Ataxia‐telangiectasia (A‐T) is an autosomal‐recessive disorder characterized by cerebellar ataxia, oculocutaneous telangiectasia, immunodeficiency, radiosensitivity, increased prevalence of malignancies, and increased level of alpha‐fetoprotein (AFP).1 The responsible gene A‐T mutated (ATM), localized to chromosome 11q22‐2, is a serine/threonine protein kinase that is involved in the cellular response to DNA damage. Whereas the classic form, genetically marked by truncating mutations of the ATM, is a severe, fast progressive disease with no residual ATM kinase activity, patients with variant A‐T show a milder form of the disease, often presenting with a plethora of different extrapyramidal manifestations—choreoathetosis, resting tremor, and myoclonus‐dystonia (M‐D) and still have some residual ATM‐kinase activity; they are usually carriers of at least one missense or leaky splice site mutation.1, 2 We present a case of a variant A‐T with mainly M‐D presenting features and a favorable outcome after bilateral DBS of the globus pallidus pars interna (GPi‐DBS).
Case Report
A 40‐year‐old right‐handed man from Irish descent had been noticed to have “clumsy” gait and poor balance from early childhood. At the age of 11, he developed abnormal movements of the head. In subsequent years, his balance and walking as well as his writing became worse to a degree when he could hardly walk and write because of generalized jerky and twisting movements, which were in the upper limbs superimposed on a background of bilateral resting tremor and an even more pronounced postural and kinetic tremor that further complicated his condition. This led to a diagnosis of, in the first instance, generalized dystonia and subsequently M‐D at the age of 20. He was tested for the presence of DYT‐1, which was shown to be negative. A direct bidirectional sequencing of the coding exons in the ε‐sarcoglycan (SGCE, DYT‐11) gene detected no mutation. In addition, no deletion or duplication within or including the SGCE gene was found by multiplex ligation‐dependent probe amplification.3 MRI of the brain was normal and did not show signs of cerebellar atrophy. Despite his condition, he managed to enrol into university and qualify as a professional accountant. After testing for hemochromatosis because of pathological liver functional tests (LFT) and high serum ferritin (SF) values, he was found to be heterozygous for H63D mutation of the hemochromatosis (HFE) gene that was proven by a liver biopsy. There were no other systemic manifestations of his disease. In the family, his sister, who had similar neurological problems, died because of hepatocellular carcinoma at the age of 26. His father, who died from a cancer of unknown origin at the age of 73, has also had pathological LFT and high SF values. Both of his brothers have never had any neurological or medical problems.
In 2010, at the age of 35, after the finding of elevated AFP of 485 IU/mL, he was investigated for A‐T. Genetic testing for ATM mutation revealed a missense mutation c.743G>T; p.(Agr248Leu), and a nonsense mutation c.8266A>T; p.(Lys2‐756X). The karyotype search did not reveal translocations (t 7;14). A large range of medications, including trihexyphenidyl, diazepam, clonazepam, baclofen, carbamazepine, levodopa, tetrabenazine, and levetiracetam, were tried during the disease course without benefit. In 2013, because of the abundance of myoclonic and dystonic movements that considerably interfered with the activities of daily living, he was referred for operative treatment and underwent bilateral GPi DBS in 2014.
The DBS electrodes were placed in the posteroventral aspects of both GPi (Fig. 1) using an MRI‐guided MRI‐verified approach.4 Ten days after the operation, there was a considerable improvement of the dystonia (36%) and myoclonus at rest (86.2%); an increase on the action myoclonus was noted early after the operation, which markedly improved 8 months later (72.3%), when further improvement of dystonia and a complete resolution of the myoclonus at rest as well as functional improvement, with a considerable shift toward self‐dependence, were also noted (Table 1; see Video 1). Furthermore, there was a complete resolution of the resting tremor; the severity of both, the postural and kinetic tremor, was considerably reduced. His gait, which before the operation was mainly impaired because of severe myoclonic jerks, improved markedly after the operation. There was also improvement of his swallowing and, importantly, no speech deterioration was noted postoperatively.
Figure 1.
