Graphical abstract
1. Introduction
Stem cell therapy has been a major focus of biomedical research over the last decade. Delivery of stem cells systemically to diseased patients or locally to injury sites is currently investigated by numerous pre-clinical and clinical studies to treat various conditions. Unfortunately, current stem cell-based regenerative strategies are restricted by inadequate cellular distribution, survival, and integration after transplantation, in part due to unfavorable methods of cell delivery to the site of tissue injury. Mesenchymal stem cells (MSCs), for instance, are anchorage-dependent cells; their survival and engraftment will be significantly impaired when they are delivered in suspension [1]. Moreover, it has been reported that after systemic delivery, many stem cells are entrapped in the lungs and capillaries of various tissues [2]. Direct delivery of stem cells to or near the injury site requires a biocompatible delivery vehicle to maintain cell viability and integration and combat detrimental environmental factors [3].
Biomaterial-based scaffolds provide a three-dimensional (3D) environment for cell growth, enabling cell morphology, physiology and functionality unobtainable in two-dimensional (2D) conditions. Of these, hydrogels have become an attractive vehicle for stem cell culture and delivery due to their biocompatibility, flexibility of physical characteristics, structural similarities to native extracellular matrix (ECM) and ability to support cellular adhesion, survival and proliferation [4]. Hydrogel-based biomatrices could mimic the natural ECM environment and provide necessary binding sites for cell functions as well as nutrient and paracrine signaling access. Stem cells embedded in hydrogel scaffolds are also easily transplantable and can be injected or applied topically. Despite the wide range of synthetic and naturally derived biomaterials, high manufacturing costs, inadequate supply and host immune response have prompted researchers to look for better alternatives [5]. Fibrin and collagen-based matrices are among the most common materials for use in tissue repair and regeneration due to their good biocompatibility, ease of manipulation for implant formation, and ability to degrade through natural processes [6].
Recently, it was shown that decellularized tissues can be digested with pepsin and form hydrogels with mechanical and physiological properties that are compatible with cell culture [7]. Hydrogels from enzymatically degraded ECM retain cell adhesion proteins (collagens and elastin), glycosaminoglycans and growth factors [8]. Tissue-derived ECM is optimal for cell culture and delivery because of its tissue-relevant composition, structure and presence of bioactive molecules. However, detrimental human immune response against mammalian antigens, such as α-gal epitope on ECM glycoproteins and proteoglycans in mammalian ECM products [9], may be developed when applied in humans, and various techniques have been developed to resolve this problem [10].
We have expanded upon these findings and developed a cost-effective method to produce an optimized hydrogel scaffolding system from easily accessible human amniotic membranes. Amnion membrane (AM), a part of placenta, is a sac filled with amniotic fluid that encapsulates the developing fetus. Postpartum placentas are readily available sources of AM that possess beneficial health factors and are usually discarded when babies are born. Furthermore, studies have shown that in addition to supporting cell adhesion, distribution and assimilation, our delivery system is also characterized by gelation properties similar to those of existing hydrogels. Placenta also plays a unique role in fetomaternal tolerance, preventing the fetal “allograft” from being rejected. Therefore, amnion may have an additional application in reducing host immune response during allogeneic transplantation. Ability to use this human-derived tissue can greatly decrease the need for xenogeneic scaffolds.
Mesenchymal stem cells (MSCs) are the most widely studied type of stem cells for cell therapy. According to the publicly available database on clinical studies (www.clinicaltrials.gov), there are over 450 registered clinical trials that utilize MSC-based therapies. MSCs are being applied in a wide range of disease models, including brain and spinal cord injury, bone, cartilage and connective tissue diseases, graft versus host disease, cardiovascular disease, and diabetes [1]. Recent studies suggest that the beneficial outcomes of stem cell transplantation are due to a paracrine modulatory effect rather than the direct replacement of damaged tissue near the site of injury [11]. Several works have demonstrated that MSCs promote tissue repair by secreting a variety of cytokines and growth factors, which enhance the process of repair and regeneration [12]. Paracrine and autocrine activity of MSCs was also shown to decrease immune response and scar formation, stimulate angiogenesis, and activate differentiation and proliferation of tissue-specific stem cells [13]. A number of recent publications suggest that placenta-derived mesenchymal stem cells (PMSCs) have a wide range of cell-based therapeutic applications [14-16] and can serve as an effective alternative to bone marrow-derived MSCs (BM-MSCs). While PMSCs and BM-MSCs share similarities in their phenotype and immunomodulatory functions [17], PMSCs were shown to surpass BM-MSCs in secretion profile [15], proliferation rate, maximum number of passages and plasticity [18]. Considering their inherent anatomical affiliation, we tested the biocompatibility of AM-ECM hydrogel with PMSCs, as well as other cells, and compared its efficacy as a delivery vehicle to conventional collagen and fibrin hydrogel matrices.
2. Materials and methods
2.1 Cell cultures
2.1.1 Placenta tissue and cells
Discarded, de-identified placental tissue was collected at the University of California, Davis Medical Center in Sacramento, CA. Protocols were submitted to the UC Davis Institutional Review Board (IRB) and deemed exempt from review. PMSCs were isolated via explant culture of chorionic villus tissue from early gestation placentas using established protocol [19]. Chorionic villus tissue was carefully dissected into fragments <5 mm and washed in sterile phosphate-buffered saline (PBS) containing 100 units/mL of penicillin and 100 μg/mL of streptomycin. Dissected tissue was spread evenly across sterile tissue culture-treated dishes and PMSCs were allowed to migrate from the tissue explants. Cells were harvested at 3-4 weeks and then re-plated as a monolayer. Culture media for PMSCs consisted of DMEM high glucose supplemented with 5% fetal bovine serum, 100 units/mL of penicillin (Invitrogen), 100 μg/mL of streptomycin (Invitrogen), 20 ng/mL recombinant human bFGF (Peprotech), and 20 ng/mL recombinant human EGF (Peprotech).
2.1.2 C2C12, BM-MSCs
C2C12 myoblasts were donated from Nobuko Hagiwara, Ph.D. from the Dept. of Internal Medicine, Division of Cardiovascular Medicine, UC Davis. BM-MSCs were a gift from Claus Sondergaard, Ph.D., Dept. of Surgery, UC Davis. All cells were cultured in 37°C, 5% CO2. SH-SY5Y cell line was purchased from ATCC (# CRL-2266). BM-MSCs and C2C12 cells were expanded in DMEM supplemented with 10% FBS, no growth factors. SH-SY5Y were cultured in F12 supplemented with 10% FBS, no growth factors.
2.1.3 Endothelial progenitor cells
Outgrowth endothelial cells (OECs), a subtype of endothelial progenitor cell (EPC), are widely investigated for vascular regeneration and tissue engineering applications. They can be isolated from peripheral blood or cord blood. In this study, the OECs were isolated from human umbilical cord blood as previously described [20, 21], with modification. Human umbilical cord blood was obtained from the UC Davis Umbilical Cord Blood Collection Program (UCBCP) and diluted 1:1 with phosphate-buffered saline (PBS), without calcium and magnesium, pH 7.2 (Hyclone). 15 mL Ficoll-Paque PLUS (Amersham Biosciences) was laid at the bottom of 20 mL diluted blood and centrifuged at 2000 rpm for 30 minutes at room temperature. The hazy layer of mononuclear cells (MNCs) that seat at the interface between the Ficoll and serum was collected and dispensed into a 50 mL conical tube containing 10 mL EBM-2 (Lonza) and centrifuged at 1500 rpm for 10 min at room temperature. The pellets were treated with red blood cell lysis buffer (eBioscience, Inc.) and then sorted by CD34 microbead magnetic sorting (Miltenyi Biotec GmbH). The CD34+ cells were seeded on rat-tail collagen I (BD Biosciences Discovery) coated tissue culture plates and cultured in EGM-2 media (Lonza). After 24 h, non-adherent cells were removed. Endothelial colonies appeared between day 4 and day 7 of culture and OECs formed thereafter. Individual colonies were isolated, expanded and allowed to grow to 80% to 90% confluency before first passage. OECs were used between P3 and P5 for all experiments.
