Abstract
Background
The Burke‐Fahn‐Marsden Dystonia Rating Scale is a universally applied instrument for the quantitative assessment of dystonia in both children and adults. However, immature movements by healthy young children may also show “dystonic characteristics” as a consequence of physiologically incomplete brain maturation. This could implicate that Burke‐Fahn‐Marsden scale scores are confounded by pediatric age.
Objective
In healthy young children, we aimed to determine whether physiologically immature movements and postures can induce an age‐related effect on Burke‐Fahn‐Marsden movement and disability scale scores.
Methods
Nine assessors specializied in movement disorders (3 adult neurologists, 3 pediatric neurologists, and 3 MD/PhD students) independently scored the Burke‐Fahn‐Marsden movement scale in 52 healthy children (4–16 years of age; 2 boys and 2 girls per year of age). Independent of that, parents scored their children's functional motor development according to the Burke‐Fahn‐Marsden disability scale in another 52 healthy children (4–16 years of age; 2 boys and 2 girls per year of age). By regression analysis, we determined the association between Burke‐Fahn‐Marsden movement and disability scales outcomes and pediatric age.
Results
In healthy children, assessment of physiologically immature motor performances by the Burke‐Fahn‐Marsden movement and disability scales showed an association between the outcomes of both scales and age (until 16 years and 12 years of age, β = −0.72 and β = −0.60, for Burke‐Fahn‐Marsden movement and disability scale, respectively [both P < 0.001]).
Conclusions
The Burke‐Fahn‐Marsden movement and disability scales are influenced by the age of the child. For accurate interpretation of longitudinal Burke‐Fahn‐Marsden Dystonia Rating Scale scores in young dystonic children, consideration of pediatric age‐relatedness appears advisory.
Keywords: Burke‐Fahn‐Marsden Dystonia Rating Scale, child, dystonia, validation, age
Dystonia is a movement disorder characterized by sustained or intermittent muscle contractions, causing abnormal, often repetitive, movements or postures.1, 2, 3 The neuroanatomical substrate for dystonia is ascribed to dysfunctional networks of the basal ganglia, cerebellum, thalamus, cerebral cortex, and brainstem.4 The term early onset dystonia is used to denounce the initiation of dystonia before the twenty‐sixth year of life.1 As the characterization spans distinctly different developmental stages, pediatric subdivision into subgroups of infancy (0–2 years), childhood (3–12 years), and adolescence (13–20 years) has been advocated.1
The Burke‐Fahn‐Marsden Dystonia Rating Scale (BFMDRS) is a universally applied biomarker for the severity of dystonia. The scale consists of a movement and disability subscale (Burke‐Fahn‐Marsden Movement Scale [BFMMS] and Burke‐Fahn‐Marsden Disability Scale [BFMDS], respectively).5 The BFMMS measures dystonia in nine body regions (including the eyes, mouth, speech and swallowing, neck, trunk, arms, and legs) with scores ranging from 0 (minimum) to 120 (maximum). The BFMDS is a functional marker consisting of parental‐ or self‐reported daily activities (involving speech, handwriting, feeding, eating, swallowing, hygiene, dressing, and walking), with scores ranging from 0 (completely independent) to 30 (completely dependent). Although BFMDRS was originally developed as an instrument for the measurement of primary torsion dystonia in adults, the scale is now uniformly being applied to quantify dystonia severity in children as well.6
In healthy young children, it was demonstrated that incomplete maturation of pediatric cerebral networks (involving the basal ganglia, cerebellum, brainstem, and cortex)4, 7, 8, 9, 10, 11, 12, 13 is reflected by developmental movements and postures.6, 14, 15, 16, 17, 18, 19, 20, 21, 22 These physiological, immature movements and postures can transiently show features that fulfill the criteria for “dystonia” or “ataxia” (such as the asymmetrical tonic neck reflex before 6 months of age17 or the scissoring grasp in toddlers14).6, 14, 15, 16, 17 Complex motor tasks by healthy school children may also show dystonic characteristics such as during writing, playing the piano, finger or foot tapping, or the fog test.18, 19 Because these physiological features are attributed to incomplete maturation of the central nervous system, they are likely to disappear when the child matures.6, 20, 21, 22, 23 For adequate interpretation of longitudinal BFMDRS scores from pediatric to adult age, this would thus implicate that one may first need to consider the effect by age (i.e, by physiologic cerebral maturation) on the scores.
