Abstract
Heat shock proteins (HSPs) are generally considered as important molecular chaperones; they are known to perform critical functions in plant development and abiotic stress response processes. In this study, we examined the role of a HSP, the Medicago sativa DnaJ-like protein (MsDJLP), in alfalfa and its potential application for the development of abiotic stress tolerance in plants. We found that expression of the MsDJLP gene was induced by chilling (4 °C) and heat (42 °C), but not by cadmium (500 µM) or arsenic (500 µM) stresses. We then cloned the MsDJLP gene downstream of the strong constitutive CaMV 35S promoter and transformed it into tobacco plants. Ectopic expression of MsDJLP conferred enhanced tolerance to both chilling and heat stresses in transgenic tobacco plants. Under chilling stress, the transgenic tobacco plants showed lower H2O2 accumulation and electrolyte leakage (EL) activity, and better photosystem II efficiency than wild-type (WT) plants, indicating that photoinhibition was less severe in transgenic compared to WT plants. Following heat treatment, the transgenic plants showed better relative chlorophyll and water contents, and lower malondialdehyde accumulation than WT plants. Our study provides evidence for a pivotal role of MsDJLP for chilling and heat stress tolerance in transgenic tobacco plants.
Keywords: Alfalfa, MsDJLP, transgenic tobacco, chilling, heat stress
1. Introduction
Plants are often exposed to multiple abiotic stresses in nature, with low and high temperature stresses the most serious determining factors for agricultural crop production (Hatfield and Prueger, 2015). Chilling is one of the critical constraints of plant performance; it triggers a series of molecular and physiological responses in plants that negatively impact the whole developmental process (Yadav, 2010) . Chilling has been shown to inhibit photosystem II (PSII) activity, reduce plant growth, and cause leaf wilting and membrane damage in plants (Kong et al., 2014b; Hatfield and Pruege, 2015) . In addition, plant responses to high temperature include the generation of reactive oxygen species (ROS) that induce oxidative stress and subsequently cellular injury and lipid peroxidation (Hasanuzzaman et al., 2013). Consequently, the discovery of new genes/enzymes with roles in stress response mechanisms in plants that could be related to stress tolerance mechanisms is of great importance and the subject of intense research interest.
Heat shock proteins (HSPs)/molecular chaperones are normally found to be involved in the response to increased temperatures in plants species (Vierling, 1991; Wang et al., 2004) . The DnaJ proteins are ubiquitously found in plants and other organisms and are important molecular chaperones, referred to as cellular stress sensors, that are involved in signal transduction, cellular protein homeostasis, and tolerance to multiple stresses in plants (Rajan and D’Silva, 2009; Kong et al., 2014b) . To date, several DnaJ gene homologs from a variety of plant species have been cloned and characterized, including from Arabidopsis thaliana (Rajan and D’Silva, 2009) , Glycine max (Liu and Whitham, 2013) , and Solanum lycopersicum (Wang et al., 2014) .
Consecutive studies have documented that DnaJ proteins play a significant role in tolerance to abiotic stresses. For example, AtDjA2 and AtDjA3, two homologs of DnaJ protein genes, were demonstrated to enhance heat tolerance in Arabidopsis plants (Li et al., 2007) . The expression of a thermosensitive male sterile (TMS1) DnaJ protein conferred thermotolerance of pollen tubes in Arabidopsis plants (Yang et al., 2009) . Significantly, the expression of AtDjB1 enhanced thermotolerance by defending cells against heat-induced stress injury in Arabidopsis (Zhou et al., 2012) . LeCDJ1, a chloroplast targeted-DnaJ protein, provides enhanced PSII-efficiency in tomato plants when under chilling stress (Kong et al., 2014b) , and the overexpression of LeCDJ2 confers drought tolerance in transgenic tobacco (Wang et al., 2014) . Recently, it has been demonstrated that chloroplast DnaJ protein SICDJ2 protects RuBisCo activity during heat stress in transgenic tomato plants (Wang et al., 2015) . The above studies have revealed the various biological functions of DnaJ-like protein candidates in conferring abiotic stress tolerance in plants.
The objective of this research was to discover new stress-response genes that would provide potential candidates for conferring abiotic stress tolerance during crop production. For this reason, in this study, we isolated the DnaJ-like protein (MsDJLP; accession no. AKA20389) from alfalfa and transformed the MsDJLP gene construct into tobacco plants. Alfalfa is an important forage legume. In fact, in recent years, proteomic and microarray data showed that many candidate genes including DnaJ-like heat shock proteins are differentially expressed in alfalfa under abiotic stresses (Li et al., 2013; Rahman et al., 2015; Zhou et al., 2016; Li et al., 2017) . In order to develop an understanding of the molecular response of the MsDJLP gene to abiotic stresses, we characterized the MsDJLP gene in transgenic tobacco plants under chilling and heat stresses.
2. Materials and methods
2.1. Plant materials and stress treatments
Alfalfa (Medicago sativa L. ‘Vernal’) plants were used as the gene source for this experiment. Alfalfa seedlings were grown in pots containing horticulture potting mix in a growth chamber where the temperature was maintained at 25 °C with a 16-h light/8-h dark period. Two-week-old alfalfa seedlings were then exposed to either 4 °C or 42 °C in the chamber under the same photoperiod conditions. A further two groups of seedlings were immersed in 100 mM sodium chloride (NaCl), 500 µM cadmium chloride (CdCl2), and 500 µM arsenic (Na2HAsO4.7H2O) solutions, respectively. All of the above treatments were maintained for up to 12 h and leaves were harvested at 0, 1, 6, and 12 h after initiation of the applied treatments. Harvested samples were quickly frozen in liquid N2 and stored at –80 °C until further use. At least three independent replications (n = 3) were conducted for each treatment.
