Abstract
Background
Cataract surgery is practiced widely, and substantial resources are committed to an increasing cataract surgical rate in low‐ and middle‐income countries. With the current volume of cataract surgery and future increases, it is critical to optimize the safety and cost‐effectiveness of this procedure. Most cataracts are performed on older individuals with correspondingly high systemic and ocular comorbidities. It is likely that routine preoperative medical testing will detect medical conditions, but it is questionable whether these conditions should preclude individuals from cataract surgery or change their perioperative management.
Objectives
1. To investigate the evidence for reductions in adverse events through preoperative medical testing
2. To estimate the average cost of performing routine medical testing
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) (2018, Issue 6); Ovid MEDLINE; Embase.com; PubMed; LILACS BIREME, the metaRegister of Controlled Trials (mRCT) (last searched 5 January 2012); ClinicalTrials.gov and the WHO ICTRP. The date of the search was 29 June 2018, with the exception of mRCT which is no longer in service. We searched the references of reports from included studies for additional relevant studies without restrictions regarding language or date of publication.
Selection criteria
We included randomized clinical trials in which routine preoperative medical testing was compared to no preoperative or selective preoperative testing prior to age‐related cataract surgery.
Data collection and analysis
Two review authors independently assessed abstracts to identify possible trials for inclusion. For each included study, two review authors independently documented study characteristics, extracted data, and assessed risk of bias.
Main results
We identified three randomized clinical trials that compared routine preoperative medical testing versus selective or no preoperative testing for 21,531 cataract surgeries. The largest trial, in which 19,557 surgeries were randomized, was conducted in Canada and the USA. Another study was conducted in Brazil and the third in Italy. Although the studies had some issues with respect to performance and detection bias due to lack of masking (high risk for one study, unclear for two studies), we assessed the studies as at overall low risk of bias.
The three randomized clinical trials included in this review reported results for 21,531 total cataract surgeries with 707 total surgery‐associated medical adverse events, including 61 hospitalizations and three deaths. Of the 707 medical adverse events reported, 353 occurred in the pre‐testing group and 354 occurred in the no‐testing group (odds ratio (OR) 1.00, 95% confidence interval (CI) 0.86 to 1.16; high‐certainty evidence). Most events were cardiovascular and occurred during the intraoperative period. Routine preoperative medical testing did not reduce the risk of intraoperative (OR 0.99, 95% CI 0.71 to 1.38) or postoperative ocular adverse events (OR 1.11, 95% CI 0.74 to 1.67) when compared to selective or no testing (2 studies; 2281 cataract surgeries; moderate‐certainty evidence). One study evaluated cost savings, estimating the costs to be 2.55 times higher in those with preoperative medical testing compared to those without preoperative medical testing (1 study; 1005 cataract surgeries; moderate‐certainty evidence). There was no difference in cancellation of surgery between those with preoperative medical testing and those with selective or no preoperative testing, reported by two studies with 20,582 cataract surgeries (OR 0.97, 95% CI 0.78 to 1.21; high‐certainty evidence). No study reported outcomes related to clinical management changes (other than cancellation) or quality of life scores.
Authors' conclusions
This review has shown that routine preoperative testing does not increase the safety of cataract surgery. Alternatives to routine preoperative medical testing have been proposed, including self administered health questionnaires, which could substitute for health provider histories and physical examinations. Such avenues may lead to cost‐effective means of identifying those at increased risk of medical adverse events due to cataract surgery. However, despite the rare occurrence, adverse medical events precipitated by cataract surgery remain a concern because of the large number of elderly patients with multiple medical comorbidities who have cataract surgery in various settings. The studies summarized in this review should assist recommendations for the standard of care of cataract surgery, at least in low‐ and middle‐income settings. Unfortunately, in these settings, medical history questionnaires may be useless to screen for risk because few people have ever been to a physician, let alone been diagnosed with any chronic disease.
Plain language summary
Routine preoperative medical testing for cataract surgery
What is the aim of this review? The aim of this Cochrane Review was to determine whether it is necessary to perform preoperative medical testing before cataract surgery.
Key messages Preoperative medical testing did not reduce the risk of medical adverse events during or after cataract surgery when compared to selective or no testing.
What was studied in the review? Cataract surgery is practiced widely, and substantial resources are being committed to increasing the cataract surgical rate in low‐ and middle‐income countries. With the current volume of cataract surgery and future increases, it is critical to be able to optimize the safety, but also the cost‐effectiveness of this procedure. Most cataracts are age‐related, and therefore surgeries are performed on older individuals with other health and eye conditions. It is likely that preoperative medical testing will detect medical conditions, but it is questionable whether these conditions should preclude these individuals from cataract surgery or change their perioperative management.
What are the main results of the review? We included three studies in this review. One study was from Canada and the USA, another from Brazil, and the third from Italy. These studies compared routine preoperative medical testing versus selective or no testing. Study participants were followed from one week to two months after surgery.
The review shows the following.
• Preoperative medical testing did not reduce the risk of medical adverse events during or after cataract surgery when compared to selective or no testing (high‐certainty evidence). The three studies reported results for 21,531 total cataract surgeries with 707 total surgery‐associated medical adverse events, including 61 hospitalizations and three deaths. Of the 707 medical adverse events reported, 353 occurred in the pre‐testing group and 354 occurred in the no‐testing group. • No clear difference was observed for the occurrence of eye‐related adverse events during or after surgery (moderate‐certainty evidence). • One study evaluated cost, estimating the cost to be 2.55 times higher in those who had routine preoperative medical testing compared to those who had selective preoperative testing (moderate‐certainty evidence). • There was no difference in the cancellation of surgery between those with routine preoperative medical testing and those with no or selective preoperative testing (high‐certainty evidence). • No study reported changes in surgical management (other than cancellation of surgery) or quality of life measures (evidence gaps).
How up‐to‐date is this review? We searched for studies that had been published up to 29 June 2018.
Summary of findings
Summary of findings for the main comparison. Routine preoperative medical testing compared with selective or no testing for cataract surgery.
| Routine preoperative medical testing compared with selective or no testing for cataract surgery | |||||
|
Population: adults with age‐related cataract Settings: hospital or clinic Intervention: routine preoperative medical testing Comparison: selective or no preoperative medical testing | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Selective or no testing | Preoperative medical testing | ||||
|
Medical adverse events up to 2 months after surgery |
33 per 1000 | 33 per 1000 (28 to 38) | OR 1.00 (0.86 to 1.16) | 21,531 (3 studies) | ⊕⊕⊕⊕ high |
| Ocular adverse events up to 2 months after surgery | Intraoperative | OR 0.99 (0.71 to 1.38) | 2281 (2 studies) | ⊕⊕⊕⊝ moderate1 | |
| 69 per 1000 | 68 per 1000 (49 to 96) | ||||
| Postoperative | OR 1.11 (0.74 to 1.67) | ||||
| 43 per 1000 | 48 per 1000 (32 to 72) | ||||
|
Cost for preoperative testing prior to surgery |
BRL 4.32 per patient | BRL 11.00 per patient | Ratio 2.55 | 1005 (1 study) | ⊕⊕⊕⊝ moderate2 |
|
Cancellation of cataract surgery prior to surgery |
16 per 1000 | 16 per 1000 (13 to 20) | OR 0.97 (0.78 to 1.21) | 20,582 (2 studies) | ⊕⊕⊕⊕ high |
|
Clinical management changes (other than cancellation) prior to surgery |
Not reported by any included study | ||||
|
Quality of life outcomes at any follow‐up time point |
Not reported by any included study | ||||
| *The basis for the assumed risk is the comparison group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention group (and its 95% CI). CI: confidence interval; OR: odds ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Downgraded for imprecision of the estimate (i.e. wide confidence intervals). 2Downgraded for reporting bias due to lack of information regarding the confidence interval around the effect estimate.
Background
Description of the condition
Cataract surgery is a highly cost‐effective means of vision restoration, and approximately 10 million surgeries are performed each year around the world (Foster 2007). In economically well‐developed countries, cataract surgeries are performed at a rate of 4000 to 6000 per million population annually (Foster 2007). It is estimated that approximately 100 million eyes are blind due to cataract, and three to four times that number are visually impaired. Aside from the direct impact of blindness and visual impairment, the risk of physical injury, such as hip fracture, increases for people with cataracts (Ivers 2003). Continued independent living and general quality of life are reduced in individuals with unoperated cataracts (Taylor 2006).
In mild cataract, vision can be optimized through good lighting, however with progression the cataract becomes dense enough to cause functional visual impairment or blindness. There are other problems encountered with unoperated cataract. The lenticular changes associated with cataract can lead to index myopia; refractive error increases rapidly and at a different rate in each eye leading to significant anisometropia. Refractive correction becomes problematic in these circumstances and is best managed in the long term by surgical intervention (Dandona 2001). Surgery is the only long‐term remedy for cataract blindness, and the best postoperative result occurs when a replacement intraocular lens is implanted (Fletcher 1998; Riaz 2006).
As discussed above, cataract surgery is practiced widely, and substantial resources are being committed to increasing the cataract surgical rate in low‐ and middle‐income countries. With the current volume of cataract surgery and future increases, it is critical to be able to optimize the safety, but also the cost‐effectiveness of this procedure. Surveys have shown that the majority of clinicians involved order a range of pre‐surgical medical tests, despite suspicion that the tests are unnecessary (Bass 1995). The focus of this review was the medical effectiveness of pre‐surgical medical testing.
The primary outcome of this review was medical adverse events that resulted in death or hospitalization and that had a plausible, causal relationship to the cataract surgery. In addition to adverse events resulting in death or hospitalization, we also investigated adverse events requiring initiation of medical treatment including hypertension and new or worsening cardiac arrhythmia, myocardial infarction, myocardial ischemia, congestive heart failure, hypotension, stroke, respiratory failure, and hypoglycemia. These events were defined by accepted clinical or laboratory criteria, or both. In this review we included both intraoperative and postoperative events in the definition of medical adverse events secondary to cataract surgery.
