Abstract
Background:
The literature on brain imaging in premature infants, is mostly made up of studies that evaluate neonates, yet the most dynamic time of brain development happens from birth to one year of age. This study was designed to obtain quantitative brain measures from Magnetic Resonance Imaging (MRI) scans of infants born prematurely at 12 months of age.
Methods:
The subject group was designed to capture a wide range of gestational age (GA) from premature to full term infants. An age-specific atlas generated quantitative brain measures. A regression model was used to predict effects of gestational age, sex, on brain measures.
Results:
There was a primary effect of sex on: 1) intracranial volume (ICV), males > females; 2) proportional cerebral cortical gray matter (females > males) and 3) cerebral white matter (males> females). GA predicted cerebral volume and cerebral spinal fluid (CSF). GA also predicted cortical gray matter in a sex specific manner with GA having a significant effect on cortical volume in the males, but not in females.
Conclusions and Relevance:
Sex differences in brain structure are large early in life. GA had sex specific effects highlighting the importance evaluating sex effects in neurodevelopmental outcomes of premature infants.
Introduction:
Advances in perinatal medicine and neonatal intensive care have successfully resulted in improved survival rates of preterm infants, in particular for those infants born with very low birth weight (VLBW, ≤1500 g) and extremely low birth weight (ELBW, <1000g) [1]Yet improvements in survival have not been accompanied by proportional reductions in the incidence of disability in this population. Major morbidities for this patient group include neurodevelopmental and behavioral abnormalities [2]. There is increasing knowledge that preterm infants are at higher risk for deficits later in life in multiple areas of cognitive function, and affecting as many as 50-70% of VLBW infants [3].
In response to this issue, there has been a major increase in the number of brain imaging studies in premature populations, striving to better understand the structural and functional neuroanatomy of these deficits. Most of these studies have utilized MRI imaging due to its lack of radiation exposure for infants and children. This is a large literature where most of the studies evaluate the subjects as neonates, though some also evaluate them as children or adults. In contrast, there are only a few brain imaging studies on premature infants in the first year of life. One group has evaluated the effects of intrauterine growth restriction (IUGR) on brain structure in preterm infants [4, 5], and another group has evaluated connectivity in preterm infants at 1-2 years of age [6].
One clear reason for the lack of studies at 12 months corrected age is that performing research scans on infants beyond the neonatal period is difficult at best. As sedation is not appropriate in this setting, children must be scanned during natural sleep. Some large groups have done this well [7]. However, without substantial infrastructure to support things such as night-time scanning and multiple attempts at scans, few researchers have attempted scans in this age range. Yet on the other hand, there is no other time in brain development that has as much dramatic change than the window between birth and one year of age [8]. The insults to the premature brain, incurred within the first few weeks and months of life, set the stage for an altered developmental trajectory that plays out throughout the remainder of development and maturation. Therefore, this time period between birth and one year is important to better understand how the premature brain develops. Also, this time period is characterized by plasticity suggesting that this is an epoch in which interventions may be most beneficial In addition, sex differences in brain structure and brain function are: 1) large in effect size [9], 2) ubiquitous from ion channel to brain volume [10], 3) present at birth [8], and 4) under-recognized [11]. In particular, genetic determinants of brain development have been shown to have sex-specific effects [12] suggesting that factors impinging upon brain development, such as preterm birth, act in the context of sex differences. Most imaging studies commonly control for the primary effect of sex on brain structure. Yet whether there are sex-specific effects of preterm birth is rarely evaluated, leaving a substantial void in an important aspect of our understanding of the effects of preterm birth on brain development. In fact, although there is one study of 8-year-olds born prematurely showing sex-specific brain findings (pre-term boys more likely to have lower white matter volume)[13], there has yet to be a study of preterm neonates or infants that addresses the issue of sex-specific findings in brain structure. To clarify, primary effects of sex on brain structure are often reported. For instance, in a recent study on a large sample of term-equivalent premature infants, male sex was associated with volumetric differences in brain regions[14]. However, these are considered to be indicative of sex differences seen in the brain that are part of normal development, which is how the findings were interpreted by the authors - a primary sex effect. However, no study has evaluated the interaction of sex and prematurity. That is, given the normal sex differences in brain morphology, what is the effect of gestational age on brain structure. Does gestational age impact different structures in the female brain than in the male brain?
The current study was designed to evaluate brain structure with MRI in a group of infants at 12 months of age (corrected for prematurity), utilizing a newly developed age-specific atlas. The sample was collected to represent a wide variance of GA (23 weeks through 41 weeks), and primary effects of GA and sex, as well as their interaction, were investigated.
Methods:
Infants were recruited through (1) the University of Iowa Children’s Hospital High-Risk Infant Follow-Up Program, (2) the hospital’s neonatal admissions registry, (3) e-mail advertisement to University affiliated employees, and (4) posted recruitment letters on pediatric nurse bulletins and outpatient clinics. Exclusionary criteria included significant co-morbidities such as epilepsy, major birth defects, or a history of neonatal surgical closure of a patent ductus arteriosus (PDA) by metal clip (a contraindication to MRI). The recruitment goal was to capture a wide variance in gestational age (GA), so we solicited for both preterm (defined as less than 37 weeks) and full term infants (define as at least 38 weeks or more GA) who were 12 months of age at the time of assessment. For infants born less than 40 weeks, the time at which they participated in the protocol was corrected for prematurity (corrected age). The protocol was approved by the Institutional Review Board (IRB) and all mothers signed informed consent. Families were instructed to wake their child a little earlier than normal, and to refrain from giving the child a morning nap or allowing the child to fall asleep during the trip to the hospital in order to maximize the chance of a successful scanning session.
