Abstract
The natural history of schistosome infection in the mammalian host is determined by CD4+ T helper responses mounted against different parasite life cycle stages. A T helper 2 (TH2) response to schistosome eggs is required for host survival and establishment of chronic infection. However, a TH2 cell-derived cytokine also contributes to an immune milieu that is conducive to schistosome growth and development. Thus, the same responses that allow for host survival have been co-opted by schistosomes to facilitate parasite development and transmission, underscoring the significance of CD4+ T cell responses to both worms and eggs in the natural history of schistosome infection. Here we show that a cathepsin B1 cysteine protease secreted by schistosome worms not only induces TH2 responses, but also TH1 and TH17 responses, by a mechanism that is dependent on the proteolytic activity of the enzyme. Further investigation revealed that, in addition to the expected TH1 and TH2 responses, acute schistosome infection also induces a transient TH17 response that is rapidly down-regulated at the onset of oviposition. TH17 responses are implicated in the development of severe egg-induced pathology. The regulation of worm-induced TH17 responses during acute infection could therefore influence the expression of high and low pathology states as infection progresses.
Author summary
Schistosomiasis, a neglected tropical disease caused by parasites of the genus Schistosoma, is prevalent throughout the developing world, with more than 230 million people infected. Left untreated, schistosome infection may cause relatively mild disease with some morbidity, or, in a minority of cases, result in severe pathology and death. These variable outcomes are recapitulated in animal models, where the natural history of schistosome infection is profoundly influenced by the responses of host CD4+ T helper cells. Type 2 CD4+ T cell (TH2) responses, which allow for host survival by limiting pathology, have ironically also been co-opted by schistosomes to promote parasite development. On the other hand, TH17 responses have been implicated in the development of severe pathology, in both experimentally infected animals and naturally infected humans. Here we show that a schistosome proteolytic enzyme (SmCB1), produced in the parasite gut and released into the bloodstream, induces both TH2 and TH17 responses by a mechanism that requires the enzyme’s inherent proteolytic activity. Further investigation revealed that acute schistosome infection also induces a transient TH17 response that is rapidly down-regulated once parasite egg-laying commences. Regulation of TH17 responses during early infection may help determine whether mild or severe pathology develops as the infection progresses.
Introduction
Trematodes of the genus Schistosoma infect at least 230 million people worldwide, causing hepatointestinal and urogenital schistosomiasis [1]. Schistosomes are the most significant helminthic cause of human morbidity—in 2010, the Institute for Health Metrics and Evaluation’s Global Burden of Disease Study estimated that schistosome infections accounted for over 3.3 million disability-adjusted life years (DALYs) worldwide [2,3]. The relative ease by which schistosome infection is acquired likely contributes to the high global prevalence of schistosomiasis. Unlike other trematodes that infect humans, schistosomes have a truncated life cycle that omits the metacercaria stage, and the cercariae shed by the snail intermediate host can directly infect the definitive mammalian host. Furthermore, the infectious stage does not require ingestion in order to enter the host, instead penetrating the body directly through the skin by secreting proteases that breach the skin’s barrier defenses [4]. Once inside the host, larval schistosomes enter blood vessels, where growth and development into mature adult schistosomes occurs. The developing parasites also use the vasculature to migrate through the body [5], to the vessels that are the preferred final destination for each schistosome species—the mesenteric veins of the hepatic portal system in the case of most species, or the veins draining the urinary bladder in the case of S. haematobium, the etiologic agent of urogenital schistosomiasis. In these vessels adjacent to mucosal sites, adult schistosomes produce eggs that traverse the mucosal tissues and return to the environment, continuing the life cycle and disseminating the infection to new hosts.
The natural history of schistosome infection in the mammalian host is determined by host immune responses mounted against different parasite life cycle stages. A CD4+ T helper 2 (TH2) response to schistosome eggs deposited in host tissues is required for host survival and establishment of chronic infection, as this response mediates formation of protective granulomas around parasite eggs [6,7], while also limiting detrimental pro-inflammatory processes [8–10]. Furthermore, this TH2-mediated granulomatous reaction is also required for passage of the eggs through mucosal tissues and their release into the environment [11,12], a necessary event in life cycle propagation.
In mouse models of schistosome infection, CD4+ T cells also play an enigmatic role in the development of schistosomes during the pre-patent period of infection, i.e., prior to the onset of oviposition [13,14]. In the specific absence of CD4+ T cells, developing schistosomes exhibit reduced growth rates, delayed reproductive maturation, and impaired reproductive fitness [13,15]. Precisely how CD4+ T cells influence schistosome development is not clear, but our data suggest a role for CD4+ T cell-derived cytokines in contributing to an immune milieu that is conducive to schistosome development [16]. While important roles for other cytokines cannot be excluded, the principal TH2 cytokine interleukin (IL-) 4 can, by itself, restore parasite development and reproduction in the absence of CD4+ T cells by a mechanism that, again, involves down-regulating pro-inflammatory signals [17]. TH2 responses, therefore, play a significant dual role in the natural history of schistosome infection. Whereas TH2 responses allow host survival in the face of schistosome egg-induced inflammation, these same responses have been co-opted by schistosomes to promote parasite development and transmission to new hosts.
Schistosome eggs are the principal inducers of TH2 responses during schistosome infection [18,19]. However, the possible contribution of TH2 cells to facilitating parasite development during pre-patent infection [17] led us to investigate how these responses are induced prior to the onset of oviposition. We previously showed that infection with schistosome worms alone was sufficient to induce systemic, antigen-specific TH2 responses that were accompanied by production of antigen-specific immunoglobulin E (IgE) and sensitization of circulating basophils to produce additional IL-4 in response to schistosome worm antigens [20]. We also demonstrated that a schistosome cysteine protease, S. mansoni cathepsin B1 (SmCB1), which is secreted from the gut of the parasite, was a principal target of the IgE response [21], suggesting this worm-secreted antigen may contribute significantly to TH2 polarization of the nascent CD4+ T cell response during early infection.
Cysteine proteases possess TH2-polarizing properties and may be key TH2-inducing components of many helminths and allergens [22,23]. One hypothesis for the immunostimulatory properties of cysteine proteases stems from the observation that vertebrate hosts typically maintain cysteine proteases under tight control in intracellular compartments and do not release these enzymes into the extracellular space [24]. In contrast, many helminths utilize cysteine proteases in critical processes such as host invasion and nutrient acquisition and, as a consequence of these activities, release cysteine proteases in large quantities into the extracellular environment [25,26]. The vertebrate immune system may, therefore, have evolved mechanisms to detect extracellular protease activity and to interpret this as a danger signal associated with helminth infection [27]. The mechanism by which the host immune system senses and responds to foreign protease activity has not been fully elucidated. Protease-activated receptors (PARs) have been proposed to serve as receptors for extracellular protease activity [28–30]. PAR activation can stimulate cells to exocytose adenosine triphosphate (ATP) [31], which then acts as an endogenous danger signal by activating the P2Y2 purinergic receptor and stimulating release of IL-33, a TH2-promoting alarmin [32]. Alternatively, proteases may damage host cells directly, causing release of IL-33, thymic stromal lymphopoietin (TSLP) and IL-25, which together promote TH2 cell differentiation by stimulating type 2 cytokine release by innate lymphoid type 2 cells (ILC2s) (reviewed in [33]).
In addition to the potential role of SmCB1 in the natural history of schistosome infection, this protease is also of interest as a novel anti-schistosome vaccine candidate [34,35]. When administered to laboratory rodents in the absence of adjuvant, either alone or in combination with other antigens, active SmCB1 confers significant protection against challenge infection [36,37], which is dependent on SmCB1 proteolytic activity [37], underlining the innate adjuvanticity of active SmCB1. We therefore sought to test the contribution of SmCB1 activity to CD4+ T cell induction during natural infection and to test the immunostimulatory properties of active SmCB1 directly. Our results provide fresh insights into the immune responses induced by SmCB1 and by schistosome worms during pre-patent infection, and may have relevance for understanding the pathogenesis of severe pathology later in infection.
Materials and methods
Ethics statement
All animal procedures were performed according to the current edition of the National Research Council’s Guide for the Care and Use of Laboratory Animals (The National Academies Press, 2011) and pre-approved by the Institutional Animal Care and Use Committee at the Uniformed Services University of the Health Sciences, permit number A3448-01. Prior to sample collection, all mice were euthanized with an overdose of pentobarbital, or by carbon dioxide inhalation, followed by cervical dislocation or bilateral thoracotomy, in accordance with guidelines issued by the American Veterinary Medical Association’s Panel on Euthanasia.
Mice, parasite infection and antigen preparation
C.129-Il4tm1Lky/J (4get; BALB/c genetic background) breeding pairs were purchased from The Jackson Laboratory (Bar Harbor, ME) and bred at the Uniformed Services University Central Animal Facility to produce offspring for experiments. Wild type 6–8 week old BALB/cJ and C57BL/6J mice were purchased from The Jackson Laboratory. Age- and sex-matched mice (4–5 animals per group) were infected percutaneously by immersion of the tail for 30 min in water containing 120–160 S. mansoni cercariae (NMRI strain) shed from infected Biomphalaria glabrata (NMRI strain) snails. At 4, 6 or 8 weeks post infection, mice were euthanized and blood, spleen and lymph nodes harvested for analysis. In some experiments, worms were perfused from the portal circulation, fixed in 4% neutral buffered formaldehyde, counted, imaged and measured using ImageJ software (https://imagej.nih.gov/ij/). For preparation of soluble schistosome worm antigen (SWAP), adult S. mansoni were perfused from the portal veins of infected mice and homogenized in sterile phosphate buffered saline on ice. Insoluble material was removed by centrifugation at 16,100 x g for 30 min at 4 °C and the resulting supernatant stored at -80 °C until use, after filter sterilization through 0.2 μm syringe filters and determination of protein concentration by Bradford assay.
