Abstract
Pseudomonas sp. sp48, a marine bacterium isolated from Bahary area (Alexandria, Egypt), showed a high potency for oil degradation up to 1.5%. Additionally, it showed an ability to consume aromatic hydrocarbons (phenol & naphthalene) and aliphatic (pentadecane) reaching to 79; 73; 62%, respectively. In the current study, Plackett-Burman factorial design was applied to evaluate culture conditions affecting the degradation potency. Analysis of Plackett-Burman design results revealed that, the most significant variables affecting oil removal were magnesium sulfate, inoculum size, glucose and Triton X-100. To optimize the levels of these significant variables Response Surface Methodology (RSM) was followed. In this respect, the three-level Box–Behnken design was employed and a polynomial model was created to correlate the relationship between the three variables and oil removal. The optimal combinations of the major constituents of media that was evaluated from the non-linear optimization algorithm of EXCEL-Solver was as follows: (w/v%) 1 crude oil, 0.5 peptone, 0.5 yeast-extract, 1 ammonium chloride, 0.7418 D-glucose, 0.5 MgSO4·7H2O, 0.1 Triton X-100 and inoculums size 4.18 ml% in natural sea water at pH 7; 30 °C incubation temperature, 200 rpm for 6 days. The predicted optimum oil removal was 89%, which is 2.4 times more than the basal medium.
Keywords: Factorial design, Pseudomonas sp., Crude oil, Biodegradation, Hydrocarbons
1. Introduction
Water and soil pollution by industrial chemical waste and crude oil represent a relevant topic with a growing awareness nowadays, due to its continuous release in environment, which is a prospective reason of social and environmental problems [1].
Spillage of thousands tons of crude oil from different site all over the world is drawing attention of the world on the hazards of oil pollution. The surging global oil demand has driven the transportation of large volume of oil through the busy sea lanes. The heavy marine movement susceptible the marine environment to the danger of oil spills [2]. The oil spills into the marine environment causes thick crude oil damaged the coats, skin, beaks, and appendages of hundreds of animals as it happened in the oil spill, located in north of Refugio State Beach in Santa Barbara County, California at 2015 [3].
Up till now, there are many techniques to remove oil contamination from the sediment and wastewater, such as, solvent extraction and chemical dispersant. However, these processes do not totally remove the oil contaminants from the environment. The use of microorganisms can transform the hydrocarbons into carbon dioxide. Biodegradation is one of the promising treatments for combating oil pollution. It costs less and contributed less destruction to the environment as compared to other treatments. On the other hand, biological remediation is appropriate techniques to remediate the oil-contaminated sediment [4], [5], [6], [7].
Highly hydrophobic hydrocarbons such as mineral oil hydrocarbons and polycyclic aromatic hydrocarbons have low aqueous solubility which negatively affects their bioavailability, sorption characteristics and accessibility to microbial attack. The use of fertilizers (Urea and K2HPO4) and surfactants as sodium dodecyl sulfate (SDS) or triton X-100 rapidly emulsify the oil by reducing the interfacial tension between hydrocarbons and surfactant solutions and increase mobility and surface area available for microbial cell contact with hydrocarbons; these factors may promote biodegradation [8] and therefore facilitate fast microbial growth and once the cell number reaches its peak, nutrient limitation would not be a problem to precede the biodegradation process [9].
The optimization of culture conditions is of importance for the progress of any biotreatment processes as crude oil degradation and hence it is imperative to evaluate the nutritional requirements toward enhancing biodegradation. The conventional approach ‘one factor per trial’ employed for medium optimization is time-consuming, expensive and often impractical when large number of variables needs to be investigated. Whereas, statistical optimization facilitates drawing a deep insight on the interactive effect of numerous factors that influence biodegradation process. Among the statistical methods accessible, factorial design and response surface analysis are recognized as important tools that enable accurate determination of optimal process conditions [10].
The Plackett–Burman design (PBD) has been frequently used to screen variables that have a major influence on the process [11]. The Response Surface Methodology (RSM) with Box–Behnken design is regularly used to statistically estimate the main and interactive effects of variables and to optimize the parameters of biotreatment processes [12], [13]. The response surface plots can be employed to study the surfaces and locate the optimum. In several industrial processes, RSM is almost regularly used to assess the results and efficiency of the operations [14].
Consequently, this study aims to isolate and identify bacteria efficient in degrading crude oil, assess effectiveness of the these bacteria in degrading different hydrocarbons, study the effect of using fertilizers and surfactants in increasing bacterial ability to degrade crude oil and finally apply a factorial design analysis to screen the significant factors which influence the degradation of crude oil by the tested bacteria.
2. Materials and methods
2.1. Sample collection
Four samples were collected namely: sample A (soil contaminated by mechanic oil spill site in the Abo-Quir Alexandria); sample B (water contaminated by mechanic oil spill site in Bahary Alexandria); sample C (soil around gasoline area); and sample D (soil contaminated by oil spill in the Red sea near the oil refining region).
2.2. Physical characterization of samples
2.2.1. pH determination
The pH of each sample was determined by stirring 10 g of each soil sample in 20 ml of distilled water, allowing it to settle and decanting the supernatant into a test tube. The glass electrode of an Orion Research Digital pH meter, was then dipped into it and the pH value read of the digital display; as described by APHA [15].
2.2.2. Moisture content analysis
Moisture content of all the samples was determined by placing 10 g of each soil sample into a pre-weighed crucible and the new weight was recorded. The crucible containing the soil was then pla ced in an oven at 105 °C for 1 h, after which it was removed and allowed to cool completely on the laboratory bench before it was reweighed [15].
2.2.3. Organic matter content analysis
To determine the organic matter content, the resulting dried soil form the moisture determination procedure, was placed in a muffle at 575 °C for 8 h, after which it was removed and allowed to cool completely on the laboratory bench before it was reweighed. The difference in weight was recorded as its organic matter content and converted to percentages [16].
2.3. Enrichment method for the bacterial isolation
Soil enrichment technique was used for the isolation of crude oil degrading microorganisms. In this method, 10 g of soil or 10 ml of water samples (oily polluted) were added to 200 ml of sterile mineral salt media MSM in 500 ml capacity Erlenmeyer flasks then 2 ml (1%) v/v of crude oil were added. The same enrichment experiment was carried out using natural sea water (NSW) amended with 0.1% glucose. The flasks were incubated for 7 days at 30 °C in an orbital shaker operated at 200 rpm. Three successive subculturing were done on the same media containing crude oil (1%) each for 7 days. Serial dilutions of the culture were prepared, a sample of it was spread onto two sets of duplicate MSM oil agar plates supplemented with 1% (v/v) crude oil as a substrate and 2% NaCl was used for the growth of marine bacteria. The MSM had the following composition (g/l) 1.8 K2PO4, 1.2 KH2PO4, 4.0 NH4Cl, 0.2 MgSO4. 7H2O, 0.1 NaCl and 0.01 FeSO4. 7H2O (pH 7.4). Oil agar is MSM supplemented with 1% crude oil and 20 g/l of agar. Afterwards the plates were incubated at 30 °C for 3 days. Colonies on the oil agar plates were randomly picked and pure isolates obtained by repeated sub-culturing on nutrient agar (Oxoid) [17], [18]. Finally 70 bacterial isolates were obtained.
2.4. Biodegradation studies
Individual quantitative estimation of oil degradation by collected isolates (70) was carried out by the gravimetric analysis [19], [20], [21]. Experiments were performed using flasks of sterile mineral salt medium (MSM), supplemented with 1% (v/v) of initially sterilized crude oil. The pre-culture inocula suspensions (24 h growth) were added at 2% (v/v) of the MSM in each flask. Crude oil was sterilized in autoclave in a sealed container (121 °C, 15 min). A control experiment without addition of isolates was also performed. The flasks were incubated at 30 °C for 6 days in an orbital shaker operated at 200 rpm [17]. The potent isolates sp29, sp48 and sp50 were selected for further experiment.
2.5. Biodegradation capability of selected isolates at different crude oil concentrations
Oil degradation was measured gravimetrically to confirm the potency of isolates sp29, sp48 and sp50. The biodegradation tested at different oil concentrations 0.2, 0.5, 1, and 1.5% (as sole carbon source) using MSM medium.
