Abstract
Background:
Pre-pregnancy cardiometabolic risk factors are associated with increased risks of adverse pregnancy outcomes. Neighborhood features may reflect pre-pregnancy exposures that contribute to poor cardiometabolic health before pregnancy and may contribute to racial disparities in pregnancy outcomes.
Methods:
Early pregnancy measurements from 1,504 women enrolled in the Prenatal Exposures and Preeclampsia Prevention study were linked to a 2000 Census-based measure of neighborhood socioeconomic status and commercial data (food, alcohol, and retail density) during 1997–2001. Multilevel random-intercept linear regression was used to separately estimate the association between levels of neighborhood assets (low, mid-low, mid-high, high) and C-reactive protein (CRP), systolic blood pressure (SBP), and body mass index (BMI) in cross-sectional analyses. Low neighborhood assets have high-poverty/low-retail, whereas, high neighborhood assets have low-poverty/high-retail. Models were adjusted for individual-level factors (age and race), and we assessed effect modification by race.
Results:
Low compared with high neighborhood assets were associated with higher BMI (β 1.95 kg/m2, 95% CI 0.89, 3.00), after adjusting for individual-level covariates. After adjusting for BMI and other covariates, low compared with high assets were associated with higher CRP concentrations (β 0.20 ng/mL, 95% CI 0.01, 0.39). Neighborhood assets were not associated with SBP. Race did not modify the association between neighborhood assets and cardiometabolic risk factors.
Conclusions:
Early pregnancy adiposity is related to neighborhood features independent of individual factors. Further, inflammation beyond accounting for adiposity is related to neighborhood features. Strategies that address neighborhood assets during pre-conception and interconception may be promising approaches to improve pre-pregnancy health.
Keywords: neighborhood, early pregnancy, cardiometabolic health
INTRODUCTION
Pregnancy can be regarded as a cardiometabolic stress test,1 with failure of maternal adaptation and pre-existing risk factors converging to contribute to adverse pregnancy outcomes.2, 3 Furthermore, adverse pregnancy outcomes may also unveil susceptibility to future cardiovascular disease in women.1, 4, 5 Recent meta-analyses have reported nearly a 2-fold increased risk of cardiovascular disease in women with a history of preterm birth (delivery <37 weeks gestation)6 and nearly a 2-to 4-fold increased risk of future heart failure, coronary heart disease, cardiovascular disease death, and stroke in women with a history of preeclampsia.7 Although the exact mechanisms that contribute to adverse pregnancy outcomes such as preterm birth and preeclampsia are poorly understood, pre-conception health is thought to be important.8–10 Few longitudinal studies have individual-level cardiometabolic risk factors measured during pre-conception.4, 11–13
Adverse cardiometabolic risk profiles including elevated blood pressure, high body mass index, and inflammation are not randomly distributed in society and tend to cluster in low-income and minority neighborhoods.14–16 Further, these same neighborhoods also have a disproportionate burden of adverse pregnancy outcomes and cardiovascular disease. For example, the percentage of infants born preterm is nearly 50% higher in black as compared with white mothers (13.4% vs. 8.9%).17 Black women ≥20 years old also have higher prevalence of cardiovascular disease as compared with white women (47.7% vs 35.1%).18 Yet, the extent to which neighborhood features may have an independent association with adverse cardiometabolic risk profiles beyond individual-level risk factors have been relatively understudied in pregnant women,19–21 with almost no studies incorporating commercial data.21 Neighborhood-level data that describe the density of retail businesses, food outlets, and alcohol sales have been largely absent from the body of literature examining the role of neighborhood environments on maternal health. The inclusion of commercial data is important because inequities in the distribution of retail, food, and alcohol outlets may contribute to poor diet quality or inadequate gestational weight gain or loss. Further, these data can provide insight in understanding racial and socioeconomic disparities in the overall burden of cardiometabolic risk.
Measurements from the Prenatal Exposures and Preeclampsia Prevention study were linked to Census-based measure of neighborhood socioeconomic status and commercial data (food, alcohol, and retail density) to explore the role of neighborhood assets on cardiometabolic risk factors in pregnant women during 1997–2001. The hypothesis of the study was that lower levels of neighborhood assets (high-poverty/low-retail) would be separately associated with higher systolic blood pressure (SBP), body mass index (BMI), and C-reactive protein (CRP) concentrations. Effect modification by maternal race was also assessed, given the excess burden of adverse pregnancy outcomes and cardiovascular disease in black women.
