Abstract
Dyeing effluents have become a vital source of water pollution. Due to the xenobiotic properties and toxicity to all life forms including humans, removal of undesirable color and associated toxicity is crucial. In this study, five dye decolorizing bacteria were isolated from dyeing effluent using selective enrichment culture in Bushnell-Haas (BH) medium amended with co-substrate (i.e. glucose, yeast extract) and 100 mg L−1 of each commercially available reactive dyes viz. Novacron Orange FN-R, Novacron Brilliant Blue FN-R, Novacron Super Black G, Bezema Yellow S8-G and Bezema Red S2-B. The isolated bacteria were identified and assigned as Neisseria sp., Vibrio sp., Bacillus sp., Bacillus sp. and Aeromonas sp. based on their phenotypic (cultural, morphological, physiological and biochemical characteristic) observation. The dye decolorization efficiency was estimated spectrophotometrically up to 6 days of static incubation at 37 °C and observed that all of the isolates were unable to induce decolorization in absence of co-substrate. In case of monoculture, decolorization percentage varies from no visible decolorization (Bezema Red S2-B by Ek-5) to highest 90% decolorization (Novacron Brilliant Blue FN-R by Ek-13) whereas the decolorization percentage of bacterial consortium varies from 65% (Bezema Yellow S8-G) to 90% (Novacron Brilliant Blue FN-R and Novacron Super Black G). The study outlines the co-substrates mediated decolorization process where bacterial consortium proved as efficient dye decolorizer than that of the monocultures. This finding confers possibility of using novel microbial consortium for biological treatment of disreputable dyeing effluents.
Keywords: Dyeing effluent, Decolorization, Reactive dye, Bacterial consortium, Biological treatment
1. Introduction
Reactive dyes are extensively used in textile industry because of their large size which results in high affinity to bind with cellulosic fiber. With the increased demand for textile products, the worldwide annual growth rates of reactive dyes are also increasing and accounts for four times as much as for conventional dyes [1]. Simultaneously, the textile industry and its waste waters have increased proportionally making it one of the main sources of severe pollution problems now-a-days. High amount of water used during dyeing and washing textiles, ultimately finds their way to the surface water system carrying a considerable amount of organic dyes. For example, a dark-color reactive dyeing process for one kilogram of cotton material consumes 70 L of fresh and soft water [2]. Also, from the dyes wasted (10–25% of the total dyes produced) during the textile processes, 2–20% are directly released to the surface water system [3]. These high colors renders the water unfit for use at the downstream of the disposal point and may hinder light penetration thereby affecting aquatic life and continuously threatening the biodiversity [4], [5], [6]. However, beyond color, the presence of these dyes in aqueous ecosystems presents serious environmental and health concerns as a result of the toxicity of the free dyes themselves and their transformation into toxic, mutagenic and carcinogenic amines, primarily as result of anaerobic microbial reductive cleavage of the azo bond [7], [8], [9] or even caused by products obtained after oxidation via cytochrome P450 [10], [11], [12]. The existing physical, chemical and photochemical approaches for the treatment of such dyeing effluents have disadvantages of being highly expensive, associated operational and technical difficulties, and production of large amounts of sludge as well as toxic substances [13], [14]. In order to overcome these drawbacks, over the past few decades, biological approaches such as biodegradation and biosorption by both live and dead microbial biomass in aerobic, anaerobic or combined treatment processes with a number of bacteria, fungi, yeasts and algae [15], have been investigated. Efforts to isolate bacterial cultures capable of degrading azo dyes started in the 1970s with reports of Bacillus subtilis [16] and since then numerous bacteria and fungi capable of dye decolorization, either in pure cultures or in consortia, have been reported [17], [18], [19], [20], [21]. Several studies on the decolorization and degradation of reactive azo dyes have been indicated the involvement of various extracellular reductive enzymes such as azoreductase and oxidative enzymes such as laccase, tyrosinase, lignin and manganese peroxidases [22]. Advantages of biological processes over conventional processes include conversion of organic compounds to non-toxic products (water and carbon dioxide), low cost, sustainability and easy to use [23]. Although still, the detail metabolic demands of different microbes are complex, impeding their utilization for the remediation of huge amount of colored wastewater. As restrictive environmental legislation, the ecological problem and the high cost of conventional technologies have resulted in the search of environmental friendly and economically viable wastewater treatment plants, it is thus important to explore the possibilities of isolating efficient dye decolorizing microbial communities as well as their consortia for their use under favorable environmental conditions in biological treatment of noxious dying effluent.
Keeping in view of the above background, the present study was focused on the screening and characterization of potent indigenous bacterial isolates from textile dying effluents and utilization of these isolates as monoculture and consortium for decolorization of some commercially available textile reactive dyes as well as dye mixture.
2. Materials and methods
2.1. Dyestuff, media and chemicals
Dyes used throughout the present study were commercially available reactive dyes viz. Novacron Orange FN-R, Novacron Brilliant Blue FN-R, Novacron Super Black G, Bezema Yellow S8-G and Bezema Red S2-B which were collected from KDS Textile Mills Ltd., Chittagong, Bangladesh. These dyes are of the same chemical class and function having different reactive systems in order to form reactive bonds with the substrates. Stock solution of the experimental dyes were prepared by dissolving 1 g of each dye into 100 mL of sterile distilled water, filtered and stored in brown bottle at room temperature. From the stock, working solution was prepared to give a final concentration of 100 mg L−1. A mixture of all dyes was prepared by adding each of the dye solution in 1:1 proportion by maintaining a final concentration of 100 mg L−1 for each dye and used for enrichment, isolation and screening of the potent dye decolorizing bacteria. All media ingredients and reagents were of analytical grade with desired purity and purchased from Hi-Media Laboratories, India; Merck, Germany and Sigma-Aldrich Ltd., USA.
