Anti‐N‐methyl‐D‐aspartate receptor (anti‐NMDAR) encephalitis can exhibit a wide spectrum of clinical features, including psychiatric symptoms, movement disorders, and autonomic dysfunction.1, 2, 3, 4 We report an unusual presentation of anti‐NMDAR encephalitis in a 31‐year‐old woman. The patient had no preceding flu‐like symptom and no history of psychotic episode or drug before symptom onset. She had experienced visual and auditory hallucinations 1 year preceding admission. Her auditory hallucinations were about a strange old woman who forced her to repeat what she said. After 6 months from onset, she had taken risperidone 8 mg at a local clinic because of suspicion of schizophrenia. Her auditory hallucination improved, but dystonia of both arms and catatonia gradually developed 3 months after risperidone treatment. Risperidone was stopped because these features were considered extrapyramidal symptoms induced by risperidone. Nevertheless, the patient's dystonia worsened over time, despite stopping risperidone treatment. After admission, the oculogyric crisis, dystonia, gait disturbance, tachycardia, and hypersalivation gradually developed and severe dysphagia caused aspiration pneumonia resulting in respiratory failure. Upon neurological examination, the patient showed rigidity and dystonia of all extremities, decreased awareness, and speech disturbance. She was then transferred to the intensive care unit. Brain T2‐weighted MRI showed increased signal intensities in the bilateral caudate nucleus, medial temporal lobe, and right insula (Fig. 1). EEG showed diffuse slow activity. Cerebrospinal fluid (CSF) analysis showed white blood cell 3/μL, red blood cell 0/μL, protein 21.7 mg/dL, glucose 78 mg/dL, and unmatched oligoclonal bands. Viral antibody (Ab) tests of CSF were negative. Serum ceruloplasmin and copper levels, 24‐hour urine copper level, serum tumor markers (alpha‐fetoprotein, carbohydrate‐antigen 19‐9, and β2 microglobulin), thyroid function test, and serum parathyroid hormone level were within normal limits. Anti‐cardiolipin Ab, anti‐double‐stranded DNA Ab, anti‐nuclear Ab, anti‐Ro/SSA and anti‐La/SSB Ab, and rheumatoid factors were all negative. There was no evidence of underlying malignancy. The patient was treated with baclofen 10 mg three times daily (TID) and clonazepam 0.5 mg TID for dystonia, but no clinical improvement was observed. Antibiotics for aspiration pneumonia and prednisolone (30 mg/day) were administered (1 year after symptom onset). Her serum and CSF was sent to Dr. Dalmau's laboratory (Department of Neurology, Hospital Clinic, University of Barcelona, Barcelona, Spain) to confirm anti‐NMDAR encephalitis, and anti‐NMDAR Ab in serum and CSF was positive. Intravenous (IV) steroid (methylprednisolone 1,000 mg) was administered for 3 days, and then IV immunoglobulin was administered for 5 days with 30 mg of oral prednisolone. In addition, plasmapheresis was performed every other day for 10 days, and cyclophosphamide (750 mg/m2) was administered 3 times every 2 weeks. The patient was then taken off the ventilator and moved to the general ward (see Video 1). The patient started to obey simple verbal commands 2 months after immunotherapy (IT). Additional doses of cyclophosphamide (750 mg/m2) were administered 6 times monthly, and prednisolone was tapered for 10 months. Seven months after IT, the patient began uttering some meaningless words, and a full range of motion was possible in all extremities. Nine months after IT, her score on the Korean version of the Mini–Mental State Examination (K‐MMSE) was 17/30. Ten months after IT, the patient could play mobile phone games. Thirteen months after IT, she could perform all daily activities independently (see Video 2). On full neuropsychological tests performed 15 months after IT, she showed normal attention and visuospatial frontal‐executive function, but abnormal language and memory functions remained, as indicated by a K‐MMSE score of 28. In patients with anti‐NMDAR encephalitis, oro‐lingual‐facial dyskinesias are the most characteristic movements, but choreoathetosis, oculogyric crisis, dystonia, rigidity, and opisthotonic postures might occur simultaneously.2 This case showed isolated psychiatric symptoms for 6 months, followed by oro‐lingual and limb dystonia and oculogyric crisis. These extrapyramidal symptoms were initially thought to be side effects of risperidone, but these symptoms continued after stopping risperidone treatment. This important clinical course suggested the possibility of anti‐NMDAR encephalitis. During the first month after disease onset, 87% of patients with anti‐NMDR encephalitis develop four or more of the following symptoms: behavioral changes, cognitive changes, memory deficit, speech disorder, loss of consciousness, movement disorders, and seizures.5, 6, 7, 8, 9 Isolated psychiatric symptoms may also exist in anti‐NMDAR encephalitis, but are uncommon, especially on the first presentation. The time from symptom onset until treatment in patients with isolated psychiatric symptoms of anti‐NMDAR encephalitis has been reported to range from 2 to 60 weeks (median, 9 weeks).8 In contrast to previous reports,5, 6, 7, 8, 9 the present case involved nearly 1 year from the onset of psychiatric symptoms to IT due to delayed diagnosis of anti‐NMDAR encephalitis. This case had an interesting clinical presentation, with good recovery despite delayed treatment, and a long duration of psychiatric symptoms alone until movement disorders developed. Early recognition of anti‐NMDAR encephalitis is important because delayed treatment is associated with poor outcome.9 Physicians should be aware that isolated psychiatric symptoms can last for months before neurological symptoms and should remain hopeful for a good prognosis because continuous IT can achieve a favorable outcome despite delayed diagnosis of anti‐NMDAR encephalitis.
