Abstract
Background:
Eosinophil-associated RNases (EARs) are stored preformed in eosinophil cytoplasmic secretory granules and have a key role in eosinophil effector functions in host defense and inflammatory disorders. However, the secretion mechanisms of EARs are poorly understood.
Objective:
Our study aimed to understand the involvement of cytoskeleton machinery in EAR secretion.
Methods:
Fresh human and mouse eosinophils were stimulated with CCL11 and the secretion of enzymatically active EARs was detected using an RNAse activity assay. The involvement of cytoskeletal elements or microtubules was probed using specific inhibitors.
Results:
We found that dynamic polymerization of microtubules and cytoskeletal elements, such as Rho and Rac, are required for chemokine-mediated EAR secretion from human and mouse eosinophils. However, inhibition of ROCK (Rho-associated protein kinase) increased EARs secretion in human and mouse eosinophils even in the absence of chemokine stimulation, suggesting ROCK negatively regulates EARs secretion.
Conclusions:
Collectively, these data suggest a cytoskeleton-dependent mechanism of EARs secretion from eosinophils, findings that are pertinent to host defense, allergy and other eosinophil-associated diseases.
Keywords: degranulation, ECP, EDN, microtubules, signaling
Introduction
Eosinophil-associated RNases (EARs) are stored in eosinophil cytoplasmic granules together with other highly basic proteins such as, major basic protein (MBP), eosinophil peroxidase (EPO), enzymes and cytokines (1). Human EARs (hEARs) include eosinophil-derived neurotoxin (EDN) and eosinophilic cationic protein (ECP). The mouse EARs (mEARs) consist of 13 RNases, at least six of which are localized within cytoplasmic granules (2).
The capacity to rapidly secrete preformed inflammatory mediators, such as EARs enables eosinophils to modulate the immune response or alternatively to eliminate pathogens (2). In pathologic situations, such as in allergic inflammation, these preformed inflammatory mediators, such as EARs, contribute to exacerbation of inflammation. Therefore, mechanisms of granule secretion (degranulation) are of primary interest.
Two main types of degranulation are described in vivo for human and mouse eosinophils: 1) piecemeal degranulation (PMD), whereby granule contents are selectively mobilized in small packets into granule-derived secretory vesicles that further carry granule contents to the cell surface for extracellular secretion, and 2) cell cytolysis whereby upon the plasma membrane ruptures and intact granules are released (3, 4). Recently we showed that extracellularly released, intact granules have the ability to secrete their contents in response to stimulation in a cell free context (3, 5).
CCL11 (eotaxin-1) and CCL24 (eotaxin-2) are major chemokines that recruit eosinophils to sites of inflammation, by binding to their G-protein coupled receptor, CCR3 (1). We have previously shown that CCL11 and CCL24 can stimulate PMD in vitro in human and mouse eosinophils (5, 6). CCL11-mediated PMD of human and mouse eosinophils share common signaling effectors, such as phosphatidylinositol-4,5-bisphosphate 3-Kinases (PI3K), extracellular signal-regulated kinases (ERK) and p38 MAPK (7). In addition, integrin-mediated cell spreading was found to be crucial for EAR secretion and degranulation (7). Since the cytoskeletal dynamics that lead to spreading seem to be required for preparing the cells for degranulation, we sought to investigate the role of actin and microtubule dynamics, as well as cytoskeletal elements, such as Rho, ROCK, and Rac, in EAR secretion from human and mouse eosinophils.
Important key players in cytoskeleton machinery are the members of the small G-protein Ras-superfamily: Rho, Rac and CDC42. RhoA is a crucial player in the regulation of the cytoskeleton as well as in cell division, survival, migration, and adhesion (8). Known downstream effectors of Rho A are the Rho-associated protein serine/threonine kinase (ROCK) family of proteins: ROCK-I (p160ROCK) and ROCK-II. ROCK is involved in cytoskeletal reorganization, stress fiber and focal adhesion formation (9), and required for eosinophil chemotaxis (10–12). However, roles for Rho or ROCK in secretion of EAR and other granule proteins from eosinophils have not been addressed. Uncovering a function for ROCK in PMD is essential, since the ROCK inhibitor Y27632, and other ROCK inhibitors, have been suggested as anti-asthmatic agents (13) due to their effects on smooth muscle cell-mediated broncho-constriction in murine models of acute allergic inflammation (14–16) and interference with leukocyte (including eosinophil) migration (16–18).
The small GTPase protein, Rac, is involved in the regulation of actin dynamics. The isoforms of Rac (1, 2, and 3) are highly homologous, but differ in their tissue expression and intracellular localization, as well as their involvement in cellular pathways such as F actin formation, actin reorganization, lamellipodia formation, adhesion, and chemotaxis. Rac1 is ubiquitously expressed, while Rac2 expression is specific to hematopoietic cells, and Rac 3 is highly expressed in the nervous system, but not exclusively. Previous studies have shown that the Rac1 and Rac2 isoforms have distinct roles in the regulation of neutrophil functions: chemotaxis regulation is mediated by Rac1, and actin polymerization is predominantly by Rac2 (19). In addition, Rac is essential for superoxide generation (19) and primary granule exocytosis in neutrophils (20). With regard to eosinophils, Rac was previously shown to be activated (Rac-GTP) in response to CCL11 and to induce actin polymerization in mouse eosinophils (21). Moreover, Rac2 was found to be involved in ionophore-mediated EPO secretion from mouse eosinophils (22). However, the involvement of Rac in EARs secretion induced by physiological stimulation, such as chemokines, in human or mouse eosinophils was not studied.
We have shown that CCL11 can induce secretion of enzymatically active EARs, as measured by a sensitive RNase activity assay, as well as other granule proteins from human and mouse eosinophils (5, 7). The RNase assay allows us to measure eosinophil degranulation with the same method that is suitable for both species. Although RNases can be found in other compartments of the cells, in addition to granules and secretory vesicles, our subcellular fractionation studies have shown that in response to CCL11, only granule fractions showed a decrease in RNase activity, while vesicle-enriched fractions increased their RNase activity, suggesting mobilization of granule EARs out of granules, into secretion-competent compartments (i.e. vesicles) in response to chemokine stimulation (5).
