and inconsistency |
Compared to AL, PY‐AS may have fewer unadjusted failures at day 42. |
| Serious adverse events (42 days) | 3 per 1000 | 3 per 1000 (1 to 12) | RR 0.90 (0.19 to 4.22) | 2004 (3 RCTs) | ⊕⊕⊝⊝
LOWf Due to imprecision |
We do not know if there is a difference in serious adverse events between PY‐AS and AL. |
| First treatment, ALT increase > 5 × ULN (42 days) | 3 per 1000 | 9 per 1000 (3 to 22) | RR 2.92 (1.15 to 7.41) | 3341 (4 RCTs) | ⊕⊕⊝⊝
LOWa,g Due to indirectness and imprecision |
Compared to AL, PY‐AS may lead to higher events of ALT increase > 5 × ULN. (Aggregate analysis indicates this estimate may be accurate). |
| First treatment, AST increase > 5 × ULN (42 days) | 4 per 1000 | 9 per 1000 (3 to 23) | RR 2.20 (0.83 to 5.82) | 3327 (4 RCTs) | ⊕⊝⊝⊝
VERY LOWa,b,h Due to indirectness, inconsistency, and imprecision |
We do not know if there is a difference in AST between PY‐AS and AL. |
| First treatment, bilirubin increase > 2.5 × ULN (42 days) | 6 per 1000 | 5 per 1000 (2 to 12) | RR 0.82 (0.33 to 2.04) | 3130 (3 RCTs) | ⊕⊕⊝⊝
LOWa,b Due to indirectness and imprecision |
We do not know if there is a difference in bilirubin between PY‐AS and AL. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: AL: artemether‐lumefantrine;ALT: alanine aminotransferase; AST: aspartate transaminase; CI: confidence interval; PCR: polymerase chain reaction; PY‐AS: pyronaridine‐artesunate; RCT: randomized controlled trial; RR: risk ratio; ULN: upper limit of normal | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by 1 for serious indirectness: the trials included adults and children and had sites in Africa and Asia. However across the trials, only 115 children and 0 adults were randomized to pyronaridine‐artesunate in Asia. Further adequately powered studies in adults and children in Asia would be needed to fully apply this result. bDowngraded by 1 for serious imprecision: the CI includes both no effect and clinically significant effect. cCertainty of the evidence grade differs from the 2014 review version due to additional data: the previous review reported no substantial difference between PY‐AS and AL in reference to this outcome and therefore did not downgrade for imprecision. In this update we report a reduced rate in the PY‐AS arm. Because we concluded that there may be a difference, we necessarily downgraded for the imprecision. dCertainty of the evidence grade differs from the 2014 review version due to additional data: the introduction of more data increased the heterogeneity between the included trials. eDowngraded by 1 for serious inconsistency: there was quantitative heterogeneity between studies. fDowngraded by 2 for very serious imprecision: the low number of events recorded in the studies is insufficient for confidently estimating the effect size. gDowngraded by 1 for serious imprecision: the CI is wide and includes a clinically significant effect, and almost no effect. hDowngraded by 1 for serious inconsistency: there was qualitative heterogeneity between studies.
Summary of findings 2. Pyronaridine‐artesunate (PY‐AS) compared to artesunate‐amodiaquine (AS‐AQ) for adults and children with uncomplicated Plasmodium falciparum malaria.
| Pyronaridine‐artesunate (PY‐AS) compared to artesunate‐amodiaquine (AS‐AQ) for adults and children with uncomplicated Plasmodium falciparum malaria | ||||||
| Patient or population: adults and children with uncomplicated P falciparum malaria Setting: malaria transmission settings Intervention: pyronaridine‐artesunate (PY‐AS) Comparison: artesunate‐amodiaquine (AS‐AQ) | ||||||
| Outcomesa | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with artesunate‐amodiaquine (AS‐AQ) | Risk with pyronaridine‐artesunate (PY‐AS) | |||||
| Total failure: day 28 (PCR‐adjusted) | 8 per 1000 | 4 per 1000 (1 to 22) | RR 0.55 (0.11 to 2.77) | 1245 (1 RCT) | ⊕⊕⊝⊝
LOWb,c Due to indirectness and imprecision |
Compared to AS‐AQ, PY‐AS may have fewer PCR‐adjusted failures at day 28. |
| Total failure: day 42 (PCR‐adjusted) | 6 per 1000 | 5 per 1000 (1 to 27) | RR 0.98 (0.20 to 4.83) | 1091 (1 RCT) | ⊕⊕⊝⊝
LOWb,d Due to indirectness and imprecision |
There may be little or no difference in PCR‐adjusted failures at day 42 between PY‐AS and AS‐AQ. |
| Total failure: day 28 (unadjusted) | 75 per 1000 | 37 per 1000 (22 to 61) | RR 0.49 (0.30 to 0.81) | 1257 (1 RCT) | ⊕⊕⊕⊝
MODERATEb Due to indirectness |
Compared to AS‐AQ, PY‐AS probably has fewer unadjusted failures at day 28. |
| Total failure: day 42 (unadjusted) | 195 per 1000 | 192 per 1000 (152 to 240) | RR 0.98 (0.78 to 1.23) | 1235 (1 RCT) | ⊕⊕⊕⊝
MODERATEb Due to indirectness |
There is probably little or no difference in unadjusted failures at day 42 between PY‐AS and AS‐AQ. |
| First treatment, ALT increase > 5 × ULN (42 days) | 1 per 1000 | 1 per 1000 (0 to 7) | RR 1.41 (0.28 to 7.09) | 1317 (1 RCT) | ⊕⊕⊝⊝
LOWb,e Due to indirectness and imprecision |
Compared to AL, PY‐AS may have lead to higher events of ALT increase > 5 × ULN. (Aggregate analysis indicates this estimate may be accurate). |
| First treatment, AST increase > 5 × ULN (42 days) | 4 per 1000 | 2 per 1000 (0 to 8) | RR 0.43 (0.08 to 2.07) | 1317 (1 RCT) | ⊕⊝⊝⊝
VERY LOWb,f Due to indirectness and imprecision |
We do not know if there is a difference in AST between PY‐AS and AS‐AQ. |
| First treatment, bilirubin increase > 2.5 × ULN (42 days) | 1 per 1000 | 1 per 1000 (0 to 16) | RR 0.99 (0.06 to 15.76) | 1317 (1 RCT) | ⊕⊝⊝⊝
VERY LOWb,f Due to indirectness and imprecision |
We do not know if there is a difference in bilirubin between PY‐AS and AS‐AQ. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: ALT: alanine aminotransferase; AS‐AQ: artesunate‐amodiaquine; AST: aspartate transaminase; CI: confidence interval; PCR: polymerase chain reaction; PY‐AS: pyronaridine‐artesunate; RCT: randomized controlled trial; RR: risk ratio; ULN: upper limit of normal | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
aSerious adverse events data were not available disaggregated by site to allow inclusion in this comparison. bDowngraded by 1 for serious indirectness: the data are drawn from one study, conducted in six sites in three countries in West Africa. Further studies in Asia would be needed to fully apply this result. cDowngraded by 1 for serious imprecision: the CI is large and includes both no effect and clinically important effects. dDowngraded by 1 for serious imprecision: the effect estimate is close to no effect, but the CI is wide. eDowngraded by 1 for serious imprecision: the low number of events recorded in the study is insufficient for confidently estimating the effect size. However, aggregate analysis of ALT increase across different comparator drugs provides indirect evidence that the point estimate may be accurate. fDowngraded by 2 for very serious imprecision: the CI is very large and includes both no effect and clinically important effects.
Summary of findings 3. Pyronaridine‐artesunate (PY‐AS) compared to mefloquine plus artesunate (MQ+AS) for adults and children with uncomplicated Plasmodium falciparum malaria.
| Pyronaridine‐artesunate (PY‐AS) compared to mefloquine plus artesunate (MQ+AS) for adults and children with uncomplicated Plasmodium falciparum malaria | ||||||
| Patient or population: adults and children with uncomplicated P falciparum malaria Setting: malaria transmission settings Intervention: pyronaridine‐artesunate (PY‐AS) Comparison: mefloquine plus artesunate (MQ+AS) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with mefloquine plus artesunate (MQ+AS) | Risk with pyronaridine‐artesunate (PY‐AS) | |||||
| Total failure: day 28 (PCR‐adjusted) | 22 per 1000 | 8 per 1000 (3 to 23) | RR 0.37 (0.13 to 1.05) | 1117 (1 RCT) | ⊕⊕⊝⊝
LOWa,b,c Due to indirectness and imprecision |
Compared to MQ+AS, PY‐AS may have fewer PCR‐adjusted failures at day 28. |
| Total failure: day 42 (PCR‐adjusted) | 29 per 1000 | 53 per 1000 (27 to 105) | RR 1.80 (0.90 to 3.57) | 1037 (1 RCT) | ⊕⊕⊝⊝
LOWa,b Due to indirectness and imprecision |
Compared to MQ+AS, PY‐AS may have more PCR‐adjusted failures at day 42. |
| Total failure: day 28 (unadjusted) | 41 per 1000 | 15 per 1000 (7 to 32) | RR 0.36 (0.17 to 0.78) | 1120 (1 RCT) | ⊕⊕⊕⊝
MODERATEa Due to indirectness |
Compared to MQ+AS, PY‐AS probably has fewer unadjusted failures at day 28. |
| Total failure: day 42 (unadjusted) | 83 per 1000 | 70 per 1000 (45 to 109) | RR 0.84 (0.54 to 1.31) | 1059 (1 RCT) | ⊕⊕⊝⊝
LOWa,b,d Due to indirectness and imprecision |
There is probably little or no difference in unadjusted failures at day 42 between PY‐AS and MQ+AS. |
| Serious adverse events (42 days) | 7 per 1000 | 7 per 1000 (2 to 28) | RR 1.00 (0.25 to 3.97) | 1271 (1 RCT) | ⊕⊕⊝⊝
LOWa,b Due to indirectness and imprecision |
There may be little or no difference in serious adverse events between PY‐AS and MQ+AS |
| First treatment, ALT increase > 5 × ULN (42 days) | 2 per 1000 | 18 per 1000 (2 to 133) | RR 7.48 (0.99 to 56.45) | 1271 (1 RCT) | ⊕⊕⊝⊝
LOWa,e Due to indirectness and imprecision |
Compared to MQ+AS, PY‐AS may lead to higher events of ALT increase > 5 × ULN. (Aggregate analysis indicates this estimate may be accurate). |
| First treatment, AST increase > 5 × ULN (42 days) | 0 per 1000 | 0 per 1000 (0 to 0) | RR 9.49 (0.55 to 162.64) | 1271 (1 RCT) | ⊕⊝⊝⊝
VERY LOWa,f Due to indirectness and imprecision |
We do not know if there is a difference in AST between PY‐AS and MQ+AS. |
| First treatment, bilirubin increase > 2.5 × ULN (42 days) | 2 per 1000 | 8 per 1000 (1 to 67) | RR 3.49 (0.43 to 28.29) | 1271 (1 RCT) | ⊕⊝⊝⊝
VERY LOWa,f Due to indirectness and imprecision |
We do not know if there is a difference in bilirubin between PY‐AS and MQ+AS. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: ALT: alanine aminotransferase; AST: aspartate transaminase; CI: confidence interval; MQ+AS: mefloquine plus artesunate; PCR: polymerase chain reaction; PY‐AS: pyronaridine‐artesunate; RCT: randomized controlled trial; RR: risk ratio; ULN: upper limit of normal | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by 1 for serious indirectness: of the 1271 children and adults aged greater than 5 years enrolled in this trial, 81.3% (1033) were enrolled and treated in trial sites in Asia (Cambodia, India, Thailand, and Vietnam), and only 18.7% (237) in Africa (Burkina Faso, Ivory Coast, and Tanzania). Further studies in African children are necessary to fully apply this result. bDowngraded by 1 for serious imprecision: the CI is large and includes both no effect and clinically important effects. cCertainty of the evidence grade differs from the 2014 review version due to additional data: the previous review reported no substantial difference between PY‐AS and MQ+AS in reference to this outcome and therefore did not downgrade for imprecision. In this update we report a reduced rate in the PY‐AS arm. Because we concluded that there may be a difference, we necessarily downgraded for the imprecision. dCertainty of the evidence grade differs from the 2014 review version due to alterations in the data extraction protocol: the CI has become less precise, and our decision has greater consistency with other outcome certainty grades. eDowngraded by 1 for serious imprecision: the low number of events recorded in the study is insufficient for confidently estimating the effect size. However, aggregate analysis of ALT increase across different comparator drugs provides indirect evidence that the point estimate may be accurate. fDowngraded by 2 for very serious imprecision: the CI is very large and includes both no effect and clinically important effects.
Summary of findings 4. Pyronaridine‐artesunate (PY‐AS) compared to other antimalarials for adults and children with uncomplicated malaria.
| Pyronaridine‐artesunate (PY‐AS) compared to other antimalarials for adults and children with uncomplicated malaria | ||||||
| Patient or population: adults and children with uncomplicated malaria Setting: high and low transmission settings for P falciparum and P vivax malaria Intervention: pyronaridine‐artesunate (PY‐AS) Comparison: other antimalarials | ||||||
| Outcomesa,b,c | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with other antimalarials | Risk with pyronaridine‐artesunate (PY‐AS) | |||||
| Serious adverse events | 5 per 1000 | 7 per 1000 (3 to 15) | RR 1.24 (0.54 to 2.84) | 3941 (7 RCTs) | ⊕⊕⊕⊝
MODERATEd Due to imprecision |
There was probably little or no difference in the rate of serious adverse events with PY‐AS compared to other antimalarials. |
| First treatment, ALT increase > 5 × ULN | 2 per 1000 | 7 per 1000 (3 to 14) | RR 3.34 (1.63 to 6.84) | 6614 (8 RCTs) | ⊕⊕⊕⊕ HIGHe | ALT increase > 5 × ULN is more frequent with PY‐AS compared to other antimalarials. |
| First treatment, AST increase > 5 × ULN | 3 per 1000 | 5 per 1000 (3 to 11) | RR 1.80 (0.89 to 3.65) | 6614 (8 RCTs) | ⊕⊕⊕⊝
MODERATEf Due to imprecision |
There is probably a small increased risk of AST increase > 5 × ULN with PY‐AS compared to other antimalarials. |
| First treatment, bilirubin increase > 2.5 × ULN | 4 per 1000 | 4 per 1000 (2 to 9) | RR 1.03 (0.49 to 2.18) | 6417 (7 RCTs) | ⊕⊕⊕⊝
MODERATEd Due to imprecision |
There is probably little or no difference for bilirubin between PY‐AS and other antimalarials. |
| Subsequent treatment(s), ALT > 5 × ULN | 4 per 1000 | 8 per 1000 (3 to 23) | RR 2.18 (0.76 to 6.27) | 1649 (1 RCT) | ⊕⊕⊝⊝
LOWd,f Due to imprecision and indirectness |
There may be an increased risk of raised ALT with subsequent treatments with PY‐AS compared to other antimalarials. |
| Subsequent treatment(s), AST > 5 × ULN | 6 per 1000 | 11 per 1000 (4 to 27) | RR 1.82 (0.74 to 4.44) | 1649 (1 RCT) | ⊕⊕⊝⊝
LOWd,f Due to imprecision and indirectness |
There may be an increased risk of raised AST with subsequent treatments with PY‐AS compared to other antimalarials. |
| Subsequent treatment(s), bilirubin > 5 × ULN | 8 per 1000 | 9 per 1000 (3 to 24) | RR 1.13 (0.42 to 3.01) | 1649 (1 RCT) | ⊕⊕⊝⊝
LOWd,f Due to imprecision and indirectness |
There may be little or no difference for bilirubin between PY‐AS and other antimalarials. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: ALT: alanine aminotransferase; AST: aspartate transaminase; CI: confidence interval; PY‐AS: pyronaridine‐artesunate; RCT: randomized controlled trial; RR: risk ratio; ULN: upper limit of normal | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
aOnly adverse event outcomes were considered for this comparison. bA comparison of pyronaridine‐artesunate versus other antimalarials for frequency of ECG abnormalities is reported in Table 9. cThe length of follow‐up varies between specific studies. Follow‐up times are reported for individual studies in the ‘Characteristics of included studies' tables. dDowngraded by 1 for serious imprecision: the CI includes both no effect and clinically important effects. eNot downgraded: although the CI is wide, there were few events. fDowngraded by 1 for serious indirectness: only 232 children aged less than five years were included in this study.
Comparison 1. Pyronaridine‐artesunate versus artemether‐lumefantrine
Four studies with 3341 participants contributed data to this comparison (Kayentao 2012; Roth 2018; Sagara 2018; Tshefu 2010).
Total treatment failure (PCR‐adjusted)
In the pooled analysis, there were fewer PCR‐adjusted treatment failures at day 28 following treatment with pyronaridine‐artesunate compared to artemether‐lumefantrine, but the CI crossed the line of no effect (risk ratio (RR) 0.59, 95% CI 0.26 to 1.31; 4 trials, 3068 participants; Analysis 1.2). There was little or no difference at day 42 (RR 0.86, 95% CI 0.49 to 1.51; 4 trials, 2575 participants; Analysis 1.1).
