Abstract
Background
Legalization of the medical use of cannabis for Parkinson's disease (PD) has bypassed the traditional drug‐approval process, leaving physicians with little evidence with which to guide patients.
Objective
The goal of this study was to gather data on the cannabis‐related prescribing practices and views regarding potential risks and benefits of cannabis among experts caring for patients with PD.
Methods
An anonymous, 73‐item online survey was conducted through an online service (SurveyMonkey) and included neurologists at all National Parkinson Foundation Centers of Excellence.
Results
Fifty‐six responders represented centers across 5 countries and 14 states. 23% reported some formal education on cannabis. Eighty percent of responders had patients with PD who used cannabis, and 95% were asked to prescribe it. Fifty‐two percent took a neutral position on cannabis use with their patients, 9% discouraged use, and 39% encouraged it. Most believed that the literature supported use of cannabis for nausea (87%; n = 48), anxiety (60%; n = 33), and pain (86%; n = 47), but responses were divided with regard to motor symptoms. Most respondents expected that cannabis would worsen motivation (59%; n = 32), sleepiness (60%; n = 31), and hallucinations (69%; n = 37). In addition, most feared negative effects on short‐term memory (75%; n = 42), long‐term memory (55%; n = 31), executive functioning (79%; n = 44), and driving (96%; n = 54). Although many did not believe that cannabis should be recreational (50%; n = 28), most believed that it should be legalized for medicinal purposes (69.6%; n = 39).
Conclusions
This study provides data on the cannabis‐related practices, beliefs, and attitudes of expert PD physicians. There is a lack of consensus that likely reflects a general knowledge gap and paucity of data to guide clinical practice.
Keywords: Parkinson's disease, cannabis, marijuana
The legal medicinal use of cannabis is rapidly expanding in the United States. In a recent poll of over 1000 physicians practicing in 72 countries, 76% favored the use of cannabis for medicinal purposes.1 As of January 2015, 20 US states have legalized the therapeutic medical use of marijuana for patients with various debilitating conditions.2 Parkinson's disease (PD) is one of the conditions for which cannabis may now be prescribed in these states. There is a discrepancy between the lack of data and new regulations as well as requests to prescribe cannabis. The science has been relatively stagnant compared with the rapidly changing regulations over the last few years.
Cannabis is a plant substance that has been used for recreational and medicinal purposes over many centuries. Cannabis extract contains over 400 chemical compounds, some of which have been shown to have activity on central and peripheral nervous system receptors. These receptors exist for endogenous fatty acid ligands known as endocannabinoids, which impact various functions ranging from immunity and inflammation, to appetite, pain, and neuroexcitability.3 The main function of the endocannabinoid system is to regulate synaptic neurotransmission of excitatory and inhibitory circuits. In the central nervous system, CB1 (the first cannabinoid receptor) is the most abundant G‐protein–coupled receptor and is strongly expressed in the basal ganglia, cerebellum, and hippocampus.3, 4 As a regulator of neurotransmission, the cannabinoid system influences many different functions. For instance, within the basal ganglia, CB1 receptors tend to increase γ‐aminobutyric acid (GABA)ergic and inhibit glutamatergic transmission—these effects may result in reduced convulsions, dyskinesias, and tremors. CB1 is also highly expressed in afferent pathways in the dorsal spinal cord, which may produce a reduction in pain signal transmission.3
The first endocannabinoid to be discovered was anandamide. Consequently, synthetic cannabinoid‐based medicines (mostly CB1 receptor agonists), which include nabilone, nabiximols (Sativex), and dronabinol, have been approved for use in pain, anorexia, spasticity, Dravet syndrome, and chemotherapy‐induced nausea.5 Cannabis plant strains are mostly derived from species sativa or indica. The cannabis plant contains many compounds, but tetrahydrocannabinol (THC) and its breakdown product cannabinol are the main psychoactive ingredients and have the strongest CB1 agonist activity. Sativa strains have higher THC concentrations and thus produce more euphoria, but they also may produce anxiety and paranoia. Cannabidiol is the main nonpsychoactive ingredient and has lower affinity for some cannabinoid receptors as well as some antagonist properties. Cannabidiol may also modulate the effects of THC. Indica strains have more cannabidiol and result in more sedating and analgesic properties.5 Other effects, such as vasodilation, amotivation, and impaired reaction time, have been described with both strains. Discussions of potential antioxidant, anti‐inflammatory, and neuroprotective effects of the cannabis substance are mainly related to CB2 receptor activity.
