Abstract
Both isolated axial dystonia and stiff person syndrome (SPS) are rare conditions that can look deceivingly similar. Here, we present three cases of axial dystonia resembling SPS with video documentation in order to illustrate the phenomenological similarities. We discuss clinical and paraclinical approaches to help distinction with its obvious implications for further management.
Keywords: stiff person syndrome, axial dystonia
Difficulties in diagnosing and differentiating clinical syndromes arise when disorders are rare, have phenocopies, and lack reliable biomarkers. In a nutshell, this is the problem when facing patients with axial dystonia leading to lumbar hyperlordosis. Both isolated axial dystonia and stiff person syndrome (SPS) are rare conditions that can look as similar as to pose difficulties even to movement disorder experts familiar with both conditions. The results of laboratory and electrophysiological investigations need to be interpreted with care given that there is hardly any definitve finding.
Here, we present three cases of axial dystonia with video documentation in order to illustrate the phenomenological similarities and discuss clinical and paraclinical approaches to help in the distinction with its obvious implications for treatment and management.
Case 1
At age 51, this gentleman presented with lower back pain and eventually developed an arched back. His back muscles would tighten up on standing, and spasms of the abdominal muscles would bulge his belly. There was no family history neither of dystonia nor endocrinopathies.
On examination, he walked with a stiff hyperlordotic gait with a tendency to lean over to the left. There was constant stiffness of the paraspinal and abdominal muscles, which did not even out when lying or bending down. The remainder of the examination was normal.
Blood tests, including thyroid hormones, blood glucose, and vitamin B12 levels, and antibodies against glutamic acid decarboxylase (GAD) were normal/negative. Electrophysiological testing showed continuous motor unit activity (CMUA) in the lumbar paraspinal muscles, but no enhanced exteroceptive reflexes and no exaggerated startle response. MRI of head and spine was normal.
At that stage, the clinical picture was deemed to most likely to represent SPS, even though axial dystonia was considered as a differential diagnosis. The finding of CMUA was thought to be supportive of this notion, even though the lack of antibodies and other characteristic electrophysiological findings was acknowledged. On two further occasions, CMUA was absent. A trial of intravenous immunoglobulin did not have any effect. A trial of levodopa did not provide any benefit. The patient received symptomatic treatment with baclofen, trihexyphenidyl, clonazepam, and tizanidine. Eventually, a combination of clonazepam and tizanidine was found to be helpful. The patient is now 70 years old (see Video 1). His symptoms have improved over the years, and no new symptoms have developed. His serological workup was recently completed for glycine receptor (GlyR) antibodies, which tested negative.
Case 2
At age 42, this gentleman developed arching back spasms after having moved furniture and tripped off the pavement. Subsequently, he was unable to walk normally because of jerky back spasms, which gradually worsened. They were mostly evident when walking, but did not interfere with swimming or water skiing. A spinal canal stenosis at L4/L5 was detected, and he underwent laminectomy 1 year later without any benefit. His gait was considered bizarre, and in fact, a referring specialist wrote: “Monty Python had nothing on him.” He was given a first diagnosis of “hysterical spinal posture,” and a treatment course with phenothiazine and chlorpromazine was suggested. There was no relevant past medical history apart from a back injury 15 years ago. There was no family history.
Examination at age 46 revealed a stiff back with hyperlordosis and occasional extension spasms of the trunk. Symptoms disappeared when he lied flat, and he was still able to bend down and touch his toes. Thus, a diagnosis of axial dystonia was thought to be more probable than SPS and treatment with trihexyphenidyl started. Over the years, however, he developed prolonged spasms of the paraspinal muscles, and the hyperlordosis would not even out completely when lying down. SPS was reconsidered, but GAD antibodies tested negative. MRI depicted some mild brain atrophy, whereas the spinal cord had normal appearance. Nine years after onset, he developed orofacial dystonia, and after a further 2 years cervical dystonia and blepharospasm (see Video 1). At age 70, he was diagnosed with sensorineuronal hearing loss. Temporarily, he had been on mexiletine, amitriptyline, fluoxetine prothiaden, haloperidol, and tetrabenazine. Eventually, a combination of trihexyphenidyl, lorazepam, and botulinum toxin injections was found to work best for him (see Video 1, taken at age 72).