By measuring EAR secretion the current study revealed for the first time the requirement of Rac and Rho but not ROCK in human and mouse eosinophil degranulation.
Materials and methods:
Isolation of eosinophils
Isolation of mouse eosinophils:
Hypereosinophilic IL-5 transgenic BALB/c mice (Mus musculus), were used as a source of mature mouse eosinophils, due to the large numbers of eosinophil cells in their blood, spleen and bone marrow (23). IL-5 transgenic BALB/c mice were housed in a pathogen-free facility. Experimental protocols were approved by the Institutional Animal Care and Use Committee of the Beth Israel Deaconess Medical Center, Boston, MA (ethical approval #043–2012). Mouse eosinophils were isolated from mechanically disrupted spleens of IL-5 transgenic mice as previously described (24). Purity and viability of >98% were determined by microscopic analyses after Hema 3 staining and trypan blue exclusion, respectively.
Isolation of human eosinophils:
Eosinophils were purified from healthy donor blood by negative selection, as described (25). Written informed consent was obtained from donors in accordance with the Declaration of Helsinki, and Institutional Review Board (IRB) approval was obtained from the Beth Israel Deaconess Medical Center (ethical approval #82-03-12-0174/E-82-0001-FB). Purity and viability of >98% were determined by microscopic analyses after Hema 3 staining and trypan blue exclusion, respectively.
Stimulation and inhibition of eosinophils
Human and mouse eosinophils were resuspended in RPMI medium 1640 without phenol red (BioWhittaker, Walkersville, MD, USA), supplemented with 0.1% ovalbumin (OVAT, SigmaTT-Aldrich, TSt. Louis, MO, USA) at a final concentration of 5 ×10PPP5 cells/0.125 ml and were stimulated at 37°C for one h with the indicated concentrations of reccombinant human CCL11 (hCCL11, R&D Systems, Inc., Minneapolis, MN, USA) or the mouse CCL11 or CCL24 (mCCL11, mCCL24, Peprotech, Rocky Hill, NJ, USA), respectively. In some experiments, cells were pretreated for two hrs at 37°C with the Rho blocker, C3 transferase (5 and 10 μg/ml, Cytoskeleton Inc., Denver, CO, USA), or for 20 min at 37°C with the vesicle formation inhibitor, brefeldin A (10 μM, Enzo Life Sciences LTD., Exeter, UK), the ROCK inhibitor, Y27632 (30, 50 and 100 μM, Calbiochem-EMD), the Rac inhibitor, EHT 1684 (5 and 10 μM, Tocris Bioscience, Bristol, UK) and the microtubule polymerization blocker, nocodazole (10 and 100 μM, Calbiochem-EMD), before and during chemokine stimulation. As controls, cells were pretreated with equivalent concentrations of vehicle. Cell viability after pretreatment with inhibitors or vehicle and stimulation, detected by trypan blue exclusion or propidium iodide staining, was >93% unless other mentioned. Supernatants were obtained by centrifugation at 300 x g for 5 min at 4°C, followed by centrifugation at 20,000 x g for 10 min at 4°C.
RNase activity assay
In brief, we measured the presence of mEARs in 1:8000 diluted supernatants of stimulated eosinophils by an RNase activity assay using a fluorescent single-stranded RNA oligonucleotide (RNaseAlert QC system, Ambion, Austin, TX, USA), according to the manufacturer’s instructions. Cleavage of the probe by RNases allows fluorescent emission that is detected by fluorometry. The total cellular content of mEARs was assessed by measuring RNase activity in eosinophils lysed in 0.2% Triton X-100. Data were acquired after 50 min by the 7300 thermocycler (Applied Biosystems, Austin, TX, USA) and were within the linear range. All reactions were performed in triplicate wells. Relative fluorescence units (x10PP4 PPRFU) represent RNase activity levels from stimulated samples minus RNase activity levels from non-stimulated samples (as detailed in Shamri et al. (5) Fig S1 and Fig 1). Ten x 10PP4PP relative fluorescence units represent enzyme activity that is equivalent to the activity of 4.0 ± 1.8 nano U RNases (~0.0398 pg), as calibrated with bovine pancreatic RNase A (Ambion). One unit is the amount of RNase equivalent to 0.1177 Kunitz Units. We confirmed that inhibitors used in this study did not interfere with our RNase activity assay.
Figure 1.
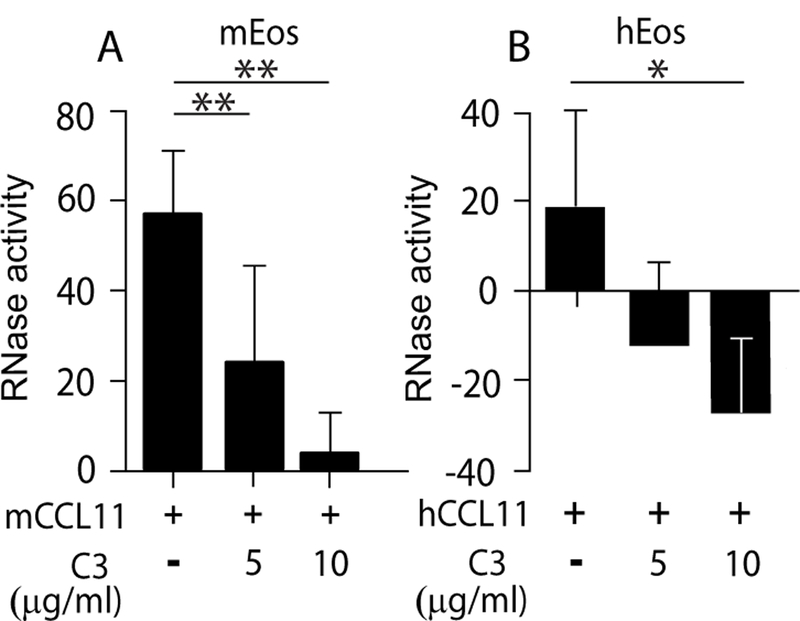
CCL11-mediated EAR secretion from eosinophils requires Rho activity. RNase activity was measured in supernatants of mCCL11-stimulated mouse (A) and hCCL11-stimulated human (B) eosinophils in the presence of Rho GTPase blocker, C3-transferase, as described in Material and Methods. Data are mean ± SD of five independent experiments (A) or mean ± SD from triplicate wells and are representative of four independent experiments (B). Asterisks represent P values < 0.05 (*), or < 0.01 (**).
