Isolated methylmalonic aciduria (MMA) is an autosomal‐recessive disorder of amino acid metabolism caused by impaired activity of the methylmalonyl‐coenzyme A mutase enzyme.1, 2, 3 Although it is primarily a pediatric disorder, undiagnosed cases may present to adult neurologists. Movement disorders (dystonia, chorea, myoclonus, and tremor) occur in 30% to 45% of cases.1, 2, 3 Recognition is important because early treatment with dietary modification and vitamin B12 and L‐carnitine supplementation can improve outcome.1, 2, 3 We describe a patient with isolated MMA presenting to our adult neurology service.
Clinical History and Examination
A 28‐year‐old Indian woman presented with seizures, generalized dystonia, and ataxia. Her parents were first cousins, but there was no other relevant family history. She had a normal birth, but at 6 months of age she was hospitalized after a febrile seizure and was found to have global developmental delay. She was able to walk with one‐person assistance around the age of 5 years, but was slow and unsteady. She could speak in short phrases and feed herself, but was dependent on caregivers for personal care. From her teenage years, she was hospitalized for bouts of severe vomiting and dehydration, as well as generalized tonic‐clonic seizures. In her mid‐twenties, she developed worsening renal function. Examination findings are shown in Video 1.
Investigations
Brain MRI showed striking pallidal T2‐hyperintensity (Fig. 1) which, taken with her clinical presentation, led us to suspect MMA.1, 2, 3, 4 The diagnosis was confirmed by urinary organic acid analysis, which revealed a very large peak of methylmalonic acid (1,229 μmol/mmol of creatinine). Serum ammonia was also elevated (49.46 μmol/L). Full blood count, liver function tests, serum folate, vitamin B12, homocysteine and lactate, and blood gases were normal. Serum creatinine was 574 mmol/L, with small kidneys documented by ultrasonography.
Figure 1.
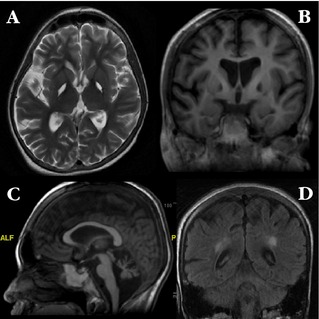
Axial T2‐weighted brain MRI (A), showing striking hyperintensity in the pallidum bilaterally, with corresponding areas of hypointensity on T1 sequence (B). There is also prominent cerebral (B) and cerebellar atrophy (C) as well as hyperintensity in the posterior periventricular regions bilaterally on T2 FLAIR (D). There were no areas of abnormal signal on gradient‐echo sequence (not shown).
Discussion
Isolated MMA has a prevalence of approximately 1 in 50,000 to 100,000.1, 2 Clinical manifestations include vomiting, dehydration, hypotonia, lethargy, failure to thrive, developmental delay, encephalopathy, learning difficulties, psychiatric disorders, epileptic seizures, pyramidal signs, movement disorders, and chronic renal failure.1, 2, 3 Catabolic events or protein overload can trigger episodes of acute metabolic decompensation. The characteristic neurological lesion involves the pallidum and patients often have dystonia.1, 2, 3, 4 The brainstem (including the nigra), cerebellum, and cerebral cortex and white matter may also be involved.1, 2, 3, 4 These changes are thought to be caused by an accumulation of toxic compounds proximal to the metabolic block and perhaps also by other mechanisms.1, 2, 3
Mutations in several genes have been implicated, but molecular genetic testing was not pursued in this patient.1, 2 The diagnosis was established by a profound increase in urinary methylmalonic acid with normal blood levels of vitamin B12 and homocysteine. Some cases respond to treatment with oral vitamin B12 and L‐carnitine supplementation and a low‐protein diet (in our patient, clinical improvement was accompanied by a drop in urinary methylmalonic acid level to 84 μmol/mmol of creatinine. Other treatments that have been reported with success in a small number of patients include liver or combined liver‐kidney transplantation and DBS surgery.1, 2, 3, 5
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Manuscript: A. Writing of the First Draft, B. Review and Critique.
