Abstract
Objectives:
Global and regional myocardial deformation have not been well described in fetuses with pulmonary atresia and intact ventricular septum (PA/IVS). Speckle tracking echocardiography (STE), an angle independent technique for assessing global and regional strain, may be a more sensitive marker of ventricular systolic dysfunction compared to traditional 2D measurements. The aim of this study was to assess myocardial deformation in fetuses with PA/IVS relative to control fetuses and to determine if strain differs between PA/IVS fetuses with and without right ventricular dependent coronary circulation (RVDCC).
Methods:
This was a retrospective analysis of fetuses with PA/IVS imaged at two medical centers from June 2005 to October 2017. LV and RV regional and global longitudinal strain (GLS) and strain rate were obtained using STE, and comparisons were performed between PA/IVS fetuses and gestational age (GA) matched controls. Postnatal outcome was assessed, including the presence of RVDCC.
Results:
Fifty-seven PA/IVS fetuses and 57 controls were analyzed at a mean GA of 26.5±5 weeks. LV GLS was significantly decreased in PA/IVS fetuses compared to controls (−17.4±1.7% vs. −23.7±2.0%, p<0.001). LV strain rate was also significantly decreased (−1.01±0.21s−1 vs. −1.42±0.20s−1, p<0.001). Fetuses with PA/IVS had decreased strain in all segments. Similarly, RV strain was also significantly decreased in fetuses with PA/IVS (−11.6±3.8 vs. −24.6±2.5, p<0.0001). Thirty-six patients had postnatal cardiac catheterization performed to define coronary anatomy: 10 fetuses had RVDCC. Fetuses with RVDCC had decreased LV strain compared to fetuses who did not (−15.8±1.1% vs. −17.9±1.7%, p=0.009). RV strain was also decreased in fetuses with RVDCC vs. fetuses without RVDCC (−7.0±2.9 vs. −12.1±3.2, p=0.0004).
Conclusions:
Fetuses with PA/IVS have decreased global and regional LV and RV strain compared to controls. The finding of decreased LV strain may be due to altered ventricular mechanics in the context of a hypertensive right ventricle and/or abnormal coronary perfusion. Moreover, fetuses who were found to have RVDCC postnatally had decreased LV and RV strain compared to fetuses who did not. These results encourage further investigation to assess whether fetal ventricular strain could be a prenatal predictor of RVDCC.
Keywords: pulmonary atresia with intact ventricular septum, speckle tracking echocardiography, right ventricular dependent coronary circulation, fetal echocardiography, global longitudinal strain
Introduction
Pulmonary atresia with intact ventricular septum (PA/IVS) is a form of congenital heart disease with a wide range of anatomic morphologies, including varying degrees of tricuspid valve (TV) and right ventricle (RV) hypoplasia1,2. There is a high incidence of abnormal connections between the RV and coronary arteries, and in the most severe cases a RV dependent coronary circulation (RVDCC) is present. RVDCC, in which myocardial perfusion depends on fistulous connections from the RV with inadequate antegrade coronary blood flow, has been shown to lead to ischemia if the RV is decompressed. RV to coronary artery fistulas occur in 35-70% of patients with PA/IVS, while RVDCC is present in 9-34%3-6. Management strategies in PA/IVS range from biventricular repair to single ventricle palliation or heart transplantation. The presence of RVDCC is associated with a worse outcome as it necessitates a single ventricle palliation or heart transplantation, and there is a higher incidence of sudden death. 2,5-10
Although PA/IVS may be diagnosed prenatally, the postnatal outcome may be difficult to predict given the heterogeneity of the lesion and the inability to identify RVDCC in utero. Strain analysis, which measures myocardial deformation, has been shown in some studies to be a more sensitive marker of ventricular systolic dysfunction compared to standard 2D measurements.11-15 Global and regional myocardial deformation have not been well described in fetuses with PA/IVS, and could provide a more sensitive assessment of ventricular function in utero. These subtle changes in myocardial deformation may provide insight into the likelihood of RVDCC allowing for a more accurate prediction of prognosis and refined prenatal counseling.
The objectives of this study were to determine whether RV and LV myocardial deformation were different in PA/IVS fetuses compared to control fetuses and whether fetal ventricular strain was different in patients with and without RVDCC.
Methods:
Study Population
We performed a retrospective study that included all fetuses diagnosed with PA/IVS from January 2005 to October 2017 at two institutions: Columbia University Medical Center and Johns Hopkins Children’s Center. We selected gestational age (GA)-matched control fetuses from over the same time period with no structural or functional heart disease identified. Inclusion criteria for PA/IVS fetuses included those with a complete fetal echocardiogram with an adequate four chamber view and the qualitative appearance of normal LV systolic function. Fetuses were excluded if they had persistent non-sinus rhythm, or maternal or fetal systemic disease that could alter LV systolic or diastolic function. Fetuses with tricuspid atresia and pulmonary atresia were also excluded. For fetuses that were followed serially throughout gestation, the first study performed was used for analysis. This study was approved by the Columbia University Medical Center and the Johns Hopkins Institutional Review Boards.