2.2. Enrichment, isolation and screening of dye decolorizing bacteria
Samples viz. inlet and outlet effluent of the effluent treatment plant, dye contaminated soil and nearby canal effluent collected from the vicinity of the KDS. Textile Mills Ltd., Chittagong, Bangladesh were used to isolate dye decolorizing bacteria. The physico-chemical and microbiological analysis of the collected samples were reported previously [24]. The isolation was carried out in Bushnell-Haas (BH) medium [25]. The medium composition was KH2PO4 – 0.1%, K2HPO4 – 0.1%, MgSO4.7H2O – 0.02%, CaCl2.2H2O – 0.002%, NH4Cl – 0.1%, NH4NO3 – 0.1%, NaCl – 0.01%, and FeCl3⋅6H2O – 0.005%. The medium was supplemented with 0.4% glucose and yeast extract respectively as a co-substrate and the pH was adjusted to 7.0. For enrichment of dye decolorizing bacteria, 10 mL of the effluent and 10 g soil from each sample was inoculated in 250 mL conical flask containing 90 mL sterilized BH medium supplemented with filter sterilized 100 mgL−1 dye mixture. After 3 days of incubation (37 °C, 150 rpm) 10 mL enriched broth was transferred to fresh dye supplemented BH medium and incubated at the same condition for another 3 days. Such serial transfers were performed for 3cycles for the enrichment of putative dye decolorizing bacteria. Serial dilutions of the enriched broth were made up to 10−6 dilutions and aliquots from each dilution were placed on BH agar medium supplemented with 100 mg L−1 of dye mixture and incubated at 37 °C for 48 h. After incubation, morphologically distinct and prominent colonies were picked up and purified through repeated streaking on the same medium. The purified bacterial isolates were maintained in nutrient agar slants for routine works and preserved for long term in 30% glycerol broth at − 80 °C.
2.3. Identification of the selected isolates
Bacterial isolates able to grow profusely at 100 mgL−1 of dye mixture were validated as promising dye decolorizer and subsequently, characterized based on their cultural (e.g., colony color, form, margin, surface and elevation); morphological (e.g., cell shape and arrangement, sporulation); physiological (e.g., growth response at different temperature, pH and salt concentration) and biochemical (e.g., motility test, catalase, oxidase, urease, MR-VP, Indole test, citrate utilization, nitrate reduction, H2S production, enzymatic hydrolysis and different sugar fermentation test) characteristics as described in the Cowan and Steel’s Manual for the identification of Medical Bacteria [26]. The isolates were then provisionally identified up to genus level by comparing the test results with the standard descriptions given in Bergey's Manual of Determinative Bacteriology [27].
2.4. Dye decolorization assay
2.4.1. Determination of absorption maxima (λmax) and plotting of standard curve
Absorption maxima (λmax) of each dye as well as dye mixture were determined in decolorizing medium through scanning the absorption of light within the visible range (400–700 nm) at an intervals of 10 nm using double beam UV–Vis Spectrophotometer (Optima sp 3000 nano, Japan). The wavelength reflecting the highest optical density was regarded as their corresponding maximum wavelength (λmax) and after determining the λmax i.e., Novacron Orange FN-R at 490 nm, Novacron Brilliant Blue FN-R at 610 nm, Novacron Black G at 600 nm, Bezama Yellow S8-G at 410 nm, Bezema Red S2-B at 530 nm and Dye mixture at 510 nm, standard curve for each dye as well as dye mixture was obtained. Briefly, standard solutions of the known concentrations (0.0, 0.1, 1, 10, 100 mgL−1) were prepared in dye decolorizing medium and centrifuged (10,000g, 15 min at 4 °C). The absorbance of the supernatant was recorded against a blank to avoid media interference and was plotted against dye concentration in Microsoft® Office Excel 2007. Using “scatter” function, trend line was added and then the R2 value was calculated. For the experimental values obtained through the same procedure, the same equation was used to calculate the percentage (%) of dye decolorization.
2.4.2. Inoculums preparation and development of bacterial consortium
Much importance was given on inoculums preparation as variation in the physiology and growth condition of microorganisms may influence on process variability. Bacterial isolates were inoculated in Luria Bertani (LB) broth (Tryptone 5 g, Yeast extract 10 g, NaCl 10 g and Distilled water 1 L) and incubated for 24 h at 37 °C under agitation (160 rpm). The broth culture was harvested by centrifugation (10,000g, 15 min at 4 °C), washed with 0.85% NaCl solution and resuspended in the same. Optical density was adjusted to 0.5 MacFarland standards (1.5 × 108 cells/mL) to get uniform cell density. Finally, Bacterial consortium was developed by mixing all isolates in 1:1 ratio by maintaining uniform cell density (1.5 × 108 cells/mL) for each isolates.
