Abstract
Background
The test for semantic verbal fluency is quick and easy to administer. Decreases in semantic verbal fluency would suggest executive dysfunction among individuals with Parkinson's disease (PD).
Methods
The National Parkinson Foundation's Outcomes Project is a multicenter study that seeks to determine best practices in PD management. We analyzed data from the baseline and two annual follow‐up visits to determine the annual rate of verbal fluency change and determinants of that change. Linear mixed modeling was used to assess relationships between verbal fluency, clinical characteristics, quality of life, and caregiver burden.
Results
There were 1,322 participants with an average age of 67.3 years, of whom 37% were women. Mean baseline verbal fluency scores at baseline were 18.81 (standard deviation = 6.25). Verbal fluency scores did not change among patients who were at our cohort's average age and average PD duration (8.4 years) and who had no other associated conditions (beta = −0.02; P = 0.80). Verbal fluency, however, did decrease for individuals with PD duration greater than the average (beta = −0.25; P = 0.03), age greater than the average (beta = −0.022; P < 0.01), a Hoehn and Yahr >=3 (beta = −0.31; P = 0.04), and in those with cardiovascular disease (beta = −0.32; P = 0.01) or psychiatric symptomatology (beta = −0.34; P = 0.01). Individuals with higher verbal fluency scores had better quality of life (P < 0.01) and decreased caregiver burden (P < 0.01).
Conclusions
Clinicians should monitor verbal fluency scores to evaluate cognitive decline among individuals with PD. Modifiable risk factors for verbal fluency changes include psychiatric symptomatology and cardiovascular disease. Clinicians may use verbal fluency testing to identify individuals at risk for decreased quality of life and increased caregiver burden, allowing for focused interventions.
Keywords: Parkinson's disease, Cognition, verbal fluency
Parkinson's disease (PD) is a progressive neurodegenerative disorder with profound global impact.1 Although PD has been traditionally characterized as a motor disorder, cognitive impairment is increasingly recognized as a disabling nonmotor symptom. Patients with PD‐related cognitive impairment experience reduced ability to work, diminished quality of life, and greater mortality.2, 3, 4
A wide variety of neuropsychological tests have been used to evaluate cognitive decline. Verbal fluency assessment has become one of the most frequently used neuropsychological tests in clinics and research studies because it reliably detects abnormalities in attention and executive function in PD cohorts.5, 6 Verbal fluency performance is also consistent with informant‐reported executive dysfunction in PD patients.7 Although PD patients are known to suffer from difficulties with both phonemic and semantic verbal fluency, the latter is typically affected to a greater degree.8
The aim of this study was to determine the rate of change in semantic verbal fluency scores and the factors that contribute to these changes in a heterogeneous PD population. We hypothesized that age and PD duration would be the primary contributors to changes in verbal fluency scores. We further theorized that individuals with higher verbal fluency scores would experience better quality of life and decreased caregiver burden.
Patients and Methods
Patients
Individuals were selected from the National Parkinson Foundation Parkinson's Outcome Project. The initial database query of individuals with at least three visits resulted in 1,974 PD patients. We subsequently disqualified 652 individuals (314 with DBS surgery; 46 with follow‐up time of less than 20 months; 237 with diagnostic uncertainty of idiopathic PD; and 55 without verbal fluency scores). The final analysis included 1,322 patients (Fig. 1).
Figure 1.