A modified Hollingshead scale documented parental socio-economic status (SES). From the medical record we obtained the measures of gestational age (GA), and birth weight. In addition, the Score for Neonatal Acute Physiology-II (SNAP2) was calculated from 6 physiologic measures (on the day of birth). The SNAP2 has been validated as a measure of newborn illness severity [15]. Mothers of the infants were asked to complete the Social-Emotional and Adaptive Behavior Questionnaire from the Bayley Scales of Infant and Toddler Development III (Bayley-III) [16]. Measures used were the Social-Emotional composite score and the General Adaptive Composite (GAC) score based on corrected age at the time of testing. Most mothers finished the questionnaire during the assessment of the infant; however, others chose to take the questionnaire home to complete it and return it by mail.
A total of 67 MRI eligible infants were enrolled in the protocol. Of the 67 participants, 35 infants slept soundly and completed the scan; the remainder awoke at the initiation of or during the scan, which was then aborted. A total of 46 (69%) of the Bayley Questionnaires were completed, 29 of 35 for those infants who completed the scan and 17 of 32 for those infants who did not complete the scan. The remaining variables of GA, birthweight and SNAP2 scores were available for all infants. There were no differences in GA, birthweight, corrected age at time of testing, SNAP2, parental SES, or the Bayley Questionnaire scores between the group of infants that completed a scan and those that did not (see Table 1).
Table 1:
Demographic information on the group of infants who completed an MRI scan compared to the group who did not complete a scan.
| Completed MRI | No Scan | |||||
|---|---|---|---|---|---|---|
| (n=35) | (n=32) | |||||
| Mean | (SD) | Mean | (SD) | t | P | |
| GA, weeks | 33.32 | 4.95 | 33.51 | 4.63 | 0.16 | 0.87 |
| Birth weight, grams | 2064.8 | 1045.0 | 2014.81 | 1002.25 | 0.20 | 0.84 |
| Age (months)# | 12.79 | 1.46 | 12.87 | 1.25 | 0.25 | 0.80 |
| SNAP2$ | 12.03 | 16.5 | 9.93 | 9.90 | 0.60 | 0.54 |
| Parental SES* | 2.68 | 0.63 | 2.71 | 0.68 | 0.21 | 0.83 |
| Social-Emotional Scale@ | 102.2 | 18.27 | 101.9 | 12.73 | 0.05 | 0.96 |
| General Adaptive Composite (GAC)@ | 95.48 | 12.59 | 97.17 | 11.91 | 0.45 | 0.65 |
Corrected for weeks premature in all infants with GA <39 weeks
Score for Neonatal Acute Physiology-II
Based on modified Hollingshead scale of 1-5 with lower numbers indicating higher SES
Social-Emotional and Adaptive Behavior Questionnaire from the Bayley Scales of Infant and Toddler Development III (Bayley-III) completed by 29/35 mothers of those infants who completed a scan and 17/32 mothers of those infants who did not complete a scan
Of the 35 completed scans, 2 were of poor quality due to motion artifact making the final data set 33 infants with high-quality scans. For the 33 infants with a high-quality scan, demographics are displayed by sex in Table 2. The Bayley questionnaire was completed for 29 of 35 mothers of infants who completed the scan (16 of the 19 male subjects and 12 of the 14 female subjects). One infant (male) was listed as ‘probably’ small for gestational age (SGA) and one infant (male) listed as definitely SGA. As expected, the male infants had significantly higher birthweights compared to female infants. In addition, the male infants had a higher average GA (35.7 weeks) compared to female infants (30.6 weeks). Consistent with this, the males had significantly higher scores on the Bayley GAC (p = 0.03) score and lower SNAP2 scores, although the SNAP2 scores were not statistically significantly different (p = 0.09). There were no differences between male and female infants in age at time of testing, parental SES or the Bayley Social-Emotional Scale score.
Table 2:
Demographic information on male and female babies with a high quality scan
| Total of 33 MRI Scans | ||||||
|---|---|---|---|---|---|---|
| Males (n=19) GA range 28.00 – 41.14 wks |
Females (n=14) GA range 24.00 – 40.00 wks |
|||||
| Mean | (SD) | Mean | (SD) | t | P | |
| GA, weeks | 35.71 | 4.15 | 30.56 | 4.16 | 3.51 | <0.01 |
| Birth weight, grams | 2545.62 | 1015.65 | 1519.10 | 760.40 | 3.18 | <0.01 |
| Age (months)# | 12.65 | 1.42 | 12.71 | 1.44 | 0.12 | 0.90 |
| SNAP2$ | 6.88 | 7.79 | 17.07 | 22.66 | 1.74 | 0.09 |
| Parental SES* | 2.63 | 0.68 | 2.78 | 0.64 | 0.68 | 0.50 |
| Social-Emotional Scale@ | 105.60 | 12.76 | 96.67 | 24.52 | 1.26 | 0.22 |
| General Adaptive Composite (GAC)@ | 100.9 | 9.79 | 90.41 | 14.00 | 2.28 | 0.03 |
Corrected for weeks premature in all infants with GA <39 weeks
Score for Neonatal Acute Physiology-II
Based on modified Hollingshead scale of 1-5 with lower numbers indicating higher SES
Social-Emotional and Adaptive Behavior Questionnaire from the Bayley Scales of Infant and Toddler Development III (Bayley-III) completed by 29/35 mothers of those infants who completed a scan and 17/32 mothers of those infants who did not complete a scan
MR Image Acquisition
In order to reduce risks to the children, no sedation was used and a behavioral protocol was utilized to obtain images while the infant was asleep. Total scan time was approximately 30 minutes. All MRI data were acquired on a 3T Siemens Trio scanner (Siemens, Erlangen, Germany). The protocol acquired a 3-dimensional T1-weighted magnetization-prepared rapid-acquisition gradient-echo sequence in the coronal plane with 1-mm isotropic resolution. A turbo spin-echo T2-weighted sequence was also obtained in the coronal plane with 1-mm isotropic resolution.