In a large cohort of healthy children, we therefore aimed to evaluate the influence of age on BFMDRS (BFMMS and BFMDS) scores. To our best knowledge, BFMDRS scores have never been studied for potential age‐relatedness in children before. We reasoned that forthcoming insight in potential BFMDRS age dependency could provide information for: (1) reliable longitudinal treatment evaluation in young children (such as for longitudinal dystonia databases and for longitudinal evaluation of innovative therapies (such as deep brain stimulation [DBS])24, 25; (2) understanding of dystonia progression in different “age‐of‐onset” groups1; and (3) adequate phenotypic discrimination between “immature” and “dystonic” motor patterns for adequate interpretation of next‐generation sequencing (NGS) panels.3, 26
Methods
Participants
After informed consent by the parents and children (when older than 12 years of age), we included a total of 104 healthy children for the investigation of BFMDRS age‐relatedness. In the absence of existing quantitative age‐related BFMDRS outcomes in children, we based sample size selection on previously published data on interobserver agreement in dystonic children.27 Detecting an Intraclass Correlation Coefficient (ICC) of 0.80 for the total score or over the null hypothesis of a moderate ICC of 0.60 (0.86 published for children),27 a sample size of 36 children would be needed. Using a significance level (alpha) of 0.05 would imply that inclusion of 52 children would be sufficient.
For the investigation of potential BFMDRS age‐relatedness, we thus included 104 healthy children (4–16 years of age; 2 boys and 2 girls per year of age, n = 52 children for each BFMMS and BFMDS subscale), following mainstream education at school. Before decision on study inclusion, the parents of the child completed a detailed questionnaire concerning the health of their child. This questionnaire involved neurological and/or skeletal diagnoses, prescribed medication, school performances, sporting activities, and parental education level. Participants were excluded from the study if they: (1) were diagnosed with a neurological or skeletal disorder; (2) showed a positive Gower's sign; (3) received medication with known side‐effects on motor behavior; (4) presented with developmental delay or cognitive impairment imposing the need for extra support by special schools. We recruited participants by open advertisements at regional schools. Analogous to previous age validation studies of ataxia rating scales,21 we did not exclude pediatric behavioral diagnoses such as Attention Deficit Hyperactive Disorder (ADHD) or Attention Deficit Disorder (ADD). For subject characteristics, see Table S1.
Procedure
The study was approved by the medical ethical committee of the University Medical Center Groningen, the Netherlands. Collected physiognomic data included length, weight, and head circumference.
Test and Scoring Methods
1. BFMMS
In a set of 52 healthy children (4–16 years of age; 2 boys and 2 girls per year of age), we video‐recorded BFMMS in a quiet place, in accordance with a standardized video protocol (see Table S2).28 Nine independent assessors from the movement‐disorder team (involving experienced pediatric neurologists [n = 3] and adult neurologists [n = 3] and less experienced [but weekly trained] MD/PhD research students [n = 3]) scored the BFMMS video recordings offline. Before the study, pilot data on BFMMS interobserver agreement in dystonic children (scored by one pediatric neurologist [D.A.S.], one adult neurologist [M.A.J.T.], and one MD/PhD student [H.E.]) showed appropriate interobserver agreement (ICC > 0.90, i.e,. excellent when interpreted according to Cicchetti29 and almost perfect when interpreted according to Landis and Koch30). All assessors received the same protocol and the written information indicating that they should assess the children's motor behavior according to the definition of dystonia,1 following BFMMS instructions,5 identical to the way they would assess the same motor behavior in an adult patient. The assessors were aware that their BFMMS scores should not include other immature, developmental features that do not fulfill criteria for dystonia. We determined the mean outcome of nine assessments per child, resulting in four data points per year of age (in the age range of 4–16 years). We subsequently associated the mean BFMMS scores with age and we determined inter‐ and intraobserver agreement and test–retest reliability (after a latent time interval of more than 3 weeks).
2. BFMDS
In a second set of 52 healthy children (4–16 years of age; 2 boys and 2 girls per year of age), we obtained BFMDS scores by parental reports on their children's performances. We thus obtained four data points per year of age (in the age range of 4–16 years). We subsequently associated BFMDS scores with age.
