Abstract
The present study was aimed at determining total phenolic and flavonoid contents and studying the antioxidant activity of ginger (Zingiber officinale Rosc.) rhizome and callus, 6-gingerol and 6-shogaol and callus treated with elicitors. Petroleum ether (PE) and chloroform: methanol (1:1, v/v) (CM) extracts were prepared by maceration. Highest total phenolic content was obtained from the CM extract (60.34 ± 0.43 mg gallic acid/g) of rhizome while callus showed lower content detected in the CM extract (33.6 ± 0.07 mg gallic acid/g). Flavonoids were only detected in rhizome (CM extract 40.25 ± 0.21 mg quercetin/g). Both rhizome extracts exhibited good antioxidant activity with higher activity recorded in PE extract (IC50 value 8.29 ± 1.73 μg/mL). Callus extracts revealed lower antioxidant activity (IC50 value 1265.49 ± 59.9 μg/mL obtained from CM extract). 6-gingerol and 6-shogaol displayed high antioxidant activity in both assays with IC50 4.85 + 0.58DPPH and 5.35 ± 0.33ABTS μg/mL for the former and IC50 7.61 ± 0.81DPPH and IC50 7.05 ± 0.23ABTS μg/mL for the latter. Treatment of callus with elicitors showed significant (p < 0.05) effects in enhancing phenolic content and related antioxidant activity. The highest significant increase in phenolic content (37% and 34%) and antioxidant activity in DPPH assay (34% and 30%) was observed in callus treated with 100 mg/L yeast extract and 50 mg/L salicylic acid respectively. Therefore, studying the effect of the elicitation of ginger cultured tissues in phenolic accumulation would be of immense importance for pharmacological, cosmetic and agronomic industries.
Keywords: Ginger, Antioxidant activity, Elicitors, Yeast extract, Salicylic acid, Glycine
1. Introduction
Zingiber officinale Rosc. (ginger) is a rhizomatous herb belonging to the family Zingiberaceae. The rhizome is extensively used around the world as spice in culinary, beverages and herbal medicinal practices to treat a wide range of diseases such as rheumatic disorders, cold symptoms, fevers, gastrointestinal complications, motion sickness, bronchitis, diabetes, cancer, etc [1], [2], [3].
The pharmacological activities of ginger were mainly attributed to its active phytocompounds 6-gingerol, 6-shogaol, zingerone beside other phenolics and flavonoids [4], [5]. 6-gingerol was reported as the most abundant bioactive compound in ginger with various pharmacological effects including antioxidant, analgesic, anti-inflammatory and antipyretic properties [6], [7]. Also, other studies showed that 6-shogaol with lowest concentration in ginger represent more biologically actives compared to 6-gingerol [8], [9].
Plant cells cultivated in vitro such as callus or cells suspension culture could potentially be competitive systems for effective production of marketable secondary metabolites possessing biological activities [10]. Moreover, the production of bioactive secondary metabolites can be enhanced by the treatment of the undifferentiated cells with elicitors [11]. Salicylic acid [12], yeast extract [13] and glycine [14] are examples of important elicitors that has the capability to induce the secondary metabolites from in vitro cultures.
The demand for Z. officinale metabolites, mainly with a higher bioactive compounds content, prompted more directed tissue culturing efforts. However, there is no report in the effect of elicitors on secondary metabolite content of ginger callus. Hence, the present study aimed at studying the total phenolic and flavonoid contents and antioxidant capacity of ginger rhizome and their callus as well as callus treated with different concentrations of yeast extract, glycine and salicylic acid.
2. Materials and methods
2.1. Plant materials
Healthy fresh rhizomes of Z. officinale Rosc were collected from the botanical garden at the Department of Biology, Faculty of Science and Technology, Al-Neelain University, Khartoum, Sudan. Rhizomes were cleaned, cut into thin slices and dried at room temperature.
2.2. Preparation of explants
Explants were prepared according to the method described by Ali et al. [15]. Healthy and clean rhizomes were incubated in the dark at 25 ± 2 °C for three weeks for sprouting buds. Then shoot tips (about 0.5 cm) from the sprouting buds were excised and used as explants for callus induction.