Automated Pipeline for Tissue Classification and Quantitative Volumetric Data
To provide unbiased quantitative volumetric data for structural analysis, an automated atlas-based segmentation approach to tissue classification was implemented. The automated pipeline consisted of an initial registration to bring the atlas into subject-specific coordinates. Tissue classification was done using an expectation maximization (EM) algorithm followed by a level set segmentation developed to enhance white/gray matter differentiation in the cerebral cortex, and isolation of results to functionally specific regions for analysis. With the exception of the level set segmentation, all programs used for the pipeline are available through the open source software suite BRAINSTools [17]. An age appropriate atlas was developed to account for the spatial likelihood of structural morphology specific to the stage of development of interest. The atlas was comprised of eight manually corrected tissue maps of one-year-old subjects taken randomly from the sample population. The pipeline was applied to the images from each subject with successful T1- and T2-weighted magnetic resonance scans. Quantitative volumetric data were obtained by examining tissue measurements within the entire head as well as within functionally distinct regions (e.g., the temporal lobe) determined by Talairach atlas space.
To apply the spatial likelihoods of each tissue type contained in the atlas to a subject, the atlas and the subject must be registered to a common space. A stepwise process of incrementally improving registration was utilized. Each subject was first aligned in anterior commissure – posterior commissure (AC-PC) space, where the origin is defined as the center point of the anterior commissure (AC) and the anterior to posterior axis runs through the posterior commissure (PC) and lies in the mid sagittal plane (MSP). This was done with BRAINSConstellationDetector, a program which automatically identifies predefined anatomical landmarks of interest [18]. After AC-PC alignment, the atlas was registered to the subject using an affine transformation optimized for Mattes mutual information metric. This was done by comparing the T1-weighted subject image to a T1 reference image contained in the atlas. The results of the affine transformation were applied to the atlas as the input for a more accurate, high-dimensional registration, symmetric image normalization (SyN) registration [19]. The results from each registration were concatenated to obtain a combined transform.
The tool used for primary tissue classification was BRAINSABC [20]. BRAINSABC utilizes an EM algorithm for segmentation and iteratively improves bias correction, tissue classification, and atlas registration. The algorithm was given the atlas in subject space as well as T1- and T2-weighted images. Multimodal information greatly enhances segmentation results. Due to the large variability of distal cortical white and gray matter structure, the spatial priors included in the atlas were not sufficient for obtaining satisfactory segmentations in those areas. To improve segmentation a level set segmentation method was developed. An approach optimal for images with the intensity inhomogeneity commonly found in MRI was adapted for three-dimensional volumes and specified for white/gray matter differentiation [21]. The level set method iteratively evaluated the T1-weighted image and was initialized with results from the EM algorithm and the distal cortical region of the resulting white and gray matter labels were used to the correct the original label map. Figure 1 shows the results of a typical segmentation using the new atlas.
Figure 1: One year old atlas.
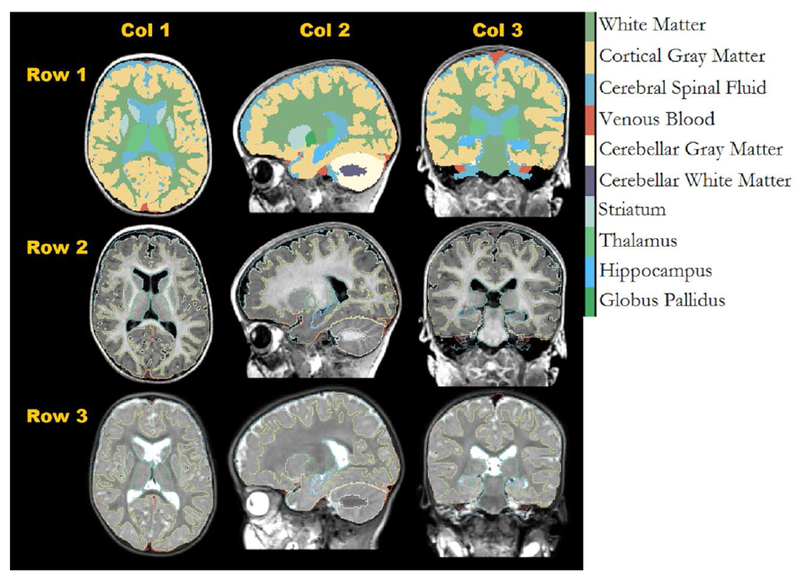
Axial (col 1), Sagittal (col 2), and coronal (col 3) views of a typical classification (row 1), with outlines overlay on T1 (row 2) and T2 (row3) images.
Brain Structure Measures
Intracranial volume (ICV) is a measure that represents tissue within the cranium. In order to control for total cranial size, all regional brain measures (cerebrospinal fluid (CSF), cerebrum, cerebellum, cortical gray, cerebral white, striatum, thalamus, and hippocampus) were adjusted for ICV by using a brain measure:ICV ratio, multiplied by 100 to represent a percentage of tissue proportional to ICV. In addition, regional measures of cerebral lobes (frontal, temporal, parietal, occipital) white and gray matter are obtained.
Statistical Analysis:
Sex differences in brain structure are robust and well-documented. Therefore, we performed all analyses on the combined sample (males and females) as well as separately for males and females. All analyses were performed by using Statistical Analysis System (SAS) procedures. The General Linear Models procedure was used to run regression models predicting quantitative measures of brain structure (dependent variables) based on GA and sex, controlling for parental SES. The distribution of GA was skewed (Shapiro-Wilk <0.05) and therefore was transformed to rank scores for normalization and to limit the influence of outlying GA values. The regression was run on the combined sample as well as for males and females separately. For the combined sample, sexes by GA interactions were entered into the model. A 2-tailed alpha level of 0.05 was used for significance tests. Given the exploratory nature of the analysis and the limited sample size, there was no correction for multiple comparisons.
