Abstract
Background
Critically ill people are at increased risk of malnutrition. Acute and chronic illness, trauma and inflammation induce stress‐related catabolism, and drug‐induced adverse effects may reduce appetite or increase nausea and vomiting. In addition, patient management in the intensive care unit (ICU) may also interrupt feeding routines. Methods to deliver nutritional requirements include provision of enteral nutrition (EN), or parenteral nutrition (PN), or a combination of both (EN and PN). However, each method is problematic. This review aimed to determine the route of delivery that optimizes uptake of nutrition.
Objectives
To compare the effects of enteral versus parenteral methods of nutrition, and the effects of enteral versus a combination of enteral and parenteral methods of nutrition, among critically ill adults, in terms of mortality, number of ICU‐free days up to day 28, and adverse events.
Search methods
We searched CENTRAL, MEDLINE, and Embase on 3 October 2017. We searched clinical trials registries and grey literature, and handsearched reference lists of included studies and related reviews.
Selection criteria
We included randomized controlled studies (RCTs) and quasi‐randomized studies comparing EN given to adults in the ICU versus PN or versus EN and PN. We included participants that were trauma, emergency, and postsurgical patients in the ICU.
Data collection and analysis
Two review authors independently assessed studies for inclusion, extracted data, and assessed risk of bias. We assessed the certainty of evidence with GRADE.
Main results
We included 25 studies with 8816 participants; 23 studies were RCTs and two were quasi‐randomized studies. All included participants were critically ill in the ICU with a wide range of diagnoses; mechanical ventilation status between study participants varied. We identified 11 studies awaiting classification for which we were unable to assess eligibility, and two ongoing studies.
Seventeen studies compared EN versus PN, six compared EN versus EN and PN, two were multi‐arm studies comparing EN versus PN versus EN and PN. Most studies reported randomization and allocation concealment inadequately. Most studies reported no methods to blind personnel or outcome assessors to nutrition groups; one study used adequate methods to reduce risk of performance bias.
Enteral nutrition versus parenteral nutrition
We found that one feeding route rather than the other (EN or PN) may make little or no difference to mortality in hospital (risk ratio (RR) 1.19, 95% confidence interval (CI) 0.80 to 1.77; 361 participants; 6 studies; low‐certainty evidence), or mortality within 30 days (RR 1.02, 95% CI 0.92 to 1.13; 3148 participants; 11 studies; low‐certainty evidence). It is uncertain whether one feeding route rather than the other reduces mortality within 90 days because the certainty of the evidence is very low (RR 1.06, 95% CI 0.95 to 1.17; 2461 participants; 3 studies). One study reported mortality at one to four months and we did not combine this in the analysis; we reported this data as mortality within 180 days and it is uncertain whether EN or PN affects the number of deaths within 180 days because the certainty of the evidence is very low (RR 0.33, 95% CI 0.04 to 2.97; 46 participants).
No studies reported number of ICU‐free days up to day 28, and one study reported number of ventilator‐free days up to day 28 and it is uncertain whether one feeding route rather than the other reduces the number of ventilator‐free days up to day 28 because the certainty of the evidence is very low (mean difference, inverse variance, 0.00, 95% CI ‐0.97 to 0.97; 2388 participants).
We combined data for adverse events reported by more than one study. It is uncertain whether EN or PN affects aspiration because the certainty of the evidence is very low (RR 1.53, 95% CI 0.46 to 5.03; 2437 participants; 2 studies), and we found that one feeding route rather than the other may make little or no difference to pneumonia (RR 1.10, 95% CI 0.82 to 1.48; 415 participants; 7 studies; low‐certainty evidence). We found that EN may reduce sepsis (RR 0.59, 95% CI 0.37 to 0.95; 361 participants; 7 studies; low‐certainty evidence), and it is uncertain whether PN reduces vomiting because the certainty of the evidence is very low (RR 3.42, 95% CI 1.15 to 10.16; 2525 participants; 3 studies).
Enteral nutrition versus enteral nutrition and parenteral nutrition
We found that one feeding regimen rather than another (EN or combined EN or PN) may make little or no difference to mortality in hospital (RR 0.99, 95% CI 0.84 to 1.16; 5111 participants; 5 studies; low‐certainty evidence), and at 90 days (RR 1.00, 95% CI 0.86 to 1.18; 4760 participants; 2 studies; low‐certainty evidence). It is uncertain whether combined EN and PN leads to fewer deaths at 30 days because the certainty of the evidence is very low (RR 1.64, 95% CI 1.06 to 2.54; 409 participants; 3 studies). It is uncertain whether one feeding regimen rather than another reduces mortality within 180 days because the certainty of the evidence is very low (RR 1.00, 95% CI 0.65 to 1.55; 120 participants; 1 study).
No studies reported number of ICU‐free days or ventilator‐free days up to day 28. It is uncertain whether either feeding method reduces pneumonia because the certainty of the evidence is very low (RR 1.40, 95% CI 0.91 to 2.15; 205 participants; 2 studies). No studies reported aspiration, sepsis, or vomiting.
Authors' conclusions
We found insufficient evidence to determine whether EN is better or worse than PN, or than combined EN and PN for mortality in hospital, at 90 days and at 180 days, and on the number of ventilator‐free days and adverse events. We found fewer deaths at 30 days when studies gave combined EN and PN, and reduced sepsis for EN rather than PN. We found no studies that reported number of ICU‐free days up to day 28. Certainty of the evidence for all outcomes is either low or very low. The 11 studies awaiting classification may alter the conclusions of the review once assessed.
Plain language summary
Delivery of nutrition (food) to critically ill adults other than by the person eating and swallowing the food/nutrition
Background
Critically ill adults in the intensive care unit (ICU) are at an increased risk of malnutrition because the body responds to serious illness or injury by increasing the metabolic rate. Also, the person's feeding routine may be disrupted because they are unconscious or too ill to feed themselves or eat normally. This means alternative ways to ensure people receive adequate nutrition must be used. People may be given artificial nutrition in three ways: enteral feeding (through a tube placed into the stomach or small intestine; parenteral feeding (through a tube inserted into a vein whereby nutrients enter the bloodstream directly); or by a combination of both routes. This review compared the effects of these routes.
Study characteristics
The evidence is current to 3 October 2017. We included 25 studies with 8816 participants who had trauma, emergency, medical or postsurgical conditions and were in the ICU. Eleven studies are awaiting classification (because we did not have enough details to assess them) and two studies are ongoing. Included studies compared enteral feeding with parenteral feeding, or with combined enteral and parenteral feeding.
Key results
Studies reported the number of people who died from any cause at different time points. We found no evidence that enteral feeding compared to parenteral feeding or compared to a combination of routes was more or less likely to reduce the number of deaths in hospital, within 90 days and 180 days. We found evidence from three small studies that fewer people died within 30 days when feeding was given through combined enteral and parenteral routes. No studies reported number of ICU‐free days up to day 28 (i.e. length of stay in the ICU by taking account of expected participant loss because of death) and one study reported that the feeding route did not affect the number of ventilator‐free days.
We found no evidence that enteral feeding compared to parenteral feeding was likely to increase or decrease cases of aspiration (the entry of materials such as food from the digestive system to the lungs) or pneumonia (swelling of the tissue in one or both lungs that is usually caused by a bacterial infection). Enteral nutrition may reduce sepsis (a life‐threatening condition that arises when the body's response to infection causes injury to its own tissues and organs), although evidence was from studies of people with different conditions (such as trauma, medical, or postsurgical conditions). We found that fewer participants vomited if they were given parenteral feeding rather than enteral feeding, although there were few studies with very few reported events.
Certainty of the evidence
It was not possible for researchers to mask the ICU staff to the type of feeding route, which may have biased the findings, and study authors did not consistently report good study methods. People in each study had different types of critical illness (such as trauma, medical, or postsurgical conditions) which may have affected how they responded to the type of feeding route, and there were limited data for many of our measurements. We believed that the certainty of the evidence was low or very low.
Conclusion
We found insufficient evidence to determine with confidence whether one feeding route was better at reducing the number of deaths, the number of ventilator‐free days, and side effects. No studies reported number of ICU‐free days up to day 28. Evidence was of low and very low certainty, and we could not be confident in the findings of our review.
Summary of findings
Background
Description of the condition
Malnutrition is associated with increased mortality and morbidity to include susceptibility to infectious complications, such as pulmonary infections, urinary infections, wound infections, and sepsis, and susceptibility to non‐infectious complications, such as respiratory failure and cardiac arrhythmias (Correia 2003; Mogensen 2015).
Acute and chronic illness, trauma, and inflammation induce stress‐related catabolism, increasing the metabolic rate at which the body breaks down food. In addition to this, drug‐related side effects may affect ingestion and lead to loss of appetite or nausea and vomiting, or both, and it has been suggested that hospital routines and lack of awareness among nursing staff may affect nutritional care (Norman 2008). Critically ill people, who may be unconscious, unable to feed themselves or unable to receive oral nutritional support, or both, are at increased susceptibility to malnutrition.
Nutritional support is a complex aspect of care for critically ill people. This systematic review aimed specifically to address the route of delivery that will optimize uptake of nutrition. It did not deal with supplementation of specific nutrients as a number of these are reviewed already (Allingstrup 2016; Dushianthan 2016; Tao 2014).
Description of the intervention
Enteral nutrition (EN) refers to the delivery of a nutritionally complete feed via a tube into the stomach, duodenum, or jejunum (NICE 2006). This method is suitable for people who have inadequate oral intake but a functional gastrointestinal tract, and some evidence suggests that it is an effective method of providing nutrition to particular patient groups (e.g. people with sepsis (Elke 2013); people with acute pancreatitis (Al‐Omran 2010)). EN may help to maintain the function and integrity of the gut barrier (Altintas 2011; King 1999; Kyle 2006), and is associated with increased immunoglobulin A production, which in turn may provide increased protection against airway infections. However, critically ill people may not tolerate enteral feeding well, and side effects such as nausea and vomiting may occur (Harvey 2015), and non‐occlusive bowel necrosis (Marvin 2000). In addition, high volumes of gastric residual may allow bacteria to colonize, and increase the risk of aspiration and complications, such as ventilator‐associated pneumonia (Altintas 2011), although one study assessing monitoring of gastric residual volume showed no difference in ventilator‐associated pneumonia with absence of monitoring (Reignier 2013). Furthermore, EN can be disturbed by patient care and diagnostic interventions, particularly among people receiving respiratory support (Corley 2017), and this may affect the capacity for EN to maintain nutritional goals (Kyle 2006; Seres 2013). Some benefit has been found from placing the tube into the duodenum or jejunum rather than the stomach (Alkhawaja 2015).
Parenteral nutrition (PN) is unphysiological and bypasses the gastrointestinal tract and portal venous system. It delivers a nutritionally complete feed intravenously via a central or peripheral venous catheter, and may be used as an alternative for people in need of nutritional support. It confers the advantage of ease of administration to the person (Seres 2013), often with no further intervention needed to provide nutritional support when all components are administered via an 'all in one bag' system. Whilst interrupting feeding during patient care is not necessary, PN may increase the risk of overfeeding (Singer 2009). PN is associated with a higher rate of hyperglycaemia; subsequently, people may require glycaemic control alongside PN. Earlier studies have reported increased susceptibility to infectious complications, such as catheter‐related bloodstream infections (Peter 2005).
PN may be used to supplement EN to achieve target energy requirements when EN alone is inadequate (Singer 2011).
Research findings are unclear regarding sufficient caloric intake needed to meet the energy requirements of critically ill people, and no evidence currently supports the assumption that these people benefit from a normocaloric intake (80% to 100% of energy requirements) rather than permissive underfeeding (less than 70% of energy requirements) (Marik 2016). Similarly, a target time for initiation of nutrition has been debated by researchers, with large randomized controlled trials (RCTs) (e.g. the EDEN (Early versus delayed enteral feeding to treat people with acute lung injury or acute respiratory distress syndrome) study (ARDS Clinical Trials Network 2012); the EPaNIC (Early Parenteral Nutrition Completing Enteral Nutrition in Adult Critically Ill Patients) study (Casaer 2011)), providing evidence that conflicts with European nutrition guidelines (ESPEN; Singer 2009), which advise early feeding during critical illness (Casaer 2014). Whilst this review aimed specifically to address the route of nutrition, both caloric intake and initiation time are also important considerations.
Why it is important to do this review
The most current American Society for Parenteral and Enteral Nutrition (ASPEN) guidelines recommend use of EN over PN (Taylor 2016), suggesting a reduction in infectious morbidity and length of stay in the intensive care unit (ICU) for people given EN. This is comparable with guidelines of the European Society for Parenteral and Enteral Nutrition (ESPEN) (Kreymann 2006; Singer 2009), and the UK National Institute for Health and Care Excellence (NICE) (NICE 2006). However, these guidelines reflect only research findings of small RCTs published prior to these guidelines, and current evidence contradicts some outcomes, for example, risk of infectious complications with PN.
It is highly debated if, how, and when nutritional support may contribute to improved patient outcomes (Casaer 2014; Preiser 2015; Schetz 2013). Nutrition for critically ill people has global relevance, achieving benefits for the patient and reducing impact on healthcare resources. This review aimed specifically to consider whether the route of delivery of nutrition is a significant factor in the treatment of critically ill adults, and incorporates recent findings to assess both evidence of benefit and risk of adverse events.
Objectives
To compare the effects of enteral versus parenteral methods of nutrition, and the effects of enteral versus a combination of enteral and parenteral methods of nutrition, among critically ill adults, in terms of mortality, number of ICU‐free days up to day 28 and adverse events.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials (RCTs), including quasi‐randomized studies (e.g. studies in which the method of assignment was based on alternation, date of birth, or medical record number) and cluster‐randomized studies.
Types of participants
We included all adults, over 16 years of age, who had been in an ICU for at least 24 hours.
We included participants admitted for all conditions, except acute pancreatitis as this patient group is reviewed elsewhere (Al‐Omran 2010). We aimed to include studies that had a mixed population that included acute pancreatitis, if fewer than 50% of participants had acute pancreatitis; we aimed to contact study authors to request additional information if necessary.
We included trauma, emergency, medical, and elective postsurgical participants. We included mechanically ventilated and non‐mechanically ventilated participants.
If studies included participants of which not all were in the ICU, we included the study if study authors reported that more than 75% of participants were in the ICU.
Types of interventions
We included studies that compare EN versus PN, and studies that compared EN versus EN and PN. These represent two comparison groups; we analysed separately data from studies comparing EN versus PN and studies comparing EN versus EN and PN.
We included EN that was given via a tube into the stomach, duodenum, or jejunum, and PN that was given via a central venous catheter or a peripheral venous catheter. We anticipated that the protocol used to administer nutrition would differ between studies. We included EN and PN initiated early or delayed, and given to meet a normocaloric or hypocaloric goal.
Types of outcome measures
We aimed to establish whether one type of feeding method reduced the rate of mortality among study participants and considered data gathered at different time points, up to 180 days. Length of stay in the ICU was an important outcome for this review topic. Given that rates of mortality may be high in the included population, and to avoid the effect of death on this outcome, we planned to report data presented as ICU‐free days. Similarly, we planned to assess duration of mechanical ventilation as the number of ventilator‐free days. These outcomes account for the number of days that a person is alive or is no longer using mechanical ventilation; therefore, a participant who has died would be counted as having zero ICU‐free or ventilator‐free days. Adverse events represent an important outcome for this review, and EN and PN may lead to different adverse events. For each adverse event reported by study authors, we collected data during the study follow‐up period.
Primary outcomes
Mortality (measured: in‐hospital, within 30 days, within 90 days, and within 180 days).
Secondary outcomes
Number of ICU‐free days up to day 28.
Number of ventilator‐free days up to day 28.
Adverse events as reported by study authors (to include hyperglycaemia, aspiration pneumonia, catheter‐related bloodstream infections, and gastrointestinal events).
Search methods for identification of studies
Electronic searches
We identified RCTs through literature searching with systematic and sensitive search strategies as outlined in Chapter 6.4 of the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011). We applied no restrictions to language or publication status.
We searched the following databases for relevant trials:
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 9);
MEDLINE (OvidSP, 1946 to 3 October 2017);
Embase (OvidSP, 1974 to 3 October 2017).
We developed a subject‐specific search strategy in MEDLINE and used that as the basis for the search strategies in the other listed databases. The search strategy was developed in consultation with the Information Specialist. Search strategies can be found in Appendix 1; Appendix 2; and Appendix 3.
We scanned the following trial registries for ongoing and unpublished trials (8 January 2018):
World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp/en/);
ClinicalTrials.gov (clinicaltrials.gov).
Searching other resources
We carried out citation searching of identified included studies in Web of Science (apps.webofknowledge.com), on 24 March 2017 and conducted a search of grey literature through Opengrey (www.opengrey.eu./), on 27 April 2017. We scanned reference lists of relevant systematic reviews to search for additional trials. We did not contact study authors or organizations to ask if they were aware of other completed or ongoing studies.
Data collection and analysis
Two review authors (SL and OSR) independently completed all data collection and analyses before comparing results and reaching consensus. We consulted with a third review author (AS) to resolve conflicts when necessary.
Selection of studies
We used reference management software to collate the results of searches and to remove duplicates (Endnote). We used Covidence software to screen results of the search of titles and abstracts and identify potentially relevant studies (Covidence). We sourced the full texts of all potentially relevant studies and considered whether they meet the inclusion criteria (see Criteria for considering studies for this review). We reviewed abstracts at this stage and included these in the review only if they provided sufficient information and relevant results that included denominator figures for each intervention/comparison group. We recorded the number of papers retrieved at each stage and reported this information using a PRISMA flow chart. We reported in the review brief details of closely related but excluded papers.
Data extraction and management
We used Covidence software to extract data from individual studies (Covidence). A basic template for data extraction forms is available at www.covidence.org. We adapted this template to include the following information.
Methods: type of study design; setting; dates of study; funding sources.
Participants: number of participants randomized to each group; baseline characteristics (to include "Acute Physiology and Chronic Health Evaluation II" (APACHE II) scores, whether mechanically ventilated and length of time in the ICU before study commencement).
Interventions: details of intervention and comparison nutrition (kilocalories per kilogram received, time of initiation, duration of delivery, use of glycaemic controls).
Outcomes: all relevant review outcomes as measured and reported by study authors.
Outcome data: results of outcome measures.
We considered the applicability of information from individual studies and the generalizability of data to our intended study population (i.e. the potential for indirectness in our review). If we found associated publications from the same study, we created a composite dataset based on all eligible publications.
Assessment of risk of bias in included studies
Two review authors (SL and OSR) independently assessed study quality, study limitations, and the extent of potential bias by using the Cochrane ’Risk of bias’ tool (Higgins 2011). We considered the following domains.
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants, personnel, and outcome assessors (performance and detection bias).
Incomplete outcome data (attrition bias).
Selective outcome reporting (reporting bias).
Other: use of concomitant drugs.
We anticipated that it would not be feasible for studies to blind participants and personnel and, in the absence of any description of personnel blinding, we assumed that no blinding occurred. However, we anticipated that it was feasible to blind outcome assessors and we considered risk of detection bias (outcome assessor blinding) by each outcome. We considered whether investigators used standard criteria for diagnosis of outcomes, for example, aspiration pneumonia or ventilator‐acquired pneumonia, which may be subject to clinician bias.
For each domain, we judged whether study authors had made sufficient attempts to minimize bias in their study design. We made judgements using three measures; high, low, and unclear risk of bias. We recorded this judgement in ’Risk of bias’ tables and presented a summary ’Risk of bias’ figure.
Measures of treatment effect
We collected dichotomous data for mortality and adverse events, and continuous data for number of ICU‐free days and number of ventilator‐free days. We reported dichotomous data as risk ratios (RR) to compare groups, and continuous data as a mean difference (MD). We reported 95% confidence intervals (CI).
Unit of analysis issues
(See Differences between protocol and review.) We conducted separate analysis for the comparison arms PN, and EN and PN; this method avoided double‐counting in multi‐arm studies.
In the event of cluster trials, we would have defined the unit of allocation as the ICU or the hospital rather than the individual participant and analysed data accordingly, calculating effect estimates using the generic inverse variance method (Higgins 2011).
Dealing with missing data
In the event that study authors did not account for missing data, we would have contacted them for information. We considered data to be complete if losses were reported and explained by study authors and we combined no incomplete data in the meta‐analysis.
Assessment of heterogeneity
We assessed whether evidence of inconsistency was apparent in our results by considering heterogeneity. We assessed clinical heterogeneity by comparing similarities in our included studies between study designs, participants, interventions, and outcomes, and used the data collected as stated under Data extraction and management. We assessed statistical heterogeneity by calculating the Chi² test or I² statistic and judged any heterogeneity above an I² value of 60% and a Chi² P value less than or equal to 0.05 to indicate moderate to substantial statistical heterogeneity (Higgins 2011).
In addition to looking at statistical results, we considered point estimates and overlap of CIs. If CIs overlap, then results are more consistent. Combined studies may show a large consistent effect but with significant heterogeneity. Therefore, we planned to interpret heterogeneity with caution (Guyatt 2011a).
Assessment of reporting biases
We attempted to source published protocols for each of our included studies by using clinical trials registers. We compared published protocols with published study results to assess the risk of selective reporting bias. If we identified sufficient studies reporting on an outcome (i.e. more than 10 studies (Higgins 2011)), we planned to generate a funnel plot to assess risk of publication bias in the review; an asymmetrical funnel plot may suggest publication of only positive results (Egger 1997).
Data synthesis
We completed meta‐analyses of outcomes for which we had comparable effect measures from more than one study, and when measures of heterogeneity indicated that pooling of results was appropriate. We did not pool studies that had a high level of clinical heterogeneity and moderate to high statistical heterogeneity indicated by I² statistics and Chi² P values. We used the statistical calculator provided in Review Manager to perform meta‐analysis (Review Manager 2014).
For dichotomous outcomes, for example, mortality rate, we calculated the RR using summary data presented in each trial. We used the Mantel‐Haenszel effects model. If events had been extremely rare (one per 1000), we would have used the Peto odds ratio (Higgins 2011). For continuous outcomes, we aimed to use the MD. We used a fixed‐effect statistical model. In the event of finding evidence of moderate statistical or clinical heterogeneity, we would have investigated this by performing subgroup analyses, as below, and analysed data using a random‐effects model to incorporate unexplained heterogeneity.
We calculated CIs at 95% and used a P value less than or equal to 0.05 to judge whether a result was statistically significant. We considered imprecision in the results of analyses by assessing the CI around an effects measure; a wide CI would suggest a higher level of imprecision in our results. A small number of identified studies may also reduce precision (Guyatt 2011b).
Subgroup analysis and investigation of heterogeneity
Study designs may differ in relation to time of initiation of feeding and target energy requirements given to participants. Therefore, we considered these subgroups for each of our outcomes. We used cut‐offs for time of feeding from the most recent ASPEN guidelines (Taylor 2016), and cut‐offs for target energy requirements from Marik 2016. Critically ill people who are elderly may have different nutritional requirements and metabolism (ASPEN 2002), leading to different responses to EN and PN methods as compared with younger participants. We did not supply a cut‐off age for this subgroup but aimed to separate participants described as 'frail elderly' by study authors from remaining participants. Heterogeneity may be introduced by the types of procedures that participants have undergone or by their reason for admission; people who have had abdominal or bowel surgery and people admitted with gastrointestinal complications may have greater difficulty with ingestion and digestion. In summary, we aimed to perform subgroup analysis as follows.
Early initiation of feeding (less than 48 hours) versus late initiation of feeding (48 hours or greater).
Normocaloric intake (to match 80% to 100% of energy expenditure) versus hypocaloric intake (less than 70% of energy expenditure).
'Frail elderly' versus other participants.
Gastrointestinal medical or surgical participants versus non‐gastrointestinal medical or surgical participants.
We performed subgroup analysis only when study authors reported outcome data for identified subgroups. In the absence of numerical data, we planned to present qualitative analysis of these factors as a possible source of heterogeneity.
We aimed to perform subgroup analyses on the following outcomes: mortality, number of ICU‐free days up to day 28, and number of ventilator‐free days up to day 28.
Sensitivity analysis
We explored the potential effects of decisions made as part of the review process as follows.
We excluded all studies that we judged at high or unclear risk of selection bias.
We assessed decisions made regarding missing data, excluding studies that provided incomplete data.
We conducted meta‐analysis using the alternate meta‐analytical effects model (fixed‐effect or random‐effects).
We compared effect estimates from the above results with effect estimates from the main analysis. We aimed to report differences that altered interpretation of effects.
We aimed to perform sensitivity analyses on the following outcomes: mortality, number of ICU‐free days up to day 28, and number of ventilator‐free days up to day 28.
'Summary of findings' table and GRADE
Two review authors (SL and OSR) independently used the GRADE system to assess the certainty of the body of evidence associated with the following outcomes (Guyatt 2008):
mortality (at time points: in‐hospital, 30 days, 90 days, 180 days);
number of ICU‐free days up to day 28;
number of ventilator‐free days up to day 28;
adverse events as reported by study authors (aspiration, sepsis, pneumonia, and vomiting).
The GRADE approach appraises the certainty of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. Evaluation of the certainty of a body of evidence considers within‐study risk of bias, directness of the evidence, heterogeneity of the data, precision of effect estimates, and risk of publication bias.
We constructed two 'Summary of findings' tables using the GRADE profiler software for the following comparisons in this review (www.guidelinedevelopment.org/):
EN versus PN for adults in the ICU;
EN versus EN and PN for adults in the ICU.
We reached consensus without consulting a third review author.
Results
Description of studies
Results of the search
We screened 4173 titles and abstracts, of which we identified 1369 through forward and backward citation searches. We screened titles from clinical trials registers and grey literature searches. We assessed 147 full texts for eligibility. See Figure 1.
