Abstract
A point-prevalence study of antimicrobial use among inpatients at 5 public hospitals in Sri Lanka revealed that 54.6% were receiving antimicrobials: 43.1% in medical wards, 68.0% in surgical wards, and 97.6% in intensive care wards. Amoxicillin-clavulanate was most commonly used for major indications. Among patients receiving antimicrobials, 31.0% received potentially inappropriate therapy.
Antimicrobial resistance is a global public health crisis and is largely driven by antimicrobial use.1 In the United States, up to 30% of antimicrobials used in hospitals are unnecessary or are prescribed incorrectly. In low- or middle-income countries (LMICs), antimicrobial overuse appears to be greater.2 Point-prevalence surveys offer an initial feasible step for describing antimicrobial use and identifying targets to reduce inappropriate use.1,3 The objective of this study was to use point-prevalence surveys to identify the prevalence, patterns, and indications of antimicrobial use among patients admitted to public hospitals in the Southern Province, Sri Lanka.
Materials and Methods
Study design and setting
A point-prevalence study of antimicrobial use was conducted using single-day cross-sectional surveys among inpatients at 5 public hospitals in the Southern Province, Sri Lanka, from June 14, 2017, to August 10, 2017. Surveys were conducted at 1 tertiary-care hospital (1,745 beds), 1 secondary-care hospital (365 beds), and 3 primary-care hospitals (26 beds, 60 beds, and 104 beds).4
Study population and data collection
Trained research assistants visited the prespecified wards at 8:00 A.M. on survey days. All patients hospitalized on the ward at the time of survey were included. Patient medical records were used to collect sociodemographic information and clinical data including antimicrobials prescribed at the time of survey.5 Antibiotics, antifungals, and antivirals prescribed via the intravenous or oral route were recorded. On average, a research assistant spent 10–15 minutes reviewing each patient chart.
The Ruhuna University Ethical Review Committee and the Duke University Institutional Review Board approved this study. The directors of each hospital and the Regional Director of Health Services, Galle, Sri Lanka also approved this study.
Data analysis
Data were entered into a Research Electronic Data Capture (REDCap) database and statistical analyses were performed using R version 3.4.1 software (R Foundation for Statistical Computing, Vienna, Austria). Overall prevalence of antimicrobial use was defined as the number of patients receiving ≥1 antimicrobial agent at the time of survey divided by the total number of patients. Demographic and clinical characteristics associated with antimicrobial use were assessed using the χ2 and Kruskall-Wallis tests. Potentially inappropriate antimicrobial use was defined as (1) antimicrobial use discordant with the Sri Lanka College of Microbiologists’ guidelines for common indications and (2) redundant combinations of antimicrobials.6 Guideline-discordant therapy was defined as the use of any antimicrobial not recommended as primary therapy for lower respiratory tract infection, cellulitis/soft-tissue infection, urinary tract infection, surgical prophylaxis, or upper respiratory infection. Redundant therapy was defined as the concurrent use of ≥2 β-lactam antibiotics or ≥2 antibiotics active against anaerobes, Pseudomonas aeruginosa, or methicillin-resistant Staphylococcus aureus.
Results
Study population
In total, 1,709 patients were included in the point-prevalence surveys: 1,190 (69.6%) from the tertiary-level hospital, 371 (21.7%) from the secondary-level hospital, and 148 (8.7%) from the primary-level hospitals. Among the screened patients, 943 (55.2%) were in medical wards, 465 (27.2%) were in surgical wards, 221 (12.9%) were in pediatric wards, and 80 (4.7%) were in intensive care units. Most patients (55.6%) were male, and the median age was 42.2 years (interquartile range [IQR], 21–63 years) (Supplemental Table 1). Only 25 patients (1.5%) had a reported antimicrobial allergy, with most common allergies being to β-lactam antibiotics. Median hospitalization duration at the time of the survey was 5.9 days (IQR, 2–6 days).
Prevalence of antimicrobial use
Among 1,709 patients enrolled in the study, 54.7% (95% CI, 52.3%–57.1%) were receiving ≥1 antimicrobial agent at time of survey. Prevalence of antimicrobial use did not vary across hospitals of different care levels (P = .439) but did vary significantly across ward types: 43.1% in medical wards, 68.0% surgical wards, 61.1% in pediatric wards, and 97.6% in intensive care units (P < .001).