Description of the intervention
The intervention under review was routine pre‐surgical medical testing to identify patients who could not safely undergo cataract surgery.
Preoperative testing: any diagnostic testing performed as part of the preoperative medical‐testing process, including complete blood counts and various serum measurements, chest x‐ray, or electrocardiography that is not done for the direct purpose of managing a pre‐existing medical condition.
How the intervention might work
Most cataracts are age‐related, and therefore surgeries are performed on older individuals with correspondingly high systemic and ocular comorbidities. In a national study in the UK, the mean age was 76 years, and 57% had a medical disorder at the time of cataract surgery (Desai 1999). It is likely that preoperative medical testing will detect medical conditions, but it is questionable whether these conditions should preclude these individuals from cataract surgery or change their perioperative management.
A successful intervention would identify, with reasonable specificity and sensitivity, those individuals at significant risk of a perioperative adverse medical event whose outcome could be favorably affected by postponing surgery or altering the perioperative medical management (Katz 2001).
Why it is important to do this review
The large volume of cataract surgeries performed now and projected for the future provides sufficient rationale to investigate the utility of routine pre‐surgical medical testing.
There is evidence from at least three randomized clinical trials, Cavallini 2004; Lira 2001; Schein 2000, suggesting that preoperative medical testing for cataract surgery does not protect against medical adverse events. Furthermore, there are substantial cost savings when redundant medical testing is avoided (Imasogie 2003). In the majority of cases, cataract surgery involves local anesthesia (Guay 2015), which is in some cases combined with intravenous sedation. Surgeries are usually performed on an outpatient basis, and medical complications are very rare (Schein 2000).
Unwarranted postponement or cancellation of surgery delays visual rehabilitation for cataract surgery candidates and misuses resources, particularly if surgery is canceled on the day it is scheduled. Conversely, routine preoperative testing may be beneficial for detecting health conditions that could preclude patients from safely undergoing cataract surgery.
Objectives
To investigate the evidence for reductions in adverse events through preoperative medical testing
To estimate the average cost of performing routine medical testing
Methods
Criteria for considering studies for this review
Types of studies
We included randomized clinical trials in the review.
Types of participants
We included all individuals who required cataract surgery due to age‐related cataract. We excluded participants with congenital cataract.
Types of interventions
We included trials in which routine pre‐surgical medical testing was compared to no routine preoperative or selective preoperative testing prior to cataract surgery. Examples of preoperative medical testing included electrocardiography, complete blood counts, and various serum measurements. Selective preoperative medical testing was limited to health status questionnaires.
Types of outcome measures
Primary outcomes
The primary outcome of the review was the risk of medical adverse events that occurred within seven days of surgery and had a plausible causal relationship to the surgery. Medical adverse events were classified as intraoperative or postoperative as defined by each study. We assessed risks of death and hospitalization individually.
Secondary outcomes
Ocular adverse events, as reported.
Cost‐effectiveness of medical testing.
The proportion of participants for which surgery was postponed or canceled on the basis of the medical screening. We measured the impact of these actions by the cost of rescheduling surgery and delay in receiving visual rehabilitation.
The proportion of participants who underwent a change in the clinical management of their underlying medical condition due to findings on routine preoperative testing.
Quality of life data, measured by any validated scale.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist searched the following electronic databases for RCTs and controlled clinical trials. We imposed no language or publication year restrictions.
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 6) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 29 June 2018) (Appendix 1).
MEDLINE Ovid (1946 to 29 June 2018) (Appendix 2).
Embase.com (1947 to 29 June 2018) (Appendix 3).
PubMed (1948 to June 2018) (Appendix 4).
LILACS BIREME (Latin American and Caribbean Health Science Information Database) (1982 to 29 June 2018) (Appendix 5).
metaRegister of Controlled Trials (mRCT) (last searched 5 January 2012) (Appendix 6).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 29 June 2018) (Appendix 7).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched 29 June 2018) (Appendix 8).
Searching other resources
We reviewed the reference lists from included studies to identify additional studies. We used the Science Citation Index to search for studies that have cited publications from the included trials (last searched June 2018).
Data collection and analysis
Selection of studies
Two review authors independently assessed the abstracts from the electronic literature searches and the manual search to identify possible trials of interest according to the Criteria for considering studies for this review. We classified the abstracts as (a) relevant, (b) possibly relevant, or (c) not relevant for this review. We retrieved full‐text copies of the articles if either review author classified an abstract as (a) or (b). Two review authors then independently assessed and classified each article as (1) include in review, (2) awaiting assessment, or (3) exclude from review. Discrepancies between authors were resolved by a third review author. For studies classified initially as (2), we contacted the study authors for further information to enable us to make a determination on the study.
Data extraction and management
Two review authors independently extracted data using the data extraction forms created by Cochrane Eyes and Vision. We extracted data on study characteristics, interventions, outcomes, cost and quality of life, and other relevant information. One review author entered the data into Review Manager 5 (RevMan 5) (Review Manager 2014), and a second review author verified the data entry. Discrepancies between review authors were resolved by a third review author. In the case of unclear or unreported data, we attempted to contact authors of the study.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias of the included studies based on the methods in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Sources of bias affecting the findings of a study included selection bias, performance bias, attrition bias, detection bias, and reporting bias. We assessed the risk of bias of each included study as low, high, or unclear. Discrepancies between review authors were resolved by a third review author. For 'Risk of bias' domains classified as unclear due to incomplete or unreported information, we contacted the authors of the study for further information in an attempt to reclassify the 'Risk of bias' assessment. If we received no response within eight weeks, we classified the study using the information available.
Measures of treatment effect
The primary outcome of the review was the risk of medical adverse events, including the risk of death and the risk of hospitalization. As the outcome was rare in the included studies, we summarized it as an odds ratio. We calculated the risk difference by estimating the total number of candidates for surgery who needed to be screened in order to prevent one adverse event.
Dichotomous data
We reported dichotomous data analysis (deaths or hospitalizations after cataract surgery) as a summarized odds ratio with 95% confidence intervals (CI).
Continuous data
We reported continuous data analysis (economic and quality of life), if evaluated, as a weighted mean difference with standard deviations.
Unit of analysis issues
The unit of analysis for this review was an individual cataract surgery in one eye.
Dealing with missing data
All three included studies reported sufficient data on the primary outcome of this review. If data were missing, we contacted the authors of the study in an attempt to obtain the missing data or imputed data from existing data. We set the response time at eight weeks.
Assessment of heterogeneity
We assessed statistical heterogeneity using forest plots and the I2 statistic. In addition, we evaluated the distribution of results for clinical heterogeneity.
Assessment of reporting biases
We used funnel plots to assess potential small‐study effects, however as only three trials were included, examination of the funnel plots did not yield any meaningful interpretations.
Data synthesis
As the review included only three trials, we used the fixed‐effect model. Should future updates of the review include additional studies, we will perform meta‐analyses using the random‐effects model if we detect no heterogeneity. If we do detect heterogeneity, we will meta‐analyze trial results by subgroups if sufficient data are available, otherwise we will describe the results in tabular form.
Subgroup analysis and investigation of heterogeneity
We detected no heterogeneity as evaluated either statistically or clinically. If sufficient data become available in the future, we will conduct subgroup analyses for age, gender, race, and medical comorbidities.
Sensitivity analysis
We did not undertake sensitivity analyses since the review included only three studies. Should future updates of the review include additional studies, we will conduct sensitivity analyses to investigate the impact of studies with poor methodological quality or missing data and the impact of unpublished studies.
Summary of findings
Two review authors independently judged the certainty of evidence for each outcome using the GRADE approach (GRADEpro 2015). Any discrepancies were resolved by discussion. We assigned a grade of very low, low, moderate, or high certainty of evidence for each outcome. Our judgements were based on the following five criteria.
Risk of bias in individual trials
Indirectness
Heterogeneity
Imprecision of estimate (wide confidence intervals)
Publication bias
We also produced a 'Summary of findings' table with the assumed risks and corresponding risks for the six outcomes evaluated in this review (risk of medical adverse events; risk of ocular adverse events; cost of medical testing; risk of postponed or canceled surgery; risk of change in the clinical management; and change in quality of life scores).
Results
Description of studies
Results of the search
The initial electronic search of the literature conducted in December 2008 identified 1232 unique references (Keay 2009), of which 21 were assessed as relevant or possibly relevant. Full‐text assessment of the 21 references resulted in the exclusion of 12 references from 11 studies, and the inclusion of nine references from three studies. A manual search of the reference lists from the nine included publications identified 21 additional references, of which six were assessed as relevant or possibly relevant. We excluded five of these references, and one was an additional reference to an already included study. The 10 included study references were entered into the Science Citation Index, yielding 75 additional references, all of which were assessed as not relevant.
We performed an updated search in December 2011 (Keay 2012). After de‐duplication, the search identified a total of 535 references consisting of three abstracts from clinical trial registers and 532 abstracts from journals. Two review authors independently assessed the abstracts, but none met our inclusion criteria. We also performed an updated search of the Science Citation Index for the original 10 included study references. We found and assessed a further 89 references, but none were relevant to the review.
For this review update, we conducted an updated search on 29 June 2018 that identified a total of 3843 new, unique references (Figure 1). Two review authors independently assessed these references and excluded 3841 non‐relevant records, among which two were assessed in full and excluded as not evaluating eligible interventions (Dessy 2017; NCT02903485). An updated search of the Science Citation Index for the 10 included study references identified 320 references, none of which were relevant to the review.
1.
