Myoclonus is a disabling disorder with a heterogenous etiology in which the causing structure can range from the peripheral nervous system to the cerebral cortex.1 Its diagnosis is often challenging especially because of the dynamic, and sometimes infrequent, character of the myoclonic jerks. The use of additional neurophysiological investigations can contribute to the diagnostic process.2 Brainstem reticular myoclonus has been described in postanoxic patients, and electrophysiological characteristics have only been described in a few cases. In this report, we present a remarkable case of symptomatic unilateral reticular myoclonus, with electrophysiological and imaging data compatible with a brainstem generator.3
Case
A 55‐year‐old male patient presented with progressive right‐sided jerks. The jerks started abruptly 18 months ago and gradually intensified until approximately 100 times a day. Prodromal symptoms were not perceived nor could the jerk be suppressed. The jerk could occur at every moment of the day and interfered with activities of daily living, such as eating and drinking. The jerks could not be induced by action or by auditory or sensory stimuli. The patient had a past medical history of hypothyroidism and hypertension for which he received levothyroxine and metoprolol, respectively. An obstructive sleep apnea syndrome was diagnosed 4 years ago and improved after tonsillectomy. Family history was negative for myoclonus. Neurological examination revealed no focal abnormalities, but infrequent short‐lasting flexion jerks of the right arm with co‐occurring flexion of the right hip. Directly after the jerks, the leg was positioned in endorotation for a few seconds. Differential diagnosis included cortical myoclonus, functional myoclonus, and reticular myoclonus. A combined polymyography of the right arm and leg with EEG was performed (see Video). No cortical preceding potential compatible with cortical myoclonus or a readiness potential was found after jerk‐locked back‐averaging of 103 segments. Additional somatosensory‐evoked potential (SSEP) analysis revealed no “giant SSEP” associated with cortical myoclonus.2 A successive, more exhaustive, polymyography revealed a myoclonus with brief burst duration between 50 and 80 ms with a consistent craniocaudal recruitment sequence.1, 2 After averaging of 37 epochs (with the right trapezius muscle as a reference), a consistent recruitment sequence was noted in which subsequently the lower brainstem musculature (trapezius, N XI), other lower brainstem musculature (sternocleidomastoid, N XI), higher brainstem musculature (orbicularis oris, N VII), and muscles of the arm and leg (with a proximal‐distal gradient) were activated (Fig. 1B). The onset of muscle recruitment was based on the first negative deflection of the electromyography (EMG) signals larger than noise level. However, for the masseter muscle, no onset could be determined because of artefacts. The reproducibility of this recruitment sequence was confirmed by “odd‐even” averaging in which the averaged 19 uneven and deaveraged 18 even epochs showed almost similar potentials, compared to all averaged epochs. This characteristic recruitment sequence strongly suggests a lower brainstem origin of the jerks and is in line with the recruitment sequence of reticular myoclonus.3, 4 The rapid efferent conduction velocity of approximately 40 m/s supported this and was in line with a pyramidal conduction velocity associated with reticular myoclonus.5
Figure 1.
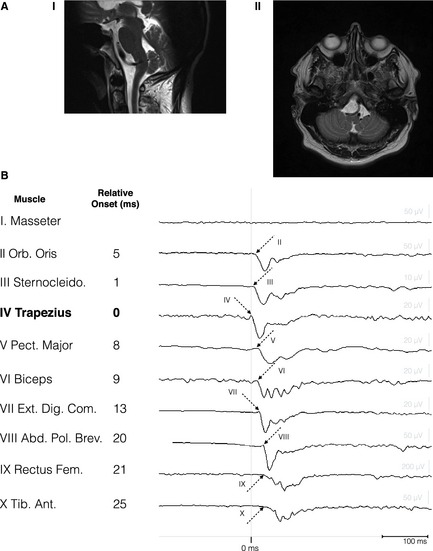
(A) Saggital (1) and transversal (2) T2‐weighted MR images showing elongation of the left vertebral artery with left‐sided compression of the lateral medulla oblongata. (B) Recruitment sequence of 10‐channel polymyography. Right‐sided muscles are specified on the left. Arrows with corresponding (latin) muscle number represent the onset of muscle recruitments. Corresponding latencies (in ms), relative to the trapezius muscle (bold), are depicted on the right.