1.
Flow diagram of search strategy.
Included studies
See Characteristics of included studies table.
We included 25 studies (37 publications), with 8816 participants (Abdulmeguid 2007; Abrishami 2010; Adams 1986; Altintas 2011; Bauer 2000; Bertolini 2003; Borzotta 1994; Casaer 2011; Cerra 1988; Chiarelli 1996; Dunham 1994; Engel 1997; Fan 2016; Gencer 2010; Hadfield 1995; Harvey 2014; Heidegger 2013; Justo Meirelles 2011; Kudsk 1992; Peterson 1988; Radrizzani 2006; Rapp 1983; Xi 2014; Young 1987). Two studies were quasi‐randomized (Altintas 2011; Fan 2016), and the remaining studies were RCTs. We found no cluster‐randomized studies. Reports for Bertolini 2003 and Radrizzani 2006 were for the same study, but participants were divided according to criteria for sepsis (severe sepsis in Bertolini 2003; and non‐severe sepsis in Radrizzani 2006), and we included them as separate studies for the purpose of this review. We included one study for which we could only source the abstract (Abdulmeguid 2007); we sourced the full text of all remaining studies.
Study population
Participants had a wide variety of primary diagnoses but all were critically ill. Sixteen studies reported that participants were mechanically ventilated (Abdulmeguid 2007; Adams 1986; Altintas 2011; Bertolini 2003; Borzotta 1994; Cerra 1988; Chiarelli 1996; Dunham 1994; Harvey 2014; Heidegger 2013; Justo Meirelles 2011; Kudsk 1992; Radrizzani 2006; Rapp 1983; Wischmeyer 2017; Xi 2014); nine studies did not describe mechanical ventilation status as part of the inclusion or exclusion criteria (Abrishami 2010; Bauer 2000; Casaer 2011; Engel 1997; Fan 2016; Gencer 2010; Hadfield 1995; Peterson 1988; Young 1987).
Study setting
All studies were conducted in the ICU (Abdulmeguid 2007; Abrishami 2010; Adams 1986; Altintas 2011; Bauer 2000; Bertolini 2003; Casaer 2011; Cerra 1988; Chiarelli 1996; Dunham 1994; Fan 2016; Gencer 2010; Hadfield 1995; Harvey 2014; Heidegger 2013; Justo Meirelles 2011; Kudsk 1992; Peterson 1988; Radrizzani 2006; Rapp 1983; Wischmeyer 2017; Xi 2014), or assumed to be in the ICU (Borzotta 1994; Engel 1997; Young 1987). Eight studies were undertaken in the USA (Adams 1986; Borzotta 1994; Cerra 1988; Dunham 1994; Kudsk 1992; Peterson 1988; Rapp 1983; Young 1987); three were in Italy (Bertolini 2003; Chiarelli 1996; Radrizzani 2006); two were in the UK (Hadfield 1995; Harvey 2014); two were in Turkey (Altintas 2011; Gencer 2010); two were in China (Fan 2016; Xi 2014); and one each in Iran (Abrishami 2010); France (Bauer 2000); Belgium (Casaer 2011); Germany (Engel 1997); Switzerland (Heidegger 2013); and Brazil (Justo Meirelles 2011). One international study was undertaken in Belgium, Canada, France, and the USA (Wischmeyer 2017). One study did not report the country in which it was conducted (Abdulmeguid 2007).
Intervention and comparisons
Seventeen studies compared an EN feeding protocol to a PN feeding protocol (Abdulmeguid 2007; Adams 1986; Altintas 2011; Bertolini 2003; Borzotta 1994; Cerra 1988; Engel 1997; Gencer 2010; Hadfield 1995; Harvey 2014; Justo Meirelles 2011; Kudsk 1992; Peterson 1988; Radrizzani 2006; Rapp 1983; Xi 2014; Young 1987); Engel 1997 was a multi‐arm study with two EN groups (one standard EN formula and one formula supplemented with arginine, omega‐3 fatty acid, nucleotide, and selenium). Six studies compared an EN feeding protocol to a protocol in which EN was supplemented with PN (Abrishami 2010; Bauer 2000; Casaer 2011; Chiarelli 1996; Heidegger 2013; Wischmeyer 2017). Two studies compared EN versus PN and versus combined EN and PN (Dunham 1994; Fan 2016).
Study authors reported initiation of both EN and PN within 48 hours of ICU admission in 13 studies (Adams 1986; Altintas 2011; Bauer 2000; Bertolini 2003; Dunham 1994; Engel 1997; Fan 2016; Harvey 2014; Justo Meirelles 2011; Kudsk 1992; Peterson 1988; Radrizzani 2006; Wischmeyer 2017). One study reported that all participants were given PN within 24 to 36 hours and that EN was initiated in one group at four days (Chiarelli 1996). One study reported that initiation of EN in one group was after at least 14 days of fasting, and study authors did not state at which time point PN was initiated (Xi 2014). Two studies initiated supplemental PN after all participants had been given EN for three days (Casaer 2011; Heidegger 2013). The remaining five study authors did not report time of initiation of feeding (Abdulmeguid 2007; Abrishami 2010; Gencer 2010; Hadfield 1995; Young 1987).
Outcomes
All studies, except Engel 1997, reported participant deaths; some studies did not clearly report time points and some studies reported deaths as participant losses with mortality as a reason for withdrawal from the study. No studies reported data for number of ICU‐free days up to day 28, and one study reported data for number of ventilator‐free days up to day 28 (Harvey 2014). Study authors reported adverse events which were: mechanical (Borzotta 1994; Casaer 2011; Harvey 2014); metabolic (Borzotta 1994; Fan 2016; Harvey 2014); gastrointestinal (Adams 1986; Altintas 2011; Bauer 2000; Borzotta 1994; Casaer 2011; Cerra 1988; Chiarelli 1996; Fan 2016; Harvey 2014); and infective (Abdulmeguid 2007; Adams 1986; Altintas 2011; Borzotta 1994; Casaer 2011; Engel 1997; Fan 2016; Gencer 2010; Justo Meirelles 2011; Heidegger 2013; Kudsk 1992; Rapp 1983; Wischmeyer 2017; Xi 2014; Young 1987).
Funding sources
Study authors reported funding sources in 11 studies (Abrishami 2010; Adams 1986; Bertolini 2003; Borzotta 1994; Casaer 2011; Hadfield 1995; Harvey 2014; Heidegger 2013; Radrizzani 2006; Rapp 1983; Wischmeyer 2017). Three studies noted no involvement in trial management from funders (Casaer 2011; Heidegger 2013; Radrizzani 2006); remaining studies reported no details of funders' involvement.
Excluded studies
We excluded 99 articles after reading the full text. We reported details of 32 of these studies in Characteristics of excluded studies. Of these 32 studies, we excluded studies in which the setting was not reported and we could not assume it was the ICU (Arefian 2007; Baigrie 1996; Braga 1996; Braga 1998; Braga 2001; Chen 2004; DiCarlo 1999; Dong 2010; Hermann 2004; Kim 2012; Klek 2008; Klek 2011; Malhotra 2004; McArdle 1981; Moore 1989; Reynolds 1997; Ryu 2009; Sand 1997; Suchner 1996; Van Barneveld 2016; Xiao‐Bo 2014; Yu 2009; Zhang 2016; Zhu 2012), or too few participants were in the ICU (Woodcock 2001). We excluded two studies of participants with acute pancreatitis (Abou‐Assi 2002; Pupelis 2001). One study compared EN versus PN in people in the ICU (Zhang 2005), but reported that participants in the EN group were also given PN as required for the first three to four days and it was therefore ineligible for our review, and one study was described as an RCT but not all participants randomized to the control group received EN (Doig 2013). We excluded one study that compared early‐goal directed nutrition (EGDN) versus EN in people in the ICU; the EGDN group were given EN supplemented with PN; however, the supplemented PN was only given if required to meet feeding goals and study authors did not report which participants had and did not have PN (Allingstrup 2017). One study compared EN versus PN but feeding took place for only one day in the ICU and feeding was continued for an additional six days on the ward (Fujita 2012). We excluded one abstract that had not been published as a full report (Zanello 1992); the abstract included no outcomes of interest and was published in 1992.
Studies awaiting classification
We were unable to assess eligibility in 11 studies (Braga 1995; Cao 2014; Chen 2011; NCT00522730; NCT01802099; Ridley 2015; Soliani 2001; Theodorakopoulou 2016; Xiang 2006; Xiu 2015; Yi 2015). We identified one study in clinical trials registers that was completed without published results (NCT00522730). From database searches we identified one protocol for a terminated study (NCT01802099), and one study that had been completed but not published (Ridley 2015). We were unable to source full texts for three trials and could not assess eligibility from abstracts (Braga 1995; Chen 2011; Soliani 2001). Four trials were published only as abstracts with insufficient information to assess eligibility (Cao 2014; Theodorakopoulou 2016; Xiu 2015; Yi 2015), and one study report requires translation before we assess eligibility (Xiang 2006). See Characteristics of studies awaiting classification table.
Ongoing studies
We identified two ongoing studies from clinical trials registers (NCT00512122; NCT02022813). See Characteristics of ongoing studies table.
Risk of bias in included studies
We have included a summary of risk of bias assessments in Figure 2 and Figure 3. Blank spaces in the risk bias summary figures indicate that study authors did not report the review outcome.
2.
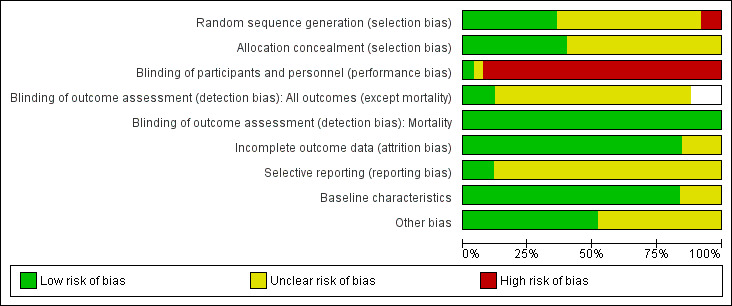
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. Blank spaces in tables indicated that study authors did not report the review outcome.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study. Blank spaces in tables indicate that study authors did not report the review outcome.
Allocation
All studies were described as randomized and nine studies provided sufficient information on the method of randomization (Bertolini 2003; Borzotta 1994; Casaer 2011; Harvey 2014; Heidegger 2013; Kudsk 1992; Peterson 1988; Radrizzani 2006; Wischmeyer 2017). Two studies randomized participants according to hospital record number (Altintas 2011; Fan 2016), and we judged these to have high risk of bias. The remaining 14 studies reported insufficient information on method of randomization and we recorded these as having unclear risk of bias (Abdulmeguid 2007; Abrishami 2010; Adams 1986; Bauer 2000; Cerra 1988; Chiarelli 1996; Dunham 1994; Engel 1997; Gencer 2010; Hadfield 1995; Justo Meirelles 2011; Rapp 1983; Xi 2014; Young 1987).
Ten studies described adequate allocation concealment methods, and we judged these to have low risk of selection bias (Altintas 2011; Bertolini 2003; Borzotta 1994; Casaer 2011; Harvey 2014; Heidegger 2013; Kudsk 1992; Peterson 1988; Radrizzani 2006; Wischmeyer 2017) The remaining 15 studies reported no description of methods to conceal allocation and we recorded these as having unclear risk of bias (Abdulmeguid 2007; Abrishami 2010; Adams 1986; Bauer 2000; Cerra 1988; Chiarelli 1996; Dunham 1994; Engel 1997; Fan 2016; Gencer 2010; Hadfield 1995; Justo Meirelles 2011; Rapp 1983; Xi 2014; Young 1987).
Blinding
One study reported that participants in the enteral group were given a placebo parenteral solution and we judged this study to have low risk of performance bias (Bauer 2000). One study reported that personnel were not blinded to the intervention group (Heidegger 2013). The remaining studies did not report whether attempts had been made to blind personnel; adequate blinding would involve intrusive procedures (e.g. central line placement or nasogastric tube placement) and if these procedures were not described we assumed that blinding had not occurred (Abdulmeguid 2007; Abrishami 2010; Adams 1986; Altintas 2011; Bertolini 2003; Borzotta 1994; Casaer 2011; Cerra 1988; Chiarelli 1996; Dunham 1994; Engel 1997; Fan 2016; Gencer 2010; Hadfield 1995; Harvey 2014; Justo Meirelles 2011; Kudsk 1992; Peterson 1988; Radrizzani 2006; Rapp 1983; Wischmeyer 2017; Xi 2014; Young 1987). We judged these studies, and Heidegger 2013, to have high risk of performance bias.
We did not believe that lack of blinding of outcome assessors would influence data for the mortality outcome and we judged all studies to have low risk of detection bias for mortality, regardless of whether study authors reported blinding of outcome assessors. Three studies adequately reported blinding of outcome assessors for the remaining review outcomes and we judged these to have low risk of detection bias (Casaer 2011; Dunham 1994; Heidegger 2013). Nineteen studies reported insufficient details of outcome assessor blinding and we judged these to have unclear risk of detection bias (Abdulmeguid 2007; Adams 1986; Altintas 2011; Bauer 2000; Bertolini 2003; Borzotta 1994; Cerra 1988; Chiarelli 1996; Engel 1997; Fan 2016; Gencer 2010; Harvey 2014; Justo Meirelles 2011; Kudsk 1992; Peterson 1988; Rapp 1983; Wischmeyer 2017; Xi 2014; Young 1987). Three studies included data only for mortality and our assessment of detection bias was limited to this outcome (Abrishami 2010; Hadfield 1995; Radrizzani 2006). We noted whether studies had included criteria for diagnoses of outcomes and we were not concerned that lack of information or type of measurement tools had introduced risk of clinician bias.
Incomplete outcome data
We judged 21 studies to have low risk of attrition bias, as there appeared to be no reported losses (Abdulmeguid 2007; Adams 1986; Altintas 2011; Bertolini 2003; Chiarelli 1996; Engel 1997; Fan 2016; Gencer 2010; Hadfield 1995; Justo Meirelles 2011; Rapp 1983; Wischmeyer 2017; Xi 2014), or losses were few and adequately explained by study authors (Abrishami 2010; Bauer 2000; Casaer 2011; Cerra 1988; Dunham 1994; Harvey 2014; Kudsk 1992; Radrizzani 2006). Four studies had a large number of losses or losses were unevenly distributed between groups and we were unclear whether these losses could influence outcome data (Borzotta 1994; Heidegger 2013; Peterson 1988; Young 1987).
Selective reporting
We were able to source prospective clinical trials registration reports for four studies, of which we judged three studies to have low risk of reporting bias (Casaer 2011; Harvey 2014; Wischmeyer 2017). We noted changes to the clinical trials registration documents after completion of the study with regard to data collection time points and we could not be certain whether these changes may have introduced bias to the results; we judged this study to have an unclear risk of selective reporting bias (Heidegger 2013). We were unable to judge reporting bias for the remaining 21 studies because study authors did not report clinical trials registration reports or published protocols (Abdulmeguid 2007; Abrishami 2010; Adams 1986; Altintas 2011; Bauer 2000; Bertolini 2003; Borzotta 1994; Cerra 1988; Chiarelli 1996; Dunham 1994; Engel 1997; Fan 2016; Gencer 2010; Hadfield 1995; Justo Meirelles 2011; Kudsk 1992; Peterson 1988; Radrizzani 2006; Rapp 1983; Xi 2014; Young 1987).
Baseline characteristics
Three studies reported some baseline imbalances between groups and we did not know if these differences could influence outcome data (Altintas 2011; Bertolini 2003; Radrizzani 2006). One study was an abstract and did not provide sufficient detail on baseline characteristics (Abdulmeguid 2007). We judged 21 studies to have low risk of bias for baseline characteristics because data for characteristics were comparable between groups (Abrishami 2010; Adams 1986; Bauer 2000; Borzotta 1994; Casaer 2011; Cerra 1988; Chiarelli 1996; Dunham 1994; Engel 1997; Fan 2016; Gencer 2010; Hadfield 1995; Harvey 2014; Heidegger 2013; Justo Meirelles 2011; Kudsk 1992; Peterson 1988; Rapp 1983; Wischmeyer 2017; Xi 2014; Young 1987).
Other potential sources of bias
We considered whether differences between intervention and comparison groups could have introduced bias; in particular we considered nutritional protocols, patient management and use of concomitant medication, and glycaemic controls. We noted some differences in 12 studies (Abdulmeguid 2007; Adams 1986; Bertolini 2003; Borzotta 1994; Chiarelli 1996; Dunham 1994; Gencer 2010; Hadfield 1995; Harvey 2014; Kudsk 1992; Radrizzani 2006; Young 1987). We were not able to judge if these differences influenced study outcome data and we reported these as having unclear risk of bias. We identified no other sources of bias in the remaining studies.
Effects of interventions
Summary of findings for the main comparison. Enteral versus parenteral nutrition for adults in the intensive care unit.
| Enteral versus parenteral nutrition for adults in the intensive care unit | |||||
| Patient or population: critically ill adults admitted to the ICU for trauma, emergency, or surgical care; population excluded people with acute pancreatitis Setting: intensive care units in: Brazil, China, Germany, Iran, Italy, Turkey, UK, and USA Intervention: EN Comparison: PN | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with EN | Risk with PN | ||||
| Mortality | In‐hospital mortality | RR 1.19 (0.80 to 1.77) | 361 (6 studies) | ⊕⊕⊝⊝ Lowa | |
| Study population | |||||
| 229 per 1000 (154 to 340) | 192 per 1000 | ||||
| Mortality within 30 days | RR 1.02 (0.92 to 1.13) | 3148 (11 studies) | ⊕⊕⊝⊝ Lowb | ||
| Study population | |||||
| 304 per 1000 (274 to 336) | 298 per 1000 | ||||
| Mortality within 90 days | RR 1.06 (0.95 to 1.17) | 2461 (3 studies) | ⊕⊝⊝⊝ Very lowc | ||
| Study population | |||||
| 393 per 1000 (352 to 434) | 371 per 1000 | ||||
| Mortality within 180 days | RR 0.33 (0.04 to 2.97) | 46 (1 study) | ⊕⊝⊝⊝ Very lowd | ||
| Study population | |||||
| 130 per 1000 | 43 per 1000 (5 in 387) | ||||
| Number of ICU‐free days up to day 28 | – | – | – | – | Not measured |
| Number of ventilator‐free days up to day 28 | Mean number of ventilator‐free days: 14.2 (SD ± 12.2) | Mean difference 0 days (0.97 fewer to 0.97 more) | N/A | 2388 (1 study) | ⊕⊝⊝⊝ Very lowd |
| Adverse events: aspiration (as reported by study authors at end of study follow‐up period) | Study population | RR 1.53 (0.46 to 5.03) | 2437 (2 studies) | ⊕⊝⊝⊝ Very lowe | |
| 5 per 1000 (2 to 17) | 3 per 1000 | ||||
| Adverse events: sepsis (as reported by study authors at end of study follow‐up period) | Study population | RR 0.59 (0.37 to 0.95) | 361 (7 studies) | ⊕⊕⊝⊝ Lowf | |
| 123 per 1000 (77 to 199) | 209 per 1000 | ||||
| Adverse events: pneumonia (as reported by study authors at end of study follow‐up period) | Study population | RR 1.10 (0.82 to 1.48) | 415 (7 studies) | ⊕⊕⊝⊝ Lowf | |
| 314 per 1000 (234 to 423) | 268 per 1000 | ||||
| Adverse events: vomiting (as reported by study authors at end of study follow‐up period) | Study population | RR 3.42 (1.15 to 10.16) | 2525 (3 studies) | ⊕⊝⊝⊝ Very lowg | |
| 11 per 1000 (4 to 32) | 3 per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EN: enteral nutrition; ICU: intensive care unit; N/A: not applicable; PN: parenteral nutrition; RR: risk ratio; SD: standard deviation. | |||||
| GRADE Working Group grades of evidence High: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aAll studies had a high risk of performance bias; downgraded one level for study limitations. Studies included a variety of primary diagnoses and evidence was less direct; downgraded one level for indirectness.
bAll studies had a high risk of performance bias; downgraded one level for study limitations. Studies included a variety of primary diagnoses and study designs and evidence were less direct; downgraded one level for indirectness.
cAll studies had a high risk of performance bias; downgraded one level for study limitations. Studies included a variety of primary diagnoses and study designs and evidence were less direct; downgraded one level for indirectness. Few studies and one included study had a large number of participants relative to other included studies; downgraded one level for imprecision.
dData from only one study that had a high risk of performance bias; downgraded one level for study limitations and two levels for imprecision.
eAll studies had a high risk of performance bias; downgraded one level for study limitations. Studies included a variety of primary diagnoses and evidence was less direct; downgraded one level for indirectness. Few studies and one included study had a large number of participants relative to other included studies; downgraded one level for imprecision.
fAll studies had a high risk of performance bias; downgraded one level for study limitations. Studies included a variety of primary diagnoses and evidence was less direct; downgraded one level for indirectness.
gAll studies had a high risk of performance bias; downgraded one level for study limitations. Studies included a variety of primary diagnoses and study designs and evidence were less direct; downgraded one level for indirectness. Few studies, with very few events, and one included study had a large number of participants relative to other included studies; downgraded one level for imprecision.
Summary of findings 2. Enteral versus enteral and parenteral nutrition for adults in the intensive care unit.
| Enteral versus enteral and parenteral nutrition for adults in the intensive care unit | |||||
| Patient or population: critically ill adults admitted to the ICU for trauma, emergency, or post‐surgical care; population excludes participants with acute pancreatitis Setting: intensive care units in: France, Italy, Switzerland, Turkey, and USA Intervention: EN Comparison: EN + PN | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with EN | Risk with EN + PN | ||||
| Mortality | In‐hospital mortality | RR 0.99 (0.84 to 1.16) | 5111 (5 studies) | ⊕⊕⊝⊝ Lowa | |
| Study population | |||||
| 106 per 1000 (90 to 124) | 107 per 1000 | ||||
| Mortality within 30 days | RR 1.64 (1.06 to 2.54) | 409 (3 studies) | ⊕⊝⊝⊝ Very lowb | ||
| Study population | |||||
| 216 per 1000 (140 to 335) | 132 per 1000 | ||||
| Mortality within 90 days | RR 1.00 (0.86 to 1.18) | 4760 (2 studies) |
⊕⊕⊝⊝ Lowc | ||
| Study population | |||||
| 115 per 1000 (99 to 135) |
115 per 1000 | ||||
| Mortality within 180 days | RR 1.00 (0.65 to 1.55) | 120 (1 RCT) |
⊕⊝⊝⊝ Very lowd | ||
| Study population | |||||
| 400 per 1000 (260 to 620) |
400 per 1000 | ||||
| Number of ICU‐free days up to day 28 | – | – | – | – | Not measured |
| Number of ventilator‐free days up to day 28 | – | – | – | – | Not measured |
| Adverse events: aspiration (as reported by study authors at end of study follow‐up period) | – | – | – | – | Not measured |
| Adverse events: sepsis (as reported by study authors at end of study follow‐up period) | – | – | – | – | Not measured |
| Adverse events: pneumonia (as reported by study authors at end of study follow‐up period) | 350 per 1000 (228 to 538) |
250 per 1000 | RR 1.40 (0.91 to 2.15) | 205 (2 studies) |
⊕⊝⊝⊝ Very lowd |
| Adverse events: vomiting (as reported by study authors at end of study follow‐up period) | – | – | – | – | Not measured |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EN: enteral nutrition; ICU: intensive care unit; PN: parenteral nutrition; RCT: randomized controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aAll studies had high risk of performance bias; downgraded one level for study limitations. Studies included a variety of primary diagnoses and evidence was less direct; downgraded one level for indirectness.
bAll studies had high risk of performance bias; downgraded one level for study limitations. Studies included a variety of primary diagnoses and evidence was less direct; downgraded one level for indirectness. Few studies with increased risk of imprecision; downgraded one level.
cBoth studies had high risk of performance bias; downgraded one level for study limitations. Studies included a variety of primary diagnoses and evidence was less direct; downgraded one level for indirectness.
dData from only one study that had a high risk of performance bias; downgraded one level for study limitations and two levels for imprecision.
Enteral nutrition versus parenteral nutrition
Primary outcome
1. Mortality
We noted that some studies reported loss of randomized participants from analysis due to death (Borzotta 1994; Dunham 1994; Kudsk 1992; Peterson 1988; Young 1987). We included participants from three of these studies (Borzotta 1994; Dunham 1994; Kudsk 1992), as data for our primary analysis; we did not include mortality data from Young 1987 or Peterson 1988 because study authors did not report to which intervention group these participants belonged. Data were grouped according to time point.
In hospital
Deaths were reported in Abrishami 2010 during the seven‐day study period, at various time points up to day 18 in Borzotta 1994, and at four days in Kudsk 1992 and we assumed that these occurred in hospital. Three studies reported ICU mortality and did not report hospital mortality (Bertolini 2003; Cerra 1988; Heidegger 2013); in this instance, we included data for ICU mortality in this analysis.
One feeding route rather than the other may make little or no difference to in‐hospital mortality (RR 1.19, 95% CI 0.80 to 1.77; 361 participants; 6 studies; I² = 3%; low‐certainty evidence; Analysis 1.1). We used GRADE to downgrade by two levels; we were concerned by study limitations and indirectness. See Table 1.
1.1. Analysis.
Comparison 1 Enteral (EN) versus parenteral nutrition (PN), Outcome 1 In‐hospital mortality.
Within 30 days
Six studies did not specify time points and we reported outcome data for these studies with mortality at 30 days (Abdulmeguid 2007; Chiarelli 1996; Fan 2016; Gencer 2010; Hadfield 1995; Justo Meirelles 2011).