Flow cytometry
Inhibited and stimulated eosinophils (as described above) were exposed to antibodies against murine CCR3, mouse CD11b (clone M1/70), human CD11b (clone ICRF44) or activated human CD11b (clone CBRM1/5) (BioLegend), or their isotype controls, as previously described in Shamri et al. (5). Expression levels were calculated as ΔMFI fold increase. TΔTMFI (mean fluorescence intensity) was calculated by subtracting the geometric mean fluorescence of stained samples from that of the non-stained (isotype control) samples. ΔMFI fold increases is the ratio of stimulated cell sample ΔMFI out of non-stimulated and non-inhibited sample ΔMFI.
Statistical analysis
Levels of significance between groups were analyzed by two-tailed paired Student’s t tests. P values < 0.05 were considered statistically significant.
Results:
Rho is required for CCR3-mediated EAR secretion from eosinophils
Recently we have shown that functional integrins are required for mouse eosinophil degranulation and EAR secretion (7), similar to previous findings in human eosinophils (21, 26, 27). This requirement for intact integrin signaling was accompanied by cell spreading of stimulated eosinophils. We found that this cell spreading was essential for degranulation from both human and mouse eosinophils (7).
Rho is an important key player in the regulation of the cytoskeleton and cell adhesion. Moreover, Rho is expressed in eosinophils [(10–12) and Fig. S1A] and is instrumental in CCL11-mediated eosinophil migration (12). Therefore, we evaluated the involvement of Rho in EAR secretion, a crucial effector function of eosinophils. Blocking Rho activity can be achieved by the commonly used inhibitor exoenzyme C3 transferase, derived from Clostridium botulinum. This inhibitor selectively inactivates the GTPases RhoA, RhoB, and RhoC proteins by ADP-ribosylation on asparagine 41 in the effector binding domain of the GTPase.
We used a cell permeable C3-transferase covalently linked to a proprietary cell penetrating moiety that is released upon cell penetration. Pretreatment of mouse eosinophils with C3-transferase robustly blocked mCCL11-stimulated EAR secretion (Fig. 1A). Similarly, EAR secretion from human eosinophils was also dependent upon Rho, as mEAR secretion was also blocked by C3-transferase (Fig. 1B). Human eosinophils were found to be more sensitive to C3-transferase since the highest concentration affected the basal levels of secretion in the absence of CCL11 as indicated by the negative RNase activity values of inhibited cells.
ROCK is not required for EAR secretion from mouse or human eosinophils
ROCK, a known downstream effector of Rho A, is involved in cytoskeletal reorganization, stress fiber and focal adhesion formation (9) and is thus required for CCL11-mediated eosinophil chemotaxis (10–12). However, its role in EAR secretion from eosinophils has not been addressed. Therefore, we examined the involvement of ROCK-I and -II in chemokine-stimulated EAR secretion by using the highly potent and selective cell permeable inhibitor, Y27632, that blocks ROCK-I and -II with similar potencies, by binding to their catalytic sites (28). Fifty μM of Y27632 abolished mouse eosinophil chemotaxis toward CCL11 (Fig. S2A) and significantly reduced ROCK kinase activity (Fig S2B) in the presence and absence of chemokine stimulation, as measured by the ability of Y27632 to inhibit the phosphorylation of the ROCK substrate, the myosin-binding subunit of myosin phosphate. Increasing the concentration to 100 μM or prolonging the preinhibition time (up to 2 hours) did not further increase inhibition of migration (Fig. S2A and unpublished data). In contrast to chemotaxis, mCCL11- and mCCL24-mediated EAR secretion was not blocked and even increased by at least 1.7 fold with 30 or 50 μM concentrations of Y27632 (Fig. 2A and B), as measured by RNase activity in supernatants. This increase in mEAR secretion was also observed with a higher concentration of Y27632 (100 μM) or longer preinhibition (2 hr, unpublished data). In addition, the inhibitor Y27632 also increased the spontaneous secretion of mEARs (Fig. 2C). Therefore, ROCK inhibitor Y27632 increased both chemokine-mediated and spontaneous secretion of mEARs. Hence, our results suggest that ROCK is playing a negative regulatory role in EAR secretion from mouse eosinophils.
Figure 2.
