Summary
Introduction
The gastrointestinal (GI) microbiome has emerged as a potential regulator of metabolism. However, the precise mechanisms of how microorganisms may influence physiology remain largely unknown. Interestingly, GI microorganisms, including methanogens, are localized within the same regions as the glucagon‐like peptide‐1 (GLP‐1) secreting L cells. GLP‐1 plays key roles appetite and glucose regulation. Furthermore, both methane and GLP‐1 levels are altered in obese humans with metabolic disease. We predict that high‐fat diet‐induced obesity alters the abundance of GI methanogens and that methane may play a role in the GLP‐1 secretory response from the L cell.
Methods
To demonstrate this, GLP‐1 secretion response and faecal methanogens were examined in mice given a high‐fat diet for 14 weeks. In addition, the direct effect of methane on GLP‐1 secretion was assessed in two L‐cell models (NCI‐H716 and GLUTag).
Results
High‐fat diet caused a significant increase in both GLP‐1 secretion and faecal methanogen content. There was a direct correlation between GLP‐1 secretion response and faecal methanogen levels. In L cells, methane stimulated GLP‐1 secretion and enhanced intracellular cAMP content.
Conclusion
These results indicate that alterations in the methanogen communities occurring in obesity may play a vital role in directly enhancing GLP‐1 secretion, and that methane can directly stimulate the secretion of GLP‐1.
Keywords: GLP‐1, gut hormones, microbiome, obesity
1. INTRODUCTION
The gastrointestinal (GI) microbiome comprises trillions of microbial organisms and has emerged as an important regulator of metabolic health. In‐depth examination of the GI microbiome has shown clear changes in the microbial community during obesity,1 and importantly, that these changes can drive metabolic changes.2 Recently, the methanogenic archaea (methanogens) have been identified as a potential regulator of metabolic function. These microorganisms metabolize a variety of electron sources including dihydrogen gas and small fatty acids within the gut milieu to produce methane (reviewed in3). While not all humans have detectable levels of breath methane, studies have shown that methane production is altered in disease states. Indeed, It appears that faecal methanogen numbers are elevated during human obesity and that disrupting methanogens through antibiotic treatment of their bacterial syntrophs also corresponds to reduced insulin secretion.4 These studies suggest that methanogens and methane might have a role to play in the regulation of glucose homeostasis during obesity, though whether linkages are direct or spurious is unclear, and our understanding of how methane might drive metabolic changes is unknown.
One potential mechanism explaining methane's action on metabolic health may be through direct action on the GI endocrine system. Indeed, in addition to housing methanogens, the GI tract is also home to a variety of metabolic hormone‐secreting cells, known collectively as the enteroendocrine system. Specifically, glucagon‐like peptide‐1 (GLP‐1) is secreted from the L cells of the lower GI epithelium and plays a vital role in post meal insulin secretion and appetite suppression (reviewed in5). Similar to the L cells, methanogens are most abundant in the lower small intestine and colon,6 and recently, bacterial metabolites including short‐chain fatty acids were shown to stimulate GLP‐1 secretion.7 These metabolites can also be methane precursors, and it is unclear if short‐chain fatty acids alone influence metabolic activities while concomitantly stimulating methanogenesis or if methane is also directly involved in secretion. Here, we test if methane plays a direct role in the regulation of GLP‐1 secretion from the L cell, and if increased methanogen numbers occurring in obesity could drive altered levels of GLP‐1 in vivo. We examined this by the direct treatment of GLP‐1 secreting cell lines with methane and by exploring altered methanogen abundance and GLP‐1 profiles that occur during diet‐induced obesity.
2. METHODS
2.1. Animal study
Animal work was approved by the University of Toronto Animal care committee in compliance with the Canadian Council for Animal care. Samples were collected as part of a study reported in Ref 8. Four‐week C57Bl6 male mice (Charles River Labs, Montreal, QC, Canada) were placed on a 60% calories from fat, high‐fat diet or standard laboratory chow diet (Research Diets, New Brunswick, NJ, USA) for 14 weeks. GLP‐1 secretion was examined from blood sampled at 0, 10, 60 minutes after oral glucose (2 g/kg) using an Active GLP‐1 rev. 2 EIA kit (Mesoscale Ltd., Rockville, MD, USA). Faecal and caecal samples were collected from overnight‐fasted animals at the end of the study. Caecal collection was carried out directly from the surgically removed caecum after the mice had been euthanized.