Image acquisition and analysis:
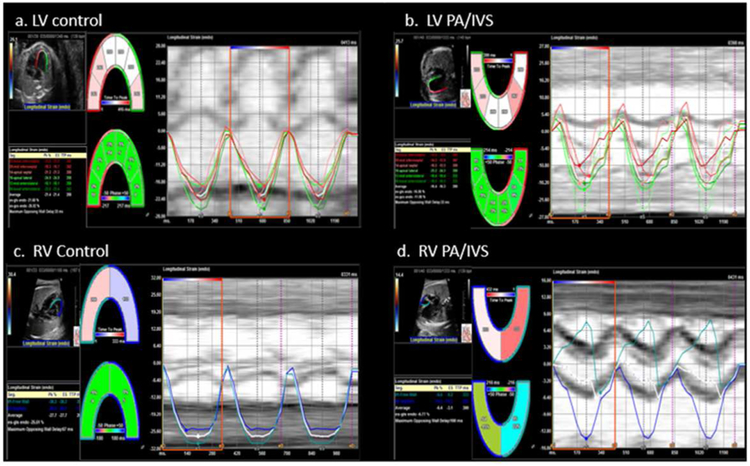
The fetal echocardiograms were obtained on Phillips iU22, 7500, Epiq 7 (Phillips Medical systems, Bothell, WA) and Siemens Sequoia (Siemens Medical Solutions, Mountain View, Calif., USA) cardiac ultrasound machines. Images with optimal 2D quality, particularly of the myocardium, were chosen. All images had a frame rate ≥ 30Hz, with a median frame rate of 42Hz (range: 30Hz-75Hz). The Digital Imaging and Communications in Medicine (DICOM) images from the selected fetal echocardiograms were imported into TomTec (Tomtec Corp USA, Chicago Illinois). The TomTec algorithm measures Lagrangian strain using speckle tracking echocardiography (STE), which is an angle independent technique for assessing global and regional cardiac deformation. This speckle-tracking algorithm was used to analyze left and right ventricular regional and global longitudinal strain (GLS) and strain rate from the 4-chamber view. M-mode tracings through the mitral valve were used to define end-systole and end-diastole. Tracking accuracy was visually confirmed and the tracing was corrected until consistent endocardial tracking was obtained. Three cardiac cycles were used for analysis when possible. Figure 1 depicts examples of LV and RV segmental longitudinal strain curves for a fetus with PA/IVS and a control fetus. Strain is reported as a negative number, with lower absolute values indicating worse strain.
Figure 1:
Segmental strain curves of the fetal left ventricle (LV) (a,b) and right ventricle (RV) (c,d). Each curve represents each segments’ strain and the average strain. In the examples shown here, the pulmonary atresia with intact ventricular septum (PA/IVS) fetuses (b,d) have more dyssynchrony between segments compared to control fetuses (a,c).
Degree of tricuspid regurgitation was assessed qualitatively by a single reader and its relationship to GLS was analyzed. TR was qualitatively classified as minimal (none to mild) versus significant (moderate to severe). The tricuspid valve and mitral valves were measured at end-diastole. RV and LV length were also measured at end-diastole from the level of the atrioventricular valve annulus to the ventricular apex. Z-scores were calculated based on gestational age using Boston z-scores.
Outcomes:
One of the primary clinical outcomes of interest was the presence of RVDCC which was determined by cardiac catheterization after birth. RVDCC was defined as stenosis in two or more main coronary arteries (left main, left anterior descending, circumflex and right coronary artery) or the presence of coronary ostial atresia. Medical record review was performed to determine outcome at last follow-up, including type of surgical repair (single ventricle palliation, 1.5 ventricle repair or biventricular repair), heart transplantation and survival. A biventricular repair was defined as the RV supporting the entire pulmonary circulation with separated systemic and pulmonary circulations. A 1.5 ventricle repair was defined as separated systemic and pulmonary circulations with a superior cavopulmonary connection “offloading” some of the pulmonary circulation from the right ventricle.
Data Analysis:
Statistical analysis was performed using Stata software, version 14.0 (StatCorp, College Station, Texas). Clinical variables were described using mean and standard deviation for normally distributed data, and median and interquartile range (IQR) for non-parametric data. Student’s t-test was used for normally distributed variables and Wilicoxon rank-sum test was used for non-parametric variables. Correlation between echocardiographic measures and strain was performed using Pearson’s correlation coefficient. A p-value of <0.05 was considered statistically significant. Intraobserver and interobserver reliability were assessed by intraclass correlation coefficient (ICC) in a randomly selected subset of 15 PA/IVS fetuses and 15 control fetuses.
