Abstract
Background:
Abiraterone acetate suppresses adrenal androgens and glucocorticoids by inhibition of CYP17; however, given the risk of mineralocorticoid excess, it is administered with glucocorticoids. We performed a phase 2, single-arm study, designed to assess the safety of abiraterone acetate without steroids in castration-resistant prostate cancer (CRPC).
Patient and Methods:
Eligible patients had CRPC with controlled blood pressure and normal potassium. Patients initially received abiraterone acetate (1000mg daily) alone. Those with persistent or severe mineralocorticoid toxicity were initiated on prednisone (5mg twice daily). Therapy was continued until radiographic progression, toxicity, or withdrawal. The primary objective was to determine the proportion of men requiring prednisone to manage mineralocorticoid toxicity.
Results:
Fifty-eight patients received at least one dose of abiraterone acetate; the majority had metastases (n=53, 91.4%). Sixteen patients (27.6%) received prior chemotherapy, six (10.3%) prior enzalutamide, and four (6.9%) prior ketoconazole. Grade 3–4 adverse events of interest included hypertension (n=9, 15.5%) and hypokalemia (n=4, 6.7%). There was no grade ≥3 edema. Seven patients (12%) initiated prednisone for mineralocorticoid toxicity: hypertension (n=3, 5%), hypokalemia (n=4, 7%). Two patients initiated prednisone for fatigue (3%). Forty patients (67%) experienced a ≥50% PSA decline with abiraterone acetate alone. Patients with lower baseline androstenedione (p=0.04), androsterone (p=0.01), dehydroepiandrosterone (p=0.03), and 17-hydroxyprogesterone (p=0.03) levels and were more likely to develop mineralocorticoid toxicity.
Conclusions:
Abiraterone acetate without steroids is feasible, however clinically significant adverse events can occur in a minority of patients. Use of abiraterone acetate without prednisone should be balanced with potential of toxicity and requires close monitoring.
Keywords: Abiraterone, Hypertension, Hypokalemia, Mineralocorticoids, Prostate cancer, Steroids
Precis:
Abiraterone acetate, a CYP17 inhibitor which suppresses adrenal androgens, is associated with an increased risk of mineralocorticoid excess and is administered with exogenous glucocorticoids. In this phase 2 study, we demonstrate that abiraterone acetate without steroids is feasible, however mineralocorticoid toxicity can occur in a minority of patients.
Introduction:
Cytochrome P450 17A (CYP17A) is an important target in prostate cancer given its central role in the production of tumor-derived and adrenal androgens[1]. Abiraterone acetate, a pro-drug of abiraterone, is a rationally designed irreversible potent inhibitor of CYP17[1]. Abiraterone acetate results in reduction of androgens and also cortisol, which activates a negative feedback loop resulting in compensatory elevated adrenocorticotropic hormone (ACTH) and mineralocorticoid excess[1, 2]. Abiraterone acetate has been developed with the co-administration of glucocorticoids, which has reduced the risk of secondary mineralocorticoid excess[3, 4].
Abiraterone acetate has demonstrated improved overall survival (OS) in patients with metastatic castration-resistant prostate cancer (CRPC) both post-(COU-301) and pre-chemotherapy (COU-302)[3, 4]. In the initial phase 1/2 trials, abiraterone acetate was tested without concomitant steroids[2, 5]. Though mineralocorticoid toxicity (MT) was common (55–90% of patients), symptoms were generally well-managed with eplerenone[2, 5]. In subsequent phase 2/3 trials, steroids (10 mg/day prednisone in COU-301/COU-302) were mandated for all patients[3, 4, 6, 7].
More recently, the LATITUDE and STAMPEDE trials demonstrated that abiraterone acetate increased OS among men with locally advanced or hormone-sensitive metastatic prostate cancer[6, 7]. The LATITUDE trial included patients with de novo high-risk metastatic disease; while STAMPEDE had a more heterogeneous patient population including men with non-metastatic prostate cancer (prednisone equivalent 5mg/daily)[6, 7]. Men treated with abiraterone acetate and corticosteroids at stages of disease earlier than metastatic CRPC had a longer duration of abiraterone acetate-corticosteroid and consequently longer exposure for potential side effects from both agents.
Glucocorticoids have a complex role in the management, treatment, and progression of prostate cancer. Historically, glucocorticoids have resulted in symptom palliation and occasional cancer responses in advanced disease[8]. However, unexpected iatrogenic stimulation of prostate cancer growth has been observed [9, 10]. Additionally, as glucocorticoids are being utilized earlier in the disease, patients are at risk of the adverse events (AEs) associated with long-term use. To study the profile of toxicities from abiraterone acetate without steroids, we performed a multicenter, phase 2 study and we explored hormone levels as potential biomarkers for secondary MT.