2.2 AM decellularization and preparation of AM ECM hydrogel
2.2.1 AM decellularization
AM was mechanically delaminated from the fresh placenta and washed in saline solution. Next, the membrane was decellularized according to a decellularization protocol for porcine dermal tissue [22], with modification. Briefly, after 3 freeze-thaw cycles, AM underwent the following treatments: 3 cycles of membrane massaging on a mesh filter in running water for 10 min; rinsing the membrane with 0.05% Trypsin/0.02% EDTA (Life Technology) on shaker (Incu-Shaker Mini, Bechmark Scientific) for 1 hour; 2 cycles of rinsing the membrane with 3% Triton (Sigma-Aldrich) on shaker for 1 hour; 2 cycles of rinsing the membrane with 3% Triton on shaker for 1 hour; 2 cycles of rinsing the membrane with 4% sodium deoxycholic acid on shaker for 1 hour; rinsing the membrane with 0.1% paracetic acid/4% ethanol (EMD Millipore) solution on shaker for 4 hours, followed by rinsing with sterile PBS for 15 min and 2 cycles of rinsing the membrane with sterile DI water for 15 min. Decellularized AM-ECM was lyophilized and stored at room temperature.
2.2.2 AM-ECM hydrogel preparation
Decellularized AM-ECM was digested in a solution of 1 mg/ml porcine pepsin (Sigma-Aldrich) and 0.01 N HCl followed by agitation on a shaker at 200 RPM for 72 hours at room temperature. AM-ECM pepsin digests with a final concentration of 10 mg ECM/ml were stored at -20°C. AM-ECM digests were neutralized by adding appropriate volumes of 0.1 N NaOH and then 10× PBS to provide an isotonic solution. AM-ECM hydrogel polymerization was initiated by neutralization of pH to 7.0 and incubation at 37°C.
2.3 Preparation of fibrin and collagen hydrogels
The fibrin glue was prepared from the commercially available FDA-approved TISEEL fibrin sealant kit, which consists of human plasma fibrinogen solution (∼85 mg/mL), aprotinin (2250 - 3750 KIU/mL), Thrombin (Human, ∼500U/mL), and calcium chloride (36 –44 μmol/m). Fibrinogen was dissolved in PBS to prepare 5 mg/ml and 20 mg/mL concentrations. Thrombin was dissolved in PBS to reach the final concentration of 10U/ml. Collagen was prepared using PureCol® EZ Gel purified Type I bovine collagen in DMEM/F-12 medium at concentrations of 5 mg/ml and 3 mg/ml. Collagen gelation was initiated by incubation at 37°C for 1 hour.
2.4 Histology of decellularized AM-ECM
AM was decellularized as described above. After fixing in 4% paraformaldehyde, the decellularized and control samples were dehydrated, embedded in O.C.T compound, and sectioned at 10 μm thickness using a microtome. Hematoxylin and eosin (H&E) and Trichrome (Masson) stains were employed to detect the presence of nucleated cells and compare tissue morphology. Stained samples were imaged using a Carl Zeiss Axio Observer D1 inverted microscope.
2.5 AM-ECM cytocompatibility
Decellularized AM-ECM was placed in a 100 mm culture dish and soaked in 2 ml of culture medium overnight at 37°C. The next day, PMSCs were seeded on the membrane surface and cultured for 72 hours. Cell culture viability was evaluated with LIVE/DEAD® Viability/Cytotoxicity assay (Life Technologies). Part of the cell-seeded membrane was fixed with 4% paraformaldehyde, dehydrated, lyophilized, sputter coated (Pelco Auto Sputter Coater SC-7) and imaged with a scanning electron microscope (Phillips XL30 TMP) at 1000×.
2.6 Hydrogel gelation time testing
Gelation time of fibrin glue was determined by quantifying changes in turbidity upon the combination of solutions with various concentrations of fibrinogen and thrombin. Collagen and amnion hydrogels were prepared on ice. For each group 100μl/well of gel was added in a 96-well plate in triplicate. The turbidity of material then was measured spectrophotometrically with a microplate reader at 550nm every minute for 60 minutes at 37°C.
2.7 Biochemical composition of AM-ECM hydrogel
In order to analyze the components of decellularized amnion ECM, a series of biochemical analysis was performed. Two different batches of AM-ECM derived pH neutralized gel were assayed for collagen, elastin and GAG proteins. The concentration of acid-pepsin soluble collagens (Types I to V) was determined using Sircol™ soluble collagen assay (Biocolor, Northern Ireland). Elastin was quantified using Fastin™ Elastin Assay (Biocolor, Northern Ireland), while sulfated proteoglycans and glycosaminoglycans were measured by Blyscan™ assay (Biocolor, Northern Ireland) upon digestion with papain extraction reagent at 65°C for 3 hours according to the manufacturer's instructions.
2.8 Evaluation of hydrogel nanostructure
Scanning Electron Microscopy was utilized to evaluate scaffold morphology. Hydrogel molds were prepared by pouring 500ul of gel per well in a 48-well plate and allowed to polymerize at 37°C for 1 hour. Next they were fixed overnight in a solution of 2.5% glutaraldehyde in cacodylate buffer at 4°C. Next gels were washed for 10 minutes in series of ethanol concentrations in distilled water for dehydration: 40%, 60%, 75%, 85%, 90%, 95%, and 100%. After critical point drying, gels were coated with metal 3 times using a sputter coater (Pelco Auto Sputter Coater SC-7) and imaged with a scanning electron microscope (Phillips XL30 TMP).
2.9 In vitro PMSC viability and proliferation tests
PMSCs were loaded in hydrogels at a final concentration of 1 × 104 cells/200ul/well of a 96-well non TC-treated plate. Following gelation, cell-loaded hydrogel constructs were placed in a 37°C CO2 incubator for 1 day, 4 days and 6 days periods. CellTiter 96 AQueous One Solution Cell Proliferation MTS Assay (Promega Corporation, Madison, WI) was used to assess the relative number of viable cells. Cell proliferation measurements were performed in triplicate.
2.10 Analysis of the presence of bioactive molecules in AM-ECM hydrogel
The presence of angiogenesis-related factors in hydrogel derived from decellularized amnion ECM was also assessed. Amnion hydrogel was prepared as discussed above without final polymerization at 37°C. Hydrogel solution was analyzed with Proteome Profiler™ Human Angiogenesis Antibody Array kit according to the manufacturer's instructions (R&D Systems). Membrane images were analyzed using ImageJ software with the Dot Blot Analysis plugin and integrated density values obtained from images were then plotted using Microsoft Excel. Reference spot densities are also included to estimate relative protein concentrations.