Case 3
This patient was referred with a diagnosis of SPS for a second opinion. He had been previously in good health apart from a 15‐year history of intermittent aches resulting from disc problems. At age 62, he started having spasms, which would begin in the lumbar paraspinal muscles and spread to the shoulders and neck. He noted that the resulting neck extension could be attenuated if he touched the back of his head with his hands. Spasms would be mainly provoked by physical activation. He was taking diazepam 5 mg od. Previously, he had tried various antispastic drugs as well as botulinum toxin injections, however without much benefit. One year after onset, he developed painless proximal weakness of the right arm (suspected brachial amyotrophy). There was no family history.
On examination (see Video 1), there was indeed marked stiffness of paravertebral and abdominal muscles, but also a mild retro‐ and torticollis to the right and scoliosis. On longer standing, a fine tremor of the back muscles was noted. The head retraction reflex was mildly positive. There was a weakness and wasting of the C5 and C6 innervated muscles on the right with atrophy and reflex loss. Electromyography (EMG) did not show CMUA, but a synchronous, waxing and waning 5‐ to 7‐Hz tremor, which developed in the lumbar paraspinal muscles and spread to the neck and dorsal back muscles; it could be intermittently blocked by interlinking his hands behind the back of his head.
Apart from the striking stiffness, this patient lacked additional features typical for SPS, but had instead signs of dystonia involving the cervical region and the geste antagoniste. Tremor of the back and neck muscles is not a feature of SPS, but is well compatible with the diagnosis of axial dystonia.
Discussion
Here, we present three cases of axial dystonia closely resembling SPS (see Fig. 1; Videos 1 and 2).
Figure 1.
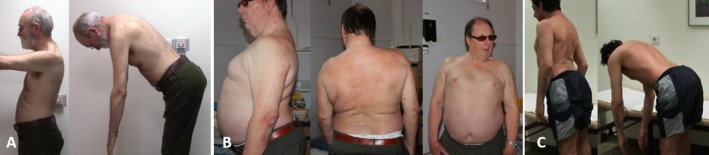
Stiff, hyperlordotic back resulting from axial dystonia (A, patient 1; B, patient #2) and SPS (C).
The core symptoms were trunk stiffness, lumbar hyperlordosis, and back spasms, starting at age 51, 42, and 62, respectively. Even though stiffness in SPS is (albeit slowly) fluctuating, it is typically more mobile in dystonia and, for example, evens out when lying down, which was an important clue in case 2. However, the hyperlordosis was fixed from the beginning in patient 1 and became relatively fixed in patient 2 during the course of the disease. Case 3, however, featured clearly a mobile element to his trunk stiffness, namely, a tremor of the back muscles, which is not observed in SPS. Scoliosis was noted in cases 1 and 3, but can also be observed in SPS and is not a differentiating feature.1 Spasms occur in both axial dystonia and SPS, but differ in their triggers. In SPS, they can be easily induced by exteroceptive stimuli, such as touch, pain, cold, or passive movement, or even by negative emotions (fear or anger). In our patients with axial dystonia, spasms occurred spontaneously or with activation (e.g., walking), but could not be elicited by the examiner.
