Abstract
Moringa oleifera Lam. and Centella asiatica (L.) Urb. leaves have been previously reported to exhibit antioxidant activity. The objective of the present study is to determine the in vitro antioxidant activity of the combined extracts of M. oleifera and C. asiatica (TGT-PRIMAAGE) and its effect on hydrogen peroxide (H 2O2)-induced oxidative stress in human dermal fibroblasts. TGTPRIMAAGE acted on the mechanism of hydrogen transfer as it showed scavenging activity in the DPPH assay. This is due to the presence of phenolics and flavonoids in TGT-PRIMAAGE. TGT-PRIMAAGE effectively reduced cellular generation of reactive oxygen species induced by H O2. The activities of superoxide dismutase and catalase were also increased in cells treated with TGT-PRIMAAGE. 2 Treatment with TGT-PRIMAAGE showed significant reduction (P < 0.05) in the number of senescent cells. Significant reduction (P < 0.05) of malondialdehyde was also seen in cells treated with TGT-PRIMAAGE. The p53 protein level was reduced in TGT-PRIMAAGEtreated cells, which indicates its potential in protecting the cells from oxidative stress induced by H2O2.
Keywords: Moringa oleifera, Centella asiatica, senescence, oxidative stress, antioxidant
1. Introduction
Reactive oxygen species (ROS), which include superoxide radical, hydrogen peroxide (H2O ), and hydroxyl radical, are continuously generated inside human cells due to various endogenous and exogenous metabolism processes (Sreelatha and Padma, 2009) . The ROS generated are detoxified by the antioxidant systems present in the body and an equilibrium between ROS and antioxidants is reached to prevent cell injury and damage. However, owing to ROS overproduction and/or inadequate antioxidant defense, this equilibrium can be hampered, favoring the ROS upsurge that culminates in oxidative stress (Kohen and Gati, 2000) . Antioxidants play an important role in scavenging and neutralizing free radicals, thus providing protection to humans against diseases that are induced by radicals.
Senescence is the process of cellular aging in which cells lose the ability to proliferate by continuous cell division and this activity is observed in all eukaryotes (Sherr and DePinho, 2000) . The changes in senescent cells are induced by extrinsic and intrinsic factors (Jung et al., 2011) . One of the causes is oxidative stress (Chen et al., 2004) and p53 is one of the key regulators of H2O2-induced senescence (Itahana et al., 2004) .
There are ongoing efforts to identify natural antioxidants that originate from plants (Sreelatha and Padma, 2009) . Plants with medicinal values are used traditionally and regarded as safe (Junsi and Siripongvutikorn, 2016) . In this study, the antioxidant properties of combined extracts of Moringa oleifera Lam. and Centella asiatica (L.) Urb. and their effects in senescence or protecting cells from the damaging effects of radicals were investigated. M. oleifera is well known in the Moringaceae family (Paliwal and Sharma, 2011) . This plant is widely distributed and cultivated in the Middle East, Africa, and Asia. Almost all parts of the plant can be eaten (Abdulkarim et al., 2005) and the leaves have been used for preventive and curative purposes in traditional medicine (Ganguly, 2013) . Important bioactive compounds were found in the leaves of M. oleifera, which include vitamins, carotenoids, polyphenols, phenolic acids, flavonoids, glucosinolates, isothiocyanates, tannins, and saponins (Leone et al., 2015) . M. oleifera leaves have been tested and proved to exhibit in vitro (Siddhuraju and Becker, 2003; Iqbal and Bhanger, 2006; Vongsak et al., 2013; Nouman et al., 2016) and in vivo (Verma, et al., 2009; Luqman et al., 2012) antioxidant potentials. M. oleifera also exhibited antiinflammatory (Coppin et al., 2013) , hepatoprotective (Sharifudin et al., 2013) , and anticancer (Purwal et al., 2010) properties. C. asiatica (Apiaceae) is widely cultivated (Miyako et al., 2005) and is known as one of the most commonly used green leafy vegetables in Asia. This plant has potential wound-healing properties (Azis et al., 2017) and may improve cognition (Gray et al., 2016) . Phytochemical analysis showed that C. asiatica contains triterpenoid glycosides, vallerin, tannins, alkaloid, volatile oil, and pectin (Brinkhaus et al., 2000) .
In the present study, water-soluble M. oleifera and C. asiatica leaf extracts were combined (TGT-PRIMAAGE) and the potential of this product in preventing H2O2 induced senescence was investigated.
2. Materials and methods
2.1. Plant materials
M. oleifera leaves were obtained from Borneo Moringa Sdn. Bhd. in Tenom, Sabah, Malaysia, with voucher identification number Bm_mo_191012_1. C. asiatica leaves were obtained from MitoMasa Sdn. Bhd. in Ranau, Sabah, Malaysia, with voucher identification number Mtt_ ca_150113_1. Both plants were identified by the Sandakan Herbarium Sabah Forestry Department, Sabah, Malaysia. The leaves were soaked in 95% ethanol at room temperature for 120 h and then water was added to the mixture and boiled for 2 h. The liquid was then concentrated to remove ethanol and water. The mixture was filtered and separated from its residue and then subjected to a spray-drying process. This water-soluble combination of extracts of M. oleifera leaves and C. asiatica leaves (TGT-PRIMAAGE) was prepared by MitoMasa Sdn. Bhd.
2.2. Phytochemical screening
A stock concentration of 1% (w/v) TGT-PRIMAAGE was prepared. TGT-PRIMAAGE along with negative (blank) and positive controls were tested for the presence of phytochemicals as described by Harborne (1998) and Kokate (2005) . The change of colors or the formation of precipitate was used as an indication of positive response to these tests. The intensity of color change was compared to the positive controls used.
2.3. Total phenolic and flavonoid contents
The total phenolic content of TGT-PRIMAAGE was determined by Folin–Ciocalteu method with minor modifications. Briefly, 0.1 mL of sample (1 mg/mL) was diluted with distilled water (4.5 mL) and subsequently Folin–Ciocalteu reagent (0.1 mL) was added with shaking for 3 min. A 2% (w/v) solution of sodium carbonate (0.3 mL) was added and the mixture was stirred and left to stand for 3 h. An aliquot of the mixture (200 µL) was transferred to a 96-well plate and the absorbance was measured at 760 nm against a blank using a microplate reader (BMG POLARstar Omega, Ortenberg, Germany). All readings were performed in triplicate. The total phenolic content was expressed as µg of gallic acid equivalent per mg of dry weight of the sample, using an equation obtained from the gallic acid standard curve.