Results:
General characteristics:
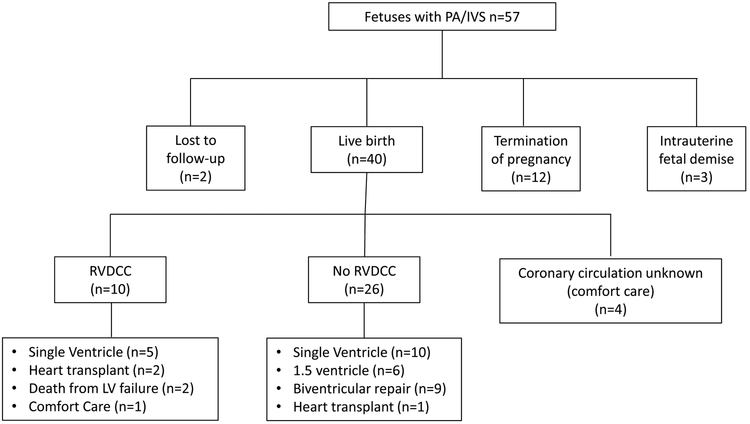
Sixty-two PA/IVS fetuses were identified over the time period; three were excluded due to decreased LV systolic function on qualitative 2D assessment and two had inadequate images for proper speckle tracking. Thus, 57 fetuses met inclusion criteria at a mean GA of 26.5 ± 5.2 weeks. The mean was 26.3± 5.2 weeks for GA-matched controls, which was not significantly different. Fetal and postnatal outcome are outlined in Figure 2. There was termination of pregnancy in 12 fetuses, intrauterine fetal demise in three fetuses and loss of follow-up in two fetuses. Forty of the PA/IVS fetuses had postnatal follow-up; 36 had coronary anatomy delineated by cardiac catheterization. All four patients with unknown coronary circulation received comfort care and were excluded from postnatal outcome analysis. One patient with RVDCC ultimately received comfort care and was included in analysis comparing strain in RVDCC vs. non-RVDCC, though excluded in the outcome analysis.
Figure 2:
Fetal pulmonary atresia with intact ventricular septum (PA/IVS) prenatal and postnatal outcomes, 2005-2017.
Footnote: RVDCC, Right ventricular dependent coronary circulation; left ventricle, LV.
LV strain:
LV GLS was significantly decreased in PA/IVS fetuses compared to controls (−17.4±1.7% vs. −23.7±2.0%, p<0.001). This relationship held across all gestational ages as shown in Figure 3a. Strain rate was also significantly decreased in PA/IVS fetuses compared with controls (−1.01±0.21s−1 vs.−1.42±0.20 s−1, p<0.001). Regional LV deformation is shown in Table 1. All cardiac segments had decreased strain in fetuses with PA/IVS compared to controls. LV septal strain was significantly decreased compared to LV free wall strain (−16.5±3.6 vs. −18.0±3.7, p=0.03) in PA/IVS fetuses.
Figure 3:
Left ventricular (LV) and right ventricular (RV) global longitudinal strain (GLS) in fetuses with pulmonary atresia with intact ventricular septum (PA/IVS) and controls shown across gestational age. (a) LV GLS is decreased in PA/IVS fetuses compared to controls across all gestational ages. (b) RV GLS is decreased in PA/IVS fetuses compared to controls across all gestational ages. Trendlines for each data set are shown.
Footnote: *P-values represent the difference between PA/IVS fetuses vs. control fetuses using students t-test.
Table 1:
Left ventricular (LV) regional and global longitudinal strain (GLS) and strain rate in fetuses with pulmonary atresia with intact ventricular septum (PA/IVS) and control fetuses.
| PA/IVS (mean±SD) n=57 |
Control (mean±SD) n=57 |
p-value | ||
|---|---|---|---|---|
| LV GLS (%) | −17.4±1.7 | −23.7±2.0 | <0.001 | |
| LV Strain Rate (s−1) | −1.0±0.2 | −1.4±0.2 | <0.001 | |
| LV Free Wall Peak Strain (%) | Basal | −14.5±6.0 | −22.9 ±4.5 | <0.001 |
| Mid | −15.5±7.9 | −23±4.5 | <0.001 | |
| Apical | −24.0±8.5 | −27.0±5.5 | 0.04 | |
| LV Septum Peak Strain (%) | Basal | −15.5±6.2 | −24.0±4.3 | <0.001 |
| Mid | −15.0±7.6 | −23.4±7.4 | <0.001 | |
| Apical | −19.1±8.3 | −26.1±7.1 | <0.001 | |
Intra and inter-observer reliability for LV GLS were excellent, with an intraclass correlation coefficient (ICC) between observers of 0.92 (95% confidence interval (CI) 0.83-0.96), and an intraobserver ICC of 0.93 (95% CI 0.84-0.97).
