Abstract
Background
Alcohol dependence displays a wide variety of clinical phenotypes. Various typology classifications of alcoholism include age of onset of alcohol abuse as one of the major phenotypic features. Serotonergic changes have been associated with alcoholism, while serotonin receptors type 1B (5-HT1B) play an important role in regulating serotonergic neurotransmission. The rs13212041 polymorphism modulates the expression of HTR1B gene coding for 5-HT1B receptor. This study examined the association of platelet serotonin (5-HT) and HTR1B gene with the onset of alcohol abuse in alcohol-dependent subjects.
Materials and methods
Determination of platelet 5-HT concentration and genotyping of rs13212041 HTR1B gene polymorphism were performed in 613 alcohol-dependent patients, subdivided according to early/late onset (before/after 25 years of age) of alcohol abuse.
Results
Alcohol-dependent individuals with CC genotype were more frequent in the group with early onset of alcohol abuse compared to carriers of T allele. Besides HTR1B genotype, age and gender, but not platelet 5-HT, were major variables associated with the onset of alcohol abuse. Platelet 5-HT concentration was not significantly different between patients with early and late onset of alcohol abuse, or patients carrying various HTR1B genotypes. Although we observed no influence of co-variables such as age, gender, or somatic and psychiatric comorbidities, platelet 5-HT concentration was significantly affected by smoking.
Conclusion
These findings support potential involvement of 5-HT1B receptors in the onset of alcohol abuse and development of alcohol dependence. Additionally, the results of our study emphasize the importance of controlling for smoking status, as one of the significant confounding factors influencing platelet 5-HT concentration.
Keywords: alcoholism, age of onset, 5-HT1B, gene variants, platelet serotonin, smoking
Introduction
Patients with alcohol dependence display individual differences in clinical symptoms and phenotypic characteristics, including disease severity, course and prognosis, comorbidity, and treatment response, which may be the result of different underlying neurobiological mechanisms, such as genetic and environmental influences.1,2 In order to reduce the heterogeneity of this complex disease, different authors proposed various typology classifications to divide alcohol-dependent subjects into more specific subtypes or endophenotypes.3 Many proposed classifications of alcohol dependence include the age of onset of alcohol abuse as one of the major phenotypic features with potential usefulness in elucidating underlying neurobiological pathways and improvement of the therapy.4,5 The age of onset of alcohol abuse might be due to the individual’s genetic predisposition, as well as to a number of environmental factors.6 The Cloninger classification,7 among the most widely used classification schemes for alcohol dependence,8 also uses onset of alcohol abuse to differentiate between alcohol-dependent subjects type I and II, which differ in the course of the disease, extent of inheritance, and personality traits.9
Subjects with an early onset (before 25 years of age) of alcohol abuse (type II alcoholism) are more likely to drink heavily, to have more alcohol-related problems, more severe symptoms, and higher rates of somatic and psychiatric comorbidity.4,10 In addition, early onset alcoholism has been often associated with elevated levels of antisocial behavior and delinquency during adolescence and characterized as strongly heritable and male-limited.7 These subjects use alcohol for its euphoric effects and demonstrate high novelty seeking and low harm-avoidance personality traits.11 On the other hand, type I alcoholics have later onset of alcohol abuse (after 25 years of age) and are more strongly influenced by social environmental factors. Moreover, alcoholism with later onset affects both men and women, who drink alcohol for its anxiolytic properties and have high harm-avoidance personality traits.7 These two subtypes of alcohol-dependence are suggested to have different neurobiological backgrounds. Namely, it has been proposed that type I and type II alcohol-dependent individuals, differing in the age of alcoholism onset, have a deficit in the dopaminergic or serotonergic neurotransmission, respectively.12 This has been supported by studies demonstrating that type II alcoholics seem to have reduced levels of serotonin transporter (5-HTT) already in early age, whereas in type I alcoholics, the serotonergic alterations may be secondary and possibly reflecting the alterations in the dopaminergic system.13,14
Variations in the 5-HTT gene15 and tryptophan hydroxylase gene16 have been associated with an increased risk of alcohol dependence with an early onset as well as with alcohol treatment response.17 Moreover, decreased serotonergic neurotransmission18 and reduced concentrations of 5-hydroxyindoleacetic acid (5-HIAA), the major metabolite of serotonin (5-HT), have been found in the cerebrospinal fluid of early onset alcohol-dependent subjects.19 Low plasma ratio of tryptophan to large neutral amino acids (TRYP/LNAA ratio), a marker of serotonergic function, has also been observed in patients with an early onset of alcohol dependence.20 The findings suggest that these individuals might have a preexisting 5-HT deficit and therefore increased vulnerability to fluctuations in precursor availability, leading to higher alcohol intake early in life.10 In line with these results, low activity of monoamine oxidase (MAO), the enzyme degrading 5-HT, has been associated with type II subtype of alcoholism.21 In addition, the classification based on the age of onset of alcohol abuse seems to be an effective predictor of treatment response to various serotonergic drugs.22 Namely, ondansetron has been useful in therapy of early onset alcoholic patients,23 whereas sertraline has shown positive effects in the late onset alcohol-dependent subjects.24 Conversely, fluoxetine reduced the beneficial effects of cognitive–behavioral therapy in early onset alcoholics.25
The involvement of 5-HT system in the onset-based alcoholism typologies has been supported by both animal and human studies demonstrating that serotonergic disturbances have been closely related to reduced inhibitory control of behavior, including extensive alcohol use.10,26 Low central serotonergic activity has been mostly associated with increased alcohol consumption and alcohol dependence.26 Central 5-HT type 1B (5-HT1B) receptors have an important role in the physiologic synaptic feedback mechanism and regulation of serotonergic neurotransmission27 and are involved in various neurological disorders such as migraine.28 Moreover, 5-HT1B receptors have been suggested to modulate the activity of dopaminergic neurons in the ventral tegmental area, which is involved in the reward mechanisms underlying addictions, including alcohol dependence.29 Various animal studies have also implicated 5-HT1B receptor in the control of alcohol preference and consumption.30,31
Previous human studies reported the associations between different polymorphisms in the gene coding for 5-HT1B receptor (HTR1B) and alcohol dependence,32,33 especially alcoholism with early onset and antisocial behavior.4,34,35 The majority of reports assessing the association of HTR1B gene and alcoholism investigated G-861C polymorphism,33,34 which is in strong linkage disequilibrium with other functional polymorphisms in the HTR1B gene promoter, such as T-261G, A-161T, and C-129T.33,36,37 On the other hand, other authors failed to confirm these findings,37–39 perhaps due to the variability of alcohol-dependent phenotypes. However, only few studies investigated rs13212041 polymorphism, shown to modulate HTRIB gene expression,40 and its role in alcohol dependence.41,42
As the majority of peripheral 5-HT is stored in platelets, which possess many other components of serotonergic system, including 5-HTT, MAO-B as well as 5-HT1A, 5-HT2A, 5-HT3A, and 5-HT4 receptors,43–48 platelets are suggested as a limited peripheral model of central serotonergic functions.49 Moreover, some reports indicated that human platelets also contain 5-HT1B receptors,50 although their function in platelets is not known. However, since upon activation, platelets release 5-HT into circulation,51 one might speculate that similar to its role in the brain, platelet 5-HT1B receptors might regulate 5-HT levels at the periphery. It is generally accepted that serotonergic components in both platelets and neurons are encoded by the same genes and have similar structural and functional properties.52,53 Hence, the rs13212041 HTR1B gene polymorphism via its influence on the 5-HT1B receptor expression might subsequently affect central as well as peripheral 5-HT turnover.
