Abstract
Background
Episodic muscular hypertonicity in Norwich terrier dogs was first reported in a brief communication in 1984. Since then, the condition has remained poorly characterized.
Objectives
The aims of this study were to characterize the phenomenology, clinical course, and family history of paroxysmal dyskinesia in the Norwich terrier and to estimate its prevalence in the United Kingdom.
Methods
The owners of Norwich terrier dogs born since January 1, 2000 were invited to complete a specifically designed questionnaire aimed at identifying affected and unaffected dogs and investigating the clinical characteristics of this paroxysmal dyskinesia. Pedigrees were collected and reviewed.
Results
The questionnaire was returned for 198 Norwich terrier dogs. Of these, 26 (13%) were classified as affected by paroxysmal dyskinesia after revision of the questionnaires and after obtaining videos of the episodes, veterinary medical records, and telephone interviews with the owners. All dogs were neurologically normal between episodes. No significant abnormalities were detected on diagnostic investigations. Mean age at the first episode was 3 years. The episodes were characterized by sustained muscular hypertonicity in the pelvic limbs, lumbar region, and thoracic limbs, impairing posture and locomotion without loss of consciousness. Episode frequency varied both between and within individuals. Stress, anxiety, excitement, and variation in daily routine were recognized as episode triggers in 13 dogs. Episode duration generally was from 2 to 5 minutes (range, from < 2 to 30 minutes). The majority of affected dogs were related.
Conclusions
Paroxysmal dyskinesia segregates in an extended pedigree of Norwich terrier dogs and thus is potentially an inherited disorder in this breed.
Keywords: Norwich terrier dogs, paroxysmal dyskinesia, movement disorder, genetic
Paroxysmal dyskinesias (PxDs) are a heterogeneous group of disorders in humans and animals characterized by recurrent episodes of sudden, abnormal, involuntary movements of variable duration.1, 2, 3 PxDs can be inherited or acquired and can be primary or secondary to various underlying disorders.1, 2 Patients with primary PxDs have normal neurologic examination between episodes. The clinical diagnosis is based on episode description, personal observation or video recording of episodes, and the exclusion of other neurologic conditions by diagnostic investigations.1 PxDs have been classified according to the precipitating factors into 3 subtypes, paroxysmal kinesigenic, nonkinesigenic, and exercise‐induced dyskinesia; and, most recently, a novel classification combining clinical and genetic characteristics has been proposed.2 Paroxysmal hypnogenic dyskinesia in which episodes occur during sleep has been reclassified as nocturnal frontal lobe epilepsy and removed from the PxDs classification system.2
Primary PxDs have been recognized with increasing frequency in various canine breeds and are generally characterized by episodic hyperkinesis that impairs posture and locomotion without loss of consciousness.3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 Age at onset, episode phenomenology, duration and frequency, clinical course, and response to treatment vary between canine breeds.3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 The majority of canine PxDs seem to be comparable to paroxysmal nonkinesigenic dyskinesia in humans.3
Episodic muscle hypertonicity in Norwich terrier (NT) dogs was first reported in a brief letter in The Veterinary Record in 1984.23 Since then, there have been anecdotal reports by breeders; however the condition has remained poorly characterized. Therefore, this study aimed to describe the phenomenology, clinical course, and family history of PxD in NT dogs and to estimate its prevalence in the United Kingdom.
Materials and Methods
This study was approved by the Ethics Committee of the Animal Health Trust, England (approval number AHT06‐2014).
