Abstract
Background
Extracorporeal membrane oxygenation (ECMO) is a form of life support that targets the heart and lungs. Extracorporeal membrane oxygenation for severe respiratory failure accesses and returns blood from the venous system and provides non‐pulmonary gas exchange. Extracorporeal membrane oxygenation for severe cardiac failure or for refractory cardiac arrest (extracorporeal cardiopulmonary resuscitation (ECPR)) provides gas exchange and systemic circulation. The configuration of ECMO is variable, and several pump‐driven and pump‐free systems are in use. Use of ECMO is associated with several risks. Patient‐related adverse events include haemorrhage or extremity ischaemia; circuit‐related adverse effects may include pump failure, oxygenator failure and thrombus formation. Use of ECMO in newborns and infants is well established, yet its clinical effectiveness in adults remains uncertain.
Objectives
The primary objective of this systematic review was to determine whether use of veno‐venous (VV) or venous‐arterial (VA) ECMO in adults is more effective in improving survival compared with conventional respiratory and cardiac support.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (Ovid) and EMBASE (Ovid) on 18 August 2014. We searched conference proceedings, meeting abstracts, reference lists of retrieved articles and databases of ongoing trials and contacted experts in the field. We imposed no restrictions on language or location of publications.
Selection criteria
We included randomized controlled trials (RCTs), quasi‐RCTs and cluster‐RCTs that compared adult ECMO versus conventional support.
Data collection and analysis
Two review authors independently screened the titles and abstracts of all retrieved citations against the inclusion criteria. We independently reviewed full‐text copies of studies that met the inclusion criteria. We entered all data extracted from the included studies into Review Manager. Two review authors independently performed risk of bias assessment. All included studies were appraised with respect to random sequence generation, concealment of allocation, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias.
Main results
We included four RCTs that randomly assigned 389 participants with acute respiratory failure. Risk of bias was low in three RCTs and high in one RCT. We found no statistically significant differences in all‐cause mortality at six months (two RCTs) or before six months (during 30 days of randomization in one trial and during hospital stay in another RCT). The quality of the evidence was low to moderate, and further research is very likely to impact our confidence in the estimate of effects because significant changes have been noted in ECMO applications and treatment modalities over study periods to the present.
Two RCTs supplied data on disability. In one RCT survival was low in both groups but none of the survivors had limitations in their daily activities six months after discharge. The other RCT reported improved survival without severe disability in the intervention group (transfer to an ECMO centre ± ECMO) six months after study randomization but no statistically significant differences in health‐related quality of life.
In three RCTs, participants in the ECMO group received greater numbers of blood transfusions. One RCT recorded significantly more non‐brain haemorrhage in the ECMO group. Another RCT reported two serious adverse events in the ECMO group, and another reported three adverse events in the ECMO group.
Clinical heterogeneity between studies prevented meta‐analyses across outcomes. We found no completed RCT that had investigated ECMO in the context of cardiac failure or arrest. We found one ongoing RCT that examined patients with acute respiratory failure and two ongoing RCTs that included patients with acute cardiac failure (arrest).
Authors' conclusions
Extracorporeal membrane oxygenation remains a rescue therapy. Since the year 2000, patient treatment and practice with ECMO have considerably changed as the result of research findings and technological advancements over time. Over the past four decades, only four RCTs have been published that compared the intervention versus conventional treatment at the time of the study. Clinical heterogeneity across these published studies prevented pooling of data for a meta‐analysis.
We recommend combining results of ongoing RCTs with results of trials conducted after the year 2000 if no significant shifts in technology or treatment occur. Until these new results become available, data on use of ECMO in patients with acute respiratory failure remain inconclusive. For patients with acute cardiac failure or arrest, outcomes of ongoing RCTs will assist clinicians in determining what role ECMO and ECPR can play in patient care.
Plain language summary
Extracorporeal (external to the body) membrane oxygenation (ECMO) for critically ill adults
Review question: Effect of ECMO on survival in critically ill adults.
Background: Extracorporeal membrane oxygenation is a form of life support that targets the heart and lungs. For patients with severe lung failure, ECMO provides extracorporeal gas exchange. For those with severe heart failure or cardiac arrest, ECMO (extracorporeal cardiopulmonary resuscitation (ECPR)) provides gas exchange and systemic blood circulation. Use of ECMO is associated with several risks (e.g. bleeding, clot formation).
Study characteristics: We found four studies that randomly allocated 389 patients to receive ECMO versus conventional lung support. All studies comprised patients with acute lung failure. We found no completed study in patients with acute heart failure or arrest. We found one ongoing study in patients with acute lung failure and two ongoing studies in patients with acute heart failure (arrest). The evidence is current to August 2014.
Key results: Clinical differences in the care provided for patients with acute lung failure prevented us from combining the results of individual studies. Individual studies reported no differences in all‐cause death at or before six months in patients given ECMO compared with those who were not. In one study survival was low in both groups but none of the patients who survived had limitations in their daily activities six months after discharge. Another study found improved survival without severe disability in patients transferred to an ECMO centre for consideration of ECMO six months after study entry. In three studies, patients in the ECMO group received greater numbers of blood transfusions. One study reported more non‐brain bleeding in the ECMO group, and another study reported two serious adverse events in the ECMO group. Another study reported three adverse events in the ECMO group.
Quality of the evidence: Clinical practice, study planning and ways of using ECMO have varied considerably among studies. Technological developments (circuits, pumps and mechanical lungs) have improved performance and patient safety with ECMO applications over time. These clinical differences in the care provided for patients with acute lung failure prevented us from combining the results of individual studies. In critically ill adults, ECMO may or may not be more effective in improving survival compared with conventional lung support. Results from ongoing studies will help us better understand the role of ECMO and ECPR in the treatment of patients with acute lung or heart failure.
Background
Description of the condition
Among critically ill adult patients, mortality rates are as high as 50% for those with acute cardiac failure and 30% to 40% for those diagnosed with severe respiratory failure (Douglas 2008). Severe hypoxaemia and/or hypercapnia as a result of acute respiratory distress syndrome (ARDS), primary graft dysfunction following lung transplant and direct trauma are common conditions treated with respiratory extracorporeal membrane oxygenation (ECMO) support (Allen 2011). Acute respiratory distress syndrome is a potentially reversible clinical syndrome of lung inflammation caused by numerous direct and indirect lung insults (Brodie 2011). Pulmonary and extrapulmonary infection, aspiration and trauma are common causes of ARDS (Rubenfeld 2007).
Cardiogenic shock unresponsive to moderate‐ or high‐dose inotropic support and refractory cardiac arrest are two clinical syndromes for which cardiac ECMO is used as rescue therapy (Chen 2008; Mirabel 2011). Chronic cardiomyopathy, acute myocardial infarction, myocarditis, primary graft dysfunction following transplant, toxic/drug causes and pulmonary embolism are conditions that may result in cardiogenic shock or cardiac arrest (Allen 2011; Marasco 2008). However, many patients have mixed conditions and die of sepsis or multi‐organ failure (MOF) rather than from isolated respiratory or cardiac failure alone (Sidebotham 2009).
When conventional treatment algorithms fail, ECMO may be a rescue therapy option for patients with severe failure of these organs (Marasco 2008). Extracorporeal membrane oxygenation is well established as a treatment for infants with respiratory and cardiac failure, and evidence supports its use in adults with life‐threatening refractory hypoxaemia or hypercapnia, severely impaired cardiac pump function or both (Cooper 2007; Gattinoni 2011; Lindstrom 2009; Mugford 2008). For example, guidelines on clinical triggers for initiation of ECMO in patients with respiratory failure include the following (ELSO 2014).
In hypoxic respiratory failure due to any cause (primary or secondary), extracorporeal life support (ECLS) should be considered when the risk of mortality is 50% (partial pressure of arterial oxygen for a given fraction of inspired oxygen (PaO2/FiO2) < 150 mmHg on FiO2 > 0.9; or Murray score 2 to 3 or greater) and is indicated when the risk of mortality is 80% or greater (80% mortality risk can be identified by a PaO2/FiO2 < 80 mmHg on FiO2 > 0.9; or Murray score 3 to 4).
Carbon dioxide (CO2) retention due to asthma or permissive hypercapnia with PaCO2 > 80 mmHg or inability to achieve safe inflation pressures (plateau pressure < 30 cm H2O) is an indication for ECLS.
Severe air leak syndromes are an indication for ECLS.
Description of the intervention
Extracorporeal membrane oxygenation is a form of extracorporeal life support that can provide complete (or partial) support of the heart and lungs (Sidebotham 2009). It consists of specialized cannulae that connect to the circulation, as well as circuit tubing, a membrane that oxygenates the blood and removes carbon dioxide and a blood pump that drives circuit blood flow (Douglas 2008). Current ECMO systems are rapidly deployable and mobile and can provide support over days to months (Forrest 2011). For patients with severe respiratory failure, ECMO accesses and returns blood from the venous system (veno‐venous (VV) ECMO). It provides non‐pulmonary gas exchange, facilitates protective lung ventilation and provides time for lung recovery from acute processes or bridging to lung transplant (Sorbo 2014). For those with severe cardiac failure or refractory cardiac arrest, ECMO provides systemic circulation (venous‐arterial (VA) ECMO) and prevents further organ injury secondary to low blood flow (Allen 2011). This may allow time for cardiac recovery or bridging to longer‐term cardiac support modalities.
In contrast to these pump‐driven ECMO circuits, other gas exchange systems are in clinical use. These pump‐free systems provide mainly arteriovenous carbon dioxide elimination (pumpless arteriovenous extracorporeal CO2 removal (avECCO2‐R) or pumpless extracorporeal lung assist (PECLA) or interventional lung assist (iLA)) and use an arterovenous (AV) circuit in which blood flow through the artificial lung fully relies on native blood pressure, which limits gas exchange and its usage in critically ill patients (Bein 2006; Bein 2013; Sorbo 2014).
With pump‐driven circuits, gas exchange rates and cardiac support depend on circuit configuration (VV or VA) and the flow of blood pumped through the artificial lung (normally 50 to 100 mL/kg/min) (Park 2011). Available blood flow in turn depends on the calibre of the drainage cannulae (23 to 25 French (F)) and of the returning cannulae (17F to 21F) (Sidebotham 2010). When sufficient blood flow rates cannot be established, central cannulation is an option. The right atrium and the ascending aorta are directly cannulated via an open chest or closed chest (tunnelled through the subcostal abdominal wall) approach; this allows bigger cannulae and increased blood flow (Marasco 2008).
A variety of significant risks are associated with use of ECMO, regardless of the configuration applied. Reported adverse effects may be patient related (e.g. haemorrhage, extremity ischaemia) or circuit related (e.g. pump failure, oxygenator failure, thrombus formation) (Allen 2011).
How the intervention might work
Extracorporeal membrane oxygenation offers the possibility of supporting patients with life‐threatening heart or lung conditions when conventional management is failing and thereby increases the time available to treat underlying illnesses (Gattinoni 2011). During the course of ECMO, gas exchange does not depend on native lung function; this allows the lungs to rest while lung‐protective mechanical ventilation strategies are used (Petrucci 2007). In VA ECMO, the extracorporeal circuit also provides cardiac support for patients who are unable to maintain sufficient cardiac output; this is seen in patients immediately after heart surgery or before or after heart transplantation (Chung 2010; D'Alessandro 2011; MacLaren 2012; Sidebotham 2009). Recently, VA ECMO has been used to maintain cardiopulmonary circulation during advanced cardiac life support (extracorporeal cardiopulmonary resuscitation (ECPR)) (Chen 2008; Dalton 2011). However, the primary indication for VA ECMO remains the patient’s condition and not, for example, specific types of heart surgery for which circulation needs to be bridged as a requirement of surgery.
Why it is important to do this review
Use of ECMO in adults has been controversial since it was first successfully introduced as a treatment option for critically ill patients (Chalwin 2008). For patient cohorts with predominantly respiratory failure, older randomized controlled trials (RCTs) (those published before the year 2000) report no advantage of the intervention compared with conventional treatment strategies (Morris 1994; Zapol 1979). In contrast, a more recent RCT (Peek 2009) reported improved survival without severe disability at six months in patients with acute respiratory failure referred for ECMO treatment. Several case series have reported high survival rates in cohorts that used ECMO during the worldwide H1N1 influenza A pandemic in 2009, during which clinicians faced a large number of severely hypoxaemic patients who did not respond to maximal ventilatory support (Davies 2009; Gattinoni 2011). Survival rates among patients with H1N1 influenza are generally high (Sorbo 2014), but advancements in technology regarding the extracorporeal circuit and better patient management are likely to have led to improved patient outcomes over past decades (Chalwin 2008; Sidebotham 2009).
Objectives
The primary objective of this systematic review was to determine whether use of VV or VA ECMO in adults is more effective in improving survival compared with conventional respiratory and cardiac support.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs that compared adult ECMO versus conventional support were eligible for inclusion in this review. Quasi‐randomized controlled trials and cluster‐randomized trials were also eligible for inclusion, although cross‐over trials were not.
Types of participants
We included in this review all studies that included adult participants (18 years of age or older) with cardiac or respiratory failure, or both. We excluded all studies that included participants who underwent surgery for whom ECMO was established as a planned procedure for the purpose of surgery.
Types of interventions
We included in this review ECMO using pump‐driven veno‐venous (VV) and venous‐arterial (VA) or pump‐free arteriovenous (AV) circuits versus all forms of conventional management (e.g. intermittent positive‐pressure ventilation). We excluded studies that compared other forms of mechanical support specifically designed to support heart function such as ventricular assist devices.
Types of outcome measures
Primary outcomes
The primary outcome of this review was rate of all‐cause mortality closest to 30, 60 or 90 days and/or at six months.