2.11 Cytokine array analysis of PMSC secreted proteins
Angiogenesis-related cytokines were analyzed from the culture supernatant. PMSCs were seeded at a density of 1 × 105 per 1ml of hydrogel per well in a 6-well plate. Culture supernatants were collected at 24 hours, centrifuged to remove particulates and then assessed for the relative levels of 55 angiogenesis-related cytokines using Proteome Profiler™ Human Angiogenesis Antibody Array kit according to the manufacturer's instructions (R&D Systems). Supernatants from a monolayer cell culture as well as from AM-ECM hydrogel and growth media (no cells) were also collected as negative controls. Stained membrane blots were imaged on a Bio-Rad ChemiDoc MP, and images were analyzed using ImageJ software with the Dot Blot Analysis plugin. Integrated density values obtained from membrane images were then plotted using Microsoft Excel.
2.12 Amnion hydrogel cytocompatibility
The cytocompatibility of AM-ECM hydrogel was tested with representative stem cells, including PMSCs, BM-MSCs, C2C12 myoblasts and SH-SY5Y neuroblastoma cells. Briefly, cells were mixed with decellularized AM digests (6 mg/ml) to form cell-hydrogel constructs containing 1 × 105 cells per 1ml of hydrogel per well of a 6-well plate. Cell-hydrogel constructs were then placed in a 37°C 5% CO2 incubator for 1 hour to gel before the addition of 2 mL respective culture media to cover the hydrogel. Cell-seeded hydrogel constructs were incubated for 3 days before cell viability was assessed using LIVE/DEAD® Viability/Cytotoxicity assay (Life Technologies). Stained constructs were imaged using a Carl Zeiss Axio Observer D1 inverted microscope, and images were processed with ImageJ software.
2.13 In vivo biocompatibility
Twelve-week-old male Sprague-Dawley rats were obtained from Harlan Laboratories and acclimated 1 week prior to exposure. All animal experiments were performed under protocols approved by the University of California Davis IACUC in accordance with National Institutes of Health guidelines. A total of 4 rats were used in the study. AM-ECM hydrogel and collagen hydrogel, both at 10mg/mL concentration, were implanted into the back of rats by subcutaneous injection. After 14 days, the rats were sacrificed and the residual hydrogels with adjacent tissues were collected. The specimens were fixed in 10% formalin, washed in DI water and dehydrated in 30% sucrose before being embedded in O.C.T compound (Tissue Tek®), and sliced at 20 μm thickness using a freezing microtome. Hematoxylin and eosin (H&E) staining was employed to detect the residual bulk and the cell infiltration into the AM-ECM hydrogel and collagen gel. To identify the infiltration cell type, macrophage marker CCR7 and EPC marker CD34 were used. The sections were incubated with 10% (w/v) bovine serum albumin in PBS for 30 minutes to inhibit nonspecific binding of IgG. Next, the sections were incubated with rabbit anti-rat CCR7 (Epitomics) and goat anti-rat CD34 (R&D) antibodies for 2 hours and detected with Alexa Fluor 488 mouse anti-rabbit and Alexa Fluor 546 donkey anti-goat (Invitrogen, ThermoFisher Scientific) secondary antibodies. Lastly, the sections were counterstained with DAPI for 1 minute to identify cell nuclei. All the sections were observed with a Carl Zeiss Axio Observer D1 inverted microscope. ImageJ software was employed to quantify the cell infiltration of the AM-ECM hydrogel and collagen.
2.14 Statistical analysis
For two-sample comparison, Student's t-test was used. For multiple-sample comparison, analysis of variance (ANOVA) was performed to detect whether a significant difference existed between groups with different treatments, and Tukey's multiple comparisons test was used for post-analysis. A p-value of 0.05 or less indicates significant difference between samples in comparison.
3. Results
3.1 AM morphology
HE and trichrome stains reveal that amniotic membrane is comprised of several major layers: a single layer of columnar epithelial cells, a dense basement membrane, an acellular compact collagen rich layer, and underlying fibroblast and spongy layers (Figure 1A, 1C). The decellularization process was successful since no cells were detected after the procedure (Figure 1B, 1D). This is a novel effective method for removing all cells from human amniotic membrane without the use of toxic SDS.
Fig. 1.
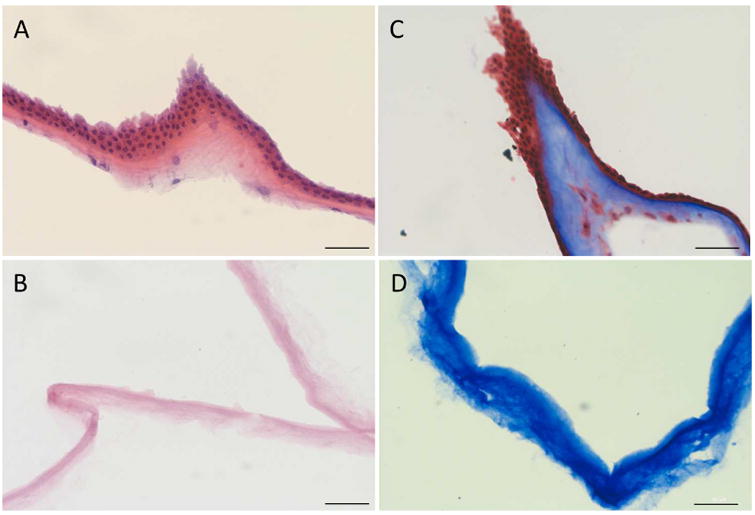
Decellularization of AM. H&E stain of intact (A) and decellularized (B) AM. Trichrome stain of intact (C) and decellularized (D) AM. Scale bar= 50 μm.
3.2 AM biocompatibility
We also tested the cytocompatibility of the acellular human AM. Live-dead assay showed that placenta derived MSCs remain metabolically active after 72 hours of culture on the membrane (Figure 2B), while electron microscopy revealed normal morphology of cells attached to the scaffold (Figure 2C). This opens an array of possible applications for this human derived biomaterial.
Fig. 2.
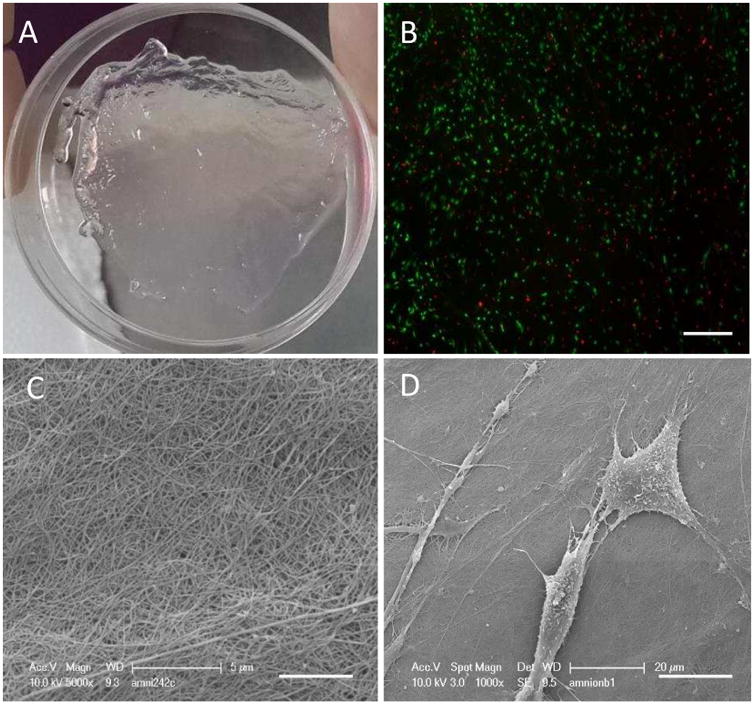
Cyto compatibility of decellularized AM. (A) AMused as a scaffold for cell culture. (B) Live-dead assay of the72 h PMSC culture, 100× magnification, scalebar = 100 μm. (C) Scanning electron microscopy of theamnion membrane surface, 5000× magnification, scalebar = 5 μm. (D) Scanning electron microscopy of cell seeded membrane, 1000× magnification. Scalebar = 20 μm.
