Abstract
Sazetidine-A [6-(5(((S)-azetidine-2-yl)methoxy)pyridine-3-yl)hex-5-yn-1-ol] is a selective α4β2 nicotinic receptor desensitizing agent and partial agonist. Sazetidine-A has been shown in our previous studies to significantly reduce nicotine and alcohol self-administration in rats. The question arises whether sazetidine-A would reduce self-administration of other addictive drugs as well. Nicotinic receptors on the dopaminergic neurons in the ventral tegmental area play an important role in controlling the activity of these neurons and release of dopamine in the nucleus accumbens, which is critical mechanism for reinforcing value of drugs of abuse. Previously, we showed that the nonspecific nicotinic antagonist mecamylamine significantly reduces cocaine self-administration in rats. In this study, we acutely administered systemically sazetidine-A and two other selective α4β2 nicotinic receptor-desensitizing agents, VMY-2–95 and YL-2–203, to young adult female Sprague-Dawley rats and determined their effects on IV self-administration of cocaine and methamphetamine. Cocaine self-administration was significantly reduced by 0.3 mg/kg of sazetidine-A. In another set of rats, sazetidine-A (3 mg/kg) significantly reduced methamphetamine self-administration. VMY-2–95 significantly reduced both cocaine and methamphetamine self-administration with threshold effective doses of 3 and 0.3 mg/kg, respectively. In contrast, YL-2–203 did not significantly reduce cocaine self-administration at the same dose range and actually significantly increased cocaine self-administration at the 1 mg/kg dose. YL-2–203 (3 mg/kg) did significantly decrease methamphetamine self-administration. Sazetidine-A and VMY-2–95 are promising candidates to develop as new treatments to help addicts successfully overcome a variety of addictions including tobacco, alcohol as well as the stimulant drugs cocaine and methamphetamine.
Keywords: Sazetidine-A, VMY-2-95, YL-2-203, Stimulants, Addiction, Treatment
1. Introduction
Nicotinic acetylcholine interactions with dopaminergic (DA) systems play important roles in addiction. The DA projection from the ventral tegmental area (VTA) to the nucleus accumbens has been very well documented to play a key role in the reinforcing and rewarding effects of drugs of abuse (McFarland and Kalivas, 2001). It has become clear that the VTA to accumbens dopamine projection interacts with a variety of neural systems with which it is interconnected (Mansvelder and McGehee, 2000). Cholinergic innervation of the VTA from the pedunuclopontine nucleus and the dorsolateral tegmental nucleus plays a key role in controlling the activity of DA neurons (Mansvelder et al., 2003; Pidoplichko et al., 2004; Sziraki et al., 2002). Application of α4β2 nicotinic cholinergic antagonists to the VTA blocks this stimulation of dopamine neurons from cholinergic innervation from the pedunuclopontine nucleus and the dorsolateral tegmental nucleus and decreases self-administration of nicotine (Lanca et al., 2000) and blocks behavioral sensitization to cocaine (Champtiaux et al., 2006). Treatments focused on nicotinic receptors, particularly the α4β2 receptor subtype, hold promise to be new effective treatments of a variety of addictive drugs in addition to nicotine.
The central hypothesis of this study is that a nicotinic ligand that selectively desensitizes α4β2 nicotinic receptors in the VTA-nucleus accumbens dopaminergic system will be potential therapeutic agent for treating cocaine and methamphetamine addiction. It has been known for many years that acetylcholine, nicotine and other nicotinic agonists have dual actions to activate and then desensitize nicotinic receptors (Giniatullin et al., 2005; Katz and Thesleff, 1957; Kellar et al., 1999; Langley and Dickenson, 1889; Quick and Lester, 2002). In 1990, Kellar and colleagues proposed that the predominant in vivo effect of nicotine is that of a “time-averaged antagonist” from the receptor stimulation then desensitization to explain how a single injection of nicotine blocks subsequent nicotine-stimulated release of dopamine for several h (Hulihan-Giblin et al., 1990). In 2006, based on studies of in vitro pharmacological properties of sazetidine-A, a ligand that preferentially desensitizes α4β2 nicotinic receptors, making it resistant to further stimulation, Xiao et al. proposed to develop novel nicotinic therapeutics based on desensitization of nicotinic receptors, rather than on activation of them (Xiao et al., 2006). It should be noted that sazetidine-A has alsobeen found tohave actions at α7 nicotinic receptors, activating and then desensitizing them (Brown and Wonnacott, 2015). In subsequent studies in animal models by our group and others, sazetidine-A showed strong efficacies in inducing analgesia (Cucchiaro et al., 2008), generating antidepressant-like effects (Kozikowski et al., 2009; Turner et al., 2010), reducing nicotine self-administration (Johnson et al., 2012; Levin et al., 2010; Rezvani et al., 2010), decreasing alcohol intake (Rezvani et al., 2010), and reversing attentional impairments in rats (Rezvani et al., 2011; Rezvani et al., 2012).
The α4β2 nicotinic receptors in the VTA play key roles in the effects of DA acting stimulant drugs such as cocaine (Champtiaux et al., 2006). Of course, systemically administered drugs affect all parts of the brain, but actions in the VTA appear to be particularly relevant. Given the efficacy of sazetidine-A in reducing nicotine and alcohol self-administration, the current studies were conducted to determine its efficacy for reducing cocaine and methamphetamine self-administration. In addition, we tested two other compounds VMY-2–95 and YL-2–203 that also desensitize α4β2 nicotinic receptors and have shown efficacy for decreasing nicotine and/or alcohol self-administration (Liu et al., 2013; Yenugonda et al., 2013) to determine how different drugs in this class may have similar or diverse effects. Nicotinic α4β2 receptor desensitization with sazetidine-A and similar drugs may provide an effective approach to treating stimulant addiction.
