Abstract
A gene encoding chitinase from B. subtilis has been isolated after optimization of PCR conditions. It was cloned with two different prometers, T7 promoter of the pJET1.2/blunt cloning vector and the SP6 promoter of pGEM®-T Easy vector. After transforming E. coli DH5α, two transformants were selected, CHI-NRC-4 from the first vector and T-CHI-NRC-6 from the second vector, and used for further studies. The complete CDS sequence of chitinase gene was determined and submitted to GenBank with the accession number KX268692.1. Culture supernatants of E. coli (CHI-NRC-4) and E. coli (T-CHI-NRC-6) were investigated for their inhibitory effect on M. javanica egg hatch under laboratory conditions. Result showed up to 96% inhibition in egg hatching due to both E. coli transformants as compared to control which reflect the same expression efficiency of both used prometers. A greenhouse experiment was carried out to evaluate the nematicidal effect of culture supernatants of the two transformts E. coli (CHI-NRC-4) and E. coli (T-CHI-NRC-6) against M. javanica infected eggplant. Obtained results showed a significant reduction in nematode population in soil and roots and enhancement in eggplant growth parameters as compared to control.
Keywords: Gene cloning, Chitinase, Nematode, Meloidogyne javanica, Eggplant
1. Introduction
Solanaceous plants represent a great economic importance all over the world. Among them, eggplant (Solanum melongena L.), is one of the most vegetable crop with nutritional and medicinal properties as well as it is a good source of vitamins A and C, in addition to other nutritional elements i.e. iron, phosphors, potassium and calcium [19]. Plant parasitic nematodes are one of the most harmful common pests which cause a reduction in the quality and quantity of eggplant production [11]. Moreover, the nematode serves as agent predisposing to the development of complex disease with fungi, bacteria and viruses.
Plant parasitic nematode can be managed effectively by chemical nematicides but many of these chemicals are not only becoming more and more expensive but also source of human and environmental risks, though it should only be used when necessary [8], [1]. Consequently, chemical control may no longer be a good option [12], [15]. The recent drive to produce vegetables free of chemical residues and increased concern for the environment and human health led to innovate and develop alternative control approaches which are environmentally safe. One plausible alternative is the use of biocontrol which involve the application of antagonistic microbes to nematodes.
Such findings open the door for using the recombination DNA technology in construction of strain that combine desired properties. Genetic manipulation could result in initiate new biocontrol agent with the ability to increase the production of toxic compounds or lytic enzymes. Both gene doses for the enzyme, and repressor and regulator molecules can be altered to achieve high concentration of enzyme with minimal detrimental by products.
Biological control tactics have being a field of interest of many researchers as they play a remarkable role in controlling diseases caused by plant-parasitic nematodes. Tikhonov et al. [18] reported that, the chitinase and protease enzymes of several plant growth-promoting rhizobacteria are responsible in degrading the nematode wall and the action of chitinase enzyme is more damaging to nematode eggshell. Woo-Jin et al. [20] detected that, P. illinoisensis having strong chitinolytic activity on the egg hatch of M. incognita that in 7 days of incubation with P. illinoisensis, none of the eggs hatched, whereas (39.8%) egg hatching rate was observed in the water control.
Chitinase gene had been cloned and characterized from different microorganisms. Some of these were cloned to plant and bacterial strains to augment their ability to control plant pathogens; others were highly expressed in E. coli to enhance their activity [4].
This study, concerning with enhancement of nematicidal potential through cloning and expression of chitinase gene from Bacillus subtilis subsp. subtilis BTN7A strain.
2. Materials and methods
2.1. Bacterial strains
Bacillus subtilis BTN7a [9] was used for chitinase gene isolation. E. coli DH5α was used in transformation trails.
2.2. Medium and growth conditions
Lauria-Bartani (LB) medium was used for bacterial growth. Growth temperature of used bacterial strains was 37 °C.
2.3. Extraction of M. javanica eggs
Extraction of M. javanica eggs was performed as in [10] using infected tomato roots. Eggs were then transferred into a clean beaker with sterilized water.