Patients and Methods:
Patients:
This is a phase 2, single arm, open-label study of abiraterone acetate without glucocorticoids in CRPC (NCT02025010). Sixty patients were enrolled between 6/2014–8/2016: DFCI (n=40, Boston, MA) and MSKCC (n=20, New York, NY). Eligible patients had CRPC by Prostate Cancer Working Group (PCWG) 2 criteria[11]. Patients were not required to have radiologic metastases.
Patients had controlled blood pressure (<140/90 on ≤3 anti-hypertensive agents) and normal potassium without supplementation. Patients may have received prior hormonal therapies and up to two cytotoxic therapies. Prior androgen synthesis inhibitors were not allowed, excluding ketoconazole. Other eligibility criteria included an Eastern Cooperative Oncology Group performance status ≤2 and normal organ and bone marrow function. Patients were excluded if they required chronic corticosteroids. The study was conducted after institutional review board approval at each of the participating institutions in accordance with the principals outlined in the Declaration of Helsinki. All patients provided written informed consent.
Treatment:
Patients received abiraterone abiraterone (1,000mg/daily) without glucocorticoids and were monitored for MT. Patients who developed hypertension (≥140/90 on three determinations) were treated with anti-hypertensives and/or a mineralocorticoid antagonist (eplerenone preferred, though spironolactone allowed) (Supplement Figure 1). Hypokalemia was treated with potassium supplementation and/or a mineralocorticoid antagonist (Supplement Figure 2). Patients with persistent or severe hypertension or hypokalemia, as defined in Supplement Figure 1 and 2, were initiated on prednisone (5mg/twice daily).
In patients not requiring prednisone for toxicity management, prednisone (5mg/twice daily) was added at PSA progression, as defined by PCWG2 criteria[11], and subsequent PSA response was evaluated. Safety laboratories were checked every two weeks for the first 12 weeks and then every 4 weeks. PSA and imaging assessment occurred every 4 and 12 weeks, respectively. Treatment was continued until radiographic progression, significant toxicity or patient/physician requested withdrawal.
Serum Hormone Levels:
Hormones were measured at baseline, cycle 2/day 1 and treatment discontinuation. The Supplement Data details the methodology for serum hormone determinations.
Statistical Analysis:
The primary endpoint was the proportion of patients requiring prednisone to manage MT. The criteria for identifying an excessive number of patients requiring prednisone was based on the sequential probability test with α=0.10, β=0.10, po=0.10 and pa=0.25. Every time a participant was classified as having persistent or severe mineralocorticoid excess, the cumulative number of participants with persistent or severe mineralocorticoid excess was compared with the number of participants evaluable for toxicity and an associated cutoff of evaluable participants. If the true underlying proportion of patients requiring prednisone is 10% (based on COU-301/COU-302 grade 3–4 hypertension, hypokalemia, and edema of 1–4%, 2–3%, and <1–2%, respectively, and any grade hypertension, hypokalemia, and edema of 10–22%, 17%, 28–31%, respectively), then the probability of identifying excessive patients requiring prednisone is 0.082 whereas if the true underlying proportion is 25% the probability is 0.907[3, 4]. If more than 12 events were observed in 60 patients, then we would reject the null hypothesis that persistent and severe mineralocorticoid excess could be managed without the addition of prednisone. Secondary objectives included toxicity, PSA and radiographic response, time to PSA and radiographic progression. Correlative objectives included assessment of baseline and change in hormone levels and association with MT.
Radiographic response as defined by Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1 was summarized with 95% confidence interval (CI) using an exact binomial test. Toxicity was summarized using Common Terminology Criteria for Adverse Events version 4.0. PSA response and progression were defined by PCWG2 criteria[11]. Radiographic progression was defined by RECIST version 1.1 for soft tissue and visceral disease and PCWG2 for bone disease[11, 12]. Time to PSA progression and radiographic progression was summarized using the Kaplan Meier method.
Hormone levels were summarized as median and interquartile range (IQR). Association between levels at baseline and 1) any grade MT, 2) grade 3–4 MT, and 3) initiation of prednisone for toxicity management were summarized using Wilcoxon’s rank sum test. Associations between the change in hormones between cycle 1 and cycle 2 and the development of any grade MT were summarized using Wilcoxon’s rank sum test, excluding patients who developed MT during cycle 1. Given the exploratory nature of this analysis, we did not correct for multiple hypotheses.