2.2. RNA isolation and cDNA cloning
Total RNA was extracted using RNAiso plus (TaKaRa Bio Inc., Japan) and an RNeasy-mini kit (QIAGEN, USA). First-strand cDNA of MsDJLP, using primers designed for the MsDJLP sequences (Table), was synthesized using a cDNA Synthesis Kit (TaKaRa Bio Inc., Korea) following the manufacturer’s instructions. The PCR amplifications were carried out to amplify the full-length transcribed sequence of MsDJLP. The PCR products were purified and then ligated by TA cloning into the pCR2.1-TOPO vector (Invitrogen, USA). Finally, the products were transformed into E.coli TOP10 competent cells (Invitrogen, USA), which were grown overnight, and then the plasmid DNA was isolated and sequenced (Bioneer, Seoul, Korea).
Table.
Gene-specific primers used for genomic and q-RT-PCR analysis.
| cDNA | Forward primer | Reverse primer |
|---|---|---|
| (Genomic PCR) | ||
| HPT | CCTGAACTCACCGCGACG | GACCAATGCGGAGCATATA |
| MsDJLP | CGGTACCATGGCTTCTTCTGTTGCAGTC | GCTCTAGACTACTCAACATTGACATTAATCACATC |
| (q-RT-PCR) | ||
| MsDJLP | GTCTGCCATCCTGATGTGGC | CGGAAGACACCATCGACGAC |
| Actin | TGAACGTTTCCGATGCCCTG | CGTCGCACTCCATGATCGAG |
The underlined bases represent the restriction sites used for cloning.
2.3. DNA sequence analysis
The sequence identity of our candidate gene was confirmed by an online BLAST search (http://www.ncbi. nlm.nih.gov/) using the full-length nucleotide sequences, deduced amino acids sequences, and open reading frame (ORF). The hypothetical pI and Mw of the deduced protein were matched using a search tool (http://web.expasy.org/ compute_pi/). The protein sequence was investigated further through the Interpro resource (http:// www.ebi. ac.uk/interpro/scan.html). Multiple alignments of amino acid sequences of the MsDJLP gene and homologs of DnaJ proteins were carried out using the Clustal Omega tool (https://www.ebi.ac.uk/Tools/msa/clustalo/). A phylogenetic tree was constructed by the neighbor-joining method using MEGA7 software (Kumar et al., 2016) .
2.4. Northern blot analysis
A northern blot was performed to study the temporal expression patterns of the MsDJLP gene in alfalfa leaves in response to several abiotic stress treatments. Total RNA was extracted from alfalfa leaf tissues using the Plant RNeasy Mini Kit (QIAGEN, USA). Ten micrograms of isolated RNA was separated on 1.2% agarose formaldehyde gel and then transferred to a nylon membrane (GE Healthcare, UK). Subsequently, the membrane was exposed to ultraviolet (UV) light for RNA-cross linking, and then the fixed membrane was hybridized using a labeled probe. In this process, the complete cDNA sequence of MsDJLP (gene-specific probe) was labeled with [α-32P] dATP using random primer labeling (GE Healthcare, UK). Hybridization was performed overnight (24 h) and then the filter was washed using 2X salinesodium citrate buffer (0.15 M NaCl and 0.015 Na C H O , at pH 7.0) with 1% (w/v) SDS solution for 15 min. The membrane was placed on a paper towel and allowed to air-dry for several minutes. Finally, autoradiography was performed at –80 °C. GelQuantNET software (version 1.8.2, BiochemLabSolutions, USA) was applied for the densitometric analysis of induced bands after northern blot analysis. By using this program the specific band intensities with densitometric values of gene expression were detected, and finally these values were exported in a format compatible with MS Excel and presented graphically.
2.5. Construction of the plant transformation vector
The full-length MsDJLP cDNA sequence (Accession No. AKA20389) was ligated with the translation initiation codon downstream of the 5′-untranslated sequence of the CaMV 35S promoter that confers constitutive expression of the gene in the plant in the cloning vector. The chimeric gene cassette was then cut out and ligated into the KpnI/ XbaI site of the pCAMBIA1300 plant transformation vector. The sequence and the correct orientation of the cDNA sequence in the recombinant plasmid were confirmed by sequence analysis. The recombinant plasmid, designated as pCam: MsDJLP, was introduced into competent cells of an Agrobacterium tumefaciens strain (EHA105) ready for plant transformation.
2.6. Plant transformation, regeneration, and selection
Transgenic tobacco plants (Nicotiana tabacum L. ‘SR-1’) were developed by the Agrobacterium-mediated plant transformation technique as described by Horsch et al. (1985) . Briefly, 4-week-old fully expanded tobacco leaves were surface-sterilized with 70% ethanol followed by 1%–1.5% NaClO3 and rinsed thoroughly. Leaf disks (0.6 cm in diameter) were punched out and then precultured on Murashige and Skoog (MS) medium in a plant growth chamber with a 16-h/8-h light/dark cycle at 25 °C for 2 days. Subsequently, leaf disks were soaked in Agrobacterium suspension (grown overnight) for 10–15 min for infection. The infected leaf disks were placed on MS medium and maintained for 3 days of cocultivation. After cocultivation, the disks were placed on MS medium supplemented with 20 mg L–1 hygromycin (Hyg) for selection and 250 mg L–1 cefotaxime to kill the Agrobacterium. Shoots were regenerated from calli on MS medium containing 0.1 mg L–1 1-naphthalene acetic acid (NAA), 1 mg L–1 benzylaminopurine (BAP), 250 mg L–1 cefotaxime, and 20 mg L–1 Hyg. Hyg-resistant putatively transformed shoots were selected after about 60 days of maintenance of plantlets on selection medium (Hyg). Regenerated shoots were moved to MS medium without BAP for rooting. The rooted tobacco plantlets were transferred to pots containing potting medium (organic soil and perlite; 1:1), acclimatized in a plant growth chamber for 7 days, and moved to the greenhouse.