In order to investigate potential changes in the serotonergic activity associated with different alcoholism subtypes, the aim of this study was to examine the association of platelet 5-HT and rs13212041 HTR1B gene polymorphism, with the early/late onset of alcohol abuse in alcohol-dependent subjects. To our knowledge, this is first such study, with appropriate sample size and carefully determined alcohol-related phenotypes, including possible confounding factors. The findings of our study might provide novel insights in the serotonergic regulation mechanisms underlying the early/late onset of alcohol abuse, which could help in further differentiation of alcoholism subtypes and potential improvement of the therapy for this complex disease.
Materials and methods
Subjects
The study enrolled 613 medication-free patients (499 males and 114 females) with alcohol dependence, recruited from the Center of Alcoholism and Other Addictions, University Psychiatric Hospital Vrapce, Zagreb, Croatia. Patients with alcohol dependence were admitted to hospital due to acute intoxication or alcohol-related withdrawal symptoms, and were left overnight to sober up. Demographic and clinical characteristics of the alcohol-dependent patients are listed in Table 1. The alcohol dependence as well as comorbid psychiatric disorders were diagnosed using a structured clinical interview (SCID), based on the DSM-IV criteria.54 If alcohol-dependent patients had any other comorbid substance abuse or dependence (except nicotine addiction) or had used any kind of drugs in the previous 12 months, they were excluded from the study. The alcohol-dependent patients were divided according to the onset of alcohol abuse into subjects with early (before 25 years of age) and late (after 25 years of age) onset of alcohol abuse.7 The data regarding somatic history, including current somatic complaints, were collected from hospital medical records and obtained by an interview. Participants were Caucasians of the European origin from Zagreb County, Croatia, and they all agreed to give a blood sample, participate in the study, and gave their written informed consent. The study was approved by the Ethics Committee of University Psychiatric Hospital Vrapce, Zagreb, Croatia and carried out in accordance with the Helsinki Declaration (1964).
Table 1.
The demographic and clinical characteristics of alcohol-dependent patients
| Alcohol-dependent patients | Patients with early onset of alcohol abuse 215 (35.07%) | Patients with late onset of alcohol abuse 398 (64.93%) | Total 613 (100.00%) | Statistics |
|---|---|---|---|---|
| Age (years) mean ± SD | 45.12±10.09 | 52.33±9.46 | 49.80±10.27 | t=8.79, df =611, P<0.0001; Student’s t-test |
| Smokers, N (%) | 145 (67.44%) | 225 (56.53%) | 370 (60.36%) | χ2=6.943, df =1, P=0.0084; χ2-test |
| Nonsmokers, N (%) | 70 (32.56%) | 173 (43.47%) | 243 (39.64%) | |
| Males, N (%) | 193 (38.68%) | 306 (61.32%) | 499 (81.40%) | χ2=15.30, df =1, P<0.0001; χ2-test |
| Females, N (%) | 22 (19.30%) | 92 (80.70%) | 114 (18.60%) | |
| Patients with somatic comorbidities, N (%) | 128 (59.53%) | 267 (67.08%) | 395 (64.44%) | χ2=3.473, df =1, P=0.0624; χ2-test |
| Patients with liver disease, N (%) | 125 (58.14%) | 261 (65.58%) | 386 (62.97%) | χ2=3.312, df =1, P=0.0688; χ2-test |
| Patients with psychiatric comorbidities, N (%) | 112 (52.09%) | 167 (41.96%) | 279 (45.51%) | χ2=5.780, df =1, P=0.0162; χ2-test |
| Patients with depression, N (%) | 10 (4.65%) | 24 (6.03%) | 34 (5.55%) | χ2=0.5067, df =1, P=0.4766; χ2-test |
| Platelet 5-HT concentration (nmol/mg protein) mean ± SD | 0.88±0.48 | 0.79±0.48 | 0.82±0.48 | U=38035, df =611, P=0.023; Mann–Whitney test |
Blood collection and processing
The samples of blood (8 mL) from alcohol-dependent patients were collected in the morning after overnight fasting by using a plastic syringe containing 2 mL anticoagulant (acid citrate dextrose). The whole blood was centrifuged at 1100 g for 3 minutes at 4°C. Obtained platelet-rich plasma was further centrifuged at 5087 g for 15 minutes, 4°C to sediment the platelets. The platelet pellet was washed with saline and centrifuged again. The obtained pellet was stored at −20°C.
Platelet 5-HT measurement
Platelet 5-HT concentration was measured by the spectrofluorometric method.55 Briefly, platelets were broken down by sonication. Specimens of standard, blank (water), and platelet sonicates were analyzed in duplicates. All samples were deproteinized with 10% ZnSO4 and 1N NaOH, and then 0.1% L-cysteine and 0.05% orthophthalaldehyde were added for the preparation of fluorophore. The 5-HT fluorescence was measured on a Varian Spectrophotofluorimeter Cary Eclipse (Varian Optical Spectroscopy Instruments, Mulgrave, VIC, Australia) (exciting wavelength: 345 nm; emitted wavelength: 485 nm). Protein concentrations in platelets were determined by the method of Lowry et al.56
Genotyping
Genomic DNA was isolated from peripheral blood leukocytes by a salting out method.57 Genotyping was performed according to the procedure described by Applied Biosystems, using TaqMan-based allele-specific polymerase chain reaction on ABI Prism 7000 Sequencing Detection System apparatus. The rs13212041 (C__32252506_10) single nucleotide polymorphism in the HTR1B gene, located on chromosome 6, was analyzed. Briefly, 20 ng of genomic DNA was PCR amplified in 96-well plates using a 10 µL reaction volume. The conditions of PCR reaction were: 40 cycles at 92°C for 15 seconds and 60°C for 60 seconds.
