Blepharospasm, a focal dystonia characterized by involuntary orbicularis oculi muscle spasms,1, 2 may vary in severity from a slightly bothersome condition to a disabling disorder that renders patients functionally blind.3 Botulinum toxin (BTX) injection, the gold‐standard treatment,1 has a beneficial effect in 95% of patients.4 Oral medication is of limited efficacy. For individuals that do not respond, eyelid myectomy or upper‐lid orbicularis muscle strip with subsequent eyebrow suspension are possible therapeutic options, although not always with acceptable results.1, 3, 4 Herein, we present the first case report of globus pallidus interna (GPi)‐DBS surgery for isolated blepharospasm.
Case Report
A 63‐year‐old man with a 1‐year history of involuntary contractions of right periorbital muscles, spreading subsequently to both eyes, was presented for the first time to our movement disorder clinic in September 2009. His past medical history was unremarkable, with no family history of dystonia and no exposure to antidopaminergic drugs. His neurological examination was normal with the exception of a blepharospasm of mild severity. Pharmacological treatment was started (clonazepam till 1.5 mg/day) as well as BTXA injection in orbicularis and tarsalis (Botox, 100 U; Dysport, 360 U) and subsequently BTXB (Neurobloc, 7,000 U) with a moderate effect. With time, there was a progressive worsening of blepharospasm. Electromyography (levator palpebrae superioris, orbicularis oculi) showed dystonic discharges of orbicularis, consistent with a blepharospasm diagnosis. Brain MRI was normal. During a 4‐year period, he received repeated BTX injections and was submitted to two oculopastic surgeries (myectomy and frontal suspension). Nonetheless, his condition was aggravated and rendered him functionally blind with a Jankovic rating scale of 4 (severity and frequency) bilaterally (see Video 1, Segment A). In October 2013, he was considered refractory to medical therapy and bilateral GPi‐DBS was performed. As described previously,5 stereotactic surgery was undertaken under general anesthesia with a Leksell G frame (Elekta Instruments AB, Stockholm, Sweden). Images of stereotactic CT scan and previous MRI were fused. Target coordinates were chosen by direct visualization in MRI. Standard burr holes and dura mater incisions were made, and central, anterior, and lateral recording electrodes were introduced. Intraoperative microrecording was used to define the neurophysiological borders of GPi. Macrostimulation was performed to find the threshold for internal capsular response. The lateral trajectory was the most favorable bilaterally. Definitive electrodes (model 3389; Medtronic, Inc., Fridley, MN) and an Activa RC IPG (Medtronic) were implanted. An implantable pulse generator was programmed on the first postoperative day (left: GPi 8 ‐ 3V / 60μs / 130Hz; right: GPi 0 ‐ 3V / 60μs / 130Hz) and blepharospasm improved gradually over the subsequent weeks achieving a Jankovic rating scale of 1 (severity) and 2 (frequency) on the left eye and of 1 (severity and frequency) on the right eye (see Video 1, Segment B). However, a progressive loss of benefit for the left eye was noted at the seventh month postsurgery. Fusion of postoperative stereotactic CT to preoperative MRI using FrameLink and OPTIVISE (Medtronic) demonstrated a correct positioning of the left electrode within the posteroventral lateral GPi, whereas the right electrode took a more lateral trajectory ending within the GPe (Fig. 1). Based on image fusion, we selected the two contacts of the right electrode in closest proximity to the GPi and tried an interleaving stimulation protocol (right: GPi 0 ‐ 3.5V / 60μs / 125Hz; 1 ‐ 4V / 90μs / 125Hz; left: GPi 8 ‐ 5.5V / 90μs / 125Hz). After changing the stimulation parameters, the patient's condition improved, achieving a Jankovic rating scale of 1 (severity) and 2 (frequency) on the left eye and of 1 (severity and frequency) on the right eye. The progressive reduction of symptoms granted him total independence for activities of daily living. Hence, up to the time of writing (21 months after surgery), it was not necessary to proceed to electrode reposition given that the same score on the Jankovic scale was maintained.
Figure 1.
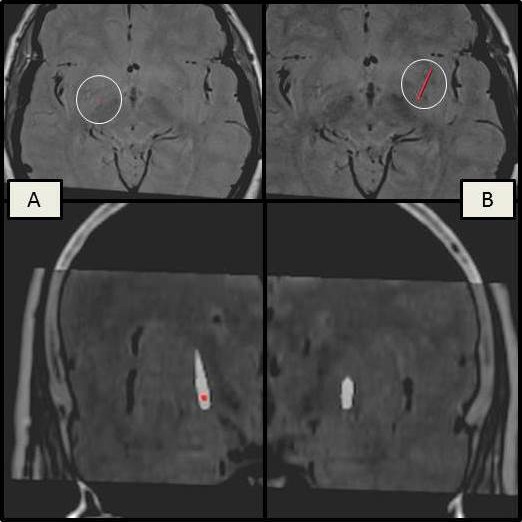
Images, using FrameLink, of the preoperative MRI fused with postoperative stereotactic CT for electrode position evaluation, showing a correct positioning of the left electrode (A) within the posteroventral lateral GPi and a lateral trajectory of the right electrode ending within the GPe (B).