3. BFMMS and BFMDS
We compared the age‐dependency of mean total BFMMS scores and BFMDS scores.
Statistical Analysis
We performed statistical analyses using PASW Statistics 20 for Windows (SPSS Inc., Chicago IL). We assessed normality of the distribution of the BFMMS and BFMDS total score, both graphically and using the Kolmogorov‐Smirnov test. With multivariable regression analysis we analyzed the influence of age, gender, school performances, sporting activities, and parental education level on BFMMS and BFMDS scores. When the variables significantly influenced the model, we calculated unstandardized (B) and standardized (β) regression coefficients. We examined outliers' ≥3 SD in more detail by calculating DFbèta. When outliers were present (DFbèta > 1), they were removed from the regression model. We also performed logarithmic analysis to assemble the best‐fitted trend line.
To check for the reliability of BFMMS scores, we determined inter‐ and intraobserver agreement and test–retest reliability of BFMMS outcomes by ICCs, using the two‐way mixed model and single measurement coefficients. According to Cicchetti,29 official cutoffs for qualitative rating of ICC values are as follows: ICC < 0.40: poor; 0.40 to 0.59: fair; 0.60 to 0.74: good; 0.75 to 1.00: excellent. For uniformity reasons with previously published data,21, 22 we also interpreted outcomes by Landis and Koch criteria,30 which are originally described for categorical data. According to Landis and Koch30 we characterized ICC outcomes as follows: ICC < 0.20: slight; 0.21 to 0.40: fair; 0.41 to 0.60: moderate; 0.61 to 0.80: substantial; >0.81: almost perfect.
To compare the age‐dependency of the BFMMS and BFMDS scores, we fitted two linear regression models in the first and second set of children, respectively. To allow meaningful comparison between the age‐dependency of the scales, the scores were transformed into z scores in advance of the analyses. Subsequently we calculated the difference between the two regression coefficients and tested its statistical significance using the Z‐test.31
All statistical tests were two‐sided. The P values of <0.05 (two‐sided) were considered as statistically significant.
Results
Characteristics of Included Children
At inclusion, only a minority of the children was diagnosed with a medical condition (involving asthma [n = 2], bowel problems [n = 1], and ADHD [n = 1]). For patient characteristics, see Table S1. The included children participated more frequently in sports (48–69% > 2 h/week) than the average Dutch population.32 Most parents of included children (58–69%) had achieved academic grades, compared to a minority (26–30%) of the average Dutch population.33 Additionally, only 4% of the included children exhibited school performances below average, compared to 25% of the average Dutch population.33
1. BFMMS
Total BFMMS Scores
Total BFMMS scores were not normally distributed (P < 0.05). The criteria of multivariable linear regression were met. Total BFMMS scores were significantly predicted by age, both for the total observer group (β = −0.72; P < 0.001), as for the three observer subgroups (pediatric neurologists: β = −0.64; P < 0.001; adult neurologists: β = −0.64; P < 0.001; research students: β = −0.57; P < 0.001). Age explained 51.9% of difference in scores of the total observer group (P < 0.001). Effects of gender, sporting activities, school performances, and the educational level of the parents did not significantly influence BFMMS scores (see Table S3). As age was the only significant predictor for total BFMMS scores, the inverse relation between age and mean total scores was determined by logarithmic analysis with a logarithmic trend (log–log line). The consistency between age and mean total BFMMS scores showed an age‐related effect until 16 years of age (Fig. 1A).
Figure 1.
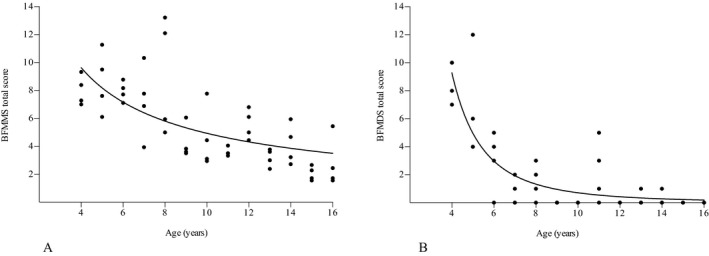
BFMMS (A) and BFMDS (B) scores related to age. Data points represent (mean) individual scores per child. BFMMS and BFMDS are age‐dependent until 16 and 12 years of age, respectively. BFMMS, Burke‐Fahn‐Marsden Movement Scale; BFMDS, Burke‐Fahn‐Marsden Disability Scale.