1.2. Analysis.
Comparison 1 Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 2 Total failure: day 28 (PCR‐adjusted).
1.1. Analysis.
Comparison 1 Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 1 Total failure: day 42 (PCR‐adjusted).
The PCR‐adjusted treatment failure rate for pyronaridine‐artesunate was less than 5% in all trials at day 28. At day 42, the PCR‐adjusted treatment failure rate for pyronaridine‐artesunate was slightly greater than 5% in two studies (Kayentao 2012, 18 events for 275 evaluable patients, 6.5%; Roth 2018, 4 events for 77 evaluable patients, 5.2%).
Total treatment failure (PCR‐unadjusted)
In the pooled analysis, there were fewer PCR‐unadjusted treatment failures following treatment with pyronaridine‐artesunate compared to artemether‐lumefantrine at day 28 (RR 0.27, 95% CI 0.13 to 0.58; 4 trials, 3149 participants; Analysis 1.3) and at day 42 (RR 0.61, 95% CI 0.46 to 0.82, 4 trials, 3080 participants; Analysis 1.4).
1.3. Analysis.
Comparison 1 Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 3 Total failure: day 28 (unadjusted).
1.4. Analysis.
Comparison 1 Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 4 Total failure: day 42 (unadjusted).
Early treatment failure
There were two events of early treatment failure which occurred in one trial (Kayentao 2012), both in the pyronaridine‐artesunate arm (RR 2.53, 95% CI 0.12 to 52.39; 4 trials, 3149 participants; Analysis 1.5).
1.5. Analysis.
Comparison 1 Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 5 Early treatment failure.
Serious adverse events
We were unable to include the data on serious adverse events from one multicentre trial in the meta‐analysis because the data were not disaggregated by trial site (Sagara 2018), and participant randomization did not take place independently from site. Instead, we have summarized the number and nature of the serious adverse events in the trial in Table 6. Across the other trials included in the quantitative synthesis, there were six serious adverse events, with four occurring in patients in the pyronaridine‐artesunate arm and two in patients in the artemether‐lumefantrine arm. There was no significant difference between treatments (RR 0.90, 95% CI 0.19 to 4.22; 3 trials, 2004 participants; Analysis 1.6).
2. Serious adverse events.
| Study | Pyronaridine‐artesunate | Comparator(s) |
| Kayentao 2012 | Severe malaria (1)a |
Artemether‐lumefantrine ‐ |
| Nelwan 2015 | Head trauma (1)a Typhoid fever (1)b Nephrolithiasis (1)b |
Artesunate only Metacarpal fracture (1)a Acute gastroenteritis (1)a Suspected ureteric stone (1)b Dihydroartemisinin‐piperaquine Dengue fever (1)a |
| Poravuth 2011 | Pyrexia (1)a Typhoid fever (1)a |
Chloroquine ‐ |
| Ringwald 1996 | ‐ | ‐ |
| Ringwald 1998 | ‐ | ‐ |
| Roth 2018 | ‐ | ‐ |
| Rueangweerayut 2012 | Autoimmune haemolytic anaemia (1)a Cholera (1)a Pneumonia (1)a Acute pyelonephritis (1)a Wound infection (1)a Abortion (1)a Depression (1)a |
Mefloquine plus artesunate Cerebral malaria (1)a Seizure (1)c Grand mal seizure (1)c |
| Tshefu 2010 | Parotitis (1)a Typhoid fever (1)a Urinary tract infection (1)a |
Artemether‐lumefantrine Cerebral malaria (1)a Immunosuppression (1)a |
| Sagara 2018d | Elevated ALT (2)c Elevated AST (2)c Transaminases increased (4)c Drug‐induced liver injury (1)c Hypercreatininemia (1)c |
Artemether‐lumefantrine Drug‐induced liver injury (1)c Toxic epidermal necrolysis (1)c Artesunate‐amodiaquine Drug‐induced liver injury (1)c Transaminases increased (2)c |
Abbreviations: ALT: alanine aminotransferase; AST: aspartate aminotransferase.
aStudy authors judged as unrelated to drug. bStudy authors judged as unlikely related to drug. cStudy authors judged as treatment‐related. dAuthors do not report the nature of the serious adverse events they judged to be unrelated to drug. Some of the listed events in the comparator groups may have occurred in comparisons with dihydroartemisinin‐piperaquine, but we were unable to extract data in relation to this.
1.6. Analysis.
Comparison 1 Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 6 Serious adverse events.
Adverse events leading to withdrawal from treatment
We were unable to include data from one trial (Sagara 2018), for the same reason as given above. Across the other trials included in quantitative synthesis, there were 37 events leading to withdrawal from treatment, with 27 occurring in patients in the pyronaridine‐artesunate arm and 10 in patients in the artemether‐lumefantrine arm. There was no significant difference between treatments (RR 1.41, 95% CI 0.68 to 2.90; 3 trials, 2004 participants; Analysis 1.7).
1.7. Analysis.
Comparison 1 Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 7 Adverse events leading to withdrawal.
Elevated liver function tests
Following first treatment, the proportion of participants with raised alanine aminotransferase (ALT) greater than five times the upper limit of normal (> 5 x ULN) was higher in those treated with pyronaridine‐artesunate compared to artemether‐lumefantrine (RR 2.92, 95% CI 1.15 to 7.41; 4 trials, 3327 participants; Analysis 1.8, Figure 3). There were 0 events in either arm of one study (Roth 2018), so this did not contribute to the relative risk calculation.
1.8. Analysis.
Comparison 1 Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 8 First treatment, ALT increase > 5 × ULN.
3.
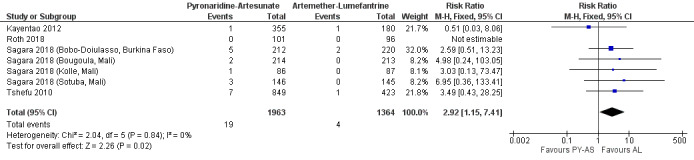
Forest plot of comparison 1: Pyronaridine‐artesunate versus artemether‐lumefantrine, outcome 1.8: ALT increase > 5 × ULN, first treatment.
There was no significant difference in raised aspartate transaminase (AST) > 5 x ULN (RR 2.20, 95% CI 0.83 to 5.82; 4 trials, 3327 participants; Analysis 1.9) or bilirubin > 2.5 x ULN (RR 0.82, 95% CI 0.33 to 2.04; 3 trials, 3130 participants; Analysis 1.10) between pyronaridine‐artesunate and artemether‐lumefantrine.
1.9. Analysis.
Comparison 1 Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 9 First treatment, AST increase > 5 × ULN.
1.10. Analysis.
Comparison 1 Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 10 First treatment, bilirubin increase > 2.5 × ULN.
One trial investigated the rate of elevated liver function tests in patients receiving second or subsequent treatments with pyronaridine‐artesunate compared to artemether‐lumefantrine (Sagara 2018). The rates of such events were low in each treatment arm. In a pooled analysis across the trial sites we detected no significant differences in the number of raised ALT (> 5 x ULN), AST (> 5 x ULN), or bilirubin (> 2.5 x ULN) events between pyronaridine‐artesunate and artemether‐lumefantrine (1 trial, 865 participants; Analysis 1.11; Analysis 1.12; Analysis 1.13).
1.11. Analysis.
Comparison 1 Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 11 Subsequent treatment(s), ALT increase > 5 × ULN.
1.12. Analysis.
Comparison 1 Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 12 Subsequent treatment(s), AST increase > 5 × ULN.
1.13. Analysis.
Comparison 1 Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 13 Subsequent treatment(s), bilirubin increase > 2.5 × ULN.
Subgroup analysis
When we included only the two trials which studied paediatric populations exclusively (Kayentao 2012; Roth 2018), we did not find differences in efficacy or safety outcomes between pyronaridine‐artesunate and artemether‐lumefantrine (2 trials, 732 participants in safety analysis; Analysis 1.14; Analysis 1.15; Analysis 1.16; Analysis 1.17; Analysis 1.18; Analysis 1.19). We were unable to extract disaggregated data for children from the other two trials (Sagara 2018; Tshefu 2010).
1.14. Analysis.
Comparison 1 Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 14 Paediatric trials ‐ total failure: day 28 (PCR‐adjusted).
1.15. Analysis.
Comparison 1 Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 15 Paediatric trials ‐ total failure: day 42 (PCR‐adjusted).
1.16. Analysis.
Comparison 1 Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 16 Paediatric trials ‐ total failure: day 28 (unadjusted).
1.17. Analysis.
Comparison 1 Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 17 Paediatric trials ‐ total failure: day 42 (unadjusted).
1.18. Analysis.
Comparison 1 Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 18 Paediatric trials ‐ first treatment, ALT increase > 5 × ULN.
1.19. Analysis.
Comparison 1 Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 19 Paediatric trials ‐ first treatment, AST increase > 5 × ULN.
There were not enough studies to perform further subgroup analyses or investigation of heterogeneity.
Narrative synthesis of other reported outcomes
Three studies also reported fever and parasite clearance times, which were broadly comparable between pyronaridine‐artesunate and artemether‐lumefantrine (Table 7). Differences in reporting precluded quantitative synthesis.
3. Pyronaridine‐artesunate (PY‐AS) versus artemether‐lumefantrine (AL): other reported outcomes.
| Trial | Fever clearance time | Parasite clearance time | ||
| PY‐AS | AL | PY‐AS | AL | |
| Kayentao 2012 | Median 8.1 h (95% CI 8.0 to 8.1) | Median 8.1 h (95% CI 8.0 to 15.8) | Median 24.1 h (95% CI 24.0 to 24.1) | Median 24.2 h (95% CI 24.1 to 32.0) |
| Roth 2018 | Median 1 day (1‐1) | Median 1 day (1‐1) | Median 1 day (1‐2) | Median 2 days (1‐2) |
| Tshefu 2010 | Mean 13.6 h (SD 8.9) | Mean 14.8 h (SD 10.1) | 23.3 h (SD 8.8) | 26.5 h (10.1) |
Abbreviations: AL: artemether‐lumefantrine; PY‐AS: pyronaridine‐artesunate.
Comparison 2. Pyronaridine‐artesunate versus artesunate‐amodiaquine
One study with 1336 participants contributed data to this comparison (Sagara 2018). We extracted data disaggregated by site as described in Results of the search, and presented them separately in our meta‐analyses.
Total treatment failure (PCR‐adjusted)
In the pooled analysis across the multiple sites, there were fewer PCR‐adjusted treatment failures at day 28 for pyronaridine‐artesunate compared to artesunate‐amodiaquine, but the CI crossed the line of no effect (RR 0.55, 95% CI 0.11 to 2.77; 1 trial, 1245 participants; Analysis 2.1). There was little or no difference in PCR‐adjusted treatment failure at day 42 (RR 0.98, 95% CI 0.20 to 4.83; 1 trial, 1091 participants; Analysis 2.2).
2.1. Analysis.
Comparison 2 Pyronaridine‐artesunate versus artesunate‐amodiaquine, Outcome 1 Total failure: day 28 (PCR‐adjusted).
2.2. Analysis.
Comparison 2 Pyronaridine‐artesunate versus artesunate‐amodiaquine, Outcome 2 Total failure: day 42 (PCR‐adjusted).
The PCR‐adjusted treatment failure rate for pyronaridine‐artesunate was less than 5% in all sites at both day 28 and day 42.
Total treatment failure (PCR‐unadjusted)
In pooled analysis, pyronaridine‐artesunate had fewer PCR‐unadjusted treatment failures compared to artesunate‐amodiaquine at day 28 (RR 0.49, 95% CI 0.30 to 0.81; 1 trial, 1257 participants; Analysis 2.3). At day 42, there was little or no difference (RR 0.98, 95% CI 0.78 to 1.23; 1 trial, 1235 participants; Analysis 2.4).
2.3. Analysis.
Comparison 2 Pyronaridine‐artesunate versus artesunate‐amodiaquine, Outcome 3 Total failure: day 28 (unadjusted).
2.4. Analysis.
Comparison 2 Pyronaridine‐artesunate versus artesunate‐amodiaquine, Outcome 4 Total failure: day 42 (unadjusted).
Early treatment failure
There was no early treatment failure reported in either the pyronaridine‐artesunate arm or the artesunate‐amodiaquine arm across all study sites (1336 participants, 1 trial).
Serious adverse events, adverse events leading to withdrawal from treatment
We were unable to include the trial's data on serious adverse events and adverse events leading to withdrawal in a meta‐analysis, for the reason given above. We summarized the number and nature of the serious adverse events in the trial in Table 6.
Elevated liver function tests
Following first treatment, there was no significant difference in raised ALT > 5 x ULN (RR 1.41, 95% CI 0.28 to 7.09; 1 trial, 1317 participants; Analysis 2.5), raised AST > 5 x ULN (RR 0.40, 95% CI 0.08 to 2.07; 1 trial, 1317 participants, Analysis 2.6), or raised bilirubin > 2.5 x ULN (RR 0.99, 95% CI 0.06 to 15.76; 1 trial, 1317 participants; Analysis 2.7) between pyronaridine‐artesunate and artesunate‐amodiaquine.
2.5. Analysis.
Comparison 2 Pyronaridine‐artesunate versus artesunate‐amodiaquine, Outcome 5 First treatment, ALT increase > 5 × ULN.
2.6. Analysis.
Comparison 2 Pyronaridine‐artesunate versus artesunate‐amodiaquine, Outcome 6 First treatment, AST increase > 5 × ULN.
2.7. Analysis.
Comparison 2 Pyronaridine‐artesunate versus artesunate‐amodiaquine, Outcome 7 First treatment, bilirubin increase > 2.5 × ULN.
Similarly, on second or subsequent treatments, we detected no significant difference in the number of raised ALT (> 5 x ULN), AST (> 5 x ULN) or bilirubin (> 2.5 x ULN) events between pyronaridine‐artesunate and artesunate‐amodiaquine treatment arms (784 participants, 1 trial (Analysis 2.8; Analysis 2.9; Analysis 2.10).
2.8. Analysis.
Comparison 2 Pyronaridine‐artesunate versus artesunate‐amodiaquine, Outcome 8 Subsequent treatment(s), ALT increase > 5 × ULN.
2.9. Analysis.
Comparison 2 Pyronaridine‐artesunate versus artesunate‐amodiaquine, Outcome 9 Subsequent treatment(s), AST increase > 5 × ULN.
2.10. Analysis.
Comparison 2 Pyronaridine‐artesunate versus artesunate‐amodiaquine, Outcome 10 Subsequent treatment(s), bilirubin increase > 2.5 × ULN.
Comparison 3. Pyronaridine‐artesunate versus mefloquine plus artesunate
One study with 1271 participants contributed data to this comparison (Rueangweerayut 2012).
Total treatment failure (PCR‐adjusted)
There were fewer PCR‐adjusted treatment failures at day 28 for pyronaridine‐artesunate compared to mefloquine plus artesunate, but the CI crossed the line of no effect (RR 0.37, 95% CI 0.13 to 1.05; 1 trial, 1117 participants; Analysis 3.1). There were more PCR‐adjusted treatment failures at day 42 for pyronaridine‐artesunate compared to mefloquine plus artesunate, but the CI crossed the line of no effect (RR 1.80, 95% CI 0.90 to 3.57; 1 trial, 1037 participants; Analysis 3.2).
3.1. Analysis.
Comparison 3 Pyronaridine‐artesunate versus mefloquine plus artesunate, Outcome 1 Total failure: day 28 (PCR‐adjusted).
3.2. Analysis.
Comparison 3 Pyronaridine‐artesunate versus mefloquine plus artesunate, Outcome 2 Total failure: day 42 (PCR‐adjusted).
The PCR‐adjusted treatment failure rate for pyronaridine‐artesunate was less than 5% at day 28. At day 42, the PCR‐adjusted treatment failure rate for pyronaridine‐artesunate was slightly greater than 5% (37 events for 698 evaluable patients, 6.5%).
Total treatment failure (PCR‐unadjusted)
Pyronaridine‐artesunate had fewer PCR‐unadjusted treatment failures compared to mefloquine plus artesunate at day 28 (RR 0.36, 95% CI 0.17 to 0.78; 1 trial, 1120 participants; Analysis 3.3). At day 42, there was little or no difference between pyronaridine‐artesunate and mefloquine plus artesunate (RR 0.84, 95% CI 0.54 to 1.31; 1 trial, 1059 participants; Analysis 3.4).
3.3. Analysis.
Comparison 3 Pyronaridine‐artesunate versus mefloquine plus artesunate, Outcome 3 Total failure: day 28 (unadjusted).
3.4. Analysis.
Comparison 3 Pyronaridine‐artesunate versus mefloquine plus artesunate, Outcome 4 Total failure: day 42 (unadjusted).
Early treatment failure
There was one early treatment failure in the mefloquine plus artesunate arm of the study, and none in the pyronaridine‐artesunate arm.
