Abstract
Lead (Pb) has wide-ranging effects on various essential physiological processes in plants including seed germination, root/ shoot growth, photosynthetic efficiency, water status, and activities of enzymes. The effect of combined treatment of 24-epibrassinolide (10-7 M, EBL) and salicylic acid (1 mM, SA) on growth, photosynthetic attributes, and phenolic compounds in 30-, 60-, and 90-day-old plants of Brassica juncea L. under Pb stress (0.25 mM, 0.50 mM, and 0.75 mM) were studied. It was observed that Pb toxicity resulted in lowered growth and photosynthetic efficiency. The expressions of CHLASE, PSY, CHS, and PAL genes were altered. Presoaking treatment with the combination of EBL and SA for metal-stressed plants mitigated the adverse effects of metal stress by improving growth and levels of pigment and phenolic compounds.
Keywords: Brassinosteroids, chlorophyll, qRT-PCR, root growth, CHLASE gene
1. Introduction
Photosynthesis is an imperative biological phenomenon, which bestows growth and development of plants. It converts light energy to useful chemical energy required for many metabolic processes (Pan et al., 2012; Ashraf and Harris, 2013) . Disturbed photosynthesis and alteration of the ultrastructure of chloroplasts are prime symptoms of metal toxicity. Metal ions restrict the functioning of several photosynthetic enzymes and the electron transport chain (Garg and Aggarwal, 2011). Abiotic stresses result in retardation of the photosynthetic rate by modulating stress-induced stomatal and nonstomatal limitations (Rahnama et al., 2010; Ahmad et al., 2011) . Inhibition of photosynthesis in response to abiotic stresses such as drought, temperature, salinity, and heavy metal stress is well documented (Pirzad et al., 2011) . Heavy metals, when present at higher concentrations than the optimal level, are considered toxic to plants (Garg and Aggarwal, 2011). Irreversible inactivation of electron transport and phosphorylation of thylakoid membranes have been reported under the effect of high concentrations of inorganic salts (Mittal et al., 2012) . Heavy metals are reported to have a direct association with acceptor sites of photosystem II (PSII) and eventually result in disruption of the electron transport chain (Patsikka et al., 2001). Lead is a highly toxic heavy metal that is persistent in the environment for a very long period of time due to its nonbiodegradable nature and is a major concern for environmentalists (McComb et al., 2012) . Significant effects of Pb on various plant processes have long been reported, the most prominent being a decline in seed germination and retarded growth of roots and shoots. The lowered photosynthetic rate in response to Pb toxicity is attributed to disruption of organelles’ ultrastructures, retardation of chlorophyll and carotenoid content, and inhibition of electron transport (Stefanov et al., 1995) .
Brassinosteroids (BRs) have an extensive range of physiological functions under stressed and even normal environmental conditions (Sharma et al., 2008) . Studies on the application of 24-epibrassinolide (EBL) and 28-homobrassinolide show significant changes in plant metabolism, growth, and productivity of crops (Sharma et al., 2011) . Membrane stability, increased photosynthetic efficiency, and osmotic regulation are major mechanisms by which BRs are known to increase abiotic stress tolerance (Bajguz et al., 2010) . Salicylic acid (SA) belongs to a unique class of endogenous plant growth regulators that are phenolic in nature and has an important role in modulating various physiological and metabolic processes (Dong et al., 2015). It plays a significant role in signal transduction in order to magnify the tolerance to heavy metal stress. SA has been reported to have antistress effects under biotic and abiotic stresses. Lee et al. (2014) reported that SA has a prominent role in modulating the photosynthetic machinery by affecting stomatal closure, chloroplast structure, and activities of photosynthetic enzymes such as carbonic anhydrase and RuBisCo (ribulose-1,5-bisphosphate carboxylase/oxygenase). Plant hormones have been reported to interplay in stressed conditions, including abscisic acid, gibberellins, SA, jasmonic acid, ethylene, and BRs (Vicente and Plasencia, 2011) . Application of BRs has been reported to enhance two significant components of the SA biosynthetic pathway, including NPR-1 (nonexpressor of pathogenesisrelated genes 1) and the WRKY70 transcription factor, in Arabidopsis thaliana plants under temperature and salinity stress (Divi et al., 2010). These components elevate the tolerance of plants to heat and salt stress. Another important observation by Deng et al. (2016) suggested the significant role of BRs in enhancing tolerance to biotic stress by enhancing the activity of SIPK (SA-induced protein kinases)m eventually resulting in activation of the Nb(MEPK2-SIPK) pathway (Nicotiana benthamiana plants mitogen activated protein kinases-2-SA-induced protein kinases pathway).
A number of studies have been conducted to observe the potential of EBL and SA individually as ameliorators of metal toxicity. However, the interactive potential of EBL and SA is yet to be studied in Brassica juncea plants under Pb metal stress. Keeping in mind the influence of the interplay of BRs and SA in modulating physiological and defense response mechanisms in plants under abiotic and biotic stress conditions, the present work was designed to observe the synergistic effects of EBL and SA treatments in B. juncea L. plants under Pb stress with changes in photosynthetic efficiency and gene expression.
2. Materials and methods
2.1. Study material
Seeds of Brassica juncea L. var. RLC-1 were procured from the Seed Technology Division of Punjab Agriculture University, Ludhiana, India.
2.2. Plant cultivation/growth
The seeds were surface-sterilized with 0.01% HgCl 2 for 2 min followed by rinsing 5 times with distilled water. The surface-sterilized seeds were then presoaked in three different hormonal solutions including 10 –7 M EBL, 1 mM SA, and a combination of EBL + SA for 8 h. The exogenous concentrations of both hormones were selected on the basis of growth promotion of seedlings. These were then sown in earthen pots containing a mixture of soil, sand, and manure at a ratio of 3:1:1. These pots were of uniform size and contained 5 kg of soil mixture. Before sowing the seeds, treatment with different concentrations of lead (0.25 mM, 0.50 mM, and 0.75 mM, selected on the basis of IC50 value) was given to soil. The plants were allowed to grow in earthen pots under natural conditions (5–10 °C) and they were harvested after 30, 60, and 90 days. The experiment was conducted in triplicates under the same conditions.
2.3. Growth parameters
Shoot and root lengths were observed 30, 60, and 90 days after seed sowing.
2.4. Photosynthetic pigment content
Total chlorophyll content was estimated by the method given by Arnon (1949) . The absorbance of the supernatant was recorded at 645 nm and 663 nm.
Carotenoid content was measured according to the method proposed by Maclachlan and Zalik (1963) . The procedure of carotenoid estimation was similar to chlorophyll estimation except that the absorbance was recorded at 480 nm and 610 nm.