METHODS
Study Population
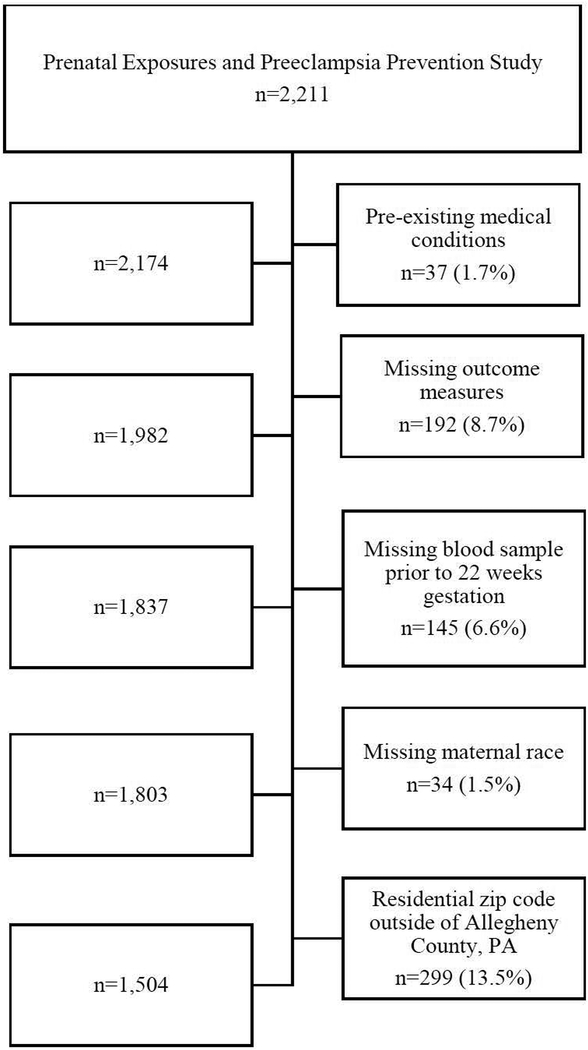
The study population was selected from the Prenatal Exposures and Preeclampsia Prevention study, which enrolled 2,211 women from clinics and private practices during 1997 to 2001 in western Pennsylvania. Briefly, this prospective study recruited healthy pregnant women (<16 weeks gestation) ages 14–44 years and followed them to delivery. Participant demographic characteristics, individual-level socioeconomic factors, cigarette smoking, alcohol consumption, medical history, and other lifestyle factors were collected via structured questionnaire. For our secondary cross-sectional analysis, we excluded women with pre-existing medical conditions such as chronic hypertension, chronic diabetes, and HIV, to avoid large variations in CRP concentrations (n=37).22 Since we were interested in early pregnancy cardiometabolic risk factors, we also excluded women who were missing these measures (n=192) or without a blood sample taken prior to 22 weeks of gestation (n=145). Given that we were interested in exploring effect measure modification by race, we excluded those with missing maternal race (n=34). Additionally, we excluded women who had a maternal residential zip code outside of Allegheny County, PA since our indices for the commercial data were restricted to this geographic area (n=299). There were 1,504 women included in this cross-sectional analysis (Figure 1). This study was approved by the Institutional Review Board at the University of Pittsburgh, and written and informed consent was obtained from all participants.
Figure 1.
Flowchart of Exclusions for Analysis of Level of Neighborhood Assets and Early Pregnancy Cardiometabolic Risk Factors, Allegheny County, 1997–2001
Early pregnancy measures
SBP measurements during each prenatal visit before 20 weeks gestation (mean= 13.8 weeks, SD=1.8 weeks; range 6 to 20) were abstracted from the prenatal record and averaged for each woman (mean=112.5 mmHg, SD=8.3, range =70–155). Maternal BMI, a measure of adiposity (weight in kg/height m2), was determined using weight and height measured at the enrollment visit (mean=9.5 weeks, SD=3.6 weeks; range 3.5–21.9 weeks) or at time of blood draw (mean=10.1 weeks, SD=3.8 weeks; range 3.4–21.9 weeks). High sensitivity CRP concentrations were measured in non-fasting blood samples collected before 22 weeks gestation (mean=10.1 weeks, SD=3.8; range 3.8–21.9) and frozen at −80°C as previously described using enzyme-linked immunosorbent assay (ELISA) on the SpectraMax Me analyzer (Molecular Devices, United States).23 The limit of detection of the assay was 0.03 ng/mL, with an intra-assay coefficient of variability of 7%. CRP is an acute and chronic marker of inflammation and has been used to predict incident cardiovascular disease events in women such as peripheral arterial disease, stroke, and sudden cardiac death.24 Moreover, CRP has also been associated with an increased risk of preterm birth and preeclampsia.23, 25 All outcomes were measured at Magee-Womens Hospital by trained research staff.
Neighborhood food and alcohol density
We used commercial data from InfoUSA and Dun&Bradstreet (D&B) for the years 2009 and 2003 for Allegheny County, PA. InfoUSA and D&B are sales and marketing companies that provide data of businesses and contain information about the name, address, and type of establishment. Types of establishments and associated classifications are based on SIC (Standard Industrial Classification) and NAICS (North American Industry Classification) taxonomy. Density measures were calculated by first categorizing various establishments according to SIC codes.26 We created the density measures based on primary SIC code and any SIC code (e.g., primary, secondary, or tertiary) and summed the number of establishments within each census tract by category and calculated the final density measures based on per capita (per 10,000 residents) and per area (square mile) from population and area data generated from the 2000 US Census.
Neighborhood-level socioeconomic status
Each mother’s residential address at time of delivery was geocoded using ArcGIS software, version 10.6 and assigned a corresponding census tract. Census tracts have been used to construct area-level socioeconomic indices and neighborhood characteristics within the context of perinatal epidemiology and have been used to approximate neighborhood in previous studies.27, 28 Census tract variables were selected based on the approach of Messer et al.29 and included the following variables: median household income, percent of black/African American residents, percent below poverty level, percent of households with public assistance, percent of male population, percent of unemployed males, percent <25 years old with at least a HS diploma, and percent of female headed households.
