Abstract
Background
Lymph node involvement is a fundamental prognostic factor in head and neck squamous cell carcinoma (SCC). Lymph node yield (LNY), which is the number of lymph nodes retrieved after neck dissection, and lymph node ratio (LNR), which is the ratio of positive lymph nodes out of the total removed, are measurable indicators that may have the potential to be used as prognostic factors. The present study is designed to define the exact role of LNY and LNR regarding the overall and specific survival of patients affected by oral cavity and oropharyngeal SCC. It has been registered on clinicaltrials.gov database (NCT03534778).
Methods
This is a multicenter study involving tertiary care referral centers in Europe and North America. Patients affected by oral cavity, HPV+ and HPV- oropharyngeal SCC undergoing neck dissection will be consecutively enrolled and followed-up for up to 5 years. Patients and disease characteristic will be properly recorded and centrally analyzed. The primary end-point is to define reliable cut off-values for LNY and LNR which may serve as prognosticators of survival. This will be achieved through the use of ROC curves. Secondary outcomes will be the Overall survival (OS), Disease Specific Survival (DSS), and Progression Free Survival Hazard Ratios (HR) at 2-, 3- and 5 years, which will be evaluated through the Kaplan-Meier method and the difference in survival attested by the log-rank test. Univariate and multivariate analysis will be performed to understand the association of various outcomes with LNY and LNR.
1. Background
Lymph node involvement is a fundamental element to consider in the prognosis of Head and Neck squamous cell carcinoma. Local spread of the tumor occurs mostly through the lymphatic vasculature to the lymph nodes of the neck. For this reason, the primary assessment of lymph nodes is a fundamental aspect of the staging system. The American Joint Committee on Cancer Staging Manual stratifies groups based on anatomic and non-anatomic criteria [1]. The 8th edition of the AJCC manual implemented some changes for oral cavity, pharynx, and larynx compared to the previous edition. Tumor depth and extranodal extension (ENE) are now factors to consider in the staging system together with the historical factors such as laterality, number, and size of the involved lymph nodes. On the other hand, it is still unclear if other elements may be of significant importance in head and neck cancer patients.
In detail, lymph node yield (LNY) and lymph node ratio (LNR) are measurable factors whose role may have a potential prognostic implication. LNY is defined as the number of lymph nodes retrieved after neck dissection, whereas LNR is defined as the ratio of pathologically positive lymph nodes out of the total number of retrieved lymph nodes after neck dissection. It is likely that a higher LNY means that more potential, occult, pathological tissue has been removed and for this reason, this should be a favorable prognostic factor.
On the other hand, a lower LNR may signify that few lymph nodes are positive out of the total removed and in consequence, the lower the value the higher the survival rate should be. Various potentially optimal cut-off values of LNY and LNR have been proposed in many retrospective studies and in two published systematic reviews [2,3], although no definitive results have been drawn yet. Elective neck dissection for cN0 oral and oropharyngeal cancer is routinely performed in many centers, considering the risk of occult metastasis. The management of cN+ neck instead routinely involves the execution of a therapeutic neck dissection according to the levels involved and the characteristic of the disease.
In order to improve survival and better tailor treatment choices, it would be important to take into account the specificity of cancers affecting specific subsites. It is now clear that HPV+ oropharyngeal carcinoma has a better prognosis than HPV- disease. The development of risk stratification should take into account these and other factors in order to understand if adjuvant treatments such as radiotherapy/chemotherapy should be performed. LNY and LNR may have a predictive value which may help to drive treatment choices in specific population of patients. The majority of the evidence on this topic comes from retrospective studies or cancer database registries [3], which are undoubtedly a valid source of information but are also predisposed to biases due to the retrospective collection of information, such as patient selection and incomplete data acquisition. For this reason, a routine evaluation of the two parameters is not part of current clinical practice.
This prospective observational study aims to validate the results coming from the currently available retrospective studies and meta-analyses. It should account for potential biases and should provide proper stratification of the analyzed outcomes in order to finally define the exact role and the precise cut-off values for LNY and LNR in oral cavity and oropharynx squamous cell carcinoma.
The protocol of this study has been compiled following the STROBE statement check-list (appendix document 1) [4]. It has been preliminary registered on clinicaltrials.gov (NCT03534778).