Results:
Baseline Characteristics:
Sixty patients were enrolled of whom 58 received at least one dose of abiraterone acetate (Table 1). The median age was 68 years. Sixteen patients (27.6%) received prior chemotherapy, six (10.3%) prior enzalutamide, and four (6.9%) prior ketoconazole. Most patients had metastases (n=53, 91.4%). Twenty-six patients (44.8%) were on at least one anti-hypertensive agent and no patient was on a mineralocorticoid antagonist at enrollment.
Table 1.
Baseline patient and disease characteristics.
| Characteristic | N | Median (q1-q3) or % |
|---|---|---|
| Age at baseline (years) | 58 | 68 (61-76) |
| ECOG performance status | ||
| 0 | 42 | 72.4% |
| 1 | 16 | 27.6% |
| Body mass index (kg/m2) | 58 | 30.3 (27.5-31.1) |
| Gleason score at diagnosis | ||
| ≤ 6 | 5 | 8.6% |
| 7 | 22 | 37.9% |
| ≥ 8 | 27 | 45.0% |
| Unknown | 4 | 6.9% |
| PSA at diagnosis (ng/mL) | 53 | 12.3 (7.1-45.4) |
| Prior chemotherapy | 16 | 27.6% |
| Prior ketoconazole | 4 | 6.9% |
| Prior enzalutamide | 6 | 10.3% |
| Presence of metastases at baseline | 53 | 91.4% |
| Sites of metastasis | ||
| Bone | 50 | 86.2% |
| Lymph nodes | 19 | 32.8% |
| Lung | 2 | 3.4% |
| Liver | 1 | 1.7% |
| Laboratory data at baseline | ||
| PSA (ng/mL) | 58 | 15.8 (4.4-49.4) |
| Alkaline phosphatase (U/L) | 58 | 84 (63, 119) |
| Calcium (mg/dL) | 58 | 9.4 (9.1-9.7) |
| Hemoglobin (g/dL) | 58 | 13.0 (12.1-13.8) |
| Platelets (K/UL) | 58 | 210 (182-244) |
| HgA1c (%) | 58 | 5.7 (5.4-6.1) |
| Baseline anti-hypertensive use | 26 | 44.8% |
| 1 agent | 15 | 57.7% |
| 2 agents | 8 | 30.8% |
| 3 agents | 3 | 11.5% |
ECOG=Eastern Oncology Cooperative Group, PSA=prostate specific antigen.
Mineralocorticoid Toxicity and Prednisone Initiation:
Any grade AEs of MT occurred in 66% of patients (n=38): hypertension (n=28, 48.3%), hypokalemia (n=15, 25.9%), and edema (n=11, 19.0%) (Table 2). The median days from abiraterone acetate initiation to any-grade hypertension or hypokalemia was 56 (range 14–492) and 39 days (range 17–534), respectively (Figure 1). Grade 3–4 MT occurred in 21% of patients (n=12): hypertension (n=9, 15.5%) and hypokalemia (n=4, 6.7%) (no grade ≥3 edema). Of the patients who developed hypertension, 42.9% (n=12/28) had a prior history of hypertension and were on anti-hypertensives at baseline.
Table 2.
Adverse events observed in >10% of patients overall.