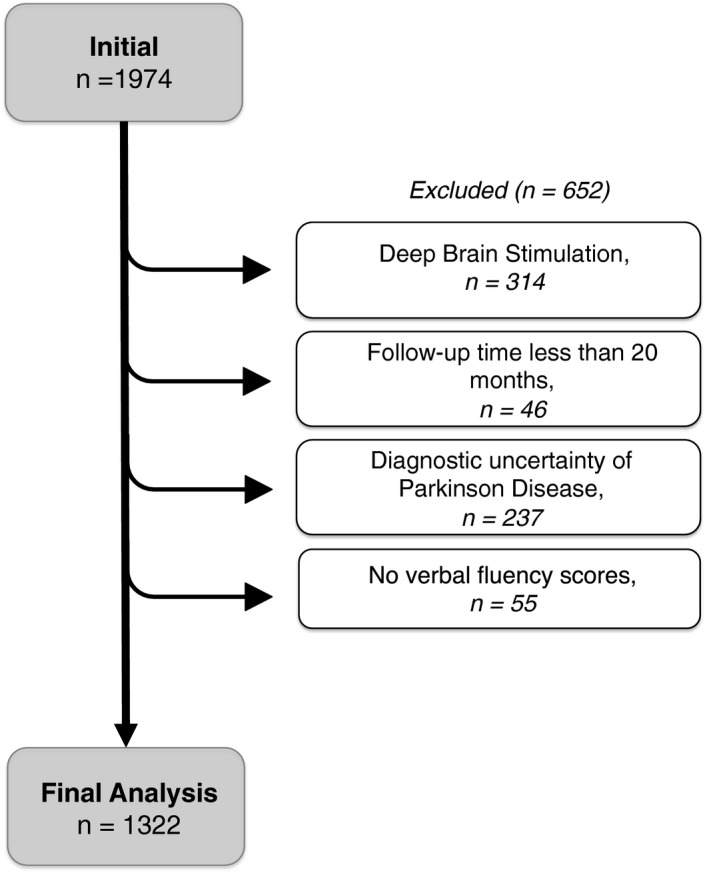
Cohort of individuals included in our analysis.
Semantic Verbal Fluency Task
Participants were asked to name as many animals as they could in 1 minute. The final score was the total number of unique animals named.
Determinants of Changes in Verbal Fluency Scores
Variables were included in the model if we suspected a relationship between the variables available in the Parkinson's Outcome Project database9 and cognitive decline. The variables considered were PD duration, age, H & Y stage, psychiatric symptomatology, prescribed cognitive enhancing medication, cardiovascular disease, and other neurological comorbidities. Because of its impact on socioeconomic status and education, race was also included in the model.10
Variables
Variables that were clinically similar were grouped: (1) Psychiatric symptomatology included individuals prescribed antidepressants, antipsychotics, and individuals referred to mental health treatment; (2) a history of heart disease and a history of diabetes were grouped under cardiovascular disease; and (3) cognitive‐enhancing medications included individuals taking rivastigmine, donepezil, galantamine, and/or memantine. In addition, (4) race was divided into white and nonwhite because of the very few individuals who identified as nonwhite. For the primary analysis, PD duration was treated as a continuous variable. We also compared the basic demographic variables of those with PD duration <10 years and those with >=10 years. This is clinically meaningful because at 10 years’ PD duration, approximately half of PD patients have developed dementia.11 We also performed two analyses: one that included all patient data and another that excluded patient data for individuals who were more than 4 standard deviations (SDs) above normative verbal fluency scores for individuals 60 to 69 years old (i.e., scores greater than 36).6 The 39‐item Parkinson's Disease Questionnaire (PDQ‐39) summary index12 was used to assess quality of life, and the Modified Caregiver Strain Index (MCSI) was used to assess caregiver burden.13
Statistical Analysis
The prediction model for verbal fluency score was a linear mixed‐effects model with two levels: verbal fluency score at each visit (level 2), grouped by patients (level 1). A random intercept for patients was used to account for the correlations among the verbal fluency scores from the same patient. A random slope for each patient was included to represent a patient‐specific progression trend. The unstructured variance‐covariance structure of the random effects was used in the model to estimate unique variances and unique pair‐wise covariances within the same‐level grouping for all random effects. The random effects and the error term were independent of each other.
The independent variables were indicators for race, sex, baseline H & Y stage, baseline disease status for cardiovascular disease, other neurological disease, psychiatric symptomatology, baseline age, baseline PD duration, and number of years since the baseline visit. To capture the potential change in the annual rate of progression, the model also used the interaction terms between the follow‐up time and the following variables, including: baseline H & Y stage, baseline disease status for cardiovascular disease, other neurological disease, and psychiatric symptomotology, baseline age, and baseline PD duration.