2.3. Callus induction and proliferation
Ginger callus was induced and proliferated according to the method described by Ali et al. [15]. Sterilized shoot tip explants were planted in MS (Murashige and Skoog) medium supplemented with 1.00 mg/L of 2,4-D (2,4-dichlorophenoxyacetic acid) as plant growth regulator and were incubated at 25 ± 2 °C and photoperiod of 16/8h light/dark for two months Callus was then regenerated on the same medium fortified with 0.5 mg/L of 2,4-D.
2.4. Elicitor’s treatments
Pieces of fresh weight (200 mg) of proliferated callus were transferred to MS medium supplemented with 0.5 mg/L of 2,4-D and treated with different concentration of elicitors namely; yeast extract (100, 300 and 500 mg/L), glycine (100, 200 and 300 mg/L) and salicylic acid (50 and 100 mg/L) separately. All cultures of the elicitor treatment were maintained at 25 ± 2 °C, photoperiod of 16/8h light/dark for three weeks. Calli were freeze dried and powdered.
2.5. Preparation of extracts
Five grams of the dry powder from rhizome, callus and treated callus were macerated separately in petroleum ether (PE) and chloroform: methanol (1:1, v/v) (CM) at room temperature for 72 h. Extracts were evaporated under vacuum to dryness and stored in dried bottles at 4 °C.
2.6. Determination of total phenolics
Total phenol contents of the extracts were determined using modified method of Wolfe et al. [16]. The extract (1 mg/mL) was mixed with 5 mL Folin–Ciocalteu reagent diluted with water 1:10 v/v and 4 mL of 7.5% sodium carbonate. The mixture was vortexed for 15 s and allowed to stand for 30 min at 40 °C for colour development. Absorbance was then measured at 765 nm using Shimadzu model 1800 double beam spectrophotometer. A calibration curve was prepared using gallic acid (25–250 mg/L) as standard and used for calculation of total phenolic compound. The total phenolic contents were expressed as gallic acid equivalents (mg/g) using the following equation based on the calibration curve: y = 0.008x + 0.1904, where y was the absorbance.
2.7. Determination of total flavonoids
Estimation of the total flavonoids in the plant extracts was carried out using the method of Ordon Ez et al. [17]. To 0.5 mL of sample, a volume of 0.5 mL of 2% AlCl3 ethanol solution was added. After one hour at room temperature, the absorbance was measured at 420 nm. A yellow colour indicated the presence of flavonoids. Extract samples were evaluated at a final concentration of 0.1 mg/mL. A calibration curve was constructed, using quercetin (5–100 mg/L) as standard. Total flavonoid contents were expressed as quercetin (mg/g) using the following equation based on the calibration curve: y = 0.0018x + 0.0122, where y was the absorbance.
2.8. Antioxidant activity
2.8.1. 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging assay
Antioxidant activity of the extracts were estimated using DPPH in vitro method Mensor et al. [18]. Test samples were dissolved separately in methanol to get test solution of 1 mg/mL. Series of extract/ pure components (6-gingerol and 6-shogaol; purchased from (Sigma-Aldrich GmbH, Germany) solutions of different concentrations (100, 200, 400, 600, 800 and 1000 µg/mL) were prepared by diluting with methanol. Assays were performed in 96-well, microtiter plates. 140 µL of 0.6 × 10−6 mol/L DPPH were added to each well containing 70 µL of sample. The mixture was shaken gently and left to stand for 30 min in dark at room temperature. The absorbance was measured spectrophotometrically at 517 nm using Cecil-Elect Spectrophotometer. Blank was done in the same way using methanol and sample without DPPH and control was done in the same way but using DPPH and methanol without sample. Ascorbic acid was used as reference antioxidant compound. Every analyse is done in triplicate. The ability to scavenge DPPH radical was calculated by the following equation:
where
Abssample is the absorbance of DPPH radical + sample;
Absblank is the absorbance of sample + methanol;
Abscontrol is the absorbance of DPPH radical + methanol.