Secondary outcomes
Length of hospital stay.
Survival to discharge.
Disability as reported by study authors.
Adverse outcomes.
Health‐related quality of life, as reported by study authors.
Longer‐term health status and well‐being, as reported by study authors.
Cost‐effectiveness.
Outcomes did not form part of the study eligibility assessment, so studies that met participant, intervention and comparison criteria were included in the review even if they reported no relevant outcomes.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 7) (Appendix 1), MEDLINE (Ovid) (1971 to 18 August 2014) (Appendix 2) and EMBASE (Ovid) (1980 to 18 August 2014) (Appendix 3).
Searching other resources
We searched conference proceedings, meeting abstracts and databases of ongoing trials such as Current Controlled Trials (http://www.controlled‐trials.com/) and Clinical Trials (http://clinicaltrials.gov). The search was performed in August 2014. In January 2014, we contacted experts at specialized treatment centres in Australia, Brazil, Canada, France, Germany, New Zealand, the United Kingdom and the United States to request results from ongoing or completed research in this area.
Data collection and analysis
Selection of studies
Two review authors (RT, DI) independently screened the titles and abstracts of all retrieved citations against the inclusion criteria. On the basis of this approach, studies were categorized into two groups.
Possibly included (studies that met the inclusion criteria and warranted full‐text access to gather further information).
Excluded (studies that clearly did not meet the inclusion criteria).
RT and DI independently reviewed all full‐text articles.
Data extraction and management
RT and DI independently used the modified data collection form of the Cochrane Anaesthesia Review Group (CARG) (Appendix 4) for data extraction from all included studies. This data extraction form includes information on sample participants (including demographic characteristics), study methods (setting, intervention, method of delivery) and reported results. All data from the included studies were extracted into Review Manager (RevMan 5.3). We resolved interrater differences by consensus and by discussion with three of the other review authors (AD, CH, VP).
Assessment of risk of bias in included studies
Two review authors (RT, DI) independently performed risk of bias assessment using the tool of The Cochrane Collaboration for assessing risk of bias (Higgins 2011). All included studies were appraised with respect to random sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias. Each of these criteria was assessed as having 'low risk of bias,' 'high risk of bias' or 'unclear risk of bias.' We considered a trial as having 'high risk of bias' if the domain 'random sequence generation' or 'allocation concealment' was assessed as inadequate or unclear. We included a 'Risk of bias' table as part of the Characteristics of included studies table and a 'Risk of bias summary' figure, which details all of the judgements made for all studies included in the review.
Measures of treatment effect
We transferred trial results using Review Manager (RevMan 5.3) and followed the recommendations given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We expressed dichotomous data (e.g. mortality) as risk ratios (RRs) with 95% confidence intervals (CIs). We used mean differences (MDs) or standardized mean differences (SMDs) with 95% CIs for continuous data (e.g. length of hospital stay).
Unit of analysis issues
We performed our analysis at the level of the individual.
Dealing with missing data
We contacted study authors in the event that not all relevant data were presented in the text of a study.
Assessment of heterogeneity
We noted clinical heterogeneity with respect to technical and medical advances in ECMO applications over the designated time period. We performed no assessment of statistical heterogeneity, as we conducted no meta‐analyses.
Assessment of reporting biases
We did not test for publication bias by using a funnel plot or other similar analytical methods because fewer than 10 studies were included in this review.
Data synthesis
We decided not to proceed with meta‐analyses in this review because of clinical heterogeneity observed between studies. In future versions of this review, we will revisit this decision as more trials are completed. We intend to use the following approach. We will analyse pooled results of continuous and dichotomous outcomes using an inverse variance random‐effects or fixed‐effect model, depending on the level of heterogeneity. We will use a random‐effects model in the event of moderate or high heterogeneity, and will apply a fixed‐effect model in cases of low heterogeneity.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses if sufficient data were available (i.e. data from two or more studies).
ECMO application.
Time periods.
Equipment used.
Experience with ECLS among centres or studies.
Participation of centres in the Extracorporeal Life Support Organization (ELSO) registry as a quality control group.
Indications.
Risk factors (age, gender, time on ECMO).
We performed no subgroup analyses or investigations of heterogeneity.
Sensitivity analysis
We did not perform a sensitivity analysis to identify the robustness of results with respect to sequence generation, concealment of allocation, blinding of outcome assessors and presence of missing data because no meta‐analysis was performed.
Summary of findings
We planned to use the principles of the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system (Guyatt 2008) in our review to assess the quality of the body of evidence associated with specific outcomes of mortality, length of hospital stay, survival to discharge, disability, adverse outcomes, health‐related quality of life, longer‐term health status, well‐being and cost‐effectiveness, and to construct a standard 'Summary of findings' table. The GRADE approach is used to appraise the quality of a body of evidence on the basis of the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence reflects within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias. We did not generate a standard 'Summary of findings' table because too few studies were available for inclusion. We summarized individual study outcomes in an additional table (Table 1) but did not use the GRADE system because no meta‐analysis was performed.
1. Results reported from included studies.
| Outcome | Zapol 1979* | Morris 1994*† | Peek 2009‡ | Bein 2013§ |
| Intervention/Population | VA ECMO + ventilation/ARDS patients | VV ECCO2‐R + ventilation/ARDS patients | VV ECMO + ventilation/ARDS patients | AV ECCO2‐R + ventilation/ARDS patients |
| All‐cause mortality (number (%)) Intervention group (IG) vs control group (CG) |
38 of 42 (91) vs 44 of 48 (92) | 14 of 21 (66) vs 11 of 19 (57) | 33 of 90 (37) vs 45 of 90 (50) | 7 of 40 (17.5) vs 6 of 39 (15.4) |
| All‐cause mortality difference IG vs CG (RR, 95% CI) | RR 0.99, 95% CI 0.87 to 1.12 Not statistically significant |
RR 1.15, 95% CI 0.71 to 1.88 Not statistically significant |
RR 0.73, 95% CI 0.52 to 1.03 Not statistically significant |
RR 1.14, 95% CI 0.42 to 3.08 Not statistically significant |
| Length of hospital stay (LOS) days (± SD)a IG vs CG |
Not reported | 26.9 (4.9) vs 28.8 (5.7) Not statistically significant |
35 (15.6 ‐74) vs 17 (4.8 ‐45.3)b | 46.7 (33) vs 35.1 (17) Not statistically significant |
| Survival to discharge (number (%)) IG vs CG |
Not reported | 7 (33)c vs 8 (42)c Not statistically significant |
Not reported | 33 (82.5) vs 33 (84.6) Not statistically significant |
| Disability as reported by study authors | Normal lung function: 7 of 8 (both groups) No limitations in daily activities for all survivors |
Not reported | No severe disabilityd at 6 months: IG: 57 of 90 (63%) CG: 41 of 87 (47%) Statistically significant‡ |
Not reported |
| Adverse outcomes | IG and CG: septicaemia (20%), pneumothorax (45%) Not statistically significant IG: lower blood platelet and white blood cell concentration and greater blood/ plasma infusion reported with 1 to 2.5 liters Statistically significant |
Major complicationse IG = 34 vs CG = 16 Not statistically significant Non‐brain haemorrhage IG = 21 vs CG = 0 Statistically significant IG: transfusion exceeded 0.8 L/d in 10 patients and led to bypass disconnection in 7 patients |
IG = 2 vs CG = 0
|
IG = 3 (7.5%) vs CG =0
Transfusion of blood unitsf IG = 3.7 units vs CG = 1.5 Statistically significant |
ARDS = acute respiratory distress syndrome.
AV ECCO2‐R = arteriovenous extracorporeal membrane carbon dioxide removal.
VV ECCO2‐R = veno‐venous extracorporeal membrane carbon dioxide removal.
aSD = Standard deviation.
b LOS days (interquartile range (IQR)).
cSurvival at 30 days after randomization.
dSevere disability was determined by the first 2 items of the EQ‐5D survey (item Mobility = unable to walk around, in addition to item Personal Care = unable to wash or dress).
eCentral nervous system (CNS), peripheral vascular and other.
fRed blood cell units until day 10.
*The ECMO configuration and respirator settings in the ECMO group and/or the control group are outdated.
†The trial was stopped after 40 participants.
‡3 patients in the control group had unknown disability status. If the 3 patients were severely disabled, RR of the primary outcome (death or severe disability) was 0.67 (95% CI 0.48 to 0.94; P value 0.017); it was 0.72 (95% CI 0.51 to 1.01; P value 0.051) if they were not severely disabled. Only 68 (75%) of participants randomly assigned to the intervention group actually received ECMO.
§Feasability study that used pump‐free avECCO2‐R to achieve very low tidal volume (3 mL/kg) in established ARDS compared with standard mechanical ventilation (6 mg/kg).
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies
Results of the search
The initial search (April 2013) returned 111 citations from CENTRAL, 260 citations from MEDLINE and 302 citations from EMBASE. After duplicates (225 citations) were eliminated from the original 673 citations, 448 citations and two additional citations remained for screening (Figure 1). Four clinical trials (Bein 2013; Morris 1994; Peek 2009; Zapol 1979) and an economic evaluation of one of the trials (Peek 2009) passed the initial screening and were clear inclusions for this review (Included studies). Eight studies (Aus. Safety Reg 2011; Bartlett 2000; Bein 2011; Benzing 1997; Bonastre 2012; Crucean 2010; Cypel 2010; Gille 1974) could not be excluded on the basis of title or abstract; the full papers were retrieved and discussed during a consensus meeting. None met the inclusion criteria (Excluded studies). LR and RT updated the search on 18 August 2014 with no new results. The search for conference proceedings, meeting abstracts and ongoing trials in databases such as Current Controlled Trials (http://www.controlled‐trials.com/) and Clinical Trials (http://clinicaltrials.gov) yielded three ongoing RCTs relevant to this review (Ongoing studies). Contact with 16 experts at specialized treatment centres in eight countries resulted in no additional ongoing or completed RCT reports relevant to this review.
1.
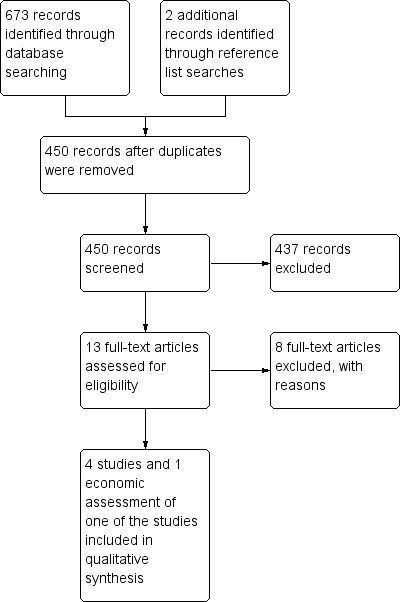
Study flow diagram.
Included studies
We included in this review four RCTs (Bein 2013; Morris 1994; Peek 2009; Zapol 1979) and an economic evaluation of one of the clinical trials (Peek 2009). One multi‐centre trial (10 hospitals) was conducted in Germany and Austria (Bein 2013). Another multi‐centre trial recruited patients from 68 hospitals across the United Kingdom (Peek 2009). Nine medical centres contributed patients to a multi‐centre trial in the United States (Zapol 1979), and another American study included a number of hospitals that were transferring patients and originally admitting patients to their single‐centre trial (Morris 1994). All trials combined included 389 participants; age inclusion criteria were adults aged > 18 years in two trials (Bein 2013; Peek 2009) and aged > 12 years in the other two trials (Morris 1994; Zapol 1979). None of these studies reported participants aged > 12 and < 18 years of age. Diagnostic variations in the included trials (published from 1979 to 2013) meant that all participants had acute respiratory failure with diagnostic entry modifications of low PaO2, high FiO2 and positive end‐expiratory pressure (PEEP) roughly comparable with the indications for ECMO as outlined in the Description of the condition section of this review. The intervention comprised venous‐venous (VV) ECMO in two trials (Morris 1994; Peek 2009), venous‐arterial (VA) ECMO in one trial (Zapol 1979) and avECCO2‐R in the remaining trial (Bein 2013). All interventions were compared with various forms of conventional mechanical ventilation as practiced at the time of the study (Table 2). For further descriptive information about these studies, please refer to the Characteristics of included studies section.
2. Study characteristics.
| Zapol 1979 | Morris 1994 | Peek 2009 | Bein 2013 | |
| Study type | Multi‐centre | Single‐centre | Multi‐centre | Multi‐centre |
| Number of participants randomly assigned | 90 | 40 | 180 | 79 |
| Condition | ARDS | ARDS | ARDS | ARDS |
| ECMO type | VA | VV | VV | AV |
| Intervention group | ECMO + mechanical ventilation | ECCO2‐R + LFPPV | ECMO + mechanical ventilation | avECCO2‐R + mechanical ventilation |
| Control group | Mechanical ventilation | Mechanical ventilation | Mechanical ventilation | Mechanical ventilation |
| Protective lung ventilation* | No | No | Yes | Yes |
| Modern ECMO‡ | No | No | Yes | Yes |
ARDS = acute respiratory distress syndrome.
avECCO2‐R = arteriovenous extracorporeal membrane carbon dioxide removal.
LFPPV‐ECCO2 = low‐frequency positive‐pressure ventilation/extracorporeal carbon dioxide removal.
*Protective lung ventilation: Since the year 2000, significant changes in the treatment of ventilated patients known as protective lung ventilation with low tidal volumes have changed the standard of care for patients with acute respiratory distress syndrome (ARDS Network 2000).
‡Modern ECMO: usage of polymethylpentene oxygenators and heparin‐coated circuits (Terragni 2014; Zampieri 2013).