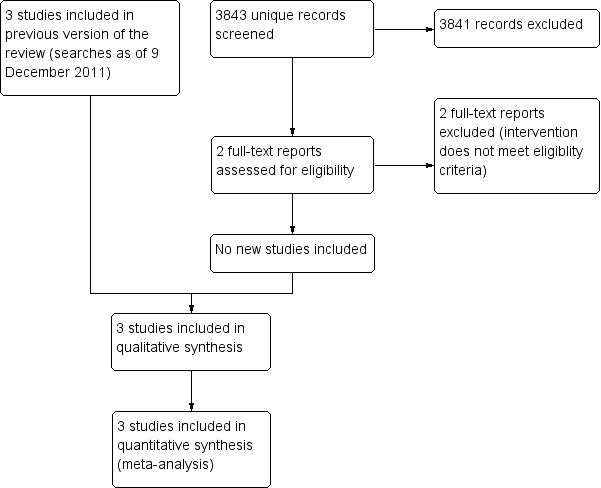
Study flow diagram.
Included studies
We included three randomized clinical trials that examined the impact of routine pre‐surgical medical testing on the risk of medical adverse events. The first was a large, multicenter study in the USA and Canada where 19,557 cataract surgeries were randomized to either routine preoperative testing or no routine testing (Schein 2000). If there was a new or changing problem identified on preoperative clinical examination that would have generated testing in the absence of the planned surgery, then specific tests were conducted in the “control” group as per the direction of the attending physician. A second study was conducted in Brazil at a single center where 1025 patients needing first‐eye cataract surgeries were randomized to either routine or selective testing (Lira 2001). The authors of this study noted that 20 patients who were randomized in this study had their operations cancelled and not subsequently rescheduled; thus, only 1005 participants underwent cataract surgery. Finally, Cavallini and colleagues reported in 2004 on a single‐center study in Italy in which 1276 participants scheduled for ambulatory cataract surgery were randomly assigned to either a group whose results of routine preoperative tests were reviewed or a group whose routine medical tests were completed but kept in a sealed envelope (Cavallini 2004).
Two studies assessed intraoperative and postoperative ocular adverse events (Cavallini 2004; Lira 2001), which are reported as secondary analyses (see Effects of interventions). Schein and colleagues examined the total rate of ocular hemorrhages in relation to anticoagulant use (Schein 2000), but did not compare the routine pre‐surgical testing and no routine pre‐surgical testing groups.
Excluded studies
We excluded 17 studies after full‐text review. Reasons for their exclusion are provided in the Characteristics of excluded studies table. In summary, 16 studies were not randomized trials, and one did not evaluate routine preoperative testing versus no preoperative testing.
Risk of bias in included studies
The risk of bias in the three included studies was generally low (Figure 2). In all studies the interventions were randomly allocated in a systematic fashion, and in two studies the allocation was known to be adequately concealed from the study personnel (Cavallini 2004; Schein 2000). The fact that participants are aware of receiving preoperative medical testing means that masking (blinding) of the participants is generally not possible. The exception was Cavallini 2004, where all participants received pre‐surgical testing, but only those in the intervention group had the test results disclosed to their physician. It was possible to mask the outcome assessors to the intervention group, and this process was confirmed in the studies reported by Schein 2000 and Lira 2001. We assessed all three studies as at low risk of attrition bias and reporting bias.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1
The meta‐analysis of these studies was dominated by the large sample size in Schein 2000, which had 8.5 times more participants than the other two studies combined. While the results were therefore strongly influenced by this one study, this study was methodologically sound and had the lowest potential for bias of the three included studies. Furthermore, the conclusions from each study were in agreement.
Medical adverse events
The three included studies reported results for 21,531 total cataract surgeries. There were 707 total medical adverse events associated with cataract surgeries, including 61 hospitalizations and three deaths, in the three studies (Table 2). Of the 707 medical adverse events reported, 353 occurred in the pre‐testing group and 354 occurred in the no‐testing group (odds ratio (OR) 1.00, 95% confidence interval (CI) 0.86 to 1.16; Analysis 1.1). Most events were cardiovascular and occurred during the intraoperative period (Table 3).
1. Medical adverse events.
| Event | Number of studies* | Routine‐testing group | No‐testing group | Odds ratio (95% confidence interval) |
| Number of events (n) | Number of events (n) | |||
| Overall | ||||
| Total | 3 | 353 | 354 | 1.00 (0.86, 1.16) |
| Death | 3 | 1 | 2 | 0.50 (0.05, 5.52) |
| Hospitalization | 1 | 28 | 33 | 0.85 (0.51, 1.40) |
| Intraoperative: day of surgery, prior to discharge | ||||
| Total | 3 | 242 | 238 | 1.02 (0.85, 1.22) |
| Death | 3 | 0 | 0 | N/A |
| Hospitalization | 1 | 3 | 5 | 0.60 (0.14, 2.51) |
| Postoperative: during study follow‐up period after discharge | ||||
| Total | 2 | 116 | 121 | 0.96 (0.74, 1.24) |
| Death | 2 | 1 | 2 | 0.50 (0.05, 5.52) |
| Hospitalization | 1 | 25 | 30 | 0.83 (0.49, 1.42) |
*Event reported by three studies (routine‐testing group: n = 10,764; no‐testing group: n = 10,767); event reported by two studies: Cavallini 2004 and Schein 2000 (routine‐testing group: n = 10,262; no‐testing group: n = 10,264); event reported by one study: Schein 2000 (routine‐testing group: n = 9624; no‐testing group: n = 9626).
1.1. Analysis.
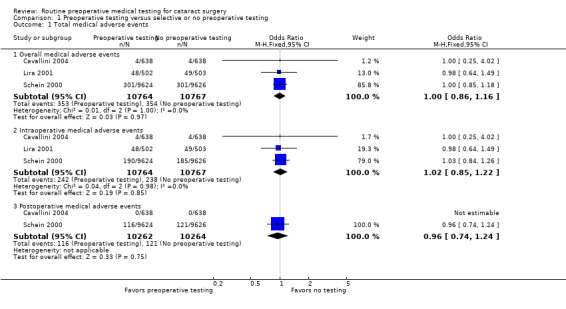
Comparison 1 Preoperative testing versus selective or no preoperative testing, Outcome 1 Total medical adverse events.
2. Types of medical adverse events.
| Adverse event | Intraoperative events | Postoperative events | ||||
| Reported by |
Routine‐testing group Number of events |
No‐testing group Number of events |
Reported by |
Routine‐testing group Number of events |
No‐testing group Number of events |
|
| Cardiovascular | ||||||
| Hypertension | Cavallini 2004; Lira 2001; Schein 2000 | 162 | 147 | Cavallini 2004; Schein 2000 | 16 | 13 |
| Hypotension | Schein 2000 | 10 | 12 | Schein 2000 | 4 | 8 |
| Arrhythmia | Lira 2001; Schein 2000 | 66 | 60 | Schein 2000 | 10 | 13 |
| Myocardial infarction | Schein 2000 | 0 | 0 | Schein 2000 | 5 | 3 |
| Myocardial ischemia | Lira 2001; Schein 2000 | 4 | 8 | Schein 2000 | 3 | 3 |
| Congestive heart failure | Schein 2000 | 0 | 0 | Schein 2000 | 5 | 5 |
| Cerebrovascular | ||||||
| Stroke | Schein 2000 | 0 | 0 | Schein 2000 | 4 | 2 |
| Transient ischemic attack | Lira 2001; Schein 2000 | 1 | 0 | Schein 2000 | 1 | 0 |
| Pulmonary | ||||||
| Respiratory failure | Schein 2000 | 0 | 0 | Schein 2000 | 1 | 1 |
| Bronchospasm | Lira 2001; Schein 2000 | 4 | 10 | Schein 2000 | 0 | 2 |
| Oxygen desaturation | Schein 2000 | 4 | 3 | Schein 2000 | 1 | 4 |
| Upper respiratory tract infection | Schein 2000 | 0 | 1 | Schein 2000 | 19 | 14 |
| Pneumonia | Schein 2000 | 0 | 0 | Schein 2000 | 6 | 5 |
| Metabolic | ||||||
| Hypoglycemia | Schein 2000 | 0 | 2 | Schein 2000 | 0 | 0 |
| Anemia | Schein 2000 | 0 | 0 | Schein 2000 | 1 | 1 |
| Hypokalemia | Schein 2000 | 0 | 0 | Schein 2000 | 2 | 0 |
| Other | ||||||
| Anxiety | Lira 2001; Schein 2000 | 2 | 2 | Schein 2000 | 2 | 0 |
| Musculoskeletal problem | Schein 2000 | 0 | 0 | Schein 2000 | 15 | 24 |
| Urinary tract infection | Schein 2000 | 0 | 0 | Schein 2000 | 9 | 11 |
| Dermatitis | Schein 2000 | 0 | 0 | Schein 2000 | 7 | 7 |
| Gastrointestinal disturbance | Schein 2000 | 0 | 0 | Schein 2000 | 12 | 11 |
| All others* | Cavallini 2004; Schein 2000 | 2 | 3 | Cavallini 2004; Schein 2000 | 8 | 6 |
Cavallini 2004: routine‐testing group: n = 638; no‐testing group: n = 638 Lira 2001: routine‐testing group: n = 502; no‐testing group: n = 503 Schein 2000: routine‐testing group: n = 9624; no‐testing group: n = 9626
*Includes atypical chest pain, chills, depression, syncope, vasovagal episode, dizziness, hyponatremia, amnesia, hyperventilation, dyspnea, and psychomotor agitation.
Preoperative medical testing did not reduce the rate of intraoperative (OR 1.02, 95% CI 0.85 to 1.22) or postoperative medical adverse events (OR 0.96, 95% CI 0.74 to 1.24) compared to selective or no testing. Lira 2001 did not evaluate postoperative medical events, therefore the latter estimate included results from only two studies.
We assessed the certainty of the evidence for medical adverse events as high, finding no reason to downgrade.
