Abstract
Background
Cerebral small vessel disease (SVD) is cross‐sectionally associated with gait disturbances, however, the relation between baseline SVD and gait decline over time is uncertain. Furthermore, diffusion tensor imaging (DTI) studies on gait decline are currently lacking.
Objective
To investigate the association between baseline imaging SVD markers and gait decline.
Methods
In 2006, 310 participants from the RUN DMC cohort, a prospective cohort with older adults aged 50–85 years with SVD, were included. Gait variables were assessed using a computerized walkway during baseline and follow‐up. Linear and logistic regression analyses were used to investigate the relation between imaging measures and gait decline and incident gait impairment (speed ≤ 1.0 m/s). Tract‐based spatial statistics (TBSS) was used to identify possible differences in DTI measures of white matter tracts between participants with and without incident gait impairment.
Results
Mean age was 63.3 years (SD: 8.4) and mean follow‐up duration 5.4 years (SD: 0.2). No significant associations between imaging measures and gait decline were found. TBSS analysis revealed no significant differences in DTI measures between participants with and without incident gait impairment after additional adjustment for SVD. In sub‐analyses, a high total WMH volume (OR: 2.8 for highest quartile, 95% CI: 1.1–7.1) and high infratentorial WMH volume (OR: 1.8 per SD increase, 95% CI: 1.1–2.9) were associated with an increased 5‐year risk of gait impairment, although this was not significant after correction for multiple testing.
Conclusion
Baseline imaging SVD markers were not associated with gait decline or incident gait impairment after 5 years. Future studies should investigate if SVD progression is related to gait deterioration.
Keywords: cerebral small vessel disease, gait, magnetic resonance imaging, diffusion tensor imaging
Introduction
Gait impairment has a major impact on the quality of life of older adults and is associated with adverse outcomes including decline in activities of daily living, falls, cognitive impairment, hospitalization, and death.1, 2, 3 Cerebral small vessel disease (SVD) has been identified as a possible risk factor of gait impairment, albeit mainly in cross‐sectional studies.4, 5 Few studies have investigated the relation between SVD and gait decline over time, often only by taking white matter hyperintensities (WMHs) into account, whereas the spectrum of traditional SVD markers also includes lacunes, microbleeds, and brain atrophy. These previous studies showed conflicting results, reporting no,6 or weak positive associations,7, 8, 9 of which some found a dose‐dependent effect,8, 9 while others postulated a threshold effect of WMHs7 after which gait decline became apparent.
Possibly, the underlying microstructural integrity of the white matter (WM), which can be assessed by diffusion tensor imaging (DTI), plays a role in gait decline. It has been suggested that changes in WM integrity precede the development of WMHs.10 Gait impairment has been cross‐sectionally associated with WM integrity,11 however, studies on gait decline using DTI are currently lacking.
There is limited evidence about a clinical relevant change in gait speed.2 However, a gait speed <1.0 m/s has been consistently associated with major adverse health‐related outcomes in well‐functioning older adults,3 which might therefore be a clinical useful cutpoint for the development of gait impairment in clinical practice. To our knowledge, no previous studies have investigated the relation between SVD and the development of gait impairment (speed < 1.0 m/s).
The aim of this study was to investigate whether baseline SVD, including conventional MRI and DTI markers of SVD, is associated with gait decline and incident gait impairment after 5 years of follow‐up. This study may provide insight into the role of SVD and gait deterioration and could possibly help to identify adults with SVD at highest risk for gait decline and incident gait impairment.
Methods
Study Population
This study is part of the Radboud University Nijmegen Diffusion tensor and Magnetic resonance Cohort study (RUN DMC study), which studies the risk factors and clinical consequences of brain changes as assessed by MRI in 503 participants with SVD. The recruitment, study rationale, and protocol of the RUN DMC study have been described in detail elsewhere.12 A SVD diagnosis was made based on the results of brain imaging and included the presence of WMHs and/or lacunes of presumed vascular origin.13 In 2006, baseline data collection was performed. Inclusion criteria were age 50–85 years and SVD on brain imaging. Main exclusion criteria were: parkinsonism, dementia, life expectancy <6 months, non‐SVD related WM lesions (e.g., multiple sclerosis), and MRI contra‐indications.12
Follow‐up assessment was performed in 2011–2012. Of the 503 baseline participants, 398 participated in the follow‐up examination. For the present study, we excluded 88 participants, yielding a final sample of 310 (see flowchart Fig. 1). All participants signed an informed consent form. The Medical Review Ethics Committee region Arnhem‐Nijmegen approved the study.
Figure 1.