As a follow-up to the regression analysis and as a method to visualize the effect more easily, each of the two samples (female and male) was split into an “Early GA” and a “Late GA” group based on the median GA of the group. Only the measures shown to have significant associated with GA, or significant sexes by GA interaction were analyzed. Z-scores of each measure were calculated and the Early and Late groups were compared to each other using analysis of covariance (ANCOVA), controlling for parental SES.
Results:
Primary Effects of Sex on Brain Structure.
Table 3 displays the results of the analysis evaluating the primary effect of sex on brain structure. The global measure of Intracranial Volume (ICV) was substantially greater in males compared to females. After controlling for ICV, females had proportionately greater volumes of cerebral gray matter and males had proportionately greater volumes of cerebral white matter. These effects are very robust given the small sample size. There were no effects of sex on volume of the cerebellum, CSF, or subcortical structures (striatum, thalamus, globus pallidus, and hippocampus).
Table 3:
Effects of Sex and Gestational Age on Brain Structure
| Brain Tissue Volumes (cm3) Females | Brain Tissue Volumes (cm3) Males | Primary Effect of Sex* (combined Sample) | Effect of GA Females | Effect of GA Males | Primary Effect of GA# (combined sample) | Sex*GA Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brain Structure Measures | Adjusted Mean (s.d.) | Adjusted Mean (s.d.) | F | (s.e.) | P | (s.e.) | p | (s.e.) | p | (s.e.) | p | |
| Intracranial Volume, ICV (cc) | 1,017.78 (103.17) | 1,121.24 (91.16) | 7.60 | <0.01 | 959.20 (7741.59) | 0.90 | −5595.42 (3588.50) | 0.13 | −2525.40 (2009.10) | 0.21 | −4364.60 (3964.02) | 0.28 |
| Cerebrum | 74.80 (1.18) | 74.13 (1.75) | 1.22 | 0.27 | 0.09 (0.08) | 0.29 | 0.15 (0.06) | 0.02 | 0.07 (0.02) | 0.01 | 0.05 (0.05) | 0.39 |
| Cerebellum | 9.08 (0.72) | 9.23 (0.56) | 0.22 | 0.64 | 6.73 ×103 (0.05) |
0.90 | 0.02 (0.02) | 0.41 | 0.01 (0.01) | 0.35 | 0.01 (0.02) | 0.55 |
| CSF | 10.19 (1.30) | 10.55 (1.81) | 0.36 | 0.55 | −0.13 (0.09) | 0.16 | −0.17 (0.06) | 0.02 | −0.97 (0.03) | <0.01 | −0.05 (0.06) | 0.41 |
| Cortical Gray | 50.80 (2.08) | 49.8 (2.24) | 8.67 | <0.01 | −0.05 (0.15) | 0.71 | 0.24 (0.07) | <0.01 | −0.03 (0.06) | 0.05 | 0.20 (0.08) | 0.02 |
| Cerebral White | 23.78 (1.86) | 24.21 (1.76) | 5.25 | 0.02 | 0.15 (0.11) | 0.21 | −0.09 (0.07) | 0.24 | 0.08 (0.08) | 0.86 | −0.15 (0.07) | 0.04 |
| Striatum | 1.58 (0.11) | 1.55 (0.12) | 0.05 | 0.82 | 11.77 × 103 (8.11 × 10−3) |
0.17 | −4.21 × 10−3 (4.76 × 10−3) |
0.38 | 7.29 × 10−3 (3.89 × 10−3) |
0.57 | −9.88 × 10−3 (4.99 × 10−3) |
0.05 |
| Thalamus | 1.15 (0.08) | 1.17 (0.10) | 0.29 | 0.59 | 1.74 × 10−3 (6.64 × 10−3) |
0.79 | −1.09 × 10−3 (4.77 × 10−3) |
0.82 | 0.26 × 10−3 (2.20 × 10−3) |
0.90 | −0.07 × 10−3 (4.43 × 10−3) |
0.86 |
| Globus Pallidus | 0.20 (0.04) | 0.21 (0.03) | 0.45 | 0.45 | −1.53 × 10−3 (3.01 × 10−3) |
0.61 | −2.15 × 10−3 (1.28 × 10−3) |
0.11 | −9.01 × 10−3 (0.80 × 10−3) |
0.26 | −1.36 × 10−3 (1.59 × 10−3) |
0.39 |
| Hippocampus | 0.44 (0.08) | 0.46 (0.11) | 0.09 | 0.76 | −1.97 × 10−3) (5.97 × 10−3) |
0.74 | −8.39 × 10−3 (4.48 × 10−3) |
0.07 | −3.09 × 10−3 (2.30 × 10−3) |
0.18 | −6.28 × 10−3 (4.48 × 10−3) |
0.17 |
controlling for parent SES, GA, and GA*sex when significant
controlling for parent SES and Sex
Effects of GA on Brain Structure.
Table 3 also displays the results of the analysis evaluating the effects of GA on brain structure. GA had no significant effect on ICV, the cerebellum, or any of the subcortical structures. However, GA did predict cerebrum volumes; cerebral volume increased with GA. Also, GA predicted CSF volumes; CSF volume decreased with increasing GA. These associations were found after controlling for ICV, indicating that an infant with lower GA has smaller cerebral volumes and therefore greater CSF volumes compared to an infant with higher GA. The effect of GA on cerebral volume and CSF was statistically significant in the male sample; although the females had the same pattern, the effect was not as strong and did not reach statistical significance. The sex by GA interaction terms for cerebral volume and CSF were not significant, indicating that although this effect was stronger in males, it was not an effect unique or specific to males only.
The two major tissue types within the cerebrum – cortical gray matter and cerebral white matter – had a sex-specific effect of GA. For the cortex, GA had a large effect with higher GA predicting higher volumes. This finding was specific to males as GA did not affect cortical volume in females (see Figure 2, panel A).