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total |
|---|---|---|---|---|---|
| Fatigue | 37 (63.9%) | 5 (8.6%) | 1 (1.7%) | 0 (0%) | 43 (74.1%) |
| Hypertension | 8 (13.8%) | 11 (19.0%) | 8 (13.8%) | 1 (1.7%) | 28(48.3%) |
| Hot flashes | 25 (43.1%) | 1 (1.7%) | 0 (0%) | 0 (0%) | 26 (44.8%) |
| Pain | 19 (32.8%) | 2 (3.4%) | 1 (1.7%) | 0 (0%) | 22 (37.9%) |
| Hypokalemia | 9 (15.5%) | 2 (3.4%) | 4 6.9%) | 0 (0%) | 15 (25.9%) |
| Back pain | 11 (19.0%) | 2 (3.4%) | 0 (0%) | 0 (0%) | 13 (22.4%) |
| Limb edema | 10 (17.2%) | 1 (1.7%) | 0 (0%) | 0 (0%) | 11 (19.0%) |
| Urinary frequency | 7 (12.1%) | 4 (6.9%) | 0 (0%) | 0 (0%) | 11 (19.0%) |
| Aspartate aminotransferase increased | 5 (8.6%) | 4 (6.9%) | 1 (1.7%) | 0 (0%) | 10 (17.2%) |
| Cough | 8 (13.8%) | 1 (1.7%) | 0 (0%) | 0 (0%) | 9 (15.5%) |
| Constipation | 8 (13.8%) | 0 (0%) | 0 (0%) | 0 (0%) | 8 (13.8%) |
| Dyspnea | 8 (13.8%) | 0 (0%) | 0 (0%) | 0 (0%) | 8 (13.8%) |
| Nausea | 6 (10.3%) | 1 (1.7%) | 0 (0%) | 0 (0%) | 7 (12.1%) |
| Renal and urinary disorders - Other | 7 (12.1%) | 0 (0%) | 0 (0%) | 0 (0%) | 7 (12.1%) |
| Dizziness | 6 (10.3%) | 0 (0%) | 0 (0%) | 0 (0%) | 6 (10.3%) |
| Musculoskeletal and connective tissue disorder - Other | 6 (10.3%) | 0 (0%) | 0 (0%) | 0 (0%) | 6 (10.3%) |
| Limb pain | 6 (10.3%) | 0 (0%) | 0 (0%) | 0 (0%) | 6 (10.3%) |
| Peripheral sensory neuropathy | 4 (6.9%) | 2 (3.4%) | 0 (0%) | 0 (0%) | 6 (10.3%) |
| Maculopapular rash | 6 (10.3%) | 0 (0%) | 0 (0%) | 0 (0%) | 6 (10.3%) |
One patient experienced grade 2 heart failure, which was possibly treatment-related to treatment.
Figure 1.
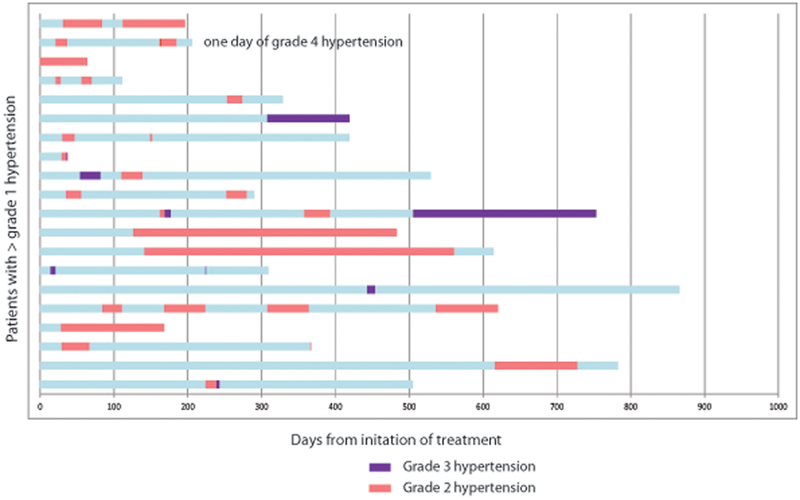
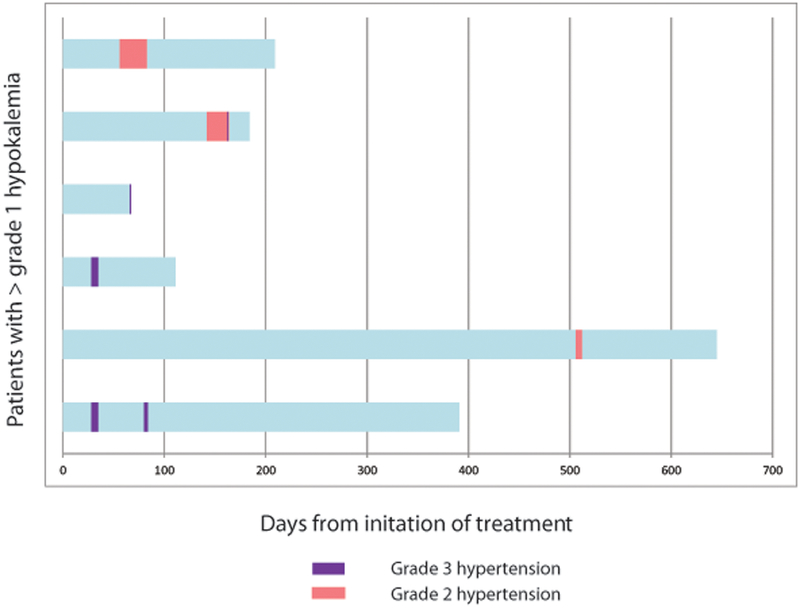
Swimmer plot of timing of onset of A) hypertension or B) hypokalemia for patients developing grade >1 toxicity. Each bar on the y-axis represents an individual patient. Pink bar represents grade 2 toxicity. Purple bar represents grade 3 toxicity.