2. Materials and methods
2.1. Subjects
Young adult female Sprague-Dawley albino rats were housed under approved standard laboratory protocols. To minimize stress induced by transportation, the rats were housed in a vivarium facility next to the behavioral testing rooms. The rats were kept on a 12:12 h reversed day-night cycle (lights on 18:00–6:00) so that they were in their active phase during behavioral testing. All rats had ad lib access to water and were fed rodent chow once daily to keep them at approximately 85% of their ad lib body weight. All procedures used in this study were approved by the Duke University Animal Care and Use Committee and conform to the Animal Care Guide.
2.2. Drug Preparation:
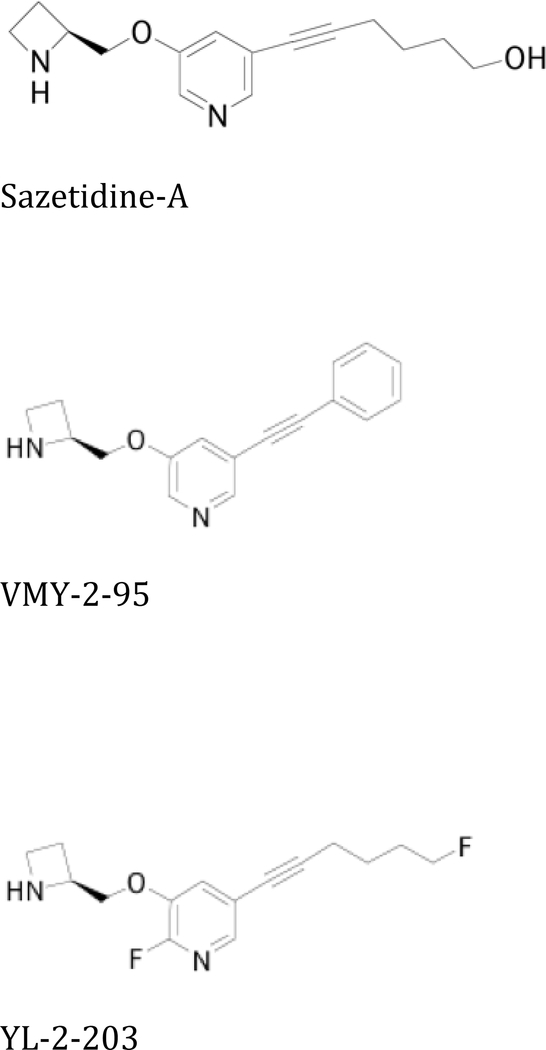
Solutions of sazetidine-A, VMY-2–95, YL-2–203, cocaine and methamphetamine were prepared in pyrogen-free glassware in sterile saline. The dose ranges tested were selected because in previous studies they have been shown to reduce self-administration of nicotine and/or alcohol (Levin et al., 2010; Liu et al., 2013; Rezvani et al., 2010; Yenugonda et al., 2013) Cocaine was supplied by NIDA, methamphetamine from Sigma-Aldrich (St. Louis, Mo, USA) and sazetidine-A,VMY-2–95 and YL-2–203 were synthesized by our co-authors Venkata M. Yenugonda, Yong Liu, and Milton L. Brown (Fig. 1).
Fig. 1:
Chemical structures of Sazetidine-A, VMY-2–95 and YL-2–203
2.3. Cocaine and Methamphetamine Self-Administration:
Following a week of acclimation, rats were placed in dual lever test chambers for behavioral testing. Each chamber was equipped with two levers, a tone generator, a house light and a cue light above each lever. Initially, rats were trained to press the levers on a FR-1 schedule for pellet reinforcement. Either the right or left lever was designated active for each rat such that half the animals received reinforcement for responding on the right lever and half for responding on the left. The cue light over the active lever was illuminated to indicate which side was correct. Responses on the active lever resulted in the immediate delivery of one 45 mg pellet and activation of the feedback tone for 0.5 s. Each session lasted for one h. Cessation of lever press training occurred when the rat had pressed the active lever a minimum of 50 times for pellet reinforcement during three consecutive sessions. Then, the rats had sterile surgery to implant IV catheters. The animals were anesthetized with a mixture of ketamine at a dose of 60 mg/kg and medetomidine at a dose of 15 mg/kg given IP. A plastic SoloPort was attached intraoperatively to a polyurethane catheter (Strategic Application Inc., Libertyville, IL, USA) and inserted into a subcutaneous interscapular pocket and sutured to underlying fascia of the rat. The rats were housed singly after surgery. Then the rats proceed to cocaine or methamphetamine training.
Cocaine (0.32 mg/kg/infusion) or methamphetamine (0.06 mg/kg/infusion) solutions were passed through a 0.22 μ filter (Millipore Corp, Billerica, MA USA) for sterilization before use. During each session a lever press on the active lever resulted in the activation of the feedback tone for 0.5 s, the immediate delivery of a 45 mg pellet, and the delivery of one 50 μl infusion of cocaine or methamphetamine in less than one s. Each infusion was immediately followed by a one-min timeout in which the house and cue lights went out and responses were recorded, but not reinforced. During the next phase, the rats completed one-h sessions in which pressing the active lever resulted in the activation of the feedback tone for 0.5 s, and the infusion of cocaine or methamphetamine (in < 1 s), without any pellet reinforcement. Dependent measures include the number of cocaine or methamphetamine infusions/session and the number of lever presses on the active and inactive levers/session. A computer programmed with MED-PC software controlled experimental events and data collection.