Data analyses
Statistical analyses were performed with GraphPad Prism version 4.00 for Windows (GraphPad Software, Inc., San Diego, CA, USA) and MedCalc Statistical Software version 14.8.1 (MedCalc Software bvba, Ostend, Belgium). Normality of distribution was assessed with the D’Agostino–Pearson omnibus normality test. Age of male and female subjects enrolled in the study was compared using Student’s t-test. The frequency of smoking and early/late onset of alcohol abuse between male and female alcohol-dependent patients was evaluated by a chi-squared test of independence. Platelet 5-HT concentrations were analyzed by Kruskal–Wallis test followed by Dunn’s multiple comparison test or with Mann–Whitney test, as normality of the data failed. Possible deviations from Hardy–Weinberg equilibrium (HWE) were tested using goodness of fit chi-squared test. Genotype and allele frequencies (presented as numbers and percentages) were evaluated by a chi-squared test of independence. Multiple regression analysis assessed the influence of various demographic and clinical data (independent variables), such as age, gender, smoking, somatic and psychiatric comorbidities, on alcohol abuse onset and platelet 5-HT concentration (dependent variables).
G*Power 3 Software58 was used for conducting power analyses, ie, to determine a priori sample size and to post hoc compute the achieved power. For F test (Kruskal–Wallis test) involving three groups (with α=0.025; power =0.80; a small effect size =0.30), total desired sample size was 132. For F test (Kruskal–Wallis test), evaluating four groups (with α=0.025; power =0.80; a small effect size =0.30), total desired sample size was 152. For Mann–Whitney test (with α=0.025; power =0.80; a median effect size =0.30), total desired sample size was 368. For analyses with a χ2-test (with α=0.025; power (1-β)=0.80 and a small effect size (ω=0.20)), for df =2 total desired sample size was 288, and for df =1, total desired sample size was 238. As the actual total sample size was 613, the power analysis revealed that the study included appropriate sample size and statistical power to detect significant differences.
Results
Alcohol-dependent participants were predominantly male (81.40%). The group of female subjects with alcohol dependence (51.89±11.62) was significantly older (t=2.42, df =611, P=0.016; Student’s t-test) than the group of male patients (49.32±9.89). There were no significant differences in frequency of smoking (χ2=2.01, df =1, P=0.148) between male (61.72%) and female (54.39%) alcohol-dependent patients. Our study demonstrated that 395 (64.44%) of alcohol-dependent patients (67.13% of males and 52.63% of females) had some somatic comorbidity, with liver diseases present in 386 (62.97%) of enrolled subjects. Hence, most (92.72%) somatic diseases in subjects with alcoholism were liver diseases. Moreover, 279 (45.51%) alcohol-dependent patients, ie, 223 (44.69%) males and 56 (49.12%) females reported some psychiatric comorbidity. These psychiatric comorbidities include personality disorders (49.10%), stress-related disorders (37.27%) including anxiety, acute stress reaction and post-traumatic stress disorder, mood disorders (12.19%) primarily depression, and psychotic disorders such as schizophrenia (1.43%). There were no significant differences in the frequency of psychiatric comorbidities between male and female patients (χ2=0.73, df =1, P=0.391); however, male patients (67.13%) had more often somatic comorbidities (χ2=8.52, df =1, P=0.0035) than female patients (52.63%).
As shown in Table 1, alcohol-dependent subjects enrolled in the study had more often late (64.93%) rather than early (35.07%) onset of alcohol abuse. However, we observed significantly lower frequency of female patients with early, rather than with late, onset of alcohol abuse in comparison with male subjects (χ2=15.30, df =1, P<0.0001). Moreover, there were significantly more smokers in the group of early-onset alcoholics than in subjects with late onset of alcohol abuse (χ2=6.94, df =1, P=0.008). There were no significant differences in the frequency of somatic comorbidities (χ2=3.47, df =1, P=0.062) or liver diseases (χ2=3.31, df =1, P=0.069) between patients with early and late onset of alcohol abuse. However, early onset alcohol-dependent subjects more often had psychiatric comorbidities (52.09%) than patients with late onset alcohol abuse (41.96%) (χ2=5.78, df =1, P=0.016), although they did not differ in frequency of depression (χ2=0.51, df =1, P=0.477) (Table 1).
Genotype distributions for rs13212041 HTR1B polymorphism in alcohol-dependent subjects were in HWE (χ2=0.03, P=0.866). No significant differences in the frequency of the genotypes (χ2=2.13, df=2, P=0.344) or alleles (χ2=1.98, df =1, P=0.160) between male and female alcohol-dependent patients were detected using χ2-test (Table 2); therefore, in further analysis, patients were not divided according to gender. The genotype distribution for HTR1B gene polymorphism was significantly different (χ2=7.54, df =2, P=0.023) between patients with early and late onset of alcohol abuse (Table 2). Namely, the individuals carrying the CC genotype were significantly more frequent (χ2=7.25, df =1, P=0.007) in the group of alcohol-dependent subjects with early onset of alcohol abuse than in carriers of T allele. This strong evidence of HTR1B was also confirmed using multiple regression analysis (P=0.024), with age (P<0.0001) and gender (P=0.001) as major variables influencing the onset of alcohol abuse.
Table 2.
Genotype and allele counts and frequencies of rs13212041 HTR1B gene polymorphism in alcohol-dependent patients subdivided according to the gender and early/late onset of alcohol abuse
| HTR1B SNP | Genotype N (%) | Statistics | Allele N (%) | Statistics | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| rs13212041 | CC | CT | TT | df =2 | C | T | df =1 |
|
| |||||||
| Males, N (%) | 21 (4.21) | 157 (31.46) | 321 (64.33) | χ2=2.135, P=0.344; χ2-test | 199 (19.94) | 799 (80.06) | χ2=1.977, P=0.160; χ2-test |
| Females, N (%) | 6 (5.26) | 43 (37.72) | 65 (57.02) | 55 (24.12) | 173 (75.88) | ||
|
| |||||||
| Early onset of alcohol abuse, N (%) | 16 (7.44) | 65 (30.23) | 134 (62.33) | χ2=7.539, P=0.023; χ2-test | 97 (22.56) | 333 (77.44) | χ2=1.366, P=0.243; χ2-test |
| Late onset of alcohol abuse, N (%) | 11 (2.76) | 135 (33.92) | 252 (63.32) | 157 (19.72) | 639 (80.28) | ||
As recent analysis suggested that the early onset of alcohol dependence is best defined as beginning before the age of 22 years,5 we additionally divided alcohol-dependent patients into subjects with early and late onset of alcohol abuse by using cut-off point at 22 years. Although the number of patients with early onset of alcohol abuse was, in this case, significantly lower (N=107), the results obtained after making cut-off at 22 years supported and even strengthened the findings obtained with cut-off at 25 years. Both the genotype (χ2=12.07, df=2, P=0.0024) and allele (χ2=8.456, df=1, P=0.0036) distribution for HTR1B gene polymorphism was significantly different between patients with early and late onset of alcohol abuse. Namely, the individuals carrying the CC genotype (χ2=10.63, df =1, P=0.0011) or C allele (χ2=4.269, df =1, P=0.0388) were significantly more frequent in the group of alcohol-dependent subjects with early onset of alcohol abuse than in carriers of the T allele or TT genotype, respectively.