2.5. Gas exchange parameters
Gas exchange parameters such as net photosynthetic rate (Pn), intercellular CO2 concentration (Ci), stomatal conductance (gs), and transpiration rate (E) were recorded using a portable photosynthetic system unit, LI-COR 6400 (LI-COR Instruments, USA). The measurements were taken between 1000 and 1200 hours on a sunny day.
2.6. Phenolic compound analysis
Total phenol content was determined by the method given by Singleton and Rossi (1965) . The absorbance of the sample was read at 765 nm.
Flavonoids were estimated by the method proposed by Zhishen et al. (1999) . Absorbance was read at 510 nm.
Anthocyanin content was determined by following the method of Mancinelli (1984) . The absorbance of the supernatant was read at 530 nm and 657 nm.
2.7. Gene expression analysis
Gene expression analysis of the chlorophyllase enzyme [CHLASE gene, AAN51934.1 (forward primer- 5’ GAATATCCGGTGGTGATGCT 3’, backward primer- 5’ TCCGCCGTTGATTTTATCTC 3’], phytoene synthase enzyme [PSY gene, AEN94302.1 (forward primer- 5’ TGGGTTGGTAAGGGCTGTAG 3’, backward primer5’ CGCTCGAAGACACAACACTC 3’], chalcone synthase [CHS gene, ABQ95969.1 (forward primer- 5’ CAAGGCGGAGAAGATGAGAG 3’, backward primer- 5’ CATCTTCCGCAGACTTCCTC 3’], and phenylalanine ammonia-lyase [PAL gene, GQ505065.1 (forward primer5’ AAACTCCGTCAACGACAACC 3’, backward primer5’ AGCGAACATGAGCTTCCCTA 3’] was done on 30-day-old samples. A fresh plant sample of 100 mg was used to extract total RNA by the TRIzol method as given by the manufacturer’s instructions (Invitrogen). Total RNA was extracted, out of which 1 µg of total RNA was further used for reverse transcription with RNA to cDNA with a cDNA kit (Invitrogen).
Primers were designed via GenBank and EMBL mRNA sequences. An actin gene (HM565958.1, forward primer- 5’ CTTGCACCTAGCAGCATGAA 3’, backward primer- 5’ GGACAATGGATGGACCTGAC 3’) was used as a control (Sharma et al., 2017) . Processing of genes was done in triplicate. This was followed by performing qualitative real-time PCR (qRT-PCR) with the Step One real-time detection system of Applied Biosystems and Power SYBR Green PCR Master Mix of Thermo Fisher Scientific. At the termination of the PCR cycle, a melting curve was derived. The quantification of mRNA was done by the method given by the Livak and Schmittgen (2001).
2.8. Statistical analysis
To apply valid statistical analysis, three replicates of each treatment for independent experiments were taken. The results were statistically analyzed using two-way analysis of variance (ANOVA) and Tukey’s honestly significant difference (HSD) test. Values are presented in the form of mean ± standard deviation (SD) of the mean and were considered significant at P ≤ 0.05 and 0.01.
3. Results
3.1. Growth parameters
Exposure of 30-, 60-, and 90-day-old B. juncea L. plants to 0.75 mM Pb resulted in a decline in root lengths by 32.08%, 29.37%, and 30.94% and shoot lengths by 49.78%, 23.61%, and 21.69%, respectively, in comparison to control plants. Combined treatment with EBL and SA resulted in enhancement in root lengths as well as shoot lengths in 0.75 mM Pb-treated plants. Root lengths were elevated by 45.08% in 30-day-old plants, 113.93% in 60-day-old plants, and 54.68% in 90-day-old plants. Similarly, shoot lengths were elevated by 137.29% (30-day-old plants), 166.66% (60-day-old plants), and 53.05% (90-day-old plants) (Figure 1).
Figure 1.
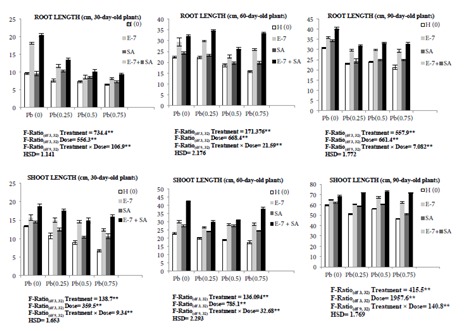
Effect of different concentrations of Pb (0.25 mM, 0.50 mM, and 0.75 mM), EBL (10–7 M), and SA (1 mM) and their combinations on root length and shoot length of 30-, 60-, and 90-day-old plants of Brassica juncea L. (values show the means of three replicates ± SD; Tukey’s test was performed and significance was checked at P ≤ 0.05 designated with * and at P ≤ 0.01 designated with **).
3.2. Plant pigment contents
The present study revealed that exposure to Pb resulted in the decline of pigment contents including chlorophyll and carotenoids. Total chlorophyll content of 30-, 60-, and 90-day-old plants was lowered by 35.92%, 43.61%, and 33.14%, respectively, in 0.75 mM Pb-treated plants in comparison to the control. A similar decline in carotenoid content was observed in 0.75 mM Pb-treated plants. Combined treatment of EBL and SA resulted in elevation in contents of chlorophyll and carotenoid. Chlorophyll content was enhanced by 82.17% in 30-day-old plants, 137.15% in 60-day-old plants, and 203.18% in 90-dayold plants. Similarly, carotenoid content was enhanced by 308.3%, 208.33%, and 288.52% in 30-, 60-, and 90-day-old plants, respectively (Figure 2).
Figure 2.
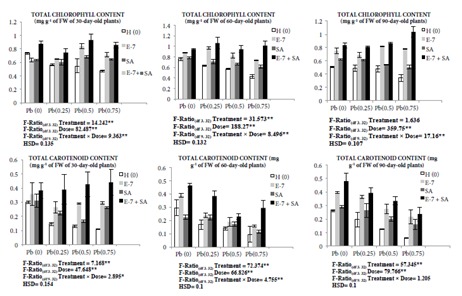
Effect of different concentrations of Pb (0.25 mM, 0.50 mM, and 0.75 mM), EBL (10–7 M), and SA (1 mM) and their combinations on total chlorophyll and carotenoid content of 30-, 60-, and 90-day-old plants of Brassica juncea L. (values show the means of three replicates ± SD; Tukey’s test was performed and significance was checked at P ≤ 0.05 designated with * and at P ≤ 0.01 designated with **).
