Abstract
Despite considerable advances in guidance of radiofrequency ablation (RFA) therapy for the treatment of cardiac arrhythmias, success rates have been hampered by a lack of tools for precise intraoperative evaluation of lesion extent. Near-infrared spectroscopic (NIRS) techniques are sensitive to tissue structural and biomolecular properties, characteristics that are directly altered by RF treatment. In this work, a combined NIRS-RFA catheter is developed for real-time monitoring of tissue reflectance during RF energy delivery. An algorithm is proposed for processing NIR spectra to approximate non-irrigated lesion depth in both atrial and ventricular tissues. The probe optical geometry was designed to bias measurement influence toward absorption enabling enhanced sensitivity to changes in tissue composition. A set of parameters termed “lesion optical indices” are defined encapsulating spectral differences between ablated and unablated tissue. Utilizing these features, a model for real-time tissue spectra classification and lesion size estimation is presented. Experimental validation conducted within freshly excised porcine cardiac specimens showed strong concordance between algorithm estimates and post-hoc tissue assessment.
Keywords: arrhythmia, atrial fibrillation, diffuse reflectance, near-infrared spectroscopy, lesion, radiofrequency ablation
Graphical Abstract
1. Introduction
Atrial fibrillation (AFib), characterized by rapid disorganized electrical activity in the upper heart chambers, is associated with a fivefold increase in stroke risk, accounting for over 15% of stroke cases in the US 1, 2. Radiofrequency ablation (RFA) therapy has become an indispensable tool for treating drug-resistant AFib. Despite its widespread use, however, single procedure success rates have been low. Arrhythmia resurgence following initial successful ablation has been reported to occur in as many as 47% of patients, requiring additional procedures to achieve a sustained effect 3. The aim of RFA therapy is to modify the underlying cardiac tissue substrate by strategic anatomical lesion placement in order to disrupt arrhythmogenic electrical pathways and restore sinus rhythm. In principle, effective treatment is directly dependent on lesion characteristics such as continuity and transmurality. Current methods for validating lesion adequacy examine regional differences in electrical activity while attempting to provoke an arrhythmic event, either pharmacologically or through pacing4. However, partial lesions may also exhibit reduced excitability and short-term electrical quiescence, elusively suggesting effective treatment, yet these pathologic tissues can eventually recover and conduct4. Despite its unquestionable significance for ensuring treatment success, currently no method exists to directly assess the extent of lesion formation in the acute setting. Such a method could potentially improve procedural efficacy by enabling intraoperative detection of undertreated sites despite transient effects.
Several groups have proposed optical methods for evaluating acute thermal injury immediately following RF treatment. In ventricular tissue, direct visualization of the myocardium by optical coherence tomography (OCT) has been shown to reliably discriminate between ablated, necrotic tissue and untreated tissue5–9. However, the inherent depth limitation of OCT (<1mm in cardiac tissue) renders the technique unsuitable for lesion transmurality assessment. NADH autofluorescence imaging has been demonstrated to correspond well with epicardial lesion boundaries10, 11. The technique relies on the fact that ablated tissues exhibit impaired mitochondrial function compared to viable tissues. More recently, atrial lesion assessment has been demonstrated based on spectral signatures of UV-excited autofluorescence using a benchtop hyperspectral imaging system 12, 13. Although there have been several reports on optical lesion assessment, few studies have shown lesion size estimation within a configuration conducive for deploying in an intraoperative setting.
Alternatively, diffuse reflectance spectroscopy (DRS), using fiber-integrated ablation catheters, has been previously proposed as a method for assessing the degree of RF treatment to cardiac tissue. Demos et. al. observed a correlation between scattering-induced changes in reflectance slope and lesion depth in bovine ventricular samples14. This technique is contingent upon changes in tissue microstructure and cellular morphology occurring as a result of RF treatment. An alternative approach is to examine variations in tissue absorption; absorption related changes within diffuse reflectance spectra reflect changes in tissue biomolecular composition, which may indicate permanent change in viability. Fiber optical geometries could be adjusted to balance the relative sensitivity of DR measurements to absorption verses scattering changes15–17. Recently our group has demonstrated a strong relationship between near-infrared spectroscopy (NIRS) derived absorption and chromophore concentrations and endocardial lesion size within porcine atrial specimens18–20. However, this technique requires the computationally intensive step of solving an inverse problem to recover tissue optical properties for feature extraction, which may limit its applicability for real-time lesion assessment.
In this work, we propose a method for rapid processing of NIR spectra to approximate lesion depth in various cardiac tissue regions including the right (RA) and left atria (LA) and right ventricular (RV) regions. We define a set of parameters termed “lesion optical indices” (LOI) encapsulating observations of spectral morphological differences between ablated and unablated tissue. Utilizing these features, we demonstrate classification of NIR spectral integrity and subsequent estimation of lesion depth. We then apply the technique for real-time monitoring of lesion progression in ex vivo swine specimens using an NIRS-integrated non-irrigated ablation catheter. The proposed method is capable of performing NIRS-facilitated lesion estimation in <5ms using un-optimized code on a commercial laptop. Experimental validation performed by comparing algorithm estimates to vital stained cross-sections for various lesion sizes showed strong correspondence. Microscopic evaluation of NIRS-predicted moderate tissue treatment using high-resolution OCT (HR-OCT) and histopathology were coincident with markers for irreversible damage; conversely, little to no evidence of irreversible damage was noted in NIRS-predicted mild treatment or untreated tissue.
2. Methods
2.1. Near-infrared reflectance spectroscopy system
A schematic diagram of the experimental setup and spectral acquisition protocol is depicted in Figure 1. Optically integrated ablation catheters were fabricated in-house to allow for simultaneous sampling of tissue diffuse reflectance at the ablation site during RF energy delivery. Custom aluminum catheter tips were designed to house an illumination and collection fiber pair separated by a distance of ρ = 2.31 +/−0.05mm (Fig 1b). This separation was selected to yield particular sensitivity to tissue absorption effects 15–17. The fiber-embedded custom tip was mounted onto a commercially available RF catheter (Biosense Webster, Diamond Bar, CA) and electrically coupled using conductive epoxy. Impedance comparisons for the fiber-integrated catheter with an unmounted identical catheter yielded values within 6% of each other. The final integrated catheter diameter was <13F. Typical ablation catheters range on the order of 6F-14F.