Ocular adverse events
Two of the three included studies reported the rate or types of ocular adverse events among 2281 cataract surgeries (Cavallini 2004; Lira 2001). There were 157 intraoperative ocular adverse events reported, 78 in the pre‐testing group and 79 in the selective‐ or no‐testing group (OR 0.99, 95% CI 0.71 to 1.38; Analysis 1.4). The most frequent intraoperative ocular adverse event was posterior capsule rupture (Table 4).
1.4. Analysis.
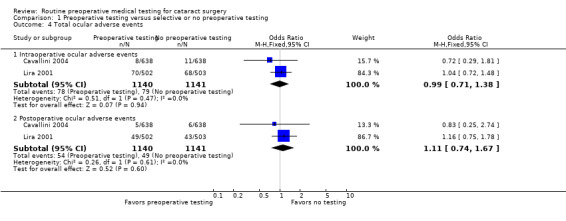
Comparison 1 Preoperative testing versus selective or no preoperative testing, Outcome 4 Total ocular adverse events.
3. Types of intraoperative ocular adverse events.
| Adverse event | Reported by |
Routine‐testing group Number of events |
No‐testing group Number of events |
| Partial dislocations of the nucleus; dislocations of nuclear fragments; cortical material in the vitreous | Cavallini 2004 | 3 | 5 |
| Anterior capsule ruptures | Cavallini 2004 | 2 | 2 |
| Posterior capsule ruptures | Cavallini 2004; Lira 2001 | 35 | 38 |
| Posterior capsule ruptures with vitreous loss | Lira 2001 | 32 | 32 |
| Retained lens fragment | Lira 2002 | 1 | 0 |
| Intraocular lens in the vitreous | Lira 2001 | 2 | 0 |
| Iridodialysis | Lira 2001 | 1 | 1 |
| Zonular rupture | Lira 2001 | 2 | 1 |
Cavallini 2004: routine‐testing group: n = 638; no‐testing group: n = 638 Lira 2001: routine‐testing group: n = 502; no‐testing group: n = 503
Of 103 postoperative ocular adverse events, 54 occurred in the pre‐testing group and 49 in the selective‐ or no‐testing group (OR 1.11, 95% CI 0.74 to 1.67; Analysis 1.4). Postoperative ocular adverse events included cystoid macular edema, increased intraocular pressure, wound leak, and others (Table 5).
4. Types of postoperative ocular adverse events.
| Adverse event | Reported by |
Routine‐testing group Number of events |
No‐testing group Number of events |
| Bullous keratopathy | Lira 2001 | 7 | 4 |
| Cystoid macular edema | Cavallini 2004; Lira 2001 | 13 | 12 |
| Increased intraocular pressure | Lira 2001 | 12 | 12 |
| Chronic iritis | Lira 2001 | 4 | 2 |
| Retinal detachment | Cavallini 2004; Lira 2001 | 4 | 5 |
| Corneal decompensation | Cavallini 2004 | 2 | 2 |
| Wound leak | Lira 2001 | 10 | 11 |
| Vitreous hemorrhage | Lira 2001 | 1 | 1 |
| Endophthalmitis | Lira 2001 | 1 | 0 |
Cavallini 2004: routine‐testing group: n = 638; no‐testing group: n = 638 Lira 2001: routine‐testing group: n = 502; no‐testing group: n = 503
We assessed the certainty of the evidence for ocular adverse events as moderate, downgrading for imprecision due to the small number of events.
Cost outcomes
Lira 2001 evaluated cost, estimating the cost to be 2.55 times higher in those who had routine preoperative medical testing compared to those who had selective preoperative testing (Table 6).
5. Cost data for preoperative medical testing.
| Study | Treatment group | Total number of exams | Average number of exams per patient | Total cost for preoperative testing | Total cost for preoperative testing per patient |
Ratio of preoperative testing cost per patient Pre‐testing: no pre‐testing |
| Lira 2001 | Preoperative testing group | 1536 | 3.00 | BRL 5632.00 | BRL 11.00 | 2.55 |
| Selective or no preoperative testing group | 604 | 1.18 | BRL 2214.66 | BRL 4.32 |
We assessed the certainty of the evidence for cost of preoperative testing as moderate, downgrading for reporting bias due to lack of information regarding the confidence interval around the effect estimate.
Surgical postponements or cancellations
Lira 2001 and Schein 2000 reported the total rate of cancellation. There was no difference in the rate of cancellation between those with routine preoperative medical testing and those with no or selective preoperative testing (OR 0.97, 95% CI 0.78 to 1.21; Analysis 1.5). Only the multisite study by Schein reported the rate of postponement or cancellation of surgeries for medical reasons, and the rate was similar in the two groups: 2.5% in the no‐testing group and 2.3% in the routine‐testing group (Schein 2000).
1.5. Analysis.
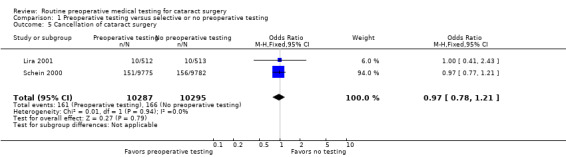
Comparison 1 Preoperative testing versus selective or no preoperative testing, Outcome 5 Cancellation of cataract surgery.
We assessed the certainty of the evidence for medical adverse events as high, finding no reason to downgrade.
Clinical management changes
None of the included studies measured the rate of change in surgical management other than cancellation of surgery.
Quality of life outcomes
None of the included studies measured quality of life outcomes.
Discussion
Summary of main results
The three studies included in this review support the notion that preoperative medical testing in cataract surgery is not protective against medical adverse events (Table 1). Likewise, no clear difference was observed between groups in occurrence of ocular adverse events. One study estimated the cost of preoperative medical testing to be 2.55 times higher than selective testing. The rates of cancellations did not differ between the two studies that reported this outcome. Approximately 2% of surgeries were canceled regardless of whether or not the participant had routine preoperative testing. In addition to cancellation, some surgeries were postponed. No evidence was available to evaluate whether preoperative medical testing leads to unnecessary delays or withholding of cataract surgery services or whether it affects quality of life measures before, during, or after cataract surgery.
Overall completeness and applicability of evidence
All three of the included studies reported data for medical adverse events, the primary outcome for this review. The studies included participants who were scheduled to undergo cataract surgery and used limited exclusion criteria, thus the study populations included participants with comorbidities, as would be expected in real‐world clinical practice. Furthermore, while adverse events tend to be higher in patients with medical comorbidities undergoing surgical procedures, Schein 2000 reported no benefit in providing routine testing to groups of patients with co‐existing illness.
One of the motivating forces for investigating the usefulness of preoperative medical testing is cost‐containment in health care. If no clinical benefit is gained from routine preoperative testing, then such testing is redundant and not cost‐effective. Using information from their randomized clinical trial at a single academic medical center in Brazil, Lira 2001 estimated the increase in the cost of preoperative medical testing as 2.55 times higher than for selective testing. Imasogie 2003 reported larger cost savings when policy eliminating routine preoperative testing for ambulatory cataract surgery patients was enacted at a single hospital in Canada, finding a reduction in preoperative testing costs of almost 90% per patient, from CAD 39.67 to CAD 4.01 per patient.
Routine preoperative medical testing may also be criticized if it leads to unnecessary or excessive actions. Routine testing will yield a significant number of positive results in an older population with high rates of comorbidities (Desai 1999; Riley 2002). Preoperative testing might increase the burden on health care through the follow‐up of unanticipated abnormalities, some of which may be minor or have limited clinical relevance (Smetana 2003). It was beyond the scope of this review to investigate how test results are interpreted and the actions resulting from routine preoperative testing, however we did examine the rate of cancellation of surgery and found no difference between routing testing and no testing. Schein 2000 reported that the combined rate of cancellations or postponement of surgery specifically for medical reasons was a little over 2% of the total surgeries, and the rate did not differ with preoperative testing.
It is reasonable that positive results for preoperative testing do not always influence surgical management for low‐risk procedures such as cataract surgery (ACC/AHA Guidelines 2014; Smetana 2003). A case‐control analysis of cataract surgeries canceled for medical reasons (n = 34) and surgeries that proceeded found no predictive value in the preoperative testing results for hemoglobin, serum glucose, and electrocardiogram (Lira 2002). This supports the hypothesis that information from routine preoperative medical testing has limited impact on surgical management.
Quality of the evidence
We graded the certainty of the evidence as moderate to high for outcomes reported by the studies included in this review. While medical adverse events are rare in low‐risk procedures such as cataract surgery, one of the studies alone, Schein 2000, and the three studies in combination produced a sufficient sample size and statistical power to investigate this claim. Only two studies reported ocular adverse events, which resulted in a smaller sample size and more imprecise estimate for this outcome. We downgraded the certainty of the evidence for ocular adverse events to moderate due to imprecision. We also downgraded the certainty of the evidence for cost to moderate because no information was provided to calculate the confidence interval for the effect estimate.
Potential biases in the review process
We aimed to minimize potential biases in the review process by following the methods prespecified in our protocol (Keay 2008). Two review authors independently selected and assessed studies, and we contacted trial investigators for unclear or unreported information.
Agreements and disagreements with other studies or reviews
Reviews of the literature and practice guidelines related to routine pre‐surgical testing support the finding that commonly performed preoperative laboratory tests in adults preparing for elective surgeries have generally low predictive value (ASA Task Force 2012; Smetana 2003). Although the number of studies included in this review was low, the three studies were in agreement and were supported by a subsequent report from Canada on experiences with policy change to stop routine preoperative testing before ambulatory cataract surgery (Imasogie 2003). At the Toronto Western Hospital, a review was completed of consecutive ambulatory cataract surgeries in a four‐month period preceding policy change in 2000 and in a second four‐month period post‐discontinuation of preoperative testing in 2001. This study examined 1231 surgeries and found no difference in the rate of intraoperative or postoperative events with the change in policy.