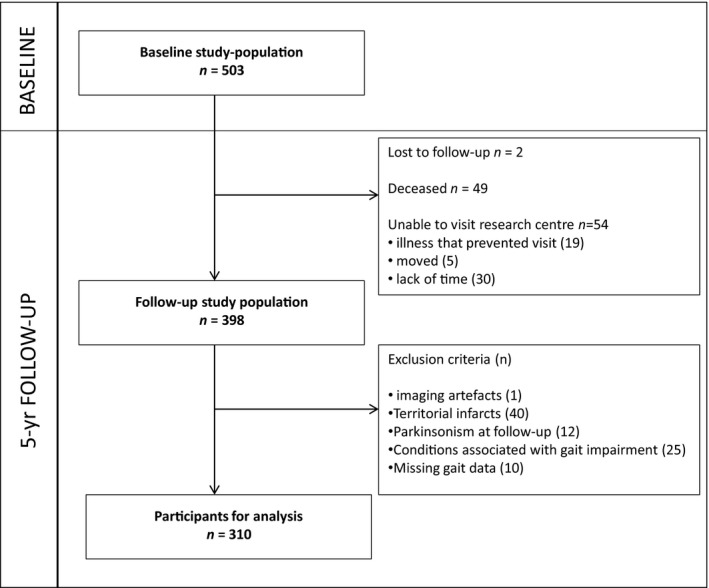
Flowchart of the Study Population. Of the 503 baseline participants, two participants were lost to follow‐up, 49 had died and 54 refused an in‐person follow‐up examination, but their clinical endpoints were available; 398 participated in the follow‐up assessment. For the present study, we included 310 participants, 88 participants were additionally excluded because of (1) baseline T1‐T2 artifacts (n = 1), (2) territorial infarcts at baseline imaging (n = 40), because these infarcts were considered as a potential confounder, (3) parkinsonism during follow‐up examination (n = 12), because apart from SVD other pathologies as amyloidopathy, Lewy body pathology and nigrastriatal dopaminergic loss could play a role in gait deterioration in these patients, (4) other conditions associated with gait impairment which prevent participants from walking unaided (n = 25) (e.g., joint fusion, severe arthritis, severe polyneuropathy, leg amputation, severe vision problems, severe cardiac or respiratory diseases, severe peripheral arterial disease, and psychogenic gait disturbance) at baseline and/or follow‐up and (5) missing data on follow‐up GAITRite (n = 10) (because they were wheelchair bound, because of home visit or technical problems), yielding a final sample of 310 participants.
Gait Measurement and Gait Impairment
Quantitative gait analysis was performed using a 5.6 meter electronic portable walkway (GAITRite, MAP/CIR Inc., Havertown, PA), which has an excellent test‐retest reliability and validity.14, 15 Each participant was instructed to walk twice over the walkway at a self selected usual gait speed. In order to measure steady‐state walking, participants started 2 m before the walkway and stopped 2 m behind it. The following gait parameters were averaged over two walks: gait speed (m/s) and its components stride length (m) (the distance between the heel points of two consecutive footprints of the same foot) and cadence (number of steps per minute). Changes over time in these gait parameters were calculated as the difference between follow‐up and baseline assessment. We considered a gait speed decline of ≥0.1 m/s as a significant decline.1, 16 Gait impairment was defined as a gait speed <1.0 m/s.3
MRI Protocol
All participants underwent a cerebral MRI on a 1.5‐Tesla Magnetom Sonata scanner (Siemens Medical Solutions, Erlangen, Germany) at baseline. The protocol included the following scans: a T1‐weighted, 3D magnetization‐prepared rapid gradient‐echo (MP‐RAGE) imaging (time repetition [TR] = 2250 ms, time echo [TE] = 3.68 ms, inversion time [TI] = 850 ms, flip angle = 15°, voxel size 1.0 × 1.0 × 1.0 mm); a Fluid‐attenuated inversion recovery (FLAIR) sequence (TR = 9000 ms, TE = 84 ms, TI = 2200 ms, voxel size 1.0 × 1.2 × 5.0 mm, with an interslice gap of 1 mm); a transversal T2*weighted gradient echo sequence (TR = 800 ms, TE = 26 ms, voxel size 1.3 × 1.0 × 6.0 mm and interslice gap of 1 mm) and a DTI sequence (TR = 10100 ms, TE = 93 ms, voxel size 2.5 × 2.5 × 2.5 mm; 4 unweighted scans, 30 diffusion weighted scans with b‐value = 900 s/mm²).
MRI Analysis
WMHs were manually segmented on the FLAIR images and total WMH volume was calculated by summing all segmented areas multiplied by slice thickness, with a good interrater variability (intraclass correlation coefficient: 0.99). WMHs were also determined in predefined regions taken from an inversely normalized Talairach‐based atlas,17 and included frontal, parietal, occipital, temporal lobes; and sublobar (basal ganglia, thalamus, internal and external capsule, insula), limbic (cingulate gyrus), and infratentorial (brainstem and cerebellum) areas. The ratings of lacunes and microbleeds were rated according to the recently published Standards for Reporting Vascular changes on nEuroimaging (STRIVE)18 by trained raters blinded to clinical information (intrarater and interrater reliabilities: for lacunes: weighted kappa values 0.87 and 0.95, respectively, and for microbleeds: 0.85 and 0.86, respectively).19
To obtain gray matter (GM) and WM and cerebrospinal fluid (CSF) volume automated segmentation on T1 images was done using Statistical Parametric Mapping 12 unified segmentation routines (SPM12; Wellcome Department of Cognitive Neurology, University College London, United Kingdom; http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). The volumes were calculated by summing all the voxel volumes belonging to that tissue class. All images were visually checked for co‐registration errors and motion and/or segmentation artifacts. All volumes were normalized to the total intracranial volume (sum of GM, WM, and CSF)20 to adjust for head size. GM volume was composed of the volume of the neocortex, basal ganglia, and thalamus.
