CAPSULE SUMMARY
Der p1-coated microneedles after just three minutes of application led to efficient allergen delivery into skin and prevented development of airway allergy in a mouse. Allergen-coated microneedles may have potential for minimally-invasive allergen vaccination and immunotherapy.
Keywords: allergy immunotherapy, allergy vaccine, CpG adjuvant, microneedle immunotherapy, microneedle allergy vaccine, microneedle vaccination, prophylactic allergy vaccination, preventive allergy vaccination, skin immunotherapy
To the Editor:
Allergies are increasing worldwide and millions of people are struggling against a host of airway allergens. 1 To cure allergies, allergen specific immunotherapy (ASI) is the primary approach. However, ASI is performed only after allergy symptoms have manifested in patients. A true mode of allergy vaccination in the form of ‘preventive immunotherapy’ like that of infectious disease vaccination could be used to stop the allergy epidemic. Prevention of allergy onset in healthy individuals is called ‘primary prevention’, and prevention in individuals who are already sensitized but have yet to develop allergy symptoms is called ‘secondary prevention’.2 Proof-of-concept for secondary prevention has been clinically demonstrated. In one multi-center preventive allergy treatment study (PAT), children aged 6–14 years and who were suffering from seasonal rhinoconjunctivitis caused by birch and/or grass pollen were given allergen specific subcutaneous immunotherapy (SCIT) for three years. Even two years after treatment was stopped, a significant preventive effect on development of asthma was seen.3
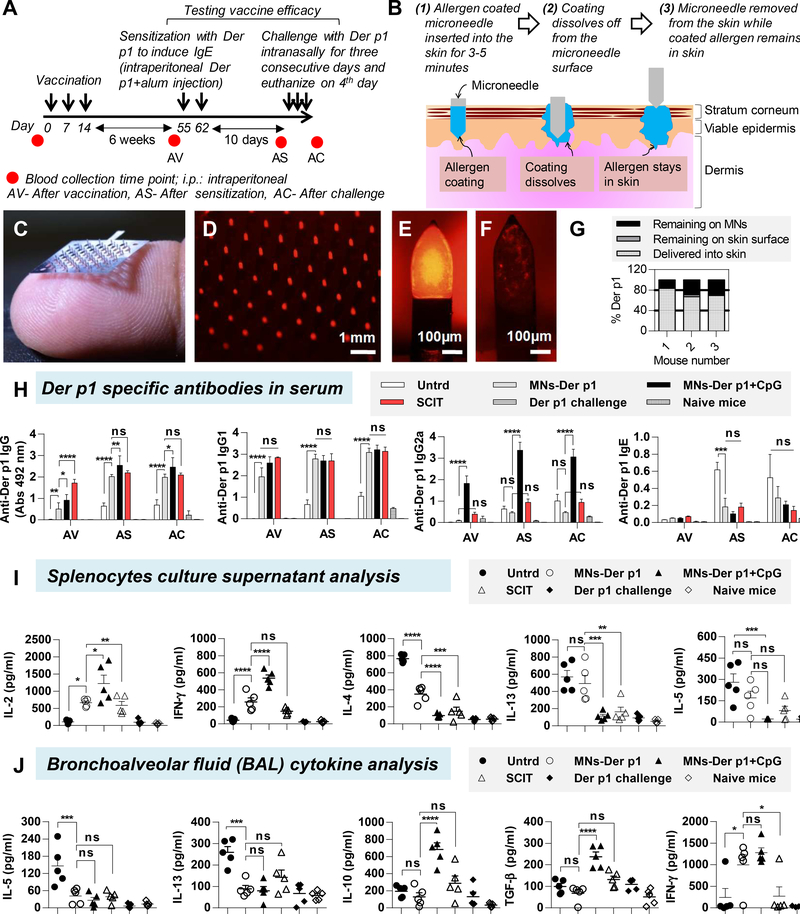
We have previously shown that micron-sized needles widely known as microneedles (MNs) can be used to deliver allergens into the skin. 4 MNs are painless 5 and can bypass the stratum corneum layer to directly deliver the allergen in to the skin. As a result, unlike conventional skin patches, MN-based delivery is not affected by differences in skin permeabilities that naturally exist amongst individuals. Hence, MNs can provide better dosage control and could improve consistency of therapeutic response as compared to use of a conventional skin patch for allergen delivery. In this study, we examined for the first time the efficacy of MNs to deliver Der p1, a house dust mite (HDM) allergen into mouse skin and determined its ability to prevent development of Der p1-induced airway allergy. A direct comparison was made against SCIT. Vaccination and challenge schedule is shown in Figure 1A, and Supplementary Table 1 describes the different groups. Concept of coated MN-based allergen delivery in to the skin is shown in Figure 1B. Der p1 (purified natural Der p1, > 95% pure, 24 kD, Indoor Biotechnologies Inc., USA) was successfully coated on the MN patches (Figure 1C, D) with uniform surface coverage (Figure 1E). Endotoxin in Der p1 was 0.17 EU/μg, which is less than the FDA allowable limit of 14 EU/μg (assuming 70 kg human and 25 μg allergen dose). Any potential adjuvant effect of the endotoxin would be equally applicable to coated MNs and SCIT groups and should not bias this study. Hair from mouse skin was removed, a coated MN patch was manually inserted, held in place for three minutes and removed. A small fraction of the coating remained on the MN surface after removal (Figure 1F). Using calibrated fluorescence spectroscopy, it was found that 73.3% (±7.4) of coated Der p1 was delivered in to the skin, while 2.6% (±1.1) Der p1 was found on the skin, and 24.1% (±6.5) Der p1 remained on the MN surface (Figure 1G).