Neighborhood assessment and asset index
There was a total of 416 census tracts with a mean of 5 (range:1–22) women per tract and 277 tracts with ≤5 women. All continuous variables were standardized by converting them to z-scores, with a mean of zero and standard deviation of one prior to conducting principal components analysis. Standardization was important so that variables measured in different units and at disproportionate ranges could contribute equally to the composite index. Principal components analysis of 8 census-tract and 8 commercial variables were used to construct a neighborhood asset index (Table 1). Scree plots were used to help ascertain the number of components to extract. Four components were extracted and accounted for 78.6% of the total variance of the data (Table 1). Variable loadings were generally higher for the demographic component with an index Cronbach’s alpha of 0.73 as compared with food and alcohol density (α= 0.53), service stations (α= 0.65), and retail businesses (α=0.62). Regression coefficients from the principal components analysis were used to weight the individual variables for each component and subsequently were combined to create a neighborhood asset index. Neighborhood asset index was categorized into quartiles as high, mid-high, mid-low, and low, with low scores indicating low levels of assets (i.e., high poverty/low retail) and higher scores indicating high level of assets (i.e., low poverty/high retail).
Table 1.
Factor Loadings and Variance of Examined Neighborhood Asset Indices
| Component | Eigenvalue | Proportion of Variance Explained | Cronbach’s Alpha |
|---|---|---|---|
| Food and alcohol densitya | 10.2 | 33% | 0.53 |
| Demographicb | 8.3 | 27% | 0.73 |
| Service stationsc | 3.5 | 11% | 0.65 |
| Retail businessesd | 2.3 | 7% | 0.62 |
Grocery stores, restaurants, on premise alcohol sale, off premise alcohol sale, and combined alcohol sale
Median household income, percent of black/African American residents, percent below poverty level, percent of households with public assistance, percent of male population, percent of unemployed males, percent <25 years old with at least a HS diploma, and percent of female headed households
Gas stations
General convenience stores and pharmacies.
Individual-level covariates
Covariates were obtained from the baseline study questionnaire and included potential confounders of the association between neighborhood assets and cardiometabolic risk factors based on previously published literature as well as change-in-estimate procedures. Maternal characteristics included age (years), race (white, black), education (<high school, high school diploma, less than 4-year college, bachelor’s degree or higher), parity (primiparous or multiparous), smoking during early pregnancy (yes or no), and gestational age at time of enrollment or first blood draw (weeks). Other potential confounders including alcohol consumption, multivitamin/prenatal vitamin use, and household income were also evaluated. However, these factors did not change the parameter estimates by more than 10% and were not included in the final regression models. Education, parity, and smoking were not included in the main analysis since they may be on the causal pathway from neighborhood features to cardiometabolic outcomes.
Statistical Analysis
The distributions of early pregnancy cardiometabolic risk factors, neighborhood asset index, and covariates were examined using medians and interquartile ranges for continuous variables and proportions for categorical variables. We used multilevel random-intercept linear regression to separately estimate the mean difference in SBP, maternal BMI, and CRP associated with neighborhood asset levels. CRP had a skewed distribution and was log transformed and was presented as geometric means for interpretation. First, we fit an unconditional means model to estimate the proportion of variance in each outcome explained by clustering at the census tract level (i.e., intraclass correlation coefficients). Second, we fit an unadjusted model with neighborhood asset alone (level 2). Third, we fit adjusted models that included both neighborhood assets (level 2) and individual-level (level 1) characteristics. Further, we considered the non-linear effect of neighborhood assets on early pregnancy cardiometabolic risk factors by using penalized B-splines, a non-parametric smoothing method. Given the evidence that the association between neighborhood environment and cardiometabolic risk factors may differ in blacks and whites,14, 15 we tested effect measure modification between neighborhood asset levels and maternal race by including an interaction term in our regression models. There was no evidence that the association between levels of neighborhood assets and SBP (P = 0.91), BMI (P =0.82), or CRP (P = 0.44) varied by race.
To maintain consistency with previously published literature,19–21 we included education, parity, and smoking status in our regression models. An additional adjustment was made to account for gestational age when samples were collected and when women entered the study. Given the evidence that obesity is associated with systemic inflammation,30, 31 we included BMI as a covariate in our sensitivity analysis when examining the association between neighborhood assets and CRP as done in previous studies.16, 32–34 Conversely, BMI was not included as covariate in our SBP analyses because we were interested in assessing the direct effect sizes associated with neighborhood assets. Although, we accounted for potential residual correlation by modelling intercepts and regression coefficients as random, this did not allow us to account for the correlation between individual and neighborhood levels (i.e., individual education and neighborhood socioeconomic status). Therefore, we conducted a sensitivity analysis using generalized estimating equations. Last, we evaluated the importance of including commercial data to the models that only used census data by comparing −2LogLikelihood (−2LL) to assess model fit. From each model, we report the unadjusted and adjusted beta coefficients and 95% confidence intervals for all women and do not stratify by race. High neighborhood asset was the referent for all analyses. We used SAS version 9.4 (SAS Institute, Inc. Cary, NC) to construct all datasets and perform all statistical analyses.