2.6. Screening of isolates on different aromatic hydrocarbons and different aliphatic hydrocarbons
For the biodegradation versatility test, several aromatic and aliphatic hydrocarbons were tested. They were phenol, phenanthrene, naphthalene 2- sulfonate and naphthalene as aromatic hydrocarbon and hexane, heptane and pentadecane as aliphatic hydrocarbon. The Bushnell Hass Mineral Salts medium (BHSM) supplemented with 2% NaCl was used for the growth of marine bacteria. BHMS contains (g/l) 0.2 of MgSO4.7H2O, 0.02 of CaCl2, 1 of KH2PO4, 1 of K2HPO4, 1 of NH4NO3 and 2 drops of FeCl3 60% pH (7.5). For evaluation of biodegradation potential of selected isolates, the BHMS medium supplemented with (500 mg/L) of different hydrocarbons and 2% (v/v) bacterial pre-culture of isolates sp29, sp48 and sp50 incubate at 30 °C and 200 rpm in the shaker incubator for 5 days. Bacterial growth was monitored through measuring the optical density OD at 600 nm against sterile control. The residual naphthalene, phenantherene and the residual of all aliphatic hydrocarbons were measured by GC. The residual phenol, and naphthalene 2-sulfonate were measured by HPLC [18]. GC and HPLC results were converted into percentage based on the initially used concentrations of tested hydrocarbons.
2.7. Molecular identification of the selected bacterial isolate
Cells from overnight culture were collected by centrifugation. The genomic DNA was isolated by a modified method of Sambrook et al. [22]. Amplification was done using prokaryotes 16S rRNA specific forward primer: 5′-AGAGTTTGATCMTGGCTCAG-3′ and reverse primer: 5′-TACGGYACCTTGTTACGACTT-3′. The PCR product was sequenced using the same PCR primers. Blast program (www.ncbi.nlm.nih.gov/blast) was used to assess the DNA similarities, multiple sequence alignment and molecular phylogeny were performed using BioEdit software [23]. The obtained sequence was submitted into GenBank under accession number (ac: KP202717).
2.8. Phenotypic characterization of selected isolate
For Scanning Electron microscopy (SEM), a thin film of selected isolate sp48 was spread on clean slide and left to dry in air. The dried film was coated with a thin layer of gold using sputtering device (JFC-1100 E JOEL, USA) for 12 min. A micrograph was obtained using (JSM 5300 JOEL, USA) Scanning Electron Microscope at 20 kV in a centre laboratory-City of Scientific Research and Technological Applications.
2.9. Effect of different media on oil degradation using isolate sp48
This experiment was carried out to investigate the effect of different media on the oil degradation using isolate sp48. Nine media (M1-M9) as presented in Table 1 were supplemented with 0.5% and or 1% of crude oil, inoculated with 2% inoculum, incubated at 30 °C (under shaking 200 rpm.) for 6 days to evaluate the oil degradation efficiency of the isolate sp48. This test was done in replica and the average results were taken. Crude petroleum oil consumption was determined gravimetrically in percentage as described by [19], [20], [21].
Table 1.
Different media used for testing the potency of the isolate sp48 in crude petroleum oil degradation.
| Medium Code | Medium composition |
|---|---|
| 1 | BHMS (Bushnell Hass Mineral Salts) |
| 2 | NSW + 0.5% Yeast extract |
| 3 | NSW + 0.5% Glucose |
| 4 | NSW + 0.5% Yeast extract + 0.5% Glucose |
| 5 | MSM (Mineral Salt Medium) |
| 6 | SWM (Sea Water mineral Medium) |
| 7 | NSW + 0.1% Yeast extract |
| 8 | NSW + 0.1% Glucose |
| 9 | NSW + 0.1% Glucose + 0.1% Yeast extract |
NSW = natural sea water.
2.10. Effect of different concentrations of fertilizer and surfactant on crude oil degradation
Biodegradation experiments were carried out in 500 ml Erlenmeyer flasks with 100 ml of natural sea water medium (NSW) amended with 0.5% yeast extract and 0.5% glucose with 0.5% (v/v) of crude oil. Sterilized culture medium was inoculated with 2% (v/v) inoculum of isolate sp48 pre-culture. The effect of fertilizer and surfactant concentrations on biodegradation of crude oil was evaluated with different concentrations of fertilizer and surfactant (0.1%, 0.5%, 1.0%, 1.5%, and 2.0% w/v). Urea and K2HPO4 (1:1, w/w) were used as fertilizer and sodium dodecyl sulphate (SDS) and Triton X-100 as a surfactant. The culture flasks were incubated with shaking at 200 rpm, 30 °C for 6 days. All the experiments were carried out in replica and the mean values were calculated.
2.11. Statistical optimization of medium components for oil degradation by isolate sp48
During the course of our study, it was observed that the marine isolate sp48 degrade crude oil with good efficiency in sea water media supplemented with yeast extract and glucose. Hence, statistical optimization of medium components for maximum oil degradation was carried out in two stages employing Plackett–Burman (PB) design followed by RSM using Box–Behnken design. The software JMP-Discovery (Version 4.0.4, SAS Inst. Inc., SAS Campus Drive, USA) was used for experimental design and interpretations [10], [24].
2.11.1. Plackett–Burman experimental design
Plackett–Burman (PB) design [25], a two-level factorial design method, was used to identify the medium components that have significant effects on oil removal. Plackett-Burman experimental design is based on the first order model:
where Y is the response, β0 is the model intercept and βi is the linear coefficient, Xi is the coded level of the independent variable, and k is the number of involved variables.
This model describes no interaction among factors and it is used to screen and evaluate the important factors that influence petroleum oil bioremediation. Eleven factors which were reported in literature to have a considerable influence on crude oil removal were studied in this experiment. A set of 14 experiments was used to determine the relative effect of 11 factors, including 2 physical factors (pH and inoculum size) and 9 nutritional factors (glucose, yeast extract, peptone, ammonium chloride, magnesium chloride, calcium chloride, arabic gum, Triton X-100 and fertilizer) as shown in Table 2. All factors were investigated at two widely spaced levels designated -1 (low level) and +1 (high level). The response was expressed as the percent removal of crude oil by the tested isolate. Shaking incubator speed and temperature were kept constant at 200 rpm and 30 °C, respectively. In all trials, the natural sea water was used as a basal medium and the oil concentration was kept constant at 1%. All experiments were carried out in triplicates and data represented are their means which used as the response variable [10], [24]
Table 2.
Variables and their levels employed in Plackett-Burman design for screening of culture conditions affecting on oil degradation.
| Variable code | Variable | Value |
|
|---|---|---|---|
| −1 | 1 | ||
| X1 | Glucose | 0.05 | 0.5 |
| X2 | Peptone | 0.05 | 0.5 |
| X3 | Yeast-extract | 0.05 | 0.5 |
| X4 | Ammonium chloride | 0.1 | 1 |
| X5 | Calcium chloride | 0 | 0.5 |
| X6 | Magnesium sulfate | 0 | 0.5 |
| X7 | Arabic gum | 0 | 0.1 |
| X8 | Triton X-100 | 0 | 0.1 |
| X9 | Fertilizer (K2HPO4+urea) | 0 | 0.1 |
| X10 | pH | 5 | 7 |
| X11 | Inoculum size | 1 | 3 |
2.11.2. Box-Behnken design
Further experiment was carried out to find out the optimum level of each of the significant variables that bring out maximum utilization or consumption of crude oil. This was performed through construction of structured quadratic model and determining true values of model coefficient in 26 trial design matrix for tested bacterium. After estimating the relative significance of independent variables, the most significant variables were selected for further determination of their optimal level. For this reason Box-Behnken Design, which is a Response Surface Methodology [26], was applied. This optimization step involves three main steps: performing the statistically designed experiments, estimating the coefficients in a mathematical model predicting the response and checking the adequacy of the model [27].