BFMMS Subscale Scores
Subscale scores were not normally distributed (P < 0.05). The “arms” subscales (i.e, during pronation and supination, finger tapping, writing/drawing, and/or at rest) showed the highest scores in comparison with the other subscales (P < 0.001). Legs, trunk, and mouth also contributed significantly to the total scores (P < 0.01). With multivariable linear regression analysis we observed a significant, inverse age‐effect on the subscale scores of the arms, legs, mouth, and trunk (P < 0.05). An age‐relationship was absent for the subscale scores of the eyes, speech and swallowing, and neck. For video examples of age‐related BFMMS performances, see the included video recordings.
Observer Agreement and Test–Retest Reliability of BFMMS
Interobserver agreement showed statistically significant ICCs of 0.40 (total group, P < 0.001), 0.62 (pediatric neurologists, P <0.001), 0.24 (adult neurologists, P = 0.002), and 0.47 (research students, P < 0.001). ICC outcomes revealed a “fair” interobserver agreement for the total group (according to criteria from both Cicchetti29 and Landis and Koch30). The ICC for the three individual observer‐subgroups varied between “poor to good” and/or “fair to substantial” (according to both Cicchetti's29 and Landis's and Koch's30 criteria, respectively). For further information on inter‐ and intraobserver agreement and test–retest reliability, see Table S4.
2. BFMDS
Total BFMDS Scores
Total BFMDS scores were not normally distributed (P < 0.001). The criteria of multivariable linear regression were met. Total BFMDS scores were significantly predicted by age (β = −0.60; P < 0.001). Age explained 36.2% of difference in scores (P < 0.001)). Effects of gender, sporting activities, school performances, and the educational level of the parents did not significantly influence BFMDS scores (see Table S3). As age was the only significant predictor for total BFMDS scores, the inverse relation between age and total scores was determined by logarithmic analysis with a logarithmic trend (log–log line). The consistency between age and total BFMDS scores showed an age‐related effect until about 12 years of age (Fig. 1B).
Association between BFMMS and BFMDS Age‐Dependency
The unstandardized regression coefficients between BFMMS (B = −0.19) and BFMDS age‐dependency (B = −0.18) revealed no significant difference (P = 0.75).
Discussion
In healthy children, we investigated the potential influence by age on BFMDRS scores. Our results indicate that both movement and disability subscales (i.e, BFMMS and BFMDS) are influenced by age (until 16 and 12 years of age, respectively). Additionally, BFMMS and BFMDS scores showed a similar pattern of age‐dependency, suggesting that BFMMS age‐relatedness has functional implications.
The present study indicates that BFMMS scores are age‐dependent, at least until 16 years of age. Significantly, this result was provided by all observer‐subgroups, that is, by the total‐observer group, by the experienced pediatric and adult neurologists, and also by the less‐experienced research students. Because dystonic pediatric outcome parameters should be interpretable against healthy reference values, this would implicate that insight in pediatric age‐related BFMMS reference values would be needed first. When measured against the theoretically maximal BFMMS score, it appears that the quantitative age‐related BFMMS effect seems relatively small. However, because young children are often at an early disease stage (i.e, remote from the theoretical maximum), consideration of the age‐related effect appears advisory. Furthermore, BFMMS and BFMDS scores showed a similar age‐related effect, suggesting that the BFMMS age‐relatedness is also reflected by functional changes. Because functional assessments are increasingly advocated as the best treatment outcome parameters,34, 35 we would therefore suggest that both BFMDRS subscales should be interpreted for age. Such data may appear of special interest for longitudinal treatment trials in small, heterogeneous groups of dystonic children, measuring relatively small effects throughout time (such as for dystonic children receiving deep brain stimulation at an increasingly younger age). Under intraindividually, longitudinally assessed conditions, small quantitative changes in BFMMS and BFMDS scores could thus run the theoretical risk of being overinterpreted, as “therapeutically” and/or “functionally” effective.36