2.8.2. 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid (ABTS) radical-scavenging assay
A second in vitro method was performed to estimate antioxidant potential of the extract: ABTS assay, based on the method of Re et al. [19]. Test samples were dissolved separately in methanol to get test solution of 1 mg/mL. Series of extract/pure components (6-gingerol and 6-shogaol) solutions of different concentrations (100, 200, 400, 600, 800 and 1000 µg/mL) were prepared by diluting with methanol. The ABTS radical cation (ABTS∗+) was produced by reacting 7 mM stock solution of ABTS with 2.45 mM potassium persulfate and allowing the mixture to stand in the dark at room temperature for 12 h before use. The obtained ABTS∗+ solution was diluted with ethanol to an absorbance of 0.700 ± 0.02 at 734 nm. 190 µL of ABTS∗+ solution were added to each well containing 10 µL of sample. The mixture was shaken gently and left to stand for 15 min in dark at room temperature. Butylated hydroxytoluene (BHT) was used as reference antioxidant compound. The absorbance was measured spectrophotometrically at 734 nm. Every analyse is done in triplicate. The ABTS∗+ scavenging capacity of the extract was calculated as:
where
Abscontrol is the absorbance of ABTS∗+ (=0.700 ± 0.02);
Abssample is the absorbance of sample + ABTS∗+.
2.9. Statistical analysis
Experiment data were statistically analyzed using SPSS version 19 (Chicago, IL, USA). All experiments were performed in triplicate, and the results were expressed as mean ± standard deviation (SD) values. The IC50 value was calculated from the linear regression of plots of concentration of the test sample against the mean percentage of the antioxidant activity. The IC50 values obtained from the regression plots (Sigma PlotsR 2001, SPSS Science) had a good coefficient of correlation, (R2 = 0.998 DPPH and R2 = 0.9926 ABTS). Significant differences between samples were analyzed using analysis of variance (ANOVA) and Duncan’s multiple-range test (P < 0.05).
3. Results and discussion
3.1. Total phenolic and flavonoid contents of ginger rhizome and callus
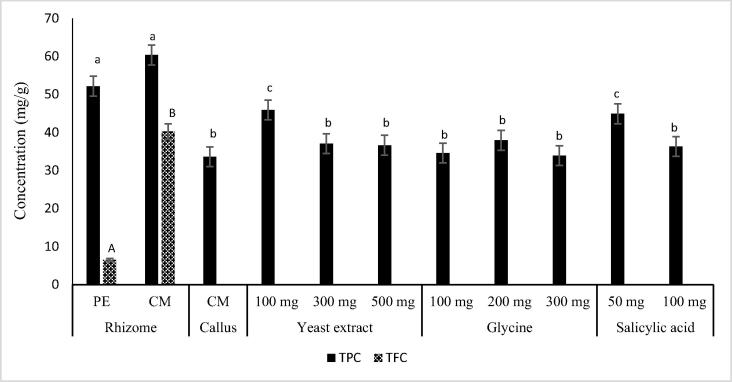
Total phenolic and flavonoid content in PE and CM extracts of dried ginger rhizome and callus were determined spectrophotometrically and results are presented in Fig. 1. The total phenolic content in the CM extract of ginger rhizome was slightly higher (60.34 ± 0.43 mg gallic acid/g), but not significantly, than that obtained in the PE extract (52.17 ± 2.41 mg gallic acid/g). These values were lower than those reported by El-ghorab et al. [20] (95.2 ± 6.2 and 87.5 ± 2.3 mg gallic acid/g) from the methanolic and hexane extracts of fresh ginger rhizome and by Mošovská et al. [21] (181.41 mg gallic acid/g) in methanolic extract of ginger roots but, were higher than those obtained in the young rhizome from two varieties of Malaysia ginger (Halia Bara and Halia Bentong) (13.5 ± 2.26 and 10.22 ± 0.87 mg gallic acid/g respectively) [22]. However, the PE extract of the callus was devoid of phenolics while its CM extract showed lower content (33.6 ± 0.07 mg gallic acid/g) than that obtained from the rhizome. Buchanan and Jones [23] and Dias et al. [24] reported that, phenolic compounds are usually generated as products of defense against pathogen attack or in response to a stressful environment and they are not directly related with the growth functions and development of plant tissue.
Fig. 1.
Total phenolic content (TPC) and total flavonoid content (TFC) on extracts of ginger rhizome, callus and callus treated with elicitors. PE; petroleum ether; CM, chloroform: methanol (1:1, v/v); elicitors (mg/L); Different letters indicate a significant difference (p < 0.05) according to Duncan’s multiple range test.
