Abstract
The central autonomic nervous system (ANS) is essential for maintaining cardiovascular and respiratory homeostasis in the newborn and has a critical role in supporting higher cortical functions. At birth, the central ANS is maturing and is vulnerable to adverse environmental and physiologic influences. Critical connections are formed early in development between the ANS and limbic system to integrate psychological and body responses. The Polyvagal Theory, developed by Stephen Porges, describes how modulation of the autonomic vagal impulse controls social responses and that a broad range of neuropsychiatric disorders may be due to impaired vagal balance, with either deficient vagal tone or excessive vagal reactivity. Under additional circumstances of prematurity, growth restriction, and environmental stress in the fetus and newborn, the immature ANS may undergo dysmaturation. Maternal stress and health as well as the intrauterine environment are also quite important and have been implicated in causing ANS changes in the infant and neuropsychiatric diseases in children. This review will cover the aspects of ANS development and maturation that have been associated with neuropsychiatric disorders in children.
Introduction
The autonomic nervous system (ANS) is essential for maintaining physiologic functions involving cardiovascular, respiratory, and gastrointestinal systems, but is also intricately connected to higher brain systems involved in the emotional and psychological aspects of life that make us uniquely human. In the mature brain, the central ANS maintains a background level of functioning with connections to the brains’ limbic structures that are involved in mood, memories, and emotional state regulation. Differences in child and adult psychological morbidity and stress resilience may be related to differences in central ANS function.(1–3) Disturbed integration between central autonomic and limbic systems play a role in the development of childhood neuropsychiatric disorders.(1) In fact, epigenetic factors in the early preconception and intrauterine periods begin to shape the development of the ANS (Figure 1).(4)
Figure 1. Factors Influencing Autonomic Nervous System (ANS) Development.
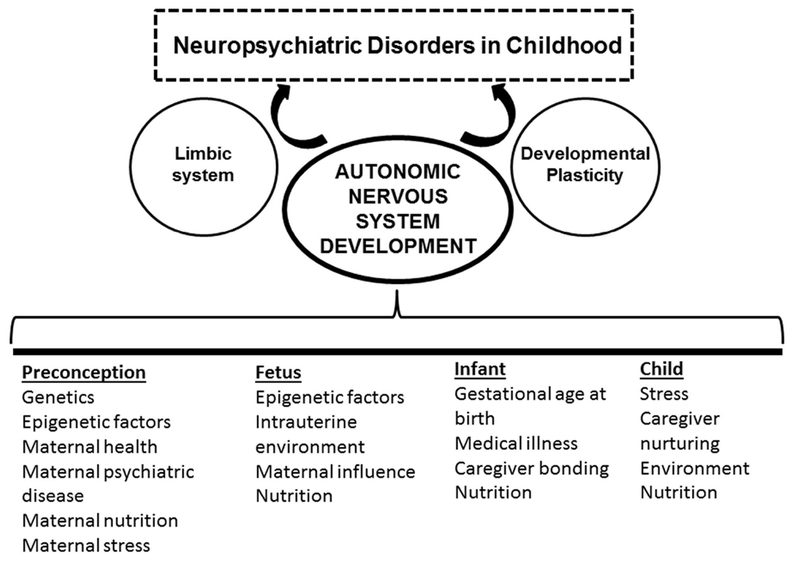
Multiple factors influence autonomic nervous system development during the preconceptional and fetal periods, as well as during infancy and childhood. Acting through developmental plasticity of the limbic system these factors may influence the development of neuropsychiatric disorders in childhood.
The developmental basis for inter-individual differences in ANS function relates in part to the prenatal, fetal, and neonatal experience. The ANS undergoes a prolonged period of development and maturation during which it remains vulnerable to developmental disruption from a variety of influences. The early disruption of autonomic development may significantly influence the developmental trajectory of the ANS system, limit its capacity to respond to physiologic changes and to the environment, and has been implicated in later neuropsychiatric disorders.(1, 4) In this review, we discuss the epidemiologic links between childhood neuropsychiatric disorders and earlier ANS ‘dysmaturation’. We then describe fetal and postnatal conditions that may disturb early maturation of the central ANS. Finally, we explore potential interventions to support normal or improved development of the central ANS in high-risk infants.
Epidemiology of Neuropsychiatric Disorders in Childhood
Over the last decade the reported incidence of neuropsychiatric disorders in childhood has increased.(5) Although the reasons for this trend are unclear, it is likely multifactorial and potentially related to factors that alter ANS maturation and limbic system function.(4, 6) Many neuropsychiatric disorders have their onset in childhood, including depression, anxiety, behavioral dysfunction, attention-deficit hyperactivity disorder (ADHD), autism spectrum disorder, and others.(5) Depression has an incidence of about five percent in children and adults age 12 years and older.(7) Similarly, ADHD has a prevalence of around 10% (and increasing) during childhood and adolescence,(8, 9) and is associated with adverse childhood experiences.(10) In two thirds of children with ADHD, the disorder is compounded by additional mental, emotional, or behavioral disorders.(8) Childhood-onset ADHD can transition to disabling internalizing psychological disorders in adulthood.(11)
The risks for the development of neuropsychiatric disorders in childhood are not well understood. The causes of neuropsychiatric disorders include neurobiological, genetic, endocrinological (hypothalamic-pituitary-adrenal axis functioning), and psychosocial factors all with varying contributions. Specific children at increased risk include those with a history of prematurity and other neonatal conditions, such as congenital heart disease, fetal growth restriction, neonatal abstinence syndrome, and maternal stress/neuropsychiatric illness.(12–15) For example, adults born preterm are at increased risk for mental health disorders including anxiety, depression, and reduced social engagement.(11) Fetal and maternal epigenetic mechanisms likely contribute to the risk for depression, bipolar disorder, and schizophrenia in these early-life conditions.(4) Nutrition may also influence psychiatric disease.(16) In a susceptible individual, the risks may act cumulatively resulting in neuropsychiatric manifestations beyond a given threshold. Under specific circumstances and depending on factors such as epigenetic influences, the phenotype may include neuropsychiatric disorders of varying severity.
The Central ANS and Limbic Connections
It is the complex interplay between the central ANS (brainstem) and the limbic system which creates a platform on which physical and emotional experiences can shape behavior, emotional, and neuropsychiatric health from the prenatal period into adulthood.(2, 6, 17) The ANS centers involved in neuropsychiatric health/disease and physiologic body responses are located in the brainstem and limbic system. The central ANS has two major components, the sympathetic nervous system responsible for the well-known “fight-or-flight” response activated from a sense of danger or elevated stress, and the parasympathetic nervous system, involved in vegetative functions, and moderating sympathetic activity. This is a rather simplified view of the competing roles of these systems, as their relationship is considerably complex, with multiple interconnections and modulation by higher cerebral centers, including the limbic system (Figure 2). The complex functions and responsiveness of the ANS is dependent upon multiple levels of interactions between the two branches.(18) The nucleus of the solitary tract and the paraventricular hypothalamic nucleus are principal regulators of sympathetic and parasympathetic ANS activity.(18)
Figure 2. Anatomy of the Central Autonomic Nervous System (ANS) and Limbic System Connections.