Due to the increasing availability of cannabis in the absence of a regulatory label, physicians must educate themselves about evidence‐based practices to better guide PD patients toward safe and appropriate decisions. Although reviews of the evidence are valuable, barriers to best‐practice adoption include lack of awareness on the part of the physician and lack of familiarity or agreement with the evidence.6 To address these barriers, an effort to better understand current cannabis‐related practices, experiences, beliefs, and attitudes of expert physicians who treat patients with PD is necessary.
In a survey of family physicians in Colorado, 31% of respondents reported recommending medical marijuana to their patients for various indications7; however, current data are limited on practices with specific regard to both movement disorders physicians and patients with PD. Small observational studies predominantly using self‐reports on the effects of cannabis use in PD, as well as small randomized controlled trials (RCTs) of oral cannabinoids, have yielded inconsistent results.8, 9, 10, 11, 12, 13 Unfortunately, there have been no PD‐specific RCTs on the effects of smoked cannabis compared with placebo. In a recent observational assessment of the effects of smoked cannabis among 22 individuals with PD who were identified as cannabis users, improvements in motor and nonmotor outcome measures were described after cannabis was smoked14; however, the open‐label design of that study has limited the generalizability of the conclusions. In 2015, Kluger et al.5 published a review of preclinical and clinical data on cannabinoids in movement disorders. Collectively, current data suggest that there is not sufficient evidence to recommend the use of cannabis for the motor symptoms in PD, and there is even less evidence on its safety in PD. The American Academy of Neurology recently concluded that oral cannabis extract is probably ineffective for treating levodopa‐induced dyskinesias and that there was a lack of evidence to comment on any other indications.15 Our aim in the current study was to assess practices, beliefs, and attitudes among expert physicians across National Parkinson Foundation (NPF) Centers of Excellence (COEs).
Materials and Methods
We conducted an online survey of PD experts from around the world with the aim of obtaining information about cannabis‐related practices, experiences, beliefs, and attitudes on the management of patients with PD. The study involved a one‐time completion of a 73‐item online survey. Participants were recruited from NPF COEs, of which there are 40 international locations (23 in the United States). An electronic request from the NPF was sent to center directors to complete the survey. Each center director sent a request for participation among all movement disorders specialists in their department. Physicians were eligible for the study whether or not they had recommended cannabis in the last 12 months. Eligibility criteria included an ability to read and speak English fluently and having been in practice for at least a full year (physicians in training were not eligible). Survey responses were anonymous. Surveys were self‐administered via on‐line forms (SurveyMonkey, Palo Alto, CA). The survey took approximately 20 minutes to complete.
After collecting demographic information, the survey included two sections: (1) clinical use of cannabis and (2) perceived effects, beliefs, and attitudes about clinical use of cannabis. Section 1 screened respondents for clinical use (“Did you recommend cannabis at least once in the last 12 months?”) and followed with 26 questions focused on familiarity with the evidence on cannabis use.14, 15, 16, 17 Section 2 of the survey was composed of 33 questions designed to assess perceived risks and benefits of cannabis for their patients with PD and four questions on respondents’ policy positions. The questions in Section 2 were designed using a modified Delphi process drawing on input from expert movement disorders neurologists who identified common symptoms of PD that might be treated with cannabis and potential risk factors of its use. Questions regarding policy positions were designed based on a review of the literature.1, 3, 5, 7, 8, 9, 10, 12, 15 Data from the survey were coded and exported into a database for analysis. Psychometric analysis was conducted to assess the reliability of the survey questions from Section 2 on perceived risks of cannabis use. Cronbach's α was calculated as a measure of the internal consistency and reliability of these questions with the criteria of 0.7 ≤ α < 0.8 as “acceptable,” 0.8 ≤ α < 0.9 as “good,” and α ≥ 0.9 as “excellent.”