Figure 2:
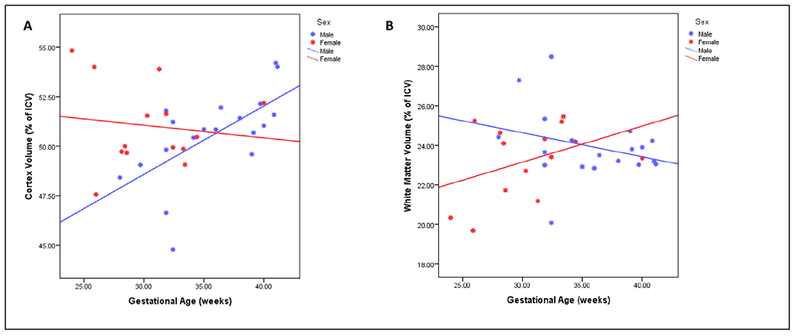
Relationship Between Brain Tissue Volume and Gestational Age: Panel A on the left shows the relationship between gestational age (X axis) and volume of the cortex (Y axis), separated by sex. For males, the higher the gestational age, the higher the cortex volume at 12 months (corrected) age. Panel B on the right shows the relationship between white matter volume and gestational age. Higher gestational age predicts greater white matter in females, but low volumes in males. This effect for both sexes was not statistically significant for either group, though the interaction is.
Given previous reports of SGA status specifically affecting cortical gray matter in premature infants [5], the two infants (both male) that were listed as probable or definite SGA were removed from the sample and the analysis repeated. Removing them did not change the any of the results.
In cerebral white matter, there was also a sex-specific finding. Higher GA predicted greater white matter in females, and in males it was the opposite pattern with higher GA predicting lower white matter volumes. This effect of GA on white matter for both sexes was not strong and was not statistically significant for either group. However, the direction of the effect being positive for females and negative for males resulted in the sex by GA interaction being significant (see Figure 2, panel B).
Because of the association of GA with cortical volume in males, a follow-up regional analysis was done evaluating the effect of GA on cortical volume within the 4 cerebral lobes (frontal, parietal, temporal, occipital). The results of this analysis are shown in Table 4. In general, the effect of GA on cortical volume was generalized with a positive coefficient for all 4 lobes. However, the effect followed a pattern in which the effect was greatest in the frontal lobe, followed in order by the parietal, temporal, and occipital lobes. The effects in the occipital and temporal lobes were so weak as to be statistically non-significant.
Table 4:
Regional Cortical Lobe Analysis in Male Infants
| Cortical Lobe Gray Matter Volume* | (s.e.)** | p |
|---|---|---|
| Frontal | 107.26 ×10−3 (32.8 × 10−3) |
<0.01 |
| Parietal | 53.19 ×10−3 (23.16 × 10−3) |
0.03 |
| Temporal | 46.36 ×10−3 (25.59 × 10−3) |
0.08 |
| Occipital | 23.31 ×10−3 (24.51 × 10−3) |
0.35 |
expressed as % of ICV
Linear Regression controlling for parent SES
Although the primary effects of age on brain structure as well as the sex specific interactions with gestational age are significant, the male infants are substantially larger in size than the female infants. This is likely due to the fact that the males have, on average, a higher gestational age, but mostly reflects the basic sex difference in size. When birth weight was entered into the models and controlled for, all primary effects of sex and sex interactions with gestational age remained significant. This supports the notion that the sex effects in the brain are primary and not simply due to a sex effect of difference of overall size of the infant.
Median Split Analysis:
Each sex was split into two groups defined by the median GA of that group. For the females, the median GA was 31 weeks, and the median GA for males was 36.0 weeks. This resulted in an Early GA group (n=7 females, n=9 males) and a Late GA group (n=7 females, n=10 males). Direct comparison of the Early and Late group for each sex was done using the brain measures that had significant effects of GA (cerebrum volume, CSF) or GA by sex interaction (cortical gray and cerebral white matter volume). Brain measures were transformed to z-scores in order to standardize the data and compare effects between males and females. Results are displayed in Figure 3 and show that for females, the Early GA group had lower cerebral volumes, lower white matter volumes, and higher CSF volumes compared to the females in the Late GA group. Importantly, these effects are modest with none of the comparisons being statistically significant. For the males, the Early GA group had substantially lower cerebral volumes, cortical gray volumes and increased CSF volumes compared to the Late GA males. All three of these measures reached statistical significance. Early GA males had somewhat higher volumes of white matter compared to Late GA males, though this did not reach significance. In general, all findings are more robust in the males compared to the females. This is most evident in the effect of GA on cortical volume which affected only the males. Yet in measures such as cerebral volume and CSF volume, GA had an effect on both males and females, but the effect was much stronger in the males. This is despite the fact that the female sample had an earlier mean GA, higher SNAP2 scores at birth, and lower scores on the Bayley Questionnaires compared to the males.