Figure 1.

(a) Schematic diagram of experimental setup. L, lamp; SM, spectrometer; RFG, radiofrequency generator; OF, optical fiber; EC, electrical cable; RE, reference electrode; PBS, phosphate buffered saline. (b) Close up of distal end of the NIRS-integrated ablation catheter housing optical fibers. S, source fiber; D, detection fiber. (c) Measurement timeline for real-time spectral data acquisition.
Broadband light from a tungsten halogen source (HL-2000-HP, Ocean Optics Inc., Dunedin, Florida) was delivered onto the tissue via a 200μm optical fiber. A 450nm longpass filter was placed between the lamp output and the source fiber input to avoid tissue and operator exposure to UV light. Diffusely backscattered light was received by an identical collection fiber and routed to a spectrometer (600–1000nm) (C9405CB, Hamamatsu, Bridgewater, New Jersey). Spectral measurements were recorded at 30–50Hz. A custom LabVIEW program was used to facilitate data acquisition. Wavelength dependent NIR measurements were converted to relative reflectance spectra, RRel, using a similar process as described in 19, 21; which included dark subtraction, removal of instrument response, and normalization at 650nm from TiO2-based, silicone phantom measurement of known optical properties.
2.2. Sample preparation
A total of nineteen fresh swine hearts were acquired (Green Village, New Jersey). Experiments were conducted within 24 hours of sacrifice. Wedges were surgically resected from LA, RA, and RV regions and submerged in 37°C maintained PBS (phosphate buffered saline) under pulsatile flow. Unless stated otherwise, catheter ablation and simultaneous optical measurements were performed using non-irrigated settings on the endocardial surface in atrial samples and on the epicardial surface in RV samples.
Lesions were sagittally bisected immediately after spectral data acquisition. To evaluate the extent of microscopic tissue injury, one half was preserved in formalin for 24 hours and paraffin embedded for further histopathological assessment. Hematoxylin and eosin (H & E) staining in addition to Masson’s Trichrome staining was performed on adjacent 5¼m sections to evaluate markers for tissue injury. A set of lesions were imaged prior to bisection under HR-OCT (2.72 μm-5.52μm axial-lateral resolution) to examine microscopic features while the specimen remained intact. Details of the HR-OCT system have been described elsewhere22. The remaining half of the gross specimen was immersed in 1% 2,3,5-triphenyl-2H-tetrazolium chloride (TTC) vital stain for 25 minutes at room temperature to delineate tissue injury. To avoid the variation in tissue size caused by histological preparation, lesion size was evaluated from manual segmentation of digitized camera images of gross, TTC-stained specimens. Agreement between optical measurements and lesion depth values were quantified in terms of the Pearson correlation coefficient.
2.3. Optical measurement of RF ablated samples
The fiber-integrated catheter was connected to a commercial RF generator (Stockert 70, Biosense Webster, Diamond Bar, CA) under the manual unipolar, power-controlled mode. Target power settings were varied between 3–25W for durations between 10–120s to vary the extent of tissue injury. Tissue bioelectrical impedance and delivered power were recorded continuously throughout the ablation process using a commercial DAQ system (NI USB-6218 BNC, National Instruments, Austin, Texas).
Preliminary experiments were first conducted to evaluate possible features in spectral morphology that were distinct in treated and untreated specimens. In these lesions, continuous data acquisition was maintained from three to five seconds prior to application of RF energy until several seconds post ablation. Spectra retrospectively chosen from confirmed lesions with depths >= 5mm in RV samples (n=6) were used to guide LOI choices. In atrial preparations (n=6 each) spectra taken from confirmed transmural lesions were used. These lesions were not included in the final analysis. A similar ablation-optical measurement protocol was applied for generating a total of 24 epicardial lesions in the RV and 33 and 31 endocardial lesions in RA and LA samples, respectively. To assess the limitations of lesion size estimation, a separate test set of RV lesions were created with treatment depths ranging up to 6mm. Additionally, interrupted lesion lines were created within the atrial samples. To assess whether the technique could reveal gaps in treatment, optical measurements were acquired using a 2-axis motorized stage to translate the optical catheter across the lesion site at a step resolution of 0.5mm. All scanning was performed while samples were submerged in blood.
2.4. Feature extraction from NIR spectra
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
where λa and λb is 600nm and 1000nm, respectively. Λ is taken to be the set of wavelengths between 730–800nm region. It should be noted that these parameters are self-contained and do not require baseline normalization from untreated tissue.
Thermal treatment of the myocardium induces changes in the underlying physiological and chemical properties. Therefore, we hypothesized that features derived from a physical model could be used for further enhance lesion assessment. In addition to LOI parameters, absorption and reduced scattering spectra were derived from NIR spectra using an inverse Monte Carlo (iMC) method19. The technique is described in detail previously 19. Briefly, a look up table-based forward model was generated through Monte Carlo simulations run for the catheter optical geometry over a range of absorption (0–10 cm−1) and reduced scattering (2–60 cm−1) values. Absorption was modelled as a weighted sum of dominant cardiac chromophores in the near-infrared region, namely oxygenated and deoxygenated hemoglobin (HbO, Hb) and myoglobin (MbO, Mb), metmyoglobin (Mmb), lipid and water (Eq. 6).
| (6) |
Reduced scattering was assumed to exhibit a power law dependence with wavelength and was modelled to accommodate both Rayleigh and Mie scattering contributions, as follows:
| (7) |
where A and fRay is the scattering amplitude and Rayleigh fraction, respectively. bMie is the unitless scattering slope parameter and gives an indication on Mie equivalent radii of spherical scatterers. These parameters, along with absorber concentrations were determined using a Levenberg-Marquardt optimization scheme. To reduce the effects of local minima convergence, a series of 6 optimizations were run per spectra with different initial guesses. The optimal solution was taken to be the result which achieved the greatest R2 value. An example fit is shown in Figure 3.