One feeding route rather than the other may make little or no difference to mortality within 30 days (RR 1.02, 95% CI 0.92 to 1.13; 3148 participants; I² = 32%; low‐certainty evidence; Analysis 1.2). We used GRADE to downgrade by two levels; we were concerned by study limitations and indirectness. See Table 1.
1.2. Analysis.
Comparison 1 Enteral (EN) versus parenteral nutrition (PN), Outcome 2 Mortality at 30 days.
Within 90 days
Three studies reported mortality within 90 days (Harvey 2014; Rapp 1983; Young 1987). It is uncertain whether one feeding route rather than another reduces mortality within 90 days because the certainty of the evidence is very low (RR 1.06, 95% CI 0.95 to 1.17; 2461 participants; I² = 55%; Analysis 1.3). We used GRADE to downgrade by three levels; we were concerned by study limitations, indirectness, and imprecision. See Table 1.
1.3. Analysis.
Comparison 1 Enteral (EN) versus parenteral nutrition (PN), Outcome 3 Mortality at 90 days.
Within 180 days
One study comparing EN versus PN reported mortality from one to four months; we assumed that there were no earlier deaths and we included it as mortality data within 180 days (Adams 1986). Study authors reported one death in the EN group (23 participants) and three deaths in the PN group (23 participants). We used the Review Manager 5 calculator to calculate an effect estimate (RR 0.33, 95% CI 0.04 to 2.97) (Review Manager 2014). It is uncertain whether either feeding route affects number of people who die within 180 days because the certainty of this evidence is very low; we were concerned by study limitations and imprecision. See Table 1.
Secondary outcomes
1. Number of intensive care unit‐free days up to day 28
No studies reported data for number of ICU‐free days.
2. Number of ventilator‐free days up to day 28
One study (2388 participants included in the analysis) reported data for number of ventilator‐free days up to day 28 (Harvey 2014). Study authors reported little or no difference in number of days free of respiratory support (EN group: mean 14.2 (standard deviation (SD) ± 12.2) days versus PN group: mean 14.2 (SD ± 12.1) days; P = 0.94). It is uncertain whether either feeding route affected the number of ventilator‐free days up to day 28 because the certainty of the evidence is very low; we were concerned by study limitations and imprecision. See Table 1.
3. Adverse events as reported by study authors
Study authors did not always describe outcomes as 'adverse events.' We collected outcomes as described by study authors, which we categorized as mechanical events, metabolic events, gastrointestinal events, and infective events. We combined data when more than one study reported an event, and when data were reported as 'number of participants' with an event rather than number of events. We reported single study data of adverse events in Table 3.
1. Adverse events for single studies: enteral nutrition versus parenteral nutrition.
| Study ID | Description of event | EN group (n/N) | PN group (n/N) |
| Mechanical events | |||
| Adams 1986 | Clogged jejunostomy tube | 9/23 | N/A |
| Disconnected line | N/A | 1/23 | |
| Line eroded into right upper lobe bronchus | N/A | 1/23 | |
| Malfunctioned line | N/A | 7/23 | |
| Dunham 1994 | Transpyloric tube occlusion | 2/12 | 0/15 |
| Failure to intubate | 0/12 | 0/15 | |
| Withdrawal of tube by participant | 1/12 | N/A | |
| Metabolic events | |||
| Adams 1986 | Hepatic failure | 1/23 | 1/23 |
| Acute renal failure | 1/23 | 1/23 | |
| Pancreatitis | 2/23 | 1/23 | |
| Fan 2016 | Hypoproteinaemia | 22/40 | 32/40 |
| Harvey 2014 | Electrolyte disturbance | 5/1197 | 8/1191 |
| Gastrointestinal events | |||
| Adams 1986 | Nausea, cramps, bloating | 19/23 | 16/23 |
| Gastrointestinal bleeding | 0/23 | 0/23 | |
| Dunham 1994 | Gastric reflux | 0/12 | 0/15 |
| Ileus | 1/12 | 0/15 | |
| Small bowel ileus | 0/12 | 1/15 | |
| Fan 2016 | Stress ulcer | 7/40 | 19/40 |
| Harvey 2014 | Elevated liver enzymes | 7/1197 | 3/1191 |
| Jaundice | 1/1197 | 1/1191 | |
| Ischaemic bowel | 0/1197 | 1/1191 | |
| Xi 2014 | Anastomotic leak | 2/22 | 6/23 |
| Infective events | |||
| Adams 1986 | Persistent fever without obvious cause | 1/23 | 5/23 |
| Altintas 2011 | Catheter infection | 2/30 | 4/41 |
| Borzotta 1994 | Meningitis | 2/28 | 0/21 |
| Sinusitis | 3/28 | 6/21 | |
| Bronchitis | 6/28 | 6/28 | |
| Clostridium difficile | 2/28 | 4/21 | |
| Peritonitis | 0/28 | 1/21 | |
| Fan 2016 | Intracranial infection | 7/40 | 13/40 |
| Pyaemia | 3/40 | 19/40 | |
| Gencer 2010 | Pulmonary infection | 2/30 | 2/30 |
| Kudsk 1992 | Empyema | 1/51 | 4/45 |
| Young 1987 | Aspiration pneumonia | 9/28 | 3/23 |
| Infection (type of infection not described) | 5/28 | 4/23 | |
EN: enteral nutrition; n: number of participants with an event; N: total number randomized to group; N/A: not applicable; PN: parenteral nutrition.
Mechanical events
Two studies comparing EN versus PN reported data for aspiration (Borzotta 1994; Harvey 2014). It is uncertain whether one feeding route rather than another reduces aspiration because the certainty of this evidence is very low (RR 1.53, 95% CI 0.46 to 5.03; 2437 participants; I² = 0%; Analysis 1.4). We used GRADE to downgrade by three levels; we were concerned by study limitations, indirectness, and imprecision. See Table 1.
1.4. Analysis.
Comparison 1 Enteral (EN) versus parenteral nutrition (PN), Outcome 4 Aspiration.
Two studies comparing EN versus PN reported data for pneumothorax, with little or no difference in incidences of pneumothorax according to feeding group (RR 1.46, 95% CI 0.19 to 11.22; 2437 participants; I² = 0%; Analysis 1.5) (Borzotta 1994; Harvey 2014).
1.5. Analysis.
Comparison 1 Enteral (EN) versus parenteral nutrition (PN), Outcome 5 Pneumothorax.
Single studies reported data for malfunctioned line, clogged jejunostomy tube, accidental disconnected line, and eroded line (Adams 1986), and one study reported data for transpyloric tube occlusion, failure to intubate, and withdrawal of tube by participant (Dunham 1994). See Table 3.
Metabolic events
Two studies comparing EN versus PN reported data for hyperglycaemia (Borzotta 1994; Harvey 2014). We found fewer people had hyperglycaemia who were given EN (RR 0.57, 95% CI 0.35 to 0.93; 2437 participants; I² = 0%; Analysis 1.6). We noted that very few people in either group had hyperglycaemia in one large study (Harvey 2014).
1.6. Analysis.
Comparison 1 Enteral (EN) versus parenteral nutrition (PN), Outcome 6 Hyperglycaemia.
Single studies reported data for hepatic failure, acute renal failure, and pancreatitis (Adams 1986), electrolyte disturbance (Harvey 2014), and hypoproteinaemia (Fan 2016). See Table 3.
Gastrointestinal events
Three studies comparing EN versus PN reported data for vomiting (Altintas 2011; Cerra 1988; Harvey 2014). It is uncertain whether PN leads to a reduction in vomiting because the certainty of this evidence is very low (RR 3.42, 95% CI 1.15 to 10.16; 2525 participants; I² = 0%; Analysis 1.7). We used GRADE to downgrade by three levels; we were concerned by study limitations, indirectness, and imprecision. See Table 1.
1.7. Analysis.
Comparison 1 Enteral (EN) versus parenteral nutrition (PN), Outcome 7 Vomiting.
Six studies comparing EN versus PN reported data for diarrhoea (Adams 1986; Altintas 2011; Borzotta 1994; Cerra 1988; Fan 2016; Young 1987). We found that fewer people had diarrhoea when given PN (RR 2.17, 95% CI 1.72 to 2.75; 363 participants; I² = 57%; Analysis 1.8).
1.8. Analysis.
Comparison 1 Enteral (EN) versus parenteral nutrition (PN), Outcome 8 Diarrhoea.
Three studies comparing EN versus PN reported data for abdominal distension, with little or no difference in incidence of abdominal distension according to feeding regimen (RR 1.53, 95% CI 0.34 to 6.96; 2505 participants; I² = 0%; Analysis 1.9) (Altintas 2011; Harvey 2014; Peterson 1988).
1.9. Analysis.
Comparison 1 Enteral (EN) versus parenteral nutrition (PN), Outcome 9 Abdominal distension.
Single studies also reported data for nausea, bloating or cramps, and gastrointestinal bleeding (Adams 1986); gastric reflux, ileus, and small bowel ileus (Dunham 1994); stress ulcer (Fan 2016); jaundice, ischaemic bowel, and elevated liver enzymes (Harvey 2014); and anastomotic leak (Xi 2014). See Table 3.
Infective events
Seven studies comparing EN versus PN reported data for sepsis (Altintas 2011; Engel 1997; Justo Meirelles 2011; Kudsk 1992; Peterson 1988; Xi 2014; Young 1987). EN may reduce incidences of sepsis (RR 0.59, 95% CI 0.37 to 0.95; 361 participants; I² = 27%; low‐certainty evidence; Analysis 1.10). We used GRADE to downgrade by two levels; we were concerned by study limitations and indirectness. See Table 1.
1.10. Analysis.
Comparison 1 Enteral (EN) versus parenteral nutrition (PN), Outcome 10 Sepsis.
Seven studies comparing EN versus PN reported data for pneumonia, or aspiration pneumonia, or ventilator‐acquired pneumonia (Adams 1986; Altintas 2011; Borzotta 1994; Fan 2016; Justo Meirelles 2011; Kudsk 1992; Young 1987). One study reported data for pneumonia and aspiration pneumonia and we included only data for pneumonia in the analysis (Young 1987). One feeding regimen rather than another may make little or no difference to pneumonia (RR 1.10, 95% CI 0.82 to 1.48; 415 participants; I² = 55%; low‐certainty evidence; Analysis 1.11). We used GRADE and downgraded by two levels; we were concerned by study limitations and indirectness. See Table 1.
1.11. Analysis.
Comparison 1 Enteral (EN) versus parenteral nutrition (PN), Outcome 11 Pneumonia.
Three studies comparing EN versus PN reported data for intra‐abdominal infection or intra‐abdominal abscess (Adams 1986; Gencer 2010; Kudsk 1992). We found fewer intra‐abdominal infections when EN was given (RR 0.26, 95% CI 0.07 to 0.89; 202 participants; I² = 0%; Analysis 1.12).
1.12. Analysis.
Comparison 1 Enteral (EN) versus parenteral nutrition (PN), Outcome 12 Intra‐abdominal infection.
Three studies reported data for wound infection (Adams 1986; Borzotta 1994; Gencer 2010). We found little or no difference between groups in number of participants with a wound infection (RR 1.45, 95% CI 0.55 to 3.82; 155 participants; I² = 55%; Analysis 1.13).
1.13. Analysis.
Comparison 1 Enteral (EN) versus parenteral nutrition (PN), Outcome 13 Wound infection.
Three studies reported data for urinary tract infection (Borzotta 1994; Gencer 2010; Young 1987). We found little or no difference between groups in number of participants with a urinary tract infection (RR 1.48, 95% CI 0.65 to 3.40; 160 participants; I² = 49%; Analysis 1.14).
1.14. Analysis.
Comparison 1 Enteral (EN) versus parenteral nutrition (PN), Outcome 14 Urinary tract infection.
Single studies also reported data for: persistent fever without obvious cause (Adams 1986); catheter infections (Altintas 2011); meningitis, sinusitis, bronchitis, Clostridium difficile, and peritonitis (Borzotta 1994); intracranial infection and pyaemia (Fan 2016); pulmonary infection (Gencer 2010); empyema (Kudsk 1992); and aspiration pneumonia and infection (type of infection not described) (Young 1987). See Table 3.
Bertolini 2003 reported that there were no severe adverse events related to the intervention or comparison and Radrizzani 2006 reported no severe adverse events related to the intervention. Hadfield 1995 did not report adverse events. Abdulmeguid 2007 collected data for nosocomial bloodstream infections and septic morbidity but these were not clearly reported in the abstract. Rapp 1983 reported data for some participants who had sepsis but this was not clearly reported.
Subgroup analysis
1. Early initiation of feeding (less than 48 hours) versus late initiation of feeding (48 hours or greater)
Eleven studies comparing EN versus PN initiated feeding within 48 hours (Adams 1986; Altintas 2011; Bertolini 2003; Dunham 1994; Engel 1997; Fan 2016; Harvey 2014; Justo Meirelles 2011; Kudsk 1992; Peterson 1988; Radrizzani 2006). No studies reported late initiation of EN and late initiation of PN, therefore we could not conduct subgroup analysis for this comparison.
2. Normocaloric intake (to match 80% to 100% of energy expenditure) versus hypocaloric intake (less than 70% of energy expenditure)
We considered possible subgroup analysis based on terms used by study authors to describe whether intake was formulated to be normocaloric or hypocaloric; we did not make judgements based on other information such as target rates (measured as kilocalories/kilogram). No studies described intake as normocaloric or hypocaloric, therefore, we did not conduct subgroup analysis.
3. 'Frail elderly' versus other participants
We identified no studies that specified inclusion of frail elderly participants, or subdivided participant characteristics by this description.
4. Gastrointestinal medical or surgical participants versus non‐gastrointestinal medical or surgical participants
Three studies comparing EN versus PN included participants with only abdominal injury or who had gastrointestinal surgery (Gencer 2010; Kudsk 1992; Peterson 1988). For the relevant outcomes, we compared these with studies in which participants did not have a primary diagnosis of gastrointestinal medical or surgical conditions. We could not be certain of primary diagnoses in Abdulmeguid 2007, and did not include this study in subgroup analysis.
Subgroup analysis showed little or no difference in rates of in‐hospital mortality (Chi² = 0.05, degrees of freedom (df) = 1 (P = 0.83), I² = 0%) based on whether participants were gastrointestinal surgical or medical participants (RR 0.88, 95% CI 0.06 to 13.74; 98 participants; 1 study), or participants who did not have a primary diagnosis of a gastrointestinal surgical or medical condition (RR 1.19, 95% CI 0.80 to 1.77; 361 participants; 6 studies; I² = 22%; Analysis 1.15).
1.15. Analysis.
Comparison 1 Enteral (EN) versus parenteral nutrition (PN), Outcome 15 In‐hospital mortality: gastrointestinal (GI) medical/surgical vs non‐GI medical/surgical.
Subgroup analysis showed no difference in rates of mortality at 30 days (Chi² = 0.25, df = 1 (P = 0.62), I² = 0%) based on whether participants were gastrointestinal surgical or medical participants (RR 0.67, 95% CI 0.12 to 3.71; 60 participants; 1 study), or participants who did not have a primary diagnosis of a gastrointestinal surgical or medical condition (RR 1.03, 95% CI 0.93 to 1.15; 3008 participants; 9 studies; I² = 41%; Analysis 1.16).
1.16. Analysis.
Comparison 1 Enteral (EN) versus parenteral nutrition (PN), Outcome 16 Mortality at 30 days: GI medical/surgical vs non‐GI medical/surgical.
Sensitivity analysis
1. Selection bias
We assessed six studies as having low risk of selection bias for both sequence generation and allocation concealment (Bertolini 2003; Borzotta 1994; Harvey 2014; Kudsk 1992; Peterson 1988; Radrizzani 2006). In sensitivity analysis, we excluded studies that had high or unclear risk of both sequence generation and allocation concealment. For in‐hospital mortality, this altered the effect estimate with fewer deaths for participants given PN (RR 2.66, 95% CI 1.04 to 6.85; 196 participants; 3 studies; I² = 0%). There was no difference in effect estimates for mortality within 30 days, and within 90 days.
2. Attrition bias
We judged two studies to have unclear risk of attrition bias and performed sensitivity analysis by excluding them from appropriate analyses (Borzotta 1994; Young 1987). For the comparison EN versus PN, we noted no change in effect for in‐hospital mortality, mortality at 30 days, and mortality at 90 days.
3. Effects model
We reanalysed our mortality data using a random‐effects model; this did not change the effect.
Enteral nutrition versus enteral nutrition and parenteral nutrition
Primary outcome
1. Mortality
One study reported loss of randomized participants from analysis due to death (Dunham 1994), and we included these participants for our primary analysis.
In hospital
Five studies comparing EN versus EN and PN reported data for in‐hospital mortality (Abrishami 2010; Casaer 2011; Dunham 1994; Heidegger 2013; Wischmeyer 2017). One feeding regimen rather than the other may make little or no difference to in‐hospital mortality (RR 0.99, 95% CI 0.84 to 1.16; 5111 participants; I² = 0%; low‐certainty evidence; Analysis 2.1). We used GRADE to downgrade by two levels; we were concerned by study limitations and indirectness. See Table 2.
2.1. Analysis.
Comparison 2 Enteral (EN) versus combined EN and parenteral nutrition (PN), Outcome 1 In‐hospital mortality.
Within 30 days
One study did not report the time point for mortality and we included this study in the analysis as mortality within 30 days (Fan 2016). We included three studies in the analysis comparing EN versus EN and PN (Chiarelli 1996; Fan 2016; Heidegger 2013). It is uncertain whether combined EN and PN reduced mortality at 30 days because the certainty of the evidence is very low (RR 1.64, 95% CI 1.06 to 2.54; 409 participants; I² = 0%; Analysis 2.2). We used GRADE to downgrade by three levels; we were concerned by study limitations, indirectness, and imprecision. See Table 2.
2.2. Analysis.
Comparison 2 Enteral (EN) versus combined EN and parenteral nutrition (PN), Outcome 2 Mortality at 30 days.
Within 90 days
Two studies comparing EN versus EN and PN reported data for mortality within 90 days (Bauer 2000; Casaer 2011). One feeding regimen rather than the other may make little or no difference to mortality at 90 days (RR 1.00, 95% CI 0.86 to 1.18; 4760 participants; I² = 0%; low‐certainty evidence; Analysis 2.3). We used GRADE to downgrade the evidence by two levels; we were concerned by study limitations and indirectness. See Table 2.
2.3. Analysis.
Comparison 2 Enteral (EN) versus combined EN and parenteral nutrition (PN), Outcome 3 Mortality at 90 days.
Within 180 days
One study (120 participants) comparing EN versus EN and PN reported mortality at two years; interpretation of a figure of cumulative survival over time reported by study authors showed that all deaths were within 180 days (Bauer 2000). Study authors reported 24 deaths in each group (60 participants per group). We used the Review Manager 5 calculator to obtain the effect estimate (RR 1.00, 95% CI 0.65 to 1.55) (Review Manager 2014). It is uncertain whether one feeding regimen rather than another reduces mortality within 180 days because the certainty of this evidence is very low. We used GRADE to downgrade by three levels; we were concerned by study limitations (one level) and imprecision (two levels). See Table 2.
Secondary outcomes
1. Number of intensive care unit‐free days up to day 28
No studies reported data for number of ICU‐free days.
2. Number of ventilator‐free days up to day 28
No studies reported data for number of ventilator‐free days.
3. Adverse events as reported by study authors
Study authors did not always describe outcomes as 'adverse events.' We collected outcomes as described by study authors, which we categorized as mechanical events, metabolic events, gastrointestinal events, and infective events. We combined data when more than one study reported an event. We reported single study data of adverse events in Table 4. Abrishami 2010 did not report adverse event outcomes.
2. Adverse events for single studies: enteral nutrition versus enteral nutrition and parenteral nutrition.
| Study ID | Description of event | EN group (n/N) | EN + PN group (n/N) |
| Mechanical events | |||
| Casaer 2011 | CVC obstruction | 9/2328 | 15/2312 |
| Nasal bleeding | 18/2328 | 14/2312 | |
| Pneumohaemothorax after CVC placement | 0/2328 | 2/2312 | |
| Subclavian artery puncture | 0/2328 | 2/2312 | |
| Dunham 1994 | Withdrawal of tube | 1/12 | 0/10 |
| Failure to intubate | 0/12 | 2/10 | |
| Metabolic events | |||
| Fan 2016 | Hypoproteinaemia | 22/40 | 7/40 |
| Gastrointestinal events | |||
| Casaer 2011 | Vomiting or aspiration | 284/2328 | 295/2312 |
| Dunham 1994 | Gastric reflux | 0/12 | 2/10 |
| Fan 2016 | Stress ulcer | 7/40 | 9/40 |
| infective events | |||
| Fan 2016 | Pyemia | 3/40 | 10/40 |
| Intracranial infection | 7/40 | 5/40 | |
| Wischmeyer 2017 | Catheter bloodstream infection | 0/73 | 7/52 |
| Intra‐abdominal infection | 0/73 | 4/52 | |
| Upper urinary tract infection | 0/73 | 1/52 | |
| Surgical deep infection | 0/73 | 1/52 | |
CVC: central venous catheter; EN: enteral nutrition; EN + PN: combined enteral and parenteral nutrition; n: number of participants with an event; N: total number randomized to group.
Mechanical events
Two studies comparing EN versus EN and PN reported data for feeding tube obstruction (Casaer 2011; Dunham 1994). There was little or no difference in events between groups (RR 0.96, 95% CI 0.70 to 1.32; 4662 participants; I² = 0%; Analysis 2.4).
2.4. Analysis.
Comparison 2 Enteral (EN) versus combined EN and parenteral nutrition (PN), Outcome 4 Feeding tube obstruction.
One study reported data for failure to intubate, and withdrawal of tube by participant (Dunham 1994), and one study reported data for nasal bleeding, central venous catheter obstruction, pneumohaemothorax, and subclavian artery puncture (Casaer 2011). See Table 4.
Metabolic events
One study comparing EN versus EN and PN reported data for hypoproteinaemia (Fan 2016). See Table 4.
Gastrointestinal events
Four studies comparing EN versus EN and PN reported data for diarrhoea (Bauer 2000; Casaer 2011; Chiarelli 1996; Fan 2016). We noted substantial statistical heterogeneity between studies (I² = 88%) and did not pool data (Analysis 2.5).
2.5. Analysis.
Comparison 2 Enteral (EN) versus combined EN and parenteral nutrition (PN), Outcome 5 Diarrhoea.
Single studies reported data for vomiting or aspiration (Casaer 2011), gastric reflux (Dunham 1994), and stress ulcer (Fan 2016). See Table 4.
Infective events
Two studies reported pneumonia (aspirated pneumonia in Fan 2016; pneumonia in the ICU in Wischmeyer 2017). It is uncertain whether one feeding regimen rather than another reduced pneumonia because the certainty of this evidence is very low (RR 1.40, 95% CI 0.91 to 2.15; 205 participants; I² = 31%; Analysis 2.6). We used GRADE to downgrade the evidence by three levels; we were concerned by study limitations (one level) and imprecision (two levels). See Table 2.
2.6. Analysis.
Comparison 2 Enteral (EN) versus combined EN and parenteral nutrition (PN), Outcome 6 Pneumonia.
Two studies reported wound infections (Casaer 2011; Wischmeyer 2017); we used data for skin/soft tissue wounds in Wischmeyer 2017. We found little or no difference in events between groups (RR 0.67, 95% CI 0.50 to 0.92; 4765 participants; I² = 46%; Analysis 2.7).
2.7. Analysis.
Comparison 2 Enteral (EN) versus combined EN and parenteral nutrition (PN), Outcome 7 Wound infection.
Two studies reported bloodstream infections (Casaer 2011; Wischmeyer 2017); we used data for primary bloodstream infections in Wischmeyer 2017. We found little or no difference in events between groups (RR 0.81, 95% CI 0.66 to 1.01; 4765 participants; I² = 0%; Analysis 2.8).
2.8. Analysis.
Comparison 2 Enteral (EN) versus combined EN and parenteral nutrition (PN), Outcome 8 Bloodstream infection.
Three studies reported urinary tract infections (Bauer 2000; Casaer 2011; Wischmeyer 2017); we used data for 'lower urinary tract infections' in Wischmeyer 2017. We found little or no difference in events between groups (RR 0.87, 95% CI 0.65 to 1.17; 4885 participants; I² = 52%; Analysis 2.9).
2.9. Analysis.
Comparison 2 Enteral (EN) versus combined EN and parenteral nutrition (PN), Outcome 9 Urinary tract infection.
Three studies reported airway infections (Bauer 2000; Casaer 2011; Wischmeyer 2017); we used data for 'lower respiratory tract infection' in Wischmeyer 2017, and 'respiratory infection' in Bauer 2000. We noted substantial statistical heterogeneity between studies (I² = 78%) and did not pool data (Analysis 2.10).
2.10. Analysis.
Comparison 2 Enteral (EN) versus combined EN and parenteral nutrition (PN), Outcome 10 Airway infection.
Single studies reported data for pyaemia and intracranial infection (Fan 2016); and surgical deep infections, catheter bloodstream infections, upper urinary tract infections, and intra‐abdominal infections (Wischmeyer 2017). See Table 4. One study reported number of infections after day nine and reported this as number of events rather than by participant; we did not include these data because we could not be certain whether participants had more than one infection (Heidegger 2013).
Subgroup analysis
1. Early initiation of feeding (less than 48 hours) versus late initiation of feeding (48 hours or greater)
Four studies comparing EN versus EN and PN initiated feeding with 48 hours (Bauer 2000; Dunham 1994; Fan 2016; Wischmeyer 2017). One study comparing EN versus EN and PN initiated a late feeding protocol for the EN group after four days of all participants being given PN (Chiarelli 1996); PN was initiated early and weaning to EN was initiated late. Two studies comparing EN versus EN and PN initiated a late feeding protocol for the PN group after three days of all participants being given EN (Casaer 2011; Heidegger 2013); EN was initiated early and supplemental PN was initiated late. We did not conduct subgroup analysis for this comparison because there were few studies.