3.3. Gas exchange parameters
The present study revealed significant effects of elevated levels of Pb concentration as well as combined treatment with EBL and SA on gas exchange parameters of all field trials. Net photosynthetic rate, intercellular CO2 concentration, stomatal conductance, and transpiration rate were observed to be lowered by 62.96%, 28.56%, 49.18%, and 58.20% in 30-day-old plants; by 43.80%, 20.12%, 17.66%, and 65.10% in 60-day-old plants; and by 45.51%, 22.59%, 25.00%, and 51.57% in 90-day-old plants, respectively. Combined treatment of EBL and SA led to further enhancement in all gas exchange parameters of 0.75 mM Pb-stressed plants. Net photosynthetic rate, intercellular CO2 concentration, stomatal conductance, and transpiration rate were elevated by 94.49%, 20.82%, 91.61%, and 25.61% in 30-day-old plants; by 66.57% 10.99%, 6.27%, and 42.51% in 60-day-old plants; and by 65.29%, 34.39%, 14.1%, and 80.35% in 90-day-old plants, respectively (Tables 1 and 2).
Table 1.
Effect of different concentrations of Pb (0.25 mM, 0.50 mM, and 0.75 mM) and the combination of EBL (10–7 M) and SA (1 mM) on net photosynthetic rate and stomatal conductance of 30-, 60-, and 90-day-old plants of B. juncea L. **: P < 0.01.
| Treatment | Net photosynthetic rate (µmol m2 s–1) (mean ± SD) | Stomatal conductance (mmol CO m2 s–1) (mean ± SD) | ||||||
|---|---|---|---|---|---|---|---|---|
| Pb | EBL | SA | 30 DAS | 60 DAS | 90 DAS | 30 DAS | 60 DAS | 90 DAS |
| 0 | 0 | 0 | 13.23 ± 0.252 | 22.83 ± 0.702 | 38.23 ± 0.153 | 0.305 ± 0.004 | 0.504 ± 0.003 | 0.416 ± 0.005 |
| 0 | 10–7 M | 0 | 14.33 ± 0.252 | 29.53 ± 0.351 | 45.90 ± 0.361 | 0.320 ± 0.002 | 0.534 ± 0.003 | 0.455 ± 0.005 |
| 0 | 0 | 1 mM | 13.97 ± 0.208 | 25.90 ± 0.265 | 42.13 ± 0.569 | 0.308 ± 0.003 | 0.528 ± 0.006 | 0.432 ± 0.003 |
| 0 | 10–7 M | 1 mM | 16.63 ± 0.306 | 37.83 ± 0.503 | 53.47 ± 0.603 | 0.327 ± 0.004 | 0.552 ± 0.004 | 0.495 ± 0.004 |
| 0.25 mM | 0 | 0 | 8.87 ± 0.351 | 21.60 ± 0.361 | 33.63 ± 0.231 | 0.247 ± 0.003 | 0.490 ± 0.005 | 0.393 ± 0.002 |
| 0.25 mM | 10–7 M | 0 | 12.23 ± 0.252 | 25.13 ± 0.451 | 42.33 ± 0.961 | 0.274 ± 0.002 | 0.525 ± 0.004 | 0.427 ± 0.006 |
| 0.25 mM | 0 | 1 mM | 11.47 ± 0.351 | 24.03 ± 0.603 | 35.03 ± 0.503 | 0.272 ± 0.003 | 0.515 ± 0.004 | 0.409 ± 0.001 |
| 0.25 mM | 10–7 M | 1 mM | 13.43 ± 0.306 | 27.37 ± 0.611 | 48.10 ± 0.854 | 0.292 ± 0.004 | 0.550 ± 0.003 | 0.393 ± 0.002 |
| 0.50 mM | 0 | 0 | 6.70 ± 0.200 | 18.10 ± 0.400 | 26.73 ± 0.473 | 0.198 ± 0.004 | 0.425 ± 0.003 | 0.323 ± 0.002 |
| 0.50 mM | 10–7 M | 0 | 9.63 ± 0.404 | 22.60 ± 0.656 | 35.20 ± 0.361 | 0.251 ± 0.003 | 0.475 ± 0.005 | 0.374 ± 0.003 |
| 0.50 mM | 0 | 1 mM | 8.50 ± 0.300 | 19.00 ± 0.458 | 31.03 ± 0.416 | 0.233 ± 0.003 | 0.454 ± 0.004 | 0.359 ± 0.004 |
| 0.50 mM | 10–7 M | 1 mM | 10.43 ± 0.208 | 12.83 ± 0.611 | 39.70 ± 0.721 | 0.355 ± 0.004 | 0.487 ± 0.002 | 0.386 ± 0.005 |
| 0.75 mM | 0 | 0 | 4.90 ± 0.361 | 15.53 ± 0.351 | 20.83 ± 0.306 | 0.155 ± 0.001 | 0.416 ± 0.005 | 0.312 ± 0.004 |
| 0.75 mM | 10–7 M | 0 | 7.80 ± 0.265 | 18.03 ± 0.611 | 29.33 ± 0.208 | 0.205 ± 0.004 | 0.434 ± 0.003 | 0.336 ± 0.005 |
| 0.75 mM | 0 | 1 mM | 7.47 ± 0.321 | 15.53 ± 0.351 | 26.00 ± 0.794 | 0.195 ± 0.003 | 0.429 ± 0.004 | 0.327 ± 0.004 |
| 0.75 mM | 10–7 M | 1 mM | 9.53 ± 0.351 | 21.37 ± 0.758 | 34.43 ± 0.833 | 0.297 ± 0.003 | 0.416 ± 0.005 | 0.356 ± 0.004 |
| F-ratio(df 3, 32) treatment | 1324.9** | 1042.9** | 2103.7** | 2465.2** | 1710.5** | 2725.4** | ||
| F-ratio(df 3, 32) dose | 384.5** | 563.3** | 1365.7** | 1768.4** | 314.8** | 643.3** | ||
| F-ratio(df 9, 32) treatment × dose | 7.95** | 38.06** | 10.60** | 239.40** | 11.33** | 16.85** | ||
| HSD | 0.904 | 1.673 | 1.727 | 0.009 | 0.012 | 0.011 | ||
Table 2.