The owners of NT dogs born since January 1, 2000 and registered with the UK kennel club or the NT breed club were invited to complete a detailed questionnaire either online or on paper between October 2014 and March 2015. The study was also promoted through social media, the breed club's newsletter, and the annual dog show. The letter of invitation to the study emphasized the importance of completing the initial section of the questionnaire whether or not the NT dog was affected by PxD in order to estimate the prevalence of the condition in the breed. The invitation letter stated that information on individual dogs was confidential to the investigators only. The questionnaire was designed by the first author (L.D.R.), a board‐certified veterinary neurologist, based on examination of affected NT dogs, information provided by owners of affected NT dogs and the NT breed club, and previous literature on canine movement disorders. The questionnaire contained an initial section asking for date of questionnaire completion, the dog's and owner's details, and whether or not the dog had ever experienced episodes of muscle cramping (prolonged muscle spasm/stiffness/ rigidity) of 1 or more limbs and trunk that resulted in difficulty standing up and walking, similar to an affected NT shown in a linked video. The responders had the option to answer “yes,” “no,” or “not sure” to this question on whether their NT dog was affected. The second section of the questionnaire was reserved for those who thought or were unsure that their NT dog was affected by PxD and included 58 questions on various aspects of the condition. Information on episode phenomenology was obtained by an open‐ended description of the event and, subsequently, by answers to numerous specific questions. Video recordings of the episodes of PxD were collected and reviewed. Consent to contact the NT dog's owner and the primary veterinarian for further information and data verification was sought. Telephone interview with the owners of affected or suspected NT dogs were performed by the first author (L.D.R.) between May 4 and May 20, 2015. Pedigrees of all kennel club‐registered dogs were collected and reviewed. An extended pedigree was constructed using the pedigree drawing and genetic analysis program Progeny Clinical version 7 (Progeny Software LLC, Delray Beach, FL; www.progenygenetics.com).
Descriptive statistics were performed for investigated parameters. The Pearson chi‐square test of independence and the Mann‐Whitney U test were used to compare categorical and continuous variables, respectively. P values < 0.05 were considered statistically significant.
Results
Study Population
The questionnaire was returned for 198 NT dogs. After revision of the questionnaires and after obtaining videos of the PxD episodes, veterinary medical records, and telephone interviews with the NT owners, 26 NT dogs (13%; 95% confidence interval, 8.8%–18%) were classified as affected. Three dogs reportedly were affected by their owners and were classified as unclear cases in this study due to incomplete questionnaire responses, unavailability of the veterinary medical records, and unavailability of the dog's owner for telephone interview. The remaining 169 NT dogs had not demonstrated episodes consistent with PxD at the time of questionnaire completion and were classified as unaffected. Information on sex, neuter status, and age in affected, unaffected, and unclear dogs is summarized in Table 1. The median age at the time of the study in affected dogs was significantly older (P < 0.001) than that in unaffected dogs. There was no significant difference in sex between affected and unaffected dogs (P = 0.168). However, neuter status was significantly different between affected and unaffected dogs (P = 0.001).
Table 1.
Sex, neuter status, and age in 198 Norwich terrier dogs
| Affected | Unaffected | Unclear | |
|---|---|---|---|
| Total number of dogs | 26 | 169 | 3 |
| Sex | |||
| Male | 14 | 66 | 1 |
| Female | 12 | 101 | 2 |
| Neuter status | |||
| Male neutered | 5 | 12 | 0 |
| Male entire | 9 | 54 | 1 |
| Female neutered | 8 | 21 | 1 |
| Female entire | 4 | 80 | 1 |
| Unknown | 0 | 2 | 0 |
| Age at the time of the study, y | |||
| Median | 8.4 | 4.6 | 13 |
| Mean ± SD | 8.3 ± 3.1 | 5.3 ± 4.1 | 13.5 ± 6.2 |
| Range | 4.2–14.7 | 0.1–19.5 | 12.5–15.0 |
SD, standard deviation.
Affected Population
In the 26 affected NT dogs, the median age at the first observed episode of PxD was 3 years (mean ± standard deviation, 3.0 ± 1.3 years; range, 0.8–5.4 years). The median age at episode onset in female affected dogs (median, 3.1 years) was not significantly different (P = 0.574) from that in male affected dogs (median, 2.5 years).