The factors of the highest confidence levels as elucidated through Plackett-Burman experimental design, Glucose (X1); Magnesium sulfate (X2); Triton X-100 (X3) and inoculum size (X4) were prescribed into three different levels low, medium and high, coded −1, 0, +1 (Table 3). The four variable experiments were tested in 26 different combinations.
Table 3.
The levels of variables chosen for Box-Behnken design.
| Variables | Variable code | −1 | 0 | 1 |
|---|---|---|---|---|
| Glucose (g%) | X1 | 0.5 | 1 | 1.5 |
| Magnesium sulfate (g%) | X2 | 0.5 | 1 | 1.5 |
| Triton X-100 (g%) | X3 | 0.1 | 0.3 | 0.5 |
| Inoculum size (ml%) | X4 | 3 | 5 | 7 |
For predicting the optimal point, a second order polynomial function was fitted to correlate relationship between independent variables and oil removal by tested isolate.
For the four factors, in respective ordering the equation:
where Y is the predicted response, β0 model constant; X1, X2, X3 and X4 independent variables; β1, β2, β3 and β4 are linear coefficients; β12, β13,β14, β23, β24 and β34 are cross product coefficients and β11, β22, β33 and β44 are the quadratic coefficients. Microsoft Excel 97 was used for the regression analysis of the experimental data obtained. The quality of fit of the polynomial model equation was expressed by the coefficient of determination R2.
2.11.3. Statistical analysis of the data
The data regarding oil degradation by tested microorganism was subjected to multiple regressions to estimate t-value, P-value. The significance level (P-value) was determined using the Students t-test. The t-test for any individual effect allows the evaluation of the probability of finding the observed effect purely by chance. If this probability is sufficiently small, the idea that is the effect was caused by varying the level of the variable under test is accepted. Confidence level is an expression of the P-value in percent. Optimal values of oil degradation were estimated using the SOLVER function of MICROSOFT EXCEL tools. The simultaneous effects of the four most significant independent factors on each response were visualized using three dimensional generated by STATISTICA 5.0 software.
3. Results and discussion
3.1. Physical characterization of samples
Samples physical characterization results were illustrated in Table 4, which included analysis of pH, moisture and organic matter contents.
Table 4.
Physicochemical properties of the collected samples.
| Sample A (soil) | Sample B (sea water) | Sample C (soil) | Sample D (soil) | |
|---|---|---|---|---|
| pH | 7.42 | 7.52 | 7.84 | 6.74 |
| Moisture content (%) | 9.04 | Not determined | 26.71 | 2.444 |
| Organic carbon content (%) | 0.76 | Not determined | 13.05 | 11.355 |
3.2. Preliminary test for degradation ability of selected isolates
Out of 70 crude oil degrading bacterial isolates, three were identified as efficient crude oil degraders based on rapid growth on agar medium containing oil and confirmed by growing on 1% of crude oil. The illustrated results (data not shown) based on gravimetric method showed that many bacterial isolates can grow on crude oil plates but few of them can degrade it efficiently.
3.3. Investigating biodegradation capability of selected isolates at different crude oil concentrations
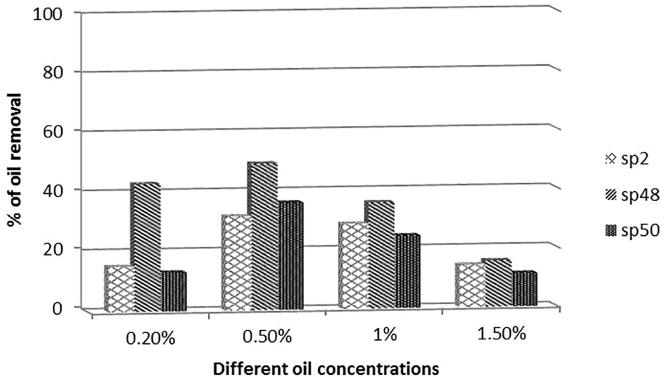
The most potent isolates for oil degradation namely: sp2, sp48 and sp50 were grown at different oil concentrations 0.2%, 0.5%, 1%, and 1.5%. The results show that the maximum percentage of oil removal was found at 0.5% (v/v) of crude oil in the MSM medium. The percentage of removal decreased with increasing the concentration likely due to the toxicity of oil on tested isolates. At low oil concentration (0.2%) the removal percentage decreased due to degradation of all available hydrocarbons in crude oil. Generally, the isolate sp48 is more potent than the other isolates in its capability for oil removal as represented in Fig. 1.
Fig. 1.
Biodegradation efficiency of isolates sp29, sp48 and sp50 at different crude oil concentrations.
3.4. Utilization of different aromatic and aliphatic hydrocarbons by selected isolates
The biodegradation adaptability of the selected isolates sp29, sp48 and sp50 for the degradation of different hydrocarbons as phenol, phenanthrene, naphthalene 2- sulfonate and naphthalene as aliphatic hydrocarbon and hexane, heptane and pentadecane as aliphatic hydrocarbon was verified.
Table 5 shows that isolate sp48 was the most potent in reference to phenol and naphthalene degradation, where the removal percentages (as described in method part) were 79.7 and 73.4, respectively. However the isolate sp50 is efficient on growing on naphthalene 2- sulfonate, hexane and heptane. On the other hand, phenanthrene did not support the growth of the three isolates.
Table 5.
Growth and biodegradation efficiency of selected isolates using different organic compounds as a sole carbon source.
| Tested hydrocarbons (500 mg/l) | sp29 |
sp48 |
sp50 |
|||
|---|---|---|---|---|---|---|
| OD at 600 nm | Removal% | OD at 600 nm | Removal% | OD at 600 nm | Removal% | |
| Phenol | 0.163 | 36.47 | 0.609 | 79.77 | 0.141 | 31.76 |
| Naphthalene | 0.192 | 30.69 | 0.509 | 73.46 | 0.16 | 37.4 |
| Naphthalene.2.sulfonate N2S | 0.19 | 28.7 | 0.08 | 7.3 | 0.242 | 23.53 |
| Phenantherene | 0.00 | 0.8 | 0.037 | 6.7 | 0.024 | 5.2 |
| Hexane | 0.181 | 42.2 | 0.144 | 41.6 | 0.188 | 52.5 |
| Heptane | 0.246 | 53.1 | 0.158 | 43.7 | 0.276 | 54.7 |
| Pentadecane | 0.215 | 48.38 | 0.344 | 62.32 | 0.327 | 58.1 |
Among the three bacterial isolates, only sp48 was capable of utilizing the aromatic fraction of crude petroleum oil. As reported in literature, bacteria and fungi were found to be the major microorganisms responsible for biodegradation of petroleum hydrocarbon. The genera to which hydrocarbon degrading bacteria belong are Pseudomonas, Alcaligenes, Micrococcus, Nocardia, Corynobacterium, Rhodococcus, Enterobacter, Eschrechia, Arthrobacter, Bacillus, Streptomyces, Closterdium, and Proteus [28], [29].
Biodegradation of aromatic hydrocarbons by oil-degrading bacteria involved multi-component enzymes [30]. The heterotrophic bacteria must have the specific enzymes systems necessary for degradation of polycyclic aromatic hydrocarbons, especially a high molecular weight one [7].
3.5. Identification of the most potent oil degrading isolate
It was found that the isolate sp48 is the most potent isolate in oil degradation and also highly efficiency in hydrocarbon degradation. Therefore, the identification of this isolate was performed using molecular sequencing of the conserved part of the 16Sr RNA. According to sequence similarities the isolate sp48 exhibited a close relation to Pseudomonas sp. The partial sequence of 16Sr RNA of this isolate has been deposited into GeneBank under accession number (ac: KP202717).