Even in the absence of a large number of randomized trials, the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation found that routine preoperative tests do not make an important contribution to patient management. The task force’s recommendations favor ordering tests on a selective basis for the purposes of guiding or optimizing perioperative management (ASA Task Force 2012). This recommendation certainly applies to a low‐risk procedure such as cataract surgery. The studies summarized in this review contribute to the research evidence in guiding recommendations for the standard of care of cataract surgery.
Authors' conclusions
Implications for practice.
Prior to the conduct of the three studies included in this review, surveys of ophthalmologists in the USA, Bass 1995, and Canada, Bellan 1994, in 1992 indicated that among ophthalmologists, ordering preoperative screening tests was common. It was also found that routine preoperative tests were often ordered despite a lack of belief in their clinical value. Tests were sometimes ordered because it was thought that other physicians required the test results or based on medico‐legal concerns.
Although research evidence is available, it does not directly follow that practices will change. It was predicted at the outset of this area of research that in order to change behavior there will need to be a consensus of research evidence across more than one medical specialty and that there will be incentives to change policy at institutions and at individual practices (Schein 1996).
There are few reports in the literature of changes in policy on pre‐medical surgical testing, and surveys on institutional policy and physicians involved in cataract care have not been completed since those reported from the early 1990s. The exception is one report of a successful and cost‐effective change to institutional policy at a single hospital in Toronto, Canada (Imasogie 2003).
While standards for pre‐surgical testing can be mandated by the institution where the surgery is undertaken, there are additional forces that can direct policy. Change in policy can result from change in health insurance coverage rather than physician‐directed change and may or may not be linked to the evidence in support of such a change. While the American Academy of Ophthalmology preferred practice guidelines recommend testing on indication rather than routine preoperative medical testing (AAO Guidelines 2016), the Centers for Medicare and Medicaid Services (CMS), which covers the majority of cataract surgeries in the USA, currently covers preoperative services that assess a beneficiary's fitness for surgery. In the UK, the Royal College of Ophthalmologists guidelines, RCO Guidelines 2010, and National Health Service, NICE Guidelines 2017, do not recommend routine preoperative medical testing (i.e. blood tests and electrocardiograms) prior to cataract surgery. Additional information on current practice trends regarding preoperative testing would be valuable in assessing the impact of this research evidence.
Implications for research.
Alternatives to pre‐surgical testing have been proposed including a self administered health questionnaire (Reeves 2003), which could substitute for health provider history and physical examination. Such avenues may lead to a cost‐effective means of identifying those at increased risk of medical adverse events due to cataract surgery.
Once ‘at risk’ patients are identified, a safe means to deliver cataract rehabilitation to these individuals is required. Kelly and Astbury discuss patient safety issues in cataract care in the UK, and their recommendations include that access to resuscitation equipment and arrangements for transfer to high‐level care should always be available (Kelly 2006). Of note is that their discussion does not include routine preoperative testing as part of the recommendations.
Despite the rare occurrence, adverse medical events that might be precipitated by cataract surgery remain a concern because of the large number of elderly patients with medical comorbidities who have cataract surgery in a variety of settings. Another direction for research is to be able to control the level of risk through variation in anesthetic management. The mechanism for intraoperative medical events has been explored in the observational data from The Study of Medical Testing for Cataract Surgery (Katz 2001).
What's new
| Date | Event | Description |
|---|---|---|
| 2 January 2019 | New citation required but conclusions have not changed | Issue 1, 2019: We added no new studies to the review. |
| 2 January 2019 | New search has been performed | Issue 1, 2019: We updated the electronic searches. |
History
Protocol first published: Issue 3, 2008 Review first published: Issue 2, 2009
| Date | Event | Description |
|---|---|---|
| 5 January 2012 | New citation required but conclusions have not changed | Issue 3, 2012: We added no new studies to the review. |
| 5 January 2012 | New search has been performed | Issue 3, 2012: We updated the electronic searches. |
Acknowledgements
We thank Lori Rosman, Information Specialist for Cochrane Eyes and Vision (CEV), for devising and implementing the electronic search strategy for the review. We thank Elizabeth Ssemanda, a methodologist with the CEV US Project, and the peer reviewers for assisting with the preparation of the review.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Cataract] explode all trees #2 MeSH descriptor: [Cataract Extraction] explode all trees #3 MeSH descriptor: [Capsulorhexis] explode all trees #4 MeSH descriptor: [Phacoemulsification] explode all trees #5 (extract* or aspirat* or operat* or remov* or surg* or excis* or implant*) near/4 (lens*) #6 (extract* or aspirat* or operat* or remov* or surg* or excis* or implant*) near/4 (cataract*) #7 (phakectom* or zonulolys* or catarectom*) #8 (pha*oemulsif* or pha?o or capsulor*hexis or lensectom*) #9 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8) #10 MeSH descriptor: [Diagnostic Tests, Routine] explode all trees #11 MeSH descriptor: [Physical Examination] explode all trees #12 MeSH descriptor: [Medical History Taking] explode all trees #13 MeSH descriptor: [Preoperative Period] explode all trees #14 MeSH descriptor: [Preoperative Care] explode all trees #15 (preoperat* or "pre operative" or "pre operation" or presurg* or "pre surgical" or "pre surgery" or medic* or premedic* or routine*) near/4 (test*) #16 (preoperat* or "pre operative" or "pre operation" or presurg* or "pre surgical" or "pre surgery" or medic* or premedic* or routine*) near/4 (eval*) #17 (preoperat* or "pre operative" or "pre operation" or presurg* or "pre surgical" or "pre surgery" or medic* or premedic* or routine*) near/4 (assessment*) #18 #10 or #11 or #12 or #15 or #16 or #17 #19 #9 and #18
Appendix 2. MEDLINE Ovid search strategy
1. Randomized Controlled Trial.pt. 2. Controlled Clinical Trial.pt. 3. (randomized or randomised).ab,ti. 4. placebo.ab,ti. 5. drug therapy.fs. 6. randomly.ab,ti. 7. trial.ab,ti. 8. groups.ab,ti. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp animals/ not humans.sh. 11. 9 not 10 12. exp cataract/ 13. exp cataract extraction/ 14. exp capsulorhexis/ 15. exp phacoemulsification/ 16. ((extract* or aspirat* or operat* or remov* or surg* or excis* or implant*) adj4 lens*).tw. 17. ((extract* or aspirat* or operat* or remov* or surg* or excis* or implant*) adj4 cataract*).tw. 18. (Phakectom* or Zonulolys* or catarectom*).tw. 19. (pha*oemulsif* or pha?o or Capsulor*hexis or lensectom*).tw. 20. or/12‐19 21. exp diagnostic tests, routine/ 22. exp physical examination/ 23. exp medical history taking/ 24. exp preoperative care/ 25. exp Preoperative Period/ 26. ((preoperat* or pre operat* or presurg* or pre surg* or medic* or premedic* or routine*) adj4 test*).tw. 27. ((preoperat* or pre operat* or presurg* or pre surg* or medic* or premedic* or routine*) adj4 eval*).tw. 28. ((preoperat* or pre operat* or presurg* or pre surg* or medic* or premedic* or routine*) adj4 assessment*).tw. 29. or/21‐28 30. 20 and 29 31. 11 and 30
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase.com search strategy
#1 'randomized controlled trial'/exp #2 'randomization'/exp #3 'double blind procedure'/exp #4 'single blind procedure'/exp #5 random*:ab,ti #6 #1 OR #2 OR #3 OR #4 OR #5 #7 'animal'/exp OR 'animal experiment'/exp #8 'human'/exp #9 #7 AND #8 #10 #7 NOT #9 #11 #6 NOT #10 #12 'clinical trial'/exp #13 (clin* NEAR/3 trial*):ab,ti #14 ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti #15 'placebo'/exp #16 placebo*:ab,ti #17 random*:ab,ti #18 'experimental design'/exp #19 'crossover procedure'/exp #20 'control group'/exp #21 'latin square design'/exp #22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #22 NOT #10 #24 #23 NOT #11 #25 'comparative study'/exp #26 'evaluation'/exp #27 'prospective study'/exp #28 control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti #29 #25 OR #26 OR #27 OR #28 #30 #29 NOT #10 #31 #30 NOT (#11 OR #23) #32 #11 OR #24 OR #31 #33 'cataract'/exp #34 'cataract extraction'/exp #35 'capsulorhexis'/exp #36 'phacoemulsification'/exp #37 ((extract* or aspirat* or operat* or remov* or surg* or excis* or implant*) NEAR/4 (lens*)):ab,ti #38 ((extract* or aspirat* or operat* or remov* or surg* or excis* or implant*) NEAR/4 (cataract*)):ab,ti #39 phakectom*:ab,ti OR zonulolys*:ab,ti OR catarectom*:ab,ti #40 pha*oemulsif*:ab,ti OR phaco:ab,ti OR phako:ab,ti OR capsular*hexis:ab,ti OR lensectom*:ab,ti #41 #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 #42 'diagnostic test'/exp #43 'physical examination'/exp #44 'anamnesis'/exp #45 'preoperative period'/exp #46 ((preoperat* or "pre operat*" or presurg* or "pre surg*" or medic* or premedic* or routine*) NEAR/4 (test*)):ab,ti #47 ((preoperat* or "pre operat*" or presurg* or "pre surg*" or medic* or premedic* or routine*) NEAR/4 (eval*)):ab,ti #48 ((preoperat* or "pre operat*" or presurg* or "pre surg*" or medic* or premedic* or routine*) NEAR/4 (assessment*)):ab,ti #49 #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 #50 #41 AND #49 #51 #32 AND #50
Appendix 4. PubMed search strategy