Exaggerated startle to acoustic or tactile stimulation (i.e., hyperekplexia) and head retraction reflexes are frequent features of SPS.2 A mildly positive head retraction reflex, however, can occasionally be observed in dystonia (case 3).3
There are a few additional distinctive symptoms that may guide diagnosis. In SPS, these are vegetative crises and the characteristic task‐specific phobia of walking unaided (see video example of SPS patient [Video 2]). Similarly, falls or blocking episodes (often related by the patient as “freezing”) are strong indicators of SPS. In contrast, the geste antagoniste is pathognomonic of dystonia (case 3). Often, minor dystonia is present in other body parts, though subtle or with a delayed onset (case 1: dystonic shoulder elevation; case 2: craniocervical dystonia; case 3: cervical dystonia).4
Of note, in both axial dystonia and classical SPS, the absence of firm neurological signs might lead to a misdiagnosis as a psychogenic disorder (case 2). Neuropsychiatric problems, such as the task‐specific phobia in SPS or depressive comorbidity in dystonia, might further fuel this misperception.5, 6 The presence of firm neurological signs would widen the differential diagnosis to stiff‐person‐plus syndromes, such as progressive encephalomyelitis with rigidity and myoclonus, or complicated dystonia syndromes as, for example, observed in neurodegeneration with brain iron accumulation7, 8; besides this, axial myopathies with contractures and fibrosis can superficially resemble the discussed phenotype, but are as well beyond the focus of this article.7
Autoimmune predisposition (either in the patient or in the family) is a useful clue indicating SPS. A positive family history of stiffness or spasms might be a pitfall, given that axial dystonia is usually a sporadic disorder,4 but we have come across four cases of familial SPS. Previous exposure to neuroleptic drugs should prompt consideration of tardive dystonia, which also can give rise to hyperextension of the trunk.
One difficulty is that investigations are not definite. MRI will not be helpful to differentiate between primary dystonia and SPS. There is no electrophysiological finding that is evidentiary for dystonia. CMUA is considered classical for SPS, but is unspecific and can also be observed in patients with, for example, dystonia (case 1), Parkinson′s disease, or even muscular tension resulting from back pain.9 More useful to diagnose SPS are abnormal exteroceptive reflexes10, 11 and increased brainstem excitability with reflex spread and loss of inhibitory reflex components.2, 9
Laboratory findings such as antibody tests and cerebrospinal fluid (CSF) studies can further substantiate a suspicion of SPS. Approximately 90% of our patients with SPS and variants harbor antibodies against GAD, GlyR, amphiphysin, or dipeptidyl aminopeptidase‐like protein 6 (DPPX).12 The small caveat here is that antibodies, particularly those directed against GAD, can be detected also in 0.5% to 1.0% of healthy controls.13, 14 On the other hand, there is a subgroup of (so far) seronegative SPS patients. Overall, an inflammatory CSF with raised cell count or intrathecal immunoglobulin G synthesis is supportive, but not mandatory, for a diagnosis of SPS.15 It should, however, be kept in mind that CSF‐specific oligoclonal bands can rarely be present in healthy subjects. Calculation of the antibody index can provide the key information to differentiate between relevant and incidental antibody findings.14, 16 In contrast, there are hardly any lab tests to substantiate a diagnosis of dystonia: Genetic testing, for example, for DYT1 or DYT11 might be considered, but the vast majority of the cases of axial dystonia are idiopathic.4
In conclusion, axial dystonia and SPS can look deceivingly similar on clinical grounds (see Videos 1 and 2) and also, laboratory and electrophysiological investigations are not entirely unambiguous. However, careful clinical examination for additional signs (e.g., inducible spasms and excessive startle suggestive of SPS; geste antagoniste and dystonic features in other body parts indicative for dystonia) will light the way. The accuracy and positive predictive value of antibody results can be increased by CSF analysis and calculation of the antibody index. Enhanced exteroceptive reflexes, rather than CMUA, are characteristic for SPS and therefore a helpful tool in the diagnostic workup. A summary of the clinical and paraclinical clues that may help in differentiating both conditions is provided in Table 1.
Table 1.