Total flavonoid content of TGT-PRIMAAGE was determined by the aluminum calorimetric method, using quercetin as the reference standard. Briefly, 150 µL of the test sample (0.3 mg/mL) was mixed with 150 µL of 2% (w/v) AlCl3 in a 96-well plate. After 15 min of incubation at room temperature, the absorbance was measured at 435 nm by microplate reader (BMG POLARstar Omega). All determinations were performed in triplicate. The content of the total flavonoids was expressed as µg of quercetin equivalent per mg of dry weight of the sample, using an equation obtained from the quercetin standard curve.
2.4. Antioxidant assays
2.4.1. DPPH (2,2-diphenyl-1-picrylhydrazyl) radicalscavenging activity
The DPPH assay was done according to Yang et al. (2011) with minor modifications. A series of different concentrations of TGT-PRIMAAGE and Trolox were prepared. The reaction mixtures were prepared in a 96well plate with 100 µL of sample and 100 µL of 0.2 mM DPPH. The mixture was stirred and left to stand for 15 min in the dark. Then the absorbance was measured at 517 nm against a blank. All determinations were performed in triplicate. The percentage of scavenging activity was calculated as:
Scavenging (%) = (A0 – A1) / A0] × 100 , where A0 is the absorbance of the control (DPPH radical) and A1 is the absorbance of the DPPH radical in the presence of samples. The scavenging ability of the samples was expressed as IC50 value, which is the effective concentration at which 50% of DPPH radicals were scavenged. The IC 50 values were calculated from the relationship curve of scavenging activities (%) versus concentrations of respective sample.
2.4.2. Ferric reducing antioxidant power (FRAP) assay
The FRAP assay was carried out according to Yang et al. (2011) with slight modifications. The FRAP reagent was prepared by mixing 300 mM acetate buffer (pH 3.6), a solution of 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ) in 40 mM hydrochloric acid, and 20 mM ferric chloride at 10:1:1 (v/v/v). FRAP reagent (270 µL) and the sample solution (0.1 mg/mL, 30 µL) were mixed in a 96-well plate and warmed at 37 °C for 4 min. The absorbance was taken at 593 nm. A standard calibration curve was plotted using different concentrations of Trolox ranging from 0.78 to 100 µg/mL. The results were calculated using the standard calibration curve and expressed as FRAP values. All determinations were performed in triplicate.
2.5. Cell culture
Human dermal fibroblasts (HDFs; CC-2511) were purchased from Lonza (Basel, Switzerland). Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Biowest, Nuaillé, France) supplemented with 10% FBS (Biowest) and 1% penicillin-streptomycin (SigmaAldrich, St. Louis, MO, USA) at 37 °C in a humidified 5% CO2 atmosphere.
2.6. Cell viability assay
The cytotoxicity of TGT-PRIMAAGE was determined using the MTT assay. Exponentially growing cells were harvested, counted, and diluted with DMEM (Biowest). The cells (1 × 10 5 cell/ mL) were prepared and seeded (100 µL) into each well of a 96-well plate. The cells were cultured overnight prior to treatment with TGT-PRIMAAGE. Cells were treated with TGT-PRIMAAGE or positive controls, which were ascorbic acid (Sigma-Aldrich) and Trolox (Sigma-Aldrich), at concentrations of 6.25, 12.5, 25, 50, 100, 200, and 1000 µg/mL for 24 h. Only the cells treated with TGT-PRIMAAGE were observed up to 72 h. At each time point, MTT (Sigma-Aldrich) solution (5 mg/mL) was added to the cultured cells and incubated at 37 °C for 3 h. Crystallized MTT was dissolved in DMSO and the optical density was measured at 570 nm using a microplate reader (BMG POLARstar Omega). The percentages of viable cells were plotted against concentrations and the IC50 values were determined. All experiments were performed in triplicate.
OD sample (mean) Percentage of cell viability (%) = OD control (mean) # 100
2.7. Induction of oxidative stress
The effects of TGT-PRIMAAGE on H 2O2-induced premature senescence using HDFs were evaluated. HDFs were cultured for 24 h and pretreated with TGTPRIMAAGE for 4 h, then exposed to H O2 (0.2 mM) for 2 h. After induction of senescence by oxidative stress, the cells were rinsed with phosphate-buffered saline (PBS), the medium was replaced, and the cells were further incubated for 48 h before being subjected to subsequent assays.
2.8. Reactive oxygen species assay
Cellular ROS production was quantified using the OxiSelect In Vitro ROS/RNS Assay Kit (Green Fluorescence) (Cell Biolabs Inc., San Diego, CA, USA) based on the ROS-dependent oxidation of DCFH-DA to DCF. Cell lysates were prepared in ice-cold PBS and sonicated on ice prior to centrifugation at 10,000 × g for 5 min at 4 °C. The homogenates were collected and assayed directly for cellular ROS production according to the manufacturer’s protocol. Fluorescence signals were read using a fluorescence plate reader (POLARstar OMEGA) at 480 nm excitation and 530 nm emission.
2.9. Catalase (CAT) and superoxide dismutase (SOD) assays
CAT and SOD enzyme activities in each sample were determined using Cayman’s Catalase Assay Kit (Cat. No. 707002) and Cayman’s Superoxide Dismutase Assay Kit (Cat. No. 706002) (Cayman Chemicals, Ann Arbor, MI, USA).
2.10. Senescence-associated β-galactosidase assay
Senescence-associated β-galactosidase staining was performed using a Cellular Senescence Assay Kit (Cat No. KAA002, Chemicon International, Temecula, CA, USA).
2.11. Lipid peroxidation assay (OxiSelect TBARS Assay Kit)
The level of the lipid peroxidation product, malondialdehyde (MDA), was determined using the OxiSelect TBARS Assay Kit (MDA Quantitation, Cat No. STA-330, Cell Biolabs Inc.).
