Abstract
Foot and Mouth disease (FMD) is a contagious disease leads to economically loss in livestock production all over the world. This serious disease is caused due to the infection of the animal with a single-stranded RNA virus (FMDV). This study aimed to investigate the genetic polymorphism of BoLA-DRB3 gene in Egyptian buffalo as a candidate genetic marker included in multi-factorial process of FMD resistance/susceptibility. Also this work aimed to genetically characterization and serotyping of circulating FMD virus in Egypt during 2016.
For serotyping of FMDV, RT-PCR was used for FMDV-positive samples and the results declared the presence of serotype O in all tested animals. The sequence analysis of FMDV samples revealed five different patterns for the detected serotype O which were submitted to GenBank under the accession Nos.: MG017361–MG017365.
The 302-bp amplified fragments from BoLA-DRB3 exon 2 were digested with HaeIII endonuclease and the results showed that the presence of five BoLA-DRB3 genotypes, among them the genotype AA might be associated with FMD-resistance (P < 0.01). On the other hand, genotype AC could be correlated with susceptibility (P < 0.01) to FMD in Egyptian buffaloes where it was absent in resistant group. The five detected genotypes of BoLA-DRB3 exon 2 were submitted to GenBank with the accession Nos.: MF977316–MF977320. In conclusion, our findings suggested that the detection of different BoLA-DRB3 genotypes may be has a promising role for raising the resistance of Egyptian buffalo against FMDV especially serotype O which is prevalent in Egypt with preferring genotype AA.
Keywords: BoLA-DRB3, FMDV, Egyptian buffalo, Qt-PCR, RFLP
1. Introduction
Foot and Mouth disease virus (FMDV) is one of the most effective viruses in livestock industry, causing Foot and Mouth disease (FMD) which is a contagious disease results in serious production losses and is considered a major constraint to international trade of livestock and their products. FMDV is a single-stranded RNA virus which affects cloven-hoofed animals including cattle, pigs, sheep, goats and buffalo. FMDV is a member of the genus Aphthovirus in the family Picornaviridae and exists in seven immunologically and serologically distinct serotypes: O, A, C, SAT 1, SAT 2, SAT 3 and Asia 1 [2], [5], [6].
In Egypt, there are currently over 7 millions buffaloes from the type of river buffalo (Bubalus bubalis) distributed all over the country representing one of the most important sources of meat and milk and other related products. (http://www.oie.int/wahis_2/public/wahid.php/Countryinformation/Animalpopulation. FMD is an endemic disease and predominantly associated with cattle and buffalo with currently FMDV serotypes O, A and SAT2 (http://www.wrlfmd.org/fmd_genotyping/africa/egy.htm). In FMDV endemic regions, control measures mainly involve regular vaccination with inactivated virus vaccines. The raised biosecurity fears of the accidental release of the virus during vaccine preparation and storage [7] in addition to the low efficacy of some used vaccines which require regular boosts [3] lead to investigation of alternative strategies to control FMD.
Some immunogenic studies focused on major histocompatibility complex (MHC) due to its crucial role in the host immune response. The main function of MHC class II molecules is to present processed pathogen derived peptides to CD4+ T lymphocytes [29]. MHC class II alleles are numerous and highly polymorphic, increasing the range of epitopes that an individual can recognize [22]. The bovine leukocyte antigen (BoLA) class II genes play a significant role in the genetic control of immune responses. Two MHC (BoLA) class II molecules are expressed, a DR molecule and one or more DQ molecules [17]. A considerable attention has been paid to investigate the polymorphism in BoLA-DRB3 gene and its association with resistance against several animal diseases such as bovine leukemia virus [32], mastitis [10] and FMD in Wanbei cattle [15].
Unlike cattle, few genetic studies have been conducted on functionally important loci in buffalo especially the loci related to disease resistance and susceptibility such as the major histocompatibility complex (MHC) class II genes [1]. Depending on this fact, this study aimed to investigate the genetic polymorphism of BoLA-DRB3 exon 2 in Egyptian buffalo with the assessment of its association with FMD resistance/susceptibility in addition to the genetic characterization and serotyping of circulating FMD virus in Egyptian buffaloes during 2016.