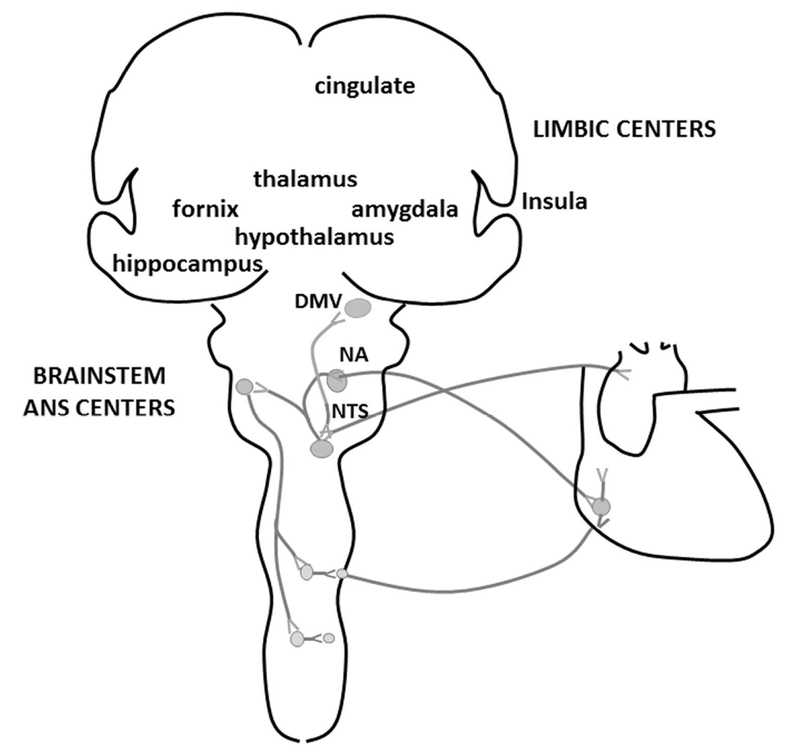
Together, the limbic and brain stem ANS centers regulate emotional, learned, and physiological body responses to the environment. The limbic system includes the amygdala, thalamus, fornix, hippocampus, hypothalamus, and cingulate gyrus. Parasympathetic tone and vagal activity is generated from the dorsal motor nucleus of the vagus (DMV) and from the nucleus ambiguus (NA). The nucleus of the solitary tract (NTS) is an important brainstem ANS center with both sympathetic and parasympathetic system functions. It receives peripheral afferent input from peripheral chemoreceptors and pulmonary mechanoreceptors to control cardiovascular and respiratory system functions. A behavioral response or stress triggers the limbic system to affect ANS tone which results in physiologic effects on heart rate, respiratory rate, and blood pressure.
The limbic system includes the amygdala, thalamus, fornix, olfactory cortex, hippocampus, hypothalamus, and cingulate gyrus (Figure 2). Early in brain development these structures develop multiple interconnections and connections to the brainstem ANS centers.(19) The higher level diencephalic/forebrain limbic centers connect to the ANS centers of the brainstem to modulate changes in cardiovascular, respiratory, and gastrointestinal system functions. Throughout the lifetime, limbic system connections strengthen or weaken depending upon the environment, stress, and other exposures.(20, 21) The early structure/volume of the developing limbic system may be influenced by the “intrauterine milieu”, including maternal preconception health and stress hormone levels and during pregnancy.(4) This plasticity is important for the individual, and environmental changes can affect limbic structure and connections even in the mature individual.(14)
The amygdala is a limbic center with important functions related to the development of neuropsychiatric disorders. It is important for memory association, survival instincts, and mood and “records” the emotion of a memory to enable the individual to react in a beneficial way in the event of a repeated exposure. The amygdala receives sensory inputs from the thalamus and cerebral cortex. A stressor or memory of a negative event activates connections to brainstem sympathetic centers that increase heart rate, blood pressure, and muscle responsiveness. The parasympathetic centers may dampen the physiologic response, functioning like a “vagal brake”.(6, 17)
The nuclei of the amygdala also make connections to the cerebral cortex, hippocampus, thalamus, and brainstem. The hippocampus is important for memory, mood regulation, and can shut off the stress response when needed. Chronic stress is associated with increased amygdala volume and has been associated with mood disorders and aggression.(22) Conversely, chronic stress is associated with volume reduction in other limbic structures, such as hippocampus and prefrontal cortex.(14, 21) Depression is associated with excessive cortisol stimulation of the amygdala with hypothalamic activation and inhibitory effects on hippocampal activity.(14) Abnormalities in amygdala and limbic system function can manifest as anxiety disorders.(23)
The Development of the Central ANS from the Fetal Period to Childhood
The ANS matures throughout the fetal period and in infancy.(24, 25) The unmyelinated, more primitive, vagal nerve is the first autonomic branch to develop in fetal life, but does not have significant function prior to birth.(26) Next to develop is the sympathetic division of the ANS, which shows steady development throughout the fetal period.(27) During fetal life and in prematurely born newborns, natural variability in sympathetic tone, as opposed to parasympathetic tone, plays an important role in heart rate and blood pressure variability.(28) The parasympathetic division of the ANS, on the other hand, including the myelinated vagus (emanating from the nucleus ambiguus), develops later in the fetal period.(26) The parasympathetic division does not exert much influence prior to 25–30 weeks gestation; the normal steep increase in vagal tone occurs around 37–38 weeks, at a time when premature newborns may already have been in an ex utero environment for some time.(27, 29) In such prematurely born infants, this normal third trimester increase in parasympathetic tone may be dampened in the ex utero environment, compared to that of the in-utero third trimester fetus.(30) Under conditions of fetal stress and hypoxia, the primitive unmyelinated vagal systems contribute to bradycardia since, depending on gestational age, the newer nucleus ambiguus has not yet fully developed to provide a more finely-tuned ‘vagal brake’.(2, 31) The normal increase in parasympathetic tone is evidenced in the increased high-frequency heart rate variability (HRV, see below) in infants born at term.(32) With increasing postnatal age, and maturation of the ANS, there is progressive parasympathetic influence on resting heart rate.(28) Ex utero third trimester development in the preterm newborn, may lead to altered autonomic development with potential short- and long-term implications for health.(33)
Evaluation of the ANS using Heart Rate Variability (HRV)
Autonomic function can be measured non-invasively from physiologic signals of heart rate, respiratory rate, and blood pressure. HRV, or the fluctuation in the length of time between heart beats (R-R intervals), provides a measure of sympathetic and parasympathetic interplay, and therefore ANS functional maturation.(24, 34) High-frequency variability reflects parasympathetic function and is influenced by the respiratory rate, while low-frequency variability is due to a combination of sympathetic and parasympathetic inputs and baroreflex-induced changes in heart rate.(35) Interestingly, there is discussion over the contribution of the sympathetic nervous system to HRV, since administration of atropine blocks almost all HRV.(36) As the ANS matures, there should be increased parasympathetic function and thus greater high-frequency variability.(37) HRV can thus be used as a marker for autonomic function in the newborn and may be associated with neurodevelopmental outcome.(38–40) The function of the myelinated vagal pathways (high-frequency HRV) is most accurately captured by quantifying respiratory sinus arrhythmia (RSA).(2) RSA is the amplitude of oscillations caused by spontaneous respiration in the beat-to-beat variability of the heart rate.(2)
Polyvagal Theory and Impaired Vagal Balance
The Polyvagal Theory was first proposed by Porges in 1995 and relates the development of the vagal system to social/emotional development.(2, 17, 26) The theory focuses on the role of the two main branches of the vagal nerve (cranial nerve X). The older branch arises from the unmyelinated dorsal motor nucleus of the vagus, and the newer branch from the myelinated, nucleus ambiguus (Figure 2). The social responses to our environment are mediated either by vagal input or vagal withdrawal through the components of the limbic system.(41) At six months of age and older, vagal development begins to influence social behavior and mood regulation of behavioral state.(2) The infant develops a ‘face-heart’ connection or Social Engagement System, whereby he/she engages muscle activity of the face/neck to communicate feelings and behavioral reactions, in concert with brainstem mediated responses in cardiovascular function.(2) These muscles are innervated by special visceral efferent pathways associated with the myelinated vagus and enable the infant to display social cues and build parental/care-giver attachment. It is the step-wise maturation of the both the cerebral cortical structures and of the ANS that enables the development of the individuals’ Social Engagement System.
