Abstract
IMPORTANCE
Cardiac magnetic resonance (CMR) imaging can identify unrecognized myocardial infarction (UMI) in the general population. Unrecognized myocardial infarction by CMR portends poor prognosis in the short term but, to our knowledge, long-term outcomes are not known.
OBJECTIVE
To determine the long-term outcomes of UMI by CMR compared with clinically recognized myocardial infarction (RMI) and no myocardial infarction (MI).
DESIGN, SETTING, AND PARTICIPANTS
Participants of the population-based, prospectively enrolled ICELAND MI cohort study (aged 67–93 years) were characterized with CMR at baseline (from January 2004-January 2007) and followed up for up to 13.3 years. Kaplan-Meier time-to-event analyses and a Cox regression were used to assess the association of UMI at baseline with death and future cardiovascular events.
MAIN OUTCOMES AND MEASURES
The primary outcome was all-cause mortality. Secondary outcomes were a composite of major adverse cardiac events (MACE: death, nonfatal MI, and heart failure).
RESULTS
Of 935 participants, 452 (48.3%) were men; the mean (SD) age of participants with no MI, UMI, and RMI was 75.6 (5.3) years, 76.8 (5.2) years, and 76.8 (4.7) years, respectively. At 3 years, UMI and no MI mortality rates were similar (3%) and lower than RMI rates (9%).At 5 years, UMI mortality rates (13%) increased and were higher than no MI rates (8%) but still lower than RMI rates (19%). By 10 years, UMI and RMI mortality rates (49% and 51%, respectively) were not statistically different; both were significantly higher than no MI (30%) (P <.001). After adjusting for age, sex, and diabetes, UMI by CMR had an increased risk of death (hazard ratio [HR], 1.61; 95% CI, 1.27–2.04), MACE (HR, 1.56; 95% CI, 1.26–1.93), MI (HR, 2.09; 95% CI, 1.45–3.03), and heart failure (HR, 1.52; 95% CI, 1.09–2.14) compared with no MI and statistically nondifferent risk of death (HR, 0.99; 95% CI, 0.71–1.38) and MACE (HR, 1.23; 95% CI, 0.91–1.66) vs RMI.
CONCLUSIONS AND RELEVANCE
In this study, all-cause mortality of UMI was higher than no MI, but within 10 years from baseline evaluation was equivalent with RMI. Unrecognized MI was also associated with an elevated risk of nonfatal MI and heart failure. Whether secondary prevention can alter the prognosis of UMI will require prospective testing.
The prevalence of unrecognized myocardial infarction (UMI) varies with the study population and methods of detection.1–7 Cardiovascular magnetic resonance imaging (CMR) identifies UMI more accurately than electrocardiograms.6 Unrecognized myocardial infarction by CMR is more prevalent than recognized myocardial infarction (RMI) in older populations. Additionally, UMI by CMR portends poor survival rates, although long-term outcomes are not known.6 We investigated the long-term prognosis of UMI by CMR. We hypothesized that participants with UMI would have higher risk of long-term mortality, nonfatal myocardial infarction (MI), and heart failure than those without MI.
Methods
A subset of the Age, Gene/Environment Susceptibility (AGES)-Reykjavik prospective cohort (5764 Icelandic, community-dwelling, older individuals) was characterized by gadolinium-enhanced CMR to form the ICELAND MI study.8 After a phase of randomized recruitment, individuals with diabetes were selectively recruited. Baseline variables were collected between January 2004 and January 2007. Electrocardiogram-gated cardiac computed tomography was also performed to determine coronary artery calcium (CAC) scores.
The National Institutes of Health, the Icelandic Heart Association, and the Icelandic Parliament funded this study. The study was approved by the National Institute on Aging intramural institutional review board and the National Bioethics Committee in Iceland. All participants provided informed consent.