3.6. Microscopic examination of Pseudomonas sp. sp48
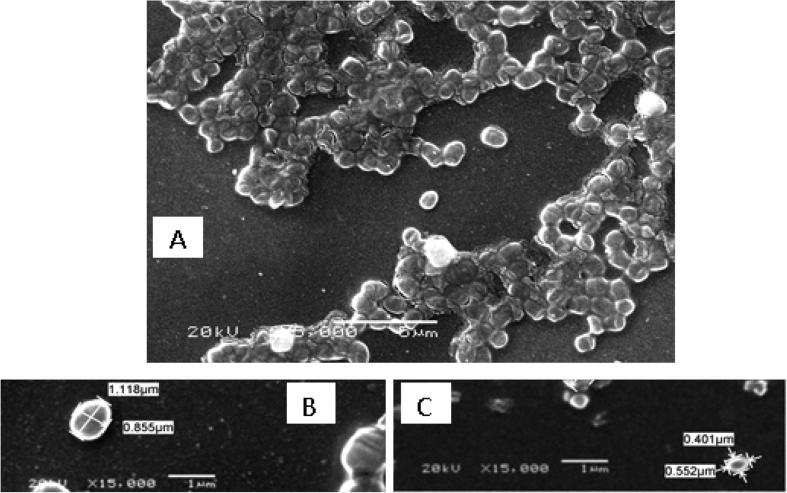
The cellular morphology of the Pseudomonas sp. sp48 was examined under scanning electron microscope, when grown in two different media: NSW supplemented with 0.5% yeast extract and 0.5% glucose and the same medium with 1% oil. It could be seen in the Fig. 2 that cells grown in medium without oil looks more healthy and bigger in diameter (1.1–0.85 µm), whereas in the other medium with oil its diameter was reduced (0.55–0.4 µm) due to the effect of oil on cell surface.
Fig. 2.
Scanning Electron Micrograph (SEM) of Pseudomonas sp. sp48, A: at X5,000, B, C: at X15,000 for isolate without oil and isolate with oil, respectively.
3.7. Effect of different media on oil degradation using Pseudomonas sp. sp48
Seeking for optimum media for biodegradation was always a critical step that requires a good study of the physiological needs of the biodegrader and the degradation process itself. For this purpose, a set of nine media (Table 1) was supplemented with 0.5% or 1% of crude oil was used to evaluate the oil degradation efficiency of the strain Pseudomonas sp. sp48.
As it could be seen in Table 6, media 4 and 9 containing natural sea water supplemented with glucose and yeast extract supported the bacterial growth as well as petroleum oil consumption. On the other hand the medium that contains yeast extract alone or glucose alone showed low levels of oil consumption percentage. However, addition of yeast and glucose together to the medium enhanced the biodegradation capacity. Medium 6 (SWM) didn’t support the oil consumption. It could be concluded that medium 4 (natural sea water supplemented with 0.5% yeast and 0.5% glucose) is the medium of choice for crude petroleum oil degradation by the strain Pseudomonas sp. sp48.
Table 6.
Crude petroleum oil consumption% in different media by Pseudomonas sp. sp48 at 0.5% and 1% crude oil concentrations.
| Different media | % of oil removal using 0.5% oil | % of oil removal using 1% oil |
|---|---|---|
| BHMS | 26.9 | 30.49 |
| NSW + 0.5% Y | 18.4 | 31.8 |
| NSW + 0.5% G | 14.4 | 33.77 |
| NSW + 0.5% Y + 0.5% G | 46.7 | 55.4 |
| MSM | 32.2 | 46.5 |
| SWM | 0.657 | 25.9 |
| NSW + 0.1%Y | 13.8 | 26.4 |
| NSW + 0.1%G | 17.89 | 29.5 |
| NSW + 0.1%Y + 0.1%G | 37.8 | 38.6 |
NSW = natural sea water.
3.8. Assessment of biodegradation capability of strain Pseudomonas sp. sp48 using different crude oil concentrations
Oil degradation was confirmed gravimetrically using the most potent strain for oil degradation (Pseudomonas sp. sp48). The best medium for crude petroleum oil degradation that was identified in the previous section was used to perform this study. The medium was supplemented with different oil concentrations (0.2%, 0.5%, 0.75, 1%, and 1.5%) as sole carbon source. The culture flasks were maintained in the shaker at 200 rpm, 30 °C for 6 days.
The results (Table 7) show that the maximum percentage of oil removal using strain sp48 was 69% at 0.2% (v/v) of crude oil in the medium and with increasing the concentration the removal percentage decreased (55% at 1% crude oil concentration) which could be rendered to the slow rate of biodegradation or the inhibition effect of higher crude oil concentrations on the bacteria.
Table 7.
Biodegradation efficiency of Pseudomonas sp. sp48 at different crude oil concentrations.
| Crude oil concentrations (%) | Oil removal (%) |
|---|---|
| 0.2% | 69.072 |
| 0.5% | 46.350 |
| 0.75% | 52.780 |
| 1% | 55.409 |
| 1.5% | 53.290 |
3.9. Effect of different concentrations of fertilizer and surfactant on crude oil degradation
Experiments conducted with different concentrations (0.1%, 0.5%, 1%, 1.5%, and 2% w/v) of fertilizer (Urea and K2HPO4 (1:1) w/w) revealed that, 0.1% of fertilizer concentration produced maximum degradation of crude oil (54.07%) as shown in Table 8. The increase of fertilizer concentration doesn't significantly improve the biodegradation of oil. Similar results were obtained by Vyas and Dave [31] who reported that addition of excess nutrients beyond certain limit in bioremediation would have no impact on cell growth and biodegradation process and excess nutrient content can be toxic to cell growth. Thus, 0.1% fertilizer concentration was used as the optimum level in degradation of oil. These results are in agreement with those obtained by Thavasi et al. [32].
Table 8.
Effect of different concentrations of surfactants (TritonX-100 and SDS) and fertilizer on biodegradation of 0.5% crude oil by Pseudomonas sp. sp48.
| Surfactant concentration (w/v) g% | Oil removal% using |
||
|---|---|---|---|
| SDS | Triton X-100 | Fertilizer | |
| 0.10 | 41.70 | 57.23 | 54.07 |
| 0.50 | 20.65 | 43.6 | 53.28 |
| 1.00 | 15.97 | 39.4 | 49.34 |
| 1.50 | 13.67 | 35.5 | 46.70 |
| 2.00 | 12.12 | 38.1 | 46.84 |
Among the different surfactant concentrations (0.1%, 0.5%, 1%, 1.5%, and 2% w/v) used in this study, maximum crude oil degradation was observed with 0.1% surfactant concentration (Table 8). At 0.1% of triton X-100 degradation of crude oil was 57.23% but for SDS was 41.7%, while the blank (without surfactants) was 46.35%. There was a significant decrease in degradation activity observed at concentrations above 0.1% with both surfactants, indicating sufficient emulsification of the crude oil occurred making it more available for biodegradation. Triton X-100 was more efficient in oil degradation than SDS as presented in Table 8. Similar results were obtained by Margesin and Schinner [8] who reported that the total hydrocarbon loss in soil was significantly inhibited in presence of SDS; the higher the SDS concentration, the higher was the inhibition, while 38% biodegradation was noticed in the absence of SDS and biodegradation in presence of SDS was only 20–30%. Aronstein and Alexander [33] found that low concentrations of SDS (50–100 mg l−1) to stimulate hydrocarbon biodegradation in liquid culture significantly, without affecting the abiotic hydrocarbon loss. Remarkably, this stimulation occurred only very late when the SDS was already fully degraded and when hydrocarbon loss had already reached a “saturation plateau”, possibly SDS is able to enhance hydrocarbon bioavailability on a long-term-basis.
3.10. Application of the plackett-Burman factorial design followed by Response Surface Methodology for optimization of crude oil biodegradation
From the previous steps, Pseudomonas sp. sp48 degraded crude oil with good efficiency in sea water media supplemented with yeast extract and glucose. Additionally the tested fertilizer and surfactant relatively enhanced the oil% removal up to 54 and 57% at 0.1% concentration, respectively.
The statistical optimization of medium components for maximum oil degradation was carried out in two stages employing Plackett–Burman (PB) design followed by Response Surface Methodology (RSM) using Box–Behnken design. Plackett-Burman design addresses the evaluation of variables' effects on crude petroleum oil degradation in terms of calculating the p-value of each studied component.