As discussed above, a broad range of neuropsychiatric disorders may be influenced by impairment in vagal balance, with either deficient vagal tone or excessive vagal reactivity.(41) Autonomic imbalance and in particular decreased parasympathetic tone is implicated in anxiety, depression, post-traumatic stress disorder, and schizophrenia.(6) In these conditions, the sympathetic-mediated responses to stressors/fear by the amygdala and pre-frontal cortex may be under-opposed by the parasympathetic system.(6) In ex-prematurely born infants, immaturity of the Social Engagement System from lower vagal activity may cause a lack of proper social cues to trigger normal co-regulation with the parents/care-giver.(2)
Under most circumstances, the body seeks to establish homeostasis by maintaining a stable functioning.(42) In a changing environment, the body may need to adapt to have optimal function, a phenomenon known as allostasis.(42, 43) Allostasis describes the attempt to keep optimal functioning despite differing physiologic and environmental demands.(42) For example, under states of chronic stress, the body may anticipate the changing environment and may enter an allostatic-state in which it maintains a specific level of function.(44, 45) There may also be the opportunity for “homeostatic” plasticity, whereby the nervous system maintains the ability to return to a prior state when the environment returns to a more balanced/normal (pre-stressor) state.(46)
Prenatal Programming and the Potential for ANS Dysmaturation
Our understanding of how maternal preconception physical and mental health, genetic influences, and epigenetic factors from the intrauterine environment shape the long-term health through adulthood, is increasing. Even prior to conception, these factors may influence offspring ANS function through prenatal programming.(4, 47) Alan Lucas, PhD coined the term ‘programming’ in 1991 to describe the long term health effects of early nutritional exposures in infants.(47, 48) Similarly, ‘developmental plasticity’ is the means by which early life environmental exposures during critical periods may influence development to the extent that it permanently affects interactions with the environment.(47) Developmental plasticity can also be viewed as the phenomenon whereby one genotype can give rise to more than one phenotype.(47) Neuropsychiatric disorders are heterogeneous and have moderate heritability, so the phenotype is variable and dependent upon many other factors.(49)
Since the ANS interacts closely with the limbic system and provides the physiologic output of emotions, happiness, and fear, the ANS is amenable to developmental alterations and prenatal programming. Epigenetic changes are believed to be the basis of the change in programming phenotype. These changes can be due to maternal factors such as nutritional deficiencies (zinc, iron), toxicants (alcohol, environmental pollutants), maternal stress, maternal disease (depression, diabetes, obesity), and placental dysfunction (Figure 1).(4) In a study of maternal depression and its influence on offspring, the cord blood T lymphocytes of newborns whose mothers reported depression had a distinct DNA methylation pattern.(50) These changes in DNA methylation seemed to persist in the adult hippocampus.(50) Fetal and neonatal factors that can also result in epigenetic changes from chronic hypoxemia as may be seen in congenital heart disease and fetal growth restriction, prematurity, medical illness, and nutritional deficiency (Figure 1).(4) ANS tone is also influenced in a top-down manner by the prefrontal cortex and brain-derived neurotrophic factor which is associated with stress resilience.(51) In a population based cohort evaluation of newborn dried blood spots, preterm newborns with higher levels of brain-derived neurotrophic factor showed improved neurodevelopment.(52)
Since the ANS matures throughout fetal life and into childhood, and since it maintains complex interactions with the limbic system that are involved in numerous brain-body connections for the developing individual, it has a sustained vulnerability to conditions that can impair its normal development (i.e. developmental programming or plasticity). Premature birth is one such condition which may negatively impact ANS development. In premature birth, the ANS becomes “engaged” with the extrauterine environment in an immature state when parasympathetic tone is typically underdeveloped. Premature engagement of the ANS under conditions of preterm birth can therefore result in dysmaturation, or a shift in the temporal program of ANS maturation due to aberrant programming. In autonomic dysmaturation, the normal developmental trajectory of the sympathetic and parasympathetic divisions is altered by abnormal or unexpected exposures/experiences during critical periods in development. ANS dysmaturation can be measured through functional, physiological, and anatomical dimensions by evaluation of psychologic/behavioral/stress responses, effect on heart rate (HR), blood pressure, and respiratory rate, and by alterations in brainstem and brain structure volume, respectively. One example of such autonomic dysmaturation in ex-preterm infants is seen in those developing later apparent life threatening events (ALTE).(53) In a study of premature newborns that developed ALTE after discharge from the neonatal intensive care unit (NICU), there was a paradoxical increase in parasympathetic tone and lower sympathetic tone by HRV analysis in the weeks prior to NICU discharge, likely restricting the ability of these infants to auto-resuscitate by increasing heart rate and blood pressure in response to an ALTE.(54) While immaturity itself is a major contributor to ANS dysmaturation in premature infants, the multitude of ‘unnatural’ stimuli in the NICU may create a challenging environment for the maturation of the ANS (Figure 1). HRV measures of ANS function may predict neurologic outcome in preterm newborns. In a study of 30 premature newborns, HRV and cardiac vagal tone were positively related to improved neurologic outcomes at three years of age in the areas of mental processing, social skills, motor development, and these children had fewer reports of behavioral problems.(55) Adolescents born preterm show ANS dysfunction compared to adolescents born at term indicating a long-standing difference in vagal tone.(56)