2.12. DNA damage assay
Cells were collected using trypsin-EDTA and DNA was purified using a DNA extraction kit. DNA samples were first converted from dsDNA to ssDNA by heat followed by digestion to single nucleotides using nuclease P1 (Sigma N8630, Sigma-Aldrich). Subsequently, 5–10 units of alkaline phosphatase was added and incubated at 37 °C for 60 min and the reaction mixture was centrifuged for 5 min at 6000 × g to collect the supernatant.. All reagents, samples, and standards were then added into precoated wells according to the manufacturer’s protocol. At the end, the stop solution was added to each well and results were measured immediately at 450 nm.
2.13. p53 protein assay
The level of p53 protein was determined using an enzymelinked immunosorbent assay kit for tumor protein p53 (Cat. No. SAE928Hu, Cloud-Clone Corp., Houston, TX, USA).
2.14. Statistical analysis
The data were expressed as mean ± SEM values and tested with one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. P < 0.05 was considered significant.
3. Results
3.1. Phytochemical screening of TGT-PRIMAAGE
Referring to Table 1, phytochemicals detected in TGTPRIMAAGE include tannins, flavonoids, phenols. and anthraquinone glycosides.
Table 1.
Phytochemical screening of TGT-PRIMAAGE. The phytochemical evaluation of TGT-PRIMAAGE was graded as very high (++++), high (+++), moderate (++), low (+), or not detected (-) based on the intensity of the colored reaction observed compared with the positive control for the respective chemical reactions. Positive controls: a) Tannins - Five drops of 5% ferric chloride solution were added to the extract. Formation of blue color indicated the presence of tannins. Tannic acid was used as the positive control. b) Triterpenoids - The extract was mixed with chloroform and concentrated sulfuric acid was added to the solution. Formation of redbrown color at the interface indicated the presence of triterpenoids. Cholesterol was used as the positive control. c) Flavonoids - 0.05 g of magnesium turnings and 5 drops of concentrated HCl were added to the extract. Appearance of pink, scarlet crimson, red/green to blue color indicated the presence of flavonoids. Quercetin was used as the positive control. d) Saponins - Ten drops of olive oil were added to the extract. Formation of soluble emulsion indicated the presence of saponins. Oat powder was used as the positive control. e) Anthraquinone glycosides - Five drops of 10% potassium hydroxide solution were added to the extract. Formation of red color indicated the presence of anthraquinone glycosides. Hydroquinone was used as positive control. f) Phenols - The extract was mixed with 10% ferric chloride solution and formation of bluish black color indicated the presence of phenols. Quercetin was used as the positive control. g) Steroids - The extract was dissolved with chloroform and then concentrated sulfuric acid was added. Appearance of red color at the upper layer of the solution and yellow/green fluorescence color at the bottom of the solution indicated the presence of steroids. Cholesterol was used the positive control. h) Alkaloids - Dragendorff’s reagent was added to the extract. Formation of orange/orange reddish brown precipitate showed a positive result. Quinine sulfate was used as the positive control.
| Color intensity | |||
|---|---|---|---|
| Tests | Blank | Positive control | TGT-PRIMAAGE |
| a) Tannins | - | ++++ | ++++ |
| b) Triterpenoids | - | ++++ | - |
| c) Flavonoids | - | ++++ | ++ |
| d) Saponins | - | +++ | - |
| e) Anthraquinone glycosides | - | ++++ | + |
| f) Phenols | - | ++++ | ++++ |
| g) Steroids | - | ++++ | - |
| h) Alkaloids | - | ++++ | - |
3.2. Total phenolics, flavonoid contents, and antioxidant activity of TGT-PRIMAAGE
Based on Table 2, the total flavonoids and phenolic contents present in TGT-PRIMAAGE were 5.96 ± 0.02 µg quercetin equivalent/mg of extract and 81.83 ± 0.00 µg gallic acid equivalent/mg of extract, respectively. The concentrations of TGT-PRIMAAGE and positive controls (Trolox and ascorbic acid) that reduced free radical DPPH• to about 50% (IC50) were 19, 1.2, and 3.0, respectively. FRAP value was expressed as Trolox equivalents (µg Trolox/µg sample dry weight). The FRAP value of TGT-PRIMAAGE was FRAP value (µg Trolox/µg sample dry weight) found to be low (7.01 ± 0.66) as compared to ascorbic acid (172.41 ± 12.6). This indicates that TGT-PRIMAAGE required 100 µg/mL to exert the same reducing activity as 7.01 ± 0.66 µg/mL Trolox.
Table 2.
Antioxidant capacities of TGT-PRIMAAGE and its total flavonoids and phenolic contents.
| Antioxidant tests | ||||
|---|---|---|---|---|
| DPPH scavenging Activity (IC50, μg/mL) | FRAP value (μg Trolox/μg sample dry weight) | Total flavonoids content (μg of quercetin equivalent/ mg of extract ± SD) | Total phenolic content (μg of gallic acid equivalent/ mg of extract ± SD) | |
| M. oleifera | 5.40 ± 0.23 | 41.28 ± 1.9 | ||
| C. asiatica | 2.65 ± 0.10 | 35.72 ± 4.80 | ||
| TGT-PRIMAAGE | 19.0 | 7.01 ± 0.66 | 5.96 ± 0.02 | 81.83 ± 0.00 |
| Trolox | 1.2 | - | - | - |
| Ascorbic acid | 3.0 | 172.41 ± 12.6 | - | - |
3.3. Cytotoxicity of TGT-PRIMAAGE in HDFs
At 1000 µg/mL, TGT-PRIMAAGE did not aefct cell viability, while cells treated with Trolox had 55% viability and 38% of the cells treated with ascorbic acid were viable. As shown in Figure 1, viability of cells treated with ascorbic acid and Trolox was reduced with increased concentrations. Viability of cells treated with TGT-PRIMAAGE was high and independent of concentrations. The effects of TGTPRIMAAGE on HDFs were further observed at 48 and 72 h (Figure 2). There were no significant (P > 0.05) effects of TGT-PRIMAAGE at various concentrations until 72 h of incubation. Therefore, three different concentrations were chosen to investigate the inhibitory effect of TGT
Figure 1.