Protease inhibitor treatment
Beginning on the day of infection, the cysteine protease inhibitor K11777 (N-methylpiperazine-phenylalanyl-homophenylalanyl-vinylsulfone-phenyl, solubilized in sterile distilled water at a concentration of 10 mg/ml) was administered once daily (SID) by intraperitoneal (IP) injection in volumes of 20 μl/mouse, at a dose of approximately 10 mg/kg [38]. Control mice received equal volumes of vehicle (water) alone. Daily administration was continued until the mice were euthanized at 4 weeks post infection.
SmCB1 immunization
Active recombinant S. mansoni cathepsin B1 (SmCB1) was expressed in Pichia pastoris and purified by NTA-affinity chromatography as previously described [39,40]. Prior to immunization, batches of SmCB1 were divided in roughly equal portions of approximately 1 mg. One portion was inactivated by incubation with 10 μM E-64 on ice for 1 h. The inactivated portion and the remaining active portion were then desalted into sterile 0.1 M phosphate buffer, pH 5.5, using PD SpinTrap G-25 columns (GE Healthcare Life Sciences) and protein concentration checked by the BCA Protein Assay (Thermo Fisher Scientific). Endotoxin was not detectable in protease preparations using a Limulus amebocyte lysate assay (Lonza Inc). No protease activity was detectable in the E-64-inactivated enzyme using the fluorogenic peptidyl substrate, Z-Phe-Arg-7-amino-4-methylcoumarin (Z-FR-AMC, Sigma Aldrich Chemical Co.), as previously described [41]. Active and inactivated enzyme was administered to separate groups of mice on day 0, 14 and 21 by (i) subcutaneous (SC) injection into the footpad (10 μg/mouse), (ii) by IP injection (25 μg/mouse), or (iii) by intravenous (IV) injection into a lateral tail vein (25 μg/mouse). Twenty-four hours after the third injection, spleens and mesenteric, popliteal and inguinal lymph nodes were collected for analysis.
Quantification of antigen-specific IgE
Plasma concentrations of SWAP-specific IgE were determined as described previously [21]. Briefly, Immulon 4HBX plates (Thermo Fisher Scientific) were coated with SWAP in borate buffered saline (BBS) for 2 h at room temperature, then blocked with BBS containing 1% fetal bovine serum (FBS). Plasma samples were first adsorbed by incubating with GammaBind G Sepharose (GE Healthcare Life Sciences) overnight at 4°C, to deplete antigen-specific IgG that might out-compete the IgE for antigen binding, then applied to the assay plates in serial dilutions in BBS containing 0.02% Tween-20 (Sigma). After washes and incubation with alkaline phosphatase-conjugated goat anti-mouse IgE (BD Biosciences), the reaction was developed by addition of p-nitrophenyl phosphate disodium salt substrate (PNPP, Thermo Fisher Scientific), stopped with 2 N NaOH and the absorbance measured at 405 nm.
Flow cytometric analysis
Single cell suspensions of spleen and lymph node cells were first incubated with anti-murine Fcγ receptor II/III monoclonal antibody (mAb) 2.4G2 for 10 min and then stained with saturating concentrations of Alexa Fluor 488-conjugated, allophycocyanin-conjugated, PE-conjugated, FITC-conjugated, PerCPCy5.5-conjugated, Alexa Fluor 700-conjugated and Pacific Blue-conjugated mAbs to CD3, CD4, CD8, and CD62L, purchased from either BD Biosciences (San Jose, CA), BioLegend (San Diego, CA), eBioscience (San Diego, CA), or Invitrogen (Carlsbad, CA). Ex vivo intracellular staining for IFN-γ, IL-4, GATA-3, IL-17A, and RORγt was performed using antibodies and reagents purchased from BD Biosciences (San Jose, CA) or Biolegend (San Diego, CA) and staining performed according to the manufacturer’s instructions. A full list of antibodies used is provided in S1 Table. Following completion of the staining protocol, cells were analyzed immediately using a BD LSRII flow cytometer (BD Biosciences, San Jose, CA). Lymphocytes were gated by forward and side scatter, and fluorescence data were collected for a minimum of 10,000 gated cells. A representative gating strategy is outlined in S1 Fig
In vitro restimulation of T cells with antigen
Spleen and lymph node cells were washed, resuspended in medium, and plated at 10 × 106 cells (splenocytes) or 2 × 106 cells (lymph node cells) per well in 96-well plates. All cells were cultured in complete medium which consisted of RPMI-1640 supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 50 μM 2- mercaptoethanol, 100 U/mL penicillin and 100 μg/mL streptomycin. For antigen stimulation, SWAP was added to a final concentration of 50 μg/ml. Cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2 and were monitored daily. Cells and supernatant were harvested for analysis after 72 hours.
IFN-γ and IL-17A ELISA
Cytokine concentrations in cell culture supernatants were measured by sandwich enzyme linked immunoassay (ELISA), using ELISA kits from Invitrogen. Assays were performed according the manufacturer’s protocols. Briefly, Costar 9018 ELISA plates were coated with capture antibody overnight at 37°C and then blocked with ELISA/ELISASPOT Diluent. Supernatant (100 μl) was added to the blocked wells and incubated for 2 h at room temperature. After washing, 100 μl/well of detection antibody was applied for 1 h at room temperature. Avidin-horseradish peroxidase (HRP) was applied for 30 min after extensive washing. Finally, 3,3',5,5'-Tetramethylbenzidine (TMB) solution was added for 30 min after washing, the reaction was stopped by Stop Solution (Invitrogen) and absorbance was measured with a Spectramax M2 plate reader (Molecular Devices).
Cytokine expression by real time PCR
Splenocytes (10 x 106) and cells from mesenteric lymph nodes (5 x 106) were homogenized in 1 ml of RNA-STAT-60 (Tel-Test, Friendswood, TX). cDNA was synthesized from mRNA using the TaqMan Reverse Transcription Reagents kit (Applied Biosystems, Foster City, CA). Real-time PCR was performed using TaqMan Gene Expression Assays and TaqMan Universal PCR Master Mix (Applied Biosystems) for the following targets: IFN-γ, IL-17A and IL-4, with 18s rRNA as an internal control. The calculation of relative gene expression differences was done by the comparative 2-ΔΔCT method [42]. The result was expressed as the -fold change in the experimental groups compared to equivalent cells from non-infected control mice.
Statistical analysis
Statistical analyses were performed using Prism 7.0 (GraphPad Software, San Diego, CA). F tests were used to compare variances between two data groups, and Brown-Forsythe and Bartlett’s tests were used to compare standard deviations in experiments with three data groups. Where parametric tests were appropriate, the means of two data groups were compared using unpaired t tests, and the means of three groups were compared by one-way ANOVA followed by Tukey’s multiple comparisons test. Where non-parametric tests were required, the means of two data groups were compared using the Mann Whitney test, and the means of three groups were compared using the Kruskal-Wallis test followed by Dunn’s multiple comparisons test. P values < 0.05 were considered significant. Experimental groups comprised 4–5 mice and all experiments were performed at least twice.
Results
In vivo cysteine protease inhibition impairs CD4+ T cell responses induced by acute schistosome infection
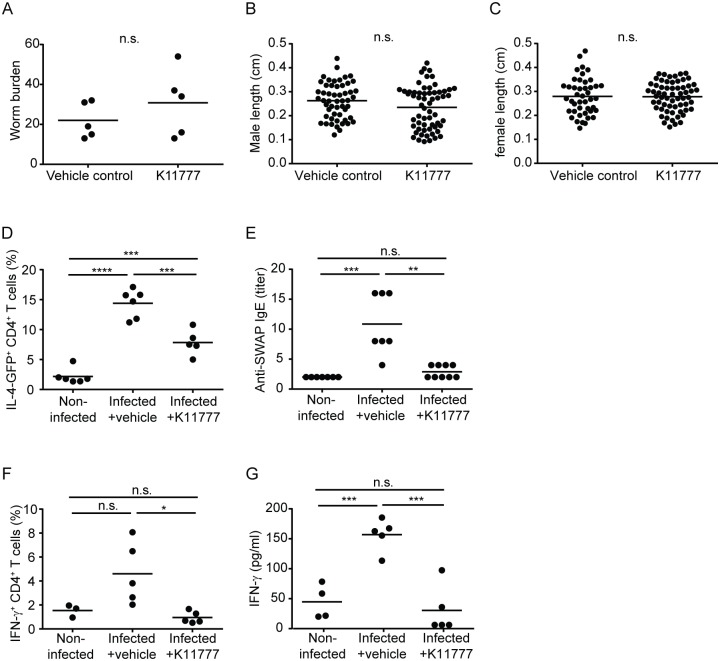
To test whether the catalytic activity of cysteine proteases was necessary to the induction of TH2 responses during pre-patent schistosome infection, we treated infected IL-4-eGFP reporter (4get) mice with the cysteine protease inhibitor, K11777, beginning on the day of infection and continuing daily for four weeks. Previous studies showed that this vinyl sulfone is well tolerated by schistosome-infected mice and decreases the amount of active SmCB1 detectable in those parasites recovered post-treatment [38]. At higher doses (25–50 mg/kg twice daily [BID]), K11777 reduced worm and egg burdens in infected mice [38]. For the purpose of these experiments, we used a lower dose (10 mg/kg SID) that did not interfere with schistosome establishment or growth in the mammalian host. In our studies, K11777-treated mice harbored similar worm burdens to vehicle control animals (Fig 1A) and the male and female worms from K11777-treated mice were the same size as those from control animals (Fig 1B and 1C). Consistent with a role for SmCB1 activity in driving TH2 responses in mice, K11777 treatment reduced the frequency of eGFP+ CD4+ T cells in the spleens of infected mice at 4 weeks post infection (Fig 1D), and reduced the titers of worm-specific IgE in the plasma of infected mice (Fig 1E). However, the inhibitory effect of K11777 was not specific to TH2 responses. Inhibitor-treated mice also exhibited reduced frequencies of splenic IFN-γ+ CD4+ T cells (Fig 1F), and their splenocytes produced less IFN-γ when cultured with parasite antigen in vitro (Fig 1G). These findings suggested that SmCB1 is involved in the induction of other TH phenotypes, in addition to TH2. However, we could not rule out the possibility that K11777 was modulating immune responses via off-target effects, e.g. by inhibiting other parasite proteases, by interfering with parasite development (and the subsequent immune response) in aspects that were not apparent from our analysis of parasite morphology, or by interfering with host proteases. To address these issues, we next examined the immunological role of SmCB1 proteolytic activity by comparing immune responses to active and inactive recombinant SmCB1.