Excluded studies
We excluded eight studies because they were not randomized controlled trials. See Characteristics of excluded studies for further details.
Risk of bias in included studies
Assessment of risk of bias for each included study is described in the Characteristics of included studies section. Risk of bias was determined as follows for each included study.
Bein 2013: low risk of bias (half of domains were assessed as low risk; 'blinding of outcome assessment', 'incomplete outcome data' and 'selective reporting' were assessed as unclear risk).
Morris 1994: high risk of bias ('allocation of concealment', 'selective reporting' and 'other bias' were assessed as unclear risk; all other domains were assessed as low risk).
Peek 2009: low risk of bias (most domains were assessed as low risk; 'other bias' was assessed as unclear risk).
Zapol 1979: low risk of bias (half of domains were assessed as low risk; 'blinding of outcome assessment', 'selective reporting' and 'other bias' were assessed as unclear risk).
Risk of bias is presented graphically in Figure 2 and Figure 3.
2.
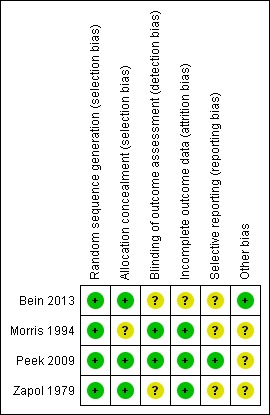
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
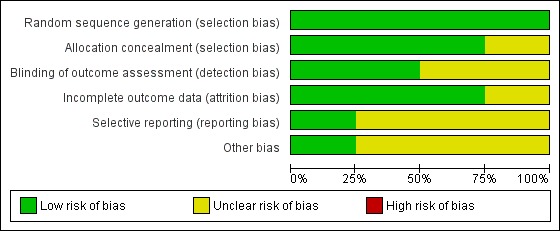
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Sequence generation and allocation concealment were clearly identified in three of the four studies (Bein 2013; Peek 2009; Zapol 1979). One study (Morris 1994) did not provide an explicit statement with respect to how allocation was concealed.
Blinding
Blinding to the intervention was not possible. In one of the studies (Peek 2009), outcomes were assessed at six months by trained researchers blinded to the intervention, and participants covered their neck to mask cannulation status. Another trial (Zapol 1979) reported follow‐up at six months with no information about blinding.
Incomplete outcome data
All studies reported the numbers of withdrawals and dropouts.
Selective reporting
In one of the studies (Peek 2009), risk of selective reporting was assessed as low. In the other trials (Bein 2013; Morris 1994; Zapol 1979), risk was assessed as unclear.
Other potential sources of bias
Other bias was observed in three trials (Morris 1994; Peek 2009; Zapol 1979). This related to variations in or absence of specific treatment and/or ventilation protocols in the intervention group and/or the control group during the study period. In one trial (Bein 2013), which hypothesized that mechanical ventilation using lower tidal volumes (3 mL/kg) assisted by avECCO2‐R would enhance lung protection and increase ventilator‐free days, the condition of 50 participants deteriorated during the screening phase; they were treated with VV ECMO, but no ECMO data or outcomes were reported. Three studies (Bein 2013; Peek 2009; Zapol 1979) were multi‐centre trials; this contributed to performance bias in two trials (Peek 2009; Zapol 1979).
Effects of interventions
A meta‐analysis was not performed because of clinical heterogeneity noted across the included studies (Table 2). Clinical aspects of the interventions varied considerably, given that the RCTs were published between 1979 (Zapol 1979) and 2013 (Bein 2013). Issues such as violation of the treatment protocol (Morris 1994), variations in the selection criteria (Zapol 1979) and outdated application of the intervention contributed to clinical heterogeneity. The oldest two RCTs (Morris 1994; Zapol 1979) were conducted before the year 2000 and do not represent the current standard of care such as protective lung ventilation or use of modern polymethylpentene oxygenators (Zampieri 2013). In one study (Bein 2013), mortality rate was not a primary outcome, randomization occurred 24 hours after a stabilization phase and the condition of 50 potential participants deteriorated; they were treated with VV ECMO. In the largest RCT (Peek 2009), only 68 (75%) of the participants randomly assigned to the intervention group actually received ECMO. Thus, this RCT evaluated referral strategies and outcomes in ARDS populations rather than isolated ECMO effectiveness. Below we provide a descriptive analysis of the effects of interventions across individual studies (Table 1).
All‐cause rate of mortality closest to 30, 60 and 90 days and at six months
The oldest RCT (Zapol 1979) reported six‐month all‐cause mortality rates of 38 of 42 participants (91%) in the intervention group compared with 44 of 48 (92%) in the control group with no statistically significant differences between groups. The risk ratio (RR) was 0.99 (95% confidence interval (CI) 0.87 to 1.12). Another study (Morris 1994) reported rate of all‐cause mortality within 30 days of randomization as 14 of 21 (66%) participants in the intervention group compared with 11 of 19 (57%) in the control group and no differences between the two groups (RR 1.15, 95% CI 0.71 to 1.88). Non‐significant differences in in‐hospital mortality were reported by another study (Bein 2013), as seven of 40 (17.5%) in the intervention group compared with six of 39 (15.4%) in the control group (RR 1.14, 95% CI 0.42 to 3.08). One study (Peek 2009) reported a rate of all‐cause mortality at or before six months of 33 of 90 participants (37%) in the intervention group compared with 45 of 90 (50%) in the control group. The difference was non‐significant (RR 0.73, 95% CI 0.52 to 1.03; P value 0.07).
Length of hospital stay (LOS)
All RCTs but one (Zapol 1979) reported data on length of hospital stay (LOS). In one study (Morris 1994), LOS (days ± standard deviation (SD)) in the intervention group was 26.9 (± 4.9) compared with 28.8 (± 5.7) in the control group. The mean difference of 1.9 days was not significant (P value 0.09). In another study (Bein 2013), LOS was 46.7 (± 33) in the avECCO2‐R group and 35.1 (± 17) in the control group. The mean difference of 11.6 days was not significant (P value 0.113). In one study (Peek 2009), participants in the intervention group had longer (median 18 days) LOS (days (interquartile range (IQR)) compared with participants in the control group (35 (15.6 to 74.0) vs 17.0 (4.8 to 45.3)).
Survival to discharge
Two RCTs reported data on survival to discharge (Bein 2013; Morris 1994). One study (Morris 1994) reported survival at 30 days after randomization. In the intervention group, seven of 21 (33%) survived compared with eight of 19 (42%) in the control group. No differences were noted between groups (P value 0.8). Another study (Bein 2013) found a non‐significant survival to discharge rate of 33 of 40 (82.5%) in the intervention group compared with 33 of 39 (84.6%) in the control group (P value 1.00).
Disability as reported by study authors
Two studies (Peek 2009; Zapol 1979) supplied data on disability. One study (Zapol 1979) followed up on pulmonary function of survivors after discharge; for both groups combined (four participants in the intervention group and four in the control group), pulmonary function was normal in seven participants. No participants had limitations in their daily activities six months after discharge. The other study (Peek 2009) assessed "severe disability" at six months. Severe disability was determined by the first two items of the EQ‐5D survey (standardized instrument from the EuroQoL Group used to measure health outcomes) (item Mobility = unable to walk around, in addition to item Personal Care = unable to wash or dress). A total of 63% (57/90) of participants allocated to consideration for treatment by ECMO survived to six months without severe disability compared with 47% (41/87) of those allocated to conventional management (RR 0.69, 95% CI 0.05 to 0.97; P value 0.03). Three participants in the control group had unknown disability status. Questions were answered by proxies for five participants in the intervention group and for seven in the control group.
Adverse outcomes
All RCTs provided data on adverse outcomes. In one study (Zapol 1979), adverse outcomes including septicaemia (20%) and pneumothorax (45%) were similar in both groups. Participants in the intervention group had lower blood platelet and white blood cell concentrations and greater blood/plasma infusion (reported at 1 to 2.5 L/d). In another study (Morris 1994), recorded major complications other than organ failure were divided into central nervous system (CNS), peripheral vascular system and other. Investigators noted 34 major complications in the intervention group and 16 in the control group, but the overall difference was not statistically significant (P value 0.12). However, non‐CNS haemorrhage occurred significantly more often (21 vs 0) in the intervention group, and transfusion of packed red blood cells (RBCs) exceeded 0.8 L/d in 10 participants, leading to bypass disconnection in seven participants in the intervention group. Another study (Peek 2009) reported two serious adverse events in the intervention group and none in the control group. One was death due to mechanical failure of oxygen supply during ambulance transport. Vessel perforation during cannulation was the other serious adverse event; however, this did not result in death of the participant. The latest study (Bein 2013) reported adverse outcomes in three participants (7.5%). One had transient ischaemia of the lower limb, and two developed a "false" aneurysm from arterial cannulation. Study authors reported significantly higher transfusion of RBCs during the time between randomization and day 10 in the intervention group when compared with the control group (3.7 ± 2.4 vs 1.5 ± 1.3 units RBCs; P value < 0.05).
Health‐related quality of life
One study (Peek 2009) reported data on health‐related quality of life using the Short Form (SF)‐36 and EQ‐5D surveys. Study authors reported no differences between intervention and control groups when EQ‐5D data or any other follow‐up assessments were compared at six months. For 17 participants EQ‐5D data were missing.
Longer‐term health status and well‐being
No RCTs provided data on longer‐term health status and well‐being.
Cost‐effectiveness
Two studies (Morris 1994; Peek 2009) reported data on cost‐effectiveness. One study of cost‐effectiveness was conducted in the United States (Morris 1994). Study authors concluded that hospital costs for participants in the intervention group were USD120,800 compared with USD97,200 in the control group. Cost calculations excluded expenses for research staff members and the senior clinical physician (on‐call for the first two to three years of the trial) as well as costs of extracorporeal equipment and disposals.
In the British context, one study (Peek 2009) reported that patient referral for ECMO more than doubled mean healthcare costs compared with healthcare costs for patients given conventional management (GBP73,979 vs GBP33,435). Study authors saw the potential for cost reduction in the transport of participants given ECMO and in the provision of ECMO itself. Additionally, quality‐adjusted life‐years (QALYs) were calculated from UK tariff values and were based on EQ‐5D survey results. Study authors regarded the lifetime predicted cost utility of GBP19,000 per QALY in the ECMO group as showing cost‐effectiveness according to measures of health technology assessment organizations.
Discussion
Summary of main results
A limited number of studies on this topic have been published. We included in this systematic review four randomized controlled trials (RCTs) that met our inclusion criteria to evaluate extracorporeal membrane oxygenation (ECMO) for critically ill adults. The four RCTs randomly assigned 389 participants. All RCTs reported data on rate of all‐cause mortality closest to 30, 60 and 90 days or at six months. One RCT (Peek 2009) found improved survival without severe disability at six months (risk ratio (RR) 0.69, 95% confidence interval (CI) 0.05 to 0.97; P value 0.03) but non‐significant all‐cause mortality at or before six months (RR 0.73, 95% CI 0.52 to 1.03; P value 0.07). On this basis, study authors recommended the transfer of participants with severe but potentially reversible respiratory failure to a centre with an ECMO‐based management protocol. The other three RCTs were inconclusive and found no difference in all‐cause mortality between intervention and control groups: Zapol 1979: RR 0.99, 95% CI 0.87 to 1.12; Morris 1994: RR 1.15, 95% CI 0.71 to 1.88; Bein 2013: RR 1.14, 95% CI 0.42 to 3.08. No currently available RCT has investigated ECMO in the context of cardiac failure or extracorporeal cardiopulmonary resuscitation (ECPR).
Overall completeness and applicability of evidence
Interventions used in the RCTs varied considerably because the RCTs were published between 1979 (Zapol 1979) and 2013 (Bein 2013) (Table 2). Two studies (Morris 1994; Zapol 1979) were conducted before the year 2000 and do not represent the current standard of care such as protective lung ventilation or use of modern polymethylpentene oxygenators and heparin‐coated circuits (Terragni 2014; Zampieri 2013). Therefore, severe complications such as barotrauma and haemorrhage occurred more frequently in these older trials (Combes 2012a). Methodological issues such as protocol violations were also present in both trials (Morris 1994; Zapol 1979). In one study (Morris 1994), ECMO was removed after five days if no improvement was noted. This study was finally stopped after 40 participants and before the targeted randomization of 60 participants. The largest RCT, the CESAR trial (Conventional Ventilation or ECMO for Severe Adult Respiratory Failure) (Peek 2009), aimed to clarify whether ECMO is beneficial for selected adult patients with respiratory failure. Study authors reported significant differences in survival without severe disability at six months among 57 of 90 (63%) in the intervention group (participants transferred to a specialist centre for consideration for ECMO) and 41 of 87 (47%) in the control group (RR 0.69, 95% CI 0.05 to 0.97; P value 0.03) and recommended the transfer of participants with severe but potentially reversible respiratory failure to a centre with an ECMO‐based management protocol. Three of the participants in the control group were alive at six months but had unknown disability status. If these three participants were severely disabled, the RR of the primary outcome (death or severe disability) was 0.67 (95% CI 0.48 to 0.94; P value 0.017); it was 0.72 (95% CI 0.51 to 1.01; P value 0.051) if they were not severely disabled. Additionally, only 68 (75%) of the participants randomly assigned to the ECMO group actually received ECMO. Therefore, positive outcomes of this RCT are hampered by methodological issues that make interpretation and general recommendations for clinicians difficult (Moran 2010; Pellegrino 2010; Sidebotham 2011; Zwischenberger 2009). Among other new prospective trials, the EOLIA trial (Extracorporeal Membrane Oxygenation to Rescue Lung Injury in Severe Acute Respiratory Distress Syndrome) potentially addresses the methodological issues observed in the CESAR trial and aims to clarify whether ECMO is beneficial for patients with ARDS (Abrams 2013; MacLaren 2012). EOLIA (NCT01470703) is an international, multi‐centre, randomized open trial that will evaluate the impact of ECMO instituted early after diagnosis of ARDS (intubation and mechanical ventilation for < seven days) for study participants not evolving favourably after three to six hours under optimal ventilatory management and maximal medical treatment. The primary outcome measure is all‐cause mortality on day 60 following randomization. Until new results become available, data on ECMO use in patients with respiratory failure remains inconclusive.