CMR Analysis
Cardiovascular magnetic resonance imaging was performed on a 1.5-T scanner (General Electric Healthcare) using a 4-element cardiac-phased array coil. Cardiac function was assessed using cinesteady state-free precession imaging (pixel dimension, 1.8 × 2.1 mm; slice thickness, 8 mm; gap, 3 mm; 30 images per cycle). Prospective electrocardiogram- gated, segmented, phase-sensitive gradient echo and inversion recovery sequences for late gadolinium enhancement (LGE) imaging were obtained 6 to 25 minutes after 0.1 mmoL/kg intravenous gadolinium (Magnevist; Berlex) contrast administration.9
Definitions
Recognized MI was defined by a history of MI before enrollment as supported by hospital records. Unrecognized MI required no history of MI but did require LGE findings involving the subendocardium in a coronary artery distribution by a consensus of experienced cardiologists.10
All-cause mortality and secondary outcomes, including nonfatal MI, heart failure, and major adverse cardiac events (MACE, a composite of mortality, MI, and heart failure), were evaluated. Outcomes were derived and adjudicated from a national database of deaths and hospital, nursing home, and home care records.
Statistical Analysis
Baseline variables were compared with the analysis of variance, Kruskal-Wallis, or χ2 tests. The t test, Wilcoxon rank sum, or χ2 was used for pairwise comparisons. Outcomes were compared with a Kaplan-Meier time-to-event analysis and Cox proportional hazards models. We calcuated 95% confidence intervals and P values were 2-sided. Statistical significance was set at P < .05.
Results
The mean (SD) age of the study population (n = 935) was 76 (5.2) years (range, 67–93 years), and 483 (52%) were women. At baseline, UMI (156 [17%]) was more prevalent than RMI (91 [10%]) (Table 1). Unrecognized MI and RMI were more common in men than women.
Table 1.
Baseline Characteristics of the Comparison Groupsa
| Characteristic | No. (%) | P Value | ||
|---|---|---|---|---|
| No MI (n = 688) |
UMI (n = 156) |
RMI (n = 91) |
||
| Age, mean (SD), y | 75.6 (5.3) | 76.8 (5.2)b | 76.8 (4.7) | .01 |
| Male | 294 (42.7) | 99 (63.5))b | 59 (64.8) | <.001 |
| BMI | 27.5 (4.3) | 27.8 (4.1) | 27.5 (4.4) | .77 |
| Blood pressure, mm Hg | ||||
| Systolic | 143 (2) | 147 (19)b | 143 (18) | .08 |
| Diastolic | 74 (10) | 74 (9) | 74 (9) | .98 |
| Risk factors | ||||
| Hypertension | 549 (79.8) | 141 (90.4)b | 88 (96.7) | <.001 |
| Diabetes | 227 (33) | 72 (46.2)b | 37 (40.7) | .01 |
| Current smoking | 73 (10.6) | 23 (14.7) | 9 (9.9) | .31 |
| Prior smoking | 324 (47.1) | 75 (48.1) | 56 (61.5) | .03 |
| Metabolic syndrome | 299 (43.5) | 85 (54.5)b | 52 (57.1) | .01 |
| Prior cardiovascular disease | ||||
| Revascularization, PCI/CABG | 43 (6.3) | 41 (26.3)b,c | 53 (58.2) | <.001 |
| Stroke | 26 (3.8) | 9(5.8) | 6 (6.6) | .31 |
| Heart failure | 6 (0.9) | 7 (4.5)b | 9 (9.9) | <.001 |
| Medications | ||||
| Aspirin | 215 (31.3) | 81 (51.9)b,c | 74 (81.3) | <.001 |
| Statin | 153 (22.2) | 56 (35.9)b,c | 66 (72.5) | <.001 |
| ACE-I/ARB | 164 (26.3) | 47 (31.8) | 27 (29.8) | .37 |
| β-Blockers | 236 (34.3) | 70 (44.9)b,c | 70 (76.9) | <.001 |
| Insulin/oral hypoglycemic agent | 119 (19.1) | 43 (29.1)b | 19 (20.9) | .02 |
| Laboratory values | ||||
| Total cholesterol, mg/dL | 216 (185–243) | 201 (168–239)b,c | 177.6 (153.7–204.6) | <.001 |
| Cholesterol, mg/dL | ||||
| LDL | 134 (108–162) | 120 (91–157)b,c | 98.3 (76.8–127.8) | <.001 |
| HDL | 58 (48–70) | 53 (45–63)a | 50.6 (42.5–59.1) | <.001 |
| Triglycerides, mg/dL | 95 (73–132) | 108 (79–150)b | 103.5 (73.5–146.9) | .01 |
| Hemoglobin A1C, % | 5.7 (5.4–6) | 5.9 (5.5–6.4)b | 5.8 (5.5–6.2) | .001 |