Platelet 5-HT concentrations (mean±SD) were not significantly different between male (0.84±0.49) and female (0.75±0.46) alcohol-dependent subjects (U=25795, df =611, P=0.121; Mann–Whitney test). Moreover, the Kruskal–Wallis test failed to detect any significant differences in platelet 5-HT concentration between carriers of CC (0.84±0.46), CT (0.79±0.48), or TT (0.84±0.48) HTR1B rs13212041 genotypes, in the whole sample of alcohol-dependent patients (H=1.556, df=610, P=0.459), as well as when alcohol-dependent patients were subdivided into subjects with early and late onset of alcohol abuse (H=6.643, df =607, P=0.249).
Significant differences in platelet 5-HT concentrations were found between alcohol-dependent patients with early (0.88±0.48) and late (0.79±0.48) onset of alcohol abuse (U=38035, df=611, P=0.023; Mann–Whitney test; Table 1), and when these patients were additionally subdivided according to smoking status (H=36.49, df =609, P<0.0001; Kruskal–Wallis ANOVA; Figure 1). However, as shown in Figure 1, the results of post-hoc Dunn’s multiple comparison test revealed significant differences only between smokers and nonsmokers, both in the group with early (P=0.0004) and late (P=0.0005) onset of alcohol abuse, suggesting an effect of smoking on platelet 5-HT concentration. There were no significant differences in platelet 5-HT levels between smokers with early and late onset of alcohol abuse (P=0.309) or between nonsmokers with early and late onset of alcohol abuse (P>0.9999) (Dunn’s multiple comparison test; Figure 1). Multiple regression analysis also confirmed that there was no association of platelet 5-HT levels (P=0.104) with the onset of alcohol abuse. Therefore, observed differences in platelet 5-HT concentrations between alcohol-dependent patients with early and late onset of alcohol abuse were probably due to higher frequency of smokers in the group of alcoholics with early onset of alcohol abuse.
Figure 1.
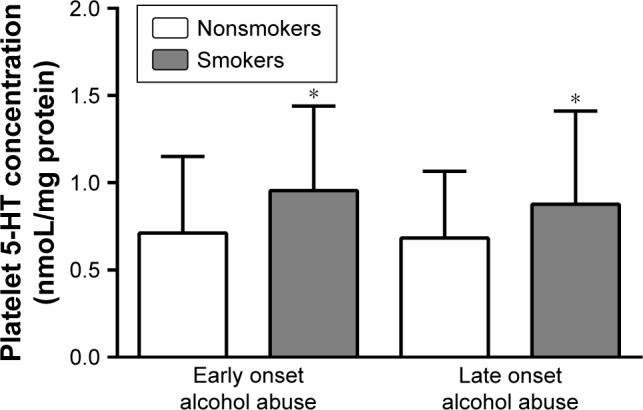
Platelet 5-HT concentration in alcohol-dependent smokers and nonsmokers subdivided according to the early/late onset of alcohol abuse.
Note: *P<0.0005 smokers vs nonsmokers (Kruskal–Wallis test and post-hoc Dunn’s multiple comparison test).
Our findings also suggested that there was significant difference in the smoking frequency between alcohol-dependent subjects with and without comorbid depression (χ2=6.71, df=1, P=0.0096; χ2-test). Namely, 81.82% of alcohol-dependent subjects with comorbid depression were smokers in comparison with 59.14% smokers in the group of alcoholic patients without comorbid depression. However, there was no significant difference in the frequency of comorbid depression between patients with early and late onset of alcohol abuse (Table 1), as well as no significant difference in the platelet 5-HT concentration between alcohol-dependent subjects with (0.94±0.57) and without (0.81±0.48) comorbid depression (U=8368, df=611, P=0.2257; Mann–Whitney test). In general, we observed no significant differences in platelet 5-HT concentration between alcohol-dependent patients with and without psychiatric (U=45371, df=611, P=0.576; Mann–Whitney test) as well as somatic comorbidities (U=42972, df=611, P=0.969; Mann–Whitney test).
Finally, multiple regression analysis demonstrated no significant influence of age (P=0.632), gender (P=0.270), onset of alcohol abuse (P=0.157), HTR1B rs13212041 genotype (P=0.473), somatic (P=0.584), and psychiatric comorbidities (P=0.993), but significant effect of smoking (P<0.0001) on platelet 5-HT concentration in patients with alcoholism.
Discussion
The main finding of our study is an association of rs13212041 HTR1B gene polymorphism with an onset of alcohol abuse in alcohol-dependent patients, demonstrating that homozygous individuals with CC genotype were more frequent in the group with early onset of alcohol abuse compared to carriers of T allele. Besides HTR1B genotype, age and gender, but not platelet 5-HT, were major variables influencing the onset of alcohol abuse. Platelet 5-HT concentration was not significantly different between subjects with early and late onset of alcohol abuse as well as between patients carrying different HTR1B genotypes. Although we observed no influence of co-variables such as age, gender, or somatic and psychiatric comorbidities, platelet 5-HT concentration was significantly affected by smoking.
Only few studies investigated the association of rs13212041 HTR1B gene polymorphism with alcohol dependence,41,42 but not with early- and late-onset alcoholism subtypes. This transition substitution (A1997G) in the distal 3′-untranslational region (UTR) of HTR1B messenger RNA disrupts a binding site for the microRNA, miR-96.40 MiR-96 is expressed in the brain,59 and various environmental factors, such as stress,60 can modulate its expression and epigenetically underlie some of the diversity in human behavior,40 including alcoholism endophenotypes. As A-allele carriers of rs13212041 HTR1B gene polymorphism demonstrate reduced HTR1B expression compared to G-allele carriers,40 we can presume that subjects with early onset of alcohol abuse, more frequently carrying the CC genotype, have higher levels of 5-HT1B receptors than patients with late onset of alcohol abuse.