2.4.3. Analysis of decolorization efficiency
For dye decolorization experiment, Erlenmeyer flasks (250 mL) containing 50 mL of sterilized BH medium (pH 7.0) amended with each experimental dye as well as dye mixture to a final concentration of 100 mg L−1 were inoculated with 10% (v/v) inoculums of each isolate as well as the developed consortium and incubated for 6 days (at 37 °C with 160 rpm). BH broth without co-substrate i.e., Glucose and yeast extract was also inoculated to assess the utilization of dyes as a sole source of energy. Control was maintained without inoculation. After 2, 4 and 6 days of incubation the culture broth was centrifuged (10,000g, 15 min at 4 °C) and absorbance of the supernatant was recorded at the corresponding λmax of each dye solution as mentioned earlier by a double beam UV–Visible spectrophotometer (Kuobta 6930, Japan). The decolorization activity in terms of (%) decolorization was calculated from standard curve of the dyes according to the formula given by Chen et al. [28]:
2.5. Statistical analysis
All experiments were conducted in triplicate and mean ± standard deviation values were expressed. Data was analyzed by using GraphPad Prism Software 6 (GPPS 6).
3. Results and discussion
3.1. Isolation, screening and identification of dye decolorizing bacterial isolates
In order to isolate dye decolorizing bacteria, the enriched culture broth were inoculated in BH agar medium amended with the dye mixture. A total of 30 morphologically distinct bacterial colonies were isolated and screened out by repeated streaking on dye supplemented BH agar medium. Based on vigorous growth on the medium four bacterial isolates designated as EK5, EK6, EK7, EK9 and EK13 were selected for further studies. During screening process no zone of clearance around bacterial colonies was observed as reported previously [29], [30]. Hence, growth on dye supplemented BH agar medium as white colonies considered as a positive result for screening potent dye decolorizers. According to Chen et al. [28] cell mat coloring occurs as a result of biosorption of dye, whereas retaining the original mat color indicates biodegradation. Thus retaining the original colony color presumptively suggests that dye decolorization was an enzymatic process rather than adsorption of dyes.
The selected bacterial isolates were characterized on the basis of their cultural, morphological physiological and biochemical characteristics as presented in Table 1. All these characteristics were then compared with the standard description of Bergey’s Manual of Determinative Bacteriology [27] and the isolates were provisionally identified up to the genus as Neisseria sp. (EK5), Vibrio sp. (EK6), Bacillus sp. (EK7), Bacillus sp. (EK9) and Aeromonas sp. (EK13).
Table 1.
The cultural, morphological, physiological and biochemical characteristics of the selected bactrial isolates.
| Test Parameters | Observations |
|||||
|---|---|---|---|---|---|---|
| Isolate EK-5 | Isolate EK-6 | Isolate EK-7 | Isolate EK-9 | Isolate EK-13 | ||
| Colony on Nutrient agar | Form-Circular Elevation-Convex Margin- Entire Surface-Concentric, Color- Whitish | Form- Circular Elevation- Raised Margin- Erose Surface- Concentric, Color- Whitish | Form- Circular Elevation- Convex Margin-Erose Surface-Radiate, Color- whitish | Form- Circular Elevation-Raised Margin-Undulate Surface-Concentric, Color- Whitish | Form- circular, Elevation- convex, Margin-erose, Surface-smooth, Color- whitish | |
| Slant characteristics | Form-Filiform Growth-little Opacity-translucent | Form- Echinulate Opacity- opaque Growth-moderate | Form-Echinulate Opacity-opaque Growth-Moderate | Form-Echinulate Opacity-opaque Growth-Moderate | Form-Arborescent, Opacity-opaque, Growth-Moderate | |
| Gram staining | Negative | Negative | Positive | Positive | Negative | |
| 3% KOH string test | Positive | Positive | Negative | Negative | Positive | |
| Cell morphology | Cocci, Single, pair (occasionally), vacculated cell | Short rod. Single, pair and slightly curved | Short rod. Single, pair, swollen cell | Long rod, Single, pair and long chain (filamentous), swollen cell | Short rod Single, pair | |
| Cell size | Diameter: 1.08–2.24 µm (avg. 1.54 ± 0.38 µm) | Length: 3.17–10.51 µm (avg. 6.21 ± 2.43 µm); Width: 1.17–1.76 µm (avg: 1.54 ± 0.21 µm) | Length: 3.91–8.65 µm (avg. 5.68 ± 1.40 µm); Width: 0.74–1.33 µm (avg. 1.07 ± 0.24 µm) | Length: 2.42–8.15 (avg. 6.24 ± 2.04); width: 1.05 ± 1.68 (avg. 1.31 ± 0.21) | Length: 1.89–3.42 µm (avg. 2.74 ± 0.47 µm); Width: 0.74–1.57 µm(avg. 1.13 ± 0.26 µm) | |