2. Normocaloric intake (to match 80% to 100% of energy expenditure) versus hypocaloric intake (less than 70% of energy expenditure)
We considered possible subgroup analysis based on terms used by study authors to describe whether intake was formulated to be normocaloric or hypocaloric; we did not make judgements based on other information such as target rates (measured as kilocalories/kilogram). No studies described intake as normocaloric or hypocaloric and we did not conduct a subgroup analysis.
3. 'Frail elderly' versus other participants
We identified no studies that specified inclusion of frail elderly participants, or subdivided participant characteristics by this description.
4. Gastrointestinal medical or surgical participants versus non‐gastrointestinal medical or surgical participants
Two studies comparing EN versus EN and PN included participants who were only non‐gastrointestinal surgical or medical participants (Abrishami 2010; Heidegger 2013). Two studies included participants with a mix of primary diagnoses which included gastrointestinal medical or surgical conditions (Casaer 2011; Wischmeyer 2017). One study did not report whether participants had gastrointestinal medical or surgical conditions (Fan 2016). We did not conduct a subgroup analysis because there were few studies.
Sensitivity analysis
1. Selection bias
We assessed five studies as having high or unclear risk of sequence generation (Abrishami 2010; Bauer 2000; Chiarelli 1996; Dunham 1994; Fan 2016). We excluded these studies from the analysis and found no difference in interpretation of effect estimates for in‐hospital mortality. It was not feasible to conduct sensitivity analysis for mortality at 30 days and mortality at 90 days because only one study remained.
2. Attrition bias
We judged one study to have unclear risk of attrition bias and performed sensitivity analyses by excluding it from appropriate analyses (Heidegger 2013). There was no difference in effect for mortality in hospital and at 30 days.
3. Effects model
We reanalysed our mortality data using a random‐effects model; this did not change the effect.
Discussion
Summary of main results
We included 25 studies comparing EN versus PN or versus EN and PN given to critically ill adults in the ICU. In addition, we identified nine studies awaiting classification (three completed or terminated studies without publication of full report, two studies published only as abstracts with insufficient information, three studies for which we were unable to access full reports, one study requires translation), and two ongoing studies.
We found low‐ and very low‐certainty evidence showing no difference between EN versus PN in mortality in hospital, within 30 days, within 90 days, and within 180 days. No studies reported number of ICU‐free days up to day 28. One study reported number of ventilator‐free days up to day 28 and it is uncertain whether one feeding route rather than another altered the number of ventilator‐free days because certainty of the evidence is very low. We found low‐ and very low‐certainty evidence showing no difference between EN versus combined EN and PN in mortality in hospital, within 90 days, and within 180 days. It is uncertain whether combined EN and PN reduces mortality at 30 days because certainty of the evidence is very low.
Studies reported adverse events, these were: mechanical (aspiration, pneumothorax, nasal bleeding, subclavian artery puncture, tube or line obstruction, line malfunctions, failure to intubate); metabolic (hyperglycaemia, hypoproteinaemia, and electrolyte disturbance); gastrointestinal (diarrhoea, vomiting, abdominal distension, nausea, bloating or cramps, jaundice, stress ulcer, elevated liver enzymes, and gastric reflux); and infective (sepsis, pneumonia, catheter infections, pulmonary infection, intracranial infection, primary bloodstream infections, wound infections, intra‐abdominal infection, urinary tract infections, surgical infections, airway infection, pyaemia, empyema, and line sepsis).
We found low‐ and very low‐certainty evidence showing no difference between EN versus PN in participants with aspiration or pneumonia. We found that EN may reduce sepsis (low‐certainty evidence) and it is uncertain whether PN reduces vomiting because certainty of the evidence is very low. In addition, we found no evidence of a difference between EN versus PN in: incidences of pneumothorax, abdominal distension, wound infections, and urinary tract infections. We found fewer people who were given EN had hyperglycaemia and had intra‐abdominal infections, and fewer people who were given PN had diarrhoea. We did not use GRADE to assess the certainty of the evidence for these additional adverse events, and noted that evidence was from few studies.
It is uncertain whether combined EN and PN compared to PN reduces pneumonia because the certainty of the evidence is very low. In addition, we found little or no difference between EN versus combined EN and PN in participants with wound infections, bloodstream infections, urinary tract infections, or feeding tube occlusion.
Overall completeness and applicability of evidence
We identified 25 studies including 8816 participants who were admitted to the ICU with a wide range of diagnoses. Whilst we noted limited statistical heterogeneity in most of the review outcome analyses, it is possible that the range of primary diagnoses may have introduced heterogeneity and reduced the applicability of these findings, and we used GRADE assessment to reduce our certainty in the estimates of effect. Despite the number of included studies, we were unable to conduct subgroup analyses on some of our proposed subgroups, and this limited exploration of differences between included studies. We also noted that studies ranged in date of publication from 1983 to 2017, and, whilst we did not assess the potential influence of date on our results, it is possible that changes in management of people in the ICU may mean that some study data may not be generalizable to the current ICU context.
Quality of the evidence
Enteral nutrition versus parenteral nutrition
We noted that all personnel were aware of the type of feeding regimen for each group of participants and for all outcomes; we believed that this introduced a high risk of performance bias. Using the GRADE approach, we downgraded the certainty of the evidence for mortality at each time point, for the number of ventilator‐free days up to day 28, and each adverse event (aspiration, sepsis, pneumonia, and vomiting) by one level for study limitations.
Studies included participants with varied primary diagnoses (e.g. gastrointestinal or non‐gastrointestinal medical or surgical patients, and whether participants were being mechanically ventilated). We believed that this reduced the directness of the evidence for some outcomes; it was possible that participants with some diagnoses may have responded differently to each feeding. Using the GRADE approach, we downgraded the certainty of evidence for in‐hospital mortality, mortality within 30 days and 90 days, and adverse events by one level for indirectness.
We noted that most studies included a small number of participants, and two studies included large sample sizes (Casaer 2011; Harvey 2014); these studies introduced a larger weighting to the effect estimates across some analyses, and was particularly noticeable in the analyses of aspiration and vomiting in which only two studies reported data for several adverse event outcomes. We considered the effect of these large studies on our results and, using the GRADE approach, we downgraded the certainty of the evidence of aspiration and vomiting by one level for imprecision. For mortality at 180 days, we found only one small study and we believed that this result alone gave an imprecise effect and we downgraded the certainty of the evidence for this outcome by one level for imprecision.
Enteral nutrition versus enteral nutrition and parenteral nutrition
We noted that all personnel were aware of the type of feeding regimen for each group of participants and for all outcomes, we believed that this introduced a high risk of performance bias. Using the GRADE approach, we downgraded the certainty of the evidence for mortality (at each time point) and for pneumonia by one level for study limitations.
Studies included participants with varied primary diagnoses (e.g. gastrointestinal or non‐gastrointestinal medical or surgical patients, and whether participants were being mechanically ventilated). We believed that this reduced the directness of the evidence for some outcomes; it is possible that participants with some diagnoses may have responded differently to each feeding. Using the GRADE approach, we downgraded the certainty of evidence for in‐hospital mortality, and mortality within 30 days and 90 days, by one level for indirectness.
For mortality at 180 days, we found only one small study, and for pneumonia we found two small studies. We believed that these results alone gave an imprecise effect and downgraded the certainty of evidence for these outcomes by one level for imprecision.
Potential biases in the review process
We conducted a thorough search and used two review authors to assess study eligibility, extract data, and assess risk of bias in included studies, and, therefore, we reduced potential bias in the review process. However, we reached some decisions on eligibility based on information presented only in study reports and we did not contact study authors for clarification; we excluded some studies that did not state clearly that participants were in the ICU. We followed a protocol decision to include outcome data reported as number of ICU‐free days, and number of ventilator‐free days, up to day 28 (Lewis 2016). Many of the included studies had reported length of stay in ICU, or duration of mechanical ventilation, and we did not include this outcome data in the review.
Agreements and disagreements with other studies or reviews
We noted that reviews by other review authors used different criteria for deciding whether participants were critically ill and, therefore, these reviews did not include all the same studies (Elke 2016; Simpson 2005). Simpson 2005 reported reduced mortality with PN, however this contradicted the more recent review by Elke 2016 whose findings were consistent with our review findings of no effect on mortality for EN versus PN.
We found no evidence of a difference in number of participants with adverse events. Reviews by Elke 2016 and Simpson 2005 reported reduced infectious complications when PN was given. Whilst these reviews included some different studies to our review, review authors presented composite data for number of infections as reported by study authors. We reported data for infections by the number of participants who had particular types of infection, rather than a composite figure, and therefore our review differed in the type of data reported for infections.
Authors' conclusions
Implications for practice.
Comparing enteral nutrition (EN) versus parenteral nutrition (PN), we found that one feeding regimen rather than another may make little or no difference to mortality in hospital or within 30 days. We are uncertain whether either of these feeding regimens reduces mortality within 90 days or within 180 days because the certainty of the evidence is very low. We are uncertain whether either of these feeding regimens reduces the number of ventilator‐free days up to 28 days, or reduces the incidence of aspiration, because the certainty of the evidence is very low. We found low‐certainty evidence that using either feeding regimen may make little or no difference to pneumonia. We found low‐certainty evidence that EN may reduce sepsis, and we are uncertain whether PN reduces vomiting because the certainty of the evidence is very low.
Comparing EN versus EN and PN, we found that one feeding regimen rather than another may make little or no difference to mortality in hospital or within 90 days. We are uncertain whether either of these feeding regimens reduces mortality within 30 days or within 180 days, or reduces incidences of pneumonia, because the certainty of the evidence is very low.
Evidence is from 25 studies with 8816 participants with a wide range of diagnoses; all participants were critically ill in the intensive care unit (ICU). The 11 studies in the Characteristics of studies awaiting classification table may alter the conclusions of the review once assessed.
Implications for research.
Research continues in the field of ICU nutrition and we have included two ongoing studies, and 11 studies that are awaiting classification, which may contribute to future updates of this review. We acknowledge the difficulty in reducing performance bias in future studies, but propose that studies should introduce methods to reduce detection bias, and improve methods of allocation concealment. Large studies with a mixed ICU population would increase generalizability to the intensive care setting. We propose that future studies consider measure of outcomes in terms of number of ICU‐free days, and number of ventilator‐free days, up to day 28 because these measures reflect the expected loss of participants in this setting due to death. Also, we propose that future studies assess the impact of nutrition on long‐term functional outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 3 January 2019 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Acknowledgements
We would like to thank Anna Lee (Content Editor), Jing Xie (Statistical Editor), Michael Casaer, Ronald L Koretz, Paul Marik (Peer Reviewers), Brian Stafford (Consumer Referee) for their help and editorial advice during the preparation of this systematic review.
We would like to thank Anna Lee (Content Editor); Asieh Golozar (Statistical Editor); and Carol Braunschweig, Michael P Casaer, Paul Marik, and Todd Rice (Peer Reviewers) for help and editorial advice provided during preparation of the protocol (Lewis 2016), for the systematic review.
We would like to thank Andrew Butler who contributed to the protocol for this review. We would like to thank Dr Rachel Markham (Consultant Anaesthetist, Intensive Care) for advice during preparation of the 'Summary of findings' tables.
The following editors screened the protocol (Lewis 2016): Marialena Trivella, Anna Lee, Arash Afshari, Bronagh Blackwood, Asieh Golozar, Nathan Pace, Jane Cracknell, and Nuala Livingstone (CEU Editor).
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Enteral Nutrition] explode all trees #2 feeding tube* or PEG line* or EN or ((enteral or enteric) near (nutrition or feeding)) #3 #1 or #2 #4 MeSH descriptor: [Parenteral Nutrition] explode all trees #5 MeSH descriptor: [Plasma Substitutes] explode all trees #6 (nutrition adj5 venous line*) or PN or ((parenteral or intravenous) near (nutrition or feeding)) #7 #4 or #5 or #6 #8 ICU or (critical* near (ill* or care)) or septic* or sepsis or feeding therap* or plasma substitute* #9 MeSH descriptor: [Sepsis] explode all trees #10 MeSH descriptor: [Shock, Septic] explode all trees #11 MeSH descriptor: [Intensive Care Units] explode all trees #12 MeSH descriptor: [Multiple Organ Failure] explode all trees #13 MeSH descriptor: [Systemic Inflammatory Response Syndrome] explode all trees #14 MeSH descriptor: [Critical Illness] explode all trees #15 MeSH descriptor: [Critical Care] explode all trees #16 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 #17 #3 and #7 and #16 in Trials
Appendix 2. MEDLINE (OvidSP) search strategy
(feeding tube* or PEG line* or EN or ((enteral or enteric) adj5 (nutrition or feeding))).mp. or exp Enteral Nutrition/
((nutrition adj5 venous line*) or PN or ((parenteral or intravenous) adj5 (nutrition or feeding))).mp. or exp Parenteral Nutrition/ or Plasma Substitutes/ or plasma substitute*.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
(ICU or (critical* adj5 (ill* or care))).mp. or (septic* or sepsis).ti,ab. or Sepsis/ or Shock, Septic/ or exp Intensive Care Units/ or exp Critical Illness/ or exp Critical Care/ or Multiple Organ Failure/ or Systemic Inflammatory Response Syndrome/ or feeding therap*.ti,ab.
((randomized controlled trial or controlled clinical trial).pt. or randomi?ed.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh.
1 and 2 and 3 and 4
Appendix 3. Embase (OvidSP) search strategy
(feeding tube* or PEG line* or EN or ((enteral or enteric) adj5 (nutrition or feeding))).mp. or exp enteric feeding/
((nutrition adj5 venous line*) or PN or ((parenteral or intravenous) adj5 (nutrition or feeding))).mp. or exp Parenteral Nutrition/ or Plasma Substitute/ or plasma substitute*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word]
(patient* adj5 (ICU or (critically adj3 (ill* or care)))).mp. or ICU.ti,ab. or (critical* adj3 ill*).ti,ab. or (septic* or sepsis).ti,ab. or Sepsis/ or septic shock/ or exp Intensive Care Unit/ or Critical Illness/ or Multiple Organ Failure/ or Systemic Inflammatory Response Syndrome/ or exp Intensive Care/ or feeding therap*.ti,ab.
((crossover procedure or double blind procedure or single blind procedure).sh. or (crossover* or cross over*).ti,ab. or placebo*.ti,ab,sh. or (doubl* adj blind*).ti,ab. or (controlled adj3 (study or design or trial)).ti,ab. or allocat*.ti,ab. or trial*.ti,ab. or randomized controlled trial.sh. or random*.ti,ab.) not ((exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.))
1 and 2 and 3 and 4
Data and analyses
Comparison 1. Enteral (EN) versus parenteral nutrition (PN).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 In‐hospital mortality | 6 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.80, 1.77] |
| 2 Mortality at 30 days | 11 | 3148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.92, 1.13] |
| 3 Mortality at 90 days | 3 | 2461 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.95, 1.17] |
| 4 Aspiration | 2 | 2437 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.46, 5.03] |
| 5 Pneumothorax | 2 | 2437 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.19, 11.22] |
| 6 Hyperglycaemia | 2 | 2437 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.35, 0.93] |
| 7 Vomiting | 3 | 2525 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.42 [1.15, 10.16] |
| 8 Diarrhoea | 6 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [1.72, 2.75] |
| 9 Abdominal distension | 3 | 2505 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.34, 6.96] |
| 10 Sepsis | 7 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.37, 0.95] |
| 11 Pneumonia | 7 | 415 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.82, 1.48] |
| 12 Intra‐abdominal infection | 3 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.07, 0.89] |
| 13 Wound infection | 3 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.55, 3.82] |
| 14 Urinary tract infection | 3 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.65, 3.40] |
| 15 In‐hospital mortality: gastrointestinal (GI) medical/surgical vs non‐GI medical/surgical | 6 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.80, 1.77] |
| 15.1 GI medical/surgical | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.06, 13.74] |
| 15.2 Non‐GI medical/surgical | 5 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.80, 1.79] |
| 16 Mortality at 30 days: GI medical/surgical vs non‐GI medical/surgical | 10 | 3068 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.93, 1.14] |
| 16.1 GI medical/surgical | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.71] |
| 16.2 Non‐GI medical/surgical | 9 | 3008 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.93, 1.15] |
Comparison 2. Enteral (EN) versus combined EN and parenteral nutrition (PN).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 In‐hospital mortality | 5 | 5111 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.84, 1.16] |
| 2 Mortality at 30 days | 3 | 409 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.06, 2.54] |
| 3 Mortality at 90 days | 2 | 4760 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.86, 1.18] |
| 4 Feeding tube obstruction | 2 | 4662 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.70, 1.32] |
| 5 Diarrhoea | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Pneumonia | 2 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.91, 2.15] |
| 7 Wound infection | 2 | 4765 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.50, 0.92] |
| 8 Bloodstream infection | 2 | 4765 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.66, 1.01] |
| 9 Urinary tract infection | 3 | 4885 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.65, 1.17] |
| 10 Airway infection | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdulmeguid 2007.
| Methods | RCT, 2‐arm, parallel design | |
| Participants |
Total number of randomized participants: 80 Inclusion criteria
Exclusion criteria
Baseline characteristics No details reported in abstract Country: not reported (published in Croatian journal) Setting: ICU |
|
| Interventions |
EN group n = 40 Details: nutritional requirements based on Harris‐Benedict equation. Formula consisted of fat, carbohydrate, and protein. Identical to PN group PN group n = 40 Details: nutritional requirements based on Harris‐Benedict equation. Formula consisted of fat, carbohydrate, and protein. Identical to EN group |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported We were unable to source the full‐text of this study, and did not have the study authors' contact details to attempt contact. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details. Abstract only |
| Allocation concealment (selection bias) | Unclear risk | No details. Abstract only |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details. Abstract only. We assumed investigators made no attempts to blind personnel |
| Blinding of outcome assessment (detection bias) All outcomes (except mortality) | Unclear risk | No details. Abstract only |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | No details. Abstract only. Lack of blinding unlikely to influence outcome data for mortality |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No details. Abstract only. Outcome data were well reported and we assumed there were no losses |
| Selective reporting (reporting bias) | Unclear risk | No details. Abstract only |
| Baseline characteristics | Unclear risk | Study authors described participants as matched on SAPS II, age, and primary diagnoses. No baseline characteristics tables or additional detail available |
| Other bias | Unclear risk | Study authors described nutritional formula of both groups as identical. Insufficient detail in abstract to make judgement on other sources of bias |
Abrishami 2010.
| Methods | RCT, single‐centre, 2‐arm, parallel design | |
| Participants |
Total number of randomized participants: 20 Inclusion criteria
Exclusion criteria
Primary diagnoses
Baseline characteristics EN group
EN + PN group
Country: Iran Setting: ICU |
|
| Interventions |
EN group n = 10; 0 losses Details: nasogastric tube feeding for 7 days. Feeding formula was Fresubin Original (Fresenius Kabi, Germany) given in solution as 1 kcal/mL. Average 70 kg participant initially received 50 mL every 3 hours, increased with 50 mL increments to maximum 300 mL every 3 hours at rate of 100 mL/h. GRV threshold at 300 mL with delay of feeding for 3 hours if threshold was reached. Glycaemic management not reported. EN + PN group n = 10; loss of 1 participant on day 3 (move to different hospital); number analysed assumed to be 9. Details: EN as above. PN consisted of 500 mL of 10% AA solutions (B Braun, Germany), 500 mL of 50% dextrose, infused over 24 hours. Equivalent management of GRV. Duration of feeding for 7 days |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: partly supported by grant from Tehran University of Medical Sciences Study dates: November 2007 to May 2009 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details and we assumed investigators made no attempts to blind personnel |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | No details. Lack of blinding unlikely to influence outcome data for mortality |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant moved to another hospital after 3 days, not clear whether data for this participant were sourced but small loss unlikely to influence outcome data |
| Selective reporting (reporting bias) | Unclear risk | No protocol or clinical trials registration reported. Therefore, not feasible to make judgement on selective outcome reporting bias |
| Baseline characteristics | Low risk | Appeared to be equivalent between groups |
| Other bias | Low risk | Glycaemic management equivalent for each group. No other sources of bias identified |
Adams 1986.
| Methods | RCT, single‐centre, 2‐arm, parallel design | |
| Participants |
Total number of randomized participants: 46 Inclusion criteria
Exclusion criteria
Primary diagnoses
Baseline characteristics EN group
PN group
Country: USA Setting: medical centre |
|
| Interventions |
EN group n = 23; 0 losses; 4 participants required conversion to PN; assume ITT analysis Details: jejunostomy tube placement. Feeding started on first postoperative day. Feeding assumed to be for duration of study period (14 days). Target rate changed during study as participants in both groups appeared to have insufficient nitrogen balance; Phase 1: target rate calculated as Harris‐Benedict BEE x 1.68, Phase 2: target rate calculated as Harris‐Benedict BEE x 2.0 plus an additional 20%. Formula consisted of polymeric feeding solution (5 participants received Isocal HCN: 15% protein calories, 45% carbohydrate calories, 49% lipid calories. 18 participants received Traumacal: 22% protein calories, 40% carbohydrate calories, 48% lipid calories) (Mead Johnson Nutritional Division, Evansville, IN, USA). Participants given insulin to manage blood glucose levels. Metabolic or gastrointestinal intolerances were treated by physician as required. Caloric intake received, mean: Phase 1: 2088 calories; Phase 2: 2678 calories PN group n = 23; 0 losses Details: subclavian line placement. Feeding started on first postoperative day, assumed duration of study period (14 days). Phase 1: target rate calculated as Harris‐Benedict BEE x 1.68, Phase 2: target rate calculated as Harris‐Benedict BEE x 2.0. Formula consisted of 25% dextrose, 4.25% crystalline AAs (Travasol: Baxter Healthcare Corporation, Deerfield, IL, USA). Additional caloric prescriptions of 500 mL of 10% lipid, twice weekly, were optional. 50 mL per hour for first 24 hours, then advanced as tolerated at physician's discretion. Caloric intake received, mean: Phase I: 2572 calories; Phase 2: 2876 |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: supported, in part, by a grant from Mead Johnson Nutritional Division, Evansville, IN, USA Study dates: January 1982 to June 1984 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized by surgical team in operating theatre. No additional information provided |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details and we assumed that investigators made no attempts to blind personnel |
| Blinding of outcome assessment (detection bias) All outcomes (except mortality) | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | No evidence of blinding. Lack of blinding unlikely to influence outcome data for mortality |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Clinical trial registration or prospectively written protocol not reported. Not feasible to judge selective outcome reporting bias |
| Baseline characteristics | Low risk | Appeared comparable |
| Other bias | Unclear risk | Changes to feeding protocol during study, but target rates were comparable between groups. No other sources of bias identified |
Altintas 2011.
| Methods | RCT, single‐centre, 2‐arm, parallel design | |
| Participants |
Total number of randomized participants: 71 Inclusion criteria
Exclusion criteria
Primary diagnoses
Baseline characteristics EN group
PN group
Country: Turkey Setting: university hospital, medical ICU |
|
| Interventions |
EN group n = 30; 0 losses; ITT analysis Details: preference for postpyloric tube placement, otherwise gastric feeding, feeding initiated within 48 hours, at target rate of 25 to 30 kcal/kg/day using ideal bodyweight, formula with proteins, carbohydrates, and lipids. 20 mL/hour of standard solution, increased every 4 to 6 hours by 20 mL/hour if GRV < 150 mL. Continuous infusion of insulin as needed with target level of 100‐140 mg/kL. Caloric intake received, reported as mean (SD) percentage of target calories given: 46.48 (± 19.34) PN group n = 41; 0 losses; deviations from protocol for 3 participants who were changed to EN feeding due to clinical needs; ITT analysis Details: preference for central or venous route, according to participant condition and contraindications to central venous line insertion, otherwise peripheral route. Target rate of delivery and glycaemic management same as EN group. Feeding started at full dose, unless participant at risk of refeeding syndrome, or severely malnourished and already had electrolyte disturbance. Caloric intake received, reported as mean (SD) percentage of target calories given: percentage of target calories given 66.78 (SD ± 18.85) |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: no details Study dates: February 2004 to January 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomized method of sequence generation. Quote: "Patients were randomized to receive either EN or PN according to the last digit of their assigned hospital record number: odd numbers received PN, and even numbers received EN" |
| Allocation concealment (selection bias) | Low risk | Adequate concealment despite inadequate sequence generation. Hospital record numbers were assigned by staff independent of the ICU team |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details and we assumed investigators made no attempts to blind personnel |
| Blinding of outcome assessment (detection bias) All outcomes (except mortality) | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | No details. Lack of blinding unlikely to influence assessment of outcomes for mortality |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses but deviations from protocol in 3 participants in PN group. ITT analysis used |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration not reported. Not possible to make assessment of selective outcome reporting bias |
| Baseline characteristics | Unclear risk | More sepsis and acute pathology in PN group; not reported as statistically significant. Unclear if these differences could influence outcome data. Also, we noted that number of participants in each group was not equivalent (41 in PN group; 30 in EN group) and this was not explained |
| Other bias | Low risk | Glycaemic controls equivalent between groups. Nutritional goals appeared to be equivalent. No other sources of bias identified |
Bauer 2000.