All 26 NT dogs were neurologically normal between episodes. No significant abnormalities were detected on diagnostic investigations (when performed), including hematology and serum biochemistry (n = 10), bile acid stimulation test (n = 3), urinalysis (n = 2), magnetic resonance imaging of the brain and cerebrospinal fluid analysis (n = 3), electroencephalography (performed between episodes in 2 dogs without pharmacologic restrain), and electromyography and motor nerve‐conduction velocity (n = 2).
Characterization of Episodes
The owners of 12 NT dogs (46%) reported that the dog was aware of the imminent occurrence of an episode, because he/she would seek the owner's reassurance and would walk or attempt to walk toward the owner as the muscle tone progressively increased in the affected body parts. Level of consciousness and awareness remained normal during the entire episode in all 26 NT dogs. The episodes were characterized by sustained hypertonicity of the muscles in the pelvic limbs (n = 26; 100%) (Video S1), lumbar region (n = 20; 77%), thoracic limbs (n = 18; 69%), abdomen (n = 4; 15%), and tail (n = 3; 12%). The affected limbs were rigidly extended and sometimes lifted off the ground. Sustained flexion of 1 or more limb was reported in 13 dogs (50%). The lumbar region reportedly became kyphotic (n = 11; 42%), and the tail arched (n = 3; 12%). Side‐to‐side swaying movements of the caudal half of the body or of the entire body were reported in 6 (23%) and 14 (54%) NT dogs, respectively. Thirteen dogs (50%) were unable to stand up during the entire episode, 10 (38%) could stand up during part of the episode, and 3 (12%) were able to stand up during the entire episode. Fifteen dogs (58%) were unable to walk during the entire duration of the episode, 9 (34%) could walk during part of the episode, and 2 (8%) (Video S2) were able to walk during the entire episode. Repeated licking movements were observed in 10 NT dogs (38%).
Episode duration was < 2 minutes (n = 3; 12%), 2 to 5 minutes (n = 18; 69%), and 6 to 30 minutes (n = 5; 19%). Episodes were self‐limiting in all dogs. The owners of 4 NT dogs (15%) felt that reassuring their dog could limit episode duration and severity. The reminder were not sure (n = 4; 15%) or did not think that any action could alter the episode course (n = 18; 70%).
When asked whether they thought that their dog was in discomfort during the episode of PxD, 14 owners (54%) replied yes, 7 (27%) replied no, and 5 (19%) were not sure. The dog's quality of life was not considered to be negatively affected by the PxD in all but 1 dog. None of the dogs were killed because of the PxD.
Occurrence of Episodes
One or more of following factors were reported as episode triggers in 18 NT dogs (69%): excitement (n = 12; 46%), stress (n = 11; 42%), anxiety (n = 6; 23%), exercise (n = 6; 23%), change or extremes of weather or temperature (n = 6; 23%), change in routine (n = 3; 12%), and waking from sleep (n = 2; 8%). No episode trigger was identified or reported in 8 NT dogs (31%).
Episodes reportedly occurred anytime during the day (n = 19; 73%) or predominantly in the afternoon (n = 4; 15%), evening (n = 2; 8%), and morning (n = 1; 4%).
Episode frequency varied both between and within individuals and ranged from 2 per day to 2 per year. Two NT dogs ages 12.5 and 14.7 years, respectively, at the time of the telephone interview, used to have 3 or 4 episodes a year and had become episode‐free during the 3.8 and 4.7 years prior to the study, respectively. The remaining 24 NT dogs had 2 or more episodes in the 12 months prior to the study. Overall, there was no consistent pattern in episode occurrence; however, 11 NT dogs (42%) generally had 2 or more episodes over a few days and then no episodes for 3 or 4 months. The median time the NT dog spent with the owner was 18 hours per day (mean ± standard deviation, 17.0 ± 6.2 hours per day; range, 7.5–24 hours per day).