RV Strain:
RV strain measurement was attempted in all 57 PA/IVS fetuses and was successfully measured in 42 fetuses (74%). The remaining 15 fetuses had either inadequate images or unsatisfactory speckle tracking on visual assessment. As shown in Table 2, RV GLS was significantly decreased in fetuses with PA/IVS compared to controls (−11.6±3.8 vs. −24.6±2.5, p<0.001). This held true at all gestational ages (Figure 3b). There was not a significant difference between RV septal strain and RV free wall strain (−12.9±7.3 vs. −11.3 ±5.1, p=0.26.). RV strain rate was also significantly decreased in PA/IVS fetuses compared to controls (−0.75±0.67 vs. −1.53±0.29, p<0.001).
Table 2:
Right ventricular (RV) global longitudinal strain (GLS) and strain rate in pulmonary atresia with intact ventricular septum (PA/IVS) and control fetuses
| PA/IVS (mean±SD) |
Control (mean±SD) |
p-value | |
|---|---|---|---|
| RV GLS (%) | −11.6±3.8 | −24.6±2.5 | <0.0001 |
| RV Strain Rate (s−1) | −0.75±0.7 | −1.53±0.30 | <0.0001 |
| RV Free Wall Peak Strain (%) |
−11.3±5.1 | −23.8±4.1 | <0.0001 |
| RV Septal Wall Peak Strain (%) |
−12.9±7.3 | −25.2±4.1 | <0.0001 |
SD, standard dviation
GLS and RVDCC:
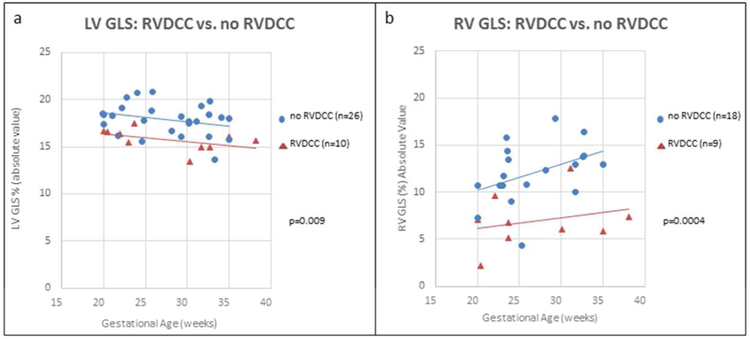
Of the 36 PA/IVS fetuses with known coronary artery anatomy, ten had RVDCC. Fetuses with RVDCC had decreased LV GLS compared to fetuses who did not (−15.8±1.2% vs. −17.9±1.7%, p=0.009). Of the 26 fetuses without RVDCC, 8 had RV to coronary connections. Mean LV GLS for this cohort did not significant differ from the fetuses without RV to coronary connections (−17.3±2.1 vs. −18.1±1.5, p=0.26). RV strain was able to be measured in 27 of the fetuses with known coronary artery anatomy. RV strain was decreased in fetuses with RVDCC (n=9) compared to fetuses without RVDCC (n=18): −7.0±2.9 vs. −12.1±3.2, p=0.0004. Figure 4 demonstrates LV and RV GLS versus gestational age in fetuses with and without RVDCC.
Figure 4:
Left ventricular (LV) and right ventricular (RV) global longitudinal strain (GLS) in fetuses with postnatally confirmed right ventricular dependent coronary circulation (RVDCC) vs. those without RVDCC shown across gestational age. (a) LV GLS is worse in fetuses with postnatally diagnosed RVDCC compared to fetuses without RVDCC. (b) RV GLS is worse PA/IVS fetuses with postnatally diagnosed RVDCC compared to fetuses without RVDCC.
Footnote : *P-value represent the difference between fetuses with RVDCC vs. without RVDCC using students t-test.
GLS and Outcome:
Median follow-up time was 3.4 years (range: 4 months to 11.4 years). Seven patients died, five of whom received comfort care. All five comfort care patients were excluded from outcome analysis. The four comfort care patients with unknown coronary circulation were all born prematurely (25-35 weeks). One patient received comfort care after cardiac catheterization showing RVDCC. For the two patients who died with intention to treat, one died at 84 days old from an ischemic event while listed for heart transplant, while the other died as a result of decreased function and ischemia after attempted patent ductus arteriosus stent placement at four days old.
At last follow-up, 15 patients had single ventricle palliations, six had 1.5 ventricle repair, nine underwent biventricular repair and three were living with a heart transplant. Seven patients died, five of whom received comfort care. Table 3 shows LV and RV GLS for various outcomes. LV GLS was decreased in patients with the most severe outcomes including those who died, received a heart transplant, or had single ventricle palliation, as compared to those with 1.5 or 2 ventricle repair (LV GLS −16.8%±1.9 vs. −18.0% ±1.5, p=0.05.). There was not a significant difference in LV GLS between individual outcomes, including comparing biventricular repair versus single ventricle palliation. The association of RV GLS with outcome was also analyzed. There were 31 PA/IVS fetuses in which RV strain was feasible and outcome was known. There was a significant difference in RV GLS between single and biventricular repair (−8.6±4.0% vs −12.6±2.7%, p=0.034).