3.3 Hydrogel nanostructure
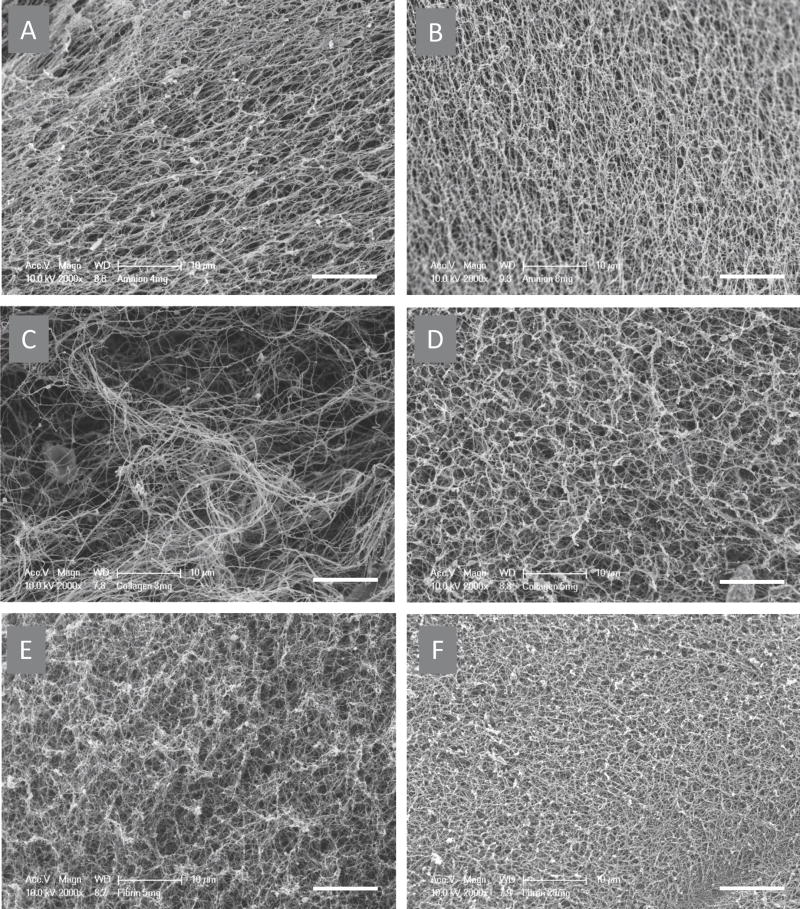
Hydrogel prepared from fibrin, collagen and AM-ECM exhibit similar surface topology and density (Figure 3). Scanning electron microscopy of collagen, fibrin and amnion gels revealed their fibrous structure and changes in fiber morphology with alteration of ECM concentration (Figure 4 A-F). The pore size is inversely proportional to the concentration of hydrogel. Also, at higher ECM concentration one can observe increased thickness of strands.
Fig. 3.
Surface topology of hydrogels. Fibrin hydrogel (A) was prepared at 20 mg/mLconcentration, collagen hydrogel (B) at 5 mg/mL and amnion derived hydrogel (C) at8 mg/mL. Scale bar =10 mm.
Fig. 4.
Scanning electron microscopy images of hydrogels.Images of amnion hydrogel with concentrations of 4 mg/mL (a) and 8 mg/mL (b). Collagen hydrogel with concentrations of 3 mg/mL (c) and 5 mg/mL (d). Fibrin hydrogel with concentrations of 5 mg/mL (e) and 20 mg/mL(f) were taken at 2000× magnification. Hydrogels exhibit comparable morphology, fiber thickness and density. Scalebar = 10 μm.
3.4 Gelation kinetics
Fibrin gel becomes solid quickly within 1-2 minutes even with very low (10U/ml) thrombin concentration (Figure 5A). This low concentration is useful when quick polymerization is required for cell delivery into the fluid abundant compartments. Amnion hydrogel reached 90% of its rigidity within 30 minutes after incubation initiation (Figure 5B). While increased concentration played a role in physical gel density, it did not affect its gelation time. When compared to Type I collagen hydrogel, amnion derived hydrogel polymerizes at least 15 minutes earlier. This phenomenon can be attributed to the presence of cross-linkable components in addition to Type I collagen and/or molecular interaction between collagen and other components of the diverse amnion scaffold.
Fig. 5.
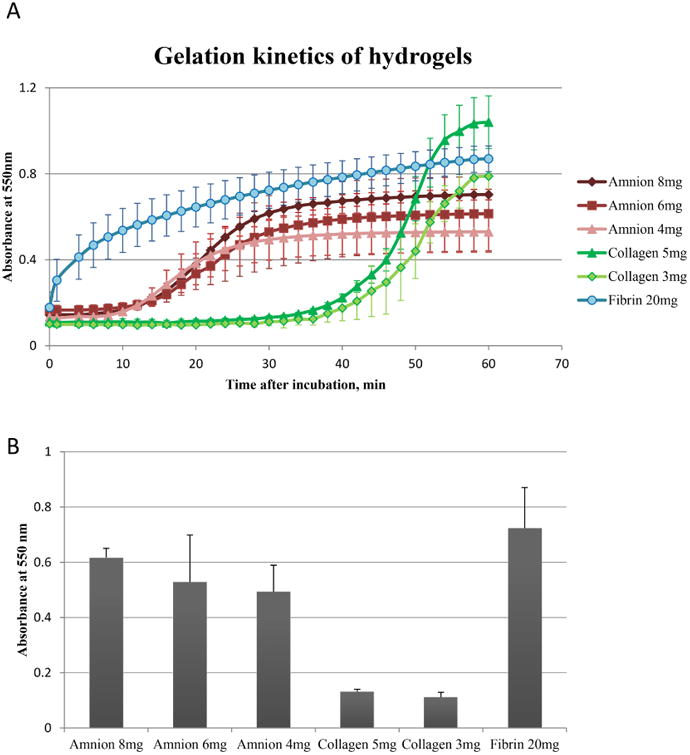
Gelation kinetics of hydrogels. (A) Hydrogel absorbancewas measured spectrophotometrically at 550 nm. (B) Optical density of gels measured at 30 min after incubation, n=4. At 37 °C amnion hydrogel solidifies faster than collagengel, but slower than fibrin clot. Error bars represent SD.
3.5 Biochemical composition of amnion hydrogel
The soluble collagen content of AM-ECM hydrogel with a concentration of 10 mg/mL was 33.5±0.9% and 41.0±0.9% for batch 1 and 2 (n=3 each). The elastin content was 24.1±0.9% and the total sGAG content was 0.40±0.03% and 0.43±0.03% for each batch (n=3) (Figure 6).
Fig. 6.