Fig 1. Treatment of mice with the cysteine protease inhibitor, K11777, inhibits T helper responses during pre-patent schistosome infection.
S. mansoni-infected 4get IL-4 reporter mice were treated with cysteine protease inhibitor (K11777) or vehicle alone throughout the first 4 weeks of infection. A, parasite burden, B length of male worms, and C, length of female worms in animals treated with K11777 or vehicle alone, at 4 weeks post infection. D, frequency of IL-4-GFP+ CD4+ T cells in spleens of non-infected control mice, and vehicle-treated and K11777-treated infected mice at 4 weeks post infection. E, Plasma titers of anti-SWAP IgE in non-infected control mice, and vehicle-treated and K11777-treated infected mice at 4 weeks post infection. F, frequency of IFN-γ+ CD4+ T cells in spleens of non-infected control mice, and vehicle-treated and K11777-treated infected mice at 4 weeks post infection. G, SWAP-stimulated IFN-γ secretion by splenocytes from non-infected control mice, and vehicle-treated and K11777-treated infected mice at 4 weeks post infection. n.s., P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < <0.0001.
Induction of TH2 cells by SmCB1 requires its endogenous proteolytic activity
To examine whether the proteolytic activity inherent to SmCB1 was necessary to induce TH2 cells, IL-4-eGFP reporter mice were injected in the hind footpad with either active or E-64-inactivated recombinant SmCB1, and the accumulation of eGFP+ cells in the draining popliteal and inguinal lymph nodes was compared. Immunization with active SmCB1 resulted in modest but significant increases in the frequency (Fig 2A) and absolute number (Fig 2B) of TH2 cells in the draining nodes. In contrast, immunization with inactivated SmCB1 had no effect, comparable to mice injected with PBS alone (Fig 2A and 2B). However, the low yield of cells from these nodes precluded the analysis of other TH phenotypes that might be present, preventing us from testing whether SmCB1 is involved in the induction of other TH phenotypes, in addition to TH2, as suggested by the results obtained with in vivo administration of K11777 (Fig 1). We therefore sought to immunize mice by other routes to allow for the collection of larger numbers of responding cells, and to use conventional intracellular cytokine and transcription factor staining, so that multiple TH phenotypes could be examined simultaneously.
Fig 2. SmCB1 proteolytic activity induces IL-4 expression in CD4+ T cells.
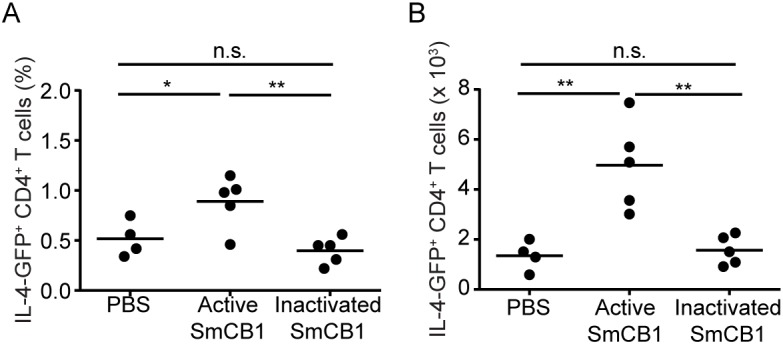
4get IL-4 reporter mice were immunized subcutaneously in the footpad with either PBS, active SmCB1, or inactivated SmCB1 on day 0, 14 and 21. On day 22, the draining popliteal and inguinal nodes were removed and the frequency (A) and total number (B) of IL-4-GFP+ CD4+ T cells were determined by flow cytometry. n.s., P > 0.05; *, P < 0.05; **, P < 0.01.
Active SmCB1 induces TH1, TH2 and TH17 cells
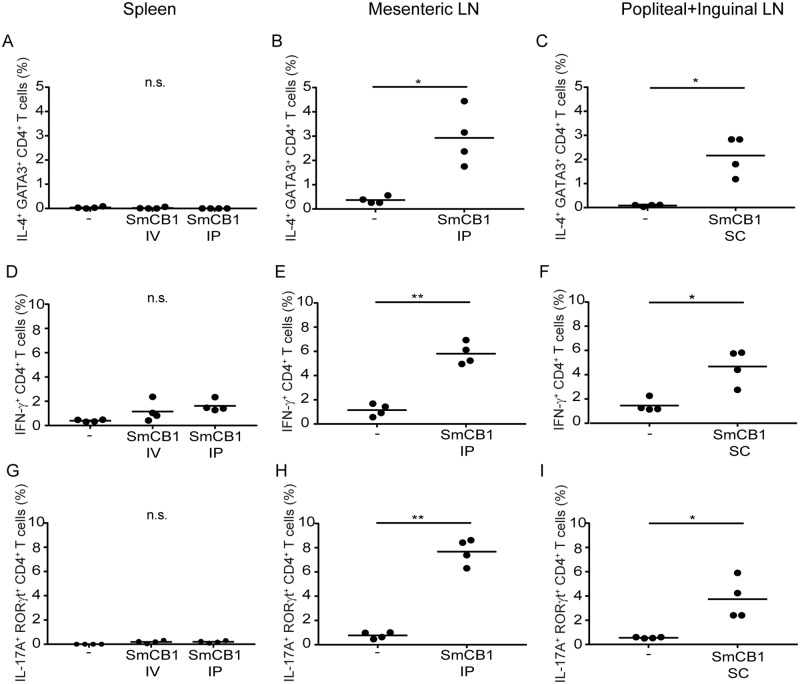
So that our data would be directly comparable to our previous studies on immune responses to SmCB1 [21], and to test whether SmCB1-induced responses were influenced by the genetic background of the mice used, further immunization experiments were conducted with wild type C57BL/6J mice (the 4get mice utilized in Figs 1 and 2 have a BALB/c genetic background). To evaluate the effect of active SmCB1 when administered by different routes in these mice, SmCB1 was administered to separate groups of C57BL/6J mice on day 0, 14 and 21 by (i) IV injection into a lateral tail vein (25 μg/mouse), or (ii) by IP injection (25 μg/mouse), or (iii) SC injection into the footpad (10 μg/mouse; identical to the route of administration employed in Fig 2). On day 22 (the day after the third injection), the mice were euthanized and flow cytometry was used to compare the TH cell frequencies in the draining popliteal and inguinal nodes of the SC group with the frequencies in the spleens of the IV and IP groups and the mesenteric lymph nodes of the IP group. TH2 cells were defined as IL-4+ GATA3+ CD4+ T cells, TH1 cells were defined as IFN-γ+ CD4+ T cells, and TH17 cells were defined as IL-17A+ RORγt+ CD4+ T cells. Representative fluorescence histograms are provided in S2 Fig Neither IV nor IP administration of active SmCB1 induced any increase in the frequency of TH2 cells in the spleen (Fig 3A). In contrast, IP administration of active SmCB1 induced a significant increase in TH2 cell frequency in the mesenteric lymph nodes (Fig 3B), comparable to that seen in the draining popliteal and inguinal lymph nodes of mice immunized by the SC route (Fig 3C). Likewise, IV and IP administration of SmCB1 did not induce a significant increase in TH1 cells in the spleen (Fig 3D), but did increase the frequency of TH1 cells in the mesenteric (Fig 3E) and popliteal/inguinal nodes (Fig 3F) when administered by the IP and SC routes, respectively. Lastly, active SmCB1 also induced TH17 cells—no induction was apparent in the spleen after IV and IP administration (Fig 3G), but IP and SC administration induced significant increases in TH17 cell frequency in the mesenteric (Fig 3H) and popliteal/inguinal nodes (Fig 3I), respectively. Together these findings suggest that active SmCB1 induces a pleiotropic CD4+ T cell response, consisting of TH1, TH2 and TH17 components, by multiple routes of administration (IP and SC).
Fig 3. Active SmCB1 induces TH2, TH1 and TH17 cells when administered by intraperitoneal and subcutaneous routes.