For patients with acute cardiac failure, no data from RCTs are available, although one systematic review (Nichol 2006), one meta‐analysis (Cardarelli 2009) and two reviews (Fagnoul 2014; Wang 2013) have assessed observational evidence in this setting. Nichol et al (Nichol 2006) included 84 non‐randomized studies in a review of ECMO/ECPR and cardiac shock or arrest. Fifty‐two studies included 533 participants with cardiogenic shock. The mean proportion of participants who survived to discharge was 51.6 ± 6.5%. Fifty‐four studies included 675 participants with cardiac arrest. The mean proportion of participants who survived to discharge was 44.9 ± 6.7%. The overall proportion of survival to discharge was 47.4 ± 4.1%. Statistically significant heterogeneity among studies was noted across all subgroups. In light of little improvement in rates of survival to discharge over time, Nichol et al concluded that percutaneous bypass is an efficacious intervention for patients with cardiogenic shock and cardiac arrest. However, study authors called for adequately designed RCTs in this new field. Cardarelli et al (Cardarelli 2009) included 11 clinical series and nine case reports in a meta‐analysis of 135 participants given ECPR. Five of these studies were also included in the previous review (Nichol 2006). Overall survival to hospital discharge for participants who received ECMO support after cardiac arrest was 40%. Older age, more days of ECMO support and manual cardiopulmonary resuscitation (CPR) > 30 minutes increased mortality. Wang et al (Wang 2013) included six other clinical studies that reported survival or neurological outcomes. Study authors reported survival to discharge for intrahospital cardiac arrest (IHCA) in 34% to 36%, and survival to discharge for out‐of‐hospital cardiac arrest (OHCA) in 4% to 36%. However, these authors stressed that data were only observational and were obtained from highly selected participant groups with non‐validated ECPR indications (Wang 2013). In the latest review, Fagnoul et al (Fagnoul 2014) included studies that reported on IHCA, OHCA and mixed locations of cardiac arrest and concluded that good neurological outcomes were seen in 40% to 50% of IHCA participants and in 15% to 30% of OHCA participants, respectively, whereas the international registry of the Extracorporeal Life Support Organization (ELSO) reported 40% survival to discharge (or transfer) among participants with cardiac failure and 29% survival to discharge (or transfer) in those treated with ECPR (ELSO 2014). However, an increase of 41% in combined registered cardiac shock and cardiac arrest case numbers between 2012 and 2014 within the registry indicates the current clinical interest in ECMO and ECPR for cardiac patients worldwide (ELSO 2014). Although contributions to the registry are voluntary and therefore may not be representative, all presented data contrast with previously reported values of 22% for survival to discharge in non‐ECPR IHCA participants and 10% for survival to discharge in non‐ECPR OHCA participants (Wang 2013). Outcomes of ongoing RCTs (NCT01511666; NCT01605409) will help to clarify the role of ECMO and ECPR in this new clinical area.
Quality of the evidence
The quality of the evidence was assessed using the approach outlined in Characteristics of included studies. The body of evidence was classified as having 'high,' 'low,' or 'unclear' risk of bias for each outcome. Overall, the evidence was assessed as having 'low' risk of bias.
Since the year 2000, significant advancements in the treatment of ventilated patients known as protective lung ventilation with low tidal volumes have changed the standard of care for patients with acute respiratory distress syndrome (ARDS Network 2000). Technological developments (circuits, pumps and oxygenators) have also improved the performance and patient safety of ECMO applications and have reduced adverse outcomes in ECMO cohorts (Combes 2012; Pellegrino 2010; Zampieri 2013). Methodological issues such as timing of recruitment, participant selection and protocol violation (Sorbo 2014) in the two older trials (Morris 1994; Zapol 1979) do not support aggregation of data with those of newer trials (Bein 2013; Peek 2009). However, although the older trials have been excluded from meta‐analysis by some review authors (Zampieri 2013), other review authors have included them (Chalwin 2008).
Methodological issues relevant to this review were also present in the newer RCTs. In the CESAR trial (Peek 2009), mechanical ventilation and conventional care were not specifically predefined for the control group; therewith intersite variations may have had an impact on outcomes (Brodie 2011; Combes 2012a; Hirshberg 2013). In the latest RCT (Bein 2013), mortality rate was not a primary outcome because researchers investigated the effects of combining a very low tidal volume (3 mL/kg) with avECCO2‐R in established ARDS compared with standard mechanical ventilation (6 mg/kg). Randomization occurred after a 24‐hour stabilization phase, and a total of 226 participants who were screened during this phase were excluded. The condition of 50 of these participants deteriorated, and they were treated with VV ECMO, but no data or outcomes have yet been reported. The newer trials also used different applications of the intervention (VV ECMO vs avECCO2‐R), leading to exclusion of aggregated data.
Evidence from observational studies suggests improved outcomes of ECPR compared with CPR in patients with acute cardiac failure, but no completed RCT has yet been published.
Potential biases in the review process
This review consisted of published data. Future versions of this review will include further details on primary and secondary outcomes as they become available through continuing publication of included studies and studies that have been identified as in progress.
Agreements and disagreements with other studies or reviews
Five systematic reviews (Fitzgerald 2014; Mitchell 2010; Munshi 2014; Zampieri 2013; Zangrillo 2013), one meta‐analysis (Zangrillo 2013a) and one review with quantitative analysis (Chalwin 2008) published between 2008 and 2014 have recently reviewed the literature with respect to respiratory failure, ARDS and mixed ECMO populations. Chalwin et al (Chalwin 2008) identified and aggregated data from two of the trials also included in this review (Morris 1994; Zapol 1979) and found no evidence of benefit or harm (odds ratio (OR) 1.28, 95% CI 0.24 to 6.55). The review authors concluded that analysis of RCTs did not support the application of ECMO, and that evidence from case series suggested otherwise. That review differs from this systematic review, in that it included only two of the four available studies. Additionally, we have not performed a meta‐analysis because of the clinical heterogeneity that we observed across studies. Mitchell et al (Mitchell 2010) included three of the four identified RCTs (Morris 1994; Peek 2009; Zapol 1979) in their meta‐analysis and found significant heterogeneity in methods and populations across studies; they reported that evidence was insufficient to reveal recommendations for ECMO use in patients with H1N1 ARDS (summary RR 0.93, 95% CI 0.71 to 1.22). The systematic review of Zampieri et al (Zampieri 2013) included three (Morris 1994; Peek 2009; Zapol 1979) of the four studies included in this systematic review but excluded two studies (Morris 1994; Zapol 1979) that were considered outdated on the basis of the analysis. Instead, the review authors included two case control studies that paired participants with H1N1 influenza according to severity (Noah 2011; Pham 2012). In their main meta‐analysis of 353 (179 ECMO) participants, ECMO did not reduce in‐hospital mortality (OR 0.71, 95% CI 0.34 to 1.47). In one subanalysis that included participants who really received ECMO and another subanalysis that used propensity scoring with replacements, ECMO reduced in‐hospital mortality (OR 0.52, 95% CI 0.35 to 0.76; OR 0.46, 95% CI 0.33 to 0.66). The review authors concluded that the benefit of ECMO for hospital mortality was unclear. Our group previously assessed the evidence with respect to H1N1 influenza–related acute respiratory failure and came to the same conclusion (Cooper 2013). The systematic review of Zangrillo et al (Zangrillo 2013) also focused on patients with H1N1 influenza and included results from eight observational studies with 266 participants. These review authors included in their quantitative analysis one study previously discussed (Noah 2011). Using random‐effects aggregated estimates and noting considerable heterogeneity, the review authors reported overall in‐hospital mortality of 27.5% (95% CI 18.4% to 36.7%) and noted that exploratory meta‐regression did not identify any significant moderator of mortality. From the results, the review authors concluded that ECMO is feasible and effective in patients with respiratory failure due to H1N1 infection. They also conducted a meta‐analysis of complications and mortality in VV and VA ECMO populations (Zangrillo 2013a). These review authors included 12 studies that reported mortality data from registries with more than 100 participants and included only studies that also described fatal and non‐fatal complications in detail. Overall mortality was 54% (95% CI 47% to 61%) after a median follow‐up of 30 days. None of the trials included in our review was included in the meta‐analysis. Fitzgerald et al (Fitzgerald 2014) collated evidence from 14 trials including two RCTs included in this review (Bein 2013; Morris 1994) and assessed the effects of VV and AV ECCO2‐R on mortality. The review authors concluded that available evidence suggested no mortality benefit of ECCO2‐R. However, they noted that ECCO2‐R technology is rapidly evolving and is effective in enabling protective lung ventilation (Fitzgerald 2014). Munshi et al (Munshi 2014) included in their systematic review (the latest) all four studies that form part of this review and added to their meta‐analysis six other observational studies (including Noah 2011 and Pham 2012).These review authors found no association of a mortality benefit for ECLS but reported a mortality benefit when the meta‐analysis was restricted to higher‐quality studies of VV ECLS. Our review suggests that more data from RCTs are needed before meta‐analysis of trials can be performed and recommendations can be made regarding the effectiveness of ECMO in reducing overall mortality.
Authors' conclusions
Implications for practice.
Extracorporeal membrane oxygenation remains a rescue therapy for critically ill adult patients. Over the past four decades, only four RCTs published results that compared the intervention versus conventional treatment at the time of the study. All studies comprised participants with acute respiratory failure. Meta‐analysis of data from included RCTs was not possible because of significant clinical heterogeneity amongst the included RCTs. Until new comparable results become available, data on ECMO use in patients with respiratory failure remains inconclusive. For patients with acute cardiac failure, ECMO may confer benefits, but only observational evidence is available and no RCT has been published. Outcomes of ongoing RCTs will help to clarify the role of ECMO and ECPR in this new clinical area.
Implications for research.
Results from ongoing research studies (NCT01470703) will further clarify the benefit or harm of ECMO for critically ill adults with acute respiratory failure. Current research into mortality risk prediction in this complex patient cohort will help clinicians more clearly identify patients who may benefit from the intervention in the future (Brogan 2009; Pappalardo 2013; Schmidt 2013; Schmidt 2014). Protective mechanical lung ventilation is permitted by VV and AV ECCO2‐R, and future trials such as SUPERNOVA (Strategy of UltraProtective lung ventilation with Extracorporeal CO2 Removal for New‐Onset moderate to seVere ARDS) will validate the benefit of these non–full‐flow ECMO approaches in the treatment of patients with acute respiratory failure (Terragni 2014).
Ongoing and planned RCTs will clarify the role of ECMO and ECPR in patients with acute cardiac failure (NCT01511666; NCT01605409). We have noted that indications and applications of the intervention are much broader than they used to be. Future systematic reviews must account for this diversity in their search strategies and analyses.
What's new
| Date | Event | Description |
|---|---|---|
| 3 January 2019 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Acknowledgements
We would like to thank Harald Herkner (content editor), Cathal Walsh (statistical editor) and Giles Peek and Alain Combes (peer reviewers) for help and editorial advice provided during preparation of this systematic review.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Extracorporeal Membrane Oxygenation] explode all trees #2 ((extracor* or extra‐cor*) and membra* and oxygen*) or ECMO:ti,ab or ((carbon dioxide or CO2) near remov*) or ((pump?free or pumpless or interventional) near lung?assist*) or (PECLA or iLA):ti,ab #3 #1 or #2 #4 ((lung near assist*) or (severe near (respiratory or cardiac or lung or heart) near (insufficiency or failure))) #5 (lung near assist*):ti,ab #6 (#3 and #4) or #5
Appendix 2. MEDLINE (Ovid) search strategy
1. exp Extracorporeal Membrane Oxygenation/ or ((extracor* or extra‐cor*) and membra* and oxygen*).mp. or ECMO.ti,ab. or ((carbon dioxide or CO2) adj3 remov*).mp. or ((pump?free or pumpless or interventional) adj3 lung?assist*).mp. or (PECLA or iLA).ti,ab. 2. ((lung adj5 assist*) or (severe adj3 (respiratory or cardiac or lung or heart) adj3 (insufficiency or failure))).af. 3. (lung adj5 assist*).ti,ab. 4. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. 5. ((1 and 2) or 3) and 4
Appendix 3. EMBASE (Ovid) search strategy
1 exp extracorporeal oxygenation/ or ((extracor* or extra‐cor*) and membra* and oxygen*).mp. or ECMO.ti,ab. or ((carbon dioxide or CO2) adj3 remov*).mp. or ((pump?free or pumpless or interventional) adj3 lung?assist*).mp. or (PECLA or iLA).ti,ab. 2 ((lung adj5 assist*) or (severe adj3 (respiratory or cardiac or lung or heart) adj3 (insufficiency or failure))).af. 3 (lung adj5 assist*).ti,ab. 4 (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh. 5 ((1 and 2) or 3) and 4
Appendix 4. Data collection form
Data collection form
| Review title or ID |
| Extracorporeal membrane oxygenation for adults in intensive care units CARG Review Number 283 |
| Study ID(surname of first author and year first full report of study was published, e.g. Smith 2001) |
| Report IDs of other reports of this study(e.g. duplicate publications, follow‐up studies) |
| Notes: |
1. General information
| Date form completed(dd/mm/yyyy) | |
| Name/ID of person extracting data | |
|
Report title (title of paper/abstract/report from which data are extracted) |
|
|
Report ID (ID for this paper/abstract/report) |
|
| Reference details | |
| Report author contact details | |
|
Publication type (e.g. full report, abstract, letter) |
|
|
Study funding sources (including role of funders) |
|
|
Possible conflicts of interest (for study authors) |
|
| Notes: | |
2. Study eligibility
| Study characteristics |
Eligibility criteria (Insert eligibility criteria for each characteristic as defined in the Protocol) |
Yes | No | Unclear |
Location in text (pg & ¶/fig/table) |
|
| Type of study | Randomized controlled trial | |||||
| Participants | ||||||
| Types of interventions | ||||||
| Types of outcome measures | ||||||
| INCLUDE | EXCLUDE | |||||
| Reason for exclusion | ||||||
| Notes: | ||||||
DO NOT PROCEED IF STUDY EXCLUDED FROM REVIEW.