2.4. In vitro evaluation of nematotoxicity
The supernatants of the transformed E. coli (NRC-CHI-4) and E. coli (T-CHI-NRC-6) were evaluated for their inhibitory effect against M. javanica egg hatch under laboratory conditions in comparison with B. subtilis. Four ml of egg suspensions containing about 100 M. javanica eggs were transferred into Petri dish of 6 cm diam. to which one ml of the supernatants of the two transformed E. coli or B. subtilis cultures were added separately. One ml of LB medium transferred to Petri dish supplied with four ml of eggs suspension served as control. All dishes kept at room temperature. Each preparation and the control were replicated five times. Emerged juveniles were counted with the aid of a stereoscopic microscope every 24 h for three days. All treatments were then transferred to distilled water for 24 h to ascertain whether the eggs had been permanently or temporarily inactivated.
2.5. In vivo evaluation of nematotoxicity
Greenhouse trial was conducted to evaluate the nematicidal efficiency of the supernatants of the transformed E. coli (NRC-CHI-4) and E. coli (T-CHI-NRC-6) and B. subtilis against M. javanica. One month-old eggplant seedlings were transplanted in 25 cm diam. pots filled with autoclaved mixed soil from loamy-sand: clay (1:1 v/v). Pots were treated with 10 ml/pot from each of the previous supernatants as soil drenched and received 2000 M. javanica eggs in 10 ml distilled water. Pots transplanted with one month old eggplant seedlings received 2000 M. javanica eggs in 10 ml distilled water served as control. Each treatment replicated eight times, pots were arranged in randomized complete block design in greenhouse at 27 ± 3 °C. The experiment was terminated 60 days after nematode infection. Data on nematode reproduction, plant growth parameters and statistically analysis were done.
2.6. DNA manipulations
Unless specified, all molecular biology manipulations were performed according to standard protocols [17] and kit supplier’s instructions.
The bacterial genomic DNA was extracted using fresh crude extract method [5] and used as DNA template in PCR amplification.
2.7. Polymerase chain reaction (PCR)
Chitinase gene amplification was performed in a thermal cycler Amplitronyx™ (NYXTECHNIK, USA) programmed for one cycle of DNA denaturation at 94 °C (5 min), 35 cycles of 94 °C (2 min), 45 °C (1 min) and 72 °C (1.5 min), plus one additional cycle of a final chain elongation at 72 °C (10 min). At the end of PCR program a sample of the reaction mixture was analyzed by agarose gel electrophoreses and the rest of the reaction mixture was held at -20 °C until used for cloning and for sequencing. Sequencing was performed at Macrogen Co., Korea.
2.8. Bioinformatics and primer design
Different internet sites have been used through this study. They included: The National Center for Biotechnology Information (NCBI), Webcutter 2.0 software, primer design (Primer3), Plasmid Mapping [6] and SnapGene® Viewer program.
The GenBank DNA sequence database of B. subtilis was accessed (NCBI Reference Sequence: NC_016047.1) and 1216 bp of the complete chitinase CDS and its surrounding bases, was analyzed using Webcutter 2.0 software and Primer3 software to select the appropriate primers which amplify the Chi complete CDS. The selected primers were Chi-bs-F (GGT TAT GAG ATG TTT CAA ATG AGC) and chi-bs-R (GGA TTT TGC TTA GAA CTA TAA ATC CTT). The expected chitinase amplicon size was 970 bp.
2.9. Chitinase gene amplification
Several attempts have been done to isolate the chitinase gene of B. subtilis BTN7a; they include different DNA concentrations, different PCR programs and different MgCl2 concentrations.
After the program was completed, 10 µl of amplified DNA were analyzed by gel electrophoreses and compared with 100 bp DNA ladder as DNA marker.
Chitinase (Chi) gene was purified from gel using MEGAquick-spin™ Total Fragment DNA purification kit (iNtRON Biotechnology).
2.10. Cloning of chitinase
Two different protocols were used to clone the chitinase gene using two vectors the pJET1.2/blunt cloning vector and pGEM®-T Easy vector.
Chitinase gene was cloned into pJET1.2/blunt Cloning vector using CloneJET™ PCR Cloning Kit (Thermo scientific, USA). The amplified chitinase gene was converted to blunt ended by using the DNA blunting enzyme and then ligated with pJET1.2/blunt Cloning Vector.