2. Methods
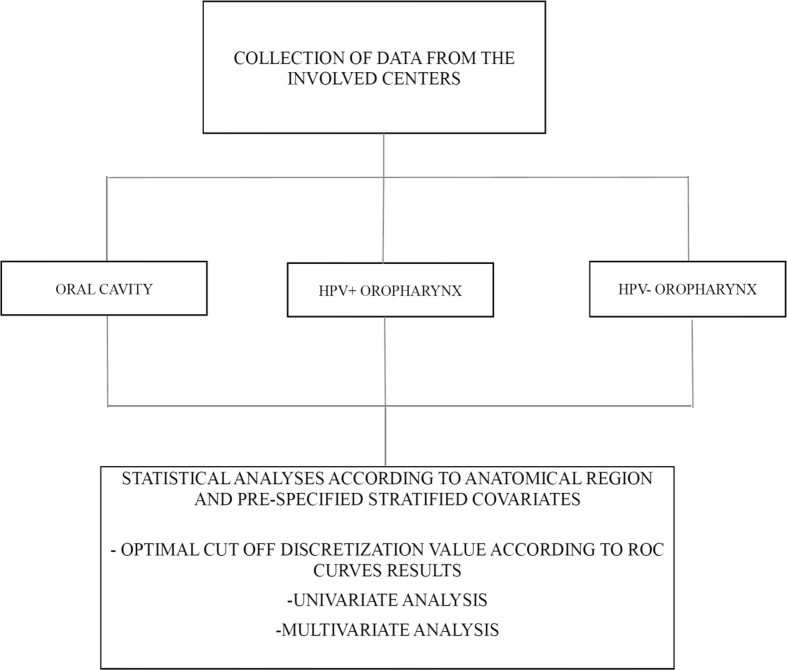
This is a multicenter study in which the participating institutions are tertiary care centers in Europe and North America (Humanitas Research Hospital - Milano, Italy; University of Pennsylvania - Philadelphia, USA; Regina Elena National Cancer Institute - Rome, Italy; Poznan University of Medical Sciences, Poland; University of Zielona Gora, Poland) who accept to conform to the present protocol for participation. Centers involved were selected on the basis of the high level of specialization in head and neck cancer surgery. Moreover, all the participating institutions are high volume centers that routinely perform surgery on oral cavity and oropharyngeal cancers. This prospective study will recruit consecutive patients diagnosed with squamous cell carcinoma of the oral cavity and HPV+ and HPV- oropharynx, whose treatment plan includes neck dissection at any level (see Fig. 1). This study focus on those two clinical entities because it is assumed that it is better to analyze data on two cancer subtypes in the head and neck region. First, because it will be possible to understand if a difference exist between LNY/LNR in oral versus oropharynx carcinoma. Second, enrolling patients affected by two subtypes will allow to reach a substantial number of patients in a shorter time frame. For each patient, a complete diagnostic workup will be conducted and detailed information will be collected. The workup includes the collection of demographic and clinical characteristics, conventional risk factors and radiographic examination as necessary. Patients will sign a uniform consent form in all the participating institutions (appendix document 2). A uniform chart describing all the included characteristics will be provided to all the participating institutions through a dedicated spreadsheet function created in googledocs as described by Rosenberg and coll. [5], in which a designated researcher in every participating institution is provided with access information and will be responsible for the input of data. The investigator at each participating institutions have personally met the principal investigator (OI) in order to discuss how to collect and input the data through the dedicated spreadsheet function. Quality assurance has been performed discussing with the local researchers the surgical protocols in every clinical scenario, the proper management of the data, and strict adherence to this protocol.
Fig. 1.
Schematization of data collection.
Through this system, all the information will be returned completed to the principal investigator, who will have access to all the information provided by every participating institution and who is ultimately responsible for the collection of data and coordination of the statistical analysis together with the involved statisticians.
To avoid any issue regarding patient data confidentiality, the designated researcher will input patient's data inserting just the initials of the treated patient in the googledocs precompiled spreadsheet and will number each one consecutively. It will be her/his care to keep record of the association between the initials/consecutive number with the patient's chart, which may be needed to be retrieved at a successive time if data are missing or incompletely reported.