Table 3:
Left ventricular (a) and right ventricular (b) global longitudinal strain by outcome
|
a* | |
| Outcome | LV GLS (%) mean±SD (95% CI) |
| Biventricular repair | −17.8 ±1.5 |
| (n=9) | (−14.8 to −20.8) |
| 1.5 ventricle | −18.4 ±1.6 |
| physiology (n=6) | (−15.2 to −21.6) |
| Single ventricle | −17.1 ±2.1 |
| (n=15) | (−12.9 to −21.5) |
| Heart transplant | −15.2 ±0.4 |
| (n=3) | (−14.4 to −16.0) |
| LV failure | −16.4 ±0.2 |
| (n=2) | (−16.0 to −16.8) |
| Comfort Care | −16.7 ±1.7 |
| (n=5) | (−13.3 to −20.1) |
|
b | |
| Outcome | RV GLS (%) mean±SD (95% CI) |
| Biventricular | −12.6±2.7 |
| repair (n=7) ¶ | (−7.2 to −18.0) |
| 1.5 ventricle | −13.2±2.7 |
| physiology (n=6) | (−5.4to 18.6) |
| Single ventricle | −8.6±3.0 |
| (n=10) ¶ | (−2.6 to −14.6) |
| Heart transplant | −5.9±1.2 |
| (n=2) | (−3.5 to −8.3) |
| LV failure | −10.0±3.7 |
| (n=2) | (−2.6 to −17.4) |
| Comfort Care | −11.2±3.7 |
| (n=4) | (−3.8 to −18.6) |
There was no significant difference when comparing LV GLS between individual outcomes
There was a significant difference when comparing RV GLS between those with single ventricle repair and biventricular repair (p=0.034).
LV, left ventricle; GLS, global longitudinal strain; SD, standard deviation; CI, confidence interval; RV, right ventricle.
LV/RV strain ratio
The median LV/RV strain ratio of PA/IVS fetuses was 1.47 (IQR 1.22-2.07). There was no significant difference in LV/RV free wall strain ratio between fetuses with RVDCC and without RVDCC [1.45 (IQR 1.39-1.89) vs. 1.70 (IQR 1.41-2.13), p=0.12]. There was also no significant difference in LV/RV free wall strain ratio between various outcomes.
Tricuspid Regurgitation:
TR on fetal echocardiogram was qualitatively classified as minimal (none to mild) versus significant (moderate to severe). Thirty-five fetuses had minimal TR and 22 fetuses had significant TR. Fetuses with minimal TR had decreased LV GLS compared with those with significant TR (−16.8±1.5 vs. −18.2±1.8 p=0.003). Twenty-three fetuses with minimal TR and 19 fetuses with significant TR were able to have RV strain analyzed. Fetuses with minimal TR had decreased RV GLS compared to those with significant TR (−10.1 ±4.0 vs. −13.2±2.9, p=0.01). All of the 10 patients with RVDCC had minimal TR. In addition, none of the patients with minimal TR went on to have a biventricular repair.
Additional Echocardiographic Measurements:
Median tricuspid valve (TV) z-score was −2.9 (range −5.22 to 2.71) and median right ventricular end diastolic (RVED) long axis z-score was −3.6 (range −6.45 to 1.92) in PA/IVS fetuses. There was a modest correlation between tricuspid valve z-score and LV GLS (r= −0.45, p=0.0005) and RV GLS (r= −0.52 p=0.0004). There was also a modest correlation between RVED long axis z-score and LV GLS (r= −0.44, p=0.006) and RV GLS (r= −0.46, p=.002). TV z-score, TV/MV annulus ratio, RVED long axis z-score and RV/LV long axis ratio were all decreased in fetuses that had RVDCC compared with those that did not have RVDCC (Table 4).
Table 4:
Left ventricular global longitudinal strain (GLS) and echocardiographic measurements in fetuses with right ventricular dependent coronary circulation (RVDCC) compared to no RVDCC.
| RVDCC n=10 |
No RVDCC n=26 |
p-value* | |
|---|---|---|---|
| LV GLS mean ±SD (%) 95% confidence interval |
−15.8±1.2 (−13.4 to −18.2) |
−17.9±1.7 (−14.5 to −21.3) |
0.009 |
| TV annulus z-score median IQR Range |
−5.6 (−4.6 to −5.2) (−3.7 to−5.2) |
−1.1 (−0.7 to −3.5) 1.9 to −4.4 |
<0.0001 |
| TV/MV annulus ratio median IQR Range |
0.39 (0.33 to 0.49) (0.3 to 0.5) |
0.75 (0.60 to 0.83) (0.38 to 1.0) |
0.0006 |
| RV end-diastolic length (z-score) median IQR Range |
−5.5 (−5.3 to −6.0) (−5.1 to−6.5) |
−3.1 (−1.8 to −4.5) (−6.0 to 0.16) |
<0.0001 |
| RV/LV length ratio median IQR Range |
0.34 (0.32 to 0.40) (0.29 to 0.44) |
0.69 (0.45 to 0.80) (0.37 to 1.05) |
<0.0001 |
p-value calculated using Student’s t-test for parametric data and Wilicoxon rank-sum test for non-parametric data. LV, left ventricle; GLS, global longitudinal strain; SD, standard deviation; TV, tricuspid valve; IQR, interquartile range; MV, mitral valve; RV, right ventricle; right ventricular dependent coronary circulation (RVDCC).