Results
The survey was sent to 40 NPF centers. It was sent first to 26 neurologists at NPF centers in the United States and then to 89 neurologists at NPF COEs around the world. The original 26 invitees received a second e‐mail, because they were included in the group of 89 neurologists. The total number of individuals invited to respond to the survey was 89, and 56 physicians responded, for a response rate of 63%. Responder demographics are presented in Table 1. Fifty‐five percent of responders were male, 57% had been in practice for more than a decade, and 52% were aged <45 years (80% were aged <55 years). The survey responders represented NPF COEs in 14 states throughout the United States (8 of which had passed laws legalizing medicinal cannabis for PD) and across 5 countries. Only 23% of responders described having any formal education on cannabis. Instead, sources of information through which responders formulated their opinions and beliefs about cannabis were most commonly “medical literature” and “personal experience.” Other sources included “the media,” “personal opinion,” and “other physicians” (Fig. 1). A large majority (80%) of responders reported cannabis use among their patients with PD, and almost all (95%) had been requested to prescribe cannabis (Table 1). Only a small minority (10%) had recommended the use of cannabis to their patients. When patients asked about cannabis, a majority of responders (52%) reported taking a neutral position on the use of cannabis by their patients, but only 9% discouraged it, whereas 39% encouraged it. Notably, practicing in a state in which the medical use of cannabis was legal was not significantly associated with encouraging its ongoing use by patients in the responses; however, there was an association with actively recommending its use (P < 0.05).
Table 1.
Demographics and Features of the Survey Responders (N = 56)*
| Variable | Responders, % |
|---|---|
| Men | 55 |
| US states represented where medicinal use is legal | n = 8 |
| US states represented where medicinal use is illegal | n = 6 |
| No. of countries represented | n = 5 |
| Responders aged ≤54 y | 80 |
| Responders in practice >10 y | 57 |
| Formal education on cannabis | 23.20 |
| Have patients that use cannabis | 80.40 |
| Asked to prescribe cannabis in last 12 mo | 94.60 |
| Tend to ask about patients’ use of cannabis | 60.70 |
| Have recommended cannabis use in last 12 mo | 10.70 |
| Have encouraged cannabis use in the last 12 mo | 39.30 |
| Have discouraged cannabis use in the last 12 mo | 8.90 |
* as of January 2015
Figure 1.
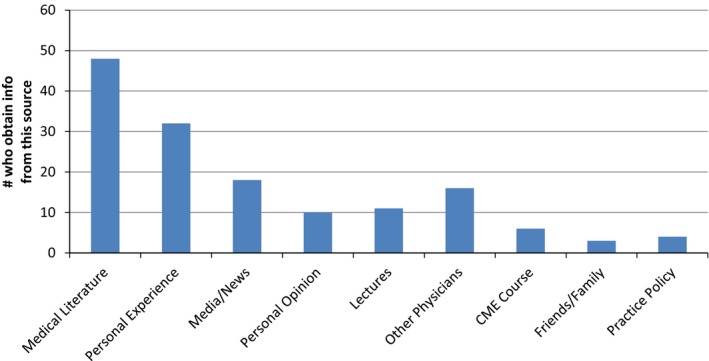
Source of Information on Cannabis. The majority of responders selected more than one source from which they obtained information to formulate opinions/beliefs about cannabis. Medical literature and personal experience were the most frequent sources of information. It is worth noting that, even at centers of excellence, there are physicians who describe their source of information as media, friends, and personal opinion. CME indicates continuing medical education.
Physicians were asked to indicate the degree to which the use of cannabis is evidence‐based for various symptoms of PD. The majority of physicians felt that the literature supported the use of cannabis for nausea (87%; n = 48), anxiety (60%; n = 33), and pain (86%; n = 47). Responses were divided with regard to motor symptoms, such as tremor (36%; n = 20) and dyskinesia (35%; n = 19). Participants were then asked what effect they expected or believed cannabis would have on various symptoms in PD (Fig. 2). The majority of physicians expected cannabis to lead to improvements in appetite (93%; n = 50), pain (85%; n = 46), nausea (80%; n = 44), and anxiety (67%; n = 37). Some positive effects were also expected for tremor, rigidity, and dyskinesias. The majority of physicians expected that cannabis would worsen motivation (59%; n = 32), sleepiness (60%; n = 31), balance (50%; n = 27), forgetfulness (76%; n = 41), and hallucinations (69%; n = 37). Physicians were then asked to indicate their beliefs regarding specific side effects of cannabis use. On average, physicians felt that cannabis would have negative effects on short‐term memory (75%; n = 42), long‐term memory (55%; n = 31), executive functioning (79%; n = 44), and driving (96%; n = 54). They also felt it could be addictive (84%; n = 47). In general, respondents were less concerned about overdose, lung cancer, or general risk to physical or mental health. The estimated Cronbach α value for the 17 questions dealing with perceived risks was 0.80 (good reliability) after two items with poor item‐total correlation were removed. Inclusion of those two items reduced the α value to 0.76, which is still acceptable reliability.
Figure 2.