DTI Analysis
Diffusion data were preprocessed and analyzed according to a previous described procedure.12 The diffusion‐weighted images of each participant were realigned on the mean of the unweighted image using mutual information based co‐registration routines from SPM5. The diffusion tensor21 and its eigenvalues were estimated using linear regression using an SPM5 add‐on (http://sourceforge.net/projects/spmtools). Spurious negative eigenvalues were set to zero, after which the tensor derivates fractional anisotropy (FA) and mean diffusivity (MD) were calculated.22 The mean unweighted image was used to compute the co‐registration parameters to the anatomic T1 reference image, which were then applied to all diffusion‐weighted images and results. All images were visually checked for motion artifacts and co‐registration errors. The mean FA and MD were then calculated in the total WM.
For the tract‐based spatial statistics (TBSS) analysis, DTIFit within the FSL toolbox was used to generate FA and MD images, which were imported into the TBSS pipeline.23 To create a FA skeleton, the mean FA image was thinned and subsequently this skeleton was thresholded at 0.3 to include major WM tracts.
Cognition and Other Measurements
Global cognitive function was evaluated by the Mini‐Mental State Examination (MMSE)24 and the Cognitive Index, a constructed compound score. The cognitive index was calculated as the mean of the z‐scores of the Speed‐Accuracy Tradeoff (SAT) score of the 1‐letter subtask of the Paper‐Pencil Memory Scanning Task, the mean of the SAT score of the reading task of the Stroop test, the mean of the Symbol‐Digit Substitution task and the mean of the added score on the three learning trials of the Rey Auditory Verbal learning test and the mean of the delayed recall of this test.25 To adjust for the number of faults in the Paper‐Pencil Memory Scanning Task and the Stroop test, we used SAT scores (accuracy [%]/reaction time). Barthel index (range: 0–20) was used to assess functional independence.26
Statistical Analysis
Statistical analyses were performed with IBM SPSS Statistics 20 for Windows.
To compare the baseline characteristics between participants who were included in this study and those who dropped out, age and sex‐adjusted ANCOVA or logistic regression were used. Gait parameters assessed during baseline and follow‐up were compared using paired t‐tests. The associations between baseline imaging measures and changes in gait parameters were assessed using multiple linear regression analysis. Adjustments were made for follow‐up duration and baseline age, sex, height, gait parameters, cognitive index and GM volume (when investigating conventional SVD markers and DTI measures) and/or conventional SVD markers (when investigating GM volume and DTI measures). WMH volume was log transformed, because of the skewed distribution. To ensure that multicollinearity was not present, variance inflation factor (VIF) was calculated for all regression models presented. The VIF scores were low (<3) for all models (VIF‐scores >5 are considered to reflect high multicollinearity). Data were presented as standardized beta's.
Logistic regression analysis was used to calculate odds ratios (OR) and 95% confidence intervals (CI) to quantify the relation between baseline imaging measures and incident gait impairment (<1.0 m/s), adjusted for the same confounders as described above.
Results with a P‐value <0.05 were considered significant. Bonferroni corrections were used to correct for multiple testing.
To compare voxel‐wise analyses of DTI measures (FA and MD) between participants with incident gait impairment (n = 48) and those without gait impairment (n = 240) a two‐sample t‐test was performed, using a permutation‐based statistical interference as part of FSL toolbox (‘randomize’), with a standard number of permutation tests set a 5000. Adjustments were made for follow‐up duration, baseline age, sex, height, gait speed, cognitive index and total brain volume and additionally for conventional SVD markers. Four participants were excluded because of missing values of microbleeds and DTI artifacts.
Results
Characteristics of the study population are shown in Table 1. Mean age of the study population at baseline was 63.3 years (SD: 8.4) and mean follow‐up duration was 5.4 years (SD: 0.2). Those who were excluded were older, had a slower gait, had smaller GM and WM volumes, higher WMH volume, higher presence of lacunes and lower FA and higher MD parameters at baseline in comparison to those who participated (Table 1).
Table 1.