Therapeutic Interventions
Changes in diet (n = 5), pharmacologic treatment (n = 5), and administration of vitamins and supplements (n = 2) were performed alone or in combination in attempts to decrease the number and severity of the episodes of dyskinesia in 8 NT dogs (31%). The new diet was a gluten‐free and beef‐free diet (n = 1), a hypoallergenic diet (n = 1), a home‐prepared raw diet with no chicken (n = 1), and a low‐protein diet (n = 2). Pharmacologic treatment consisted of carbamazepine (n = 1), phenobarbital (n = 1), phenobarbital and potassium bromide (n = 1), phenobarbital and imepitoin (n = 1), and hyoscine (n = 1). Diet changes and pharmacologic treatment (eg, hyoscine [n = 1] or carbamazepine [n = 1]) were performed concurrently in 2 dogs. Vitamins and supplements were administered concurrently with the diet change in 2 dogs and included vitamin E, vitamin B and kelp tablets in 1 dog, and epitaur supplement and vitamin E in another dog. Episode frequency decreased from 5 episodes in 5 months (before the diet change) to 2 episodes in 7 months in the NT dog on the gluten‐free and beef‐free diet and from 4 episodes per year (before the diet change) to 1 episode in 4.8 years in the NT dog on the home‐prepared raw diet with no chicken plus epitaur supplement and vitamin E. In 1 NT dog, episode frequency decreased by one‐third after the change to a hypoallergenic diet 2.9 years before this study. In the remaining 2 NT dogs, the diet changes and concurrent administration of vitamin and supplements were not reported to affect episode frequency. None of the pharmacologic treatments used alone or in combination reportedly decreased episode frequency by 50% or more in the long term (e.g., > 6 months of treatment).
Clinical Progression
When asked about disease progression over time, episode severity was considered unchanged (n = 14; 54%), decreased (n = 6; 23%), or increased (n = 2; 8%). The frequency of the episodes of PxD reportedly was overall unchanged (n = 10; 38%), increased (n = 4; 15%), or decreased after diet change (n = 3; 12%) and decreased as the dog became older (n = 3; 12%). The owners of some NT dogs were unsure about changes in episode severity (n = 4; 15%) and frequency (n = 6; 23%) from disease onset to questionnaire completion.
Median follow‐up duration from date of first episode of PxD to date of the telephone interview with the primary investigator was 4.5 years (range, 0.3–12.7 years; mean ± standard deviation, 5.3 ± 3.5 years). All affected NT were alive at the time of the study.
Pedigree Analysis
Pedigrees were available for 25 affected and 162 unaffected NT dogs. Pedigree analysis revealed clustering of cases (Fig. 1), which suggests a potential inherited component to PxD in NT dogs.
Figure 1.
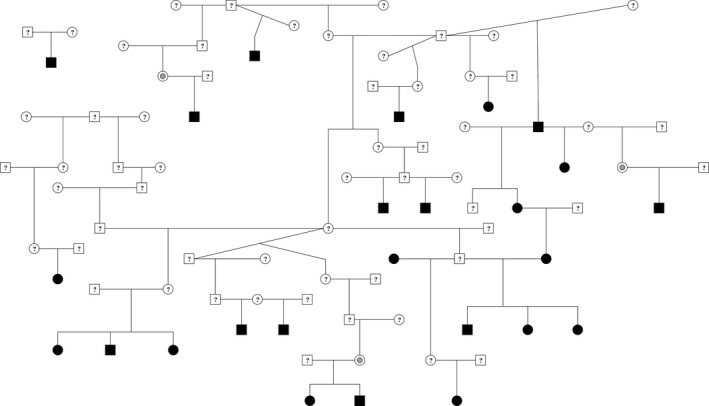
Extended pedigree containing UK Kennel Club‐registered Norwich terrier dogs. Affected cases are marked in solid black, individuals of unknown phenotype are indicated with question marks, and 3 clinically unaffected dogs are marked with gray dots. All but 1 of the affected cases are connected through the extended pedigree, potentially suggesting an inherited component to the disease. The separate family of 3 could not be linked to the main pedigree with the 5‐generation pedigree data available for each individual.