Inclusion criteria are as follows: age of at least 18 years, histologically confirmed diagnosis of squamous cell carcinoma, surgically resectable disease at any stage, neck dissection included in the treatment plan either for N0 or N+ disease. Exclusion criteria are recurrent carcinoma after surgery, previous irradiation, metastatic carcinoma, ECOG performance status >3.
This protocol will be submitted to each ethical committee of involved institutions. Given the purely observational, non experimental design of this study, which involves just a rigorous examination of data coming from standard of care reports, no particular ethical issues must be outlined.
3. Treatment
Surgical resection of the primary tumor will be performed according to the disease and the surgical protocol of the involved institutions. Monolateral or bilateral, radical, modified radical, selective, or extended neck dissection will be performed according to the treatment plan for the specific tumour and will be carefully reported according to AAO-HNS criteria (Table 1) [6]. Lymph node levels will be sampled and analyzed separately. Variables recorded will be: exact anatomic location of the tumour, size of the primary tumor, pathologic depth of invasion of the tumor, grade of differentiation, status of resection margins, type of lymph node dissection performed, lymph node involvement, extracapsular spread (ECS), perineural invasion, tumor infiltrating lymphocytes, exact lymph node yield per level, number of lymph nodes involved by disease per level (Table 2). Chemoradiotherapy or radiotherapy treatment plan will be recorded in detail, if performed. Surgical specimens will be examined by pathologists with a special interest in head and neck pathology. Specimen preparation, dissection, sampling, and microscopical examination will be performed in a standardized way. Care will be taken to report the characteristics of the resected lymph nodes, including the exact number, presence of metastasis, and ECS.
Table 1.
Type of neck dissection as defined by AAO-HNS criteria [5].
| Radical | Removal of all ipsilateral cervical lymph nodes extending from the inferior border of the mandible to the clavicle, from the lateral border of the sternohyoid muscle medially, to the anterior border of the trapezius muscle. Spinal accessory nerve, Sternocleidomastoid muscle, and internal jugular vein are also removed. |
| Modified radical | Excision of all lymph nodes removed with the radical neck dissection with preservation of one or more of non lymphatic structures, such as internal jugular vein, sternocleidomastoid muscle, or spinal accessory nerve. Preserved structures should be specified. |
| Selective | Preservation of one or more lymphnode groups that are routinely removed in the radical neck dissection. |
| Extended | Removal of one or more additional lymph nodes group or non lymphatic structures not included in the definition of radical neck dissection (e.g. parapharyngeal lymph nodes, buccinator muscle etc.). The additional structures should be specified. |
Table 2.
Form used for collection of pathology data.
| Data element | Response |
|---|---|
| Fresh tissue received | Yes/No Description of pathology techniques used |
| Procedure performed | Brief description of the surgery on primary tumour and neck dissection performed |
| Pathological depth of invasion | In millimeters |
| Laterality of neck dissection | Monolateral/Bilateral |
| Type of neck dissection | Reported according to AHNS/AAOHNS [4] |
| Specimen dimensions | In millimeters |
| Description of anatomical components included | e.g. Submandibular gland, spinal accessory nerve etc. |
| Lymph nodes retrieved (Lymph Node Yield) | number |
| Number of positive lymph nodes at microscopic examination | number |
| Number of positive lymph nodes over Lymph nodes retrieved (Lymph Node Ratio) | number |
| Presence of extracapsular spread (ECS) | Yes/no |
| In case of ECS, distance of closest margin in mm | In millimeters |
| Perineural invasion | Yes/no |
| Additional observations |
4. Statistical analysis
The primary endpoint of this study is the assessment of reliable cut-offs for LNY and LNR. To do so, the sum of specificity and sensitivity will be maximized as a function of possible cut-offs. Regarding the identification of the best cut off values for LNY and LNR, the ability of the variables to discriminate the most favorable outcomes will be performed using the time-dependent receiver operating characteristics (ROC) curves. ROC curves show sensitivity versus 1 – specificity, such that the area under the curve is able to demonstrate higher discriminatory ability in defining different risk groups for a given value. We can conservatively estimate the standard error of the sum as 1.4 times the standard error of specificity and sensitivity, and an upper bound for this is therefore 0.7 divided by the square root of the total sample size. We can then fix a minimal sample size of 400 subjects per anatomical subsite in order to be able to distinguish the performance of cut-offs with a difference in sum of 3.5%.