The interaction terms with the time since baseline allowed us to estimate differences in the progression trend over time based on specific patient conditions. For example, the estimation of the interaction terms between psychiatric symptomatology and the time since baseline shows the difference in progression over time among patients with and without psychiatric symptomatology.
To determine the association between verbal fluency scores and MCSI and PDQ‐39, linear mixed‐effects models with a random intercept for patients were used by regressing MCSI and PDQ‐39 on the verbal fluency scores, respectively. P values ≤0.05 were considered statistically significant. Data analysis was performed using Stata software (version 14.0; StataCorp LP, College Station, TX).
Results
Table 1 summarizes the demographic data for study participants. Participants averaged 67 years old (SD = 9.2; range = 46–90) with a follow‐up time of approximately 2.3 years. Women comprised 37.4% of the overall sample, and 6.4% of the sample were under‐represented minorities. Although duration of PD symptoms ranged from 1 to 30 years, 64.5% (n = 853) of the participants had PD <10 years at the time of the first clinic visit. Among the entire cohort, most individuals (60.9%) were H & Y stage 2. The most prevalent comorbidities were psychiatric symptomatology (32.0%) and cardiovascular disease (39.2%). Only a small percentage of the cohort was taking cognitive‐enhancing medications (6.4%).
Table 1.
Demographic data, clinical characteristics, and verbal fluency test scores
| PD Duration <10 Years | PD Duration ≥10 Years | Total | P Value | |
|---|---|---|---|---|
| (n = 853) | (n = 469) | (n = 1,322) | ||
| Demographics | ||||
| Age, mean ± SD | 66.59 ± 9.42 | 68.60 ± 8.67 | 67.30 ± 9.21 | <0.01 |
| Sex, % female | 36.93 | 38.38 | 37.44 | 0.60 |
| Race, % nonwhite | 6.80 | 5.54 | 6.35 | 0.37 |
| Mean time, visits 1 and 3, mean ± SD | 2.28 ± 0.39 | 2.29 ± 0.40 | 2.28 ± 0.39 | 0.69 |
| Median time, visits 1 and 3, median (range) |
2.17 (1.66–3.67) |
2.24 (1.66–3.83) |
2.16 (1.66–3.83) |
0.79 |
| Baseline clinical characteristics | ||||
| H & Y stage, % of cohort at each stage | <0.01 | |||
| 1: 1.5 | 15.90 | 4.22 | 11.80 | |
| 2: 2.5 | 64.34 | 54.67 | 60.94 | |
| 3: 3.5 | 16.51 | 33.11 | 22.34 | |
| ≥4 | 3.25 | 8.00 | 4.92 | |
| Cognitive enhancing medication, % yes | 4.35 | 10.06 | 6.38 | <0.01 |
| Psychiatric symptomatology, % yes | 26.51 | 41.95 | 31.98 | <0.01 |
| Cardiovascular disease, % yes | 40.40 | 37.12 | 39.24 | 0.24 |
| (heart disease or diabetes) | ||||
| Verbal fluency, excluding scores higher than 36 | ||||
| Verbal fluency total score, first visit, mean ± SD | 19.14 ± 5.91 | 17.95 ± 6.36 | 18.71 ± 6.10 | <0.01 |
| Verbal fluency total score, second visit, mean ± SD | 19.10 ± 6.18 | 17.39 ± 6.22 | 18.49 ± 6.22 | <0.01 |
| Verbal fluency total score, third visit, mean ± SD | 18.55 ± 6.52 | 16.55 ± 6.69 | 17.84 ± 6.65 | <0.01 |
There were significant differences in the demographic data, clinical characteristics, and verbal fluency test scores between those with PD less than 10 years and those with PD greater than or equal to 10 years. Bold = P value <0.05.