Discussion
This is the first study evaluating phenomenology, clinical course, pedigree analysis, and prevalence of PxD in NT dogs in the United Kingdom. This condition appears to be an inherited primary PxD in the NT dog.
PxD in NT dogs may be comparable to paroxysmal nonkinesigenic dyskinesia in humans, because excitement and stress were the most commonly reported triggering factors, whereas sudden movement and sleep were not precipitants in any of the dogs (Table 2). Exercise reportedly triggered episodes in 6 NT dogs (23%); however, stress and excitement were also reported as episode triggers in 5 of those 6 dogs. Another similarity with paroxysmal nonkinesigenic dyskinesia in humans is the overall episode frequency, which ranges from 2 per day to 2 per year, with months of episode‐free intervals.24 Several patients with paroxysmal nonkinesigenic dyskinesia report an aura‐like sensation immediately prior to an episode characterized by limb paresthesias or stiffness or by an undefined feeling that patients recognize as the onset of another attack.24 Although evaluating the presence of similar symptoms in dogs is challenging, it is interesting to note that the owners of nearly half of the NT dogs in this study reported that their dog was aware of the imminent occurrence of an episode, because he/she would seek the owner's reassurance just before the onset of the muscle hypertonicity.
Table 2.
Summary of clinical and genetic data of primary paroxysmal dyskinesia in Norwich terrier dogs and in peoplea
| NTDPD | PNKD | PKD | PED | |
|---|---|---|---|---|
| Trigger (most commonly) | Excitement, stress, anxiety | Emotional stress, excitement, caffeine, alcohol | Sudden voluntary movement, startle | Prolonged exercise |
| Episode phenomenology | Dystonia of the pelvic limbs ± thoracic limbs and trunk | Dystonia with or without chorea or athetosis of the limbs, trunk, face | Dystonia with or without chorea or ballism of 1 or more extremities ± face | Dystonia sometimes in combination with chorea of 1 or more foot/leg |
| Episode duration | <2 to 30 min | 2 min to 4 h | <1 to 5 min | 5 min to 2 h |
| Episode frequency | 2/day to 2/y | A few/day to a few/y | 1/mo to 100/day | 1/day to a few/mo |
| Premonitory sensation | Nearly 50% | 50% or more | Sometimes | No |
| Improvement with age | Sometimes | Sometimes | Sometimes | Unknown |
| Treatment response | Avoidance of precipitating factors and diet change may benefit some dogs; no response to phenobarbital, potassium bromide, or imepitoin | Avoidance of precipitating factors, benzodiazepines (diazepam or clonazepam) | Carbamazepine, phenytoin, phenobarbital, levetiracetam | Avoid strenuous exercise, ketogenic diet, levodopa, acetazolamide |
| Inheritance | Unknown | Autosomal dominant | Autosomal dominant | Autosomal dominant |
| Affected gene (most commonly) | Unknown | PNKD (formerly referred to as MR‐1) | PRRT2 | SLC2A1 |
NTDPD, Norwich terrier dog paroxysmal dyskinesia; PNKD, paroxysmal nonkinesigenic dyskinesia; PKD, paroxysmal kinesigenic dyskinesia; PED, paroxysmal exercise‐induced dyskinesia; MR‐1, myofibrillogenesis regulator‐1; PRRT2, proline‐rich transmembrane protein 2; SLC2A1, solute carrier family 2 (facilitated glucose transporter), member 1.