2.4. Locomotor Activity
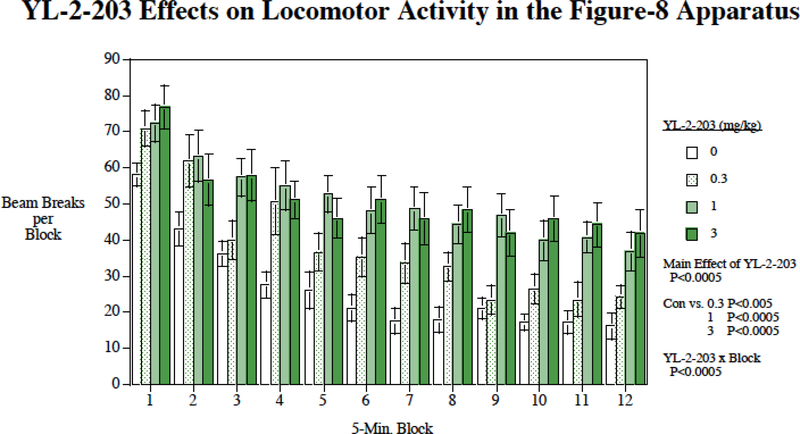
Acute YL-2–203 effects on locomotor activity were tested in a figure-8 maze over the course of a one-h session in young adult female Sprague-Dawley rats (N=20). The mazes had continuous enclosed alleys 10 ×10 cm in the shape of a figure-8. The overall dimensions of the apparatus were 70 cm long and 42 cm wide, with a 21×16 cm central arena, a 20-cm high ceiling and two blind alleys extending 20 cm from either side. Eight infrared photobeams, which crossed the alleys, indexed locomotor activity. One photobeam was located on each of the two blind alleys and three were located on each of two loops of the figure-8. The number of photobeam breaks was tallied during the one-h session with the data recorded in five-min time bins.. The linear and quadratic trends across twelve five-min blocks in each session were calculated to determine the habituation of locomotor activity over the course of the session.
Fig. 8:
Acute YL-2–203 effects on locomotor activity (mean±S.E.M.).
2.5. Food-Motivated Responding
Adult female rats (N=20) were assessed for the same YL-2–203 dose effects on food motivated responding. The test doses were administered in a repeated measures counterbalanced order. The behavioral paradigm used FR1, with activation of a feedback tone for 0.5 s after reinforcement. Cue lights were on throughout the session with no house light illumination and no time out after reinforcement. The rewards were 45-mg food pellets. As with cocaine SA the sessions for food SA were 1-hr. long. Sazetidine-A and VMY-2–95 were tested for effects on food motivated responding and locomotor activity in previous studies (Levin et al., 2010; Yenugonda et al., 2013).
2.6. Data Analysis:
The data were evaluated with analysis of variance (ANOVA) for within subjects factors. Drug dose (including vehicle control) and repetition of drug dose were repeated measures. Planned comparisons were made between the vehicle control dose and each of the drug treatment doses when there was a significant (P < 0.05) treatment effect. Alpha of P < 0.05 (two-tailed) was used as the threshold for statistical significance.
3. Results
3.1. Sazetidine-A
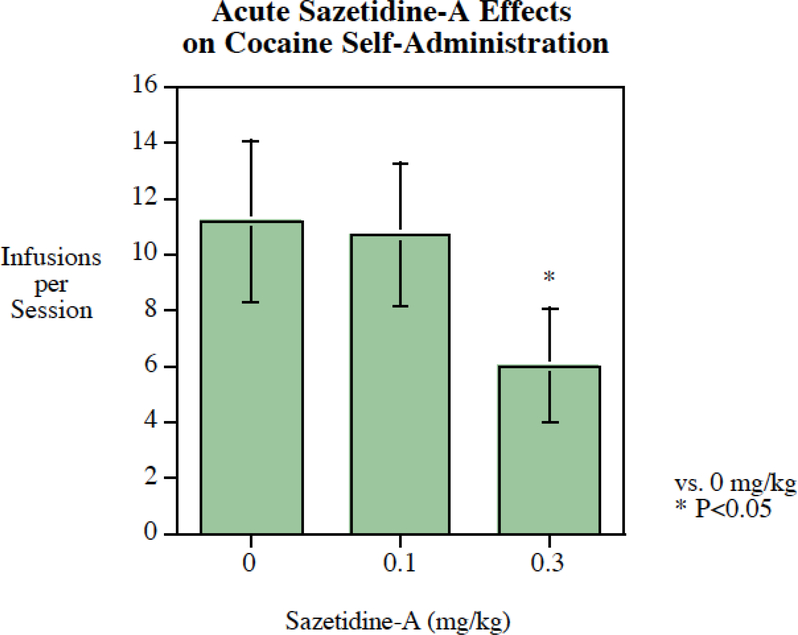
Acute sazetidine-A treatment (SC) significantly (P < 0.025) reduced cocaine self-administration in rats (Fig. 2) with an effective dose of 0.3 mg/kg. After four sessions of pre-training, the nine rats in this study averaged 8.8 ± 1.0 (mean ± S.E.M.) cocaine infusions per session with no treatment (i.e baseline). The rats averaged 11.2 ± 2.9 infusions after saline pretreatment, 10.7 ± 2.5 infusions after 0.1 mg/kg sazetidine-A pretreatment and 6.0 ± 2.0 infusions after 0.3 mg/kg sazetidine-A pretreatment. The main effect of sazetidine-A treatment was significant (P < 0.05). Follow-up planned comparisons of the drug treatments vs. saline showed that the 0.3 mg/kg dose of sazetidine-A resulted in a significant (P < 0.025) decrease in cocaine self-administration compared to the same rats when given saline pretreatment. This represented a 46% reduction in cocaine self-administration with an acute dose of 0.3 mg/kg of sazetidine-A.