Interestingly, the BFMMS age‐dependency lingered until 16 years of age, revealing no optimum score (zero) in the oldest included children (16 years of age). However, in the absence of reference values in healthy adults, it is tempting to speculate that BFMMS scores of 16‐year‐old children are likely to approach adult optimality as a reflection of physiologic brain maturation. The basal ganglia receive signals from several cortical areas (i.e, motor, somatosensory and [pre]frontal cortex, and the limbic system) and the cerebellum. They modulate and transport these signals via the thalamus, to the brainstem, cortical motor areas (such as primary motor, premotor, and oculomotor cortex), posterior parietal cortex, and the temporal cortex.7, 8, 9 Throughout childhood (until about 17 years of age), these networks mature by physiologic neurodevelopmental processes, such as selective elimination of neuronal connections and myelination.13, 37, 38, 39, 40 As implicated by the interconnecting brain networks between the basal ganglia and cerebellum,10, 11, 12 we also recognized a striking similarity between the presently reported BFMMS age‐relatedness and the previously reported age‐relatedness of the Scale for the Assessment and Rating of Ataxia (SARA).21 Comparing age‐relatedness between BFMDRS and SARA scales showed that the BFMMS age‐relatedness lingered for a longer time course than that of BFMDS and SARA (≥16 years versus 12 and 10 years21 of age, respectively). This could theoretically be attributed to the more detailed subdivision of the BFMMS compared to that of BFMDS and SARA (120 versus 30 and 40 units, respectively).
We recognize several limitations to this study. In studies with (presumably) healthy control children, one could never provide a 100% proof that the included children are really healthy. However, before entering the study, all children fulfilled the predefined inclusion criteria and 2 years after inclusion we checked whether the included children still did. In this 2‐year interval after inclusion, two children had developed a neurological diagnosis, consisting of migraine (n = 1) and a hernia nuclei pulposi leading to a radicular syndrome (n = 1). We checked whether retrospective exclusion of these two children would have changed the outcomes, which was not the case. Furthermore, the pediatric neurologists had also independently provided phenotypic assessments, indicating suspicion of a potential dystonic movement disorder in one child (indicated by two of three pediatric neurologists and subsequently confirmed by M.A.J.T.). The parents of this child had reported no medical complaints. Two years after inclusion, we subsequently checked for the emergence of a dystonic movement disorder by repeating BFMMS scores. In this child, all “dystonic” features had disappeared. As this child still fulfilled the inclusion criteria, it is tempting to speculate that the transiently observed dystonic features are developmental in origin. Another potential weakness of the study is that the included children exhibited above average educational attainment and that they also participated more frequently in sporting activities than the average Dutch population. However, because we did not observe a correlation between these factors and BFMDRS scores, we would not expect that this has influenced the results. Finally, all assessors had access to the study protocol, implicating that they were aware that they were scoring presumably healthy children. However, we checked the outcomes of all assessors by determining interobserver agreement, both for the total group and also for each assessor subgroup. Furthermore, we obtained a similar age‐relatedness from the functional scores that were provided by parents (who were not aware of age‐related motor score outcomes). In this perspective, we would therefore suggest that the presented BFMMS age‐relatedness can be regarded as indicative.
In conclusion, pediatric BFMDRS outcomes are influenced by age. For optimal interpretation of longitudinal BFMDRS scores in young dystonic children, consideration of pediatric BFMDRS age‐relatedness appears advisory. In young children, we hope that further insight in the BFMDRS construct may contribute to adequate and uniform interpretation of longitudinal BFMDRS scores and may facilitate unanimous data entry in international dystonia databases, from pediatric to adult age.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique; 4. Video Assessment.
M.J.K.: 1B, 1C, 2A, 2B, 2C, 3A, 4
L.V.: 1B, 1C, 2A, 2B, 2C, 3A, 4
R.B.: 1B, 2A, 2C, 3B, 4
R.J.L.: 3B, 4
H.B.: 2C, 3B
H.E.: 3B, 4
K.J.P.: 3B, 4
M.F.C.: 3B, 4
J.D.S.: 3B, 4
M.A.J.T.: 1A, 3B
D.A.S.: 1A, 1B, 1C, 3A, 3B, 4
Disclosures
Funding Sources and Conflict of Interest: K.J. Peall has received the clinical starter grant from the Academy of Medical Sciences. M.A.J. Tijssen received financial support from NeuroSIPE NWO Medium Fund, Nuts‐Ohra Fund, Prinses Beatrix Fund, Gossweiler Foundation, Science Fund of the Dystonia Society. The authors report no conflicts of interest.