3.10.1. Screening for the most significant factors affecting petroleum bioremediation by Pseudomonas sp. sp48 using plackett–burman experimental design
The measured response in this experiment was the residual petroleum oil. Table 9 illustrates that the highest crude petroleum removal percentage by Pseudomonas sp. sp48 was obtained in the trials 2 and 11 in which the oil removal is 77.3 and 70.1, respectively. The least removal percentage was obtained at the trial 12 with a value of 21.6% oil removal. As a result, it can be said that the variability created in the petroleum bioremediation results in the different trials reflects the importance of studying the effect of different variables (either nutritional or physical) on this microbiological process.
Table 9.
Plackett-Burman experimental design for evaluation of factors affecting oil degradation using Pseudomonas sp. sp48.
| Trial No. | X1 | X2 | X3 | X4 | X5 | X6 | X7 | X8 | X9 | X10 | X11 | Oil removal% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | 1 | 1 | 1 | −1 | 1 | 1 | −1 | 1 | 55.08 |
| 2 | 1 | −1 | 1 | 1 | −1 | 1 | −1 | −1 | −1 | 1 | 1 | 77.38 |
| 3 | −1 | 1 | 1 | −1 | 1 | −1 | −1 | −1 | 1 | 1 | 1 | 35.08 |
| 4 | −1 | −1 | 1 | 1 | 1 | −1 | 1 | 1 | −1 | 1 | −1 | 23.61 |
| 5 | 1 | 1 | −1 | 1 | −1 | −1 | −1 | 1 | 1 | 1 | −1 | 51.15 |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 24.26 |
| 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 30.77 |
| 8 | 1 | −1 | −1 | −1 | 1 | 1 | 1 | −1 | 1 | 1 | −1 | 27.87 |
| 9 | −1 | 1 | −1 | −1 | −1 | 1 | 1 | 1 | −1 | 1 | 1 | 61.31 |
| 10 | −1 | 1 | 1 | 1 | −1 | 1 | 1 | −1 | 1 | −1 | −1 | 22.62 |
| 11 | 1 | 1 | 1 | −1 | 1 | 1 | −1 | 1 | −1 | −1 | −1 | 70.16 |
| 12 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 21.64 |
| 13 | 1 | −1 | 1 | −1 | −1 | −1 | 1 | 1 | 1 | −1 | 1 | 34.10 |
| 14 | 1 | 1 | −1 | 1 | 1 | −1 | 1 | −1 | −1 | −1 | 1 | 43.61 |
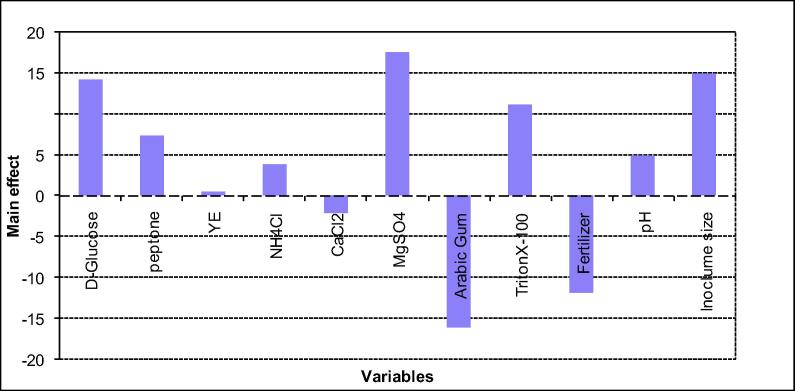
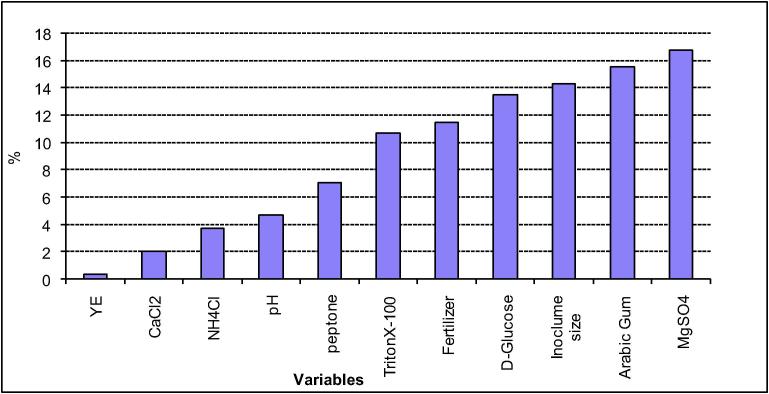
Statistical analysis of these data revealed that the value of determination coefficient R2, that measures the goodness of model fitting, is 0.897. This indicates that less than 11% of the total variations are not explained by the model, which ensures the good adjustment of the model to the experimental results. Data analysis of the Plackett-Burman design is represented in Table 10. The factors tested in this design contributed differently to crude oil bioremediation by Pseudomonas sp. sp48, which means that some of them had positive and others negatively effects. Fig. 3 shows that calcium chloride, arabic gum and fertilizer affect negatively on oil degradation while other factors: glucose, yeast, peptone, ammonium chloride, magnesium chloride, Triton X-100, pH and inoculum size affected positively and enhance the crude oil degradation. Similar report was stated by Farag and Soliman [34] concerning glucose that showed enhancement of crude oil removal by Candida tropicals strain A. Fig. 4 shows the ranking of factor estimates in a Pareto chart. The Pareto chart displays the magnitude of each factor estimate (independent on its contribution, either positive or negative) and is a convenient way to view the results of a Plackett-Burman design [35]. On the model level, the R2 = 0.90 which is a considerable coefficient of determination that explains the variability of the data. It is worthwhile to mention that Plackett-Burman design is useful not only in evaluating the significance of some variables on the bioprocess, but also in comparing between different categories, which is difficult to compare between their effects in conventional experiments, and hence maintain a comprehensive evaluation of the overall process. Recently, this design was used intensively for pre-screening nutritional and cultural conditions affecting the production of many enzymes and other metabolites [36], [37].
Table 10.
Statistical analysis of Plackett-Burman experimental design showing coefficient values, t- and p- values for each variable affecting on oil degradation.
| Variables | Coefficients | Main- effect | Standard error | t-Stat | P-value | Confidence level (%) |
|---|---|---|---|---|---|---|
| Intercept | 41.33183817 | 4.081772 | 10.12584 | .009613 | 99.0387415 | |
| D-Glucose | 7.076502732 | 14.1530055 | 4.408817 | 1.60508 | .249691 | 75.0309174 |
| Peptone | 3.68852459 | 7.37704918 | 4.408817 | 0.836625 | .490841 | 50.9159063 |
| Yeast extract | 0.191256831 | 0.38251366 | 4.408817 | 0.043381 | .96934 | 3.0660245 |
| NH4Cl | 1.93989071 | 3.87978142 | 4.408817 | 0.440003 | .702918 | 29.708192 |
| CaCl2 | −1.06557377 | −2.1311475 | 4.408817 | −0.24169 | .831541 | 16.8459292 |
| MgSO4 | 8.770491803 | 17.5409836 | 4.408817 | 1.989307 | .184966 | 81.5033656 |
| Arabic Gum | −8.1147541 | −16.229508 | 4.408817 | −1.84057 | .20704 | 79.2959602 |
| TritonX-100 | 5.601092896 | 11.2021858 | 4.408817 | 1.27043 | .331722 | 66.8277882 |
| Fertilizer | −5.98360656 | −11.967213 | 4.408817 | −1.35719 | .307589 | 69.2411241 |
| pH | 2.431693989 | 4.86338798 | 4.408817 | 0.551552 | .636649 | 36.3350514 |
| Inoculum size | 7.459016393 | 14.9180328 | 4.408817 | 1.691841 | .232749 | 76.725102 |
Fig. 3.
Effect of different culture conditions on crude oil removal (represented as oil removal percentage) by Pseudomonas sp. sp48 in Plackett-Burman experimental design.
Fig. 4.
Pareto plot for Plackett-Burman parameter estimates.
3.10.2. Optimization of petroleum oil consumption by Pseudomonas sp. sp48 using Response Surface Method (Box- Behnken design)
In order to approach the optimum response region of the oil consumption, significant independent variables (D-Glucose, X1; MgSO4.7H2O, X2; Triton-X100, X3; and inoculum size, X4) were further explored using strain sp48, each at three levels as explained in Table 3. The Box-Behnken design matrix of the variables in both coded and natural units together with the experimental results of the oil degradation was presented in Table 11.