2.7. PCR and Southern analysis
Genomic DNA was extracted from putative transgenic and wild-type (WT) tobacco leaves using a Nucleon PhytoPure Kit (GE Healthcare, USA) according to the manufacturer’s protocol. For transgenic validation, the PCR was performed using PCR primers designed to amplify the hygromycin phosphotransferase (HPT) and MsDJLP genes (Table). The amplification program was set as: 94 °C for 2 min (one cycle); 94 °C for 30 s, 62 °C for 30 s, and 72 °C for 1 min (30 cycles); and an extension step at 72 °C for 10 min. PCR products were separated on 1% agarose gel, stained with nucleic acid cleaning solution (Red Safe, Intron, Korea), and visualized under a UV light. Southern blot analysis was performed to confirm the transgene copy number. Aliquots of 10 µg of genomic DNA were digested with Hind III and separated by electrophoresis on 1% agarose gel. The size-fractionated DNA fragments were transferred and fixed to a Hybond N + membrane (Amersham, UK). The blots were hybridized with the α 32P-labeled MsDJLP PCR probe. The probe was hybridized to the membrane overnight at 65 °C in a rotating rotisserie tube containing 0.5 M sodium phosphate buffer (pH 7.2), 7% SDS, and 1 mM EDTA and then washed and visualized. All subsequent experiments were conducted on the T1 generation of transgenic plants.
2.8. Chilling and heat treatments of transgenic and WT tobacco plants
Transgenic and WT tobacco plants were grown for 6 weeks at 25 °C with a 16-h light/8-h dark period, 320 µmol m–2 s–1 light intensity, and 65% relative humidity. Two transgenic lines (TDJ1 and TDJ3) were subjected to further analyses due to exhibition of MV-mediated oxidative stress tolerance. Transgenic and WT plants were initially maintained under normal conditions (25 °C) for 24 h prior to chilling and heat treatments. eThn plants were exposed to chilling (4 °C) for 36 h or to 42 °C for 24 h in a chamber where all other conditions were maintained. Both groups of plants were then maintained in normal culture conditions (25 °C) for recovery. Each experiment was conducted with three independent replications. Following chilling and heat treatments, plant samples were harvested for further analyses.
2.9. Expression analysis of MsDJLP by qRT-PCR
To investigate expression profiles of MsDJLP, total RNA was extracted from transgenic and WT tobacco leaves. The RNAs were converted into first-strand cDNAs, which acted as templates for qRT-PCR analysis (CFX96 System, USA). The reference gene, actin (EU938079), was used as an internal control for each reaction. The gene-specific primers for reference and target genes were designed as shown in the Table. The PCR amplifications were performed in a reaction volume of 20 µL including 1 µL of cDNA, 2X qPCR Master Mix (10 µL), 50X ROX reference dye (0.4 µL), and gene-specific primers (0.4 µL, 10 µM). The samples were subjected to qRT-PCR conditions as follows: the initial denaturation step was maintained at 95 °C for 5 min, followed by 42 cycles of 95 °C for 3 s and annealing at 55 °C for 15 s, and the final extension period was set at 72 °C for 15 s. The 2 −∆Ct method (Livak and Schmittgen, 2001) was used for measuring relative gene expression levels.
2.10. Determination of photosynthetic efficiency
The PSII efficiency was estimated using a portable ulforometer FMS2 (Hansatech, UK) as described previously (van Kooten and Snel, 1990) . Treated tobacco plants were adapted to the dark for 15 min prior to data collection. The minimal florescence (F 0), with all PSII reaction centers open, was measured under modulating light (10 µmol m–2 s–1). eThn the maximum florescence (Fm) was measured when saturating light was applied (7000 µmol m–2 s–1). The Fv/Fm of tobacco PSII was calculated by Fv/Fm = (Fm – F0)/Fm.
2.11. Measurement of electrolyte leakage
Electrolyte leakage (EL) activity was measured by a leaf disk assay following the method of Bajji et al. (2002) . The fourth leaves from the top of transgenic and WT tobacco plants were collected and then rinsed in distilled water for three times. Leaf disks (6 mm in diameter) were punched from three different seedlings of the same line. Leaf disks were floated in a solution containing 5 µM methyl viologen (MV) and 0.3% (w/v) sorbitol. Disks were incubated in the dark for 1 h, followed by illumination with a light intensity of 200 µE cm–2 at 25 °C for 9 h. The EL of the solution was measured using a conductivity meter (M-455C, Isteck Co., Korea) at different time intervals. eThn the leaf samples were autoclaved at 121 °C for 15 min and the percentage of EL attributed to the MV treatment was calculated as: conductivity of experimental samples/conductivity after autoclaving × 100.
2.12. Determination of H2O2 level
Hydrogen peroxide (H2O2) accumulation was measured spectrophotometrically as described previously by Rahman et al. (2016) . Briefly, 200 mg of tobacco leaf sample was homogenized in 2 mL of phosphate buffer (50 mM, pH 6.8). Next, the homogenate was centrifuged (10,000 × g for 20 min), 1.5 mL of extracted supernatant was taken in a new tube, and 0.5 mL of 0.1% titanium sulfate containing 20% (v/v) H2SO4 was added. The mixture was centrifuged again for 15 min and then the intensity of the supernatant was quantified at 410 nm. The H 2O2 content was calculated by its extinction coefficient of 0.28 µmol –1 cm–1.
2.13. Measurement of MDA content
Malondialdehyde (MDA) accumulation was determined by 2-thiobarbituric acid-reactive substances (TBARS) using the method of Shohael et al. (2006) . Briefly, 300 mg of tobacco leaf tissue was homogenized in 1.5 mL of 0.1% (w/v) trichloroacetic acid (TCA). eThn the homogenate was centrifuged (10,000 × g) for 5 min and 2 mL of 20% TCA containing 0.5% thiobarbituric acid (TBA) was mixed with 0.5 mL of supernatant. This mixture was heated at 95 °C for 30 min and then swiftly cooled on ice for 5 min. The mixture was centrifuged again for 5 min, 1 mL of supernatant was taken, and absorbance was measured at 532 nm followed by 600 nm to correct for nonspecific turbidity. Finally, the MDA level was measured using its extinction coefficient of 155 mM –1 cm–1.