Verbal fluency scores ranged from 0 to 61 words per minute. Mean verbal fluency score at baseline for the entire population was 18.8 words per minute (SD = 6.3). The second analysis that excluded outliers (i.e., those results that are greater than 4 SDs above age and education normative scores) eliminated 17 scores. Given that including the outlying raw scores provides somewhat different coefficient estimates for the variables, we wanted to ensure that our findings were based on the bulk of the data and that the outliers were not driving significant results. Therefore, we report only the analysis with the outliers excluded and we based our conclusions on this second analysis. All of the patients remain part of our investigation because they scored within acceptable ranges at other visits (i.e., reporting the analysis that excludes the outliers does not change our population size).
After excluding the outliers, the mean verbal fluency for the entire population was 18.71 (SD = 6.1). The change in verbal fluency scores between visits 1 and 3 varied from −19 to 21, indicating that some individuals improved between visits 1 and 3. However, 53.6% of the overall population had a decline in scores. Significant predictors of total verbal fluency scores included race (classification as “white” race increased scores by 2.95 points over classification as “nonwhite”; beta = 2.95; P < 0.01), H & Y stage >=3 (beta = −1.82; P < 0.01) and age at baseline visit (beta = −0.23; P < 0.01). Our average patient, that is, a patient of average age (67.3 years old) and average PD duration (8.4 years), but no other conditions, had no significant annual rate of change in verbal fluency scores (beta = −0.02; P = 0.80). Rather, the verbal fluency total scores decreased when any other conditions are present. Specifically, if H & Y stage was >=3, verbal fluency decreased by an additional 0.31 points (beta = −0.31; P = 0.04). Cardiovascular disease (beta = −0.32; P = 0.01), psychiatric symptomatology (beta = −0.34; P = 0.01), each additional year of PD duration above the mean at baseline (P = –0.025; P = 0.03), and each additional year of age above the mean at baseline (beta = −0.022; P < 0.01) are associated with a decrease in verbal fluency scores. Table 2 describes these predictors of verbal fluency.
Table 2.
Predictors of verbal fluency scores
| Predictors | Beta | 95% CI | P Value | |
|---|---|---|---|---|
| Intercept | 16.33 | 15.13 | 17.54 | <0.001 |
| White vs. nonwhite | 2.95 | 1.80 | 4.09 | <0.001 |
| Female vs. male | 0.31 | −0.26 | 0.87 | 0.286 |
| H & Y stage >=3 vs. <3 | −1.82 | −2.55 | −1.09 | <0.001 |
| Cardiovascular yes vs. no | 0.52 | −0.10 | 1.13 | 0.101 |
| Other neurological comorbidities | −0.03 | −1.03 | 0.97 | 0.954 |
| Depression yes vs. no | −0.01 | −0.67 | 0.64 | 0.97 |
| Cognitive‐enhancing medication yes vs. no | −0.76 | −1.68 | 0.16 | 0.107 |
| PD duration at baseline visit | −0.04 | −0.10 | 0.01 | 0.139 |
| Age at baseline visit | −0.23 | −0.26 | −0.19 | <0.001 |
| Annual rate of VF change | −0.02 | −0.21 | 0.16 | 0.803 |
| Change in annual rate of VF change when | ||||
| H & Y stage >=3 | −0.31 | −0.60 | −0.02 | 0.035 |
| Cardiovascular disease | −0.32 | −0.57 | −0.08 | 0.01 |
| Other neurological comorbidities | −0.33 | −0.73 | 0.06 | 0.098 |
| Depression | −0.34 | −0.60 | −0.08 | 0.011 |
| Cognitive‐enhancing medication | −0.32 | −0.80 | 0.15 | 0.18 |
| Additional year of PD duration at baseline | −0.025 | −0.047 | −0.002 | 0.03 |
| Additional year of age at baseline | −0.022 | −0.036 | −0.008 | 0.002 |
The variables listed on the upper part of the table were part of our model of the predictors of verbal fluency scores. Significant variables were those that had an impact on total verbal fluency scores. The lower part of the table enumerates which variables had an impact on change in verbal fluency scores when patients had specific characteristics. The beta coefficient is the annual rate of change for each variable. Bold rows = statistically significant predictors of verbal fluency scores.