2.2. Methanogen detection
DNA from caecal and faecal samples (25 mg) were isolated using the PowerFecal® Isolation Kit (MoBio, Carlsbad, CA, USA). Quantitative PCR was performed targeting the mcrA gene encoding a subunit of the methyl coenzyme‐M reductase, a key enzyme in methanogenesis, using the primer pair described in.9 A standard curve of template methanogen DNA with known copy number was used for downstream absolute quantification.
2.3. Cell culture
Mouse (GLUTag) and human (NCIH716) GLP‐1 secreting cells were grown under standard conditions (5% CO2, 37C). For cAMP and GLP‐1 secretion experiments, cells (5 00 000) were seeded into 6 well plates for 48 hours. Methane treatments were prepared in 0.5% serum as previously described in.10 Methane concentrations were verified in gas‐phase following a liquid‐headspace equilibration using a gas chromatograph with a flame ionization detector. GLP‐1 secretion was examined in cell culture media after 2 hours using a commercial total GLP‐1 ELISA (Sigma Aldrich, St. Louis, Missouri, MO, USA). Intracellular cAMP levels were assayed after 30 minutes using a Cyclic AMP ELISA Kit (Cayman Chemical, Ann Arbor, MI, USA).
2.4. Analysis
All data are presented as the mean ± standard error of the mean. Methyl coenzyme‐M reductase is presented as copy number per 30 mg dry faecal/material. Comparisons between two groups are analysed by the student's t test. Comparisons between multiple doses are analysed by a one‐way ANOVA with a Dunnett's post hoc test between doses.
3. RESULTS
3.1. Elevated methanogens and GLP‐1 secretion in high‐fat fed mice
Methanogen numbers in the faecal extracts were significantly higher than in the caecal extracts (Figure 1A), as such faecal DNA data were used for subsequent analysis. To determine the effect of high‐fat diet on methanogen abundance and GLP‐1 secretion, faecal mcrA numbers and glucose‐stimulated GLP‐1 levels were compared in mice following a 14‐week high‐fat diet and a 14‐week chow diet. Mice receiving the high‐fat diet had significantly elevated body weight (average weight 44.6 g vs 29.2 g) and significantly elevated faecal methanogen numbers compared to chow‐fed controls (Figure 1B). GLP‐1 response to oral glucose was also significantly elevated in the high‐fat diet group (Figure 1C‐D). Further, GLP‐1 secretion varied significantly and positively with methanogen abundance (P < .0351, r = .4641, Figure 1E).