Fig. 2:
Acute sazetidine-A administration significantly (P < 0.025) reduced cocaine self-administration, infusions per session (mean± S.E.M.) with a threshold of effect at 0.3 mg/kg, N=9
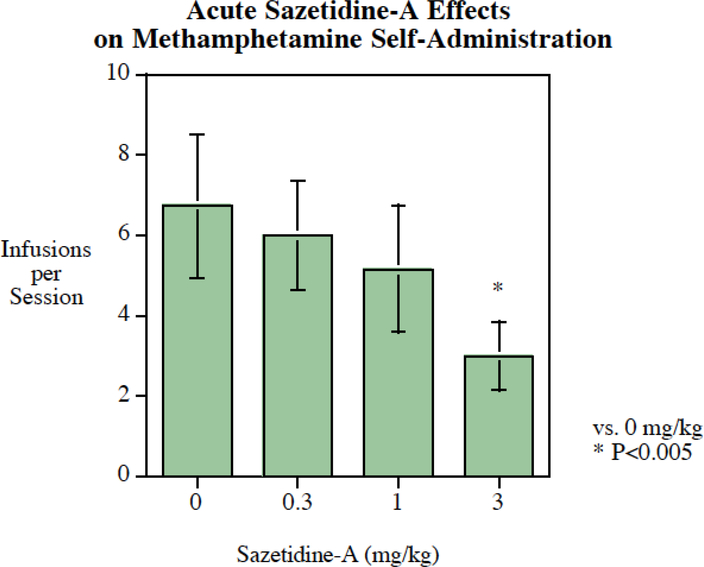
Acute sazetidine-A also reduced methamphetamine self-administration in rats with a significant (P < 0.025) main effect (Fig. 3). Planned comparisons of each dose to control showed that an effective dose of 3 mg/kg (P < 0.005). This represented a 56% reduction in methamphetamine self-administration when given 3 mg/kg sazetidine-A compared to the same rats when given saline pretreatment.
Fig. 3:
Acute sazetidine-A administration significantly (P < 0.005) reduced methamphetamine self-administration, infusions per session (mean± S.E.M.) with a threshold of effect at 3 mg/kg, N=14.
3.2. VMY-2–95
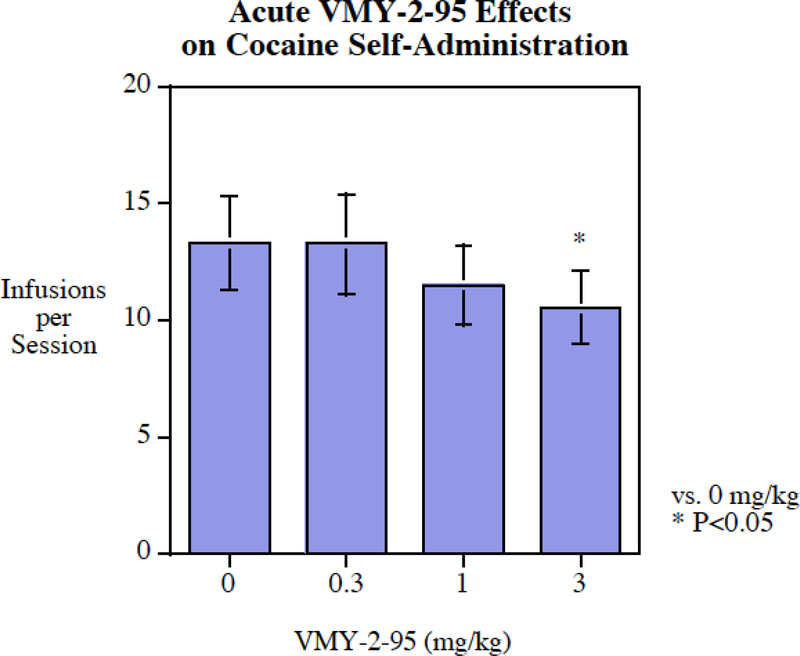
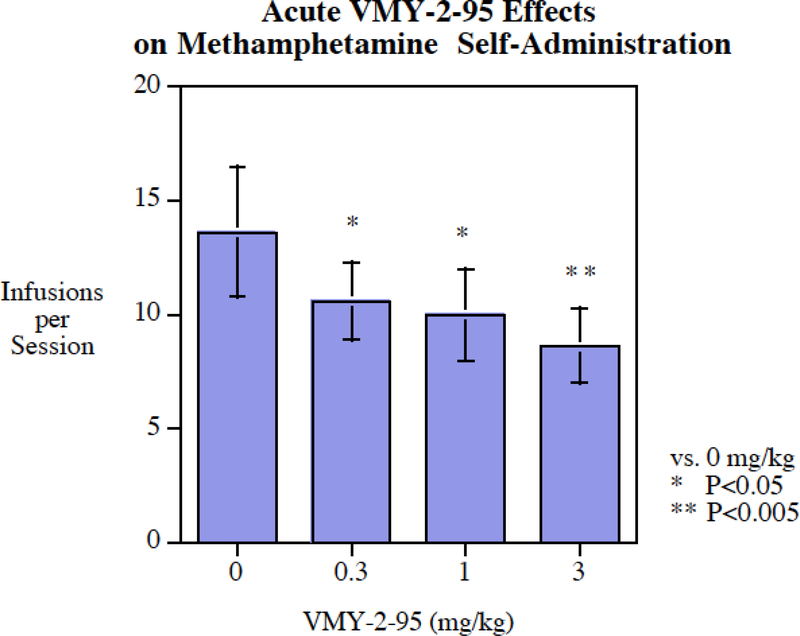
Acute injection of VMY-2–95 significantly reduced cocaine self-administration (Fig. 4). The 3 mg/kg VMY-2–95 was the minimal effective dose (P < 0.05). The effect was modest with only a 21% decrease. The same dose range was more effective in reducing methamphetamine self-administration (Fig. 5) with significant reductions at the 0.3 mg/kg (P < 0.05), 1 mg/kg (P < 0.05) and 3 mg/kg (P < 0.005) doses. The magnitude of methamphetamine reduction was greater than that of cocaine, with a reduction of 37% at the 3 mg/kg dose.
Fig. 4:
Acute VMY-2–95 administration significantly (P < 0.025) reduced cocaine self-administration, infusions per session (mean± S.E.M.) with a threshold of effect at 0.3 mg/kg, N=17
Fig. 5:
Acute VMY-2–95 administration significantly (P < 0.005) reduced methamphetamine self-administration, infusions per session (mean± S.E.M.) with a threshold of effect at 1 mg/kg, N=18.