Effect of different concentrations of Pb (0.25 mM, 0.50 mM, and 0.75 mM), EBL (10–7 M), and SA (1 mM) and their interactions on intercellular CO2 concentration and transpiration rate of 30-, 60-, and 90-day-old plants of B. juncea L. *: P < 0.05, **: P < 0.01.
| Treatment | Cellular CO2 concentration (µmol mol–1) (mean ± SD) | Transpiration rate (mmol m2 s–1) (mean ± SD) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pb | EBL | SA | 30 DAS | 60 DAS | 90 DAS | 30 DAS | 60 DAS | 90 DAS | ||
| 0 | 0 | 0 | 304 ± 2.5 | 444 ± 3.0 | 273 ± 2.0 | 1.18 ± 0.061 | 2.10 ± 0.051 | 3.95 ± 0.132 | ||
| 0 | 10–7 M | 0 | 327 ± 5.8 | 463 ± 2.5 | 294 ± 3.512 | 1.39 ± 0.025 | 2.35 ± 0.035 | 4.44 ± 0.099 | ||
| 0 | 0 | 1 mM | 316 ± 5.6 | 456 ± 4.6 | 290 ± 4.5 | 1.29 ± 0.060 | 2.23 ± 0.085 | 4.17 ± 0.092 | ||
| 0 | 10–7 M | 1 mM | 354± 3.5 | 478 ± 4.0 | 305 ± 4.0 | 1.59 ± 0.035 | 2.58 ± 0.040 | 4.88 ± 0.070 | ||
| 0.25 mM | 0 | 0 | 255 ± 3.5 | 405 ± 3.5 | 246 ± 7.5 | 0.85 ± 0.003 | 1.54 ± 0.042 | 3.27 ± 0.057 | ||
| 0.25 mM | 10–7 M | 0 | 295 ± 4.0 | 426 ± 2.1 | 275 ± 2.5 | 0.88 ± 0.024 | 1.74 ± 0.050 | 3.75 ± 0.04 | ||
| 0.25 mM | 0 | 1 mM | 284 ± 3.5 | 424 ± 3.5 | 265 ± 2.5 | 0.88 ± 0.027 | 1.66 ± 0.114 | 3.68 ± 0.070 | ||
| 0.25 mM | 10–7 M | 1 mM | 305 ± 4.0 | 435 ± 2.1 | 285 ± 3.5 | 0.91 ± 0.004 | 1.84 ± 0.076 | 3.86 ± 0.046 | ||
| 0.50 mM | 0 | 0 | 242 ± 4.0 | 395 ± 5.0 | 225 ± 4.0 | 0.74 ± 0.006 | 0.99 ± 0.009 | 2.91 ± 0.066 | ||
| 0.50 mM | 10–7 M | 0 | 275 ± 3.0 | 415 ± 4.0 | 254 ± 3.1 | 0.80 ± 0.006 | 1.23 ± 0.072 | 3.50 ± 0.207 | ||
| 0.50 mM | 0 | 1 mM | 264± 4.0 | 402 ± 2.1 | 245 ± 3.0 | 0.76 ± 0.009 | 1.18 ± 0.023 | 3.29 ± 0.045 | ||
| 0.50 mM | 10–7 M | 1 mM | 271± 3.1 | 425 ± 3.0 | 272 ± 3.5 | 0.82 ± 0.012 | 1.33 ± 0.060 | 3.77 ± 0.057 | ||
| 0.75 mM | 0 | 0 | 217 ± 1.8 | 354 ± 3.5 | 211 ± 3.5 | 0.49 ± 0.010 | 0.73 ± 0.008 | 1.91 ± 0.091 | ||
| 0.75 mM | 10–7 M | 0 | 247 ± 2.0 | 382 ± 2.1 | 232 ± 2.5 | 0.58 ± 0.017 | 0.88 ± 0.018 | 2.63 ± 0.104 | ||
| 0.75 mM | 0 | 1 mM | 236 ± 5.0 | 378 ± 3.0 | 234 ± 3.5 | 0.55 ± 0.035 | 0.86 ± 0.018 | 2.46 ± 0.123 | ||
| 0.75 mM | 10–7 M | 1 mM | 263 ± 4.0 | 393 ± 7.6 | 284 ± 3.5 | 0.62 ± 0.006 | 1.05 ± 0.046 | 3.45 ± 0.183 | ||
| F-ratio(df 3, 32) treatment | 1017.9** | 991.2** | 396.3** | 1756.9** | 1998.8** | 616.1** | ||||
| F-ratio(df 3, 32) dose | 265.9** | 161.2** | 308.2** | 79.0** | 117.3** | 178.6** | ||||
| F-ratio(df 9, 32) treatment × dose | 8.66** | 2.50* | 16.64** | 18.72** | 3.99** | 14.6** | ||||
| HSD | 11.864 | 11.496 | 11.751 | 0.085 | 0.146 | 0.315 | ||||
3.4. Phenolic compounds
Increase in Pb concentration resulted in enhancement of phenolic contents including total phenols, flavonoids, and anthocyanin. The total phenol, flavonoid, and anthocyanin contents were elevated by 6.22%, 102.48%, and 139.63% in 30-day-old plants, respectively, in 0.75 mM Pb-treated plants in comparison to the control. Similar elevations by 81.81%, 55.06%, and 68.97% were recorded in 60-day-old plants and by 15.22%, 39.75%, and 159.04% in 90-day-old plants, respectively. Combined treatment with EBL and SA resulted in further enhancement in contents of phenolic compounds. Total phenols were elevated by 74.41% in 30-day-old plants, by 29.40% in 60-day-old plants, and by 88.19% in 90-dayold plants under 0.75 mM Pb stress with EBL and SA combination treatment. Similar elevations in flavonoid and anthocyanin contents were observed. They were elevated by 78.55% and 26.35% in 30-day-old plants, by 56.53% and 23.32% in 60-day-old plants, and by 109.32% and 39.81% in 90-day-old plants, respectively. Variation in contents of total phenol and flavonoids is represented in Table 3 and anthocyanin content is represented in Figure 3.
Table 3.
Effect of different concentrations of Pb (0.25 mM, 0.50 mM, and 0.75 mM), EBL (10–7 M), and SA (1 mM) and their interactions on total phenolic and flavonoid content of 30-, 60-, and 90-day-old plants of B. juncea L. *: P < 0.05, **: P < 0.01.