Figure 1. Microneedle mediated preventive allergy immunotherapy.
(A) Immunotherapy schedule. (B) Concept of allergen delivery into skin with an allergen-coated microneedle (MN). (C) Digital photograph of a MN patch containing 57 total MNs resting on a human fingertip. Fluorescence micrographs of (D) MNs coated with rhodamine conjugated Der p1, (E) zoomed image of a single MN before insertion in to mouse skin, and (F) zoomed image of a single MN after insertion in to mouse skin. (G) Delivery efficiency from a MN patch coated with rhodamine-conjugated Der p1 after insertion in to mouse skin. (H) Der p1 specific antibodies in serum after vaccination (AV), after sensitization (AS), and after challenge (AC). Serum dilutions: 1:500 for IgG, IgG1 and IgG2a, and 1:20 for IgE. n=5 mice per group. (I) Cytokine expression in splenocyte supernatants after Der p1 restimulation. (J) Cytokine expression in BAL fluid after challenge. One way ANOVA was used for statistical comparisons between groups. Bars denote Mean ± SEM. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001, and ns: not significant.
Here, a MN patch was coated with 25 μg Der p1 with or without 25 μg CpG as adjuvant. However, a MN patch with about 60 MNs can be readily coated with 100–200 μg of allergen and is thus adequate for human use since for most allergens including house dust mite the maintenance doses range between 5 and 20 μg.6
After vaccination (AV), MNs-Der p1 group induced an anti-Der p1 IgG response (Figure 1H), which was considerably higher than the Untrd group (untreated negative control with no Der p1 coated on MNs). Since purified Der p1 protein was used, we included adjuvants in the formulations to increase its immunogenicity. We used alum in the SCIT group because it is approved for allergen immunotherapy in Europe. For MN coatings we used CpG as an adjuvant because it is a known stimulator of the Th1 pathway. As expected, addition of CpG in to Der p1 coatings (MNs-Der p1+CpG group) increased the IgG response over MNs-Der p1 group, but it was not statistically significant (Figure 1H). SCIT induced the highest IgG response (Figure 1H). Anti-Der p1 IgG1 response was similar in the Der p1 vaccinated groups (MNs-Der p1, MNs-Der p1+CpG and SCIT) and was significantly higher than the Untrd group (Figure 1H). In contrast, anti-Der p1 IgG2a response was found to be significantly higher in the MNs-Der p1+CpG group as compared to both MNs-Der p1 group and SCIT groups (Figure 1H). This can be attributed to CpG, which is a known stimulator of Th1 type immune response. The anti-Der p1 IgE response in all vaccinated groups was low (Figure 1H). After sensitization (AS) the IgG, IgG1, and IgG2a antibody responses increased across all groups but the relative trend between them did not change considerably (Figure 1H). No major change was seen after challenge (AC). However, AS the anti-Der p1 IgE response in the Untrd group (no Der p1 in formulation) increased significantly as compared to Der p1 vaccinated groups (Figure 1H). This is indicative of the development of a type 1 hypersensitive reaction in the control Untrd group. Naïve mice and mice that received just the Der p1 challenge (without any vaccination or sensitization) did not show any considerable anti-Der p1 antibody response in serum across all points.