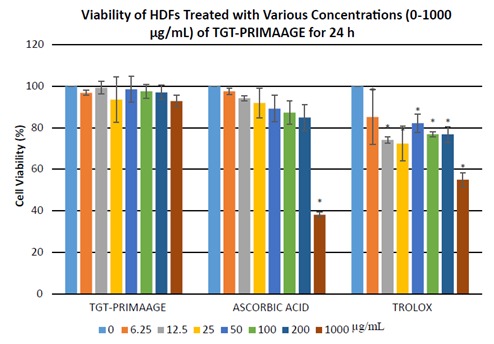
Viability of HDFs treated with various concentration of TGT-PRIMAAGE, ascorbic acid, and Trolox for 24 hours. Values are mean ± SEM. All tests were performed in triplicate. *: P < 0.05 compared to control (untreated) by one-way ANOVA followed by Tukey’s multiple comparison test. HDFs were treated with TGT-PRIMAAGE, ascorbic acid, and Trolox at 7 different concentrations. After 24 h, MTT solution (5 mg/mL) was added to the cultured cells and incubated at 37 °C for 3 h. Crystallized MTT was dissolved in DMSO and the optical density was measured at 570 nm.
Figure 2.
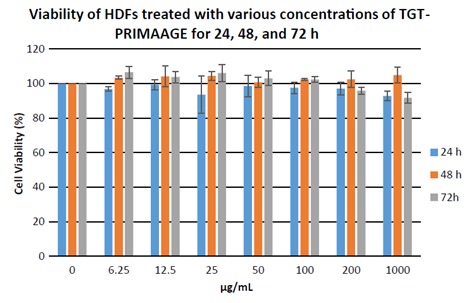
Dose- and incubation time-dependent study of TGT-PRIMAAGE on HDFs. Viability of HDFs treated with various concentration of TGT-PRIMAAGE for 24, 48, and 72 h. Values are mean ± SEM. All tests were performed in triplicate. *: P < 0.05 compared to control (untreated) by one-way ANOVA followed by Tukey’s multiple comparison test. HDFs were treated at 7 different concentrations of TGT-PRIMAAGE and incubated over 24, 48, and 72 h. After the respective incubation hours MTT solution (5 mg/ mL) was added to the cultured cells and incubated at 37 °C for 3 h. Crystallized MTT was dissolved in DMSO and the optical density was measured at 570 nm.
3.4. Effect of TGT-PRIMAAGE on ROS, SOD, and CAT levels after H 2O2-induced premature senescence
Significant production of cellular ROS was demonstrated in HDFs treated with H O2 compared to the control group (P < 0.05) as shown in Figure 3. Incubation with TGTPRIMAAGE at concentrations of 125, 250, and 500 µg/mL reduced cellular ROS production induced by H O2. Similar effects were seen in HDFs treated with ascorbic acid and Trolox. At concentrations of 125 and 250 µg/mL, TGTPRIMAAGE showed the best ROS scavenging activity compared to the same doses of ascorbic acid and Trolox. However, at 500 µg/mL, ROS scavenging activity was best seen in Trolox. HDFs treated with 0.2 mM H2O2 required significantly high amounts of enzyme to exhibit 50% dismutation of the superoxide radical (low SOD activity) compared to the untreated group (control). Among the different doses of TGT-PRIMAAGE, ascorbic acid, and Trolox, TGT-PRIMAAGE at 250 µg/mL showed the best protective effects (Figure 4). HDFs treated with H 2 2 caused a significant decrease in CAT activity compared to the control group (P < 0.05). Meanwhile, HDFs pretreated with TGT-PRIMAAGE at a concentration of 250 µg/mL showed a significant increase (P < 0.05) in CAT activity compared to the H O2 group. Ascorbic acid at 250 µg/mL showed the highest CAT activity (Figure 5).
Figure 3.
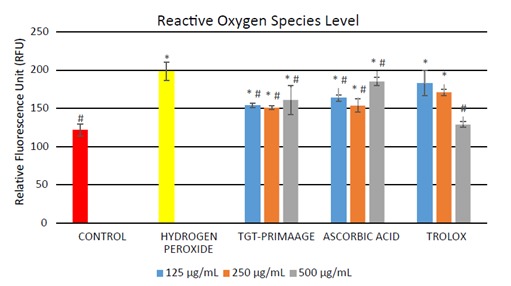
Level of ROS in H2O2-induced premature senescence in HDFs. Results are expressed as relative fluorescence unit (RFU) ± SEM. All tests were performed in triplicate. The significance of differences as compared with control group (*P < 0.05) and the group with hydrogen peroxide alone (#P < 0.05) was assessed using ANOVA. Cellular ROS production was quantified using the OxiSelect In Vitro ROS/RNS Assay Kit (Green Fluorescence) (Cell Biolabs Inc.) based on the ROS-dependent oxidation of DCFH-DA to DCF. The details were explained in Section 2.
Figure 4.
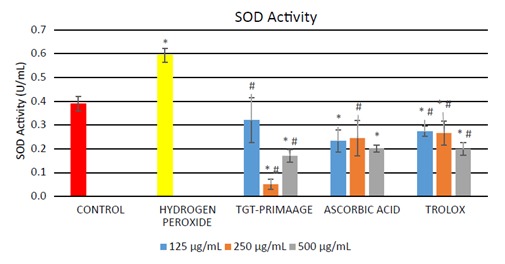
SOD activity in H2O2-induced premature senescence in HDFs. One unit of SOD represents the amount of enzyme needed to exhibit 50% dismutation of superoxide radical. All tests were performed in triplicate. The significance of differences as compared with control group (*P < 0.05) and the group with hydrogen peroxide alone (#P < 0.05) was assessed using ANOVA. Mixtures containing cell lysate samples and tetrazolium salt solution were prepared. Xanthine oxidase was added to all samples to initiate the reaction, after which absorbances were measured for each sample at 440–460 nm. A standard curve was calibrated using the SOD standards prepared.
Figure 5.
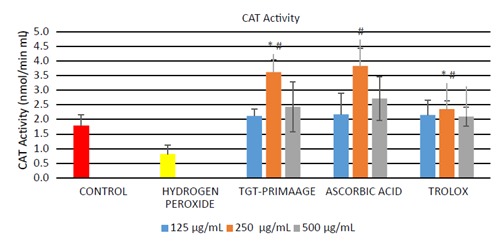
CAT activity in H2O2-induced premature senescence in HDFs. One unit of CAT represents the amount of enzyme that will cause the formation of 1 nmol of formaldehyde/min. All tests were performed in triplicate. The significance of differences as compared with control group (*P < 0.05) and the group with hydrogen peroxide group (#P < 0.05) was assessed using ANOVA. Mixtures containing cell lysate samples and diluted assay buffer and methanol were prepared. The reaction was then initiated by adding hydrogen peroxide. Diluted potassium hydroxide was added to terminate the reaction, after which absorbances were measured for each sample at 540 nm.