On day 22, intracellular cytokine/transcription factor staining and flow cytometry were used to determine the frequencies of IL4+ GATA3+ CD4+ T cells (A-C), IFN-γ+ CD4+ T cells (D-F), and IL-17A+ RORγt+ CD4+ T cells (G-I), in the spleens (A, D, G), mesenteric nodes (B, E, H), and popliteal and inguinal nodes (C, F, I) of wild type C57BL/6 mice that received injections of active SmCB1 on days 0, 14 and 21, by intravenous (IV; A, D, G), intraperitoneal (IP; A-B, D-E, G-H) and subcutaneous (SC; C, F, I) routes. Frequencies were compared to those in the comparable tissues of animals that received no antigen. n.s., P > 0.05; *, P < 0.05; **, P < 0.01.
SmCB1 proteolytic activity is required for the induction of TH1, TH2 and TH17 cells
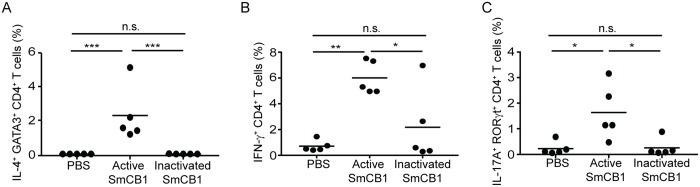
Because IP administration of active SmCB1 induced robust CD4+ T cell responses in the mesenteric nodes, which yield significantly more cells than the popliteal and inguinal nodes, we used this route of administration to test whether the catalytic activity of SmCB1 was required for TH cell induction. Either active or E-64-inactivated SmCB1 was administered to separate groups of C57BL/6J mice on day 0, 14 and 21 by IP injection (25 μg/mouse), and the TH cell frequencies in the mesenteric nodes the day after the last injection were compared with those in mice that received PBS alone. Catalytic inactivation of SmCB1 significantly reduced the induction of TH2 (Fig 4A), TH1 (Fig 4B) and TH17 cells (Fig 4C) compared to active SmCB1, and in the case of TH2 and TH17 cells, inactivated SmCB1 appeared to have no stimulatory capacity at all (Fig 4A and 4C). These data suggest that the ability of SmCB1 to induced TH1, TH2 and TH17 cells is dependent on the enzyme’s proteolytic activity.
Fig 4. The capacity of SmCB1 to induce TH2, TH1 and TH17 cells is dependent on its proteolytic activity.
On day 22, intracellular cytokine/transcription factor staining and flow cytometry were used to determine the frequencies of IL4+ GATA3+ CD4+ T cells (A), IFN-γ+ CD4+ T cells (B), and IL-17A+ RORγt+ CD4+ T cells (C), in the mesenteric lymph nodes of wild type C57BL/6 mice that received injections of PBS, active SmCB1, or inactivated SmCB1, on days 0, 14 and 21 by the intraperitoneal route. n.s., P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Acute schistosome infection induces a transient TH17 response
The finding that active SmCB1 induced a robust TH17 response led us to hypothesize that acute schistosome infection may also induce a hitherto unappreciated TH17 response. To test this hypothesis, we infected groups of wild type C57BL/6J mice with S. mansoni by percutaneous exposure to cercariae, and then evaluated the frequency of TH1, TH2 and TH17 cells in the spleens and mesenteric lymph nodes at 4, 6 and 8 weeks post infection. Consistent with our previous findings [20], the frequency of IL-4+ CD4+ T cells was significantly increased at all time points in the spleen (S3 Fig, part A) and mesenteric nodes (S3 Fig, part B), with the frequency increasing as the infection progressed. In the spleen, elevated frequencies of GATA3+ CD4+ T cells were detected at 6 and 8 weeks post infection (S3 Fig, part C), and at all three time points in the mesenteric nodes (S3 Fig, part D), with frequencies again increasing as the infection progressed. Using the most stringent definition of both IL-4 and GATA3 positivity, increased frequencies of TH2 cells were detected in the spleen at week 8 (S3 Fig, part E) and at all three time points in the mesenteric nodes (S3 Fig, part F).
For TH1 cells, elevated frequencies were detected at 6 and 8 weeks post infection in both spleen (S3 Fig, part G) and mesenteric nodes (S3 Fig, part H), with frequencies reduced at week 8 compared to week 6, consistent with the down-modulation of the TH1 response that occurs at the onset of oviposition [18].
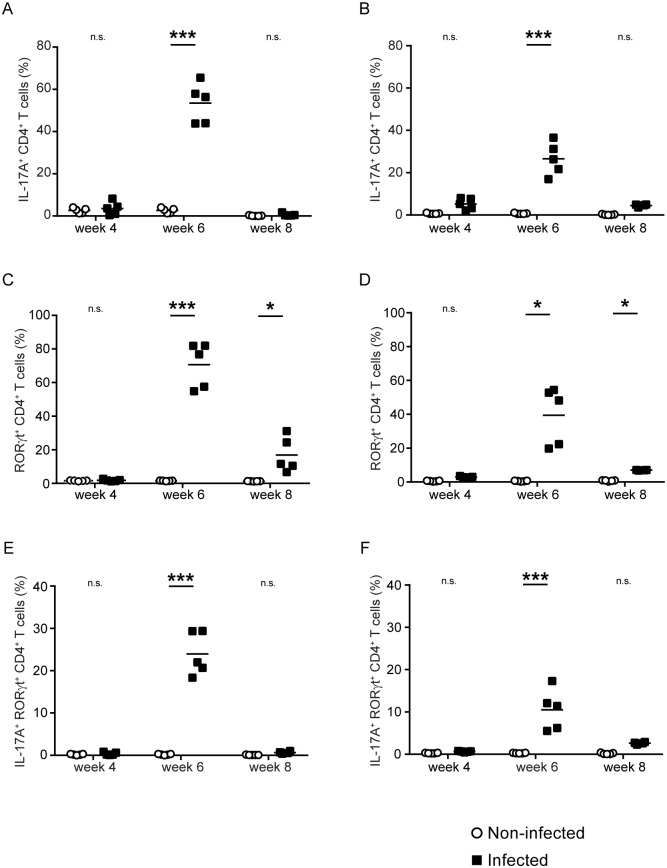
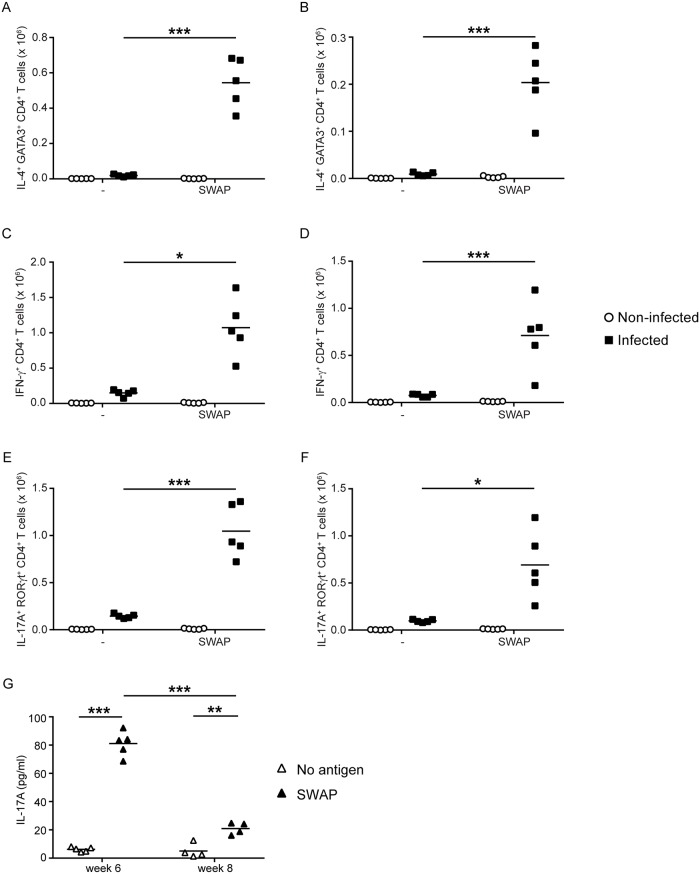
For TH17 cells, significantly elevated frequencies of IL-17A+ CD4+ T cells were detected in the spleen (Fig 5A) and mesenteric nodes (Fig 5B) at week 6, but not at week 4 or 8. Significantly elevated frequencies of RORγt+ CD4+ T cells were detected in spleen (Fig 5C) and mesenteric nodes (Fig 5D) at 6 and 8 weeks post infection, with frequencies at 8 weeks substantially decreased relative to week 6. Using the most stringent definition of both IL-17A and RORγt positivity, frequencies of TH17 cells were elevated in spleen (Fig 5E) and mesenteric nodes (Fig 5F) at week 6 only. These data suggest that acute schistosome infection induces a transient TH17 response that, like the TH1 response, is down-regulated at the onset of oviposition.
Fig 5. Acute schistosome infection induces a transient TH17 response in the spleen and mesenteric lymph nodes.
Using intracellular cytokine/transcription factor staining and flow cytometry, the frequency of IL-17A+ CD4+ T cells (A, B), RORγt+ CD4+ T cells (C-D), and double-positive IL-17A+ RORγt+ CD4+ T cells (E, F) in the spleens (A, C, E) and mesenteric lymph nodes (B, D, F) of S. mansoni-infected C57BL/6 mice, was determined at 4, 6 and 8 weeks post infection. Non-infected mice were included at each time point, as negative controls. n.s., P > 0.05; *, P < 0.05; ***, P < 0.001.