RESULTS
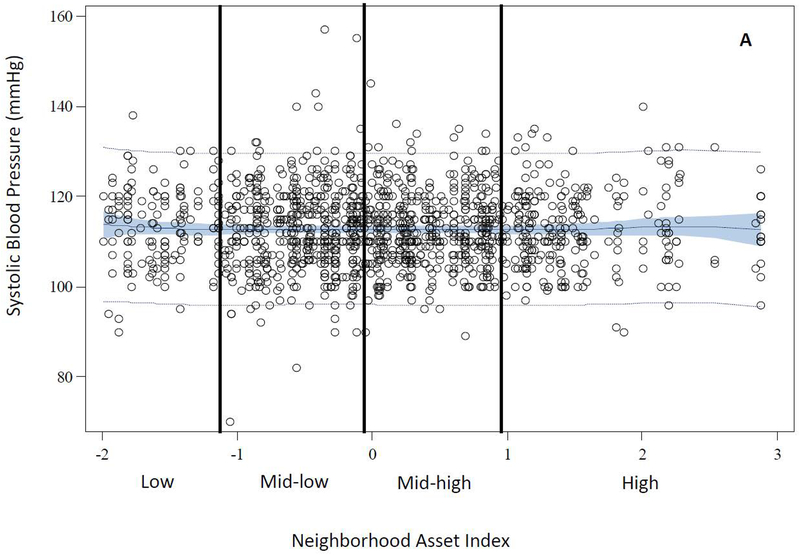
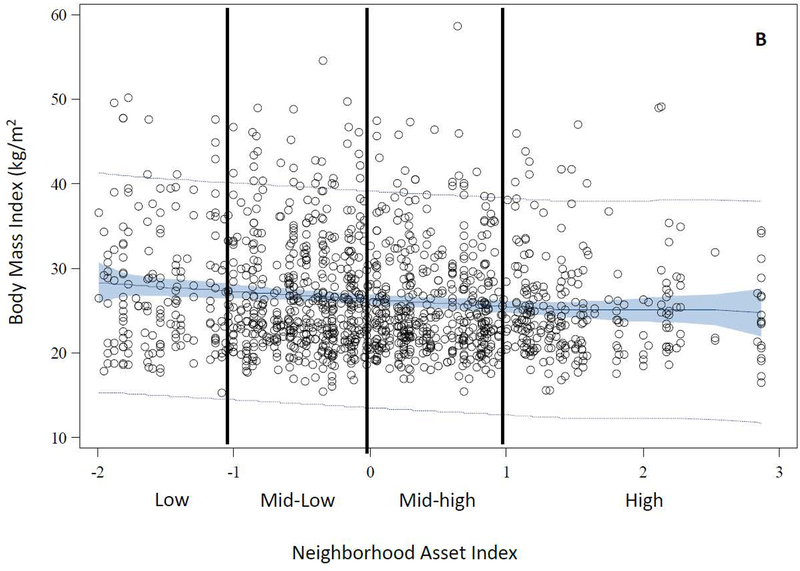
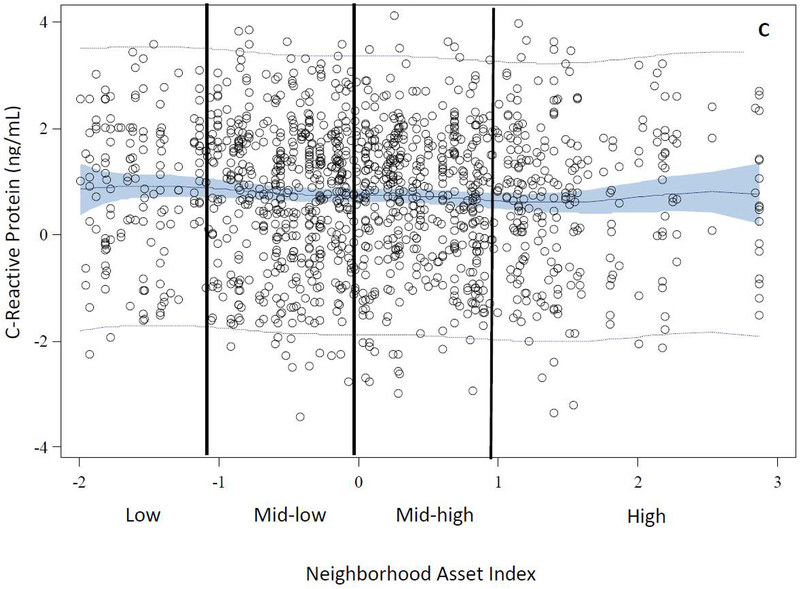
The distributions of age, race, education, primiparity, and smoking differed across levels of neighborhood assets. Compared with women in high asset neighborhoods, those in low asset neighborhoods were generally younger [median (IQR) = 21.5 (5.7) vs. 26.2 (10.3)], more likely to be black (70% vs. 21%), have less than a high school education (31% vs. 14%), and were less primiparous (55% vs. 63%). Additionally, women in low asset areas were more likely to be smokers compared with those in high asset areas (34% vs. 23%) (Table 2). The distributions of BMI and CRP differed by levels of neighborhood assets. However, there was no difference in the distribution of median systolic blood pressure by levels of neighborhood assets. Women in low asset neighborhoods had higher BMI [median (IQR) = 26.3 (10.9) vs. 23.9 (6.6)] and concentrations of CRP [median (IQR) = 1.2 (2.0) vs. 0.7 (2.0)] compared with women in high asset neighborhoods (Table 2). Figure 2 illustrates the observed data and predicted values of each outcome plotted as a function of a penalized B spline transformation of the neighborhood asset index. As anticipated, low levels of neighborhood assets were associated with increases in BMI and CRP concentrations (Panels B and C), whereas, neighborhood assets appeared to be unrelated to SBP (Panel A).