While prematurity is a prevalent cause of ANS dysmaturation, it is often comorbid with other maternal, fetal, and neonatal conditions that disrupt normal ANS maturation (Figure 1). One such condition is maternal nutritional deficiency during pregnancy. For example, Zinc has been shown to support normal ANS development, and maternal Zinc deficiency in an animal model is associated with increased anxiety and impaired social behavior in offspring.(57, 58) Congenital heart disease and fetal growth restriction are complicated by in utero chronic hypoxemia which can affect ANS maturation as shown by differences in HRV.(12, 13) In these conditions, ANS tone is reduced at term compared to non-affected newborns. In fetuses with congenital heart disease, the early difference in ANS tone is associated with 18 month motor and cognitive outcome.(59) Maternal stress also may exert a powerful influence on fetal/neonatal ANS development and its limbic connections.(14)
The model of ANS dysmaturation leading to neuropsychiatric disorders is also supported by deficits in ANS regulation in older individuals with neuropsychiatric diagnoses. For example, reduced HRV in adults is a sensitive marker for major depressive disorder.(60) These clinical populations also have low amplitude RSA (or high frequency HRV) and have deficits in the function of the muscles regulated by special visceral efferent pathways, which regulate sucking, swallowing, and vocalizations and in the coordination of these actions with breathing. Consistent with the proposed model and Polyvagal Theory, preterm infants have difficulties in these functional domains, which normally play an important role in the Social Engagement System and influence social behavior.(2)
Other Causes of ANS Dysmaturation
In a study of autonomic function in premature newborns, those infants whose mothers smoked during pregnancy showed higher sympathetic function, lower parasympathetic function, and had less cardiac autonomic adaptability compared to control newborns whose mothers did not smoke during pregnancy.(61) Infants exposed to prenatal opiates had higher HRV and higher sympathetic and parasympathetic tone compared to control, non-exposed, newborns during feeding,(15) however the duration of this difference in autonomic tone due to prenatal opiate exposure is not entirely known. In another study, children exposed to opiates in utero were found to have reduced social maturity at three years of age, compared to non-opiate exposed children.(62) Children born to opiate-addicted mothers also demonstrate increased hyperactivity, aggressiveness, and ADHD at school-age.(63) The ANS and limbic structures are under strong environmental influence, so this difference in social skills is likely related to a combination of environment and ANS dysmaturation. The intrauterine and extrauterine environmental exposures early in life can be the origins of adult diseases that take years to manifest.(3)
Maternal and Infant Stress, the ANS, and Neuropsychiatric Outcome
Stress, of varying levels, is a constant part of our environment and impacts fetal brain and ANS maturation. Conditions of stress which cause a disruption of the maternal hypothalamic-pituitary-adrenal-axis may in turn affect the developing fetal hypothalamic-pituitary-adrenal-axis and lead to alterations in pathways important for mood regulation, autonomic nervous system development, growth, metabolism, and cardiovascular function in the infant that may last a lifetime. This ‘prenatal stress-immune programming’ is found to increase risk for depression and obesity.(64) Not surprisingly, impaired ANS function is implicated in the pathophysiology of obesity and weight reduction may improve autonomic balance.(65)
During pregnancy, maternal toxic stress can influence fetal cortisol levels which, may affect the newborn’s response to stress after birth, and is associated with gray matter volume changes.(66) Mothers with a history of toxic-stress are at higher risk for spontaneous preterm labor, and have infants of lower birth weight and of lower gestational age compared to pregnant women without a high stress history.(66) Hair cortisol levels in mothers who delivered preterm compared to term, were lower at delivery indicating that reduced hypothalamic-pituitary-adrenal axis activity may itself be associated with premature delivery.(67) In a prospective cohort study of pregnant women, perceived stress at 16 weeks gestation was correlated with hair cortisol level in the second trimester and subsequent premature delivery.(68)
The Polyvagal Theory can also be applied in the setting of the NICU for understanding the maternal and infant reactions to stress.(26, 66) For both the mother and her newborn, stressors during this period include maternal anxiety, lack of physical closeness, iatrogenic factors, and medical illness. Parents of premature infants report higher levels of depression and social isolation and this stress may negatively affect bonding with their infant,(69, 70) but also the infants development of their Social Engagement System.(2) Maternal mood disorders may also result in larger amygdala volume in offspring.(14)
Infants requiring care in the NICU can experience stress which may impact ANS development. Infants born very preterm are at risk for impaired brain growth, since the late fetal period is a time of substantial increase in brain volume.(71) They are also vulnerable to brain injury and impaired neurodevelopmental outcomes which may complicate the infant stress response.(38) Stressors in the NICU in very preterm infants may affect DNA methylation (epigenetic regulation) of the serotonin transporter gene (SLC6A4).(72) Higher SLC6A4 methylation was seen at term equivalent age in ex-very preterm infants and this was associated with reduced anterior temporal lobe volume and lower Griffith Mental Development Scales at 12 months corrected age.(72) These findings suggests that early infant stress from preterm birth may contribute to altered programming of socio-emotional development in very preterm infants by mechanisms of epigenetic modification and structural changes in the developing brain.(72) Specialized programs in the NICU to address stress in premature newborns can have lasting effects on cerebral volumes and later childhood stress responses.(66)
The early life effects of prematurity on ANS development and long-term neuropsychiatric health can be seen in adulthood. Having a low birth weight increases risk for depression and mood disorders.(73) In a meta-analysis of adults born very preterm, higher levels of internalizing and avoidant personality problems were found, with less externalizing, rule-breaking, antisocial personality problems compared to term-born adults.(11) Adults born preterm are at increased risk for mental health disorders including anxiety, depression, and reduced social engagement.(11) Childhood problems such as attention/ADHD may transition to these other internalizing psychological disorders in adulthood.(11)
Nutrition for ANS Development and Neuropsychiatric Outcome
The period of fetal brain development has not received much attention for clinical interventions to improve brain development beyond routine prenatal care and standard prenatal vitamin supplementation.(16) Data are emerging that indicators of maternal health such as substance abuse and smoking, quality of nutrition and nutritional body stores, and mental health may effect fetal brain development and risk for long-term neuropsychiatric disorders in offspring.