VF, verbal fluency.
Utilizing linear mixed‐effect methodology, we also modeled the relationship between verbal fluency and caregiver burden and quality of life. Individuals with higher verbal fluency scores had better quality of life, as indicated by lower scores on the PDQ‐39 (beta = −0.43; P < 0.01), and lower caregiver burden, as indicated by lower scores on the MCSI (beta = −0.50; P < 0.01).
Discussion
Decreases in verbal fluency were associated with PD disease severity, PD disease duration, age, cardiovascular disease, and psychiatric symptomatology. Each of the factors in the model had a similar effect size, indicating that no variable was a primary contributor toward verbal fluency decrease. Rather, each variable contributed a relatively equal amount toward decrease in verbal fluency. In addition, patients with higher verbal fluency scores reported better quality of life and decreased caregiver burden.
The rate of decline among this heterogeneous group of individuals with PD provides a standard that can be used in additional research studies and in clinical practice. Though verbal fluency is certainly variable on an individual basis, we would expect large groups of individuals in a study to decline at a rate similar to that presented here. The significant variability in verbal fluency scores between visits for some of the participants speaks to the many factors associated with cognitive testing scores, including possible practice effects,14 psychiatric symptomatology, and whether the participant is in the OFF time with regard to motor symptoms.15 This individual variability identifies the need to recruit large cohorts of participants in research studies in which verbal fluency is used as an outcome. Moreover, in clinical practice, physicians can identify many of the reasons for this individual variability and therefore use this easy‐to‐administer test to monitor the cognition of their patients over time.
Cardiovascular disease is an interesting variable to be associated with a more‐rapid rate of decrease of verbal fluency. Numerous investigators have identified an additive or synergistic interaction between Alzheimer's disease pathology and cardiovascular pathology leading to increased cognitive impairment.16 There is less evidence for cardiovascular disease potentiating PD pathology. The importance of cardiovascular disease as a contributor to verbal fluency decline, however, adds to growing evidence that cardiovascular disease may be contributing to the risk of dementia in PD. Most important, cardiovascular disease is a modifiable risk factor that could be improved with increased emphasis on vascular risk factor management.
Psychiatric symptomatology is also potentially modifiable with medications and therapeutic counseling.17 Researchers have demonstrated that individuals with depression and/or anxiety perform more poorly on cognitive testing. Furthermore, treatment of the psychiatric symptoms improves the cognitive testing profile.18
The dynamic of age and PD duration as contributors toward cognitive decline is a debated area in the literature. In brief, individuals of older age and longer PD duration are both at an increased risk of cognitive impairment. In our analysis, these two factors contributed with equal effect to verbal fluency changes. According to the Braak hypothesis, Lewy body pathology eventually spreads to the cortex during the later PD stages. These cortical Lewy bodies are thought to correlate with cognitive decline. Additionally, the likelihood of Alzheimer's disease increases with age. Finally, growing evidence suggests that alpha‐synuclein and tau pathology may potentiate each other, thereby leading to faster rates of cognitive decline with both increasing age and increasing PD duration.
As previously stated, increases in verbal fluency are directly associated with increased quality of life. Importantly, however, a single‐point increase in verbal fluency is likely not clinically significant. Rather, the effect size is such that approximately 4‐point changes of verbal fluency are associated with a clinically meaningful change in PDQ‐39.19 To our knowledge, clinically significant changes in the MCSI have not been established.
The major limitation of this analysis is that education was not included in the National Parkinson Foundation registry. Typically, age accounts for around 23% of the variance in categorical verbal fluency, and education accounts for approximately 11% of the variance.6 In addition, participants in the registry did not have additional cognitive testing such as the Mini–Mental State Examination or the Montreal Cognitive Assessment to correlate with our verbal fluency findings and identify those participants who have dementia.