2. Materials and methods
2.1. Animals
Samples used in this study were collected from 33 buffaloes reared in a farm that was suspected to be infected with FMDV in Giza governorate in Egypt during an outbreak in 2016. The animals were divided clinically into two groups; a susceptible (S) group included sixteen diseased animals which had severe extensive vesicular lesions in mouth, tongue, gums and/or feet and a resistance (R) group included seventeen apparently healthy animals with no lesions which were in contact with the diseased animals.
2.2. Detection and serotyping of circulating FMDV
2.2.1. Samples
For clinically diseased animals, the samples were collected from epithelial tissue and/or vesicular fluid from recently ruptured or un-ruptured vesicles which were collected from tongue, buccal mucosa or feet. The samples were placed in a transportation medium which is a mixture of equal amounts of sterile glycerol and phosphate-buffered saline (PBS, pH 7.2–7.4) with antibiotics and processed according to Kitching and Donaldson [12] and OIE recommendations. For healthy animals, the samples of Oro Pharyngeal (OP) fluid were collected by means of a probang (sputum) cup and placed in a transportation medium as OIE recommended [19]. Samples were kept on ice till reached to lab and then stored in −80 °C freezer till work.
2.2.2. Viral RNA extraction
Total RNA was extracted from collected samples using total RNA Purification Kit (Jena Bioscience, Germany), according to the manufacturer’s instructions. The RNA was eluted in 50 ul of elution buffer provided with the kits and stored at −20 °C.
2.2.3. Detection of FMDV by real time PCR
3D gene of FMDV was amplified by Real Time PCR (qtPCR) described by Callahan et al. [4] using QuantiNova Probe RT-PCR Kit (Qiagen, GmbH, Germany) with the universal probe and primers. Cycle threshold (CT) for each sample was determined according to Reid et al. [25].
Forward primer: 5′-ACTGGGTTTTACAAACCTGTGA-3′
Reverse primer: 5′-GCGAGTCCTGCCACGGA-3′
TaqMan probe: 5′-FAM-TCCTTTGCACGCCGTGGGAC-TAMRA-3′
2.2.4. Serotyping of detected FMDV by RT-PCR and sequencing
The serotyping of detected FMDV was done by RT-PCR according to the protocol described by Knowles et al. [14] using serotype specific primers (Table 1) and one-step RT-PCR Pre Mix Kit (Intron Biotechnology Inc., Korea). A thermo cycler (Bio-Rad, USA) was used and the PCR cycling program was selected according to the manufacturer's instructions of kit. The PCR products were subjected to electrophoresis on a 1.5% agarose-tris-borate-EDTA (TBE) gel containing ethidium bromide (Applichem, USA) and using GeneRuler 100-bp DNA (Thermo Scientific, Germany) as size marker [13].
Table 1.
List of oligonucleotide primers used for RT-PCR and sequencing of FMDV.
| Serotype | Name | Sequence (5′–3′) | Gene |
|---|---|---|---|
| O | O–1C244F | GCAGCAAAACACATGTCAAACACCTT | VP3 |
| EUR–2B52R | GACATGTCCTCCTGCATCTGGTTGAT | 2B | |
| O | O–1C283F | GCCCAGTACTACACACAGTACAG | VP3 |
| EUR–2B52R | GACATGTCCTCCTGCATCTGGTTGAT | 2B | |
| A | A–1C562F | TACCAAATTACACACGGGAA | VP3 |
| EUR–2B52R | GACATGTCCTCCTGCATCTGGTTGAT | 2B | |
| A | A–1C612F | TAGCGCCGGCAAAGACTTTGA | VP3 |
| EUR–2B52R | GACATGTCCTCCTGCATCTGGTTGAT | 2B | |
| SAT2 | SAT2–445F | TGGGACACMGGIYTGAACTC | VP3 |
| SAT2–2B208R | ACAGCGGCCATGCACGACAG | 2B | |
| SAT2 | SAT2–1223F | TGAACTACCACTTCATGTACACAG | VP3 |
| SAT2–2B208R | ACAGCGGCCATGCACGACAG | 2B |
2.2.5. Sequencing of 1D (VP1) gene of FMD virus
Five samples of PCR products of viral protein 1 (VP1) representing the five detected Bola-DRB3 genotypes were subjected for sequencing. The PCR products purified and sequenced by Macrogen Incorporation (Seoul, Korea). For sequencing, the primer O-1C244F (Table 1) was used as forward primer which designed to anneal within the VP3 coding region while the reverse primer was the universal primer NK72 (GAAGGGCCCAGGGTTGGACTC) which annealing the 2B coding region and thus, the full length of the FMDV VP1 coding region could be amplified.