Baseline characteristics of the study sample
| Characteristics | Participants included | Participants not included | P‐ value for difference |
|---|---|---|---|
| Demographics | n = 310 | n = 193 | |
| Age (SD), years | 63.3 (8.4) | 69.5 (8.1) | <0.001a |
| Male sex, No. (%) | 173 (55.8) | 111 (57.5) | 0.80b |
| MMSE score, mean (SD) | 28.4 (1.5) | 27.7 (1.8) | 0.005a |
| Cognitive index, mean (SD) | 0.24 (0.74) | −0.42 (0.67)c | <0.001a |
| Barthel index, mean (SD) | 19.8 (0.5) | 19.5 (1.2) | 0.002a |
| Gait characteristics | n = 310 | n = 189 d | |
| Gait speed, mean (SD), m/s | 1.37 (0.22) | 1.13 (0.29) | <0.001a |
| Gait impairment (gait speed <1.0 m/s), No. (%) | 18 (5.8) | 52 (27.5) | <0.001b |
| Imaging measures d | n = 310 | n = 192e | |
| WMH volume, median (IQR), mL | 5.1 (2.9–12.0) | 13.3 (5.9–25.5) | <0.001a |
| Lacunes, presence, No. (%) | 56 (18.1) | 78 (40.6) | <0.001b |
| Microbleeds, presencef, No. (%) | 43 (14.0) | 38 (19.9) | 0.82b |
| WM volume, mean (SD), mL | 472.9 (37.9) | 450.6 (50.3) | 0.02a |
| GM volume, mean (SD), mL | 628.4 (46.9) | 596.0 (50.4) | <0.001a |
| WM global FAg, mean (SD) | 0.33 (0.02) | 0.32 (0.02) | 0.007a |
| WM global MDg, mean (SD), x10‐3 mm2/s | 0.88 (0.04) | 0.91 (0.04) | 0.008a |
FA, fractional anisotropy; GM, gray matter; IQR , interquartile range; MD, mean diffusivity; MMSE, Mini Mental State Examination; WM, white matter; WMH, WM hyperintensity
Age and sex adjusted using ANCOVA.
Age and sex adjusted using logistic regression.
1 participant was excluded because of missing cognitive data.
4 participants had missing values of baseline gait speed.
Brain volumes are represented normalized to the total intracranial volume.
1 participants was excluded because of imaging artifacts
Respectively 3 (in group included in analysis) and 1 participant(s)(in group not included in analysis) were excluded because of missing values of baseline microbleeds.
Respectively 2 (in group included in analysis) and 1 participant(s) (in group not included in analysis) were excluded because of baseline DTI artifacts.
Of 310 participants, 48 (15.5%) developed gait impairment during follow‐up, 18 had already an impaired gait at baseline and 244 participants maintained a gait speed above 1.0 m/s at follow‐up. In total, 11.6% showed no gait decline and 71.9% had gait decline of ≥0.1 m/s. After 5 years of follow‐up, there was a significant reduction in gait speed, stride length and cadence in the total study population (Table 2).
Table 2.
Comparison of GAITRite parameters at baseline and follow‐up
| GAITRite parameters | Total study population | P‐valuea | No gait impairment | p‐valuea | Incident gait impairment | P‐valuea | Baseline gait impairment | P‐valuea |
|---|---|---|---|---|---|---|---|---|
| n = 310 | n = 244 | n = 48 | n = 18 | |||||
| Gait speed (m/s) | ||||||||
| Baseline | 1.37 (0.22) | <0.001 | 1.43 (0.18) | <0.001 | 1.22 (0.17) | <0.001 | 0.92 (0.08) | 0.011 |
| Follow‐up | 1.18 (0.22) | 1.26 (0.15) | 0.89 (0.08) | 0.78 (0.23) | ||||
| Stride length (m) | ||||||||
| Baseline | 1.45 (0.19) | <0.001 | 1.50 (0.16) | <0.001 | 1.33 (0.16) | <0.001 | 1.11 (0.12) | <0.001 |
| Follow‐up | 1.25 (0.19) | 1.32 (0.13) | 1.03 (0.11) | 0.92 (0.23) | ||||
| Cadence (steps/min) | ||||||||
| Baseline | 113.7 (9.4) | 0.01 | 115.3 (8.9) | 0.64 | 110.6 (8.2) | <0.001 | 100.5 (5.8) | 0.81 |
| Follow‐up | 112.6 (9.8) | 115.1 (8.4) | 104.9 (8.8) | 99.7 (10.2) | ||||
Data represent mean (SD).
P‐value for difference between baseline and follow‐up gait parameters calculated with a paired t‐test.
There were no significant associations between the baseline conventional SVD markers (WMH volume, WM and GM volume and the number of lacunes and microbleeds) and DTI measures of the WM and changes in gait parameters (including gait speed, stride length, and cadence) (Table 3) and incident gait impairment (Table 4).
Table 3.
Relation between baseline imaging measures and changes in gait
| Baseline imaging characteristics (n = 310) | Change in gait parameters | ||
|---|---|---|---|
| Δ Gait speed (m/s) | Δ Stride length (m) | Δ Cadence (steps/min) | |
| WMH volumea, per SDb, mL | −0.04 | −0.09 | 0.03 |
| Lacunes, per numberb | 0.01 | 0.01 | −0.02 |
| Microbleedsc, per numberb | 0.04 | 0.03 | 0.06 |
| WM volume, per SDb | 0.10 | 0.09 | 0.07 |
| GM volume, per SDd | 0.07 | 0.09 | −0.02 |
| WM global FAe, per SDb , d | −0.02 | −0.05 | 0.04 |
| WM global MDe, per SDb , d | 0.05 | 0.04 | 0.01 |
FA, fractional anisotropy; GM, gray matter; MD, mean diffusivity (x10−4 mm2/s); WM, white matter; WMH, WM hyperintensity
Data are standardized beta‐values.