Figure 3:
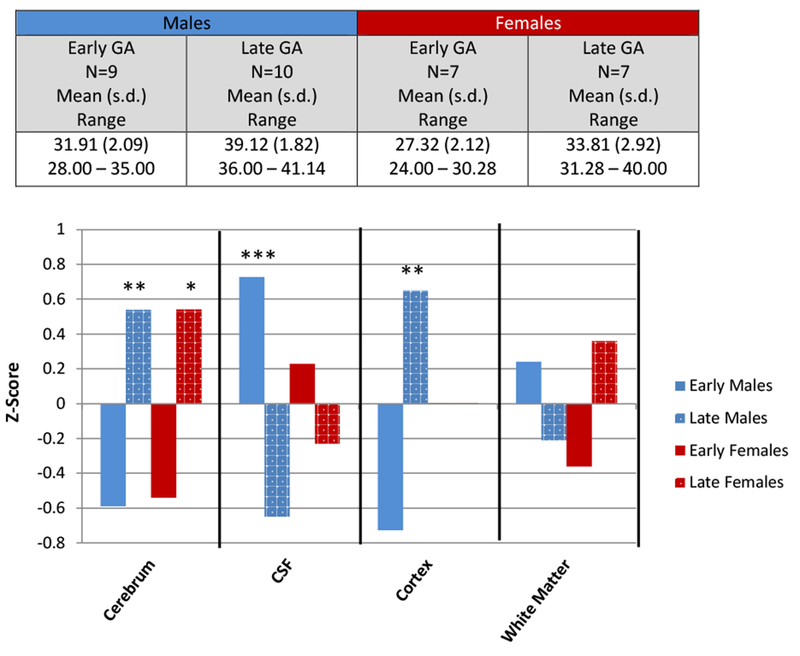
Median-Split Group Analysis
*p <0.10
**p <0.05
***p>0.01
Discussion:
Despite a massive growth in brain imaging studies on the premature brain, this study is one of very few reporting the effects of prematurity on brain development in infants at 12 months corrected age. The results indicate that prematurity has a global effect on cerebral volume, and within the cerebrum, the tissue affected is sex-specific with gestational age predicting lower volumes of cortex in males and lower volumes of cerebral white matter in females. In general, all effects of gestational age on brain development are more robust in male infants compared to female infants.
Most of the literature evaluating the brain in prematurity has utilized imaging done in the neonatal period, or MRIs obtained in long-term follow-up when the infants are children or adults. In just the past 2 years, at least 48 MRI brain imaging studies on premature neonates utilized a variety of assessments including qualitative readings of clinical scans, quantitative structure analysis, functional resting state MRI, diffusion tensor imaging (DTI) including tractography, quantitative measures of cortical structure, and assessment of specific regions such as the hippocampus, and cerebellum. This new body of literature continues to confirm the fact that white matter abnormalities (qualitative and quantitative) are commonly associated with prematurity.[22] In addition, a recent large (420 neonatal MRI scans from 338 infants) quantitative study showed decreased volumes of whole brain, cerebral white matter, cerebral cortical volume and subcortical volumes, and increases in CSF volumes in very preterm infants (defined as less than 30 weeks gestation) compared to full term infants, confirming that all tissues and regions within the cerebrum are affected by prematurity [23].
Many imaging studies of prematurity have utilized the statistical model of group comparison with ‘pre-term’ and ‘full-term’ being defined by weeks of gestational age, or by birth weight in which groups are defined as VLBW or ELBW. Yet, in reality, gestational age and birth weight are continuous variables with no clear biological ‘cut-off’ of what is normal. We recently reported that brain structure and developmental trajectory is altered in what is considered late preterm birth (34–36 weeks gestation) [24]. Therefore, results of group comparisons may vary depending upon what criteria the groups are made of. However, it is clear that the brain changes of prematurity occur along a spectrum in which lower gestational ages exhibit the greatest changes with late preterm birth subjects showing the mildest changes [23]. Instead of group comparisons (especially when samples are small), using the strategy used here of gestational age (GA) as a continuous variable predicting structural or functional brain measures may be a more appropriate approach than a group comparison analysis.
Although brain development in utero is rapid and dynamic, the period between birth and one year of age shows this period to be also dramatic in terms of both structural and functional change. These changes slow substantially in the second year. As shown in elegant work by the Gilmore lab, total brain volume increases 101% in the first year compared to only 15% in the second year of life [8]. Also, the majority of this increase between birth and one year is in gray matter which increases 149% compared to white matter which increases only 11% [7]. This suggests that in the time epoch between birth and one year, abnormalities in cortical development may be affected disproportionately compared to white matter given that the tissue undergoing the most dramatic change is the gray matter. Rapid growth means both increased vulnerability to insults (such as nutritional deficiency) as well as increased plasticity indicating an ability to recover [25]. In all, this is an extremely important time in neurodevelopment to evaluate, understand, and potentially intervene.
An important focus of the current study is on that of primary sex effects in brain structure as well as sex-specific effects of the neurobiology of prematurity. The primary sex effects seen here of larger ICV in males is one of the most frequently replicated sex differences in brain structure; it was first reported in 1892 [26], hundreds of times since then, [27] present at birth, [8] throughout the lifespan, [28] and also seen in primates [29]. Moreover, in addition to this global sex effect, the proportional difference in cortical and white matter volumes with females in general having greater gray matter volumes and males having greater white matter volume has also been reported[30].
However, more important than the primary sex effects are the sex-specific effects of prematurity on brain development. In the current study, the generalized effect of decreased cerebral volume and increased CSF was not sex specific, but was much more robust in the male sample despite the fact that compared to the males, the female infants were substantially more premature, more ill at birth (higher SNAP2 scores), and at the time of assessment, scored more poorly on parental developmental assessment tools (Bayley-III). In addition, effects of GA on cortical gray matter volume were seen in males only, a sex-specific finding. Although the effects of GA on the female brain were less severe than the males, the tissue type most affected in the females was the white matter.
It is well established, even epidemiologic dogma, that the male fetus is more vulnerable to developmental aberration such that male sex is an independent factor for any unfavorable perinatal outcome of pregnancy and delivery, including prematurity. Although well established in past literature, three recent studies evaluating a combined number of 4 million births confirmed this fact [31–33]. Moreover, within prematurity, male infants are more likely to suffer greater mortality and morbidity including abnormal neurodevelopmental outcomes [34]. Further, it is not just the vulnerability of males to adverse perinatal outcomes in general, but in particular, the male brain that appears to be most vulnerable to developmental aberration. The majority of neuropsychiatric syndromes with their origins in abnormal development are more common and more severe in males including all forms of Intellectual Disability (ID), autism, Tourette’s, attention deficit hyperactivity disorder and all learning disorders (especially language based ones such as dyslexia) [35]. In addition, studies in brain imaging on premature infants have shown greater qualitative ratings of abnormal brain structure, as well as volumetric abnormalities in quantitative studies, in males compared to females in both neonatal[36, 37] and childhood samples[13]. Given this background, it seems likely and even expected that the effects of prematurity on brain development would be more severe in males.