2.14. Relative water content
In response to heat treatment, the relative water content (RWC) of transgenic and WT leaves was measured following the method of González and González-Vilar (2001). The RWC was calculated using the formula RWC (%) = [(FW – DW)/(TW – DW)] × 100, where FW represents the fresh weight, TW signifies the turgid weight (flooded with light at 20 °C for 5 h), and DW indicates the dry weight (same leaves dried at 70 °C for 48 h) of tobacco leaves, respectively.
2.15. Relative chlorophyll content
The foliar chlorophyll contents of tobacco plants were determined using a chlorophyll meter (SPAD-502, Ireland) according to the method of Coste et al. (2010) . The fully expanded fourth leaves from the top were selected for this experiment. The relative chlorophyll contents of heat-treated leaves were analyzed and compared to the chlorophyll content under normal conditions. Three independent tobacco plants of the same line were subjected to this measurement.
2.16. Statistical analysis
Data were statistically analyzed using SPSS 22.0. All of the results are represented as means ± SE of three independent replications (n = 3). The significance refers to the statistical level at P ≤ 0.05.
3. Results
3.1. Cloning, sequencing, and bioinformatics analyses of the MsDJLP gene
A full-length cDNA of MsDJLP (Accession No. AKA20389) was obtained and analyzed. The cDNA of MsDJLP consisted of a 495-bp ORF that encoded a deduced protein of 165 amino acid residues (Figure S1). BLAST analysis of the amino acid sequence revealed that the MsDJLP sequence shared greatest identity (94%) with a chaperone protein (DnaJ) from Medicago truncatula (XP_003612176.1). The MsDJLP sequence shared 81%, 74%, and 52% identities with DnaJ 11 chaperone proteins from Cicer arietinum (XP_004512137), Glycine max (XP_003517172.1), and Vitis vinifera (XP_002265844.2), respectively, and 68% identity with the DnaJ-like protein (MsDJLP) from Phaseolus vulgaris (AAB36543.1). A multiple sequence alignment of MsDJLP with the DnaJlike amino acid sequences of the above plants species is shown in Figure S2.
A phylogenetic tree was established using molecular evolutionary genetics analysis software (MEGA7; Kumar et al., 2016) based on the DnaJ amino acid sequences of these six plant species obtained from the NCBI database (Figure S3). In the phylogenic tree, the Vitis vinifera sequence separated into a separate clade from the other DnaJ subgroups, whereas DnaJ sequences from M. sativa, M. truncatula, and Cicer arietinum clustered in the same subgroup. Further, M. sativa and M. truncatula were segmented and clustered as isoforms in this subgroup. The G. max and P. vulgaris sequences clustered in a different subgroup.
3.2. Expression of MsDJLP under abiotic stresses
As mentioned earlier the DnaJ proteins are often induced by abiotic stresses and we evaluated the expression of MsDJLP in alfalfa leaves under multiple abiotic stresses. We observed that MsDJLP gene expression rapidly increased in response to chilling (4 °C) and heat (42 °C) treatments. In contrast, the MsDJLP transcript decreased under cadmium (Cd; 500 µM) and arsenic (As; 500 µM) treatments (Figure 1a), whereas the MsDJLP transcript level was mostly unchanged in response to salt (NaCl; 100 mM) stress (data not shown). However, densitometric analyses of northern blot results are presented by a graph that shows fold differences among treatments (Figure 1b). Northern blot analysis clearly indicated that MsDJLP is involved in the response to abiotic stresses in alfalfa. However, given these results, we needed to check the expression of the MsDJLP gene further in transgenic tobacco plants.
Figure 1.
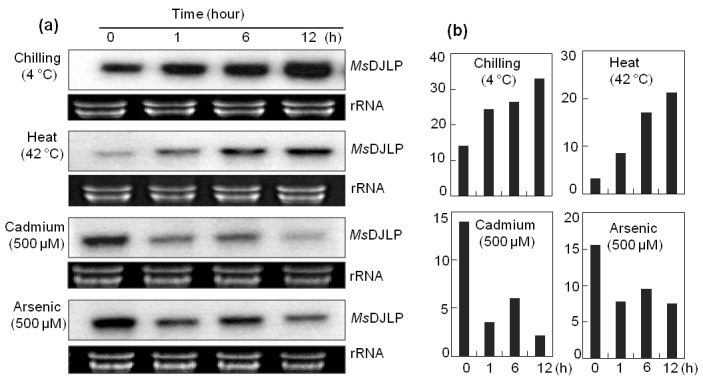
Temporal expression of MsDJLP in alfalfa leaves analyzed by northern blot analysis. a) Alfalfa seedlings were exposed to 4 °C chilling, 42 °C heat, 500 μM cadmium (Cd), and 500 μM arsenic (As) treatments. Leaves were harvested at different time intervals (0, 1, 6, and 12 h). Ten micrograms of total RNA was loaded in each lane. Equal loading was verified by ethidium bromide staining of the gel. The gene-specific probe (cDNA of MsDJLP) was labeled with [α-32P] dATP using random primer labeling. b) Densitometric analysis of MsDJLP gene expression after exposure of alfalfa plants to chilling, heat, cadmium, and arsenic stresses.
3.3. Integration of MsDJLP in tobacco plants
Tobacco plants expressing the MsDJLP gene under the control of the 35S promoter were successfully generated by Agrobacterium-mediated plant transformation (Figure 2a). A total of seven independent Hyg-resistant transgenic tobacco lines (TDJs) were randomly selected from Hyg (20 mg L–1) containing medium and four lines (TDJ1, TDJ3, TDJ4, and TDJ7) were determined to be positive by PCR amplification from plant genomic DNA of the predicted 0.8-kb and 0.495-kb internal fragments of the HPT and MsDJLP genes, respectively (Figures 2b and 2c). The PCRpositive transformants were checked for Southern blot analysis to determine which lines contained a single-copy gene insertion. The gene (MsDJLP)-specific probe showed one copy of the transgene in the transgenic lines TDJ1, TDJ3, and TDJ4, whereas at least two copies were detected in line TDJ7 (Figure 2d).