#1 ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomised[tiab] OR randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh]) #2 (cataract*[tiab]) NOT Medline[sb] #3 (lens*[tiab]) NOT Medline[sb] #4 (Phakectom*[tiab] OR Zonulolys*[tiab] OR catarectom*[tiab]) NOT Medline[sb] #5 (phaco*[tiab] OR phako*[tiab] OR Capsulorhexis[tiab] OR Capsulorrhexis[tiab] OR lensectom*[tiab]) NOT Medline[sb] #6 (#2 OR #3 OR #4 OR #5) #7 (preoperat*[tiab] OR pre operat*[tiab] OR presurg* OR pre surg*[tiab] OR medica*[tiab] OR premedic*[tiab] OR routine*[tiab]) AND (test[tiab] OR tests[tiab] OR tested[tiab] OR testing[tiab]) NOT Medline[sb] #8 ((preoperat*[tiab] OR pre operat*[tiab] OR presurg* OR pre surg*[tiab] OR medica*[tiab] OR premedic*[tiab] OR routine*[tiab]) AND eval*[tiab]) NOT Medline[sb] #9 ((preoperat*[tiab] OR pre operat*[tiab] OR presurg* OR pre surg*[tiab] OR medica*[tiab] OR premedic*[tiab] OR routine*[tiab]) AND assessment*) NOT Medline[sb] #10 #7 OR #8 OR #9 #11 #6 AND #10 #12 #1 AND #11
Appendix 5. LILACS search strategy
((cataract$ OR catarata$ OR lens OR capsulor$ OR phaco OR phacoemulsif$ OR phako OR phakoemulsif$ OR facoemulsif$ OR phakectom$ OR Zonulolys$ OR catarectom$ OR MH:C11.510.245$ OR MH:E04.540.825.249$ OR MH:E04.943.875$) AND (MH:E01.370.395$ OR MH:E01.370.600$ OR MH:E01.370.510$ OR MH:E04.614.937$ OR MH:E02.760.795 OR MH:E04.604.750 OR MH:N02.421.585.795$ OR ((preoperat$ OR "pre operative" OR "pre operation" OR presurg$ OR "pre surgery" OR "pre surgical" OR medical$ OR premedic$ OR routine$) AND (test$ OR eval$ OR assessment$))))
Appendix 6. metaRegister of Controlled Trials search strategy
cataract and preoperative testing
Appendix 7. ClinicalTrials.gov search strategy
(cataract OR phacoemulsification OR capsulorhexis OR phaco OR phako) AND (preoperative OR preoperation OR presurgery OR presurgical OR premedical) AND (testing OR evaluation OR assessment)
Appendix 8. WHO ICTRP search strategy
cataract AND preoperative AND testing OR cataract AND preoperative AND evaluation OR cataract AND preoperative AND assessment OR cataract AND presurgical AND testing OR cataract AND presurgical AND evaluation OR cataract AND presurgical AND assessment
Data and analyses
Comparison 1. Preoperative testing versus selective or no preoperative testing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total medical adverse events | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Overall medical adverse events | 3 | 21531 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.86, 1.16] |
| 1.2 Intraoperative medical adverse events | 3 | 21531 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.85, 1.22] |
| 1.3 Postoperative medical adverse events | 2 | 20526 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.74, 1.24] |
| 2 Total hospitalizations | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Overall hospitalizations | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Intraoperative hospitalizations | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Postoperative hospitalizations | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Total deaths | 2 | 20526 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.52] |
| 4 Total ocular adverse events | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Intraoperative ocular adverse events | 2 | 2281 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.71, 1.38] |
| 4.2 Postoperative ocular adverse events | 2 | 2281 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.74, 1.67] |
| 5 Cancellation of cataract surgery | 2 | 20582 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.78, 1.21] |
1.2. Analysis.
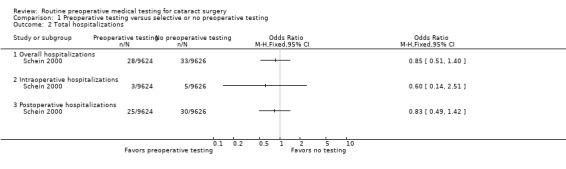
Comparison 1 Preoperative testing versus selective or no preoperative testing, Outcome 2 Total hospitalizations.
1.3. Analysis.
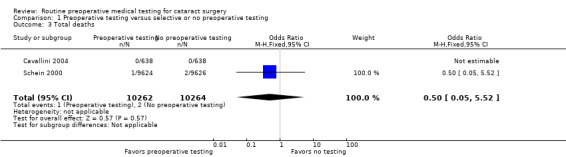
Comparison 1 Preoperative testing versus selective or no preoperative testing, Outcome 3 Total deaths.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cavallini 2004.
| Methods | Study design: randomized clinical trial Number of study centers: 1 (University of Modena and Reggio Emilia) Number randomized: 1276 (sample size calculations based on risk of adverse events) Study follow‐up: 1 month postsurgery |
|
| Participants | Country: Italy Age: not reported Gender: included men and women Inclusion criteria: patients admitted to the day surgery section at the Institute of Ophthalmology for outpatient cataract surgery under local anesthesia Exclusion criteria: ongoing treatment with anticoagulants and subcutaneous insulin therapy |
|
| Interventions | Intervention: physician review of preoperative testing, defined as routine medical tests and electrocardiograms (n = 638) Comparison: no physician review of preoperative testing, test results kept in sealed envelopes (n = 638) |
|
| Outcomes | Primary outcome: ocular adverse events, including intraoperative or postoperative adverse events Secondary outcomes: systemic adverse events defined as intra‐ or postoperative occurrence of acute respiratory, cardio‐circulatory, or neuropsychiatric disease; or decompensation in analogous, established chronic disease |
|
| Notes | Study date: 1 October 2002 to 30 November 2003 Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization list generated by Randomization Center, which was separate from the study center. |
| Allocation concealment (selection bias) | Low risk | Medical staff at study center called into Randomization Center for participant allocation after patients were enrolled in study. |
| Masking (performance bias) Were the participants masked to the treatment group? | Low risk | Participants were informed of the aims and methods for the study at enrollment, however all participants underwent preoperative testing. |
| Masking (performance bias) Were the physicians performing the preoperative tests masked to the treatment group? | Unclear risk | The physicians evaluating the preoperative tests were not masked to the participants in the testing group, however they only received sealed envelopes for the participants in the non‐testing group and were not informed of participants' identities or surgery dates. It is unclear if the physician evaluating the preoperative tests was also the physician performing the surgery. |
| Masking (detection bias) Were the primary outcome assessors masked to the treatment group? | Unclear risk | Ocular outcomes were assessed by clinical records at the time of discharge (intraoperative outcomes) and by telephone interviews 1 month after surgery (postoperative outcomes). It is unclear if the clinical records contained the treatment assignment or if the interviewers were informed of the treatment assignment. |
| Masking (detection bias) Were the secondary outcome assessors masked to the treatment group? | Unclear risk | Systemic outcomes were assessed by clinical records at the time of discharge (intraoperative outcomes) and by telephone interviews and primary care examinations 1 month after surgery (postoperative outcomes). It is unclear if the clinical records contained the treatment assignment or if the interviewers or primary care physicians were informed of the treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up is reported. Reported results are based on total number randomized. |
| Selective reporting (reporting bias) | Low risk | Reported all ocular and systemic adverse events that occurred intraoperatively or postoperatively |
Lira 2001.
| Methods | Study design: randomized clinical trial Number of study centers: 1 (State University of Campinas) Number randomized: 1025 (sample size calculations based on risk of adverse events) Study follow‐up: up to 60 days postsurgery |
|
| Participants | Country: Brazil Age: 66.5 ± 11.6 years, range 40 to 97 years (routine‐testing group = 66.4 ± 11.9 years; selective‐testing group = 66.7 ± 11.4 years) Gender: 547 men, 478 women (routine‐testing group: men = 279, women = 233; selective‐testing group: men = 268, women = 245) Inclusion criteria: people scheduled to undergo cataract surgery Exclusion criteria: less than 40 years old; undergoing surgery on the 2nd eye; were receiving general anesthesia; had a myocardial infarction within the preceding 3 months |
|
| Interventions | Intervention: routine testing with a 12‐lead electrocardiogram, a complete blood count, and measurements of serum glucose (n = 512) Comparison: selective testing defined by no preoperative testing unless the participant presented with a new or worsening condition that would warrant medical testing even if no surgery was scheduled (n = 513) |
|
| Outcomes | Primary outcome: rate of complications during the perioperative period Secondary outcomes: rate of cancellation of surgery; visual acuity |
|
| Notes | Study date: 10 February 2000 to 10 January 2001 Publication languages: English and Portuguese Surgery: extra capsular extraction performed by residents under training |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization using blocks of 4 participants |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Masking (performance bias) Were the participants masked to the treatment group? | High risk | Participants either had preoperative testing done or did not. |
| Masking (performance bias) Were the physicians performing the preoperative tests masked to the treatment group? | High risk | Physicians performing the preoperative medical assessment knew for which participants to conduct preoperative testing. |
| Masking (detection bias) Were the primary outcome assessors masked to the treatment group? | Low risk | Medical events and treatments were recorded by an ophthalmologist or nurse using a standardized form during surgery. The researchers reviewing the forms for classifying adverse events were masked to the treatment assignments. |
| Masking (detection bias) Were the secondary outcome assessors masked to the treatment group? | Unclear risk | It was unclear who made the decision to cancel surgeries, or when those decisions were made. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome data are presented for all participants who underwent surgery, thus for all participants at risk for complications due to cataract surgery. |
| Selective reporting (reporting bias) | Low risk | Reported the results for adverse medical events defined in methods section using a standardized form |
Schein 2000.