Table 2.
Characteristics by Level of Neighborhood Asset During Early Pregnancy in Women with Live Singleton Births Delivered at Magee-Womens Hospital, Pittsburgh, Pennsylvania, 1997–2001
| Characteristics | Low (Q1) | Mid-Low (Q2) | Mid-High (Q3) | High (Q4) |
|---|---|---|---|---|
| n=372 | n=377 | n=380 | n=375 | |
| n (%) | n (%) | n (%) | n (%) | |
| Neighborhood asset score | ||||
| Median (IQR) | −1.59 (1.0) | −0.36 (0.36) | 0.29 (0.44) | 1.29 (0.81) |
| Age (years) | ||||
| Median (IQR) | 21.5 (5.7) | 23.4 (7.4) | 23.6 (8.7) | 26.2 (10.3) |
| Race | ||||
| Black | 262 (70) | 139 (37) | 86 (23) | 79 (21) |
| White | 110 (30) | 238 (63) | 294 (77) | 296 (79) |
| Education | ||||
| <High school | 117 (31) | 64 (17) | 79 (21) | 52 (14) |
| High school diploma | 159 (43) | 172 (46) | 142 (37) | 105 (28) |
| Less than 4-year college | 79 (21) | 88 (23) | 91 (24) | 89 (24) |
| Bachelor’s degree or higher | 17 (5) | 53 (14) | 68 (18) | 129 (34) |
| Primiparity | 204 (55) | 235 (62) | 249 (66) | 237 (63) |
| Smoking | 126 (34) | 136 (36) | 116 (31) | 87 (23) |
| Gestational age at time of enrollment or sample collection (weeks), median (IQR) | 8.9 (4.7) | 9.3 (5.3) | 9.0 (4.5) | 9.4 (4.1) |
| Cardiometabolic risk factors | ||||
| SBP mmHg, median (IQR) | 113.0 (12.0) | 113.0 (11.0) | 112.0 (10.0) | 112.0 (11.0) |
| BMI kg/m2, median (IQR) | 26.3 (10.9) | 25.2 (8.4) | 24.2 (7.7) | 23.9 (6.6) |
| CRP (ng/L), median (IQR) | 1.2 (2.0) | 0.88 (1.8) | 0.92 (1.8) | 0.7 (2.0) |
Chi-squared tests used for associations among categorical variables.
Kruskal-Wallis test used for associations among continuous variables.
Figure 2.
Observed and Predicted Values of Early Pregnancy Cardiometabolic Risk Factors as a Function of Penalized B-Spline Transformations of Neighborhood Asset Index
The intraclass correlation coefficient for the unconditional means models for SBP, BMI, and CRP was 0.7%, 5.0%, and 3.4%, respectively. Women with low (β 1.95 kg/m2, 95% CI 0.89, 3.00) or mid-low neighborhood assets (β 1.13 kg/m2, 95% CI 0.16, 2.10) compared with high assets had higher BMI after adjusting for age and race (Table 3). Related, low assets were associated with higher CRP concentrations in both unadjusted (β 0.31 ng/mL, 95% CI 0.11, 0.52) and adjusted models (β 0.38 ng/mL, 95% CI 0.17, 0.60). In contrast, we observed no association between level of neighborhood assets and SBP at less than 20 weeks gestation, in both unadjusted models and models adjusting for age and race (Table 3).
Table 3.