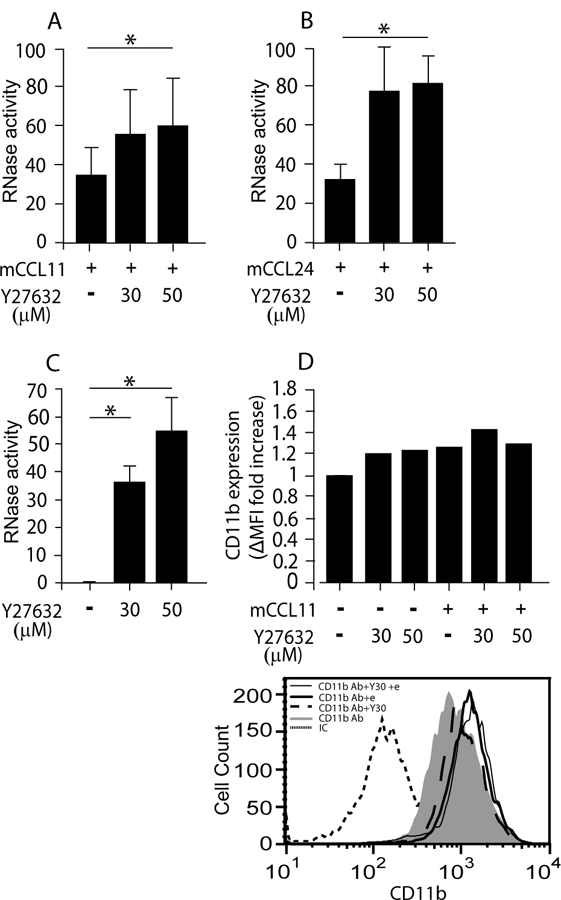
EAR secretion from mouse eosinophils is increased in the presence of ROCK inhibitor. (A-C) RNase activity was measured in supernatants of non stimulated (C) or mCCL11- (A), or mCCL24-(B) stimulated mouse eosinophils, in the presence of the ROCK inhibitor, Y27632, as described in Material and Methods. Data are mean ± SD from five (A), and three (B, C) independent experiments. (D) Expression of CD11b (αM) integrin on mouse eosinophils in response to 30 μM Y27632 (Y30) in the presence or absence of CCL11 (e). The expression was measured by flow cytometry, using specific antibodies (Ab) or their isotype control (IC) and calculated as detailed in supplemental Materials and Methods. Data (D) are mean of two independent experiments. The lower panel is a representative experiment of D upper panel. Asterisks represent P values < 0.05 (*), or < 0.01 (**).
mEAR secretion enhancement by ROCK inhibition likely does not stem from an increase in CCR3 expression (Fig. S2C), due to blocking receptor recycling, or due to an effect on actin polymerization (Fig. S2D), suggesting a different mechanism by which ROCK inhibitor affects mEAR secretion.
Similar to mouse eosinophils, 30 μM Y27632, an effective concentration that blocked hCCL11-mediated chemotaxis of human eosinophils (Fig. S3A), as well as ROCK activity (Fig. S3B), enhanced hEAR secretion from human eosinophils in the presence or absence of CCL11 (Fig. 3A). Blockade of ROCK with 30 and 50 μM Y27632 hardly affected actin polymerization in human eosinophils (an increase of 6% and 16% , respectively, over non-stimulated cells) (Fig. S3C).
Figure 3.
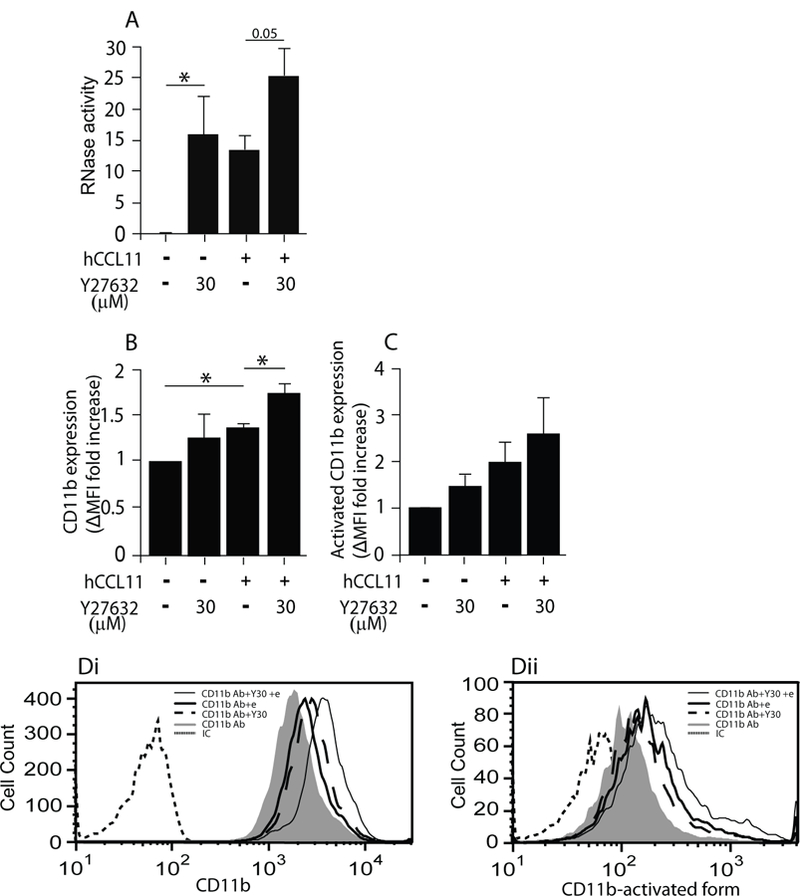
ROCK involvement in EAR secretion from human eosinophils. (A) RNase activity was measured in supernatants of hCCL11-stimulated or non-stimulated human eosinophils in the presence of the ROCK blocker, Y27632, as described in Material and Methods. Data are mean ± SEM of six independent experiments. (B-D) Expression of CD11b (αM) integrin and its activated conformation on human eosinophils in response to 30 μM Y27632 (Y30) in the presence or absence of CCL11 (e). The expression was measured by flow cytometry, using specific antibodies (Ab) or their isotype control (IC) and calculated as detailed in supplemental Materials and Methods. Data (B,C) are mean ± SD of three independent experiments. Di is a representative of B, and Dii of C. Asterisks represent P values < 0.05 (*), or < 0.01 (**).
ROCK was previously shown to be involved in de-adhesion and tail contraction of granulocytes to enable leukocyte migration, by increasing integrin avidity and cell spreading (29). Interestingly, we have previously shown (7) that β2 integrin-mediated spreading is essential for CCL11-mediated EAR secretion from mouse eosinophils. This spreading requirement was also observed in human eosinophils. Therefore, we quantified the surface expression of CD11b (αΜ chain of αΜβ2 integrin) in the presence of Y27632 to determine if ROCK inhibition enhances EAR secretion via an effect on integrin expression. Indeed, Y27632 pretreatment of human eosinophils increased the surface expression of CD11b (Fig. 3B and Di), as well as its active conformation (Fig. 3C and Dii) in the presence and absence of CCL11. Similar results with CD11b surface expression were also found with mouse eosinophils (Fig. 2D). These results suggest that ROCK inhibition affects EAR secretion by its effect on CD11b expression and activation.