There is a growing interest in using DBS on patients with craniocervical dystonia (Meige syndrome) who became refractory to other forms of therapy.6, 7, 8, 9, 10, 11 In 2003, Capelle6 reported on the first case of pallidal DBS for isolated Meige syndrome with marked improvement of oromandibular dystonia and blepharospasm (2 years after surgery Burke‐Fahn‐Marsden Dystonia Rating Scale [BFMDRS] score improved by 92% for eyes). Reese7 demonstrated sustained benefit (BFMDRS subscore for eyes was improved by 47%) of pallidal neurostimulation in 12 patients with this condition for up to 6 years. In the case series of Ghang,8 (11 patients), Ostrem9 (6 patients), Sako10 (5 patients), and Limotai11 (6 patients), positive results were also achieved in patients with Meige syndrome treated with DBS; blepharospasm improved in all them (improvement of 63% in BFMDRS subscores for the eyes at 12 months, 72% in BFMDRS total movement score at 6 months, 84% in BFMDRS total movement score at 49 ± 43.7 months, and 61.8 ± 30.9% in BFMDRS total movement score at 12 months, respectively).
BTX is still the main treatment option for blepharospasm. When it fails, there are not many options. Given the experience of blepharospasm improving with bilateral GPi‐DBS in patients with Meige syndrome, DBS surgery can be an acceptably effective therapy for patients with isolated blepharospasm, like it was in our case. The risk of procedure should be weighed cautiously against the potential benefit.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
A.F.S.: 1A, 1B, 1C, 3A, 3B
A.V.: 1A, 1B, 1C, 3A, 3B
L.A.: 1B, 1C, 3B
R.V.: 1A, 1B, 1C, 3B
M.J.R.: 1A, 1B, 1C, 3B
J.V.: 1A, 1B, 1C, 3B
Disclosures
Funding Sources and Conflicts of Interest: J.V. has received support (grant support and consulting fees) for this research and work regardless of date from Medtronic and Boston Scientific. The authors report no sources of funding and no conflicts of interest.
Financial Disclosures for previous 12 months: J.V. has received support (lecture fees) unrelated to this research from Medtronic, Boston Scientific, and St. Jude.
Supporting information
A video accompanying this article is available in the supporting information here.
Video 1. Video captured and broadcasted with the patient's written consent, illustrating the most relevant clinical aspects. Segment A: preoperative video showing a bilateral blepharospasm with Jankovic rating scale of 4 (severity and frequency) on both eyes, rendering the patient functionally blind. Segment B: postoperative video showing much improved bilateral blepharospasm with a Jankovic rating scale of 1 (severity) and 2 (frequency) on the left eye and of 1 (severity and frequency) on the right eye.
Acknowledgments
The authors thank the other members of the Movement Disorders and Functional Surgery Unit (Carolina Garrett, Paulo Linhares, Clara Chamadoira, Cláudia Sousa, Joana Lima, Carina Reis, Margarida Basto, Celeste Silveira, and João Pedro Costa) for their exceptional collaboration in managing this case.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Hallett M, Evinger C, Jankovic J, Stacy M. Update on blepharospasm: report from the BEBRF International Workshop. Neurology 2008;71:1275–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Defazio G, Hallett M, Jinnah HA, Berardelli A. Development and validation of a clinical guideline for diagnosing blepharospasm. Neurology 2013;81:236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peckham EL, Lopez G, Shamim EA, et al. Clinical features of patients with blepharospasm: a report of 240 cases. Eur J Neurol 2011;18:382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anwar MS, Zafar H. Efficacy of botulinum toxin in benign essential blepharospasm: desirable & undesirable effects. Pak J Med Sci 2013;29:1389–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. França S, Massano J, Linhares P, Rosas MJ, Volkmann J. Pallidal deep brain stimulation in DYT6: significant long‐term improvement of dystonia and disability. Mov Disord Clin Pract 2014;1:118–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Capelle HH, Weigel R, Krauss JK. Bilateral pallidal stimulation for blepharospasm‐oromandibular dystonia (Meige syndrome). Neurology 2003;60:2017–2018. [DOI] [PubMed] [Google Scholar]
- 7. Reese R, Gruber D, Schoenecker T, et al. Long‐term clinical outcome in Meige syndrome treated with internal pallidum deep brain stimulation. Mov Disord 2011;26:691–698. [DOI] [PubMed] [Google Scholar]
- 8. Ghang JY, Lee MK, Jun SM, Ghang CG. Outcome of pallidal deep brain stimulation in Meige syndrome. J Korean Neurosurg Soc 2010;48:134–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ostrem JL, Marks WJ Jr, Volz MM, Heath SL, Starr PA. Pallidal deep brain stimulation in patients with cranial‐cervical dystonia (Meige syndrome). Mov Disord 2007;22:1885–1891. [DOI] [PubMed] [Google Scholar]
- 10. Sako W, Morigaki R, Mizobuchi Y, et al. Bilateral pallidal deep brain stimulation in primary Meige syndrome. Parkinsonism Relat Disord 2011;17:123–125. [DOI] [PubMed] [Google Scholar]
- 11. Limotai N, Go C, Oyama G, et al. Mixed results for GPi‐DBS in the treatment of cranio‐facial and cranio‐cervical dystonia symptoms. J Neurol 2011;258:2069–2074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A video accompanying this article is available in the supporting information here.
Video 1. Video captured and broadcasted with the patient's written consent, illustrating the most relevant clinical aspects. Segment A: preoperative video showing a bilateral blepharospasm with Jankovic rating scale of 4 (severity and frequency) on both eyes, rendering the patient functionally blind. Segment B: postoperative video showing much improved bilateral blepharospasm with a Jankovic rating scale of 1 (severity) and 2 (frequency) on the left eye and of 1 (severity and frequency) on the right eye.