TH17 cells induced by schistosome infection are responsive to schistosome antigens
To test whether the TH17 cells induced by schistosome infection were specific for and responsive to schistosome antigens, spleen and mesenteric lymph node cells from non-infected mice and mice infected for 6 weeks with S. mansoni were cultured in vitro with SWAP for 3 days. At the end of the culture period, the numbers of TH1, TH2 and TH17 cells in the cultures were compared to cultures where the antigen was omitted. The numbers of TH2 cells expanded significantly in response to SWAP in both spleen (Fig 6A) and mesenteric node (Fig 6B). Likewise, TH1 cells expanded significantly in response to SWAP stimulation, in both spleen (Fig 6C) and mesenteric node (Fig 6D). Finally, TH17 cells from the spleen (Fig 6E) and the mesenteric nodes (Fig 6F) also expanded in numbers in response to SWAP. These data suggest that, like the TH1 and TH2 cells induced during schistosome infection, the TH17 cells induced by acute schistosome infection are specific for schistosome worm antigens.
Fig 6. TH2, TH1 and TH17 cells induced by acute schistosome infection proliferate and produce cytokine in response to schistosome worm antigens.
At 6 weeks post infection, cells from the spleens (A, C, E) and mesenteric lymph nodes (B, D, F) of S. mansoni-infected mice were cultured in vitro in the absence (-) or presence of S. mansoni worm antigens (SWAP). Spleen and mesenteric lymph node cells from non-infected mice were included as controls and cultured under identical conditions. After three days in culture, intracellular cytokine/transcription factor staining and flow cytometry were used to determine the number of IL4+ GATA3+ CD4+ T cells (A, B), IFN-γ+ CD4+ T cells (C, D), and IL-17A+ RORγt+ CD4+ T cells (E, F) in the cultures. G, spleen cells from mice that were infected with S. mansoni for 6 and 8 weeks were cultured in vitro either in the presence or absence of S. mansoni worm antigens (SWAP). After three days of culture, the concentration of IL-17A in the culture supernatant was determined by ELISA. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Lastly, splenocytes from mice that were infected with S. mansoni for 6 and 8 weeks were cultured in vitro for 3 days either with or without SWAP, and the accumulation of IL-17A in the supernatant was measured by ELISA. We found that SWAP stimulated significant secretion of IL-17A by splenocytes at 6 and 8 weeks post infection (Fig 6G), with the 6 week splenocytes producing more IL-17A than the 8 week cells. These data further support the conclusion that the TH17 cells induced by acute schistosome infection are specific for schistosome worm antigens and that this response is subject to down-modulation by 8 weeks post infection.
Cytokine transcript abundance mirrors the frequency of TH phenotypes during acute schistosome infection
To corroborate our flow cytometry data on the relative abundance of TH1, TH2 and TH17 cells using another independent methodology, we estimated the relative abundance of IL-4, IFN-γ and IL-17A transcripts in RNA extracted from splenocytes at 6 and 8 weeks post infection. IL-4 transcript was most abundant at week 8 (Fig 7A), while IFN-γ (Fig 7B) and IL-17A transcripts (Fig 7C) were most abundant at week 6, consistent with the conclusion that the TH1 and TH17 responses induced by acute schistosome infection are down-modulated after the onset of oviposition.
Fig 7. The abundance of IL-4, IFN-γ and IL-17A mRNA mirrors the relative frequency of TH2, TH1 and TH17 cells at 6 and 8 weeks post infection with S. mansoni.
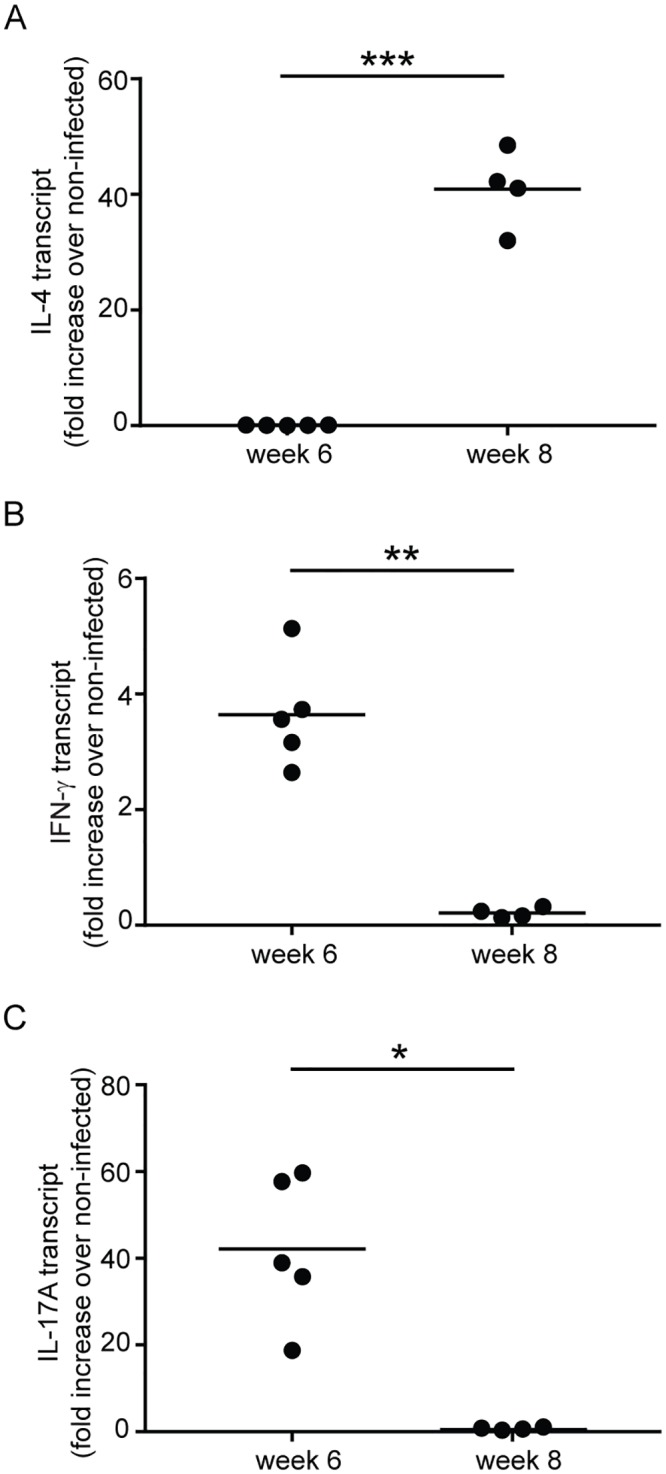
The relative abundance of IL-4 (A), IFN-γ (B) and IL-17A (C) mRNA in spleen cells from S. mansoni-infected mice at 6 and 8 weeks post infection was determined by quantitative real-time PCR. Results are expressed as fold change in the experimental groups compared to equivalent cells from non-infected control mice. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Discussion
Because proteolytic activity is considered to be essential to the TH2-promoting capacity of proteases [30], we attempted to test the contribution of SmCB1 activity to the induction of TH2 responses during the first four weeks of schistosome infection. Previously, in vivo administration of the cysteine protease inhibitor, K11777, to schistosome-infected mice was found to be highly effective at ablating SmCB1 activity [38]. We therefore investigated pre-patent CD4+ T cell responses in infected mice under K11777 treatment. Our finding that both TH2 and TH1 responses were significantly reduced by K11777 treatment could be interpreted as evidence that SmCB1 activity contributes broadly to initiation of CD4+ T cell responses during schistosome infection and not specifically to TH2 cell induction. However, this interpretation is confounded by the potential off-target effects that K11777 treatment may have. For example, we cannot rule out the possibility that other schistosome proteases with potential immunological roles, such as S. mansoni cathepsins L and E [34], were not also inhibited. Also, host lysosomal cysteine proteases are important in the presentation of antigens by antigen-presenting cells (APCs) to CD4+ T cells via the major histocompatibility complex (MHC) class II pathway, being required both for antigenic peptide processing and for degradation of the invariant chain [43,44]. Interference by K11777 with APC protease activity could therefore have potentially broad effects on CD4+ T cell responses. Finally, despite specifically selecting doses of K11777 that do not interfere with parasite establishment or alter gross parasite morphology, we cannot rule out the possibility that K11777 had some effect on schistosome development or antigen expression and thus altered parasite immunogenicity. Given these caveats, we focused instead on testing the immunological significance of SmCB1 activity by comparing the immunogenicity of active and chemically inactivated recombinant SmCB1.
The native proteolytic activity of SmCB1 was previously shown to induce protection against challenge infection with cercariae, as irreversible inhibition of SmCB1 significantly diminished, though did not ablate, the protection conveyed by SmCB1 immunization [37]. Consistent with these results, we found that SmCB1 had robust TH2-inducing capacity that was dependent on the enzyme’s protease activity. TH2 cell induction by SmCB1 was independent of the detection method employed (IL-4 reporter mice, intracellular cytokine and transcription factor staining), the genetic background of the mice (BALB/c, C57BL/6), and the route of SmCB1 administration (subcutaneous, intraperitoneal). Interestingly, no cytokine responses were detected to SmCB1 administered intravenously. We hypothesize that, when administered by this route, perhaps SmCB1 is rapidly inactivated by circulating protease inhibitors such as α-2-macroglobulin [45], before any immunological effects can be exerted.