Figure 1.
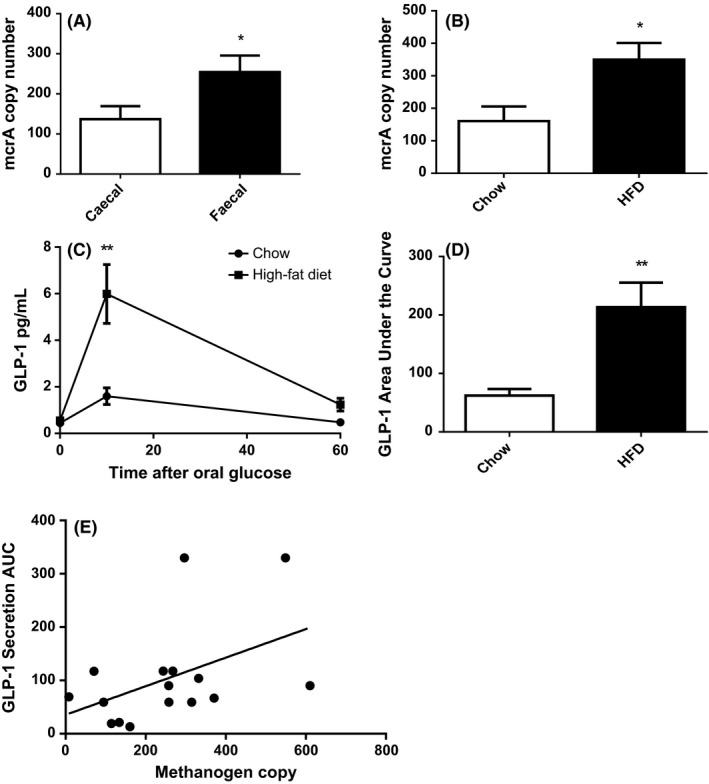
High‐fat diet increases faecal methanogens and circulating GLP‐1. Quantitative PCR of the mcrA gene was used to compare methanogen copy number (A), and the effect of 14 wks of high‐fat diet in faeces (B). GLP‐1 secretion after oral glucose gavage after 14 wks of high‐fat diet (C) and (D). Pearson correlation between GLP‐1 secretion activity and methanogen numbers (E). N = 16 *= P < .05, **= P < .01
3.2. Methane directly stimulates the secretion of GLP‐1
Mouse (GLUTag) and human (NCI‐H716) L cells were treated with methane to determine whether methane was directly involved in GLP‐1 secretion. In both GLUTag and NCI‐H716 cells, methane had an overall significant effect (P < .05 1 way ANOVA, Figure 2A‐B). In GLUTag cells, strong stimulatory effects were observed at 27 μmol/L (1.95‐fold of control) and 2.7 μmol/L (1.58‐fold of control) (Figure 2A). In NCI‐H716 cells, the strongest stimulation was observed at 2.7 μmol/L methane (2.19‐fold of control). To determine a possible mechanism for increased GLP‐1 secretion, the level of intracellular cAMP was measured and shown to increase substantially under 2.7 and 27 μmol/L methane concentrations (1.65‐ and 2.33‐fold of control respectively, Figure 2C).
Figure 2.
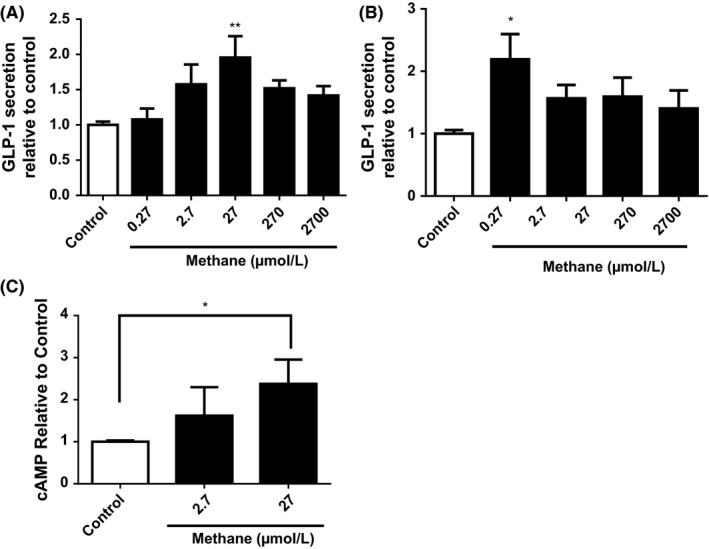
Methane directly stimulates GLP‐1 secretion in L cells. GLP‐1 media content (relative to vehicle control) from mouse GLUTag (A) and human NCI‐H716 (B) cells was examined after a 2‐hour methane treatment. Intracellular cAMP was examined in NCI‐H716 cells after 30‐min methane treatment (C). *= P < .05, **= P < .01
4. CONCLUSIONS
While changes in the microbiome during obesity and diabetes have been examined in several studies, the precise mechanism of how microbial products or metabolites can influence metabolic function remains largely unknown. As both methanogens and GLP‐1 secreting L cells are primarily localized in the distal small intestine and colon, we aimed to determine whether methane played a direct role in GLP‐1 regulation.