A)
B)
Thirty-three patients (56.9%) required use of anti-hypertensives: 48.5% (n=16) received one agent, 18.2% (n=6) received two, 21.2% (n=7) received three, and 6.9% (n=4) received ≥4. Twenty-seven (46.6%) patients required the addition of more anti-hypertensive agents compared to baseline, while 16 (27.6%) required less anti-hypertensives. Of the 11 patients (19.0%) using ≥3 anti-hypertensives, seven (12.1%) were using anti-hypertensives at baseline. Eleven patients (19.0%) required potassium supplementation. Mineralocorticoid antagonists were utilized in 28 (48.3%) patients (eplerenone n=23; spironolactone n=3; both n=2). In total, seven patients (12.1%) initiated prednisone for MT: hypokalemia (n=4, 6.9%), hypertension (n=3, 5.2%), meeting the trials primary endpoint that MT could be managed without the addition of prednisone. Two patients initiated prednisone for fatigue (3.4%), one of whom experienced grade 3 fatigue.
PSA and Radiographic Response:
The median baseline PSA was 15.8 g/mL. Median PSA nadir was 2.1ng/mL (IQR 0.2–8.8ng/mL) and median time to nadir was 3.0 months (IQR 1.5–6.4 months) (Figure 2). Forty patients (69.0%) achieved a ≥50% PSA reduction. Of the 53 patients with metastatic disease, 23 (43.4%) had measurable disease. Eleven patients (20.1%, 95% CI 10.4–26.7) experienced an objective response: complete response n=3 (5.5%, 95% CI 1.1–15.1) and partial response n=8 (14.5%, 95% CI 6.5–26.7). Stable disease was observed in 63.9% of patients (n=35).
Figure 2.

Waterfall plot of best PSA response to therapy with abiraterone acetate alone. Forty patients (69.0%) achieved a ≥50% PSA reduction, of whom 29 did not receive prior chemotherapy, 39 did not receive prior enzalutamide, and 38 who did not receive prior ketoconazole, and 21 (36.2%) achieved a ≥90% reduction. Five patients (8.6%) did not experience any PSA decline. Each bar represents an individual patient. Patients marked with an * did not have radiographic evidence of metastatic disease.
PSA and Radiographic Progression:
Twenty-eight (48.3%) patients experienced PSA progression at a median time of 10.2 months (95% CI 6.5–19.4). Of these individuals, twenty-one (36.2%) initiated prednisone for PSA progression. Median time to initiation of prednisone for PSA progression was 6.4 months (IQR 4.6–11.1). Of patients initiated on prednisone for PSA progression, six (28.6%) experienced a PSA decline (median decline 20%), with one patient achieving a >50% decline (89% decline). In patients with some PSA decline following the addition of prednisone, the median time to radiographic progression was 11 months from the time prednisone was initiated. In patients without any PSA decline, the median time to radiographic progression was 11 months. Of the 53 patients with metastases, 17 (32.1%) experienced radiographic progression and median time to radiographic progression was 22.1 months.
Serum Hormone Levels:
There was an association between lower baseline androgens including androstenedione (p=0.04), androsterone (p=0.01), and DHEA (p=0.03) and lower 17-hydroxyprogesterone (p=0.03) and the development of any grade MT (n=38) (Table 3). Baseline hormones did not correlate with grade 3–4 MT (n=12) or the addition of prednisone for hypertension or hypokalemia (n=7) (Supplemental Table 1/2). Supplement Figure 3 denotes changes in hormones during the first cycle. More pronounced declines in cortisone were associated with MT (p=0.01) (Supplement Table 3).
Table 3.
The association between baseline serum hormone levels and the development of any grade hypertension, hypokalemia or edema. Bolded values are statistically significant (p<0.05) using Wilcoxon’s rank sum test. All units are ng/dL. DHEA=Dehydroepiandrosterone, DHT=Dihydrotestosterone.