| Methods | Study design: randomized clinical trial Number of study centers: 9 Number randomized: 19,557 operations (18,189 participants) (sample size calculations based on risk of adverse events) Study follow‐up: 1 week postsurgery |
|
| Participants | Country: USA and Canada Age (per operation): routine‐testing group = 73 ± 8 years; no‐testing group = 74 ± 8 years Gender (per operation): 7631 men; 11,926 women (routine‐testing group: men = 3769, women = 6006; no‐testing group: men = 3862, women = 5920) Inclusion criteria: people scheduled to undergo cataract surgery Exclusion criteria: less than 50 years old; were receiving general anesthesia; had a myocardial infarction within the preceding 3 months; had any preoperative medical testing done during the 28 days prior to enrollment; could not speak English or Spanish; 2nd eye not eligible if surgery was within 28 days of surgery in 1st randomized eye |
|
| Interventions | Intervention: routine testing with electrocardiography, complete blood count, and measurement of serum levels of electrolytes, urea nitrogen, creatinine, and glucose (operations scheduled: operations: n = 9775, participants: n = 9456; operations performed: operations: n = 9624, participants: n = 9411) Comparison: no preoperative testing unless the participant presented with a new or worsening condition that would warrant medical testing even if no surgery was scheduled (operations scheduled: operations: n = 9782, participants: n = 9445; operations performed: operations: n = 9626, participants: n = 9408) |
|
| Outcomes | Primary outcome: adverse medical events and interventions on the day of surgery and up to 7 days after surgery Secondary outcome: whether preoperative testing could have prevented the adverse event from occurring |
|
| Notes | Study date: 1 June 1995 to 30 June 1997 Publication language: English Participation rate: 94% Funding source: Agency for Health Care Policy and Research |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was stratified according to clinical center, age (in decades), and health status reported by participants using blocks of 4. |
| Allocation concealment (selection bias) | Low risk | Randomization was done by computer at time of enrollment. |
| Masking (performance bias) Were the participants masked to the treatment group? | High risk | Participants were informed of group assignment and given a letter and study brochure to present to the healthcare provider performing the preoperative assessment. |
| Masking (performance bias) Were the physicians performing the preoperative tests masked to the treatment group? | High risk | Healthcare providers performing the preoperative tests received a letter and study brochure from the participant at the time of the preoperative assessment. |
| Masking (detection bias) Were the primary outcome assessors masked to the treatment group? | Low risk | Medical events and treatments were recorded by an anesthesiologist or nurse anesthetist using a standardized form during surgery, and by a standardized telephone interview conducted by a study co‐ordinator 1 week following surgery. Additional patient information was recorded by nursing staff before discharge. 2 investigators reviewed medical charts to verify adverse events, and a 3rd investigator who was masked to the treatment assignment made the final clinical judgement. |
| Masking (detection bias) Were the secondary outcome assessors masked to the treatment group? | Low risk | 2 investigators reviewed medical charts to verify adverse events, and a 3rd investigator who was masked to the treatment assignment made the final clinical judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis was used. Data were 100% from day of surgery and 99.8% for 1 week after surgery. |
| Selective reporting (reporting bias) | Low risk | Reported the results for adverse medical events defined in methods section using a standardized form and standardized telephone interview |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Blery 1986 | Observational study of selective preoperative testing for any surgery requiring general or regional anesthesia; no control group |
| Brown 2001 | Comment and summary of Dr Schein’s study |
| Bruns 2001 | Review of lab testing in outcome studies |
| Coleman 2002 | Editorial on applying results of trials to practice |
| Dessy 2017 | Intervention does not meet eligibility criteria: abstract compares onsite same‐day mandatory pre‐admission testing to offsite pre‐admission testing. |
| Francis 1996 | Comment on report of local vs general anesthesia for cataract surgery |
| Gao 2006 | Retrospective review of age‐related cataract patients with cardiovascular disease |
| Gibson 2000 | Comment and summary of Dr Schein’s study |
| Gimbel 2000 | Review of cataract surgery at the Gimbel Eye Surgical Centre in Alberta, Canada |
| Imasogie 2003 | Not a randomized trial; 4 months pre‐ and 4 months post‐discontinuation of routine testing |
| Johnson 1988 | Observational study of routine preoperative testing for ambulatory surgery patients; no control group |
| Lira 2002 | Retrospective case‐control study to identify factors associated with cancelling cataract surgery; cases were cataract patients whose surgeries were canceled due to medical events, while controls were patients who underwent surgery |
| Macpherson 1993 | Review of pre‐surgical tests commonly used for general surgeries |
| Maltzman 1981 | Retrospective review of results from pre‐admission evaluations in a cohort that underwent cataract extraction |
| NCT02903485 | Randomized clinical trial comparing pre‐surgical assessment and surveillance during cataract surgery performed by nurses versus anesthetists |
| Smithen 2003 | Comment and summary of Reeves 2003 cohort analysis of Dr Schein's study |
| Tallo 2007 | Retrospective review of cataract patients in Brazil, 2004 |
| Walters 1997 | Study of whether or not doctors involved in peribulbar local anaesthetic surgery reviewed results of preoperative tests for patients |
Differences between protocol and review
We conducted the assessment of methodological quality using Cochrane's updated 'Risk of bias' format (Higgins 2017). We added ocular adverse events to the secondary outcomes and extended the period for medical adverse events to the length of follow‐up. We incorporated GRADE assessments and a 'Summary of findings' table in the review in accordance with updated Cochrane requirements.
Contributions of authors
LK conceived the review question. LK and KL co‐ordinated the review, screened search results, organized retrieval of papers, screened retrieved paper against inclusion criteria, appraised quality of papers, extracted data from papers, provided additional data about papers, obtained and screened data on unpublished studies, analyzed data, and provided a methodological perspective. KL entered data into Review Manager 5, and LK verified the data entry. LK, OS, JT, and JK provided clinical, policy, and consumer perspectives as well as general advice on the review. LK, OS, and KL wrote the review. OS secured funding for the review. OS, JT, and JK performed previous work that was the foundation of the current review.
LK and KL screened search results for the update of the review and revised the text of the review. OS, JT, and JK provided feedback for the update of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
National Eye Institute, National Institutes of Health, USA.
Australian National Health and Medical Research Council Post Doctoral Research Fellowship, Australia.
George Institute for International Health, University of Sydney, Australia.
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the NIHR to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- This review update was supported by the NIHR, via Cochrane Infrastructure funding to the CEV UK editorial base.
The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service, or the Department of Health.
Declarations of interest
Lisa Keay: none known. Kristina Lindsley: none known. James Tielsch, Joanne Katz, and Oliver Schein were co‐investigators in a trial examining preoperative medical testing and cataract surgery funded by a grant from the Agency for Health Care Policy and Research (RO1‐HSO‐8331).
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Cavallini 2004 {published data only}
- Cavallini GM, Saccarola P, D'Amico R, Gasparin A, Campi L. Impact of preoperative testing on ophthalmologic and systemic outcomes in cataract surgery. European Journal of Ophthalmology 2004;14(5):369‐74. [PubMed] [Google Scholar]
Lira 2001 {published data only}
- Arieta CE, Nascimento MA, Lira RP, Kara‐Jose N. Waste of medical tests in preoperative evaluation for cataract surgery [Desperdício de exames complementares na avaliação pré‐operatória em cirurgias de catarata]. Cadernos de Saude Publica 2004;20(1):303‐10. [DOI] [PubMed] [Google Scholar]
- Lira RP. Preoperative tests in ambulatory cataract surgery in adults: is a routine necessary? [Testes pré‐operatórios na cirurgia de cataracta ambulatorial em adultos: É necessária uma rotina?]. Tese de Doutorado (Doctoral Thesis); Campinas: Faculdade de Ciências Médicas da UNICAMP 2002.
- Lira RP, Nascimento MA, Moreira‐Filho DC, Kara‐Jose N, Arieta CE. Are routine preoperative medical tests needed with cataract surgery?. Pan American Journal of Public Health 2001;10(1):13‐7. [DOI] [PubMed] [Google Scholar]
- Nascimento MA, Lira RP, Kara‐Jose N, Arieta CE. Predictive value of preoperative fasting glucose test of diabetic patients regarding surgical outcome in cataract surgery [Valor preditivo da glicemia de jejum pré‐operatória de pacientes diabéticos quanto ao resultado cirúrgico da cirurgia de catarata]. Arquivos Brasileiros de Oftalmologia 2005;68(2):213‐7. [DOI] [PubMed] [Google Scholar]
- Nascimento MA, Lira RP, Soares PH, Spessatto N, Kara‐Jose N, Arieta CE. Are routine preoperative medical tests needed with cataract surgery? Study of visual acuity outcome. Current Eye Research 2004;28(4):285‐90. [DOI] [PubMed] [Google Scholar]
Schein 2000 {published data only}
- Katz J, Feldman MA, Bass EB, Lubomski LH, Tielsch JM, Petty BG, et al. Risks and benefits of anticoagulant and antiplatelet medication use before cataract surgery. Ophthalmology 2003;110(9):1784‐8. [DOI] [PubMed] [Google Scholar]
- Katz J, Schein OD, Tielsch JM, Lubomski LH, Feldman M, Petty BG, et al. Effects of randomizing second eyes in a trial to evaluate preoperative medical testing for cataract surgery. Ophthalmic Epidemiology 1997;4(2):101‐5. [DOI] [PubMed] [Google Scholar]
- Reeves SW, Tielsch JM, Katz J, Bass EB, Schein OD. A self‐administered health questionnaire for the preoperative risk stratification of patients undergoing cataract surgery. American Journal of Ophthalmology 2003;135(5):599‐606. [DOI] [PubMed] [Google Scholar]
- Schein OD, Katz J, Bass EB, Tielsch JM, Lubomski LH, Feldman MA, et al. The value of preoperative medical testing for cataract surgery. American Academy of Ophthalmology. 1999:256. [DOI] [PubMed]
- Schein OD, Katz J, Bass EB, Tielsch JM, Lubomski LH, Feldman MA, et al. The value of routine preoperative medical testing before cataract surgery. Study of Medical Testing for Cataract Surgery. New England Journal of Medicine 2000;342(3):168‐75. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Blery 1986 {published data only}
- Blery C, Szatan M, Fourgeaux B, Charpak Y, Darne B, Chastang CL, et al. Evaluation of a protocol for selective ordering of preoperative tests. Lancet 1986;1(8473):139‐41. [DOI] [PubMed] [Google Scholar]
Brown 2001 {published data only}
- Brown MM. The value of routine preoperative medical testing before cataract surgery. Evidence‐Based Eye Care 2001;2(3):186‐7. [Google Scholar]
Bruns 2001 {published data only}
- Bruns DE. Laboratory‐related outcomes in healthcare. Clinical Chemistry 2001;47(8):1547‐52. [PubMed] [Google Scholar]
Coleman 2002 {published data only}
- Coleman AL. Applying evidence‐based medicine in ophthalmic practice. American Journal of Ophthalmology 2002;134(4):599‐601. [DOI] [PubMed] [Google Scholar]
Dessy 2017 {published data only}
- Dessy A, Mayro E, Bailey R, Wisner D, Koka A, Hark L, et al. A proposed intervention to decrease resident‐performed cataract surgery cancellation. Investigative Ophthalmology and Visual Science 2017; Vol. 58, issue 8:5054.