| Creatinine, mg/dL | 1 (0.8–1.1) | 1 (0.9–1.2)b | 1 (0.9–1.3) | <.001 |
| Estimated GFR, mL/min/1.73 m2 | 58 (49–70) | 53 (44–64)b | 50.5 (41.1–58.8) | <.001 |
| Imaging | ||||
| Coronary calcium score, AUd | 227 (50–694) | 778 (262–1684)b,c | 1133 (654–2159) | <.001 |
| LV ejection fraction, % | 63 (58–67) | 60 (51–65)b,c | 53 (42–61) | <.001 |
| LV end-diastolic volume index, mL/m2 | 97 (85–113) | 109 (90–131)b | 116 (92–151) | <.001 |
| LV mass index, gm/m2 | 71 (59–86) | 84 (71–100)b | 84 (69–109) | <.001 |
| Infarct size, % | 0 | 4 (2.2–8.1)b,c | 9.6 (0.6–22.8) | <.001 |
Abbreviations: ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; AU, Agatston units; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CABG, coronary artery bypass grafting; GFR, glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LV, left ventricular; MI,myocardial infarction; PCI, percutaneous coronary intervention; RMI, recognized myocardial infarction; UMI, unrecognizedmyocardial infarction. SI Conversion Units: To convert creatinine to micromoles per liter, multiply by 88.4; for HDL, LDL, and total cholesterol to millimoles per liter, multiply by 0.0259; for hemoglobin A1c to the proportion of total hemoglobin, mulitply by 0.01.
Categorical variables are represented as No. (%), parametric continuous variables as mean (SD), and nonparametric continuous variables as median (interquartile range). P values are derived from comparing the 3 groups using analysis of covariance, Kruskal-Wallis, or χ2 tests. The t test, Wilcoxon rank sum test, or χ2 test was used for pairwise comparisons.
Pairwise comparisons show statistically significant differences between no MI and UMI (P <.03 using Bonferroni correction).
Pairwise comparisons show statistically significant differences between UMI and RMI (P <.03 using Bonferroni correction).
Coronary calcium score was derived from a noncontrast, electrocardiogram-gated, cardiac computed tomography scan and reported as AU. Left ventricular ejection fraction, end-diastolic volume, mass, and infarct size were derived from gadolinium contrast–enhanced cardiac magnetic resonance imaging.
Traditional cardiovascular risk factors were more prevalent in individuals with UMI and RMI. There were signs of riskfactor modification, with the RMI group having the lowest smoking rate, lower cholesterol levels, and more prescriptions for guideline-based medical therapy. Coronary artery calcium scores in UMI were intermediate between RMI and no MI.
Left ventricular ejection fraction of UMI (60%) was intermediate between RMI (53%) and no MI (63%). Unrecognized MI infarct size, on LGE results, was significantly smaller than RMI (4% vs 9.6% of the left ventricle).
Outcomes
The average follow-up was 10.5 years (95% CI, 10.3–10.8) for all-cause mortality, 9.4 years (95% CI, 9.1–9.6) for MACE, 11.8 years (95% CI, 11.6–12.0) fornonfatal MI, and 11.4 (95% CI, 11.1–11.6) for heart failure. There was no loss to follow-up.
There were 424 deaths. At 3 years, UMI mortality rates (3%) were not significantly different from no MI rates (3%;P = .62) and were significantly lower than RMI rates (9%;P = .03) (Figure 1). By 5 years, UMI mortality rates (13%) were intermediate between no MI rates (8%) and RMI rates (19%). However, at 10 years, UMI and RMI mortality rates were not statistically different (49% and 51%, respectively;P = .99) and were significantly higher than no MI rates (30%; P <.001). After adjusting for age, sex, and diabetes, the UMI mortality risk remained higher than no MI (hazard ratio [HR], 1.61; 95% CI, 1.27–2.04) and statistically not different from RMI (HR, 0.99; 95% CI, 0.71–1.38) (Table 2).
Figure.1. Kaplan-Meier Survival Analysis.