3.3. YL-2–203
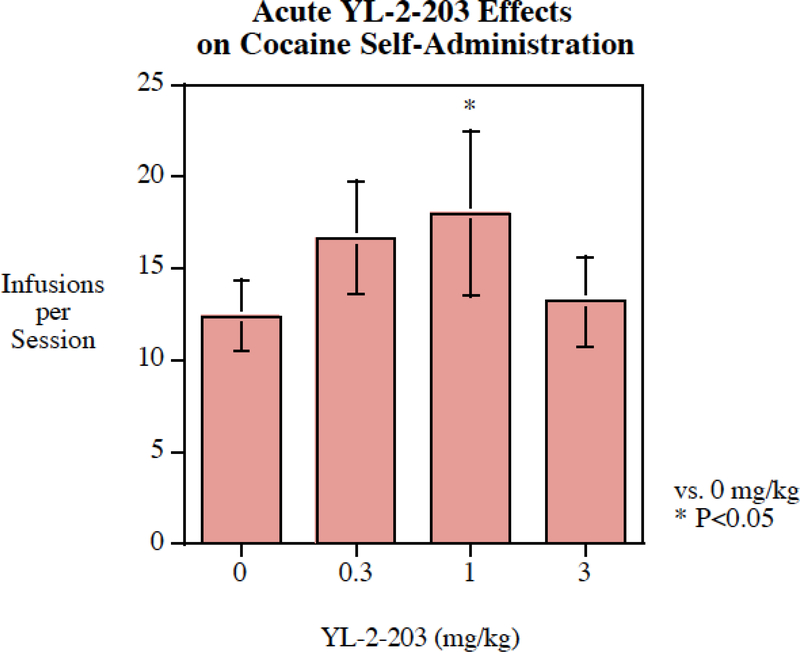
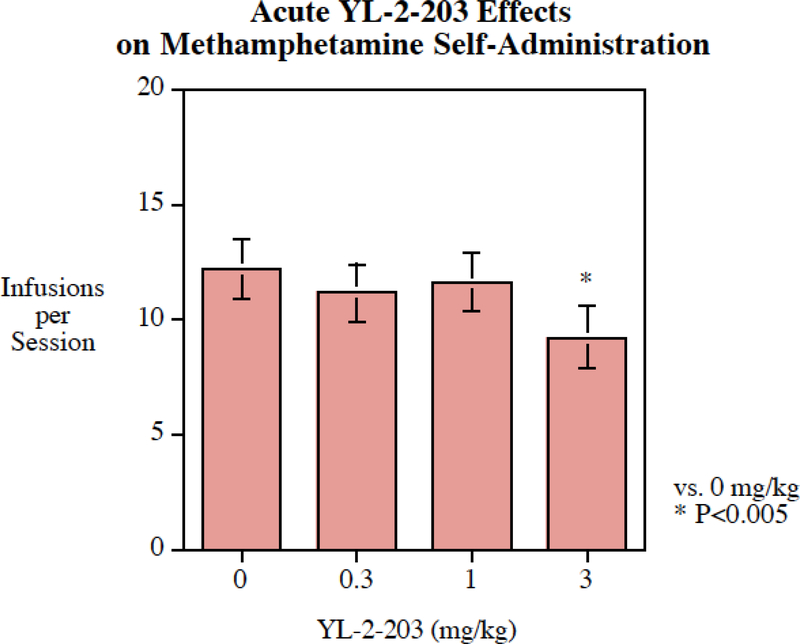
Acute administration of YL-2–203 (0.3–3 mg/kg) did not decrease cocaine self-administration (Fig. 6). In fact, the 1 mg/kg YL-2–203 significantly increased cocaine self-administration (P < 0 .05). Acute administration of 3 mg/kg of YL-2–203 (Fig. 7) did significantly decrease methamphetamine self-administration. The main effect of YL-2–203 on methamphetamine self-administration was significant (F(3,75) = 3.32, P < 0.025). Planned comparisons of each dose with the vehicle control showed that only the highest dose of 3 mg/kg YL-2–203 significantly (P < 0.005) reduced methamphetamine self-administration, with a 25% decrease from 12.2 ± 1.3 infusions per session to 9.2 ± 1.4 infusions per session (Fig. 7).
Fig. 6:
Acute YL-2–203 administration significantly (P < 0.025) increased cocaine self-administration, infusions per session (mean± S.E.M.) at the dose of 1 mg/kg, N=18
Fig. 7:
Acute YL-2–203 administration significantly (P < 0.005) reduced methamphetamine self-administration, infusions per session (mean± S.E.M.) with a threshold of effect at 1 mg/kg, N=18.
There was no significant effect of acute YL-2–203 on food-motivated responding. After vehicle administration the rats earned 106.0 ± 5.9 food pellets, after 0.3 mg/kg of YL-2–203 108.1 ± 5.9, after 1 mg/kg 95.7 ± 5.2 and after 3 mg/kg 99.4 ± 6.3. There was a significant increase in locomotor activity caused by acute YL-2–203. The main effect of YL-2–203 was significant (F(3,45) = 22.84, P < 0.0005) with significant increases in activity caused by 0.3 mg/kg (F(1,45) = 11.70, P < 0.005), 1 mg/kg (F(1,45) = 49.31, P < 0.0005) and 3 mg/kg (F(1,45) = 49.83, P < 0.0005). There was also a significant YL-2–203 × block interaction (F(33,495) = 1.68, P < 0.025). As shown in Fig. 8 there was an attenuated habituation over the 1-h session when YL-2–203 was administered.