| Methods | RCT, single‐centre, 2‐arm, parallel design | |
| Participants |
Total number of randomized participants: 120 Inclusion criteria
Exclusion criteria
Primary diagnoses
Baseline characteristics EN group
EN + PN group
Country: France Setting: 2 ICUs (medical and surgical) at same hospital |
|
| Interventions |
EN group n = 60; 7 losses; ITT analysis Details: feeding for 4 to 7 days with target rate of delivery 25 kcal/kg bodyweight/day = 100 kcal carbohydrates‐fat per gram of nitrogen. Typical 70 kg person received 100 mL initially, with an increased amount in 50 mL steps to a maximum of 350 mL every 4 hours. Feeding solution consisted of protein (20%), polyunsaturated fats (30%), carbohydrates (50%), non‐soluble fibres, sodium chloride, potassium chloride, hydrosoluble and liposoluble vitamins. All participants in the EN group also received placebo PN formula, consisting of: sodium chloride, Intravit, Soluvit (Pharmacia and Upjohn, St Quentin‐Yvelines, France). GRV measured before each feed; delayed feeding if > 300 mL and cisapride added. Glucose level checked every 4 hours and maintained around 1.6 to 2 g/L with insulin using a sliding scale Caloric intake received, mean (SD): EN: 9.9 (± 3.9) kcal/kg/day; PN: 1.4 (± 0.3) kcal/kg/day EN + PN group n = 60; 6 losses; ITT analysis Details: EN composition as above. PN feeding through central line access. Feeding solution consisted of EN: protein (20%), polyunsaturated fats (30%), carbohydrates (50%), non‐soluble fibres, sodium chloride, potassium chloride, hydrosoluble and liposoluble vitamins. PN: 3‐in‐1 solution of carbohydrates, lipids, and protein; Vitrimix (Fresenius Kabi); hydrosoluble vitamins; Soluvit (Pharmacia and Upjohn, St Quentin‐Yvelines, France). Caloric intake received, mean (SD): EN: 11 (± 3.3) kcal/kg/day; PN: 13.9 (± 2.5) kcal/kg/day Note: study authors reported that increases in EN were the same. PN increases were the same in theory, but not in actuality due to the lack of fat content in the EN group placebo feed |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: no reported details Study dates: August 1996 to May 1997 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization carried out at central pharmacy but methods were not adequately described |
| Allocation concealment (selection bias) | Unclear risk | Use of sealed envelopes; no additional details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both participants and personnel were blind to the intervention. Quote: "The bags were prepared under the label A or B. Neither the health care providers nor the patients were aware of their content. Both types of bags were opalescent by the adjunction of small amount of fat and vitamins." |
| Blinding of outcome assessment (detection bias) All outcomes (except mortality) | Unclear risk | No details. Statistician was blinded |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | No details; lack of blinding unlikely to influence outcome data for mortality |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Some participants received sufficient nutrition by day 4, and some died by day 4 but no difference in these losses between groups and participants included in analysis as ITT |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration not reported. Not possible to make assessment on selective outcome reporting bias |
| Baseline characteristics | Low risk | Baseline characteristics were very similar between groups |
| Other bias | Low risk | Protocol for glycaemic management was the same for both groups. Other differences in nutritional protocol are due to study design (EN vs EN + PN). No other sources of bias identified |
Bertolini 2003.
| Methods | RCT, multi‐centre, 2‐arm, parallel design | |
| Participants |
Total number of randomized participants: 36 Inclusion criteria
Exclusion criteria
Primary diagnoses
Baseline characteristics EN group
PN group
Country: Italy Setting: 33 ICUs |
|
| Interventions |
EN group n = 17; note 1 participant wrongly randomized to non‐septic group (see Radrizzani 2006) and then analysed in this report; ITT analysis. 18 participants analysed. Details: feeding initiated within 48 hours of ICU admission. Feeding started at 10 kcal/kg/day, rising to 25 to 28 kcal/kg/day by 4th day. Nutritional formula consisted of 55% carbohydrates, 25% fat, 21% protein, 1.3 kcal/mL, containing per 100 mL: L‐arginine 0.8 g, omega‐3 fatty acids 0.15 g, omega‐6 fatty acids 0.7 g, vitamin E 2.9 mg, β‐carotene 0.75 mg, zinc 2.2 mg, and selenium 7 μg (Perative Abbott). Caloric intake received, mean (SD): 19.1 (± 7.6) kcal/kg/day over the first 6 days PN group n = 19; note 2 participants wrongly randomized to non‐septic group (see Radrizzani 2006) and then analysed in this report; ITT analysis. 21 participants analysed Details: feeding protocol as for EN group. Nutritional formula consists of equivalent carbohydrates, fats, and proteins (59% carbohydrates, 23% fat, 18% protein) but does not appear to include additional vitamins/minerals Caloric intake received, mean (SD): 25.9 (± 6.4) kcal/kg/day over the first 6 days |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: Abbott Italia Study dates: November 1999 to April 2001 Note: study was described as an interim analysis. It is the same study as Radrizzani 2006 but data included were for a subgroup of participants with sepsis only. Also, study authors considered the difference in caloric intake and differences in some baseline characteristics to be significant, and conducted an adjusted analysis after which they concluded that these factors were potentially confounding and subsequently ceased randomization into this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate sequence generation. Use of computer‐generated randomization code |
| Allocation concealment (selection bias) | Low risk | Adequate concealment. Quote: "The randomisation code was generated by a computer programme at the co‐ordinating centre and was revealed to investigators by telephone at the moment of randomisation, once baseline data collection was completed." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details and we assumed investigators made no attempts to blind personnel |
| Blinding of outcome assessment (detection bias) All outcomes (except mortality) | Unclear risk | Study authors reported that outcome data analysts were not blinded; study authors did not report whether outcome assessors were blinded |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | Lack of blinding unlikely to influence outcome data for mortality |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or prospectively published protocol not reported. Not possible to make assessment on reporting bias |
| Baseline characteristics | Unclear risk | Study authors noted an imbalance in baseline characteristics (PN group had more women, more participants aged > 60 years, more participants with cardiovascular and respiratory failure) and completed adjusted analysis for these variables. We were unclear whether these differences may affect results for our outcomes |
| Other bias | Unclear risk | Participants in PN group were given more calories in first 3 days because of study design and study authors completed adjusted analysis for this variable. Participants in EN group given additional supplements of minerals/vitamins which are not included in PN formula. We were unclear whether these differences may affect results for our outcomes |
Borzotta 1994.
| Methods | RCT, single‐centre, 2‐arm, parallel design | |
| Participants |
Total number of randomized participants: 59 Inclusion criteria
Exclusion criteria
Primary diagnosis
Baseline characteristics EN group
PN group
Country: USA Setting: level 1 trauma centre |
|
| Interventions |
EN group n = 36; 8 losses; 28 analysed. Per‐protocol analysis (except for mortality) Details: jejunal tube placement, and gastrotomy tubes placed to drain stomach. Feeding started within 24 hours of randomization. Target rates calculated with Harris Benedict formula BEE + 50%. Initiated at 20% of target rate for 12 hours; 40% for 12 hours; 60% for 12 hours; 80% for 12 hours; then target rate. Formula was a Vivonex solution TEN (Norwich Eaton Pharmaceuticals, Inc, Norwich, NY, USA) consisting of 4.9 g/L glutamine, carbohydrates, fat, AAs, other minerals, and Travasol solution (Baxter Healthcare Corp, Deerfield, IL, USA) consisting of 3.21 g/L glutamine, carbohydrates, fat, AAs, other minerals. If extra protein was required then Travasol 10% was given to EN solution. At day 9 to 11, EN group converted from Vivonex TEN to Isotein HN via jejunal tube ("thus keeping both groups identical except for route") PN group n = 23; 2 losses; 21 analysed. Per‐protocol analysis (except for mortality) Details: central venous catheter placement. Feeding continued for 5 days and then attempts to convert to gastric feeding by any routes at the discretion of the clinician. Target rates calculated with Harris Benedict formula BEE + 50%. Feeding initiated 40% target rate of 24 hours; 60% for 12 hours, 80% for 12 hours, then target rate. Formula was an Isotein HN solution (Sandoz Nutrition Corp: Minneapolis, MN, USA) consisting of carbohydrates, fat, AAs, and other minerals. If extra protein was required then Travasol 10% was given to PN solution |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: financial support from Norwich Eaton Pharmaceuticals, NY, USA. Study dates: July 1990 to December 1991 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate sequence generation. Computer‐generated random number tables |
| Allocation concealment (selection bias) | Low risk | Computer‐generated numbers and we assumed that allocation was concealed from investigators |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details and we assumed investigators made no attempts to blind personnel |
| Blinding of outcome assessment (detection bias) All outcomes (except mortality) | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | No details. Lack of blinding unlikely to influence outcome data for mortality |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Some loss of participant data: 2 participants in EN group, 8 participants in PN group. Reasons for losses were explained and mortality data included these (death was one of reasons for loss) |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or prospectively published protocol was not reported. Therefore, not feasible to make judgement on selective outcome reporting bias |
| Baseline characteristics | Low risk | Appeared comparable, although we noted a large difference in sample size between groups which was unexplained |
| Other bias | Unclear risk | Some possible differences in nutritional formula. Unclear if this was likely to influence data |
Casaer 2011.
| Methods | RCT, multi‐centre, 2‐arm, parallel design | |
| Participants |
Total number of randomized participants: 4640 Inclusion criteria
Exclusion criteria
Primary diagnoses
Baseline characteristics EN group
EN + PN group
Country: Belgium Setting: 7 ICUs |
|
| Interventions |
EN group (study authors referred to this group as "late‐initiation group") n = 2328; 15 discontinued intervention owing to protocol violation (inadvertent administration of ≥ 1 L/day PN for ≥ 2 days during intervention period); 2328 analysed as ITT Details: IV 20% glucose solution (target for total energy intake was 400 kcal/day on 1st ICU day, and 800 kcal/day on 2nd ICU day), and EN via duodenal feeding tube. If EN was insufficient after 7 days, PN was initiated on day 8 to reach caloric goal. Continuous insulin infusion adjusted to obtain blood glucose level 80 to 100 mg/dL EN + PN group (study authors referred to this group as "early‐initiation group") n = 2312; 0 losses; 2312 analysed Details: IV 20% glucose solution (target for total energy intake was 400 kcal/day on first ICU day, and 800 kcal/day on second ICU day). On day 3, PN initiated with dose targeted to 100% of caloric goal through combined EN + PN (except when clinicians predicted that participant would tolerate sufficient EN or oral feeding on day 3). Amount of PN was calculated as amount that was not effectively delivered by EN. Calculations of caloric goal included protein energy and based on ideal bodyweight, age, and gender. PN was reduced and eventually stopped if participant was able to meet > 80% caloric goal with EN or able to resume normal oral feeding. Continuous insulin infusion adjusted to obtain blood glucose level 80 to 100 mg/dL |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: supported by Methusalem programme of the Flemish government, Research Fund of the Catholic University of Leuven, Belgium; the Research Foundation Flanders, Belgium; and the Clinical Research Fund of the University Hospitals Leuven, Belgium. In addition, the Catholic University of Leuven received unrestricted research grant from Baxter Healthcare for less than one‐third of study costs. Baxter Healthcare were not involved in the design of study, collection, analysis, or interpretation of data, in preparation of manuscript for publication. Study dates: August 2007 to November 2010 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of a digital system to prepare order of envelopes |
| Allocation concealment (selection bias) | Low risk | Use of sequentially numbered, sealed, opaque envelopes, and block size was concealed from treating physicians and nurses |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details and we assumed that investigators made no attempts to blind personnel |
| Blinding of outcome assessment (detection bias) All outcomes (except mortality) | Low risk | All outcome assessors were blinded to group allocation |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | All outcome assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Small number of losses in 1 group, unlikely to influence outcome data |
| Selective reporting (reporting bias) | Low risk | Prospective clinical trials registration (NCT00512122). Outcomes reported same as clinical trials registration documents. We noted that duration of ICU was reported but not listed in the registration documents, but primary outcomes were generally all reported |
| Baseline characteristics | Low risk | Appeared comparable |
| Other bias | Low risk | Protocol for glycaemic management was the same for both groups. No other sources of bias identified |
Cerra 1988.
| Methods | RCT, single‐centre, 2‐arm, parallel design | |
| Participants |
Total number of randomized participants: 70 Inclusion criteria
Exclusion criteria
Primary diagnosis
Baseline characteristics EN group
PN group
Country: USA Setting: surgical ICU |
|
| Interventions |
EN group n = 33; 2 losses; ITT analysis = 31 analysed Details: nasoduodenal feeding, started 4 to 6 days after sepsis and surgery, target rate of 1.5 g protein/kg/day. 30 NPC/kg/day, carbohydrates, protein, fats, salts, minerals etc. Duration of feeding assumed to be for 8 to10 days Note: 10 participants given PN during period prior to randomization Caloric intake received, mean (SD): protein: 80 (± 26) g/day. Non‐protein: 1684 (± 573) kcal/day PN group n = 37; 2 losses: ITT analysis = 35 analysed Details: feeding formula described as "identical composition" to EN group, with same target rate of delivery Note: 10 participants given PN during period prior to randomization Caloric intake received, mean (SD): protein: 88 (± 20) g/day. Non‐protein: 2000 (± 20) kcal/day |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: no details reported Study dates: not reported Note: participants in study were a subgroup of a larger epidemiological study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details and we assumed investigators made no attempts to blind personnel |
| Blinding of outcome assessment (detection bias) All outcomes (except mortality) | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | No details. Lack of blinding unlikely to influence outcome data for mortality |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of 4 participants, with reasons reported by study authors; small number of losses unlikely to influence data |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration not reported. Not feasible to judge risk of reporting bias |
| Baseline characteristics | Low risk | Appeared comparable |
| Other bias | Low risk | No details of glycaemic controls, limited detail in paper but nutritional composition is described as identical. We noted more calories in PN group, but study authors reported "no statistical difference." |
Chiarelli 1996.
| Methods | RCT, single‐centre, 2‐arm, parallel design | |
| Participants |
Number of randomized participants: 24 Inclusion criteria
Exclusion criteria
Primary diagnosis
Baseline characteristics EN group
EN + PN group
Country: Italy Setting: ICU |
|
| Interventions |
EN group n = 12: no apparent losses Details: nutrition initiated 24 to 36 hours after admission. All participants fed with PN for 4 days, then 'weaned' to EN. Nasogastric tube placement, duration of feeding for 7 days, with formula consisting of high protein content with high ratio of calories/nitrogen. Caloric intake received, mean (SD): 33 (± 9) kcal/kg EN with PN group n = 12; no apparent losses Details: nutrition initiated 24 to 36 hours after admission. All participants given PN for 4 days, then given mixed feeding of 50% PN and 50% EN Caloric intake received, mean (SD): 31 (± 6) kcal/kg |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Study report published in Italian |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomly assigned to groups but no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details and we assumed investigators made no attempts to blind personnel |
| Blinding of outcome assessment (detection bias) All outcomes (except mortality) | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | No details. Lack of blinding unlikely to influence outcome data for mortality |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Clinical trial registration or publication of prospective protocol not reported; not feasible to judge risk of reporting bias |
| Baseline characteristics | Low risk | Study authors reported that baseline characteristics were comparable |
| Other bias | Unclear risk | No other sources of bias identified |
Dunham 1994.
| Methods | RCT, single‐centre, 3‐arm, parallel design | |
| Participants |
Total number of randomized participants: 38 Inclusion criteria
Excluded criteria
Primary diagnoses
Baseline characteristics EN group
PN group
EN + PN group
Country: USA Setting: trauma centre |
|
| Interventions |
EN group n = 12; 0 losses Details: transpyloric tube placement, feeding started within 24 hours of randomization and continued for 7 days. Investigators used Harris Benedict formula BEE x 1.3 to calculate caloric intake. Aimed to provide 50% projected calories by 24 hours after randomization; and 100% by 48 hours. Formula was Traumacal (Mead‐Johnson), NPC given in form of lipids (30%) and carbohydrates (70%). Ratio of NPC to nitrogen was 105:1. Protein load was 1.75 g/kg/day. Mean caloric intake: 1789 calories/day for 7 days PN group n = 16; 1 participant died on 4th study day, not included in study analysis but we have included in review outcome data. Details: feeding started within 24 hours of randomization and continued for 7 days. Investigators used Harris Benedict formula BEE x 1.3 to calculate caloric intake. Aim to provide 50% projected calories by 24 hours after randomization; and 100% by 48 hours. Formula consisted of dextrose‐lipid‐AA mixture; soybean solution, multi‐vitamin mixture. Mixture was 6.7% AA and 23.1% dextrose, soybean solution provided 30% of the NPC. Mean caloric intake: 1961 calories/day for 7 days EN + PN group n = 10; 0 losses Details: feeding started within 24 hours of randomization and continued for 7 days. Investigators used Harris Benedict formula BEE x 1.3 to calculate caloric intake. Aim to provide 50% projected calories by 24 hours after randomization; and 100% by 48 hours. EN formula provided 50% of calories and PN formula provided 50% of calories. EN consisted of Traumacal (Mead‐Johnson), NPC given in form of lipids (30%) and carbohydrates (70%). PN formula consisted of dextrose‐lipid‐AA mixture; soybean solution, multi‐vitamin mixture. Mean caloric intake: 2030 calories/day for 7 days |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details and we assumed investigators made no attempts to blind personnel |
| Blinding of outcome assessment (detection bias) All outcomes (except mortality) | Low risk | Most outcome data were taken from the trauma registry. Quote: "All data from the trauma registry and the finance officers were blinded, since these sources had no knowledge of the patient's status relative to the research arm assigned." |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | Outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant was not included in data for PN group due to death during 7‐day intervention period; we have included this event in review analysis. No other loss of data |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or prospectively published protocol not reported; not feasible to judge risk of selective outcome reporting bias |
| Baseline characteristics | Low risk | Some usual baseline characteristics not reported (age, gender) but other characteristics all appeared comparable |
| Other bias | Unclear risk | Target rate of nutrition the same. Unclear if differences in formula were equivalent |
Engel 1997.
| Methods | RCT, single‐centre, 3‐arm, parallel design | |
| Participants |
Number of randomized participants: 20 Inclusion criteria
Exclusion criteria
Primary diagnoses
Baseline characteristics EN group (standard)
EN group (supplemented)
PN group
Country: Germany Setting: ICU |
|
| Interventions |
EN group (standard) n = 10; 0 losses Details: nasojejunal tube with feeding pump, feeding started within 24 hours of trauma, "Oligopeptide standard diet" (Survimed OPD, Frensenius). Target energy of 25 kcal/kg/day. Initial rate of 25 mL/hour. Infusion rate increased at rate of 25 mL/hour up to the 4th day and a minimum of 75 mL/hour. Caloric intake received: not reported EN group (supplemented) n = 10; 0 losses Details: nasojejunal tube with feeding pump, feeding started within 24 hours of trauma. Formula consisted of Impact (Fa. Sandoz), supplemented with arginine, omega‐3 fatty acids, nucleotide, and selenium. Target energy of 25 kcal/kg/day. Initial rate of 25 mL/hour. Infusion rate increased at rate of 25 mL/hour up to the 4th day and a minimum of 75 mL/hour. Caloric intake received: not reported PN group n = 10; 0 losses Details: isocaloric and isonitrogenous total PN Caloric intake received: not reported |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note: for the purpose of review analysis, we combined data for the 2 EN groups. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details and we assumed investigators made no attempts to blind personnel |
| Blinding of outcome assessment (detection bias) All outcomes (except mortality) | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | No details. Lack of blinding unlikely to influence outcome data for mortality |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration of prospectively published protocol not reported; not feasible to assess risk of reporting bias |
| Baseline characteristics | Low risk | Appear comparable |
| Other bias | Low risk | No other sources of bias identified |
Fan 2016.
| Methods | RCT, single‐centre, 3‐arm, parallel design | |
| Participants |
Total number of randomized participants: 120 Inclusion criteria
Exclusion criteria
Primary diagnosis
Baseline characteristics EN group
PN group
EN + PN group
Country: China Setting: NICU |
|
| Interventions |
EN group n = 40; 0 losses Details: all participants had nasogastric tube intubation and central venous catheterization within 48 hours of admission. EN given via nasogastric tube accompanied by suctioning gastric tube, within 48 hours of admission. Increasing dose to a maximum of 1500 mL/day in 7 days, with a pumping speed < 75 mL/hour. Normal sodium, glucose, and saline given IV. Energy as 105 to 126 kJ/kg/day. Supplements of vitamins, micro‐elements, natrium, and kalium given if required PN group n = 40; 0 losses Details: all participants had nasogastric tube intubation and central venous catheterization within 48 hours of admission. Participants given PN through central venous catheter within 48 hours. Ratio of 2:1 for carbohydrates to lipids, and ratio of 100:1 for calorie nitrogen ratio. Energy as 105 to 126 kJ/kg/day. Supplements of vitamins, micro‐elements, natrium, and kalium given if required EN + PN group n = 40; 0 losses Details: all participants had nasogastric tube intubation and central venous catheterization within 48 hours of admission. EN given via nasogastric tube accompanied by suctioning gastric tube, within 48 hours of admission. Increasing dose to a maximum of 1000 mL/day in 7 days, with a pumping speed < 50 mL/hour. Insufficient energy was given by PN. Energy as 105 to 126 kJ/kg/day. Supplements of vitamins, micro‐elements, natrium, and kalium given if required |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: supported by the Natural Science Foundation of Shandong province, and Technology Supporting Program of Qingdao Study dates: January 2009 to May 2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomized method of sequence generation. Participants were randomly allocated according to hospital record numbers |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details and we assumed investigators made no attempts to blind personnel |
| Blinding of outcome assessment (detection bias) All outcomes (except mortality) | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | No details. Lack of blinding unlikely to influence outcome data for mortality |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration of prospectively published protocol not reported; not feasible to assess risk of reporting bias |
| Baseline characteristics | Low risk | Appeared comparable |
| Other bias | Low risk | No other sources of bias identified |
Gencer 2010.
| Methods | RCT, single‐centre, 2‐arm, parallel design | |
| Participants |
Number of randomized participants: 60 Inclusion criteria
Exclusion criteria
Primary diagnoses
Baseline characteristics EN group
PN group
Country: Turkey Setting: ICU |
|
| Interventions |
EN group n = 30; no apparent losses Details: nasogastric or jejunum feeding, initiated within 12 hours of surgery with target rate of delivery at 35 kcal/kg/day PN group n = 30; no apparent losses Details: standard formula of TPN (75% carbohydrate, 25% fat), with target rate of delivery at 35 kcal/kg/day |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: funding not reported. Study authors declared no conflicts of interest. Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to groups; no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | We assumed investigators made no attempts to blind personnel |
| Blinding of outcome assessment (detection bias) All outcomes (except mortality) | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | Lack of blinding unlikely to influence outcome data for mortality |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration not reported; not feasible to assess risk of reporting bias |
| Baseline characteristics | Low risk | Appeared comparable |
| Other bias | Unclear risk | No other sources of bias identified |
Hadfield 1995.
| Methods | RCT, single‐centre, 2‐arm, parallel design | |
| Participants |
Total number of randomized participants: 24 Inclusion criteria
Exclusion criteria
Primary diagnoses
Baseline characteristics Overall gender, M/F: 17/7 EN group
PN group
Country: UK Setting: adult ICU |
|
| Interventions |
EN group n = 13; 0 losses Details: nasogastric tube placement, formula used was Alitraq (Abbott Laboratories), supplemented with glutamine, delivered at 30 mL/hour (rate increased to meet nutritional requirements during 24 to 36 hours). Sucralfate (for stress ulcers) also given when required. PN group n = 11; 0 losses Details: formula described as standard regimen (Kabi 1 or Kabi 2 ‐ Kabi Pharmacia, Ltd, Milton Keynes, UK). Did not include glutamine supplement. Sucralfate (for stress ulcers) also given when required. |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: Abbott Laboratories Ltd Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details and we assumed investigators made no attempts to blind personnel |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | Lack of blinding unlikely to influence outcome data for mortality |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration not reported. Not possible to make judgement on risk of bias for selective outcome reporting |
| Baseline characteristics | Low risk | Appeared comparable |
| Other bias | Unclear risk | Glutamine supplement given to EN group but not PN group |
Harvey 2014.
| Methods | RCT, multi‐centre, 2‐arm, parallel design | |
| Participants |
Total number of participants: 2400 Inclusion criteria
Exclusion criteria
Primary diagnoses
Baseline characteristics EN group
PN group
Country: UK Setting: 33 ICUs |
|
| Interventions |
EN group n = 1200; 1195 analysed; participants lost to follow‐up were not included in analysis, but protocol deviations were. See note Details: nasogastric or nasojejunal tube feeding for 5 days. Time of initiation of feeding: median 22 (IQR 16 to 28) hours. Target rate of 25 kcal/kg bodyweight/day, with goal to reach target within 48 to 72 hours. Prokinetics given for GRV cut‐offs at 200 to 500 mL. Use of international guidelines for glycaemic management, plus target level for serum glucose of < 180 mg/dL (10 mmol/L) Caloric intake received, mean (SD): 74 (± 44) kcal/kg PN group n = 1200; 1188 analysed; participants lost to follow‐up were not included in analysis, but protocol deviations were Details: central venous catheter placement. Target rate of delivery as for EN. Equivalent nutritional formula as EN, with same glycaemic management. IV feeding for 5 days, then weaned to gastric feeding. Time of initiation of feeding of feeding: median 24 (IQR 17 to 30) hours Caloric intake received, mean (SD): 89 (SD ± 44) kcal/kg |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: NIHR Study dates: June 2011 to March 2014 Protocol deviations: EN: 30 did not receive assigned nutritional support, 26 received no nutritional support, 4 received PN. PN: 36 did not receive assigned nutritional support, 24 received no nutritional support, 12 received EN. All analysed as ITT. Additionally, there was a cross‐over of 18 participants in the EN group and 81 participants in the PN group, but these occurred towards the end of feeding and did not constitute protocol deviations. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate sequence generation. 24‐hour telephone randomization system with computer algorithm used to balance groups in ICU |
| Allocation concealment (selection bias) | Low risk | Telephone randomization system and we assumed that allocation was concealed from investigators |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details and we assumed investigators made no attempts to blind personnel |
| Blinding of outcome assessment (detection bias) All outcomes (except mortality) | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | Lack of blinding unlikely to influence outcome data for mortality |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Small number lost to follow‐up and not included in ITT analysis. Some protocol deviations but less than 10%; ITT analysis used for these |
| Selective reporting (reporting bias) | Low risk | Prospective clinical trials registration ISRCTN17386141. Protocol outcomes were consistent with outcomes reported in published study |
| Baseline characteristics | Low risk | Appear comparable |
| Other bias | Unclear risk | Not enough information on nutritional formula to make judgement. "Standard stock supply" used. Glycaemic controls appeared the same |
Heidegger 2013.