MRI of the brain revealed an elongation and dilatation of the left vertebral artery (dolichoectasia), which impressed and displaced the medulla oblongata (Fig. 1A). Clonazepam was started (daily dose: 1 mg) and reduced the burden of the myoclonus by approximately 50%.
Discussion
In this unique case report, we show the electrophysiological features of a unilateral reticular myoclonus resulting from vascular compression of the lower brainstem. It is the first time that such a clear anatomical correlation has been shown in this type of myoclonus.
Given its direct anatomical relation with the ventral medulla oblongata, where the pyramidal tract runs, and the neurophysiological findings compatible with a reticular myoclonus, we postulated that this appears to be the most obvious cause of the hemimyoclonus.
Reticular Myoclonus
Brainstem myoclonus is a type of myoclonus originating from the lower brainstem. The hallmark of brainstem myoclonus is an initial muscle activation in muscles innervated in the lower brainstem (sternocleidmastoid or trapezius muscles, NXI) followed by a sequence of both higher brainstem innervated muscles and muscles innervated further down the spinal cord.6 A reflex closely related to the reticular reflex is the startle reflex, also originating in the lower brainstem. The electrophysiological difference is the efferent conduction time. In the reticular myoclonus, these spinal efferent velocities are rapid compatible with a rapid conduction through the pyramidal tract.4 In the startle reflex, the conduction time is slower, and in addition, the latency to the hand muscles is relatively delayed.
Reticular myoclonus was first described by Hallett et al.3 In total, 4 cases have been described in which an anoxic etiology was present.3, 4, 7, 8 Our case appears to be the first case of reticular myoclonus with a clear anatomical anomaly, namely, a dolichovertebral artery, that may very well cause the myoclonus. In the literature, 1 case has been described with vertebral artery dolichoectasia associated with unilateral cervical and facial myoclonus.6 However, this was a more focal myoclonus without the spreading of a reticular myoclonus. To our knowledge, this is the first case with documented electrophysiological data in which reticular myoclonus has a cause other than hypoxia.3, 4, 7, 8
In our patient, the reticular myoclonus was unilateral. In the other described cases, myoclonus was either described as generalized or symmetrical.3, 4, 7, 8 This might be because of the fact that only unilateral compression was present in our case. This unique unilateral activation pattern supports the relation with the vascular compression and therefore the clear origin in the lower brainstem of reticular myoclonus.
Stimulus sensitivity was absent in our case and contrasts with the description in previously described cases.3, 4, 7, 8 The absence of reflex sensitivity might be because of the fact that stimulation comes from direct compression and not from generalized, putatively GABAA‐based, disinhibition, which is present in posthypoxic myoclonus.9 Possibly, the compression could spare the disinhibited afferent component of reticular myoclonus. This is further supported by other disinhibition phenomenon, namely, co‐occurring cortical myoclonus, in patients with posthypoxic reticular myoclonus.4
Dolichoectasias
Dolichoectasia means both elongation and dilatation of an artery resulting from weakening of the tunica media and is associated with vascular risk factors, such as hypertension. Dolichoectasia most often occurs in the vertebrobasilar arteries,10, 11 and cranial nerves or the brainstem can be compressed as in our patient. Endovascular reconstruction of the dolichoectasia or microvascular decompression could be considered.12 However, given the positive effect of clonazepam and the risks associated with an endovascular reconstruction, we retained the patient from surgery at this point.
Conclusion
Reticular myoclonus is rare in patients without an anoxic period. Electrophysiological testing is helpful in making the diagnosis and important to determine the cause of the myoclonus and best treatment options. Our finding of a brainstem compression supports the initial of hypothesis of hyperactivity in the reticular formation and medulla oblongata by Hallett et al.3
Author Roles
(1) Case Report Project: A. Conception, B. Organization, C. Execution; (2) Manuscript: A. Writing of the First Draft, B. Review and Critique.
M.B.: 1C, 2A
J.W.J.E.: 1C, 2B
M.W.C.v.d.B.: 2B
M.U.: 1C, 2B
M.A.J.T.: 1A, 1C, 2B
Disclosures
Funding Sources and Conflicts of Interest: There is no conflict of interest with any financial organization from any of the authors regarding the material discussed in the manuscript.