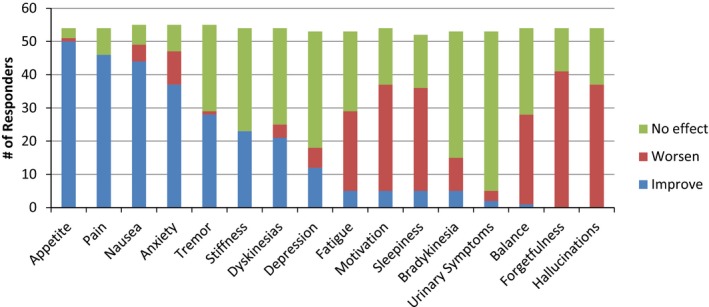
Expected Effect of Cannabis by Symptom. Physicians were asked about the extent to which they expected cannabis would improve or worsen the following Parkinson's disease‐related symptoms: “improve” included the responses “much improved” and “little improved”; “worsen” included the responses “much worse” and “a little worse”; and “no effect” represented the response “no effect.” The majority of physicians expected that cannabis would lead to improvement in appetite, pain, nausea, and anxiety. Some positive effects also were expected for tremor, rigidity, and dyskinesias. The majority of physicians expected that cannabis would worsen fatigue, motivation, sleepiness, balance, forgetfulness, and hallucinations.
Finally, respondents were asked to comment on cannabis‐related policy (Fig. 3). On average, physicians agreed that cannabis deserved more attention in the medical school curriculum (93%; n = 52). They were divided on whether the status as a Schedule I substance should be reclassified, with a slight majority agreeing that it should be reclassified (52%; n = 29). Most physicians did not feel strongly that cannabis should be made recreational (50%; n = 28), but many felt it should be allowed to be prescribed for medicinal purposes (69.6%; n = 39). Physicians from outside the United States were not significantly different from their US colleagues in their likelihood to encourage or discourage the use of cannabis.
Figure 3.
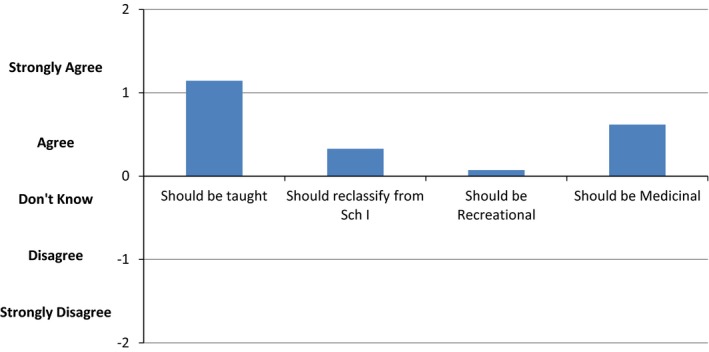
Beliefs About Policy. On average, physicians agreed that cannabis should be taught in medical schools. There was a tendency toward agreement that the status as a Schedule I (Sch I) substance should be reclassified. Most physicians did not feel strongly that cannabis should be made recreational, but many believed it should be allowed to be prescribed for medicinal purposes.
Discussion
To the best of our knowledge, this is the first study to provide data on the practices, beliefs, and attitudes of expert PD physicians with regard to cannabis use in PD. Our data demonstrate that, despite increasing use of cannabis for medicinal purposes, there is a lack of consensus among providers regarding the efficacy and scope of adverse effects of the drug as well as variability in the approach to recommendations made. The survey covered about 16 symptoms that cannabis use might improve, worsen, or not affect, and 50 respondents answered that they expected a certain effect of cannabis on all 16 symptoms. However, only two responded with the same answers to all 16 questions; of 50 respondents, there were 49 different answers. Only 4 of 16 symptoms had at least 75% agreement on the direction of the effect (improvement of appetite, pain, and nausea; no effect on urinary symptoms). We also found that, although the majority of physicians polled had been asked to prescribe cannabis, most did not provide any opinion about whether it should be used. The clear lack of consensus in practice likely reflects a general lack of knowledge and paucity of high‐quality data to guide practice. Only 23% described any formal education on the subject of cannabis, although the survey was limited in deciphering what exactly was meant by this (i.e., a whole course, a single lecture, etc.). In fact, many physicians described obtaining their information from secondary sources, such as the media and from personal experience.