Figure 3.

Example model fits to experimental data for untreated (blue) and treated (green) RA spectra.
2.5. Contact classification
A preliminary contact classification stage was implemented to filter out spectra that were unsuitable for lesion size estimation (i.e. blood contaminated). For this, a linear discriminant analysis (LDA) classifier was employed to categorize spectra into either one of three classes: blood (or non-contact), contact-untreated, and contact-treated. An unbiased estimate of classification performance was obtained using “leave one out” cross-validation using the cvpartiton and crossval functions within MATLAB. A goal of this work was to develop a rapid (sub-second) spectral analysis for lesion assessment. Therefore, the feature set for classification was limited to parameters that did not require iterative optimization for extraction (i.e. LOIs). These parameters were also analyzed using repeated measure ANOVA and Tukey’s multiple comparison tests to evaluate statistical significance between groups and diagnostic potential for treatment discrimination. All statistical analyses were performed in Prism 6 (GraphPad Software, San Diego, California).
2.6. Lesion size estimation
A regression model approximating the extent of thermal treatment was derived for each chamber utilizing spectra originating from treated and untreated tissues. Lesion depth values for spectra classified as in contact and untreated were set to zero. Spectra determined to be in contact and treated underwent further processing to determine the corresponding extent of treatment. To accomplish lesion size estimation, a lesion regression model was generated by obtaining solution weights, W, to the second order normal equation in Eq. 8, where X is an Nx2M feature vector comprised of LOI values and their squares, and Y is the Nx1 lesion depth.
| (8) |
A quadratic relationship was chosen based on prior studies in ventricular tissue that demonstrated a second order correspondence14. Due to anatomical differences between the chambers, a set of weights were computed separately for each chamber. Finally, the regression model output was passed through a rectified linear unit (ReLU) to prevent negative lesion size estimates.
In order to compare the influence of optical parameter inclusion on estimation performance, a separate estimation model was determined which consisted of both the LOI values, in addition to μa,630nm and b. These were selected based on previous literature examining optical changes within the thermally treated myocardium 19, 23, 24. All calculations were performed on a 2013 Macbook Air equipped with a 1.7GHz Intel i7 CPU and 8GB RAM.
3. Results
3.1. Effect of RFA on NIR measurements
The effect of RFA treatment on measured tissue reflectance was evaluated and compared to that of untreated cardiac tissue. Spectral measurements were retrospectively taken from TTC-confirmed transmural lesions in the atria and ventricular lesions extending beyond 5mm. Figure 3 shows the wavelength dependent response in tissue reflectance with progressive RF energy deposition for representative atrial and ventricular specimens. Marked differences in spectral shape were apparent throughout the entire spectral range and became more prominent with treatment. A distinct and broad reduction in RRel spectral shape was noted between 600–700nm. A broad increase in reflectance was observed > ~800nm in ventricular specimens and > ~870nm in atrial tissues. This rise was concurrent with a dip in reflectance at approximately 835nm and an ~18nm red shift in the local minima lying between 730–800nm. Ablated spectra also exhibited a subtle sharpening of the peak near 960nm. Overall the relative ratio between mean RRel values in the 600–700nm region and 830–965nm region were considerably lower in untreated spectra compared with increased treatment. These observations were consistent across chambers and were used as a basis to parameterize LOIs.
Changes in spectral shape in ablated tissue exhibited characteristic features primarily in regions coinciding with prominent Mmb absorption (Fig. 3), in addition to a scattering-induced spectral offset and tilting. Although generally similar in spectral shape, RV samples exhibited lower mean RRel values over the entire wavelength range for untreated preparations compared to atrial tissues. In addition, RV treated spectral changes were more dramatic with a larger reduction centered at 630 nm and a greater rise in RRel at longer wavelengths, followed by RA, then LA. We attribute this to the inherently greater amounts of myoglobin present in the RV as compared to atrial samples. Because Mmb is a large contributor to spectral shape changes seen with ablation treatment, a greater myoglobin reservoir is likely to absorb more at baseline and generate a greater measured response during ferrous to ferric state conversions. It is also likely that the increased endocardial thickness within atrial samples may limit myocardial sampling and hence scattering-induced reflectance changes therein. The relatively larger confidence intervals range observed in the atria as compared to ventricular samples (Fig. 3) could be explained by the interplay between the optical sampling volume and tissue wall thickness. In atrial samples, the selection criterion was lesion transmurality, which may not imply uniform wall thickness across samples. Because typical atrial wall thicknesses are within the range of longitudinal sampling for this optical geometry, measurements are likely to be affected by wall thickness as well as lesion extent. In RV samples however, the inherently greater wall thickness is likely to exceed the sampling depth and mimic a semi-infinite geometry thus less susceptible to these factors.
LOI parameters were then computed for lesions created on each tissue region, their corresponding unablated baseline spectra, as well as spectra acquired from whole swine blood. Statistical analysis revealed significant differences (p<0.0001) between untreated and treated tissue values as well as treated tissue and blood for all LOIs in both ventricular and atrial cohorts (Fig 5). These results suggested that these LOIs were potentially suitable for spectra classification and lesion size approximation.
Figure 5.