Comparison of clinical and paraclinical findings in SPS and axial dystonia
| Clues From | SPS | Idiopathic Axial Dystonia |
|---|---|---|
| Clinical features |
Stiffness more constant Anxiety specifically related to standing and walking unaided (and marked improvement if only little or negligible support is offered) Exaggerated startle/positive head retraction reflex Falls, “freezing‐like episodes” Vegetative crises |
Stiffness usually more mobile, evens out when lying down or bending down Spasms mostly spontaneous or with activation Anxiety and depression as part of the nonmotor symptoms in dystonia Dystonia affecting other body parts Geste antagoniste |
| History | Autoimmune predisposition in patient or family | (Family history usually negative) |
| Lab |
Antibodies: GAD, GlyR, amphiphysin, DPPX, GABAAR Inflammatory CSF including antibody index |
(Consider genetic testing, but most cases are idiopathic) |
| Electrophysiology |
Enhanced exteroceptive reflexes CMUA (not specific for SPS!) Brainstem reflexes: spread of brainstem reflexes into pericranial muscles, reflex spasms; attenuated reflex inhibition (loss of S2 component of masseter inhibitory reflex; enhanced blink reflex recovery cycle) |
Cocontraction of agonist and antagonist muscles (but can also be present in SPS) Overflow of cocontraction to adjacent or remote muscles (but can also be present in SPS) Resolution of EMG findings with sensory trick |
| MRI | No pathognomonic findings | No pathognomonic findings |
GABAAR, gamma‐aminobutyric acid type A and type B receptors.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the First Draft, B. Review and Critique.
B.B.: 1A, 1B, 1C, 3A, 3B
H.‐M.M.: 1B, 3B
K.P.B.: 1A, 1B, 3B
Disclosures
Funding Sources and Conflicts of Interest: The authors report no sources of funding and no conflicts of interest.
Financial Disclosures for previous 12 months: K.P.B. has received honoraria/financial support to speak/attend meetings from GlaxoSmithKline, Boehringer Ingelheim, Ipsen, Merz, Sun Pharma, Allergan, Teva Lundbeck, and Orion pharmaceutical companies and holds grants from MRC Welcome Strategic grant (ref. no.: WT089698) and PD UK (ref. no.: G‐1009). K.P.B. and B.B. hold a grant from the Gossweiler Foundation. B.B. has received travel grants from the International Parkinson and Movement Disorder Society (MDS), EFNS, ENS, and AAN. H.‐M.M. has received speaker′s honoraria from Euroimmun and MDS.
Supporting information
Videos accompanying this article are available in the supporting information here.
Video 1. Cases 1, 2, and 3, with truncal stiffness and lumbar hyperlordosis resulting from axial dystonia. Case 1 has marked stiffness of the lumbar spinal and abdominal muscles. Stiffness impedes him bending forward and lumbar lordosis does not even out. There is a mild tendency to lean over to the left, but no other overt signs of dystonia. Case 2 has marked lumbar hyperlordosis, which leads to a transverse skin crease (often considered typical for SPS). The lumbar hyperlordosis evens, however, out when bending forward or lying down, albeit incompletely. Please also note other dystonic features, such as blepharospasm and cervical dystonia. Case 3 has lumbar hyperlordosis, which does not even out at all on attempted bending, and marked abdominal stiffness. Please note the mild retrocollis, which improves with a geste antagoniste.