4. Discussion
Our current data demonstrate that the compounds that preferential desensitize nicotinic α4β2 receptors can significantly reduce cocaine and methamphetamine self-administration in rats. We previously shown that sazetidine-A, a preferential nicotinic α4β2 receptor desensitizing agent, reduced nicotine self-administration in rats (Johnson et al., 2012; Levin et al., 2010; Rezvani et al., 2010). Interestingly, sazetidine-A also significantly reduces alcohol self-administration in selectively-bred alcohol preferring rats (Rezvani et al., 2010). The current study extends this line of research to show that sazetidine-A also significantly reduces self-administration of the stimulants, cocaine and methamphetamine.
The threshold for an effective dose of sazetidine-A for significantly reducing cocaine self-administration was an order of magnitude lower than the threshold dose for reducing nicotine self-administration or causing effects on locomotor activity. This was also lower than the effective doses for reducing alcohol and methamphetamine self-administration. We hypothesize that this may reflect the greater reliance of nicotine self-administration on conditioned sensory stimuli making it more challenging for pharmacological intervention to immediately alter self-administration patterns. It is true that nicotine self-administration is critically dependent on conditioned sensory cues (Caggiula et al., 2001). Sazetidine-A- induced reduction of the stimulants cocaine and methamphetamine may be related to desensitization of nicotinic receptors on dopaminergic neurons in the ventral tegmental area, which consequently reduces the rewarding effects of drugs by reducing the drug-induced dopamine release in the nucleus accumbens. Previously, we tested the effects of this dose range of sazetidine-A on food motivated responding and locomotor activity (Levin et al., 2010). We found that 3 mg/kg of sazeetidine-A but not lower doses caused a significant decrease in food motivated responding and hypoactivity. Given that a significant reduction in cocaine self-administration was seen in the current study at a dose of 0.3 mg/kg it seems unlikely that this effect was due possible sedative effects of more general effects on any sort of motivated responding. The effective dose for significantly reducing methamphetamine self-administration was 3 mg/kg, in the range that we also found to reduce nicotine and food-motivated responding and to cause modest hypoactivity in a test of locomotor activity in the Figure-8 apparatus (Levin et al., 2010) so that effect may be associated with a more pervasive behavioral action.
VMY-2–95 also significantly decreased cocaine self-administration, but this was rather a modest effect at a higher threshold dose of 3 mg/kg, ten times higher than an effective dose of sazetidine-A. As with sazetidine-A, VMY-2–95 had a greater effect on decreasing methamphetamine self-administration. For VMY-2–95 the minimal effective dose (0.3 mg/kg) for reducing methamphetamine was an order of magnitude lower than for reducing cocaine self-administration. Previously, we tested the effects of this dose range of VMY-2–95 on locomotor activity and found that the dose range that significantly reduced cocaine and methamphetamine self-administration did not cause locomotor hypoactivity. In fact, this dose range caused modest hyperactivity in the Figure-8 apparatus (Yenugonda et al., 2013). Thus, the observed reductions in cocaine and methamphetamine seen in the current studies was likely not secondary to sedative effects of VMY-2–95.
YL-2–203, in contrast to sazetidine-A and VMY-2–95, caused a significant elevation of cocaine self-administration at the 1 mg/kg dose. Similar to sazetidine-A and VMY-2–95, YL-2–203 caused a significant, but modest reduction in methamphetamine self-administration with the 3 mg/kg dose. The increase in cocaine self-administration may have resulted from the rats reacting to diminished reinforcement with greater response to overcome it. However, it is curious that the YL-2–203 induced increase in responding is seen with cocaine, but not with methamphetamine. Further research with chronic YL-2–203 could help determine whether compensatory responding is resulting from acute desensitization and then reduces with further testing.
In previous studies we have shown that administration of the noncompetitive nicotinic antagonist mecamylamine in rats causes a significant reduction in cocaine self-administration (Levin et al., 2000). Others have replicated this effect in rodents (Blokhina et al., 2005) and earlier studies had shown that mecamylamine given to cocaine addicts significantly reduces cocaine cue-elicited craving (Reid et al., 1999). However, the nonspecific nature of mecamylamine blockade across all nicotinic receptor subtypes compromises its use as therapeutic treatment due to pronounced side effects including sedation, constipation and orthostatic hypotension (Taylor, 2001).
The key nicotinic receptor in the VTA controlling dopaminergic activity is the α4β2 subtype. It has been shown that local application of the selective α4β2 antagonist dihydro-β-erythroidine (DHβE) to the VTA causes a significant decrease in dopaminergic activity and self-administration of nicotine (Lanca et al., 2000). However, DHβE is not a good drug candidate because of its very limited penetrance of the blood-brain barrier. Other selective nicotinic α4β2 subtype ligands may be more useful.
Nicotinic receptors are easily desensitized rendering them resistant to subsequent stimulation and this effect may be a useful mechanism for drug development (Buccafusco et al., 2009; Picciotto et al., 2008). We have developed a lead compound, sazetidine-A, in a novel class of nicotinic receptor desensitizers. Sazetidine-A is very selective to the α4β2 receptor subtype (Xiao et al., 2006). In a series of studies, we have shown that acute and chronic treatment with sazetidine-A significantly reduces self-administration of nicotine in the rat model (Johnson et al., 2012; Levin et al., 2010; Rezvani et al., 2010). We have also found that acute and chronic sazetidine-A treatment significantly reduces alcohol intake and preference in rats selectively bred for high alcohol intake and preference (Rezvani et al., 2010). The current study examined this line of treatment for use in combating stimulant addiction focusing on the two most widely abused illegal stimulants, cocaine and methamphetamine.
