Abstract
Purpose
To obtain protein hydrolysates from fresh water green algae Scenedesmus obliquus by three different enzymes and evaluate its antioxidant and antiviral activity.
Methods
Enzymatic hydrolysates of green algae Scenedesmus obliquus protein were prepared by treatment with: 1.2% solution of pepsin, trypsin or papain. Protein was extracted from S. obliquus by three different extraction methods. Protein extracts and hydrolysates were assessed from stained gels following SDS–PAGE of samples. Antioxidant activity of protein hydrolysates was investigated.
Results
S. obliquus cells and protein extracts were rich in Arg, Lys, Asp, Ala, and His. Protein hydrolyzed by papain (Sd1pa) and protein hydrolyzed by trypsin (Sd2Try) induced highest antioxidant activity based on 1,1-diphenyl-2-picryl-hydrazyl (DPPH) radical-scavenging (41.41% and 40.62%) respectively, and on 2,2′-azinobis 3-ethyl-benzothiazoline-6-sulphonate (ABTS) radical (87.03% and 45.12%) respectively, at 150 µg/ml. The inhibitory effect and mode of action of protein hydrolysates were evaluated against Coxsackie B3 virus (CVB3). Protein hydrolyzed by papain (Sd2pa) and protein hydrolyzed by pepsin (Sd1pep) at 100 µg/ml exhibited antiviral activity (66.2% and 57.6%, respectively), against (CVB3) from all protein hydrolysates.
Conclusion
S. obliquus protein hydrolysates have a potential as antioxidative neutraceutical ingredients and a potential therapeutic agent against CVB3.
Abbreviations: Sd1, proteins extracted using NaOH pH 12 with neutralization; Sd2, proteins extracted using NaOH pH 12; Sd1pa, second extract hydrolyzed with papain; Sd1try, second extract hydrolyzed with trypsin; Sd1pep, second extract hydrolyzed with pepsin; Sd2pa, third extract hydrolyzed with papain
Keywords: Green algae, Scenedesmus obliquus, Protein hydrolysates, Antioxidant property, Antiviral activity, Pepsin hydrolysis, Papain hydrolysis
1. Introduction
The biotechnology involving microalgae have great evolution in early 1950s with developments in processing technologies relevant to the industrial applications of certain metabolites. The environmental and nutritional supplies alter the cell composition inducing the production of specific metabolites that are used for different industries. Different microalgae strains awake or acquire more attention for its ease in cultivation, economically credible advantages, and high biomass productivity [1]. Green microalgae are unicellular, eukaryotic, photosynthetic microorganisms living in fresh, saline or brackish water environments. Green algae (Chlorophyta) contain chlorophyll A and B, and chloroplasts responsible by photosynthesis and pyrenoids which are involved in the storage of starch. The formation of this storage product occurs in chloroplast instead of cytoplasm; differing from other eukaryotic algae. They express exclusive features to produce pharmaceuticals, nutraceuticals, cosmetics and biofuel manufacture due to its components as: proteins, essential amino acids, fatty acids, pigments, vitamins and other bioactive compounds [2].
The genus Scenedesmus is the common green algae, that often occurring as unique population in plankton. Cells in the colony occur in multiples of two with four or eight cells being most common. The species differ mostly in the texture of the wall, the number and type of spines on the cells. The uni-nucleate cells have a single laminate chloroplast. The morphology of the colony can vary, by varying the medium in which the cells are growing [3].
Enzymatic protein hydrolysates can be obtaining from traditional sources by using papain, pepsin, and trypsin [4]. Protein hydrolysates have many advantages such as using it as protein supplement in food and drinks. It can be used for individuals who suffer from digestibility problems such as gastrointestinal malfunction or cystic fibrosis, [5]. For the growing demand of global population there are considering the need of alternative sustainable food sources, microalgal hydrolysates are also emerging as promising functional protein nutrition products [6], [7].
The aim of this study was to cultivate S. obliquus in large scale to obtain protein hydrolysates produced by enzymatic hydrolysis using (pepsin, papain and trypsin). Identification of protein and peptides contents, were conducted by means of its amino acid profile. SDS–PAGE for S. obliquus and its protein hydrolysate was applied to recognize their molecular weights. Estimation of antioxidant and antiviral activity for all protein hydrolysates were performed by DPPH and ABTS methods.
2. Materials and methods
2.1. Algae source
Scenedesmus obliquus (Turpin) Kützing was obtained was obtained from the Culture Collection of Texas University, Austin, Texas, USA, and the Culture developed at Plant Biochemistry Department, National Research Centre. Cairo, Egypt.
2.2. Cultivation conditions
The culture of S. obliquus was initially inoculated in Bold Basil Media (BBM), by adding the microalgae into eight culture flasks (2 liters each) from the stock (Optical density at 620 nm = 0.20) to get 10% suspension of S. obliquus. All the flasks were kept under of direct fluorescent light exposition. The culture flasks were continuously aerated using electric aerator. Samplings were taken every 48 h from each flask to observe cell density, chlorophyll A content, and optical density of culture media. The amount of inseminated S. obliquus in samples was 5 mg dried substance per 2 liter of the Bold basal medium (BBM) (Table 1) was used as control medium [8]. The algae were cultured at optimal conditions using mono white color (3500 ± 350 Lux) with a photoperiod of 24 h, at 28 ± 2 °C and pH = 7.5–8 [9]. The S. obliquus were collected from the medium after 14 days when they were at the end of their logarithmic growth phase and when they were at their maximum nutritional value and density.
Table 1.
Composition of Bold Basal medium (BBM) for Scenedesmus culture.
| Chemicals/Compounds | Concentration in stock solution (g l−1) | Amount in culture medium (ml l−1) |
|---|---|---|
| NaNO3 | 25 | 10.0 |
| MgSO4. 7H2O | 7.5 | 10.0 |
| NaCI | 2.5 | 10.0 |
| K2HPO4 | 7.5 | 10.0 |
| KH2PO4 | 17.5 | 10.0 |
| CaCI2. 2H2O | 2.5 | 10.0 |
| Trace elements | 1.0 | |
| (a) ZnSO4. 7H2O | 8.82 | – |
| (b) MnCl2. 4H2O | 1.44 | – |
| (c) MoO3 | 0.71 | – |
| (d) CuSO4. 5H2O | 1.57 | – |
| (e) Co(NO3)2.6H2O | 0.94 | – |
| H3BO3 | 11.4 | 1.0 |
| EDTA-KOH solution | 1.0 | |
| (a) EDTA Na2 | 50 | – |
| (b) KOH | 31 | – |
| FeSO4·7H2O | 4.98 | 1.0 |
| Conc. H2SO4 | 1.0 ml. l−1 | 1.0 |
2.3. Growth measurement
Biomass concentration (g l−1) was calculated by measuring optical density at 620 nm to produce a standard curve relating dry weight of S. obliquus biomass (g l−1) to optical density. To monitor biomass changes in cultures, samples were taken every 48 h using aseptic technique [10].
2.4. Harvesting
At the end of each batch run, replicate cultures were pooled, filtered, washed with distilled water to remove soluble salts, centrifuged at 4830g for 15 min at 4 °C and frozen at -25 °C.
2.5. Determination of dry weight
A known weight of cells (0.5 g) was dried at 100 °C for 16 h, after filtration and washing, the filter paper was placed in desiccators and cooled to room temperature. The mass was recorded by using analytical balance until constant weight [11].
2.6. Specific growth rate
The specific growth rate (SGR) (µg/day) of cultured S. obliquus was calculated by Eq. (1) [12]:
| (1) |
where
X1 = Biomass concentration at the end of selected time interval.
X2 = Biomass concentration at the beginning of selected time interval, and
t2–t1 = Elapsed time between selected times in the day.