Serious adverse events, adverse events leading to withdrawal from treatment
There was little or no difference in serious adverse events between pyronaridine‐artesunate and mefloquine plus artesunate (RR 1.00, 95% CI 0.25 to 3.97; 1 trial, 1271 participants; Analysis 3.5). There was no significant different in adverse events leading to withdrawal from treatment (1271 participants, 1 trial; Analysis 3.6).
3.5. Analysis.
Comparison 3 Pyronaridine‐artesunate versus mefloquine plus artesunate, Outcome 5 Serious adverse events.
3.6. Analysis.
Comparison 3 Pyronaridine‐artesunate versus mefloquine plus artesunate, Outcome 6 Adverse events leading to withdrawal.
Elevated liver function tests
There were higher rates of raised ALT > 5 x ULN in the pyronaridine‐artesunate arm compared to the mefloquine plus artesunate arm, but the CIs crossed the line of no effect (RR 7.48, 95% CI 0.99 to 56.45; 1 trial, 1271 participants; Analysis 3.7). We did not find a significant difference for the rate of raised AST > 5 x ULN (RR 9.49, 95% CI 0.55 to 162.64; 1 trial, 1271 participants; Analysis 3.8) or bilirubin > 2.5 x ULN (RR 3.49, 95% CI 0.43 to 28.29; 1 trial, 1271 participants; Analysis 3.9).
3.7. Analysis.
Comparison 3 Pyronaridine‐artesunate versus mefloquine plus artesunate, Outcome 7 First treatment, ALT increase > 5 × ULN.
3.8. Analysis.
Comparison 3 Pyronaridine‐artesunate versus mefloquine plus artesunate, Outcome 8 First treatment, AST increase > 5 × ULN.
3.9. Analysis.
Comparison 3 Pyronaridine‐artesunate versus mefloquine plus artesunate, Outcome 9 First treatment, bilirubin increase > 2.5 × ULN.
Narrative synthesis of other reported outcomes
The Rueangweerayut 2012 study also reported fever, parasite and gametocyte clearance times, which were broadly comparable between pyronaridine‐artesunate and mefloquine plus artesunate (Table 8).
4. Pyronaridine‐artesunate (PY‐AS) versus mefloquine plus artesunate (MQ+AS): other reported outcomes.
| Trial | Fever clearance time | Parasite clearance time | Gametocyte clearance time | |||
| PY‐AS | MQ+AS | PY‐AS | MQ+AS | PY‐AS | MQ+AS | |
| Rueangweerayut 2012 | Mean 19.3 h (SD 12.9) | Mean 19.2 h (SD 12.5) | Mean 35.9 h (SD 19.8) | Mean 38.5 h (SD 20.1) | Mean 25.5 h (SD 23.3) | Mean 30.9 h (SD 19.9) |
Abbreviations: MQ+AS: mefloquine plus artesunate; PY‐AS: pyronaridine‐artesunate.
Comparison 4. Pyronaridine‐artesunate versus any other antimalarial
Eight RCTs with 6614 participants contributed data to a safety meta‐analysis in which we compared pyronaridine‐artesunate to any other antimalarial. The comparators were artesunate alone, artemether‐lumefantrine, dihydroartemisin‐piperaquine, chloroquine, mefloquine plus artesunate, and artesunate‐amodiaquine. For the Sagara 2018 trial, we extracted data disaggregated by site, as described in Results of the search, and we present them separately in meta‐analysis.
An additional two RCTs compared pyronaridine monotherapy to chloroquine (Ringwald 1996; Ringwald 1998), and contributed to the quantitative and qualitative synthesis of other adverse events (excluding serious adverse events or liver enzymes).
All trials contributing to the safety meta‐analysis excluded participants with baseline hepatic impairment.
Serious adverse events
We detected little or no difference in the rate of serious adverse events with pyronaridine‐artesunate compared to other antimalarials (RR 1.24, 95% CI 0.54 to 2.84; 7 trials, 3941 participants; Analysis 4.1). We were unable to include data from the Sagara 2018 trial in this meta‐analysis, as explained above.
4.1. Analysis.
Comparison 4 Pyronaridine‐artesunate versus other antimalarials for all malaria subtypes (safety outcomes only), Outcome 1 Serious adverse events.
To provide a narrative synthesis, we summarized the nature and number of serious adverse events in Table 6. In the pyronaridine‐artesunate arm, we judged 10 of 26 serious adverse events across 10 trials to be related to treatment with pyronaridine‐artesunate or pyronaridine alone (Kayentao 2012; Nelwan 2015; Poravuth 2011; Ringwald 1996; Ringwald 1998; Roth 2018; Rueangweerayut 2012; Sagara 2018; Shin 2011; Tshefu 2010), with each of these coming in the Sagara 2018 trial. In comparison, we judged two of four events in the artemether‐lumefantrine arm (Kayentao 2012; Sagara 2018; Tshefu 2010), three of three in the artesunate‐amodiaquine arm (Sagara 2018), and two of three in the mefloquine plus artesunate arm (Rueangweerayut 2012), to be related to the treatment. We did not judge the sole serious adverse event seen with dihydroartemisinin‐piperaquine and the three seen in the artesunate only arm to be related to treatment (Nelwan 2015). No serious adverse events were seen in the chloroquine arm (Poravuth 2011; Ringwald 1996; Ringwald 1998; Roth 2018).
Adverse events leading to withdrawal from treatment
We detected little or no differences in the rate of adverse events leading to withdrawal from treatment with pyronaridine‐artesunate compared to other antimalarials (RR 1.06, 95% CI 0.58 to 1.94; 6 trials, 3911 participants; Analysis 4.2).
4.2. Analysis.
Comparison 4 Pyronaridine‐artesunate versus other antimalarials for all malaria subtypes (safety outcomes only), Outcome 2 Adverse events leading to withdrawal.
Elevated liver function tests
Quantitative synthesis
Different studies defined rises in ALT, AST, and bilirubin as important at different levels, ranging from 3 x ULN to 10 x ULN. The differences between definitions of important rises are shown in Table 5.
Following first treatment, pyronaridine‐artesunate was associated with a greater incidence of raised ALT > 5 x ULN compared to other antimalarials (RR 3.34, 95% CI 1.63 to 6.84; 8 trials, 6581 participants; Analysis 4.3, Figure 4). Pyronaridine‐artesunate was also associated with a greater incidence of raised AST > 5 x ULN compared to other antimalarials, but the CIs crossed the line of no effect (RR 1.80, 95% CI 0.89 to 3.65; 8 trials, 6581 participants; Analysis 4.4). We detected little or no difference in raised bilirubin > 2.5 x ULN events (RR 1.03, 95% CI 0.49 to 2.18; 7 trials, 6384 participants; Analysis 4.5).
4.3. Analysis.
Comparison 4 Pyronaridine‐artesunate versus other antimalarials for all malaria subtypes (safety outcomes only), Outcome 3 First treatment, ALT increase > 5 × ULN.
4.
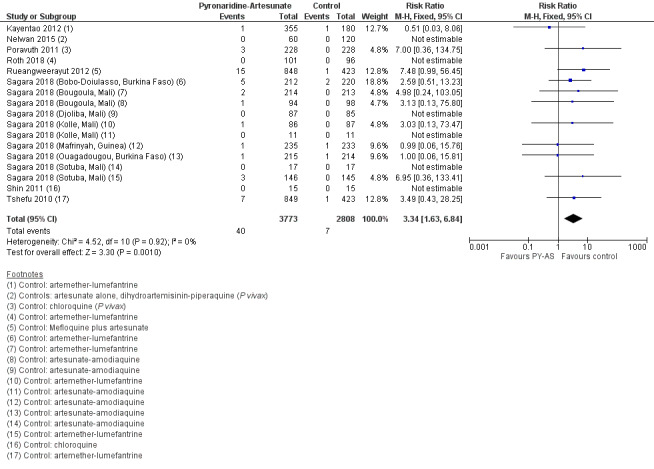
Forest plot of comparison 4: Pyronaridine‐artesunate versus other antimalarials, outcome: 4.3 ALT increase > 5 × ULN, first treatment.
4.4. Analysis.
Comparison 4 Pyronaridine‐artesunate versus other antimalarials for all malaria subtypes (safety outcomes only), Outcome 4 First treatment, AST increase > 5 × ULN.
4.5. Analysis.
Comparison 4 Pyronaridine‐artesunate versus other antimalarials for all malaria subtypes (safety outcomes only), Outcome 5 First treatment, bilirubin increase > 2.5 × ULN.
For second or subsequent treatments, we detected no significant differences in the number of raised ALT (> 5 x ULN), AST (> 5 x ULN) or bilirubin (> 2.5 x ULN) events between pyronaridine‐artesunate and other antimalarials (1649 participants, 1 trial; Analysis 4.6; Analysis 4.7; Analysis 4.8). There were small numbers in each arm.
4.6. Analysis.
Comparison 4 Pyronaridine‐artesunate versus other antimalarials for all malaria subtypes (safety outcomes only), Outcome 6 Subsequent treatment(s), ALT increase > 5 × ULN.
4.7. Analysis.
Comparison 4 Pyronaridine‐artesunate versus other antimalarials for all malaria subtypes (safety outcomes only), Outcome 7 Subsequent treatment(s), AST increase > 5 × ULN.
4.8. Analysis.
Comparison 4 Pyronaridine‐artesunate versus other antimalarials for all malaria subtypes (safety outcomes only), Outcome 8 Subsequent treatment(s), bilirubin increase > 2.5 × ULN.
A sensitivity analysis confined to only those trials which used the same grading for severely raised ALT also found that those treated with pyronaridine‐artesunate had a greater incidence of raised ALT > 5 x ULN compared to other antimalarials (RR 4.07, 95% CI 1.83 to 9.05; 4 trials, 5672 participants; Analysis 4.9).
4.9. Analysis.
Comparison 4 Pyronaridine‐artesunate versus other antimalarials for all malaria subtypes (safety outcomes only), Outcome 9 Sensitivity analysis: first treatment, ALT increase > 5 × ULN.
Qualitative (narrative) synthesis
Ringwald 1996 reported that five out of 40 participants given pyronaridine had elevated bilirubin levels compared to zero out of 41 given chloroquine. It should be noted, however, that this study used pyronaridine monotherapy, and a higher dose than that which is currently recommended. The report did not give any further details of the extent of the increase.
As an indication of the magnitude of ALT increases, the highest reported ALT values in individual studies were 612 IU/L in Rueangweerayut 2012 and 1229 IU/L in Sagara 2018. These were in individual patients, and are not indicative of the population as a whole. The study with the longest follow‐up recruited 180 participants (Nelwan 2015). In the 60 participants receiving pyronaridine‐artesunate, observed increases in the median ALT and AST values had returned to baseline by day 14, and no clinical consequences of these liver enzyme increases were reported over one year of follow‐up. None of the 60 participants experienced raised ALT or AST > 5 x ULN. It should be noted, however, that this was a study of pyronaridine‐artesunate plus primaquine, which could impact the findings. Sagara 2018 reported one case in which raised ALT occurred with raised bilirubin in a two‐year old girl. The safety monitoring board concluded that this event was an acute hepatocellular liver injury, and was reported as a serious adverse event (Table 6).
Other adverse events
We have summarized all other reported adverse events in Analysis 4.10. To enable graphical display of these adverse events by MedDRA 2018 system organ class and higher level term, we present the results in a forest plot based on meta‐analysed subtotals in Figure 5, based on PRISMA guidance (Zorzela 2016). There was a lower risk of electrocardiograph (ECG) abnormality, including QT prolongation, with pyronaridine‐artesunate compared to each of the other antimalarials used as comparator drugs. A summary of the proportion of participants experiencing ECG abnormalities in each treatment group is provided in Table 9. The greatest differences were seen in the Sagara 2018 study when pyronaridine‐artesunate was compared to artemether‐lumefantrine and artesunate‐amodiaquine. The rates of observed QT prolongation were much higher in this study compared to other included studies.
4.10. Analysis.
Comparison 4 Pyronaridine‐artesunate versus other antimalarials for all malaria subtypes (safety outcomes only), Outcome 10 Other adverse events.
5.
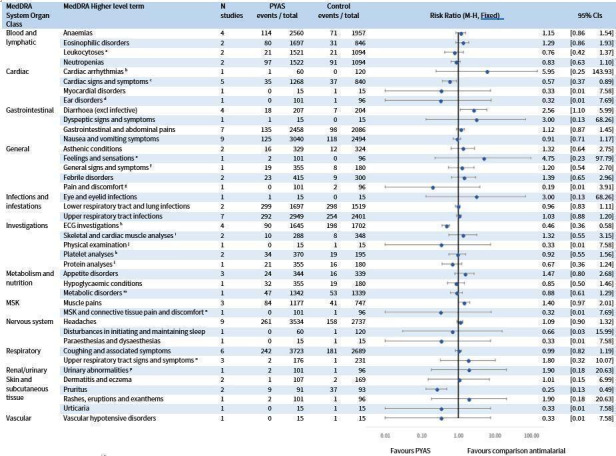
A comparison of adverse events following treatment with pyronaridine‐artesunate versus other antimalarials, based on the reporting guidelines in PRISMA harms (Zorzela 2016). Adverse events are categorized according to MedDRA 2018 system organ class and higher level terms. Where specific lower level terms were reported, we have used footnotes to indicate the condition described. Where trials reported more than one lower level term belonging to the same higher level term, we reported the lower level term with the highest frequency. aIncludes basophilia and monocytosis. bAsymptomatic unifocal ventricular ectopics. cIncludes dizziness and palpitations. dEar pain. e"Chills". fInfluenza‐like illness. gChest pain. hProlonged QTc, t wave inversion. iElevated CPK. jWeight decreased. kThrombocytopaenia. lHypoalbuminaemia. mRaised creatinine. nNeck pain. oThroat pain, cold, postnasal drip. pDark urine.
5. Pyronaridine‐artesunate (PY‐AS) versus other antimalarials: electrocardiogram (ECG) abnormalities.
| Comparator drug | Trial | Pyronaridine‐artesunate | Comparator | ||
| ECG abnormalities | Number of participants | ECG abnormalities | Number of participants | ||
| Artemether‐lumefantrine | Sagara 2018 | 55 | 673 | 99 | 671 |
| Amodiaquine‐artesunate | Sagara 2018 | 34 | 669 | 91 | 668 |
| Chloroquine | Poravuth 2011a | 1 | 228 | 6 | 228 |
| Shin 2011a | 0 | 15 | 1 | 15 | |
| Dihydroartemisinin‐piperaquine or artesunate alone | Nelwan 2015b | 0 | 60 | 1 | 120 |
| Total | 90 | 1645 | 198 | 1702 | |
aP vivax participants only. bPyronaridine alone used as study drug.
For most types of adverse event, rates were similar between pyronaridine‐artesunate and comparators. Differences observed included the following.
Lower risk of cardiac symptoms. This category included dizziness and palpitations. The difference observed related to high instances of dizziness in the control group (mefloquine plus artesunate) in one study (Rueangweerayut 2012).
Lower risk of pruritis. This occurred in comparisons to chloroquine, for which pruritis is a commonly recognized adverse event.
Higher risk of diarrhoea: most cases of diarrhoea were contributed by one study, which was a monotherapy study using higher doses of pyronaridine than currently recommended (Ringwald 1996).
Discussion
Summary of main results
See Table 1; Table 2; Table 3; Table 4.
Summary of efficacy findings
Overall, pyronaridine‐artesunate appears to have similar efficacy to other artemisinin‐based combination therapies (ACTs) (artemether‐lumefantrine, artesunate‐amodiaquine, mefloquine plus artesunate). In most included trials, pyronaridine‐artesunate had a lower than 5% PCR‐adjusted treatment failure rate at day 28 and day 42.
Treatment with pyronaridine‐artesunate may lead to fewer PCR‐adjusted failures at day 28 compared to artemether‐lumefantrine, artesunate‐amodiaquine, and mefloquine plus artesunate (low‐certainty evidence). In all these instances, the CIs cross the line of no effect.
Treatment with pyronaridine‐artesunate may lead to fewer PCR‐unadjusted failures at day 28 compared to artemether‐lumefantrine (low certainty evidence), and probably leads to fewer failures compared to artesunate‐amodiaquine and mefloquine plus artesunate (moderate‐certainty evidence). The PCR‐unadjusted outcome reflects the post‐treatment effect of the drug in preventing new infections.
Pyronaridine‐artesunate may have little or no difference on the rate of PCR‐adjusted failure at day 42 compared to artemether‐lumefantrine or artesunate‐amodiaquine (low‐certainty evidence), but may lead to higher rates of failure than mefloquine plus artesunate (low‐certainty evidence).
Pyronaridine‐artesunate may lead to a lower rate of PCR‐unadjusted treatment failure at day 42 compared to artemether‐lumefantrine (low‐certainty evidence), suggesting that the drug combination may reduce the likelihood of reinfection during the treatment period. Pyronaridine may little or no difference compared to mefloquine plus artesunate (low‐certainty evidence), and probably makes little or no difference compared to artesunate‐amodiaquine (moderate‐certainty evidence).