2.2.6. Phylogenetic analysis
VP1 nucleotide sequences were aligned together using BioEdit v7.1.3 [11] and ClustalW 1.83 method [28]. Midpoint-rooted Neighbor-joining phylogenetic tree was constructed using Mega 6.06 software [27].
2.3. BoLA-DRB3 genetic polymorphism
2.3.1. Genomic DNA extraction
Genomic DNA was extracted from the whole blood according to the method described by Miller et al. [21] with minor modifications. Briefly, blood samples were mixed with cold 2× sucrose-triton and centrifuged at 5000 rpm for 15 min at 4 °C. The nuclear pellet was suspended in lysis buffer, sodium dodecyl sulfate and proteinase K and incubated overnight in a shaking water bath at 37 °C. Nucleic acids were extracted with saturated NaCl solution. The DNA was picked up and washed in 70% ethanol and dissolved in 1× TE buffer. DNA concentration was determined using Nano Drop1000 Thermo Scientific spectrophotometer and diluted to the working concentration of 50 ng/μl.
2.3.2. Polymerase chain reaction (PCR)
BoLA-DRB3 exon 2 fragments were amplified by conventional PCR using the primers described by Xu et al. [32]. The PCR was performed in a final volume of 25 ul, using MyTaq™ Red DNA Polymerase kit (Bioline, GmbH, Germany). The reaction was cycled for 1 min. at 94 °C, 1 min at 60 °C and 1 min. at 72 °C for 30 cycles. The PCR products were electrophoresed on 2% agarose gel stained with ethidium bromide using a Gene Ruler™ 50-bp DNA Ladder (Thermo Scientific, Germany).
Forward primer: 5′-ATCCTCTCTCTGCAGCACATTTCC-3′
Reverse primer: 5′-CTTGAATTCGCGCTCACCTCGCCGCTG-3′
2.3.3. Restriction fragment length polymorphism (RFLP) analysis
Twenty ul of PCR products for BoLA-DRB3 exon 2 were digested with 10 unites of HaeIII restriction enzyme (Promega, USA) for 15 min at 37 °C. The digested PCR products were electrophoresed on a 4% agarose gel containing ethidium bromide using Gene Ruler™ 50-bp DNA Ladder (Thermo Scientific, Germany) as a molecular marker. Gels were visualized under UV light and documented in FX Molecular Imager apparatus (BIO-RAD).
2.3.4. Sequencing of BoLA-DRB3 gene
Two representative PCR products for each detected genotype were purified and sequenced by Macrogen Incorporation (Seoul, Korea). Sequence analysis and alignment were carried out using NCBI/BLAST/blastn suite. Results of endonuclease restriction were carried out using FastPCR. The nucleotide sequences of detected genotypes were submitted to GenBank (NCBI, BankIt).
2.3.5. Statistical analyses
The different genotypes were investigated in the animals of both groups; susceptible (S) and resistant (R). The genotypes and alleles frequencies were calculated and these frequencies were compared between both susceptible and resistant animals. The statistical significances of associations between different genotypes and the resistance/susceptibility to FMD were analyzed using SPSS 14.0 software and the difference is considered statistically significant when P was <0.05.
3. Results
3.1. FMDV detection, serotyping and genetic characterization
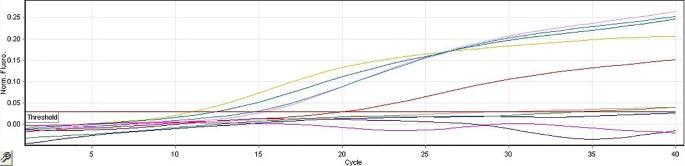
For FMDV detection, RT-PCR (using universal primers and probe of FMDV) was performed on all collected samples. Samples were considered FMDV positive when Ct values were ≤25 which indicate high viral genomic load. Only thirty-three samples showed positive results were included in this study while the negative samples were excluded (Fig. 1).
Fig. 1.
Representative results obtained from real time RT-PCR. FMDV positive (above the threshold line) and negative (below the threshold line) samples.