Successful allergen immunomodulation involves activation of the Th1 pathway. Therefore, to assess the nature of T helper pathway, splenocytes were cultured and re-stimulated with Der p1. Th1 cytokines, IL-2 and IFN-γ were significantly higher in the Der p1 vaccinated groups (MNs-Der p1, MNs-Der p1+CpG, and SCIT) as compared to the Untrd group (Figure 1I). In contrast, Th2 cytokines IL-4, IL-13, and IL-5 in the MNs-Der p1+CpG and SCIT groups were low and similar to the naïve control group. In the Untrd group, the Th2 cytokines were higher, and the IgE levels were also higher. Naïve mice and mice that received just the Der p1 challenge showed low expressions of both Th1 and Th2 cytokines.
To examine the effectiveness of vaccination, the mice AS were challenged intranasally with Der p1. After challenge (AC), the groups vaccinated with Der p1 (MNs-Der p1, MNs-Der p1+CpG, and SCIT) exhibited protection from development of an allergic reaction. Pro-inflammatory cytokines IL-5 and IL-13 in BAL fluid were significantly lower in mice vaccinated with Der p1 than the Untrd group (Figure 1J). Interestingly, the anti-inflammatory cytokines IL-10 and TGF-β in the Untrd and MN-Der p1 groups were not different from each other (Figure 1J), however, when CpG was added to the MN-Der p1 coatings (i.e., in MNs-Der p1+CpG group) it led to a significantly higher IL-10 and TGF-β secretion as compared to both Untrd and MNs-Der p1 groups (Figure 1J). This indicates a beneficial role of CpG as an adjuvant for allergy vaccination. IFN-γ in the lungs was significantly higher in the MNs-Der p1 and MNs-Der p1+CpG vaccinated groups as compared to the Untrd group and even the SCIT group (Figure 1J). This is consistent with IFN-γ secretion pattern in splenocyte cultures.
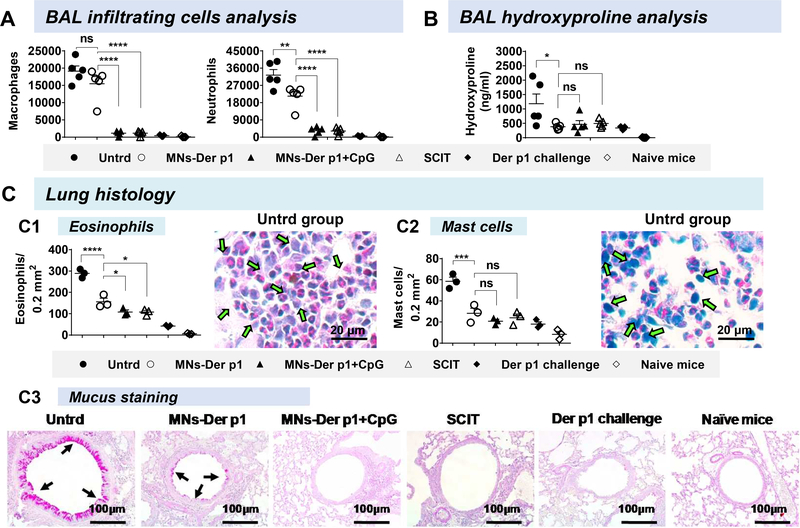
The protective nature of coated MN-based vaccination was reconfirmed when we analyzed effector cells (macrophages and neutrophils) in the BAL fluid. The Der p1 vaccinated groups (MNs-Der p1, MNs-Der p1+CpG, and SCIT) had fewer macrophage and neutrophil cells in their BAL fluid as compared to Untrd group (Figure 2A). Of note, with Untrd group as the high datum, the fold reduction in macrophages and neutrophils was higher in the MNs-Der p1+CpG group over that in the MNs-Der p1 group, reinforcing that CpG exerts a beneficial effect as an adjuvant (Figure 2A). Neutrophils have been implicated with increased steroid resistance in humans7 and mice8.Collagen is one of the indicators of lung remodeling. Hydroxyproline amount, which reflects collagen amount, was significantly lower in BAL fluids of all groups vaccinated with Der p1 (including MNs-Der p1) and was only higher in the Untrd group (Figure 2B). Lower collagen is indicative of protective effect of MN based Der p1 vaccination because collagen deposition can contribute to thickening of the bronchiole wall and ultimately lead to a narrowing of airway tract and bronchial hyper-responsiveness.
Figure 2. Lung histopathology.