2.7. Chlorophyll A
Optical densities of the prepared samples were determined at 664, 647 and 630 nm wave length by using UV spectrophotometer. A blank with 100% acetone was run simultaneously. Chlorophyll A content was calculated by Eq. (2) [13]:
| (2) |
2.8. Extraction of protein
Protein was extracted from S. obliquus by means of two extraction methods:
2.8.1. Extraction with SDS-mercaptoethanol
Fresh algae (0.1 g) was mixed with the sample buffer consists of: 10% w/v SDS, 10 mM β-mercaptoethanol, 20% v/v glycerol, 0.2 M Tris–HCl (pH 6.8), 0.05% w/v bromo-phenol blue. Urea (8 M) should add for really hydrophobic proteins. Heat sample by boiling for 5–10 min. Later, this extract (SDS/ME) used for electrophoresis analysis [14].
2.8.2. Extraction with NaOH, 2 mol/l, pH 12 with neutralization
Freeze-dried biomass (0.5 g) was added to 25 mL, 2 mol/l NaOH solution and then stirred for 2 h at 40 °C. The supernatant was separated from the pellet by centrifugation at 13,416g for 10 min at 20 °C and adjusted to pH 3 with HCl 1 mol/l in order to precipitate the proteins. The protein isolate (Sd1) was collected after centrifugation at 13,000g for 10 min at 20 °C and the pellets were neutralized with NaOH 0.1 mol/l [15]. Samples were taken for protein analysis. Solubilized protein was dialyzed against water for 24 h.
2.8.3. Extraction with NaOH, 2 mol/l, pH 12
Protein was extracted using the alkali solubilization method described by Hultin and Kelleher [16]. The dried algae was mixed and homogenized with nine parts cold (6 °C) deionized water for each part of algae using a Bio- Homogenizer at a high speed for 1 min. In the alkaline solubilization step, the pH of the homogenized sample was adjusted to 11.0 using NaOH 2 mol/l and incubated at 4 °C for 30 min. After centrifuging the mixture at 13,416g for 20 min at 4 °C, the supernatant containing the soluble proteins was separated by centrifugation at 13,000g for 10 min at 20 °C and adjusted to pH 5.5 using HCl 1 mol/l to precipitate the proteins. The mixture was then centrifuged at 13,416g for 20 min at 4 °C to recover the proteins in the pelleted material (Sd2).
2.9. Enzymatic hydrolysis
Protein extracts from previous two methods Sd1 and Sd2 were mixed with 0.05 M phosphate buffer solution (PBS, pH 7.2) at liquid–material ratio 500:1. The mixtures were digested with pepsin, trypsin and papain 1.2% at 37 and 50 °C, pH 2.0, 7.0, 8.0 respectively, for 2 h [17]. After hydrolysis, the samples were heated in a boiling water bath for 20 min to inactivate the enzyme. The hydrolysates were centrifuged at 13,000g for 15 min and the supernatants were lyophilized and stored at −20 °C.
2.10. Total nitrogen
The total nitrogen was determined using semi-micro Kjeldahl method [18]. Approximately 2 g of sample were weighed into a digestion flask together with a combined catalyst of 5 g K2SO4 and CuSO4 and 15 ml of concentrated H2SO4. The percentage nitrogen was converted into protein percentage using the conversion factor obtained established for each microalga in previous study [18].
2.11. Determination of protein by Bradford method
Protein content of all extracts was estimated according to Bradford [19] at 595 nm, using Coomassie brilliant blue G-250 as a protein binding dye. Bovine serum albumin (BSA) was used as a protein standard. Protein concentrations in the samples were calculated from the calibration curve, in µg protein in µl extract. From µg protein versus µl extract calibration curve.
2.12. Amino acid analysis
The procedure for amino acid [aspartic acid (Asp), threonine (Thr), serine (Ser), glutamic acid (Glu), proline (Pro), glycine (Gly), alanine (Ala), valine (Val), isoleucine (Ile), leucine (Leu), phenylalanine (Phe), tyrosine (Tyr), histidine (His), lysine (Lys), arginine (Arg), methionine (Met) and cysteine (Cys) ] analysis was essentially the same as reported by Gerloff [20] Small samples (30–50 mg) were weighed into glass tubes and 2 ml of 6 mol/l HCl were added for each mg of crude protein (nitrogen × 5.89) in the samples. Tubes were opened and the hydrolysates were dried at 40 °C in a rotary evaporator. The residue was dissolved in a known amount of citrate buffer and an aliquot was analyzed for its amino acid content using a Beckman Spinco amino acid analyzer.
2.13. Electrophoresis
Proteins were separated by 12% PAA using sodium dodecylsulphate poly-acrylamide gel electrophoresis (SDS–PAGE) with a Mini Protean II Dual Slab Cell (Bio-Rad) according to Laemmli [14]. Samples with protein quantity equivalent were loaded for all variants. Gels were stained with Coomassie brilliant blue R-250. The markers used as MW referential are 250, 148, 98, 64, 59 and 36.
2.14. Antioxidant property
The antioxidant property of S. obliquus protein extracts and its hydrolysates was determined by means of following methods:
2.14.1. Determination of antioxidant activity of protein extracts and its hydrolysates by DPPH. free radical-scavenging activity
The DPPH. (2,2-diphenyl-1-picrylhydrazyl) free radical-scavenging activity of S. obliquus samples were determined according to the method described by Molyneux, [21]. Briefly, a 0.1 mM of ethanolic DPPH· solution was prepared. The initial absorbance of DPPH in ethanol absolute was measured at 517 nm and did not change throughout the period of assay. An aliquot (0.1 ml) of each sample (with appropriate dilution if necessary) was added to 3.0 ml of ethanolic DPPH solution. Discolorations were measured at 517 nm after incubation for 30 min in the dark. Measurements were performed in triplicate. Results were compared with butylated hydroxyanisol (BHA) and butylated hydroxytoluene (BHT) solutions, used as reference standard. The percentage of DPPH which was scavenged was calculated using Eq. (3):
| (3) |
Ethanol (3.0 ml) plus sample solution (0.1 ml) were used as a blank and 3 ml of DPPH–ethanol solution plus absolute ethanol (0.1 ml) were used as a negative control.
2.14.2. ABTS.+ free radical-scavenging activity
The antioxidant activity of S. obliquus samples was determined by the ABTS+ (2,2′-azinobis(3-ethyl-benzothiazoline-6-sulfonic acid) radical cation decolorization assay [22], involving preformed ABTS+ radical. A solution of (7 mM) ABTS was obtained and next ABTS+ radical was obtained by reacting ABTS with potassium per-sulfate. Before the assay, the mixture was diluted with ethanol absolute at a ratio of 1:100 to give an absorbance at λ = 734 nm of 0.70 ± 0.02. All samples from S. obliquus were dissolved in ethanol absolute at a concentration of 50, 100 and 150 µg/ml were added to ethanolic ABTS+ to measure absorbance after 6 min. Trolox (6-hydroxy-2, 5, 7, 8-tetramethylchroman-2-carboxylic acid) was used as antioxidant standard reference. Antioxidant activity measurements were carried out in triplicate and expressed as percentage of the absorbance of the uninhibited radical solution according to Eq. (4):
| (4) |
Being:
Absc: Absorbance of ethanolic ABTS.+.
Abs0: Absorbance of samples.