Video 2. For comparison, a patient with classic SPS. He has marked lumbar hyperlordosis, leading to a transverse skin crease and impaired range of movement when attempting to bend forward. The striking improvement when (slightly) holding on to something is a typical accompaniment of the characteristic fear of walking/standing unaided in SPS patients.4
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Balint B, Jarius S, Nagel S, et al. Progressive encephalomyelitis with rigidity and myoclonus: a new variant with DPPX antibodies. Neurology 2014;82:1521–1528. [DOI] [PubMed] [Google Scholar]
- 2. Khasani S, Becker K, Meinck HM. Hyperekplexia and stiff‐man syndrome: abnormal brainstem reflexes suggest a physiological relationship. J Neurol Neurosurg Psychiatry 2004;75:1265–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foerster O. Zur Analyse und Pathophysiologie der striären Bewegungsstörungen. Zeitschr f d ges Neurol u Psychiatrie; 1921;73:1–30. [Google Scholar]
- 4. Bhatia KP, Quinn NP, Marsden CD. Clinical features and natural history of axial predominant adult onset primary dystonia. J Neurol Neurosurg Psychiatry 1997;63:788–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Henningsen P, Clement U, Küchenhoff J, Simon F, Meinck HM. Psychological factors in the diagnosis and pathogenesis of stiff‐man syndrome. Neurology 1996;47:38–42. [DOI] [PubMed] [Google Scholar]
- 6. Stamelou M, Edwards MJ, Hallett M, Bhatia KP. The non‐motor syndrome of primary dystonia: clinical and pathophysiological implications. Brain 2012;135(Pt 6):1668–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meinck HM, Thompson PD. Stiff man syndrome and related conditions. Mov Disord 2002;17:853–866. [DOI] [PubMed] [Google Scholar]
- 8. Stamelou M, Lai SC, Aggarwal A, et al. Dystonic opisthotonus: a “red flag” for neurodegeneration with brain iron accumulation syndromes? Mov Disord 2013;28:1325–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meinck HM. Neurophysiological findings in the stiff man syndromes. Klin Neurophysiol 2014;45:207–213. [Google Scholar]
- 10. Meinck HM, Ricker K, Conrad B. The stiff‐man syndrome: new pathophysiological aspects from abnormal exteroceptive reflexes and the response to clomipramine, clonidine, and tizanidine. J Neurol Neurosurg Psychiatry 1984;47:280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meinck HM, Ricker K, Hülser PJ, Solimena M. Stiff man syndrome: neurophysiological findings in eight patients. J Neurol 1995;242:134–142. [DOI] [PubMed] [Google Scholar]
- 12. Balint B, Blöcker IM, Unger M, et al. Antibody spectrum in stiff person syndrome and related disorders. Mov Disord 2015;30:S568–S633. [Google Scholar]
- 13. Meinck HM, Faber L, Morgenthaler N, et al. Antibodies against glutamic acid decarboxylase: prevalence in neurological diseases. J Neurol Neurosurg Psychiatry 2001;71:100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jarius S, Stich O, Speck J, Rasiah Ch, Wildemann B, Meinck HM, Rauer S. Qualitative and quantitative evidence of anti‐glutamic acid decarboxylase‐specific intrathecal antibody synthesis in patients with stiff person syndrome. J Neuroimmunol 2010;229:219–224. [DOI] [PubMed] [Google Scholar]
- 15. Meinck HM, Ricker K, Hülser PJ, Schmid E, Peiffer J, Solimena M. Stiff man syndrome: clinical and laboratory findings in eight patients. J Neurol 1994;241:157–166. [DOI] [PubMed] [Google Scholar]
- 16. Carvajal‐González A, Leite MI, Waters P, et al. Glycine receptor antibodies in PERM and related syndromes: characteristics, clinical features and outcomes. Brain 2014;137(Pt 8):2178–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Videos accompanying this article are available in the supporting information here.
Video 1. Cases 1, 2, and 3, with truncal stiffness and lumbar hyperlordosis resulting from axial dystonia. Case 1 has marked stiffness of the lumbar spinal and abdominal muscles. Stiffness impedes him bending forward and lumbar lordosis does not even out. There is a mild tendency to lean over to the left, but no other overt signs of dystonia. Case 2 has marked lumbar hyperlordosis, which leads to a transverse skin crease (often considered typical for SPS). The lumbar hyperlordosis evens, however, out when bending forward or lying down, albeit incompletely. Please also note other dystonic features, such as blepharospasm and cervical dystonia. Case 3 has lumbar hyperlordosis, which does not even out at all on attempted bending, and marked abdominal stiffness. Please note the mild retrocollis, which improves with a geste antagoniste.
Video 2. For comparison, a patient with classic SPS. He has marked lumbar hyperlordosis, leading to a transverse skin crease and impaired range of movement when attempting to bend forward. The striking improvement when (slightly) holding on to something is a typical accompaniment of the characteristic fear of walking/standing unaided in SPS patients.4