3.2. Effect of elicitors on the total phenolic content of ginger callus
The phenolic content of CM extract of callus elicited with different concentrations of elicitors (yeast extract (100, 300 and 500 mg/L), glycine (100, 200 and 300 mg/L) and salicylic acid (50 and 100 mg/L)) is presented in Fig. 1. The results showed that the production of polyphenols was significantly (p < 0.05) affected by the type and concentration of elicitors used during the cell growth. The highest significant increase (37% and 34%) in phenolic content was observed in callus treated with 100 mg/L yeast extract and 50 mg/L salicylic acid respectively. This result supported the finding of El-Nabarawy et al. [25] who reported that, the culture medium supplemented with low concentrations of yeast extract increased the phenolic content in plant cells grown in vitro, whereas a higher amount of yeast extract did not act as elicitor for phenolic production. Moreover, Gorni and Pacheco [26] found that yarrow (Achillea millefolium) treated with 0.50 and 1.00 mM salicylic acid showed a marked increase in the total phenolic content as compared to control plants. A slight insignificant (p < 0.05) increase (13%, 10%, 9% and 8%) was observed in total phenolic content in callus treated with 200 mg/L glycine, 300 and 500 mg/L yeast extracts and 100 mg/L salicylic acid respectively. Treatment with 100 and 200 mg/L glycine did not improve the production of phenolic content of the callus. The production of phenolic compounds in callus cultures is related to mitochondrial activity; that is, while the cell dehydrogenase activity (including FADH 2/NADH dehydrogenases) and the cytochrome Coxidase declined, the concentration of phenolic compounds increases, which occurs during growth and stabilization of callus cultures [24], [27].
Thus, in this study different elicitors enhanced the phenolic content of ginger callus as follows: callus treated with 100 mg/L yeast (45.91 ± 1.8 mg gallic acid/g) > 50 mg/L salicylic acid (44.89 ± 0.86 mg gallic acid/g) > 200 mg/L glycine (37.92 ± 0.07 mg gallic acid/g) > 300 mg/L yeast (37.05 ± 1.24 mg gallic acid/g) > 500 mg/L yeast (36.63 ± 0.78 mg gallic acid/g) > 100 mg/L salicylic acid (36.32 ± 0.06 mg gallic acid/g) > 100 mg/L glycine (34.59 ± 0.00 mg gallic acid/g) 300 mg/L glycine (33.90 ± 1.37 mg gallic acid/g) > untreated callus (33.6 ± 0.07 mg gallic acid/g).
Flavonoids were only detected in ginger rhizome with highest amount found in CM extract (40.25 ± 0.21 mg quercetin/g) while PE extract contained small amount (6.55 ± 0.20 mg quercetin/g). No flavonoid was detected in both callus extracts.
3.3. Antioxidant activity of ginger rhizome and callus
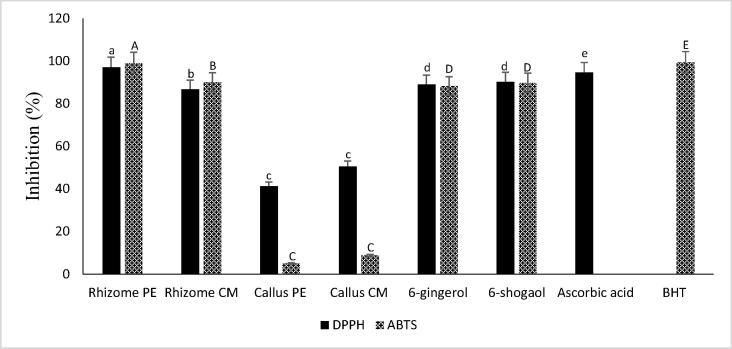
Ginger PE and CM extracts prepared from rhizome and callus in addition to pure components 6-gingerol and 6-shogaol were investigated to determine their in vitro antioxidant activity using the DPPH and ABTS assays. Results are summarized in Fig. 2. Both rhizome extracts exhibited good antioxidant activity and the highest DPPH and ABTS radicals inhibition was recorded in PE extract (97.47 ± 0.93% and 99.06 ± 1.00% respectively) and then followed by the CM extract (86.7 ± 0.99% and 90.02 ± 0.08% respectively). These results were generally in agreement with the findings of El Bedawey et al. [28], Al-Tahtawy et al. [29], Eleazu et al. [30] and Aziz et al. [31].
Fig. 2.