Financial Disclosures for the previous 12 months: H. Burger received an honorarium as a statistical consultant for Plos One. M.F. Contarino is on the advisory boards of Medtronic and Boston Scientific and received speaking fees from Abbvie, Medtronic, Boston Scientific, and ECMT. J.D. Speelman advised Medtronic and Ipsen Pharmaceuticals. M.A.J. Tijssen received fees from Ipsen and Allergan Famaceutics and from Medtronic and Merz. The remaining authors report no financial disclosures.
Supporting information
A video accompanying this article is available in the supporting information here:
Table S1. Population Characteristics. Sporting activities are indicated in h/week; school performances are indicated as mean achievements. Dutch population numbers were determined from Trimbos Institute, 32 Central Statistical Office of the Netherlands, and National Kompas. 33 SD, standard deviation; pop., population.
Table S2. Video Recording Examination Protocol. *Speech interview: standard sentences and questions, including questions about problems and the ability to swallow. F, frontal view; P, profile view.
Table S3.Multivariable Regression Analysis for the Prediction of BFMMS and BFMDS Scores. Regression analysis results for the effects of the variables on BFMMS and BFMDS scores; when the variables significantly influenced the model (F change), we calculated B (unstandardized coefficient with standard error in parenthesis) and β (standardized regression coefficient). *P < 0.001.
Table S4. Intraclass Correlation Coefficients (ICCs) for BFMMS. Inter‐ and intraobserver agreement and test–retest reliability for BFMMS (ICCs); latent time interval for determining intraobserver agreement and test–retest reliability was more than 3 weeks. *P < 0.05. ns, not significant; =, agreement is 1.0 (total agreement).
Video S1. Immature motor performances: “drawing a spiral” by a healthy child, 4 years of age.
Video S2. Immature motor performances: “finger tapping” by a healthy child, 6 years of age.
Video S3. Immature motor performances: “walking” by a healthy child, 8 years of age.
Video S4. Developed motor performances: “finger tapping” and “drawing a spiral” by a healthy child, 16 years of age.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord 2013;28:863–873. doi: 10.1002/mds.25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fung VSC, Jinnah HA, Bhatia K, Vidailhet M. Assessment of patients with isolated or combined dystonia: an update on dystonia syndromes. Mov Disord 2013;28:889–898. doi: 10.1002/mds.25549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jinnah HA, Teller JK, Galpern WR. Recent developments in dystonia. Curr Opin Neurol 2015;28:400–405. doi: 10.1097/WCO.0000000000000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Neychev VK, Gross RE, Lehéricy S, Hess EJ, Jinnah HA. The functional neuroanatomy of dystonia. Neurobiol Dis 2011;42:185–201. doi: 10.1016/j.nbd.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burke RE, Fahn S, Marsden CD, Bressman SB, Moskowitz C, Friedman J. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology 1985;35:73–77. [DOI] [PubMed] [Google Scholar]
- 6. Mink JW. Special concerns in defining, studying, and treating dystonia in children. Mov Disord 2013;28:921–925. doi: 10.1002/mds.25548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nakano K. Neural circuits and topographic organization of the basal ganglia and related regions. Brain Dev 2000;22(Suppl 1):S5–S16. http://www.ncbi.nlm.nih.gov/pubmed/10984656. Accessed October 25, 2015. [DOI] [PubMed] [Google Scholar]