All covariates are adjusted for time between baseline and follow‐up assessment and the following baseline covariates: age, sex, height, gait parameters, cognitive index.
log transformed.
Adjusted in addition for gray matter volume.
3 participants were excluded because of missing values of microbleeds at baseline.
Adjusted in addition for SVD markers (WMH volume, number of lacunes and microbleeds and WM volume).
2 participants were excluded for DTI analyses because of baseline DTI artifacts.
P < 0.05.
Table 4.
Relation between baseline imaging measures and the risk of incident gait impairment at follow‐up
| Baseline imaging characteristics (n = 292)a | Odds ratio (95% CI) for incident gait impairmentb (n = 48) | P‐value |
|---|---|---|
| WMH volume, per SD | 1.35 (0.93–1.96)c | 0.12 |
| Lacunes, presence | 0.90 (0.33–2.48)c | 0.84 |
| Microbleeds, presenced | 1.55 (0.57–4.24)c | 0.39 |
| WM volume, per SD | 0.98 (0.63–1.51)c | 0.92 |
| GM volume, per SD | 0.89 (0.52–1.53)e | 0.68 |
| WM global FA, per SDf | 0.98 (0.58–1.23)c , e | 0.95 |
| WM global MD, per SDf | 0.98 (0.51–1.88)c , e | 0.94 |
FA, fractional anisotropy; GM, gray matter; MD, mean diffusivity (*10‐4mm2/s); per SD, odds ratios per standard deviation difference from the mean; WM, white matter; WMH, WM hyperintensity.
All covariates are adjusted for time between baseline and follow‐up assessment and the following baseline covariates: age, sex, height, gait speed, cognitive index .
18 participants with baseline gait speed impairment were excluded from this analysis.
Defined as a gait speed <1.0 m/s at follow‐up.
Adjusted in addition for gray matter volume.
3 participants were excluded because of missing values of microbleeds at baseline.
Adjusted in addition for SVD markers (WMH volume, number of lacunes and microbleeds and WM volume).
2 participants were excluded for DTI analyses because of baseline DTI artifacts.
The TBSS analysis revealed higher MD values in multiple WM tracts in participants with incident gait impairment compared to those without (Fig. 2). However, these differences were not significant after additional adjustment for conventional SVD markers. For FA values no significant differences were found between both groups (data not shown).
Figure 2.
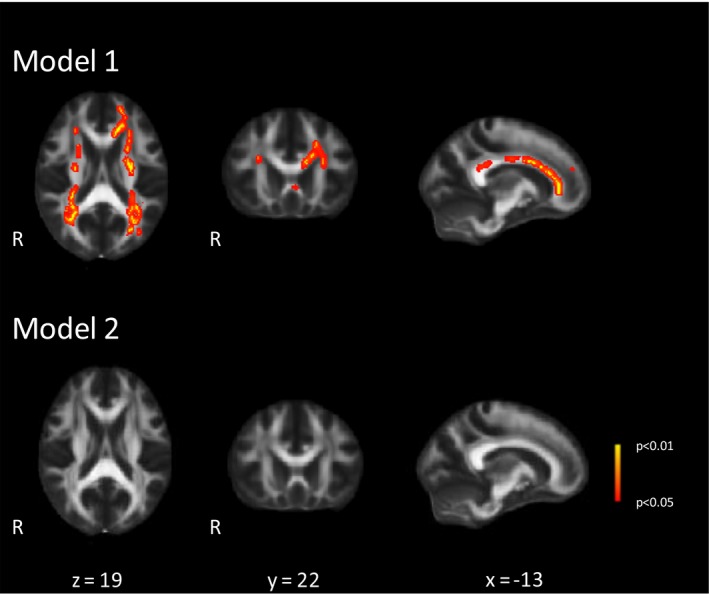
Differences in baseline mean diffusivity (MD) values between participants with and without incident gait impairment. Voxel‐wise analysis of the differences in MD values between participants with incident gait impairment (n = 48) and without gait impairment (n = 240; four participants were additionally excluded because of missing values of microbleeds and DTI artifacts). Adjusted for follow‐up duration, baseline age, sex, height, gait speed, cognitive index and normalized total brain volume (Model 1) and additionally for conventional SVD markers (WMH volume, number of lacunes and microbleeds) (Model 2), performed with a two sample t‐test, thresholded at P < 0.05 and corrected for multiple comparisons. These images are superimposed onto the spatially normalized Montreal Neurological Institute (MNI) stereotactic space FA map. R indicates right side. The x, y, and z coordinates represent the MNI coordinates of each slide.
In sub‐analyses, a possible threshold effect was seen for WMH volume; participants with the highest quartile of WMH volume (>11.6 mL) had an increased 5‐year risk for the development of gait impairment (OR: 2.8, 95% CI: 1.1–7.1, P = 0.03 in comparison to participants with the 1st–3rd quartiles of WMH volume, range: 0.6–11.6 mL), although this remained not significant after correction for multiple testing (data not shown). No threshold effects were found for the other imaging measures.