2.15. Antiviral activity
A known weight of protein extracts of S. obliquus and its hydrolysates (100 mg) was dissolved in 10% DMSO, (1 ml final volume), and stored at −20 °C for antiviral test.: For these biological assays, Coxsackie B3 virus (CVB3) (Nancy strain) was obtained from Dr. Mohamed Naser, Slovak Medical University, Slovakia Republic. The CVB3 virus was cultured and propagated in a cell culture medium and stored in small aliquots at −70 °C for use in research at Virology Lab at NRC, Egypt. Cell line: Vero cell line obtained from Holding Company for Biological Products and Vaccines, Egypt in 75 cm2 tissue culture flasks as monolayer sheets. This cell line was cultured in Dulbecco’s Modification of Eagle’s Medium (DMEM).
2.15.1. Determination of viral titer
Using Endpoint (limiting) dilution method, stock of viral solutions (100 µl) was tenfold dilute. Confluent cells (104 cells/well) prepared in 24-well plates were inoculated with 100 µl of each dilution in quadruple. The plates of inoculated cells were then incubated at 37 °C (5% CO2) and observed daily for development of characteristic cytopathogenic effect (CPE). The lowest dilution of viral stock suspension that could still induce 50% of a CPE was taken as the maximum dilution that would lead to infection. Median tissue culture infective dose (TCID50) as it was the minimum concentration of virus required to detect viral CPE, and gave a rough indication of the overall concentration of virus present within the stock suspension. And the endpoint dilution, which expressed as TCID50/ml, calculated by using Reed-Muench formula [23].
2.15.2. Cell lines toxicity to tested metabolites
Different dilutions of tested S. obliquus samples were added to evaluate the cytoxicity. Thus, the maximum concentration of tested extracts at which the cells displayed no morphological changes was determined to ensure that all morphological changes recorded due to the activity of the virus and not to the test compounds. Vero cell suspensions (100 µl) were seeded in 96-well plates and left in 5% CO2 incubator at 37 °C for 24 h. Once confluent, different dilutions from 0% to 100% of tested extracts diluted, in maintenance medium were added. The plates were then incubated at 37° C (5% CO2) for a few days and observed daily for changes in cellular morphology. Evidence of morphological changes (such as loss of monolayer, granulation, and vacuolization in cytoplasm) were recorded using the CPE scoring system.
2.15.3. Mode of action of extracts against Coxsackie B3 virus using CPE inhibition assay
2.15.3.1. Cytopathic effect (CPE) inhibition assay
Vero confluent 24-well plate were infected with 100 µl of stock Coxsackie B3 virus for 90 min at 37 °C. Virus was removed and 100 µl of tested extracts were added. Four wells were used for each dilution and 1000 µl of maintenance medium added per well. Then plats were incubated until complete CPE observed through 3 days. Antiviral activity was determined by the inhibition of CPE compared to control, expressed through formula of Reed and Muench [23].
2.15.3.2. Effect of tested extracts before virus infection
Confluent monolayer of Vero cells was grown in 24-well plates and inoculated with 100 µl of tested extracts for 90 min at 37 °C in 5% CO2 incubator. Four wells were used for each dilution. The medium was aspirated and 100 µl of Coxsackie B3 virus were inoculated and incubated for 90 min at 37 °C in 5% CO2 incubator. Then 1000 µl of maintenance medium were added and further incubation time for 3 days at 37 °C in 5% CO2 incubator. Antiviral activity was determined by the inhibition of CPE compared to control [24] and expressed through formula of Reed and Muench [23].
2.15.3.3. Effect of tested extracts on viral attachment
Coxsackie B3 virus (100 µl) was inoculated with equal volume of tested extracts for 90 min at 37 °C in 5% CO2 incubator. Mixed suspension (100 µl) was added to confluent monolayer of HEp-2 cells which were grown in 24-well plates. After 90 min of incubation for virus adsorption, mixed suspension was replaced by 1000 µl of maintenance medium. Four wells were used for each dilution. The plates were incubated for 3 days until CPE was observed. Antiviral activity was determined by the inhibition of CPE compared to control and expressed through formula of Reed and Muench [23].
2.15.3.4. Effect of tested extracts post-Coxsackie B3 virus infection
This experiment was carried out as stated in the above sections with the following differences: Confluent of HEp-2 cell line was grown in 24-well plates and treated with 100 µl of Coxsackie B3 virus for 90 min. Cells were washed and overlaid with 100 µl of different dilutions of tested extracts. Four wells were used for each dilution. Then, 1000 µl of maintenance medium were added. The plates were incubated for 3 days until CPE was observed. Determine CPE and viral evaluation following the procedures described by Reed and Muench [23].
2.16. Statistical analysis
Results are expressed as mean ± standard deviation (SD). Comparison between the mean values of different parameters in the different concentrations was done using one way analysis of variance (ANOVA). Excel 2010 was used for data analysis. P ≤ .05 was considered significant. A Pearson product-moment correlation coefficient was used to describe the relationships between antiviral as well as antioxidant and digestion with enzymes.
3. Results and discussion
In S. obliquus culture, pH of the media increase with the age of culture and on 14th day, it ranged from 7.3 to 8.5. The SGR, (µg/day) of cell and chlorophyll a were recorded in the range of 0.32 to 0.42 and 0.33 to 0.41, respectively [25]. Chlorophyll a content, which is also directly related to cell density, was also significantly (P ≤ 0.05) higher for BBM (6.14 mg/l).
3.1. Growth rate, chlorophyll a, total biomass, %N and protein content
Specific growth rate, chlorophyll a, total biomass, %N and protein content are presented in Table 2. These parameters were determined every 24 h. Total biomass of S. obliquus was 411.16 mg/l which somehow supported with the findings of Habib, [26]. The pH range (7.03–8.07) of the study reported by Toyub [25] supported the range (7.00–8.01) observed in present work.
Table 2.
Specific growth rate (µg/day) of cell, chlorophyll a (chll-a) and total biomass (mg/l) of Scenedesmus obliquus grown in BBM.
| Parameter | Scenedesmus obliquus |
|---|---|
| SGR of cell | 0.039 ± 0.01 µg/day |
| SGR of Chll-a | 2.7 ± 0.01 µg/day |
| %N | 6.91 ± 0.014 |
| %Crude protein | 40.69 ± 0.021 |
| N-T-P factora | 5.89 |
| Total biomass | 411.16 mg/l |
N-T-P: nitrogen to protein factor.
Protein content of S. obliquus was 40.69%. Toyub [25] found the protein percentage of S. obliquus cultivated in BBM was 34.39%. This difference may be due to the growth period of algae cells.
As shown in (Table 3), % protein determined through Bradford method was 34.45% in Sd1 extracted with NaOH pH 12 and neutralized. While, it was 39.55% in Sd2 extracted with NaOH pH 11. Nevertheless, it can be seen in Table 3 that the amount of amino acids in all protein extracts was reduced according to the extraction method. It’s noted that the total nitrogen estimation includes other nitrogenous compounds, such as intracellular inorganic materials. Pigments, nucleic acids, glucosamine and amines that could account for about 10% of the overall nitrogen content in S. obliquus.
Table 3.
Protein percentage of Scenedesmus obliquus protein extracts with Bradford assay.
| Protein extracts | % protein |
|---|---|
| Sd1 | 34.45 ± 0.01 |
| Sd2 | 39.55 ± 0.02 |
3.2. Amino acids content
The amino acid profile for crude S. obliquus and for Sd1 and Sd2 protein extracts are presented in Table 4. S. obliquus was evaluated as a source of essential (Thr, Val, Met, Ile, Leu) and non-essential (His, Arg, Ala, Pro, Asp) amino acids. All amino acids were presented in lower concentrations in Sd1 and Sd2 protein extracts. The most abundant essential amino acid was lysine, while arginine was evaluated as the most abundant non-essential amino acid. And, leucine and phenylalanine were found in the lowest concentrations within a group of essential amino acids.
Table 4.