Micronutrients are critical for brain development including that of the autonomic and limbic systems.(74) Some micronutrients have received specific attention and have been studied in regards to maternal levels and associated child outcome. Folic acid is widely known to decrease risk of neural tube defects;(75, 76) however taking folic acid prior to 10 weeks of pregnancy may also boost the child’s attention, social skills, and behavior at 18 months of age compared to children whose mothers did not take folic acid supplementation prior to 10 weeks gestation.(16) Low maternal folic acid levels are associated with impaired child emotional development.(16) Folic acid supplementation may therefore improve limbic structure function and vagal tone which is important for social and emotional engagement of the child.(2) Zinc has also been found to be important for ANS regulation, hippocampal, and cerebellar development.(57) Phosphatidylcholine may improve child emotional development and attention, through effects on limbic structure and function.(16, 77) Schizophrenia risk, which may include autonomic imbalance and lower parasympathetic tone, may be reduced by phosphatidylcholine, although prospective randomized trials are needed.(16) Vitamin A and D supplementation during pregnancy also seem to reduce risk of schizophrenia and have been studied more extensively.(78) Interestingly, fatty acid supplementation shows mixed findings. In a study of pregnant women, fatty acid levels at 36 weeks gestation were positively associated with infant autonomic function at four months of age.(79) Meanwhile, in a 7-year follow-up study of docosahexaenoic (DHA) supplementation in pregnancy, those women randomized to DHA reported higher behavioral problems and executive dysfunction in their children.(16, 80) Further studies are needed to determine the impact of these micronutrients on autonomic development, as well as the dosage needed, and whether they should be incorporated into current nutritional recommendations for pregnant women to improve neuropsychological health in their offspring.
Neonatal nutrition also plays an important role in supporting ANS development. The physical act of being held to breastfeed improves an infant’s social development likely through the visual, olfactory, and sensory experience, but also by early ability to regulate the feeding by controlling flow of milk and feeding duration.(81) The feeding experience in turn supports strengthening of the vagal complex to regulate state by social engagement.(2) The nutrient component of the milk/formula is also important. Preterm newborns fed-nutrient enriched specialized formula, showed better social maturity and motor function at 18 months of age, compared to those fed a standard term newborn formula.(82) It is these early nutritional observations that were the basis for implicating altered programming as a cause of long-term neuropsychiatric and health disorders in children.(48, 83)
Long-Term Outcome Related to ANS Dysmaturation
The preschool years represent a critical time period for the development of the neurotransmitter systems (noradrenergic, serotonergic, dopaminergic) that are important for behavioral control.(41) Follow-up studies of HRV analysis in ex-premature newborns show differing findings depending upon the age at which children are studied. In a study evaluating early child ANS activity in preterm newborns compared to term newborns, the initial lower ANS activity seen in preterm newborns at term gestational age, resolved by two-years of age.(84) Possible explanations for this include excessive early sympathetic tone in premature newborns due to high stress associated with prematurity, sensory stimulation in the NICU, inadequate nutrition, ventilation, and prenatal exposures to tobacco.(84) Parasympathetic tone seems to mature long after birth in premature newborns and by two years of age may “pseudo-normalize”.(84)
Differences in ANS function may be more easily identified in older children. In a study of ex-preterm adolescents compared to ex-term born adolescents, preterm adolescents had lower autonomic tone and longer heart rate recovery following exercise compared to term-born adolescents showing a prolonged autonomic dysfunction due to prematurity.(56) It has also been shown that adults born preterm are more likely to develop cardiovascular disease at an earlier age(85) and have an increased risk for death in middle age from cardiovascular disease compared to adults born at term.(86)
Interventions to Support ANS Development and Improve Neuropsychiatric Outcome
Given the complexity and multifactorial nature of neuropsychiatric disorders in children, there is not a universal intervention which would dramatically reduce the incidence of these disorders. There are, however, interventions which may promote autonomic development.(57, 87, 88) As discussed throughout, an intimate mother-infant connection plays an important role in the developing neuropsychological phenotype of the infant. Consequently, any intervention aimed at supporting autonomic development of the infant needs to include measures that promote the mental and physical health of the mother, beginning in the prenatal period. Potentially modifiable factors include nutrition, environment, and psychological well-being.
Pregnant women should have optimal nutrition, which goes beyond a prenatal multivitamin. In future, prenatal assessment for micronutrient deficiencies may inform supplementation that decreases the risk of adverse autonomic and neuropsychological outcomes.(16) Stress in pregnancy may increase the risk for preterm birth; therefore, addressing prenatal toxic stress may have the additional effect of improving autonomic-limbic regulation through a reduction in the incidence of prematurity.(68) Strategies for reducing prenatal stress effectively may therefore improve ANS and limbic system development in infants. For example, mindfulness-based meditation has been shown to help reduce stress in mothers of premature children, and has a beneficial effect on infant brain development.(14)
Fortunately, the prolonged critical period for ANS development (which increases its vulnerability), also extends the window during which developmental plasticity may be exploited to promote recovery and optimize normal/improved ANS development in early infancy. Such interventions may be relatively simple, including changes in caregiving and the nursery environment.(88) For example, skin-to-skin contact with kangaroo care in premature newborns improves vagal tone and autonomic functioning into childhood.(66, 89) Infants randomized to maternal singing during kangaroo care compared to kangaroo care without singing, showed even greater improvements in autonomic regulation.(90) As a secondary benefit, maternal anxiety was reduced,(90) again supporting the connection between the mother and baby. Infants are born knowing their mothers voice,(91) so it is not surprising for maternal voice to have an effect on autonomic regulation. Even in preterm newborns, maternal voice lowers heart rate during care and promotes infant relaxation.(92) Pacifier use in preterm newborns also modifies ANS tone and improves infant blood pressure and heart rate.(87) However, the potential long-term benefit of pacifier use in promoting ANS development remains unknown.
After NICU discharge, interventions for the mother and young child during infancy may improve neuropsychiatric outcome. Child psychosocial interventions can be successful in prevention of ADHD and conduct disorder in vulnerable children with heritable risk.(41) At preschool age, interventions aimed at improving parenting responses may help children better regulate emotion and executive function skills at older ages.(41)
Conclusion
Maternal factors, epigenetics, and the intrauterine milieu as well as the early postnatal experience all shape the developing ANS and limbic system. Dysfunction or dysmaturation of these systems are implicated in the subsequent development of neuropsychiatric disorders in childhood. To address the high and increasing prevalence of these disorders in children, a careful review of strategies to promote normal development of the ANS under adverse conditions such as prematurity is clearly indicated. Improved support of the mother, fetus, and infant by means of stress reduction, nutrition, and environmental improvements, may improve the ANS-mediated pathways associated with mental health in children and improve their long-term outcome. Further studies are needed to guide clinical and environmental management of the sick newborn with the goal of enhancing ANS development.