The region‐specific sub‐analyses of WMHs, showed that baseline WMH volume in the infratentorial region (brainstem and cerebellum) was associated with gait decline (gait speed decline β = −0.22, P = 0.008; stride length decline β = −0.18, P = 0.03; cadence decline β = −0.24, P = 0.01) (Table 5) and incident gait impairment after 5 years of follow‐up (OR: 1.8 per SD increase, 95% CI: 1.1–2.9, P = 0.02) (Table 6), although this either was not significant after correction for multiple testing.
Table 5.
Relation between baseline WMH volume per location and changes in gait
| Baseline WMH volume per location (n = 310) | Change in gait parameters | ||
|---|---|---|---|
| Δ Gait speed (m/s) | Δ Stridelength (m) | Δ Cadence (steps/min) | |
| Frontal lobe WMH volumea | −0.06 | −0.09 | 0.15 |
| Parietal lobe WMH volumea | 0.01 | 0.05 | −0.02 |
| Temporal lobe WMH volumea | −0.04 | −0.03 | −0.10 |
| Occipital lobe WMH volumea | −0.05 | −0.05 | −0.06 |
| Sublobar WMH volumea | 0.22 | 0.18 | 0.17 |
| Limbic WMH volumea | −0.01 | 0.03 | −0.03 |
| Infratentorial WMH volumea | −0.22b | −0.18b | −0.24b |
WMH, white matter hyperintensity.
Data are standardized beta‐values.
All covariates are adjusted for time between baseline and follow‐up assessment and the following baseline covariates: age, sex, height, gait parameters, cognitive index, gray matter volume and total WMH volume.
log transformed.
P < 0.05.
Bold values indicate significance after Bonferonni correction (P < 0.007).
Table 6.
Relation between baseline WMH volume per location and the risk of incident gait impairment at follow‐up
| Baseline imaging characteristics (n = 292)a | Odds ratio (95% CI) for incident gait impairmentb (n = 48) | P‐value |
|---|---|---|
| Frontal lobe WMH volume, per SD | 0.34 (0.09–1.22) | 0.34 |
| Parietal lobe WMH volume, per SD | 0.75 (0.34–1.64) | 0.47 |
| Temporal lobe WMH volume, per SD | 1.49 (0.73–3.03) | 0.27 |
| Occipital lobe WMH volume, per SD | 1.20 (0.74–1.96) | 0.46 |
| Sublobar lobe WMH volume, per SD | 0.63 (0.28–1.45) | 0.63 |
| Limbic lobe WMH volume, per SD | 1.13 (0.42–3.07) | 0.81 |
| Infratentorial WMH volume, per SD | 1.77 (1.10–2.85) | 0.02 |
Per SD, odds ratios per standard deviation difference from the mean; WMH, white matter hyperintensity.
All covariates are adjusted for time between baseline and follow‐up assessment and the following baseline covariates: age, sex, height, gait speed, cognitive index, gray matter volume and total WMH volume.
8 participants with baseline gait speed impairment were excluded from this analysis.
defined as a gait speed <1.0 m/s at follow‐up.
Bold values indicate significance after Bonferonni correction (P < 0.007).
Discussion
In this cohort study with older adults with SVD we found no significant associations between baseline imaging markers of SVD and gait decline or incident gait impairment after 5 years, even though our population showed a mean gait decline of 0.2 m/s in 5 years and a considerable amount of participants developed gait impairment (15.5%). In our TBSS analysis, we found higher baseline MD values in multiple WM tracts in participants with incident gait impairment compared to those without. However, this remained not significant after additional adjustment for conventional SVD markers. In sub‐analyses, we found that participants with the highest quartile of baseline WMH volume had an increased 5‐year risk of incident gait impairment. Furthermore, region‐specific analyses revealed that WMHs in the infratentorial region were associated with gait decline and incident gait impairment after 5 years. Although, results of these sub‐analyses were not significant after correction for multiple testing.
Major strengths of our study include the single center design, the quantitative measurement of gait, the inclusion of multiple imaging markers of SVD, including DTI measures, and the follow‐up duration of 5 years. Furthermore, all imaging data were analyzed by raters blinded to clinical information and adjustments for several confounders, including cognitive performance, were made. No adjustments were made for cardiovascular risk factors, as we considered them part of the causal chain of SVD.
A methodological consideration include the occurrence of attrition bias, because a considerable number of participants could not be included in the present study. As these participants were older, more disabled and had a higher load of SVD, it is possible that the strength of the associations have been underestimated.