Statistical analysis will be performed by a single center, where data will be gathered by the principal investigator from the multiple centers involved in the study. Variables examined will include patient and tumor characteristics and their association with recurrence and death. Analyzed outcomes will include Overall Survival (OS), Disease-specific survival (DSS) and Progression Free Survival (PFS) Hazard Ratios. The 2, 3 and 5 year OS, DSS and PFS will be evaluated using the Kaplan-Meier method and the difference in survival assessed by the log-rank test. OS will be measured by the time of surgery to the date of death or last follow-up. DSS will be calculated from the time of diagnosis to cancer-related death. Subgroup analysis will be performed differentiating among oral, HPV+, and HPV- oropharyngeal cancer. Further stratification of patients will be performed in order to account for possible biases in the interpretation of the results and also to properly evaluate the prognostic significance of LNY and LNR on various categories of patients. Categorization of variables will be performed as follows: age, primary site of the tumor, cN stage, pN stage, cT stage, pT stage, ECS, tumor depth of infiltration, type of neck dissection, number of levels dissected, adjuvant treatment, median lymph node yield (this one just for the LNR analysis). Univariate Cox regression analyses will be performed to examine the association between unfavorable outcomes and the LNY and LNR. A multivariate analysis using the Cox proportional hazards model will then be conducted to determine independent predictors of survival adjusted for the previously defined categorized variables. The sample size of 400 patients also satisfies the rule of thumb as described by Ogundimu and coll. [7], in which an estimated number of events per variable should be > 20 in order to eliminate any bias in regression coefficients. Patients accrual started in November 2018. Accrual is expected to be completed in 2 years. Two-sided p-value <0.05 will be considered to represent a statistically significant difference.
5. Follow-up
Patients participating in the study will be followed-up periodically for recording of death (overall and cancer related) and recording of recurrence of disease. Patients lost to follow-up will be considered as censored.
The designated researcher in each institution will insert the required data in the precompiled form on googledocs server once the event occurs. If the patient will complete the follow-up period or is still alive at completion of the study it will be inserted in the form as terminally censored.
Follow-up after surgery will be performed at each single participating institution according to their standards. Anyway, a follow-up after surgery at 1 month, 3 months, 6 months, 1 year and subsequently every 6 months up to 5 years is the minimum requested.
6. Results
Results of the study will be presented in synthesis tables, graphs will be provided to better illustrate the outcomes of the statistical analyses.
ROC curves identifying the likely best cut-off values of LNY and LNR for OS, DSS and PFS will be constructed. Tables for the univariate and the multivariate analyses will be provided.
2-, 3- and 5- years OS, DSS and PFS graphs calculated using the Kaplan-Meier method will be presented. The different Kaplan-Meier curves will depict results according to the various pre-specified, stratified variables.
7. Discussion
Lymph node status of the neck may be considered the main prognostic indicator in patients affected by HNSCC. Laterality, size and number of the involved lymph nodes are established factors to consider in the evaluation of patients. On the other hand, LNY and LNR are measurable factors whose role as prognosticators is unclear.
The literature is rich in reports that suggest that LNY and LNR may be used as prognosticators of survival [2]. Data from retrospective and registry studies indicate that they can be both useful in defining the prognosis of patients undergoing neck dissection in the context of the treatment for HNSCC. The results of a meta-analysis and meta-regression conducted by our group (submitted for publication) further confirm this assumption.
Ebrahimi and coll. [8], in a retrospective study, concluded that a minimum lymph node yield of ≥18 conferred a survival advantage in clinically node negative oral cavity cancer patients. A database registry study by Kuo and coll. [9] stratified patients in 8 groups and established that the greatest survival advantage in cN0 patients was achieved at values ≥ 16. The same study also evaluated cN+ patients and in this case a cut-off of ≥26 was necessary to confer a greater survival.
Divi and coll [10]. performed a cancer database study on all head and neck subsites and including all N stages, confirming that a value ≥ 18 likely gives a better survival compared to patients with lower LNY values. The same value was confirmed by Bottcher and coll [11]. regarding larynx cancer patients. Literature confirms that a higher yield is associated with a improvement in prognosis, although the exact cut-off values are variable from study to study.