- 8. Bostan AC, Strick PL. The cerebellum and basal ganglia are interconnected. Neuropsychol Rev 2010;20:261–270. doi: 10.1007/s11065-010-9143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol 2007;64:20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- 10. Filip P, Lungu OV, Bareš M. Dystonia and the cerebellum: a new field of interest in movement disorders? Clin Neurophysiol 2013;124:1269–1276. doi: 10.1016/j.clinph.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 11. Prudente CN, Hess EJ, Jinnah HA. Dystonia as a network disorder: what is the role of the cerebellum? Neuroscience 2014;260:23–35. doi: 10.1016/j.neuroscience.2013.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berman BD, Jinnah HA. Dystonia: five new things. Neurol Clin Pract 2015;5:232–240. doi: 10.1212/CPJ.0000000000000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 14. Hempel MS. The Neurological Examination for Toddler‐Age. Groningen: Thesis; University Medical Center, University of Groningen, the Netherlands; http://irs.ub.rug.nl/ppn/10820832Xs.n. 1993:47–57. [Google Scholar]
- 15. Prechtl HF. Continuity of Neural Functions from Prenatal to Postnatal Life. Cambridge: Cambridge University Press; 1991:179–197. [Google Scholar]
- 16. Singer HS, Mink JW, Gilbert DL, Jankovic J. Transient and Developmental Movement Disorders in Children In: Brigido A, Ball T, eds. Movement Disorders in Childhood. Philadelphia: Saunders Elsevier; 2015:70. [Google Scholar]
- 17. Volpe J. Neurology of the Newborn. vol. 5 Philadephia: Saunders Elsevier; 2008:130–134. [Google Scholar]
- 18. Szatmari P, Taylor DC. Overflow movements and behaviour problems: scoring and using a modification of fogs' test. Dev Med Child Neurol 1984;26:297–310. doi: 10.1111/j.1469-8749.1984.tb04446.x. [DOI] [PubMed] [Google Scholar]
- 19. Largo RH, Caflisch JA, Hug F, Muggli K, Molnar AA, Molinari L. Neuromotor development from 5 to 18 years. Part 2: associated movements. Dev Med Child Neurol 2007;43:444–453. doi: 10.1111/j.1469-8749.2001.tb00740.x. [DOI] [PubMed] [Google Scholar]
- 20. Sival DA, Brunt ER. The international cooperative ataxia rating scale shows strong age‐dependency in children. Dev Med Child Neurol 2009;51:571–572. doi: 10.1111/j.1469-8749.2009.03334.x. [DOI] [PubMed] [Google Scholar]
- 21. Brandsma R, Spits AH, Kuiper MJ, et al. Ataxia rating scales are age‐dependent in healthy children. Dev Med Child Neurol 2014;56:556–563. doi: 10.1111/dmcn.12369. [DOI] [PubMed] [Google Scholar]
- 22. Kuiper MJ, Brandsma R, Lawerman TF, et al. Assessment of speech in early‐onset ataxia: a pilot study. Dev Med Child Neurol 2014;56:1202–1206. doi: 10.1111/dmcn.12517. [DOI] [PubMed] [Google Scholar]
- 23. Mink JW. The impact of development on the interpretation of movement disorders rating scales. Dev Med Child Neurol 2014;56:511–512. doi: 10.1111/dmcn.12464. [DOI] [PubMed] [Google Scholar]
- 24. Air EL, Ostrem JL, Sanger TD, Starr PA. Deep brain stimulation in children: experience and technical pearls. J Neurosurg Pediatr 2011;8:566–574. doi: 10.3171/2011.8.PEDS11153. [DOI] [PubMed] [Google Scholar]
- 25. Bertucco M, Sanger TD. Current and emerging strategies for treatment of childhood dystonia. J Hand Ther 2015;28:185–194. doi: 10.1016/j.jht.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dan B, Baxter P. Paediatric neurology: a year of DNA technology. Lancet Neurol 2014;13:16–18. doi: 10.1016/S1474-4422(13)70283-7. [DOI] [PubMed] [Google Scholar]
- 27. Monbaliu E, Ortibus E, Roelens F, et al. Rating scales for dystonia in cerebral palsy: reliability and validity. Dev Med Child Neurol 2010;52:570–575. doi: 10.1111/j.1469-8749.2009.03581.x. [DOI] [PubMed] [Google Scholar]
- 28. Comella CL, Leurgans S, Wuu J, Stebbins TGT, Chmura T. Rating scales for dystonia: a multicenter assessment. Mov Disord 2003;18:303–312. doi: 10.1002/mds.10377. [DOI] [PubMed] [Google Scholar]
- 29. Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess 1994;6:284–290. [Google Scholar]
- 30. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–174. http://www.ncbi.nlm.nih.gov/pubmed/843571. Accessed July 20, 2014. [PubMed] [Google Scholar]
- 31. Clogg CC, Petkova E, Haritou A. Statistical methods for comparing regression coefficients between models. Am J Sociol 1995;100:1261–1293. [Google Scholar]
- 32. Trimbos Institute . Sporten en psychische gezondheid; resultaten van “the Netherlands Mental Healthy Survey and Incidence Study” 2009. http://www.trimbos.nl/webwinkel/productoverzicht-webwinkel/psychische-gezondheid/af/~/media/files/inkijkexemplaren/af0927 sporten en psych gezondheid compleet.ashx.