Francis 1996 {published data only}
- Francis JG, Barker JP. Postoperative morbidity following cataract surgery. Anaesthesia 1996;51(9):888‐9. [DOI] [PubMed] [Google Scholar]
Gao 2006 {published data only}
- Gao ZY, Jin M, Hu YF, Yang WZ, Wang XY, Hu YJ, et al. Clinical value of preoperative comprehensive evaluation of cataract surgery in age‐related cataract. Chinese Journal of Ophthalmology 2006;42(6):522‐5. [PubMed] [Google Scholar]
Gibson 2000 {published data only}
- Gibson N. Routine testing before cataract surgery did not reduce medical adverse events. Evidence‐Based Medicine 2000;5(5):151. [Google Scholar]
Gimbel 2000 {published data only}
- Gimbel HV, Hamilton RC. The value of medical testing before cataract surgery. Clinical and Surgical Ophthalmology 2003;21(12):487‐90. [Google Scholar]
- Gimbel HV, Hamilton RC. The value of medical testing before cataract surgery. Ophthalmic Practice 2000;18(4):166‐71. [Google Scholar]
Imasogie 2003 {published data only}
- Imasogie N, Wong DT, Luk K, Chung F. Elimination of routine testing in patients undergoing cataract surgery allows substantial savings in laboratory costs. A brief report. Canadian Journal of Anaesthesia 2003;50(3):246‐8. [DOI] [PubMed] [Google Scholar]
Johnson 1988 {published data only}
- Johnson H, Knee‐Loli S, Butler TA, Munoz E, Wise L. Are routine preoperative laboratory screening tests necessary to evaluate ambulatory surgical patients?. Surgery 1988;104(4):639‐45. [PubMed] [Google Scholar]
Lira 2002 {published data only}
- Lira RP, Covolo GA, Monsanto AR, Kara‐Jose N, Arieta CE. Influence of preoperative testing on cancellation of ambulatory cataract surgery in adults. Annals of Ophthalmology 2002;34(3):203‐5. [Google Scholar]
Macpherson 1993 {published data only}
- Macpherson DS. Preoperative laboratory testing: should any tests be "routine" before surgery?. Medical Clinics of North America 1993;77(2):289‐308. [DOI] [PubMed] [Google Scholar]
Maltzman 1981 {published data only}
- Maltzman BA, Cinotti AA, Calderone JP Jr. Preadmission evaluation and elective cataract surgery. Journal of the Medical Society of New Jersey 1981;78(7):519‐20. [PubMed] [Google Scholar]
NCT02903485 {unpublished data only}
- NCT02903485. Bypassing anesthesiologist assessment before cataract surgery: a non inferiority study (CATARIDE). clinicaltrials.gov/ct2/show/NCT02903485 (first received 16 September 2016).
Smithen 2003 {published data only}
- Smithen L, Spaide R. A self‐administered health questionnaire for the preoperative risk stratification of patients undergoing cataract surgery. Evidence‐Based Eye Care 2003;4(4):190‐1. [DOI] [PubMed] [Google Scholar]
Tallo 2007 {published data only}
- Tallo FS, Soriano ES, Alvarenga LS. Preoperative evaluation and cataract surgery [Avaliação pré‐operatória na cirurgia de catarata]. Arquivos Brasileiros de Oftalmologia 2007;70(4):633‐7. [DOI] [PubMed] [Google Scholar]
Walters 1997 {published data only}
- Walters G, McKibbin M. The value of pre‐operative investigations in local anaesthetic ophthalmic surgery. Eye 1997;11(Pt 6):847‐9. [DOI] [PubMed] [Google Scholar]
Additional references
AAO Guidelines 2016
- American Academy of Ophthalmology. Cataract in the adult eye preferred practice pattern. www.aao.org/preferred‐practice‐pattern/cataract‐in‐adult‐eye‐ppp‐2016 (accessed 17 January 2018).
ACC/AHA Guidelines 2014
- Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary. Journal of the American College of Cardiology 2014;64(22):2373‐405. [DOI] [PubMed] [Google Scholar]
ASA Task Force 2012
- American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology 2012;116(3):522‐38. [DOI] [PubMed] [Google Scholar]
Bass 1995
- Bass EB, Steinberg EP, Luthra R, Schein OD, Tielsch JM, Javitt JC, et al. Do ophthalmologists, anesthesiologists, and internists agree about preoperative testing in healthy patients undergoing cataract surgery?. Archives of Ophthalmology 1995;113(10):1248‐56. [DOI] [PubMed] [Google Scholar]
Bellan 1994
- Bellan L. Preoperative testing for cataract surgery. Canadian Journal of Ophthalmology 1994;29(3):111‐4. [PubMed] [Google Scholar]
Dandona 2001
- Dandona R, Dandona L. Refractive error blindness. Bulletin of the World Health Organization 2001;79(3):237‐43. [PMC free article] [PubMed] [Google Scholar]
Desai 1999
- Desai P, Reidy A, Minassian DC. Profile of patients presenting for cataract surgery in the UK: national data collection. British Journal of Ophthalmology 1999;83(8):893‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fletcher 1998
- Fletcher A, Vijaykumar V, Selvaraj S, Thulasiraj RD, Ellwein LB. The Madurai Intraocular Lens Study. III: Visual functioning and quality of life outcomes. American Journal of Ophthalmology 1998;125(1):26‐35. [DOI] [PubMed] [Google Scholar]
Foster 2007
- Foster A. Vision 2020: the cataract challenge. Community Eye Health Journal 2007;13(34):17‐9. [PMC free article] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version (accessed 17 January 2018). Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guay 2015
- Guay J, Sales K. Sub‐Tenon's anaesthesia versus topical anaesthesia for cataract surgery. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD006291.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Ivers 2003
- Ivers RQ, Cumming RG, Mitchell P, Simpson JM, Peduto AJ. Visual risk factors for hip fracture in older people. Journal of the American Geriatrics Society 2003;51(3):356‐63. [DOI] [PubMed] [Google Scholar]
Katz 2001
- Katz J, Feldman MA, Bass EB, Lubomski LH, Tielsch JM, Petty BG, et al. Study of Medical Testing for Cataract Surgery Study Team. Adverse intraoperative medical events and their association with anesthesia management strategies in cataract surgery. Ophthalmology 2001;108(10):1721‐6. [DOI] [PubMed] [Google Scholar]
Kelly 2006
- Kelly SP, Astbury NJ. Patient safety in cataract surgery. Eye 2006;20(3):275‐82. [DOI] [PubMed] [Google Scholar]
NICE Guidelines 2017
- National Institute for Health and Care Excellence. Cataracts in adults: management. www.nice.org.uk/guidance/ng77 (accessed 17 January 2018).
RCO Guidelines 2010
- The Royal College of Ophthalmologists. Cataract surgery guidelines. www.rcophth.ac.uk/wp‐content/uploads/2014/12/2010‐SCI‐069‐Cataract‐Surgery‐Guidelines‐2010‐SEPTEMBER‐2010.pdf (accessed 17 January 2018).
Reeves 2003
- Reeves SW, Tielsch JM, Katz J, Bass EB, Schein OD. A self‐administered health questionnaire for the preoperative risk stratification of patients undergoing cataract surgery. American Journal of Ophthalmology 2003;135(5):599‐606. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riaz 2006
- Riaz Y, Mehta JS, Wormald R, Evans JR, Foster A, Ravilla T, et al. Surgical interventions for age‐related cataract. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD001323.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Riley 2002
- Riley AF, Malik TY, Grupcheva CN, Fisk MJ, Craig JP, McGhee CN. The Auckland Cataract Study: co‐morbidity, surgical techniques, and clinical outcomes in a public hospital service. British Journal of Ophthalmology 2002;86(2):185‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schein 1996
- Schein O. Assessing what we do: the example of preoperative medical testing. Archives of Ophthalmology 1996;114(9):1129‐31. [DOI] [PubMed] [Google Scholar]
Smetana 2003
- Smetana GW, Macpherson DS. The case against routine preoperative laboratory testing. Medical Clinics of North America 2003;87(1):7‐40. [DOI] [PubMed] [Google Scholar]
Taylor 2006
- Taylor HR, Pezzullo ML, Keeffe JE. The economic impact and cost of visual impairment in Australia. British Journal of Ophthalmology 2006;90(3):272‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Keay 2008
- Keay L, Lindsley K, Tielsch J, Katz J, Ssemanda E, Schein O. Preoperative medical testing for cataract surgery. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD007293] [DOI] [Google Scholar]
Keay 2009
- Keay L, Lindsley K, Tielsch J, Katz J, Schein O. Routine preoperative medical testing for cataract surgery. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007293.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Keay 2012
- Keay L, Lindsley K, Tielsch J, Katz J, Schein O. Routine preoperative medical testing for cataract surgery. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD007293.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