Summary of safety findings
Raised ALT > 5 x ULN is more frequent with pyronaridine‐artesunate compared to other antimalarials (high‐certainty evidence). Raised AST > 5 x ULN is probably also more frequent (moderate‐certainty evidence), but the CI crosses the line of no effect. There is probably little or no difference for raised bilirubin > 2.5 x ULN between pyronaridine‐artesunate and other antimalarials (moderate‐certainty evidence). There was one reported case in which raised ALT occurred with raised bilirubin. Qualitative evidence from one trial, with a cohort of 180 participants and a follow‐up of one year, indicated that raised liver enzymes were not prolonged and did not lead to clinical sequelae, though it should be noted that pyronaridine‐artesunate was administered concurrently with primaquine.
ECG abnormalities were less commonly seen with pyronaridine‐artesunate compared to other antimalarials. For the remaining safety outcomes, there appears to be little or no difference in the safety of pyronaridine‐artesunate compared to other ACTs (artemether‐lumefantrine, artesunate‐amodiaquine, mefloquine plus artesunate, dihydroartemisinin‐piperaquine) or non‐ACT antimalarials (artesunate alone, chloroquine).
Overall completeness and applicability of evidence
Completeness and applicability of efficacy findings
Five trials contributed 5711 participants to quantitative synthesis for efficacy analyses in this review. There were 4465 participants from 13 trial sites in Africa (Burkina Faso, DRC, Gabon, Cote d'Ivoire, Kenya, Mali, Tanzania, The Gambia, Ghana, Mozambique, Senegal, Guinea, Mali), and 1246 participants from five sites in Asia (the Philippines, Cambodia, Indonesia, Thailand, Vietnam). The large number of included sites broadens the applicability of the efficacy findings. The actual number of participants recruited at country level was small, precluding evaluation of efficacy at country level.
A key limitation on the applicability of review findings on efficacy is the age of the participants, as the included trials mostly recruited older children and adults. The two new studies included in the efficacy analysis in this update have contributed an additional 375 children under five years who received pyronaridine‐artesunate and 409 who received comparator drugs. However, the total number of participants definitively aged less than five years remains at just 527 in the pyronaridine‐artesunate arm and 472 in the comparator arm.
Trials additionally reported a number of outcomes relating to fever clearance, parasite clearance, and gametocyte carriage, which did not form a priori outcomes for this version of the review, in a change to the previously published protocol. We encountered different modes of reporting these outcomes, different units of measurement, and incomplete reporting of these outcomes. This limits the contribution of these outcomes to the evidence.
Completeness and applicability of safety findings
Eight trials contributed to quantitative synthesis for key safety outcomes in this review (serious adverse events and liver function tests), and a further two trials contributed data to an analysis of all safety outcomes. All trials contributing to the safety meta‐analysis excluded participants with baseline hepatic impairment, and most trials listed viral hepatitis as a specific exclusion criterion. Similarly, Sagara 2018 excluded patients with raised liver enzymes from second and subsequent treatments. Screening for baseline hepatic impairment, or for hepatic impairment during treatment, may not be feasible in many malaria‐endemic settings where resources are limited. This may limit the applicability of the findings.
Four trials explicitly listed HIV as an exclusion criterion (Kayentao 2012; Poravuth 2011; Sagara 2018; Tshefu 2010). Such exclusions represent standard practice for phase III trials. Given the high seroprevalence of such conditions in malaria‐endemic areas, this may limit the applicability of the safety findings. However, there was no reported screening for these conditions in any of the trials, meaning that it is likely that some patients with undiagnosed HIV were included. In a safety study in healthy volunteers (Morris 2012), of 17 healthy volunteers receiving pyronaridine‐artesunate and ritonavir, five volunteers experienced ALT > 3 x ULN. The WHO recommends ritonavir as part of second‐line antiretroviral therapy. Thus a commonly prescribed antiretroviral may increase the risk of raised ALT.
Certainty of the evidence
We assessed the certainty of the evidence in this review using the GRADE approach and presented it in Table 1, Table 2, Table 3, and Table 4.
With respect to the efficacy of pyronaridine‐artesunate, the studies included in this review provided moderate‐ to low‐certainty evidence due to inconsistency, with frequent quantitative and qualitative heterogeneity between studies, and indirectness, given that children under five years are under‐represented (especially in Asia).
With respect to the safety of pyronaridine‐artesunate, we judged the certainty of evidence in relation to raised ALT to be high, and did not downgrade for imprecision, as although the CI is wide, there were few events. We found moderate‐certainty evidence that pyronaridine‐artesunate increases the proportion of patients experiencing raised AST > 5 x ULN, and moderate‐certainty evidence that treatment has little or no difference on the proportion of patients experiencing raised bilirubin > 2.5 x ULN. We downgraded both outcomes for imprecision.
Potential biases in the review process
This represents the third update of this review. For this review we repeated the screening process, increasing the likelihood that we identified all relevant studies.
The largest included trial in this review, Sagara 2018, did not randomize patients to all comparisons at all sites, and we were unable to obtain disaggregated data for all outcomes at all sites. However, we were able to do so for the outcomes most pertinent to this review (efficacy and liver function data) so do not consider that this introduces significant bias to the review process.
As shown in Table 5, different trial authors used different grading for the severity of adverse events. Most trial authors considered ALT > 5 x ULN as important, and reported at this threshold. The three studies which reported at a lower threshold recorded zero events, so we retained these in the analysis for completeness (Nelwan 2015; Roth 2018; Shin 2011). Kayentao 2012 reported at a higher threshold, and so may under‐detect events. When we performed a sensitivity analysis excluding these four trials, the risk ratio (RR) was similar (Analysis 4.9). The reader should note that the grading for raised ALT does not correspond with international definitions for drug‐induced liver injury (Aithal 2011; Appendix 1). Similarly, the thresholds for raised bilirubin also differed, both from each other and from the 2 x ULN used in international definitions of moderate or severe drug‐induced liver injury (Aithal 2011; Appendix 1). As we are unable to provide case‐by‐case analysis for raised ALT > 5 x ULN, we cannot exclude the possibility that in some instances these occurred in conjunction with symptoms of bilirubin > 2 x ULN (given that the reported threshold was 2.5 in most studies). Qualitative synthesis did not suggest this was the case.
We had planned to conduct a sensitivity analysis altering the denominator for the efficacy outcomes according to Appendix 4. However, we were unable to reliably extract data pertinent to missing or indeterminate PCR values. As PCR is unlikely to differentially misclassify recrudescences as reinfections between comparison groups, we do not feel this is likely to introduce bias to the main outcome of PCR‐adjusted treatment failure.
For the safety analysis we used MedDRA 2018 to create analogous definitions to allow comparison. This may lead to misclassification and loss of detail. However, we feel that the overview is more useful and meaningful to the clinical reader. The methodology of this review is not a primary adverse effects review, and as such does not include safety data from non‐comparative or non‐randomized studies, which such a review might include. This limits the depth to which this review can comment on the extent and implications of safety findings, but does not effect the certainty of the evidence presented.
Agreements and disagreements with other studies or reviews
Our search identified an individual patient data analysis published by authors from Medicines for Malaria Venture and Shin Poong Pharmaceuticals, who developed the pyronaridine‐artesunate combination (Duparc 2013). The efficacy analysis in Duparc 2013 included four of the RCTs included in our review (Kayentao 2012; Poravuth 2011; Rueangweerayut 2012; Tshefu 2010). The safety analysis included an additional two studies: a non‐randomized dose‐finding study excluded from this review (Ramharter 2008), and a randomized dose‐finding study that was published as a conference abstract which assessed pyronaridine‐artesunate without a comparator (Looareesuwan 2007). This integrated analysis is not a formal systematic review, and as such did not have an a priori protocol or a formal search strategy. It did not make assessments of risk of bias. It is unclear whether the authors have included all potentially relevant studies or whether it represents a convenience sample of available data at the time of the analysis. The safety findings of this integrated analysis do not report relative risks or anticipated absolute effects of raised ALT, instead reporting incidence at day three and day seven. The day seven incidence of 0.9% (24/2709) is similar to our anticipated absolute effect of 0.8%.
Our review agrees with the conclusion from this integrated analysis (Duparc 2013), that pyronaridine‐artesunate has good efficacy for uncomplicated P falciparum malaria. The authors conclude that pyronaridine‐artesunate was well tolerated with a similar adverse event profile to comparators, and note that pyronaridine‐artesunate was associated with transient increases in transaminases in a relatively small proportion of patients, although do not caveat this statement with reference to the exclusion criteria within the clinical trials. When interpreting the conclusion in Duparc 2013, readers should consider that the further data from Sagara 2018 excluded participants who had raised liver enzyme events on first treatment from any subsequent treatments, and this may limit applicability.
Authors' conclusions
Implications for practice.
Pyronaridine‐artesunate was efficacious against uncomplicated P falciparum malaria, achieved a PCR‐adjusted treatment failure rate of < 5% at days 28 and 42, and may be at least as good as or better than existing artemisinin‐based combination therapies (ACTs).
Pyronaridine‐artesunate causes a three‐fold increase in the risk of raised ALT > 5 x ULN. This meets clinical chemistry criteria for drug‐induced liver injury (Aithal 2011; Appendix 1). In the absence of raised bilirubin > 2 x ULN, and in the absence of symptoms, this corresponds to mild drug‐induced liver injury only. There was one reported case in which raised ALT occurred with raised bilirubin, meeting criteria for moderate drug‐induced liver injury.
We conclude that although raised ALT is a safety signal indicating a theoretical risk of severe drug‐induced liver injury, no such cases have been reported amongst over 6000 otherwise healthy trial participants to date. As such, the raised ALT may reflect a capacity for pyronaridine‐artesunate to cause only mild, asymptomatic, and reversible injury. WHO 2015 guidelines stated that pyronaridine‐artesunate may be considered in areas of multiple drug resistance. Our review indicates that this is an appropriate recommendation, as the benefit in this clinical scenario clearly outweighs a theoretical risk.
The findings of this review cannot fully inform a risk‐benefit assessment for an unselected population. Readers should remain aware of this uncertainty when considering use of pyronaridine‐artesunate in patients with known or suspected pre‐existing liver dysfunction, and when co‐administering with other medications which may cause liver dysfunction.
Implications for research.
Raised ALT > 5 x ULN is a safety signal which warrants further evaluation in cohort studies. Of note, Shin Poong Pharmaceutical Company Ltd and Medicines for Malaria Venture are conducting an ongoing hepatic safety study (NCT03201770). This aims to recruit 8572 malaria episodes and will analyse safety in people with baseline ALT elevations.
There is limited published data in children aged less than five years of age. NCT03201770 intends to recruit a greater number of children who are < 1 year of age, which may help redress this.
What's new
| Date | Event | Description |
|---|---|---|
| 8 January 2019 | New citation required and conclusions have changed | We included four new studies: three published after the previous version of this review, Bukirwa 2014, and one that was not identified in the previous review. During re‐extraction of data from the previous studies we used a transparent, itemized, and replicable procedure that differed from Bukirwa 2014. The inclusion of new studies has allowed a new comparison of pyronaridine‐artesunate and artesunate‐amodiaquine, and has led to changes in the certainty of the evidence in relation to the primary outcomes. This review update gives higher‐certainty evidence in relation to the effect of pyronaridine‐artesunate on raised alanine aminotransferase (ALT). |
| 8 January 2019 | New search has been performed | There is a new author team: Joseph Pryce, Paul Hine (new contact person). We use the term pyronaridine‐artesunate in preference to artesunate‐pyronaridine to reflect how most authors refer to the intervention. We updated the background to reflect changes in global epidemiology, current World Health Organization (WHO) guidelines, and other developments. We added the term ‘pyramax' to the search strategy. We replaced the quantitative analysis of secondary outcomes ‘parasite clearance', ‘fever clearance', and ‘gametocyte carriage' with a narrative synthesis, and comment on the reasons for doing so. We simplified the ‘Adverse events' outcomes to reflect that elevated liver function tests are a primary area of interest. We itemized the procedure for data extraction to ensure that the process is transparent and replicable in future review updates. We have added further commentary regarding the data extraction process. We simplified the ‘Risk of bias' assessment for adverse events. We abbreviated the content of tables to enable clear and succinct presentation. We incorporated risk of bias for adverse events assessments into the main ‘Risk of bias' tables. We added an appendix to summarize our ‘Risk of bias' assessment process. |
History
Protocol first published: Issue 1, 2007 Review first published: Issue 3, 2014
| Date | Event | Description |
|---|---|---|
| 11 November 2008 | Amended | We converted to the new review format with minor editing. |
Notes
Not applicable.
Acknowledgements
Dr Hellen Gelband is the Academic Editor of this review.
We thank the authors of the previously published versions of this Cochrane Review (Bukirwa 2014; Unnikrishnan 2007).
We thank Paul Garner who commented on drafts of this review update. Paul Garner is a member of the Expert Committee on Malaria Chemotherapy at the World Health Organization (WHO), and is Co‐ordinating Editor of the Cochrane Infectious Diseases Group (CIDG).
JP and PH were supported by the Research, Evidence and Development Initiative (READ‐It) project. READ‐It and the CIDG editorial base are funded by UK aid from the UK government for the benefit of low‐ and middle‐income countries (project number 300342‐104). The views expressed do not necessarily reflect the UK government’s official policies.
Appendices
Appendix 1. Case definition in drug‐induced liver injury
Aithal 2011 is a consensus statement from an Expert Working Group of clinicians and scientists on the case definition of drug‐induced liver injury. The criteria are as follows.
Clinical chemistry criteria for drug‐induced liver injury
Any one of the following.
More than or equal to five‐fold elevation above the ULN for ALT.
More than or equal to two‐fold elevation above the ULN for ALP (particularly with accompanying elevations in concentrations of 5′‐nucleotidase or γ‐glutamyl transpeptidase in the absence of known bone pathology driving the rise in ALP level).
More than or equal to three‐fold elevation in ALT concentration and simultaneous elevation of bilirubin concentration exceeding 2 × ULN.
Drug‐induced liver injury severity index
| Category | Severity | Description |
| 1 | Mild | Elevated ALT/ALP concentration reaching clinical chemistry criteria for drug‐induced liver injury, but bilirubin concentration < 2 × ULN |
| 2 | Moderate | Elevated ALT/ALP concentration reaching criteria for drug‐induced liver injury, and bilirubin concentration ≥ 2 × ULN, or symptomatic hepatitis |
| 3 | Severe | Elevated ALT/ALP concentration reaching criteria for drug‐induced liver injury, bilirubin concentration ≥ 2× ULN, and one of the following.
|
| 4 | Fatal or transplantation | Death or transplantation due to drug‐induced liver injury |
Abbreviations: ALP: alkaline phosphatase; ALT: alanine aminotransferase; ULN: upper limit of normal.
Appendix 2. Search strategy
| Search set | CIDG SRa | CENTRAL | MEDLINEb | Embaseb | LILACSb |
| 1 | Malaria | Malaria ti, ab, kw | Malaria ti, ab, Mesh | Malaria.mp Emtree | malaria |
| 2 | pyronaridine | Pyronaridine ti, ab, kw | "pyronaridine"ti, ab, Supplementary Concept | pyronaridine/ or artesunate plus pyronaridine/ or pyronaridine.mp. | pyronaridine |
| 3 | pyramax | Pyramax ti, ab, kw | "Naphthyridines" Mesh | Pyramax.mp | pyramax |
| 4 | 2 or 3 | 2 or 3 | Pyramax ti, ab | 2 or 3 | 2 or 3 |
| 5 | 1 and 4 | 1 and 4 | 2 or 3 or 4 | 1 and 4 | 1 and 4 |
| 6 | — | — | 1 and 5 | — |
aCochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by Cochrane (Lefebvre 2011).
Appendix 3. Authors' judgement on risk of bias
| Potential bias | Authors' judgementa |
| Random sequence generation (selection bias) | High – not randomized or quasi‐randomized Unclear – states "randomized", but does not report method Low – describes method of randomization |
| Allocation concealment (selection bias) | High – not concealed, open‐label trial for individually randomized, method of concealment not adequate Unclear – details of method not reported or insufficient details Low – central allocation, sequentially numbered opaque sealed envelopes |
| Blinding (performance bias and detection bias) | High – personnel, participants or outcome assessors not blinded Unclear – no details reported, insufficient details reported Low – personnel, participants and outcome assessors blinded |
| Incomplete outcome data (attrition bias) | High – losses to follow‐up not evenly distributed across intervention and control group Unclear ‐ no details reported, insufficient details reported Low – losses to follow‐up evenly distributed across groups, reasons for loss to follow‐up and exclusions clearly stated |
| Selective reporting (reporting bias) | High – did not fully report measured or relevant outcomes Unclear – not enough information reported to judge Low – all stated outcomes reported |
| Other bias | High – other source of bias identified by review authors Low – no obvious other source of bias of concern to review authors |
| Adverse event monitoring (detection bias) | High – passive methods relying on spontaneous patient report only, undefined adverse events Unclear – not enough information reported to judge Low – key adverse events defined, prespecified active detection method |
| Incomplete adverse event reporting (reporting bias) | High – adverse event severity undefined, combination of treatment groups, post hoc cut‐offs for reporting adopted Unclear – not enough information reported to judge Low – clear reporting on important adverse events with numerical data by intervention group |
aIndicative reasons for judgements only; this list is not intended to be exhaustive.