Our group has previously reported on sex differences in the long-term outcome (average age 12 years at time of assessment) of premature infants randomized to liberal or restricted transfusions. In that study, we found that females who received liberal transfusions were more likely to have adverse outcomes in brain structure and function [38]. Moreover, it was the white matter that was most affected in the liberally transfused females. Although the findings for that study were evaluating effects on the brain specifically in the context of transfusion, the fact that the white matter in females was the tissue most affected is in line with the current findings of premature females having possibly more developmental vulnerability of the white matter compared to males.
Although findings from the current study do not specify any mechanisms that could cause the sex-specific findings, both animal and human studies provide some clues. In our study, male infants were more likely to have GA affect gray matter. Iron deficiency in infancy has been shown to be detrimental to brain development and in particular affecting gray matter structures such as the hippocampus[39]. Moreover, studies have shown that male infants have lower iron stores than females [40]. For females, the current findings suggest that it is white matter that is more vulnerable to the effects of GA. Several studies in mice models have shown that female mice, compared to male mice, have a much greater rise in the chemokine Monocyte Chemoattractant Protein_2 (MCP_1) in response to lung injury [41]. In premature infants, MCP_1 has been shown to be specifically elevated and also associated with adverse outcomes such as Intraventricular Hemorrhage (IVH), Necrotizing Enterocolitis (NEC) and sepsis [42]. In fact, MCP_1 has been shown to be uniquely elevated in association with white matter brain injury in premature infants[43]. In sum, the sex-specific tissue differences in response to prematurity may also have sex-specific mechanisms with gray matter in boys being primarily affected by issues such as anemia and iron deficiency while white matter in females may be vulnerable to specific inflammatory chemokines.
Limitations to the current study include a small sample size and some inequalities in gestational age between the male and the female groups. Future research should include larger samples with male and female groups that are comparable, if not matched, on important variables such as gestational age and SNAP2 score. Despite the small samples of the current study, significant sex-specific differences were found suggesting that the effect sizes are large, diminishing the need for very large samples. Another limitation is that the findings from this study represent effects of sex on the brain at a single point in development, i.e. 12 months old. Further investigation is required to fully elucidate the trajectory of these differential sex effects on brain development over time, such as from birth and/or term-equivalent age, or even as compared to later in childhood and adolescence. Additionally, this study aimed to identify the specific brain structural areas where altered development in the preterm infant was mediated by sex. Future studies will be critical to identify possible mechanisms for these sex-specific brain findings. Additionally, further investigation is needed to understand how early environmental factors, as well as early interventional therapies, may impact this brain development in the first year of life, with particular attention to investigating what role sex may have in mediating those potential effects.
Conclusion:
Development of an age-specific atlas has allowed for detailed quantitative measurements of brain structure in a sample of infants with a wide range of GA, all assessed at 12 months of age. Primary effects of sex are strong at this age, and in addition we report effects of GA on brain structure. The effects of GA on brain structure are both generalized to both sexes (cerebral volume), but also sex-specific with GA affecting cortical volume only in males.
Acknowledgements:
We would like to thank all of the families who participated in our study. In addition, we acknowledge Corinne Hamlin, research coordinator for her time and effort.
There are no conflicts of interest for any author.
Funding:
The current study was funded by 2P01 HL046925 (Widness, PI), National Institutes of Heart, Lung and Blood and 1 U54 TR001013 (Rosenthal, PI), NCATS which funds our Clinical Research Unit.
Financial Support: This study was supported by NIH grant 2P01 HL046925, National Institute for Heart, Lung and Blood (NHLBI). Preterm Transfusions: Brain Structure and Function Outcomes.
Footnotes
Category of study: clinical, population study
Disclosure Statement: There are no conflicts of interest for any author.
References
- 1.Horbar JD, et al. , Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics, 2012. 129(6): p. 1019–26. [DOI] [PubMed] [Google Scholar]
- 2.Hintz SR, et al. , Early-childhood neurodevelopmental outcomes are not improving for infants born at <25 weeks’ gestational age. Pediatrics, 2011. 127(1): p. 62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aylward GP, Neurodevelopmental outcomes of infants born prematurely. J Dev Behav Pediatr, 2014. 35(6): p. 394–407. [DOI] [PubMed] [Google Scholar]
- 4.Padilla N, et al. , Differential effects of intrauterine growth restriction on brain structure and development in preterm infants: a magnetic resonance imaging study. Brain Res, 2011. 1382: p. 98–108. [DOI] [PubMed] [Google Scholar]
- 5.Padilla N, et al. , Differential vulnerability of gray matter and white matter to intrauterine growth restriction in preterm infants at 12 months corrected age. Brain Res, 2014. 1545: p. 1–11. [DOI] [PubMed] [Google Scholar]
- 6.Pandit AS, et al. , Whole-brain mapping of structural connectivity in infants reveals altered connection strength associated with growth and preterm birth. Cereb Cortex, 2014. 24(9): p. 2324–33. [DOI] [PubMed] [Google Scholar]
- 7.Knickmeyer RC, et al. , A structural MRI study of human brain development from birth to 2 years. J Neurosci, 2008. 28(47): p. 12176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilmore JH, et al. , Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J Neurosci, 2007. 27(6): p. 1255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cahill L, Why sex matters for neuroscience. Nat Rev Neurosci, 2006. 7(6): p. 477–84. [DOI] [PubMed] [Google Scholar]