Financial Disclosures for previous 12 months: M.B., J.W.J.E., M.U., and M.A.J.T. were employed at the University Medical Center in Groningen and M.W.C.v.d.B. at the St. Jansdal Hospital during the past 12 months.
Supporting information
Video. Simultaneous video (right) and EEG‐EMG coregistration (left) of hemimyoclonic jerks. EEG comprises the upper 19 channels in a 10 to 20 montage, EMG the nine channels below in a craniocaudal sequence. Additional accelerometers (ACC1 and ACC2) and electrocardiography analyses comprise the lower channels. The time base between vertical bars is 1 second.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Shibasaki H, Thompson PD. Milestones in myoclonus. Mov Disord 2011;26:1142–1148. DOI: 10.1002/mds.23673. [DOI] [PubMed] [Google Scholar]
- 2. Caviness JN, Brown P. Myoclonus: current concepts and recent advances. Lancet Neurol 2005;3:598–607. DOI: 10.1016/S1474-4422(04)00880-4. [DOI] [PubMed] [Google Scholar]
- 3. Hallett M, Chadwick D, Adam J, Marsden CD. Reticular reflex myoclonus: a physiological type of human post‐hypoxic myoclonus. J Neurol Neurosurg Psychiatr 1977;40:253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown P, Thompson PD, Rothwell JC, Day BL, Marsden CD. A case of postanoxic encephalopathy with cortical action and brainstem reticular reflex myoclonus. Mov Disord 1991;6:139–144. DOI: 10.1002/mds.870060209. [DOI] [PubMed] [Google Scholar]
- 5. Brown P, Rothwell JC, Thompson PD, Britton TC, Day BL, Marsden CD. The hyperekplexias and their relationship to the normal startle reflex. Brain 1991;114(Pt 4):1903–1928. [DOI] [PubMed] [Google Scholar]
- 6. Muñoz EJ, Vila N, Valls‐Solé J, Tolosa E. Cervical and facial myoclonus associated with dolichoectasia of the left vertebral artery. Mov Disord 1997;12:790–793. DOI: 10.1002/mds.870120529. [DOI] [PubMed] [Google Scholar]
- 7. Inoue M, Kojima Y, Kinboshi M, Kanda M, Shibasaki H. A case of post‐anoxic reticular reflex myoclonus. Rinsho Shinkeigaku 2012;52:557–560. [DOI] [PubMed] [Google Scholar]
- 8. Oguro K, Kobayashi J, Aiba H, Kobayashi S, Hojo H. Electrographic study of brainstem reflex myoclonus. Electromyogr Clin Neurophysiol 1997;37:99–106. [PubMed] [Google Scholar]
- 9. Hallett M. Physiology of human posthypoxic myoclonus. Mov Disord 2000;15(Suppl 1):8–13. [DOI] [PubMed] [Google Scholar]
- 10. Passero SG, Rossi S. Natural history of vertebrobasilar dolichoectasia. Neurology 2008;70:66–72. DOI: 10.1212/01.wnl.0000286947.89193.f3. [DOI] [PubMed] [Google Scholar]
- 11. Ubogu EE, Zaidat OO. Vertebrobasilar dolichoectasia diagnosed by magnetic resonance angiography and risk of stroke and death: a cohort study. J Neurol Neurosurg Psychiatr 2004;75:22–26. [PMC free article] [PubMed] [Google Scholar]
- 12. Wu X, Xu Y, Hong B, Zhao W‐Y, Huang Q‐H, Liu J‐M. Endovascular reconstruction for treatment of vertebrobasilar dolichoectasia: long‐term outcomes. AJNR Am J Neuroradiol 2013;34:583–588. DOI: 10.3174/ajnr.A3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video. Simultaneous video (right) and EEG‐EMG coregistration (left) of hemimyoclonic jerks. EEG comprises the upper 19 channels in a 10 to 20 montage, EMG the nine channels below in a craniocaudal sequence. Additional accelerometers (ACC1 and ACC2) and electrocardiography analyses comprise the lower channels. The time base between vertical bars is 1 second.