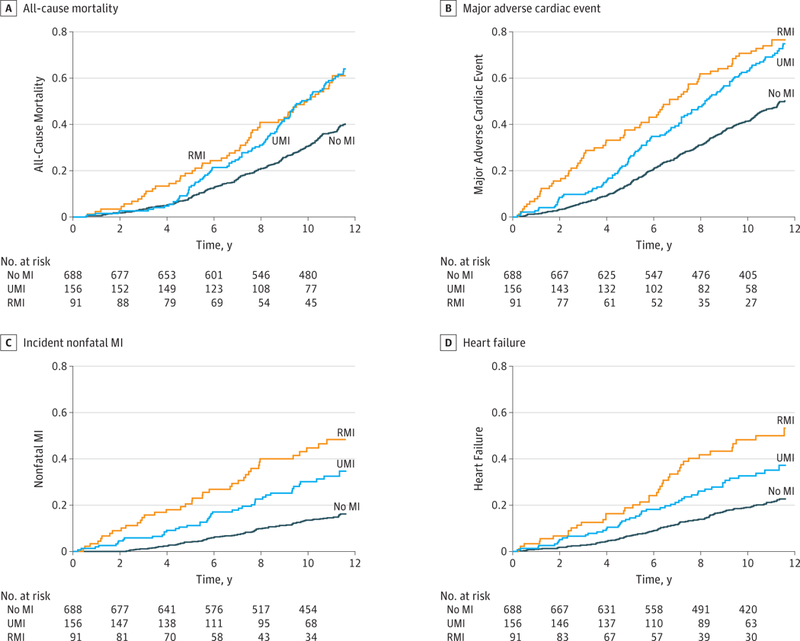
All-cause mortality (A), major adverse cardiac events (B), incident nonfatalmyocardial infarction (MI) (C), and incident heart failure (D) in participants with unrecognized MI (UMI), recognized MI (RMI), and no MI at baseline.
Table 2.
Association of Unrecognized and Recognized Myocardial Infarction Compared With No Myocardial Infarction With Primary and Secondary Outcomes
| Characteristic | Hazard Ratio (95% CI)a | ||
|---|---|---|---|
| Model 1b | Model 2c | Model 3d | |
| All-Cause Mortality | |||
| UMI vs no MI | 1.90 (1.51–2.39) | 1.61 (1.27–2.04) | 1.60 (1.26–2.03) |
| RMI vs no MI | 1.86 (1.39–2.49) | 1.59 (1.18–2.14) | 1.47 (1.07–2.03) |
| RMI vs UMI | 0.98 (0.70–1.37) | 0.99 (0.71–1.38) | 0.92 (0.65–1.30) |
| Myocardial Infarction | |||
| UMI vs no MI | 2.44 (1.70–3.51) | 2.09 (1.45–3.03) | 1.87 (1.28–2.73) |
| RMI vs no MI | 4.17 (2.86–6.09) | 3.56 (2.42–5.23) | 2.89 (1.87–4.44) |
| RMI vs UMI | 1.71 (1.10–2.64) | 1.70 (1.10–2.63) | 1.54 (0.98–2.43) |
| MACE | |||
| UMI vs no MI | 1.85 (1.50–2.29) | 1.56 (1.26–1.93) | 1.49 (1.19–1.85) |
| RMI vs no MI | 2.24 (1.73–2.90) | 1.92 (1.47–2.49) | 1.72 (1.29–2.29) |
| RMI vs UMI | 1.21 (0.90–1.63) | 1.23 (0.91–1.66) | 1.16 (0.85–1.58) |
| Heart Failure | |||
| UMI vs no MI | 1.84 (1.32–2.56) | 1.52 (1.09–2.14) | 1.40 (1.00–2.00) |
| RMI vs no MI | 3.07 (2.15–4.37) | 2.63 (1.83–3.77) | 2.18 (1.47–3.23) |
| RMI vs UMI | 1.67 (1.10–2.55) | 1.72 (1.13–2.63) | 1.55 (1.00–2.41) |
Abbreviations: MACE, major adverse cardiac events; MI, myocardial infarction; RMI, recognized myocardial infarction; UMI, unrecognized myocardial infarction.