| Hormone | Toxicity | N | Min | Q1 | Median | Q3 | Max | P-value |
|---|---|---|---|---|---|---|---|---|
| Androstenedione | No | 20 | 0.13 | 0.23 | 0.39 | 0.70 | 1.36 | 0.04 |
| Yes | 38 | 0.11 | 0.17 | 0.28 | 0.40 | 1.04 | ||
| All | 58 | 0.11 | 0.18 | 0.29 | 0.44 | 1.36 | ||
| Androsterone | No | 20 | 0.02 | 0.05 | 0.07 | 0.10 | 0.16 | 0.01 |
| Yes | 38 | 0.01 | 0.02 | 0.04 | 0.07 | 0.13 | ||
| All | 58 | 0.01 | 0.03 | 0.05 | 0.09 | 0.16 | ||
| Corticosterone | No | 20 | 0.33 | 0.98 | 1.35 | 2.58 | 7.35 | 0.99 |
| Yes | 38 | 0.38 | 0.98 | 1.35 | 2.24 | 9.62 | ||
| All | 58 | 0.33 | 0.98 | 1.35 | 2.58 | 9.62 | ||
| Cortisol | No | 20 | 31.08 | 82.26 | 112.10 | 164.21 | 239.82 | 0.57 |
| Yes | 38 | 46.32 | 88.85 | 103.52 | 140.88 | 204.87 | ||
| All | 58 | 31.08 | 88.85 | 103.52 | 147.28 | 239.82 | ||
| Cortisone | No | 20 | 72.09 | 156.91 | 211.48 | 250.49 | 310.90 | 0.25 |
| Yes | 38 | 81.06 | 146.37 | 181.49 | 214.58 | 317.20 | ||
| All | 58 | 72.09 | 151.07 | 187.85 | 237.27 | 317.20 | ||
| DHEA | No | 20 | 0.20 | 0.77 | 1.54 | 2.13 | 6.10 | 0.03 |
| Yes | 38 | 0.17 | 0.44 | 0.73 | 1.49 | 4.50 | ||
| All | 58 | 0.17 | 0.60 | 0.90 | 1.72 | 6.10 | ||
| DHT | No | 20 | 0.01 | 0.01 | 0.02 | 0.02 | 0.05 | 0.17 |
| Yes | 38 | 0.01 | 0.01 | 0.01 | 0.02 | 0.03 | ||
| All | 58 | 0.01 | 0.01 | 0.01 | 0.02 | 0.05 | ||
| 11-Deoxycorticosterone | No | 20 | 0.01 | 0.01 | 0.01 | 0.03 | 0.08 | 0.40 |
| Yes | 38 | 0.00 | 0.01 | 0.01 | 0.02 | 0.08 | ||
| All | 58 | 0.00 | 0.01 | 0.01 | 0.02 | 0.08 | ||
| 11-Deoxycortisol | No | 19 | 0.08 | 0.13 | 0.22 | 0.45 | 0.73 | 0.19 |
| Yes | 38 | 0.06 | 0.12 | 0.15 | 0.28 | 0.78 | ||
| All | 57 | 0.06 | 0.12 | 0.17 | 0.30 | 0.78 | ||
| 17-Hydroxypregnenolone | No | 20 | 0.19 | 0.19 | 0.63 | 1.31 | 4.78 | 0.08 |
| Yes | 38 | 0.19 | 0.19 | 0.19 | 0.70 | 3.67 | ||
| All | 58 | 0.19 | 0.19 | 0.20 | 1.08 | 4.78 | ||
| 17-Hydroxyprogesterone | No | 20 | 0.05 | 0.07 | 0.13 | 0.21 | 0.46 | 0.03 |
| Yes | 38 | 0.03 | 0.05 | 0.09 | 0.13 | 0.38 | ||
| All | 58 | 0.03 | 0.06 | 0.09 | 0.17 | 0.46 | ||
| Pregnenolone | No | 19 | 0.13 | 0.20 | 0.33 | 0.49 | 1.13 | 0.06 |
| Yes | 38 | 0.11 | 0.17 | 0.26 | 0.32 | 0.97 | ||
| All | 57 | 0.11 | 0.17 | 0.27 | 0.35 | 1.13 | ||
| Progesterone | No | 20 | 0.01 | 0.02 | 0.02 | 0.03 | 0.06 | 0.05 |
| Yes | 38 | 0.01 | 0.01 | 0.02 | 0.02 | 0.09 | ||
| All | 58 | 0.01 | 0.01 | 0.02 | 0.03 | 0.09 | ||
| Testosterone | No | 20 | 0.04 | 0.05 | 0.08 | 0.09 | 0.21 | 0.32 |
| Yes | 38 | 0.02 | 0.04 | 0.07 | 0.10 | 0.34 | ||
| All | 58 | 0.02 | 0.05 | 0.07 | 0.09 | 0.34 |
Discussion:
In this study, we demonstrated that administration of abiraterone acetate without glucocorticoids was feasible, though 21% (n=12) of patients experienced grade 3–4 MT and 66% (n=38) experienced any grade MT. In most patients, toxicities were effectively abrogated with anti-hypertensives, potassium supplementation, and/or mineralocorticoid antagonists. In total, 15.5% (n=9/58) of patients required prednisone for toxicity management.