3. Population and setting
|
Description (Include comparative information for each group (i.e. intervention and controls) if available) |
Location in text (pg & ¶/fig/table) |
||
|
Population description (from which study participants are drawn) |
|||
|
Setting (including location and social context) |
|||
| Inclusion criteria | |||
| Exclusion criteria | |||
| Method/s of recruitment of participants | |||
| Informed consent obtained | Yes No Unclear | ||
| Notes: | |||
4. Methods
| Descriptions as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Aim of study | |||
| Design(e.g. parallel, cross‐over, cluster) | |||
|
Unit of allocation (by individuals, clusters/groups or body parts) |
|||
| Start date | |||
| End date | |||
| Total study duration | |||
| Ethical approval needed/obtained for study | Yes No Unclear | ||
| Notes: | |||
5. Risk of bias assessment
| Domain | Risk of bias | Support for judgement |
Location in text (pg & ¶/fig/table) |
||
| Low risk | High risk | Unclear risk | |||
|
Random sequence generation (selection bias) |
|||||
|
Allocation concealment (selection bias) |
|||||
|
Incomplete outcome data (attrition bias) |
|||||
|
Selective outcome reporting? (reporting bias) |
|||||
| Other bias | |||||
| Notes: | |||||
6. Participants
Provide overall data and, if available, comparative data for each intervention or comparison group.
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Total no. randomly assigned (or total population at start of study for NRCTs) |
||
|
Clusters (if applicable, no., type, no. of people per cluster) |
||
| Baseline imbalances | ||
|
Withdrawals and exclusions (if not provided below by outcome) |
||
| Age | ||
| Sex | ||
| Race/Ethnicity | ||
| Severity of illness | ||
| Co‐morbidities | ||
| Other treatment received(additional to study intervention) | ||
| Other relevant sociodemographics | ||
| Subgroups measured | ||
| Subgroups reported | ||
| Notes: | ||
7. Intervention groups
Intervention group
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
| Group name | ||
|
No. randomly assigned to group (specify whether no. of people or clusters) |
||
| Theoretical basis(include key references) | ||
| Description(include sufficient detail for replication, e.g. content, dose, components) | ||
| Duration of treatment period | ||
| Timing(e.g. frequency, duration of each episode) | ||
| Delivery(e.g. mechanism, medium, intensity, fidelity) | ||
|
Providers (e.g. no., profession, training, ethnicity, etc., if relevant) |
||
| Co‐interventions | ||
| Economic variables (i.e. intervention cost, changes in other costs as a result of intervention) | ||
|
Resource requirements to replicate intervention (e.g. staff numbers, cold chain, equipment) |
||
| Notes: | ||
Comparison group
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
| Group name | ||
|
No. randomly assigned to group (specify whether no. of people or clusters) |
||
| Theoretical basis(include key references) | ||
| Description(include sufficient detail for replication, e.g. content, dose, components) | ||
| Duration of treatment period | ||
| Timing(e.g. frequency, duration of each episode) | ||
| Delivery(e.g. mechanism, medium, intensity, fidelity) | ||
|
Providers (e.g. no., profession, training, ethnicity, etc., if relevant) |
||
| Co‐interventions | ||
| Economic variables (i.e. intervention cost, changes in other costs as a result of intervention) | ||
|
Resource requirements to replicate intervention (e.g. staff numbers, cold chain, equipment) |
||
| Notes: | ||
8. Outcomes
Copy and paste table for each outcome.
Outcome 1
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Outcome name | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear | ||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
| Notes: | |||
9. Results
Copy and paste the appropriate table for each outcome, including additional tables for each time point and subgroup as required.
Dichotomous outcomes
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Time point (specify whether from start or end of intervention) | ||||||
| Results | Intervention | Comparison | ||||
| No. of events | No. of participants | No. of events | No. of participants | |||
| No. of missing participants and reasons | ||||||
| No. of participants moved from other group and reasons | ||||||
| Any other results reported | ||||||
| Unit of analysis(by individuals, clusters/groups or body parts) | ||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||
| Reanalysis required?(specify) | Yes No Unclear | |||||
| Reanalysis possible? | Yes No Unclear | |||||
| Reanalysed results | ||||||
| Notes: | ||||||
Continuous outcomes
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Time point (specify whether from start or end of intervention) | ||||||||||
| Post intervention or change from baseline? | ||||||||||
| Results | Intervention | Comparison | ||||||||
| Mean | SD (or other variance) | No. of participants | Mean | SD (or other variance) | No. of participants | |||||
| No. of missing participants and reasons | ||||||||||
| No. of participants moved from other group and reasons | ||||||||||
| Any other results reported | ||||||||||
|
Unit of analysis (individuals, clusters/groups or body parts) |
||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Reanalysis required?(specify) | Yes No Unclear | |||||||||
| Reanalysis possible? | Yes No Unclear | |||||||||
| Reanalysed results | ||||||||||
| Notes: | ||||||||||
Other outcomes
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Time point (specify whether from start or end of intervention) | ||||||
| Results | Intervention result | SD (or other variance) | Control result | SD (or other variance) | ||
| Overall results | SE (or other variance) | |||||
| No. of participants | Intervention | Control | ||||
| No. of missing participants and reasons | ||||||
| No. of participants moved from other group and reasons | ||||||
| Any other results reported | ||||||
| Unit of analysis(by individuals, clusters/groups or body parts) | ||||||
| Statistical methods used and appropriateness of these methods | ||||||
| Reanalysis required?(specify) | Yes No Unclear | |||||
| Reanalysis possible? | Yes No Unclear | |||||
| Reanalysed results | ||||||
| Notes: | ||||||
10. Applicability
| Have important populations been excluded from the study?(consider disadvantaged populations and possible differences in the intervention effect) | Yes No Unclear | |
| Is the intervention likely to be aimed at disadvantaged groups?(e.g. lower socioeconomic groups) | Yes No Unclear | |
|
Does the study directly address the review question? (any issues of partial or indirect applicability) |
Yes No Unclear | |
| Notes: | ||
11. Other information
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
| Key conclusions of study authors | ||
| References to other relevant studies | ||
| Correspondence required for further study information(from whom, what and when) | ||
| Notes: | ||
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bein 2013.
| Methods | This multi‐centre randomized clinical trial conducted in Germany (8 sites) and Austria (2 sites) hypothesized that mechanical ventilation using lower tidal volumes (3 mL/kg) assisted by avECCO2‐R would enhance lung protection and hence increase 28‐day and 60‐day ventilator‐free days compared with mechanical ventilation using conventional tidal volumes (6 mL/kg). After screening for inclusion of patients with a PaO2/FiO2 ratio ≤ 200 mmHg, eligible patients entered a 24‐hour stabilization phase with the following targets: VT 6 mL/kg/IBW; "high peep" as per ARDSNet, CVP 10 to 16 mmHg; MAP ≥ 70 mmHg and haemodynamic evaluation via echocardiography. Participants who remained with a PaO2/FiO2 ≤ 200 mmHg were randomly assigned via phone using a random number table generated by the involved statistician. Researchers assessed ventilator‐free days at 28 and 60 days, non‐pulmonary organ failure–free days at 60 days, lung injury score at day 10, length of hospital/ICU stay and in‐hospital mortality. |
|
| Participants | Patients with ARDS according to the American‐European Consensus Conference in intensive care units (ICUs) in Germany and in Austria. Inclusion criteria: ARDS according to the American‐European Consensus Conference with bilateral infiltrates on chest x‐ray and PaO2/FiO2 < 200 mmHg for at least 2 hours. Screening exclusion criteria: left ventricular failure; age > 18 years; history of mechanical ventilation > 7 days; plateau pressure > 25 cm H2O at defined ventilator settings (PEEP/FiO2‐table + VT = 6 mL/kg) and absence of severe haemodynamic instability with high demand for vasopressors (MAP ≥70 mmHg with continuous norepinephrine infusion < 0.4 mcg/kg/min). Exclusion criteria: decompensated heart insufficiency, acute coronary syndrome, severe chronic obstructive pulmonary disease, advanced malignancy with life expectancy < 6 months, long‐term dialysis treatment, lung transplant, proven heparin‐induced thrombocytopenia (HIT), morbid obesity (BMI 40 kg/m2), cirrhosis of the liver, Child class ≥ B (Child–Pugh scores > 7) or acute fulminant hepatic failure, severe peripheral arterial occlusive disease, absence of limb doppler pulse and acute brain injury (Glasgow Coma Scale < 9). Of 79 randomly assigned participants, 40 were assigned to the intervention group (avECCO‐R) and 39 to the control group. |
|
| Interventions | The 40 participants in the intervention group received percutaneous cannulation and initiation of pumpless extracorporeal lung assist (iLA AV; Novalung, Heilbronn, Germany). Unlike "classic" pump‐driven extracorporeal carbon dioxide removal, iLA does not require a blood pump because the extremely low resistance of this circuit allows flows of about 1 to 2 L/min with normal arterial pressures. After initiation of avECCO2‐R, the ventilation strategy according to study protocol was adapted as follows: rapid titration down to VT 3 mL/kg/PBW, PEEP following ARDSNet "high‐PEEP/FiO2" table, respiratory rate 10 to 25/min with an inspiratory/expiratory ratio of 1:1. Termination of avECCO2‐R therapy and decannulation were performed according to a defined algorithm. The 39 participants in the control group received ventilatory management that followed the algorithm of the study group except for the use of VT = 6 mL/kg/PBW. Target blood gases for both groups were as follows: PaO2 > 60 mmHg and arterial pH > 7.2. Use of buffering (tris‐(hydroxymethyl) aminomethane (TRIS)) was permitted if the participant had hypercapnia and respiratory acidosis (pH < 7.2). |
|
| Outcomes | The primary outcome parameter was the proportion of days without assisted ventilation in a 28‐day period ("ventilator‐free" days within 28 days (28‐VFD)) and in a 60‐day period ("ventilator‐free" days within 60 days (60‐VFD)). Secondary outcomes included inspiratory plateau pressure levels (Pplat), proportion of spontaneous breathing as a percentage of the minute ventilation (automatically calculated by the ventilator’s software), RASS score, haemodynamic changes, incidence of complications or adverse reactions, frequency and duration of other adjunctive therapeutic measures, transfusion requirements (packed red blood cell transfusions (units), fresh frozen plasma units, platelet transfusion), daily cumulative doses of analgesic and sedative agents, cumulative catecholamine requirements/24 h throughout the study period, frequency and duration of renal replacement therapy, number of failing organs, "organ‐failure‐free days" within 28 days after randomization and "in‐hospital" mortality. |
|
| Notes | Screening was followed by a stabilization period of 24 hours, characterized by lung‐protective mechanical ventilation with high PEEP (≥ 12 cm H2O), use of supportive measures and haemodynamic evaluation (echocardiography). Participants who met ARDS criteria (PaO2/FiO2< 200 mmHg) after 24 hours despite optimal supportive treatment were identified as those with established ARDS and were randomly assigned. 50 participants were excluded and were treated with VV ECMO. This study was supported by a grant from Novalung, Heilbronn, Germany. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomized through phone hot line by a random number table generated by the involved statistician" |
| Allocation concealment (selection bias) | Low risk | "Randomized through phone...by the involved statistician with respect to the stratum pulmonary/non‐pulmonary ARDS" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "At the time of screening (24 hr prior to randomization) physiologic data were recorded and relevant laboratory, radiographic and clinical findings were collected. Throughout the complete study period, data on ventilator settings, laboratory, physiologic, radiographic and interventional data were recorded" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Primary outcome ("ventilator‐free" days within 28/60 days (28‐VFD/60‐VFD)) is reported for the full cohort. No withdrawals from the 2 groups were cited. However, further analyses report outcomes in surviving participants only. "Post‐hoc analysis: probability of successful weaning in patients presenting with PaO2/FIO2 = / < 150 versus > 150 (only surviving patients)" |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit a judgement |
| Other bias | Low risk | |
Morris 1994.