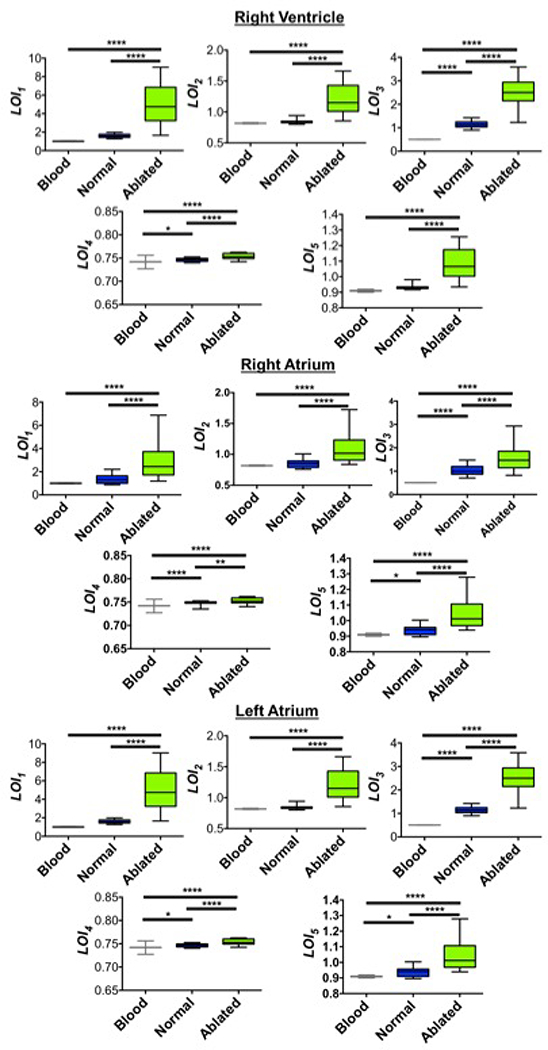
Statistical results for LOI values derived from both atrial and ventricular samples. Comparison of parameter values showed significant differences across the various spectra groups. blood (n = 60); normal and ablated each (RV, n=24; RA, n=33; LA, n=31). *p<0.05, **p<0.01, **** p<0.0001.
3.2. Physical model fitting results
In addition to parameters based on spectral morphology, tissue optical properties were determined using an inverse MC method. Overall, good agreement between estimated and experimental data was observed as judged by low residuals (Fig 3). Mean R2 values for untreated and treated spectral fits were 0.995 and 0.985, respectively. The slightly reduced R2 value for the treated spectra could be explained by greater residuals caused by the apparent broadening and redshifted local minima lying between 750nm-800nm region. This discrepancy could be attributed to RF-induced Mmb formation and increases in pH25.
3.3. Contact classification accuracy
A LDA classifier was generated in order to ensure the fidelity of spectra prior to lesion size estimation. The classifier was designed to accept all seven LOIs as features and determine whether measurements had originated from normal tissue, ablated tissue, or were blood contaminated. Performance of the classifier is depicted in Table 1 for determining contact (either untreated or treated) vs noncontact (blood). Good classification accuracy (>90%) was observed across all chambers and was best in RA specimens (n=66). Nevertheless, slight errors (<3%) were observed RV (n=48) and LA (n=62) specimens in discriminating ablated vs untreated tissue.
Table 1.
Performance of tissue contact classification.
| Region | CVCR[%] | Sensitivity[%] | Specificity[%] |
|---|---|---|---|
| RA | 99.1 | 100 | 100 |
| LA | 97.5 | 100 | 96.7 |
| RV | 98.1 | 100 | 97.9 |
CVCR – cross-validated classification rate
3.4. Regression model performance
A LOI-based, quadratic regression model was trained to carry out lesion size estimation. Figure 6 shows regression model results (prior to ReLU application) for predicted lesion depth values in comparison to actual values obtained through digitized lesion cross- sections. In RV samples, a test set of 17 additional lesions were created from 0 to 6mm in depth within the swine RV to test the limitations of depth estimation (Figure 6e, triangles). Strong prediction agreement was observed for lesions below ~4mm. Treatment resulting in lesion depths above these values were systematically underestimated. This can be explained in part due to limitation within the sampling volume. Another contributing factor to be considered is the decorrelation between endogenous markers expressed within the lesion core and the extent to which it correlates with deeper treatment. This influence is discussed further within the Discussion section. Table 2 shows Pearson correlation coefficient values for RV, RA, and LA for LOI-based and LOI+iMC-based regression models demonstrating strong concordance with experimental data (R>0.9) on all chambers tested. It was observed that the inclusion of optical parameters slightly enhanced agreement increasing correlation coefficients at most 2%. Because the addition of iMC-derived terms only showed marginal improvement in lesion size estimation, it may be difficult to justify the additional cost in computational time (1–10s per spectra depending on lesion properties) required as a result of iterative optimization and thereby limiting its application for real-time use; LOI-based estimates were computed within 2–5ms on average. However, this technique could potentially be used for investigating the underlying temporal dynamics in endogenous myocardial chromophores and ultrastructure as a result of RF ablation. The variability of lesion size estimates was greater within atrial samples than ventricular specimens. This can be attributed to the greater variability in endocardial thickness within atrial tissues compared to ventricular samples, which is likely to influence estimation. The impact of local endocardial thickness on lesion size estimation must be assessed further. However, these effects at lesion sizes of zero (i.e. untreated tissue) could be address in part during the preceding classification step, which sets lesion depth values of untreated tissue to zero.
Figure 6.

Lesion estimation results using both LOI-based and inverse MC-based regression models in (a-d) atrial and (e)(f) ventricular tissue. Triangles in (e) shows prediction for the additional lesion test set with extended range up to 6mm. Solid and dashed line represents the line of best fit, and confidence interval, respectively.
Table 2.
Pearson correlation coefficients across chambers.
| Region | LOI-based | LOI+iMC-based |
|---|---|---|
| RA | 0.932 | 0.952 |
| LA | 0.912 | 0.925 |
| RV | 0.969 | 0.977 |
3.5. Real-time monitoring of lesion formation
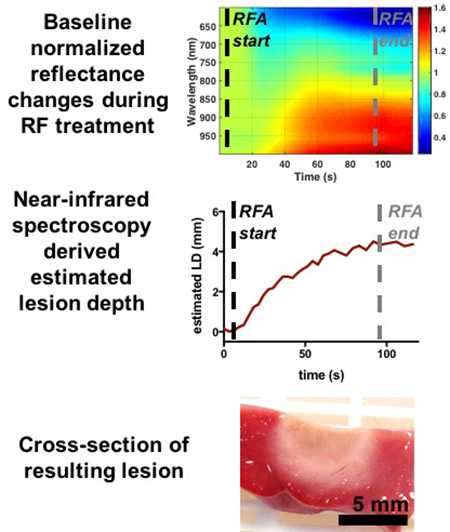
Dynamic lesion size estimates were computed for a set of lesions with varied doses of applied RF energy delivery (Figure 7, 8). In general, a short delay following ablation onset was noted followed by a monotonic increase with progressive treatment. Continuous monitoring after ablation offset demonstrated reasonable stability in estimated values. Resulting lesion depth values for both LOI- and LOI+iMC-based regression models were within 10% of the actual depths measured from digitized TTC stained images.