- 33. CBS ‐ Nederlanders (25–64 jaar) naar opleidingsrichting, opleidingsniveau, leeftijd en geslacht—Maatwerk. http://www.cbs.nl/nl-NL/menu/themas/onderwijs/cijfers/incidenteel/maatwerk/2007-maatwerktabel-onderwijs2343.htm. Accessed August 6, 2015.
- 34. Gimeno H, Tustin K, Lumsden D, Ashkan K, Selway R, Lin J.‐P. Evaluation of functional goal outcomes using the Canadian Occupational Performance Measure (COPM) following Deep Brain Stimulation (DBS) in childhood dystonia. Eur J Paediatr Neurol 2014;18:308–316. doi: 10.1016/j.ejpn.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 35. Gimeno H, Tustin K, Selway R, Lin JP. Beyond the Burke‐Fahn‐Marsden Dystonia rating scale: deep brain stimulation in childhood secondary dystonia. Eur J Paediatr Neurol 2012;16:501–508. doi: 10.1016/j.ejpn.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 36. Lumsden DE, Kaminska M, Gimeno H, et al. Proportion of life lived with dystonia inversely correlates with response to pallidal deep brain stimulation in both primary and secondary childhood dystonia. Dev Med Child Neurol 2013;55:567–574. doi: 10.1111/dmcn.12117. [DOI] [PubMed] [Google Scholar]
- 37. Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Taki Y, Hashizume H, Thyreau B, et al. Linear and curvilinear correlations of brain gray matter volume and density with age using voxel‐based morphometry with the Akaike information criterion in 291 healthy children. Hum Brain Mapp 2013;34:1857–1871. doi: 10.1002/hbm.22033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chugani HT. A critical period of brain development: studies of cerebral glucose utilization with PET. Prev Med (Baltim) 1998;27:184–188. doi: 10.1006/pmed.1998.0274. [DOI] [PubMed] [Google Scholar]
- 40. Durston S, Hulshoff Pol HE, Casey BJ, Giedd JN, Buitelaar JK, van Engeland H. Anatomical MRI of the developing human brain: what have we learned? J Am Acad Child Adolesc Psychiatry 2001;40:1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A video accompanying this article is available in the supporting information here:
Table S1. Population Characteristics. Sporting activities are indicated in h/week; school performances are indicated as mean achievements. Dutch population numbers were determined from Trimbos Institute, 32 Central Statistical Office of the Netherlands, and National Kompas. 33 SD, standard deviation; pop., population.
Table S2. Video Recording Examination Protocol. *Speech interview: standard sentences and questions, including questions about problems and the ability to swallow. F, frontal view; P, profile view.
Table S3.Multivariable Regression Analysis for the Prediction of BFMMS and BFMDS Scores. Regression analysis results for the effects of the variables on BFMMS and BFMDS scores; when the variables significantly influenced the model (F change), we calculated B (unstandardized coefficient with standard error in parenthesis) and β (standardized regression coefficient). *P < 0.001.
Table S4. Intraclass Correlation Coefficients (ICCs) for BFMMS. Inter‐ and intraobserver agreement and test–retest reliability for BFMMS (ICCs); latent time interval for determining intraobserver agreement and test–retest reliability was more than 3 weeks. *P < 0.05. ns, not significant; =, agreement is 1.0 (total agreement).
Video S1. Immature motor performances: “drawing a spiral” by a healthy child, 4 years of age.
Video S2. Immature motor performances: “finger tapping” by a healthy child, 6 years of age.
Video S3. Immature motor performances: “walking” by a healthy child, 8 years of age.
Video S4. Developed motor performances: “finger tapping” and “drawing a spiral” by a healthy child, 16 years of age.