Hazard ratios (95% CI) are derived from Cox proportional hazards modeling. Because the mortality hazard of UMI changes overtime, the Cox proportional hazards assumptions are violated. Hazard ratios should therefore only be interpreted as a weighted average over the entire follow-up duration.
Model 1 is the unadjusted analysis.
Model 2 adjusts for age, sex, and diabetes.
Model 3 adjusts for age, sex, diabetes, smoking, hypertension, total cholesterol, high-density lipoprotein cholesterol, statin use, body mass index, and estimated glomerular filtration rate.
There were 174 nonfatal MI and 220 heart failure events, with 198 individuals (21.2%) having either and 98 (10.5%) having both events. Unrecognized MI had an intermediate risk of nonfatal MI and heart failure vs no MI and RMI (Figure 1). After statistical adjustment for age, sex, and diabetes, UMI had significantly higher risk of MACE (HR, 1.56; 95% CI, 1.26–1.93), nonfatal MI (HR, 2.09; 95% CI, 1.45–3.03), and heart failure (HR, 1.52; 95% CI, 1.09–2.14) compared with no MI. When compared with RMI, the risk of death (HR, 0.99; 95% CI, 0.71–1.38) and MACE (HR, 1.23; 95% CI, 0.91–1.66) in UMI were not statistically different.
A subgroup analysis (eFigure in the Supplement) showed higher hazard ratios for mortality in the UMI group for men and in those with diabetes, whereas opposite trends were seen with RMI. Participants younger than 70 years appeared to be at the highest risk of death from UMI, especially when compared with the RMI group; however, the confidence intervals were wide because there were only 24 deaths in this age category. The mortality risk of both UMI and RMI increased with a larger infarct size. No effect modification was seen when stratified by left ventricular systolic function. Most participants (935 [77%]) had an ejection fraction of 55% or more and only 28 (3%) had an ejection fraction ofless than 35%.
Discussion
In a cohort of community-dwelling, elderly individuals, UMI by CMR had higher rates of death, nonfatal MI, and heart failure than no MI at 10-year follow-up. After an initial period of relative quiescence, the UMI mortality rate increased substantially, catching up to RMI mortality.
During the initial 4 years, the mortality rate of UMI was low and not significantly different from the no MI group. This may reflect a lower short-term clinical effect of the smaller infarct size in UMI. Between 4 and 9 years, the UMI mortality rate climbed significantly faster than no MI, and UMI mortality rates equaled RMI mortality rates by 10 years. The progressive convergence of the UMI and RMI mortality curves may have 2 possible mechanisms. First, as suggested in previous studies, UMI may represent a different coronary disease phenotype with more small-vessel involvement and atrial fibrillation than RMI and thus chart a different natural course.11–13 Because of a lower epicardial plaque burden (lower CAC) than RMI at baseline, UMI event rates may lag behind RMI and increase after a delay. It is also plausible that additional UMI events over time accelerate the mortality rate in this group.
Second, preventive therapy with aspirin, statin, and β-blockers presumably attenuated RMI mortality rates. The associations of statins and diet appear evidenced by the lower cholesterol levels in this group. The recognition of an MI may have changed risky behaviors, as individuals with RMI were less likely to continue smoking. High mortality rates in the UMI group might be explained by fewer prescriptions of preventive treatments. Survival bias is another possible mechanism for comparatively lower mortality in the RMI group because those with more severe MI events may have died before enrollment.
Strengths and Limitations
Unrecognized MI by CMR in our study and UMI by ECG in a study by Dehgan et al14 show mortality rates that approach those of RMI on long-term follow-up. However, the initial quiescence of mortality is unique to UMI by CMR, possibly from the effect of small infarctions picked up by CMR but not by an ECG.6,14 Barbier et al15 showed a higher risk of MACE but not of mortality or MI in UMI by CMR. This may be due to the smaller sample size and lower event rates in their study.
Men, individuals with diabetes, and those younger than 70 years had a higher risk of death from UMI but comparatively lower mortality risk with RMI. These directionally opposite stratified outcomes of UMI and RMI may be due to distinct pathophysiological mechanisms or may represent a treatment effect. These findings should be considered hypothesis generating. Both UMI and RMI mortality risks rose with increasing terciles of infarct size.