| Treatment | Total phenolic content (mg g–1 of DW) (mean ± SD) | Flavonoid content(mg g–1 of DW) (mean ± SD) | ||||||
|---|---|---|---|---|---|---|---|---|
| Pb | EBL | SA | 30 DAS | 60 DAS | 90 DAS | 30 DAS | 60 DAS | 90 DAS |
| 0 | 0 | 0 | 10.41 ± 0.373 | 12.91 ± 0.151 | 14.32 ± 0.979 | 0.525 ± 0.013 | 0.543 ± 0.085 | 0.722 ± 0.014 |
| 0 | 10–7 M | 0 | 13.63 ± 0.072 | 18.83 ± 0.180 | 18.99 ± 0.355 | 0.812 ± 0.012 | 0.847 ± 0.041 | 1.016 ± 0.076 |
| 0 | 0 | 1 mM | 10.84 ± 0.256 | 16.11 ± 0.284 | 16.99 ± 0.087 | 0.762 ± 0.090 | 0.639 ± 0.010 | 0.908 ± 0.050 |
| 0 | 10–7 M | 1 mM | 18.68 ± 0.219 | 20.89 ± 0.259 | 21.50 ± 0.293 | 1.108 ± 0.018 | 0.561 ± 0.035 | 1.289 ± 0.018 |
| 0.25 mM | 0 | 0 | 11.26 ± 0.155 | 18.16 ± 0.175 | 15.87 ± 0.222 | 0.660 ± 0.02 | 0.726 ± 0.135 | 0.689 ± 0.019 |
| 0.25 mM | 10–7 M | 0 | 16.92 ± 0.154 | 24.58 ± 0.276 | 18.46 ± 0.155 | 0.791 ± 0.009 | 0.900 ± 0.049 | 1.303 ± 0.026 |
| 0.25 mM | 0 | 1 mM | 13.81 ± 0.137 | 20.69 ± 0.615 | 16.76 ± 0.196 | 0.764 ± 0.031 | 0.666 ± 0.019 | 0.914 ± 0.070 |
| 0.25 mM | 10–7 M | 1 mM | 18.17 ± 0.098 | 26.31 ± 0.103 | 21.52 ± 0.123 | 0.919 ± 0.156 | 0.948 ± 0.011 | 1.189 ± 0.010 |
| 0.50 mM | 0 | 0 | 12.43 ± 0.154 | 20.35 ± 0.083 | 17.59 ± 0.231 | 0.893 ± 0.015 | 0.890 ± 0.079 | 0.729 ± 0.062 |
| 0.50 mM | 10–7 M | 0 | 16.35 ± 0.393 | 23.41 ± 0.118 | 21.69 ± 0.193 | 1.075 ± 0.011 | 1.034 ± 0.009 | 1.258 ± 0.023 |
| 0.50 mM | 0 | 1 mM | 13.41 ± 0.221 | 21.99 ± 0.154 | 19.16 ± 0.226 | 0.960 ± 0.046 | 0.923 ± 0.106 | 1.084 ± 0.023 |
| 0.50 mM | 10–7 M | 1 mM | 19.55 ± 0.264 | 26.75 ± 0.143 | 26.35 ± 0.165 | 1.748 ± 0.012 | 1.204 ± 0.016 | 1.513 ± 0.014 |
| 0.75 mM | 0 | 0 | 11.06 ± 0.190 | 23.47 ± 0.196 | 16.50 ± 0.136 | 1.063 ± 0.007 | 0.842 ± 0.084 | 1.009 ± 0.089 |
| 0.75 mM | 10–7 M | 0 | 16.34 ± 0.738 | 28.29 ± 0.122 | 22.88 ± 0.180 | 1.263 ± 0.013 | 1.221 ± 0.050 | 1.533 ± 0.061 |
| 0.75 mM | 0 | 1 mM | 13.50 ± 0.150 | 25.21 ± 0.154 | 20.43 ± 0.262 | 1.141 ± 0.011 | 1.015 ± 0.049 | 1.167 ± 0.029 |
| 0.75 mM | 10–7 M | 1 mM | 19.28 ± 0.190 | 30.36 ± 0.257 | 31.05 ± 0.761 | 1.898 ± 0.622 | 1.318 ± 0.011 | 2.112 ± 0.008 |
| F-ratio(df 3, 32) treatment | 135.4** | 2643.7** | 768.8** | 34.7** | 86.6** | 277.5** | ||
| F-ratio(df 3, 32) dose | 1974.1** | 2019.2** | 2122.1** | 34.5** | 86.4** | 620.6** | ||
| F-ratio(df 9, 32) treatment × dose | 32.27** | 101.27** | 106.39** | 2.43* | 2.64* | 36.62** | ||
| HSD | 0.819 | 0.720 | 0.873 | 0.491 | 0.187 | 0.136 | ||
Figure 3.
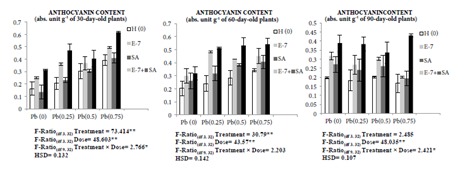
Effect of different concentrations of Pb (0.25 mM, 0.50 mM, and 0.75 mM), EBL (10–7 M), and SA (1 mM) and their combinations on anthocyanin content of 30-, 60-, and 90-day-old plants of Brassica juncea L. (values show the means of three replicates ± SD; Tukey’s test was performed and significance was checked at P ≤ 0.05 designated with * and at P ≤ 0.01 designated with **).
3.5. Gene expression analysis
The observations on gene expression analysis reflected a drastic elevation in CHLASE gene expression in 0.75 mM Pb-treated plant samples in comparison to the control. An enhancement of 3.211-fold was observed in the expression of the CHLASE gene. Similar elevations in expression of the CHS and PAL genes were observed at 2.483-fold and 2.297-fold, respectively. Gene expression analysis of the PSY gene showed a decline of 0.822-fold in 0.75 mM Pb-treated plants in comparison to the control. Treatment with the combination of EBL and SA in 0.75 mM Pbstressed plants resulted in lowering the expression of the CHLASE gene by 1.879-fold in comparison to plants treated with 0.75 mM Pb alone. Expression of the PSY, CHS, and PAL genes were further enhanced by 6.357fold, 6.053-fold, and 6.042-fold, respectively, in similar treatments (Figure 4).
Figure 4.
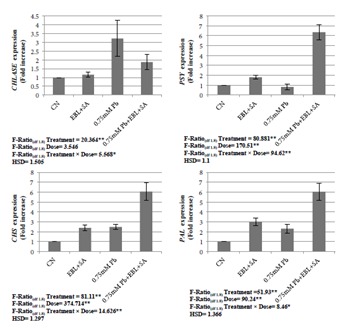
Effect of different concentrations of Pb (0.25 mM, 0.50 mM, and 0.75 mM) and combination of EBL (10–7 M) and SA (1 mM) on gene expression analysis of CHLASE, PSY, CHS, and PAL genes of 30-day-old plants of Brassica juncea L. (values show the means of three replicates ± SD; Tukey’s test was performed and significance was checked at P ≤ 0.05 designated with * and at P ≤ 0.01 designated with **).