Table 11.
Box-Behnken factorial experimental design, representing the response of oil degradation as influenced by D-Glucose, X1; MgSO4, X2; Triton X-100, X3; and inoculum size, X4 for Pseudomonas sp. sp48.
| Trials | X1 | X2 | X3 | X4 | Oil removal experimental (g%) | Oil removal predicted (g%) |
|---|---|---|---|---|---|---|
| 1 | 0 | −1 | 0 | 1 | 63.28 | 72.7404372 |
| 2 | 0 | 0 | −1 | −1 | 92.30 | 86.0724044 |
| 3 | −1 | 1 | 0 | 0 | 74.07 | 71.5259563 |
| 4 | −1 | 0 | 0 | −1 | 57.74 | 68.6789617 |
| 5 | −1 | 0 | −1 | 0 | 92.72 | 84.4398907 |
| 6 | 1 | 1 | 0 | 0 | 66.59 | 73.7937158 |
| 7 | −1 | 0 | 1 | 0 | 35.41 | 43.9043716 |
| 8 | 0 | 1 | 0 | 1 | 72.16 | 74.0300546 |
| 9 | −1 | −1 | 0 | 0 | 79.51 | 72.2035519 |
| 10 | 0 | 0 | 1 | −1 | 47.48 | 50.5040984 |
| 11 | −1 | 0 | 0 | 1 | 63.18 | 61.8702186 |
| 12 | 0 | 0 | 1 | 1 | 39.80 | 45.9248634 |
| 13 | 0 | 1 | 1 | 0 | 80.36 | 67.7281421 |
| 14 | 0 | 0 | 0 | 0 | 71.93 | 75.5191257 |
| 15 | 1 | 0 | −1 | 0 | 86.82 | 80.4945355 |
| 16 | 0 | −1 | −1 | 0 | 92.16 | 102.728142 |
| 17 | 1 | −1 | 0 | 0 | 65.57 | 68.0122951 |
| 18 | 0 | 0 | 0 | 0 | 69.90 | 75.5191257 |
| 19 | 0 | −1 | 1 | 0 | 57.34 | 41.8811475 |
| 20 | 0 | −1 | 0 | −1 | 73.77 | 74.0737705 |
| 21 | 0 | 1 | −1 | 0 | 68.59 | 81.9849727 |
| 22 | 1 | 0 | 1 | 0 | 35.48 | 45.9262295 |
| 23 | 0 | 1 | 0 | −1 | 85.18 | 77.8879781 |
| 24 | 0 | 0 | −1 | 1 | 88.59 | 85.4603825 |
| 25 | 1 | 0 | 0 | 1 | 78.13 | 65.1215847 |
| 26 | 1 | 0 | 0 | −1 | 64.26 | 63.5040984 |
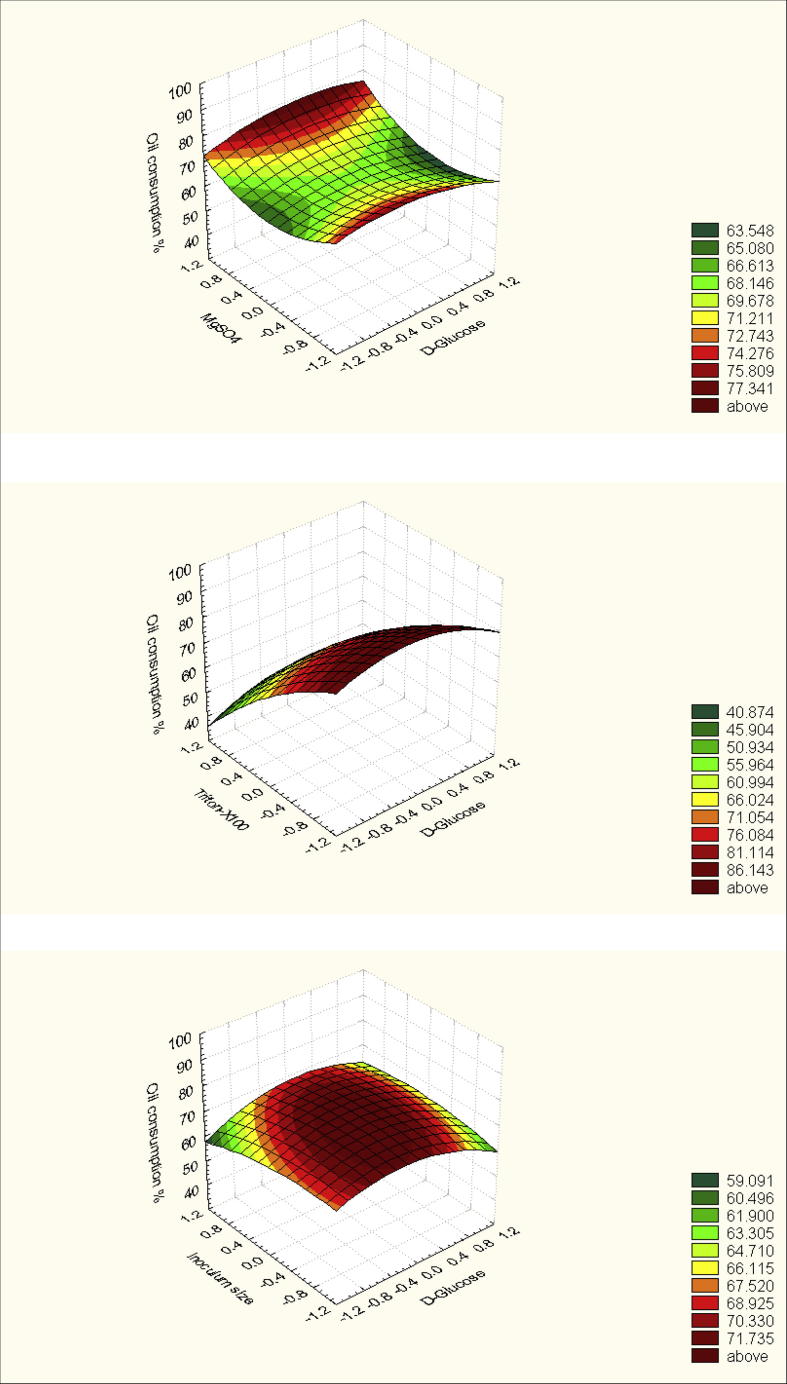
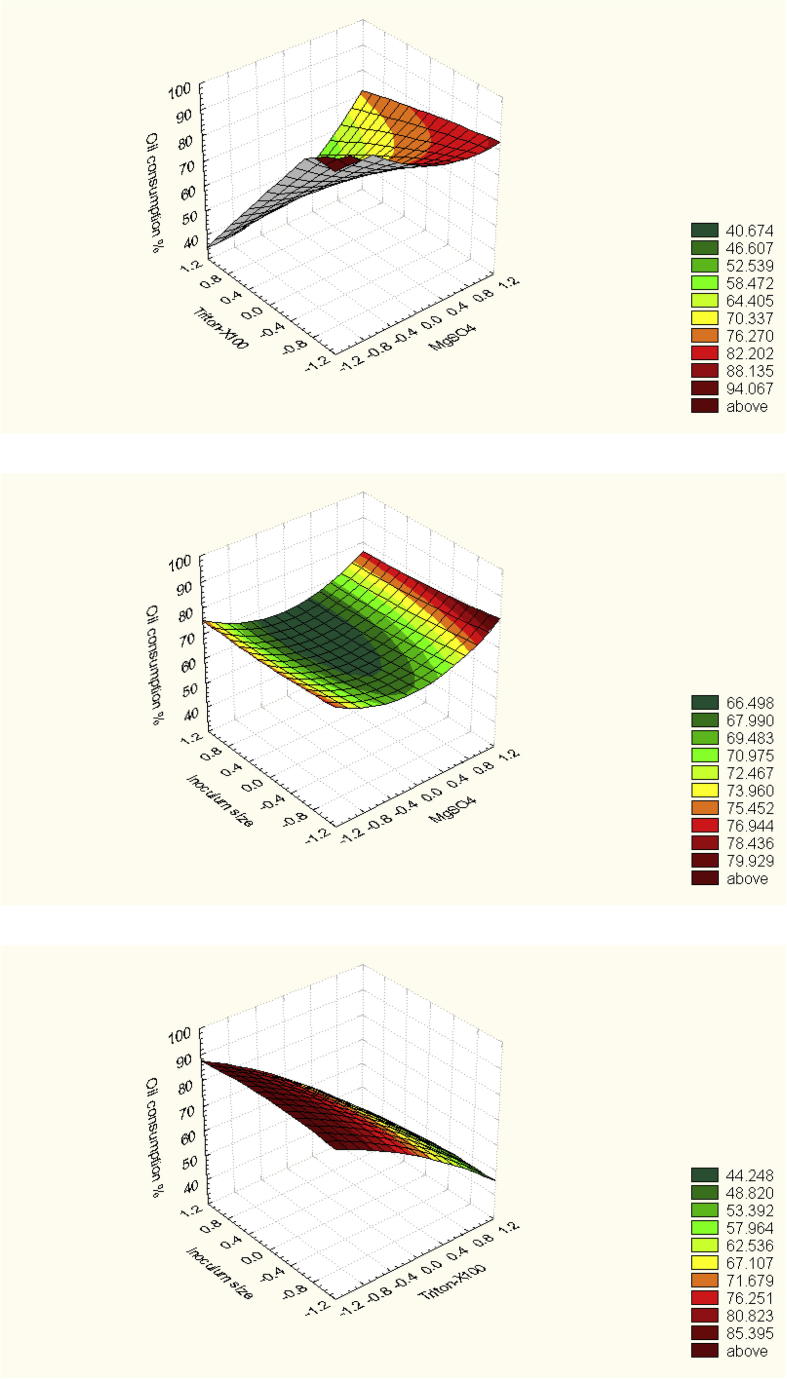
Presenting the results in the form of surface plot (Fig. 5) for predicting the optimal points within experimental constrains, a second order polynomial function was fitted to the experimental results of oil consumption by strain sp48 and the results represented in Table 12:
Fig. 5.