Obesity has previously been correlated with increased breath methane levels in humans11; therefore, we aimed to stimulate a similar scenario in mice using a high‐fat diet. Our diet intervention led to a significant increase in faecal methanogen abundance as assessed by mcrA gene copy numbers. Interestingly, these same animals had an enhanced glucose‐stimulated GLP‐1 secretion and a significant positive correlation between methanogen abundance and GLP‐1 secretion response. This enhanced GLP‐1 secretion in obesity may seem in contrast to the known reduction in GLP‐1 response found in obese and type 2 diabetic humans.12 However, studies examining early 6‐week diet‐induced weight gain in rats have observed a gradual increase in the GLP‐1 secretion response to nutrients.13 Furthermore, GLP‐1 levels in adolescent humans are higher in obese non diabetics vs diabetics.14 This suggests that that an elevated GLP‐1 secretion response in obesity may be protective against the development of T2DM. As diet‐induced changes in the gut microbiome are also known to occur rapidly (in the span of days to weeks),15 the timing for an interaction between methanogens or methane and GLP‐1 secretion is possible. It should be noted that our study examined faecal methanogen levels and not absolute faecal methane. Future work establishing an increase in methane gas in obesity (alongside increased GLP‐1 responses) will strengthen the connection between these 2 molecules.
To demonstrate a direct link between methane and GLP‐1 secretion, as opposed to indirect effects of methane precursors such as short‐chain fatty acids stimulating GLP‐1 secretion and methanogenesis concomitantly,6 we tested the effect of methane alone on 2 GLP‐1 secreting cell lines. Both human (NCI‐H716) and mouse (GLUTag) cells exhibited increased GLP‐1 secretion when treated with methane. The most effective doses were in the low μM range. To our knowledge, precise colonic methane levels in mice have not yet been determined, however in humans, mean breath methane levels are known to be in the range of 10 ppm16 (equivalent to low μmol/L), and methane in levels in flatus has been calculated upwards of 30 μmol/L.17 Interestingly, in our study, the most effective doses of methane on in vitro GLP‐1 secretion were within the physiological range (2.7‐27 μmol/L), while supraphysiological doses of methane did not impact GLP‐1 secretion. Future work on potential toxicity at these higher doses may shed light on this. Our finding of increased intracellular cAMP in human GLP‐1 secreting cells treated with methane is in line with the previous reports demonstrating a role for this second messenger in GLP‐1 secretion. There was some discrepancy in these experiments as the most effective methane dose for stimulating cAMP was 27 μmol/L while GLP‐1 secretion from the same cell line was highest at 2.7 μmol/L. This difference may be due to the timing of these 2 experiments (cAMP was a 30‐minute treatment while GLP‐1 secretion was 120 minutes). Nevertheless, an overall effect of methane on cAMP levels was detected in the 1 way ANOVA. Despite a connection between methane, cAMP and GLP‐1 release, the precise mechanism for methane's action remains to be determined. Previous work demonstrated methane can stimulate anti‐inflammatory pathways and redox balance in the cell (reviewed in18). As GLP‐1 secretion is impaired by the inflammatory cytokine TNFα,8 future studies should examine the potential restorative role of methane in this setting. Additional in vivo work with exogenous (methane‐saturated saline) or endogenous (probiotics/prebiotics) methane production will determine whether methane‐based therapies can be used in the GLP‐1‐based treatment of diabetes and obesity.
GLP‐1 has emerged as a key therapeutic in the treatment of both obesity (through appetite suppression) and T2DM (through reducing circulating glucose). As the prevalence of these diseases continues to rise, novel strategies that seek to increase the level of GLP‐1 levels are needed. Together, these initial experiments demonstrate a potential beneficial role for methane in the enhancement of GLP‐1 secretion.
CONFLICT OF INTEREST
As corresponding author, I declare that none of the authors of this manuscript have any potential conflict of interest.
AUTHOR CONTRIBUTION
Rose Laverdure carried out the GLUTag cell culture and faecal QPCR experiments and wrote the first draft of the manuscript. Ania Mezouari carried out the NCI‐H716 cell culture experiments. Michael Carson supported the faecal QPCR experiments, carried out methane concentration analysis and conducted revisions of the manuscript. Nathan Basiliko provided guidance with methanogen QPCR experiments and methane analysis and conducted revisions of the manuscript. Jeffrey Gagnon designed the study, completed thorough revisions of the manuscript and responses throughout the editorial process.
ACKNOWLEDGEMENT
The authors wish to acknowledge the Natural Sciences and Engineering Research Council of Canada for their support of this research through the discovery research program RGPIN‐2016‐05905.