Antioxidant activity of ginger rhizome and callus extracts, 6-gingerol and 6-shogaol. PE; petroleum ether; CM, chloroform: methanol (1:1, v/v); Different letters indicate a significant difference (p < 0.05) according to Duncan’s multiple range test.
6-gingerol and 6-shogaol, which were known for their abundance in ginger rhizome, displayed high antioxidant activity with scavenging capacity of 88.93 ± 0.03% and 88.23 ± 0.98% in DPPH and ABTS assays respectively for 6-gingerol and 90.2 ± 0.11% and 89.81 ± 0.6% in DPPH and ABTS assays respectively for 6-shogaol. These results suggested that these two compounds play a major role on the antioxidant activity of ginger rhizome. Mošovská et al. [21] reported that, the high scavenging activity of ginger rhizome against ABTS and DPPH radicals, was due to the presence polyphenolic compounds, including gingerols, shogaols, paradols and gingerdions.
Callus extracts revealed lower antioxidant activity as compared with the rhizome. Only the CM extract of ginger callus showed moderate antioxidant activity (50.53 ± 1.03%) against DPPH radicle while the PE extract showed weak activity (41.23 ± 0.23%). In the ABTS assay both callus extracts revealed very weak antioxidant activity. This result supported the finding of Pawar et al. [32] who demonstrated that, lowest antioxidant activity was recorded in callus sample, compared to the conventionally grown ginger plants.
IC50 values were also calculated for the tested samples which displayed the ability to quench 50% of the initial DPPH and ABTS radicals (Table 1). PE extract of rhizome displayed strongest antioxidant activity in the DPPH assay with IC50 value 8.29 ± 1.73 μg/mL followed by CM extract of rhizome (IC50 value 29.87 ± 1.09 μg/mL) while the CM extract of callus displayed lower value (IC50 value 1265.49 ± 59.9 μg/mL). Moreover, the PE and CM extracts of the rhizome showed lower antioxidant activity in the ABTS assay (IC50 value 250.33 ± 13.6 and 334.86 ± 6.97 μg/mL respectively) as compared to DPPH one. 6-gingerol and 6-shogaol displayed high antioxidant activity in both assays with IC50 4.85 + 0.58DPPH and 5.35 ± 0.33ABTS μg/mL for the former and IC50 7.61 ± 0.81DPPH and IC50 7.05 ± 0.23ABTS μg/mL for the latter which is remarkable higher than that of standard BHT (IC50 64.90 ± 0.75 μg/mL) in the ABTS assay and thus supporting their major role in the antioxidant activity of the rhizome. Some researchers like Weng et al. [9] and Guo et al. [33] showed that 6-shogaol displayed higher antioxidant activity than 6-gingerol while others, Ghasemzadeh et al. [34], found that 6-gingerol was most potent than 6-shogaol. In this study, the antioxidant activity of the two compounds was not significantly different although 6-gingerol displayed lower IC50 values.
Table 1.
Antioxidant activity (IC50 values) of ginger rhizome, callus and treated callus extracts, 6-gingerol and 6-shogaol.
| Extract | IC50 (μg /mL) |
||
|---|---|---|---|
| DPPH | ABTS | ||
| Rhizome | PE | 8.29 ± 1.73a | 250.33 ± 13.6c |
| CM | 29.87 ± 1.09b | 334.86 ± 6.97d | |
| Callus | PE | ND | ND |
| CM | 1265.49 ± 59.9 g | ND | |
| Yeast extract | |||
| 100 mg/L | CM | 726.98 ± 28.92c | ND |
| 300 mg/L | CM | 803.22 ± 5.51d | ND |
| 500 mg/L | CM | 892.02 ± 6.85e | ND |
| Glycine | |||
| 100 mg/L | CM | 1100.56 ± 27.22f | ND |
| 200 mg/L | CM | 783.90 ± 7.84d | ND |
| 300 mg/L | CM | ND | ND |
| Salicylic acid | |||
| 50 mg/L | CM | 748.87 ± 7.30c | ND |
| 100 mg/L | CM | ND | ND |
| 6-gingerol | 4.85 ± 0.58a | 5.35 ± 0.33a | |
| 6-shogaol | 7.61 ± 0.81a | 7.05 ± 0.23a | |
| Ascorbic acid | 0.13 ± 0.01a | _ | |
| BHT | _ | 64.90 ± 0.75b | |
PE, petroleum ether; CM, chloroform: methanol (1:1, v/v); ND, not determined; different letters within column indicate a significant difference (p < 0.05).