Three-dimensional graphs showing the response surface affected by some culture variables during degradation of petroleum oil by Pseudomonas sp. sp48.
Table 12.
Statistical analysis of Box-Behnken design showing coefficient values, t- and p- values for each variable affecting on oil degradation.
| Term | Coefficients | Standard error | t-Stat | P-value | Confidence level (%) |
|---|---|---|---|---|---|
| Intercept | 70.91803 | 8.613102 | 8.233739 | 4.96E−06 | 99.9995 |
| x1 | −0.48087 | 3.516284 | −0.13676 | .893694 | 10.63058 |
| x2 | 1.275956 | 3.516284 | 0.362871 | .723578 | 27.64222 |
| x3 | −18.776 | 3.516284 | −5.33972 | .000238 | 99.97625 |
| x4 | −1.29781 | 3.516284 | −0.36909 | .719069 | 28.09311 |
| x1x2 | 1.614754 | 6.090383 | 0.265132 | .795811 | 20.41894 |
| x1x3 | 1.491803 | 6.090383 | 0.244944 | .811009 | 18.89909 |
| x1x4 | 2.106557 | 6.090383 | 0.345883 | .735956 | 26.40444 |
| x2x3 | 11.64754 | 6.090383 | 1.912448 | .082199 | 91.78011 |
| x2x4 | −0.63115 | 6.090383 | −0.10363 | .919328 | 8.067193 |
| x3x4 | −0.9918 | 6.090383 | −0.16285 | .873592 | 12.64083 |
| x1x1 | −4.71175 | 5.831097 | −0.80804 | .436195 | 56.3805 |
| x2x2 | 5.177596 | 5.831097 | 0.887928 | .393581 | 60.64193 |
| x3x3 | −2.51503 | 5.831097 | −0.43131 | .674572 | 32.54278 |
| x4x4 | −1.41257 | 5.831097 | −0.24225 | .813046 | 18.69544 |
where x1, x2, x3 and x4 represent codified values for glucose; Magnesium sulfate; Triton-X100; and inoculum size, respectively. At the model level, the correlation measures for the estimation of the regression equation are the multiple correlation coefficient R and the determination coefficient R2. The closer the value of R is to 1, the better is the correlation between the observed and the predicted values. In this experiment the value of R was 0.8743 for oil consumption. This value indicates the degree of correlation between the experimental and the predicted values. The value of determination coefficient R2=0.764 being a measure of fit of the model, which indicated that a satisfactory adjustment of the quadratic model to the experimental data was ensured. Approximately 87% of the variability in the dependent variable (response) could be explained by the model.
From statistical analysis, it can be concluded that, among the test variables effect on oil consumption by Pseudomonas sp. sp48, the optimal levels of the tested four variables (as obtained from the maximum point of the polynomial model) were estimated using the solver function of MICROSOFT EXCEL tools, and found to be: (g%) 0.7418 for Glucose, 0.5 MgSO4.7H2O, 0.1 Triton X-100 and the inoculum size is 4.18 ml% with a predicted oil consumption about 100%. Data analyses of Box-Behnken experiment are represented in Table 12.
3.10.3. Verification of model
The adequacy of polynomial model of Pseudomonas sp. sp48 was examined by an additional experiment using the derived optimal conditions. The experimental values were compared with the predicted ones where, the closeness between the experimental and predicted ones measures the adequacy of the model. The experiment was carried out in shake flask using the derived optimal conditions based on the previous result of Box–Behnken design. The experiment was carried out in tripled flasks and the mean value was calculated. The oil removal percentage obtained experimentally was 89%. Hence, it is concluded that the model was successfully validated. Fig. 6 shows the effect of applying the optimized medium on oil bioremediation by tested strain sp48, where one can easily recognize the disappearance of crude oil in comparison to the control.
Fig. 6.

Oil bioremediation by Pseudomonas sp. sp48 using optimized medium against negative control.
4. Conclusion
The current study investigated the factors affecting the crude oil removal by a local isolate marine bacterium Pseudomonas sp. sp.48. Eleven variables were screened through statistical experimental design (PBD) to select the most significant variables affected on oil degradation. Subsequently, further optimization using Response Surface Methodology (RSM) was applied. Successively it was found that natural sea water supplemented with (g%) 0.5 peptone, 0.5 yeast extract, 1 ammonium chloride, 0.7418 d-Glucose, 0.5 MgSO4, 0.1 Triton X-100, inoculum size 4.18 ml%, pH 7; incubation temperature 30 °C, incubation time 6 days and 200 rpm are optimal for oil removal. The oil removal increased to nearly 2.4 times higher than that obtained under the non-optimized conditions.
Acknowledgment
This work was financially supported by the project grant from the Egyptian Science and Technology Development Fund, STDF, Egypt (Grant No. 1196).
Footnotes
Peer review under responsibility of National Research Center, Egypt.
References
- 1.Beek B., Bohling S., Franke C., Johnke U., Studinger G., Thumm E. The assessment of biodegradation and persistence. Handbook Environ Chem. 2001;2:291–320. [Google Scholar]
- 2.Samanta S.K., Singh O.V., Jain R.K. Polycyclic aromatic hydrocarbons: environmental pollution and bioremediation. Trends Biotechnol. 2002;20:243–248. doi: 10.1016/s0167-7799(02)01943-1. [DOI] [PubMed] [Google Scholar]
- 3.Cooper, Lara, Oil spill reported on coast near Refugio state beach. Noozhawk; 2015. <https://en.wikipedia.org/wiki/Refugio_oil_spill> [retrieved 20 May].