Figure 7.

Radiofrequency energy dose response with real-time monitoring of lesion formation in ventricular tissue. Representative spectro-temporal responses (a-c) during RF energy delivery are shown in context with the resulting TTC-stained lesion cross-sections (d-f) for increasing levels of treatment. Time courses for (g) LOI- and (h) LOI+iMC-based estimations for lesions are shown increasing monotonically with RF treatment. Final values following ablation, as indicated by the corresponding arrows, are in good agreement with resulting lesion sizes. Black dashed line denotes ablation start. White dashed line denotes the end of ablation.
Figure 8.

Microstructural analysis corresponding to spectroscopic results for treatment extent in right atrial tissue. (a) Baseline and final spectral profiles are shown for two lesions varying in size, along with (b) NIRS-derived time courses for lesion depth for deep and superficial lesions. (c) Ablated sites in the white light camera image shows the resulting lesions as seen from the endocardial surface. Heavier treatment corresponds with regions of greater myocardial discoloration. (d) HROCT B-scans showing superficial structural features for the various tissue treatments. Blue arrows demarcate signs of irreversible damage including micro-tears and coagulated necrosis at the catheter contact point. The untreated site shows a well delineated endocardium layer and birefringence band (yellow arrows). The superficial lesion presents a similar loss of endocardium in the absence of visible signs of permanent damage. (e) and (f) shows the corresponding H&E and trichrome histological correlates, respectively for comparison. Heavier treatment is confirmed in the deeper lesion with markers of coagulated necrosis in concert with dark purple region in trichrome. Viable tissue stained red in trichrome was absent within lesion centers and gradually becomes more prominent at lesion boundaries. Corresponding lesion depth for deep and superficial lesion measured from TTC images to be 2.76mm and 0.86mm, respectively.
3.6. Microscopic evaluation of RF treatment
Following NIRS-monitored RF ablation, lesions were fixed and paraffin-embedded prepared for histological examination. In a subset of lesions, OCT imaging was additionally performed in the intact specimen immediately after ablation and prior to sample fixation. Volumetric scans obtained were taken over normal and ablated myocardial tissues to compare microstructural details with NIRS measurements. A representative RA sample following this workflow is shown in Figure 8. In general, untreated sites exhibited a stratified appearance consisting of a thick, well-delineated endocardium and a birefringence band within the myocardium within HROCT. H&E showed a thick elastin and collagen layer with an intact myocardial structure underneath. Trichrome histology correspondingly showed the grerposition of collagenous endocardium (blue) over viable myocardium (red). Regions consistent with high NIRS-estimated lesion sizes demonstrated markers for treatment and irreversible injury within both HROCT and histology. HROCT images showed evidence of sub-endocardial micro-tears and loss in cellular structure, tissue coagulation indicated by higher myocardial intensity and reduced endocardial differentiation, and the characteristic loss in birefringence band6. Trichrome images showed uniform blue hue within the myocardial layer that became diffusely integrated with red viable myocardium on the lesion boundary. H&E images similarly showed evidence of tissue coagulation at the catheter contact point. In NIRS-estimated shallow lesions, HROCT showed similarly the absence of tissue birefringence and superficial coagulation, however micro-tears were not present. H&E and Trichrome images revealed superficial coagulation and loss in viability, respectively.
3.7. Exposing discontinuities along lesion lines
Reconnection along linear ablation lines is the primary factor for arrhythmia recurrence after pulmonary vein isolation4. Therefore, we sought to assess whether the techniques proposed were sensitive to detect lesion inadequacy along the lesion segment. In a subset of atrial specimens, interrupted linear ablation lines were created with treatment gaps <1mm wide. Optical measurements were taken over the intact lesion segment over a lateral step resolution of 0.5 mm (Figure 9). Samples were then sagitally bisected and TTC-stained to reveal regions of lesion non-uniformity for comparison with measured values. The spatially-resolved classification output and subsequent lesion-size estimation showed stark correspondence with lesion placement on gross pathology. Gaps within the optical image maps appeared wider. This can perhaps be attributed to the optical measurement sensitivity to features induced by irreversible tissue damage which may not present at the rim. Preliminary correlation experiments between the extent of damage delineated by TTC and tissue structural damage with histology demonstrated over-estimation of lesion boundaries where lesion were apparent. Further work is needed to systematically compare the relationship between treatment extent as indicated by TTC and the extent of irreversible injury markers within histological correlates. In a few instances within LA maps, the appearance false lesions were present as seen in the lower left of Fig. 9g. These instances may be due in part to algorithm challenges posed by endocardial scattering which, in some regions may be comparable to that of superficial lesions. These observations were not present within right atrial and ventricular samples where the thinner endocardium may permit more reliable myocardial sampling, and thus relatively greater differentiation between untreated tissue and subtle changes induced by mild ablation.
Figure 9.

Detection of treatment discontinuity along lesion line in swine RA (a-d) and LA (e-h) tissue. Gross pathology camera images (a)(e) are shown in context with lesion placement areas in classification (b)(f) and subsequent lesion estimation (c)(g) maps. Extracted lesion sizes are in reasonable agreement with post-hoc TTC-stained cross sections (d)(h). The treatment gap is indicated by the blue arrow. Dashed green line marks the boundary between the myocardium and epicardium layers. Bar is 5mm.