Association between Level of Neighborhood Assets and Cardiometabolic Risk Factors During Early Pregnancy in Women with Singleton Live Births at Magee-Womens Hospital, Pittsburgh, Pennsylvania, 1997–2001
| Unadjusted | Adjusteda | |
|---|---|---|
| Outcome | β (95% CI) | β (95% CI) |
| Systolic blood pressure | ||
| Low (Q1) | −0.14 (−1.36, 1.08) | −0.50 (−1.82, 0.82) |
| Mid-low (Q2) | −0.07 (−1.28, 1.15) | −0.09 (−1.32, 1.14) |
| Mid-high (Q3) | −0.40 (−1.61, 0.82) | −0.23 (−1.44, 0.99) |
| High (Q4) | 0.00 (Reference) | 0.00 (Reference) |
| Body mass index | ||
| Low (Q1) | 2.76 (1.74, 3.78) | 1.95 (0.89, 3.00) |
| Mid-low (Q2) | 1.26 (0.25, 2.27) | 1.13 (0.16, 2.10) |
| Mid-high (Q3) | 0.55 (−0.46, 1.56) | 0.74 (−0.23, 1.70) |
| High (Q4) | 0.00 (Reference) | 0.00 (Reference) |
| C-reactive protein | ||
| Low (Q1) | 0.31 (0.11, 0.52) | 0.38 (0.17, 0.60) |
| Mid-low (Q2) | 0.13 (−0.08, 0.33) | 0.19 (0.00, 0.40) |
| Mid-high (Q3) | 0.11 (−0.10, 0.31) | 0.20 (0.00, 0.40) |
| High (Q4) | 0.00 (Reference) | 0.00 (Reference) |
Adjusted for maternal age and maternal race
Similar to the main analyses, in our sensitivity analyses where we further adjusted for education, parity, and smoking, we found higher BMI (β 1.67 kg/m2, 95% CI 0.62, 2.71) and CRP concentrations (β 0.34 ng/mL, 95% CI 0.12, 0.56) in women residing in neighborhoods with low assets compared with high assets (Supplemental Table 1). Further adjustment for gestational age had minimal impact on these estimates (Supplemental Table 1). After adjusting for BMI in addition to other individual-level covariates, we found that women in low asset neighborhoods had higher CRP concentrations compared with women in high asset neighborhoods (β =0.20 ng/mL, 95% CI =0.01, 0.39) (Supplemental Table 2). After accounting for correlation between individual- and neighborhood-levels, women living in areas with low assets had higher BMI (β 1.67 kg/m2, 95% CI 0.50, 2.85) and CRP concentrations (β 0.34 ng/mL, 95% CI 0.12, 0.56) compared with women living in areas with high assets (Supplemental Table 3). Based on changes in the −2LL, the model containing census and commercial data was improved compared to the model with census data alone only when examining CRP (change in −2LL= 5.1, p <0.05).
COMMENT
Principal findings
Using early pregnancy measurements from a birth cohort linked to neighborhood-level data, we observed that women with low neighborhood assets had higher BMI compared with women with high neighborhood assets, after adjusting for individual-level characteristics. Related, we also observed that women living in neighborhood areas with low assets had higher CRP concentrations compared with women living in neighborhood areas with high assets, after adjusting for the same covariates in addition to BMI. We observed no association between level of neighborhood assets and SBP Although black women had lower levels of assets compared with white women, the association between neighborhood assets and early pregnancy cardiometabolic risk factors did not vary by race. This may provide some evidence that the race-specific burden of adverse cardiometabolic risk may be associated with inequities in the distribution of neighborhood socioeconomic status.
Strengths of the study
There are several strengths to this study. First, we included a Census-based measure of neighborhood socioeconomic status and commercial data (food, alcohol, and retail density) which may better characterize the neighborhood features that shape individual risk than census tract data alone. Second, we had early pregnancy measurements for all outcomes. Body mass index was not self-report and was based on measurements at time of enrollment or time of first blood draw. Third, we considered many potential confounders including parity, alcohol consumption, multivitamin or prenatal vitamin use, household income, and smoking.
Limitations of the data
Our study is not without limitations. First, in a cross-sectional study design, we are unable to ensure temporality. Therefore, causal inferences cannot be made from our results. Second, we lacked information on maternal infection during the time of blood collection. Given that maternal infection is positively related to CRP, but may be inversely related to neighborhood assets, the effect estimates we reported are likely an underestimate of the true association. Third, we did not have information on psychosocial stressors which may mediate or modify the association between neighborhood assets and adverse cardiometabolic risk. Fourth, we did not have information on other cardiometabolic risk factors such as lipid/lipoprotein levels or glucose/insulin levels during pre-pregnancy or early pregnancy. Fifth, we were unable to assess residential mobility and lacked information on residential history. We relied on residential address at delivery and assumed that women did not move during early pregnancy which may have resulted in exposure error. Given that our study sample was drawn from a longitudinal cohort where women received prenatal care and delivered at the same hospital, we find it reasonable to believe that either most participants did not move or may have moved within the same census tract during early pregnancy.