In summary, our results suggest that although Rho is required for CCL11-mediated EAR secretion in both human and mouse, Rho signaling pathways do not require ROCK activity for secretion, and might employ different downstream effectors, other than ROCK. Moreover, blocking ROCK enhanced spontaneous and CCL11-mediated EAR secretion and αM integrin expression and activation, therefore, we suggest that ROCK negatively regulate EAR secretion by its effect on integrins.
Rac is required for CCR3-mediated EAR secretion
Rac GTPase is expressed in human and mouse eosinophils [(21) and Fig. S4] and was previously shown to be induced by CCL11 (21). To assess Rac involvement in CCL11-stimulated EAR secretion from human and mouse eosinophils, we used the Rac inhibitor EHT 1864, which selectively binds Rac isoforms 1–3 and promotes the loss of bound guanine nucleotide and guanine nucleotide exchange factor (GEF) association (30). Pretreatment of mouse eosinophils with EHT 1864 robustly blocked CCL11-stimulated EAR secretion in a dose dependent manner (Fig. 4A) and affected the basal levels of secretion in the absence of CCL11 as indicated by the negative RNase activity values of inhibited cells. Similarly, EAR secretion from human eosinophils was also dependent upon Rac, as EAR secretion was blocked by EHT-1864 (Fig. 4B). In summary, our results demonstrate a requirement for Rac in EAR secretion from human and mouse eosinophils.
Figure 4.
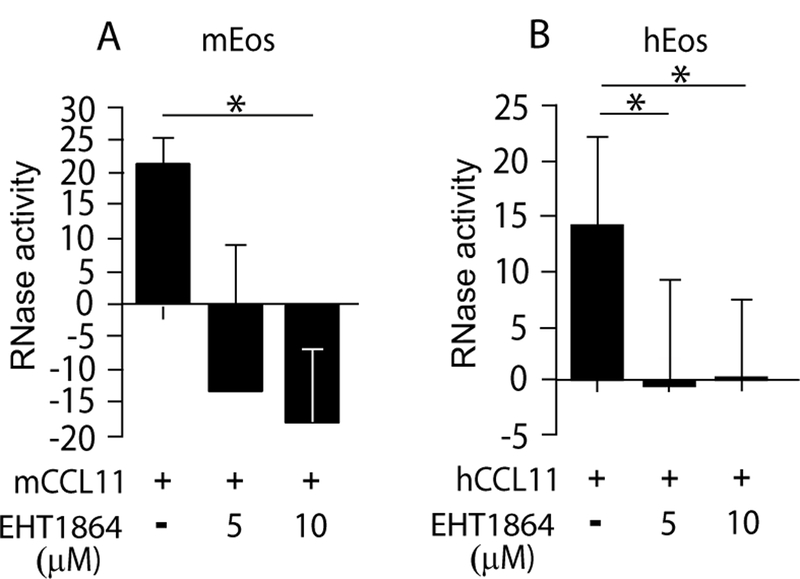
Rac is required for CCL11-mediated EAR secretion from eosinophils. (A-B) RNase activity was measured in supernatants of mCCL11-stimulated mouse (A) or hCCL11-stimulated human (B) eosinophils in the presence of the Rac blocker, EHT1864, as described in Material and Methods. Data are mean ± SD of three (A) and four (B) independent experiments, respectively. Asterisks represent P values < 0.05 (*), or < 0.01 (**).
Microtubule polymerization is required for CCR3-mediated EAR secretion
Microtubules are known to be involved in exocytotic and endocytotic processes, enabling endosome and vesicle migration toward the plasma membrane. CCL11-mediated secretion of EARs from human (6, 31) and mouse (5) eosinophils is by means of PMD, which utilizes secretory vesicles to transfer material from granules to the extracellular space. Therefore, we hypothesized that EAR secretion would be affected by microtubule depolymerization. To delineate the role of microtubules in the secretion of EARs, we used a cell permeable, specific inhibitor of microtubule polymerization, nocodazole, that binds to β-tubulin and thus blocks microtubule assembly. Pretreatment of mouse eosinophils with nocodazole robustly blocked mCCL11-stimulated EAR secretion in a dose-dependent manner (Fig. 5A). Similarly, EAR secretion from mouse eosinophils induced by mCCL24, another ligand of the CCR3 receptor, was also significantly blocked by nocodazole in a dose-dependent manner (Fig. 5B). Similarly, hCCL11-stimulated EAR secretion from human eosinophils was robustly blocked by nocodazole in a dose-dependent manner (Fig. 5C). Measuring secretion of one specific human EAR, ECP, by Elisa revealed a similar dependence on dynamic microtubules (not shown). Nocodazole pretreatment affected the basal levels of secretion in the absence of chemokine stimulation, as indicated by the negative values of inhibited human and mouse eosinophils. In addition, mCCL11 and mCCL24-mediated mEAR secretion was blocked by the vesicle formation blocker, brefeldin A (BFA) (Fig. 5D), further confirming the vesicle-mediated secretion route (i.e. PMD). These results are in agreement with our previous results showing hCCL11-mediated secretion from human eosinophils is attenuated by brefeldin A (6, 31).
Figure 5.

CCL11-mediated EAR secretion from eosinophils is nocodazole and brefeldin A- dependent (A, B) RNase activity was measured in supernatants of mCCL11- (A) or ,CCL24- (B) stimulated mouse eosinophils in the presence of the microtubule polymerization blocker, nocodazole, as described in Material and Methods. Data are mean ± SD of four independent experiments (C) RNase activity was measured in supernatants of hCCL11-stimulated human eosinophils in the presence of the microtubule polymerization blocker, nocodazole. Data are mean ± SD of three independent experiments. (D) RNase activity was measured in supernatants of mCCL11- or ,CCL24- stimulated mouse eosinophils in the presence of the microtubule polymerization blocker, brefeldin A (BFA), as described in Material and Methods. Data are mean of two independent experiments. Higher RNase activity values were obtained in D, due to higher numbers of cells (1× 106 cells per sample) used for the experiments. Asterisks represent P values < 0.05 (*), or < 0.01 (**).