| Methods | RCT, multi‐centre, 2‐arm, parallel design | |
| Participants |
Total number of participants: 305 Inclusion criteria
Exclusion criteria
Primary diagnoses
Baseline characteristics EN group
EN + PN group
Country: Switzerland Setting: 2 ICUs, medical and surgical |
|
| Interventions |
EN group n = 152; 10 participants discontinued study due to protocol violations; ITT analysis Details: nasogastric tube feeding (preferable). All participants fed EN until day 3. Participants in EN group continued with EN feeding for 5 days as part of intervention period, then remained on EN for 28 days as required. Target rate of delivery for women 25 kcal/kg of ideal bodyweight/day, for men 30 kcal/kg of ideal bodyweight/day. Protein delivery at 1.2 g/kg of ideal bodyweight/day, formula consisted of polymeric, fibre‐enriched formulas, routinely prescribed in both hospitals, containing 1.05 to 1.62 kcal/mL of energy (18% proteins, 29% lipids (8% medium‐chain triglycerides), 53% carbohydrates). Prokinetics given if GRV ≥ 300 mL. Continuous IV insulin therapy to maintain blood glucose at lower than 8.5 mmol/L. Caloric intake received, mean (SD): 20 (± 7) kcal/kg/day for days 4 to 8 EN + PN group n = 153; 20 participants discontinued study due to protocol violations; ITT analysis Details: central or peripheral catheter placement. All participants fed with EN formula until day 3. Participants in EN + PN group supplemented with PN feeding for 5 days, then resumed with only EN for 28 days as required. Target rate of delivery as for EN. EN formula as above. Formula for PN consisted of 0.62‐1.37 kcal/mL of energy (20% proteins, 29% lipids (15% medium‐chain triglycerides), and 51% carbohydrates). Caloric intake received, mean (SD): 28 (± 5) kcal/kg/day for days 4 to 8 |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: Foundation Nutrition 2000Plus, ICU Quality Funds, Baxter, and Fresenius Kabi. Study authors reported that, "sponsors of study had no role in study design, data collection, data analysis, data interpretation, or writing of the report." Study dates: December 2008 to December 2010 Note: study authors reported number of infections, rather than number of participants with an infection, and we could not report this data because we did not know if a participant had more than 1 infection |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate sequence generation. Computer‐generated randomization sequence |
| Allocation concealment (selection bias) | Low risk | Adequate allocation concealment. Sequentially numbered, sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Some attempts to reduce risk of bias; study investigators involved in decisions regarding caloric goal were blinded. However, other investigators, personnel, and participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes (except mortality) | Low risk | Quote: "The senior site investigator from each university hospital prospectively obtained information about infectious episodes in study patients from the other centre, and was unaware of the treatment groups assigned to patients." Attempts to reduce outcome assessor and statistician blinding sufficient for our review outcomes |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | Assume blinding of outcome assessors for blinding; and lack of blinding unlikely to influence outcome data for mortality |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 20 participants in EN + PN group, and 10 participants in EN group discontinued study mostly due to protocol violation. Study authors used an ITT analysis. We noted an uneven number of losses between groups, with > 10% loss in the EN + PN group, and that death before 9 days was classed as protocol violation. We assumed that participants who died were included in mortality data for this study |
| Selective reporting (reporting bias) | Unclear risk | Quote: "registered with ClinicalTrials.gov, number NCT00802503" Prospective registration. All outcomes reported in trial register documents were consistent with reported outcomes. However, we noted changes to the trial registration documents after completion of the trial to state time point for data collection of infections between day 9 to day 28. We could not be certain whether this change affected the data reported in the published study |
| Baseline characteristics | Low risk | Appeared comparable |
| Other bias | Low risk | No other sources of bias identified. No evidence of differences in glycaemic controls or nutritional protocol |
Justo Meirelles 2011.
| Methods | RCT, single‐centre, 2‐arm, parallel design | |
| Participants |
Total number of randomized participants: 22 Inclusion criteria
Exclusion criteria
Primary diagnoses
Baseline characteristics EN group
PN group
Country: Brazil Setting: ICU |
|
| Interventions |
EN group n = 12: 0 losses Details: oro‐ or naso‐feeding tube in gastric position with pump infusion. Feeding initiated as soon as participant was haemodynamically stable. Duration of feeding for 5 days, with target rate of delivery of 25 to 30 kcal/kg/day with 1.5 g/kg/day of protein. Composition of feeding solution per 100 mL: protein 3.6 g (70% soy protein), carbohydrate 14 g, lipids 3.5 g added with casein to reach 1.5 g/kg/day Caloric intake received, mean (SD): total during 5 days: 5958 (± 3619) kcal PN group n = 10; 0 losses Details: central venous access, with equivalent target rate of delivery as EN group. Composition of feeding solution per 100 mL: 3.8 g of AAs, 14 g of glucose and 3.3 g or lipids Caloric intake received, mean (SD): total during 5 days: 6586 (± 1052) kcal |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: not reported Study dates: August 2008 to June 2009 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details and we assumed investigators made no attempts to blind personnel |
| Blinding of outcome assessment (detection bias) All outcomes (except mortality) | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | Lack of blinding unlikely to influence assessment of mortality |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Details of clinical trial registration not reported. Not possible to make assessment of selective outcome reporting bias |
| Baseline characteristics | Low risk | All comparable |
| Other bias | Low risk | Glycaemic controls not reported. We noted no differences in nutritional protocol. No other sources of bias identified |
Kudsk 1992.
| Methods | RCT, single‐centre, 2‐arm, parallel design | |
| Participants |
Total number of randomized participants: 98 Inclusion criteria
Exclusion criteria
Primary diagnoses
Baseline characteristics EN group
PN group
Country: USA Setting: trauma ICU |
|
| Interventions |
EN group n = 52; 1 death within 4 days, excluded from study analysis but we included in the review analysis; 2 participants were switched to PN group at 1 week, included in ITT analysis Details: feeding tube placement in jejunum. Target rate of delivery 1.5 to 2.0 g/kg/day of protein/AAs and 30 to 35 kcal/kg/day of NPC. Participants randomized within 8 hours of surgery, mean (SD) time until initiation of feeding was 24 (± 1.7) hours. Feeding formula was Vital HN (Ross Laboratories, Columbus, OH, USA), and consisted of protein (16.7%), branched‐chain AAs (18.2%), carbohydrates (73.9%), and fat (9.4%) Caloric intake received, mean (SEM): 30.2 (± 1.2) NPC/kg/day (maximum rate). Participants in the EN group received significantly less total nutrition per day than participants in the PN group. PN group n = 46; 1 death within 4 days, excluded from study analysis but we included in the review analysis; of 40 participants, people with infections were transferred to EN group, but kept in analysis as ITT Details: central venous access. Target rate of delivery as for EN group. Mean (SD) time until initiation of feeding was 22.9 (± 1.6) hours. Pharmacy provided formula with similar concentrations of protein, carbohydrate, and fat. Caloric intake received, mean (SEM): 29.9 (± 15) NPC/kg/day |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: not reported Study dates: December 1989 to August 1991 Note: 8 participants (did not state from which groups) required return to surgery within 24 to 48 hours, then randomized to receive EN or PN. Assumed that analysis and baseline characteristics were from point of new randomization (i.e. participants were removed and then re‐introduced into the study. 2 participants were switched from EN to PN because of failure to tolerate ≥ 50% or nutritional goal; use of ITT analysis for these participants. Protocol broken for 6 participants who had candida infections (4 in PN group, 2 in EN group) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization table |
| Allocation concealment (selection bias) | Low risk | Computer randomization and we assumed that allocation was concealed from investigators |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details and we assumed investigators made no attempts to blind personnel. |
| Blinding of outcome assessment (detection bias) All outcomes (except mortality) | Unclear risk | Principal investigator, who we assumed was not blinded, was involved in data analysis (review of charts at hospital discharge). However, attempts were made to reduce bias by using a 2nd blinded surgeon to resolve discrepancies in infections diagnoses |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | Lack of blinding unlikely to influence outcome data for mortality |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants excluded from analysis due to death; included in review analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol or clinical trials registration reported, therefore, not feasible to make judgement on risk of selective reporting bias |
| Baseline characteristics | Low risk | Appeared comparable |
| Other bias | Unclear risk | Some changes to feeding protocols that were deemed clinically appropriate. Use of ITT analysis |
Peterson 1988.
| Methods | RCT, single‐centre, 2‐arm, parallel design | |
| Participants |
Total number of randomized participants: 59 Inclusion criteria
Exclusion criteria
Primary diagnosis
Baseline characteristics EN group
PN group
Country: USA Setting: ICU |
|
| Interventions |
EN group n = 29; 8 losses, participants withdrawn due to: failure to meet inclusion/exclusion criteria, presence of underlying bowel disease, mechanical failure of EN delivery, early patient transfer, death within 72 hours; 21 analysed Details: needle‐catheter jejunostomy placed at initial laparotomy. BEE calculated by Harris‐Benedict equation at 1.5 x BEE. Feeding initiated within 12 hours of surgery. Formula consisted of Vivonex TEN (Norwich Eaton Pharmaceuticals, Inc, Norwich, NY, USA); provided 2.5% fat and approximately 33% branched chain AAs with NPC to grams nitrogen ratio of 150:1 Caloric intake received, mean (SEM): day 5: 2203.7 (± 172.8) kcal/kg PN group n = 30; 5 losses, participants withdrawn due to: failure to meet inclusion/exclusion criteria, presence of underlying bowel disease, early patient transfer, death within 72 hours; 25 analysed Details: central venous catheter placed at initial laparotomy. BEE calculated by Harris‐Benedict equation at 1.5 x BEE. Feeding initiated within 12 hours of surgery, except 2 participants for which feeding was initiated within 24 to 36 hours after laparotomy. Formula consisted of a mixture of FraAmine HBC 6.9% (Kendall‐McGaw Laboratories, Irvine, CA, USA) and TrophAmine 6% (Kendall‐McGaw Laboratories, Irvine, CA, USA); provided 2.5% fat and approximately 33% branched chain AAs with NPC to grams nitrogen ratio of 150:1 Caloric intake received, mean (SEM): day 5: 2548.1 (± 85.3) kcal/kg |
|
| Outcomes |
|
|
| Notes |
Funding/declarations: not reported Study dates: February 1985 to September 1987 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized by computer assignment" |
| Allocation concealment (selection bias) | Low risk | Computer randomization and we assumed that allocation was concealed from investigators. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details and we assumed investigators made no attempts to blind personnel |
| Blinding of outcome assessment (detection bias) All outcomes (except mortality) | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | Lack of blinding unlikely to influence outcome data for mortality |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Large number of losses after randomization. Explanations given, but it was unclear if the number of losses was balanced between groups |
| Selective reporting (reporting bias) | Unclear risk | Details of clinical trial registration not reported. Not possible to make assessment of selective outcome reporting bias |
| Baseline characteristics | Low risk | Appeared comparable |
| Other bias | Low risk | No other sources of bias identified |
Radrizzani 2006.
| Methods | RCT, multi‐centre, 2‐arm, parallel design | |
| Participants |
Total number of randomized participants: 290 Inclusion criteria
Exclusion criteria
Primary diagnoses
Baseline characteristics EN group
PN group
Country: Italy Setting: 33 adult ICUs |
|
| Interventions |
EN group n = 143; 1 participant met criteria for sepsis and not analysed (baseline characteristics excluded this participant), ITT was used for remaining participants. 142 participants analysed Details: no details of feeding tube placement. Duration of feeding assumed to be 6 days, with mean (SD) time to initiation of feeding 30.1 (± 13.8) hours, started at 10 kcal/kg/day, rising to 25 to 28 kcal/kg/day by the 4th day. Nutritional formula consisted of 55% carbohydrates, 25% fat, 21% protein, 1.3 kcal/mL, containing per 100 mL: L‐arginine 0.8 g, omega‐3 fatty acids, omega‐6 fatty acids 0.7 g, vitamin E 2.9 mg, β‐carotene 0.75 mg, zinc 2.2 mg, and selenium 7 μg. Blood glucose kept < 180 mg/dL Caloric intake received, mean (SD): 20.0 (± 8.3) kcal/kg/day PN group n = 147; 2 participants met criteria for sepsis and not analysed (baseline characteristics excluded this participant), ITT was used for remaining participants. 145 participants analysed Details: mean (SD) time to initiation of feeding 32.0 (± 12.2) hours. Nutrition supplied by pump 24 hours/day, with target of 25 to 28 kcal/kg bodyweight/day. PN not supplemented with EN before day 6. Nutritional formula consisted of 59% carbohydrate, 23% fat, 18% protein, 1.2 kcal/mL Caloric intake received, mean (SD): 23.7 (± 8.6) kcal/kg Authors conducted an adjusted analysis for caloric differences and baseline differences; concluded that differences were not significant |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: partially funded by Abbott Italia. Also unrestricted educational grant from AstraZeneca Italy Study dates: November 1999 to December 2001 Participants stratified to severely septic and non‐severely septic. Early stopping of recruitment, initially of severely septic participants who had increased mortality in EN group, then of non‐severely septic due to low accrual rate |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate sequence generation, computer‐generated randomization |
| Allocation concealment (selection bias) | Low risk | Adequate allocation concealment, randomization code generated externally and communicated via telephone to ICUs |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details and we assumed investigators made no attempts to blind personnel |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | . |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | Lack of blinding unlikely to influence outcome data for mortality |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants excluded from analysis and included in the analysis of associated study (Bertolini 2003) due to misdiagnosis. Not included in review analysis |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration not reported. Not feasible to assess risk of reporting bias |
| Baseline characteristics | Unclear risk | Some baseline characteristics imbalance. Quote: "The PN group had more men than the iEN group, more patients coming from wards and fewer from emergency rooms, and more with multiple organ failure. Other baseline characteristics were similar in the two arms." According to the adjusted analysis, these baseline differences did not confound the results |
| Other bias | Unclear risk | Blood glucose protocols equivalent between groups. Overall nutritional protocols were not comparable for the first 4 days of the study; an adjusted analysis was planned for this. No other sources of bias identified |
Rapp 1983.
| Methods | RCT, single‐centre, 2‐arm, parallel design | |
| Participants |
Total number of randomized participants: 38 Inclusion criteria
Exclusion criteria
Primary diagnosis
Baseline characteristics EN group
PN group
Country: USA Setting: neurosurgical unit |
|
| Interventions |
EN group n = 18; 0 losses Details: nasogastric tube placement. Feeding started as soon as possible after randomization, when bowel sounds were present and GRV <100 mL/hour. Study authors did not report target rate of delivery. Formula was Vital (Ross Laboratories, Columbus, Ohio, USA) ‐ 42 g protein, 10.8 g fat, 185 g carbohydrates per litre Caloric intake received, mean: 685 calories and 4.0 g nitrogen per day PN group n = 20; 0 losses Details: percutaneous intraclavicular subclavian vein catheter placement. Feeding started within 48 hours of admission. Study authors did not report target rate of delivery. Formula consisted of synthetic AAs 42.5 g/L, 25% dextrose, electrolytes, vitamins, trace elements. 10% soybean oil emulsion 250 to 500 mL/day. Insulin used to control hyperglycaemia as required Caloric intake received, mean: 1750 calories and 10.2 nitrogen per day |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: supported, in part, by a grant from Baxter‐Travenol Laboratories Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no additional details |
| Allocation concealment (selection bias) | Unclear risk | No evidence of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details and we assumed investigators made no attempts to blind personnel |
| Blinding of outcome assessment (detection bias) All outcomes (except mortality) | Unclear risk | No evidence of blinding |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | Lack of blinding unlikely to influence outcome data for mortality |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration not reported. Not feasible to assess risk of reporting bias |
| Baseline characteristics | Low risk | Appeared comparable |
| Other bias | Low risk | Insulin use to control hyperglycaemia was described for the PN group and we assumed that this was the same for each group. No other sources of bias identified |
Wischmeyer 2017.
| Methods | RCT, multi‐centre, 2‐arm, parallel design. Pilot study | |
| Participants |
Total number of randomized participants: 125 Inclusion criteria
Exclusion criteria:
Primary diagnosis
Baseline characteristics EN group
EN + PN group
Country: Canada, USA, Belgium, France Setting: 11 ICUs |
|
| Interventions |
EN group n = 73; 0 losses (for clinical outcomes) Details: EN initiated at 20 mL/hour and increased by 20 mL/hour every 4 hours, until goal was reached. A standard polymeric solution with 1.2 (± 0.2) kcal/mL was used to standardize nutrition delivery. Continued for 7 days or until death EN + PN group n = 52; 0 losses (for clinical outcomes) Details: PN given via central IV access. PN solution had similar caloric density to EN solutions (1.2 kcal/mL providing 0.06 to 0.09 g protein/mL). PN initiated at 20 mL/hour and increased by 20 mL/hour every 4 hours, until goal was reached. Continued for 7 days or until death |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: The National Institutes of Health; The Royal Alexandra Hospital Foundation, Edmonton, Canada; PN solutions and funding for assistance with distribution from Baxter Inc Study dates: June 2011 to January 2015 Note: study specifically recruited participants who were underweight or overweight; BMI status was balanced between groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralized web‐based randomization system used to randomize participants to groups |
| Allocation concealment (selection bias) | Low risk | Centralized randomization system used, which would conceal allocation codes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details and we assumed investigators made no attempts to blind personnel |
| Blinding of outcome assessment (detection bias) All outcomes (except mortality) | Unclear risk | No evidence of blinding |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | Lack of blinding unlikely to influence outcome data for mortality |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses for clinical outcome data |
| Selective reporting (reporting bias) | Low risk | Prospective clinical trials registration (NCT01206166). Outcomes were reported according to clinical trials documents |
| Baseline characteristics | Low risk | Largely comparable |
| Other bias | Low risk | We identified no other sources of bias. |
Xi 2014.
| Methods | RCT, single‐centre, 2‐arm, parallel design | |
| Participants |
Total number of randomized participants: 45 Inclusion criteria
Exclusion criteria
Primary diagnoses
Baseline characteristics EN group
PN group
Country: China Setting: ICU |
|
| Interventions |
EN group n = 22; 0 losses Details: oro‐ or naso‐enteral feeding tube, in gastric position with pump infusion. All participants fed PN until randomization. EN nutrition commenced when condition of participant allowed EN feeding. Duration of feeding assumed to be 7 days. Target rate of delivery at 20 to 25 kcal/kg/day with protein 1.5 g/kg/day. Glucose adjusted to 10 mmol/L. If EN could not meet participant's caloric needs, then PN was used as supplement from 4th day. PN group n = 23; 0 losses Details: central venous access. All participants fed PN until randomization and then participants in PN continued with feed. Duration of feeding unclearly reported but assumed to be 7 days. Target rate of delivery as for EN group. Study authors reported no significant difference in the administered total calories between groups (P > 0.05) |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: not reported Study dates: February 2010 to February 2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no further detail |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details and we assumed that investigators made no attempts to blind personnel |
| Blinding of outcome assessment (detection bias) All outcomes (except mortality) | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | No details; lack of blinding unlikely to influence outcome data for mortality |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or prospectively prepared protocol not reported; not feasible to assess this domain |
| Baseline characteristics | Low risk | Appear comparable |
| Other bias | Low risk | Limited information concerning nutritional protocol or glycaemic controls. No other sources of bias identified |
Young 1987.
| Methods | RCT, single‐centre, 2‐arm, parallel design | |
| Participants |
Total number of randomized participants: 51 Inclusion criteria
Exclusion criteria
Primary diagnosis
Baseline characteristics EN group
PN group
Country: USA Setting: medical centre |
|
| Interventions |
EN group n = 28; some early participant loss but study authors do not report to which group these participants belonged. 11 participants did not tolerate tube feedings and were switched to PN group; used ITT analysis Details: nasogastric tube feeding, started as soon as feeding tube in place. Feeding assumed to be for duration of study (i.e. 18 days). Target rate of delivery set at 1.75 x Harris Benedict BEE and 1.5 g protein/kg bodyweight/day. Formula consisted of Traumacal (1.5 calories/mL and 22% protein, 40% fat, and 38% carbohydrate) or Ensure Plus (1.5 calories/mL and 14.7% protein, 32% fat, and 53.3% carbohydrate). Participants given metoclopramide 10 mg/6 hours to stimulate gastrointestinal motility. No participant was treated with corticosteroids Cumulative intake of protein, mean (SEM): 1.35 (± 0.12) g/kg/day PN group n = 23; some early participant loss but study authors did not report to which group these participants belonged; use of ITT analysis Details: feeding commenced within 48 hours of randomization. Feeding assumed to be for duration of study (18 days); however, participants were given EN once bowel sounds were present and GRV < 100 mL every 2 hours. Target rate of delivery set at 1.75 x Harris Benedict BEE and 1.5 g protein/kg bodyweight/day. Formula consisted of sterile AA/dextrose solutions, multi‐vitamins, trace elements, and IV lipids. 17% calories as protein, 41% as fat, 42% dextrose. No participant was treated with corticosteroids Cumulative intake of protein, mean (SEM): 0.91 (± 0.09) g/kg/day |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note: we assumed that study participants were in the ICU, although study authors did not report this in the paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details and we assumed that there were no attempts to blind personnel |
| Blinding of outcome assessment (detection bias) All outcomes (except mortality) | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | Lack of blinding unlikely to influence outcome data for mortality |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7 participants were entered into the study but due to exclusion criteria were excluded from analysis, included 5 deaths within 4 days. Study authors did not report to which group these participants belonged |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration or prospectively prepared protocol not reported; not feasible to assess risk of reporting bias |
| Baseline characteristics | Low risk | Appeared comparable |
| Other bias | Unclear risk | Participants in PN group received more calories until the 9th day of study, and 11 EN participants required PN |
AA: amino acid; ACCP: American College of Chest Physicians; ADL: activities of daily living; APACHE II: Acute Physiology and Chromic Health Evaluation II; ARDS: acute respiratory deficiency syndrome; ASA: American Society of Anesthesiologists; ATI: Abdominal Trauma Index; BEE: basal energy expenditure; BMI: body mass index; COPD: chronic obstructive pulmonary disease; CT: computed tomography; DNR: do not resuscitate; EN: enteral nutrition; F: female; GCS: Glasgow Coma Scale; GRV: gastric residual volume; HCN: high calorie nutrition; ICU: intensive care unit; iEN: immuno‐enteral nutrition; ISS: Injury Severity Score; ITT: intention‐to‐treat; IQR: interquartile range; IV: intravenous; LOS: length of stay; M: male; n: number of participants; NICU: neuro‐intensive care unit; NIHR: National Institute of Health Research; NPC: non‐protein calorie; NRS: nutritional risk score; PN: parenteral nutrition; RCT: randomized controlled trial; RIFLE: scoring system for acute kidney injury, risk, injury, failure, loss, end‐stage renal disease; SAPS: Simplified Acute Physiology Score; SCCM: Society of Critical Care Medicine; SD: standard deviation; SF‐36: 36‐item Short Form; SEM: standard error of the mean; SIRS: systemic inflammatory response syndrome; SOFA: Sequential Organ Failure Assessment; TBI: traumatic brain injury; TEN: total enteral nutrition; TPN: total parenteral nutrition; VAP: ventilator‐acquired pneumonia; WBC: white blood cell.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abou‐Assi 2002 | RCT. Compared EN vs PN. People with acute pancreatitis |
| Allingstrup 2017 | RCT. Early‐goal directed nutrition vs EN. Adults in ICU. Participants in the early‐goal directed nutrition group were given EN and PN; however, PN was only given if required and therefore, we excluded this study because some participants in early‐goal directed nutrition group may not have had PN, and this information was not reported by study authors. |
| Arefian 2007 | RCT. EN vs PN. People with trauma injuries. Study authors did not report that participants were in the ICU. |
| Baigrie 1996 | RCT. EN vs PN feeding. People undergoing oesophagectomy or gastrectomy. Study did not report that participants were in the ICU. |
| Braga 1996 | RCT. 3 study groups: EN vs enriched EN vs PN. People undergoing curative surgery for gastric or pancreatic cancer. Study did not report that participants were in the ICU. |
| Braga 1998 | RCT. 3 study groups: EN vs enriched EN vs PN. People undergoing curative surgery for gastric or pancreatic cancer. Study did not report that participants were in the ICU. |
| Braga 2001 | RCT. PN vs early EN feeding. People undergoing curative surgery for cancer of the upper gastrointestinal tract. Study did not report that participants were in the ICU. |
| Chen 2004 | RCT. EN vs PN feeding. People in a burns unit not an ICU |
| DiCarlo 1999 | RCT. 3 study groups: EN vs enriched EN vs PN. People undergoing curative surgery for cancer of the pancreatic head. Study did not report that participants were in the ICU. |
| Doig 2013 | RCT. People admitted to the ICU. This trial assessed early PN in people with relative contraindications to EN, not all the participants randomized to the control group received EN. |
| Dong 2010 | RCT. 3 study groups: EN vs PN vs combined EN with Shenmai injection. People with gastric cancer after surgery. Study aimed to assess postoperative fatigue. Decision made from English abstract; study did not report that participants were in the ICU. |
| Fujita 2012 | RCT. EN vs PN. Participants were in the ICU but only for 1 day as part of standard management of participants after thoracic oesophagectomy. Feeding by EN or PN continued on the ward for 6 postoperative days. |
| Hermann 2004 | RCT. Compared EN vs PN. People with acute myeloid leukaemia. Decision made from abstract as we were unable to source the full text; study did not report that participants were in the ICU |
| Kim 2012 | RCT. EN vs PN. People after gastrectomy with gastric cancer. Study authors did not report that participants were in the ICU. |
| Klek 2008 | RCT. 4 study groups: EN vs immuno‐modulating EN vs PN vs immuno‐modulating PN. Well‐nourished people undergoing resection for gastrointestinal cancer. Study did not report that participants were in the ICU. |
| Klek 2011 | RCT. 4 study groups: EN vs immuno‐modulating EN vs PN vs immuno‐modulating PN. Malnourished people undergoing resection for gastrointestinal cancer. Study did not report that participants were in the ICU. |
| Malhotra 2004 | RCT. Compared enteral nutrition with PN. People undergoing surgical intervention for peritonitis. Study setting was reported as a surgical unit, not an ICU. |
| McArdle 1981 | RCT. Compared EN vs PN. People treated in a surgical clinic. Study did not report that participants were in the ICU. |
| Moore 1989 | RCT. Compared EN vs PN. People with abdominal trauma. Study did not report that participants were in the ICU. |
| Pupelis 2001 | RCT. > 50% of participants had pancreatitis |
| Reynolds 1997 | RCT. Compared EN vs PN. People undergoing upper gastrointestinal surgery. Study did not report that participants were in the ICU. |
| Ryu 2009 | RCT. Compared EN vs PN. People undergoing surgery for laryngeal or pharyngeal cancer. Study did not report that participants were in the ICU. |
| Sand 1997 | RCT. Compared EN vs PN. People undergoing gastrectomy for gastric cancer. Study did not report that participants were in the ICU. |
| Suchner 1996 | RCT. Compared EN vs PN. People with head trauma or need for craniotomy. Study did not report that participants were in the ICU. |
| Van Barneveld 2016 | RCT. Compared EN vs PN. People undergoing surgery for rectal carcinoma. Study did not report that participants were in the ICU. |
| Woodcock 2001 | RCT. Compared EN vs PN. People requiring adjuvant nutritional support but study authors reported that only 37.4% were in the ICU and we excluded the study as this was too few and the data for participants in the ICU were not separate. |
| Xiao‐Bo 2014 | RCT. Compared EN vs PN. People undergoing oesophagectomy for oesophageal cancer. Study did not report that participants were in the ICU. |
| Yu 2009 | RCT. Compared EN vs PN. People undergoing surgery for colorectal cancer. Decision made from English abstract only; study did not report that participants were in the ICU. |
| Zanello 1992 | RCT. Compared EN vs PN. People in the ICU with severe trauma or severe postoperative complications. Published only as an abstract; insufficient information on outcomes and not possible to use data. Abstract was from 1992, and unlikely to be published as a full report. |
| Zhang 2005 | RCT. Compared EN vs PN. People in the ICU after pericardial devascularization. We noted that participants in the EN group were all given PN as a supplement for the first 3 days and, therefore, we excluded this study. |
| Zhang 2016 | RCT. Compared EN vs PN. People with burn‐induced fungal infection. Study did not report that participants were in the ICU. |
| Zhu 2012 | RCT. Compared EN vs PN. People with acute stroke. Study did not report that participants were in the ICU. |
EN: enteral nutrition; ICU: intensive care unit; PN: parenteral nutrition; RCT: randomized controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Braga 1995.