| Methods | This randomized, controlled, non‐cross‐over clinical trial was carried out in the United States (Shock Trauma/Intermountain Respiratory ICU, LOS Hospital, Salt Lake City). Participants were treated with pressure‐controlled inverse ratio ventilation followed by VV extracorporeal CO2 removal compared with continuous positive‐pressure ventilation. Inclusion criteria: PaO2 < 50 mmHg with FiO2 1.0 and PEEP ≥ 5 cm H2O for 2 hours OR PaO2 < 50 mmHg for > 12 hours with FiO2 0.6 and PEEP ≥ 5 cm H2O with a shunt fraction above 30%. Exclusion criteria: age < 12 or > 65 years; mechanical ventilation > 21 days; immunosuppression; positive human immunodeficiency virus test. |
|
| Participants | Patients with ARDS. ARDS was defined by the presence of all of the following: P(a/A)O2 < 0.2, bilateral chest radiographic infiltrates, CTH < 50 mL/cm H2O and PW < 15 mmHg (or no clinical evidence of heart failure). Patients with severe ARDS who met ECMO entry and exclusion criteria were considered candidates for the clinical trial. 249 patients with ARDS were identified, and 41 met ECMO entry criteria. 40 participants were enrolled (1 with no consent) and were randomly assigned from August 25, 1987, to April 24, 1991. Of these 40 randomly assigned participants, 5 were originally admitted to the LDS Hospital and 35 were transferred there from other hospitals: 6 from Salt Lake City hospitals, 6 from Utah hospitals outside Salt Lake City and 23 by air from out‐of‐state hospitals. Patient referrals were actively solicited from within the LDS Hospital and from other medical centres. All patients at the LDS Hospital were screened for ARDS. |
|
| Interventions | Researchers constructed an extracorporeal system with a parallel and series configuration of two 3.5‐m2 Sci‐Med membrane lungs for gas and blood flow, respectively. If PCIRV support failed, LFPPV‐ECCO2R was initiated. Failure of PCIRV was based on failure to maintain PaO2 or failure to maintain pHa. 21 participants received the intervention (LFPPV‐ECCO2R). 3 to 6 hours after initiation of LFPPV‐ECCO2R, the Ppeak was 45.4 ± 1.7 cm H2O (mean ± SEM, with the number of observations in parentheses) for the 19 new therapy participants supported extracorporeally (35.8 ± 0.5 cm H2O for the first 10 participants). The desired low Ppeak goal was maintained for the first day of LFPPVECCO2R, as Ppeak was only 41.2 cm H2O 24 to 27 hours after initiation of LFPPV‐ECCO2R in the first 10 LFPPV‐ECCO2R participants. During the entire LFPPV‐ECCO2R period, the Ppeak was 54.1 ± 0.2 cm H2O (2865). VR was reduced to 3 to 5/min in all participants during LFPPV‐ECCO2R initiation and was kept at 3.3 ± 0.1/min during the first 3 to 6 hours of LFPPV‐ECCO2R in all participants. For all 21 participants receiving new therapy, Ppeak during all mechanical ventilation support modes grouped together (PCIRVb + LFPPV‐ECCO2R + CPAP) was 49.5 ± 0.2 (6331). For the 19 control participants, Ppeak during the entire CPPV period was 57.8 ± 0.2 cm H2O. For all participants, arterial oxygenation protocols consistently reduced FiO2 and PEEP to the lowest values necessary to maintain the common PaO2 endpoint of 59 mmHg. |
|
| Outcomes | Primary outcome: survival Secondary outcomes: hospital costs; physiological data; length of hospital stay and blood product consumption |
|
| Notes | The trial was stopped after 40 participants with the conclusion that the difference between new and traditional therapies was too small for a significant survival difference to be demonstrated with 60 randomly assigned participants. The study was supported by Grant No. HL36787 from the National Institutes of Health, and by the Deseret Foundation, the Respiratory Distress Syndrome Foundation and LDS Hospital/IHC, Inc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Blinded randomization with blocking was used, and patients were assigned to receive either control therapy or the new therapy" |
| Allocation concealment (selection bias) | Unclear risk | "Blinded randomization with blocking was used, and patients were assigned to receive either control therapy or the new therapy" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment was not reported. However, it was judged unlikely that outcome assessment was influenced by lack of blinding: "The end point of this analysis was the time until death occurred" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "We randomized all patients who met entry criteria, despite the gravity of their clinical state (1 new therapy patient died rapidly before we could initiate LFPPV‐ECCO2R, and 2 patients died within 1 day after initiating LFPPV‐ECCO2R). We observed the "intention‐to‐treat" principle in our evaluation of this new therapy in the clinical trial" |
| Selective reporting (reporting bias) | Unclear risk | Information was insufficient to permit a judgement |
| Other bias | Unclear risk | "We used a minimum VT of 4 ± 0.5 ml/kg BWp rather than a Ppeak limit. We abandoned use of the Ppeak limit because of difficulty in maintaining a VT > 100 ml in some patients after several days of extracorporeal support. After the first 10 patients, we were advised to insist upon a minimum VT of about 250 ml (A. Pesenti, personal communication). Of 6 survivors receiving LFPPV‐ECCO2R, 3 were maintained with the Ppeak limit and 3 with the minimum VT after we abandoned the Ppeak limit" "We used explicit protocols to ensure uniformity of care, with equal frequency of monitoring, consistent decision‐making logic for the management of arterial oxygenation, and common PaO end points for all randomized ARDS patients from the time of randomization to extubation or death, regardless of the therapy limb" |
Peek 2009.
| Methods | This multi‐centre randomized clinical trial aimed to delineate the safety, clinical efficacy and cost‐effectiveness of extracorporeal membrane oxygenation (ECMO) compared with conventional ventilation support. | |
| Participants | 180 participants were enrolled from 3 types of centres: the ECMO Centre at Glenfield Hospital, Leicester, which treated all patients who were randomly allocated for consideration to receive ECMO; tertiary intensive care units (conventional treatment centres); and referral hospitals, which sent patients to conventional treatment centres if they were randomly allocated to receive continued conventional management. 103 hospitals obtained ethics committee approval to collaborate in the study, of which 92 were conventional treatment centres and 11 were referral hospitals. Eligible patients were aged 18 to 65 years with severe but potentially reversible respiratory failure and a Murray score (from all 4 variables—PaO2/FiO2 ratio, positive end‐expiratory pressure, lung compliance and chest radiograph appearance—and FiO2 1) of 3.0 or higher, or uncompensated hypercapnia with pH < 7.20 despite optimal conventional treatment. Reversibility was based on the clinical opinion of 1 of 3 duty ECMO consultants. Patients were also considered for inclusion if the Murray score was 2.5 or higher, so that trial entry could be accelerated if the condition of the patient continued to deteriorate. Patients were excluded if they had been on high‐pressure (peak inspiratory pressure > 30 cm H2O) or high‐FiO2 (> 0.8) ventilation for longer than 168 hours (7 days) or had signs of intracranial bleeding, any other contraindication to limited heparinization or any contraindication to continuation of active treatment. Ventilation parameters were assessed on an hourly basis for high‐pressure (peak airway pressure > 30 cm H2O) or high‐FiO2 (> 0.8) ventilation. |
|
| Interventions | Patients randomly allocated to consideration for treatment by ECMO were transferred to Glenfield Hospital. Protocol: pressure‐restricted mechanical ventilation at 30 cm H2O, positive end‐expiratory pressure titrated to optimal SaO2, FiO2 titrated to maintain SaO2 at > 90%, diuresis to dry weight, target packed cell volume of 40%, prone positioning and full nutrition. If patients did not respond to this protocol within 12 hours (FiO2 > 0.9 needed to maintain SaO2 > 90%, respiratory or metabolic acidosis < 7.2) or were haemodynamically unstable, they received cannulation and ECMO. All ECMO was done in the veno‐venous mode with percutaneous cannulation. Servo‐controlled roller pumps (Stockert, Freiburg,Germany) and polymethylpentene oxygenators (Medos Medizintechnik, Stolberg, Germany) were used. Ventilation was provided in pressure‐control mode with Siemens Servo 300 ventilators (Solna, Sweden); lung rest settings were peak inspiratory pressure 20 to 25, positive end‐expiratory pressure 10 to 15, rate 10 and FiO2 0.3. ECMO was continued until lung recovery, or until apparently irreversible multi‐organ failure. Participants randomly allocated to receive conventional management were given the best critical care practice available at their conventional treatment centres. As a pragmatic trial, a specific management protocol was not mandated, but treatment centres were advised to follow a low‐volume low‐pressure ventilation strategy—i.e. tidal volume of 4 to 8 mL/kg body weight and pressure plateau < 30 cm H2O. |
|
| Outcomes | Primary outcome: death or severe disability at 6 months after randomization (defined as death by 6 months or before discharge from hospital at any time to the end of data collection). Severe disability was defined as confinement to bed and inability to wash or dress alone; according to this definition, all patients were severely disabled at randomization. Health status at 6 months after randomization was assessed from activities of daily living, quality of life, respiratory symptoms, cognitive psychological state and lung function. |
|
| Notes | This study was supported by UK NHS Health Technology Assessment, English National Specialist Commissioning Advisory Group, Scottish Department of Health and Welsh Department of Health. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were screened and selected in accordance with the published protocol: "Patients were randomly allocated by minimization in a 1:1 ratio…" |
| Allocation concealment (selection bias) | Low risk | Concealment of allocation according to protocol: "Clinical advisor phones independent central randomisation service for random allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | At 6‐month follow‐up, testing was done in participants' homes by trained researchers who were masked to treatment allocation. Participants and their relatives were instructed not to reveal which treatment had been used. A scarf was used to cover the neck, thereby masking cannulation status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary analysis was by intention‐to‐treat |
| Selective reporting (reporting bias) | Low risk | Primary analysis was by intention‐to‐treat |
| Other bias | Unclear risk | Only 68 (75%) in the intervention group received ECMO |
Zapol 1979.
| Methods | This multi‐centre randomized clinical trial sought to define which, if any, types of patients with acute lung injury might experience increased survival rates when extracorporeal membrane oxygenation (ECMO) therapy supplements mechanical ventilation. | |
| Participants | 9 medical centres collaborated in a prospective randomized study of ICU patients with acute respiratory failure. With the exception of patients suffering from chronic and irreversible diseases, adult patients with acute respiratory failure of all causes were treated. Inclusion criteria for patients with acute respiratory failure via fast entry: PaO2< 50 mmHg for longer than 2 hours with FiO2 1 and PEEP > 5 cm H2O. Inclusion criteria for patients with acute respiratory failure via slow entry: maximal medical treatment for 48 hours: PaO2 < 50 mmHg for longer than 12 hours with FiO2 > 0.6 and PEEP > 5 cm H2O and Qs/Qt > 30% when measured at FiO2 1 and PEEP > 5 cm H2O. Patients were excluded from the study if any of the following criteria were applicable: age < 12 or > 65 years; duration of pulmonary insult > 21 days; pulmonary capillary wedge pressure > 25 mmHg; chronic systemic disease, including irreversible CNS injury, chronic pulmonary disease, rapidly fatal malignancy and chronic heart, liver or renal failure; severe body burns; or lack of physician or patient consent. |
|
| Interventions | For 42 participants, veno‐arterial partial bypass was performed at the 9 centres using 4 membrane oxygenator designs (Kolobow Scimed, Lande‐Edwards, Bramson and General Electric Peirce). Venous blood was roller‐pumped through the membrane oxygenator and was returned to the systemic circulation. All bypass participants received intravenous heparin, and their kaolin‐activated coagulation time was monitored at least hourly. To assess changes in pulmonary haemodynamics, 45 participants had a flow‐directed pulmonary artery catheter inserted. When possible, bypass flow was reduced to 0.5 L/min every 12 hours and arterial blood gas tensions were sampled without substantial extracorporeal support. 48 participants in the control group were treated with conventional mechanical ventilation. |
|
| Outcomes | Survival. | |
| Notes | This study was supported by the Division of Lung Diseases, National Heart, Lung and Blood Institute. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Therapy, either mechanical ventilation alone (control) or supplemented with partial bypass, was randomly assigned by the data centre after entry into the study" |
| Allocation concealment (selection bias) | Low risk | "…randomly assigned by the data centre after entry into the study" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Of the eight patients who survived, seven had serial pulmonary function studies" |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit a judgement |
| Other bias | Unclear risk | "Although specific mechanical ventilation patterns may have differed between centres, each patient was treated in an established intensive care unit employing broadly accepted regimens of medical therapy" |
ARDS = Acute respiratory distress syndrome.
BMI = body mass index.
cm H2O = centimetre of water.
CNS = central nervous system.
CO2 = carbon dioxide.
CPAP = continuous positive airway pressure.
CPPV = continuous positive‐pressure ventilation.
CTH = total thoracic compliance.
CVP = central venous pressure.
ECMO = extracorporeal membrane oxygenation.
FiO2 = fraction of inspired oxygen.
HIT = heparin‐induced thrombocytopenia.
IBW = ideal body weight.
ICU = intensive care unit.
iLA = interventional lung assist.
LFPPV‐ECCO2 = low‐frequency positive‐pressure ventilation/extracorporeal carbon dioxide removal.
LOS = length of stay.
MAP = mean arterial pressure.
mcg/kg/min = microgram/kilogram/minute.
mmHg = millimetre of mercury.
PAO2= partial alveolar oxygen pressure.
PaO2 = partial arterial pressure of oxygen.
PCIRV = pressure‐controlled inverse ratio ventilation.
PEEP = positive end‐expiratory pressure.
pH = potential hydrogen.
pHa = arterial potential hydrogen.
Ppeak = peak airway pressure.
Pplat = plateau pressure levels.
PW = pulmonary artery wedge pressure.
RASS = Richmond Agitation Sedation Scale.
SaO2 = arterial saturation of oxygen.
SEM = standard error of the mean.
VFD = ventilator‐free day.
VR = ventilation rate.
VT = volume tidal.