Acknowledgment of support
Dr. Mulkey receives support by Award Numbers UL1TR001876 and KL2TR001877 from the NIH National Center for Advancing Translational Sciences. The contents are solely the responsibility of the author and do not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
Disclosure statement: The authors have no conflicts of interest to disclose.
Contributor Information
Sarah B. Mulkey, Assistant Professor, Department of Pediatrics and Neurology, George Washington University School of Medicine and Health Sciences, Fetal-Neonatal Neurologist, Division of Fetal and Transitional Medicine, Children’s National Health System, Washington, District of Columbia
Adre J. du Plessis, Professor, Department of Pediatrics and Neurology, George Washington University School of Medicine and Health Sciences, Chief, Division of Fetal and Transitional Medicine, Children’s National Health System, Washington, District of Columbia
References
- 1.Montagna A, Nosarti C. Socio-Emotional Development Following Very Preterm Birth: Pathways to Psychopathology. Front Psychol 2016;7:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porges SW, Furman SA. The Early Development of the Autonomic Nervous System Provides a Neural Platform for Social Behavior: A Polyvagal Perspective. Infant Child Dev 2011;20:106–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hack M, Schluchter M, Cartar L, Rahman M. Blood pressure among very low birth weight (<1.5 kg) young adults. Pediatr Res 2005;58:677–84. [DOI] [PubMed] [Google Scholar]
- 4.Faa G, Manchia M, Pintus R, et al. Fetal programming of neuropsychiatric disorders. Birth Defects Research C, Embryo Today 2016;108:207–23. [DOI] [PubMed] [Google Scholar]
- 5.Atladottir HO, Parner ET, Schendel D, et al. Time trends in reported diagnoses of childhood neuropsychiatric disorders: a Danish cohort study. Arch Pediatr Adoles Med 2007;161:193–8. [DOI] [PubMed] [Google Scholar]
- 6.Thayer JF, Brosschot JF. Psychosomatics and psychopathology: looking up and down from the brain. Psychoneuroendocrinology 2005;30:1050–8. [DOI] [PubMed] [Google Scholar]
- 7.Pratt LA, Brody DJ. Depression in the United States household population, 2005–2006. NCHS Data Brief 2008:1–8. [PubMed] [Google Scholar]
- 8.Danielson ML, Bitsko RH, Ghandour RM, et al. Prevalence of Parent-Reported ADHD Diagnosis and Associated Treatment Among U.S. Children and Adolescents, 2016. J Clin Child Adoles Psychol 2018;47:199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Attention-Deficit/Hyperactivity Disorder (ADHD). Centers for Disease Control and Prevention; 2018. [updated 3/20/2018; cited 2018 4/12/2018]; Available from: https://www.cdc.gov/ncbddd/adhd/data.html.
- 10.Brown NM, Brown SN, Briggs RD, et al. Associations Between Adverse Childhood Experiences and ADHD Diagnosis and Severity. Acad Pediatr 2017;17:349–55. [DOI] [PubMed] [Google Scholar]
- 11.Pyhala R, Wolford E, Kautiainen H, et al. Self-Reported Mental Health Problems Among Adults Born Preterm: A Meta-analysis. Pediatrics 2017;139. [DOI] [PubMed] [Google Scholar]
- 12.Siddiqui S, Wilpers A, Myers M, et al. Autonomic regulation in fetuses with congenital heart disease. Early Hum Dev 2015;91:195–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stampalija T, Casati D, Monasta L, et al. Brain sparing effect in growth-restricted fetuses is associated with decreased cardiac acceleration and deceleration capacities: a case-control study. BJOG 2016;123:1947–54. [DOI] [PubMed] [Google Scholar]
- 14.McEwen BS, Nasca C, Gray JD. Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology 2016;41:3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hambleton MT, Reynolds EW, Sithisarn T, et al. Autonomic nervous system function following prenatal opiate exposure. Front Pediatr 2013;1:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freedman R, Hunter SK, Hoffman MC. Prenatal Primary Prevention of Mental Illness by Micronutrient Supplements in Pregnancy. Am J Psychiatry 2018. Epub 2018/03/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porges SW, Doussard-Roosevelt JA, Portales AL, et al. Infant regulation of the vagal “brake” predicts child behavior problems: a psychobiological model of social behavior. Dev Psychobiol 1996;29:697–712. [DOI] [PubMed] [Google Scholar]
- 18.Ondicova K, Mravec B. Multilevel interactions between the sympathetic and parasympathetic nervous systems: a minireview. Endocr Regul 2010;44:69–75. [DOI] [PubMed] [Google Scholar]
- 19.Barbe MF, Levitt P. The early commitment of fetal neurons to the limbic cortex. J Neurosci 1991;11:519–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci 1998;840:33–44. [DOI] [PubMed] [Google Scholar]
- 21.McEwen BS. Stress-induced remodeling of hippocampal CA3 pyramidal neurons. Brain Res 2016;1645:50–4. [DOI] [PubMed] [Google Scholar]
- 22.Drevets WC, Raichle ME. Neuroanatomical circuits in depression: implications for treatment mechanisms. Psychopharmacol Bull 1992;28:261–74. [PubMed] [Google Scholar]
- 23.Babaev O, Piletti Chatain C, Krueger-Burg D. Inhibition in the amygdala anxiety circuitry. Exp Mol Med 2018;50:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fyfe KL, Yiallourou SR, Wong FY, et al. The Effect of Gestational Age at Birth on Post-Term Maturation of Heart Rate Variability. Sleep 2015;38:1635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karin J, Hirsch M, Akselrod S. An estimate of fetal autonomic state by spectral analysis of fetal heart rate fluctuations. Pediatr Res 1993;34:134–8. [DOI] [PubMed] [Google Scholar]
- 26.Porges SW. The Polyvagal Theory. 1 ed. Schore AN, editor. New York: W. W. Norton & Company; 2011. 347 p. [Google Scholar]
- 27.Longin E, Gerstner T, Schaible T, et al. Maturation of the autonomic nervous system: differences in heart rate variability in premature vs. term infants. J Perinat Med 2006;34:303–8. [DOI] [PubMed] [Google Scholar]
- 28.Segar JL. Ontogeny of the arterial and cardiopulmonary baroreflex during fetal and postnatal life. Am J Physiol 1997;273:R457–71. [DOI] [PubMed] [Google Scholar]
- 29.F; PHBJPVMCTGDGR. Birth prematurity determines prolonged autonomic nervous system immaturity. Clin Auton Res 2004;14:391–5. [DOI] [PubMed] [Google Scholar]