| Methods | RCT |
| Participants | 77 people in a surgical ICU undergoing curative surgery for gastric or pancreatic cancer. Participants randomized into 3 groups: standard EN formula (n = 24), enriched EN formula with arginine, RNA, and omega‐3 fatty acids (n = 26), isocoloric TPN formula (n = 27) |
| Interventions | EN formula vs enriched EN formula vs isonitrogen‐isocaloric parenteral formula. EN started 12 hours following surgery. Infusion rate was gradually increased until full amount was achieved on postoperative day 4. |
| Outcomes |
Measurements were taken on postoperative days 1 and 8 |
| Notes | We were unable to source the full text for this study and the abstract contained insufficient information to decide eligibility. |
Cao 2014.
| Methods | RCT |
| Participants | 61 people in the NICU |
| Interventions | EN vs early PN and vs supplemental PN |
| Outcomes |
|
| Notes | Abstract only with insufficient information to justify inclusion |
Chen 2011.
| Methods | RCT |
| Participants | 147 elderly people in a RICU |
| Interventions | EN + PN vs EN vs PN |
| Outcomes |
|
| Notes | We were unable to source the full text for this study and the abstract contained insufficient information to decide eligibility. |
NCT00522730.
| Methods | RCT |
| Participants | 15 participants in each group |
| Interventions | Nutrition delivered continuously for 5 days to provide daily energy supply corresponding to current resting energy expenditure as determined by indirect calorimetry. Formula consisted of 35% of total energy requirements as lipids, 15% as proteins (maximum 1.2 g/kg ideal bodyweight/day), and 50% as dextrose. There was a tight glucose control strategy to avoid hyperglycaemia. |
| Outcomes |
|
| Notes | Trial was listed as completed in clinical trials register. Awaiting full publication of report to assess inclusion |
NCT01802099.
| Methods | RCT |
| Participants | Adults, ≥ 18 years of age, requiring mechanical ventilation for > 48 hours, treated with a vasoactive drug via a CVC, eligible for nutritional support started within 24 hours after endotracheal intubation (or within 24 hours after ICU admission if intubation occurred before ICU admission) |
| Interventions | Early EN formula vs PN formula. EN group given EN for 8 days, then supplemental PN if required. PN group given PN for at least 72 hours, then weaned to EN if haemodynamically stable |
| Outcomes |
|
| Notes | Clinical trials registration ID: NCT0180299 Study terminated early due to Data Safety and Monitoring Board recommendation. Report of results prior to termination not yet published |
Ridley 2015.
| Methods | Enrolled participants allocated to supplemental PN (via CVC) for 7 days post randomization or usual care with EN |
| Participants | Participants admitted to the ICU within 48‐72 hours, mechanically ventilated, ≥ 16 years of age, central venous access suitable for PN solution, ≥ 1 organ system failure related to their acute illness, renal dysfunction, intracranial pressure monitor or ventricular drain in situ, currently receiving extracorporeal membrane, currently has a ventricular assist device |
| Interventions | EN and supplemental PN formula vs standard EN formula |
| Outcomes |
|
| Notes | Clinical trials registration ID: NCT01847534 |
Soliani 2001.
| Methods | RCT |
| Participants | 171 people undergoing major abdominal and urological surgery for neoplastic pathology. Aim was to assess the effectiveness and clinical outcomes of total PN. |
| Interventions | Total PN vs early EN vs early immuno‐EN |
| Outcomes |
|
| Notes | We were unable to source the full text of this study, and its abstract contained insufficient information to decide whether participants were in the ICU. |
Theodorakopoulou 2016.
| Methods | RCT |
| Participants | 148 participants in the ICU |
| Interventions | EN vs PN |
| Outcomes |
|
| Notes | Reported as an abstract only. Study authors did not report denominator figures for each group, and, therefore, there were no useable data. |
Xiang 2006.
| Methods | RCT |
| Participants | 42 critically ill people |
| Interventions | EN vs PN vs control |
| Outcomes |
|
| Notes | English abstract did not report review outcomes. Requires translation to assess full eligibility |
Xiu 2015.
| Methods | RCT |
| Participants | 335 people who were expected to survive for > 7 days and were admitted to multiple Chinese ICUs |
| Interventions | Supplemented EN vs supplemented PN |
| Outcomes |
|
| Notes | This study was published only as an abstract that contained insufficient information to decide eligibility. |
Yi 2015.
| Methods | RCT |
| Participants | 63 liver transplant recipients |
| Interventions | Early EN formula vs PN formula. EN started within 48 hours of transplant surgery |
| Outcomes |
|
| Notes | This study was published only as an abstract that contained insufficient information to decide eligibility |
CVC: central venous catheter; EN: enteral nutrition; HDL: high‐density lipoprotein; ICU: intensive care unit; n: number of participants; NICU: neuro‐intensive care unit; PN: parenteral nutrition; RCT: randomized controlled trial; RICU: respiratory intensive care unit; RNA: ribonucleic acid; SOFA: sequential organ failure assessment; TPN: total parenteral nutrition; VAP: ventilator‐associated pneumonia.
Characteristics of ongoing studies [ordered by study ID]
NCT00512122.
| Trial name or title | Impact of early parenteral nutrition completing enteral nutrition in adult critically ill patients |
| Methods | Participants randomly divided into EN or EN and early PN group. Multi‐centre study |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
EN group: withholding PN during the first week of ICU stay. Participants will receive exclusively EN. If EN is insufficient after 7th day of ICU stay, PN will be started. EN and early PN: PN will be started the morning of 3rd day of ICU stay. Amount of PN will be calculated to cover the caloric needs of the participant, based on EN energy intake during the previous 24 hours. |
| Outcomes |
|
| Starting date | August 2007 |
| Contact information | Greet Van den Berghe, Katholieke Universiteit Leuven |
| Notes | Clinical trials registration ID: NCT00512122 |
NCT02022813.
| Trial name or title | Impact of supplemental parenteral nutrition in ICU patients on metabolic, inflammatory and immune responses (SPN2) |
| Methods | RCT. Trial aims to investigate the underlying carbohydrate and protein metabolism changes, as well as the immune and inflammatory modulations associated with these interventions. |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
EN group: EN to be progressed as soon as possible to energy target measured on day 3, and verified on day 4, using the usual facilitators (prokinetics) Supplemental PN group: addition of supplemental PN to complete the gap between energy delivered by EN feeding and energy target measured on day 4 |
| Outcomes |
|
| Starting date | April 2014 |
| Contact information | Mette M Berger, Prof, Centre Hospitalier Universitaire Vaudois |
| Notes | Clinical trials registration ID: NCT02022813 |
BMI: body mass index; DNR: do not resuscitate; EN: enteral nutrition; EPaNIC: early parenteral nutrition completing enteral nutrition in adult critically ill patients; ICU: intensive care unit; NRS: nutritional risk screening; PN: parenteral nutrition; SOFA: sequential organ failure assessment; SPN2: supplemental parenteral nutrition 2.
Differences between protocol and review
We made the following changes to the published protocol (Lewis 2016).
We changed the title to: Enteral versus parenteral nutrition and enteral versus a combination of enteral and parenteral nutrition for adults in the intensive care unit.
We added a new author, Oliver Schofield‐Robinson, who independently carried out screening of search results and data collection. Andrew Butler did not contribute to completion of the review and was removed from the review author list.
We edited the criteria for considering studies in the review: we intended to include only participants who were in the ICU. We edited the criteria to only include studies of mixed population if more than 75% of participants were in the ICU; at protocol stage we had not anticipated that studies may have a mixed participant population.
Objectives: we noted a difference between the objectives and the outcomes in our published protocol. The list of review outcomes did not include a measure of length of hospital stay. We edited the objectives to state that we compared the effect of nutrition on the number of ICU‐free stays up to day 28.
Unit of analysis: in the protocol, we stated "If multi‐arm studies compare more than one relevant intervention (e.g. EN vs PN and EN vs EN and PN), we will include both comparison groups but will split the data for the intervention group ‐ EN in this example ‐ by using a ‘halving’ method to avoid double‐counting, as recommended by Higgins 2011." In the review, we analysed outcome data as two separate comparisons (i.e. EN versus PN and EN versus EN and PN) and therefore we did not split data in multi‐arm studies.
Summary of findings: we outlined in the published protocol (Lewis 2016), that we would include the following outcomes in the 'Summary of findings' table: mortality (in hospital, at 30 days, at 90 days, at 180 days); number of ICU‐free days; number of ventilator‐free days; and adverse events (as reported by study authors). We limited number the number of adverse events in the 'Summary of findings' table to four outcomes for each comparison group (aspiration, sepsis, pneumonia, and vomiting); we had not specified these in the protocol. Selection of appropriate adverse events was taken following discussion with a Consultant Anaesthetist in Intensive Care.
Contributions of authors
Conceiving the review: ARB, SL.
Co‐ordinating the review: SL.
Undertaking manual searches: SL, OSR.
Screening search results: SL, OSR.
Organizing retrieval of papers: OSR.
Screening retrieved papers against inclusion criteria: SL, OSR.
Appraising quality of papers: SL, OSR.
Abstracting data from papers: SL, OSR.
Managing data for the review: SL.
Entering data into Review Manager 5 (Review Manager 2014): SL, OSR.
Analysing Review Manager 5 statistical data: SL, OSR.
Interpreting data: all review authors.
Writing the review: SL, OSR.
Securing funding for the review: PA, AS.
Serving as guarantor for the review (one author): AS.
Taking responsibility for reading and checking the review before submission: SL.
Sources of support
Internal sources
No sources of support supplied
External sources
-
NIHR Cochrane Collaboration Programme Grant, UK.
'Back to normal': speed and quality of recovery after surgery, major injury and critical care. Project ref. 13/89/16
Declarations of interest
SL's institution receives the National Institute for Health Research (NIHR) Cochrane Collaboration Programme Grant for programme of reviews in perioperative care, which supported her work on this review (see Sources of support).
OSR's institution receives the NIHR Cochrane Collaboration Programme Grant for programme of reviews in perioperative care, which supported his work on this review (see Sources of support).
PA's institution receives the NIHR Cochrane Collaboration Programme Grant for programme of reviews in perioperative care, which supported his work on this review (see Sources of support). Dr Alderson is employed by NICE, which has published a clinical guideline relevant to this topic (NICE 2006).
AS's institution receives the NIHR Cochrane Collaboration Programme Grant for programme of reviews in perioperative care, which supported his work on this review (see Sources of support).
Edited (no change to conclusions)
References
References to studies included in this review
Abdulmeguid 2007 {published data only}
- Abdulmeguid AM, Hassan A. Enteral versus parenteral nutrition in mechanically ventilated patients. Neurologia Croatica 2007;56:15‐24. [Google Scholar]
Abrishami 2010 {published data only}
- Abrishami R, Ahmadi A, Abdollahi M, Moosivand A, Khalili H, Najafi A, et al. Comparison the inflammatory effects of early supplemental parenteral nutrition plus enteral nutrition versus enteral nutrition alone in critically ill patients. Daru 2010;18(2):103‐6. [PUBMED: 22615602] [PMC free article] [PubMed] [Google Scholar]
Adams 1986 {published data only}
- Adams S, Dellinger EP, Wertz MJ, Oreskovich MR, Simonowitz D, Johansen K. Enteral versus parenteral nutritional support following laparotomy for trauma: a randomized prospective trial. Journal of Trauma 1986;26(10):882‐91. [PUBMED: 3095558] [DOI] [PubMed] [Google Scholar]
Altintas 2011 {published data only}
- Altintas ND, Aydin K, Türkoğlu MA, Abbasoğlu O, Topeli A. Effect of enteral versus parenteral nutrition on outcome of medical patients requiring mechanical ventilation. Nutrition in Clinical Practice: Official Publication of the American Society for Parenteral and Enteral Nutrition 2011;26(3):322‐9. [PUBMED: 21531737] [DOI] [PubMed] [Google Scholar]
Bauer 2000 {published data only}
- Bauer P, Charpentier C, Bouchet C, Nace L, Raffy F, Gaconnet N. Parenteral with enteral nutrition in the critically ill. Intensive Care Medicine 2000;26(7):893‐900. [PUBMED: 10990103] [DOI] [PubMed] [Google Scholar]
Bertolini 2003 {published data only}
- Bertolini G, Iapichino G, Radrizzani D, Facchini R, Simini B, Bruzzone P, et al. Early enteral immunonutrition in patients with severe sepsis: results of an interim analysis of a randomized multicentre clinical trial. Intensive Care Medicine 2003;29(5):834‐40. [PUBMED: 12684745] [DOI] [PubMed] [Google Scholar]
Borzotta 1994 {published data only}
- Borzotta AP, Pennings J, Papasadero B, Paxton J, Mardesic S, Borzotta R, et al. Enteral versus parenteral nutrition after severe closed head injury. Journal of Trauma 1994;37(3):459‐68. [PUBMED: 8083910] [DOI] [PubMed] [Google Scholar]
Casaer 2011 {published data only}
- Casaer MP, Hermans G, Wilmer A, Berghe G. Impact of early parenteral nutrition completing enteral nutrition in adult critically ill patients (EPaNIC trial): a study protocol and statistical analysis plan for a randomized controlled trial. Trials 2011;12:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaer MP, Langouche L, Coudyzer W, Beckevoort D, Dobbelaer B, Guiza FG, et al. Impact of early parenteral nutrition on muscle volume and integrity during the first week of critical illness. Clinical Nutrition, Supplement 2012;7(1):271‐2. [Google Scholar]
- Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, et al. Early versus late parenteral nutrition in critically ill adults. New England Journal of Medicine 2011;365(6):506‐17. [PUBMED: 21714640] [DOI] [PubMed] [Google Scholar]
Cerra 1988 {published data only}
- Cerra FB, McPherson JP, Konstantinides FN, Konstantinides NN, Teasley KM. Enteral nutrition does not prevent multiple organ failure syndrome (MOFS) after sepsis. Surgery 1988;104(4):727‐33. [PUBMED: 3140403] [PubMed] [Google Scholar]
Chiarelli 1996 {published data only}
- Chiarelli AG, Ferrarello S, Piccioli A, Abate A, Chini G, Berioli MB, et al. Total enteral nutrition versus mixed enteral and parenteral nutrition in patients at an intensive care unit [La nutrizione enterale totale verso la nutrizione mista enterale e parenterale in pazienti ricoverati in una terapia intensiva polivalente]. Minerva Anestesiologica 1996;62(1‐2):1‐7. [PUBMED: 8768018] [PubMed] [Google Scholar]
Dunham 1994 {published data only}
- Dunham CM, Frankenfield D, Belzberg H, Wiles C, Cushing B, Grant Z. Gut failure ‐ predictor of or contributor to mortality in mechanically ventilated blunt trauma patients?. Journal of Trauma 1994;37(1):30‐4. [PUBMED: 8028055] [DOI] [PubMed] [Google Scholar]
Engel 1997 {published data only}
- Engel JM, Menges T, Neuhauser G, Schaefer B, Hempelmann C. Effects of different feeding regimens on septic complications and immune parameters in polytraumatised patients [Auswirkungen verschiedener Ernahrungsregime bei polytraumatisierten Patienten auf septische Komplikationen und Immunparameter]. Anasthesiologie Intensivmedizin Notfallmedizin Schmerztherapie 1997;32(4):234‐9. [DOI: 10.1055/s-2007-995043] [DOI] [PubMed] [Google Scholar]
Fan 2016 {published data only}
- Fan MC, Wang QL, Fang W, Jiang Y, Li L, Sun P, et al. Early enteral combined with parenteral nutrition treatment for severe traumatic brain injury: effects on immune function, nutritional status and outcomes. Chinese Medical Science Journal 2016;31(4):213‐20. [DOI] [PubMed] [Google Scholar]
Gencer 2010 {published data only}
- Gencer A, Ozdemir Y, Sucullu I, Filiz AI, Yucel E, Akin ML, et al. The effects of enteral immunonutrient products and total parenteral nutrition in patients who underwent major abdominal surgery [Majör abdominal kanser cerrahisi uygulanan hastalarda total parenteral nutrisyon ve enteral immunonutrisyon karşilaştırılması]. Trakya Universitesi Tip Fakultesi Dergisi 2010;27(4):404‐10. [DOI: 10.5174/tutfd.2009.02426.1] [DOI] [Google Scholar]
Hadfield 1995 {published data only}
- Hadfield RJ, Sinclair DG, Houldsworth PE, Evans TW. Effects of enteral and parenteral nutrition on gut mucosal permeability in the critically ill. American Journal of Respiratory and Critical Care Medicine 1995;152(5: Part I):1545‐8. [PUBMED: 7582291] [DOI] [PubMed] [Google Scholar]
Harvey 2014 {published data only}
- Harvey SE, Parrott F, Harrison DA, Bear DE, Segaran E, Beale R, et al. Trial of the route of early nutritional support in critically ill adults. New England Journal of Medicine 2014;371(18):1673‐84. [PUBMED: 25271389] [DOI] [PubMed] [Google Scholar]
- Harvey SE, Parrott F, Harrison DA, Mythen M, Rowan KM. The CALORIES trial: statistical analysis plan. Critical Care and Resuscitation: Journal of the Australasian Academy of Critical Care Medicine 2014;16(4):248‐54. [PUBMED: 25437217] [PubMed] [Google Scholar]
- Harvey SE, Parrott F, Harrison DA, Sadique MZ, Grieve RD, Canter RR, et al. A multicentre, randomised controlled trial comparing the clinical effectiveness and cost‐effectiveness of early nutritional support via the parenteral versus the enteral route in critically ill patients (CALORIES). Health Technology Assessment 2016;20(28):1‐144. [PUBMED: 27089843] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN17386141. Clinical and cost‐effectiveness of early nutritional support in critically ill patients via the parenteral versus the enteral route [A phase III, open, multicentre, randomised controlled trial comparing the clinical and cost‐effectiveness of early nutritional support in critically ill patients via the parenteral versus the enteral route]. www.isrctn.com/ISRCTN17386141 (first received 25 March 2009). [DOI: 10.1186/ISRCTN17386141] [DOI]
Heidegger 2013 {published data only}
- Berger M, Brancato V, Graf S, Heidegger C, Darmon P, Pichard C. SPN study: supplemental parenteral nutrition (PN) to reach energy target does not compromise glucose control. Clinical Nutrition 2011;Suppl 6 (1):11‐12. [Google Scholar]
- Berger MM, Brancato V, Graf S, Heidegger C, Darmon P, Pichard C. SPN study: supplemental parenteral nutrition (PN) to reach energy target does not compromise glucose control. Intensive Care Medicine 2011;37:S98. [Google Scholar]
- Graf S, Berger MM, Clerc A, Brancato V, Heidegger CP, Pichard C. SPN study: supplemental parenteral nutrition (SPN) to reach energy target does not compromise glucose control. Clinical Nutrition 2012;Suppl 7 (1):138‐9. [Google Scholar]
- Heidegger CP, Berger MM, Graf S, Zingg W, Darmon P, Costanza MC, et al. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: a randomised controlled clinical trial. Lancet 2013;381(9864):385‐93. [PUBMED: 23218813] [DOI] [PubMed] [Google Scholar]
- Heidegger CP, Graf S, Thibault R, Darmon P, Berger M, Pichard C. Supplemental parenteral nutrition (SPN) in intensive care unit (ICU) patients for optimal energy coverage: improved clinical outcome. Clinical Nutrition 2011;Suppl 6 (1):2‐3. [DOI: 10.1016/S1744-1161(11)70006-0] [DOI] [Google Scholar]
- Heidegger CP, Thibault R, Methot C, Maisonneuve N, Jolliet P, Darmon P, et al. Supplemental parenteral nutrition (SPN) in ICU patients for early coverage of energy target: preliminary report. Intensive Care Medicine. 2009; Vol. 35:S149. [DOI: 10.1016/S1744-1161(09)70076-6] [DOI]
- NCT00802503. Impact of SPN on infection rate, duration of mechanical ventilation & rehabilitation in ICU patients [Impact of supplemental parenteral nutrition (SPN) on infection rate, duration of mechanical ventilation and rehabilitation in intensive care unit patients: a quality control program for the implementing of new nutrition guidelines]. clinicaltrials.gov/ct2/show/NCT00802503 (first received 3 December 2008).
Justo Meirelles 2011 {published data only}
- Justo Meirelles CM, Aguilar‐Nascimento JE. Enteral or parenteral nutrition in traumatic brain injury: a prospective randomised trial. Nutrición Hospitalaria 2011;26(5):1120‐4. [PUBMED: 22072362] [DOI] [PubMed] [Google Scholar]
Kudsk 1992 {published data only}
- Kudsk KA. Gut mucosal nutritional support ‐ enteral nutrition as primary therapy after multiple system trauma. Gut 1994;(1 Suppl):S52‐4. [PUBMED: 8125392] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudsk KA, Croce MA, Fabian TC, Minard G, Tolley EA, Poret HA, et al. Enteral versus parenteral feeding: effects on septic morbidity after blunt and penetrating abdominal trauma. Annals of Surgery 1992;215(5):503‐13. [PUBMED: 1616387] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudsk KA, Minard G, Wojtysiak SL, Croce M, Fabian T, Brown RO. Visceral protein response to enteral versus parenteral nutrition and sepsis in patients with trauma. Surgery 1994;116(3):516‐23. [PUBMED: 7521542] [PubMed] [Google Scholar]
Peterson 1988 {published data only}
- Peterson VM, Moore EE, Jones TN, Rundus C, Emmett M, Moore FA, et al. Total enteral nutrition versus total parenteral nutrition after major torso injury: attenuation of hepatic protein reprioritization. Surgery 1988;104(2):199‐207. [PUBMED: 2456626] [PubMed] [Google Scholar]
Radrizzani 2006 {published data only}
- Radrizzani D, Bertolini G, Facchini R, Simini B, Bruzzone P, Zanforlin G, et al. Early enteral immunonutrition vs. parenteral nutrition in critically ill patients without severe sepsis: a randomized clinical trial. Intensive Care Medicine 2006;32(8):1191‐8. [PUBMED: 16788808] [DOI] [PubMed] [Google Scholar]
Rapp 1983 {published data only}
- Rapp RP, Young DB, Twyman D, Bivins BA, Haack D, Tibbs PA, et al. The favorable effect of early parenteral feeding on survival in head‐injured patients. Journal of Neurosurgery 1983;58(6):906‐12. [PUBMED: 6406649] [DOI] [PubMed] [Google Scholar]
Wischmeyer 2017 {published data only}
- NCT01206166. Trial of supplemental parenteral nutrition in under and over weight critically ill patients (TOP‐UP) [A randomized trial of supplemental parenteral nutrition in under and over weight critically ill patients: the TOP UP trial (pilot)]. clinicaltrials.gov/ct2/show/NCT01206166 (first received 14 September 2010).