The ability to elicit CD4+ T cell responses in the mesenteric lymph nodes by intraperitoneal administration of SmCB1 afforded the opportunity to use intracellular cytokine and transcription factor staining and flow cytometry to detect other effector phenotypes among the responding CD4+ T cells. Unexpectedly, we found that active SmCB1, but not inactivated SmCB1, also elicited robust TH1 and TH17 responses, in addition to TH2 cells. While we were not able to detect traces of endotoxin in our SmCB1 preparations, we considered the possibility that the immunostimulatory activity was due to contaminating pattern recognition receptor (PPR) ligands of microbial origin. However, if this were the case, we would not expect the immunostimulatory capacity of SmCB1 to have been sensitive to catalytic inactivation. Furthermore, previous studies showed that, in mice immunized with SmCB1 and subsequently infected with cercariae, SmCB1 elicited IFN-γ and IL-17 production from cells in the lymph node draining the site of infection 4 days after challenge [36], suggesting that the protease may have broad CD4+ T cell-priming activity that extends beyond TH2 cell induction. How SmCB1 proteolytic activity simultaneously induces TH1, TH2 and TH17 responses is an open question, but perhaps the most parsimonious explanation is that, in this instance, each of the three effector phenotypes shares a common initiator mechanism. In support of this argument, we note that PARs have been implicated in the induction of TH1 [46] and TH17 [47] responses, as well as TH2 responses [28–30]. Alternatively, SmCB1-mediated proteolytic damage to cells and the extracellular matrix may mobilize a broad repertoire of alarmins and endogenous damage-associated molecular patterns (DAMPs) [48]. In addition to the IL-33, IL-25 and TSLP that promote TH2 cell differentiation by activating ILC2s [33], other DAMPs may activate dendritic cells and other APCs to produce the IL-12 required for TH1 induction [49], and the IL-1β, IL-6 and IL-23 that promote TH17 differentiation [50,51].
TH1 responses induced by schistosome worm antigens are an acknowledged feature of pre-patent schistosome infection, detectable within the first four weeks of infection and subsequently down-modulated at the onset of parasite egg-laying [10,18,20]. In contrast, TH17 responses have been primarily detected later in infection, driven by egg antigens, and in mouse strains prone to severe egg-induced pathology (reviewed in [52]). Our finding that active SmCB1, a worm antigen, stimulated a TH17 response prompted us to investigate whether TH17 responses are induced during acute schistosome infection. Our analysis of TH1, TH2 and TH17 cell frequency from 4 to 8 weeks post infection, in a strain not considered to be prone to pathogenic TH17 responses (C57BL/6) [52], revealed a transient TH17 response that peaked at 6 weeks post infection and was subsequently down-modulated. The timing of this induction and down-regulation resembles that of the pre-patent TH1 response, which is down-regulated by egg-induced TH2 responses [6,53], suggesting that the TH17 response we observed may be regulated by similar mechanisms. Failure to induce a TH2 response results in a persistent TH1 response and excessive inflammation and pathology [6,54]. If the TH17 response we observed is controlled by similar regulatory mechanisms, a persistent TH17 response could also contribute to the pathology and morbidity that accompanies a failure of the TH2 response [6]. TH2 responses have been shown to limit IL-17-mediated pathology in other models of helminth infection [55]. There is also a precedence from other models of TH2-mediated inflammation, e.g. allergic airway disease [56], for TH17-mediated pathology to emerge when TH2 responses are impaired.
Among inbred mouse strains, certain strains (e.g. CBA, C3H) are considered “high pathology” strains with regard to schistosome infection, as these animals develop more severe pathology upon infection, characterized by large, poorly circumscribed granulomas around parasite eggs that are driven by TH17 responses [57–59]. Imbalances in IL-1β and IL-23 production by dendritic cells in response to schistosome egg antigens were found to underlie the preferential development of TH17 responses in CBA mice [57,58] suggesting that fundamental differences in the dendritic cell response to egg antigens distinguishes high pathology strains from other mouse strains. One question arising from these findings is whether high-pathology strains also differ in their response to worm antigens during acute infection, and whether persistent TH17 responses to worm antigens contribute to pathology in these strains. Since there is evidence that immune responses to worm antigens during pre-patent infection can prime subsequent responses to eggs after the onset of oviposition, presumably due to the expression of common antigens and antigenic cross-reactivity between life cycle stages [60], persistent TH17 responses may set the stage for a subsequent pathogenic TH17 response to schistosome eggs.
Gene expression analyses that compared dendritic cells from high-pathology CBA mice and low-pathology C57BL/6 mice revealed significant differences in the expression of C-type lectin receptors (CLRs) between the two strains, with CBA dendritic cells expressing 18-fold higher levels of CD209a (SIGNR5) compared to C57BL/6 dendritic cells [61]. Furthermore, ablation of CD209a expression in CBA dendritic cells, and overexpression of CD209a in C57BL/6 dendritic cells, revealed that CD209a was required for the expression of IL-1β and IL-23 by egg-stimulated dendritic cells and for the subsequent development of TH17 cells [61]. Recently, it was shown that signaling through three CLRs, CD209a, Dectin-2 and Mincle, together augment the production of IL-1β and IL-23 by egg-stimulated dendritic cells, and is required for TH17 development and immunopathology [62]. These findings firmly implicate carbohydrate antigens, rather than proteases, in the induction of TH17 responses to schistosome eggs, and suggest that multiple different schistosome antigens, from different life cycle stages, might contribute to TH17 induction throughout infection. Indeed, although SmCB1 may contribute to TH17 induction, there may well be other worm antigens also driving TH17 polarization during pre-patent infection. We note that secretory-excretory products of schistosome worms, which contains SmCB1 but also many other antigens, also induce TH17 responses when used to immunize mice [63], suggesting that other worm antigens may contribute to this response.
We considered the possibility that the sharp increase in frequency of TH17 cells at week 6 post infection, in spleen and mesenteric lymph node, was due to bacterial translocation from the intestine and exposure to microbial PRR ligands [64]. However, the timing of the TH17 response suggests it is induced before the onset of parasite egg-laying and before significant egg-induced damage of the intestinal mucosa would occur. Furthermore, the TH17 cells present at 6 weeks post infection are clearly specific for and responsive to worm antigens, suggesting they are induced by the schistosome infection and not by microbial antigens.
In summary, our data suggest that a secreted parasite cathepsin is a broadly stimulatory immunogen with capacity to initiate TH17 as well as TH1 and TH2 responses. This led to the finding that acute schistosome infection in a low-pathology mouse strain is associated with a transient TH17 response to worm antigens that is rapidly down-regulated at the onset of egg production. Understanding the development and regulation of TH17 responses in models of schistosome infection will be relevant to elucidating the mechanisms that underlie the development of pathology in human schistosomiasis, which remain poorly understood. One recent study revealed a positive correlation between the frequency of circulating TH17 cells and the occurrence of bladder pathology in S. haematobium-infected children [65]. Furthermore, significantly elevated levels of IL-17 are detectable in the plasma of symptomatic acute schistosomiasis patients (T.A. Pereira, personal communication). Associations between pathology and TH17 responses have also been noted in other human helminth infections [66] and animal models of helminthic disease [67,68]. Our findings provide new insights into the role of TH17 responses in the natural history of schistosomiasis and suggest these potentially pathogenic responses warrant further investigation, both in animal models and naturally infected human subjects.
Supporting information
Top row, left to right: a FSC-A vs. FSC-H plot is used to gate on singlet events (left panel); a FSC-A vs. SSC-A plot is used to gate on lymphocytes (center panel); a CD19 histogram is used to exclude CD19+ cells (right panel). Bottom row, left to right: a CD4 vs. CD3 plot is used to gate on double-positive CD3+ CD4+ cells in the upper right (Q2) quadrant (left panel); a TCRβ vs. CD4 plot is used to gate on double-positive TCRβ+ CD4+ cells in the upper right (Q2) quadrant (middle panel); a GFP vs. TCRβ plot is used to identify IL-4-GFP+ cells in the upper right (Q2) quadrant (right panel).
(EPS)
On day 22, intracellular cytokine/transcription factor staining and flow cytometry were used to determine the frequencies of IL4+ GATA3+ CD4+ T cells (A), IFN-γ+ CD4+ T cells (B), and IL-17A+ RORγt+ CD4+ T cells (C), in the spleens, mesenteric lymph nodes, and popliteal and inguinal lymph nodes of wild type C57BL/6 mice that had received injections of active SmCB1 on days 0, 14 and 21, by intravenous (IV), intraperitoneal (IP) and subcutaneous (SC) routes. Cells from comparable tissues of animals that received no antigen (-) were included as negative controls.
(TIF)
The frequency of IL-4+ CD4+ T cells (A, B), GATA3+ CD4+ T cells (C, D), double-positive IL-4+ GATA3+ CD4+ T cells (E, F), and IFN-γ+ CD4+ T cells (G, H), in the spleens (A, C, E, G) and mesenteric lymph nodes (B, D, F, H), of S. mansoni-infected C57BL/6 mice at 4, 6 and 8 weeks post infection, and in non-infected mice, were compared using intracellular cytokine/transcription factor staining and flow cytometry. n.s., P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
(EPS)
Information regarding the source and format of all antibodies used in the flow cytometric analysis of immune cells is provided.