For serotyping of the detected FMD virus in positive samples, conventional RT-PCR using serotype-specific primers was performed. All the amplified positive samples revealed FMDV serotype O which was demonstrated by the presence of 1049-bp fragment (Fig. 2).
Fig. 2.
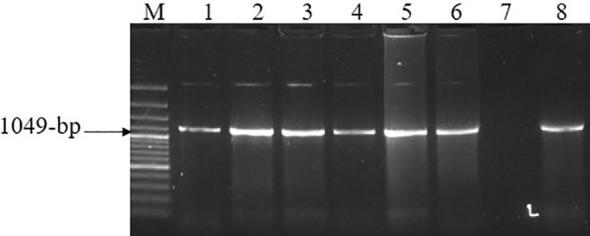
Agarose gel electrophoresis of RT-PCR products of FMDV VP1 and adjacent regions. M: 100-bp ladder. Lanes 1–6 and 8: 1049-bp PCR product of FMDV VP1 representing serotype O. Lane 7: control negative sample.
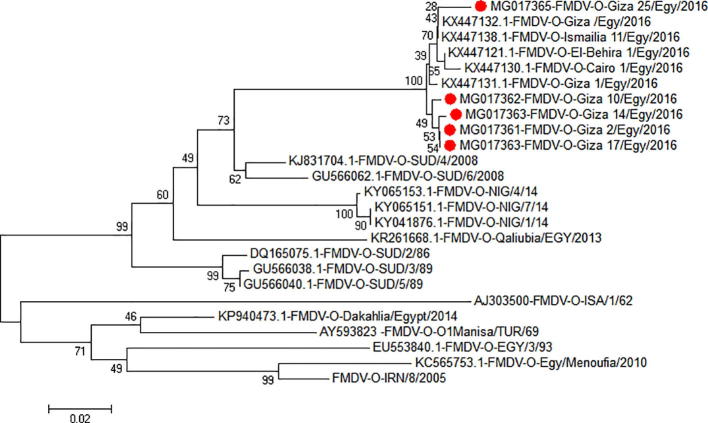
The sequences of the selected five FMDV samples (VP1 and adjacent coding regions) were submitted in GenBank under the accession Nos.: MG017361-MG017365. The phylogenetic tree of these five sequences was constructed with other published sequences (GenBank and http://www.wrlfmd.org/fmd_genotyping/prototypes.htm) which represent a list of reference sequences for FMDV serotype O using MEGA 6.06 software (Fig. 3).
Fig. 3.
Phylogenetic analysis of the detected viruses compared with other FMD Viruses serotype O.
3.2. BoLA-DRB3 genetic polymorphism
PCR products of BoLA-DRB3 exon 2 were electrophoresed in 1% agarose gel and the results showed that the amplified products were at 302-bp (Fig. 4).
Fig. 4.
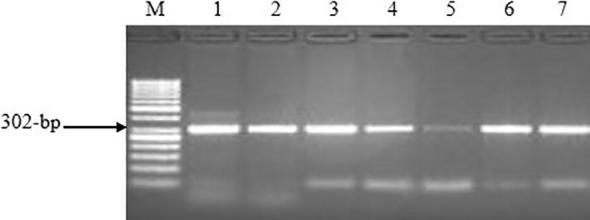
Agarose gel electrophoresis of BoLA-DRB3 exon 2 PCR products. M: 50-bp ladder. Lanes 1–7: 302-bp PCR product of targeted fragment.