3.5. Effect of TGT-PRIMAAGE on senescent cells, MDA level, DNA damage, and p53 level after H 2O2-induced premature senescence
HDFs treated with H O2 showed a significant (P < 0.05) number of positive SA-β-gal-stained cells compared to the untreated HDFs. HDFs pretreated with TGT-PRIMAAGE prior to H2O2 exposure exhibited a reduction of these phenomena (Figure 6). In this assay, Trolox failed to protect cells from oxidative stress-induced senescence. There was a significant (P < 0.05) increase in the MDA level in the H2O2 group compared to the control group. HDFs pretreated with TGT-PRIMAAGE at a concentration of 250 µg/mL resulted in a marked reduction in MDA level. However, protection against lipid peroxidation was better in cells treated with Trolox (Figure 7).
Figure 6.
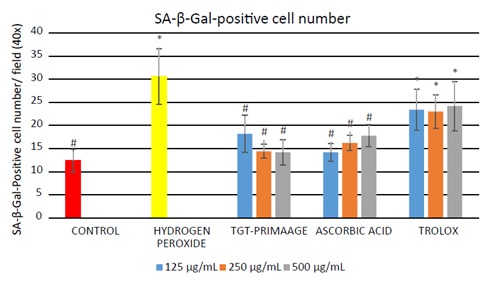
Effect of TGT-PRIMAAGE on H2O2-induced premature senescence in HDFs. The number of cells positive for SA- β-galactosidase was measured and results are expressed as mean ± SEM. All tests were performed in triplicate. The significance of differences as compared with control group (*P < 0.05) and the group with hydrogen peroxide alone (#P < 0.05) was assessed using ANOVA. Briefly, HDFs treated with extracts and hydrogen peroxide were incubated with freshly prepared 1X SA-β-gal solution. HDFs that stained blue were counted.
Figure 7.
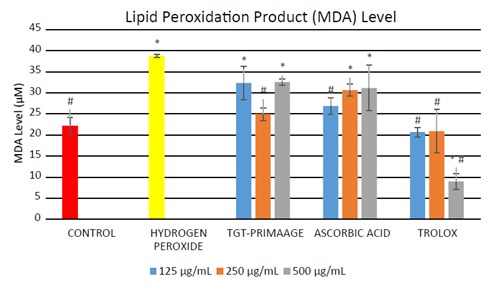
Effect of TGT-PRIMAAGE against lipid peroxidation in H2O2-induced premature senescence in HDFs. Data expressed as mean MDA level (μM) ± SEM. All tests were performed in triplicate. The significance of differences as compared with control group (* P < 0.05) and the hydrogen peroxide alone group (# P < 0.05) was assessed using ANOVA test. SDS lysis solution was added to both the samples and the MDA standards. Then TBA reagent was added to each sample and standard and incubated at 95 °C for 50 min. Absorbances were measured spectrophotometrically at 532 nm.
In Figure 8, HDFs exposed to H2O2 showed a significant increase (P < 0.05) in the level of DNA damage (measured as 8-OHdG) in comparison to the control group. Pretreatment with TGT-PRIMAAGE caused a decrease in the 8-OHdG level, but no significant reduction (P < 0.05) was seen in the H O2 group. Significant reduction (P < 0.05) of 8-OHdG were seen with 250 and 500 µg/mL ascorbic acid and 500 µg/mL Trolox. TGT-PRIMAAGE and ascorbic acid at doses of 250 µg/mL showed the lowest p53 levels. Trolox at lower doses of 125 and 250 µg/mL showed the best protection compared to TGT-PRIMAAGE and ascorbic acid. However, at a high dose of 500 µg/mL, the effect of Trolox was reversed (Figure In Figure 9).
Figure 8.
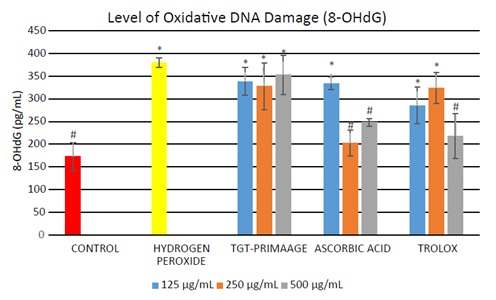
Effect of TGT-PRIMAAGE against lipid DNA damage in H2O2-induced premature senescence in HDFs. Data expressed as mean concentration of 8-OHdG (pg/mL) ± SEM. All tests were performed in triplicate. The significance of differences as compared with control group (*P < 0.05) and the group with hydrogen peroxide alone (#P < 0.05) was assessed using ANOVA. 8-OHdG levels, i.e. the oxidative DNA damage by products, were measured using the ELISA method. DNA was extracted from all the samples. The doublestranded DNA was converted to single-stranded and digested to single nucleotides of guanosine.
Figure 9.
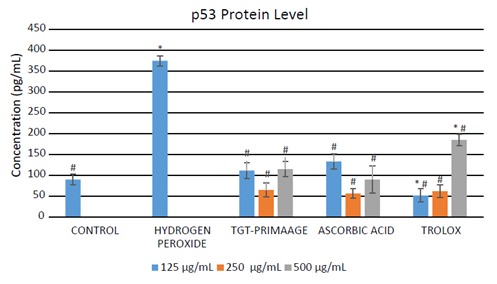
Effect of TGT-PRIMAAGE on tumor suppressor protein (p53) expression in H2O2-induced premature senescence in HDFs. Data expressed as mean concentration of p53 (pg/mL) ± SEM. All tests were performed in triplicate. The significance of differences as compared with control group (*P < 0.05) and the group with hydrogen peroxide alone (#P < 0.05) was assessed using ANOVA. The p53 level was measured using the supernatant from the cells according to the manufacturer’s instructions. The reaction mixture was read at 450 nm after adding the stop solution.