4. Discussion
The results presented in this work indicate that real-time spectroscopic measurement of absorption-biased diffuse reflectance during application of RF energy has the potential to inform on the extent of thermal injury (Fig. 6–8). Such measurements are predominantly influenced by changes in the endogenous tissue chromophore composition modified during treatment. Furthermore, a model for lesion size estimation in various chamber tissues has been proposed and validated for rapid on-line monitoring of lesion progression. Current methods for evaluating lesion adequacy rely on the mapping of local electrical activity with concurrent stimulatory provocations to verify voltage abatement. However, with such methods it is challenging to discriminate permanently damaged tissue from that which has been rendered reversibly unexcitable and functionally inactive in the acute setting. Optical measurements are sensitive to the underlying biomolecular constituents and tissue architecture which are reflected in tissue optical properties. Under normal physiological conditions, cardiac tissue is rich in ferrous myoglobin content and contains trace amounts of the ferric derivative26, 27. The application of thermal energy facilitates ferrous to ferric conversions through accelerated oxidation; in this state, these respiratory proteins lose the ability to reversibly bind to oxygen26, 27. These chromophore transitions influence the spectral morphology of optical measurements sampled at the catheter tip, in particular spectral regions of prominent Mb and Mmb absorption bands (Fig. 4). Our results indicate that extraction of the proposed indices are sensitive to these changes and could be used to infer lesion characteristics in situ (Fig. 6–8). Ascertaining these parameters during ablation treatment could potentially be used in feedback control methods for titrating RF energy dose. Furthermore, permanent tissue damage as judged by coagulative necrosis and loss in cellular structure was observed in microscopic assessment of the lesion core (Fig. 8) and was consistent with previously reported findings27, 28. This suggests that these methods could provide confirmatory measurements for lesion delivery that could be implemented together with conventional electroanatomical mapping systems to estimate long-term isolation durability and improve overall procedural efficacy using non-irrigated catheters.
Figure 4.

Effect of radiofrequency ablation on recovered RRel spectra. Representative NIR spectral measurements are shown for progressive RF treatment within RV (a), RA (b), and LA (c) tissues. Spectra were normalized to a value of one at 960nm for emphasis on spectral shape differences. (d)-(f) depicts mean +/− 95% confidence interval for normal (n=6) and moderately (n=6) treated tissue for RV, RA, and LA tissues, respectively. Lesions >= 5mm in RV tissue were selected while the criterion for moderate treatment was transmurality (2–4mm) in LA and RA samples. Characteristic differences between untreated and treated spectral shapes were used to guide the calculation of lesion optical indices.
Increases in reflectance of thermally coagulated tissues have been reported by several groups in literature which appear prima facie to contradict RRel measurements presented within this study (Fig. 4)11, 14, 29–32. However, these differences could be attributed to the dissimilarities in optical measurement geometries. In studies further deriving optical coefficients by solving the inverse problem, thermal coagulation is reported to produce an increase in tissue absorption (1.3–2 fold) and reduced scattering (2–7 fold)23, 24, 29, 30, 32. In a systematic Monte Carlo study conducted by Calabro and Bigio, the effect of reduced scattering on measured reflectance was shown to strongly depend on the product of source-detector separation and reduced scattering values (dimensionless scattering, μs’ρ); increased scattering results in decreased reflectance within the diffuse regime (μs’ρ >4) while resulting in increased reflectance when within the sub-diffuse regime (μs’ρ <2)33, 34. Measurement configurations employed in prior studies, including integrating-sphere, fiber probes with small ρ, and camera-based systems using wide-field illumination are likely to experience predominantly contributions from sub-diffuse reflectance, thereby experiencing an increase in reflected light due to ablation-induced scattering changes. Considering the range of μs’ values for the untreated and ablated myocardium reported within the visible to NIR (4 – 30cm−1) and a source-detector separation of 2.3 mm, it is likely that measurements presented within this study lie largely within the transition into the diffuse regime (1<μs’ρ <7) where the scattering influence on reflectance changes sign33.
Although model fitting accuracies in this paper showed good agreement with experimental data (R2 >=0.95), most residuals occurred in moderately treated samples near ~775nm and ~835nm. RRel spectra shown in Fig. 4 for moderately treated samples exhibited a corresponding local minimum in reflectance at ~835nm and an 18nm red shift and broadening of the minima centered at ~758nm. These observations may be due in part to potential changes in pH induced by thermal treatment that may have altered the spectral shape of distinct cardiac chromophores. Bowen et. al. reported considerable sensitivity of Mmb extinction spectra to changes in pH25. In spectrophotometric studies, formation of a broad peak near ~835nm was apparent as pH transitioned from 7.4 to 8.0 and became more pronounced with greater alkalinity. This change was also coupled with considerable reduction in absorption after 860nm. This observation was consistent with our measurements of increased reflectance noted after bands >900nm in atrial samples and >870nm in ventricular samples. The LOI1 parameter calculations were based on these changes, while further studies are needed to investigate variations in pH during RF ablation.
It is unlikely that the 2.3mm source-detector separation employed permits a sampling depth that extends beyond 1–3mm for the optical properties of ablated tissue. Therefore, we believe the apparent relationship of optically derived parameters to lesions beyond this range can be attributed to proportional changes within the sampled lesion core that correlate with amount of RF energy deposition. This hypothesis is supported by the plateauing of the regression model for deeper lesions (>4mm) within the RV, suggesting that a wider source-detector separation may be needed to extend the sampling depth for these cases. However, the current optical geometry is suitable for atrial tissue. Furthermore, because the method is sensitive to lesion size, transmurality of measurements are only ensured when the sampling volume extends beyond the wall and into the pericardial fluid. Consequently, a measurement indicating a lesion size in its current state cannot ensure lesion transmurality without comparing depth to the local wall thickness.