Unlike mortality, but consistent with previous studies,1 the rates of nonfatal MI and heart failure in UMI increased in the short term. Over 10 years, the risk of these outcomes in UMI was intermediate between no MI and RMI. Higher mortality rates but lower nonfatal MI and heart failure events in UMI are possible if the incident adverse events were more likely fatal. Second, poor symptom recognition in patients with UMI may also contribute to lower secondary event rates.
To our knowledge, this is the first epidemiological study to evaluate the long-term outcomes of UMI by CMR, including heart failure, in an adequately powered sample. Importantly, over a 13-year period, there was no loss to follow-up, which makes the study results robust. The use of multiple sources for baseline characterizations and the adjudication of all of the outcome events are other strengths of the study.
Conclusions
In conclusion, UMI detected by CMRhas similar long-term mortality risks as RMI and a significantly higher risk of death, nonfatal MI, and heart failure than individuals without evidence of MI on CMR results. Being more prevalent than RMI, UMI constitutes an underappreciated public health problem. Whether early detection of UMI by CMR could allow for the institution of risk factor management and thus reduce the associated long-term risks merits further investigation.
Supplementary Material
Key Points.
Question What is the long-term prognosis of individuals with unrecognized myocardial infarction (UMI) detected by cardiac magnetic resonance imaging compared with those with clinically recognized myocardial infarction (RMI) and those with no myocardial infaction (MI)?
Findings In this cohort study of 935 participants, UMI mortality was similar to no MI mortality in the short term but higher than no MI on intermediate-term follow-up. In the long term, mortality associated with UMI was significantly higher than for no MI, but also not statistically different from RMI.
Meaning The long-term mortality risk of UMI can be as high as RMI.
Acknowledgments
Funding/Support: This study was funded by the National Heart, Lung, and Blood Institute Intramural Research Program (Z01 HL004607–08CE and 1ZIA HL006136–06), Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament).
Role of the Funder/Sponsor: The National Heart, Lung, and Blood Institute had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Concept and design: Acharya, Schelbert,Thorgeirsson, Arai.
Acquisition, analysis, or interpretation of data: Acharya, Aspelund, Jonasson, Schelbert, Cao Sathya, Sigurdsson, Thorgeirsson, Eiriksdottir,Gudnason, Arai.
Drafting of the manuscript: Acharya.
Critical revision of the manuscript for important intellectual content: Acharya, Aspelund, Jonasson, Schelbert, Cao, Sathya, Sigurdsson, Thorgeirsson, Eiriksdottir, Gudnason, Arai.
Statistical analysis: Acharya, Aspelund.
Obtained funding: Harris, Gudnason, Arai.
Administrative, technical, or material support: Jonasson, Schelbert, Cao, Eiriksdottir, Gudnason.
Supervision: Gudnason, Arai.
Other- Adjudication ofendpoints: Thorgeirsson.
Data Sharing Statement: See Supplement 2.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Schelbert reports receiving personal fees from Merck and Bayer Healthcare as well as nonfinancial support from Bracco Diagnostics. Dr Arai receives nonfinancial support from Siemens and other support from Bayer. He has 2 patents issued (US Patent 6 031374 and 5 997 883). No other disclosures are reported.
Contributor Information
Tushar Acharya, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland.
Thor Aspelund, Department of Medicine, University of Iceland, Reykyavik, Iceland.
Torfi F. Jonasson, Akureyri Hospital, Akureyri, Iceland.
Erik B. Schelbert, Heart and Vascular Institute of UPMC, University of Pittsburgh, Pittsburgh, Pennsylvania.
Jane J. Cao, The Heart Center, St Francis Hospital, State University of New York at Stony Brook, Stony Brook.
Bharath Sathya, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland.
Christopher K. Dyke, Alaska Heart Institute, Anchorage.
Anthony H. Aletras, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland.
Sigurdur Sigurdsson, Icelandic Heart Association, Kopavogur, Iceland.
Gudmundur Thorgeirsson, Department of Medicine, University of Iceland, Reykyavik, Iceland.
Gudny Eiriksdottir, Icelandic Heart Association, Kopavogur, Iceland.
Tamara Harris, National Institute on Aging, National Institutes of Health, Bethesda, Maryland.
Lenore J. Launer, National Institute on Aging, National Institutes of Health, Bethesda, Maryland.