Discussion:
This study demonstrates that fetuses with PA/IVS have decreased global and regional LV and RV strain compared to controls. Interestingly, LV and RV strain were both significantly decreased in fetuses found postnatally to have RVDCC, compared to those without RVDCC.
This finding of decreased LV strain in PA/IVS fetuses may be due to altered ventricular mechanics in the context of a hypertensive right ventricle, abnormal coronary perfusion, and/or intrinsic abnormality of the myocardium. LV septal and free wall GLS were both decreased in fetuses with PA/IVS compared to controls; however, septal GLS was significantly decreased compared to the free wall, which may indicate that the hemodynamic effects of the hypertensive RV play a significant role in LV mechanics. In addition, GLS was decreased in fetuses with RVDCC compared to those without, indicating that abnormal coronary flow may contribute to decreased GLS in fetuses with PA/IVS. Strain analysis of the LV in this patient population was easily performed, with only two patients having been excluded for inadequate speckle tracking.
The finding of decreased RV GLS in fetuses with PA/IVS was an expected finding, given the change in RV hemodynamics associated with pulmonary atresia and an intact ventricular septum and its consequences on RV development. RV strain assessment was more difficult in PA/IVS fetuses, and we were unable to perform RV strain analysis in 15 patients due to inadequate speckle tracking. RV strain did however differ based on outcome with decreased RV strain in fetuses with RVDCC and decreased RV strain in fetuses with eventual single ventricle repair compared to biventricular repair. RV strain may become a more useful measure as algorithms improve to allow adequate speckle tracking even in very abnormal ventricles associated with congenital heart disease. LV/RV strain ratio was also assessed, with a median of 1.47 in PA/IVS fetuses, demonstrating that RV strain is affected more than LV strain, as expected. LV/RV strain ratio was not predictive of RVDCC or outcome.
Two previous studies have looked at fetal myocardial deformation of the single left ventricle. Brooks et al demonstrated that single left ventricles had no difference in peak LV GLS compared to controls as assessed by velocity vector imaging (VVI); however, only 8 of the 29 fetuses in this study had PA/IVS.16 Truong et al examined 18 fetuses with single left ventricles, of whom 6 had PA/IVS, and also found no significant difference in GLS compared to controls.17 In both studies, the majority of patients had tricuspid atresia or double inlet left ventricle. This may account for the difference in our results, given that tricuspid atresia and double inlet left ventricle do not have the hemodynamic effects of a hypertensive RV and the presence of coronary abnormalities. This hypothesis is supported by a study by Tanoue et al which compared measures of LV contractility between patients with PA/IVS and tricuspid atresia before and after Fontan operation and found that end systolic elastance as assessed by pressure-volume loops from cardiac catheterization, a measure of LV contractility, was decreased in patients with PA/IVS (both with and without RVDCC) compared to those with tricuspid atresia.18
The presence of RVDCC significantly changes postnatal outcome in PA/IVS, with multiple studies demonstrating that RVDCC is a significant risk factor for mortality. Patients with RVDCC are also at risk for sudden ischemic events, especially in the neonatal period.2-5, 7-10 Previous studies examined prenatal predictors of outcome, including RVDCC, in PA/IVS, and have shown that TV annulus size tends to be the best predictor of postnatal outcome, with varying z-score cut-offs between −3 and −4. TV/MV annulus ratio and RV/LV length ratio were also found to be useful in predicting postnatal outcome.11-13, 19-20 In our study, TV annulus z-scores were also significantly lower in fetuses with RVDCC.
The absence of TR has also been shown to be a marker of poor outcome in PA/IVS.12,20 Similarly, in our study, fetuses with mild or less TR had worse LV GLS than those with significant TR. As expected, RV strain was also worse in fetuses with minimal TR, consistent with the idea that these abnormal RVs with decreased contractility are unable to generate enough force to produce significant tricuspid regurgitation. In addition, none of the fetuses with minimal TR went on to have a biventricular repair, and none of the patients with moderate to severe TR had RVDCC. It is proposed that right ventricular hypertension may be responsible for the persistence of embryologic ventricular coronary connections, and that the high pressure and turbulent flow within these sinusoids may cause endothelial injury and intimal hyperplasia associated with coronary artery stenosis or atresia.4,5 Therefore, the presence of TR, which decompresses the RV, is protective against developing RVDCC.