Appendix 4. Planned sensitivity analysis
| Analysisa | Participants | PCRb‐unadjusted | PCR‐adjusted | ||
| Numerator | Denominator | Numerator | Denominator | ||
| Primary analysis | Exclusions after enrolment | Excludedc | Excluded | Excluded | Excluded |
| Missing or indeterminate PCR | Included as failures | Included | Excluded | Excluded | |
| New infections | Included as failures | Included | Excluded | Excluded | |
| Sensitivity analysis 1d | As ‘Primary analysis' except: missing or indeterminate PCR | — | — | Included as failures | Included |
| Sensitivity analysis 2e | As ‘Sensitivity analysis 1' except: new infections | — | — | Included as successes | Included |
| Sensitivity analysis 3f | As ‘Sensitivity analysis 2' except: exclusions after enrolment | Included as failures | Included | Included as failures | Included |
| Sensitivity analysis 4g | As ‘Sensitivity analysis 2' except: exclusions after enrolment | Included as successes | Included | Included as successes | Included |
aRemove participants that did not satisfy the inclusion criteria after randomization from all calculations. bPCR: polymerase chain reaction. c‘Excluded' means removed from the calculation. dTo reclassify all indeterminate or missing PCR results as treatment failures in the PCR‐adjusted analysis. eTo reclassify all PCR‐confirmed new infections as treatment successes in the PCR‐adjusted analysis. (This analysis may overestimate efficacy as PCR is not wholly reliable and some recrudescences may be falsely classified as new infections. Also some participants may have gone on to develop a recrudescence after the new infection). fTo reclassify all exclusions after enrolment (losses to follow‐up, withdrawn consent, other antimalarial use, or failure to complete treatment) as treatment failures. For PCR‐unadjusted total failure this represents a true worse‐case scenario. gTo reclassify all exclusions after enrolment (losses to follow‐up, withdrawn consent, other antimalarial use, or failure to complete treatment) as treatment successes.
Data and analyses
Comparison 1. Pyronaridine‐artesunate versus artemether‐lumefantrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total failure: day 42 (PCR‐adjusted) | 7 | 2575 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.49, 1.51] |
| 2 Total failure: day 28 (PCR‐adjusted) | 7 | 3068 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.26, 1.31] |
| 3 Total failure: day 28 (unadjusted) | 7 | 3149 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.13, 0.58] |
| 4 Total failure: day 42 (unadjusted) | 7 | 3080 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.46, 0.82] |
| 5 Early treatment failure | 7 | 3149 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [0.12, 52.39] |
| 6 Serious adverse events | 3 | 2004 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.19, 4.22] |
| 7 Adverse events leading to withdrawal | 3 | 2004 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.68, 2.90] |
| 8 First treatment, ALT increase > 5 × ULN | 7 | 3327 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [1.15, 7.41] |
| 9 First treatment, AST increase > 5 × ULN | 7 | 3327 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [0.83, 5.82] |
| 10 First treatment, bilirubin increase > 2.5 × ULN | 6 | 3130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.33, 2.04] |
| 11 Subsequent treatment(s), ALT increase > 5 × ULN | 4 | 865 | Risk Ratio (M‐H, Random, 95% CI) | 2.54 [0.49, 13.10] |
| 12 Subsequent treatment(s), AST increase > 5 × ULN | 4 | 865 | Risk Ratio (M‐H, Random, 95% CI) | 1.88 [0.50, 7.06] |
| 13 Subsequent treatment(s), bilirubin increase > 2.5 × ULN | 4 | 865 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.16, 3.63] |
| 14 Paediatric trials ‐ total failure: day 28 (PCR‐adjusted) | 2 | 684 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.13, 7.84] |
| 15 Paediatric trials ‐ total failure: day 42 (PCR‐adjusted) | 2 | 558 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.41, 2.59] |
| 16 Paediatric trials ‐ total failure: day 28 (unadjusted) | 2 | 693 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.50, 1.18] |
| 17 Paediatric trials ‐ total failure: day 42 (unadjusted) | 2 | 654 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.74, 1.33] |
| 18 Paediatric trials ‐ first treatment, ALT increase > 5 × ULN | 2 | 732 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.03, 8.06] |
| 19 Paediatric trials ‐ first treatment, AST increase > 5 × ULN | 2 | 732 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.16, 14.52] |
Comparison 2. Pyronaridine‐artesunate versus artesunate‐amodiaquine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total failure: day 28 (PCR‐adjusted) | 6 | 1245 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.11, 2.77] |
| 2 Total failure: day 42 (PCR‐adjusted) | 6 | 1091 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.20, 4.83] |
| 3 Total failure: day 28 (unadjusted) | 6 | 1257 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.30, 0.81] |
| 4 Total failure: day 42 (unadjusted) | 6 | 1235 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.78, 1.23] |
| 5 First treatment, ALT increase > 5 × ULN | 6 | 1317 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.28, 7.09] |
| 6 First treatment, AST increase > 5 × ULN | 6 | 1317 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.07] |
| 7 First treatment, bilirubin increase > 2.5 × ULN | 6 | 1317 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.76] |
| 8 Subsequent treatment(s), ALT increase > 5 × ULN | 6 | 784 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.48, 7.76] |
| 9 Subsequent treatment(s), AST increase > 5 × ULN | 6 | 784 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.49, 6.62] |
| 10 Subsequent treatment(s), bilirubin increase > 2.5 × ULN | 6 | 784 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.33, 9.83] |
Comparison 3. Pyronaridine‐artesunate versus mefloquine plus artesunate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total failure: day 28 (PCR‐adjusted) | 1 | 1117 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.13, 1.05] |
| 2 Total failure: day 42 (PCR‐adjusted) | 1 | 1037 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [0.90, 3.57] |
| 3 Total failure: day 28 (unadjusted) | 1 | 1120 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.17, 0.78] |
| 4 Total failure: day 42 (unadjusted) | 1 | 1059 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.54, 1.31] |
| 5 Serious adverse events | 1 | 1271 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.25, 3.97] |
| 6 Adverse events leading to withdrawal | 1 | 1271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.17, 2.31] |
| 7 First treatment, ALT increase > 5 × ULN | 1 | 1271 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.48 [0.99, 56.45] |
| 8 First treatment, AST increase > 5 × ULN | 1 | 1271 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.49 [0.55, 162.64] |
| 9 First treatment, bilirubin increase > 2.5 × ULN | 1 | 1271 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.49 [0.43, 28.29] |
Comparison 4. Pyronaridine‐artesunate versus other antimalarials for all malaria subtypes (safety outcomes only).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serious adverse events | 7 | 3941 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.54, 2.84] |
| 2 Adverse events leading to withdrawal | 6 | 3911 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.58, 1.94] |
| 3 First treatment, ALT increase > 5 × ULN | 14 | 6581 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.34 [1.63, 6.84] |
| 4 First treatment, AST increase > 5 × ULN | 14 | 6581 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.89, 3.65] |
| 5 First treatment, bilirubin increase > 2.5 × ULN | 13 | 6384 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.49, 2.18] |
| 6 Subsequent treatment(s), ALT increase > 5 × ULN | 7 | 1649 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [0.76, 6.27] |
| 7 Subsequent treatment(s), AST increase > 5 × ULN | 7 | 1649 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.74, 4.44] |
| 8 Subsequent treatment(s), bilirubin increase > 2.5 × ULN | 7 | 1649 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.42, 3.01] |
| 9 Sensitivity analysis: first treatment, ALT increase > 5 × ULN | 10 | 5672 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.07 [1.83, 9.05] |
| 10 Other adverse events | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Blood and lymphatic: anaemias | 4 | 4517 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.86, 1.54] |
| 10.2 Blood and lymphatic: eosinophilic disorders | 2 | 2543 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.86, 1.93] |
| 10.3 Blood and lymphatic: leukocytoses | 2 | 2615 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.42, 1.37] |
| 10.4 Blood and lymphatic: neutropaenias | 2 | 2616 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.63, 1.10] |
| 10.5 Cardiac: cardiac arrhythmias | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.95 [0.25, 143.93] |
| 10.6 Cardiac: cardiac signs and symptoms | 5 | 2108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.37, 0.89] |
| 10.7 Cardiac: myocardial disorders | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.58] |
| 10.8 Ear: ear disorders | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.69] |
| 10.9 Gastrointestinal: diarrhoea (excl infective) | 4 | 411 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.56 [1.10, 5.99] |
| 10.10 Gastrointestinal: dyspeptic signs and symptoms | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.26] |
| 10.11 Gastrointestinal: GI and abdominal pains | 7 | 4544 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.87, 1.45] |
| 10.12 Gastrointestinal: nausea and vomiting symptoms | 9 | 5534 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.71, 1.17] |
| 10.13 General: asthenic conditions | 2 | 653 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.64, 2.75] |
| 10.14 General: general signs and symptoms | 1 | 535 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.54, 2.70] |
| 10.15 General: feelings and sensations | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.75 [0.23, 97.79] |
| 10.16 General: febrile disorders | 2 | 715 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.65, 2.96] |
| 10.17 General: pain and discomfort | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.91] |
| 10.18 Infections and infestations: eye and eyelid | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.26] |
| 10.19 Infections and infestations: LRTI and lung | 2 | 3216 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.83, 1.11] |
| 10.20 Infections and infestations: URTI | 7 | 5350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.88, 1.20] |
| 10.21 Investigations: ECG | 4 | 3347 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.36, 0.58] |
| 10.22 Investigations: skeletal/cardiac muscle analyses | 2 | 636 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.55, 3.15] |
| 10.23 Investigations: physical exam | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.58] |
| 10.24 Investigations: platelet | 2 | 565 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.55, 1.56] |
| 10.25 Investigations: protein | 1 | 535 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.36, 1.24] |
| 10.26 Metabolism and nutrition: appetite disorders | 3 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.80, 2.68] |
| 10.27 Metabolism and nutrition: hypoglycaemia conditions | 1 | 535 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.50, 1.46] |
| 10.28 Metabolism and nutrition: metabolic disorders | 1 | 2681 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.61, 1.29] |
| 10.29 MSK and connective tissue: muscle pains | 3 | 1924 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.97, 2.01] |
| 10.30 MSK and connective tissue: pain and discomfort | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.69] |
| 10.31 Nervous system: headaches | 9 | 6271 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.90, 1.32] |
| 10.32 Nervous system: sleep disturbance | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.03, 15.99] |
| 10.33 Nervous system: paraesthesias and dysasthesias | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.58] |
| 10.34 Respiratory: coughing and assoc symptoms | 6 | 6412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.82, 1.19] |
| 10.35 Respiratory: upper respiratory tract signs and symptoms | 3 | 407 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.32, 10.07] |
| 10.36 Renal and urinary: urinary abnormalities | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.18, 20.63] |
| 10.37 Skin and subcutaneous tissue: dermatitis and eczema | 2 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.15, 6.99] |
| 10.38 Skin and subcutaneous tissue: pruritis | 2 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.13, 0.49] |
| 10.39 Skin and subcutaneous tissue: rashes, eruptions, exanthems | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.18, 20.63] |
| 10.40 Skin and subcutaneous tissue: urticaria | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.58] |
| 10.41 Vascular: vascular hypotensive disorders | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.58] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Kayentao 2012.
| Methods | RCT Duration: 1 year, November 2007 to November 2008 |
|
| Participants | Children with P falciparum malaria Number: 535, randomized 2:1 Inclusion criteria: age ≤ 12 years; bodyweight 5 kg ‐ 25 kg; fever or history of fever within 24 hours Exclusion criteria: severe/complicated malaria; mixed Plasmodium infection; other clinically significant disorder; severe vomiting; severe diarrhoea; viral hepatitis/HIV; malnutrition; QTc ≥ 450 ms; other febrile conditions; hepatic impairment (AST/ALT > 2.5x ULN); renal impairment; electrolyte imbalance; anaemia (Hb < 8 g/dL); allergy to study drugs; antimalarial therapy in previous 2 weeks, investigational drug in previous 4 weeks; taking any drug metabolized by cytochrome enzyme CYP2D6; previous participation in pyronardine‐artesunate studies; pregnancy/lactation; unable to comply with follow‐up visits Diagnosis: microscopy (asexual parasite density 1000‐200,000/µL blood) Children under 5: 152 (pyronaridine‐artesunate); 64 (artemether‐lumefantrine) |
|
| Interventions |
|
|
| Outcomes |
†Two consecutive normal readings taken between 7 and 25 hours apart ‡Not assessed in quantitative synthesis in this review |
|
| Notes | Location: Africa (n = 514, 96%) and Asia (n = 21, 4%). Africa: Burkina Faso, DRC, Gabon, Côte d’Ivoire, Kenya, Mali. Asia: the Philippines Setting: Local hospitals and clinics Malaria endemicity: high Resistance profile: not described Source of funding: Medicines for Malaria Venture, Shin Poong Pharmaceutical Company Ltd, Seoul, Republic of Korea Follow‐up: 42 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomization schedule |
| Allocation concealment (selection bias) | Low risk | Individually numbered treatment packs of similar appearance masked on allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients not blinded ("drugs given open‐label") Clinical and parasitological assessments blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Report lists reasons for attrition and exclusions |
| Selective reporting (reporting bias) | Low risk | Prospectively registered. Report includes all prestated outcomes of interest |
| Other bias | Low risk | 3 authors declared conflicting interests (employees of funders); blind to treatment allocation |
| Adverse event monitoring (detection bias) Adverse Events | Unclear risk | Authors do not fully describe method for detection of adverse events, includes biochemical and ECG monitoring. Authors do not give definitions of all adverse events. Uses higher ULN values for grading of hepatic enzyme severity compared to other studies |
| Incomplete adverse event reporting (reporting bias) Adverse events | Low risk | All adverse events reported. Supplementary tables detail laboratory variables at each assessment |
Nelwan 2015.
| Methods | RCT Duration: 1 year, 4 months. March 2013 to July 2014 |
|
| Participants | Adult soldiers with P vivax malaria Number: 180 Inclusion criteria: age 18 ‐ 65 years; travelled to NE Papua within 12 months; bodyweight 40 kg ‐ 90 kg Exclusion criteria: G6PD deficiency; P falciparum monoinfection, hospitalization, anaemia (Hb < 7 g/dL), planned absence from military base; clinically significant disorders; QTc ≥ 450 ms; family history prolonged QTc/sudden death; concomitant drugs known to prolong QT; hepatic impairment (AST/ALT > 2.5 x ULN); renal impairment; viral hepatitis; allergy to study drugs; previous participation; recent antimalarials Diagnosis: microscopy of P vivax, confirmed by a second microscopist |
|
| Interventions |
|
|
| Outcomes |
*Not assessed in quantitative synthesis in this review |
|
| Notes | Location: Indonesia, in travellers returning from Papua Setting: army base Malaria endemicity: no known risk of malaria in study site Resistance profile: the infections were by the chloroquine‐resistant and primaquine‐tolerant Chesson‐like P vivax strains Source of funding: sponsored by the ALERT Asia Foundation (Indonesia), funded by Medicines for Malaria Venture (Switzerland) Follow‐up: 1 year |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Statistician block‐allocated treatment assignments by varying blocking number at random" |
| Allocation concealment (selection bias) | Low risk | Sealed envelope |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Unclear if participants or assessing clinicians were blinded. Parasitological assessments blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Report lists reasons for attrition and exclusions |
| Selective reporting (reporting bias) | Low risk | Prospective registration, reports all a priori outcomes |
| Other bias | Low risk | MMV played an advisory role, but had no role in study conduct or analysis |
| Adverse event monitoring (detection bias) Adverse Events | Unclear risk | Authors do not fully describe schedule for detection of adverse events, includes biochemical and ECG monitoring. Authors do not clearly define adverse events, including significant raise in hepatic enzymes |
| Incomplete adverse event reporting (reporting bias) Adverse events | Unclear risk | Reports numbers of adverse events by grade, but do not define grading used |
Poravuth 2011.