Vilmundur Gudnason, Icelandic Heart Association, Kopavogur, Iceland.
Andrew E. Arai, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland.
REFERENCES
- 1.Kannel WB, Abbott RD. Incidence and prognosis of unrecognized myocardial infarction: an update on the Framingham study. N Engl J Med. 1984;311 (18):1144–1147. doi: 10.1056/NEJM198411013111802 [DOI] [PubMed] [Google Scholar]
- 2.Sheifer SE, Gersh BJ, Yanez ND III, Ades PA,Burke GL, Manolio TA. Prevalence, predisposing factors, and prognosis of clinically unrecognized myocardial infarction in the elderly. J Am Coll Cardiol. 2000;35(1):119–126. doi:10.1016/S0735-1097(99) 00524-0 [DOI] [PubMed] [Google Scholar]
- 3.Barbier CE, Bjerner T, Johansson L, Lind L, Ahlstrom H; Magnetic Resonance Imaging Detects Potential Risk Group. Myocardial scars more frequent than expected: magnetic resonance imaging detects potential risk group. J Am Coll Cardiol. 2006;48(4):765–771. doi: 10.1016/j.jacc.2006.05.041 [DOI] [PubMed] [Google Scholar]
- 4.Kwong RY, Sattar H, Wu H, et al. Incidence and prognostic implication of unrecognized myocardial scar characterized by cardiac magnetic resonance in diabetic patients without clinical evidence of myocardial infarction. Circulation. 2008;118(10): 1011–1020. doi: 10.1161/CIRCULATIONAHA.107.727826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim HW, Klem I, Shah DJ, et al. Unrecognized non-q-wave myocardial infarction: prevalence and prognostic significance in patients with suspected coronary disease. PLoS Med. 2009;6(4):e1000057. doi: 10.1371/journal.pmed.1000057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schelbert EB, Cao JJ, Sigurdsson S, et al. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA. 2012; 308(9):890–896. doi: 10.1001/2012.jama.11089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turkbey EB, Nacif MS, Guo M, et al. Prevalence and correlates of myocardial scar in a US cohort. JAMA. 2015;314(18):1945–1954. doi: 10.1001/jama.2015.14849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165(9):1076–1087. doi: 10.1093/aje/kwk115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kellman P, Arai AE, McVeigh ER, Aletras AH. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn Reson Med. 2002;47(2): 372–383. doi: 10.1002/mrm.10051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N EnglJ Med. 2000;343(20):1445–1453. doi: 10.1056/NEJM200011163432003 [DOI] [PubMed] [Google Scholar]
- 11.Ikram MA, van Oijen M, de Jong FJ, et al. Unrecognized myocardial infarction in relation to risk of dementia and cerebral small vessel disease. Stroke. 2008;39(5):1421–1426. doi: 10.1161/STROKEAHA.107.501106 [DOI] [PubMed] [Google Scholar]
- 12.Øhrn AM, Schirmer H, von Hanno T, et al. Small and large vessel disease in persons with unrecognized compared to recognized myocardial infarction: the Troms0 Study 2007–2008. Int J Cardiol. 2018;253:14–19. doi: 10.1016/j.ijcard.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 13.Krijthe BP, Leening MJ, Heeringa J, et al. Unrecognized myocardial infarction and risk of atrial fibrillation: the Rotterdam Study. Int J Cardiol. 2013;168(2):1453–1457.doi:10.1016/j.ijcard.2012.12.057 [DOI] [PubMed] [Google Scholar]
- 14.Dehghan A, Leening MJ, Solouki AM, et al. Comparison of prognosis in unrecognized versus recognized myocardial infarction in men versus women >55 years of age (from the Rotterdam Study). Am J Cardiol. 2014;113(1):1–6. doi: 10.1016/j.amjcard.2013.09.005 [DOI] [PubMed] [Google Scholar]
- 15.Barbier CE, Themudo R, Bjerner T, Johansson L, Lind L, Ahlstrom H. Long-term prognosis of unrecognized myocardial infarction detected with cardiovascular magnetic resonance in an elderly population. J Cardiovasc Magn Reson. 2016;18(1):43. doi: 10.1186/s12968-016-0264-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