Compared to phase 3 studies utilizing abiraterone acetate with 10 mg of daily prednisone, we observed a higher rate of grade 3–4 MT[3, 4, 6, 7]. In COU-301 and COU-302, rates of grade 3–4 hypertension, hypokalemia, and edema were 1–4%, 2–3%, and <1–2%, respectively.[3, 4] However, in the LATITUDE and STAMPEDE studies, which utilized lower doses of corticosteroids, rates of grade 3–4 hypertension, hypokalemia, and edema were 5–20%, 1–11%, and 0.3–1%, respectively.[6, 7] In our study, grade 3–4 hypertension, hypokalemia, and edema occurred in 15.5%, 6.7%, and 0% of patients. Most cases of MT (71%) were observed within the first 3 months, however, a small subset developed delayed toxicity after prolonged treatment (14% and 10% of patients developed toxicity after 6 and 9 months, respectively). These data highlight the need for regular monitoring of blood pressure and electrolytes throughout treatment. We do not recommend the routine use of abiraterone acetate without steroids; however, the clinician considering abiraterone acetate without steroids due to specific patient concerns, should weigh the risks described herein with the possible benefits of avoiding daily steroids. Consideration for abiraterone acetate without glucocorticoids requires an assessment of predisposing factors for MT, such as baseline hypertension, hypokalemia, or edema, and additional patient education about the potential for toxicity, coaching on home blood pressure monitoring, and close communication with the care team.
Corticosteroids are commonly used in prostate cancer and corticosteroid-related AEs associated with low-dose corticosteroids (equivalent prednisone 10mg/day) include hyperglycemia[13, 14], myopathy[14], edema[14], dyspnea[14], and cataracts[13]. Men with prostate cancer may be at increased susceptibility to toxicity due to advanced age and concurrent androgen deprivation therapy (ADT). Prospective studies have demonstrated that ADT can cause sarcopenia, increased fat mass, weight gain, increased cholesterol and triglycerides, insulin resistance, and loss of bone mineral density[15] and these overlap with corticosteroid effects. Our study gives treating physicians and patients who are concerned with corticosteroid toxicities, an assessment of the incidence of MT when abiraterone acetate is used without steroids and an algorithm for use of anti-hypertensives including eplerenone and/or potassium replacement in lieu of steroids.
In a planned exploratory analysis evaluating serum hormones, we observed a correlation between lower baseline hormones and change in hormones after one month of treatment for MT. Identification of biomarkers for secondary mineralocorticoid excess toxicity could potentially select patients at risk of MT development with abiraterone acetate alone. We found that lower baseline serum androgens and 17-hydroxyprogesterone were associated with toxicity development. Additionally, a steep decline in cortisone after one month of treatment was associated with MT. We hypothesize that patients with lower baseline hormones may be primed for increased activation of the negative feedback loop stimulating a rise in ACTH and thus a higher risk of MT. This analysis is exploratory and limited by the small sample size. We do not recommend use of these hormone levels to guide patient treatment, however this hypothesis could be tested in future studies.
Our study did not directly compare the efficacy of abiraterone acetate with prednisone versus abiraterone acetate alone. Comparisons with historical controls are confounded by differences in patient populations and methods. Our study population is heterogeneous including non-metastatic patients (8.6%) and chemotherapy (27.6%) and ketoconazole (6.9%) pretreated patients. Sixty-nine percent of all patients experienced a ≥50% PSA decline from baseline and patients with metastatic disease (n=53) had a median time to radiographic progression of 22.1 months. These metrics are comparable to historic studies (COU-301 38% with ≥50% PSA decline and radiographic PFS 5.6 months; COU-302 62% with ≥50% decline PSA and radiographic PFS 16.5 months) and we conclude that efficacy of abiraterone acetate without co-administration of steroids is not compromised[3, 4].