Figure 2.
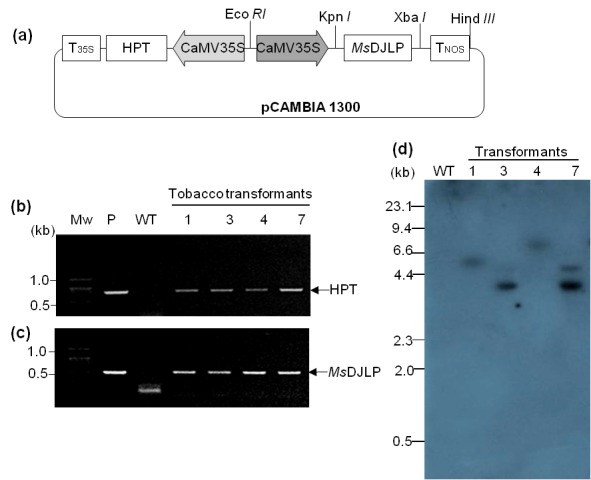
Molecular verification of transgenes in tobacco plants. a) Schematic representation of the T-DNA region of pCam: MsDJLP, the expression vector used for genetic transformation. CaMV 35S: the cauliflower mosaic 35S promoter; MsDJLP: Medicago sativa DnaJ-like protein; HPT: hygromycin phosphotransferase. b) Gel image of the PCR amplification of 0.8-kb HPT gene. c) PCR band of 0.495-kb MsDJLP fragments in tobacco transformants. Mw: molecular weight; P: plasmid DNA of expression vector pCam: MsDJLP; WT: wild-type plant. d) Southern blot analysis of tobacco plants. Ten micrograms of genomic DNA was digested with Hind III, the size-fractionated DNA fragments were transferred to a Hybond N+ membrane, and blots were hybridized with α32P-labeled PCR probe amplified from MsDJLP.
3.4. Transgenic (MsDJLP) tobacco plants show enhanced tolerance to chilling stress
In the chilling stress tolerance assay, the WT tobacco plants displayed wilting and curling symptoms, whereas the selected transgenic lines (TDJ1 and TDJ3) were only mildly affected and most of the transgenic leaves remained fully expanded (Figure 3a). The PSII photosynthetic efficiency in tobacco leaves after 36 h of chilling treatment indicated that the PSII efficiency of WT plants had reduced by approximately 30.0%, whereas it was reduced by 13.6% and 12.9% in TDJ1 and TDJ3 plants, respectively (Figure 3b). As shown in Figure 3b, after a 72-h recovery phase, the photosynthetic effectiveness of TDJ1 and TDJ3 plants reverted to near pretreatment levels, whereas the efficiency remained reduced in WT tobacco plants.
Figure 3.
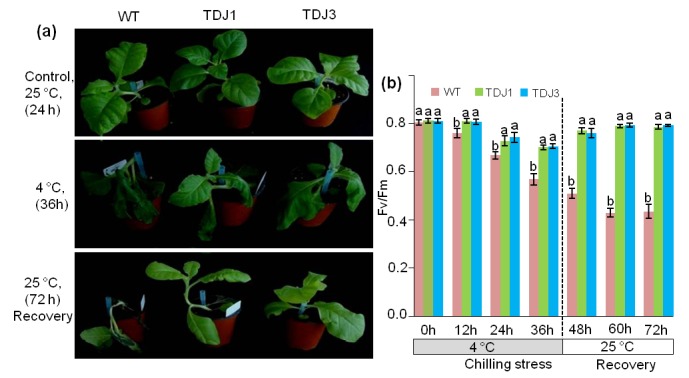
Chilling tolerance assay for wild-type (WT) and transgenic tobacco plants (TDJ). a) Plant growth before and after chilling (4 °C) treatment. Plants under normal (25 °C) conditions (upper panel). Plants exposed to chilling (4 °C) for 36 h (middle panel). Recovery of plants at 25 °C after 72 h (lower panel). b) Effect of chilling stress on chlorophyll florescence (Fv/Fm) in tobacco plants. Different letters above the error bars indicate statistically significant differences (P ≤ 0.05) according to Duncan’s multiple range test. Values represent the mean ± standard deviation of three independent experiments.
Cell membrane stability of WT and transgenic plants in response to chilling stress was assessed by the MVmediated EL test. TDJ1 and TDJ3 transgenic plants showed reduced EL responses of 23.0% and 25.7%, respectively, compared to WT plants (Figure 4a). We also investigated the correlation between MsDJLP overexpression and the amount of ROS after exposure of tobacco plants to the chilling treatment. H2O2 levels were not significantly different among WT and TDJ lines under normal conditions (25 °C). Following chilling (4 °C) treatment, the TDJ1 and TDJ3 plants accumulated 36.3% and 31.8% less H O , respectively, compared to the WT plants (Figure 4b). Under chilling stress, the transcript levels of MsDJLP significantly increased in transgenic tobacco plants but were not detected in WT plants (Figure 4c).
Figure 4.
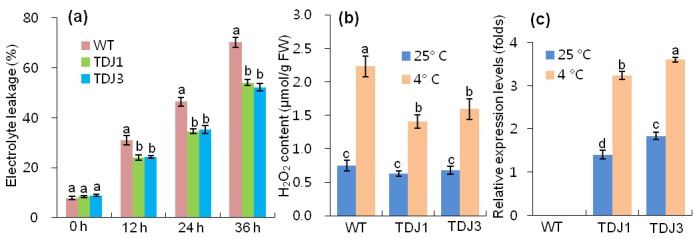
Effect on physiological indices of tobacco plants after chilling treatments. a) Electrolyte leakage analysis of tobacco plants in response to MV (5 μM) treatment. The electrical conductivity (EC) of the MV solution was compared with the total EC of the solution after tissue destruction. b) Quantitative analysis of H2O2 levels in tobacco plants after 36 h of chilling treatment. c) The expression levels of the MsDJLP genes were normalized to the tobacco (Nicotiana tabacum L.) actin gene as the internal control. Bars with different letters are significantly different (P ≤ 0.05) according to Duncan’s multiple range test.