Discussion:
Eosinophils and their cytoplasmic granule contents, including EARs, have key roles in host defense as well as in inflammatory disorders. Secretion from granules (degranulation) is a multifaceted process that requires signal transduction and cytoskeletal and vesiculotubular transport machinery. Our previous (7) and current observations show that in response to stimulation with a chemokine, such as CCL11, shape change, spreading and degranulation occur in human and mouse eosinophils. Cell spreading is essential for chemokine-mediated PMD and EAR secretion and requires actin reassembly.
Our observations reveal a common requirement of the cytoskeletal elements Rho and Rac for EAR secretion from human and mouse eosinophils, as EAR secretion was completely abolished by Rho and Rac inhibitors in both species. Interestingly, a previous study in Rac2- deficient mouse eosinophils (22) showed only a partial reduction (~30%) in ionophore-stimulated EPO secretion compared to wild type eosinophils. The different outcomes between the studies might reflect redundancy in Rac1 and 2 functions in degranulation, or might be explained by the use of different stimulators (ionophore in the Lacy et al. study, vs. physiological CCL11 used here), or the methods used to measure eosinophil granule-derived mediators (EPO activity in Lacy et al., vs. EARs activity in the current study).
We assume that the requirements of Rho and Rac for EAR secretion are due to their effect on actin reassembly, since a previous study has shown that CCL11 signaling activates Rac and that CCL11-mediated actin polymerization in mouse eosinophils requires Rac2 (21). Rho and Rac can be activated by heterotrimeric G proteins through their specific GEFs (32), such as Tiam or VAV, respectively (33). PI3K or c-Src might also be candidates for the link between Gαi and Rac and Rho (33) (Fig. 6).
Figure 6:
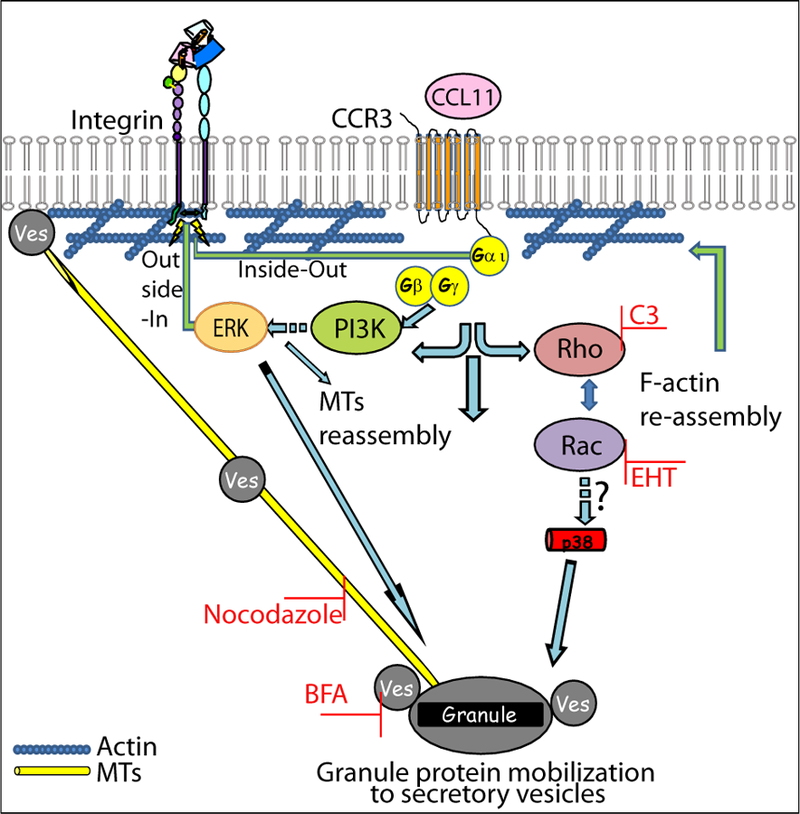
Schematic model for mechanisms leading to CCR3-induced EARs secretion: Upon stimulation, i.e. CCL11 binding to its receptor, CCR3, G-protein α subunit is activated and released from the Gβγ units. The alpha subunit or the βγ units (through PI3K) can activate small-GTPase GEFs leading to activation of Rho and Rac GTPases. Rho and Rac induce actin reassembly. This reassembly probably includes growing filaments to assist vesicles (Ves) pinching off from granules, and/or might act as tracks, in concert with myosin motors and microtubules (MTs), to drive the vesicles towards the plasma membrane (36), as well as spacing actin filaments in the cortex to allow the access of secretory vesicles to the plasma membrane. In addition, as found previously (7) CCR3 signaling leads to upregulation of β2 integrins and cell spreading, all required for EAR secretion. Integrin outside-in signaling is also likely to be involved, since β2 integrins were required for CCL11-mediated p38 and MAP kinase phosphorylation. Both ERK and p38, which are activated by CCL11 or by integrins and are associated with actin filaments and microtubules, can affect cytoskeletal elements (37–39).
Although ROCK was found to be activated by CCL11 in human eosinophils (10) and to be an essential downstream effector in chemotaxis toward CCL11 in human (10) and mouse eosinophils (our results), Rho-mediated EAR secretion is ROCK independent in both human and mouse eosinophils. Moreover, inhibition of ROCK enhanced EAR secretion, even in the absence of stimulation. Similarly, in rat mast cells Rho, but not ROCK, was found to be involved in secretion from granules (34). To determine the mechanism of ROCK inhibitor-mediated enhancement of EAR secretion, we investigated the effect of this inhibitor on cellular processes with potentials to influence secretion, such as endocytosis, pinocytosis, chemokine receptor (CCR3) expression, and actin polymerization (current study and unpublished data). However, we found no impact of ROCK inhibition on any of the above processes in human or mouse eosinophils.