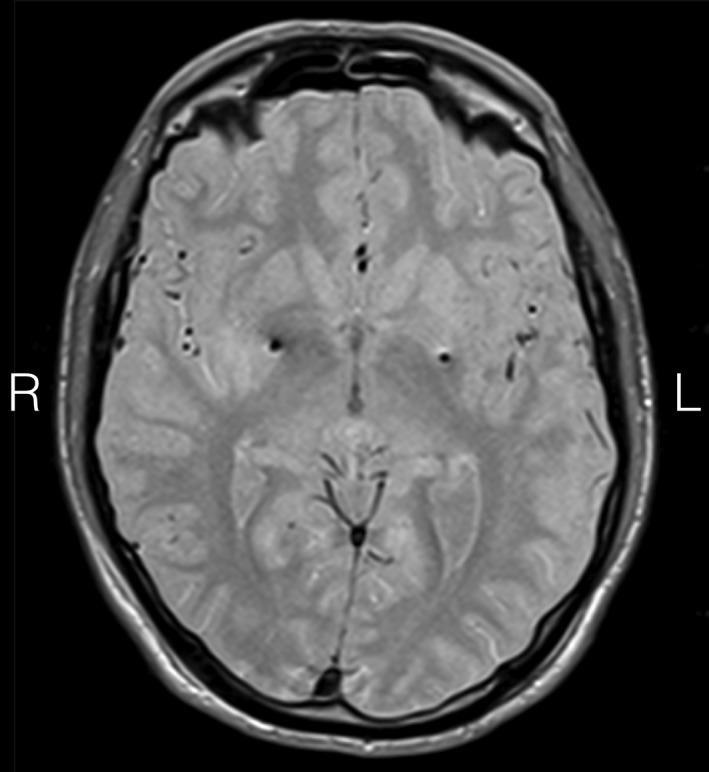
Stereotactic axial 2‐mm‐thick proton density MR scan at the level of anterior/posterior commissure showing electrode (model 3389; Medtronic, Inc., Minneapolis, MN) artifacts in the posterior GPi. Active contact coordinates measured from the mid‐commissural point were: left GPi (contact 1): x = −22.15, y = 2.63, z = −1.76; right GPi (contact 10): x = 20.86, y = 5.51, z = −1.02.
Table 1.
Results of the dystonia (BFMDRS) and myoclonus (UMRS) rating scales for the patient pre‐op, 10 days, and 8 months post‐op
| Pre‐op | 10 Days Post‐opa | 8 Months Post‐opb | % Improvement at 10 Days Post‐op | % Improvement at 8 Months Post‐op | |
|---|---|---|---|---|---|
| Dystonia (BFMDRS) | |||||
| Movement score (0–116) | 29 | 18 | 7 | 37.9 | 75.8 |
| Disability scale (0–30) | 11 | 9 | 8 | 18 | 27 |
| Σ score (0–146) | 40 | 27 | 15 | 32.5 | 62.5 |
| Myoclonus (UMRS) | |||||
| Myoclonus at rest (0–108) | 29 | 4 | 0 | 86.2 | 100.0 |
| Action myoclonus (0–160) | 62 | 71 | 15 | −14.5c | 72.3 |
| Functional tests (0–20) | 16 | 12 | 8 | 25.0 | 50.0 |
| Σ score (0–288) | 107 | 87 | 23 | 18.6 | 78.5 |
Σ score = sum score. In both scales, higher score indicate greater disability.
DBS settings 10 days after the operation: left GPi: 1‐, 2.5 V; right GPi 10‐, 1.7 V; 60 μs, 130 Hz bilaterally.
DBS settings 8 months after the operation: left GPi: 1‐, 2.4 V; right GPi 10‐, 2.0 V; 60 μs, 160 Hz bilaterally. The myoclonic component of the disorder improved better on 160 Hz.
Note that a transient worsening of the myoclonus at rest was noted directly after the operation, which improved considerably 8 months later. A transient worsening of symptoms is a known nonserious adverse effect of pallidal DBS. The mechanism of this worsening is not known, but it might be related to the lesional effect induced by electrode insertion, which abolishes the activity of the tonically active inhibitory output of the GPi that acts as a brake on the motor patterns.