Lymph node ratio of 0.13 was found to be statistically associated with a decrease in overall and disease specific survival in a retrospectively evaluated series of oral cavity cancer patients [12]. Gil and coll. [13] found that a ratio lower than 0.06 in oral cavity cancer patients, was associated to better survival outcomes. While Kunzel and coll. [13] evaluated oropharyngeal squamous cell carcinoma series establishing that a cut of 0.09 was clinically significant for improved outcomes. Other retrospective studies or cancer database registry analyses on other anatomical subsites confirmed that a lower ratio leads to improved survival, but the cut-off values varies among studies.
It is clear that current state of evidence on this topic does not allow to implement the use of LNY and LNR in routine clinical practice [14]. Retrospective and cancer registry studies do not account for proper stratification of patients. Some evaluations have been made on specific anatomic subsites only, while others on head and neck cancers as a whole.
In summary, while some authors have advocated the use of LNY values just as quality indicators of the surgical procedures performed [15], many reports arrive at the conclusion that it may be used as a real prognostic indicator of survival [[16], [17], [18]]. Regarding LNR, it has been observed that higher values are associated with poorer prognosis [[19], [20], [21]].
The current challenge is to establish an optimal cut-off value for both the indicators, given that the different studies report different values. It should be pointed out that the apparent increase in survival with higher LNY values does not contradict the fact that selective neck dissection is the best practice in specific clinical scenarios. Instead, aiming for a higher LNY also for selective neck dissections would lead to adequate examination of the lymph-nodes removed and to a more radical surgery of the levels selected for removal. This last concept should be more important than the extent of the surgery itself. For example, if just levels I-III have been dissected, it is likely more important to have all the lymph nodes removed from that specific levels rather than a greater absolute number of lymph nodes removed in levels I-V, but at the same time leaving some pathological tissue behind (eg. 15 lymph nodes removed in levels I-III are likely a better outcome than 17 lymph nodes removed in levels I-V). In other terms, it is desirable that the cut-off values would be differentiated according to the levels involved in the dissection, but this will be possible only when data coming from properly designed prospective studies will give enough information to do so.
LNR has been found useful in predicting survival, even if clear cut-off values are needed to be established. As with LNY, also for LNR different values should be determined on the basis of the neck levels involved in a neck dissection. A standardized procedure of collecting and reporting the pathology sample is required to achieve these aims.
With these premises, the present prospective study, with proper stratification of patients, can allow to achieve agreement on the use of LNY and LNR in routine clinical practice. We decided to conduct a multicenter, observational, prospective study to determine if the two indicators may be added to the TNM staging system of patients affected by HNSCC of the oral cavity and oropharynx who require neck dissection. Our study would allow correlation of the LNY and LNR cut-offs to the pre-specified categorized variables, thus leading to a more precise estimate of the real impact of these indicators on prognosis and hopefully determining better tailored treatment decisions for the patients affected by oral cavity and oropharyngeal squamous cell carcinoma.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2019.100324.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lydiatt W. Head and neck cancers-major changes in the American Joint committe on cancer eighth edition cancer staging manual. Ca - Cancer J. Clin. 2017;67:122–137. doi: 10.3322/caac.21389. [DOI] [PubMed] [Google Scholar]
- 2.Cheraghlou S. Prognostic value of lymph node yield and density in head and neck malignancies. Otolaryngol. Head Neck Surg. 2018 doi: 10.1177/0194599818756830. United States. [DOI] [PubMed] [Google Scholar]
- 3.Talmi Y.P., Takes R.P., Alon E.E. Prognostic value of lymph node ratio in head and neck squamous cell carcinoma. Head Neck. 2018;40:1082–1090. doi: 10.1002/hed.25080. [DOI] [PubMed] [Google Scholar]