In general, alcohol dependence has been associated with increased density of 5-HT1B receptors in specific brain regions.61 5-HT1B receptors have been shown to modify the effects of alcohol and regulate its voluntary intake,62 whereas 5-HT1B receptor ligands reduce extensive alcohol drinking and alcohol-related aggressive or impulsive behavior, observed in more severe forms of alcohol dependence.31,63 Therefore, our findings might suggest increased expression of 5-HT1B receptors in more severe types of alcoholism, characterized with an early onset of alcohol abuse.
Our results demonstrating that early onset alcohol-dependent patients more often have psychiatric comorbidities agree with findings that these subjects have more alcohol-related problems, more severe symptoms, and higher rates of somatic and psychiatric comorbidity.4,10 In our study, besides HTR1B genotype, age and gender were major variables influencing the onset of alcohol abuse. This is not surprising as male patients were younger and more often present in the group with an early onset of alcohol abuse, in line with suggestions that type II alcoholism is male predominant.7
The HTR1B rs13212041 polymorphism, by influencing 5-HT1B receptor expression, can subsequently affect 5-HT turnover. Namely, 5-HT1B receptors function as inhibitory autoreceptors or heteroreceptors on both serotonergic and non-serotonergic neurons, and modulate serotonergic activity. However, despite presumably higher density of 5-HT1B receptors in early-onset alcoholics, platelet 5-HT concentrations did not differ between patients with early and late onset of alcohol abuse, suggesting no association of platelet 5-HT with the onset of alcohol abuse.
Many components of serotonergic system, such as 5-HTT, MAO, and some 5-HT receptors, in both platelets and neurons are encoded by the same genes and have similar structural and functional properties.52,53 However, the role of 5-HT1B receptors might differ depending upon their specific location.64 In addition, activation of presynaptic 5-HT1B receptors inhibits 5-HT release, while 5-HT1B postsynaptic heteroreceptors are involved in the modulation of addictive behaviors.65 Therefore, platelet 5-HT1B receptors might not regulate 5-HT concentration in platelets, or their function could be affected by other 5-HT receptors66,67 or interaction with 5-HTT.68 No differences in platelet 5-HT levels, between carriers of different HTR1B rs13212041 genotypes in all alcohol-dependent patients, as well as in subjects with early and late onset of alcohol abuse, suggest that this polymorphism does not influence platelet 5-HT concentration.
Although previous studies found decreased platelet 5-HT concentration in alcohol-dependent subjects,69–71 only one small study reported higher platelet 5-HT uptake in subjects with early than with late-onset of alcohol dependence, suggesting differences in platelet 5-HTT function.72 However, in this study as well as these in many other studies, somatic and psychiatric comorbidities and tobacco smoking have been neglected as confounding factors, in spite of reports suggesting their effects on platelet 5-HT.49,73 We observed no significant influence of somatic and psychiatric comorbidities, including depression, but smoking significantly affected platelet 5-HT levels in alcohol-dependent patients in our present and previous study.74 In agreement with previous results,71 we detected higher platelet 5-HT concentrations in smoking alcoholics than in alcohol-dependent subjects who did not smoke. Patkar et al75 reported correlation of smoking with decreased density of platelet 5-HTT sites, whereas various studies reported metabolic effects of smoking on the breakdown of dopamine and 5-HT,76,77 as well as decreased platelet MAO-B activity due to cigarette smoking.78,79
In addition to appropriate sample size and statistical power, other advantages of this study include ethnically homogenous and medication-free alcohol-dependent patients and carefully determined specific alcohol-related phenotypes, including somatic and psychiatric comorbidities and other potential confounding factors such as age, gender, and smoking. However, one of the study limitations is a lack of replication of obtained results in the independent sample.
Conclusion
As far as we know, this is the first study reporting the association of rs13212041 polymorphism, located in the HTR1B gene coding for 5-HT1B receptor, with the onset of alcohol abuse in alcohol-dependent subjects. Our results suggest that homozygous individuals with CC genotype are more likely to develop a more severe form of alcoholism, characterized by the early onset of alcohol abuse, than carriers of T-allele. As rs13212041 polymorphism affects microRNA regulation of HTR1B gene expression, these data might suggest the involvement of epigenetic mechanisms in the modulation of serotonergic functions in alcohol dependence. Our findings might provide novel insights in the serotonergic regulation mechanisms underlying the early/late onset of alcohol abuse, which could help in further differentiation of alcoholism subtypes and potential improvement of the therapy for this complex disease. The results of our study also emphasize the importance of controlling for various confounding factors, such as smoking, which significantly influence platelet 5-HT levels.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Enoch MA. Genetic and environmental influences on the development of alcoholism: resilience vs. risk. Ann N Y Acad Sci. 2006;1094(1):193–201. doi: 10.1196/annals.1376.019. [DOI] [PubMed] [Google Scholar]
- 2.Litten RZ, Ryan ML, Falk DE, Reilly M, Fertig JB, Koob GF. Heterogeneity of alcohol use disorder: understanding mechanisms to advance personalized treatment. Alcohol Clin Exp Res. 2015;39(4):579–584. doi: 10.1111/acer.12669. [DOI] [PubMed] [Google Scholar]
- 3.Leggio L, Kenna GA, Fenton M, Bonenfant E, Swift RM. Typologies of alcohol dependence. From Jellinek to genetics and beyond. Neuropsychol Rev. 2009;19(1):115–129. doi: 10.1007/s11065-008-9080-z. [DOI] [PubMed] [Google Scholar]