4. Discussion
Based on the phytochemical screening tests, tannins, phenols, flavonoids, and anthraquinone glycosides were present in TGT-PRIMAAGE. This indicates that TGTPRIMAAGE may have important pharmacological properties such as antioxidant activity since it possesses important secondary metabolites such as flavonoids. Flavonoids, phenolic compounds, have been found to prevent injury caused by free radicals and exert protective effects in biological systems. One of the mechanisms is the direct scavenging of ROS. Flavonoids may scavenge free radicals directly by hydrogen atom donation ( Procházková et al., 2011 ). Plant phenolic compounds such as flavonoids are important constituents of the human diet and have been recognized largely as beneficial antioxidants that can scavenge harmful active oxygen species (Sakihama et al., 2002) . Tannic acid also acts as an antioxidant and scavenges free radicals and superoxide radicals. The ability to chelate iron and copper ions has also been attributed to its antioxidant effect as reported by Andrade et al. (2005) . This suggest that TGT-PRIMAAGE has components that may exert antioxidant activity.
The most widely used protocols for measurement of antioxidant activity capacity is DPPH radical-scavenging analysis. DPPH is a stable free radical due to its spare electron delocalization over whole molecules. Low IC50 values indicate the strong ability of the sample to act as a DPPH radical scavenger. The IC 50 value of PRIMAAGE was found to be higher than those of Trolox and ascorbic acid. The FRAP assay is a single-electron transfer method based on the reduction of a ferrictripyridyltriazine complex to its ferrous in the presence of antioxidants (Cottica et al., 2011) . The FRAP value of TGT-PRIMAAGE was found to be low as compared to ascorbic acid. TGT-PRIMAAGE may act in a hydrogen atom transfer mechanism rather than a single-electron transfer mechanism due to its scavenging activity in the DPPH assay. The results presented in this study suggest that antioxidant activity is associated with the phenolic contents in TGT-PRIMAAGE. Phenolic compounds may be responsible for the antioxidant activity of many plants as hydroxyl groups confer scavenging ability and flavonoids also exhibited free radical scavenging activity.
In the MTT assay, the viability of cells treated with TGT-PRIMAAGE was independent of concentrations. From the data presented, TGT-PRIMAAGE is therefore considered safe for HDFs. A considerable amount of literature has been published on in vitro models of HDFs for the study of aging (Park et al., 2014) . Fibroblasts are vital cellular components of human skin as they play essential roles in the regulation of skin physiology and pathology. Therefore, an investigation of the toxicity effect on HDFs is critical for pharmacological and clinical studies on aging.
The imbalanced cellular ROS production triggered by endogenous and exogenous factors may lead to certain pathological conditions. uThs, assessment of ROS levels provides an important clue about redox balance in humans and can act as a prognostic in certain diseases. TGTPRIMAAGE has significantly reduced the production of ROS in HDFs exposed to H O . This indicates the protective ability of TGT-PRIMAAGE against the damaging effect of ROS. SOD serves as the first gatekeeper in the antioxidant defense system designed to scavenge superoxide anions. This enzyme cata lyzes the dismutation of superoxide anion to molecular oxygen and H2O2. In cultured HDFs obtained from aged cells or induced by H O2 it has been found that the catalase activity is decreased. This is because CAT plays a central role in the aging process of human fibroblasts by scavenging the intracellular H O generated. It converts H2O2 to water and oxygen molecules, which provide protection from deleterious effects of ROS attacks against macromolecules. CAT is also involved in the antioxidant defense system against ROS by catalyzing conversion of ROS to less harmful species. TGT-PRIMAAGE was found to increase SOD and CAT activities compared to nontreated HDFs. The antioxidant properties of TGT-PRIMAAGE may have acted via upregulating the SOD and CAT activities and therefore protecting cells from oxidative stress. Oxidative stress contributes to a state of stress-induced premature senescence, which displays features of replicative senescence including lack of cell proliferation, increased cell volume, distinct flat morphology, and elevated expression of cell-cycle inhibitor proteins (Comings and Okada, 1970; Dimri et al., 1996) . To investigate the protective effect of TGT-PRIMAAGE on H2O2-induced premature senescence, SA-β-gal staining was performed. HDFs pretreated with TGT-PRIMAAGE prior to H O2 exposure exhibited a reduction of premature senescence similar to that with ascorbic acid. In this study, Trolox failed to protect cells from oxidative stress-induced senescence. The result suggested that TGT-PRIMAAGE may inhibit H2O2-induced premature senescence by its antioxidant capabilities.
Lipid peroxidation is one of the main events in free radical-induced cell injury. MDA is a byproduct of lipid peroxidation and is widely used as a biomarker of oxidative stress (Feng et al., 2013) . Lipid peroxidation has been implicated in the pathogenesis of several diseases such as Alzheimer and Parkinson diseases (Devasagayam et al., 2003) . The results obtained from the experimental analysis of MDA levels in HDFs showed a significant increase in the MDA level in the H2O2 group compared to the control group. Incubation with H2O2 caused lipid peroxidation via oxidative stress and the HDFs pretreated with TGTPRIMAAGE at a concentration of 250 µg/mL had a marked reduction in MDA level, which suggests the protective effect of the sample against lipid peroxidation. However, protection against lipid peroxidation was more significant in cells treated with Trolox than TGT-PRIMAAGE.
The production of ROS such as •OH radicals could lead to generation of various damaged DNA bases and strand breaks following reaction of •OH radicals with DNA bases or the deoxyribose backbone of DNA. Guanine is the base that is most prone to oxidation, and 8-hydroxy-2’-deoxyguanosine is the form of oxidized guanine that is most commonly studied. DNA damage is the major contributor to aging processes (Bagnyukova et al., 2008) . The free radical theory of aging of Harman (1956) hypothesized that the onset of aging occurred due to failure in antioxidant defense machinery to protect against prooxidants, leading to accumulation of damaged biomolecules such as DNA and loss of its functions with age. The efficacy of repair machinery seems to decline with age, allowing for continuous and rapid accumulation of DNA damage and errors in replication (Cooke et al., 2003) . Exposure to H O2 caused increased DNA damage resulting from free radical attack. However, pretreatment with TGT-PRIMAAGE caused a decrease in 8-OHdG level, but no significant reduction was seen in the H 2 2 group. Significant reduction of 8-OHdG was seen in the ascorbic acid and Trolox groups. The decrease in 8-OHdG level might be due to the defense mechanism exerted by the antioxidants present in the sample, preventing the damaging effect of •OH radicals.