Interpretation
The distribution of neighborhood features in our study reflects the distribution in the general population of women of childbearing age in Allegheny County. In a previous population-based study of 55,608 women with live births during 2003–2010, neighborhood socioeconomic deprivation was highest among women who were black (63.8%), had less than a high school education (8.1%), or multiparous (7.4%).27 Neighborhood deprivation was also highest among women <20 years old (7.4%) and smokers (18.4%).27 Our findings are generally consistent with prior studies that have found an association between the neighborhood features and pre-pregnancy obesity.19–21 For example, in a cross-sectional study examining the association between neighborhood food environment and pre-pregnancy weight in New York City, the authors observed a 10–14% increased odds of pre-pregnancy obesity for women living in a neighborhood with no food outlets as compared with two or more healthy food outlets, after adjusting for neighborhood deprivation, percentage commercial space, borough, and individual-level maternal characteristics.21 However, pre-pregnancy weight >200lbs was used as a proxy for obesity due to their inability to obtain information on maternal height which is required to calculate body mass index. Similar to the present study, a study of Swedish primiparous women in poor neighborhoods had increased odds of being obese compared with women in affluent neighborhoods, after adjusting for individual-level covariates including maternal income (OR:1.80, 95% CI=1.54, 2.19).20 Our study extends these in several important ways by incorporating neighborhood-level data that describe food and alcohol density and measurements of BMI. Further, our study also extends the notion that neighborhood deprivation may underlie disparities in obesity prevalence during early pregnancy. Clinical significance of these findings are that increases in early pregnancy weight have maternal and neonatal complications such as adverse pregnancy outcomes, metabolic dysfunction infant adiposity.3
Our results highlight the possibility that neighborhood assets may be an important factor determining inflammation during early pregnancy and warrants further investigation. Clinical significance of CRP findings is less apparent, and its relevance is likely more consequential of neighborhood factors. Previously, in a cross-sectional study of 1,160 women aged 30 to 59 years enrolled in the Midspan family study in the United Kingdom, the authors observed 4.8% (95%CI=0.4%, 9.5%) increase in CRP concentration associated with a one-unit increase in deprivation score after adjusting for age, smoking, BMI, and medication.32 They also reported a positive association which was consistent with our findings, but did not adjust for any individual-level socioeconomic indicators such as maternal education which likely confounds the association between neighborhood deprivation and inflammation.
Although our inability to observe an association between neighborhood features and SBP was inconsistent with the existing literature, it was consistent with a previous study.[40] In a cross-sectional study of 60,775 postmenopausal women aged 50 to 79 years from the Women’s Health Initiative Clinical Trial, as grocery store density increased from the 10th to 90th percentiles, SBP remained unchanged (125.5 to 125.2 mmHg), after adjusting for birth cohort, individual-level characteristics, and population density.35 Our study suggests that neighborhood assets may not be associated with SBP. Elevations in SBP are likely to be influenced by several factors [in addition to diet] including physical activity, stress, genetics, access to quality healthcare, and antihypertensive medication use, that are not directly captured in our index. Future studies are warranted to examine the association between neighborhood socioeconomic status and early pregnancy SBP.
Conclusions
Our results provide new evidence that early pregnancy adiposity and inflammation are associated with neighborhood features independent of age, education, smoking status, and parity. Further, inflammation in pregnancy is associated with neighborhood features beyond accounting for individual-level covariates, including adiposity. Strategies and interventions that increase neighborhood access to healthy foods and improve diet quality during pre-conception and interconception may be promising approaches for reducing adverse cardiometabolic risk profiles.
Supplementary Material
Acknowledgments:
The authors thank all the Prenatal Exposures and Preeclampsia study participants for their participations and Marcia Gallaher for her help measuring the C-reactive protein.
Funding: This research was supported by the American Heart Association Go Red for Women Strategic Focused Research Network Grant AHA16SFRN27810001 and 16SFRN28930000 (Catov), National Institutes of Health Grant 1-PO1 HD30367, and Centers for Disease Control and Prevention Grant U01-CE001630.
REFERENCES
- 1.Williams D Pregnancy: a stress test for life. Current Opinion in Obstetrics & Gynecology 2003; 15:465–471. [DOI] [PubMed] [Google Scholar]
- 2.Bramham K, Parnell B, Nelson-Piercy C, Seed PT, Poston L, Chappell LC. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ 2014; 348:g2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ: British Medical Journal (Online) 2017; 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanz LJ, Stuart JJ, Williams PL, Rimm EB, Missmer SA, Rexrode KM, et al. Preterm Delivery and Maternal Cardiovascular Disease in Young and Middle-Aged Adult WomenClinical Perspective. Circulation 2017; 135:578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectiveness-Based Guidelines for the Prevention of Cardiovascular Disease in Women—2011 Update. Circulation 2011; 123:1243–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heida KY, Velthuis BK, Oudijk MA, Reitsma JB, Bots ML, Franx A, et al. Cardiovascular disease risk in women with a history of spontaneous preterm delivery: A systematic review and meta-analysis. European Journal of Preventive Cardiology 2016; 23:253–263. [DOI] [PubMed] [Google Scholar]
- 7.Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circulation: Cardiovascular Quality and Outcomes 2017; 10:e003497. [DOI] [PubMed] [Google Scholar]