- 30.Padhye NS, Verklan M, Brazdeikis A, et al. A comparison of fetal and neonatal heart rate variability at similar post-menstrual ages. Conf Proc IEEE Eng Med Bio Soc 2008;2008:2801–4. [DOI] [PubMed] [Google Scholar]
- 31.Reed SF, Ohel G, David R, Porges SW. A neural explanation of fetal heart rate patterns: A test of the polyvagal theory. Developmental psychobiology 1999;35:108–18. [PubMed] [Google Scholar]
- 32.Clairambault J, Curzi-Dascalova L, Kauffmann F, et al. Heart rate variability in normal sleeping full-term and preterm neonates. Early Hum Dev 1992;28:169–83. [DOI] [PubMed] [Google Scholar]
- 33.Yiallourou SR, Witcombe NB, Sands SA, et al. The development of autonomic cardiovascular control is altered by preterm birth. Early Hum Dev 2013;89:145–52. [DOI] [PubMed] [Google Scholar]
- 34.Electrophysiology TFotESoCtNASoP. Heart Rate Variability. Standards of Measurement, Physiological Interpretation, and Clinical Use. Eur Heart J 1996;93:1043–65. [PubMed] [Google Scholar]
- 35.Malliani A, Lombardi F, Pagani M. Power spectrum analysis of heart rate variability: a tool to explore neural regulatory mechanisms. Br Heart J 1994;71:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garabedian C, Champion C, Servan-Schreiber E, et al. A new analysis of heart rate variability in the assessment of fetal parasympathetic activity: An experimental study in a fetal sheep model. PloS one 2017;12:e0180653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fyfe KL, Yiallourou SR, Wong FY, et al. The development of cardiovascular and cerebral vascular control in preterm infants. Sleep Med Rev 2014;18:299–310. [DOI] [PubMed] [Google Scholar]
- 38.Thiriez G, Mougey C, Vermeylen D, et al. Altered autonomic control in preterm newborns with impaired neurological outcomes. Clin Auton Res 2015;25:233–42. [DOI] [PubMed] [Google Scholar]
- 39.Doussard-Roosevelt JA, McClenny BD, Porges SW. Neonatal cardiac vagal tone and school-age developmental outcome in very low birth weight infants. Dev Psychobiol 2001;38:56–66. [PubMed] [Google Scholar]
- 40.Fox NA, Porges SW. The relation between neonatal heart period patterns and developmental outcome. Child Dev 1985;56:28–37. [PubMed] [Google Scholar]
- 41.Beauchaine TP, Gatzke-Kopp L, Mead HK. Polyvagal Theory and developmental psychopathology: emotion dysregulation and conduct problems from preschool to adolescence. Biol Psychol 2007;74:174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramsay DS, Woods SC. Clarifying the roles of homeostasis and allostasis in physiological regulation. Psychol Rev 2014;121:225–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav 2003;43:2–15. [DOI] [PubMed] [Google Scholar]
- 44.Schulkin J Social allostasis: anticipatory regulation of the internal milieu. Front Evol Neurosci 2011;2:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldstein DS, Kopin IJ. Evolution of concepts of stress. Stress 2007;10:109–20. [DOI] [PubMed] [Google Scholar]
- 46.Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell 2008;135:422–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gluckman PD, Hanson MA, Pinal C. The developmental origins of adult disease. Matern Child Nutri 2005;1:130–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lucas A Programming by early nutrition in man. Ciba Found Symp 1991;156:38–50; discussion −5. [PubMed] [Google Scholar]
- 49.Burmeister M, McInnis MG, Zollner S. Psychiatric genetics: progress amid controversy. Nat Rev Genet 2008;9:527–40. [DOI] [PubMed] [Google Scholar]
- 50.Nemoda Z, Massart R, Suderman M, et al. Maternal depression is associated with DNA methylation changes in cord blood T lymphocytes and adult hippocampi. Transl Psychiatry 2015;5:e545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang WH, Lee IH, Chi MH, et al. Prefrontal cortex modulates the correlations between brain-derived neurotrophic factor level, serotonin, and the autonomic nervous system. Sci Rep 2018;8:2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghassabian A, Sundaram R, Chahal N, et al. Determinants of neonatal brain-derived neurotrophic factor and association with child development. Dev Psychopathol 2017;29:1499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fyfe K, Odoi A, Yiallourou SR, et al. Preterm Infants Exhibit Greater Variability in Cerebrovascular Control than Term Infants. Sleep 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nino G, Govindan RB, Al-Shargabi T, et al. Premature Infants Rehospitalized because of an Apparent Life-Threatening Event Had Distinctive Autonomic Developmental Trajectories. Am J Resp Crit Care Med 2016;194:379–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanna BD, Nelson MN, White-Traut RC, et al. Heart rate variability in preterm brain-injured and very-low-birth-weight infants. Biol Neonate 2000;77:147–55. [DOI] [PubMed] [Google Scholar]
- 56.Haraldsdottir K, Watson AM, Goss KN, et al. Impaired autonomic function in adolescents born preterm. Physiol Rep 2018;6:e13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Georgieff MK. Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr 2007;85:614S–20S. [DOI] [PubMed] [Google Scholar]
- 58.Grabrucker S, Boeckers TM, Grabrucker AM. Gender Dependent Evaluation of Autism like Behavior in Mice Exposed to Prenatal Zinc Deficiency. Front Behav Neurosci 2016;10:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siddiqui S, Fifer WP, Ordonez-Retamar M, et al. An antenatal marker of neurodevelopmental outcomes in infants with congenital heart disease. J Perinatol 2017;37:953–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun G, Shinba T, Kirimoto T, Matsui T. An Objective Screening Method for Major Depressive Disorder Using Logistic Regression Analysis of Heart Rate Variability Data Obtained in a Mental Task Paradigm. Front Psychiatry 2016;7:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stephan-Blanchard E, Chardon K, Djeddi DD, et al. The dynamics of cardiac autonomic control in sleeping preterm neonates exposed in utero to smoking. Clin Neurophysiol 2016;127:2871–7. [DOI] [PubMed] [Google Scholar]