4.1. Study limitations
Most RF ablations today are performed using irrigated catheters. In preliminary measurements on irrigated lesions (data not shown), treatment extent was underestimated by the current algorithm. However, the insights gained from these data suggest that irrigated lesions present differences in the distribution of endogenous damage markers, particularly near the catheter contact point which differ under non-irrigated treatment. Future studies are aimed at testing whether a separate estimation model trained solely on irrigated lesions could be used to enable regression analysis of treatment extent under these conditions. Albeit, the current classification model seems robust enough to distinguish between untreated and irrigated lesion and thus may be utilized to reveal gaps within irrigated linear ablation lines. Another limitation is that our proposed method was developed with spectra acquired while the catheter was in contact with the tissue orthogonally. In practice, however, catheter steerability is limited and ablations are often performed at various catheter tissue contact angles. Ongoing work includes manipulation of optical geometries to obtain similar parameters from a wide range of contact angles. In recent years, the use of robotics assisted catheter navigation systems to enhance catheter control and maneuverability has shown promise in improving treatment safety and outcome35–37. Moreover, force-sensing catheters have been developed to allow operators to more precisely control the vector and amplitude of applied force. Fiber optic-integrated ablation catheters could be incorporated into such systems to meet contact angle requirements and enable on-line monitoring of optical parameters for reduced ambiguity in lesion durability assessment. Nonetheless, the proposed spectral analysis could potentially be applicable in non-contact ablation systems to remotely monitor tissue treatment. Previous studies have shown that optical measurements can readily discern catheter-tissue contact in measurements made with surrounding blood through the increased tissue reflectance19, 38, 39. Hemoglobin contributions were omitted from spectral analysis in this work since the method of sample preparation removed residual blood, while in vivo experiments in perfused tissue will require consideration in the absorption model. LOI definitions in this case may have to be modified to accommodate differences in physiological state such as increased hemoglobin due to tissue perfusion. However, previous works demonstrating DRS measurement in vivo in calves showed that a lumped Hb and Mb absorption spectra was sufficient to produce reasonable fitting accuracies for extraction of myocardial oxygen saturation40. Furthermore, experiments were performed in ex vivo hearts from healthy swine. It is possible that alterations in tissue ultrastructure occur in concert with arrhythmia, which may produce differences in baseline optical properties22, 41. In the future, the combination of optical spectroscopy and optical coherence tomography in a catheter based setting can provide information about the tissue ultrastructure22, 41 by OCT and lesion extent by spectroscopy.
4.2. Future work
Prospective studies are aimed at assessing the validity of the spectroscopic analysis under improved physiological conditions including in vivo experimentation in healthy pigs. These studies will help to evaluate the influence of hemodynamics and tissue oxygenation on spectral measurements and may produce additional features suitable for estimating treatment extent. Furthermore, concurrent pacing and mapping during these experiments will aid in exploring the relationship between lesion inducibility and extracted LOI and iMC parameters. Additionally, because typical treatment procedures could take several hours, extended studies are needed to investigate the stability of these parameters over time. Finally, patients with cardiovascular disease may exhibit pathological changes within atrial and ventricular substrates22. Therefore, future studies will target evaluating treatment extent in the context of modified diseased substrates presented within ex vivo human donor hearts.
5. Conclusion
A method for real-time assessment of RFA lesion size in cardiac tissue has been presented based on thermally induced changes in chromophore composition detectable by NIRS. In addition, a classification algorithm has been proposed for checking probe-tissue contact fidelity by rejection of spectra impaired by blood. These observations have the potential to improve upon current strategies and outcomes with catheter ablation using non-irrigated catheters, however further studies are needed to extend the algorithm to accommodate irrigated lesions. Direct estimation of lesion size during ablation treatment of AFib could provide useful indication regarding the likelihood of long-term isolation in the acute setting. These findings suggest a framework for rapid monitoring of lesion characteristics in situ using reflectance spectroscopic methods in the VIS-NIR region.
Figure 2.

Algorithm flow diagram for processing NIR spectra. Acquired spectra first undergoes feature extraction which involves calibration and subsequent determination of LOI values and/or inverse Monte Carlo-derived physical parameters. These values are then used to assess whether tissue contact had been established during measurement and if so, determine the corresponding lesion depth.
Acknowledgements
The authors would like to thank Arthur Autz for aid in obtaining spectrophotometric measurements. This work was supported in part by National Institutes of Health grant NIH 1DP2HL127776–01 (CPH) and National Science Foundation Career Award 1454365 (CPH).
References
- [1].Reiffel JA Am J Med. 2014, 127, e15–16. [DOI] [PubMed] [Google Scholar]
- [2].Wolf PA, Abbott RD, Kannel WB Stroke. 1991, 22, 983–988. [DOI] [PubMed] [Google Scholar]
- [3].Ganesan AN, Shipp NJ, Brooks AG, Kuklik P, Lau DH, Lim HS, Sullivan T, Roberts-Thomson KC, Sanders P Journal of the American Heart Association. 2013, 2, e004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wood MA Circ Arrhythm Electrophysiol. 2011, 4, 257–259. [DOI] [PubMed] [Google Scholar]
- [5].Fleming CP, Quan KJ, Rollins AM Journal of biomedical optics. 2010, 15, 041510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fleming CP, Quan KJ, Wang H, Amit G, Rollins AM Optics express. 2010, 18, 3079–3092. [DOI] [PubMed] [Google Scholar]
- [7].Fu X, Blumenthal C, Dosluoglu D, Wang YT, Jenkins MW, Souza R, Snyder C, Arruda M, Rollins Proc A SPIE 2016, 9697.