- 8.Bombard JM, Robbins CL, Dietz PM, Valderrama AL. Preconception care: the perfect opportunity for health care providers to advise lifestyle changes for hypertensive women. American Journal of Health Promotion 2013; 27:S43–S49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beckmann MM, Widmer T, Bolton E. Does preconception care work? Australian and New Zealand Journal of Obstetrics and Gynaecology 2014; 54:510–514. [DOI] [PubMed] [Google Scholar]
- 10.Lu MC. Recommendations for preconception care. American Family Physician 2007; 76:397–400. [PubMed] [Google Scholar]
- 11.Gunderson EP, Quesenberry CP Jr, Jacobs DR Jr, Feng J, Lewis CE, Sidney S. Longitudinal study of prepregnancy cardiometabolic risk factors and subsequent risk of gestational diabetes mellitus: the CARDIA study. American Journal of Epidemiology 2010; 172:1131–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hedderson MM, Xu F, Darbinian JA, Quesenberry CP, Sridhar S, Kim C, et al. Prepregnancy SHBG concentrations and risk for subsequently developing gestational diabetes mellitus. Diabetes care 2014; 37:1296–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace M, Bazzano L, Chen W, Harville E. Maternal childhood cardiometabolic risk factors and pregnancy complications. Annals of Epidemiology 2017; 27:429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leal C, Chaix B. The influence of geographic life environments on cardiometabolic risk factors: a systematic review, a methodological assessment and a research agenda. Obesity reviews 2011; 12:217–230. [DOI] [PubMed] [Google Scholar]
- 15.Diez-Roux AV, Nieto FJ, Muntaner C, Tyroler HA, Comstock GW, Shahar E, et al. Neighborhood environments and coronary heart disease: a multilevel analysis. American Journal of Epidemiology 1997; 146:48–63. [DOI] [PubMed] [Google Scholar]
- 16.Petersen KL, Marsland AL, Flory J, Votruba-Drzal E, Muldoon MF, Manuck SB. Community socioeconomic status is associated with circulating interleukin-6 and C-reactive protein. Psychosomatic Medicine 2008; 70:646–652. [DOI] [PubMed] [Google Scholar]
- 17.Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Drake P. Births: Final data for 2016. Hyattsville, MD: National Center for Health Statistics; 2018 [PubMed] [Google Scholar]
- 18.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 2017; 135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SK, Sobal J, Frongillo EA, Olson CM, Wolfe WS. Parity and body weight in the United States: differences by race and size of place of residence. Obesity 2005; 13:1263–1269. [DOI] [PubMed] [Google Scholar]
- 20.Sellström E, Arnoldsson G, Alricsson M, Hjern A. Obesity prevalence in a cohort of women in early pregnancy from a neighbourhood perspective. BMC Pregnancy and Childbirth 2009; 9:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janevic T, Borrell LN, Savitz DA, Herring AH, Rundle A. Neighbourhood food environment and gestational diabetes in New York City. Paediatric and perinatal epidemiology 2010; 24:249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee P-C, Talbott EO, Roberts JM, Catov JM, Sharma RK, Ritz B. Particulate air pollution exposure and C-reactive protein during early pregnancy. Epidemiology 2011; 22:524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubel CA, Powers RW, Snaedal S, Gammill HS, Ness RB, Roberts JM, et al. C-reactive protein is elevated 30 years after eclamptic pregnancy. Hypertension 2008; 51:1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003; 107:363–369. [DOI] [PubMed] [Google Scholar]
- 25.Catov JM, Bodnar LM, Ness RB, Barron SJ, Roberts JM. Inflammation and dyslipidemia related to risk of spontaneous preterm birth. American Journal of Epidemiology 2007; 166:1312–1319. [DOI] [PubMed] [Google Scholar]
- 26.Mendez DD, Duell J, Reiser S, Martin D, Gradeck R, Fabio A. A methodology for combining multiple commercial data sources to improve measurement of the food and alcohol environment: applications of geographical information systems. Geospatial Health 2014; 9:71–96. [DOI] [PubMed] [Google Scholar]
- 27.Mendez DD, Doebler DA, Kim KH, Amutah NN, Fabio A, Bodnar LM. Neighborhood socioeconomic disadvantage and gestational weight gain and loss. Maternal and Child Heatlh Journal 2014; 18:1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vinikoor-Imler L, Messer L, Evenson K, Laraia B. Neighborhood conditions are associated with maternal health behaviors and pregnancy outcomes. Social Science & Medicine 2011; 73:1302–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Messer LC, Laraia BA, Kaufman JS, Eyster J, Holzman C, Culhane J, et al. The development of a standardized neighborhood deprivation index. Journal of Urban Health 2006; 83:1041–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ndumele CE, Nasir K, Conceiçao RD, Carvalho JA, Blumenthal RS, Santos RD. Hepatic steatosis, obesity, and the metabolic syndrome are independently and additively associated with increased systemic inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology 2011; 31:1927–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. The Journal of Clinical Investigation 2017; 127:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Reilly DSJ, Upton M, Caslake M, Robertson M, Norrie J, McConnachie A, et al. Plasma C reactive protein concentration indicates a direct relation between systemic inflammation and social deprivation. Heart 2006; 92:533–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nazmi A, Roux AD, Ranjit N, Seeman TE, Jenny NS. Cross-Sectional and Longitudinal Asociations of Neighborhood Characteristics with Inflammatory Markers: Findings from the Multi-Ethnic Study of Atherosclerosis. Health & Place 2010; 16:1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cozier YC, Albert MA, Castro-Webb N, Coogan PF, Ridker P, Kaufman HW, et al. Neighborhood socioeconomic status in relation to serum biomarkers in the Black Women’s Health Study. Journal of Urban Health 2016; 93:279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dubowitz T, Ghosh-Dastidar M, Eibner C, Slaughter ME, Fernandes M, Whitsel EA, et al. The Women’s Health Initiative: The food environment, neighborhood socioeconomic status, BMI, and blood pressure. Obesity 2012; 20:862–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.