Amino acids (µmol/100 mg dw) of S. obliquus.
| Amino acid |
Scenedesmus obliquus |
||
|---|---|---|---|
| Algae | Sd 1 | Sd 2 | |
| Histidine | 2.774 | 2.399 | 2.037 |
| Arginine | 8.6 | 6.482 | 7.156 |
| Threonine | 2.101 | 1.692 | 1.716 |
| Alanine | 2.734 | 1.828 | 2.091 |
| Proline | 0.466 | 0.334 | 0.405 |
| Tyrosine | 1.34 | 1.158 | 1.184 |
| Valine | 1.663 | 1.339 | 1.416 |
| Methionine | 1.602 | 1.208 | 1.352 |
| Isoleucine | 1.653 | 1.187 | 1.008 |
| Leucine | 0.857 | 0.573 | 0.486 |
| Phenylalanine | 1.273 | 0.914 | 1.142 |
| Lysine | 2.322 | 2.062 | 1.889 |
| Aspargine | 2.261 | 2.304 | 1.956 |
| Aspartic acid | 1.714 | 1.146 | 0.973 |
| Glutamic acid | 1.613 | 1.01 | 1.291 |
Sd1: Proteins were extracted using NaOH pH 12 with neutralization.
Sd2: Proteins were extracted using NaOH pH 12.
The nutritional quality of a protein is determined basically by the content, proportion and availability of its amino acids. It can be seen that the amino acid pattern of S. obliquus compares favorably with that of the reference and the other food proteins [27].
3.3. Electrophoresis protein profiles
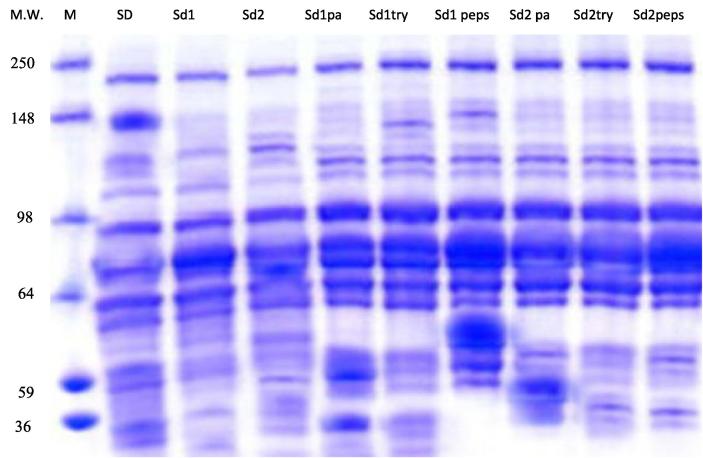
In order to evaluate the populations of proteins in different extracts and to determine their molecular weights under denaturizing conditions, electrophoresis was undertaken. Based on the electropherogram (SDS–PAGE) (Fig. 1), a qualitative comparison was made between protein extracts patterns obtained from S. obliquus. The First extract (SD) patterns showed nine major bands per lane within a range molecular weight varying between 211-kDa and 33-kDa. While the other methods of extraction obtained four major bands per lane in the second method, molecular weight range from 211-kDa and 69-kDa and the third method gave seven bands per lane molecular weight range from 217-kDa and 46-kDa.
Fig. 1.
SDS-PAGE profile of Scenedesmus obliquus while,(M) Marker, (SD) Algae extracted with first method,(Sd1) Algae extracted with second method,(Sd2)Algae extracted with third method, (Sd1pa)second extract hydrolyzed with papain, (Sd1try)second extract hydrolyzed with trypsin, (Sd1pep)second extract hydrolyzed with pepsin, (Sd2pa)third extract hydrolyzed with papain, (Sd2try)third extract hydrolyzed with trypsin, (Sd2pep)third extract hydrolyzed with pepsin.
Protein hydrolysates obtained from Sd1 by using papain showed nine major bands per lane, molecular weight range from 224-kDa and 35-KDa. While, hydrolysis with trypsin obtained eight bands per lane with molecular weight range from: 224-KDa and 66-KDa. However, hydrolysis with pepsin obtained ten bands per lane, with molecular weight range from 224-KDa and 46-KDa (Table 5).
Table 5.
Molecular weight (KD) of protein bands in Scenedesmus obliquus protein extracts and its hydrolysates.
| Lanes | MARKER |
SD |
Sd1 |
Sd2 |
Sd1pa |
Sd1try |
Sd1peps |
Sd2pa |
Sd2 try |
Sd2 peps |
|---|---|---|---|---|---|---|---|---|---|---|
| Bands | KD | |||||||||
| 1 | 250 | 211 | 211 | 217 | 224 | 224 | 224 | 224 | 224 | 224 |
| 2 | 148 | 154 | 96 | 137 | 129 | 172 | 164 | 132 | 132 | 132 |
| 3 | 98 | 93 | 80 | 96 | 98 | 132 | 98 | 101 | 101 | 101 |
| 4 | 64 | 77 | 69 | 86 | 86 | 98 | 86 | 86 | 80 | 83 |
| 5 | 59 | 61 | 77 | 80 | 86 | 80 | 72 | 72 | 72 | |
| 6 | 36 | 48 | 61 | 72 | 80 | 72 | 67 | 67 | 67 | |
| 7 | 44 | 46 | 66 | 72 | 66 | 44 | 40 | 51 | ||
| 8 | 33 | 46 | 66 | 55 | 39 | |||||
| 9 | 35 | 49 | ||||||||
| 10 | 46 | |||||||||
Protein profiles of investigated green freshwater and blue-green algae are mainly formed by polypeptides associated with photosynthesis. Protein bands from interval 4–36 kDa are supposed predominantly formed by polypeptides originating from photosystems I and II. Polypeptides from thylakoid membranes, light-harvesting complex proteins (LHC IIc, LHCPa, LHCPb) and phytochelatins, and polypeptides of P700-chlorophyll a-protein 1 from photosystem I are displayed as protein bands 8, 10, 15 and 18 kDa; molecular weight 9 kDa could belong to protein CPIII - Chlorophyll a protein; and the area around 22 kDa may indicate protein CP22 [28], [29], [30], [31], [32], [33]. Protein bands around 40 and 70 kDa may belong to the enzyme magnesium chelatase (termed I and D); moreover, bands 75 kDa are adherent to protein Toc75 [34], [35]. Polypeptides from photosystem I complex (PSI-200) are commonly displayed as protein bands 58 and 62 kDa [36]. The presence of bands around 100 and 110 kDa may be induced by chlorophyll a protein CPIV and P700-chlorophyll a protein 1 from photosystem I [28], [29].
3.4. Antioxidant activity
The antioxidant capacity of protein hydrolysates was determined by the evaluation of their capacity to scavenge DPPH and ABTS radicals.
All hydrolysates obtained have the property of scavenging DPPH radicals (Table 6). DPPH radical-scavenging property ranged from 30.9% to 41.41% at 150 µg/ml. The highest antioxidant capacities were obtained with samples extracted with the first method and hydrolyzed with Papain (41.41%) and samples extracted with the second method and hydrolyzed with Trypsin (40.62%), while the lowest capacities were obtained for the first and the second crude protein extracts (33.25%) and (30.9%) respectively.
Table 6.