With regard to the motor symptoms and motor complications of PD, there was clear division among physicians regarding their belief and expectation that cannabis could be helpful. The Cronbach α calculation showed that the survey was a reliable tool for assessing these beliefs. With regard to nonmotor symptoms, such as nausea and pain, there was greater consensus and expectation of benefit, which likely reflects the existence of a more extensive literature supporting the use of cannabis for these symptoms in other populations. Despite an equal paucity of data with regard to negative effects of cannabis in PD, physicians had more consistent concerns regarding nonmotor side effects, such as worsening fatigue, motivation, cognition, driving, and hallucinations. Most physicians did not feel that recreational use of cannabis was appropriate; however, although there was little evidence or consensus regarding specific benefits in PD, the majority did feel that physicians should have the option of prescribing it.
The survey we used contained content of a sensitive nature regarding substance use, which may have limited the validity of the results, because participants may have feared offering truthful answers due to concern over how they would be perceived and treated or because of potential legal ramifications. To address this concern, the survey was anonymous, confidential, and self‐administered. In a 2007 national survey by the US Department of Health, 35 million Americans were willing to tell government representatives that they used marijuana in the past year (available at: oas.samhsa.gov). We expect that this survey, which was conducted in a medical setting, would yield similar, if not better, response rates than a government‐sponsored survey. Another limitation of the study was that regulations on cannabis use and stigma surrounding use and prescribing practices differ from country to country, and this could impact the overall results of the study. Interpretation of the results of this survey should be done with caution, because the questions had not been previously validated. Furthermore, because this was an anonymous electronic survey, the responders themselves cannot be verified with certainty, and there is a potential for responder bias toward those people with the strongest beliefs, knowledge, or opinions about cannabis use.
This study highlights the failure of translation of scientific findings to clinical practice and the challenges associated with the introduction of a drug into clinical practice when it occurs outside of a regulatory framework. In the case of medicinal cannabis, the traditional drug‐approval process, in which a series of increasingly rigorous trials culminate in an independent review and approval, has been bypassed. In many regions, cannabis has avoided quality control, and issues related to combinations and ratios of active ingredients and sources add to the complexity of the situation.18 Ultimately, the legalization process is resulting in a much more heterogeneous level of knowledge and clinical practice patterns than is expected through the typical regulatory approval process. Seldom in the modern history of medicine has there been a similar situation.
In conclusion, the results of this survey underscore the urgent need for the widespread education of providers on the pharmacology and known risks versus benefits of cannabis. There is a need for well‐designed RTCs of cannabis in PD to establish evidence‐based data on the scope of pharmacological benefits and adverse effects in the PD population.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
D.B.: 1A, 1B, 1C, 2A, 2B, 3A, 3B
M.S.O.: 1A, 1B, 1C, 2A, 2B, 3A, 3B
X.C.: 1A, 1B, 1C, 2A, 2B, 3A, 3B
P.S.: 1A, 1B, 1C, 2A, 2B, 3A, 3B
T.S.: 1A, 1B, 1C, 2A, 2B, 3A, 3B
Disclosures
Funding Sources and Conflicts of Interest: The authors report no sources of funding and no conflicts of interest.
Financial Disclosures for the previous 12 months: Dr. Bega has received royalties from the British Medical Journal and research support from the National Parkinson Foundation (NPF); he has participated as a site principal investigator and/or coinvestigator for several National Institutes of Health (NIH)‐sponsored, foundation‐sponsored, and industry‐sponsored trials over the years but has not received honoraria. Dr. Simuni has served as a consultant and received honorarium from Novartis, Ibsen, General Electric, UCB Pharma, TEVA, IMPAX, Merz, Boehringer Ingelheim, the NPF, and GSK; in addition, she has received research support from the NIH, the Michael J. Fox Foundation, TEVA, IMPAX, the NPF, and Northwestern Memorial Foundation. Dr. Okun serves as a consultant for the NPF and has received research grants from the NIH, the NPF, the Michael J. Fox Foundation, the Parkinson Alliance, the Smallwood Foundation, the Bachmann‐Strauss Foundation, the Tourette Syndrome Association, and the UF Foundation; he has previously received honoraria but, in the past >60 months, has received no support from industry; he has received royalties for publications from Demos, Manson, Amazon, Smashwords, Books4Patients, and Cambridge (movement disorders books); he is an associate editor for the New England Journal of Medicine Journal Watch Neurology and has participated in continuing medical education and educational activities on movement disorders (in the last 36 months) sponsored by PeerView, Prime, QuantiaMD, WebMD, MedNet, Henry Stewart, and Vanderbilt University (the institution, and not Dr. Okun, receives grants from Medtronic, Abbvie, Allergan, and ANS/St. Jude, and the principal investigator has no financial interest in these grants); and he has participated as a site principal investigator and and/or coinvestigator for several NIH‐sponsored, foundation‐sponsored, and industry sponsored trials over the years but has not received honoraria. Dr. Chen and Dr. Schmidt report no sources of funding and no conflicts of interest.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Adler JN, Colbert JA. Clinical decisions. Medicinal use of marijuana—polling results [serial online]. N Engl J Med 2013;368:e30. [DOI] [PubMed] [Google Scholar]
- 2. Illinois General Assembly . Public Health (410 ILCS 130/): Compassionate Use of Medical Cannabis Pilot Program Act. Available at: http://www.ilga.gov/legislation/ilcs/ilcs3.asp?ActID=3503&ChapterID=35 last viewed 4/4/2016.