Interestingly, ROCK inhibition increased the expression of CD11b, the αΜ chain of the β2 integrin (CD11b/CD18), on both human and mouse eosinophils, as well as its activated conformation as found in human eosinophils in the presence or absence of CCL11. It is likely that the active conformation of mouse CD11b is also increased in the presence of ROCK inhibitor. However we could not confirm this due to lack of specific antibodies against the active conformation of the murine integrin. Since β2 integrin expression and activation are increased upon CCL11 stimulation and mediate spreading required for EAR secretion, these results might suggest a mechanism by which ROCK inhibitor can bypass CCL11-mediated integrin activation, and increase integrin expression and activation and as a consequence EAR secretion. Noteworthy, in addition to mediating spreading, integrins can affect secretion through their outside-in signaling, and therefore ROCK inhibitor might induce integrin signaling through integrin activation. Therefore, we suggest that ROCK negatively regulates EAR secretion via its effect on integrins. Further studies are necessary to verify this hypothesis. Since ROCK was not required for EAR secretion from either mice or human eosinophils, Rho might affect actin reassembly through different effectors (33).
PMD, in which secretory vesicles transfer material from granules to the extracellular space, is the mechanism of CCL11-mediated EAR secretion from mouse (5) and human (6, 31) eosinophils. In this study we have shown that CCL11-mediated EAR secretion from human and mouse eosinophils requires microtubule dynamics, likely due to microtubule involvement in vesicle movement toward the cytoplasmic membrane. Recently Han et al. (35) described a nucleopode structure in which the nucleus is located at the rear of IL-5-treated eosinophils with a microtubule cage-like structure around the nucleus. This nucleopode is enriched with surface expression of CD44 and activated β2 integrins. The microtuble cage structure is disrupted upon treatment with nocodazole. These structural studies might suggest that apart of directing granules to the membranes, microtuble filaments are required also for stabilizing specific cell morphology required for degranulation.
In summary, our results reveal requirements of Rho and Rac in human and mouse eosinophil degranulation. Interestingly, we have found inhibition of ROCK enhanced secretion, even in the absence of chemokine stimulation in human and mouse eosinophils, and we suggest a negative regulatory role for this effector, perhaps through its effect on integrins. Our current results complement our previous results and add more components to the signal transduction pathways (Fig. 6) that lead to EAR secretion and degranulation from eosinophils, that are pertinent to our understanding of eosinophil-mediated host responses.
Supplementary Material
Acknowledgments
We thank Dr. Lisa A. Spencer for editorial assistance.
This study was funded by grants from NIH: R37 AI020241, R01 AI051645 (P.F.W) and EU FP7-MC-CIG-630897 (RS)
Abbreviations:
- BFA
brefeldin A
- EAR
eosinophil-associate RNase
- hEAR
human EAR
- mEAR
mouse EAR
- ECP
eosinophil cationic protein
- EDN
eosinophil derived neurotoxin
- EPO
eosinophil peroxidase
- ERK
extracellular signal-regulated kinase
- GEF
guanine nucleotide exchange factor
- MBP
major basic protein
- MFI
mean fluorescence intensity
- OVA
Ovalbumin
- PI3K
phosphatidylinositol-4,5-bisphosphate 3-Kinases
- PMD
piecemeal degranulation
- ROCK
Rho-associated protein serine/threonine kinase
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References:
- 1.Shamri R, Xenakis JJ, Spencer LA. Eosinophils in innate immunity: an evolving story. Cell. Tissue Res 2011;343(1):57–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JJ, Lee NA. Eosinophil degranulation: an evolutionary vestige or a universally destructive effector function? Clin. Exp. Allergy 2005;35(8):986–94. [DOI] [PubMed] [Google Scholar]
- 3.Neves JS, Perez SA, Spencer LA, Melo RC, Reynolds L, Ghiran I, et al. Eosinophil granules function extracellularly as receptor-mediated secretory organelles. Proc. Natl. Acad. Sci. U S A 2008;105(47):18478–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ueki S, Melo RC, Ghiran I, Spencer LA, Dvorak AM, Weller PF. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood 2013;121(11):2074–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shamri R, Melo RC, Young KM, Bivas-Benita M, Xenakis JJ, Spencer LA, et al. CCL11 elicits secretion of RNases from mouse eosinophils and their cell-free granules. Faseb J 2012;26(5):2084–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bandeira-Melo C, Sugiyama K, Woods LJ, Weller PF. Cutting edge: eotaxin elicits rapid vesicular transport-mediated release of preformed IL-4 from human eosinophils. J Immunol 2001;166(8):4813–7. [DOI] [PubMed] [Google Scholar]
- 7.Shamri R, Young KM, Weller PF. PI3K, ERK, p38 MAPK and integrins regulate CCR3-mediated secretion of mouse and human eosinophil-associated RNases. Allergy 2013;68(7):880–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tybulewicz VL, Henderson RB. Rho family GTPases and their regulators in lymphocytes. Nat Rev Immunol 2009;9(9):630–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakagawa O, Fujisawa K, Ishizaki T, Saito Y, Nakao K, Narumiya S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett 1996;392(2):189–93. [DOI] [PubMed] [Google Scholar]
- 10.Adachi T, Vita R, Sannohe S, Stafford S, Alam R, Kayaba H, et al. The functional role of rho and rho-associated coiled-coil forming protein kinase in eotaxin signaling of eosinophils. J Immunol 2001;167(8):4609–15. [DOI] [PubMed] [Google Scholar]