Chitinase gene was also cloned with pGEM®-T Easy vector (Promega Co. Madison, USA).
2.11. E. coli DH5α transformation
E. coli DH5α was converted to competent cells and transformed with recombinant plasmids as described in [17]. Transformants were selected by their ability to grow in the presence of ampicilin and by white/blue screening (i.e., Xgal and IPTG method).
3. Results and discussion
3.1. Isolation of chitinase (Chi) gene
The genomic DNA of B. subtilis BTN7a was extracted and polymerase chain reaction was performed with the designed Chi-bs-F and Chi-bs-R primers. To optimize the expected PCR product amplification, different concentrations of DNA were used and different concentration of MgCl2 were tried in PCR reaction mixture (i.e., without or with 2 µl of 25 mM MgCl2) using iNtRON’s Maxime PCR PreMix Kit. After completion of PCR program, sample of PCR product was analyzed using agarose gel electrophoresis. The optimized condition for chi amplification was found to be 10 µl Master Mix iTaq, 1 µl DNA, 1 µl of each primer, without Mg Cl2 and 7 µl of H2O were added.
The agarose gel electrophoreses of the amplified DNA was analyzed using 100 bp Ladder DNA marker (AXYGEN). The 970 bp DNA amplified was then extracted, purified and used for cloning experiments using the pJET1.2/blunt cloning vector and pGEM®-T Easy vector. The obtained recombinant plasmids were used to transform E. coli DH5α. Several E. coli DH5α transformants were obtained with T-easy vector and only few with pJET1.2 vector. This results is more probable due to that cohesive DNA ends (i.e., T-easy vector) are more efficient in cloning than the blunt DNA ends (i.e., pJET 1.2 vector). Their plasmids were isolated and analyzed to confirm the existence of chitinase gene and its physical orientation. Three recombinant plasmids were used for further studies they named T-NRC-CHI-6, T-NRC-CHI-101 and CHI-NRC-4 where NRC represents National Research Centre. The vector of the first two plasmids was T Easy vector while the latter one was pJET1.2/blunt vector.
To confirm the existence of chitinase gene in the recombinant plasmids, they were used in PCR trial with chitinase primers. All three transformants presented the 970 bp ampilicons which contain the chitinase gene as shown in Fig. 1.
Fig. 1.
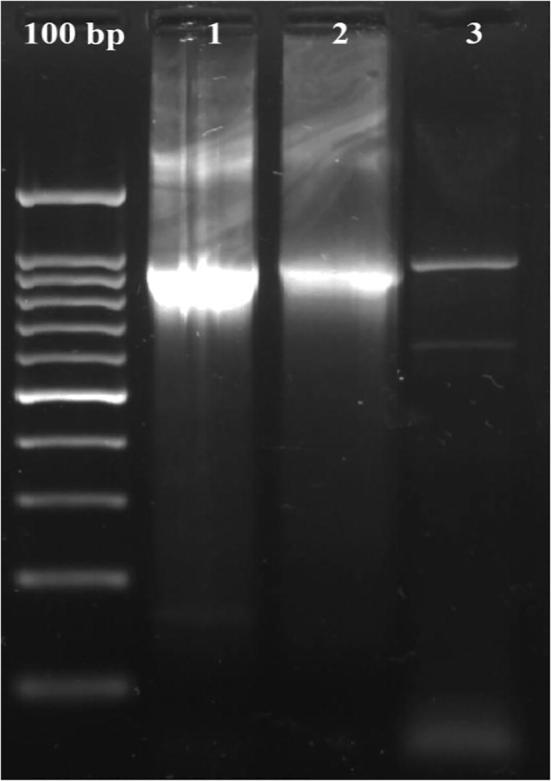
Agarose gel electrophoreses of chitinase gene amplification from recombinant plasmids. Lane 1: T-NRC-CHI-6, lane 2: T-NRC-CHI-101 and lane 3: CHI-NRC-4.