- Wischmeyer PE, Hasselmann M, Kummerlen C, Kozar R, Kutsogiannis DJ, Karvellas CJ, et al. A randomized trial of supplemental parenteral nutrition in underweight and overweight critically ill patients: the TOP‐UP pilot trial. Critical Care 2017;21:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xi 2014 {published data only}
- Xi F, Li N, Geng Y, Gao T, Zhang J, Jun T, et al. Effects of delayed enteral nutrition on inflammatory responses and immune function competence in critically ill patients with prolonged fasting. Hepato‐gastroenterology 2014;61(131):606‐12. [PUBMED: 26176044] [PubMed] [Google Scholar]
Young 1987 {published data only}
- Young B, Ott L, Twyman D, Norton J, Rapp R, Tibbs P, et al. The effect of nutritional support on outcome from severe head injury. Journal of Neurosurgery 1987;67(5):668‐76. [PUBMED: 3117982] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abou‐Assi 2002 {published data only}
- Abou‐Assi S, Craig K, O'Keefe SJ. Hypocaloric jejunal feeding is better than total parenteral nutrition in acute pancreatitis: results of a randomized comparative study. American Journal of Gastroenterology 2002;97(9):2255‐62. [PUBMED: 12358242] [DOI] [PubMed] [Google Scholar]
Allingstrup 2017 {published data only}
- Allingstrup MJ, Kondrup J, Wiis J, Claudius C, Pedersen UG, Hein‐Rasmussen R, et al. Early goal‐directed nutrition versus standard of care in adult intensive care patients: the single‐centre, randomised, outcome assessor‐blinded EAT‐ICU trial. Intensive Care Medicine 2017;43(11):1637‐47. [PUBMED: 28936712] [DOI] [PubMed] [Google Scholar]
Arefian 2007 {published data only}
- Arefian NM, Teymourian H, Radpay B. Effect of partial parenteral versus enteral nutritional therapy on serum indices in multiple trauma patients. Tanaffos 2007;6(4):37‐41. [Google Scholar]
Baigrie 1996 {published data only}
- Baigrie RJ, Devitt PG, Watkin DS. Enteral versus parenteral nutrition after oesophagogastric surgery: a prospective randomized comparison. Australian and New Zealand Journal of Surgery 1996;66(10):668‐70. [PUBMED: 8855920] [DOI] [PubMed] [Google Scholar]
Braga 1996 {published data only}
- Braga M, Vignali A, Gianotti L, Cestari A, Profili M, Carlo VD. Immune and nutritional effects of early enteral nutrition after major abdominal operations. European Journal of Surgery 1996;162(2):105‐12. [PUBMED: 8639722] [PubMed] [Google Scholar]
Braga 1998 {published data only}
- Braga M, Gianotti L, Vignali A, Cestari A, Bisagni P, Carlo V. Artificial nutrition after major abdominal surgery: impact of route of administration and composition of the diet. Critical Care Medicine 1998;26(1):24‐30. [PUBMED: 9428539] [DOI] [PubMed] [Google Scholar]
- Cestari A, Braga M, Gianotti L, Vignali A, Carlo V. Tolerance and septic complications in surgical patients fed with different routes and composition of nutritional support [abstract]. Clinical Nutrition 1996;15(Suppl 1):31. [Google Scholar]
Braga 2001 {published data only}
- Braga M, Gianotti L, Gentilini O, Parisi V, Salis C, Carlo V. Early postoperative enteral nutrition improves gut oxygenation and reduces costs compared with total parenteral nutrition (structured abstract). Critical Care Medicine 2001;29(2):242‐8. [PUBMED: 11246300] [DOI] [PubMed] [Google Scholar]
Chen 2004 {published data only}
- Chen ZY, Gu CZ, Wang SL, Yu B. Comparative study on the enteral and parenteral nutrition during early postburn stage in burn patients. Zhonghua Shao Shang za Zhi [Chinese Journal of Burns] 2004;20(4):217‐9. [PUBMED: 15447821] [PubMed] [Google Scholar]
DiCarlo 1999 {published data only}
- Carlo V, Gianotti L, Balzano G, Zerbi A, Braga M. Complications of pancreatic surgery and the role of perioperative nutrition. Digestive Surgery 1999;16(4):320‐6. [PUBMED: 10449977] [DOI] [PubMed] [Google Scholar]
Doig 2013 {published data only}
- Doig GS, Simpson F, Sweetman EA, Finfer SR, Cooper J, Heighes PT, et al. Early parenteral nutrition in critically ill patients with short‐term relative contraindications to early enteral nutrition: a randomized controlled trial. JAMA 2013;309(20):2130‐8. [PUBMED: 23689848] [DOI] [PubMed] [Google Scholar]
Dong 2010 {published data only}
- Dong QT, Zhang XD, Yu Z. Integrated Chinese and Western medical treatment on postoperative fatigue syndrome in patients with gastric cancer. Zhongguo Zhong Xi Yi Jie He za Zhi [Chinese Journal of Integrated Traditional and Western Medicine] 2010;30(10):1036‐40. [PUBMED: 21066885] [PubMed] [Google Scholar]
Fujita 2012 {published data only}
- Fujita T, Daiko H, Nishimura M. Early enteral nutrition reduces the rate of life‐threatening complications after thoracic esophagectomy in patients with esophageal cancer. European Surgical Research 2012;48(2):79‐84. [DOI] [PubMed] [Google Scholar]
Hermann 2004 {published data only}
- Hermann K, Kremer G, Sohngen D, Diehl V, Scheid C. Immune status and infectious complications in patients with acute myeloic leukemia after chemotherapy. Influence of glutamine in the parenteral nutrition [Zellularer immunstatus und infektionskomplikationen bei patienten mit akuter myeloischer leukae nach chemotherapie. Einfluss einer parenteralen ernahrung mit oder ohne zusatz von glutamin]. Chemotherapie Journal 2004;13(6):251‐6. [EMBASE: 40006938] [Google Scholar]
Kim 2012 {published data only}
- Kim HU, Chung JB, Kim CB. The comparison between early enteral nutrition and total parenteral nutrition after total gastrectomy in patients with gastric cancer: the randomized prospective study. Korean Journal of Gastroenterology 2012;59(6):407‐13. [DOI] [PubMed] [Google Scholar]
Klek 2008 {published data only}
- Klek S, Kulig J, Sierzega M, Szybinski P, Szczepanek K, Kubisz A, et al. The impact of immunostimulating nutrition on infectious complications after upper gastrointestinal surgery: a prospective, randomized, clinical trial. Annals of Surgery 2008;248(2):212‐20. [PUBMED: 18650630] [DOI] [PubMed] [Google Scholar]
Klek 2011 {published data only}
- Klek S, Sierzega M, Szybinski P, Szczepanek K, Scislo L, Walewska E, et al. Perioperative nutrition in malnourished surgical cancer patients ‐ a prospective, randomized, controlled clinical trial. Clinical Nutrition 2011;30(6):708‐13. [PUBMED: 21820770] [DOI] [PubMed] [Google Scholar]
Malhotra 2004 {published data only}
- Malhotra A, Mathur AK, Gupta S. Early enteral nutrition after surgical treatment of gut perforations: a prospective randomised study. Journal of Postgraduate Medicine 2004;50(2):102‐6. [PUBMED: 15235203] [PubMed] [Google Scholar]
McArdle 1981 {published data only}
- McArdle AH, Palmason C, Morency I, Brown RA. A rationale for enteral feeding as the preferable route for hyperalimentation. Surgery 1981;90(4):616‐23. [PUBMED: 6792730] [PubMed] [Google Scholar]
Moore 1989 {published data only}
- Moore FA, Moore EE, Jones TN, McCroskey BL, Peterson VM. TEN versus TPN following major abdominal trauma ‐ reduced septic morbidity. Journal of Trauma 1989;29(7):916‐22. [PUBMED: 2501509] [DOI] [PubMed] [Google Scholar]
Pupelis 2001 {published data only}
- Pupelis G, Selga G, Austrums E, Kaminski A. Jejunal feeding, even when instituted late, improves outcomes in patients with severe pancreatitis and peritonitis. Nutrition 2001;17(2):91‐4. [PUBMED: 11240334] [DOI] [PubMed] [Google Scholar]
Reynolds 1997 {published data only}
- Reynolds JV, Kanwar S, Welsh FK, Windsor AC, Murchan P, Barclay GR, et al. Does the route of feeding modify gut barrier function and clinical outcome in patients after major upper gastrointestinal surgery?. Journal of Parenteral and Enteral Nutrition 1997;21(4):196‐201. [PUBMED: 9252944] [DOI] [PubMed] [Google Scholar]
Ryu 2009 {published data only}
- Ryu J, Nam BH, Jung YS. Clinical outcomes comparing parenteral and nasogastric tube nutrition after laryngeal and pharyngeal cancer surgery. Dysphagia 2009;24(4):378‐86. [PUBMED: 19255706] [DOI] [PubMed] [Google Scholar]
Sand 1997 {published data only}
- Sand J, Luostarinen M, Matikainen M. Enteral or parenteral feeding after total gastrectomy: prospective randomised pilot study (structured abstract). European Journal of Surgery 1997;163(10):761‐6. [PUBMED: 9373227] [PubMed] [Google Scholar]
Suchner 1996 {published data only}
- Suchner U, Senftleben U, Eckart T, Scholz MR, Beck K, Murr R, et al. Enteral versus parenteral nutrition: effects on gastrointestinal function and metabolism. Nutrition 1996;12(1):13‐22. [PUBMED: 8838831] [DOI] [PubMed] [Google Scholar]
Van Barneveld 2016 {published data only}
- Barneveld KW, Boelens PG, Heesakkers FF, Luyer MD, Bakker JA, Rutten HJ, et al. Enteral nutrition and prevention of anastomotic leakage: a possible role for conditional essential amino acids? Results of a randomized clinical trial. European Journal of Cancer 2013;49:S483. [EMBASE: 71218432] [Google Scholar]
- Barneveld KW, Smeets BJ, Heesakkers FF, Bosmans JW, Luyer MD, Wasowicz D, et al. Beneficial effects of early enteral nutrition after major rectal surgery: a possible role for conditionally essential amino acids? Results of a randomized clinical trial. Critical Care Medicine 2016;44(6):e353‐61. [PUBMED: 26937858] [DOI] [PubMed] [Google Scholar]
Woodcock 2001 {published data only}
- Woodcock NP, Zeigler D, Palmer MD, Buckley P, Mitchell CJ, MacFie J. Enteral versus parenteral nutrition: a pragmatic study. Nutrition 2001;17(1):1‐12. [PUBMED: 11165880] [DOI] [PubMed] [Google Scholar]
Xiao‐Bo 2014 {published data only}
- Xiao‐Bo Y, Qiang L, Xiong Q, Zheng R, Jian Z, Jian‐Hua Z, et al. Efficacy of early postoperative enteral nutrition in supporting patients after esophagectomy. Minerva Chirurgica 2014;69(1):37‐46. [PUBMED: 24504222] [PubMed] [Google Scholar]
Yu 2009 {published data only}
- Yu HZ, Long X, Liu CM, Cao YL, Li SX, Wu XQ. Impact of enteral nutrition or parenteral nutrition in post‐operative colorectal cancer patients on viscera organ functions and "passing wind" time. Chinese Journal of Clinical Nutrition 2009;5:268‐70. [EMBASE: 361197093] [Google Scholar]
Zanello 1992 {published data only}
- Zanello M, Negro GC, Sangiorgi G, Ridolfi L, Martinelli G, Kahn JM. A comparison of enteral and parenteral nutrition in critically ill patients: metabolic results and effects on body composition. Clinical Nutrition 1992;11:57. [Google Scholar]
Zhang 2005 {published data only}
- Zhang K, Sun WB, Wang HF, Li ZW, Zhang XD, Wang HB, et al. Early enteral and parenteral nutritional support in patients with cirrhotic portal hypertension after pericardial devascularization. Hepatobiliary and Pancreatic Diseases International 2005;4(1):55‐9. [PUBMED: 15730920] [PubMed] [Google Scholar]
Zhang 2016 {published data only}
- Zhang Y, Gu F, Wang F, Zhang Y. Effects of early enteral nutrition on the gastrointestinal motility and intestinal mucosal barrier of patients with burn‐induced invasive fungal infection. Pakistan Journal of Medical Sciences 2016;32(3):599‐603. [PUBMED: 27375697] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhu 2012 {published data only}
- Zhu DJ, Xu ZQ, Luo BY. Clinical observation of short term prognosis of acute severe stroke patients with early enteral and parenteral nutrition. Chinese Journal of Neurology 2012;45(12):855‐60. [EMBASE: 368085043] [Google Scholar]
References to studies awaiting assessment
Braga 1995 {published data only}
- Braga M, Vignali A, Gianotti L, Cestari A, Profili M, Carlo V. Benefits of early postoperative enteral feeding in cancer patients. Infusionstherapie und Transfusionsmedizin 1995;22(5):280‐4. [PUBMED: 8924741] [DOI] [PubMed] [Google Scholar]
Cao 2014 {published data only}
- Cao J, Zhang H, Sun L, Gao L. Effects of enteral plus parenteral nutrition on early nutrition parameters and immune function in neurocritical patients. Brain Pathology 2014;24:46‐7. [Google Scholar]
Chen 2011 {published data only}
- Chen F, Wang J, Jiang Y. Influence of different routes of nutrition on the respiratory muscle strength and outcome of elderly patients in respiratory intensive care unit. [Chinese]. Chinese Journal of Clinical Nutrition 2011;19(1):7‐11. [Google Scholar]
NCT00522730 {published data only}
- NCT00522730. Safety and tolerance on lipids of parenteral and enteral nutrition in critically ill patients with liver failure (SELLIFA) [Sepsis, endothelial function, and lipids in critically ill patients with liver failure (SELLIFA). Randomized controlled trial comparing the tolerance on lipids and safety of isocaloric parenteral nutrition with enteral nutrition]. clinicaltrials.gov/ct2/show/NCT00522730 (first received 29 August 2007).
NCT01802099 {published data only}
- NCT01802099. Impact of early enteral vs. parenteral nutrition on mortality in patients requiring mechanical ventilation and catecholamines (NUTRIREA 2) [Impact of early enteral vs. parenteral nutrition on mortality in patients requiring mechanical ventilation and catecholamines: multicenter, randomized controlled trial (NUTRIREA‐2)]. clinicaltrials.gov/ct2/show/NCT01802099 (first received 27 February 2013).
Ridley 2015 {published data only}
- NCT01847534. Supplemental parenteral nutrition in critically ill adults: a pilot randomised controlled trial. clinicaltrials.gov/ct2/show/NCT01847534 (first received 22 April 2013).
- Ridley EJ, Davies AR, Parke R, Bailey M, McArthur C, Gillanders L, et al. Supplemental parenteral in critically ill patients: a study protocol for a phase II randomised controlled trial. Trials 2015;16(1):587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soliani 2001 {published data only}
- Soliani P, Dell'Abate P, Rio P, Arcuri MF, Salsi P, Cortellini P, et al. Early enteral nutrition in patients treated with major surgery of the abdomen and the pelvis [Nutrizione enterale precoce nei pazienti sottoposti a chirurgia maggiore del distretto addomino‐pelvico]. Chirurgia Italiana 2001;53(5):619‐32. [PUBMED: 11723892] [PubMed] [Google Scholar]
Theodorakopoulou 2016 {published data only}
- Theodorakopoulou M, Christodoulopoulou T, Diamantakis A, Frantzeskaki F, Kontogiorgi M, Chrysanthopoulou E, et al. Effect of enteral versus parenteral nutrition on outcome of mechanically ventilated septic ICU patients. Intensive Care Medicine Experimental. Conference: 29th Annual Congress of the European Society of Intensive Care Medicine, ESICM; 2016 Oct 1‐5; Milan, Italy. 2016.
Xiang 2006 {published data only}
- Xiang XJ. Comparative study on influence of enteral and parenteral nutrition on organ function in critically ill patients. Chinese Critical Care Medicine 2006;18(10):613‐5. [PUBMED: 17038251] [PubMed] [Google Scholar]
Xiu 2015 {published data only}
- Xiu M, Wang Y, Liu Z, Wang S, Lu Y. Enteral nutrition (EN) with supplemental parenteral nutrition (SPN) in critically ill patients in northeastern China: a randomized controlled clinical trial. American Journal of Gastroenterology 2015;110:S941. [Google Scholar]
Yi 2015 {published data only}
- Yi H, Fu B, An Y, Yi X, Lv H, Chen G. Early enteral nutrition support in patients undergoing liver transplantation decreased the incidence of postoperative infection. Transplantation 2015;1:263‐4. [Google Scholar]
References to ongoing studies
NCT00512122 {published data only}
- NCT00512122. Impact of early parenteral nutrition completing enteral nutrition in adult critically ill patients (EPaNIC). clinicaltrials.gov/ct2/show/NCT00512122 (first received 31 July 2007).
NCT02022813 {published data only}
- NCT02022813. Impact of supplemental parenteral nutrition in ICU patients on metabolic, inflammatory and immune responses (SPN2) [Impact of supplemental parenteral nutrition (SPN) on energy balance, and infection rate in intensive care patients: underlying metabolic, inflammatory and immune mechanisms]. clinicaltrials.gov/ct2/show/NCT02022813 (first received 25 November 2013).
Additional references
Al‐Omran 2010
- Al‐Omran M, AlBalawi ZH, Tashkandi MF, Al‐Ansary LA. Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD002837.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alkhawaja 2015
- Alkhawaja S, Martin C, Butler RJ, Gwadry‐Sridhar F. Post‐pyloric versus gastric tube feeding for preventing pneumonia and improving nutritional outcomes in critically ill adults. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD008875.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Allingstrup 2016
- Allingstrup M, Afshari A. Selenium supplementation for critically ill adults. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD003703.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
ARDS Clinical Trials Network 2012
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Rice TW, Wheeler AP, Thompson BT, Steingrub J, Hite RD, Moss M, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA 2012;307(8):795‐803. [PUBMED: 22307571] [DOI] [PMC free article] [PubMed] [Google Scholar]
ASPEN 2002
- ASPEN Board of Directors and the Clinical Guidelines Task Force. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. Journal of Parenteral and Enteral Nutrition 2002;26(1 Suppl):1SA‐138SA. [PUBMED: 11841046] [PubMed] [Google Scholar]
Casaer 2014
- Casaer MP, Berghe G. Nutrition in the acute phase of critical illness. New England Journal of Medicine 2014;370(13):1227‐36. [PUBMED: 24670169] [DOI] [PubMed] [Google Scholar]
Corley 2017
- Corley A, Rickard CM, Aitken LM, Johnston A, Barnett A, Fraser JF, et al. High‐flow nasal cannulae for respiratory support in adult intensive care patients. Cochrane Database of Systematic Reviews 2017, Issue 5. [DOI: 10.1002/14651858.CD010172.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Correia 2003
Covidence [Computer program]
- Veritas Health Innovation Ltd. Covidence. Version accessed 23 October 2015. Melbourne, Australia: Veritas Health Innovation Ltd.
Dushianthan 2016
- Dushianthan A, Cusack R, Grocott MP. Immunonutrition for acute respiratory distress syndrome (ARDS) in adults. Cochrane Database of Systematic Reviews 2016, Issue 1. [DOI: 10.1002/14651858.CD012041] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elke 2013
- Elke G, Kuhnt E, Ragaller M, Schadler D, Frerichs I, Brunkhorst FM, et al. Enteral nutrition is associated with improved outcome in patients with severe sepsis. A secondary analysis of the VISEP trial. Medizinische Klinik Intensivmedizin und Notfallmedizin 2013;108(3):223‐33. [PUBMED: 23455443] [DOI] [PubMed] [Google Scholar]
Elke 2016
- Elke G, Zanten AR, Lemieux M, McCall M, Jeejeebhoy KN, Kott M, et al. Enteral versus parenteral nutrition in critically ill patients: an updated systematic review and meta‐analysis of randomized controlled trials. Critical Care 2016;20(1):117. [PUBMED: 27129307] [DOI] [PMC free article] [PubMed] [Google Scholar]
Endnote [Computer program]
- Thomson Reuters. Endnote X5. Version assessed 8 September 2017. Philadelphia (PA): Thomson Reuters, 2011.
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann H. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Harvey 2015
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
King 1999
- King BK, Kudsk KA, Li J, Wu Y, Renegar KB. Route and type of nutrition influence mucosal immunity to bacterial pneumonia. Annals of Surgery 1999;229(2):272‐8. [PUBMED: 10024110] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kreymann 2006
- Kreymann KG, Berger MM, Deutz NE, Hiesmayr M, Jolliet P, Kazandjiev G, et al. ESPEN guidelines on enteral nutrition: intensive care. Clinical Nutrition (Edinburgh, Scotland) 2006;25(2):210‐23. [PUBMED: 16697087] [DOI] [PubMed] [Google Scholar]
Kyle 2006
- Kyle UG, Genton L, Heidegger CP, Maisonneuve N, Karsegard VL, Huber O, et al. Hospitalized mechanically ventilated patients are at higher risk of enteral underfeeding than non‐ventilated patients. Clinical Nutrition 2006;25(5):727‐35. [PUBMED: 16725230] [DOI] [PubMed] [Google Scholar]
Marik 2016
- Marik PE, Hooper MH. Normocaloric versus hypocaloric feeding on the outcomes of ICU patients: a systematic review and meta‐analysis. Intensive Care Medicine 2016;42(3):316‐23. [PUBMED: 26556615] [DOI] [PubMed] [Google Scholar]
Marvin 2000
- Marvin RG, McKinley BA, McQuiggan M, Cocanour CS, Moore FA. Nonocclusive bowel necrosis occurring in critically ill trauma patients receiving enteral nutrition manifests no reliable clinical signs for early detection. American Journal of Surgery 2000;179(1):7‐12. [PUBMED: 10737569] [DOI] [PubMed] [Google Scholar]
Mogensen 2015
NICE 2006
- National Institute for Health and Clinical Excellence (NICE). Nutrition support for adults. Oral nutrition support, enteral tube feeding and parenteral nutrition (CG32), 2006. www.nice.org.uk/guidance/cg32/evidence/full‐guideline‐194889853 (accessed 30 October 2015). [PubMed]
Norman 2008
Peter 2005
- Peter JV, Moran JL, Phillips‐Hughes J. A metaanalysis of treatment outcomes of early enteral versus early parenteral nutrition in hospitalized patients. Critical Care Medicine 2005;33(1):213‐20; discussion 260‐1. [PUBMED: 15644672] [DOI] [PubMed] [Google Scholar]
Preiser 2015
- Preiser JC, Zanten AR, Berger MM, Biolo G, Casaer MP, Doig GS, et al. Metabolic and nutritional support of critically ill patients: consensus and controversies. Critical Care (London, England) 2015;19:35. [PUBMED: 25886997] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reignier 2013
- Reignier J, Mercier E, Gouge A, Boulain T, Desachy A, Bellec F, et al. Effect of not monitoring residual gastric volume on risk of ventilator‐associated pneumonia in adults receiving mechanical ventilation and early enteral feeding: a randomized controlled trial. JAMA 2013;309(3):249‐56. [PUBMED: 23321763] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schetz 2013
- Schetz M, Casaer MP, Berghe G. Does artificial nutrition improve outcome of critical illness?. Critical Care (London, England) 2013;17(1):302. [PUBMED: 23375069] [DOI] [PMC free article] [PubMed] [Google Scholar]
Seres 2013
- Seres DS, Valcarcel M, Guillaume A. Advantages of enteral nutrition over parenteral nutrition. Therapeutic Advances in Gastroenterology 2013;6(2):157‐67. [PUBMED: 23503324] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simpson 2005
- Simpson F, Doig GS. Parenteral vs. enteral nutrition in the critically ill patient: a meta‐analysis of trials using the intention to treat principle. Intensive Care Medicine 2005;31(1):12‐23. [PUBMED: 15592814] [DOI] [PubMed] [Google Scholar]
Singer 2009
Singer 2011
- Singer P, Anbar R, Cohen J, Shapiro H, Shalita‐Chesner M, Lev S, et al. The tight calorie control study (TICACOS): a prospective, randomized, controlled pilot study of nutritional support in critically ill patients. Intensive Care Medicine 2011;37(4):601‐9. [PUBMED: 21340655] [DOI] [PubMed] [Google Scholar]
Tao 2014
- Tao K‐M, Li X‐Q, Yang L‐Q, Yu W‐F, Lu Z‐J, Sun Y‐M, et al. Glutamine supplementation for critically ill adults. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD010050.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2016
- Taylor BE, McClave SA, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). Critical Care Medicine 2016;44(2):390‐438. [PUBMED: 26771786] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lewis 2016
- Lewis SR, Butler AR, Alderson P, Smith AF. Enteral versus parenteral nutrition for adults in the intensive care unit. Cochrane Database of Systematic Reviews 2016, Issue 7. [DOI: 10.1002/14651858.CD012276] [DOI] [PMC free article] [PubMed] [Google Scholar]