p53 is closely related to the apoptosis mechanism, which remained at a low state under normal conditions, regulated by the p53 inhibitor Mdm2. However, the level of p53 is dramatically elevated following accumulation of DNA damage, hypoxia, or aberrant oncogene expression to enhance cell-cycle checkpoints, DNA repair, and apoptosis (Fridman and Lowe, 2003; Xu et al., 2008) . Significant increment in the level of p53 protein in HDFs treated with H2O2 shows that exposure to H O led to oxidative damage to macromolecules such as DNA (accumulation of DNA damage), which in turn raised the expression of p53. The expression of p53 rises dramatically as the level of DNA damage increases, thus activating the roles of p53 by either inducing cell-cycle arrest coupled with DNA damage repair or cell death (Fei and El-Deiry, 2003) . HDFs pretreated with TGT-PRIMAAGE caused a markedly diminished level of p53 compared to HDFs treated with H O2 alone, suggesting that less damage occurred to the cells. Similar results were observed for HDFs pretreated with ascorbic acid and Trolox, which showed significantly lower levels of p53 compared to the H2O2 group. HDF cells treated with TGT-PRIMAAGE and ascorbic acid at doses of 250 µg/ mL showed the lowest p53 levels, ROS levels, and MDA levels and therefore had the most optimum protective effects against oxidative damage. Therefore, at 250 µg/mL, TGT-PRIMAAGE could protect the HDFs from oxidative damage caused by H2O2 at a efficacy level similar to those of ascorbic acid and Trolox. This could be due to the dosedependent prooxidative potential of TGT-PRIMAAGE. At higher doses, TGT-PRIMAAGE may revert to having oxidation potential, which may be useful for the induction of apoptotic cell death.
In conclusion, this study provided information on the antioxidant activities of the combined leaf extracts of M. oleifera and C. asiatica. TGT-PRIMAAGE did not show significant reduction of DNA damage; however, the p53 protein level was reduced, which indicates its ability to protect cells from oxidative stress induced by H O .
Acknowledgments
This study was supported and funded by the Ministry of Agriculture & Agro-Based Industry of Malaysia (KP/HDO/S/34-9) and conducted at the Integrative Pharmacogenomics Institute (iPROMISE), Universiti Teknologi MARA (UiTM), Malaysia.
References
- Abdulkarim SM , Long K , Lai OM , Muhammad SKS , Ghazali HM ( 2005. ). Some physico-chemical properties of Moringa oleifera seed oil extracted using solvent and aqueous enzymatic methods . Food Chem 93 : 253 - 263 . [Google Scholar]
- Andrade RG Jr, Dalvi LT , Silva JM Jr, Lopes GK , Alonso A , HermesLima M ( 2005. ). The antioxidant effect of tannic acid on the in vitro copper-mediated formation of free radicals . Arch Biochem Biophys 437 : 1 - 9 . [DOI] [PubMed] [Google Scholar]
- Azis HA , Taher M , Ahmed AS , Sulaiman WMAW , Susanti D , Chowdhury SR , Zakaria ZA ( 2017. ). In vitro and in vivo wound healing studies of methanolic fraction of Centella asiatica extract . S Afr J Bot 108 : 163 - 174 . [Google Scholar]
- Bagnyukova TV , Powell CL , Pavliv O , Tryndyak V P , Pogribny IP ( 2008. ). Induction of oxidative stress and DNA damage in rat brain by a folate/methyl-deficient diet . Brain Res 1237 : 44 - 51 . [DOI] [PubMed] [Google Scholar]
- Brinkhaus B , Lindner M , Schuppan D , Hahn EG ( 2000. ). Chemical, pharmacological and clinical profile of the East Asian medical plant Centella asiatica . Phytomedicine 7 : 427 - 448 . [DOI] [PubMed] [Google Scholar]
- Chen JH , Stoeber K , Kingsbury S , Ozanne SE , Williams GH , Hales CN ( 2004. ). Loss of proliferative capacity and induction of senescence in oxidatively stressed human fibroblasts . J Biol Chem 279 : 49439 - 49446 . [DOI] [PubMed] [Google Scholar]
- Comings DE , Okada TA ( 1970. ). Electron microscopy of human bifroblasts in tissue culture during logarithmic and conuflent stages of growth . Exp Cell Res 61 : 295 - 301 . [DOI] [PubMed] [Google Scholar]
- Cooke MS , Evans MD , Dizdaroglu M , Lunec J ( 2003. ). Oxidative DNA damage: mechanisms, mutation, and disease . FASEB J 17 : 1195 - 214 . [DOI] [PubMed] [Google Scholar]
- Coppin JP , Xu Y , Chen H , Pan MH , Ho CT , Juliani R , Simon JE , Wu Q ( 2013. ). Determination of flavonoids by LC/MS and antiinflammatory activity in Moringa oleifera . J Funct Foods 5 : 1892 - 1899 . [Google Scholar]
- Cottica SM , Sawaya ACHF , Eberlin MN , Franco SL , Zeoula LM , Visentainer JV ( 2011. ). Antioxidant activity and composition of propolis obtained by different methods of extraction . J Brazil Chem Soc 22 : 929 - 935 . [Google Scholar]
- Devasagayam TPA , Boloor KK , Ramasarma T ( 2003. ). Methods for estimating lipid peroxidation: an analysis of merits and demerits . Indian J Biochem Bio 5 : 300 - 308 . [PubMed] [Google Scholar]
- Dimri GP , Testori A , Acosta M , Campisi J ( 1996. ). Replicative senescence, aging and growth-regulatory transcription factors . Biol Signal Recept 5 : 154 - 162 . [DOI] [PubMed] [Google Scholar]
- Fei P , El-Deiry WS ( 2003. ). P53 and radiation responses . Oncogene 22 : 5774 - 5783 . [DOI] [PubMed] [Google Scholar]
- Feng B , Ma LJ , Yao JJ , Fang Y , Mei YA , Wei SM ( 2013. ). Protective effect of oat bran extracts on human dermal bfiroblast injury induced by hydrogen peroxide . J Zhejiang Univ Sci B 14 : 97 - 105 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman JS , Lowe SW ( 2003. ). Control of apoptosis by p53 . Oncogene 22 : 9030 - 9040 . [DOI] [PubMed] [Google Scholar]
- Ganguly S ( 2013. ). Indian ayurvedic and traditional medicinal implications of indigenously available plants, herbs and fruits: a review . International Journal of Research in Ayurveda and Pharmacy 4 : 623 - 625 . [Google Scholar]
- Gray NE , Harris CJ , Quinn JF , Soumyanath A ( 2016. ). Centella asiatica modulates antioxidant and mitochondrial pathways and improves cognitive function in mice . J Ethnopharmacol 180 : 78 - 86 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harborne JB ( 1998. ). Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis . 3rd ed. New York, NY, USA: Chapman and Hall.