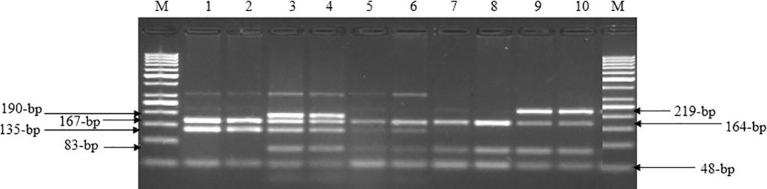
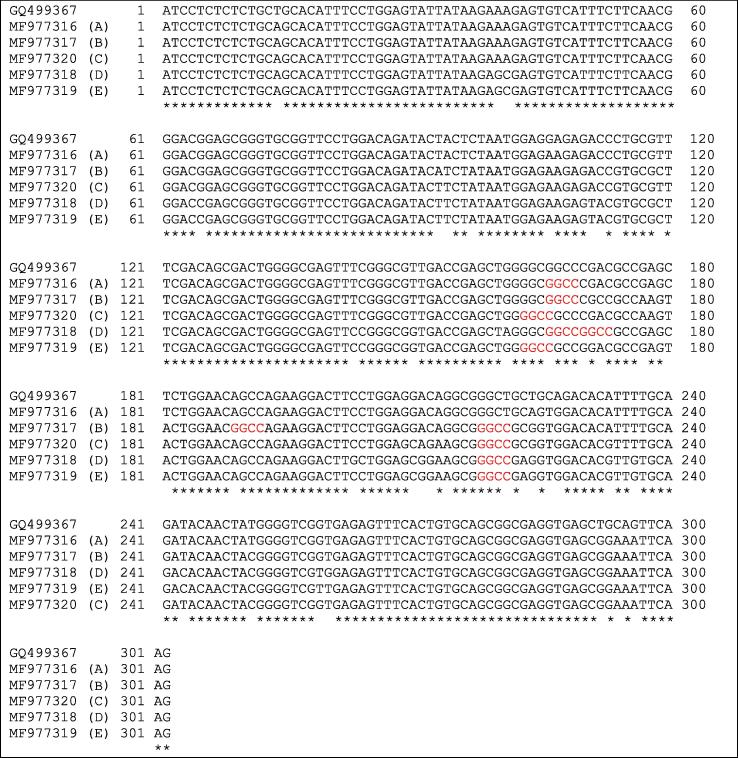
The digestion of PCR products with HaeIII endonuclease showed the appearance of five genotypes; AA, AB, AC, DD and CE (Fig. 5) resulted from the presence of five different alleles named A, B, C, D and E depending on the presence of one or more restriction sites of HaeIII restriction enzyme (GG^CC). The sequences of the five detected alleles were submitted to GenBank under the accession Nos.: MF977316 (A), MF977317 (B), MF977318 (D), MF977319 (E) and MF977320 (C). The alignment of the five detected alleles sequences with a GenBank reference (GQ499367.1) using BioEdit v7.1.3 [11] and DnaSP v5 v [18] revealed the presence of 41 nucleotide substitutions among them (Fig. 6).
Fig. 5.
RFLP patterns of BoLA-DRB3 gene digested by HaeIII restriction enzyme. M: 50-bp ladder. Lanes 1 and 2: AA genotype with two digested fragments at 167- and 135-bp. Lanes 3 and 4: AB genotype with five digested fragments at 190-, 167-, 135-, 83- and 29-bp. Lanes 5 and 6: AC genotype with five digested fragments at 167-, 164-, 135-, 83- and 55-bp. Lanes 7 and 8: DD genotype with four digested fragments at 167-, 83-, 48- and 4-bp. Lanes 9 and 10; CE genotype with four digested fragments at 219-, 164-, 83- and 55-bp. The faint bands at 302-bp are residues from the non-digested original band. The closely bands with sizes at 167- and 164-bp showed as one band on agarose gel. The bands at 55- and 48-bp appeared as one band with the primer dimer. The very small bands at 29- and 4-bp could not be shown on the agarose gel.
Fig. 6.
Alignment of the nucleotide sequences of the detected five alleles with a GenBank reference (GQ499367.1). The restriction sites GG^CC of HaeIII restriction Enzyme in red.
The five alleles A, B, C, D and E were appeared in all tested buffaloes giving four genotypes AA, AB, DD and CE in resistant buffalos and five genotypes AA, AB, AC, DD and CE in susceptible buffalos. The frequencies of different BoLA-DRB3 genotypes (Table 2) and alleles (Table 3) in both resistant and susceptible buffaloes were calculated.
Table 2.
Number and frequency of different BoLA-DRB3 genotypes.
| Genotypes | No. of buffaloes (33 animals) | Resistance buffaloes (17 animals) |
Susceptible buffaloes (16 animals) |
||
|---|---|---|---|---|---|
| No. | Frequency (%) | No. | Frequency (%) | ||
| AA | 9 | 8 | 88.89 | 1 | 11.1 |
| AB | 3 | 2 | 66.7 | 1 | 33.3 |
| AC | 7 | 0 | 0 | 7 | 100 |
| DD | 5 | 3 | 60 | 2 | 40 |
| CE | 9 | 4 | 44.5 | 5 | 55.5 |
Table 3.