| Methods | RCT Duration: 1 year, March 2007 to March 2008 |
|
| Participants | Adults and children with P vivax malaria Number: 456 Inclusion criteria: age 3 ‐ 60 years; fever or history of fever within 24 hours; bodyweight 20 kg ‐ 90 kg Exclusion criteria: severe/complicated malaria; mixed Plasmodium infection; severe vomiting; other clinical condition recurring hospitalization; other clinically significant disorder; viral hepatitis/HIV; malnutrition; QTc ≥ 450 ms; other febrile conditions; hepatic impairment (AST/ALT > 2.5 x ULN); renal impairment; anaemia (Hb < 8 g/dL); allergy to study drugs; antimalarial therapy in previous 2 weeks (or antibacterial with antimalarial effect); investigational drug in previous 4 weeks; previous participation in pyronaridine‐artesunate studies; pregnancy/lactation; unable to comply with follow‐up visits Diagnosis: microscopy of P vivax (parasite density ≥ 250/mL blood, including > 50% asexual parasites) |
|
| Interventions |
|
|
| Outcomes |
*Not assessed in quantitative synthesis in this review †Two consecutive normal readings taken between 7 and 25 hours apart |
|
| Notes | Location: Asia (Cambodia, India, Indonesia, and Thailand) Setting: local hospitals Malaria endemicity: high Resistance profile: not described Source of funding: Medicines for Malaria Venture, Shin Poong Pharmaceutical Company Ltd, Seoul, Republic of Korea Follow‐up: 42 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Doubly‐dummy ‐ study drug and matching placebo, packaged similarly All study investigators, laboratory technicians, and patients blind to treatment assignment Investigator calculated the appropriate dose and study drug was administered by a different member of staff |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Report lists reasons for attrition and exclusions |
| Selective reporting (reporting bias) | Low risk | Prospectively registered. Report includes all prestated outcomes of interest |
| Other bias | Low risk | 3 authors declared conflicting interests (employees of funders); blind to treatment allocation |
| Adverse event monitoring (detection bias) Adverse Events | Unclear risk | Authors do not fully describe method for detection of adverse events, but describe interval for ECG assessment. Authors do not give definitions of all adverse events |
| Incomplete adverse event reporting (reporting bias) Adverse events | Low risk | All‐cause adverse events enumerated. Report table only includes adverse events occurring in ≥ 2% (or ≥ 1% if judged to be drug‐related) |
Ringwald 1996.
| Methods | RCT Duration: 1 year, 1 month: April 1994 to May 1995 |
|
| Participants | Adults (> 15 years) with P falciparum malaria Number: 96 Inclusion criteria: fever or history of fever within 24 hours Exclusion criteria: severe/complicated malaria; mixed Plasmodium infection; recent self‐medication; pregnancy Diagnosis: thin film microscopy of P falciparum (asexual parasite density > 5000/µL blood) |
|
| Interventions |
|
|
| Outcomes |
*Not assessed in quantitative synthesis in this review |
|
| Notes | Location: Cameroon Setting: dispensary (outpatients) Malaria endemicity: high Resistance profile: 57% of isolates chloroquine‐resistant Source of funding: French Ministere de la Co‐operation (Grant 93A43); pyronaridine was supplied by the Institute of Parasitic Diseases, Chinese Academy of Preventive Medicine, Shangai, China Follow‐up: 14 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization (blocks of 10); from communication with authors recorded by previous version of this review |
| Allocation concealment (selection bias) | Low risk | Central randomization; from communication with authors recorded by previous version of this review |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded, but tablets were different and patients treated with chloroquine suffered pruritis; from communication with authors recorded by previous version of this review |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Report lists reasons for attrition and exclusions; unlikely to have differentially influenced liver toxicity outcomes used in this review |
| Selective reporting (reporting bias) | Low risk | Trial not prospectively registered, trial protocol not available. However, all outcomes stated in methods reported |
| Other bias | Low risk | None identified |
| Adverse event monitoring (detection bias) Adverse Events | Unclear risk | Authors do not fully describe method for detection of adverse events. Authors do not give definitions of all adverse events |
| Incomplete adverse event reporting (reporting bias) Adverse events | Unclear risk | Authors give brief narrative description of adverse events only. Report does not detail extent of elevation in liver transaminases or the proportions retested at day 14 |
Ringwald 1998.
| Methods | RCT Duration not stated. Year 1996 |
|
| Participants | Children (< 15 years) with P falciparum malaria Number: 88 Inclusion criteria: fever or history of fever within 24 hours Exclusion criteria: severe/complicated malaria; mixed Plasmodium infection; recent self‐medication; pregnancy; anaemia (Hb < 5g/dL), moderate to severe malnutrition. Diagnosis: thin film microscopy of P falciparum (asexual parasite density > 5000/µL blood) |
|
| Interventions |
|
|
| Outcomes |
*Not assessed in quantitative synthesis in this review |
|
| Notes | Location: Cameroon Setting: dispensary (outpatients) Malaria endemicity: high Resistance profile: 49% of isolates chloroquine‐resistant Source of funding: French Ministere de la Co‐operation (Grant 93A43); pyronaridine was supplied by the Institute of Parasitic Diseases, Chinese Academy of Preventive Medicine, Shangai, China Follow‐up: 14 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization (blocks of 10); from communication with authors recorded by previous version of this review |
| Allocation concealment (selection bias) | Low risk | Central randomization; from communication with authors recorded by previous version of this review |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded, but tablets were different and patients treated with chloroquine suffered pruritis; from communication with authors recorded by previous version of this review |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Report and subsequent correspondence from authors lists reasons for attrition and exclusions; unlikely to have differentially influenced liver toxicity outcomes used in this review |
| Selective reporting (reporting bias) | Low risk | Trial not prospectively registered, trial protocol not available. However, all outcomes stated in methods reported |
| Other bias | Low risk | None identified |
| Adverse event monitoring (detection bias) Adverse Events | Unclear risk | Authors do not fully describe method for detection of adverse events. Authors do not give definitions of all adverse events |
| Incomplete adverse event reporting (reporting bias) Adverse events | Unclear risk | Authors give brief narrative description of adverse events only. Report does not detail extent of elevation in liver transaminases or the proportions retested at day 14 |
Roth 2018.
| Methods | RCT Duration: 1 year, 5 months. October 2015 to June 2016, January 2017 to August 2017 |
|
| Participants | Children with P falciparum malaria Number: 197 Inclusion criteria: age 6 months ‐ 12 years; bodyweight ≥ 5 kg Exclusion criteria: severe/complicated malaria; non‐falciparum/mixed Plasmodium infection; other clinically significant disorder; malnutrition; hepatic impairment (AST/ALT not specified); renal impairment; anaemia (Hb <6 g/dL); allergy to study drugs; current participation in other antimalarial study; previous participation in study, not available for follow‐up Diagnosis: microscopically‐confirmed P falciparum monoinfection (asexual parasite density 1000 µL‐200,000/µL) Children under 5: 31 (pyronardine‐artesunate); 31 (artemether‐lumefantrine) |
|
| Interventions |
|
|
| Outcomes |
*Adequate clinical and parasitological response rate †Two consecutive normal readings taken between 7 and 25 hours apart ‡Not assessed in quantitative synthesis in this review |
|
| Notes | Location: Kenya Setting: Local clinic Malaria endemicity: high Resistance profile: not described Source of funding: EU FP7‐Health‐2013. 0‐1 Project “Translation of the direct‐on‐blood PCR‐NALFIA system into an innovative near point‐of‐care diagnostic for malaria” (DIAGMAL) [Grant Number 601714] Follow‐up: 42 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomization schedule provided by sponsor |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients probably not blinded (drugs were administered by pharmacy personnel aware of group
assignments) Clincial and parasitological assessments performed by study staff blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Report lists reasons for attrition and exclusions |
| Selective reporting (reporting bias) | Low risk | Authors report fever clearance time which was not in trial registration document. Unlikely to introduce significant bias |
| Other bias | Low risk | Target recruitment not reached Shin Poong Pharmaceutical Company (Seoul, South Korea) provided pyronaridine–artesunate tablets and granules, but had no further role in study design, data collection, data analysis and writing of the report |
| Adverse event monitoring (detection bias) Adverse Events | Unclear risk | Authors describe full schedule for ALT/AST monitoring. Due to logistic constraints, ALT and AST were only measured for the first 150 participants |
| Incomplete adverse event reporting (reporting bias) Adverse events | Unclear risk | Authors do not give reporting threshold for adverse events |
Rueangweerayut 2012.
| Methods | RCT Duration: 1 year, 10 months. January 2007 to October 2008 |
|
| Participants | Adults and children with P falciparum malaria Number: 1271 Inclusion criteria: age 3‐60 years; bodyweight 20 kg ‐ 90 kg; fever or history of fever within 24 hours Exclusion criteria: severe/complicated malaria; anaemia (Hb < 8 g/dL); severe vomiting; diarrhoea; pregnancy/lactation; other clinically significant disorder; hepatic impairment (undefined); renal impairment; antimalarial therapy in previous 2 weeks; investigational drug in previous 4 weeks; previous participation in study; allergy to study drugs Diagnosis: thick and thin film microscopy of P falciparum (asexual parasite density 1000 mm3‐ 100,000 mm3 blood) |
|
| Interventions | Randomized in a 2:1 ratio to:
|
|
| Outcomes |
*Adequate clinical and parasitological response rate †Two consecutive normal readings taken between 7 and 25 hours apart ‡Not assessed in quantitative synthesis in this review |
|
| Notes | Location: Asia (81%) and Africa (19%). Asia: Cambodia, India, Thailand, Vietnam. Africa: Bukina Faso, Ivory Coast, Tanzania Setting: local hospitals and health centres Malaria endemicity: high Resistance profile: in Cambodia, significantly extended parasite clearance times (for both treatment arms) were suggestive of in vivo resistance to artemisinin Source of funding: Medicines for Malaria Venture, Shin Poong Pharmaceutical Company Ltd, Seoul, Republic of Korea Follow‐up: 42 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomization schedule |
| Allocation concealment (selection bias) | Low risk | Individually numbered treatment packs Randomization communicated by investigator to a third party who administered the correct amount of tablets |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Unclear if patients blinded Outcome assessors blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Report lists reasons for attrition and exclusions |
| Selective reporting (reporting bias) | Low risk | Prospectively registered. Report includes all prestated outcomes of interest |
| Other bias | Low risk | Some authors employed by trial sponsors, but all authors assumed responsibility for reporting accuracy |
| Adverse event monitoring (detection bias) Adverse Events | Unclear risk | Authors do not describe methods for monitoring adverse events, but includes biochemical monitoring Authors do describe time point of assessments in protocol |
| Incomplete adverse event reporting (reporting bias) Adverse events | Unclear risk | Report percentage of adverse events. Supplementary data table provided. Reports "any cause" adverse events if they occurred in > 2% of patients. Reports "treatment related" adverse events if they occurred in > 1% of patients. Does not report method of judging relation of adverse events to treatment |
Sagara 2018.
| Methods | RCT* Duration: 4 years, 4 months. 24 October 2011 to 01 February 2016 *Different arms at different study centres; therefore we requested disaggregated data |
|
| Participants | Adults and children with P falciparum malaria Number: 4710 (2640 within pyronaridine‐artesunate subsection) Inclusion criteria: age > 2 years*; bodyweight ≥ 15 kg (decreased to ≥ 5 kg after review); fever or history of fever within 24 hours Exclusion criteria: severe/complicated malaria; severe vomiting; severe diarrhoea; other clinically significant disorder including QTc ≥ 450 ms, active TB, jaundice and others; anaemia (Hb < 70 g/dL); other febrile conditions; allergy to study drugs; antimalarial therapy in previous 2 weeks; investigational drug in previous 4 weeks; pregnancy/lactation; alcohol abuse; viral hepatitis/HIV; hepatic impairment (ALT > 2 x ULN); renal impairment (1.5 x ULN) Diagnosis: positive microscopy for Plasmodium spp. (> 0 to < 200,000 parasites/μL blood) *For pyronaridine‐artesunate group, inclusion criteria changed during the study. (i) Beginning of the study, inclusion age of 15 years or older and bodyweight of 24 kg or over, (ii) after 20 retreatments, inclusion age of 2 years or older and bodyweight of 15 kg or over, and (iii) After 40 retreatments, inclusion age of at least 6 months with bodyweight of 5 kg or over Children under 5: 344 (Pyr‐AS); 129 (AL); 249 (AS‐AQ) The total number of participants receiving at least one study treatment in each comparison are as below.
The breakdown of total participants receiving each treatment at each site is as below.
The total numbers of participants and numbers disaggregated by each site were obtained from the trial authors in response to a request for further information in May 2017 |
|
| Interventions |
*Not compared against pyronaridine‐artesunate in this trial |
|
| Outcomes |
*Not assessed in quantitative synthesis in this review |
|
| Notes | Location: West Africa (Burkina Faso, Guinea, Mali), conducted by the West African Network for Clinical Trials of Antimalarial Drugs (WANECAM). Setting: tertiary health facilities Malaria endemicity: high Resistance profile: not described Source of funding: European and Developing Countries Clinical Trial Partnership, Medicines for Malaria Venture, United Kingdom Medical Research Councils, Swedish International Development Co‐operation Agency, German Ministry for Education and Research, University Claude Bernard (France), University of Science Techniques and Technologies of Bamako, Centre National de Recherche et de Formation sur le Paludisme (Burkina Faso), Institut de Recherche en Sciences de la Santé (Bobo‐ Dioulasso, Burkina Faso), and Centre National de Formation et de Recherche en Santé Rurale (Republic of Guinea) Follow‐up: 42 days active; 2 year passive |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomization list for each site within each country was used; block size of two |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque, sequentially numbered envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Open‐label: patients and investigators not blinded to treatment allocation Microscopists assessing parasite outcomes masked to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons provided for all withdrawals across study arms. Withdrawal numbers small and balanced across the intervention arms with reasons for withdrawal similar between groups |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as listed in the trial register, however day 63 outcomes are not reported |
| Other bias | Low risk | Some authors employed by trial sponsors, but all authors assumed responsibility for reporting accuracy |
| Adverse event monitoring (detection bias) Adverse Events | Low risk | Authors report that physical examinations made and adverse events recorded at all assessments. Describes ECG and biochemistry monitoring schedule. Used Medical Dictionary for Regulatory Activities (MedDRA) |
| Incomplete adverse event reporting (reporting bias) Adverse events | Low risk | Authors enumerate adverse events clearly, and report events of interest. Provide supplementary tables. We were unable to extract adverse events by study site. |
Sagara 2018 (Bobo‐Doiulasso, Burkina Faso).
| Methods | RCT* ‐ represents disaggregated data from Sagara 2018 (see above) | |
| Participants | Number receiving at least one study treatment: 232 | |
| Interventions |
|
|
| Outcomes | ‐ | |
| Notes | Location: Bobo, Burkina Faso | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Represents disaggregated data from Sagara 2018 (see above) |
| Allocation concealment (selection bias) | Low risk | ‐ |
| Blinding (performance bias and detection bias) All outcomes | Low risk | ‐ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ‐ |
| Selective reporting (reporting bias) | Low risk | ‐ |
| Other bias | Low risk | ‐ |
| Adverse event monitoring (detection bias) Adverse Events | Low risk | ‐ |
| Incomplete adverse event reporting (reporting bias) Adverse events | Low risk | ‐ |
Sagara 2018 (Bougoula, Mali).
| Methods | RCT* ‐ represents disaggregated data from Sagara 2018 (see above) | |
| Participants | Number receiving at least one study treatment: 619 | |
| Interventions | Comparison 1
Comparison 2
|
|
| Outcomes | ‐ | |
| Notes | Location: Bougoula, Mali | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Represents disaggregated data from Sagara 2018 (see above) |
| Allocation concealment (selection bias) | Low risk | ‐ |
| Blinding (performance bias and detection bias) All outcomes | Low risk | ‐ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ‐ |
| Selective reporting (reporting bias) | Low risk | ‐ |
| Other bias | Low risk | ‐ |
| Adverse event monitoring (detection bias) Adverse Events | Low risk | ‐ |
| Incomplete adverse event reporting (reporting bias) Adverse events | Low risk | ‐ |
Sagara 2018 (Djoliba, Mali).
| Methods | RCT* ‐ represents disaggregated data from Sagara 2018 (see above) | |
| Participants | Number receiving at least one study treatment: 172 | |
| Interventions |
|
|
| Outcomes | ‐ | |
| Notes | Location: Djoliba, Mali | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Represents disaggregated data from Sagara 2018 (see above) |
| Allocation concealment (selection bias) | Low risk | ‐ |
| Blinding (performance bias and detection bias) All outcomes | Low risk | ‐ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ‐ |
| Selective reporting (reporting bias) | Low risk | ‐ |
| Other bias | Low risk | ‐ |
| Adverse event monitoring (detection bias) Adverse Events | Low risk | ‐ |
| Incomplete adverse event reporting (reporting bias) Adverse events | Low risk | ‐ |
Sagara 2018 (Kolle, Mali).
| Methods | RCT* ‐ represents disaggregated data from Sagara 2018 (see above) | |
| Participants | Number receiving at least one study treatment: 195 | |
| Interventions | Comparison 1
Comparison 2
|
|
| Outcomes | ‐ | |
| Notes | Location: Kolle, Mali | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Represents disaggregated data from Sagara 2018 (see above) |
| Allocation concealment (selection bias) | Low risk | ‐ |
| Blinding (performance bias and detection bias) All outcomes | Low risk | ‐ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ‐ |
| Selective reporting (reporting bias) | Low risk | ‐ |
| Other bias | Low risk | ‐ |
| Adverse event monitoring (detection bias) Adverse Events | Low risk | ‐ |
| Incomplete adverse event reporting (reporting bias) Adverse events | Low risk | ‐ |
Sagara 2018 (Mafrinyah, Guinea).