Our study suggests that measuring fetal LV and RV GLS may add to the ability to prenatally predict the presence of RVDCC. LV and RV strain were significantly decreased in fetuses found postnatally to have RVDCC, compared to those without RVDCC. LV strain was not significantly different when comparing individual outcomes such as biventricular repair versus single ventricle palliation. This may be due to the small number of patients in each group. RV strain was noted to be different between biventricular repair and single ventricle palliation, although this measurement was more difficult to obtain. Combining LV and RV GLS with other echocardiographic measurements (TV annulus z-score, TV/MV ratio, RV end diastolic length z-score and RV/LV length ratio) in a larger population may yield an effective scoring system to help predict RVDCC and/or the ultimate type of surgical management.
The main limitations of this study are the retrospective methodology and relatively small number of cases, despite the inclusion of two institutions. Frame rates in this study of ≥30 Hz have been reported to be acceptable for fetal STE analysis; however, higher frame rates in the range of 50-90Hz have been shown to improve accuracy. Although our measures of inter-observer reliability were satisfactory, previous studies have demonstrated significant inter-observer variability in fetal strain measurements.21-24 A prospective, multi-center study of this rare disease would permit strain analysis at higher frame rates and aid in further assessing the utility and clinical impact of measuring fetal left ventricular strain.
This is, to our knowledge, the largest description of myocardial deformation in fetuses with PA/IVS. Fetuses with PA/IVS have decreased LV and RV strain compared to controls. Our results suggest that both interventricular interactions involving a hypertensive RV and abnormal coronary connections may play an important role in left ventricular mechanics in PA/IVS, distinguishing PA/IVS from other single left ventricles. In addition, given the difference in LV and RV strain between fetuses with and without RVDCC, this study encourages further investigation as to whether fetal ventricular strain could be used as a prenatal predictor of RVDCC.
Acknowledgements:
Funding provided by the Colin’s Kids Foundation.
References:
- 1.Zuberbuhler JR, Anderson RH. Morphological variations in pulmonary atresia with intact ventricular septum. Br Heart J. 1979;41(3):281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashburn DA, Blackstone EH, Wells WJ, Jonas RA, Pigula FA, Manning PB, Lofland GK, Williams WG, McCrindle BW; Congenital Heart Surgeons Study Members. Determinants of mortality and type of repair in neonates with pulmonary atresia and intact ventricular septum. J Thorac Cardiovasc Surg 2004; 127(4): 1000–1008. doi: 10.1016/j.jtcvs.2003.11.057. [DOI] [PubMed] [Google Scholar]
- 3.Calder AL, Peebles CR, Occleshaw CJ. The prevalence of coronary arterial abnormalities in pulmonary atresia with intact ventricular septum and their influence on surgical results. Vol. 17, Cardiology in the Young. 2007. p. 387–96. [DOI] [PubMed] [Google Scholar]
- 4.Giglia TM, Mandell VS, Connor AR, Mayer JE, Lock JE. Diagnosis and management of right ventricle-dependent coronary circulation in pulmonary atresia with intact ventricular septum. Circulation. 1992;86(5): 1516–1528. doi: 10.1161/01.CIR.86.5.1516. [DOI] [PubMed] [Google Scholar]
- 5.Coles JG, Freedom RM, Lightfoot NE, Dasmahapatra HK, Williams WG, Trusler GA, Burrows PE. Long-term results in neonates with pulmonary atresia and intact ventricular septum. Ann Thorac Surg [Internet]. 1989;47(2):213–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2919904 [DOI] [PubMed] [Google Scholar]
- 6.Yoshimura N, Yamaguchi M, Ohashi H, Oshima Y, Oka S, Yoshida M, Murakami H, Tei T. Pulmonary atresia with intact ventricular septum: Strategy based on right ventricular morphology. J Thorac Cardiovasc Surg 2003;126(5):1417–1426. doi: 10.1016/S0022-5223(03)01035-3. [DOI] [PubMed] [Google Scholar]
- 7.Hanley FL, Sade RM, Blackstone EH, Kirklin JW, Freedom RM, Nanda NC. Outcomes in neonatal pulmonary atresia with intact ventricular septum. A multiinstitutional study. J Thorac Cardiovasc Surg [Internet]. 1993;105(3):406–23, 424–7-4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8445920 [PubMed] [Google Scholar]
- 8.Daubeney PEF, Wang D, Delany DJ, Keeton BR, Anderson RH, Slavik Z, Flather M, Webber SA. Pulmonary atresia with intact ventricular septum: Predictors of early and medium-term outcome in a population-based study. J Thorac Cardiovasc Surg 2005;130(4):1–9. doi: 10.1016/j.jtcvs.2005.05.044. [DOI] [PubMed] [Google Scholar]