| Spore staining | Non spore former | Non spore former | Spore former | Spore former | Non spore former | |
| Motility | Non motile | Motile | Motile | Motile | Motile | |
| Deep glucose agar test | Aerobic | Aerobic | Aerobic | Aerobic | Aerobic | |
| Catalase test | Positive | Positive | Positive | Positive | Positive | |
| Oxidase test | Positive | Positive | Positive | Negative | Positive | |
| Urease test | Negative | Positive | Negative | Negative | Negative | |
| H2S production | Positive | Positive | Negative | Negative | Positive | |
| Nitrate reduction test | Negative | Positive | Negative | Negative | Negative | |
| Citrate utilization | Negative | Positive | Negative | Negative | Negative | |
| Voges-Proskauer test | Positive | Negative | Negative | Negative | Negative | |
| Methyl red test | Negative | Negative | Negative | Negative | Positive | |
| Indole test | Negative | Negative | Negative | Negative | Negative | |
| Starch hydrolysis | Negative | Negative | Positive | Negative | Positive | |
| Gelatin hydrolysis | Positive | Positive | Positive | Positive | Positive | |
| Fermentation test | Acid but no gas from glucose, maltose, galactose Alkali without gas from sucrose, lactose, fructose, mannitol, rhamnose Non fermentative for cellobiose, raffinose, xylose |
Acid but no gas from glucose, cellobiose, maltose, sucrose, lactose, fructose, galactose, xylose Alkali without gas from raffinose, mannitol, rhamnose |
Acid but no gas from glucose, mannitol Alkali without gas from arabinose No acid and gas from cellobiose, lactose, maltose, raffinose, rhamnose, galactose, sucrose, fructose, xylose |
Alkali without gas from glucose, mannitol, arabinose No Acid and no gas from cellobiose, sucrose, maltose, raffinose, rhamnose, fructose, xylose, galactose, lactose |
Acid but no gas from glucose, maltose, sucrose, fructose, mannitol Alkali without gas from cellobiose, raffinose, lactose, xylose, galactose, rhamnose |
|
| Growth response at different pH | 4.5 | – | – | – | – | – |
| 6.5 | ++ | + | + | + | + | |
| 7.5 | +++ | +++ | +++ | +++ | +++ | |
| 8.5 | ++ | ++ | ++ | ++ | ++ | |
| Growth response at different NaCl conc. (%) | 6.5 | – | + | ++ | – | ++ |
| 7 | – | ++ | + | – | + | |
| 8 | – | ++ | – | – | – | |
| 10 | – | + | – | – | – | |
| Growth response at different temperature (°C) | 4 | – | – | – | – | – |
| 25 | ++ | + | ++ | + | ++ | |
| 37 | +++ | +++ | +++ | +++ | +++ | |
| 45 | – | +++ | +++ | +++ | +++ | |
| Isolates identified* | Neisseria sp. | Vibrio sp. | Bacillus sp. | Bacillus sp. | Aeromonas sp. | |
Note: + = Scanty, ++ = Moderate, +++ = Heavy, – = No growth.
Isolates were identified on the basis of standard description in “Bergey’s Manual of Determinative Bacteriology – 8th edn.” [27].
3.2. Dye decolorization by bacterial monoculture and consortium
In the present study selected bacterial isolates and developed consortium were tested for their ability to decolorize five commonly used textile reactive dyes (Novacron Orange FN-R, Novacron Brilliant Blue FN-R, Novacron Super Black G, Bezema Yellow S8-G and Bezema Red S2-B) as well as dye mixture at a final concentration of 100 mg L−1. It has been reported that a typical textile effluent contains a dye mass concentration of 10–50 mg L−1 [31]. Therefore, a final dye concentration of 100 mg L−1 was chosen for decolorization assay throughout the study.
After 2, 4 and 6 days of incubation period, satisfactory result of dye decolorization by selected bacterial monoculture and consortium was recorded. However, despite repeated attempts, no decolorization was observed in BH medium without co-substrate, i.e. glucose and yeast extract indicating the obligate requirement of labile carbon and nitrogen sources for induction of dye decolorization as also described in many studies [32], [33], [34], [35]. Previous studies reported that only few researches were successful in isolating bacterial culture capable of utilizing dyes as sole source of energy [36]. This may be due to co-metabolic nature of microorganisms in natural environments. In co-metabolic process, specific co-substrates are added which can induce the biodegradation process and subsequently, reduce the overall process time [37]. It was reported that medium compositions are critical to the efficiency of microbial decolorization and the reduction of azo dyes depends on the presence and availability of a co-substrate because it acts as an electron donor for the azo dye reduction [17], [38]. Many different co-substrates were found to suite as electron donor, like glucose and yeast extract, and addition of co-substrates induces dye degrading enzymes [39], [40].