| Methods | RCT* ‐ represents disaggregated data from Sagara 2018 (see above) | |
| Participants | Number receiving at least one study treatment: 468 | |
| Interventions |
|
|
| Outcomes | ‐ | |
| Notes | Location: Mafrinyah, Guinea | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Represents disaggregated data from Sagara 2018 (see above) |
| Allocation concealment (selection bias) | Low risk | ‐ |
| Blinding (performance bias and detection bias) All outcomes | Low risk | ‐ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ‐ |
| Selective reporting (reporting bias) | Low risk | ‐ |
| Other bias | Low risk | ‐ |
| Adverse event monitoring (detection bias) Adverse Events | Low risk | ‐ |
| Incomplete adverse event reporting (reporting bias) Adverse events | Low risk | ‐ |
Sagara 2018 (Ouagadougou, Burkina Faso).
| Methods | RCT* ‐ represents disaggregated data from Sagara 2018 (see above) | |
| Participants | Number receiving at least one study treatment: 429 | |
| Interventions |
|
|
| Outcomes | ‐ | |
| Notes | Location: Ouagadougou, Burkina Faso | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Represents disaggregated data from Sagara 2018 (see above) |
| Allocation concealment (selection bias) | Low risk | ‐ |
| Blinding (performance bias and detection bias) All outcomes | Low risk | ‐ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ‐ |
| Selective reporting (reporting bias) | Low risk | ‐ |
| Other bias | Low risk | ‐ |
| Adverse event monitoring (detection bias) Adverse Events | Low risk | ‐ |
| Incomplete adverse event reporting (reporting bias) Adverse events | Low risk | ‐ |
Sagara 2018 (Sotuba, Mali).
| Methods | RCT* ‐ represents disaggregated data from Sagara 2018 (see above) | |
| Participants | Number receiving at least one study treatment: 325 | |
| Interventions | Comparison 1
Comparison 2
|
|
| Outcomes | ‐ | |
| Notes | Location: Sotuba, Mali | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Represents disaggregated data from Sagara 2018 (see above) |
| Allocation concealment (selection bias) | Low risk | ‐ |
| Blinding (performance bias and detection bias) All outcomes | Low risk | ‐ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ‐ |
| Selective reporting (reporting bias) | Low risk | ‐ |
| Other bias | Low risk | ‐ |
| Adverse event monitoring (detection bias) Adverse Events | Low risk | ‐ |
| Incomplete adverse event reporting (reporting bias) Adverse events | Low risk | ‐ |
Shin 2011.
| Methods | RCT | |
| Participants | Adults and children with P vivax malaria Number: 30 |
|
| Interventions |
|
|
| Outcomes |
*Not assessed in quantitative synthesis in this review |
|
| Notes | Location: Korea Setting: not known Malaria endemicity: unstable Resistance profile: not described Source of funding: not known Follow‐up: 42 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients assigned in ascending order a randomization number according to order recruited |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and investigators blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data available for 14 of 15 patients in pyronardine‐artesunate arm, and 15 of 15 patients in chloroquine arm |
| Selective reporting (reporting bias) | Low risk | Authors provided all data requested |
| Other bias | Unclear risk | Authors from pharmaceutical company manufacturing pyronardine‐artesunate |
| Adverse event monitoring (detection bias) Adverse Events | Low risk | Full schedule of safety monitoring |
| Incomplete adverse event reporting (reporting bias) Adverse events | Low risk | Authors provided all data requested |
Tshefu 2010.
| Methods | RCT Duration: 1 year, 3 months. January 2007 to April 2008 |
|
| Participants | Adults and children with P falciparum malaria Number: 1272 Inclusion criteria: age 3‐60 years; bodyweight 20 kg ‐ 90 kg; fever or history of fever within 24 hours Exclusion criteria: severe/complicated malaria; mixed Plasmodium infection; malnutrition; anaemia (Hb < 8 g/dL); severe vomiting; severe diarrhoea; other clinically significant disorder; hepatic impairment (limit not stated); renal impairment; other febrile conditions; viral hepatitis/HIV; electrolyte imbalance; allergy to study drugs; antimalarial therapy in previous 2 weeks, investigational drug in previous 4 weeks; taking any drug metabolized by cytochrome enzyme CYP2D6; previous participation in pyronaridine‐artesunate studies; pregnancy/lactation Diagnosis: microscopy (asexual parasite density 1000 µL to 100,000/µL blood) |
|
| Interventions | Randomized in a 2:1 ratio to:
|
|
| Outcomes |
*Adequate clinical and parasitological response rate †Two consecutive normal readings taken between 7 and 25 hours apart ‡Not assessed in quantitative synthesis in this review |
|
| Notes | Location: Africa (n = 1080, 85%) and Asia (n = 192, 15%). Africa: DRC, The Gambia, Ghana, Kenya, Mali, Mozambique, Senegal. Asia: Indonesia, the Phillipines Setting: local hospitals and clinics Malaria endemicity: high Resistance profile: not described Funding: Medicines for Malaria Venture, Shin Poong Pharmaceutical Company Ltd, Seoul, Republic of Korea Follow‐up: 42 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomization schedule. Block randomization of 9 by study centre |
| Allocation concealment (selection bias) | Low risk | Individually numbered treatment packs Randomization communicated by investigator to a third party who administered the correct amount of tablets, and who was not involved in clinical assessment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients blinded: artemether‐lumefantrine placebo dosed twice daily to maintain blinding. Food not required for artemether‐lumefantrine dosing to retain blinding Outcome assessors blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Report lists reasons for attrition and exclusions |
| Selective reporting (reporting bias) | Low risk | Prospectively registered. Report includes prestated outcomes of interest. Day 42 efficacy outcomes and gametocyte counts not listed in trial registration document; listed in the report as exploratory |
| Other bias | Unclear risk | Sponsors designed the trial, were responsible for data collection and analysis, and developed the report; all authors had access to trial data Participants on artemether‐lumefantrine were not expected to take medication after food; unclear if this reduced bioavailability of lumefantrine |
| Adverse event monitoring (detection bias) Adverse Events | Low risk | Report that adverse events recorded during treatment and at all follow‐up visits |
| Incomplete adverse event reporting (reporting bias) Adverse events | Unclear risk | Authors report all‐cause adverse events as percentages. Report table only includes adverse events occurring in ≥ 5% (or ≥ 1% if judged to be drug related). Authors explain method for determining relation to study drug |
Abbreviations: ACPR: adequate clinical and parasitological response; ALT: alanine aminotransferase; AST: aspartate transaminase; ECG: electrocardiogram; Hb: haemoglobin; MMV: Medicines for Malaria Venture; PCR: polymerase chain reaction; QT: QT interval on electrocardiogram; QTc: corrected QT interval on electrocardiogram; RCT: randomized controlled trial; ULN: upper limit of normal.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Huang 1988 | Quasi‐RCT. Randomized according to order of admission |
| Huang 1989 | Quasi‐RCT. Odd and even numbers used for allocation |
| Huang 1993 | RCT: conducted in people with complicated falciparum malaria |
| Laman 2014 | Trial registration (ACTRN12610000913077) planned to use pyronaridine‐artesunate, but not available due to concerns over hepatotoxicity at time of trial |
| Leang 2016 | Not a RCT: single‐arm observational study |
| Looareesuwan 1996 | Not a RCT: clinical trial of two doses of pyronaridine monotherapy with group given second dose recruited after results of first dose were analysed |
| Looareesuwan 2007 | RCT: phase II dose ranging trial |
| Piola 2008 | Not a RCT: phase II dose ranging study |
| Ramharter 2008 | Not a RCT: open‐label dose‐escalation study |
| Sagara 2014 | Not a RCT: pooled analysis |
Abbreviations: RCT: randomized controlled trial.
Differences between protocol and review
Differences between protocol and 2014 review
We stated in the protocol that we intended to assess the methods used to generate the allocation sequence and conceal allocation concealment as adequate, inadequate, or unclear according to Jüni 2001, and note who was blinded to the interventions in each trial. However, since the introduction of Review Manager 2011, we made these assessments using the methods described in Higgins 2011.
In keeping with the Cochrane Collaboration policy to use 'Summary of findings' tables, which was introduced after publication of the protocol, we generated them using GRADE profiler (GRADE 2008) and interpreted the evidence for each outcome and comparison using the GRADE approach (Schünemann 2008).
We revised the list of outcomes to reflect current WHO standards for assessing outcomes in antimalarial trials.
Although gametocyte carriage was not included as an outcome in the protocol, we included it as a secondary outcome due to its importance in malaria transmission.
In the protocol we stated that we intended to assess the effectiveness of pyronaridine both as a monotherapy and in combination with an artemisinin. However, we revised this to focus only on pyronaridine‐artemisinin combinations. In addition, due to concerns regarding pyronaridine's effect on the liver, assessment of the effects of the comparisons on liver function now include randomized comparisons in both falciparum and vivax malaria. Accordingly, we updated the background and methods sections considerably to reflect the changing scenario in malaria policies and epidemiology.
PT and HB joined the review team. Rajeev Aravindakshan withdrew from the team due to conflicting demands on his time.
Differences between protocol and 2018 review update
There is a new author team: Joseph Pryce and Paul Hine.
We use the term pyronaridine‐artesunate in preference to artesunate‐pyronaridine to reflect how most authors refer to the intervention; we changed the title accordingly. We did not proceed with quantitative analysis of secondary outcomes ‘parasite clearance', ‘fever clearance', and ‘gametocyte carriage'. The original protocol did not clearly define these outcomes, including whether they refer to durations, rates, or proportions of patients at given time points. We encountered considerable heterogeneity in these measures between studies, and therefore present a narrative synthesis. We simplified the ‘Adverse events' outcomes to reflect areas which are of most interest.
We added the term ‘pyramax' to the search strategy. We simplified the assessment of risk of bias for adverse events.
Contributions of authors
For this update, Joseph Pryce (JP) and Paul Hine (PH) extracted data from all studies, and verified characteristics of studies and previous ‘Risk of bias' assessments. PH rewrote the Background, Methods, Discussion, and Authors' conclusions, with contributions from JP. PH and JP completed the analysis, ‘Summary of findings' tables, and results. Both review authors read and approved the final manuscript.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
External sources
-
Department for International Development, UK.
Project number 300342‐104
Declarations of interest
JP has no known conflicts of interests.
PH was previously employed full‐time by Cochrane Infectious Diseases Group (CIDG), and currently works full‐time within the UK National Health Service (NHS). He received a Registration Scholarship to attend the 23rd Annual British HIV Association Conference 2017 from ViiV healthcare. ViiV had no involvement in the selection of recipients of the scholarship. In 2018, he attended a CPD‐certified clinical research training programme organized and funded by Gilead Sciences Europe Ltd. To the best of his knowledge, neither financial or non‐financial conflicts of interests have influenced the current submitted work.
Unchanged
References
References to studies included in this review
Kayentao 2012 {published data only}
- Kayentao K. Pyronaridine‐artesunate versus artemether/lumefantrine: efficacy in malaria patients with uncomplicated acute falciparum malaria: results of a pivotal Phase III trial. American Journal of Tropical Medicine and Hygiene 2008;79(Suppl 6):114. [Google Scholar]
- Kayentao K, Doumbo OK, Pénali LK, Offianan AT, Bhatt KM, Kimani J, et al. Pyronaridine‐artesunate granules versus artemether‐lumefantrine crushed tablets in children with Plasmodium falciparum malaria: a randomized controlled trial. Malaria Journal 2012;11:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00541385. Pyronaridine artesunate 3:1 granule formulation vs. Coartem© crushed tablets in P. falciparum malaria pediatric patients. www.clinicaltrials.gov/show/NCT00541385 (first received 10 October 2007).
Nelwan 2015 {published data only}
- Nelwan EJ, Ekawati LL, Tjahjono B, Setiabudy R, Sutanto I, Chand K, et al. Randomized trial of primaquine hypnozoitocidal efficacy when administered with artemisinin‐combined blood schizontocides for radical cure of Plasmodium vivax in Indonesia. BMC Medicine 2015;13:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Poravuth 2011 {published data only}
- Duparc S, Borghini‐Fuhrer I, Craft JC, Arbe‐Barnes S, Miller RM, Shin CS, et al. Efficacy of pyronaridine/artesunate in clinical trials in patients with uncomplicated acute Plasmodium falciparum or Plasmodium vivax malaria: results of an integrated analysis. American Journal of Tropical Medicine and Hygiene 2009;81 Suppl:51‐100. [Google Scholar]
- Duparc S, Borghini‐Fuhrer I, Craft JC, Arbe‐Barnes S, Miller RM, Shin CS, et al. Safety of pyronaridine/artesunate in clinical trials in patients with uncomplicated acute Plasmodium falciparum or Plasmodium vivax malaria: results of an integrated analysis. American Journal of Tropical Medicine and Hygiene 2009;81 Suppl:101‐50. [Google Scholar]
- Poravuth Y, Socheat D, Rueangweerayut R, Uthaisin C, Pyae Phyo A, Valecha N, et al. Pyronaridine‐artesunate versus chloroquine in patients with acute Plasmodium vivax malaria: a randomized, double‐blind, non‐inferiority trial. PLoS One 2011;6(1):e14501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ringwald 1996 {published data only}
- Ringwald P, Bickii J, Basco L. Randomised trial of pyronaridine versus chloroquine for acute uncomplicated falciparum malaria in Africa. Lancet 1996;347(8993):24‐8. [DOI] [PubMed] [Google Scholar]
- Ringwald P, Meche FS, Basco LK. Short report: effects of pyronaridine on gametocytes in patients with acute uncomplicated falciparum malaria. American Journal of Tropical Medicine and Hygiene 1999;61(3):446‐8. [DOI] [PubMed] [Google Scholar]
Ringwald 1998 {published data only}
- Ringwald P, Bickii J, Basco LK. Efficacy of oral pyronaridine for the treatment of acute uncomplicated falciparum malaria in African children. Clinical Infectious Diseases 1998;26(4):946‐53. [DOI] [PubMed] [Google Scholar]
Roth 2018 {published data only}
- Roth JM, Sawa P, Makio N, Omweri G, Osoti V, Okach S, et al. Pyronaridine–artesunate and artemether–lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in Kenyan children: a randomized controlled non‑inferiority trial. Malaria Journal 2018;17(1):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rueangweerayut 2012 {published data only}
- NCT00403260. Pyronaridine artesunate (3:1) versus mefloquine artesunate in P. falciparum malaria patients. www.clinicaltrials.gov/show/NCT00403260 (first received 21 November 2006).
- Rueangweerayut R, Phyo AP, Uchaisin C, Poravuth Y, Quang Binh T, Tinto H, et al. A randomized clinical trial comparing the efficacy and safety of fixed‐dose pyronaridine‐artesunate versus mefloquine plus artesunate in uncomplicated Plasmodium falciparum malaria. Submitted under review; personal communication by email from Isabelle Borghini Fuhrer, Medicines for Malaria Venture.
- Rueangweerayut R, Phyo AP, Uchaisin C, Socheat D, Quang Binh T, Tinto H, et al. Efficacy and safety of pyronaridine/artesunate fixed‐dose combination compared with mefloquine plus artesunate in patients with acute uncomplicated Plasmodium falciparum malaria: results of a pivotal phase III trial. Tropical Medicine and International Health 2009;14 Suppl 2:30‐97. [Google Scholar]
- Rueangweerayut R, Phyo AP, Uthaisin C, Poravuth Y, Binh TQ, Tinto H, et al. Pyronaridine‐artesunate versus mefloquine plus artesunate for malaria. New England Journal of Medicine 2012;366(14):1298‐309. [DOI] [PubMed] [Google Scholar]
- Rueangweerayut R, Phyo AP, Uthaisin C, Poravuth Y, Binh TQ, Tinto H, et al. Supplement to: Pyronaridine–artesunate versus mefloquine plus artesunate for malaria. New England Journal of Medicine 2012;366(14):1298‐309. [DOI] [PubMed] [Google Scholar]
Sagara 2018 {published and unpublished data}
- Beshir KB, Diallo N, Fofana B, Traore A, Kodio A, Togo A, et al. Measuring the efficacy of four ACT regimens in Mali using qPCR‐based estimates of Plasmodium falciparum clearance time. 63rd Annual Meeting of the American Society of Tropical Medicine and Hygiene, New Orleans (LA), 2013 Nov 02‐06. American Journal of Tropical Medicine and Hygiene. 2014.