Number and frequency of different BoLA-DRB3 alleles.
| Alleles | No. of buffaloes (33 animals) | Resistance buffaloes (17 animals) |
Susceptible buffaloes (16 animals) |
||
|---|---|---|---|---|---|
| No. | Frequency (%) | No. | Frequency (%) | ||
| A | 28 | 18 | 64.3 | 10 | 35.7 |
| B | 3 | 2 | 66.7 | 1 | 33.3 |
| C | 16 | 4 | 25 | 12 | 75 |
| D | 10 | 6 | 60 | 4 | 40 |
| E | 9 | 4 | 44.5 | 5 | 55.5 |
4. Discussion
Host heterogeneity has a major role in disease resistance/susceptibility [31], [16] and was increasingly investigated by evolutionary biology during the study of infectious diseases. Immunogenetics and molecular advances provide a robust tools to understand pathogenic pathways and protective mechanisms in such infectious diseases and open the way to study the genetic basis of host resistance [8], [20]. Several studies have appeared documenting that the influence of host genetic variations implicated in protection against infectious diseases are mediated by polymorphisms in multiple genes located in different regions of the host genome.
In the last few years there has been a growing immunogenic studies focus on major histocompatibility complex (MHC) due to its crucial role in the host immune response and immunological recognition [26]. So, this study aimed to investigate the genetic polymorphism of BoLA-DRB3 gene in Egyptian buffalo with the assessment of its association with FMD resistance/susceptibility in addition to genetic characterization the circulating FMD virus in Egyptian animal population during 2016.
The FMD viral RNA was extracted from thirty-three buffalos reared in the same farm in Giza governorate that was suspected to be infected with FMDV during the outbreak in 2016. The extracted RNA was subjected for RT-PCR technique using universal primers. The conventional RT-PCR was used as an effective confirmatory diagnostic procedure in serotyping of detected FMD Viruses using serotype-specific primers. The results showed that the detected FMD viruses were belonging to serotype O. The obtained specific PCR bands which include viral VP1 and adjacent regions were sequenced for genetic characterization of the circulating FMD virus. Comparison of the obtained VP1 and partial nucleotide sequences of serotype O viruses with those of other isolates in gene bank revealed that the detected viruses in this study were closely related to FMDV – type O isolate Giza 1/Egypt/2016 VP1 gene with identity 99%, FMDV – type O isolate Ismailia 11/Egypt/2016 VP1 gene, with identity 98% and FMDV – type O isolate O/SUD/4/2008 capsid protein gene with identity 93%. These isolates are included in topotype East Africa-3 (EA-3) that differs from the previous topotype Middle East-South Africa (ME-SA) with lineage Panasia2 (O Panasia2) that was prevalent in Egypt from 2010 to 2012 [24] and also, different from the vaccinal strains (O/EGY/93) that belongs to ME-SA topotype.
The sequence comparison between the five detected alleles of BoLA-DRB3 and a GenBank reference (GQ499367.1) revealed the presence of 41 nucleotide substitutions which represents 13.58% of the amplified fragments. In resistant appeared healthy buffalos, there were five alleles of BoLA-DRB3 named A, B, C, D and E giving four genotypes: AA, AB, DD and CE whereas the same alleles gave five genotypes in susceptible diseased buffalos; four of them as in healthy buffalo in addition to a new genotype AC. The frequency of AA genotype was higher in resistant buffalos than in susceptible ones (88.89% and 11.1% respectively). On the other hand, AC genotype was existed only in susceptible buffalos. This results suggested that BoLA-DRB3 genotype HaeIII AA might be associated with resistance to FMD (P < 0.05) and may confer a relative protective effect against FMDV serotype O infection in Egyptian water buffalo. Whereas, the HaeIIIAC genotype could be associated with susceptibility to FMD (P < 0.01) in buffalo which are infected with FMDV type O. Regarding to HaeIII AB, HaeIII DD and HaeIII CE genotypes, there were no significantly differences between resistant and susceptible buffalos when they got infected with type O FMD Virus.
In our buffaloes, BoLA-DRB3 exon 2 coded ninety-three amino acids which were compared with the GenBank sequence (ABA29015.1) and revealed the presence of 19 amino acid substitutions (20.4%) among them. The results of this study suggested that the Egyptian water buffalo might have the ability to relatively resist diseases by mutation which results in changes of the translated amino acids and then the protein structure to perform the regulation of different signaling pathways used by the cell in the long process of choice evolution [30].