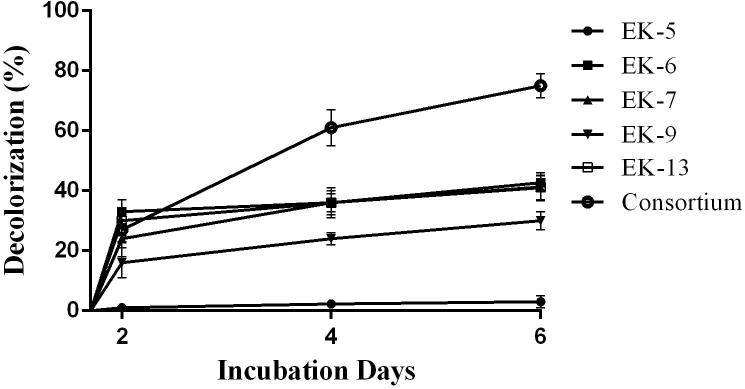
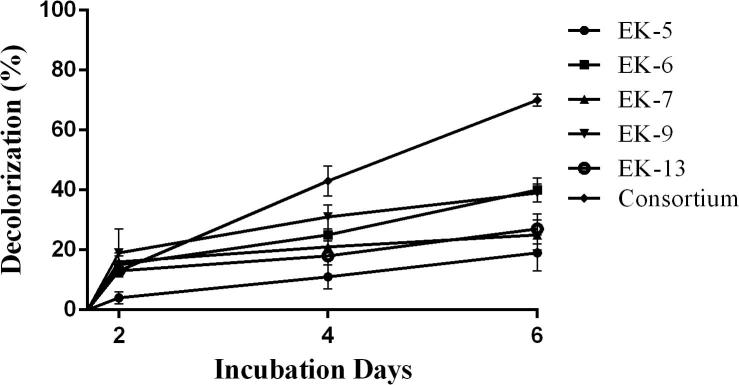
Decolorization of Bezema Red S2-B by selected bacterial isolates and consortium were recorded and highest decolorization (75%) was observed with the bacterial consortium after 6 days of incubation period (Fig. 1). The decolorization efficiency of isolate Ek6 (42%), EK7 (41%) and EK13 (41%) were almost similar within the same incubation period. Unfortunately isolate EK5 was unable to decolorize the dye even after desired incubation periods (6 days).
Fig. 1.
Decolorization of Bezema Red S2-B by bacterial isolates and consortium.
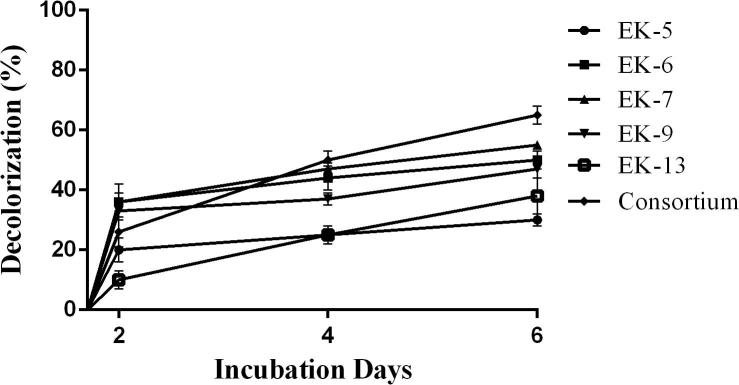
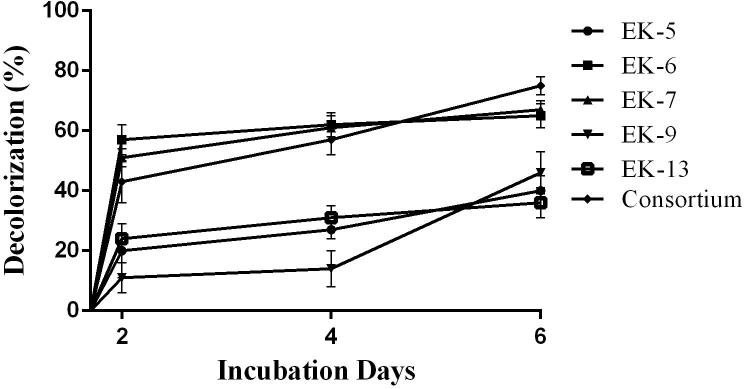
Decolorization of Bezema Yellow S8-G by selected bacterial isolates and consortium were shown in the Fig. 2. In this experiment bacterial consortium represented as the significant decolorizer (65%) of the experimental dye followed by isolate EK7 (55%), EK6 (50%), EK9 (47%), EK13 (38%) and EK5 (30%) after 6 days of incubation.
Fig. 2.
Decolorization of Bezema Yellow S8-G by bacterial isolates and consortium.
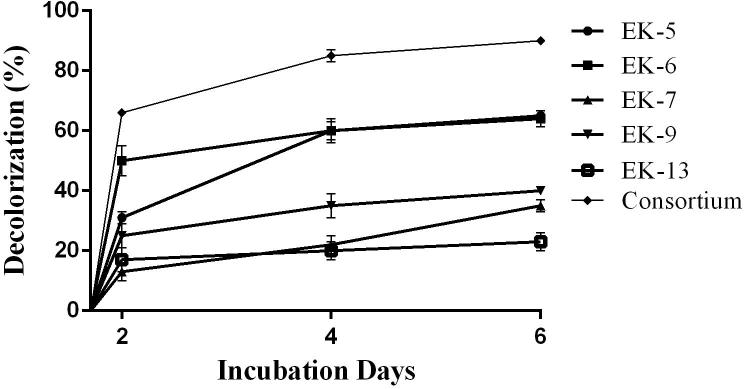
In the present study the highest decolorization (90%) was for Novacron Super Black-G dye by the bacterial consortium as shown in the Fig. 3. Others isolates followed almost similar decolorization pattern such as isolate Ek5 and EK6 (65%) followed by isolate EK9 (40%) and EK7 (35%) at the end of incubation period. Only 23% decolorization was observed for the isolate EK13.
Fig. 3.
Decolorization of Novacron Super Black-G by bacterial isolates and consortium.