At the end of allergen challenge, BAL fluid and lungs were collected for analysis. (A) Macrophages (CD11b+Gr1−) and neutrophils (CD11b+Gr1+) in BAL fluid were counted as described previously. 9 (B) Hydroxyproline content was analyzed in BAL fluids (Sigma Aldrich, Cat. No. MAK008). Each symbol represents an individual mouse (n=5 mice per group). (C) Lung histology: Multiple sections (7 μm thick) separated by 500 μm were obtained from the top and bottom of the left lung and stained with either hematoxylin and eosin (H&E) for eosinophil analysis or May-Grünwald Giemsa (MGG) stain for mast cell analysis. Each symbol represents average cells from a mouse averaged at 18 independent locations. (C1) Eosinophil cells and (C2) Mast cell. Eosinophil and mast cells for other treatment groups are presented in Supplementary Figure 1 and 2, respectively. (C3) Tissue sections were stained with periodic acid-Schiff (PAS) stain for mucus secretion inside the bronchioles. Bright field micrographs are shown. Arrows point to mucus deposition. One-way ANOVA was used for statistical comparisons between the groups. Bars denote Mean ± SEM. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001, and ns: not significant.
Considerably lower number of eosinophils (Figure 2C1, Supplementary Figure 1) and mast cells (Figure 2C2, Supplementary Figure 2) were observed in lung sections of Der p1 vaccinated groups as compared to the Untrd group. Furthermore, in the MNs-Der p1 and MNs-Der p1+CpG groups, similar to SCIT, less mucus deposition was seen inside the bronchioles, indicating suppression of inflammatory response (Figure 2C3). In contrast, hyper mucus secretion was noticed in the Untrd group (Figure 2C3). Mucus hyperplasia is one of the major characteristics of airway allergy wherein secretion of mucus in bronchioles obstructs the airflow that ultimately leads to shortness of breath.
The trend in protection generally correlated with AS IgE serum levels. The Untrd group, which had higher IgE levels exhibited an allergic reaction while the Der p1 vaccinated groups, which had lower IgE levels were better protected. In conclusion, MN-based and SCIT vaccinations with Der p1 each led to similar protective effects against sensitization in a mouse model. Because MNs are minimally invasive, painless, and easy to apply, they can offer a more patient friendly approach of allergy vaccination as compared to SCIT. Initially, allergen vaccines could be offered to individuals who are deemed at a higher risk of developing allergies due to family history, or to patients who are already sensitized but have yet to progress to a diseased state, for example to patients with allergic rhinitis to prevent their disease exacerbation to asthma. This study provides proof-of-concept that MNs have potential for allergy vaccination and immunotherapy but does not elucidate the mechanism of action. Thus, follow on studies that dissect the mechanism of action including the contribution of Tregs are required. Future studies that focus on optimization of vaccination parameters such as dose, frequency of administration, and number of treatments are also required to develop this approach further.
Supplementary Material
Acknowledgments
The following sources funded this study: National Institutes of Health (NIH) [grant number 1R01AI121322-01] and by funding from Texas Tech University.
Footnotes
Disclosure of potential conflict of interest: HS Gill is a co-inventor on a patent related to coated microneedles, and is co-founder of a startup company that is developing microneedles for food allergy immunotherapy. This potential conflict of interest has been disclosed and is being managed by Texas Tech University.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akinbami LJ, Simon AE, Schoendorf KC. Trends in allergy prevalence among children aged 0–17 years by asthma status, United States, 2001–2013. Journal of Asthma 2016; 53:356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larsen JN, Broge L, Jacobi H. Allergy immunotherapy: the future of allergy treatment. Drug Discov Today 2016; 21:26–37. [DOI] [PubMed] [Google Scholar]
- 3.Niggemann B, Jacobsen L, Dreborg S, Ferdousi HA, Halken S, Host A, et al. Five-year follow-up on the PAT study: specific immunotherapy and long-term prevention of asthma in children. Allergy 2006; 61:855–9. [DOI] [PubMed] [Google Scholar]
- 4.Shakya AK, Lee CH, Gill HS. Cutaneous vaccination with coated microneedles prevents development of airway allergy. J Control Release 2017:August 15 pii: S01683659(17)30779–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. Clin J Pain 2008; 24:585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol 2011; 127:S1–55. [DOI] [PubMed] [Google Scholar]
- 7.Ray A, Kolls JK. Neutrophilic Inflammation in Asthma and Association with Disease Severity. Trends Immunol 2017; 38:942–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ano S, Morishima Y, Ishii Y, Yoh K, Yageta Y, Ohtsuka S, et al. Transcription factors GATA-3 and RORgammat are important for determining the phenotype of allergic airway inflammation in a murine model of asthma. J Immunol 2013; 190:1056–65. [DOI] [PubMed] [Google Scholar]
- 9.Jonsson F, Mancardi DA, Kita Y, Karasuyama H, Iannascoli B, Van Rooijen N, et al. Mouse and human neutrophils induce anaphylaxis. J Clin Invest 2011; 121:1484–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.