- [8].Fu X, Wang Z, Wang H, Wang YT, Jenkins MW, Rollins AM Optics letters. 2014, 39, 5066–5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Herranz D, Lloret J, Jimenez-Valero S, Rubio-Guivernau JL, Margallo-Balbas E Biomedical optics express. 2015, 6, 3268–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mercader M, Swift L, Sood S, Asfour H, Kay M, Sarvazyan N American journal of physiology. Heart and circulatory physiology. 2012, 302, H2131–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Swift L, Gil DA, Jaimes R 3rd, Kay M, Mercader M, Sarvazyan N Circ Arrhythm Electrophysiol. 2014, 7, 929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gil DA, Swift LM, Asfour H, Muselimyan N, Mercader MA, Sarvazyan NA J Biophotonics. 2017, 10, 1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Muselimyan N, Swift LM, Asfour H, Chahbazian T, Mazhari R, Mercader MA, Sarvazyan NA PLoS One. 2016, 11, e0167760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Demos SG, Sharareh S Optics express. 2008, 16, 15286–15296. [DOI] [PubMed] [Google Scholar]
- [15].Mourant JR, Bigio IJ, Jack DA, Johnson TM, Miller HD Applied optics. 1997, 36, 5655–5661. [DOI] [PubMed] [Google Scholar]
- [16].Mourant JR, Johnson TM, Los G, Bigio IJ Physics in medicine and biology. 1999, 44, 1397–1417. [DOI] [PubMed] [Google Scholar]
- [17].Kumar G, Schmitt JM Applied optics. 1997, 36, 2286–2293. [DOI] [PubMed] [Google Scholar]
- [18].Singh-Moon RP, Hendon CP IEEE 12th International Symposium on Biomed Imaging 2015, 751–755. [Google Scholar]
- [19].Singh-Moon RP, Marboe CC, Hendon Biomed C Opt. Express 2015, 6, 2494–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Singh-Moon RP, Yao X, Marboe CC, Hendon CP Biomedical Optics Congress. 2016, Tu3A.39.pdf. [Google Scholar]
- [21].Reif R, A’Amar O, Bigio IJ Applied optics. 2007, 46, 7317–7328. [DOI] [PubMed] [Google Scholar]
- [22].Yao X, Gan Y, Marboe CC, Hendon CP Journal of biomedical optics. 2016, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Swartling J, Palsson S, Platonov P, Olsson SB, Andersson-Engels S Medical & biological engineering & computing. 2003, 41, 403–409. [DOI] [PubMed] [Google Scholar]
- [24].Thomsen SLJ, L S; Flock ST. Proc. Soc. Photo-Opt. Instrum. Eng 1202. 1990, 2–10. [Google Scholar]
- [25].Bowen WJ The Journal of biological chemistry. 1949, 179, 235–245. [PubMed] [Google Scholar]
- [26].Antonini E, Brunori M, Hemoglobin and myoglobin in their reactions with ligands, North-Holland Pub. Co., Amsterdam,, 1971. [Google Scholar]
- [27].Celik H, Ramanan V, Barry J, Ghate S, Leber V, Oduneye S, Gu Y, Jamali M, Ghugre N, Stainsby JA, Shurrab M, Crystal E, Wright GA Circ Arrhythm Electrophysiol. 2014, 7, 718–727. [DOI] [PubMed] [Google Scholar]
- [28].Dickfeld T, Kato R, Zviman M, Lai S, Meininger G, Lardo AC, Roguin A, Blumke D, Berger R, Calkins H, Halperin H Journal of the American College of Cardiology. 2006, 47, 370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Splinter R, Svenson RH, Littmann L, Tuntelder JR, Chuang CH, Tatsis GP, Thompson M Lasers in surgery and medicine. 1991, 11, 117–124. [DOI] [PubMed] [Google Scholar]
- [30].Agah R, Gandjbakhche AH, Motamedi M, Nossal R, Bonner RF IEEE transactions on bio-medical engineering. 1996, 43, 839–846. [DOI] [PubMed] [Google Scholar]
- [31].Swift LM, Asfour H, Muselimyan N, Larson C, Armstrong K, Sarvazyan NA Heart rhythm : the official journal of the Heart Rhythm Society. 2018, 15, 564–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nilsson AM, Sturesson C, Liu DL, Andersson-Engels S Applied optics. 1998, 37, 1256–1267. [DOI] [PubMed] [Google Scholar]
- [33].Calabro KW, Bigio IJ Journal of biomedical optics. 2014, 19, 75005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bigio IJ, Fantini S, Quantitative biomedical optics : theory, methods, and applications, Cambridge University Press, Cambridge, 2016. [Google Scholar]
- [35].Bai R, B. L DI, Valderrabano M, Lorgat F, Mlcochova H, Tilz R, Meyerfeldt U, Hranitzky PM, Wazni O, Kanagaratnam P, Doshi RN, Gibson D, Pisapia A, Mohanty P, Saliba W, Ouyang F, Kautzner J, Gallinghouse GJ, Natale A J Cardiovasc Electrophysiol. 2012, 23, 820–826. [DOI] [PubMed] [Google Scholar]
- [36].Di Biase L, Wang Y, Horton R, Gallinghouse GJ, Mohanty P, Sanchez J, Patel D, Dare M, Canby R, Price LD, Zagrodzky JD, Bailey S, Burkhardt JD, Natale A J Cardiovasc Electrophysiol. 2009, 20, 1328–1335. [DOI] [PubMed] [Google Scholar]
- [37].Saliba W, Reddy VY, Wazni O, Cummings JE, Burkhardt JD, Haissaguerre M, Kautzner J, Peichl P, Neuzil P, Schibgilla V, Noelker G, Brachmann J, Di Biase L, Barrett C, Jais P, Natale A Journal of the American College of Cardiology. 2008, 51, 2407–2411. [DOI] [PubMed] [Google Scholar]
- [38].Fleming CP, Rosenthal N, Rollins AM, Arruda M The Journal of Innovations in Cardiac Rhythm Management. 2011, p199–201. [Google Scholar]
- [39].Fleming CP, Wang H, Quan KJ, Rollins AM Journal of biomedical optics. 2010, 15, 030516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lindbergh T, Larsson M, Szabo Z, Casimir-Ahn H, Stromberg T Journal of biomedical optics. 2010, 15, 027009. [DOI] [PubMed] [Google Scholar]
- [41].Gan Y, Tsay D, Amir SB, Marboe CC, Hendon CP Journal of biomedical optics. 2016, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]