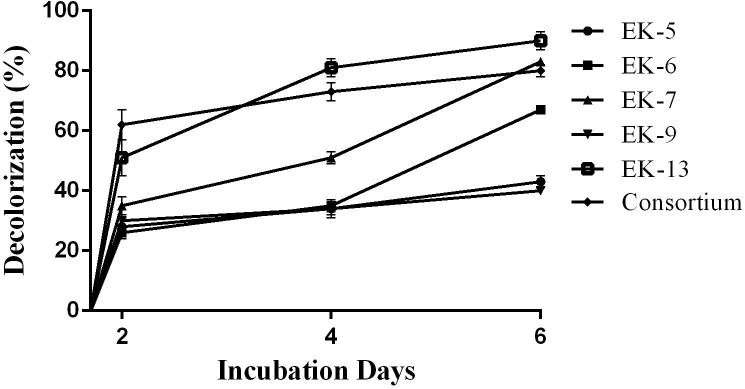
Decolorization of Novacron Brilliant Blue FN-R by bacterial isolates and consortium were also represented in the Fig. 4. In this case, isolate EK13 (90%), EK7 (83%) and also consortium (80%) were represented as the top decolorizer of the experimental dye followed by isolate EK6 (67%), EK5 (43%) and EK9 (40%) after 6 days of incubation. Among the tested dyes this is the only dye where most of the isolates as well as consortium were found to decolorize more efficiently.
Fig. 4.
Decolorization of Novacron Brilliant Blue FN-R by bacterial isolates and consortium.
Bacterial consortium decolorized about 70% of the Novacron Orange FN-R dye followed by isolate Ek6 (40%), EK9 (39%), EK13 (27%), EK7 (25%) and EK5 (19%) (Fig. 5).
Fig. 5.
Decolorization of Novacron Orange FN-R by bacterial isolates and consortium.
Finally an attempt was also made to investigate the decolorization of all experimental dye mixtures by the selected bacterial isolates as well as developed consortium (Fig. 6). This case also shown that bacterial consortium proved to be potent dye decolorizer and the highest decolorization (75%) achieved within 6 days of incubation. The decolorization efficiency of isolate EK6 (65%) and EK7 (67%) were almost similar, whereas isolate EK5, EK9 and EK13 decolorized 40%, 46% and 36% respectively within the speculated incubation period.
Fig. 6.
Decolorization of dye mixtures by bacterial isolates and consortium.
From the above interpretation, it was found that all the selected bacterial isolates have tolerance to all experimental dyes, but decolorization rates were different for each dye. In the present study, the dye Bezema Red S2-B and Novacron Orange FN-R was found to be difficult to decolorize by the monoculture and Bezema Yellow S8-G by the consortium. The difference in decolorization pattern is due to the dissimilarity in specificities, structure and complexity, particularly on the nature and position of substituent in the aromatic rings and the interaction with azo bond with different dyes as reported by many authors [41], [42].
The developed bacterial consortium was more efficient in decolorizing pure dye solutions as well as mixture of all dyes, which indicates that microbial consortium is more powerful agent to treat dying wastewater than single bacterial inoculums. All the isolates used for development of consortium had showed compatibility with each other as no reduction in decolorization percentage was observed during the decolorization cycle. Waghmode et al. [43] reported the decolorization and biodegradation of 150–200 mg L−1 Rubin GFL dye by microbial consortium GG-BL. The importance and the efficiency of bacterial consortium to decolorize reactive azo dyes than monoculturewere reported previously by many authors [44], [45], [46], [47], [48], [49].
In this study, most of the bacterial genera were reported previously for dye decolorization studies, except Neisseria sp. which is still not documented as a dye decolorizing bacteria in the literature. The presence of the genus Bacillus sp. in textile effluent is a regular finding and many authors reported the role of these bacterial genera for various dye degradation studies [50]. The capability of Vibrio sp. such as Vibrio logei for degradation of textile dyes has been exploited [51]. Sharma et al. [52] collected various soil and sludge samples from the vicinity of textile dyeing industries and waste disposal sites and identified five bacterial isolates belonging to the genera Bacillus sp., Alkaligenes sp. and Aeromonas sp. Although, bacterial isolates such as Nesseria sp., Vibrio sp. and Aeromonas sp. are known as pathogenic bacteria, their utilization in biological treatment of noxious wastewater may pose a great public health problem, if these isolates are not managed properly. The utilization of modern genome editing techniques such as CRISPR/Cas9 to eliminate potential health hazard associated genes from microbial genome or cloning of desired gene from pathogenic strain to non-pathogenic strain might be helpful in this regard, considering their broad spectrum application for sustainable environmental remediation.
4. Conclusion
Textile dyeing effluent is a great threat to sustainable environmental development and remediation of this effluent represents an arduous task. Considering this fact, in the present study, isolation, identification and decolorization of some commercially available textile reactive dyes by indigenous bacterial isolates viz. Neisseria sp., Vibrio sp., Bacillus sp., Bacillus sp., Aeromonas sp. and their consortium were investigated. The study clearly demonstrates that bacterial isolates didn’t utilize the dyes as their sole source of energy; instead, required appropriate co-substrates, i.e., glucose and yeast extract, for induction of dye decolorization and associated metabolism. The developed bacterial consortium was much more efficient in decolorizing single dyes as well as mixture of dyes than monocultures indicating the potential of mixed microbial consortium as potent bioremediation agent for cost effective removal of diverse dyes from dying effluent. Further studies on molecular characterization of the isolated bacteria, optimization of their cultural conditions and detoxification mechanism are required to validate the isolates as promising bioremediation agents.