PxDs have been described in other canine breeds.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 In the majority of breeds with PxD affecting the limbs and trunk, the first episode of PxD is observed in the first year after birth.5, 7, 11, 12, 13, 14, 15, 16, 17, 18 However, in the NT dogs in this study, the median and mean age at the first observed episode of PxD was 3 years, similar to what has been reported in the border terrier.6, 22 Other similarities with the border terrier PxD include no gender predilection in affected dogs; seeking to be near the owner at the start of an episode; certain aspects of the episode phenomenology, such as the sustained hypertonicity of the appendicular and lumbar muscles, repeated licking movements, and side‐to‐side swaying movements of the body; and episode frequency.6, 22 Similar to what has been reported in other canine breeds with PxD,3, 4, 5, 6 treatment with antiepileptic medications, such as phenobarbital and potassium bromide, did not decrease episode frequency by 50% or more. Although no definitive conclusions can be drawn due to the small number of cases and the fact that treatment modalities were various and not randomized, it seems that a change in diet can be beneficial in some NT dogs, as previously suggested in other canine breeds.6, 18, 22 Further investigations are required to clarify whether and which diet changes or other treatment modalities are most beneficial in NT dogs with PxD.
It is unlikely that the paroxysmal disorder described in the NT dogs in this study represents a form of epilepsy, considering the episode phenomenology (e.g., normal level of consciousness and awareness, lack of autonomic signs, the tendency to continue performing certain activities during the episode, and the absence of postictal signs), the similarities with PxDs in other canine breeds, the lack of response to antiepileptic drugs commonly used in canine epilepsy, and the absence of electroencephalogram (EEG) abnormalities in the 2 tested dogs. However, differentiation between PxD and epilepsy can be very challenging, and both conditions can occur in the same family or individual.1, 2, 22 None of the NT dogs included in this study underwent EEG during an episode due to logistic limitations of EEG in veterinary medicine. The stereotyped licking movements (observed in 38% of NT dogs in this study) can occur in epileptic patients.
In humans, thorough characterization of clinical phenotypes and the use of advanced methods of molecular genetics have led to the identification of the genetic cause of various forms of PxDs (Table 2).2, 25, 26 In canine PxDs, the causative mutation has been identified in the brevican (BCAN) gene in Cavalier King Charles spaniels, and an autosomal recessive mode of inheritance has been reported in the Chinook and Scottish terrier dogs.8, 9, 10, 15, 16 In the NT dogs, the pattern observed in the pedigree suggests an inherited component to the disease. Lack of phenotype information for complete families precluded an assessment of the mode of inheritance.
The prevalence of PxD in the NT dogs in this study was estimated at 13%, which seems relatively high and further supports the possibility of a genetic component of this condition. However, the prevalence of PxDs in the canine population or in a specific dog breed has not been reported previously; therefore, no comparison can be made. Epilepsy is the most common, chronic neurological condition in domestic dogs, and its prevalence in the general canine population in the United Kingdom has been estimated as 0.6%,27 which is much lower than the prevalence of PxD in the NT dogs in this study. Although the invitation letter to the study highlighted the importance to participate regardless of whether or not the dog was affected, it is possible that owners of NT dogs with PxD were more inclined to participate than owners who had no knowledge or experience with this disease. It is possible that a higher response has been achieved from the owners of NT dogs of a particular breeding line with affected dogs. Conversely, lack of response from the owners of other affected NT dogs may have occurred due to an unwillingness to disclose the presence of PxD in their breeding lines. This potential responder bias needs to be considered when interpreting prevalence and pedigree analysis results in this study. In addition, UK‐registered NT dogs form a relatively small, closed breeding population; thus, all dogs share similar ancestry.
The median age at onset of PxD in the NT dog is after the age at which a dog is generally used for breeding for the first time, making it difficult to breed out the condition. Therefore, identification of the genetic component of the disease would be an important achievement in the prevention of this disorder in the NT dog breed. The clinical characterization of PxD in the NT dog provided in this study can help to classify NT dogs as affected or unaffected for future genetic studies.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
L.D.R.: 1A, 1B, 1C, 2A, 2B, 3A, 3B
O.P.F.: 1C, 3B
C.S.M.: 3B
J.F.: 1B, 3B
Disclosures
Funding Sources and Conflicts of Interest: No specific funding was received for this work. The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the previous 12 months: In the past 12 months, Dr CS Mellersh has received funding from the United Kingdom Kennel Club, Dogs Trust, PetPlan Charitable Trust, Waltham Foundation United Kingdom, and Kennel Club Charitable Trust. Dr L De Risio has received consultancy fees from Vetoquinol and funding from PetPlan Charitable Trust. All funding is unrelated to the Norwich terrier study.