- 62.Hunt RW, Tzioumi D, Collins E, et al. Adverse neurodevelopmental outcome of infants exposed to opiate in-utero. Early Hum Dev 2008;84:29–35. [DOI] [PubMed] [Google Scholar]
- 63.Sundelin Wahlsten V, Sarman I. Neurobehavioural development of preschool-age children born to addicted mothers given opiate maintenance treatment with buprenorphine during pregnancy. Acta Paediatr 2013;102:544–9. [DOI] [PubMed] [Google Scholar]
- 64.Goldstein JM, Holsen L, Huang G, et al. Prenatal stress-immune programming of sex differences in comorbidity of depression and obesity/metabolic syndrome. Dialogues Clin Neurosci 2016;18:425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guarino D, Nannipieri M, Iervasi G, et al. The Role of the Autonomic Nervous System in the Pathophysiology of Obesity. Front Physiol 2017;8:665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sanders MR, Hall SL. Trauma-informed care in the newborn intensive care unit: promoting safety, security and connectedness. J Perinatol 2018;38:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duffy AR, Schminkey DL, Groer MW, et al. Comparison of Hair Cortisol Levels and Perceived Stress in Mothers Who Deliver at Preterm and Term. Biol Res Nurs 2018;20:292–99. [DOI] [PubMed] [Google Scholar]
- 68.Hoffman MC, Mazzoni SE, Wagner BD, et al. Measures of Maternal Stress and Mood in Relation to Preterm Birth. Obstet Gynecol 2016;127:545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chang HP, Chen JY, Huang YH, et al. Factors Associated with Post-Traumatic Symptoms in Mothers of Preterm Infants. Arch Psychiatr Nurs 2016;30:96–101. [DOI] [PubMed] [Google Scholar]
- 70.Howe TH, Sheu CF, Wang TN, et al. Parenting stress in families with very low birth weight preterm infants in early infancy. Res Dev Disabil 2014;35:1748–56. [DOI] [PubMed] [Google Scholar]
- 71.Clouchoux C, Guizard N, Evans AC, et al. Normative fetal brain growth by quantitative in vivo magnetic resonance imaging. Am J Obstet Gynecol 2012;206:173 e1–8. [DOI] [PubMed] [Google Scholar]
- 72.Fumagalli M, Provenzi L, De Carli P, et al. From early stress to 12-month development in very preterm infants: Preliminary findings on epigenetic mechanisms and brain growth. PloS one 2018;13:e0190602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thompson C, Syddall H, Rodin I, et al. Birth weight and the risk of depressive disorder in late life. Br J Psychiatry 2001;179:450–5. [DOI] [PubMed] [Google Scholar]
- 74.McGrath JJ, Feron FP, Burne TH, et al. Vitamin D3-implications for brain development. J Steroid Biochem Mol Bio 2004;89–90:557–60. [DOI] [PubMed] [Google Scholar]
- 75.Mulinare J, Cordero JF, Erickson JD, et al. Periconceptional use of multivitamins and the occurrence of neural tube defects. JAMA 1988;260:3141–5. [PubMed] [Google Scholar]
- 76.CDC Grand Rounds: additional opportunities to prevent neural tube defects with folic acid fortification. MMWR Morb Mortal Wky Rep 2010;59:980–4. [PubMed] [Google Scholar]
- 77.Ross RG, Hunter SK, Hoffman MC, et al. Perinatal Phosphatidylcholine Supplementation and Early Childhood Behavior Problems: Evidence for CHRNA7 Moderation. Am J Psychiatry 2016;173:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McGrath J, Saari K, Hakko H, et al. Vitamin D supplementation during the first year of life and risk of schizophrenia: a Finnish birth cohort study. Schizophr Res 2004;67:237–45. [DOI] [PubMed] [Google Scholar]
- 79.Drewery ML, Gaitan AV, Spedale SB, et al. Maternal n-6 and n-3 fatty acid status during pregnancy is related to infant heart rate and heart rate variability: An exploratory study. Prostaglandins Leukot Essent Fatty Acids 2017;126:117–25. [DOI] [PubMed] [Google Scholar]
- 80.Gould JF, Treyvaud K, Yelland LN, et al. Seven-Year Follow-up of Children Born to Women in a Randomized Trial of Prenatal DHA Supplementation. JAMA 2017;317:1173–5. [DOI] [PubMed] [Google Scholar]
- 81.Baumgartner C Psychomotor and social development of breast-fed and bottle-fed babies during their first year of life. Acta paediatrica Hungarica 1984;25:409–17. [PubMed] [Google Scholar]
- 82.Lucas A, Morley R, Cole TJ, et al. Early diet in preterm babies and developmental status at 18 months. Lancet 1990;335:1477–81. [DOI] [PubMed] [Google Scholar]
- 83.Lucas A Does early diet program future outcome? Acta paediatrica Scandinavica Suppl 1990;365:58–67. [DOI] [PubMed] [Google Scholar]
- 84.De Rogalski Landrot I, Roche F, et al. Autonomic nervous system activity in premature and full-term infants from theoretical term to 7 years. Auton Neurosci 2007;136:105–9. [DOI] [PubMed] [Google Scholar]
- 85.Irving RJ, Belton NR, Elton RA, et al. Adult cardiovascular risk factors in premature babies. Lancet 2000;355:2135–6. [DOI] [PubMed] [Google Scholar]
- 86.Barker DJ, Eriksson JG, Forsen T, et al. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 2002;31:1235–9. [DOI] [PubMed] [Google Scholar]
- 87.Horne RS, Fyfe KL, Odoi A, et al. Dummy/pacifier use in preterm infants increases blood pressure and improves heart rate control. Pediatr Res 2016;79:325–32. [DOI] [PubMed] [Google Scholar]
- 88.Feldman R, Eidelman AI. Skin-to-skin contact (Kangaroo Care) accelerates autonomic and neurobehavioural maturation in preterm infants. Dev Med Child Neurol 2003;45:274–81. [DOI] [PubMed] [Google Scholar]
- 89.Kommers DR, Joshi R, van Pul C, et al. Features of Heart Rate Variability Capture Regulatory Changes During Kangaroo Care in Preterm Infants. J Pediatr 2017;182:92–8 e1. [DOI] [PubMed] [Google Scholar]
- 90.Arnon S, Diamant C, Bauer S, et al. Maternal singing during kangaroo care led to autonomic stability in preterm infants and reduced maternal anxiety. Acta Paediatr 2014;103:1039–44. [DOI] [PubMed] [Google Scholar]
- 91.Kisilevsky BS, Hains SM, Brown CA, et al. Fetal sensitivity to properties of maternal speech and language. Infant Behav Dev 2009;32:59–71. [DOI] [PubMed] [Google Scholar]
- 92.Rand K, Lahav A. Maternal sounds elicit lower heart rate in preterm newborns in the first month of life. Early Hum Dev 2014;90:679–83. [DOI] [PMC free article] [PubMed] [Google Scholar]