Acknowledgments
The authors are grateful to the Ministry of Science and Technology, Government of the People’s Republic of Bangladesh for financial assistant under the project ‘‘Bio-decolorization of textile reactive dyes’’ [Grant Number: 39.012.002.01.03.019.2013-281(145)] in the fiscal year 2013–2014.
Conflict of interest
The authors declared that they have no conflict of interest.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
References
- 1.Zollinger H. 3rd ed. Wiley-VCH; Cambridge: 2003. Color chemistry: syntheses, properties and applications of organic dyes and pigments. [Google Scholar]
- 2.Marechal AML, Križanec B, Vajnhandl S, Valh JV. Textile finishing industry as an important source of organic pollutants, organic pollutants ten years after the stockholm convention – environmental and analytical update. In: Dr. Tomasz Puzyn, editor. ISBN: 978-953-307- 917-2, InTech, 2012. Available from: <http://www.intechopen.com/books/organic-pollutants-ten-years-after-thestockholm-convention-environmental-and-analytical-update/textile-finishing-industry-as-an-important-source of organic-pollutants>.
- 3.Carmen Z, Daniela S. Textile organic dyes – characteristics, polluting effects and separation/elimination procedures from industrial effluents – A critical overview, organic pollutants ten years after the stockholm convention - environmental and analytical update. In: Puzyn T, editor. ISBN: 978-953-307-917-2, InTech, 2012.
- 4.Ajayi S.O., Osibanjo O. Monogra. 1980;1:76–86. [Google Scholar]
- 5.Rao M.N., Datta A.K. Oxford & IBH Publishing Co Pvt. Ltd.; New Delhi: 1987. Wastewater treatment-rational methods of design and Industrial practices, 2nd ed. [Google Scholar]
- 6.Goncalves I.M.C., Gomes A., Bras R., Ferra M.I.A., Amorin M.T.P., Porter R.S. J Soc Dye Color. 2000;116:393–397. [Google Scholar]
- 7.Chung KT, Cerniglia CE. Mutat Res l992:277;207–20. [DOI] [PubMed]
- 8.Wesenberg D., Kyriakidesand I., Agathos S.N. Biotechnol Adv. 2003;22:161–187. doi: 10.1016/j.biotechadv.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Asad S., Amoozegar M.A., Pourbabaee A.A., Sarbolouki M.N., Dastgheib S.M.M. Bioresour Technol. 2007;98:2082–2088. doi: 10.1016/j.biortech.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Fujita S., Peisach J. J Biol Chem. 1978;253(13):4512–4513. [PubMed] [Google Scholar]
- 11.Arlt V.M., Glatt H., Muckel E., Pabel U., Sorg B.L., Schmeiser H.H., Phillips D.H. Carcinogenesis. 2002;23(11):1937–1945. doi: 10.1093/carcin/23.11.1937. [DOI] [PubMed] [Google Scholar]
- 12.Umbuzeiro G.A., Freeman H.S., Warren S.H., Oliveira D.P., Terao Y., Watanabe T., Claxton L.D. Chemosphere. 2005;60(1):55–64. doi: 10.1016/j.chemosphere.2004.11.100. [DOI] [PubMed] [Google Scholar]
- 13.Borchert M., Libra J.A. Biotechnol Bioeng. 2001;75(3):313–321. doi: 10.1002/bit.10026. [DOI] [PubMed] [Google Scholar]
- 14.Srinivasan A., Viraraghavan T. J Environ Manage. 2010;91(10):1915–1929. doi: 10.1016/j.jenvman.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Robinson T., McMullan G., Marchant R. Bioresour Technol. 2001;77:247–255. doi: 10.1016/s0960-8524(00)00080-8. [DOI] [PubMed] [Google Scholar]
- 16.Horitsu H., Takada M., Idaka E., Tomoyeda M., Ogawa T. Eur J Appl Microbiol. 1977;4:217–224. [Google Scholar]
- 17.Banat IM, Nigam P, Singh Datel, Marchant R. Biosour Technol 1996:58;217–27.