Figure 1.
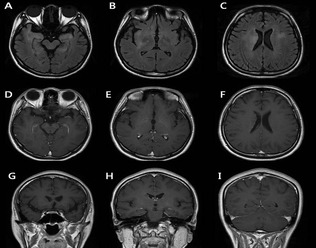
The patient's initial brain MRI. The fluid‐attenuated inversion recovery images (A–C) show hyperintensity, with no enhancement lesion on T1‐weighted images (D–I) in the bilateral medial temporal lobes, basal ganglia, right insula, and periventricular areas.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
S.W.K.: 1B, 1C, 3A, 3B
H.S.L.: 1B, 1C, 3A, 3B
P.H.L.: 1A, 1B, 1C, 3B
S.‐A.C.: 1A, 1B, 1C, 3A, 3B
Disclosures
Funding Sources and Conflicts of Interest: The authors report no sources of funding and no conflicts of interest.
Financial Disclosures for previous 12 months: Sun‐Ah Choi has received research funding from UCB BioSciences GmbH.
Supporting information
Videos accompanying this article are available in the supporting information here.
Video 1. Three months after immunotherapy, the patient still showed oculogyric crisis and dystonia of four extremities. She could not speak or walk independently, although she showed some clinical improvement compared with the previous respiratory failure status.
Video 2. Fifteen months after the immunotherapy, her dystonic posture disappeared completely and she could walk independently.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Dalmau J, Gleichman AJ, Hughes EG, et al. Anti‐NMDA‐receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008;7:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dalmau J, Lancaster E, Martinez‐Hernandez E, Rosenfeld MR, Balice‐Gordon R. Clinical experience and laboratory investigations in patients with anti‐NMDAR encephalitis. Lancet Neurol 2011;10:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Irani SR, Bera K, Waters P, et al. N‐methyl‐d‐aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non‐paraneoplastic disorder of both sexes. Brain 2010;133:1655–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Irani SR, Vincent A. NMDA receptor antibody encephalitis. Curr Neurol Neurosci Rep 2011;11:298–304. [DOI] [PubMed] [Google Scholar]
- 5. De Nayer AR, Myant N, Sindic CJ. A subacute behavioral disorder in a female adolescent. Autoimmune anti‐N‐methyl‐d‐aspartate receptor encephalitis associated with ovarian teratoma. Biol Psychiatry 2009;66:e13–e14. [DOI] [PubMed] [Google Scholar]
- 6. Tanyi JL, Marsh EB, Dalmau J, Chu CS. Reversible paraneoplastic encephalitis in three patients with ovarian neoplasms. Acta Obstet Gynecol Scand 2012;91:630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lebon S, Mayor‐Dubois C, Popea I, et al. Anti‐N‐methyl‐d‐aspartate (NMDA) receptor encephalitis mimicking a primary psychiatric disorder in an adolescent. J Child Neurol 2012;27:1607–1610. [DOI] [PubMed] [Google Scholar]
- 8. Kayser MS, Titulaer MJ, Gresa‐Arribas N, Dalmau J. Frequency and characteristics of isolated psychiatric episodes in anti‐NMDA receptor encephalitis. JAMA Neurol 2013;70:1133–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long‐term outcome in patients with anti‐NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013;12:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Videos accompanying this article are available in the supporting information here.
Video 1. Three months after immunotherapy, the patient still showed oculogyric crisis and dystonia of four extremities. She could not speak or walk independently, although she showed some clinical improvement compared with the previous respiratory failure status.
Video 2. Fifteen months after the immunotherapy, her dystonic posture disappeared completely and she could walk independently.