VV = veno‐venous.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aus. Safety Reg 2011 | Review |
| Bartlett 2000 | Review |
| Bein 2011 | Retrospective cohort study |
| Benzing 1997 | Prospective cohort study |
| Bonastre 2012 | Prospective, observational, multi‐centre study |
| Crucean 2010 | French report of the Peek 2009 results |
| Cypel 2010 | Conference paper of a prospective non‐randomized trial |
| Gille 1974 | Comparative study |
Characteristics of ongoing studies [ordered by study ID]
NCT01470703.
| Trial name or title | Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome (EOLIA) |
| Methods | A multi‐centre, randomized, open trial. 23 centres will participate in this project to be conducted within the REVA network |
| Participants | Patients with the diagnosis of acute respiratory distress syndrome (ARDS) not evolving favourably after 3 to 6 hours under optimal ventilatory management and maximal medical treatment |
| Interventions | Intervention group: ECMO (Quadrox®, Jostra®, Maquet®) Control group: conventional care |
| Outcomes | All‐cause mortality on day 60 following randomization |
| Starting date | October 2011 |
| Contact information | Combes Alain, MD, PhD Hôpitaux de Paris |
| Notes |
NCT01511666.
| Trial name or title | Hyperinvasive Approach in Cardiac Arrest/Hyperinvasive Approach to Out‐of Hospital Cardiac Arrest Using Mechanical Chest Compression Device, Prehospital Intraarrest Cooling, Extracorporeal Life Support and Early Invasive Assessment Compared to Standard of Care. A Randomized Parallel Group Comparative Study. "Prague OHCA Study" |
| Methods | Prospective randomized multi‐centre clinical study |
| Participants | Patients with witnessed OHCA without ROSC after a minimum of 5 minutes of ACLS by emergency medical service (EMS) team |
| Interventions | Intervention group: prehospital mechanical compression device, intra‐arrest cooling and in‐hospital ECLS (LUCAS, Rhinochill, PLS ECMO). Control group: Standard care as per recent guidelines will be provided |
| Outcomes | Composite endpoint of survival with good neurological outcome (CPC 1‐2) |
| Starting date | March 2013 |
| Contact information | Jan Belohlavek, MD, PhD Charles University, Czech Republic |
| Notes |
NCT01605409.
| Trial name or title | Emergency Cardiopulmonary Bypass After Cardiac Arrest With Ongoing Cardiopulmonary Resuscitation—a Pilot Randomized Trial |
| Methods | Single‐centre (university hospital), randomized, controlled and prospective pilot study |
| Participants | Patients with witnessed out‐of‐hospital cardiac arrest, with presumed cardiac cause, with immediate initiation of bystander CPR and without return of spontaneous circulation after a minimum of 15 minutes of advanced cardiac life support are eligible Patients who do achieve ROSC at first but suffer rearrest afterwards and do not achieve ROSC again after 15 minutes of advanced cardiac life support are considered eligible as well |
| Interventions | Intervention group: emergency cardiopulmonary bypass under ongoing CPR Control group: standard ACLS |
| Outcomes | Primary outcome measure is the rate of sustained restoration of spontaneous circulation according to Utstein criteria |
| Starting date | September 2012 |
| Contact information | Andreas Schober, MD Medical University of Vienna |
| Notes |
ACLS = advanced cardiovascular life support.
ARDS = acute respiratory distress syndrome.
CPC = cerebral performance categories.
CPR = cardiopulmonary resuscitation.
ECCO2‐R = extracorporeal carbon dioxide removal.
ECLS = extracorporeal life support.
ECMO = extracorporeal membrane oxygenation.
EMS = emergency medical service.
OHCA = out‐of‐hospital cardiac arrest.
REVA = Recherche en Ventilation Artificielle.
ROSC = return of spontaneous circulation.
VILI = ventilator‐induced lung injury.
Vt = volume tidal.
Differences between protocol and review
We made the following changes to the protocol (Tramm 2013).
We updated all sections of the Background of this review.
We included 'blinding of outcome assessment' in the risk of bias assessment of included studies.
We updated the search strategy in cooperation with the Cochrane Anaesthesia Review Group Trials Search Co‐ordinator (Karen Hovhannisyan).
Contributions of authors
Ralph Tramm (RT), Dragan Ilic (DI) Andrew R Davies (AD), Vincent A Pellegrino (VP), Lorena Romero (LR), Carol Hodgson (CH).
Conceiving of the review: RT, DI, AD, VP, LR, CH
Co‐ordinating the review: RT.
Undertaking manual searches: RT, DI, LR.
Screening search results: RT, DI.
Organizing retrieval of papers: RT.
Screening retrieved papers against inclusion criteria: RT, DI.
Appraising quality of papers: RT, DI.
Abstracting data from papers: RT, DI.
Writing to authors of papers for additional information: RT.
Providing additional data about papers: RT.
Obtaining and screening data from unpublished studies: RT, DI.
Managing data for the review: RT.
Entering data into Review Manager (RevMan 5.3): RT, CH, DI.
Analysing RevMan statistical data: RT, DI, CH.
Performing other statistical analyses not using RevMan: N/A.
Interpreting data: RT, DI, AD, VP, CH.
Making statistical inferences: N/A
Writing the review: RT, DI, CH, AD, VP.
Securing funding for the review: N/A.
Performing previous work that served as the foundation of the present study: N/A.
Serving as guarantor for the review (one review author): RT.
Taking responsibility for reading and checking the review before submission: RT.
Sources of support
Internal sources
-
Monash University, Australia.
RT is supported by an Australian Postgraduate Award (APA) administered by the University. In kind support of infrastructure and database access.
External sources
No sources of support supplied
Declarations of interest
Ralph Tramm (RT): none known.
Dragan Ilic (DI): none known.
Andrew R Davies (AD): none known.
Vincent A Pellegrino (VP): grant from Intensive Care Foundation (Australia and New Zealand), Effect of ECMO on long term disability in severe ARDS. Grant from Extracorporeal Life Support Organization (ELSO) (USA), Predictors of survival from VV and VA ECMO. ELSO is an international non‐profit organization of healthcare professionals and scientists who are dedicated to the development and evaluation of novel therapies for support of failing organ systems. The primary mission of the Organization is to maintain a registry of, at least, use of extracorporeal membrane oxygenation in active ELSO centres. Registry data are to be used to support clinical research, support regulatory agencies and support individual ELSO centres. ELSO provides educational programmes for active centres, as well as for the broader medical and lay communities. This grant was used to analyse data from adult patients with severe acute respiratory failure treated with ECMO from 2000 to 2012 using information derived from the ELSO international registry. VP is a co‐investigator who received funding. The grant has no relevance to the review because it developed a score from registry data, not from clinical trials. Travel/accommodations/meeting expenses: Maquet provided a travel fund for attendance at the 4th International Pediatric Symposium in Singapore, October 2013. The 4th International Pediatric Symposium was a Maquet‐sponsored scientific meeting held in Singapore that was dedicated to perfusion and ECMO (extracorporeal membrane oxygenation) matters in the care of paediatric patients. Marquet supplies extracorporeal life support (ECLS) equipment that is used to provide ECMO. This encompasses pumps (The CARDIOHELP System), circuits (HLS Set Advanced) and other supportive equipment.
Lorena Romero (LR): none known.
Carol Hodgson (CH): none known.
Edited (no change to conclusions)
References
References to studies included in this review
Bein 2013 {published data only}
- Bein T, Weber‐Carstens S, Goldmann A, Müller T, Staudinger T, Brederlau J, et al. Lower tidal volume strategy (approximately 3 ml/kg) combined with extracorporeal CO2 removal versus 'conventional' protective ventilation (6 ml/kg) in severe ARDS: the prospective randomized Xtravent‐study. Intensive Care Medicine 2013;39(5):847‐9. [PUBMED: 23306584] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morris 1994 {published data only}
- Morris AH, Wallace CJ, Menlove RL, Clemmer TP, Orme JF Jr, Weaver LK, et al. Randomized clinical trial of pressure‐controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine 1994;2(149):295‐310. [PUBMED: 8306022] [DOI] [PubMed] [Google Scholar]
Peek 2009 {published data only}
- Peek GJ, Elbourne D, Mugford M, Tiruvoipati R, Wilson A, Allen E, et al. Randomised controlled trial and parallel economic evaluation of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR). Health Technology Assessment 2010;14(35):1‐46. [PUBMED: 20642916] [DOI] [PubMed] [Google Scholar]
- Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351‐63. [PUBMED: 19762075] [DOI] [PubMed] [Google Scholar]
Zapol 1979 {published data only}
- Zapol WM, Snider MT, Hill JD, Fallat RJ, Bartlett RH, Edmunds LH, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979;242:2193‐6. [PUBMED: 490805] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aus. Safety Reg 2011 {published data only}
- Australian Safety Efficacy Register of New Interventional Procedures Surgical. Pumpless extracorporeal lung assist device (Novalung) (Project record). Health Technology Assessment Database 2011. [HTA‐32011000484]
Bartlett 2000 {published data only}
- Bartlett RH. Extracorporeal life support in the management of severe respiratory failure. Clinics in Chest Medicine 2000;21:555‐61. [PUBMED: 11019727] [DOI] [PubMed] [Google Scholar]
Bein 2011 {published data only}
- Bein T, Zimmermann M, Philipp A, Ramming M, Sinner B, Schmid C, et al. Addition of acetylsalicylic acid to heparin for anticoagulation management during pumpless extracorporeal lung assist. ASAIO Journal 2011;57:164‐8. [PUBMED: 21427564] [DOI] [PubMed] [Google Scholar]
Benzing 1997 {published data only}
- Benzing A, Mols G, Brieschal T, Geiger K. Hypoxic pulmonary vasoconstriction in nonventilated lung areas contributes to differences in hemodynamic and gas exchange responses to inhalation of nitric oxide. Anesthesiology 1997;86:1254‐61. [PUBMED: 9197293] [DOI] [PubMed] [Google Scholar]
Bonastre 2012 {published data only}
- Bonastre J, Suberviola B, Pozo JC, Guerrero JE, Torres A, Rodriguez A, et al. [Extracorporeal lung support in patients with severe respiratory failure secondary to the 2010‐2011 winter seasonal outbreak of influenza A (H1N1) in Spain]. [Spanish]. Medicina Intensiva 2012;36:193‐9. [PUBMED: 22341559] [DOI] [PubMed] [Google Scholar]
Crucean 2010 {published data only}
- Crucean AC, Peek GJ. CESAR study: Justification of extracorporeal membrane oxygenation for severe adult respiratory failure [Article in French]. IRBM 2010;31:S46‐51. [DOI: 10.1016/S1959-0318(10)70009-9] [DOI] [Google Scholar]
Cypel 2010 {published data only}
- Cypel M, Fischer S, Reynolds S, Pierre A, Perrot M, Yeung JC, et al. Safety and efficacy of the Novalung interventional lung assist (iLA) device as a bridge to lung transplantation. Journal of Heart and Lung Transplantation. 2010. [30th Annual Meeting and Scientific Sessions of the International Society for Heart and Lung Transplantation (ISHLT) Date: 21. ‐ 24.04.2010]
Gille 1974 {published data only}
- Gille JP, Miguel E, Polu JM, Lambert H, Sadoul P. [Extracorporeal circulation using a membrane artificial lung for respiratory assistance]. [French]. Poumon et Le Coeur 1974;30:63‐71. [PUBMED: 4427873] [PubMed] [Google Scholar]
References to ongoing studies
NCT01470703 {unpublished data only}
- NCT01470703. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome (EOLIA). http://clinicaltrials.gov/ct2/show/NCT01470703 (accessed 18 August 2014). [NCT01470703]
NCT01511666 {unpublished data only}
- NCT01511666. Hyperinvasive approach in cardiac arrest. http://clinicaltrials.gov/ct2/show/NCT01511666 (accessed 18 August 2014). [NCT01511666]
NCT01605409 {unpublished data only}
- NCT01605409. Emergency cardiopulmonary bypass for cardiac arrest (ECPB4OHCA). http://clinicaltrials.gov/ct2/show/study/NCT01605409 (accessed 18 August 2014). [NCT01605409]
Additional references
Abrams 2013
- Abrams D, Brodie D, Combes A. What is new in extracorporeal membrane oxygenation for ARDS in adults?. Intensive Care Medicine 2013;39(11):2028‐30. [PUBMED: 23903779] [DOI] [PubMed] [Google Scholar]
Allen 2011
- Allen S, Holena D, McCunn M, Kohl B, Sarani B. A review of the fundamental principles and evidence base in the use of extracorporeal membrane oxygenation (ECMO) in critically ill adult patients. Journal of Intensive Care Medicine 2011;26(1):13‐26. [PUBMED: 21262750] [DOI] [PubMed] [Google Scholar]
ARDS Network 2000
- Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. New England Journal of Medicine 2000;342(18):1301‐8. [PUBMED: 10793162] [DOI] [PubMed] [Google Scholar]
Bein 2006
- Bein T, Weber F, Philipp A, Prasser C, Pfeifer M, Schmid FX, et al. A new pumpless extracorporeal interventional lung assist in critical hypoxemia/hypercapnia. Critical Care Medicine 2006;34(5):1372‐7. [PUBMED: 16540950] [DOI] [PubMed] [Google Scholar]
Brodie 2011
- Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. New England Journal of Medicine 2011;365(20):1905‐14. [PUBMED: 22087681] [DOI] [PubMed] [Google Scholar]
Brogan 2009
- Brogan TV, Thiagarajan RR, Rycus PT, Bartlett RH, Bratton SL. Extracorporeal membrane oxygenation in adults with severe respiratory failure: a multi‐center database. Intensive Care Medicine 2009;35(12):2105‐14. [PUBMED: 19768656] [DOI] [PubMed] [Google Scholar]
Cardarelli 2009
- Cardarelli MG, Young AJ, Griffith B. Use of extracorporeal membrane oxygenation for adults in cardiac arrest (E‐CPR): a meta‐analysis of observational studies. ASAIO Journal 2009;55(6):581‐6. [PUBMED: 19770800] [DOI] [PubMed] [Google Scholar]
Chalwin 2008
- Chalwin RP, Moran JL, Graham PL. The role of extracorporeal membrane oxygenation for treatment of the adult respiratory distress syndrome: review and quantitative analysis. Anaesthesia and Intensive Care 2008;36(2):152‐61. [PUBMED: 18361004] [DOI] [PubMed] [Google Scholar]
Chen 2008
- Chen YS, Lin JW, Yu HY, Ko WJ, Jerng JS, Chang WT, et al. Cardiopulmonary resuscitation with assisted extracorporeal life‐support versus conventional cardiopulmonary resuscitation in adults with in‐hospital cardiac arrest: an observational study and propensity analysis. Lancet 2008;372(9638):554‐61. [PUBMED: 18603291] [DOI] [PubMed] [Google Scholar]
Chung 2010
- Chung JC, Tsai PR, Chou NK, Chi NH, Wang SS, Ko WJ. Extracorporeal membrane oxygenation bridge to adult heart transplantation. Clinical Transplantation 2010;24(3):375‐80. [PUBMED: 19744095] [DOI] [PubMed] [Google Scholar]
Combes 2012
- Combes A, Brechot N, Luyt CE, Schmidt M. What is the niche for extracorporeal membrane oxygenation in severe acute respiratory distress syndrome?. Current Opinion in Critical Care 2012;18(5):527‐32. [PUBMED: 22914430] [DOI] [PubMed] [Google Scholar]
Combes 2012a
- Combes A, Bacchetta M, Brodie D, Muller T, Pellegrino V. Extracorporeal membrane oxygenation for respiratory failure in adults. Current Opinion in Critical Care 2012;18(1):99‐104. [PUBMED: 22186218] [DOI] [PubMed] [Google Scholar]
Cooper 2007
- Cooper DS, Jacobs JP, Moore L, Stock A, Gaynor JW, Chancy T, et al. Cardiac extracorporeal life support: state of the art in 2007. Cardiology in the Young 2007;17(Suppl 2):104‐15. [PUBMED: 18039404] [DOI] [PubMed] [Google Scholar]
Cooper 2013
- Cooper DJ, Hodgson CL. Extracorporeal membrane oxygenation rescue for H1N1 acute respiratory distress syndrome: equipoise regained. American Journal of Respiratory and Critical Care Medicine 2013;187(3):224‐6. [PUBMED: 23378436] [DOI] [PubMed] [Google Scholar]
D'Alessandro 2011
- D'Alessandro C, Golmard JL, Barreda E, Laali M, Makris R, Luyt CE, et al. Predictive risk factors for primary graft failure requiring temporary extra‐corporeal membrane oxygenation support after cardiac transplantation in adults. European Journal of Cardio‐Thoracic Surgery 2011;40(4):962‐9. [PUBMED: 21414795] [DOI] [PubMed] [Google Scholar]
Dalton 2011
- Dalton HJ. Extracorporeal life support: moving at the speed of light. Respiratory Care 2011;56(9):1445‐56. [PUBMED: 21944690] [DOI] [PubMed] [Google Scholar]
Davies 2009
- Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, et al. Extracorporeal membrane oxygenation for 2009 influenza A (H1N1) acute respiratory distress syndrome. JAMA 2009;302(17):1888‐95. [PUBMED: 19822628] [DOI] [PubMed] [Google Scholar]
Douglas 2008
- Douglas JE, Schuerer DJ, Kolovos NS, Boyd KV, Coopersmith CM. Extracorporeal membrane oxygenation: current clinical practice, coding, and reimbursement. Chest 2008;134(1):179‐84. [PUBMED: 18628221] [DOI] [PubMed] [Google Scholar]
ELSO 2014
- Extracorporal Life Support Organization 2014. http://www.elso.org (accessed 18 August 2014).