BFMDRS, Burke‐Fahn‐Marsden Dystonia Rating Scale; UMRS, Unified Myoclonus Rating Scale; pre‐op, preoperation; post‐op, postoperation.
To our knowledge, this is the first report of a favorable outcome of bilateral GPi‐DBS in (variant) A‐T patient with a predominant M‐D phenotype. The role of pallidal DBS in successfully treating medically refractory primary M‐D has been reported before.5, 6 In line with findings of previous studies,5, 6 in our patient there was a rapid (within 10 days after the operation) improvement of the dystonia and myoclonus at rest; dystonia and both action myoclonus and myoclonus at rest improved further during the subsequent months. In addition to A‐T, our patient was a heterozygote (H63D) for hemochromatosis that is rarely pathological in the heterozygous state,7 but could possibly become clinically apparent when combined with A‐T mutation, as was already shown in an animal A‐T model.8 Both his sister and his father, who were never diagnosed with hemochromatosis, had also had pathological LFTs.
The basal ganglia and the pallido‐thalamo‐cortical projections have been widely implicated in the pathogenesis of dystonia and myoclonus.9 DBS of the ventral intermediate thalamic nucleus (VIM) has also been found to be as effective in the treatment of M‐D.6 Even though the precise mechanism of action of DBS is not clear, it is believed that stimulation of GPi, and also possibly VIM, leads to a stimulation‐induced regularization of neuronal patterns by preventing transmission of pathological oscillatory activity within the network.10 Indeed, the favorable outcome of GPi‐DBS in our patient suggests that this can be a possible treatment option for variant A‐T presenting with M‐D phenotype.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the First Draft, B. Review and Critique.
D.G.: 1A, 1B, 1C, 2A, 2B, 3A, 3B
D.M.: 2A, 2B, 3A
A.Z.: 1A, 1B, 1C, 3B
R.S.V.: 1A, 1B, 1C, 3B
C.M.: 1C, 2C, 3B
J.C.: 1C, 2C, 3B
E.T.: 1A, 1B, 1C, 2C, 3B
J.A.H.: 1B, 1C, 2B, 2C, 3B
L.Z.: 1B, 1C, 2C, 3B
M.H.: 1A, 1B, 1C, 2B, 2C, 3B
S.O.: 1A, 1B, 3B
T.F.: 1A, 1B, 1C, 2B, 2C, 3B
P.L.: 1A, 1B, 1C, 2A, 2B, 2C, 3B
Disclosures
Funding Sources and Conflicts of Interest: The authors report no sources of funding and no conflicts of interest.
Financial disclosures for previous 12 months: Dr Ludvic Zrinzo has received honoraria for educational purposes from Medtronic and St. Jude Medical. Prof. Marwan Hariz has received fees and travel expenses from Medtronic for speaking at meetings in educational purposes.
Supporting information
A video accompanying this article is available in the supporting information here.
Video 1. Section 1 (preoperative assessment) shows the patient before the operation: The patient presented with predominant myoclonic jerks superimposed on dystonic movements. The head and the upper limbs were most severely affected. Eye movements were abnormal with long latency to onset, slow and hypometric horizontal and vertical saccades (not shown on the video, assessed by eye‐tracker). A resting tremor and more pronounced bilateral jerky postural and kinetic tremor, with a slight past‐pointing and a few rhythmic, terminal tremulous movements, but no overt intention component on finger‐nose test, can been seen on the video. Tandem gait was difficult mainly owing to myoclonic jerks throwing him off balance. He did not have signs of peripheral neuropathy. Functionally, he was unable to perform many activities of daily living and required assistance during eating and a straw to drink. Section 2 (8 months after bilateral GPi DBS) shows a considerable improvement of the patient; there was a complete resolution of the myoclonus at rest and a substantial improvement of dystonia and action myoclonus. No resting tremor and a substantial improvement of the postural and kinetic tremor can also be seen on the video; the terminal tremor component was still present on finger‐nose testing. The patient also functionally improved after the operation—now he was able to write and use eating utensils and holding objects in his hands (e.g., he could hold and drink from a cup). His gait improved as well. He was able to perform tandem‐walking test because of the reduction of the action myoclonus.