- 3. Baker D, Pryce G, Giovannoni G, Thompson AJ. The therapeutic potential of cannabis. Lancet Neurol 2003;2:291–298. [DOI] [PubMed] [Google Scholar]
- 4. Benarroch E. Endocannabinoids in basal ganglia circuits: implications for Parkinson disease. Neurology 2007;69:306–309. [DOI] [PubMed] [Google Scholar]
- 5. Kluger B, Triolo P, Jones W, Janovic J. The therapeutic potential of cannabinoids for movement disorders. Mov Disord 2015;30:313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, Rubin HR. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999;282:1458–1465. [DOI] [PubMed] [Google Scholar]
- 7. Kondrad E, Reid A. Colorado family physicians’ attitudes toward medical marijuana. J Am Board Fam Med 2013;26:52–60. [DOI] [PubMed] [Google Scholar]
- 8. Carroll CB, Bain PG, Teare L, et al. Cannabis for dyskinesia in Parkinson disease: a randomized double‐blind crossover study. Neurology 2004;63:1245–1250. [DOI] [PubMed] [Google Scholar]
- 9. Finseth TA, Hedeman JL, Brown RP 2nd, Johnson KI, Binder MS, Kuger BM. Self‐reported efficacy of cannabis and other complementary medicine modalities by Parkinson's disease patients in Colorado [serial online]. Evid Based Complement Alternat Med 2015;2015:874849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frankel JP, Hughes A, Lees AJ, Stern GM. Marijuana for parkinsonian tremor [letter]. J Neurol Neurosurg Psychiatry 1990;53:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sieradzan KA, Fox SH, Hill M, Dick JP, Crossman AR, Brotchie JM. Cannabinoids reduce levodopa‐induced dyskinesia in Parkinson's disease: a pilot study. Neurology 2001;57:2108–2111. [DOI] [PubMed] [Google Scholar]
- 12. Venderova K, Ruzicka E, Vorisek V, Visnovsky P. Survey on cannabis use in Parkinson's disease: subjective improvement of motor symptoms. Mov Disord 2004;19:1102–1106. [DOI] [PubMed] [Google Scholar]
- 13. Chagas MH, Zuardi AW, Tumas V, et al. Effects of cannabidiol in the treatment of patients with Parkinson's disease: an exploratory double‐blind trial. J Psychopharmacol 2014;28:1088–1098. [DOI] [PubMed] [Google Scholar]
- 14. Lotan I, Treves TA, Roditi Y, Djaldetti R. Cannabis (medical marijuana) treatment for motor and non‐motor symptoms of Parkinson disease: an open‐label observational study. Clin Neuropharmacol 2014;37:41–44. [DOI] [PubMed] [Google Scholar]
- 15. Koppel BS, Brust JC, Fife T, Bronstein J, Youssof S, Gronseth G, Gloss D. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2014;82:1556–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Gastel WA, Wigman JT, Monshouwer K, Kahn RS, van Os J, Boks MP, Vollebergh WA. Cannabis use and subclinical positive psychotic experiences in early adolescence: findings from a Dutch survey. Addiction 2012;107:381–387. [DOI] [PubMed] [Google Scholar]
- 17. Webb CW, Webb SM. Therapeutic benefits of cannabis: a patient survey. Hawaii J Med Public Health 2014;73:109–111. [PMC free article] [PubMed] [Google Scholar]
- 18. Saper CB. Up in smoke: a neurologist's approach to “medical marijuana”. Ann Neurol 2015;77:13–14. [DOI] [PubMed] [Google Scholar]