3.5. Enhanced tolerance to high temperature
WT plants exhibited severe wilting in response to heat shock when exposed to high temperature, whereas the selected transgenic MsDJLP plants (TDJ1 and TDJ3) were only mildly affected (Figure 5a). Heat shock treatment reduced the chlorophyll content by 22.9% in WT plants while in transgenic lines (TDJ1 and TDJ3) it was reduced by approximately 10.0% and 8.8%, respectively (Figure 5b). In addition, RWC was reduced in WT plants by 35.8%, whereas in transgenic TDJ1 and TDJ3 lines the reduction was 7.0% and 4.3%, respectively (Figure 6a). MDA content was measured as an indicator of lipid peroxidation response to heat stress in plants. MDA accumulation increased in both WT and transgenic tobacco plants after 24 h of heat (42 °C) treatment. However, the level of MDA content increase in the WT plants was approximately 40% of that in transgenic lines (Figure 6b). Consequently, we checked the transcript level of MsDJLP in response to heat stress. The transcripts of MsDJLP increased considerably in transgenic plants but were not detected in WT plants (Figure 6c).
Figure 5.
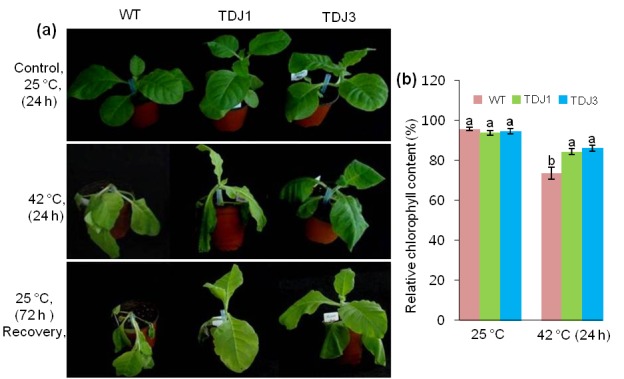
Heat shock tolerance assay of wild-type (WT) and transgenic tobacco plants (TDJ). a) Plant growth before and after heat (42 °C) treatment. Plants under normal (25 °C) conditions (upper panel). Plants exposed to heat for 24 h (middle panel). Plant recovery at 25 °C after 72 h (lower panel). b) Relative chlorophyll contents of WT and TDJ plants at 25 °C and after 42 °C heat treatment for 24 h.
Figure 6.
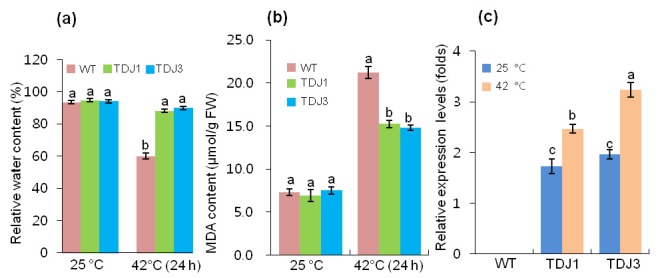
Effect of heat shock stress at the physiological and molecular level in wild-type (WT) and transgenic tobacco plants (TDJ). a) Relative water content, b) MDA content of WT and TDJ plants. Data were taken at 25 °C and after 24 h of 42 °C heat treatment. c) Relative expression of the MsDJLP gene in response to normal conditions (25 °C) and heat treatment (42 °C) for 24 h in WT and TDJ plants.
4. Discussion
The DnaJ proteins are involved in several cellular processes including plant development, protein homeostasis, and abiotic stress tolerance in plants (Wang et al., 2004; Kong et al., 2014a, 2014b) . DnaJ proteins are known as cellular stress sensors; it has been reported that the expressions of these genes are induced by MV, cold, intense light, and extreme temperatures (Rajan and D’Silva, 2009; Zhou et al., 2012) . In this study, we identified an alfalfa DnaJ-like protein (MsDJLP) and demonstrated that the discovered gene was involved in abiotic stress responses. MsDJLP was induced by chilling (4 °C) and heat (42 °C), but not by Cd (500 µM) or As (500 µM) stresses. These results support the nature of HSPs in that they are often induced by temperature in plant species (Yadav, 2010; Bokszczanin et al., 2013) . Furthermore, we checked the expression of MsDJLP in transgenic tobacco plants under chilling and heat stresses.
We developed transgenic tobacco plants expressing the MsDJLP gene under the control of an enhanced CaMV 35S promoter and subsequently revealed the role of MsDJLP under chilling and heat stresses. The transgenic and WT plants grew well under normal conditions but showed differences in physiological indices. Further, transgenic plants (TDJ1, TDJ3) showed better PSII photosynthetic efficiency (Fv/Fm), lower electrolyte leakage, and less accumulation of H O2 than the WT plants during chilling (4 °C) treatment. These results indicate that the PSII photoinhibition as well as the ROS-induced oxidative damage were less serious in transgenic plants compared to WT plants. Our results correspond to those from a previous report on photoinhibition that occurred due to blocking of the PSII photosynthetic electron transport, ROS generation, and damage of the photosynthetic pigment complex under chilling stress (Suzuki et al., 2008) . In addition, LeCDJ1, a DnaJ protein, was shown to be involved in chilling tolerance by maintaining PSII activity in tomato (Kong et al., 2014b) . These studies provide additional support for our results that demonstrate that the MsDJLP protein enhanced tolerance by maintaining PSII activity and mitigating oxidative stress-induced cellular damage in transgenic tobacco plants.