4. Discussion
Lead, highly toxic, is the most abundant ubiquitously present metal in soil (Andra et al., 2010) . It is accumulated in aboveground parts, especially leaves, and subsequently sequestered in tissues, such as in stomatal enzymes, acyl lipid membranes, and chloroplast structures, resulting in modulation of PSI and PSII (Liu et al., 2015). The present results showed that Pb treatment resulted in significant decline in the growth of shoots and roots. The results accorded with the studies of Lamhamdi et al. (2013) in wheat and spinach plants under Pb-stressed conditions. This might have resulted from a decline in the uptake of various essential elements such as Mg, Ca, Na, and K and inhibition of cellular metabolism including cell division and expansion (Jazi et al., 2011). Specifically, Pb has been reported to damage the ultrastructure of the microtubules present in actively dividing meristamatic root cells (Jiang et al., 2010). It was also revealed in the present investigation that combined treatment with EBL and SA led to enhancement in the growth of stressed plants, which corroborates with the findings of Jazi et al. (2011) in Brassica napus and Dong et al. (2015) in Gossypium hirsutum plants. The enhancement in growth by exogenous application of EBL in the present study might be due to its ability to improve cell division, expansion, and elongation (Shahid et al., 2011) .
Total chlorophyll and carotenoid content was recorded to be lowered as a result of the increase in the concentration of Pb, a pattern similar to the findings of Bhatti et al. (2013), who showed significant decline in total chlorophyll, chlorophyll a, and chlorophyll b contents in various wheat varieties under Pb metal stress. A strong decline in carotenoid content in cotton plants under Pb metal stress was reported by Bharwana et al. (2014). According to Bhatti et al. (2013), decline in chlorophyll contents can possibly be due to Pb toxicity, which resulted in inclusion of Fe ions instead of Mg ions in the central phytoporphyrin ring of chlorophyll, consequently resulting in imbalance in nutrient uptake such as Mg and Fe, which eventually results in reduction in chlorophyll content. Sarangthem et al. (2011) suggested that a decline in chlorophyll content might be due to downregulation of synthesis of δ-amino levulinic acid dehydratase (ALA-dehydratase) as well as protochlorophyllide reductase, both enzymes essential for de novo synthesis of chlorophyll. Similarly lowered ALAdehydratase activity was observed in radish leaves under Pb, Hg, Zn, and Cd stress (Morsch et al., 2002) . The decline in contents of plant pigments in the present study could be interrelated with the enhancement in expression of the CHLASE gene (chlorophyllase enzyme) and the decline in the PSY gene (phytoene synthase) in the present study.
Combined treatment with EBL and SA in stressed plants resulted in an elevation in the content of chlorophyll and carotenoids. Similar reports of enhancement in chlorophyll contents were accounted by Verma et al. (2012) in groundnut supplemented with EBL. BRs also promote xylem differentiation by increasing the sensitivity of plants to auxin, which in turn increases the photosynthetic efficiency of the plants. In addition to this, they have a role in several light- and hormone-regulated photosynthetic metabolisms such as expression of light-regulated genes, promotion of cell elongation, and floral induction (Rashid, 1991) . Moreover, Gaballah and Rady (2012) reported that exogenous application of SA ameliorates the harmful effects of Pb metal on chlorophyll content and further suggested that the mitigating effect of SA at the seedling stage is due to its ameliorative role in reducing the accumulation of ROS. The decline in expression of the CHLASE gene with combined treatment of EBL and SA led to a corresponding decline in chlorophyllase activity, which eventually resulted in increased chlorophyll content in the present study. Also, exogenous application of EBL and SA led to enhancement in carotenoid content. Elevation in carotenoid contents might be due to enhanced expression of the PSY gene.
Retardation in contents of gas exchange parameters were recorded in response to Pb metal stress. Similar retardation in contents of gas exchange parameters was reported by Bharwana et al. (2014) in cotton seedlings. It was revealed that increase in Pb stress (100 µM) resulted in decline in gas exchange parameters including photosynthetic rate, stomatal conductance, and transpiration rate. Khan et al. (2015) suggested a decline in all gas exchange attributes including cellular CO2, stomatal conductance, net photosynthetic rate, and transpiration rate in tomato plants under abiotic stress conditions. Another reason for lowered photosynthetic rate is damage caused to photosynthetic pigments eventually resulting in lowered light-absorbing capacity of both photosystems (Zhang et al., 2011) . The present study revealed that the combined treatment of EBL and SA resulted in further enhancement in all gas exchange parameters. Similar observations of enhancement in photosynthetic gas exchange parameters were reported by
Xia et al. (2009) in Cucumis sativus plants by exogenous application of BRs. Enhancement in parameters such as photosynthetic rate by 29% and stomatal conductance by 18% with exogenous supplementation with EBL in saltstressed pea plants was reported by Shahid et al. (2011) .
Upregulation of net CO2 assimilation was reported in mustard seedlings supplemented with 10 µM SA.
Farriduddin et al. (2003) suggested that the increase in net photosynthetic rate might be due to enhanced chlorophyll content and activity of photosynthetic enzymes such as nitrate reductase and carbonic anhydrase.
Phenolic compounds are believed to be the most significant group of secondary metabolites (Jung et al., 2003). The increase in total phenol content in response to Pb treatment can be attributed to enhanced levels of enzymes involved in de novo synthesis of phenolic compounds (Winkel-Shirley, 2002) . Flavonoid content was also observed to be enhanced in response to Pb metal treatment in a dose-dependent manner. Flavonoids primarily function as antimetal toxic antioxidants and bioutilizers of metals. They form conjugates with metal complexes and transition metals (Michalak, 2006) . They also play protective roles against various environmental stresses by scavenging ROS (Mourato et al., 2012) .
According to Korkina (2007), the enhanced levels of phenolic compounds might be due to metal-induced activation of the phenylpropanoid pathway. Exogenous treatment with the combination of EBL and SA led to further enhancement in contents of total phenols in stressed plants. BRs have been reported to trigger accumulation of phenolic compounds under Cr stress (Choudhary et al., 2011) in plants. Enhanced phenolic compound content in response to EBL treatment might be due to lowered ROS accumulation under Pb stress by enhancing the antioxidative capacity of plants. Recent reports also suggested the role of SA in the synthesis of secondary metabolites (Idrees et al., 2013). Chalcone synthase (CHS) plays an imperative role in de novo synthesis of anthocyanins, and in the present experiment, the expression of CHS was observed to be increased by combined treatment with EBL and SA. The enhanced contents of anthocyanins might be due to the modulation of CHS by EBL and SA. Additionally, Lauan et al. (2013) also reported that BRs upregulated the genes that are responsible for the biosynthesis of anthocyanins.