- 4.Chen YC, Prescott CA, Walsh D, et al. Different phenotypic and genotypic presentations in alcohol dependence: age at onset matters. J Stud Alcohol Drugs. 2011;72(5):752–762. doi: 10.15288/jsad.2011.72.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Strat Y, Grant BF, Ramoz N, Gorwood P. A new definition of early age at onset in alcohol dependence. Drug Alcohol Depend. 2010;108(1–2):43–48. doi: 10.1016/j.drugalcdep.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu IC, Blacker DL, Xu R, Fitzmaurice G, Tsuang MT, Lyons MJ. Genetic and environmental contributions to age of onset of alcohol dependence symptoms in male twins. Addiction. 2004;99(11):1403–1409. doi: 10.1111/j.1360-0443.2004.00877.x. [DOI] [PubMed] [Google Scholar]
- 7.Cloninger CR, Sigvardsson S, Gilligan SB, von Knorring AL, Reich T, Bohman M. Genetic heterogeneity and the classification of alcoholism. Adv Alcohol Subst Abuse. 1988;7(3–4):3–16. doi: 10.1300/J251v07n03_02. [DOI] [PubMed] [Google Scholar]
- 8.Pombo S, Lesch OM. The alcoholic phenotypes among different multidimensional typologies: similarities and their classification procedures. Alcohol Alcohol. 2009;44(1):46–54. doi: 10.1093/alcalc/agn080. [DOI] [PubMed] [Google Scholar]
- 9.Johnson BA, Cloninger CR, Roache JD, Bordnick PS, Ruiz P. Age of onset as a discriminator between alcoholic subtypes in a treatment-seeking outpatient population. Am J Addict. 2000;9(1):17–27. doi: 10.1080/10550490050172191. [DOI] [PubMed] [Google Scholar]
- 10.Buydens-Branchey L, Branchey MH, Noumair D. Age of alcoholism onset. I. Relationship to psychopathology. Arch Gen Psychiatry. 1989;46(3):225–230. doi: 10.1001/archpsyc.1989.01810030031004. [DOI] [PubMed] [Google Scholar]
- 11.Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236(4800):410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- 12.Cloninger CR. The psychobiological regulation of social cooperation. Nat Med. 1995;1(7):623–624. doi: 10.1038/nm0795-623. [DOI] [PubMed] [Google Scholar]
- 13.Storvik M, Haukijärvi T, Tupala E, Tiihonen J. Correlation between the SERT binding densities in hypothalamus and amygdala in Cloninger type 1 and 2 alcoholics. Alcohol Alcohol. 2008;43(1):25–30. doi: 10.1093/alcalc/agm157. [DOI] [PubMed] [Google Scholar]
- 14.Tupala E, Hall H, Bergström K, et al. Different effect of age on dopamine transporters in the dorsal and ventral striatum of controls and alcoholics. Synapse. 2003;48(4):205–211. doi: 10.1002/syn.10206. [DOI] [PubMed] [Google Scholar]
- 15.Hallikainen T, Saito T, Lachman HM, et al. Association between low activity serotonin transporter promoter genotype and early onset alcoholism with habitual impulsive violent behavior. Mol Psychiatry. 1999;4(4):385–388. doi: 10.1038/sj.mp.4000526. [DOI] [PubMed] [Google Scholar]
- 16.Chung IW, Kim H, Sribney W, et al. Tryptophan hydroxylase polymorphism is associated with age of onset of alcoholism related behaviors. Alcohol. 2005;36(1):1–3. doi: 10.1016/j.alcohol.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Thompson MD, Kenna GA. Variation in the serotonin transporter gene and alcoholism: risk and response to pharmacotherapy. Alcohol Alcohol. 2016;51(2):164–171. doi: 10.1093/alcalc/agv090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berglund K, Fahlke C, Berggren U, Eriksson M, Balldin J. Personality profile in type I alcoholism: long duration of alcohol intake and low serotonergic activity are predictive factors of anxiety proneness. J Neural Transm. 2006;113(9):1287–1298. doi: 10.1007/s00702-005-0412-3. [DOI] [PubMed] [Google Scholar]
- 19.Fils-Aime ML, Eckardt MJ, George DT, Brown GL, Mefford I, Linnoila M. Early-onset alcoholics have lower cerebrospinal fluid 5-hydroxyindoleacetic acid levels than late-onset alcoholics. Arch Gen Psychiatry. 1996;53(3):211–216. doi: 10.1001/archpsyc.1996.01830030029006. [DOI] [PubMed] [Google Scholar]
- 20.Swann AC, Johnson BA, Cloninger CR, Chen YR. Relationships of plasma tryptophan availability to course of illness and clinical features of alcoholism: a preliminary study. Psychopharmacology. 1999;143(4):380–384. doi: 10.1007/s002130050962. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan JL, Baenziger JC, Wagner DL, Rauscher FP, Nurnberger JI, Holmes JS. Platelet MAO in subtypes of alcoholism. Biol Psychiatry. 1990;27(8):911–922. doi: 10.1016/0006-3223(90)90473-f. [DOI] [PubMed] [Google Scholar]
- 22.Roache JD, Wang Y, Ait-Daoud N, Johnson BA. Prediction of serotonergic treatment efficacy using age of onset and type A/B typologies of alcoholism. Alcohol Clin Exp Res. 2008;32(8):1502–1512. doi: 10.1111/j.1530-0277.2008.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kranzler HR, Pierucci-Lagha A, Feinn R, Hernandez-Avila C. Effects of ondansetron in early- versus late-onset alcoholics: a prospective, open-label study. Alcohol Clin Exp Res. 2003;27(7):1150–1155. doi: 10.1097/01.ALC.0000075547.77464.76. [DOI] [PubMed] [Google Scholar]
- 24.Pettinati HM, Volpicelli JR, Kranzler HR, Luck G, Rukstalis MR, Cnaan A. Sertraline treatment for alcohol dependence: interactive effects of medication and alcoholic subtype. Alcohol Clin Exp Res. 2000;24(7):1041–1049. [PubMed] [Google Scholar]
- 25.Kranzler HR, Burleson JA, Brown J, Babor TF. Fluoxetine treatment seems to reduce the beneficial effects of cognitive-behavioral therapy in type B alcoholics. Alcohol Clin Exp Res. 1996;20(9):1534–1541. doi: 10.1111/j.1530-0277.1996.tb01696.x. [DOI] [PubMed] [Google Scholar]
- 26.Lemarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse, and dependence: Clinical evidence. Biol Psychiatry. 1994;36(5):326–337. doi: 10.1016/0006-3223(94)90630-0. [DOI] [PubMed] [Google Scholar]
- 27.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38(8):1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 28.Thompson MD, Noble-Topham S, Percy ME, Andrade DM, Ebers GC. Chromosome 1p36 in migraine with aura: association study of the 5HT(1D) locus. Neuroreport. 2012;23(1):45–48. doi: 10.1097/WNR.0b013e32834e5af3. [DOI] [PubMed] [Google Scholar]