Patients receiving antimicrobial therapy had a longer duration of hospitalization at time of survey (median, 7.0 vs 4.5 days; P < .001) and were more likely to have a history of diabetes mellitus (18.5% vs 12.7%; P= .001) or chronic pulmonary disease (10.9% vs 5.4%; P<.001) than patients not receiving antimicrobials (Supplemental Table 1).
Antimicrobials prescribed
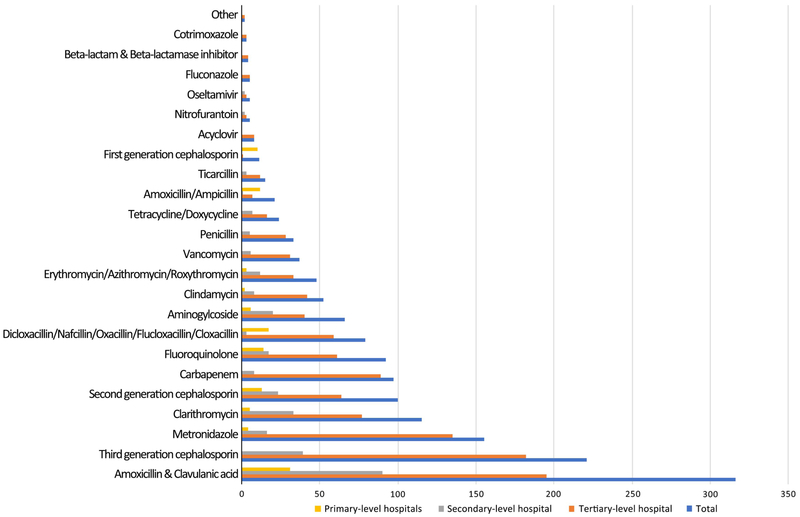
Among 935 patients who were prescribed antimicrobials, the most commonly prescribed antimicrobial was amoxicillin/clavulanic acid (n = 316, 33.8% of patients receiving antimicrobials) (Fig. 1). Other commonly used antimicrobials included third-generation cephalosporins (n = 221, 23.6%), metronidazole (n = 155, 16.6%), clarithromycin (n = 115, 12.3%), second-generation cephalosporins (n = 100, 10.7%), and carbapenems (n = 97, 10.4%). Approximately half of patients (n = 451, 48.2%) were receiving 2 or more antimicrobial agents at the time of survey (Supplemental Table 2).
Fig. 1.
Antimicrobial agents used at primary, secondary, and tertiary-level hospitals, southern Sri Lanka, 2017.
Indications for antimicrobial use
Among 935 patients on antimicrobial therapy, 350 (44.9%) had indications explicitly written in medical charts; 54.9% had indications that were inferred by research assistants based on the clinical record; and 16.6% did not have indications that were written or could be inferred. Overall, the most common indications for antimicrobial use were lower respiratory tract infection/pneumonia (20.7% of patients receiving antimicrobials), cellulitis/abscess/soft-tissue infection (19.4%), urinary tract infection/pyelonephritis (9.7%), surgical prophylaxis (7.8%), and upper respiratory tract infection (4.3%; Supplemental Table 2). Among 935 patients receiving antimicrobial therapy, only 135 (14.4%) had a culture result relevant to the indication for antimicrobial therapy at the time of survey.
Potentially inappropriate antimicrobial use
When compared with local treatment guidelines, 22.6% of antimicrobials for the 5 most common indications were potentially inappropriate (Table 1).6 In addition, 160 patients (17.1%) were receiving potentially redundant therapy (Supplemental Table 2). Overall, 290 (31.0%) of patients were receiving potentially inappropriate antimicrobial therapy that was either guideline discordant or redundant.
Table 1.