- 4.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P., STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 2008 Apr;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg J., Henriksen N.A., Jørgensen L.N. Vol. 11. 2010. Multicenter data acquisition made easy Trials; p. 49. Published online 2010 Apr 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robbins K.T., Clayman G., Levine P.A. Neck dissection classification UpdateRevisions proposed by the American head and neck society and the American academy of otolaryngology–head and neck surgery. Arch. Otolaryngol. Head Neck Surg. 2002;128(7):751–758. doi: 10.1001/archotol.128.7.751. [DOI] [PubMed] [Google Scholar]
- 7.Ogundimu E., Altman D.G., Collins G.S. Adequate sample size for developing prediction models is not simply related to events per variable. J. Clin. Epidemiol. 2016 Aug;76:175–182. doi: 10.1016/j.jclinepi.2016.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebrahimi A., Zhang W.J., Gao K., Clark J.R. vol 117. 2011. pp. 2917–2925. (Nodal Yield and Survival in Oral Squamous Cancer: Defining the Standard of Care Cancer). [DOI] [PubMed] [Google Scholar]
- 9.Kuo P., Mehra S., Sosa J.A. Proposing prognostic thresholds for lymph node yield in clinically lymph node-negative and lymph node-positive cancers of the oral cavity. Cancer. 2016;122:3624–3631. doi: 10.1002/cncr.30227. [DOI] [PubMed] [Google Scholar]
- 10.Divi V., Harris J., Harari P.M. Establishing quality indicators for neck dissection: correlating the number of lymph nodes with oncologic outcomes (NRG Oncology RTOG 9501 and RTOG 0234) Cancer. 2016;122:3464–3471. doi: 10.1002/cncr.30204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bottcher A., Dommerich S., Sander S. Nodal yield of neck dissections and influence on outcome in laryngectomized patients. Eur. Arch. Oto-Rhino-Laryngol. 2016;273:3321–3329. doi: 10.1007/s00405-016-3928-2. [DOI] [PubMed] [Google Scholar]
- 12.Shrime M.G., Bachar G., Lea J. Nodal ratio as an independent predictor of survival in squamous cell carcinoma of the oral cavity. Head Neck. 2009;31:1482–1488. doi: 10.1002/hed.21114. [DOI] [PubMed] [Google Scholar]
- 13.Gil Z., Carlson D.L., Boyle J.O. Lymph node density is a significant predictor of outcome in patients with oral cancer. Cancer. 2009;115:5700–5710. doi: 10.1002/cncr.24631. [DOI] [PubMed] [Google Scholar]
- 14.Kunzel J., Mantsopoulos K., Psychogios G., Grundtner P., Koch M., Iro H. Lymph node ratio as a valuable additional predictor of outcome in selected patients with oral cavity cancer. Oral Surg. Oral Med. Oral. Pathol. Oral Radiol. 2014;117:677–684. doi: 10.1016/j.oooo.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 15.Chassin M.R., Loeb J.M., Schmaltz S.P., Wachter R.M. Accountability measures—using measurement to promote quality improvement. N. Engl. J. Med. 2010;363:683–688. doi: 10.1056/NEJMsb1002320. [DOI] [PubMed] [Google Scholar]
- 16.Lemieux A., Kedarisetty S., Raju S., Orosco R., Coffey C. Lymph node yield as a predictor of survival in pathologically node negative oral cavity carcinoma. Otolaryngol. Head Neck Surg. 2016;154:465–472. doi: 10.1177/0194599815622409. [DOI] [PubMed] [Google Scholar]
- 17.Lee S., Kim H.J., Cha I.H., Nam W. Prognostic value of lymph node count from selective neck dissection in oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2018;47(8):953–958. doi: 10.1016/j.ijom.2018.03.007. Aug. [DOI] [PubMed] [Google Scholar]
- 18.Ebrahimi A., Clark J.R., Amit M. Minimum nodal yield in oral squamous cell carcinoma: defining the standard of care in a multicenter international pooled validation study. Ann. Surg. Oncol. 2014;21:3049–3305. doi: 10.1245/s10434-014-3702-x. [DOI] [PubMed] [Google Scholar]
- 19.Shrime M.G., Ma C., Gullane P.J. Impact of nodal ratio on survival in squamous cell carcinoma of the oral cavity. Head Neck. 2009;31:1129–1136. doi: 10.1002/hed.21073. [DOI] [PubMed] [Google Scholar]
- 20.Patel S.G., Amit M., Yen T.C. Lymph node density in oral cavity cancer: results of the International Consortium for Outcomes Research. Br. J. Canc. 2013;109:2087–2095. doi: 10.1038/bjc.2013.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki H., Matoba T., Hanai N. Lymph node ratio predicts survival in hypopharyngeal cancer with positive lymph node metastasis. Eur. Arch. Oto-Rhino-Laryngol. 2016;273:4595–4600. doi: 10.1007/s00405-016-4170-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.