It is important to note that although we first reported that sazetidine-A did not activate nicotinic receptors, as measured with ion flux assays in a mammalian cell model (Xiao et al., 2006), later studies measuring ligand-induced currents using oocyte expression systems and dopamine release measurements in rat striatal slice demonstrated that sazetidine-A has short-lasting agonist activity, possibly dependent on subunit stoichiometry (Zwart et al., 2008). Carbone at al. reported that sazetidine-A shows full agonist activity at (α4)2( β2)3 nicotinic receptors but nearly no agonist activity (less than 1% efficacy of that of acetylcholine) at (α4)3(β2)2 nicotinic receptors (Carbone et al., 2009). It is important to note that two populations of α4β2 nicotinic receptors in mouse brain display similar agonist-induced desensitization properties (Marks et al., 1994) and that both (α4)2( β2)3 and (α4)3(β2)2 nicotinic receptors are desensitized by nicotinic agonists at more than 20-fold lower concentrations than the concentrations to activate the receptors (Rollema et al., 2007). Furthermore, Rollema et al. compared the unbound brain concentration of nicotinic agonists, the concentration-activation curves and the concentration-desensitization-curves (Rollema et al., 2007; Rollema et al., 2009). They revealed that at the unbound brain concentrations of nicotine, varenicline, cytisine or dianicline, there is very little, if any, possibility for receptor activation. These in vitro and in vivo studies are highly supportive of the notion that desensitization is the predominant mechanism for effects of nicotine and other nicotinic drugs. Nicotinic receptor desensitization has received attention in the field for several years as the mechanism to develop nicotinic therapeutics (Buccafusco et al., 2009; Picciotto et al., 2008).
The relationship of neuronal nicotinic receptor systems with dopaminergic neurons in the VTA is likely the key in the effect of sazetidine-A reducing stimulant self-administration given that the stimulants act directly on neuronal dopaminergic systems. It is likely that, desensitization of α4β2 receptors in the VTA by these compounds leads to reduction of stimulant-elicit dopamine release in the nucleus accumbens and consequently leading to less rewarding effects.
It has been shown that the mesolimbic dopaminergic system is considered a principal site for nicotine-cocaine interactions. Intra-nucleus accumbens perfusion of mecamylamine or co-perfusion of methyllycaconitine (MLA) and dihydro-β-erythroidine (DHβE) decreased cocaine-elicited increase in DA perfusate levels in mice confirming the dynamic interaction of nicotinic receptors in the mesolimbic dopaminergic system and cocaine (Zanetti et al., 2007). Further research is needed to determine whether the same effects are seen with effects on methamphetamine self-administration.
Interestingly, there is evidence that sazetidine-A itself has reinforcing effects (Paterson et al., 2010). This may be due to the partial agonist effect of sazetidine-A. Alternatively, it may be the pronounce desensitizing effects of sazetidine-A that has reinforcing actions.
Overall, the current results as well as our previous findings (Johnson et al., 2012; Levin et al., 2010; Rezvani et al., 2010) support the view that nicotinic receptor desensitization, in particular desensitization of α4β2 nicotinic receptor, significantly reduces self-administration of alcohol and nicotine and stimulants cocaine and methamphetamine in rats. It is possible that nicotinic α7 receptor actions may be involved as well since sazetidine-A has been found to stimulate and then desensitize these receptors as well (Brown and Wonnacott, 2015). Sazetidine-A and similar compounds such as VMY-2–95 could be promising therapy for helping people quit using stimulants like cocaine and methamphetamine.
Acknowledgement
Research supported by NIDA U19 grant DA027990.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blokhina EA, et al. , 2005. Effects of nicotinic and NMDA receptor channel blockers on intravenous cocaine and nicotine self-administration in mice. European Neuropsychopharmacology. 15, 219–25. [DOI] [PubMed] [Google Scholar]
- Brown JA, Wonnacott S, 2015. Sazetidine-A activates and desensitizes native a7 nicotinic acetylcholine receptors. Neurochemical Research. 40, 2047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccafusco JJ, Brach JW, Terry AV, 2009. Desensitization of nicotinic acetylcholine receptors as a stategy for drug development. The Journal of Pharmacology and Experimental Therapeutics. 328, 364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, et al. , 2001. Cue dependency of nicotine self-administration and smoking. Pharmacology, Biochemistry & Behavior. 70, 515–30. [DOI] [PubMed] [Google Scholar]
- Carbone AL, et al. , 2009. Pentameric concatenated (a4)2(b2)3 and (a4)3(b2)2 nicotinic acetylcholine receptors: subunit arrangement determines functional expression. British Journal of Pharmacology. 156, 970–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N, Kalivas PW, Bardo MT, 2006. Contribution of dihydro-beta-erythroidine sensitive nicotinic acetylcholine receptors in the ventral tegmental area to cocaine-induced behavioral sensitization in rats. Behavioral Brain Research. 168, 120–126. [DOI] [PubMed] [Google Scholar]
- Cucchiaro G, et al. , 2008. Analgesic effects of Sazetidine-A, a new nicotinic cholinergic drug. Anesthesiology. 109, 512–519. [DOI] [PubMed] [Google Scholar]
- Giniatullin R, Nistri A, Yakel JL, 2005. Desensitization of nicotinic ACh receptors: shaping cholinergic signaling. Trends in Neurosciences. 28, 371–378. [DOI] [PubMed] [Google Scholar]
- Hulihan-Giblin BA, Lumpkin ML, Kellar KJ, 1990. Acute effects of nicotine on prolactin release in the rat: Agonist and antagonist effects of a single injection of nicotine. The Journal of Pharmacology and Experimental Therapeutics. 252, 15–20. [PubMed] [Google Scholar]