During 2016, FMDV outbreak has been occurred in Egypt and the disease varied in severity within buffalo herds. Severe cases characterized by extensive FMD vesicular lesions and/or mortalities, while the mild cases showed small vesicular lesions, fast recovery and absence of mortalities. Individual variations in the immune response to infectious diseases and vaccination are considerable, which are complex polygenic traits determined by infectious agent, environmental and host genetic factors [9], [23] and thus, variations in protection level against FMDV infection are expected among individual animals within the same herd.
The bovine leukocyte antigen (BoLA) class II genes play a significant role in the genetic control of immune responses. A subtle change in the nucleotide sequence of the MHC-DRB3 gene may lead to substitution in the coding amino acids and mainly results in conformational changes in the antigen binding pocket of the MHC II molecule, which affect the efficiency of the molecule to present the processed antigens for further triggering of the immune response [30].
Many studies reported the high polymorphism of BoLA-DRB3 gene and its association with the resistance/susceptibility to abroad range of diseases. Baxter et al. [3] studied the association between BoLA-DRB3 gene polymorphism and immune response to a simple peptide vaccine derived from FMDV VP1. They detected eighteen different DRB3 alleles in a crossbred (Charolaise and Holstein) cattle population, some of them showed highly significant associations with antibody response. The data in their study suggested that the DRB3 alleles had important role in determining the degree of immune response.
The results obtained by Xu et al. [32] concluded that the polymorphism in bovine MHC class II BoLA-DRB3 gene was supposed to be correlated with resistance and susceptibility to the development of persistent lymphocytosis (PL) caused by bovine leukemia virus (BLV) infection. BoLA-DRB3 gene polymorphisms and their association with resistance and susceptibility to FMD in bovine was investigated by Lei et al. [15]. They suggested that, in Wanbei cattle infected with FMDV, the genotypes HaeIII CC and HaeIII BC were associated with resistance to FMD (P < 0.01) while HaeIII AA genotype was associated with susceptibility to FMD (P < 0.01).
The findings of our study showed that BoLA-DRB3 gene and its polymorphism may play an essential role in FMD resistance/susceptibility in Egyptian buffaloes infected with serotype O FMD Virus. In spite of the presence of the same alleles in both groups, the genotype AA may be associated with FMD-resistance where its frequency was 88.89% in resistant buffalos compared to 11.1% in susceptible group. On the other hand, genotype AC could be correlated with the susceptibility (P < 0.01) to FMD in Egyptian buffalo where it absent in resistant buffaloes. The results may be has a promising role for raising the resistance of Egyptian buffalo against FMDV especially serotype O which is prevalent in Egypt with preferring genotype AA of BoLA-DRB3 gene.
Footnotes
Peer review under responsibility of National Research Centre, Egypt.
Contributor Information
Othman E. Othman, Email: othmanmah@yahoo.com.
Hussein A. Hussein, Email: husvirol@cu.edu.eg.
References
- 1.Aravindakshan T., Nainar A., Sivaselvam S. Anim Sci. 2000;70:221–226. [Google Scholar]
- 2.Bachrach H.L. Annu Rev Microbiol. 1968;22:201–244. doi: 10.1146/annurev.mi.22.100168.001221. [DOI] [PubMed] [Google Scholar]
- 3.Baxter R., Craigmile S., Haley C., Douglas A., Williams J., Glass E. Vaccine. 2009;28:28–37. doi: 10.1016/j.vaccine.2009.09.131. [DOI] [PubMed] [Google Scholar]
- 4.Callahan J.D., Brown F., Osorio F.A., Sur J.H., Kramer E., Long G.W., Lubroth J., Ellis S.J., Shoulars K.S., Gaffney K.L. J Am Vet Med Assoc. 2002;220:1636–1642. doi: 10.2460/javma.2002.220.1636. [DOI] [PubMed] [Google Scholar]
- 5.Carrillo C., Tulman E., Delhon G., Lu Z., Carreno A., Vagnozzi A., Kutish G., Rock D. J Virol. 2005;79:6487–6504. doi: 10.1128/JVI.79.10.6487-6504.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coetzer J., Thomson G., Tustin R. Onderstepoort J Vet Res. 2014;70:1–6. [Google Scholar]
- 7.Collen T., Dimarchi R., Doel T. J Immunol. 1991;146:749–755. [PubMed] [Google Scholar]
- 8.Cooke G.S., Hill A.V. Nat Rev Genet. 2001;2:967. doi: 10.1038/35103577. [DOI] [PubMed] [Google Scholar]
- 9.Davies G., Genini S., Bishop S., Giuffra E. Animal. 2009;3:415–436. doi: 10.1017/S1751731108003522. [DOI] [PubMed] [Google Scholar]
- 10.Duangjinda M., Buayai D., Pattarajinda V., Phasuk Y., Katawatin S., Vongpralub T., Chaiyotvittayakul A. J Anim Sci. 2009;87:469–476. doi: 10.2527/jas.2007-0789. [DOI] [PubMed] [Google Scholar]
- 11.Hall T.A. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 12.Kitching R, Donaldson A. Rev Sci Tech Off Int Epiz 1987;6:263–72.