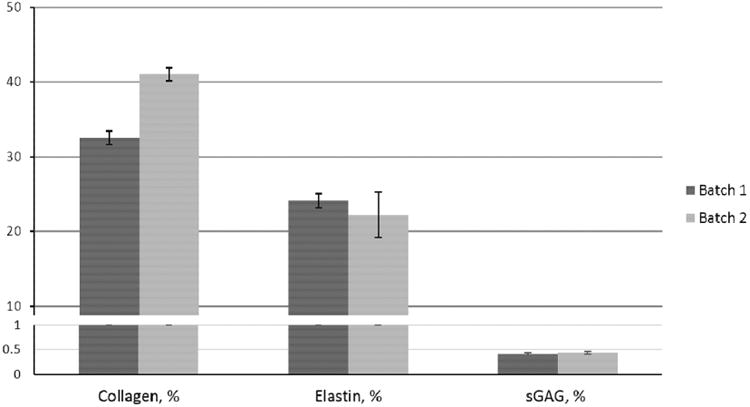
Biochemical composition of amnion hydrogel.Relative content of soluble collagen, elastin, and sulfated GAG proteins in 2 batches of amnion hydrogel. Error barsrepresent SD, n=3.
3.6 Analysis of bioactive molecules present in decellularized amnion ECM
Angiogenesis array screening revealed relatively low levels of 8 cytokines in cell-free amnion hydrogel (Figure 7A). Nevertheless, these factors can play a modulatory role in the behavior of embedded cells. The following proteins were detected: serpin E1 and F1, placenta growth factor, platelet factor 4, persephin, MMP-8, insulin-like growth factor-binding protein 1, and endocrine gland-derived vascular endothelial growth factor (Figure 7B).
Fig. 7.
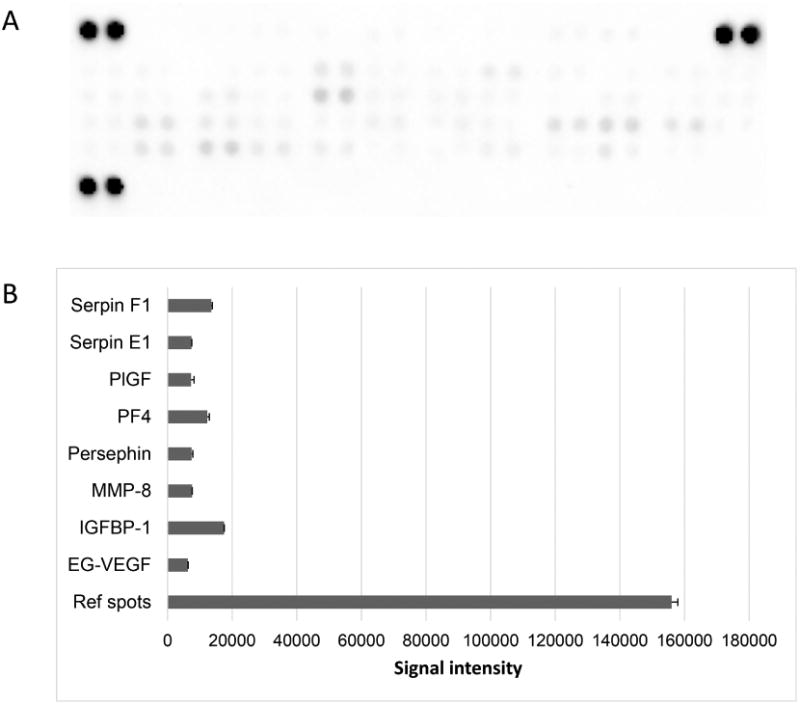
Detection of bioactive molecules in decellularizedamnion hydrogel. (A) Image of membrane from the angiogenesisarray. (B) Graph of corresponding dot integrated density values, error bars represent SD, n=2. Factors with integrated density values above 103 are shown.
3.7 In vitro MSC viability and proliferation tests
All hydrogels support proliferation of PMSCs (Figure 8A). However, at day 4 we noticed degradation of the fibrin 5 mg/ml matrix, and by day 6 most of the matrix was degraded, making it impossible to assess corresponding cellular propagation. Fibrin gel at 20 mg/ml concentration retained most of its volume, but a significant portion of it was degraded by day 6. PMSCs proliferate in amnion hydrogel significantly better than in collagen hydrogel (Figure 8B).
Fig. 8.
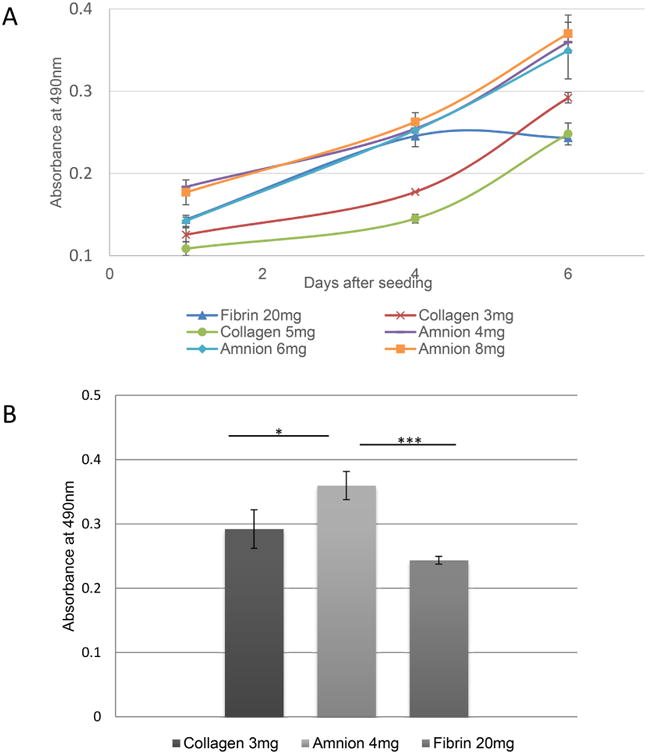
Proliferation of PMSCs in various hydrogels. (A)Absorbance values from the MTS assay represents the relative number of viable PMSCs in fibrin hydrogels with concentration of 20 mg/mL, collagen hydrogels with concentrations of 3 and 5 mg/mL and amnion hydrogels with concentration of 6 and 8 mg/mL at 1, 4 and 6 days periods. (B) Relative number of PMSCs in collagen, fibrin and amnionhydrogels at day 6. Error bars represent SEM.*p< 0.05, ***p< 0.005.
3.8 Cytokine array analysis of PMSC secreted proteins
According to results from the human angiogenesis array kit we detected 22 factors secreted by PMSCs in hydrogels (Figure 9A-B). Generally, PMSCs shared their cytokine secretion profiles when cultured in hydrogels. Compared to a monolayer culture, some factors were upregulated in cells grown in 3D matrix supported culture, including vascular endothelial growth factor (VEGF), hepatocyte growth factor, thrombospondin-2, platelet factor 4, matrix metallopeptidase 9, and angiopoietin. PMSCs cultured in amnion hydrogel expressed elevated levels of placental growth factor (PlGF), VEGF, prolactin, IL-8, FGF-7 and amphiregulin (Figure 9A). Fibrin based matrix stimulated secretion of platelet factor 4, matrix metallopeptidase 9, insulin-like growth factor-binding proteins 2 and 3, dipeptidyl peptidase 4 and angiostatin. Cells cultured in collagen based matrix expressed high levels of urokinase-type plasminogen activator (uPA), TIMP metallopeptidase inhibitor 1 (TIMP 1), and serpin peptidase inhibitor, clade E (Serpin E1).
Fig. 9.