In addition to the above, MsDJLP was shown to be highly expressed at the transcript level under chilling stress, indicating a molecular response of the MsDJLP gene in tobacco plants under chilling treatment. It has been previously reported that transgenic tobacco expressing the CfCBF3 and LeCDJ2 genes had enhanced tolerance to chilling and drought stresses, respectively (Yang et al., 2011; Wang et al., 2014) . These results support the suggestion that that the increased expression of MsDJLP confers chilling stress tolerance in transgenic tobacco plants.
A number of studies have demonstrated that high expression and accumulation of DnaJ proteins are associated with thermotolerance in plants. For example, the expression of AtDjA2 and AtDjA2 chaperone proteins improved thermotolerance in young Arabidopsis plants (Li et al., 2007) . A thermosensitive male sterile (TMS1) protein contributed to pollen tube thermotolerance in Arabidopsis plants (Yang et al., 2009) and the AtDjB1 gene conferred enhanced thermotolerance by defending plant cells from oxidative stress injury in Arabidopsis plants (Zhou et al., 2012) . Consequently, in our study we evaluated the response of MsDJLP-transgenic plants to heat (42 °C) stress. We observed that WT plants wilted after 24 h of heat treatment, whereas transgenic plants (TDJ1, TDJ3) were mildly affected by heat stress and were healthy after the stress was alleviated. This phenotypic difference in response indicates that the transgene expressed in the TDJ plants confers the ability to alleviate heat stress. In addition, the lower chlorophyll level and water contents recorded under heat treatment significantly reduced the photosynthetic capacity of WT tobacco plants. In contrast, the levels of chlorophyll and RWC were maintained in transgenic plants, presumably due to enhanced activity of the MsDJLP gene during heat stress. Transgenic activation of LeCdJ1 conferred enhanced tolerance in tomato plants under heat stress conditions (Kong et al., 2014a) . Recently, it has been reported that a DnaJ protein (SICDJ2) maintained CO2 assimilation by protecting RuBisCo activity in tomato (Wang et al., 2015) . Our findings for MsDJLP provide further evidence that support the role of DnaJ proteins in thermotolerance.
Heat stress induces ROS production and subsequent oxidative stress injury, lipid peroxidation, and cellular damage in plants (Wang et al., 2014) . A correlation has been found between the effect of heat stress and oxidative stress responses in plants (Hasanuzzaman et al., 2013), and oxidative stress-induced lipid peroxidation provides an efficient indicator to assess the extent of stress tolerance in plants (Hodges et al., 1999). In our study, we found that the MDA content was lower in transgenic plants compared to WT plants after heat stress. This result implies that the frequency of membrane damage was lower in transgenic tobacco plants than in their WT counterparts.
The DnaJ proteins are known as cellular sensors; they are upregulated in response to several stresses including MV, cold, high light, and heat (Park and Seo, 2015) . In our study, the expression profile of MsDJLP clearly revealed that the gene expression was further induced by heat stress (42 °C) in transgenic tobacco plants. The results corroborate previous reports on expression patterns of a DnaJ protein gene (LeCDJ1) in tomato, driven by the CaMV 35S promoter, which increased transcript levels under heat stress (Kong et al., 2014a) . Consequently, we propose that the elevated expression of the MsDJLP gene enhanced heat tolerance of transgenic tobacco plants.
In conclusion, this current study demonstrates that the transgenic expression of the MsDJLP gene enhances the tolerance to chilling and heat stresses in transgenic tobacco plants. The expression of the MsDJLP gene in tobacco showed significant differences between transgenic and WT plants in response to chilling and heat stresses. Interestingly, the expression of the MsDJLP gene increased significantly in the leaves of transgenic tobacco plants compared to WT ones. This study provides a new possibility of using the MsDJLP gene for stress-tolerant crop production in the face of chilling and heat stresses.
Acknowledgments
This study was funded by the Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ01193504). We are thankful to the National Institute of Animal Science, Rural Development Administration, for continuing the Postdoctoral Fellowship Program 2018, Republic of Korea.
References
- Bajji M , Kinet JM , Lutts S ( 2002. ). The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat . Plant Growth Regul 36 : 61 - 70 . [Google Scholar]
- Bokszczanin KL , Fragkostefanakis S , Bostan H , Bovy A , Chaturvedi P , Chiusano M , Firon N , Iannacone R , Jegadeesan S , Klaczynskid K et al. ( 2013. ). Perspectives on deciphering mechanisms underlying plant heat stress response and thermotolerance . Front Plant Sci 4 : 315 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste S , Baraloto C , Leroy C , Marcon É , Renaud A , Richardson AD , Roggy JC , Schimann H , Uddling J , Hérault B ( 2010. ). Assessing foliar chlorophyll contents with the SPAD-502 chlorophyll meter: a calibration test with thirteen tree species of tropical rainforest in French Guiana . Annals For Sci 67 : 607 . [Google Scholar]
- Determination of relative water content. In: Reigosa Roger MJ, editor. Handbook of Plant Ecophysiology Techniques. 1st ed. Berlin, Germany: Springer. 2001. pp. 207–212.