- 10.Jazin E and Cahill L, Sex differences in molecular neuroscience: from fruit flies to humans. Nat Rev Neurosci, 2010. 11(1): p. 9–17. [DOI] [PubMed] [Google Scholar]
- 11.Cahill L and Aswad D, Sex Influences on the Brain: An Issue Whose Time Has Come. Neuron, 2015. 88(6): p. 1084–5. [DOI] [PubMed] [Google Scholar]
- 12.Lee JK, et al. , Sex-specific effects of the Huntington gene on normal neurodevelopment. J Neurosci Res, 2017. 95(1–2): p. 398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reiss AL, et al. , Sex differences in cerebral volumes of 8-year-olds born preterm. J Pediatr, 2004. 145(2): p. 242–9. [DOI] [PubMed] [Google Scholar]
- 14.Ball G, et al. , Multimodal image analysis of clinical influences on preterm brain development. Ann Neurol, 2017. 82(2): p. 233–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson DK, et al. , SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. J Pediatr, 2001. 138(1): p. 92–100. [DOI] [PubMed] [Google Scholar]
- 16.Bayley N, The Bayley Scales of Infant and Toddler Development - Third Edition (Bayley-III). 2005, The Psychological Corporation: San Antonio, Texas. [Google Scholar]
- 17.Kim RE, et al. , Preliminary analysis using multi-atlas labeling algorithms for tracing longitudinal change. Front Neurosci, 2015. 9: p. 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghayoor A, Vaidya J, and Johnson HJ, Development of a Novel Constellation Based Landmark Detection Algorithm. Proc Spie, 2013: p. 8669. [Google Scholar]
- 19.Avants BB, et al. , A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage, 2011. 54(3): p. 2033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young Kim E and Johnson HJ, Robust multi-site MR data processing: iterative optimization of bias correction, tissue classification, and registration. Front Neuroinform, 2013. 7: p. 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li C, et al. , A level set method for image segmentation in the presence of intensity inhomogeneities with application to MRI. IEEE Trans Image Process, 2011. 20(7): p. 2007–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hintz SR, et al. , Neuroimaging and neurodevelopmental outcome in extremely preterm infants. Pediatrics, 2015. 135(1): p. e32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makropoulos A, et al. , Regional growth and atlasing of the developing human brain. Neuroimage, 2016. 125: p. 456–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brumbaugh JE, et al. , Altered brain function, structure, and developmental trajectory in children born late preterm. Pediatr Res, 2016. 80(2): p. 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Georgieff MK, Brunette KE, and Tran PV, Early life nutrition and neural plasticity. Dev Psychopathol, 2015. 27(2): p. 411–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall J, Relations between the Weight of the Brain and its Parts, and the Stature and Mass of the Body, in Man. J Anat Physiol, 1892. 26(Pt 4): p. 445–500. [PMC free article] [PubMed] [Google Scholar]
- 27.Cahill L, Fundamental sex difference in human brain architecture. Proc Natl Acad Sci U S A, 2014. 111(2): p. 577–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nopoulos P, et al. , Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Res, 2000. 98(1): p. 1–13. [DOI] [PubMed] [Google Scholar]
- 29.Knickmeyer RC, et al. , Maturational trajectories of cortical brain development through the pubertal transition: unique species and sex differences in the monkey revealed through structural magnetic resonance imaging. Cereb Cortex, 2010. 20(5): p. 1053–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koscik T, et al. , Sex differences in parietal lobe morphology: relationship to mental rotation performance. Brain Cogn, 2009. 69(3): p. 451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peelen MJ, et al. , Impact of fetal gender on the risk of preterm birth, a national cohort study. Acta Obstet Gynecol Scand, 2016. 95(9): p. 1034–41. [DOI] [PubMed] [Google Scholar]
- 32.Verburg PE, et al. , Sexual Dimorphism in Adverse Pregnancy Outcomes - A Retrospective Australian Population Study 1981–2011. PLoS One, 2016. 11(7): p. e0158807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weng YH, Yang CY, and Chiu YW, Neonatal outcomes in relation to sex differences: a national cohort survey in Taiwan. Biol Sex Differ, 2015. 6: p. 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wallace ME, et al. , Racial/ethnic differences in preterm perinatal outcomes. Am J Obstet Gynecol, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Association A.P., Daignostic and Statistical Manual of Mental Disorders (5th ed). 2013, Washington, DC. [Google Scholar]
- 36.Skiold B, et al. , Sex differences in outcome and associations with neonatal brain morphology in extremely preterm children. J Pediatr, 2014. 164(5): p. 1012–8. [DOI] [PubMed] [Google Scholar]
- 37.Rose J, et al. , Neonatal brain structure on MRI and diffusion tensor imaging, sex, and neurodevelopment in very-low-birthweight preterm children. Dev Med Child Neurol, 2009. 51(7): p. 526–35. [DOI] [PubMed] [Google Scholar]
- 38.Nopoulos PC, et al. , Long-term outcome of brain structure in premature infants: effects of liberal vs restricted red blood cell transfusions. Arch Pediatr Adolesc Med, 2011. 165(5): p. 443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bastian TW, et al. , Iron Deficiency Impairs Developing Hippocampal Neuron Gene Expression, Energy Metabolism, and Dendrite Complexity. Dev Neurosci, 2016. 38(4): p. 264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Domellof M, et al. , Sex differences in iron status during infancy. Pediatrics, 2002. 110(3): p. 545–52. [DOI] [PubMed] [Google Scholar]
- 41.Lingappan K, et al. , Sex-specific differences in neonatal hyperoxic lung injury. Am J Physiol Lung Cell Mol Physiol, 2016. 311(2): p. L481–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otsubo Y, et al. , Association of cord blood chemokines and other biomarkers with neonatal complications following intrauterine inflammation. PLoS One, 2017. 12(5): p. e0175082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu H, et al. , Relationship between premature brain injury and multiple biomarkers in cord blood and amniotic fluid. J Matern Fetal Neonatal Med, 2017: p. 1–7. [DOI] [PubMed] [Google Scholar]