Based on the presented data, it is suggested that Pb has toxic effects on growth parameters, photosynthetic attributes, and gene expression. It is obvious from the results that Pb treatment, even at low concentrations, induces large disturbances in the growth as well as physiology of plants, including a decline in photosynthetic pigments and ion uptake, consequently resulting in reduced growth. It was also demonstrated from the results that BRs and SA can be effectively used for alleviating Pb metal stress in B. juncea plants. Future studies will be aimed at searching for the mechanisms responsible for improved protection of B. juncea plants against deleterious Pb metal stress and insight into the interactive role of EBL and SA application in stressed plants.
Acknowledgments
This work was carried out under the financial assistance provided by the University Grant Commission, Government of India (Maulana Azad National Fellowship). The researchers are also thankful to the Department of Science and Technology, Government of India, for supporting the department with a DST-FIST grant.
References
- Ahmad P , Bhardwaj R , Tuteja N ( 2011. ). Plant signaling under abiotic stress environment . In: Ahmad P, Prasad MNV , editors. Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change . Berlin, Germany: Springer, pp. 297 - 323 .
- Andra SS , Datta R , Sarkar D , Makris KC , Mullens CP , Sahi SV , Bach SB ( 2010. ). Synthesis of phytochelatins in vetiver grass upon lead exposure in the presence of phosphorus . Plant Soil 326 : 171 - 185 . [Google Scholar]
- Arnon DT ( 1949. ). Copper enzymes in isolated chloroplast polyphenol oxidase in Beta vulgaris . Plant Physiol 24 : 1 - 15 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M , Harris PJC ( 2013. ). Photosynthesis under stressful environments: an overview . Photosynthetica 51 : 163 - 190 . [Google Scholar]
- Bajguz A ( 2010. ). An enhancing effect of exogenous brassinolide on the growth and antioxidant activity in Chlorella vulgaris cultures under heavy metals stress . Environ Exp Bot 68 : 175 - 179 . [Google Scholar]
- Bharwana SA Ali S Farooq MA Iqbal N Hameed A Abbas F Ahmad MS Glycine betaine-induced lead toxicity tolerance related to elevated photosynthesis, antioxidant enzymes suppressed lead uptake and oxidative stress in cotton. Turk J Bot. 2014;38:281–292. [Google Scholar]
- Bhatti KH Anwar S Nawaz AK Hussain K Siddiqi EJ Sharif RU Talat A Khalid A Effect of heavy metal lead (Pb) stress of different concentration on wheat (Triticum aestivum L.). Middle East J Sci Res. 2013;14:148–154. [Google Scholar]
- Choudhary SP Kanwar M Bhardwaj R Gupta BD Gupta RK Epibrassinolide ameliorates Cr (VI) stress via inuflencing the levels of indole-3-acetic acid, abscisic acid, polyamines and antioxidant system of radish seedlings. Chemosphere. 2011;84:592–600. doi: 10.1016/j.chemosphere.2011.03.056. [DOI] [PubMed] [Google Scholar]
- Deng XG Zhu T Peng XJ Xi DH Guo H Yin Y Zhang DW Lin DW Role of brassinosteroid signaling in modulating tobacco mosaic virus resistance in Nicotiana benthamiana. Sci Rep. 2016;6:20579. doi: 10.1038/srep20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divi UK Rahman T Krishna P Brassinosteroid-mediated stress tolerance in Arabidopsis shows interactions with abscisic acid, ethylene and salicylic acid pathways. BMC Plant Biol. 2010;10:151. doi: 10.1186/1471-2229-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong YJ Wang ZL Zhang JW Liu S He ZL He MR Interaction effects of nitric oxide and salicylic acid in alleviating salt stress of Gossypium hirsutum L. J Soil Sci Plant Nutr. 2015;15:561–573. [Google Scholar]
- Fariduddin Q Hayat S Ahmad A Salicylic acid influences net photosynthetic rate, carboxylation efficiency, nitrate reductase activity, and seed yield in Brassica juncea. Photosynthetica. 2003;41:281–284. [Google Scholar]
- Gaballah MS Rady MM Salicylic acid mitigated cadmium toxicity by attenuating the oxidative stress in pea (Pisum sativum L.) plants. Int J Biol Eco Environ Sci. 2012;1:159–165. [Google Scholar]
- Garg N Aggarwal N Effects of interactions between cadmium and lead on growth, nitrogen fixation, phytochelatin, and glutathione production in mycorrhizal Cajanus cajan (L.) Millsp. J Plant Growth Regul. 2011;30:286–300. [Google Scholar]
- Idrees M Naeem M Aftab T Khan MM Salicylic acid restrains nickel toxicity, improves antioxidant defence system and enhances the production of anticancer alkaloids in Catharanthus roseus (L.). J Hazard Mat. 2013;252:367–374. doi: 10.1016/j.jhazmat.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Jazi SB Yazdi HL Ranjbar M Effect of salicylic acid on some plant growth parameters under lead stress in Brassica napus var. Okapi. Iran J Plant Physiol. 2011;1:177–185. [Google Scholar]
- Jiang N Luo X Zeng J Yang ZR Zheng LY Wang ST Lead toxicity induced growth and antioxidant responses in Luaf cylindrical seedlings. Int J Agric Biol. 2010;12:205–210. [Google Scholar]
- Jung C Maeder V Funk F Frey B Sticher H Frossard E Release of phenols from Lupinus albus L. roots exposed to Cu and their possible role in Cu detoxification. Plant Soil. 2003;252:301–312. [Google Scholar]
- Khan MS Kanwal B Nazir S Metabolic engineering of the chloroplast genome reveals that the yeast ArDH gene confers enhanced tolerance to salinity and drought in plants. Front Plant Sci. 2015;6:725. doi: 10.3389/fpls.2015.00725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamhamdi M El Galiou O Bakrim A Nóvoa-Muñoz JC Arias-Estévez M Aarab A Lafont R Effect of lead stress on mineral content and growth of wheat (Triticum aestivum) and spinach (Spinacia oleracea) seedlings. Saudi J Biol Sci. 2013;20:29–36. doi: 10.1016/j.sjbs.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkina LG Phenylpropanoids as naturally occurring antioxidants: from plant defense to human health. Cell Mol Biol. 2007;53:15–25. [PubMed] [Google Scholar]