- Hasanuzzaman M Nahar K Alam MM Roychowdhury R Fujita M Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci. 2013;14:9643–9684. doi: 10.3390/ijms14059643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield JL Prueger JH Temperature extremes: effect on plant growth and development. Weather Clim Extremes. 2015;10:4–10. [Google Scholar]
- Hodges DM DeLong JM Forney CF Prange RK Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 1999;207:604–611. doi: 10.1007/s00425-017-2699-3. [DOI] [PubMed] [Google Scholar]
- Horsch RB , Fry JE , Hofmann NL , Eichholtz D , Rogers SG , Fraley RT ( 1985. ). A simple and general method for transferring genes into plants . Science 227 : 1229 - 1231 . [DOI] [PubMed] [Google Scholar]
- Kong F , Deng Y , Wang G , Wang J , Liang X , Meng Q ( 2014a. ). LeCDJ1, a chloroplast DnaJ protein, facilitates heat tolerance in transgenic tomatoes . J Integr Plant Biol 56 : 63 - 74 . [DOI] [PubMed] [Google Scholar]
- Kong F , Deng Y , Zhou B , Wang G , Wang Y , Meng Q ( 2014b. ). A chloroplast-targeted DnaJ protein contributes to maintenance of photosystem II under chilling stress . J Exp Bot 65 : 143 - 158 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S , Stecher G , Tamura K ( 2016. ). MEGA7: Molecular evolutionary Genetics Analysis version 7.0 for bigger datasets . Mol Biol Evol 33 : 1870 - 1874 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GL , Chang H , Li B , Zhou W , Sun DY , Zhou RG ( 2007. ). The roles of the at DjA2 and at DjA3 molecular chaperone proteins in improving thermotolerance of Arabidopsis thaliana seedlings . Plant Sci 173 : 408 - 416 . [Google Scholar]
- Li W , Wei Z , Qiao Z , Wu Z , Cheng L ( 2013. ). Proteomics analysis of alfalfa response to heat stress . PLoS One 8 : e82725 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y , Wan L , Bi S , Wan X , Li Z , Cao J , Tong Z , Xu H , He F , Li X ( 2017. ). Identification of drought-responsive microRNAs from roots and leaves of alfalfa by high-throughput sequencing . Genes 8 : 119 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JZ , Whitham SA ( 2013. ). Overexpression of a soybean nuclear localized type-III DnaJ domain-containing HSP40 reveals its roles in cell death and disease resistance . Plant J 74 : 110 - 121 . [DOI] [PubMed] [Google Scholar]
- Livak KJ , Schmittgen TD ( 2001. ). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method . Methods 25 : 402 - 408 . [DOI] [PubMed] [Google Scholar]
- Park CJ , Seo YS ( 2015. ). Heat shock proteins: a review of the molecular chaperones for plant immunity . Plant Pathol J 31 : 323 - 333 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MA , Alam I , Kim YG , Ahn NY , Heo SH , Lee DG , Liu G , Lee BH ( 2015. ). Screening for salt-responsive proteins in two contrasting alfalfa cultivars using a comparative proteome approach . Plant Physiol Biochem 89 : 112 - 122 . [DOI] [PubMed] [Google Scholar]
- Rahman MA , Kim YG , Alam I , Liu G , Lee H , Lee JJ , Lee BH ( 2016. ). Proteome analysis of alfalfa roots in response to water deficit stress . Journal of Integrative Agriculture 15 : 1275 - 1285 . [Google Scholar]
- Rajan VB , D'Silva P ( 2009. ). Arabidopsis thaliana J-class heat shock proteins: cellular stress sensors . Funct Integr Genomics 9 : 433 - 446 . [DOI] [PubMed] [Google Scholar]
- Shohael AM , Ali MB , Yu KW , Hahn EJ , Paek KY ( 2006. ). Eefct of temperature on secondary metabolites production and antioxidant enzyme activities in Eleutherococcus senticosus somatic embryos . Plant Cell Tiss Organ Cult 85 : 219 - 228 . [Google Scholar]
- Suzuki K , Nagasuga K , Okada M ( 2008. ). The chilling injury induced by high root temperature in the leaves of rice seedlings . Plant Cell Physiol 49 : 433 - 442 . [DOI] [PubMed] [Google Scholar]
- Van Kooten O , Snel JH ( 1990. ). The use of chlorophyll uflorescence nomenclature in plant stress physiology . Photosynth Res 25 : 147 - 150 . [DOI] [PubMed] [Google Scholar]
- Vierling E ( 1991. ). The roles of heat shock proteins in plants . Annu Rev Plant Phys 42 : 579 - 620 . [Google Scholar]
- Wang G , Cai G , Kong F , Deng Y , Ma N , Meng Q ( 2014. ). Overexpression of tomato chloroplast-targeted DnaJ protein enhances tolerance to drought stress and resistance to Pseudomonas solanacearum in transgenic tobacco . Plant Physiol Biochem 82 : 95 - 104 . [DOI] [PubMed] [Google Scholar]
- Wang G , Kong F , Zhang S , Meng X , Wang Y , Meng Q ( 2015. ). A tomato chloroplast-targeted DnaJ protein protects rubisco activity under heat stress . J Exp Bot 66 : 3027 - 3040 . [DOI] [PubMed] [Google Scholar]
- Wang W , Vinocur B , Shoseyov O , Altman A ( 2004. ). Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response . Trends Plant Sci 9 : 244 - 252 . [DOI] [PubMed] [Google Scholar]
- Yadav SK ( 2010. ). Cold stress tolerance mechanisms in plants. A review . Agron Sustain Dev 30 : 515 - 527 . [Google Scholar]
- Yang KZ , Xia C , Liu XL , Dou XY , Wang W , Chen LQ , Zhang XQ , Xie LF , He L , Ma X et al. ( 2009. ). A mutation in thermosensitive male sterile 1, encoding a heat shock protein with DnaJ and PDI domains, leads to thermosensitive gametophytic male sterility in Arabidopsis . Plant J 57 : 870 - 882 . [DOI] [PubMed] [Google Scholar]
- Yang S , Tang XF , Ma NN , Wang LY , Meng QW ( 2011. ). Heterology expression of the sweet pepper CBF3 gene confers elevated tolerance to chilling stress in transgenic tobacco . J Plant Physiol 168 : 1804 - 1812 . [DOI] [PubMed] [Google Scholar]
- Zhou P , Su L , Lv A , Wang S , Huang B , An Y ( 2016. ). Gene expression analysis of alfalfa seedlings response to acid-aluminum . Int J Genomics 2016. : 13 . [DOI] [PMC free article] [PubMed]
- Zhou W , Zhou T , Li MX , Zhao CL , Jia N , Wang XX , Sun YZ , Li GL , Xu M , Zhou RG et al. ( 2012. ). The Arabidopsis J -protein AtDjB1 facilitates thermotolerance by protecting cells against heatinduced oxidative damage . New Phytol 194 : 364 - 378 . [DOI] [PubMed] [Google Scholar]