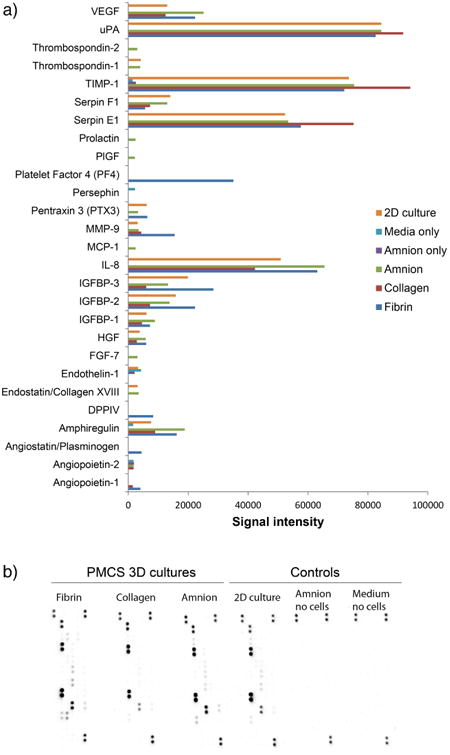
(A) Paracrine secretion of PMSCs embedded in amnion, collagen and fibrin hydrogels when compared to 2D cell culture and controls. Factors with integrated density values above 103 are shown. (B) Image of membranes from the cell secretion angiogenesis array.
3.9 Amnion hydrogel cytocompatibility
Results from the live-dead assay show that amnion derived hydrogel supports viability of various stem cell types, including C2C12 myoblasts, SH-SY5Y neuroblastoma cells, BM-MSCs, and PMSCs. Very few dead cells (in red fluorescence) were noticed, while the vast majority of cells were alive with intact membranes (in green fluorescence). All cell types exhibited normal and healthy morphology in the 3D amnion hydrogel matrix (Figure 10 A-D).
Fig. 10.
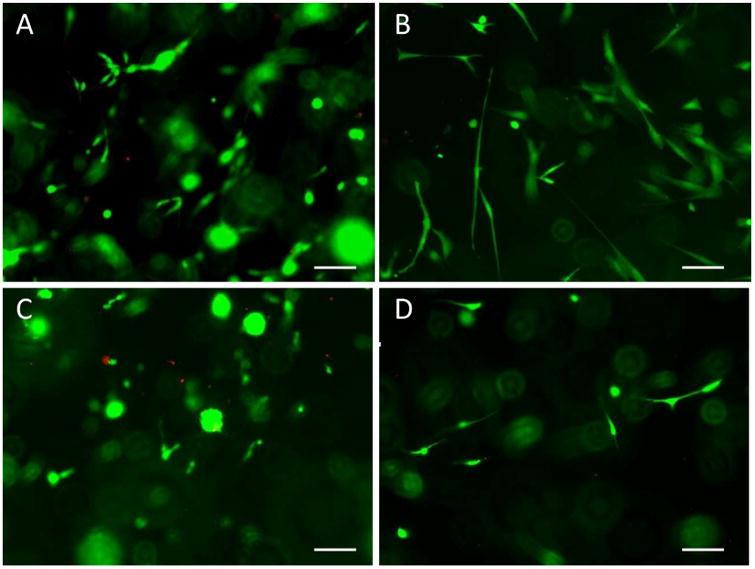
Live-dead fluorescent assay of stem cells embedded in AM-ECM hydrogel constructs. AM-ECM hydrogel with C2C12 myoblasts (A), PMSCs (B), SH-SY5Y neuroblastomacells (C), and BM-MSCs (D). Images were taken at 100× magnification. Scale bars= 100 μm.
3.10 In-vivo hydrogel compatibility
Two weeks after subcutaneous implantation of hydrogel, in vivo host response was evaluated. Hemotoxylin and eosin (H&E) staining examination showed the residual hydrogel (area between the two yellow imaginary lines, Figure 11A) was thicker than collagen (Figure 11B) and there was less cell infiltration (black arrow) in hydrogel (Figure 11C) than in collagen (Figure 11D). Immunofluorescence examination showed the infiltrated cells in hydrogel and collagen were CCR7 positive (stain green with DAPI blue nucleus stain, Figure 11E-F). The density of CCR7+ cells in hydrogel (Figure 11E) was lower than in collagen (Figure 11F) and there was a significant difference between them (p<0.01) (Figure 11G). This indicated that the amnion hydrogel had a lower degradation rate and lower inflammatory reaction in vivo than the collagen hydrogel.
Fig. 11.
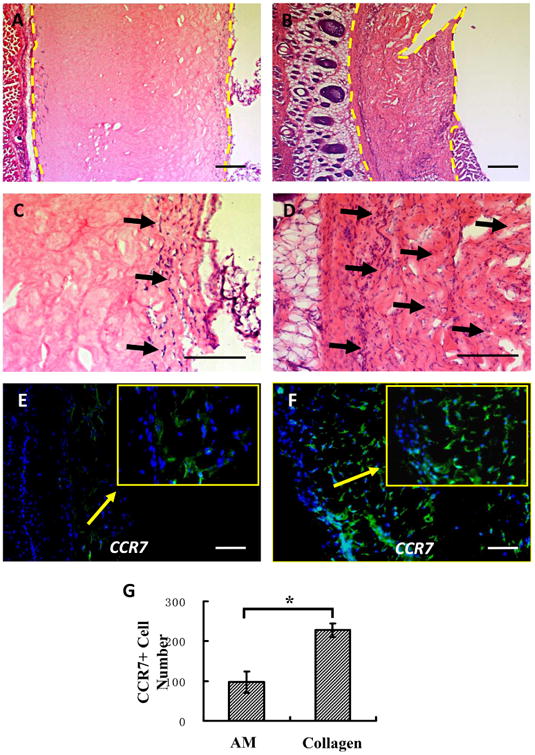
Histologic appearance of hydrogel and collagen after 14 days of in vivo implantation. Hematoxylin andeosin staining showed the remaining area (area between the two yellow imaginary lines) of hydrogel (A) was thickerthan collagen (B) and there was less cell infiltration (blackarrow) in hydrogel (C) than in collagen (D).Immunofluorescence staining examination indicated the density of CCR7+ cells (colocalization of green fluorescence with DAPI blue nucleus stain) in hydrogel (E) was lower than collagen (F) and there was a significant difference between them (G) (p< 0.01, n =3). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Biological hydrogels that mimic ECM are novel candidates that support 3D cell culture and may be utilized for research and clinical applications such as tissue engineering and stem cell therapy. A number of biocompatible hydrogels, either synthetically produced or naturally derived from plant or animal tissues, have been shown to successfully mimic ECM structure and support cell culture and delivery. They include a hyaluronan and methylcellulose blend [23], Poly(ethylene glycol)-Poly(ε-caprolactone) [23], alginate [24], collagen [25] and fibrin [4]. Animal derived collagen hydrogel is one of the most commonly used scaffolds for stem cell culture and delivery due to its abundance, accessibility and biocompatibility. Collagen is also compatible with multiple cell types and even was suggested for tissue and organ engineering [26, 27]. Fibrin based gel is another delivery system derived from human plasma and FDA-approved for surgical cosmetic and hemostatic applications [28]. It was shown that fibrin hydrogel facilitates cell attachment, proliferation, differentiation and tissue organization [29]. Nevertheless, the comparatively high cost of fibrin gel is a limiting factor for its widespread application.
Existing hydrogels are either chemically produced, which usually lacks native cell interaction capability, or extracted from animal origins that could trigger undesired immune responses from the host. Consequentially, dependent cellular therapies have also had limited efficacy, such as in cases of MSCs. Decellularization of human tissues, including the skin, liver, blood vessels and small intestinal submucosa, has shown some success in early clinical trials. However, this method is recently discovered and still relatively under-explored. In addition, application of human tissue derived products is limited by tissue availability and accessibility.