- 4.Wood P.A. Remediation methods for contaminated sites. In: Hester R.E., Harrison R.M., editors. Contaminated land and its reclamation. The Royal Society of Chemistry; Letchworth: 1997. pp. 58–72. [Google Scholar]
- 5.Yuan S.Y., Wei S.H., Chang B.V. Biodegradation of polycyclic aromatic hydrocarbons by a mixed culture. Chemosphere. 2000;41:1463–1468. doi: 10.1016/s0045-6535(99)00522-6. [DOI] [PubMed] [Google Scholar]
- 6.Oh Y., Sim D., Kim S. Effects of nutrients on crude oil biodegradation in the upper intertidal zone. Marine Poll Bull. 2001;42(12):1367–1372. doi: 10.1016/s0025-326x(01)00166-7. [DOI] [PubMed] [Google Scholar]
- 7.Hii Y.S., Law Ah.T., Shazili N.A.M., Abdul-Rashid M.K., Lee C.W. Biodegradation of Tapis blended crude oil in marine sediment by a consortium of symbiotic bacteria. Int Biodeterior Biodegrad. 2009;63:142–150. [Google Scholar]
- 8.Margesin R., Schinner F. Biodegradation of diesel oil by cold-adapted microorganisms in presence of sodium dodecyl sulfate. Chemosphere. 1999;38(15):3463–3472. doi: 10.1016/s0045-6535(98)00575-x. [DOI] [PubMed] [Google Scholar]
- 9.Thavasi R., Jayalakshmi S., Banat I.M. Effect of biosurfactant and fertilizer on biodegradation of crude oil by marine isolates of Bacillus megaterium, Corynebacterium kutscheri and Pseudomonas aeruginosa. Bioresour Technol. 2011;102:772–778. doi: 10.1016/j.biortech.2010.08.099. [DOI] [PubMed] [Google Scholar]
- 10.Basheer S.M., Chellappan S., Beena P.S., Sukumaran R.K., Elyas K.K., Chandrasekaran M. Lipase from marine Aspergillus awamori BTMFW032: production, partial purification and application in oil effluent treatment. New Biotechnol. 2011;28(6):627–638. doi: 10.1016/j.nbt.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Imandi S.B., Bandaru V.V., Somalanka S.R., Bandaru S.R., Garapati H.R. Application of statistical experimental designs for the optimization of medium constituents for the production of citric acid from pineapple waste. Bioresour. Technol. 2008;99:4445–4450. doi: 10.1016/j.biortech.2007.08.071. [DOI] [PubMed] [Google Scholar]
- 12.Mohana S., Shah A., Divecha J., Madamwar D. Xylanase production by Burkholderia sp. DMAX strain under solid state fermentation using distillery spent wash. Bioresour Technol. 2008;99:7553–7564. doi: 10.1016/j.biortech.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Yao Y., Lv Z., Min H., Lv Z., Jiao H. Isolation, identification and characterization of a novel Rhodococcus sp. Strain in biodegradation of tetrahydrofuran and its medium optimization using sequential statistics-based experimental designs. Bioresour Technol. 2009;100:2762–2769. doi: 10.1016/j.biortech.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Ahmadi M., Vahabzadeh F., Bonakdarpour B., Mofarrah E., Mehranian M. Application of the central composite design and response surface methodology to the advanced treatment of olive oil processing wastewater using Fenton’s peroxidation. J Hazard Mater B. 2005;123:187–195. doi: 10.1016/j.jhazmat.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 15.American Public Health Association (APHA), Standard methods for the examination of water and wastewater, 1995, Washington. D.C.
- 16.Gesinde A.F., Agbo E.B., Agho M.O., Dike E.F.C. Bioremediation of some Nigerian and arabian crude oils by fungal isolates. Int J Pure Appl Sci. 2008;2(3):37–44. [Google Scholar]
- 17.Ijah U.J.J. Studies on relative capabilities of bacterial and yeast isolates from tropical soil in degrading crude oil. Waste Manage. 1998;18:293–299. [Google Scholar]
- 18.Khodijah C.S., Tazaki K., Asada R., Kogure K. Bioremediation of coastal areas 5 years after the Nakhodka oil spill in the Sea of Japan: isolation and characterization of hydrocarbon-degrading bacteria. Environ Int. 2004;30:911–922. doi: 10.1016/j.envint.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Law A.T., Teo K.S. Oil biodegradation in the Straits of Malacca: phenanthrene degradation by AR-3. J Mar Biotechnol. 1997;5:162–167. [Google Scholar]
- 20.Tomasi G., Christensen J.H. A Tucker model based approach for analysis of complex oil biodegradation data. J Chromatogr A. 2009;1216:7865–7872. doi: 10.1016/j.chroma.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 21.Farag S., Soliman N.A., Abdel-Fattah Y.R. Optimization of immobilization conditions for petroleum oil biodegradation by Candida tropicalis AQ1 using wood chips and wax. Res J Pharm Biol Chem Sci. 2016;7(5):200–210. [Google Scholar]
- 22.Sambrook J., Fritsch E.F., Maniatis T. Cold Spring Harbor Laboratory; NY: 1989. Molecular cloning A. Laboratory manual. [Google Scholar]
- 23.Hall T.A. BioEdit: a user- friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 24.Srinivasan A., Viraraghavan T. Oil removal from water by fungal biomass: a factorial design analysis. J Hazard Mater. 2010;175:695–702. doi: 10.1016/j.jhazmat.2009.10.065. [DOI] [PubMed] [Google Scholar]
- 25.Plackett R.L., Burman J.P. The design of optimum multifactorial experiments. Biometrika. 1946;33:305–325. [Google Scholar]
- 26.Box G.E.F., Behnken D.W. Some new three level designs for the study of quantitative variables. Technometrics. 1960;24:455–475. [Google Scholar]
- 27.Lee M.T., Chen W.C., Chou C.C. Maximization of cholesterol oxidase production by Rhodococcus equi no. 23 by using response surface methodology. Biotechnol Appl Biochem. 1998;28:229–233. [PubMed] [Google Scholar]
- 28.Strauss J.M., du Plessis C.A. Empirical model for Biofiltration of toluene. J Environ Eng. 2000;126(7):644–648. [Google Scholar]
- 29.Bassim E.A., Walid D.S. Kinetics of indigenous isolated bacteria used for Ex-Situ bioremediation of petroleum contaminated soil. Water Air Soil Pollut. 2008;192:221–226. [Google Scholar]
- 30.Kanaly R., Harayama S. Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by bacteria. J Bacteriol. 2000;182(8):2059–2067. doi: 10.1128/jb.182.8.2059-2067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vyas T.K., Dave B.P. Effect of addition of nitrogen, phosphorus and potassium fertilizers on biodegradation of crude oil by marine bacteria. Indian J Mar Sci. 2010;39:143–150. [Google Scholar]
- 32.Thavasi R., Jayalakshmi S., Banat I.M. Application of biosurfactant produced from peanut oil cake by Lactobacillus delbrueckii in biodegradation of crude oil. Bioresour Technol. 2011;102:3366–3372. doi: 10.1016/j.biortech.2010.11.071. [DOI] [PubMed] [Google Scholar]
- 33.Aronstein B.N., Alexander M. Surfactants at low concentrations stimulate biodegradation of sorbed hydrocarbons in samples of aquifer sands and soil slurries. Environ Toxicol Chem. 1992;11:1227–1233. [Google Scholar]
- 34.Farag S., Soliman N.A. Biodegradation of Crude Petroleum Oil and Environmental Pollutants by Candida tropicalis Strain A. Braz Arch Boil Technol. 2011;54(4):821–830. [Google Scholar]
- 35.Strobel R.J., Sullivan G.R. Experimental design for improvement of fermentations. In: Demain A.L., Davies J.E., editors. Manual of industrial microbiology and biotechnology. ASM Press; Washington: 1999. pp. 80–93. [Google Scholar]
- 36.Othman E., Yusof F., Azmi A.S. Application of Plackett-Burman design for screening of parameters for the production of tetrathionate hydrolase by Thiobacillus ferrooxidans. J Engine Sci Technol. 2015;3(1):12–21. [Google Scholar]
- 37.Ashour W.E., Abd El Aty A.A., Hamed E.R., Swelim M.A., El-Diwany A.I. Applications of Plackett-Burman and central composite design for the optimization of novel brevundimonas diminuta KT277492 chitinase production, investigtion of its antifungal activity. Braz Arch Boil Technol. 2016;59 ISSN 1678-4324 Online Edition. [Google Scholar]