Previous performed studies on baseline SVD and gait decline over time are limited and their results are conflicting6, 7, 8, 9. These studies are mostly performed in ageing populations (mean age > 72 years), with often already a low gait speed (<1.0 m/s) at baseline,8, 9 which make comparison to our study difficult. Furthermore, no corrections were made for multiple testing in these previous studies. Extending our previous findings in which we showed that baseline SVD is associated with incident parkinsonism, with lower body symptoms, including gait difficulties, being the dominant feature of these patients,19 we hypothesized that baseline SVD might also be associated with gait decline over time. Surprisingly, we found no associations between baseline SVD markers and gait decline after 5 years of follow‐up, despite the observation of a considerable deterioration of gait in our participants. Several possible explanations could be proposed for finding. First, SVD is just one of multiple risk factors of gait impairment, as gait is the result of a complex interaction between many (organ) systems, including the peripheral and central nerve system, cardiovascular and pulmonary system and musculoskeletal system.27 This is in accordance with the results of a recent study, showing that a high disease burden across multiple organ systems at baseline was associated with gait decline in a community‐based population aged ≥65 years.28 No specific system was found to be primarily responsible for the observed gait decline over 6 years.28 This indicates that accumulation of pathology in multiple organ systems, which in part share the same common pathway by cardiovascular risk factors, might be a better predictor of gait decline. This might possibly also explain our result that only participants with the highest quartile of WMHs at baseline seemed to have an increased risk for the development of gait impairment, as a high WMH volume might be a reflection of increased (vascular) damage to cerebral networks and possibly also to other organ systems. However, a note of caution is needed here, due to wide confidence intervals and multiple testing. Second, by initially analyzing total burden of the different SVD markers, we could have missed region‐specific associations. We therefore performed region‐specific sub‐analyses revealing associations between WMHs in the infratentorial region and gait decline, although these associations were not significant after correction for multiple testing. Our results are in line with a cross‐sectional study, which also showed that participants with WMHs in the brainstem walked slower.29 An explanation for this finding might be that these WMHs could damage motor fibers in the corticospinal and spinocerebellar tracts, as well as numerous cerebellovestibular connections, which are centered in a relatively small area in comparison to supratentorial regions.29 Third, it may be that progression of SVD is associated with gait decline, rather than baseline burden of SVD. Most of our participants had only mild to moderate severe SVD at baseline, which may have prevented us for finding significant associations. A recent study showed that WM atrophy and WMH progression were associated with gait decline after 2.5 years of follow‐up.30 A future study of the RUN DMC is underway to further investigate this.
Our study is unique in using baseline DTI measures in relation to gait decline. Nevertheless, our TBSS analysis revealed no significant differences in baseline WM microstructural integrity between participants with and without incident gait impairment independent of conventional SVD markers. DTI might however be of interest for future research as a recent study showed that change in DTI measures could serve as a sensitive marker for SVD progression, especially MD.31 Therefore, future studies should focus on changes in DTI measures in relation to gait decline, in addition to changes in conventional MRI markers of SVD. We hypothesize that changes in diffusion measures might be a better marker of gait deterioration than traditional SVD markers, as loss of WM microstructural integrity might possibly underlie and precede the earlier observed relation between WM atrophy and WMH progression and gait decline.30
In conclusion, in older adults with SVD conventional SVD markers and WM microstructural integrity at baseline are not associated with gait decline or incident gait impairment after 5 years. This result might, however, in part be driven by the attrition bias in our study, despite the fact that a high percentage of our participants experienced a significant gait decline. Future studies should be directed at changes in these cerebral imaging markers in relation to gait decline, as this could provide more insight into the role of (progression of) SVD to gait deterioration, which more and more burdens the health care system of aging societies.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
H.M.v.d.H.: 1B, 1C, 2A, 2B, 2C, 3A, 3B
W.M.v.U.: 1B, 1C, 3B
K.F.d.L.: 1A, 1B, 1C, 3B
E.M.C.v.L.: 1C, 3B
A.G.W.v.N.: 1A, 1B, 1C, 3B
D.G.N.: 1A, 1B, 3B.
E.J.v.D.: 1A, 1B, 3B
A.M.T.: 1B, 1C, 2C, 3B
F‐E.d.L.: 1A, 1B, 1C, 2C, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: “This study was supported by the Dutch Brain Foundation and the Netherlands Organization for Scientific Research.
Financial Disclosures for Previous 12 Months: Dr. van Dijk received a personal fellowship from the Dutch Brain Foundation (H04‐12;F2009(1)‐16). Dr. de Leeuw received a personal fellowship from the Dutch Brain Foundation (H04‐12;F2009(1)‐16) and a VIDI innovational grant from the Netherlands Organization for Scientific Research (grant number 016.126.351). Drs. van der Holst reports no disclosures. Drs. van Uden reports no disclosures. Dr. de Laat reports no disclosures. Drs. Van Leijsen reports no disclosures. Dr. van Norden reports no disclosures. Prof. Norris reports no disclosures. Drs. Tuladhar reports no disclosures.