- 18.Sani R.K., Banerjee U.C. Enzyme Microb Technol. 1999;24:433–437. doi: 10.1016/s0141-0229(97)00159-2. [DOI] [PubMed] [Google Scholar]
- 19.Pearce C.I., Lioyd J.R., Guthrie J.T. Dyes Pigments. 2003;58:179–186. [Google Scholar]
- 20.Mohan S.V., Prasad K.K., Rao N.C., Sharma P.N. Chemosphere. 2005;58:1097–1105. doi: 10.1016/j.chemosphere.2004.09.070. [DOI] [PubMed] [Google Scholar]
- 21.Jadhav J.P., Parshetti G.K., Kalme S.D., Govindwar S.P. Chemosphere. 2007;68:394–400. doi: 10.1016/j.chemosphere.2006.12.087. [DOI] [PubMed] [Google Scholar]
- 22.Fu Y., Viraraghavan T. Biresour Technol. 2001;79:251–262. doi: 10.1016/s0960-8524(01)00028-1. [DOI] [PubMed] [Google Scholar]
- 23.Chang J.S., Kuo T.S. Bioresour Technol. 2000;75(1):07–11. [Google Scholar]
- 24.Karim M.E., Dhar K., Hossain M.T. IOSR J Environ Sci Toxicol Food Technol. 2015;9(7):41–45. [Google Scholar]
- 25.Bushnell Haas. J Bacteriol. 1941;41:653. doi: 10.1128/jb.41.5.653-673.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrow G.I., Feltham R.K.A. Camb. Univ. Press; New York, USA: 1993. Cowan and steel’s manual for the identification of medical bacteria. [Google Scholar]
- 27.Bergey D.H., Buchanan R.E., Gibbons N.E. 8th ed. The Williams and Wilkins Co.; Baltimore: 1974. Bergey’s manual of determinative bacteriology. [Google Scholar]
- 28.Chen C., Wu J.Y., Huang C.C., Liang Y.M., Hwang S.C.J. J Biotechnol. 2003;101:241–252. doi: 10.1016/s0168-1656(02)00362-0. [DOI] [PubMed] [Google Scholar]
- 29.Rajee O., Patterson J. Indian. J Microbiol. 2011;51(2):159–163. doi: 10.1007/s12088-011-0127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahbub K.R., Azaz M., Monzur M.A., Salma B. Asian. J Biotechnol. 2012;4(3):129–136. [Google Scholar]
- 31.Clarke E.A., Anliker R. Springer Verlag; Handbook of Environmental Chemistry: 1980. Organic dyes and pigments. [Google Scholar]
- 32.Hu T.L. Water Sci Technol. 1998;38(4–5):299–306. [Google Scholar]
- 33.O’Neill C., Lopez A., Esteves S., Hawkes R.F., Hawkes D.L., Wilcox S. Appl Microbiol Biotechnol. 2000;53(2):249–254. doi: 10.1007/s002530050016. [DOI] [PubMed] [Google Scholar]
- 34.Mariappan C., Gayathri D.T.V., Yamuna R.L., Palaniappan R., Selvamohan T. Biosci Biotechnol Res. Asia. 2003;01(2):87–91. [Google Scholar]
- 35.Karim M.E., Dhar K., Hossain M.T. Int J Environ Sci Technol. 2017;14(1):177–186. [Google Scholar]
- 36.Sarnaik S., Kanekar P. Appl Microbiol Biotechnol. 1999;52(25):1–4. doi: 10.1007/s002530051517. [DOI] [PubMed] [Google Scholar]
- 37.Jadhav S.P., Jadhav U.U., Dawkar V.V., Govindwar S.P. Biotechnol Biopro Eng. 2008;13:232–239. [Google Scholar]
- 38.Hu T.L. Water Sci Technol. 1992;26:57–366. [Google Scholar]
- 39.Swamy J., Ramsay J.A. Enzyme Microb Technol. 1999;25:278–284. [Google Scholar]
- 40.Yuri L., Park C., Lee B., Han E.J., Kim T.H., Lee J., Kim S. J Microbiol Biotechnol. 2006;16:226–231. [Google Scholar]
- 41.Radha K.V., Raghupati I., Arunagiri A., Murugesan T. Process Biochem. 2005;40:3337–3345. [Google Scholar]
- 42.Vijaykumar M.H., Vaishampayan P.V., Shouche Y.S., Karegoudar T.B. Enzyme Microb Technol. 2007;40:204–211. [Google Scholar]
- 43.Waghmode T.R., Kurade M.B., Lade H.S. Appl Biochem Biotechnol. 2012;167(6):1578–1594. doi: 10.1007/s12010-012-9585-z. [DOI] [PubMed] [Google Scholar]
- 44.Khehra M.S., Saini H.S., Sharma D.K., Chadha B.S., Chimni S.S. Water Res. 2005;39(20):5135–5141. doi: 10.1016/j.watres.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 45.Moosvi S., Kher X., Madamwar D. Dyes Pigments. 2007;74:723–729. [Google Scholar]
- 46.Dafale N., Rao N.N., Meshram S.U., Wate R.S. Bioresour Technol. 2008;99:2552–2558. doi: 10.1016/j.biortech.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 47.Chaube P., Indurkar H., Moghe S. Afr J Biotechnol Res. 2010;01:45–56. [Google Scholar]
- 48.Ayed L., Harb B., Cheref A., Bakhrouf A., Achour S. Water Sci Technol. 2010;62(12):2837–2845. doi: 10.2166/wst.2010.709. [DOI] [PubMed] [Google Scholar]
- 49.Phugare S.S., Dayanand C.K., Shripad N.S., Jyoti P.J. Ecotox Environ Saf. 2011;74:1288–1296. doi: 10.1016/j.ecoenv.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 50.Pourbabaee A.A., Malekzadeh F., Sarbolouki M.N., Najafi F. Biotechnol Bioeng. 2006;93(4):631–635. doi: 10.1002/bit.20732. [DOI] [PubMed] [Google Scholar]
- 51.Adedayo O., Javadpour S., Taylor C., Anderson W.A., Moo-Young M. World J Microbiol Biotechnol. 2004;20:545–550. [Google Scholar]
- 52.Sharma D.K., Saini H.S., Singh M., Chimni S.S., Chandha B.S. J Basic Microbiol. 2004;44(1):59–65. doi: 10.1002/jobm.200310334. [DOI] [PubMed] [Google Scholar]