(XLSX)
Acknowledgments
Infected snails were provided by Biomedical Research Institute (BRI) via the National Institute of Allergy and Infectious Diseases (NIAID) Schistosomiasis Resource Center under National Institutes of Health (NIH)-NIAID Contract No. HHSN272201000005I.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funded by National Institutes of Health/National Institute of Allergy and Infectious Diseases grant AI099734 to SJD. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Colley DG, Bustinduy AL, Secor WE, King CH (2014) Human schistosomiasis. The Lancet 383: 2253–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King CH (2015) Health metrics for helminth infections. Acta Trop 141: 150–160. 10.1016/j.actatropica.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, et al. (2012) Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2197–2223. 10.1016/S0140-6736(12)61689-4 [DOI] [PubMed] [Google Scholar]
- 4.McKerrow JH, Salter J (2002) Invasion of skin by Schistosoma cercariae. Trends Parasitol 18: 193–195. [DOI] [PubMed] [Google Scholar]
- 5.Georgi JR, Wade SE, Dean DA (1987) Schistosoma mansoni: mechanism of attrition and routes of migration from lungs to hepatic portal system in the laboratory mouse. J Parasitol 73: 706–711. [PubMed] [Google Scholar]
- 6.Brunet LR, Finkelman FD, Cheever AW, Kopf MA, Pearce EJ (1997) IL-4 protects against TNF-alpha-mediated cachexia and death during acute schistosomiasis. J Immunol 159: 777–785. [PubMed] [Google Scholar]
- 7.Fallon PG, Richardson EJ, McKenzie GJ, McKenzie AN (2000) Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J Immunol 164: 2585–2591. [DOI] [PubMed] [Google Scholar]
- 8.Stadecker MJ, Asahi H, Finger E, Hernandez HJ, Rutitzky LI, et al. (2004) The immunobiology of Th1 polarization in high-pathology schistosomiasis. Immunol Rev 201: 168–179. 10.1111/j.0105-2896.2004.00197.x [DOI] [PubMed] [Google Scholar]
- 9.Pearce EJ, MK C, Sun J, JT J, McKee AS, et al. (2004) Th2 response polarization during infection with the helminth parasite Schistosoma mansoni. Immunol Rev 201: 117–126. 10.1111/j.0105-2896.2004.00187.x [DOI] [PubMed] [Google Scholar]
- 10.Pearce EJ, MacDonald AS (2002) The immunobiology of schistosomiasis. Nature Reviews Immunology 2: 499–511. 10.1038/nri843 [DOI] [PubMed] [Google Scholar]
- 11.Doenhoff MJ (1998) Granulomatous inflammation and the transmission of infection: schistosomiasis—and TB too? Immunol Today 19: 462–467. [DOI] [PubMed] [Google Scholar]
- 12.Doenhoff MJ (1997) A role for granulomatous inflammation in the transmission of infectious disease: schistosomiasis and tuberculosis. Parasitology 115: S113–125. [DOI] [PubMed] [Google Scholar]
- 13.Davies SJ, Grogan JL, Blank RB, Lim KC, Locksley RM, et al. (2001) Modulation of Blood Fluke Development in the Liver by Hepatic CD4+ Lymphocytes. Science 294: 1358–1361. 10.1126/science.1064462 [DOI] [PubMed] [Google Scholar]
- 14.Harrison RA, Doenhoff MJ (1983) Retarded development of Schistosoma mansoni in immunosuppressed mice. Parasitology 86: 429–438. [DOI] [PubMed] [Google Scholar]
- 15.Lamb EW, Crow ET, Lim KC, Liang YS, Lewis FA, et al. (2007) Conservation of CD4+ T cell-dependent developmental mechanisms in the blood fluke pathogens of humans. Int J Parasitol 37: 405–415. 10.1016/j.ijpara.2006.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamb EW, Walls CD, Pesce JT, Riner DK, Maynard SK, et al. (2010) Blood fluke exploitation of non-cognate CD4+ T cell help to facilitate parasite development. PLoS Pathog 6: e1000892 10.1371/journal.ppat.1000892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riner DK, Ferragine CE, Maynard SK, Davies SJ (2013) Regulation of Innate Responses during Pre-patent Schistosome Infection Provides an Immune Environment Permissive for Parasite Development. PLoS Pathogens 9: e1003708 10.1371/journal.ppat.1003708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearce EJ, Caspar P, Grzych JM, Lewis FA, Sher A (1991) Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med 173: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grzych JM, Pearce E, Cheever A, Caulada ZA, Caspar P, et al. (1991) Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J Immunol 146: 1322–1327. [PubMed] [Google Scholar]
- 20.de Oliveira Fraga LA, Torrero MN, Tocheva AS, Mitre E, Davies SJ (2010) Induction of type 2 responses by schistosome worms during prepatent infection. J Infect Dis 201: 464–472. 10.1086/649841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Oliveira Fraga LA, Lamb EW, Moreno EC, Chatterjee M, Dvorak J, et al. (2010) Rapid induction of IgE responses to a worm cysteine protease during murine pre-patent schistosome infection. BMC Immunol 11: 56 10.1186/1471-2172-11-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pulendran B, Tang H, Manicassamy S Programming dendritic cells to induce T(H)2 and tolerogenic responses. Nat Immunol 11: 647–655. 10.1038/ni.1894 [DOI] [PubMed] [Google Scholar]
- 23.Ghaemmaghami AM, Gough L, Sewell HF, Shakib F (2002) The proteolytic activity of the major dust mite allergen Der p 1 conditions dendritic cells to produce less interleukin-12: allergen-induced Th2 bias determined at the dendritic cell level. Clin Exp Allergy 32: 1468–1475. [DOI] [PubMed] [Google Scholar]
- 24.Lecaille F, Kaleta J, Bromme D (2002) Human and parasitic papain-like cysteine proteases: their role in physiology and pathology and recent developments in inhibitor design. Chem Rev 102: 4459–4488. [DOI] [PubMed] [Google Scholar]
- 25.Robinson MW, Dalton JP, Donnelly S (2008) Helminth pathogen cathepsin proteases: it’s a family affair. Trends Biochem Sci 33: 601–608. 10.1016/j.tibs.2008.09.001 [DOI] [PubMed] [Google Scholar]
- 26.Caffrey CR, McKerrow JH, Salter JP, Sajid M (2004) Blood ’n' guts: an update on schistosome digestive peptidases. Trends Parasitol 20: 241–248. 10.1016/j.pt.2004.03.004 [DOI] [PubMed] [Google Scholar]
- 27.Sokol CL, Barton GM, Farr AG, Medzhitov R (2008) A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol 9: 310–318. 10.1038/ni1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Briot A, Deraison C, Lacroix M, Bonnart C, Robin A, et al. (2009) Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med 206: 1135–1147. 10.1084/jem.20082242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kouzaki H, O'Grady SM, Lawrence CB, Kita H (2009) Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol 183: 1427–1434. 10.4049/jimmunol.0900904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang G, Barker T, Xie Z, Charles N, Rivera J, et al. (2012) Naive T cells sense the cysteine protease allergen papain through protease-activated receptor 2 and propel TH2 immunity. J Allergy Clin Immunol 129: 1377–1386 e1313 10.1016/j.jaci.2012.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kreda SM, Seminario-Vidal L, van Heusden CA, O'Neal W, Jones L, et al. (2010) Receptor-promoted exocytosis of airway epithelial mucin granules containing a spectrum of adenine nucleotides. J Physiol 588: 2255–2267. 10.1113/jphysiol.2009.186643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kouzaki H, Iijima K, Kobayashi T, O'Grady SM, Kita H (2011) The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol 186: 4375–4387. 10.4049/jimmunol.1003020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris NL, Loke P (2017) Recent Advances in Type-2-Cell-Mediated Immunity: Insights from Helminth Infection. Immunity 47: 1024–1036. 10.1016/j.immuni.2017.11.015 [DOI] [PubMed] [Google Scholar]
- 34.El Ridi R, Tallima H, Dalton JP, Donnelly S (2014) Induction of protective immune responses against schistosomiasis using functionally active cysteine peptidases. Front Genet 5: 119 10.3389/fgene.2014.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ricciardi A, Visitsunthorn K, Dalton JP, Ndao M (2016) A vaccine consisting of Schistosoma mansoni cathepsin B formulated in Montanide ISA 720 VG induces high level protection against murine schistosomiasis. BMC Infect Dis 16: 112 10.1186/s12879-016-1444-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tallima H, Dvorak J, Kareem S, Abou El Dahab M, Abdel Aziz N, et al. (2017) Protective immune responses against Schistosoma mansoni infection by immunization with functionally active gut-derived cysteine peptidases alone and in combination with glyceraldehyde 3-phosphate dehydrogenase. PLoS Negl Trop Dis 11: e0005443 10.1371/journal.pntd.0005443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El Ridi R, Tallima H, Selim S, Donnelly S, Cotton S, et al. (2014) Cysteine peptidases as schistosomiasis vaccines with inbuilt adjuvanticity. PLoS One 9: e85401 10.1371/journal.pone.0085401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdulla MH, Lim KC, Sajid M, McKerrow JH, Caffrey CR (2007) Schistosomiasis mansoni: novel chemotherapy using a cysteine protease inhibitor. PLoS Med 4: e14 10.1371/journal.pmed.0040014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sajid M, McKerrow JH, Hansell E, Mathieu MA, Lucas KD, et al. (2003) Functional expression and characterization of Schistosoma mansoni cathepsin B and its trans-activation by an endogenous asparaginyl endopeptidase. Mol Biochem Parasitol 131: 65–75. [DOI] [PubMed] [Google Scholar]