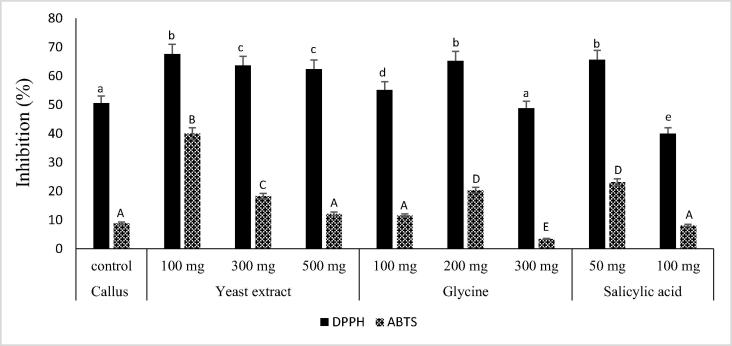
3.4. Effect of elicitors on the antioxidant activity of ginger callus
The effect of different elicitors on antioxidant activity of ginger callus were also assessed (Fig. 3). In general, treatment of ginger callus with elicitors showed significant (p < 0.05) effects in improving the antioxidant capacity of the callus when compared to control (untreated callus). Treatment of callus with yeast extract at concentrations 100, 300 and 500 mg/L increased significantly (p < 0.05) the DPPH scavenging inhibition activity by 34%, 26% and 23% respectively. Treatment of callus with glycine increased significantly (p < 0.05) the DPPH scavenging inhibition activity by 29% at concentration 200 mg/L and only by 9% at concentration 100 mg/L. Treatment of callus with salicylic acid only increased significantly (p < 0.05) the antioxidant activity in DPPH assay by 30% at concentration 50 mg/L. However, the effect of elicitors on the ABTS scavenging inhibition activity of callus was not remarkable as that observed in DPPH assay. Mošovská et al. [21] demonstrated that ginger extract showed stronger ability to scavenge DPPH radical than ABTS cation radical.
Fig. 3.
Antioxidant activity of ginger rhizome and callus CM extracts treated with elicitors. CM, chloroform: methanol (1:1, v/v); elicitors (mg/L); Different letters indicate a significant difference (p < 0.05).
In summary, the antioxidant activity in term of IC50 values of ginger callus treated with different elicitors in decreasing order was callus treated with 100 mg/L yeast (726.98 ± 28.92 μg/mL) > 50 mg/L salicylic acid (IC50 748.87 ± 7.30 μg/mL) > 200 mg/L glycine (IC50 783.90 ± 7.84 μg/mL) > 300 mg/L yeast (IC50 803.22 ± 5.5 μg/mL) > 500 mg/L yeast (892.02 ± 6.85 μg/mL) > 100 mg/L glycine (1100.56 ± 27.22 μg/mL) > untreated callus (1265.49 ± 59.9 μg/mL). Interestingly, this order coordinate well with that of total phenolic content reinforcing the significant effect of elicitors on increasing the total phenolic content of the callus and consequently its antioxidant capacity. Thus, it was clear that high production of phenolic compounds with high antioxidant activity could be achieved by determining the appropriated concentration of elicitors.
4. Conclusion
The results of the present study suggested that ginger rhizome and its callus were a potential source of phenolics with antioxidant activity. 6-gingerol and 6-shogaol displayed comparable antioxidant activity. Elicitors, namely yeast, salicylic acid and glycine significantly influenced the total phenolic content in callus culture and consequently its antioxidant potential. The study provides valuable insights into the potential manipulation of ginger callus on the production of phenolic molecules that exhibit antioxidant activity for pharmalogical, cosmetic and agronomic industries. Therefore, the elicitation of cultured tissues is necessary to improve the production of phytochemical compounds and to increase the antioxidant capacity of ginger callus culture.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
References
- 1.Afzal M., Al-Hadidi D., Menon M., Pesek J., Dhami M.S. Ginger: an ethmomedical, chemical and pharmacological review. Drug Metab Drug Interact. 2001;18:159–190. doi: 10.1515/dmdi.2001.18.3-4.159. [DOI] [PubMed] [Google Scholar]
- 2.Eid BG, Mosli H, Hasan A, El-Bassossy HM. Ginger ingredients alleviate diabetic prostatic complications: effect on oxidative stress and fibrosis. Evidence-based complementary and alternative medicine. Volume 2017, Article ID 6090269, 12 pages. DOI:10.1155/2017/6090269.