The Most Common Antimicrobials and Inappropriate Antimicrobial Therapy for the Top 5 Indications, Southern Sri Lanka, 2017
| Indication and Antimicrobial | No. (%) | No. of Patients (%) Receiving Antimicrobials Not Recommended by the Guidelines |
Total Patients |
|---|---|---|---|
| Lower respiratory infection/ pneumoniaa | |||
| Amoxicillin & clavulanic acid | 76 (39.8) | 10 (5.2) | 191 |
| Clarithromycin | 75 (39.3) | ||
| Third-generation cephalosporins | 62 (32.5) | ||
| Carbapenems | 22 (11.5) | ||
| Second-generation cephalosporins | 15 (7.8) | ||
| Cellulitis/ abscess/ soft tissue infectionb | |||
| Amoxicillin & clavulanic acid | 73 (40.6) | 59 (32.6) | 180 |
| Anti-staphylococcal penicillins | 45 (25.0) | ||
| Metronidazole | 40 (22.2) | ||
| Clindamycin | 40 (22.2) | ||
| Third-generation cephalosporins | 25 (13.9) | ||
| Urinary tract infection/ pyelonephritisc | |||
| Amoxicillin & clavulanic acid | 30 (34.9) | 26 (28.6) | 86 |
| Fluoroquinolone | 27 (31.4) | ||
| Third-generation cephalosporins | 20 (23.2) | ||
| Carbapenems | 13 (15.1) | ||
| Clarithromycin | 6 (7.0) | ||
| Surgical prophylaxisd | |||
| Amoxicillin & clavulanic acid | 32 (43.8) | 10 (13.7) | 73 |
| Metronidazole | 24 (32.9) | ||
| Second-generation cephalosporin | 23 (31.5) | ||
| Aminogylcoside | 9 (12.3) | ||
| Carbapenems | 8 (11.0) | ||
| Upper respiratory infectione | |||
| Amoxicillin & clavulanic acid | 18 (45.0) | 0 (0) | 40 |
| Clarithromycin | 7 (17.5) | ||
| Second-generation cephalosporins | 4 (10.0) | ||
| First-generation cephalosporins | 4 (10.0) | ||
| Erythromycin/azithromycin/roxythromycin | 3 (7.5) | ||
Lower respiratory infection/ pneumonia includes acute bronchitis, acute bacterial exacerbation of chronic obstructive pulmonary disease, acute infective exacerbation of bronchiectasis, empyema, lung abscess, and pneumonia.6
Cellulitis/abscess/soft-tissue infection includes animal bites, burns, cellulitis, cutaneous abscesses, diabetic foot ulcer, erysipelas, erythrasma, impetigo, mastitis, necrotizing fasciitis, paronychia, and surgical site infections.6
Urinary tract infection/pyelonephritis includes acute uncomplicated cystitis, acute uncomplicated pyelonephritis, complicated urinary tract infections, catheter-associated urinary tract infections, afebrile urinary tract infections, asymptomatic bacteriuria, and uncomplicated febrile urinary tract infections.6
Surgical prophylaxis includes cardiothoracic, vascular surgery, ENT surgery, oro-maxillo-facial surgery, gastrointestinal tract surgery, neurosurgery, obstetric and gynaecological surgery, general surgery, orthopaedic surgery, urological surgery, peritoneal dialysis catheter placement, ophthalmic surgery, solid organ transplant surgery, plastic surgery.6
Upper respiratory infection includes sinusitis, nasal vestibulitis, nasal septal abscess, acute bacterial tonsillitis/ pharyngitis, peritonsillar abscess, retropharyngeal/ parapharyngeal abscess, acute laryngitis, and acute epiglottitis.6
Discussion
Antimicrobial resistance is a growing global problem. Antimicrobial point-prevalence surveys are a feasible initial step in evaluating antimicrobial use and identifying targets for improvement.1,2,7 We report the first point-prevalence study of antimicrobial use in public hospitals in Sri Lanka.
In our study, prevalence of antimicrobial use was ~50%, similar to figures reported from other Asian and LMIC settings.9,10 We discovered that antimicrobial use was highest in surgical wards and intensive care units, perhaps due to greater acuity. Unnecessary and inappropriate broad-spectrum antimicrobial use needs to be identified and addressed in future studies.