Fagnoul 2014
- Fagnoul D, Combes A, Backer D. Extracorporeal cardiopulmonary resuscitation. Current Opinion in Critical Care 2014;20(3):259‐65. [PUBMED: 24785674] [DOI] [PubMed] [Google Scholar]
Fitzgerald 2014
- Fitzgerald M, Millar J, Blackwood B, Davies A, Brett SJ, McAuley DF, et al. Extracorporeal carbon dioxide removal for patients with acute respiratory failure secondary to the acute respiratory distress syndrome: a systematic review. Critical Care 2014;18(3):222. [PUBMED: 25033302] [DOI] [PMC free article] [PubMed] [Google Scholar]
Forrest 2011
- Forrest P, Ratchford J, Burns B, Herkes R, Jackson A, Plunkett B, et al. Retrieval of critically ill adults using extracorporeal membrane oxygenation: an Australian experience. Intensive Care Medicine 2011;37(5):824‐30. [DOI] [PubMed] [Google Scholar]
Gattinoni 2011
- Gattinoni L, Carlesso E, Langer T. Clinical review: extracorporeal membrane oxygenation. Critical Care 2011;15:243. [PUBMED: 22188792] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(5):995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hirshberg 2013
- Hirshberg E, Miller RR 3rd, Morris AH. Extracorporeal membrane oxygenation in adults with acute respiratory distress syndrome. Current Opinion in Critical Care 2013;19(1):38‐43. [PUBMED: 23222676] [DOI] [PubMed] [Google Scholar]
Lindstrom 2009
- Lindstrom SJ, Pellegrino VA, Butt WW. Extracorporeal membrane oxygenation. Medical Journal of Australia 2009;191(3):178‐82. [PUBMED: 19645652] [DOI] [PubMed] [Google Scholar]
MacLaren 2012
- MacLaren G, Combes A, Bartlett RH. Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Medicine 2012;38(2):210‐20. [PUBMED: 22147116] [DOI] [PubMed] [Google Scholar]
Marasco 2008
- Marasco SF, Lukas G, McDonald M, McMillan J, Ihle B. Review of ECMO (extracorporeal membrane oxygenation) support in critically ill adult patients. Heart, Lung and Circulation 2008;17(Suppl 4):S41‐7. [PUBMED: 18964254] [DOI] [PubMed] [Google Scholar]
Mirabel 2011
- Mirabel M, Luyt CE, Leprince P, Trouillet JL, Léger P, Pavie A, et al. Outcomes, long‐term quality of life, and psychologic assessment of fulminant myocarditis patients rescued by mechanical circulatory support. Critical Care Medicine 2011;39(5):1029‐35. [PUBMED: 21336134] [DOI] [PubMed] [Google Scholar]
Mitchell 2010
- Mitchell MD, Mikkelsen ME, Umscheid CA, Lee I, Fuchs BD, Halpern SD. A systematic review to inform institutional decisions about the use of extracorporeal membrane oxygenation during the H1N1 influenza pandemic. Critical Care Medicine 2010;38(6):1398‐404. [PUBMED: 20400902] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moran 2010
- Moran JL, Chalwin RP, Graham PL. Extracorporeal membrane oxygenation (ECMO) reconsidered. Critical Care and Resuscitation 2010;12(2):131‐5. [PUBMED: 20513222] [PubMed] [Google Scholar]
Mugford 2008
- Mugford M, Elbourne D, Field D. Extracorporeal membrane oxygenation for severe respiratory failure in newborn infants. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD001340.pub2] [DOI] [PubMed] [Google Scholar]
Munshi 2014
- Munshi L, Telesnicki T, Walkey A, Fan E. Extracorporeal life support for acute respiratory failure. A systematic review and metaanalysis. Annals of the American Thoracic Society 2014;11(5):802‐10. [PUBMED: 24724902] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nichol 2006
- Nichol G, Karmy‐Jones R, Salerno C, Cantore L, Becker L. Systematic review of percutaneous cardiopulmonary bypass for cardiac arrest or cardiogenic shock states. Resuscitation 2006;70(3):381‐94. [PUBMED: 16828957] [DOI] [PubMed] [Google Scholar]
Noah 2011
- Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A (H1N1). JAMA 2011;306(15):1659‐68. [PUBMED: 21976615] [DOI] [PubMed] [Google Scholar]
Pappalardo 2013
- Pappalardo F, Pieri M, Greco T, Patroniti N, Pesenti A, Arcadipane A, et al. Predicting mortality risk in patients undergoing venovenous ECMO for ARDS due to influenza A (H1N1) pneumonia: the ECMOnet score. Intensive Care Medicine 2013;39(2):275‐81. [PUBMED: 23160769] [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2011
- Park PK, Napolitano LM, Bartlett RH. Extracorporeal membrane oxygenation in adult acute respiratory distress syndrome. Critical Care Clinics 2011;27:627‐46. [PUBMED: 21742220] [DOI] [PubMed] [Google Scholar]
Pellegrino 2010
- Pellegrino VA, Davies AR. CESAR: deliverance or just the beginning?. Critical Care and Resuscitation 2010;12(2):75‐7. [PUBMED: 20513213] [PubMed] [Google Scholar]
Petrucci 2007
- Petrucci N, Iacovelli W. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD003844.pub3] [DOI] [PubMed] [Google Scholar]
Pham 2012
- Pham T, Combes A, Richard JCM, Rozé H, Chevret S, Roch A, et al. Extracorporeal membrane oxygenation for pandemic influenza A (H1N1)‐induced acute respiratory distress syndrome: a cohort study and propensity‐matched analysis. American Journal of Respiratory and Critical Care Medicine 2012;187(3):276‐85. [PUBMED: 23155145] [DOI] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rubenfeld 2007
- Rubenfeld GD, Herridge MS. Epidemiology and outcomes of acute lung injury. Chest 2007;131(2):554‐62. [PUBMED: 17296661] [DOI] [PubMed] [Google Scholar]
Schmidt 2013
- Schmidt M, Zogheib E, Roze H, Repesse X, Lebreton G, Luyt CE, et al. The PRESERVE mortality risk score and analysis of long‐term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Medicine 2013;39(10):1704‐13. [PUBMED: 23907497] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmidt 2014
- Schmidt M, Bailey M, Sheldrake J, Hodgson C, Aubron C, Rycus PT, et al. Predicting survival after ECMO for severe acute respiratory failure: the Respiratory ECMO Survival Prediction (RESP)‐Score. American Journal of Respiratory and Critical Care Medicine 2014;189(11):1374‐82. [PUBMED: 24693864] [DOI] [PubMed] [Google Scholar]
Sidebotham 2009
- Sidebotham D, McGeorge A, McGuinness S, Edwards M, Willcox T, Beca J. Extracorporeal membrane oxygenation for treating severe cardiac and respiratory disease in adults: Part 1—overview of extracorporeal membrane oxygenation. Journal of Cardiothoracic and Vascular Anesthesia 2009;23(6):886‐92. [PUBMED: 19944353] [DOI] [PubMed] [Google Scholar]
Sidebotham 2010
- Sidebotham D, McGeorge A, McGuinness S, Edwards M, Willcox T, Beca J. Extracorporeal membrane oxygenation for treating severe cardiac and respiratory failure in adults: Part 2—technical considerations. Journal of Cardiothoracic and Vascular Anesthesia 2010;24(1):164‐72. [PUBMED: 19875307] [DOI] [PubMed] [Google Scholar]
Sidebotham 2011
- Sidebotham D. Extracorporeal membrane oxygenation—understanding the evidence: CESAR and beyond. Journal of ExtraCorporeal Technology 2011;43(1):23‐6. [PUBMED: 21449236] [PMC free article] [PubMed] [Google Scholar]
Sorbo 2014
- Sorbo L, Cypel M, Fan E. Extracorporeal life support for adults with severe acute respiratory failure. The Lancet Respiratory Medicine 2014;2(2):154‐64. [PUBMED: 245032708] [DOI] [PubMed] [Google Scholar]
Terragni 2014
- Terragni P, Faggiano C, Ranieri VM. Extracorporeal membrane oxygenation in adult patients with acute respiratory distress syndrome. Current Opinion in Critical Care 2014;20(1):86‐91. [PUBMED: 24322337] [DOI] [PubMed] [Google Scholar]
Wang 2013
- Wang CH, Chen YS, Ma MH. Extracorporeal life support. Current Opinion in Critical Care 2013;19(3):202‐7. [PUBMED: 23587757] [DOI] [PubMed] [Google Scholar]
Zampieri 2013
- Zampieri FG, Mendes PV, Ranzani OT, Taniguchi L, Pontes Azevedo LC, Vieira Costa EL, et al. Extracorporeal membrane oxygenation for severe respiratory failure in adult patients: a systematic review and meta‐analysis of current evidence. Journal of Critical Care 2013;28(6):998‐1005. [PUBMED: 23954453] [DOI] [PubMed] [Google Scholar]
Zangrillo 2013
- Zangrillo A, Biondi‐Zoccai G, Landoni G, Frati G, Patroniti N, Pesenti A, et al. Extracorporeal membrane oxygenation (ECMO) in patients with H1N1 influenza infection: a systematic review and meta‐analysis including 8 studies and 266 patients receiving ECMO. Critical Care 2013;17(1):R30. [PUBMED: 23406535] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zangrillo 2013a
- Zangrillo A, Landoni G, Biondi‐Zoccai G, Greco M, Greco T, Frati G, et al. A meta‐analysis of complications and mortality of extracorporeal membrane oxygenation. Critical Care and Resuscitation 2013;15(3):172‐8. [PUBMED: 23944202] [PubMed] [Google Scholar]
Zwischenberger 2009
- Zwischenberger J, Lynch JE. Will CESAR answer the adult ECMO debate?. Lancet 2009;374(9698):1307‐8. [PUBMED: 19762074] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Tramm 2013
- Tramm R, Ilic D, Davies AR, Pellegrino VA, Hodgson C. Extracorporeal membrane oxygenation for critically ill adults. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD010381] [DOI] [PMC free article] [PubMed] [Google Scholar]