% Antioxidant activity of Scenedesmus protein extracts and hydrolysates by DPPH (Data are means of triplicate ± SD.).
| Protein extracts & Hydrolysates | Sample concentrations (µg/ml) |
||
|---|---|---|---|
| 50 | 100 | 150 | |
| Sd1 | 27.71 ± 0.47 | 29.57 ± 0.44 | 33.25 ± 0.54 |
| Sd1Pa | 30.64 ± 0.42 | 38.84 ± 0.39 | 41.41 ± 0.41 |
| Sd1Pep | 27.63 ± 0.41 | 32.40 ± 0.60 | 38.33 ± 0.77 |
| Sd1Try | 28.13 ± 0.88 | 31.18 ± 1.10 | 37.35 ± 0.13 |
| Sd2 | 27.99 ± 0.43 | 29.44 ± 0.22 | 30.90 ± 0.30 |
| Sd2Pa | 28.84 ± 0.73 | 30.17 ± 0.44 | 33.67 ± 0.39 |
| Sd2Pep | 28.65 ± 0.39 | 30.11 ± 0.44 | 33.29 ± 0.28 |
| Sd2Try | 30.21 ± 0.33 | 36.27 ± 0.88 | 40.62 ± 0.98 |
| BHA (butylated hydroxyanisol) | 88.54 ± 0.020 | 95.22 ± 0.02 | 99.41 ± 0.025 |
| BHT (butylated hydroxytoluene) | 85.57 ± 0.025 | 94.76 ± 0.031 | 98.15 ± 0.046 |
Data are means of triplicate ± SD.
All protein from S. obliquus and its hydrolysates exhibited good antioxidant property using the ABTS radical scavenging assay. The antioxidant property ranged from 36.95% to 68.23% at 150 µg/ml (Table 7). Pepsin was the enzyme showing the highest antioxidant property with the first extraction method (68.32%).
Table 7.
% Antioxidant activity of Scenedesmus protein extracts and hydrolysates by ABTS (Data are means of triplicate ± SD).
| Protein extracts & hydrolysates | Sample concentrations (µg/ml) |
||
|---|---|---|---|
| 50 | 100 | 150 | |
| Sd1 | 35.96 ± 0.54 | 53.02 ± 0.92 | 68.23 ± 0.59 |
| Sd1 Pa | 39.50 ± 0.73 | 57.23 ± 0.35 | 87.03 ± 0.68 |
| Sd1 Pep | 45.97 ± 0.75 | 56.03 ± 0.25 | 68.32 ± 0.75 |
| Sd1 Try | 41.12 ± 0.80 | 44.05 ± 0.76 | 46.14 ± 0.43 |
| Sd2 | 25.69 ± 0.35 | 30.70 ± 0.34 | 36.95 ± 0.08 |
| Sd2 Pa | 28.18 ± 0.32 | 37.68 ± 0.60 | 38.94 ± 0.14 |
| Sd2 Pep | 24.89 ± 0.14 | 32.94 ± 1.01 | 41.07 ± 0.02 |
| Sd2 Try | 32.02 ± 0.08 | 37.75 ± 0.25 | 45.12 ± 0.38 |
| Trolox | 92.35 ± 0.020 | 95.66 ± 0.020 | 98.23 ± 0.038 |
Data are means of triplicate ± SD.
The ABTS and DPPH assays are based on electron transfer making it possible measure the presence of hydrophilic and lipophilic antioxidant substances [37], [38].
Becker [39], suggesting that all three micro-algal residual biomasses represent good nonconventional sources of protein that can potentially be employed as feedstock for animal and human consumption. Although further studies are needed to confirm protein quality, several reports have shown micro-algal proteins to have a high nutritional value, containing all essential amino acids, and are a rich source of tyrosine, arginine, aspartic acid, glutamic acid, and leucine [40], [41], [42], [43].
In present work, the antioxidant capacity values found for from S. obliquus constituents and derivatives by means of the DPPH method showed the lowest radical scavenging values among the three antioxidant capacity determination assays. A similar effect has been previously observed in other studies, in which a diverse range of phenolic compounds was shown to react slowly with the DPPH radical reaction [44], [45]. The three protein hydrolysates analyzed exhibited antioxidant property which varied depending on the determination assay used.
The increase in antioxidant property found in protein hydrolysates can be explained, at least partially, by the fact that hydrolysis of proteins is shown to be necessary for the release of anti-oxidizing peptides, which are normally inactive within the original protein [46], [47]. The exact mechanism of action of protein hydrolysates as antioxidants has yet to be clearly elucidated, but it is recognized that their bioactivities largely depend on the peptide structure, hydrophobicity, and type and size of the amino acids generated during hydrolysis [48], [49], [50]. Previous studies have shown that low molecular weight hydrolysates generally possess higher radical scavenging capacity than high molecular weight hydrolysates [51], since small peptides can more easily access the oxidative mechanism to donate protons to free radicals, and stabilize oxygen reactive species through a direct electron transfer, thereby exhibiting better antioxidant capacity [46], [49], [52].
The mechanism of action may be that the antioxidant protein hydrolysates and peptides could easily enter into target organs through hydrophobic interactions with membrane lipid bilayers by the aid of their hydrophobicity, where they are able to exert significant capacity of scavenging radicals [53].
By assessing antioxidant properties of microalgae protein hydrolysates, we have demonstrated that the selected microalgae protein hydrolysates represent a natural source of nutraceutical compounds that could be exploited for health benefits [54]. The use of protein hydrolysates as potent bioactive compounds is a growing field in the development of nutraceutical products, as they have higher bioactivities than their parent proteins. Several studies have been conducted to identify bioactive peptides present in protein hydrolysates [50], [54], [55], [56], [57], [58].
According to Alem’an [27] all amino acids can generally interact with free radicals, but the most effective are those containing nucleophilic sulfur-containing side chains (Cys and Met) or aromatic side chains (Trp, Tyr and Phe), which can easily donate hydrogen atoms. The antioxidant activity followed a sequence order of Pro-Tyr-Ser-Phe-Lys > Gly-Phe-Gly-Pro-Glu-Leu > Val-Gly-Gly-Arg-Pro [59] for DPPH, OH radical and ABTS assays.
3.5. Antiviral activity and cytotoxicity of Scenedesmus obliquus protein hydrolysates
The cytotoxicites of S. obliquus protein hydrolysates for HEp-2 cells were evaluated (Table 8). Subconfluent monolayers treated with extracts at different concentrations under 6.25 µg/ml and 100 µg/ml did not show any visible changes in cell morphology or cell density except the first extract hydrolyzed with Trypsin and Pepsin at 100 µg/ml sample concentration.
Table 8.
Cytotoxicities of Scenedesmus protein hydrolysates on HEp-2 Cells.
| Extracts | Concentration (µg/ml) |
||||
|---|---|---|---|---|---|
| 100 | 50 | 25 | 12.5 | 6.25 | |
| Sd1Pa | − | − | − | − | − |
| Sd1Try | + | − | − | − | − |
| Sd1Pep | + | − | − | − | − |
| Sd2Pa | − | − | − | − | − |
(−) No Cytotoxicity, (+) weak cytotoxicity
Most of protein hydrolysates induced antiviral activity, in low concentration used in the assays. S. obliquus protein hydrolysates, exhibited detectable effect against Coxsackie B3 Virus with an inhibitory concentration of 66.2% at 100 μg/ml of the second protein hydrolysate hydrolyzed with papain (Table 9).
Table 9.
% Inhibitory activity of Scenedesmus protein hydrolysates (µg/ml) against Coxsackie B3 Virus.
| Extracts | Concentration (µg/ml) |
|||
|---|---|---|---|---|
| 100 | 50 | 25 | 12.5 | |
| Sd1pa | 57 | 51 | 26 | 11.5 |
| Sd1try | 49.3 | 36.6 | 16 | 4 |
| Sd1pep | 57.6 | 46.5 | 23.5 | 3.5 |
| Sd2pa | 66.2 | 52.5 | 29 | 12.4 |
Positive control was 106 TCID50/100 µl.