- Johnson JE, et al. , 2012. Chronic sazetidine-A infusion reduces nicotine self-administration in male and female rats. Psychopharmacology. 222, 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Thesleff S, 1957. A study of the desensitization produced by acetylcholine at the motor endplate. Journal of Physiology. 138, 63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellar KJ, Davila-Garcia MI, Xiao Y, 1999. Pharmacology of neuronal nicotinic acetylcholine recceptors: effects of acute and chronic nicotine. Nicotine & Tobacco Research. 1 Suppl 2, S117–20; discussion S139–40. [DOI] [PubMed] [Google Scholar]
- Kozikowski AP, et al. , 2009. Chemistry and pharmacology of nicotinic ligands based on 6-[5-(azetidin-2-ylmethoxy)pyridin-3-yl]hex-5-yn-1-ol (AMOP-H-OH) for possible use in depression. Chem Med Chem. 4, 1279–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanca AJ, et al. , 2000. The pedunculopontine tegmental nucleus and the role of cholinergic neurons in nicotine self-administration in the rat: a correlative neuroanatomical and behavioral study. Neuroscience. 96, 735–742. [DOI] [PubMed] [Google Scholar]
- Langley JH, Dickenson WL, 1889. On the local paralysis of the peripheral ganglia and on the connexion of different classes of nerve fibres with them. Proceedings of the Royal Society. 46, 423–431. [Google Scholar]
- Levin ED, et al. , 2000. The nicotinic antagonist mecamylamine preferentially inhibits cocaine vs. food self-administration in rats. Physiology & Behavior. 71, 565–70. [DOI] [PubMed] [Google Scholar]
- Levin ED, et al. , 2010. Sazetidine-A, a selective α4β2 nicotinic receptor desensitizing agent and partial agonist reduces nicotine self-administration in rats. Journal of Pharmacology and Experimental Therapeutics. 332, 933–939. [DOI] [PubMed] [Google Scholar]
- Liu Y, et al. , 2013. Chemistry and pharmacological studies of 3-alkoxy-2,5-disubstituted-pyridinyl compounds as novel selective α4β2 nAChRs ligands that reduces alcohol intake in rats. Journal of Medicinal Chemistry. 56, 3000–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS, 2000. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 27, 349–57. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, et al. , 2003. Cholinergic modulation of dopaminergic reward areas: upstream and downstream targets of nicotine addiction. European Journal of Pharmacology. 480, 117–23. [DOI] [PubMed] [Google Scholar]
- Marks MJ, et al. , 1994. Desensitization of nicotine-stimulated 86Rb+ efflux from mouse brain synaptosomes. J Neurochem. 63, 2125–2135. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW, 2001. The circuitry mediating cocaine-induced reinstatement of drugseeking behavior. Journal of Neuroscience. 21, 8655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, et al. , 2010. The high-affinity nAChR partial agonists varenicline and sazetidine-A exhibit reinforcing properties in rats. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 34, 1455–64. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, et al. , 2008. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Progress in Neurobiology. 84, 329–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoplichko VI, et al. , 2004. Nicotinic cholinergic synaptic mechanisms in the ventral tegmental area contribute to nicotine addiction. Learning & Memory. 11, 60–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick M, Lester R, 2002. Desensitization of neuronal nicotinic receptors. Journal of Neurobiology. 53, 457–478. [DOI] [PubMed] [Google Scholar]
- Reid MS, et al. , 1999. A nicotine antagonist, mecamylamine, reduces cue-induced cocaine craving in cocaine-dependent subjects. Neuropsychopharmacology. 20, 297–307. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, et al. , 2010. Sazetidine-A, a selective α4β2 nicotinic acetylcholine receptor desensitizing agent and partial agonist reduces both alcohol and nicotine self-administration in selectively-bred alcohol preferring (P) rats. Psychopharmacology. 211, 161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, et al. , 2011. Sazetidine-A, a selective α4β2 nicotinic acetylcholine receptor desensitizing agent reverses dizocilpine and scopolamine-induced attentional impairments in rats. Psychopharmacology. 215, 621–630. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, et al. , 2012. Effects of chronic sazetidine-A, a selective β2* nicotinic receptor desensitizing agent on pharmacologically-induced impaired sustained attention in rats. Psychopharmacology. 222, 269–276. [DOI] [PubMed] [Google Scholar]
- Rollema H, et al. , 2007. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 52, 985–994. [DOI] [PubMed] [Google Scholar]
- Rollema H, et al. , 2009. Preclinical pharmacology of the alpha4beta2 nAChR partial agonist varenicline related to effects on reward, mood and cognition. Biochem Pharmacol. 78, 813–24. [DOI] [PubMed] [Google Scholar]
- Sziraki I, et al. , 2002. Receptors in the ventral tegmental area mediating nicotine-induced dopamine release in the nucleus accumbens. Neurochemical Research. 27, 253–61. [DOI] [PubMed] [Google Scholar]
- Taylor P, 2001. Agents acting at the neuromuscular junction and autonomic ganglia In: Goodman & Gilman’s The Pharmacological Basis of Therapeutics. Vol., Hardman JG, Limbird LE, ed.^eds. McGraw-Hill, New York, pp. 193–213. [Google Scholar]
- Turner JR, Castellano LM, Blendy JA, 2010. Nicotinic partial agonists varenicline and sazetidine-A have differential effects on affective behavior. Journal of Pharmacology & Experimental Therapeutics. 334, 665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, et al. , 2006. Sazetidine-A, a novel ligand that desensitizes alpha4beta2 nicotinic acetylcholine receptors without activating them. Molecular Pharmacology. 70, 1454–1460. [DOI] [PubMed] [Google Scholar]
- Yenugonda VM, et al. , 2013. Design, synthesis and discovery of picomolar selective alpha4beta2 nicotinic acetylcholine receptor ligands. Journal of Medicinal Chemistry. 56, 8404–8421. [DOI] [PubMed] [Google Scholar]
- Zanetti L, Picciotto MR, Zoli M, 2007. Differential effects of nicotinic antagonists perfused into the nucleus accumbens or the ventral tegmental area on cocaine-induced dopamine release in the nucleus accumbens of mice. Psychopharmacology. 190, 189–199. [DOI] [PubMed] [Google Scholar]
- Zwart R, et al. , 2008. Sazetidine-A is a potent and selective agonist at native and recombinant alpha 4 beta 2 nicotinic acetylcholine receptors. Mol Pharmacol. 73, 1838–1843. [DOI] [PubMed] [Google Scholar]