- 3.Kundu J.K., Na H.K., Surh Y.J. Ginger-derived phenolic substances with cancer preventive and therapeutic potential. Forum Nutr. 2009;61:182–192. doi: 10.1159/000212750. [DOI] [PubMed] [Google Scholar]
- 4.Danciu C., Vlaia L., Fetea F., Hancianu M., Coricovac D.E., Ciurlea S.A., Şoica C.M. Evaluation of phenolic profile, antioxidant and anticancer potential of two main representants of Zingiberaceae family against B164A5 murine melanoma cells. Bio Med Central Biol Res. 2015;1:1–9. doi: 10.1186/0717-6287-48-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ha S.K., Moon E., Ju M.S., Kim D.H., Ryu J.H., Oh M.S. 6-Shogaol, a ginger product, modulates neuroinflammation: a new approach to neuroprotection. Neuropharmacology. 2012;2:211–223. doi: 10.1016/j.neuropharm.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Dugasani S., Pichika M.R., Nadarajah V.D., Balijepalli M.K., Tandra S., Korlakunta J.N. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J Ethnopharm. 2010;2:515–520. doi: 10.1016/j.jep.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Kundu J.K., Surh Y.J. Molecular basis of chemoprevention with dietary phytochemicals: redox-regulated transcription factors as relevant targets. Phytochem. Rev. 2009;2:333–347. [Google Scholar]
- 8.Peng F., Tao Q., Wu X., Dou H., Spencer S., Mang C. Cytotoxic, cytoprotective and antioxidant effects of isolated phenolic compounds from fresh ginger. Fitoterapia. 2012;3:568–585. doi: 10.1016/j.fitote.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 9.Weng C.J., Wu C.F., Huang H.W., Ho C.T., Yen G.C. Anti-invasion effects of 6-shogaol and 6-gingerol, two active components in ginger, on human hepatocarcinoma cells. Mol Nutr Food Res. 2010;11:1618–1627. doi: 10.1002/mnfr.201000108. [DOI] [PubMed] [Google Scholar]
- 10.Sák M., Dokupilova‘ I., Mihalik D., Lakatosova J., Gubisova M., Kraic J. Elicitation of phenolic compounds in cell culture of vitis vinifera L. by Phaeomoniella chlamydospora. Nova Biotechnol Chim. 2014;2:162–171. [Google Scholar]
- 11.Namdeo A.G. Plant cell elicitation for production of secondary metabolites: a review. Pharmacog Rev. 2007;1:69–79. [Google Scholar]
- 12.Naik P.M., Al-Khayri J.M. Impact of abiotic elicitors on in vitro production of plant secondary metabolites: a review. J Adv Res Biotech. 2016;1:1–7. [Google Scholar]
- 13.Seidel V., Windhövel J., Eaton G., Alfermann A.W., Arroo R.R.J., Medarde M., Petersen M., Wolley J.G. Biosynthesis of podophyllotoxin in Linum album cell cultures. Planta. 2002;215:1013–1039. doi: 10.1007/s00425-002-0834-1. [DOI] [PubMed] [Google Scholar]
- 14.Molnár Z., Virág E., Ördög V. Natural substances in tissue culture media of higher plants. Acta Biol Szegediensis. 2011;1:123–127. [Google Scholar]
- 15.Ali A.M.A., El-Nour M.E.M., Yagi S.M. Callus induction, direct and indirect organogenesis of ginger (Zingiber officinale Rosc) Afr J Biotech. 2016;38:2106–2114. [Google Scholar]
- 16.Wolfe K., Wu X., Liu R.H. Antioxidant activity of apple peels. J Agr Food Chem. 2003;51:609–614. doi: 10.1021/jf020782a. [DOI] [PubMed] [Google Scholar]
- 17.Ordon Ez A.A.L., Gomez J.D., Vattuone M.A., Isla M.I. Antioxidant activities of Sechium edule (Jacq.) Swart extracts. Food Chem. 2006;97:452–458. [Google Scholar]