The steroid containing control arms of several large phase 3 studies observed a PSA response in 5.5–28% of patients suggesting that steroids alone may have modest clinical activity in CPRC.[16] In this study, we evaluated the addition of prednisone in patients with PSA progression. Though declines in PSA were observed (28.6% of patients), only one patient experienced a ≥50% PSA decline. Earlier studies evaluated low-dose dexamethasone in patients with PSA progression on abiraterone acetate alone and demonstrated PSA ≥50% responses in 20% of patients (n=3/15)[5]. Glucocorticoids can potentially have dual functions in both response and resistance in CRPC. While it is hypothesized that glucocorticoids can further suppress androgen production by preventing a rise in substrates of backdoor androgen synthesis[1], other studies have demonstrated that glucocorticoids and the glucocorticoid receptor (GR) may mediate resistance through activation of mutated androgen receptor (AR) in CRPC[10]. Additionally, emerging data suggests that GR is upregulated in some CRPCs, in the context of enzalutamide, stimulating AR-target gene expression and promoting tumor growth[9, 17]. The effects of glucocorticoids are likely heterogeneous and the effect at the tumor cell level cannot be determined.
Based on the product label, abiraterone acetate should be prescribed with steroids. We demonstrate that abiraterone acetate used without corticosteroids results in secondary mineralocorticoid excess toxicities in 66% of patients and that all but 12% can be effectively treated with mineralocorticoid antagonists, anti-hypertensives and/or potassium replacement. Single-agent abiraterone acetate with prompt use of these agents may be a consideration for patients at risk for toxicities from corticosteroids and limited predisposing factors for MT, such as baseline hypertension, hypokalemia, or edema. Additional biomarker studies are warranted to identify patients at high-risk for MT.
Supplementary Material
Acknowledgements:
We would like to thank the patients and family members who participated in this clinical trial.
Funding: This study was funded by Janssen. It was also supported by the Fairweather Family Fund and Fat Boys Slim Sisters Fund at the Lank Center for Genitourinary Oncology DFCI (MET), PCCTC, PCF Challenge Award (Award Number 142016; MET), and the DH/HCC Prostate Cancer SPORE (NCI P50 CA090381).
Footnotes
Disclosures: MET, PWK serve on the Advisory Board for Janssen and receive clinical research funding from Janssen. RRM serves on the Advisory Board for Janssen. The remaining authors have no disclosures.
References:
- 1.Attard G, Reid AH, Auchus RJ et al. Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. J Clin Endocrinol Metab 2012; 97: 507–516. [DOI] [PubMed] [Google Scholar]
- 2.Attard G, Reid AH, Yap TA et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol 2008; 26: 4563–4571. [DOI] [PubMed] [Google Scholar]
- 3.de Bono JS, Logothetis CJ, Molina A et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011; 364: 1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan CJ, Smith MR, de Bono JS et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013; 368: 138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attard G, Reid AH, A’Hern R et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol 2009; 27: 3742–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fizazi K, Tran N, Fein L et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 2017; 377: 352–360. [DOI] [PubMed] [Google Scholar]
- 7.James ND, de Bono JS, Spears MR et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med 2017; 377: 338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tannock I, Gospodarowicz M, Meakin W et al. Treatment of metastatic prostatic cancer with low-dose prednisone: evaluation of pain and quality of life as pragmatic indices of response. J Clin Oncol 1989; 7: 590–597. [DOI] [PubMed] [Google Scholar]
- 9.Arora VK, Schenkein E, Murali R et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 2013; 155: 1309–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richards J, Lim AC, Hay CW et al. Interactions of abiraterone, eplerenone, and prednisolone with wild-type and mutant androgen receptor: a rationale for increasing abiraterone exposure or combining with MDV3100. Cancer Res 2012; 72: 2176–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scher HI, Halabi S, Tannock I et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008; 26: 1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura K, Nonomura N, Yasunaga Y et al. Low doses of oral dexamethasone for hormone-refractory prostate carcinoma. Cancer 2000; 89: 2570–2576. [DOI] [PubMed] [Google Scholar]
- 14.Sartor O, Weinberger M, Moore A et al. Effect of prednisone on prostate-specific antigen in patients with hormone-refractory prostate cancer. Urology 1998; 52: 252–256. [DOI] [PubMed] [Google Scholar]
- 15.Saylor PJ, Smith MR. Adverse effects of androgen deprivation therapy: defining the problem and promoting health among men with prostate cancer. J Natl Compr Canc Netw 2010; 8: 211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghatalia P, Pond GR, Templeton AJ, Sonpavde G. Effect of Single-agent Daily Prednisone on Outcomes and Toxicities in Metastatic Castration-resistant Prostate Cancer: Pooled Analysis of Prospective Studies. Clin Genitourin Cancer 2018; 16: e277–e287. [DOI] [PubMed] [Google Scholar]
- 17.Isikbay M, Otto K, Kregel S et al. Glucocorticoid receptor activity contributes to resistance to androgen-targeted therapy in prostate cancer. Horm Cancer 2014; 5: 72–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