Supporting information
Videos accompanying this article are available in the supporting information here.
Video S1. Episode of paroxysmal dyskinesia in a 4‐year‐old, female Norwich terrier dog. There is increased muscle tone and abnormal posture and movement in the pelvic limbs and, to a lesser extent, in the thoracic limbs. This results in difficulty ambulating and falling on 1 occasion. The dog is fully aware and tries to walk toward the owner, who is recording the episode. There are repeated licking movements and side‐to‐side swaying movements of the caudal half of the body during part of the episode.
Video S2. Episode of paroxysmal dyskinesia in a male, neutered Norwich terrier dog aged 4 years and 3 months. The dog is initially able to walk, although the progressive, sustained hypertonicity of the muscles of the pelvic limbs and subsequently also in the thoracic limbs results in the inability to stand up and walk. The dog is alert and aware of his owner's presence. He tries to walk toward his owner. There are repeated licking movements.
Acknowledgments
We are grateful for the cooperation of the UK kennel club, the UK Norwich terrier club, the veterinarians, and the owners of the dogs described in this paper.
Supporting information may be found in the online version of this article.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Waln O, Jankovic J. Paroxysmal movement disorders. Neurol Clin 2015;33:137–152. [DOI] [PubMed] [Google Scholar]
- 2. Erro R, Sheerin U, Bhatia K. Paroxysmal dyskinesias revisited: a review of 500 genetically proven cases and a new classification. Mov Disord 2014;29:1109–1116. [DOI] [PubMed] [Google Scholar]
- 3. Urkasemsin G, Olby N. Canine paroxysmal movement disorders. Vet Clin North Am Small Anim Pract 2014;44:1091–1102. [DOI] [PubMed] [Google Scholar]
- 4. Penderis J, Franklin R. Dyskinesia in an adult Bichon Frise. J Small Anim Pract 2001;42:24–25. [DOI] [PubMed] [Google Scholar]
- 5. Ramsey I, Chandler K, Franklin R. A movement disorder in boxer pups. Vet Rec 1999;144:179–180. [DOI] [PubMed] [Google Scholar]
- 6. Black V, Garosi L, Lowrie M, Harvey R, Gale J. Phenotypic characterisation of canine epileptoid cramping syndrome in the Border terrier. J Small Anim Pract 2014;55:102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herrtage M, Palmer A. Episodic falling in the cavalier King Charles spaniel. Vet Rec 1983;112:458–459. [DOI] [PubMed] [Google Scholar]
- 8. Forman O, Penderis J, Hartley C, Hayward L, Rickets S, Mellersh C. Parallel mapping and simultaneous sequencing reveals deletions in BCAN and FAM83H associated with discrete inherited disorders in a domestic dog breed [serial online]. PLoS Genet 2012;8:e1002462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gill J, Tsai K, Krey C, et al. A canine BCAN microdeletion associated with episodic falling syndrome. Neurobiol Dis 2012;45:130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Packer R, Patterson E, Taylor J, Coates J, Schnabel RD, O'Brien D. Characterization and mode of inheritance of a paroxysmal dyskinesia in Chinook dogs. J Vet Intern Med 2010;24:1305–1313. [DOI] [PubMed] [Google Scholar]
- 11. Woods C. Hyperkinetic episodes in two Dalmatian dogs. J Am Anim Hosp Assoc 1977;13:255–257. [Google Scholar]
- 12. Harcourt‐Brown T. Anticonvulsant responsive, episodic movement disorder in a German shorthaired pointer. J Small Anim Pract 2008;49:405–407. [DOI] [PubMed] [Google Scholar]
- 13. Clemmon R, Peters R, Meyers K. Scotty cramp: a review of cause, characteristics, diagnosis, and treatment. Compend Contin Educ Vet 1980;2:385–388. [Google Scholar]