3.2. Chi orientation of pJET-CHI-4
Since Chi cloning with pJET needed to have the insert blunt ended, the insert could be ligated to the vector in both directions. To identify the chi orientation in the recombinant plasmid pJET-CHI-4, PCR was performed using different sets of primers. The two primers of the pJET vector (pJET1.2 - For and pJET1.2- Rev) and the chitinase primers (Chi-bs-F and Chi-bs-R). The principal of this experiment was that if the two used primers are located at the same DNA strand no amplicon will be produced. The amplified DNA fragments were then detected after agarose gel electrophoresis.
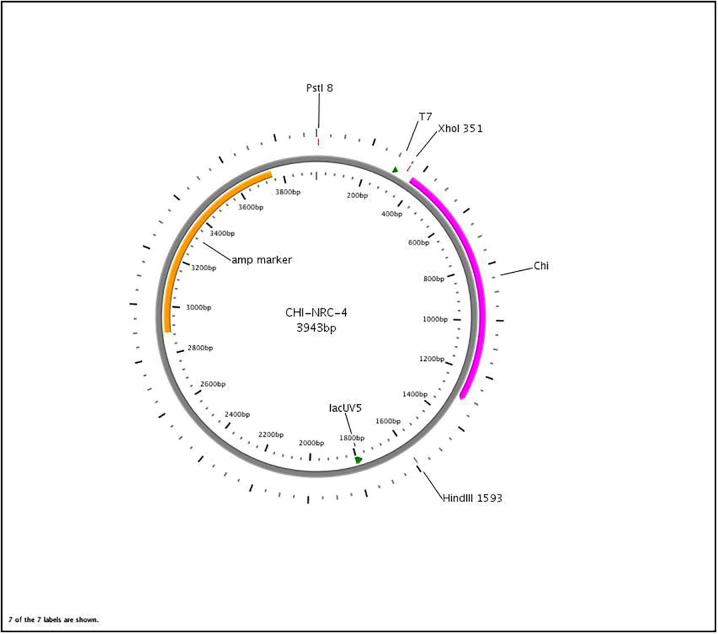
Results indicated that the orientation of the chitinase gene was clockwise (i.e., under the control of T7 promoter of the pJET vector). Where by using the primers JET- F and Chi-bs-F (F) no DNA amplifications were found. At the same time when the primers JET- F and Chi-bs-R were used DNA band with the expected size was found. Fig. 2 represents the map of the recombinant plasmid CHI-NRC-4. Plasmid CHI-NRC-4 map represented the existence of the Chi CDS location from 386 to 1321 base equal to 936 bp which represents 311 amino acids with 33.5 kDa.
Fig. 2.
Plasmid CHI-NRC-4.
The recombinant plasmid T-NRC-CHI-6 was sent for nucleotide sequence using the two vector primers M13 forward and M13 reverse primers. The complete nucleotide sequence of the amplicon was obtained and Chi CDS nucleotide sequence and its deduced amino acid sequencing were submitted to GenBank with the accession number GenBank: KX268692.1.
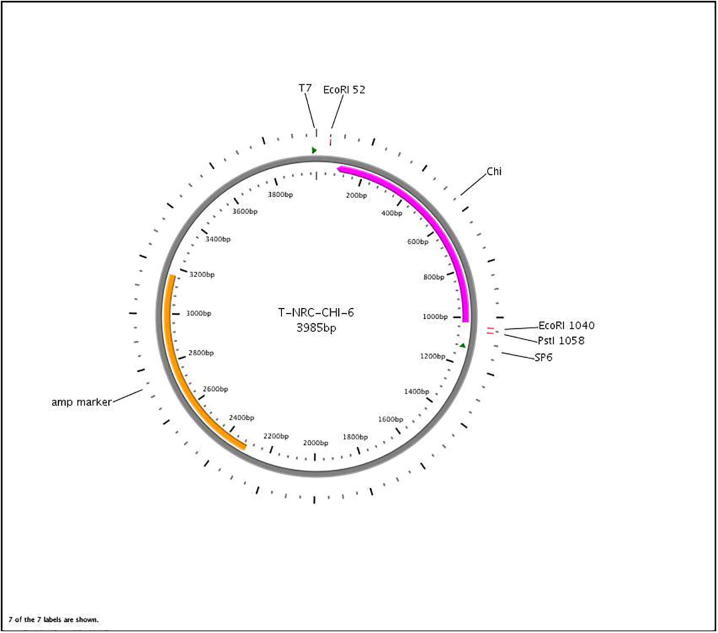
Using the complete amplicon of B. subtilis BTN7a chitinase CDS and its surrounding sequences the map of the recombinant plasmid T-NRC-CHI-6 was identified (Fig. 3). As indicated the Chi gene was under the control of SP6 promoter.