Laverdure R, Mezouari A, Carson MA, Basiliko N, Gagnon J. A role for methanogens and methane in the regulation of GLP‐1. Endocrinol Diab Metab. 2018;1:e6 10.1002/edm2.6
REFERENCES
- 1. Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pimentel M, Gunsalus RP, Rao SS, Zhang H. Methanogens in human health and disease. Am J Gastroenterol Suppl. 2012;1:28‐33. [Google Scholar]
- 4. Mathur R, Chua KS, Mamelak M, et al. Metabolic effects of eradicating breath methane using antibiotics in prediabetic subjects with obesity. Obesity (Silver Spring). 2016;24:576‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dong CX, Brubaker PL. Ghrelin, the proglucagon‐derived peptides and peptide YY in nutrient homeostasis. Nat Rev Gastroenterol Hepatol. 2012;9:705‐715. [DOI] [PubMed] [Google Scholar]
- 6. Nava GM, Carbonero F, Croix JA, Greenberg E, Gaskins HR. Abundance and diversity of mucosa‐associated hydrogenotrophic microbes in the healthy human colon. ISME J. 2012;6:57‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tolhurst G, Heffron H, Lam YS, et al. Short‐chain fatty acids stimulate glucagon‐like peptide‐1 Secretion via the G‐protein–coupled receptor FFAR2. Diabetes. 2012;61:364‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gagnon J, Sauvé M, Zhao W, et al. Chronic exposure to TNFα impairs secretion of glucagon‐like peptide‐1. Endocrinology. 2015;156:3950‐3960. [DOI] [PubMed] [Google Scholar]
- 9. Narihiro T, Sekiguchi Y. Oligonucleotide primers, probes and molecular methods for the environmental monitoring of methanogenic archaea. Microb Biotechnol. 2011;4:585‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu J, Wang R, Ye Z, et al. Protective effects of methane‐rich saline on diabetic retinopathy via anti‐inflammation in a streptozotocin‐induced diabetic rat model. Biochem Biophys Res Comm. 2015;466:155‐161. [DOI] [PubMed] [Google Scholar]
- 11. Mathur R, Amichai M, Chua KS, Mirocha J, Barlow GM, Pimentel M. Methane and hydrogen positivity on breath test is associated with greater body mass index and body fat. J Clin Endocrinol Metab. 2013;98:E698‐E702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Færch K, Torekov SS, Vistisen D, et al. GLP‐1 response to oral glucose is reduced in prediabetes, screen‐detected type 2 diabetes, and obesity and influenced by sex: the ADDITION‐PRO Study. Diabetes. 2015;64:2513‐2525. [DOI] [PubMed] [Google Scholar]
- 13. Nakajima S, Hira T, Hara H. Postprandial glucagon‐like peptide‐1 secretion is increased during the progression of glucose intolerance and obesity in high‐fat/high‐sucrose diet‐fed rats. Br J Nutr. 2015;113:1477‐1488. [DOI] [PubMed] [Google Scholar]
- 14. Manell H, Staaf J, Manukyan L, et al. Altered plasma levels of glucagon, GLP‐1 and glicentin during OGTT in adolescents with obesity and type 2 diabetes. J Clin Endocrinol Metab. 2016;101:1181‐1189. [DOI] [PubMed] [Google Scholar]
- 15. Foley KP, Denou E, Duggan BM, et al. Long‐term dysbiosis promotes insulin resistance during obesity despite rapid diet‐induced changes in the gut microbiome of mice. BioRxiv. 10.1101/116095 [DOI] [Google Scholar]
- 16. Basseri RJ, Basseri B, Pimentel M, et al. Intestinal methane production in obese individuals is associated with a higher body mass index. Gastroenterol Hepatol. 2012;8:22‐28. [PMC free article] [PubMed] [Google Scholar]
- 17. McKay LF, Eastwood MA, Brydon WG. Methane excretion in man–a study of breath, flatus, and faeces. Gut. 1985;26:69‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu W, Wang D, Tao H, Sun X. Is methane a new therapeutic gas? Med Gas Res. 2012;2:25. [DOI] [PMC free article] [PubMed] [Google Scholar]