Taken together, understanding the factors that contribute to the rate of decline in verbal fluency scores will increase clinicians’ armamentarium of tests that can identify patients at increased risk of cognitive decline. Our study reinforces the importance of treating modifiable risk factors to cognitive decline, such as cardiovascular factors and psychiatric symptomatology. We have also provided an expected rate of decline of verbal fluency scores, thereby allowing clinicians to identify individuals who are experiencing a faster rate of decline than would be expected and raising the clinical suspicion for an additional process, such as Alzheimer's disease or vascular dementia. Clinicians therefore should consider assessing verbal fluency early in the disease course to establish a baseline, and at every subsequent visit to identify patients at risk for more‐rapid cognitive decline, advise patients regarding their quality of life, and help identify resources to support their care partners. Verbal fluency assessments may be useful in both clinical research and patient care as a quick assessment of cognitive dysfunction with a predictable rate of decline over the course of PD.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
L.R.: 1A, 1B, 1C, 2A, 2C, 3A, 3B
Y.S.: 2C, 3B
G.P.: 2C, 3B
A.P.: 2C, 3B
K.M.: 2C, 3B
R.D.: 2C, 3B
J.W.: 2B, 2C, 3B
S.W.: 2B, 2C, 3B
Z.M.: 2C, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines
Funding Sources and Conflicts of Interest: The National Parkinson's Foundation Parkinson's Outcome Project is funded by the National Parkinson's Foundation. This analysis of the data generated did not receive additional support. Samuel Wu and Zoltan Mari received salary support from the National Parkinson's Foundation (NPF).
Financial Disclosures for previous 12 months: Dr. Rosenthal received salary support in the previous 12 months from the JHU Morris K. Udall Parkinson's Disease Research Center of Excellence National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS) P50NS038377, the Johns Hopkins Biomarker Initiative (NIH/NINDS U01 NS082133), the Marilyn and Edward Macklin Foundation, and the Michael J. Fox Foundation. She also received an honorarium from the Edmond J. Safra Foundation and Functional Neuromodulation. Ms. Salnikova received salary support in the previous 12 months from the Johns Hopkins Biomarker Initiative (NIH/NINDS U01 NS082133). Dr. Pontone received salary support in the previous 12 months from the NINDS. Dr. Pantelyat received salary support in the previous 12 months from the JHU Morris K. Udall Parkinson's Disease Research Center of Excellence (NIH/NINDS P50NS038377). Dr. Mills has received an honorarium from Johns Hopkins University Continuing Medical Education for being faculty in a CME activity and he received an honorarium from Medtronic. Dr. Dorsey is an advisor to, and has stock options in, Grand Rounds, is a compensated consultant to Clintrex, mc10, Lundbeck, MedAvante, Roche, and the National Institute of Neurological and Communicative Diseases and Stroke of the NIH. He is also an unpaid advisor to SBR Health and Vidyo and he receives research support from Auspex Pharmaceuticals, Davis Phinney Foundation, Great Lakes Neurotechnologies, Huntington Study Group, the Michael J. Fox Foundation, the Patient‐Centered Outcomes Research Institute, Prana Biotechnology, and Sage Bionetworks and has filed a patent application related to neurology and telemedicine. Ms. Wang receives salary support from the Bloomberg School of Public Health, Johns Hopkins. Dr. Wu receives grant support as co‐investigators from the NIH (1R01DK099334, P30AG028740, 1R01DK099334) and American Physical Therapy Association (KK‐G000790). He has also received salary support from the National Parkinson's Foundation and received consulting honoraria in the past 12 months from Bioness, Inc. Dr. Mari receives salary support from the Michael J. Fox Foundation, Avid Radiopharmaceuticals, and US Worldmeds LLC. He has also received salary support from the Dystonia Medical Research Foundation, the Morris K. Udall Parkinson's Disease Research Center of Excellence, and the JHMI Clinical Center for PD Neuroprotection Trials. He received consulting honoraria in the past 12 months from Navidea, Inc. He also received expert fees for medicolegal work