Acknowledgments
Drs. Van der Holst and Dr. De Leeuw had access to all the data and take responsibility for the data, accuracy of the data analysis, and the conduct of the research.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA 2011;305(1):50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community‐dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutrition Health and Aging. 2009;13(10):881–889. [DOI] [PubMed] [Google Scholar]
- 3. Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well‐functioning older people—results from the Health, Aging and Body Composition Study. J Am Geriatr Soc 2005;53(10):1675–1680. [DOI] [PubMed] [Google Scholar]
- 4. Zheng JJ, Delbaere K, Close JC, et al. White matter hyperintensities are an independent predictor of physical decline in community‐dwelling older people. Gerontology 2012;58(5):398–406. [DOI] [PubMed] [Google Scholar]
- 5. de Laat KF, van Norden AG, Gons RA, et al. Gait in elderly with cerebral small vessel disease. Stroke. A Journal of Cerebral Circulation. 2010;41(8):1652–1658. [DOI] [PubMed] [Google Scholar]
- 6. Aribisala BS, Gow AJ, Bastin ME, et al. Associations between level and change in physical function and brain volumes. PLoS ONE 2013;8(11):e80386. doi: 10.1371/journal.pone.0080386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Soumare A, Elbaz A, Zhu Y, et al. White matter lesions volume and motor performances in the elderly. Ann Neurol 2009;65(6):706–715. [DOI] [PubMed] [Google Scholar]
- 8. Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT Jr, Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high‐functioning older adults. J Am Geriatr Soc 2005;53(4):649–654. [DOI] [PubMed] [Google Scholar]
- 9. Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology 2008;71(2):108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Groot M, Verhaaren BF, de Boer R, et al. Changes in normal‐appearing white matter precede development of white matter lesions. Stroke A Journal of Cerebral Circulation. 2013;44(4):1037–1042. [DOI] [PubMed] [Google Scholar]
- 11. de Laat KF, Tuladhar AM, van Norden AG, Norris DG, Zwiers MP, de Leeuw FE. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain 2011;134(Pt 1):73–83. [DOI] [PubMed] [Google Scholar]
- 12. van Norden AG, de Laat KF, Gons RA, et al. Causes and consequences of cerebral small vessel disease. The RUN DMC study: a prospective cohort study. Study rationale and protocol. BMC Neurology. 2011;11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Erkinjuntti T. Subcortical vascular dementia. Cerebrovascular Diseases (Basel, Switzerland). 2002;2:58–60. [DOI] [PubMed] [Google Scholar]
- 14. Menz HB, Latt MD, Tiedemann A, Mun San Kwan M, Lord SR. Reliability of the GAITRite walkway system for the quantification of temporo‐spatial parameters of gait in young and older people. Gait Posture 2004;20(1):20–25. [DOI] [PubMed] [Google Scholar]
- 15. Bilney B, Morris M, Webster K. Concurrent related validity of the GAITRite walkway system for quantification of the spatial and temporal parameters of gait. Gait Posture 2003;17(1):68–74. [DOI] [PubMed] [Google Scholar]
- 16. Willey JZ, Scarmeas N, Provenzano FA, Luchsinger JA, Mayeux R, Brickman AM. White matter hyperintensity volume and impaired mobility among older adults. J Neurol 2013;260(3):884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. NeuroImage 2003;19(3):1233–1239. [DOI] [PubMed] [Google Scholar]
- 18. Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurology. 2013;12(8):822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van der Holst HM, van Uden IW, Tuladhar AM, et al. Cerebral small vessel disease and incident parkinsonism: The RUN DMC study. Neurology 2015;85(18):1569–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Colliot O, Chetelat G, Chupin M, et al. Discrimination between Alzheimer disease, mild cognitive impairment, and normal aging by using automated segmentation of the hippocampus. Radiology 2008;248(1):194–201. [DOI] [PubMed] [Google Scholar]
- 21. Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self‐diffusion tensor from the NMR spin echo. J Magn Reson, Ser B 1994;103(3):247–254. [DOI] [PubMed] [Google Scholar]
- 22. Basser PJ, Jones DK. Diffusion‐tensor MRI: theory, experimental design and data analysis ‐ a technical review. NMR Biomed 2002;15(7–8):456–467. [DOI] [PubMed] [Google Scholar]
- 23. Smith SM, Jenkinson M, Johansen‐Berg H, et al. Tract‐based spatial statistics: voxelwise analysis of multi‐subject diffusion data. NeuroImage 2006;31(4):1487–1505. [DOI] [PubMed] [Google Scholar]
- 24. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 25. van Uden IWM, van der Holst HM, Schaapsmeerders P, et al. Baseline white matter microstructural integrity is not related to cognitive decline after 5 years: the RUN DMC study. BBA Clinical. 2015;4:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mahoney FI, Barthel DW. Functional Evaluation: the Barthel Index. Maryland State Medical Journal. 1965;14:61–65. [PubMed] [Google Scholar]
- 27. Rosso AL, Studenski SA, Chen WG, et al. Aging, the central nervous system, and mobility. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences 2013;68(11):1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosso AL, Sanders JL, Arnold AM, et al. Multisystem physiologic impairments and changes in gait speed of older adults. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2015;70(3):319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Starr JM, Leaper SA, Murray AD, et al. Brain white matter lesions detected by magnetic resonance [correction of resosnance] imaging are associated with balance and gait speed. The Journal of Neurology, Neurosurgery, and Psychiatry. 2003;74(1):94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Callisaya ML, Beare R, Phan TG, et al. Brain structural change and gait decline: a longitudinal population‐based study. J Am Geriatr Soc 2013;61(7):1074–1079. [DOI] [PubMed] [Google Scholar]
- 31. Zeestraten EA, Benjamin P, Lambert C, et al. Application of diffusion tensor imaging parameters to detect change in longitudinal studies in cerebral small vessel isease. PLoS One 2016;11:e0147836. [DOI] [PMC free article] [PubMed] [Google Scholar]