Entero-viruses such as coxsackie virus, poliovirus, and echovirus are small, non-enveloped viruses possessing a single-stranded RNA genome in positive orientation that acts directly as mRNA in infected cells [60]. Entero-viruses are of high clinical relevance with coxsackie virus B3 (CVB3), which can cause heart-muscle infection, being an important member of this category [61]. To date, no effective antiviral therapies have been approved for either the prevention or treatment of diseases caused by viruses classified within the Picornaviridae family, including CVB3, EV71, and HRV [62]. In this regard, many trials have been conducted to find antiviral components from plants. Such trials have specifically targeted plants with intrinsic defense mechanisms in the form of secondary metabolites against a broad range of viral infections, in contrast to adaptive immunity induced in mammals.
3.6. Mode of action of antiviral activity of Scenedesmus obliquus protein hydrolysates
For All tested extracts of S. obliquus were observed antiviral activity more than 50% during virus adsorption step, except Sd2Try (27.6%) and Sd1Try (41%). Also Sd1Pep and Sd2Pa caused inhibition during viral penetration step. The others also presented activity in this stage of the viral cycle, but to a lower degree. However, during viral attachment step, the antiviral activity was increased more than in adsorption step and viral penetration step. It is ranged from 74% to 78.3% in Sd1Pep and Sd2Pa, respectively. (Table 10).
Table 10.
Mode of action of antiviral activity.
| Extracts | Attachment inhibition% | Penetration inhibition% | Adsorption inhibition% |
|---|---|---|---|
| Sd1pa | 65 | 62.5 | 54 |
| Sd1try | 61 | 58 | 41 |
| Sd1Pep | 74 | 70.3 | 63 |
| Sd2pa | 78.3 | 75.1 | 56 |
However, there have been no previous reports on the antiviral activity of protein hydrolysates against other viruses included in Picornaviridae. In the current study, was showed that protein hydrolysates Sd, Sd1, and Sd2 have evident antiviral activity against CVB3. It is believed that CVB3 is an etiological agent causing myocarditis and dilated cardiomyopathy, and outbreaks of CVB3 infection occur worldwide annually [63]. Currently, there are no effective therapeutic agents against CVB3, and only ribavirin has been shown to have weak antiviral activity against CVB3 infection [64], [65], [66]. The results of the present study demonstrating the antiviral activities of protein hydrolysates suggest that the compounds may provide a therapeutic option for the treatment of CVB3 infection; furthermore, the compounds could potentially be effective against Picornaviridae viruses in general.
One of the most likely modes of action of all these protein hydrolysates for antiviral activity might be by competitive binding of it to some regions of the viral spike protein or to cellular surfaces, blocking the membrane fusion. In a similar manner, bovine lactoferrin and peptidic fragments thereof have been described as inhibitors of early phases of naked viruses, as echovirus, which interacts in an acidic-dependent manner, characteristic of late endosomes and lysosomes [66] relevant for the pyknotic via of viral penetration.
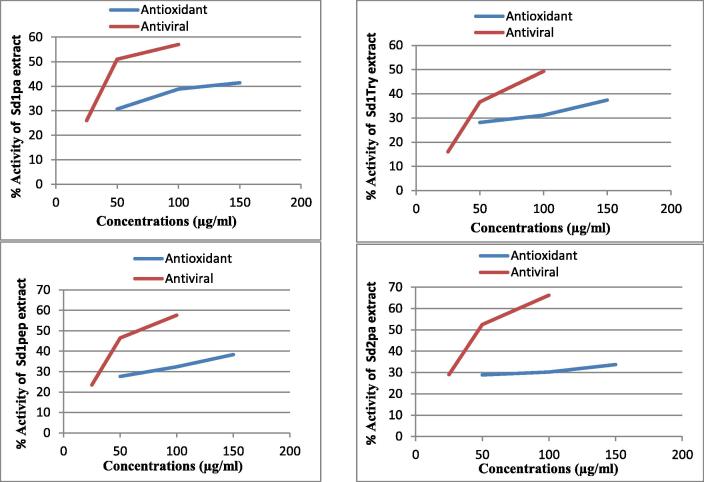
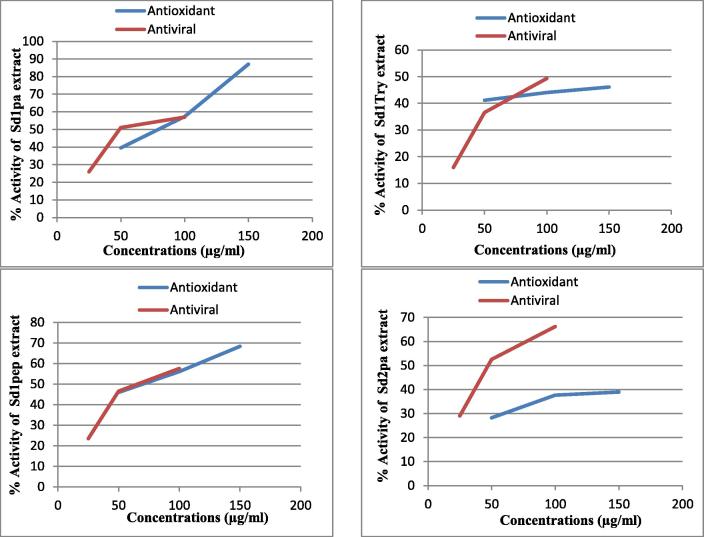
Relationship between antiviral and antioxidant property determined by means of DPPH and ABTS radicals methods (Fig. 2, Fig. 3) were shown by Pearson correlation which shows that there is a strong positive correlation (0.953 for DPPH and 0.950 for ABTS), which means there is a tendency for high concentrations variable scores go with high antiviral and antioxidant variable scores (and “vice versa”)
Fig. 2.
Relationship between antiviral and antioxidant activity by DPPH. (Sd1pa) second extract hydrolyzed with papain, (Sd1try) second extract hydrolyzed with trypsin, (Sd1pep) second extract hydrolyzed with pepsin, (Sd2pa) third extract hydrolyzed with papain.
Fig. 3.
Relationship between antiviral and antioxidant activity by ABTS. (Sd1pa) second extract hydrolyzed with papain, (Sd1try) second extract hydrolyzed with trypsin, (Sd1pep) second extract hydrolyzed with pepsin, (Sd2pa) third extract hydrolyzed with papain.
4. Conclusion
In order to characterize S. obliquus protein hydrolysates obtained in this study, protein hydrolysates gave high antioxidant activity. These results revealed that S. obliquus protein hydrolysates having antioxidant activity as well as antiviral because of the appearance of amino acid residues such as methionine and arginine as shown in our results.
In conclusion, the investigations conducted that algal biomass shows promising qualities as a novel source of protein, its quality of protein could be increased by treatments with enzymes. We think our data also demonstrate that antiviral activities are related to amino acid sequence analysis as well as releasing of small peptides. Therefore, further studies are required to explore the detailed antiviral mechanisms of S. obliquus protein hydrolysates as well as to assess in vivo antiviral activity.
A high proportion of hydrophobic amino acids has been reported in peptides with high antioxidant activity, compared to other hydrophilic amino acids, which is considered as the key factor in peptide with high antioxidant activity, compared to other hydrophilic amino acids, which is considered as the key factor in peptide ability to scavenge radicals.
Conflict of interests
None.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
Contributor Information
Abd El-Moneim M.R. Afify, Email: abdelmoneimafify@yahoo.com.
Gamal S. El Baroty, Email: elbarotys@hotmail.com.
Farouk K. El Baz, Email: fa_elbaz@hotmail.com.
Hanaa H. Abd El Baky, Email: abdelbakyhanaa@yahoo.com.
Soha A. Murad, Email: sohaahmed77@gmail.com.
