Hemichorea‐hemiballism (HC‐HB) is classically associated with stroke lesions in the STN.1, 2, 3 A large clinical study reported that some other lesions in the basal ganglia, such as in the caudate and putamen, may also cause HC‐HB,4 which is seldom the result of a cortical infarction. We observed transient HC‐HB in a patient with a cerebral infarction in the temporal‐parietal lobe without any lesions in the basal ganglia including the STN after intravenous recombinant tissue plasminogen activator (rtPA) administration.
A 72‐year‐old right‐handed female, who had neither a history of diabetes mellitus nor dopaminergic medication use, was admitted to our hospital because of sudden‐onset left‐sided hemiparesis. Neurological examination revealed spatial disorientation, conjugate eye deviation to the right, and dysarthria. She had left‐sided hemiparesis and sensory disturbance of all modalities. Deep tendon reflexes were exaggerated in the affected limbs. The plantar response was bilaterally flexor. National Institutes of Health Stroke Scale (NIHSS) was 19 points on admission. Blood sample tests were normal except for elevations of brain natriuretic peptide (717 pg/mL) and D‐dimer (10.3 μg/mL). Thyroid function was normal, and hyperglycemia was ruled out. Brain MRIs showed acute cerebral infarction in the right temporal‐parietal lobe perfused by the middle cerebral artery (MCA; Fig. 1A,B). Electrocardiogram showed atrial fibrillation. The patient was diagnosed as having cardioembolic infarction, and rtPA was administered within 3 hours after onset of symptoms. Nine hours later, her hemiparesis was improved significantly, which was reflected in the NIHSS score of 8 points (11‐point decrease). Subsequent to motor improvement, the left hand started to show choreic movements, such as stereotypic pronation and supination, which progressed into ballistic arm movements in a day's time. Eventually, the left‐lower extremity was also involved, confirming HC‐HB syndrome (Video 1). Subsequent brain MRIs confirmed no lesions in the basal ganglia, including the STN, and no changes in size of the stroke lesion (Fig. 1C,D). Single‐photon emission CT (SPECT) showed an increase of blood flow in the right basal ganglia and hypoperfusion in the affected cortical lesion (Fig. 1E). These involuntary movements persisted and disappeared on the ninth day. She no longer exhibited neurological deficits thereafter. Five months later, follow‐up brain MRIs revealed cortical laminar necrosis with atrophic changes in the stroke lesion, but no lesions in the basal ganglia (Fig. 1F).
Figure 1.
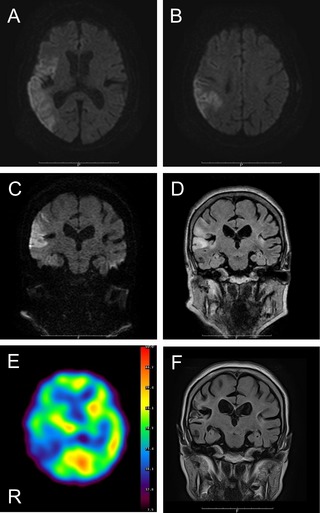
(A and B) Axial diffusion‐weighted images DWIs demonstrated acute infarction in the right temporal‐parietal lobe. (C) Coronal DWI and (D) fluid‐attenuated inversion recovery verified no lesions in the basal ganglia. (E) SPECT showed hyperperfusion in the right basal ganglia. (F) Follow‐up MRIs detected no lesions in the basal ganglia.