Acknowledgments
The authors are most grateful to the patient and his family for consenting to publish this material and sharing all the useful information regarding his condition.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Verhagen MM, Abdo WF, Willemsen MA, et al. Clinical spectrum of ataxia‐telangiectasia in adulthood. Neurology 2009;73:430–437. [DOI] [PubMed] [Google Scholar]
- 2. Taylor AM, Lam Z, Last JI, Byrd PJ. Ataxia telangiectasia: more variation at clinical and cellular levels. Clin Genet 2015;87:199–208. [DOI] [PubMed] [Google Scholar]
- 3. Grunewald A, Djarmati A, Lohmann‐Hedrich K, et al. Myoclonus‐dystonia: significance of large SGCE deletions. Hum Mutat 2008;29:331–332. [DOI] [PubMed] [Google Scholar]
- 4. Aviles‐Olmos I, Kefalopoulou Z, Tripoliti E, et al. Long‐term outcome of subthalamic nucleus deep brain stimulation for Parkinson's disease using an MRI‐guided and MRI‐verified approach. J Neurol Neurosurg Psychiatry 2014;85:1419–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cif L, Valente EM, Hemm S, et al. Deep brain stimulation in myoclonus‐dystonia syndrome. Mov Disord 2004;19:724–727. [DOI] [PubMed] [Google Scholar]
- 6. Gruber D, Kuhn AA, Schoenecker T, et al. Pallidal and thalamic deep brain stimulation in myoclonus‐dystonia. Mov Disord 2010;25:1733–1743. [DOI] [PubMed] [Google Scholar]
- 7. Ekanayake D, Roddick C, Powell LW. Recent advances in hemochromatosis: a 2015 update: a summary of proceedings of the 2014 conference held under the auspices of Hemochromatosis Australia. Hepatol Int 2015;9:174–182. [DOI] [PubMed] [Google Scholar]
- 8. McDonald CJ, Ostini L, Wallace DF, John AN, Watters DJ, Subramaniam VN. Iron loading and oxidative stress in the Atm−/− mouse liver. Am J Physiol Gastrointest Liver Physiol 2011;300:G554–G560. [DOI] [PubMed] [Google Scholar]
- 9. Foncke EM, Bour LJ, Speelman JD, Koelman JH, Tijssen MA. Local field potentials and oscillatory activity of the internal globus pallidus in myoclonus‐dystonia. Mov Disord 2007;22:369–376. [DOI] [PubMed] [Google Scholar]
- 10. Hu W, Stead M. Deep brain stimulation for dystonia. Transl Neurodegener 2014;3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A video accompanying this article is available in the supporting information here.
Video 1. Section 1 (preoperative assessment) shows the patient before the operation: The patient presented with predominant myoclonic jerks superimposed on dystonic movements. The head and the upper limbs were most severely affected. Eye movements were abnormal with long latency to onset, slow and hypometric horizontal and vertical saccades (not shown on the video, assessed by eye‐tracker). A resting tremor and more pronounced bilateral jerky postural and kinetic tremor, with a slight past‐pointing and a few rhythmic, terminal tremulous movements, but no overt intention component on finger‐nose test, can been seen on the video. Tandem gait was difficult mainly owing to myoclonic jerks throwing him off balance. He did not have signs of peripheral neuropathy. Functionally, he was unable to perform many activities of daily living and required assistance during eating and a straw to drink. Section 2 (8 months after bilateral GPi DBS) shows a considerable improvement of the patient; there was a complete resolution of the myoclonus at rest and a substantial improvement of dystonia and action myoclonus. No resting tremor and a substantial improvement of the postural and kinetic tremor can also be seen on the video; the terminal tremor component was still present on finger‐nose testing. The patient also functionally improved after the operation—now he was able to write and use eating utensils and holding objects in his hands (e.g., he could hold and drink from a cup). His gait improved as well. He was able to perform tandem‐walking test because of the reduction of the action myoclonus.