References
- 1.Pulz O., Gross W. Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol. 2004;65:635–648. doi: 10.1007/s00253-004-1647-x. [DOI] [PubMed] [Google Scholar]
- 2.Hegewald E., Bock C., Krienitz L. A phylogenetic study on Scenedesmaceae with the description of a new species of Pectinodesmus and the new genera Verrucodesmus and Chodatodesmus (Chlorophyta, Chlorophyceae) Fottea Olomouc. 2013;13(2):149–164. [Google Scholar]
- 3.Lee R. Phycology. 2008:1–27. [Cambridge University Press, UK.] [Google Scholar]
- 4.Najafian L., Babji A.S. Isolation, purification and identification of three novel antioxidative peptides from patin (Pangasius sutchi) myofibrillar protein hydrolysates. LWT Food Sci Technol. 2015;60:452–461. [Google Scholar]
- 5.Ratna parkhi M.P., Chaudhari S.P., Pandya V.A. Peptides and proteins in pharmaceuticals. Int J Curr Pharmac Res. 2011;3(2):19. [Google Scholar]
- 6.Clemente A. Enzymatic protein hydrolysates in human nutrition. Trends Food Sci Technol. 2000;11:254–262. [Google Scholar]
- 7.Wang B., Li Z.R., Chi C.F., Zhang Q.H., Luo H.Y. Preparation and evaluation of antioxidant peptides from ethanol-soluble proteins hydrolysate of S. lewini muscle. Peptides. 2012;36:240–250. doi: 10.1016/j.peptides.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Cox ER, Bold HC. Taxonomic investigation of Stigeoclonium, University of Texas, Publ. No. 6618, Austin (Texas). Phycological studies VII, 10; 1966.
- 9.Ordog V. Apparatus for laboratory algal bioassays. Hydrobiologia. 1981;16:127–136. [Google Scholar]
- 10.Payer H.D. Inst. Food Res. Product Development Kasetsar Univ; Bangkok (Thailand): 1971. First report upon the organization and experimental work of the Thailand German project on the production and utilization of single cell green algae as a protein source for human nutrition. [Google Scholar]
- 11.AOAC . 15th ed. Association of Official Analytical Chemists; 1990. Official methods of analysis. [Google Scholar]
- 12.Boyle D.T. A rapid method for measuring specific growth rate of microorganisms. Biotechnol Bioeng. 1977;(1977) doi: 10.1002/bit.260190210. [DOI] [PubMed] [Google Scholar]
- 13.Vernon LP. Spectrophotometric determination of chlorophylls and phaeophytins in plant extracts. 32(9); 1960. p. 1144–15.
- 14.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Safi C., Ursu A.V., Laroche C., Zebib B., Merah O., Pontalier P.Y. Aqueous extraction of proteins from microalgae: effect of different cell disruption methods. Algal Res. 2014;3:61–65. [Google Scholar]
- 16.Hultin HO, Kelleher SD. High efficiency alkaline protein extraction. US Patent 6136,959; 2000.
- 17.Adler-Nissen J. Elsevier Applied Science Publishers; New York (USA): 1986. Enzymic hydrolysis of food proteins. [Google Scholar]
- 18.AOAC . Association of Official Analytical Chemists; Arlington (VA): 1995. Official methods of analysis. [Google Scholar]
- 19.Bradford M.M. A rapid and sensitive method for the quantitation of microgram of protein utilizing of protein-dye binding. Anal Biochem. 1976;72:248–258. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 20.Gerloff E.D., Lima I.H., Stahmann M.A. Amino acid composition of leaf protein concentrates. J Agri Food Chem. 1965;13:139–143. [Google Scholar]
- 21.Molyneux P. The Use of the stable free radical diphenylpicryl-hydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol. 2004;26(December 2003):211–219. [Google Scholar]
- 22.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med. 1999;26(9–10):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 23.Reed L., Muench H. A simple method of estimating fifty percent end points. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 24.Hai-Rong X., Yuan-Ying S., Li L., Wei H., Fan L., Hong X., Zhan-Qiu Y. The inhibitory effect of Rheum palmatum against Coxsackie B3 in vitro and in vivo. Am J Chin Medic. 2012;40:801–812. doi: 10.1142/S0192415X12500607. [DOI] [PubMed] [Google Scholar]
- 25.Toyub M.A., Miah M.I., Habib M.A.B., Rahman M.M. Growth performance and nutritional value of scenedesmus obliquus cultured in different concentrations of sweetmeat factory waste media. Bang J Anim Sci. 2008;37(1):86–93. [Google Scholar]
- 26.Habib MAB. Culture of selected microalgae in rubber and palm oil effluents and their use in the production of enriched rotifers. Doctoral Thesis, University of Putra. Malaysia; 1998. p. 539.
- 27.Becker E.W. Micro-algae as a source of protein. Biotechnol Adv. 2007;25:207–210. doi: 10.1016/j.biotechadv.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Brandt P., Kaiser-Jarry K., Wiessner W. Chlorophyll-protein complexes. variability of CPI, and the existence of two distinct forms of LHCP and one low-molecularweightchlorophylla protein. BBA – Bioenergetics. 1982;679:404–409. [Google Scholar]
- 29.Hoj P.B., Moller B.L. The 110-kDa reaction center protein of photosystem I, P700- chlorophyll a-protein 1, is an iron-sulfur protein. J Biol Chem. 1986;261:14292–14300. [PubMed] [Google Scholar]
- 30.Ikeuchi M., Inoue Y. A new 4.8-kDa polypeptide instrictic to the PS II reaction center, as revealed by modified SDS-PAGE with improved resolution of low-molecular- weight proteins. Plant Cell Physiol. 1988;29:1233–1239. [Google Scholar]
- 31.Funk C., Schröder W.P., Green B.R., Renger G., Andersson B. The instrictic 22 kDa protein is a chlorophyll-binding subunit of photosystem II. FEBS Lett. 1994;342:261–266. doi: 10.1016/0014-5793(94)80513-x. [DOI] [PubMed] [Google Scholar]
- 32.Morishige D.T., Thornber J.P. Identification of a novel light-harvesting complex II protein (LHC IIc’) Photosynth Res. 1994;39:33–38. doi: 10.1007/BF00027140. [DOI] [PubMed] [Google Scholar]
- 33.Osman M.E.H., El-Naggar A.H., El-Sheekh M.M., El-Mazally E.E. Differential effects of Co2+ and Ni2+ on protein metabolism in Scenedesmus obliquus and Nitzschia perminuta. Environ. Toxicol. and Pharmacol. 2004;16:169–178. doi: 10.1016/j.etap.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Inoue K., Potter D. The chloroplastic protein translocation channel Toc75 and its paralog OEP80 represent two distinct protein families and are targeted to the chloroplastic outer envelope by different mechanisms. Plant J. 2004;39:354–365. doi: 10.1111/j.1365-313X.2004.02135.x. [DOI] [PubMed] [Google Scholar]
- 35.Gamini Kannangara C., Wettstein D. Magnesium Chelatase. In: Rebeiz C.A., Benning C., Bohnert H.J., Daniell H., Hoober J.K., Lichtenthaler H.K., Portis A.R., Tripathy B.C., editors. The Chloroplast, Basic and Applications. Springer; The Netherlands: 2010. pp. 79–88. [Google Scholar]
- 36.Malkin R. I. Photosynthetic Unit; The antenna system, and the photosynthetic pigments. Photosynth. Res. 1986;10:197–200. [Google Scholar]