Among patients receiving antimicrobial therapy, nearly 40% did not have a clear indication documented in the medical chart, and another 15% did not have an indication that was documented or could be inferred. Prior studies have shown that documenting an indication for antimicrobials reduces inappropriate therapy, and this action may be a target for future antimicrobial stewardship efforts.10
Nearly one-third of patients were receiving either guideline-discordant or redundant therapy. This estimate may be conservative because we did not account for dosing and duration in our assessments. In addition, upper respiratory infection, which is usually viral in etiology, was the fifth most common indication for antimicrobial use. We could not assess whether antimicrobials were warranted for the specific upper respiratory infections in this study. Finally, ~10% of patients were receiving carbapenem therapy, and this class was among the most commonly used for lower respiratory infection, urinary tract infection, and surgical prophylaxis. The need for broad-spectrum therapy with carbapenems is another area for future study.
Our study was limited by the use of medical charts, which may contain incomplete information, especially for patients admitted on the day of survey. Potentially redundant combinations of antimicrobials were identified based on spectrum of activity, and combination use may have been warranted in some patients. Finally, we only considered primary therapy as listed in the guidelines as appropriate, and some patients may have been receiving alternate or other therapy appropriately.
In conclusion, a point-prevalence study was feasible and effective at identifying potentially inappropriate antimicrobial use in southern Sri Lanka. Opportunities for improving antimicrobial use were identified and should be addressed by future antimicrobial stewardship efforts.
Supplementary Material
Acknowledgments
Financial support. This study was funded by a fieldwork grant from the Duke Global Health Institute to T Sheng. LG Tillekeratne was supported by supported by the National Institutes of Allergy and Infectious Diseases (grant no. K23AI125677). The authors also acknowledge the Duke Global Health Institute and the Hubert-Yeargan Center for Global Health for providing operational funds for the study site, the Duke-Ruhuna Collaborative Research Centre.
Footnotes
Supplementary material. To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2018.321
PREVIOUS PRESENTATION: These data were presented at a poster session at the American Society for Microbiology Microbe 2018 conference on June 7–11, 2018, in Atlanta, Georgia.
Conflicts of interest. All authors report no conflicts of interest relevant to this article
References
- 1.Laxminarayan R, Duse A, Wattal C, et al. Antibiotic resistance—the need for global solutions. Lancet Infect Dis 2013;13:1057–1098. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Antibiotic use in the United States, 2017: progress and opportunities. Atlanta, GA: US Department of Health and Human Services, CDC; 2017. [Google Scholar]
- 3.Centers for Disease Control and Prevention. The core elements of human antibiotic stewardship programs in resource-limited settings: national and hospital levels. Centers for Disease Control and Prevention; website. https://www.cdc.gov/antibiotic-use/healthcare/implementation.html. Published 2018. Accessed November 21, 2018. [Google Scholar]
- 4.Ministry of Healthcare and Nutrition, Sri Lanka. Health information unit beds and institution annual census Sri Lanka. Sri Lanka: Medical Statistics Unit, Ministry of Health, Nutrition and Indigenous Medicine; 2012. [Google Scholar]
- 5.Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA. 2014;312:1438–1446. doi: 10.1001/jama.2014.12923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sri Lanka College of Microbiologists in Collaboration with other Professional Colleges in Healthcare and The Ministry of Health, Nutrition and Indigenous Medicine. Empirical and prophylactic use of antimicrobials, national guidelines, 2016. Sri Lanka College of Microbiologists; website. http://slmicrobiology.net/download/National-Antibiotic-Guidelines-2016-Web.pdf. Published 2016. Accessed November 21, 2018. [Google Scholar]
- 7.Morgan DJ, Okeke IN, Laxminarayan R, Perencevich EN, Weisenberg S. Non-prescription antimicrobial use worldwide: a systematic review. Lancet Infect Dis 2011;11:692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie DS, Xiang LL, Li R, Hu Q, Luo QQ, Xiong W. A multicenter point-prevalence survey of antibiotic use in 13 Chinese hospitals. J Infect Public Health 2015;8:55–61. [DOI] [PubMed] [Google Scholar]
- 9.Thu TA, Rahman M, Coffin S, Rashid HO, Sakamoto J, Nguyen VH. Antibiotic use in Vietnamese hospitals: a multicenter point-prevalence study. Am J Infect Control 2012;40:840–844. [DOI] [PubMed] [Google Scholar]
- 10.Yeo JM. Antimicrobial stewardship: improving antibiotic prescribing practice in a respiratory ward. BMJ Open Quality 2016;5:u206491.w3570. doi: 10.1136/bmjquality.u206491.w3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.