In this study, we showed that human amniotic membrane can be effectively decellularized without the use of sodium dodecyl sulfate (SDS). Since SDS has adverse effects on tissue architecture and cell viability, and residual SDS can be detected upon application of common decellularization methods, we employed a SDS-free protocol. We were able to produce a mechanically durable cell-free biomaterial that can also be used as a scaffold for cell culture where rigid form of base matrix is required. Such applications include cell transplantation to epithelial tissues.
Next, acellular AM can be solubilized via pepsin digestion to form a hydrogel with biophysical characteristics similar to collagen and fibrin. On the nanoscale base, hydrogels produced with collagen at 3mg/ml, fibrin at 5mg/ml and amnion at 4 mg/ml concentrations exhibited similar fiber thickness and density as well as pore size. Fibrin clot polymerization is the fastest among hydrogels, and takes about 2-3 minutes. Amnion hydrogel solidifies within 25 minutes at 37°C, whereas collagen based gel solidifies in about 45 minutes. Faster polymerization may be desired for in vivo applications to limit the cell loss from the site of application. Amnion and collagen hydrogel polymerization rate and mechanical strength can be further manipulated with non-cytotoxic cross-linking agents, such as transglutaminase and ribose via nonenzymatic glycation, and should be further investigated in stem cell therapy applications [30, 31].
In vitro cell proliferation tests revealed the ability of amnion hydrogel to support the growth of PMSCs. Moreover, cells embedded in amnion gel propagated at significantly higher rates when compared to collagen and fibrin embedded cells (Figure 6). Fibrin hydrogel degraded quickly and was unsuitable for 3D cell cultures lasting longer than 5 days. Protease inhibitors prolong the integrity of fibrin, but may limit its in vivo application due to undesirable side effects. At the end of 6-day culture amnion hydrogel retained most of its volume, indicating its potential to protect cells in the matrix for at least 1 week.
Scientists agree that many of the therapeutic properties of MSCs are derivative of their paracrine activity [11]. Cytokine secretion assays demonstrated that, despite generally similar secretion profiles among hydrogelembedded PMSCs, extracellular scaffold derived variations in secretion levels of specific cytokines exist. Most notably, stem cells altered their secretion configurations when they were cultured in fibrin and amnion hydrogels. PMSCs that were cultured in amnion derived hydrogel were noted for expression of cytokines related to angiogenesis, such as PlGF and prolactin. PlGF along with VEGF play significant roles in angiogenesis, endothelial cell migration and proliferation [32, 33]. Also, amnion embedded PMSCs secreted higher levels of IL-8 and FGF-7. IL-8 is known for chemotaxis of granulocytes, cell survival and proliferation [34]. FGF-7, aka keratinocyte growth factor, is a mitogen, and participates in neuroprotection and wound repair [35]. This data suggests that PMSCs can affect angiogenesis related processes in response to environmental signaling, and proper considerations should be taken when selecting a cell delivery vehicle. To ensure the safety and effectiveness of stem cell therapy, cytokine secretion profiles should be carefully evaluated upon in vivo delivery of cells to the target site.
Our preliminary data support that the amnion derived hydrogel is a suitable candidate for delivery of PMSCs. This matrix is able to maintain normal cellular attachment, proliferation, and migration. Unlike collagen, amnion hydrogel is human derived, eliminating host rejection concerns exerted by xenogeneic materials.
To further investigate AM-ECM hydrogel cell delivery capabilities, we tested its cytocompatibility with several widely used cell lines. C2C12 cells are used to study myoblast differentiation, and are capable in producing skeletal muscle-like tissues [36]. The SH-SY5Y line serves as a model of neuron function and maturation, whereas BM-MSCs are the current gold standard in MSC-related therapies. All of these cells successfully adhered to the amnion hydrogel matrix, exhibited their normal morphology and proliferated at rates comparable to conventional 2D culture. The number of dead cells detected in the live-dead assay was negligible, signifying the cytocompatibility of amnion 3D matrix for cell culture.
Preliminary in vivo studies demonstrated that AM-ECM hydrogel does not cause any visible tissue inflammation or active recruitment of immune cells. Additional experiments will be needed to investigate hydrogel degradation rate, cellular migration and distribution within the target site.
5. Conclusions
Amnion hydrogel possesses a set of unique properties that makes it stand out from other biomatrices. This matrix is human derived, prepared from processed amnion ECM, and is inherently compatible with human PMSCs. This hydrogel is biocompatible with a wide range of stem cell types, supporting their normal morphology and physiology. AM-ECM hydrogel is a promising material for use in tissue repair and regeneration due to its angiogenic nature, biocompatibility, and the ability of AM-ECM to biodegrade through a natural process. To this end, extracellular matrix made from decellularized human AM (AM-ECM, in the formats of sheet and hydrogel) is a novel candidate for propagating and transplanting human stem cells. Considering that this material is readily available for both basic research and clinical applications, its development may lead to a breakthrough in stem cell therapeutic applications. So far, no human ECM based (xeno-free) hydrogel matrix for human stem cell culture, expansion and delivery is available on market. The result of this innovative advancement is a familiar and promising alternative that eliminates host rejection, decreases morbidity, promotes medical efficiency and reduces economic burdens that may otherwise arise from secondary care.
Acknowledgments
We would like to acknowledge Christopher Pivetti, Benjamin Keller, Trent You, Aireen Agulto and Ivonne Palma at the Surgical Bioengineering Laboratory at the University of California, Davis School of Medicine for their technical assistance with the project.