- 37.Huang D.J., Ou B.X., Prior R.L. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 38.Prior R., Wu X., Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 39.Becker E.W. Microalgae for human and animal nutrition. In: Richmond A., Hu Q., editors. Handbook of microalgal culture: applied phycology and biotechnology. 2nd edn. John Wiley and Sons; UK: 2013. pp. 461–503. [Google Scholar]
- 40.Brown M.R., Jeffrey J.K., Volkman J.K., Dunstan G.A. Nutritional properties of microalgae for Mari culture. Aquaculture. 1997;151:315–331. [Google Scholar]
- 41.Derrien A., Coiffard L., Coiffard C., De Roeck Y. Free amino acid analysis of five microalgae. J Appl Phycol. 1998;10:131–134. [Google Scholar]
- 42.Kolb N., Vallorani L., Milanovic N., Stocchi V. Evaluation ofmarine algae wakame (Laminaria digitata japonica) as food supplements. Food Technol Biotechnol. 2004;42:57–61. [Google Scholar]
- 43.Kang K., Qian Z., Ryu B., Karadeniz F., Kim D., Kim S. Antioxidant peptides from protein hydrolysate of microalgae Navicula incerta and their protective effects in Hepg2/CYP2E1 cells induced by ethanol. Phytother Res. 2012;26:1555–1563. doi: 10.1002/ptr.4603. [DOI] [PubMed] [Google Scholar]
- 44.Villaño D., Fernández-Pachón M.S., Moyá M.L., Troncoso A.M., García- Parrilla M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta. 2007;71:230–235. doi: 10.1016/j.talanta.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 45.Shalaby E.A., Shanab S.M. Comparison of DPPH and ABTS assay for determining antioxidant potential of water and methanol extracts of Spirulina platensis. Indian J Geomar Sci. 2013;42:556–564. [Google Scholar]
- 46.Sheih I.C., Wu T.K., Fang T.J. Antioxidant properties of a new antioxidative peptide from algae protein waste hydrolysate in different oxidation systems. Biores Technol. 2009;100:3419–3425. doi: 10.1016/j.biortech.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 47.Chen H., Muramoto K., Yamauchi F., Fujimoto K., Nokihara K. Antioxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein. J Agric Food Chem. 1998;46:49–53. doi: 10.1021/jf970649w. [DOI] [PubMed] [Google Scholar]
- 48.Cian R.E., Alaiz M., Vioque J., Drago S. Enzyme proteolysis enhanced extraction of ACE inhibitory and antioxidant compounds (peptides and polyphenols) from Porphyra columbina residual cake. J Appl Phycol. 2013;25:1197–1206. [Google Scholar]
- 49.Valdez-Flores M., Germán-Báez L.J., Gutiérrez-Dorado R., Medina-Godoy S., Norzagaray-Valenzuela C., Hernández-Verdugo S., Reyes-Moreno C., Valdez-Ortiz A. Improving bioactivities of Jatropha curcas protein hydrolysates by optimizing with response surface methodology the extrusion cooking process. Ind Crop Prod. 2016;85:353–360. [Google Scholar]
- 50.Gallegos-Tintoré S., Torres C.F., Solorza J.F., Alaiz M., Girón J.C., Martínez A.L., Guerrero L.C., Vioque J. Antioxidant and chelating activity of nontoxic Jatropha curcas L. protein hydrolysates produced by in vitro digestion using pepsin and pancreatin. J Chem. 2015;1:1–9. [Google Scholar]
- 51.Fan X., Bai L., Zhu L., Yang L., Zhang X. Marine algae-derived bioactive peptides for human nutrition and health. J Agric Food Chem. 2014;62:9211–9222. doi: 10.1021/jf502420h. [DOI] [PubMed] [Google Scholar]
- 52.Chi C.F., Hu F.Y., Wang B., Li T., Ding G.F. Antioxidant and anticancer peptides from the protein hydrolysate of blood clam (Tegillarca granosa) muscle. J Funct Foods. 2015;15:301–313. [Google Scholar]
- 53.Norzagaray-Valenzuela C.D., Valdez-Ortiz A., Shelton L.M., Jiménez-Edeza M., Rivera-López J., Valdez-Flores M.A., Germán-Báez L.J. Residual biomasses and protein hydrolysates of three green microalgae species exhibit antioxidant and anti-aging activity. J Appl Phycol. 2017;29:189–198. [Google Scholar]
- 54.Kim S.K., Wijesekara I. Development and biological activities of marine-derived bioactive peptides: a review. J Funct Foods. 2010;2:1–9. [Google Scholar]
- 55.Valdez-Ortiz A., Fuentes-Gutiérrez C.I., Germán-Báez L.J., Gutiérrez- Dorado R., Medina-Godoy S. Protein hydrolysates obtained from Azufrado (sulphur yellow) beans (Phaseolus vulgaris): nutritional, ACE-inhibitory and antioxidative characterization. LWTFood Sci Technol. 2012;46:91–96. [Google Scholar]
- 56.Luna-Vital D.A., González de Mejía E., Mendoza S., Loarca Pina G. Peptides present in the non-digestible fraction of common beans (Phaseolus vulgaris L.) inhibit the angiotensin I-converting enzyme by interacting with its catalytic cavity independent of their antioxidant capacity. Food Funct. 2015;6:1470–1479. doi: 10.1039/c5fo00190k. [DOI] [PubMed] [Google Scholar]
- 57.Alemán A., Giménez B., Pérez-Santin E., Gómez-Guillén M.C., Montero P. Contribution of Leu and Hyp residues to antioxidant and ACE-inhibitory activities of peptide sequences isolated from squid gelatin hydrolysate. Food Chem. 2011;125:334–341. [Google Scholar]
- 58.Rodríguez Saint-Jean S., Isabel Pérez Prieto S., López -Expósito I., Ramos M., de las Heras A.I., Recio I. Antiviral activity of dairy proteins and hydrolysates on salmonid fish viruses. Int Dairy J. 2012;23:24–29. [Google Scholar]
- 59.Oberste M.S., Schnurr D., Maher K., Al-Busaidy S., Pallansch M.A. Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. J Virol. 1999;73(3):1941–1948. doi: 10.1128/jvi.73.3.1941-1948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li M., Yan K., Wei L., Yang J., Lu C., Xiong F., Zheng C., Xu W. Zinc finger antiviral protein inhibits coxsackievirus B3 virus replication and protects against viral myocarditis. Antiviral Res. 2015;123:50–61. doi: 10.1016/j.antiviral.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 61.Turner R.B. The treatment of rhinovirus infections: progress and potential. Antiviral Res. 2001;49:1–14. doi: 10.1016/S0166-3542(00)00135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakayama T., Urano T., Osano M., Hayashi Y., Sekine S., Ando T., Makinom S. Outbreak of herpangina associated with Coxsackievirus B3 infection. Pediatr Infect Dis J. 1989;8:495e8. doi: 10.1097/00006454-198908000-00004. [DOI] [PubMed] [Google Scholar]
- 63.Graci J.D., Cameron C.E. Mechanisms of action of ribavirin against distinct viruses. Rev Med Virol. 2006;16:37–48. doi: 10.1002/rmv.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi H.J., Lim C.H., Song J.H., Baek S.H., Kwon D.H. Antiviral activity of raoulic acid from Raoulia australis against Picornaviruses. Phytomedicine. 2009;16:35–39. doi: 10.1016/j.phymed.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 65.Choi H.J., Song J.H., Lim C.H., Baek S.H., Kwon D.H. Anti-human rhinovirus activity of raoulic acid from Raoulia australis. J Med Food. 2010;13:326–328. doi: 10.1089/jmf.2009.1149. [DOI] [PubMed] [Google Scholar]
- 66.Ammendolia M.G., Pietrantoni A., Tinari A., Valenti P., Superti F. Bovine lactoferrin inhibits echovirus endocytic pathway by interacting with viral structural polypeptides. Antiviral Res. 2007;73:151–160. doi: 10.1016/j.antiviral.2006.09.002. [DOI] [PubMed] [Google Scholar]