A.H.T.: 1A, 1B, 1C, 2A, 2B
M.J.S.Y.: 1C, 2A
M.K.T.: 1A, 1B, 1C, 2B
S.‐Y.L.: 1A, 1B, 1C, 2B
Disclosures
Funding Sources and Conflicts of Interest: This study was supported by the Malaysian Ministry of Higher Education grant for High‐Impact Research (HIR; UM.0000017/HIR.C3 [to A.H.T. and S.Y.L.]). The authors report no conflicts of interest.
Financial Disclosures for previous 12 months: A.H.T. was awarded an International Scholarship from the American Academy of Neurology to attend its 67th Annual Meeting. S.‐Y.L. has received honoraria from the International Parkinson and Movement Disorder Society (MDS), the ASEAN Neurological Association, the Taiwan Movement Disorder Society, and Boehringer Ingelheim and has received a Malaysian Ministry of Higher Education grant for High‐Impact Research (HIR; UM.C/625/1/HIR/MOHE/CHAN/11‐H‐50001‐00‐A000025).
Supporting information
A video accompanying this article is available in the supporting information here.
Video 1. The patient is short and underweight. There is some difficulty obeying simple commands. She has mild facial grimacing, neck dystonia (with forward shift, lateral shift to the right, and left torticollis), dystonic slurred speech, hand dystonia with mild jerks in the fingers that were probably myoclonic, mild‐to‐moderate intention tremor during finger‐nose testing, curling of the toes and inturning of the feet, incoordination of foot tapping, and a dystonic and wide‐based gait (and somewhat high‐stepping with dystonic plantarflexion of the feet). Not shown: Visual system examination was normal. Limb tone and power were normal, but reflexes were brisk with extensor plantar responses.
Acknowledgment
The authors gratefully acknowledge Associate Professor Dr. Pavai Sthaneshwar from the Department of Pathology, University of Malaya, for her assistance with the laboratory diagnosis of this case.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Baumgartner MR, Horster F, Dionisi‐Vici C, et al. Proposed guidelines for the diagnosis and management of methylmalonic and propionic acidemia. Orphanet J Rare Dis 2014;9:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Manoli I, Venditti CP. Methylmalonic acidemia In: Pagon RA, Adam MP, Ardinger HH, et al., eds. GeneReviews® [Internet]. Seattle, WA: University of Washington; 1993. –2014. Available at: http://www.ncbi.nlm.nih.gov/books/NBK1231/. 16 August 2005 [updated 28 September 2010]. Accessed 25 December 2014. [Google Scholar]
- 3. Nicolaides P, Leonard J, Surtees R. Neurological outcome of methylmalonic acidaemia. Arch Dis Child 1998;78:508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baker EH, Sloan JL, Hauser NS, et al. MRI characteristics of globus pallidus infarcts in isolated methylmalonic acidemia. AJNR Am J Neuroradiol 2014;35:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chakraborti S, Hasegawa H, Lumsden DE, et al. Bilateral subthalamic nucleus deep brain stimulation for refractory total body dystonia secondary to metabolic autopallidotomy in a 4‐year‐old boy with infantile methylmalonic acidemia: case report. J Neurosurg Pediatr 2013;12:374–379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A video accompanying this article is available in the supporting information here.
Video 1. The patient is short and underweight. There is some difficulty obeying simple commands. She has mild facial grimacing, neck dystonia (with forward shift, lateral shift to the right, and left torticollis), dystonic slurred speech, hand dystonia with mild jerks in the fingers that were probably myoclonic, mild‐to‐moderate intention tremor during finger‐nose testing, curling of the toes and inturning of the feet, incoordination of foot tapping, and a dystonic and wide‐based gait (and somewhat high‐stepping with dystonic plantarflexion of the feet). Not shown: Visual system examination was normal. Limb tone and power were normal, but reflexes were brisk with extensor plantar responses.