- 40.Stack CM, Dalton JP, Cunneen M, Donnelly S (2005) De-glycosylation of Pichia pastoris-produced Schistosoma mansoni cathepsin B eliminates non-specific reactivity with IgG in normal human serum. J Immunol Methods 304: 151–157. 10.1016/j.jim.2005.07.019 [DOI] [PubMed] [Google Scholar]
- 41.Caffrey CR, Rheinberg CE, Mone H, Jourdane J, Li YL, et al. (1997) Schistosoma japonicum, S. mansoni, S. haematobium, S. intercalatum, and S. rodhaini: cysteine-class cathepsin activities in the vomitus of adult worms. Parasitol Res 83: 37–41. [DOI] [PubMed] [Google Scholar]
- 42.Livak KJ, Schmittgen TD (2001) Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 25: 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 43.Hsing LC, Rudensky AY (2005) The lysosomal cysteine proteases in MHC class II antigen presentation. Immunol Rev 207: 229–241. 10.1111/j.0105-2896.2005.00310.x [DOI] [PubMed] [Google Scholar]
- 44.Manoury B (2013) Proteases: essential actors in processing antigens and intracellular toll-like receptors. Front Immunol 4: 299 10.3389/fimmu.2013.00299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rehman AA, Ahsan H, Khan FH (2013) alpha-2-Macroglobulin: a physiological guardian. J Cell Physiol 228: 1665–1675. 10.1002/jcp.24266 [DOI] [PubMed] [Google Scholar]
- 46.Csernok E, Ai M, Gross WL, Wicklein D, Petersen A, et al. (2006) Wegener autoantigen induces maturation of dendritic cells and licenses them for Th1 priming via the protease-activated receptor-2 pathway. Blood 107: 4440–4448. 10.1182/blood-2005-05-1875 [DOI] [PubMed] [Google Scholar]
- 47.Saeed MA, Ng GZ, Dabritz J, Wagner J, Judd L, et al. (2017) Protease-activated Receptor 1 Plays a Proinflammatory Role in Colitis by Promoting Th17-related Immunity. Inflamm Bowel Dis 23: 593–602. 10.1097/MIB.0000000000001045 [DOI] [PubMed] [Google Scholar]
- 48.Schaefer L (2014) Complexity of danger: the diverse nature of damage-associated molecular patterns. J Biol Chem 289: 35237–35245. 10.1074/jbc.R114.619304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, et al. (1993) Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 260: 547–549. [DOI] [PubMed] [Google Scholar]
- 50.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, et al. (2007) IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 8: 967–974. 10.1038/ni1488 [DOI] [PubMed] [Google Scholar]
- 51.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC (2006) A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med 203: 1685–1691. 10.1084/jem.20060285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Larkin BM, Smith PM, Ponichtera HE, Shainheit MG, Rutitzky LI, et al. (2012) Induction and regulation of pathogenic Th17 cell responses in schistosomiasis. Semin Immunopathol 34: 873–888. 10.1007/s00281-012-0341-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pearce EJ, La Flamme A, Sabin E, Brunet LR (1998) The initiation and function of Th2 responses during infection with Schistosoma mansoni. Adv Exp Med Biol 452: 67–73. [DOI] [PubMed] [Google Scholar]
- 54.Brunet LR, Beall M, Dunne DW, Pearce EJ (1999) Nitric oxide and the Th2 response combine to prevent severe hepatic damage during Schistosoma mansoni infection. J Immunol 163: 4976–4984. [PubMed] [Google Scholar]
- 55.Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, et al. (2012) An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med 18: 260–266. 10.1038/nm.2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lim H, Kim YU, Yun K, Drouin SM, Chung Y (2013) Distinct regulation of Th2 and Th17 responses to allergens by pulmonary antigen presenting cells in vivo. Immunol Lett 156: 140–148. 10.1016/j.imlet.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 57.Shainheit MG, Lasocki KW, Finger E, Larkin BM, Smith PM, et al. (2011) The pathogenic Th17 cell response to major schistosome egg antigen is sequentially dependent on IL-23 and IL-1beta. J Immunol 187: 5328–5335. 10.4049/jimmunol.1101445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shainheit MG, Smith PM, Bazzone LE, Wang AC, Rutitzky LI, et al. (2008) Dendritic Cell IL-23 and IL-1 Production in Response to Schistosome Eggs Induces Th17 Cells in a Mouse Strain Prone to Severe Immunopathology. The Journal of Immunology 181: 8559–8567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rutitzky LI, Bazzone L, Shainheit MG, Joyce-Shaikh B, Cua DJ, et al. (2008) IL-23 Is Required for the Development of Severe Egg-Induced Immunopathology in Schistosomiasis and for Lesional Expression of IL-17. The Journal of Immunology 180: 2486–2495. [DOI] [PubMed] [Google Scholar]
- 60.Leptak CL, McKerrow JH (1997) Schistosome egg granulomas and hepatic expression of TNF-alpha are dependent on immune priming during parasite maturation. J Immunol 158: 301–307. [PubMed] [Google Scholar]
- 61.Ponichtera HE, Shainheit MG, Liu BC, Raychowdhury R, Larkin BM, et al. (2014) CD209a expression on dendritic cells is critical for the development of pathogenic Th17 cell responses in murine schistosomiasis. J Immunol 192: 4655–4665. 10.4049/jimmunol.1400121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kalantari P, Morales Y, Miller EA, Jaramillo LD, Ponichtera HE, et al. (2018) CD209a Synergizes with Dectin-2 and Mincle to Drive Severe Th17 Cell-Mediated Schistosome Egg-Induced Immunopathology. Cell Rep 22: 1288–1300. 10.1016/j.celrep.2018.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.El Ridi R, Tallima H, Mahana N, Dalton JP (2010) Innate immunogenicity and in vitro protective potential of Schistosoma mansoni lung schistosomula excretory—secretory candidate vaccine antigens. Microbes Infect 12: 700–709. 10.1016/j.micinf.2010.04.012 [DOI] [PubMed] [Google Scholar]
- 64.Sinkala E, Kapulu MC, Besa E, Zyambo K, Chisoso NJ, et al. (2016) Hepatosplenic schistosomiasis is characterised by high blood markers of translocation, inflammation and fibrosis. Liver Int 36: 145–150. 10.1111/liv.12891 [DOI] [PubMed] [Google Scholar]
- 65.Mbow M, Larkin BM, Meurs L, Wammes LJ, de Jong SE, et al. (2013) T-helper 17 cells are associated with pathology in human schistosomiasis. J Infect Dis 207: 186–195. 10.1093/infdis/jis654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Babu S, Bhat SQ, Pavan Kumar N, Lipira AB, Kumar S, et al. (2009) Filarial lymphedema is characterized by antigen-specific Th1 and th17 proinflammatory responses and a lack of regulatory T cells. PLoS Negl Trop Dis 3: e420 10.1371/journal.pntd.0000420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nogueira DS, Gazzinelli-Guimaraes PH, Barbosa FS, Resende NM, Silva CC, et al. (2016) Multiple Exposures to Ascaris suum Induce Tissue Injury and Mixed Th2/Th17 Immune Response in Mice. PLoS Negl Trop Dis 10: e0004382 10.1371/journal.pntd.0004382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yan C, Zhang BB, Hua H, Li B, Zhang B, et al. (2015) The Dynamics of Treg/Th17 and the Imbalance of Treg/Th17 in Clonorchis sinensis-Infected Mice. PLoS One 10: e0143217 10.1371/journal.pone.0143217 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Top row, left to right: a FSC-A vs. FSC-H plot is used to gate on singlet events (left panel); a FSC-A vs. SSC-A plot is used to gate on lymphocytes (center panel); a CD19 histogram is used to exclude CD19+ cells (right panel). Bottom row, left to right: a CD4 vs. CD3 plot is used to gate on double-positive CD3+ CD4+ cells in the upper right (Q2) quadrant (left panel); a TCRβ vs. CD4 plot is used to gate on double-positive TCRβ+ CD4+ cells in the upper right (Q2) quadrant (middle panel); a GFP vs. TCRβ plot is used to identify IL-4-GFP+ cells in the upper right (Q2) quadrant (right panel).
(EPS)
On day 22, intracellular cytokine/transcription factor staining and flow cytometry were used to determine the frequencies of IL4+ GATA3+ CD4+ T cells (A), IFN-γ+ CD4+ T cells (B), and IL-17A+ RORγt+ CD4+ T cells (C), in the spleens, mesenteric lymph nodes, and popliteal and inguinal lymph nodes of wild type C57BL/6 mice that had received injections of active SmCB1 on days 0, 14 and 21, by intravenous (IV), intraperitoneal (IP) and subcutaneous (SC) routes. Cells from comparable tissues of animals that received no antigen (-) were included as negative controls.
(TIF)
The frequency of IL-4+ CD4+ T cells (A, B), GATA3+ CD4+ T cells (C, D), double-positive IL-4+ GATA3+ CD4+ T cells (E, F), and IFN-γ+ CD4+ T cells (G, H), in the spleens (A, C, E, G) and mesenteric lymph nodes (B, D, F, H), of S. mansoni-infected C57BL/6 mice at 4, 6 and 8 weeks post infection, and in non-infected mice, were compared using intracellular cytokine/transcription factor staining and flow cytometry. n.s., P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
(EPS)
Information regarding the source and format of all antibodies used in the flow cytometric analysis of immune cells is provided.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.