- 29.O’Dell LE, Parsons LH. Serotonin1B receptors in the ventral tegmental area modulate cocaine-induced increases in nucleus accumbens dopamine levels. J Pharmacol Exp Ther. 2004;311(2):711–719. doi: 10.1124/jpet.104.069278. [DOI] [PubMed] [Google Scholar]
- 30.Crabbe JC, Phillips TJ, Feller DJ, et al. Elevated alcohol consumption in null mutant mice lacking 5–HT1B serotonin receptors. Nat Genet. 1996;14(1):98–101. doi: 10.1038/ng0996-98. [DOI] [PubMed] [Google Scholar]
- 31.Fish EW, McKenzie-Quirk SD, Bannai M, Miczek KA. 5-HT(1B) receptor inhibition of alcohol-heightened aggression in mice: comparison to drinking and running. Psychopharmacology. 2008;197(1):145–156. doi: 10.1007/s00213-007-1017-3. [DOI] [PubMed] [Google Scholar]
- 32.Lappalainen J, Long JC, Eggert M, et al. Linkage of antisocial alcoholism to the serotonin 5-HT1B receptor gene in 2 populations. Arch Gen Psychiatry. 1998;55(11):989–994. doi: 10.1001/archpsyc.55.11.989. [DOI] [PubMed] [Google Scholar]
- 33.Sun HF, Chang YT, Fann CS, et al. Association study of novel human serotonin 5-HT(1B) polymorphisms with alcohol dependence in Taiwanese Han. Biol Psychiatry. 2002;51(11):896–901. doi: 10.1016/s0006-3223(01)01366-x. [DOI] [PubMed] [Google Scholar]
- 34.Hasegawa Y, Higuchi S, Matsushita S, Miyaoka H. Association of a polymorphism of the serotonin 1B receptor gene and alcohol dependence with inactive aldehyde dehydrogenase-2. J Neural Transm. 2002;109(4):513–521. doi: 10.1007/s007020200042. [DOI] [PubMed] [Google Scholar]
- 35.Soyka M, Preuss UW, Koller G, Zill P, Bondy B. Association of 5-HT1B receptor gene and antisocial behavior in alcoholism. J Neural Transm. 2004;111(1):101–109. doi: 10.1007/s00702-003-0064-0. [DOI] [PubMed] [Google Scholar]
- 36.Duan J, Sanders AR, Molen JE, et al. Polymorphisms in the 5′-untranslated region of the human serotonin receptor 1B (HTR1B) gene affect gene expression. Mol Psychiatry. 2003;8(11):901–910. doi: 10.1038/sj.mp.4001403. [DOI] [PubMed] [Google Scholar]
- 37.Kranzler HR, Hernandez-Avila CA, Gelernter J. Polymorphism of the 5-HT1B receptor gene (HTR1B): strong within-locus linkage disequilibrium without association to antisocial substance dependence. Neuropsychopharmacology. 2002;26(1):115–122. doi: 10.1016/S0893-133X(01)00283-4. [DOI] [PubMed] [Google Scholar]
- 38.Sander T, Ostapowicz A, Samochowiec J, et al. Evaluation of an allelic association of the serotonin 5-HT1B G681C polymorphism with antisocial alcoholism in the German population. Addict Biol. 2000;5(2):167–172. doi: 10.1080/13556210050003757. [DOI] [PubMed] [Google Scholar]
- 39.Sinha R, Cloninger CR, Parsian A. Linkage disequilibrium and haplotype analysis between serotonin receptor 1b gene variations and subtypes of alcoholism. Am J Med Genet B Neuropsychiatr Genet. 2003;121B(1):83–88. doi: 10.1002/ajmg.b.20064. [DOI] [PubMed] [Google Scholar]
- 40.Jensen KP, Covault J, Conner TS, Tennen H, Kranzler HR, Furneaux HM. A common polymorphism in serotonin receptor 1B mRNA moderates regulation by miR-96 and associates with aggressive human behaviors. Mol Psychiatry. 2009;14(4):381–389. doi: 10.1038/mp.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Contini V, Bertuzzi GP, Polina ER, et al. A haplotype analysis is consistent with the role of functional HTR1B variants in alcohol dependence. Drug Alcohol Depend. 2012;122(1–2):100–104. doi: 10.1016/j.drugalcdep.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 42.Plemenitas A, Kastelic M, O Porcelli S, Serretti A, Dolžan V, Kores Plesnicar B. Alcohol dependence and genetic variability in the serotonin pathway among currently and formerly alcohol-dependent males. Neuropsychobiology. 2015;72(1):57–64. doi: 10.1159/000437432. [DOI] [PubMed] [Google Scholar]
- 43.Cook EH, Fletcher KE, Wainwright M, Marks N, Yan SY, Leventhal BL. Primary structure of the human platelet serotonin 5-HT2A receptor: identify with frontal cortex serotonin 5-HT2A receptor. J Neurochem. 1994;63(2):465–469. doi: 10.1046/j.1471-4159.1994.63020465.x. [DOI] [PubMed] [Google Scholar]
- 44.Mercado CP, Kilic F. Molecular mechanisms of SERT in platelets: regulation of plasma serotonin levels. Mol Interv. 2010;10(4):231–241. doi: 10.1124/mi.10.4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruchkin VV, Koposov RA, af Klinteberg B, Oreland L, Grigorenko EL. Platelet MAO-B, personality, and psychopathology. J Abnorm Psychol. 2005;114:477–482. doi: 10.1037/0021-843X.114.3.477. [DOI] [PubMed] [Google Scholar]
- 46.Serebruany VL, El Mouelhi M, Pfannkuche HJ, Rose K, Marro M, Angiolillo DJ. Investigations on 5-HT4 receptor expression and effects of tegaserod on human platelet aggregation in vitro. Am J Ther. 2010;17(6):543–552. doi: 10.1097/MJT.0b013e3181b63f21. [DOI] [PubMed] [Google Scholar]
- 47.Stratz C, Trenk D, Bhatia HS, Valina C, Neumann FJ, Fiebich BL. Identification of 5-HT3 receptors on human platelets: increased surface immunoreactivity after activation with adenosine diphosphate (ADP) and thrombin receptor-activating peptide (TRAP) Thromb Haemost. 2008;99(4):784–786. doi: 10.1160/TH07-10-0630. [DOI] [PubMed] [Google Scholar]
- 48.Zhang ZJ, Wang D, Man SC, et al. Platelet 5-HT(1A) receptor correlates with major depressive disorder in drug-free patients. Prog Neuropsychopharmacol Biol Psychiatry. 2014;53:74–79. doi: 10.1016/j.pnpbp.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Mück-Šeler D, Pivac N. Serotonin. Period Biol. 2011;113:29–41. [Google Scholar]
- 50.Massot O, Rousselle JC, Fillion MP, Januel D, Plantefol M, Fillion G. 5-HT1B receptors: a novel target for lithium. Possible involvement in mood disorders. Neuropsychopharmacology. 1999;21(4):530–541. doi: 10.1016/S0893-133X(99)00042-1. [DOI] [PubMed] [Google Scholar]
- 51.Warkentin TE, Arnold DM, Nazi I, Kelton JG. The platelet serotonin-release assay. Am J Hematol. 2015;90(6):564–572. doi: 10.1002/ajh.24006. [DOI] [PubMed] [Google Scholar]