- Harman D ( 1956. ). Aging: a theory based on free radical and radiation chemistry . J Gerontol 11 : 298 - 300 . [DOI] [PubMed] [Google Scholar]
- Iqbal S , Bhanger MI ( 2006. ). Effect of season and production location on antioxidant activity of Moringa oleifera leaves grown in Pakistan . J Food Compos Anal 19 : 544 - 551 . [Google Scholar]
- Itahana K , Campisi J , Dimri GP ( 2004. ). Mechanisms of cellular senescence in human and mouse cells . Biogerontology 5 : 1 - 10 . [DOI] [PubMed] [Google Scholar]
- Jung M , Lee S , Park HY , Youm, JK , Jeong S , Bae J , Kwon MJ , Park BD , Lee SH , Choi EH ( 2011. ). Anti-ageing effects of a new synthetic sphingolipid (K6EAA-L12) on aged murine skin . Exp Dermatol 20 : 314 - 319 . [DOI] [PubMed] [Google Scholar]
- Junsi M , Siripongvutikorn S ( 2016. ). uThnbergia laurifolia , a traditional herbal tea of aThiland: botanical, chemical composition, biological properties and processing inuflence . International Food Research Journal 23 : 923 - 927 . [Google Scholar]
- Kohen R , Gati I ( 2000. ) Skin low molecular weight antioxidants and their role in aging and in oxidative stress . Toxicology 148 : 149 - 157 . [DOI] [PubMed] [Google Scholar]
- Kokate CK ( 2005. ). A Text Book of Practical Pharmacognosy . 5th ed. New Delhi, India: Vallabh Prakashan.
- Leone A , Spada A , Battezzati A , Schiraldi A , Aristil J , Bertoli S ( 2015. ). Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: an overview . Int J Mol Sci 16 : 12791 - 12835 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luqman S , Srivastava S , Kumar R , Maurya AK , Chanda D ( 2012. ). Experimental assessment of Moringa oleifera leaf and fruit for its antistress, antioxidant, and scavenging potential using in vitro and in vivo assays . Evid-Based Compl Alt 2012. : 519084 . [DOI] [PMC free article] [PubMed]
- Miyako Y , Masahiro F , Tsuneatsu N , Hikaru O , Kazuhisa M , Jiro T , Yoshiharu K , Ryota, T , Junei K , Kunihide M et al. ( 2005. ). Antiproliferative constituents from Umbelliferae plants VII. Active triterpenes and rosmarinic acid from Centella asiatica . Biol Pharm Bull 28 : 173 - 175 . [DOI] [PubMed] [Google Scholar]
- Nouman W , Anwar F , Gull T , Newton A , Rosa E , Domínguez-Perles R ( 2016. ). Profiling of polyphenolics, nutrients and antioxidant potential of germplasm's leaves from seven cultivars of Moringa oleifera Lam . Ind Crops Prod 83 : 166 - 176 . [Google Scholar]
- Paliwal R , Sharma V ( 2011. ). A review on horse radish tree (Moringa oleifera): a multipurpose tree with high economic and commercial importance . Asian J Biotechnol 3 : 317 - 328 . [Google Scholar]
- Park JM , Lee JS , Lee KR , Ha SJ , Hong EK ( 2014. ). Cordyceps militaris extract protects human dermal fibroblasts against oxidative stress-induced apoptosis and premature senescence . Nutrients 6 : 3711 - 3726 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procházková D , Bousová I , Wilhelmová N ( 2011. ). Antioxidant and prooxidant properties of flavonoids . Fitoterapia 82 : 513 - 23 . [DOI] [PubMed] [Google Scholar]
- Purwal L , Pathak AK , Jain UK ( 2010. ). In vivo anticancer activity of the leaves and fruits of Moringa oleifera on mouse melanoma . Pharmacologyonline 1 : 655 - 665 . [Google Scholar]
- Sakihama Y , Cohen MF , Grace SC , Yamasaki H ( 2002. ). Plant phenolic antioxidant and prooxidant activities: phenolics-induced oxidative damage mediated by metals in plants . Toxicology 177 : 67 - 80 . [DOI] [PubMed] [Google Scholar]
- Sharifudin SA , Fakurazi S , Hidayat MT , Hairuszah I , Aris Mohd Moklas M , Arulselvan P ( 2013. ). Therapeutic potential of Moringa oleifera extracts against acetaminophen-induced hepatotoxicity in rats . Pharm Biol 51 : 279 - 288 . [DOI] [PubMed] [Google Scholar]
- Sherr CJ , DePinho RA ( 2000. ). Cellular senescence: minireview mitotic clock or culture shock ? Cell 102 : 407 - 410 . [DOI] [PubMed] [Google Scholar]
- Siddhuraju P , Becker K ( 2003. ). Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves . J Agric Food Chem 51 : 2144 - 2155 . [DOI] [PubMed] [Google Scholar]
- Sreelatha S , Padma PR ( 2009. ). Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity . Plant Foods Hum Nutr 64 : 303 - 311 . [DOI] [PubMed] [Google Scholar]
- Verma AR , Vijayakumar M , Mathela CS , Rao CV ( 2009. ). In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves . Food Chem Toxicol 47 : 2196 - 2201 . [DOI] [PubMed] [Google Scholar]
- Vongsak B , Sithisarn P , Mangmool S , oThngpraditchote S , Wongkrajang Y , Grigsonian W ( 2013. ). Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method . Ind Crops Prod 44 : 566 - 571 . [Google Scholar]
- Xu J , Lian L , Wu C , Wang X , Fu W , Xu L ( 2008. ). Lead induces oxidative stress, DNA damage and alteration of p53, Bax and Bcl-2 expressions in mice . Food Chem Toxicol 46 : 1488 - 1494 . [DOI] [PubMed] [Google Scholar]
- Yang H , Dong Y , Du H , Shi H , Peng Y , Li X. ( 2011. ). Antioxidant compounds from propolis collected in Anhui, China . Molecules 16 : 3444 - 3455 . [DOI] [PMC free article] [PubMed] [Google Scholar]