- Lee SY Damodaran PN Roh KS Influence of salicylic acid on rubisco and rubisco activase in tobacco plants grown under sodium chloride in-vitro. Saudi J Biol Sci. 2014;27:417–426. doi: 10.1016/j.sjbs.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T Sheng M Wang CY Impact of arbuscular mycorrhizal fungi on the growth, water status, and photosynthesis of hybrid poplar under drought stress and recovery. Photosynthetica. 2015;53:250–258. [Google Scholar]
- Livak KJ Schmittgen TD Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luan LY , Zhang ZW , Xi ZM , Huo SS , Ma LN ( 2013. ). Brassinosteroids regulate anthocyanin biosynthesis in the ripening of grape berries . South Afri J Enol Viticul 34 : 196 - 203 . [Google Scholar]
- Maclachlan C , Zalik S ( 1963. ). Plastid structure, chlorophyll concentration and free amino acid composition of a chlorophyll mutant of barley . Can J Bot 41 : 1053 - 1062 . [Google Scholar]
- Mancinelli AL ( 1984. ). Photoregulation of anthocyanin synthesis . VIII. Effects of light pretreatments . Plant Physiol 75 : 447 - 453 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComb J , Hentz S , Miller GS , Begonia M ( 2012. ). Effects of lead on plant growth, lead accumulation and phytochelatin contents of hydroponically-grown Sesbania exaltata . World Environ 2 : 38 - 43 . [Google Scholar]
- Michalak A ( 2006. ). Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress . Pol J Environ Stud 15 : 523 - 530 . [Google Scholar]
- Mittal S , Kumari N , Sharma V ( 2012. ). Diefrential response of salt stress on Brassica juncea: Photosynthetic performance, pigment, proline, D1 and antioxidant enzymes . Plant Physiol Biochem 54 : 17 - 26 . [DOI] [PubMed] [Google Scholar]
- Morsch VM , Schetinger MRC , Martins AF , Rocha JBT ( 2002. ). Effects of cadmium, lead, mercury and zinc on δ-aminolevulinic acid dehydratase activity from radish leaves . Biol Plant 45 : 85 - 89 . [Google Scholar]
- Mourato M , Reis R , Martins LL ( 2012. ). Characterization of plant antioxidative system in response to abiotic stresses: a focus on heavy metal toxicity . In: Montanaro G, editor. Advances in Selected Plant Physiology Aspects. Rijeka , Croatia: InTech, pp. 23 - 44 .
- Pan J , Lin S , Woodbury NW ( 2012. ). Bacteriochlorophyll excited state quenching pathways in bacterial reaction centers with the primary donor oxidized . J Phys Chem B 116 : 2014 - 2022 . [DOI] [PubMed] [Google Scholar]
- Pirzad A , Shakiba MR , Zehtab-Salmasi S , Mohammadi SA , Darvishzadeh R , Samadi A ( 2011. ). Effect of water stress on leaf relative water content, chlorophyll, proline and soluble carbohydrates in Matricaria chamomilla L . J Med Plant Res 5 : 2483 - 2488 . [Google Scholar]
- Rahnama A , Poustini K , Tavakkol-Afshari R , Tavakoli A ( 2010. ). Growth and stomatal responses of bread wheat genotypes in tolerance to salt stress . Int J Biol Life Sci 6 : 216 - 221 . [Google Scholar]
- Rashid A , Bernier M , Pazdernick L , Carpentier R ( 1991. ). Interaction of Zn2+ with the donor side of photosystem II . Photosyn Res 30 : 123 - 130 . [DOI] [PubMed] [Google Scholar]
- Sarangthem J , Jain M , Gadre R ( 2011. ). Inhibition of δ-aminolevulinic acid dehydratase activity by cadmium in excised etiolated maize leaf segments during greening . J Plant Soil Environ 57 : 332 - 337 . [Google Scholar]
- Shahid MA , Pervez MA , Balal RM , Mattson NS , Rashid A , Ahmad R , Ayyub CM , Abbas T ( 2011. ). Brassinosteroid (24-Epibrassinolide) enhances growth and alleviates the deleterious effects induced by salt stress in pea (Pisum sativum L.) . Aust J Crop Sci 5 : 500 - 510 . [Google Scholar]
- Sharma A , aThkur S , Kumar V , Kesavan AK , uThkral AK , Bhardwaj R ( 2017. ). 24 - Epibrassinolide stimulates imidacloprid detoxification by modulating the gene expression of Brassica juncea L . BMC Plant Biol 17 : 56 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma I , Pati PK , Bhardwaj R ( 2011. ). Effect of 24-epibrassinolide on oxidative stress markers induced by nickel-ion in Raphanus sativus L. Acta Physiol Plant 33 : 1723 - 1735 . [Google Scholar]
- Sharma P , Bhardwaj R , Arora HK , Arora N ( 2008. ). Effect of 28-homobrassinolide on nickel uptake, protein content and antioxidative defense system in Brassica juncea L . Biol Plant 52 : 767 - 770 . [Google Scholar]
- Singleton VL , Rossi JA ( 1965. ). Colorimetry of total phenolics with phosphomolybdicphosphotungstic acid reagents . Am J Enol Vitic 16 : 144 - 158 . [Google Scholar]
- Stefanov K , Seizova K , Popova I , Petkov V , Kimenov G , Popov S ( 1995. ). Effect of lead ions on the phospholipid composition in leaves of Zea mays and Phaseolus vulgaris . J Plant Physiol 147 : 243 - 246 . [Google Scholar]
- Verma A , Malik CP , Gupta VK ( 2012. ). In vitro effects of brassinosteroids on the growth and antioxidant enzyme activities in groundnut . ISRN Agronomy 2012. : 356485 .
- Vicente MR , Plasencia J ( 2011. ). Salicylic acid beyond defence: its role in plant growth and development . J Exp Bot 62 : 3321 - 3338 . [DOI] [PubMed] [Google Scholar]
- Winkel-Shirley B ( 2002. ). Biosynthesis of flavonoid and effects of stress . Curr Opin Plant Biol 5 : 218 - 223 . [DOI] [PubMed] [Google Scholar]
- Xia XJ , Huang LF , Zhou YH ( 2009. ). Brassinosteroids promote photosynthesis and growth by enhancing activation of Rubisco and expression of photosynthetic genes in Cucumis sativus . Planta 230 : 1185 - 1196 . [DOI] [PubMed] [Google Scholar]
- Zhang H , Gong G , Guo S , Ren Y , Xu Y , Ling KS ( 2011. ). Screening the USDA watermelon germplasm collection for drought tolerance at the seedling stage . Hort Science 46 : 1245 - 1248 . [Google Scholar]
- Zhishen J , Mengcheng T , Jianming W ( 1999. ). The determination of lfavonoid contents in mulberry and their scavenging effects on superoxide radicals . Food Chem 64 : 555 - 559 . [Google Scholar]