- 9.Powell a J, Mayer JE, Lang P, Lock JE. Outcome in infants with pulmonary atresia, intact ventricular septum, and right ventricle-dependent coronary circulation. Am J Cardiol. 2000;86(11): 1272–1274, A9. doi: 10.1016/S0002-9149(00)01220-0. [DOI] [PubMed] [Google Scholar]
- 10.Cheung EW, Richmond ME, Turner ME, Bacha EA, Torres AJ. Pulmonary atresia/intact ventricular septum: Influence of coronary anatomy on single-ventricle outcome. Ann Thorac Surg 2014;98(4): 1371–1377. doi: 10.1016/j.athoracsur.2014.06.039. [DOI] [PubMed] [Google Scholar]
- 11.Gardiner HM, Belmar C, Tulzer G, Barlow A, Pasquini L, Carvalho JS, Daubeney PE, Rigby ML, Gordon F, Kulinskaya E, Ranklin RC. Morphologic and Functional Predictors of Eventual Circulation in the Fetus With Pulmonary Atresia or Critical Pulmonary Stenosis With Intact Septum. J Am Coll Cardiol 2008;51(13): 1299–1308. doi: 10.1016/j.jacc.2007.08.073. [DOI] [PubMed] [Google Scholar]
- 12.Peterson RE, Levi DS, Williams RJ, Lai WW, Sklansky MS, Drant S. Echocardiographic predictors of outcome in fetuses with pulmonary atresia with intact ventricular septum. J Am Soc Echocardiogr 2006;19(11):1393–1400. doi: 10.1016/j.echo.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 13.Salvin JW, McElhinney DB, Colan SD, Gauvreau K, del Nido PJ, Jenkins KJ, Lock JE, Tworetzky W. Fetal tricuspid valve size and growth as predictors of outcome in pulmonary atresia with intact ventricular septum. Pediatrics. 2006;118(2):e415–e420. doi: 10.1542/peds.2006-0428. [DOI] [PubMed] [Google Scholar]
- 14.Ishii T, Mcelhinney DB, Harrild DM, Marcus EN, Sahn DJ, Truong U, Tworetzky W. Circumferential and Longitudinal Ventricular Strain in the Normal Human Fetus. 2011:1–7. doi: 10.1016/j.echo.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J [Internet]. 2015;37(15): 1196–207. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4830908&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooks PA, Khoo NS, Hornberger LK. Systolic and diastolic function of the fetal single left ventricle. J Am Soc Echocardiogr 2014;27(9):972–977. doi: 10.1016/j.echo.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Truong UT, Sun HY, Tacy TA. Myocardial deformation in the fetal single ventricle. J Am Soc Echocardiogr 2013;26(1):57–63. doi: 10.1016/j.echo.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Tanoue Y, Kado H, Maeda T, Shiokawa Y, Fusazaki N, Ishikawa S. Left ventricular performance of pulmonary atresia with intact ventricular septum after right heart bypass surgery. J Thorac Cardiovasc Surg. 2004;128(5):710–717. doi: 10.1016/jjtcvs.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 19.Roman KS, Fouron J-C, Nii M, Smallhorn JF, Chaturvedi R, Jaeggi ET. Determinants of Outcome in Fetal Pulmonary Valve Stenosis or Atresia With Intact Ventricular Septum. Am J Cardiol 2007;99(5):699–703. doi: 10.1016/j.amjcard.2006.09.120. [DOI] [PubMed] [Google Scholar]
- 20.Iacobelli R, Pasquini L, Toscano A, Raimondi F, Michielon G, Tozzi AE, Sanders SP. Role of tricuspid regurgitation in fetal echocardiographic diagnosis of pulmonary atresia with intact ventricular septum. Ultrasound Obstet Gynecol 2008;32(1):31–35. doi: 10.1002/uog.5356. [DOI] [PubMed] [Google Scholar]
- 21.Matsui H, Germaniakis I, Kulinskaya E, Gardiner HM. Temporal and spatial performance of vector velocity imaging in the human fetal heart. Ultrasound Obstet Gynecol 2011; 37 (1): 150–157. doi 10.1002/uog.8815 [DOI] [PubMed] [Google Scholar]
- 22.Germanakis I, Gardiner H. Assessment of fetal myocardial deformation using speckle tracking techniques. Fetal Diagn Ther 2012;32(1–2):39–46. doi: 10.1159/000330378. [DOI] [PubMed] [Google Scholar]
- 23.Kapusta L, Mainzer G, Weiner Z, Deutsch L, Khoury A, Haddad S, Lorber A. Second trimester ultrasound: Reference values for two-dimensional speckle tracking-derived longitudinal strain, strain rate and time to peak deformation of the fetal heart. J Am Soc Echocardiogr. 2012;25(12):1333–1341. doi: 10.1016/j.echo.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Koopman LP, Slorach C, Manlhiot C, McCrindle BW, Jaeggi ET, Mertens L, Friedberg MK. Assessment of myocardial deformation in children using Digital Imaging and Communications in Medicine (DICOM) data and vendor independent speckle tracking software. J Am Soc Echocardiogr 2011;24(1):37–44. doi: 10.1016/j.echo.2010.09.018. [DOI] [PubMed] [Google Scholar]