- 13.Knowles N, Samuel A. Inst Anim Health Pirbright. 1998;37.
- 14.Knowles N, Wadsworth J, Bachanek-Bankowska K, King D. Revue scientifique et technique de l’Office International des Epizooties; 2016. [DOI] [PubMed]
- 15.Lei W., Liang Q., Jing L., Wang C., Wu X., He H. Mol Biol Rep. 2012;39:9203–9209. doi: 10.1007/s11033-012-1793-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levin B.R., Lipsitch M., Bonhoeffer S. Science. 1999;283:806–809. doi: 10.1126/science.283.5403.806. [DOI] [PubMed] [Google Scholar]
- 17.Lewin H.A., Russell G.C., Glass E.J. Immunol Rev. 1999;167:145–158. doi: 10.1111/j.1600-065x.1999.tb01388.x. [DOI] [PubMed] [Google Scholar]
- 18.Librado P., Rozas J. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 19.Manual O.T. OIE Terrestrial Manual. 2012:145–173. [Google Scholar]
- 20.McLeod R., Buschman E., Arbuckle L.D., Skamene E. Curr Opin Immunol. 1995;7:539–552. doi: 10.1016/0952-7915(95)80100-6. [DOI] [PubMed] [Google Scholar]
- 21.Miller S., Dykes D., Polesky H. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newman M.J., Truax R.E., French D.D., Dietrich M.A., Franke D., Stear M.J. Vet Immun Immunopath. 1996;50:43–54. doi: 10.1016/0165-2427(95)05483-9. [DOI] [PubMed] [Google Scholar]
- 23.O’Neill R., Woolliams J., Glass E., Williams J., Fitzpatrick J. Vaccine. 2006;24:4007–4016. doi: 10.1016/j.vaccine.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 24.Rady A.A., Khalil S.A., Torky H.A. Alex J Vet Sci. 2014;41:120–130. [Google Scholar]
- 25.Reid S., Ferris N., Hutchings G., Zhang Z., Belsham G., Alexandersen S. Vet Rec. 2001;149:621–623. doi: 10.1136/vr.149.20.621. [DOI] [PubMed] [Google Scholar]
- 26.Sette A., Fikes J. Curr Opin Immunol. 2003;15:461–470. doi: 10.1016/s0952-7915(03)00083-9. [DOI] [PubMed] [Google Scholar]
- 27.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson J.D., Higgins D.G., Gibson T.J. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Todd J.A., Acha-orbea H., Bell J.I., Chao N., Fronek Z., Jacob C.O., Mcdermott M., Sinha A.A., Timmerman L., Steinman L. Science. 1988;240:1003–1009. doi: 10.1126/science.3368786. [DOI] [PubMed] [Google Scholar]
- 30.Wang X., Xu S., Zan L., Chen J., Gao X., Ren H. J Agric Biotechnol. 2007;15(1) 46-15. [Google Scholar]
- 31.Woolhouse M.E., Dye C., Etard J.F., Smith T., Charlwood J., Garnett G., Hagan P., Hii J., Ndhlovu P., Quinnell R. Proc Nat Acad Sci. 1997;94:338–342. doi: 10.1073/pnas.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu A., Van ejik M., Park C., Lewin H.A. J Immunol. 1993;151(12):6977–6985. [PubMed] [Google Scholar]