- 52.Oreland L. Platelet monoamine oxidase, personality and alcoholism: the rise, fall and resurrection. Neurotoxicol. 2004;25(1–2):79–89. doi: 10.1016/S0161-813X(03)00115-3. [DOI] [PubMed] [Google Scholar]
- 53.Yubero-Lahoz S, Robledo P, Farré M, de Latorre R, Torre R. Platelet SERT as a peripheral biomarker of serotonergic neurotransmission in the central nervous system. Curr Med Chem. 2013;20(11):1382–1396. doi: 10.2174/0929867311320110003. [DOI] [PubMed] [Google Scholar]
- 54.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington DC: American Psychiatric Press; 1994. [Google Scholar]
- 55.Pivac N, Mück-Seler D, Barisić I, Jakovljević M, Puretić Z. Platelet serotonin concentration in dialysis patients with somatic symptoms of depression. Life Sci. 2001;68(21):2423–2433. doi: 10.1016/s0024-3205(01)01034-7. [DOI] [PubMed] [Google Scholar]
- 56.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 57.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 59.Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004;5(3):R13. doi: 10.1186/gb-2004-5-3-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smalheiser NR, Lugli G, Rizavi HS, et al. MicroRNA expression in rat brain exposed to repeated inescapable shock: differential alterations in learned helplessness vs. non-learned helplessness. Int J Neuropsychopharmacol. 2011;14(10):1315–1325. doi: 10.1017/S1461145710001628. [DOI] [PubMed] [Google Scholar]
- 61.Furay AR, Neumaier JF, Mullenix AT, Kaiyala KK, Sandygren NK, Hoplight BJ. Overexpression of 5-HT(1B) mRNA in nucleus accumbens shell projection neurons differentially affects microarchitecture of initiation and maintenance of ethanol consumption. Alcohol. 2011;45(1):19–32. doi: 10.1016/j.alcohol.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoplight BJ, Sandygren NA, Neumaier JF. Increased expression of 5-HT1B receptors in rat nucleus accumbens via virally mediated gene transfer increases voluntary alcohol consumption. Alcohol. 2006;38(2):73–79. doi: 10.1016/j.alcohol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 63.Bannai M, Fish EW, Faccidomo S, Miczek KA. Anti-aggressive effects of agonists at 5-HT1B receptors in the dorsal raphe nucleus of mice. Psychopharmacology. 2007;193(2):295–304. doi: 10.1007/s00213-007-0780-5. [DOI] [PubMed] [Google Scholar]
- 64.Drago A, Alboni S, Brunello N, Nicoletta B, de Ronchi D, Serretti A. HTR1B as a risk profile maker in psychiatric disorders: a review through motivation and memory. Eur J Clin Pharmacol. 2010;66(1):5–27. doi: 10.1007/s00228-009-0724-6. [DOI] [PubMed] [Google Scholar]
- 65.Sari Y. Serotonin1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev. 2004;28(6):565–582. doi: 10.1016/j.neubiorev.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 66.Berg KA, Clarke WP. Regulation of 5-HT(1A) and 5-HT(1B) receptor systems by phospholipid signaling cascades. Brain Res Bull. 2001;56(5):471–477. doi: 10.1016/s0361-9230(01)00645-1. [DOI] [PubMed] [Google Scholar]
- 67.Tournois C, Mutel V, Manivet P, Launay JM, Kellermann O. Crosstalk between 5-hydroxytryptamine receptors in a serotonergic cell line. Involvement of arachidonic acid metabolism. J Biol Chem. 1998;273(28):17498–17503. doi: 10.1074/jbc.273.28.17498. [DOI] [PubMed] [Google Scholar]
- 68.Montañez S, Munn JL, Owens WA, Horton RE, Daws LC. 5-HT1B receptor modulation of the serotonin transporter in vivo: studies using KO mice. Neurochem Int. 2014;73:127–131. doi: 10.1016/j.neuint.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bailly D, Vignau J, Lauth B, et al. Platelet serotonin decrease in alcoholic patients. Acta Psychiatr Scand. 1990;81(1):68–72. doi: 10.1111/j.1600-0447.1990.tb06451.x. [DOI] [PubMed] [Google Scholar]
- 70.Pivac N, Mück-Seler D, Mustapić M, Nenadić-Sviglin K, Kozarić-Kovacić D. Platelet serotonin concentration in alcoholic subjects. Life Sci. 2004;76(5):521–531. doi: 10.1016/j.lfs.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 71.Schmidt LG, Dufeu P, Heinz A, Kuhn S, Rommelspacher H. Serotonergic dysfunction in addiction: effects of alcohol, cigarette smoking and heroin on platelet 5-HT content. Psychiatry Res. 1997;72(3):177–185. doi: 10.1016/s0165-1781(97)00102-9. [DOI] [PubMed] [Google Scholar]
- 72.Javors M, Tiouririne M, Prihoda T. Platelet serotonin uptake is higher in early-onset than in late-onset alcoholics. Alcohol Alcohol. 2000;35(4):390–393. doi: 10.1093/alcalc/35.4.390. [DOI] [PubMed] [Google Scholar]
- 73.Lowery CL, Elliott C, Cooper A, et al. Cigarette smoking-associated alterations in serotonin/adrenalin signaling pathways of platelets. J Am Heart Assoc. 2017;6(5):e005465. doi: 10.1161/JAHA.116.005465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nenadic-Sviglin K, Nedic G, Nikolac M, et al. Suicide attempt, smoking, comorbid depression, and platelet serotonin in alcohol dependence. Alcohol. 2011;45(3):209–216. doi: 10.1016/j.alcohol.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 75.Patkar AA, Gopalakrishnan R, Berrettini WH, Weinstein SP, Vergare MJ, Leone FT. Differences in platelet serotonin transporter sites between African-American tobacco smokers and non-smokers. Psychopharmacology. 2003;166(3):221–227. doi: 10.1007/s00213-002-1353-2. [DOI] [PubMed] [Google Scholar]
- 76.Fowler JS, Volkow ND, Wang GJ, et al. Inhibition of monoamine oxidase B in the brains of smokers. Nature. 1996;379(6567):733–736. doi: 10.1038/379733a0. [DOI] [PubMed] [Google Scholar]
- 77.Fowler JS, Volkow ND, Wang GJ, et al. Brain monoamine oxidase A inhibition in cigarette smokers. Proc Natl Acad Sci U S A. 1996;93(24):14065–14069. doi: 10.1073/pnas.93.24.14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Berlin I, Anthenelli RM. Monoamine oxidases and tobacco smoking. Int J Neuropsychopharm. 2001;4(01):33–42. doi: 10.1017/S1461145701002188. [DOI] [PubMed] [Google Scholar]
- 79.Whitfield JB, Pang D, Bucholz KK, et al. Monoamine oxidase: associations with alcohol dependence, smoking and other measures of psychopathology. Psychol Med. 2000;30(2):443–454. doi: 10.1017/s0033291799001798. [DOI] [PubMed] [Google Scholar]