- 14. Meyers K, Lund J, Padgett G, Dickson W. Hyperkinetic episodes in Scottish terrier dogs. J Am Vet Med Assoc 1969;155:129–133. [PubMed] [Google Scholar]
- 15. Meyers K, Padgett G, Dickson W. The genetic basis of a kinetic disorder of Scottish terrier dogs. J Hered 1970;61:189–192. [DOI] [PubMed] [Google Scholar]
- 16. Urkasemsin G, Olby N. Clinical characteristics of Scottie Cramp in 31 cases. J Small Anim Pract 2015;56:276–280. [DOI] [PubMed] [Google Scholar]
- 17. Shelton G. Muscle pain, cramps and hypertonicity. Vet Clin North Am Small Anim Pract 2004;34:1483–1496. [DOI] [PubMed] [Google Scholar]
- 18. Park H, Seo D, Song K, Seo K. Paroxysmal dyskinesia suspected as canine epileptoid cramping syndrome in a young Yorkshire terrier dog. J Vet Med Sci 2014;76:1129–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guevar J, De Decker S, Van Ham L, Fischer A, Volk H. Idiopathic head tremor in English bulldogs. Mov Disord 2014;29:191–194. [DOI] [PubMed] [Google Scholar]
- 20. Wolf M, Bruehschwein A, Sauter‐Louis C, Sewell AC, Fischer A. An inherited episodic head tremor syndrome in Doberman pinscher dogs. Mov Disord 2011;26:2381–2386. [DOI] [PubMed] [Google Scholar]
- 21. Shell L, Berezowski J, Rishniw M, Nibblett B, Kelly P. Clinical and breed characteristics of idiopathic head tremor syndrome in 291 dogs: a retrospective study [serial online]. Vet Med Int 2015;2015:165463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marioni‐Henry K, Rusbridge C, Volk HA. Clinical features in border terrier dogs with paroxysmal involuntary movements [published online ahead of print Nov 23, 2015]. Mov Disord Clin Pract. doi: 10.1002/mdc3.12232. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Furber RM. Cramp in Norwich terriers [letter]. Vet Rec 1984;115:46. [Google Scholar]
- 24. Hao S, Feng Y, Zhang G, Wang A, Wang F, Wang P. Neuropathophysiology of paroxysmal, systemic, and other related movement disorders. Eur Rev Med Pharmacol Sci 2015;19:2452–2460. [PubMed] [Google Scholar]
- 25. Brockmann K. Episodic movement disorders: from phenotype to genotype and back [serial online]. Curr Neurol Neurosci Rep 2013;13:379. [DOI] [PubMed] [Google Scholar]
- 26. Gardiner AR, Jaffer F, Dale RC, et al. The clinical and genetic heterogeneity of paroxysmal dyskinesias. Brain 2015;138(pt 12):3567–3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kearsley‐Fleet L, O'Neill DG, Volk HA, Church DB, Brodbelt DC. Prevalence and risk factors for canine epilepsy of unknown origin in the UK [serial online]. Vet Rec 2013;172:338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Videos accompanying this article are available in the supporting information here.
Video S1. Episode of paroxysmal dyskinesia in a 4‐year‐old, female Norwich terrier dog. There is increased muscle tone and abnormal posture and movement in the pelvic limbs and, to a lesser extent, in the thoracic limbs. This results in difficulty ambulating and falling on 1 occasion. The dog is fully aware and tries to walk toward the owner, who is recording the episode. There are repeated licking movements and side‐to‐side swaying movements of the caudal half of the body during part of the episode.
Video S2. Episode of paroxysmal dyskinesia in a male, neutered Norwich terrier dog aged 4 years and 3 months. The dog is initially able to walk, although the progressive, sustained hypertonicity of the muscles of the pelvic limbs and subsequently also in the thoracic limbs results in the inability to stand up and walk. The dog is alert and aware of his owner's presence. He tries to walk toward his owner. There are repeated licking movements.