- 18.Mensor L.I., Menezes F.S., Leitao G.G., Reis A.S., Santos D.O.S., Leitao S.G. Screening of Brazilian plants extracts for an antioxidant activity by the use of DPPH free radical method. Phytother Res. 2001;15:127–130. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- 19.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Bio Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 20.EL-ghorab A.H., Nauman M., Anjum F.M., Hussain S., Nadeem M. A comparative study on chemical composition and antioxidant activity of ginger (zingiber officinale) and cumin (Cuminum cyminum) J Agric Food Chem. 2010;58:8231–8237. doi: 10.1021/jf101202x. [DOI] [PubMed] [Google Scholar]
- 21.Mošovská S., Nováková D., Kaliňák M. Antioxidant activity of ginger extract and identification of its active components. Acta Chim. 2015;2:115–119. [Google Scholar]
- 22.Ghasemzadeh A., Jaafar H.Z.E., Rahmat A. Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe) Molecules. 2010;15:4324–4333. doi: 10.3390/molecules15064324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buchanan B.B., Jones R.L. vol. 40. American Society of Plant Physiologists; Rockville, MD: 2000. (Biochemistry and molecular biology of plants). [Google Scholar]
- 24.Dias M.I., Sousa M.J., Alves R.C., Ferreira I.C.F.R. Exploring plant tissue culture to improve the production of phenolic compounds: a review. Ind Crop Prod. 2016;82:9–12. [Google Scholar]
- 25.El-Nabarawy M.A., El-Kafafi S.H., Hamza M.A., Omar M.A. The effect of some factors on stimulating the growth and production of active substances in Zingiber officinale callus cultures. Ann Agric Sci. 2015;1:1–9. [Google Scholar]
- 26.Gorni, Pacheco A.C. Growth promotion and elicitor activity of salicylic acid in Achillea millefolium L. Pedro Henrique Afr J Biotech. 2016;16:657–665. [Google Scholar]
- 27.Kintzios S., Adamopoulou M., Pistola E., Delki K., Drossopoulos J. Studies on the physiological function of in vitro produced antioxidants from sage (Salvia officinalis L.): effects on cell growth and metabolism. J Herbs Spices Med Plants. 2002;9:229–233. [Google Scholar]
- 28.El-Bedawey A.A., Mansour E.H., Zaky M.S., Hassan A.A. Characteristics of antioxidant isolated from some plant sources. Food Nut Sci. 2010;1:5–12. [Google Scholar]
- 29.Al-Tahtawy R.H.M., El-Bastawesy A.M., Abdel Monem M.G., Zekry Z.K., Al-Mehdar H.A., El-Merzabani M. Antioxidant activity of the volatile oils of Zingiber officinale (ginger) Spatula DD. 2011;1:1–8. [Google Scholar]
- 30.Eleazu C.O., Amadi C.O., Iwo G., Nwosu P., Ironua C.F. Chemical composition and free radical scavenging activities of 10 elite accessions of ginger (Zingiber officinale Roscoe) J Clinic Toxicol. 2013;1:1–5. [Google Scholar]
- 31.Aziz D.M., Wsoo M.A., Ibrahim B.M. Antimicrobial and antioxidant activities of extracts from medicinal plant ginger (Zingiber officinale) and identification of components by gas chromatography. Afr J Plant Sci. 2015;10:412–420. [Google Scholar]
- 32.Pawar N., Pai S., Nimbalkar M., Dixit G. RP-HPLC analysis of phenolic antioxidant compound 6-gingerol from in vitro cultures of Zingiber officinale Roscoe. Plant Sci Today. 2015;1:24–28. [Google Scholar]
- 33.Guo J., Wu H., Du L., Zhang W., Yang J. Comparative antioxidant properties of some gingerols and shogaols, and the relationship of their contents with the antioxidant potencies of fresh and dried ginger (Zingiber officinale Roscoe) J Agric Sci Technol. 2014;5:1063–1072. [Google Scholar]
- 34.Ghasemzadeh A., Jaafar H.Z.E., Rahmat A. Optimization protocol for the extraction of 6-gingerol and 6-shogaol from Zingiber officinale var. rubrum Theilade and improving antioxidant and anticancer activity using response surface methodology. BMC Complementary Alternative Med. 2015;258:1–10. doi: 10.1186/s12906-015-0718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]