- Kabore TN, Barry N, Compaore YD, Nikiema F, Kabre Z, Fofana A. Randomized trial to assess the effect on qtc interval of repeated treatment of uncomplicated malaria with acts in Bobo‐Dioulasso, Burkina Faso: relation between parasitemia and prolonged qtc. American Journal of Tropical Medicine and Hygiene 2017;97(5 Suppl 1):94. [Google Scholar]
- Sagara I, Beavogui AH, Zongo I, Soulama I, Borghini‐Fuhrer I, Fofana B, et al. Safety and efficacy of re‐treatments with pyronaridine‐artesunate in African patients with malaria: a substudy of the WANECAM randomised trial. Lancet Infectious Diseases 2016;16(2):189‐98. [DOI: 10.1016/S1473-3099(15)00318-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagara I, Beavogui AH, Zongo I, Soulama I, Borghini‐Fuhrer I, Fofana B, et al. Pyronaridine‐artesunate or dihydroartemisinin‐piperaquine versus current first‐line therapies for repeated treatment of uncomplicated malaria: a randomised, multicentre, open‐label, longitudinal, controlled, phase 3b/4 trial. Lancet 2018;391(10128):1378‐90. [DOI: 10.1016/S0140-6736(18)30291-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulama I, Coulibaly AS, Kabore JM, Ouattara MS, Bougouma EC, Sanon S. Assessment of the dynamics of Plasmodium falciparum parasitemia regarding three artemisinin combination regimens for acute uncomplicated malaria treatment, Banfora, Burkina Faso. American Journal of Tropical Medicine and Hygiene 2017;97(5 Suppl 1):317. [Google Scholar]
- Soulama I, Coulibaly AS, Kabore MJ, Ouattara M, Bougouma EC, Sanon S. Safety and efficacy of repeated administration of pyronaridine‐artesunate or dihydroartemisinin‐piperaquine vs artesunate‐amodiaquine in children and adult patients with acute uncomplicated Plasmodium sp malaria over of two years period at banfora/niangoloko site in Burkina Faso. 65th annual meeting of the American Society of Tropical Medicine and Hygiene, Atlanta (GA), 2016 Nov 13‐17. American Journal of Tropical Medicine and Hygiene. 2017.
- Tekete M, Djimde A, Borrmann S. A phase IIIb/IV comparative, randomised, multi‐centre, open label, parallel 3‐arm clinical study to assess the safety and efficacy of repeated administration of four artemisinin‐based combination therapy (ACT) over a two‐year period in children and adult patients with acute uncomplicated Plasmodium sp. malaria. 9. Jahrestreffen der AG Malaria der Sektion Antiparasitare Chemotherapie der Paul‐Ehrlich‐Gesellschaft fur Chemotherapie. 2012.
Sagara 2018 (Bobo‐Doiulasso, Burkina Faso) {published and unpublished data}
- WANECAM trial authors. Site data: Bobo, Burkina Faso (as supplied 6 July 2017). Data on file.
Sagara 2018 (Bougoula, Mali) {published and unpublished data}
- WANECAM trial authors. Site data: Bougala, Mali (as supplied 6 July 2017). Data on file.
Sagara 2018 (Djoliba, Mali) {published and unpublished data}
- WANECAM trial authors. Site data: Djoliba, Mali (as supplied 6 July 2017). Data on file.
Sagara 2018 (Kolle, Mali) {published and unpublished data}
- WANECAM trial authors. Site data: Kolle, Mali (as supplied 6 July 2017). Data on file.
Sagara 2018 (Mafrinyah, Guinea) {published and unpublished data}
- WANECAM trial authors. Site data: Mafrinyah, Guinea (as supplied 6 July 2017). Data on file.
Sagara 2018 (Ouagadougou, Burkina Faso) {published and unpublished data}
- WANECAM trial authors. Site data: Ouaga, Burkina Faso (as supplied 6 July 2017). Data on file.
Sagara 2018 (Sotuba, Mali) {published and unpublished data}
- WANECAM trial authors. Site data: Sotuba, Mali (as supplied 6 July 2017). Data on file.
Shin 2011 {published and unpublished data}
- Shin CS, Kwak YG, Lee KD, Borghini Fuhrer I, Miller RM, Duparc S. Treatment of Korean vivax malaria patients with the fixed‐dose combination of pyronaridine:artesunate. 7th European Congress on Tropical Medicine and International Health. 2011.
Tshefu 2010 {published data only}
- Duparc S, Borghini‐ Fuhrer I, Craft JC, Arbe‐ Barnes S, Miller RM, Shin CS, et al. Safety of pyronaridine/artesunate in clinical trials in patients with uncomplicated acute Plasmodium falciparum or Plasmodium vivax malaria: results of an integrated analysis. American Journal of Tropical Medicine and Hygiene 2009;81 Suppl:101‐50. [Google Scholar]
- Duparc S, Borghini‐Fuhrer I, Craft JC, Arbe‐Barnes S, Miller RM, Shin CS, et al. Efficacy of pyronaridine/artesunate in clinical trials in patients with uncomplicated acute Plasmodium falciparum or Plasmodium vivax malaria: results of an integrated analysis. American Journal of Tropical Medicine and Hygiene 2009;81 Suppl:51‐100. [Google Scholar]
- Tshefu AK, Gaye O, Kayentao K, Thompson R, Bhatt KM, Sesay SS, et al. Efficacy and safety of a fixed‐dose oral combination of pyronaridine‐artesunate compared with artemether‐lumefantrine in children and adults with uncomplicated Plasmodium falciparum malaria: a randomised non‐inferiority trial. Lancet 2010;375(9724):1457‐67. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Huang 1988 {published data only}
- Huang ZS, Shao BR, Meng F, Zeng LH, Ye XY, Huang J, et al. Effects of combined dose of pyronaridine/sulfadoxine/pyrimethamine on falciparum malaria. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi [Chinese Journal of Parasitology and Parasitic Diseases] 1988;6(4):285‐8. [PubMed] [Google Scholar]
Huang 1989 {published data only}
- Huang ZS, Feng Z, Meng F, Zeng LH, Lin X, Zhen Y, et al. Therapeutic effect of pyronaridine in plain tablets and enteric coated tablets in falciparum malaria patients. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi [Chinese Journal of Parasitology and Parasitic Diseases] 1989;7(1):19‐21. [PubMed] [Google Scholar]
Huang 1993 {published data only}
- Huang Z, Shao B, Meng F, Shi X. Comparison of different regimen of pyronaridine and sulfadoxine combined with pyrimethamine in the treatment of malignant malaria. Chinese Journal of Infectious Diseases 1993;11(Suppl 3):175‐7. [ISSN: CN‐00256187] [Google Scholar]
Laman 2014 {published data only}
- Laman M, Moore BR, Benjamin JM, Yadi G, Bona C, Warrel J. Artemisinin‐naphthoquine versus artemether‐lumefantrine for uncomplicated malaria in Papua New Guinean children: an open‐label randomized trial. PLoS Medicine 2014;11(12):e1001773. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leang 2016 {published data only}
- Leang R, Canavati SE, Khim N, Vestergaard LS, Borghini Fuhrer I, Kim S, et al. Efficacy and safety of pyronaridine‐artesunate for the treatment of uncomplicated Plasmodium falciparum malaria in western Cambodia. Antimicrobial Agents and Chemotherapy 2016;60(7):3884‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Looareesuwan 1996 {published data only}
- Looareesuwan S, Kyle DE, Viravan C, Vanijanonta S, Wilairatana P, Wernsdorfer WH. Clinical study of pyronaridine for the treatment of acute uncomplicated falciparum malaria in Thailand. American Journal of Tropical Medicine and Hygiene 1996;54(2):205‐9. [DOI] [PubMed] [Google Scholar]
Looareesuwan 2007 {published data only}
- Looareesuwan S, Gaye O, Tjitra E, Bojang K, Socheat D, Piola P. Results of a randomized, multicentre, phase II, dose‐ranging, clinical study to assess the safety and efficacy of fixed dose, orally administered pyronaridine and artesunate in adult patients with acute uncomplicated Plasmodium falciparum malaria. American Journal of Tropical Medicine and Hygiene 2007;77(5):1004. [Google Scholar]
Piola 2008 {published data only}
- Piola P, Fleckenstein L. Pharmacokinetics, clinical and safety outcomes of pyronaridine/artesunate treatment of acute Plasmodium falciparum malaria in Uganda. American Journal of Tropical Medicine and Hygiene 2008;79(6):855. [Google Scholar]
Ramharter 2008 {published data only}
- Ramharter M, Kurth F, Schreier AC, Nemeth J, Glasenapp Iv, Bélard S, et al. Fixed‐dose pyronaridine‐artesunate combination for treatment of uncomplicated falciparum malaria in pediatric patients in Gabon. Journal of Infectious Diseases 2008;198(6):911‐9. [DOI] [PubMed] [Google Scholar]
Sagara 2014 {published data only}
- Sagara I, Piarroux R, Djimde A, Giorgi R, Kayentao K, Doumbo OK, et al. Delayed anemia assessment in patients treated with oral artemisinin derivatives for uncomplicated malaria: a pooled analysis of clinical trials data from Mali. Malaria Journal 2014;13(1):358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Aithal 2011
- Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, et al. Case definition and phenotype standardization in drug‐induced liver injury. Clinical Pharmacology & Therapeutics 2011;89(6):806‐15. [DOI] [PubMed] [Google Scholar]
Basco 1992
- Basco LK, Bras J. In vitro activity of pyronaridine against African strains of Plasmodium falciparum. Annals of Tropical Medicine and Parasitology 1992;86(5):447‐54. [DOI] [PubMed] [Google Scholar]
Chavalitshewinkoon‐Petmitr 2000
- Chavalitshewinkoon‐Petmitr P, Pongvilairat G, Auparakkitanon S, Wilairat P. Gametocytocidal activity of pyronaridine and DNA topoisomerase II inhibitors against multidrug‐resistant Plasmodium falciparum in vitro. Parasitology International 2000;48(4):275‐80. [DOI] [PubMed] [Google Scholar]
Chen 1992
- Chang C, Lin‐Hua T, Jantanavivat C. Studies on a new antimalarial compound: pyronaridine. Transactions of the Royal Society of Tropical Medicine and Hygiene 1992;86(1):7‐10. [DOI] [PubMed] [Google Scholar]
Childs 1988
- Childs GE, Häusler B, Milhous W, Chen C, Wimonwattrawatee T, Pooyindee N, et al. In vitro activity of pyronaridine against field isolates and reference clones of Plasmodium falciparum. American Journal of Tropical Medicine and Hygiene 1988;38(1):24‐9. [DOI] [PubMed] [Google Scholar]
Croft 2012
- Review of pyronaridine anti‐malarial properties, product characteristics. Croft SL, Duparc S, Arbe‐Barnes SJ, Craft JC, Shin CS, Fleckenstein L, et al. Malaria Journal 2012;11:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dondorp 2009
- Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, et al. Artemisinin resistance in Plasmodium falciparum malaria. New England Journal of Medicine 2009;351(5):455‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duparc 2013
- Duparc S, Borghini‐Fuhrer I, Craft CJ, Arbe‐Barnes S, Miller RM, Shin CS, et al. Safety and efficacy of pyronaridine‐artesunate in uncomplicated acute malaria: an integrated analysis of individual patient data from six randomized clinical trials. Malaria Journal 2013;12:70. [DOI: 10.1186/1475-2875-12-70] [DOI] [PMC free article] [PubMed] [Google Scholar]
EMA 2015
- European Medicines Agency. Summary of opinion: Pyramax (pyronaridine–artesunate). www.ema.europa.eu/docs/en_GB/document_library/Medicine_for_use_outside_EU/2015/11/WC500196738.pdf 19 November 2015.
Fu 1991
- Fu S, Xiao SH. Pyronaridine: a new antimalarial drug. Parasitology Today 1991;7(11):310‐3. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kurth 2009
- Kurth F, Pongratz P, Bélard S, Mordmüller B, Kremsner PG, Ramharter M. In vitro activity of pyronaridine against Plasmodium falciparum and comparative evaluation of anti‐malarial drug susceptibility assays. Malaria Journal 2009;8:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration.
Loke 2007
- Loke YK, Price D, Herxheimer A, Cochrane Adverse Effects Methods Group. Systematic reviews of adverse effects: framework for a structured approach. BMC Medical Research Methodology 2007;7(32):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lubell 2014
- Lubell Y, Dondorp A, Guerin PJ, Drake T, Meek S, Ashley E, et al. Artemisinin resistance‐modelling the potential human and economic costs. Malaria Journal 2014;13(452):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
MedDRA 2018 [Computer program]
MMV 2002
- TDR/Medicines for Malaria Venture/Shin Poong Pharmaceuticals Inc. WHO TDR/Medicines for Malaria Venture/Shin Poong Pharmaceuticals Inc. sign agreement for development of pyronaridine‐artesunate for treatment of malaria. www.mmv.org/newsroom/press‐releases/tdrmedicines‐malaria‐ventureshin‐poong‐pharmaceuticals‐inc‐sign‐agreement (accessed 17 April 2018).
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morris 2012
- Morris CA, Lopez‐Lazaro L, Jung D, Methaneethorn J, Duparc S, Borghini‐Fuhrer I, et al. Drug‐drug interaction analysis of pyronaridine/artesunate and ritonavir in healthy volunteers. American Journal of Tropical Medicine and Hygiene 2012;86(3):489‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT03201770
- NCT03201770. Cohort event monitoring study of Pyramax. clinicaltrials.gov/ct2/show/NCT03201770 (first posted 28 June 2017).
Noedl 2008
- Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Evidence of artemisinin‐resistant malaria in western Cambodia. New England Journal of Medicine 2008;359(24):2619‐20. [DOI] [PubMed] [Google Scholar]
Peters 1997
- Peters W, Robinson BL. The chemotherapy of rodent malaria. LV. Interactions between pyronaridine and artemisinin. Annals of Tropical Medicine and Parasitology 1997;91(2):141‐5. [DOI] [PubMed] [Google Scholar]
Pradines 1998
- Pradines B, Tall A, Parzy D, Spiegel A, Fusai T, Hienne R, et al. In‐vitro activity of pyronaridine and amodiaquine against African isolates (Senegal) of Plasmodium falciparum in comparison with standard antimalarial agents. Journal of Antimicrobial Chemotherapy 1998;42(3):333‐9. [DOI] [PubMed] [Google Scholar]
Price 2010
- Price RN, Marfurt J, Chalfein F, Kenangalem E, Piera KA, Tjitra E, et al. In vitro activity of pyronaridine against multidrug‐resistant Plasmodium falciparum and Plasmodium vivax. Antimicrobial Agents and Chemotherapy 2010;54(12):5146‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ringwald 1999
- Ringwald P, Eboumbou EC, Bickii J, Basco LK. In vitro activities of pyronaridine, alone and in combination with other antimalarial drugs, against Plasmodium falciparum. Antimicrobial Agents and Chemotherapy 1999;43(6):1525‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). GRADE handbook for grading quality of evidence and strength of recommendations. gdt.guidelinedevelopment.org/app/handbook/handbook.html (accessed 3 May 2018).
Sinclair 2009
- Sinclair D, Zani B, Donegan S, Olliaro P, Garner P. Artemisinin‐based combination therapy for treating uncomplicated malaria. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007483.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vivas 2008
- Vivas L, Rattray L, Stewart L, Bongard E, Robinson BL, Peters W, et al. Anti‐malarial efficacy of pyronaridine and artesunate in combination in vitro and in vivo. Acta Tropica 2008;105(3):222‐8. [DOI] [PubMed] [Google Scholar]
WHO 2003
- World Health Organization. Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria. December 2003. www.who.int/malaria/publications/atoz/whohtmrbm200350/en/ (accessed 1 September 2017).
WHO 2006
- World Health Organization. WHO guidelines for the treatment of malaria. 2006. http://archives.who.int/publications/2006/9241546948_eng.pdf (accessed 1 September 2017).
WHO 2009
- World Health Organization. Methods for surveillance of antimalarial drug efficacy. November 2009. www.who.int/malaria/publications/atoz/9789241597531/en/ (accessed 1 September 2017).
WHO 2011
- World Health Organization. Global plan for artemisinin resistance containment (GPARC). www.who.int/malaria/publications/atoz/9789241500838/en/index.html (accessed 4 February 2011).
WHO 2015
- World Health Organization. Guidelines for the treatment of malaria ‐ 3rd edition. April 2015. www.who.int/malaria/publications/atoz/9789241549127/en/ (accessed 1 September 2017).
WHO 2017
- World Health Organization. World Malaria Report 2017. www.who.int/malaria/publications/world‐malaria‐report‐2017/en/ (accessed 1 April 2018).
Zorzela 2016
- Zorzela L, Loke YK, Ioannidis JP, Golder S, Santaguida P, Altman DG, et al. PRISMA Harms Group. PRISMA harms checklist: improving harms reporting in systematic reviews. BMJ 2016;352(157):1‐17. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bukirwa 2014
- Bukirwa H, Unnikrishnan B, Kramer CV, Sinclair D, Nair S, Tharyan P. Artesunate plus pyronaridine for treating uncomplicated Plasmodium falciparum malaria. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD006404.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Unnikrishnan 2007
- Unnikrishnan B, Nair S, Aravindakshan R. Pyronaridine for treating uncomplicated malaria. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD006404] [DOI] [PMC free article] [PubMed] [Google Scholar]