Acknowledgment
The authors thank Ms. Angela Zhang for her assistance with figure development.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Dorsey ER, Constantinescu R, Thompson JP, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2009. Neurology 2007;68:384–386. [DOI] [PubMed] [Google Scholar]
- 2. Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson's disease? J Neurol Neurosurg Psychiatry 2000;69:308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aarsland D, Larsen JP, Tandberg E, et al. Predictors of nursing home placement in Parkinson's disease: a population‐based, prospective study. J Am Geriatr Soc 2000;48:938–942. [DOI] [PubMed] [Google Scholar]
- 4. Levy G, Tang MX, Louis ED, et al. The association of incident dementia with mortality in PD. Neurology 2002;59:1708–1713. [DOI] [PubMed] [Google Scholar]
- 5. Cholerton BA, Zabetian CP, Wan JY, et al. Evaluation of mild cognitive impairment subtypes in Parkinson's disease. Mov Disord 2014;29:756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and Animal Naming. Arch Clin Neuropsychol 1999;14:167–177. [PubMed] [Google Scholar]
- 7. Lanni KE, Ross JM, Higginson CI, et al. Perceived and performance‐based executive dysfunction in Parkinson's disease. J Clin Exp Neuropsychol 2014;36:342–355. [DOI] [PubMed] [Google Scholar]
- 8. Henry JD, Crawford JR. Verbal fluency deficits in Parkinson's disease: a meta‐analysis. J Int Neuropsychol Soc 2004;10:608–622. [DOI] [PubMed] [Google Scholar]
- 9. Okun MS, Siderowf A, Nutt JG, et al. Piloting the NP data‐driven quality improvement initiative. Parkinsonism Relat Disord 2010;16:517–521. [DOI] [PubMed] [Google Scholar]
- 10. Boone KB, Victor TL, Wen J, et al. The association between neuropsychological scores and ethnicity, language, and acculturation variables in a large patient population. Arch Clin Neuropsychol 2007;22:355–365. [DOI] [PubMed] [Google Scholar]
- 11. Williams‐Gray CH, Mason SL, Evans JR, et al. The CamPaIGN study of Parkinson's disease: 10‐year outlook in an incident population‐based cohort. J Neurol Neurosurg Psychiatry 2013;84:1258–1264. [DOI] [PubMed] [Google Scholar]
- 12. Jenkinson C, Fitzpatrick R, Petp V, et al. The Parkinson's Disease Questionnaire (PDQ‐39): development and validation of a Parkinson's disease summary index score. Age Ageing 1997;26:353–357. [DOI] [PubMed] [Google Scholar]
- 13. Thornton M, Travis SS. Analysis of the reliability of the Modified Caregiver Strain Index. The J Gerontol 2003;58B:S127–S132. [DOI] [PubMed] [Google Scholar]
- 14. Hausknecht JP, Halpert JA, Di Paolo N, et al. Retesting in selection: a meta‐analysis of coaching and practice effects for tests of cognitive ability. J Appl Psychol 2007;92:373–385. [DOI] [PubMed] [Google Scholar]
- 15. Brown RG, Marsden CD, Quinn N, Wyke MA. Alterations in cognitive performance and affect‐arousal state during fluctuations in motor function in Parkinson's disease. J Neurol Neurosurg Psychiatry 1984;47:454–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Attems J, Jellinger KA. The overlap between vascular disease and Alzheimer's disease—lessons from pathology. BMC Med 2014;12:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aarsland D, Panlhagen S, Ballard CG, Ehrt U, and Svenningsson P. Depression in Parkinson disease—epidemiology, mechanisms and management. Nat Rev Neurol 2012;8:35–47. [DOI] [PubMed] [Google Scholar]
- 18. Greer TL, Sunderajan P, Grannemann BD, et al. Does duloxetine improve cognitive function independently of its antidepressant effect in patients with major depressive disorder and subjective reports of cognitive dysfunction? Depress Res Treat 2014;2014:627863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peto V, Jenkinson C, Fitzpatrick R. Determining minimally important differences for the PDQ‐39 Parkinson's disease questionnaire. Age and Aging 2001;30:299–302. [DOI] [PubMed] [Google Scholar]