- 11.Alblas J, Ulfman L, Hordijk P, Koenderman L. Activation of Rhoa and ROCK are essential for detachment of migrating leukocytes. Mol Biol Cell 2001;12(7):2137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muessel MJ, Scott KS, Friedl P, Bradding P, Wardlaw AJ. CCL11 and GM-CSF differentially use the Rho GTPase pathway to regulate motility of human eosinophils in a three-dimensional microenvironment. J Immunol 2008;180(12):8354–60. [DOI] [PubMed] [Google Scholar]
- 13.Kume H RhoA/Rho-kinase as a therapeutic target in asthma. Curr Med Chem 2008;15(27):2876–85. [DOI] [PubMed] [Google Scholar]
- 14.Iizuka K, Shimizu Y, Tsukagoshi H, Yoshii A, Harada T, Dobashi K, et al. Evaluation of Y-27632, a rho-kinase inhibitor, as a bronchodilator in guinea pigs. Eur J Pharmacol 2000;406(2):273–9. [DOI] [PubMed] [Google Scholar]
- 15.Henry PJ, Mann TS, Goldie RG. A rho kinase inhibitor, Y-27632 inhibits pulmonary eosinophilia, bronchoconstriction and airways hyperresponsiveness in allergic mice. Pulm Pharmacol Ther 2005;18(1):67–74. [DOI] [PubMed] [Google Scholar]
- 16.Taki F, Kume H, Kobayashi T, Ohta H, Aratake H, Shimokata K. Effects of Rho-kinase inactivation on eosinophilia and hyper-reactivity in murine airways by allergen challenges. Clin Exp Allergy 2007;37(4):599–607. [DOI] [PubMed] [Google Scholar]
- 17.Schaafsma D, Bos IS, Zuidhof AB, Zaagsma J, Meurs H. The inhaled Rho kinase inhibitor Y-27632 protects against allergen-induced acute bronchoconstriction, airway hyperresponsiveness, and inflammation. Am J Physiol Lung Cell Mol Physiol 2008;295(1):L214–9. [DOI] [PubMed] [Google Scholar]
- 18.Schaafsma D, Gosens R, Zaagsma J, Halayko AJ, Meurs H. Rho kinase inhibitors: a novel therapeutical intervention in asthma? Eur J Pharmacol 2008;585(2–3):398–406. [DOI] [PubMed] [Google Scholar]
- 19.Bokoch GM. Regulation of innate immunity by Rho GTPases. Trends Cell Biol 2005;15(3):163–71. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell T, Lo A, Logan MR, Lacy P, Eitzen G. Primary granule exocytosis in human neutrophils is regulated by Rac-dependent actin remodeling. Am J Physiol Cell Physiol 2008;295(5):C1354–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fulkerson PC, Zhu H, Williams DA, Zimmermann N, Rothenberg ME. CXCL9 inhibits eosinophil responses by a CCR3- and Rac2-dependent mechanism. Blood 2005;106(2):436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lacy P, Willetts L, Kim JD, Lo AN, Lam B, Maclean EI, et al. Agonist activation of f-actin-mediated eosinophil shape change and mediator release is dependent on Rac2. Int Arch Allergy Immunol 2011;156(2):137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dent LA, Strath M, Mellor AL, Sanderson CJ. Eosinophilia in transgenic mice expressing interleukin 5. J. Exp. Med 1990;172(5):1425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang HB, Ghiran I, Matthaei K, Weller PF. Airway eosinophils: allergic inflammation recruited professional antigen-presenting cells. J. Immunol 2007;179(11):7585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akuthota P, Shamri R, Weller PF. Isolation of human eosinophils. Curr. Protoc. Immunol 2012;98:Unit7.31.1–7.31.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horie S, Kita H. CD11b/CD18 (Mac-1) is required for degranulation of human eosinophils induced by human recombinant granulocyte-macrophage colony-stimulating factor and platelet-activating factor. J. Immunol 1994;152(11):5457–67. [PubMed] [Google Scholar]
- 27.Horie S, Okubo Y, Hossain M, Momose T, Suzuki J, Isobe M, et al. Intercellular adhesion molecule-1 on eosinophils is involved in eosinophil protein X release induced by cytokines. Immunology 1997;90(2):301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishizaki T, Uehata M, Tamechika I, Keel J, Nonomura K, Maekawa M, et al. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol Pharmacol 2000;57(5):976–83. [PubMed] [Google Scholar]
- 29.Liu L, Schwartz BR, Lin N, Winn RK, Harlan JM. Requirement for RhoA kinase activation in leukocyte de-adhesion. J Immunol 2002;169(5):2330–6. [DOI] [PubMed] [Google Scholar]
- 30.Shutes A, Onesto C, Picard V, Leblond B, Schweighoffer F, Der CJ. Specificity and mechanism of action of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases. J Biol Chem 2007;282(49):35666–78. [DOI] [PubMed] [Google Scholar]
- 31.Melo RC, Perez SA, Spencer LA, Dvorak AM, Weller PF. Intragranular vesiculotubular compartments are involved in piecemeal degranulation by activated human eosinophils. Traffic 2005;6(10):866–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhattacharya M, Babwah AV, Ferguson SS. Small GTP-binding protein-coupled receptors. Biochem Soc Trans 2004;32(Pt 6): 1040–4. [DOI] [PubMed] [Google Scholar]
- 33.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol 2008;9(9):690–701. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan R, Price LS, Koffer A. Rho controls cortical F-actin disassembly in addition to, but independently of, secretion in mast cells. J Biol Chem 1999;274(53):38140–6. [DOI] [PubMed] [Google Scholar]
- 35.Han ST, Mosher DF. IL-5 induces suspended eosinophils to undergo unique global reorganization associated with priming. Am J Respir Cell Mol Biol 2014;50(3):654–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol 2006;16(10):522–9. [DOI] [PubMed] [Google Scholar]
- 37.Boehme SA, Sullivan SK, Crowe PD, Santos M, Conlon PJ, Sriramarao P, et al. Activation of mitogen-activated protein kinase regulates eotaxin-induced eosinophil migration. J. Immunol 1999;163(3):1611–8. [PubMed] [Google Scholar]
- 38.Reszka AA, Bulinski JC, Krebs EG, Fischer EH. Mitogen-activated protein kinase/extracellular signal-regulated kinase 2 regulates cytoskeletal organization and chemotaxis via catalytic and microtubule-specific interactions. Mol Biol Cell 1997;8(7):1219–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu Q, Paredes M, Zhang J, Kosik KS. Basal extracellular signal-regulated kinase activity modulates cell-cell and cell-matrix interactions. Mol Cell Biol 1998;18(6):3257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