Funding: This work was in part supported by NIH grant 5R01NS100761-02 and March of Dimes Foundation grant 5FY1682. Volodymyr Ryzhuk was supported by the California Institute for Regenerative Medicine Bridges to Stem Cell Research program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of trans differentiation and modes of tissue repair--current views. Stem cells (Dayton, Ohio) 2007;25(11):2896–902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 2.Kraitchman DL, Tatsumi M, Gilson WD, Ishimori T, Kedziorek D, Walczak P, Segars WP, Chen HH, Fritzges D, Izbudak I, Young RG, Marcelino M, Pittenger MF, Solaiyappan M, Boston RC, Tsui BM, Wahl RL, Bulte JW. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112(10):1451–61. doi: 10.1161/CIRCULATIONAHA.105.537480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manuelpillai U, Moodley Y, Borlongan CV, Parolini O. Amniotic membrane and amniotic cells: potential therapeutic tools to combat tissue inflammation and fibrosis? Placenta. 2011;32(4):S320–5. doi: 10.1016/j.placenta.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Hydrogels in regenerative medicine. Advanced Materials. 2009;21(32-33):3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark RA, Ghosh K, Tonnesen MG. Tissue engineering for cutaneous wounds. The Journal of investigative dermatology. 2007;127(5):1018–29. doi: 10.1038/sj.jid.5700715. [DOI] [PubMed] [Google Scholar]
- 6.Catelas I, Sese N, Wu BM, Dunn JC, Helgerson S, Tawil B. Human mesenchymal stem cell proliferation and osteogenic differentiation in fibrin gels in vitro. Tissue engineering. 2006;12(8):2385–2396. doi: 10.1089/ten.2006.12.2385. [DOI] [PubMed] [Google Scholar]
- 7.DeQuach JA, Mezzano V, Miglani A, Lange S, Keller GM, Sheikh F, Christman KL. Simple and High Yielding Method for Preparing Tissue Specific Extracellular Matrix Coatings for Cell Culture. PloS one. 2010;5(9) doi: 10.1371/journal.pone.0013039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolf MT, Daly KA, Brennan-Pierce EP, Johnson SA, Carruthers CA, D'Amore A, Nagarkar SP, Velankar SS, Badylak SF. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials. 2012;33(29):7028–38. doi: 10.1016/j.biomaterials.2012.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galili U. Avoiding detrimental human immune response against Mammalian extracellular matrix implants, Tissue engineering. Part B. Reviews. 2015;21(2):231–41. doi: 10.1089/ten.TEB.2014.0392. [DOI] [PubMed] [Google Scholar]
- 10.Wong ML, Griffiths LG. Immunogenicity in xenogeneic scaffold generation: antigen removal vs. decellularization. Acta biomaterialia. 2014;10(5):1806–16. doi: 10.1016/j.actbio.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsivetherapeutics for regenerative medicine. Experimental & molecular medicine. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076–84. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 13.Bollini S, Gentili C, Tasso R, Cancedda R. The regenerative role of the fetal and adult stem cell secretome. Journal of Clinical Medicine. 2013;2(4):302–327. doi: 10.3390/jcm2040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown EG, Keller BA, Lankford L, Pivetti CD, Hirose S, Farmer DL, Wang A. Age Does Matter: A Pilot Comparison of Placenta-Derived Stromal Cells for in utero Repair of Myelomeningocele Using a Lamb Model. Fetal Diagn Ther. 2016;39(3):179–85. doi: 10.1159/000433427. [DOI] [PubMed] [Google Scholar]
- 15.Wang A, Brown EG, Lankford L, Keller BA, Pivetti CD, Sitkin NA, Beattie MS, Bresnahan JC, Farmer DL. Placental mesenchymal stromal cells rescue ambulation in ovine myelomeningocele. Stem Cells Transl Med. 2015;4(6):659–69. doi: 10.5966/sctm.2014-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meierhenry JA, Ryzhuk V, Miguelino MG, Lankford L, Powell JS, Farmer D, Wang A. Placenta as a Source of Stem Cells for Regenerative Medicine. Current Pathobiology Reports. 2015;3(1):9–16. [Google Scholar]
- 17.Meierhenry J, Ryzhuk V, Miguelino M, Lankford L, Powell J, Farmer D, Wang A. Placenta as a Source of Stem Cells for Regenerative Medicine. Curr Pathobiol Rep. 2015;3(1):9–16. [Google Scholar]
- 18.Barlow S, Brooke G, Chatterjee K, Price G, Pelekanos R, Rossetti T, Doody M, Venter D, Pain S, Gilshenan K, Atkinson K. Comparison of human placenta- and bone marrow-derived multipotent mesenchymal stem cells. Stem cells and development. 2008;17(6):1095–107. doi: 10.1089/scd.2007.0154. [DOI] [PubMed] [Google Scholar]
- 19.Lankford L, Selby T, Becker J, Ryzhuk V, Long C, Farmer D, Wang A. Early gestation chorionic villi-derived stromal cells for fetal tissue engineering. World Journal of Stem Cells. 2015;7(1):195–207. doi: 10.4252/wjsc.v7.i1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104(9):2752–60. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 21.Williams PA, Stilhano RS, To VP, Tran L, Wong K, Silva EA. Hypoxia augments outgrowth endothelial cell (OEC) sprouting and directed migration in response to sphingosine-1-phosphate (S1P) PloS one. 2015;10(4):e0123437. doi: 10.1371/journal.pone.0123437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reing JE, Brown BN, Daly KA, Freund JM, Gilbert TW, Hsiong SX, Huber A, Kullas KE, Tottey S, Wolf MT, Badylak SF. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials. 2010;31(33):8626–33. doi: 10.1016/j.biomaterials.2010.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ballios BG, Cooke MJ, van der Kooy D, Shoichet MS. A hydrogel-based stem cell delivery system to treat retinal degenerative diseases. Biomaterials. 2010;31(9):2555–2564. doi: 10.1016/j.biomaterials.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Bhat A, Hoch AI, Decaris ML, Leach JK. Alginate hydrogels containing cell-interactive beads for bone formation. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27(12):4844–52. doi: 10.1096/fj.12-213611. [DOI] [PubMed] [Google Scholar]
- 25.Elgharably H, Roy S, Khanna S, Abas M, Das Ghatak P, Das A, Mohammed K, Sen CK. A modified collagen gel enhances healing outcome in a preclinical swine model of excisional wounds. Wound Repair and Regeneration. 2013;21(3):473–481. doi: 10.1111/wrr.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huh D, Hamilton GA, Ingber DE. From 3D cell culture to organs-on-chips. Trends in cell biology. 2011;21(12):745–754. doi: 10.1016/j.tcb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferreira AM, Gentile P, Chiono V, Ciardelli G. Collagen for bone tissue regeneration. Acta biomaterialia. 2012;8(9):3191–200. doi: 10.1016/j.actbio.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Man D, Plosker H, Winland-Brown JE. The use of autologous platelet-rich plasma (platelet gel) and autologous platelet-poor plasma (fibrin glue) in cosmetic surgery. Plastic and reconstructive surgery. 2001;107(1):229–37. doi: 10.1097/00006534-200101000-00037. discussion 238-9. [DOI] [PubMed] [Google Scholar]
- 29.Kim I, Lee SK, Yoon JI, Kim DE, Kim M, Ha H. Fibrin glue improves the therapeutic effect of MSCs by sustaining survival and paracrine function. Tissue Engineering Part A. 2013;19(21-22):2373–2381. doi: 10.1089/ten.tea.2012.0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orban JM, Wilson LB, Kofroth JA, El-Kurdi MS, Maul TM, Vorp DA. Crosslinking of collagen gels by transglutaminase. Journal of biomedical materials research Part A. 2004;68(4):756–62. doi: 10.1002/jbm.a.20110. [DOI] [PubMed] [Google Scholar]
- 31.Roy R, Boskey A, Bonassar LJ. Processing of type I collagen gels using nonenzymatic glycation. Journal of biomedical materials research Part A. 2010;93(3):843–51. doi: 10.1002/jbm.a.32231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luttun A, Tjwa M, Carmeliet P. Placental growth factor (PlGF) and its receptor Flt-1 (VEGFR-1): novel therapeutic targets for angiogenic disorders. Annals of the New York Academy of Sciences. 2002;979:80–93. doi: 10.1111/j.1749-6632.2002.tb04870.x. [DOI] [PubMed] [Google Scholar]
- 33.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacological reviews. 2004;56(4):549–80. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 34.Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. Journal of immunology (Baltimore, Md : 1950) 2003;170(6):3369–76. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 35.Beer HD, Gassmann MG, Munz B, Steiling H, Engelhardt F, Bleuel K, Werner S. Expression and function of keratinocyte growth factor and activin in skin morphogenesis and cutaneous wound repair. The journal of investigative dermatology Symposium proceedings / the Society for Investigative Dermatology, Inc [and] European Society for Dermatological Research. 2000;5(1):34–9. doi: 10.1046/j.1087-0024.2000.00009.x. [DOI] [PubMed] [Google Scholar]
- 36.Donnelly K, Khodabukus A, Philp A, Deldicque L, Dennis RG, Baar K. A novel bioreactor for stimulating skeletal muscle in vitro, Tissue engineering. Part C. Methods. 2010;16(4):711–8. doi: 10.1089/ten.TEC.2009.0125. [DOI] [PubMed] [Google Scholar]