Case Report
A 72‐year‐old lady presented to the neurology clinic with a constellation of symptoms that began 2 years previously with nocturnal wandering, erratic driving, and becoming withdrawn. Over the next 2 years, she developed recurrent falls, slowness and stiffness, urinary urgency, and hoarseness of voice. She was a previous heavy smoker. Family history revealed idiopathic Parkinson's disease in her father and motor neuron disease in her brother. Examination showed a broad‐based gait, stooped posture, bilateral reduced arm swing, jerky tremor on outstretched hands, mild limb rigidity, and severe symmetric bradykinesia. There was mild proximal weakness of the lower limbs with bilateral extensor plantar responses. There was dysphonia without dysarthria. Extraocular movements were normal.
A noncontrast computed tomography (CT) scan of the brain showed atrophy of the caudate nuclei and small‐vessel ischemic changes (Fig. 1A). Magnetic resonance imaging (MRI) of the brain revealed small caudate nuclei and nonspecific white matter changes on a T2‐weighted sequence (Fig. 1B) with unremarkable susceptibility‐weighted (Fig. 1C) and sagittal view fluid‐attenuated inversion recovery (Fig. 1) sequences. An 18‐fluorodeoxyglucose positron emission tomography (18FDG PET) scan of the brain showed prominent, symmetric hypometabolism in the caudate nuclei and putamina, mild left anterior temporal hypometabolism, and low‐normal cerebellar metabolic activity (Fig. 1E,F). Left and right basal ganglia activity was reduced by 6.2 and 4.7 standard deviations below the mean, respectively. Electroencephalography revealed intermittent slowing in the temporal regions. Levodopa (l‐dopa) was tried, but her condition did not respond.
Figure 1.
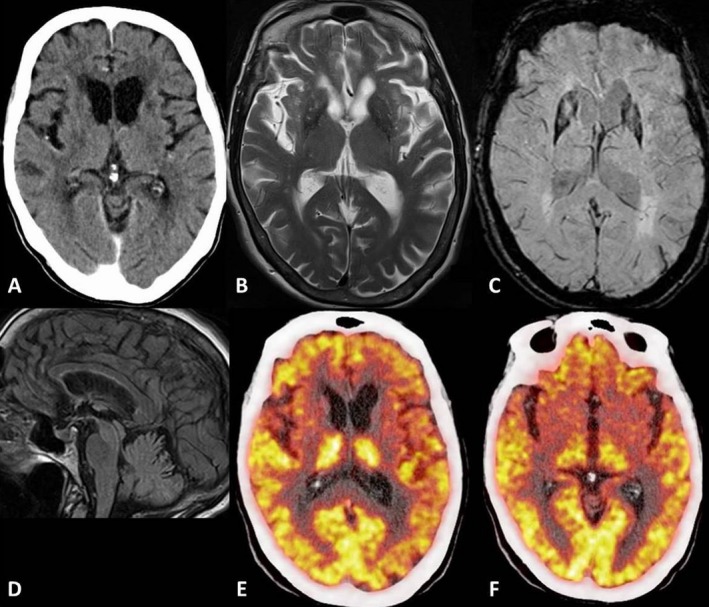
Brain images include a noncontrast computed tomography (CT) scan (A), a T2‐weighted magnetic resonance image (MRI) (B), a susceptibility‐weighted MRI (C), a sagittal view fluid attenuated inversion recovery (FLAIR) MRI (D), a fused positon emission tomography (PET) CT at the level of the caudate lobes (E), and a fused PET CT at a lower level through the basal ganglia (F).
Three years after initial symptom onset, the patient presented to hospital with dyspnea. A CT‐guided biopsy followed by pathologic analysis of a newly discovered right lung mass revealed small cell lung carcinoma. Further tests were performed. Western blot analysis of serum was positive for anticollapsin response mediator protein 5 (CRMP5) antibodies. Other onconeuronal antibodies, specifically, anti‐Hu, Yo, Ri, Ma1, Ma2, amphiphysin, Sox‐1, Zic‐4, and anti‐Tr, were negative. Antivoltage‐gated potassium channel (anti‐VGKC) and anti‐N‐methyl‐D‐aspartate (anti‐NMDA) receptor antibodies were negative. Oligoclonal immunoglobulin G bands were positive in the cerebrospinal fluid (CSF) but not in the serum, suggesting intrathecal synthesis. Further CSF analysis revealed a raised protein level at 629 mg/L and a normal white cell count of 3 cells/mL. A diagnosis was made of paraneoplastic parkinsonism. The patient commenced carboplatin and etoposide. At the time of this writing, the patient had shown clinical improvement after receiving the first three cycles of chemotherapy over 10 weeks, as demonstrated by enhanced mobility, reduced falls, and resolution of nocturnal wandering. A repeat CT scan of the thorax revealed a significant interval decrease in size of the lung lesion.