Hyperkinetic involuntary movements accompanied by a pure cortical infarction sparing the basal ganglia are extremely rare. The aforementioned large clinical study reported that only 6 cases of 5,007 consecutive stroke patients had hemichorea (0.12%), and the lesions were located in the MCA territory.4 In patients with an STN lesion, hyperkinetic disorder can be explained by dysfunction of the basal ganglia in the motor loop: Reduction of the striatopallidal inhibitory network by STN lesions leads to disinhibition of the thalamus and cortex, resulting in hyperkinetic involuntary movements.5 The mechanism of the cortical HC‐HB has yet to be elucidated. A possible hypothesis proposes that interruption of the motor loop projection from the cortex to the basal ganglia by cortical infarctions might induce disparity of the excitatory and inhibitory neural network in the basal ganglia. Mizushima et al. reported HC‐HB after a parietal lobe infarction. The brain MRI of this patient showed no basal ganglia lesions, but SPECT revealed hypoperfusion in the basal ganglia, indicating that ischemia of the basal ganglia may lead to functional imbalance and trigger HC‐HB.6 A recent case series study showed 3 HC‐HB cases that were associated with cerebral infarctions and reduction of blood perfusion in the frontal and parietal cortices sparing the basal ganglia. The researchers suggested a model of HC‐HB induced by cortical lesions in which derangement of the corticocortical network from parietal to frontal cortices may disharmonize sensorimotor integration, leading to hyperkinetic involuntary movements.7
Two recent case reports demonstrated that rtPA resulted in a good outcome for acute‐onset ballistic involuntary movements induced by ischemic stroke, and the researchers suggested that rtPA therapy should be considered for patients with HC‐HB.8, 9 In the present case, HC‐HB conspicuously appeared subsequent to improvement of the left hemiparesis by rtPA treatment. Brain MRIs revealed no lesions in the corticospinal tract. We speculated that the ballistic involuntary movement of the affected limbs was masked by corticospinal dysfunction in the acute phase, but then unmasked by pyramidal tract functional recovery from ischemic penumbra by rtPA treatment. The SPECT results proposed another possible mechanism: Increase of blood flow in the basal ganglia after rtPA administration might induce reperfusion injury, resulting in the triggering of HC‐HB. To the best of our knowledge, this is the first case report presenting with HC‐HB caused by cortical infarction sparing the basal ganglia during hemiparesis recovery by rtPA treatment.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
T.M.: 1A, 1B, 1C, 3A
T.W.: 1B, 1C
I.S.: 1B, 1C
K.Y.: 1B, 1C
M.S.: 1B, 1C
S.Ka.: 1B, 1C
A.Y.: 1B, 1C
S.Ko.: 1B, 1C
Y.S.: 1B, 1C
A.H.: 1B, 1C, 3B
Y.U.: 1A, 1B, 1C, 3B
Disclosures
Funding Sources and Conflicts of Interest: The authors reported no sources of funding and no conflicts of interest.
Financial Disclosures for previous 12 months: Dr. Murakami has received research funding from the Takeda Science Foundation, the Kanae Foundation for the Promotion of Medical Science, and the Research Project Grant‐in‐aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (no. 26860675). Dr. Hoshi was supported by grants from the Research Project Grant‐in‐aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (no. 26461314). Dr. Ugawa was supported by grants from the Research Project Grant‐in‐aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (no. 22390181), the Research Committee on Degenerative Ataxia from the Ministry of Health and Welfare of Japan, the Uehara Memorial Foundation, and the Novartis Foundation (Japan) for the Promotion of Science.
Supporting information
A video accompanying this article is available in the supporting information here.
Video 1. Ballistic involuntary movements were observed in the left extremities subsequent to choreic movements in the left hand.
This article was reported in the 4th Asian Oceanian Parkinson's Disease and Movement Disorders Congress in Pattaya, Thailand, in 2014.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Martin JP. Hemichorea resulting from a local lesion of the brain (syndrome of the body of Luys). Brain 1927;50:637–651. [Google Scholar]
- 2. Whittier JR. Ballism and the subthalamic nucleus (nucleus hypothalamicus; corpus Luysi). Arch Neurol Psychiatry 1947;58:672–692. [DOI] [PubMed] [Google Scholar]
- 3. Melamed E, Korn‐Lubetzki I, Reches A, Siew F. Hemiballismus: detection of focal hemorrhage in subthalamic nucleus by CT scan. Ann Neurol 1978;4:582. [DOI] [PubMed] [Google Scholar]
- 4. Chung SJ, Im JH, Lee MC, Kim JS. Hemichorea after stroke: clinical‐radiological correlation. J Neurol 2004;251:725–729. [DOI] [PubMed] [Google Scholar]
- 5. Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 1990;13:266–271. [DOI] [PubMed] [Google Scholar]
- 6. Mizushima N, Park‐Matsumoto YC, Amakawa T, Hayashi H. A case of hemichorea‐hemiballism associated with parietal lobe infarction. Eur Neurol 1997;37:65–66. [DOI] [PubMed] [Google Scholar]
- 7. Hwang KJ, Hong IK, Ahn TB, Yi SH, Lee D, Kim DY. Cortical hemichorea‐hemiballism. J Neurol 2013;260:2986–2992. [DOI] [PubMed] [Google Scholar]
- 8. McCollum D, Silvers S, Dawson SB, Barrett KM. Resolution of acute onset hemichorea‐hemiballismus after treatment with intravenous tissue plasminogen activator. Neurohospitalist 2013;3:131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zidverc‐Trajkovic J, Jovanovic DR, Marjanovic I, Radojicic A, Beslac‐Bumbasirevic L. Successful intravenous thrombolysis in a stroke patient with hemiballism. Neurologist 2011;17:205–207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A video accompanying this article is available in the supporting information here.
Video 1. Ballistic involuntary movements were observed in the left extremities subsequent to choreic movements in the left hand.