Discussion
We report a novel finding of prominent, symmetric caudate and putaminal hypometabolism on 18FDG PET imaging of the brain as a manifestation of paraneoplastic parkinsonism associated with anti‐CRMP5 antibodies. Paraneoplastic parkinsonism itself is very rare and is usually associated with anti‐Ri or anti‐Ma2 antibodies.1 There are only four cases described in the literature of anti‐CRMP5 antibody‐associated paraneoplastic parkinsonism.2, 3
Several key findings support paraneoplastic etiology. CSF analysis demonstrated an inflammatory process. The patient was poorly l‐dopa–responsive but improved with chemotherapy. The lung cancer was detected within 5 years of the diagnosis of the neurological disorder. Our patient fulfils the criteria for definite paraneoplastic neurological syndrome (PNS) based on diagnostic criteria proposed by Graus et al. in 2004.4 Another diagnostic consideration is dual pathology involving a coincidental lung malignancy with a separate Parkinsonian disease, such as that associated with C9Orf72 mutation,5 given the remarkable family history; these alternatives are unlikely, though not further investigated.
PNS associated with onconeuronal antibodies (e.g., Hu, Yo, CV2/CRMP5, Ri, Ma2, or amphiphysin) are rare, comprising from 0.1% to 1% of patients with cancer, and likely involve T‐cell–mediated pathophysiologic mechanisms.6 Notably, extrapyramidal syndromes are commonly associated with antibodies against neuronal cell‐surface antigens, e.g., NMDA receptor‐associated encephalitis.1 Although less common, onconeuronal antibody‐associated movement disorders have been described.1 Anti‐CRMP5 antibodies are usually associated with cerebellar ataxia, chorea, optic neuritis, encephalomyelitis, and sensorimotor neuropathy.3 However, the patient's clinical picture does not fit into these categories. CRMP5 is a neuronal cytoplasmic protein expressed throughout the nervous system and in small cell lung carcinoma.3 One study demonstrated localization of CRMP5 expression in the ganglionic eminence, neocortex, and hippocampus in the developing mouse brain.7
In the assessment of neurodegenerative disease with 18FDG PET, putaminal hypometabolism is described in multisystem atrophy and caudate hypometabolism in Huntington's disease and with progressive supranuclear palsy and widespread cortical hypometabolism in idiopathic Parkinson's disease.8 In our patient, cortical involvement was limited to mild left temporal hypometabolism and may have correlated with her behavioral change. There was no temporal lobe atrophy on brain MRI studies. It was proposed that a functional‐anatomic discordance between FDG PET and MRI studies frequently occurs in the investigation of PNS.9 This is an area worthy of further research.
In conclusion, this case illustrates a vital point that paraneoplastic syndromes may present unusually and mimic neurodegenerative disease. 18FDG PET may play a role in the investigation of paraneoplastic neurological syndromes.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft; B. Review and Critique.
S.M.Y.: 1A, 1C, 3A, 3B
T.L.: 1A, 1C, 3A, 3B
P.M.: 1A, 1B, 1C, 3B
B.M.: 1A, 1C, 3A, 3B
Disclosures
Funding Sources and Conflict of Interest: The authors report no sources of funding and no conflicts of interest.
Financial Disclosures for the previous 12 months: The authors report no sources of funding and no conflicts of interest.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Barton B, Zauber SE, Goetz CG. Movement disorders caused by medical disease. Semin Neurol 2009;29:97–110. [DOI] [PubMed] [Google Scholar]
- 2. Tada S, Furuta M, Fukada K, et al. Severe parkinsonism associated with anti‐CRMP5 antibody‐positive paraneoplastic neurological syndrome and abnormal signal intensity in the bilateral basal ganglia [published online ahead of print 15 September 2015]. J Neurol Neurosurg Psychiatry 2016; doi: 10.1136/jnnp-2015-311569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yu Z, Kryzer TJ, Griesmann GE, Kim K, Benarroch EE, Lennon VA. CRMP‐5 neuronal autoantibody: marker of lung cancer and thymoma‐related autoimmunity. Ann Neurol 2001;49:146–154. [PubMed] [Google Scholar]
- 4. Graus F, Delattre JY, Antoine JC, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry 2004;75:1135–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schottlaender LV, Polke JM, Ling H, et al. Analysis of C9orf72 repeat expansions in a large series of clinically and pathologically diagnosed cases with atypical parkinsonism [serial online]. Neurobiol Aging 2015;36:1221.e1–1221.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leypoldt F, Wandinger KP. Paraneoplastic neurological syndromes. Clin Exp Immunol 2014;175:336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McLaughlin D, Vidaki M, Karagogeos D. Localization of CRMP5 mRNA by in situ hybridisation during development of the mouse forebrain. Neurosci Lett 2008;432:117–120. [DOI] [PubMed] [Google Scholar]
- 8. Berti V, Pupi A, Mosconi L. PET/CT in diagnosis of movement disorders. Ann NY Acad Sci 2011;1228:93–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Basu S, Alavi A. Role of FDG‐PET in the clinical management of paraneoplastic neurological syndrome: detection of the underlying malignancy and the brain PET‐MRI correlates. Mol Imaging Biol 2008;10:131–137. [DOI] [PubMed] [Google Scholar]