Fig. 3.
The recombinant plasmid T-NRC-CHI-6.
3.3. Effect of cell-free culture of transformed E. coli with chitinase gene on M. javanica egg hatch
Data in Table 1 illustrated the inhibition effect of cell-free culture of the two transformed E. coli with the chitinase gene (E. coli (CHI-NRC-4) and E. coli (T-CHI-NRC-6), and B. subtilis as control, on M. javanica egg hatch during three days of exposure. Based on the bioassay results, both transformants E. coli (T-CHI-NRC-6) and E. coli (CHI-NRC-4) showed about the same inhibition activity against M. javanica eggs within three days (i.e., 96% and 95%, respectively). These indicated that both of T7 promoter (i.e., E. coli (CHI-NRC-4)) and SP6 promoter (i.e., E. coli (T-CHI-NRC-6)) have the same expression efficiency on chitinase gene.
Table 1.
Inhibition effect of cultural supernatant of E. coli transformed with chitinase gene on egg hatching of M. javanica under laboratory conditions.
| Treatments | 24 h |
48 h |
72 h |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Egg | J2s | % Inhib. | Egg | J2s | % Inhib. | Egg | J2s | % Inhib. | |
| B. subtilis | 96 | 4 | 80.95 | 91 | 9 | 84.74 | 87 | 13 | 87.00 |
| CHI-NRC-4 | 99 | 1 | 95.23 | 97 | 3 | 94.91 | 95 | 5 | 95.00 |
| T-CHI-NRC-6 | 100 | 0 | 100.00 | 98 | 2 | 96.61 | 96 | 4 | 96.00 |
| Control | 79 | 21 | – | 41 | 59 | – | 0 | 100 | – |
J2s: M. javanica second stage juveniles.
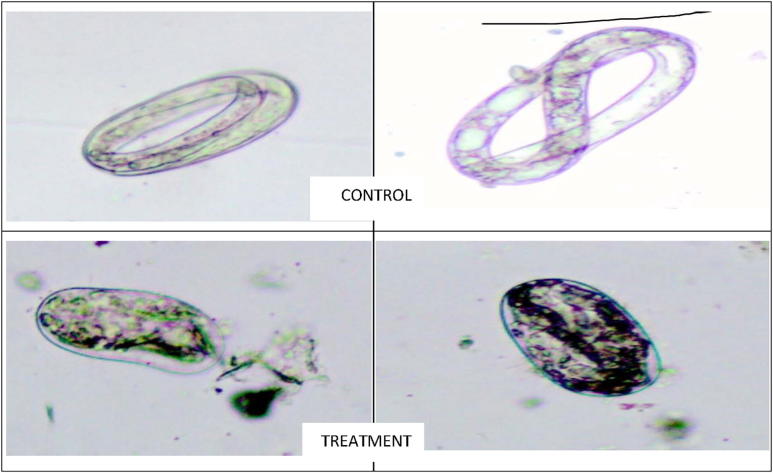
Moreover, B. subtilis resulted in 87% inhibition as compared to control. The larvae inside the egg treated with the supernatants of transformed E. coli were completely destroyed as shown in Fig. 4. These results are in agreement with [13], [7] finding that in vitro effect of fungal chitinases resulted in rupture the eggshell which confirms their role as a pathogenic factor. It was also in agreement of [20] who concluded that chitinase produced by P. illinoisensis KJA-424 caused the lysis of M. incognita eggshell and resulted in the inhibition of egg hatching in vitro.
Fig. 4.
Inhibition effect of supernatant of transformed E. coli with chitinase gene on M. javanica eggs.
3.4. Effect of cell-free culture of transformed E. coli on M. javanica reproduction under greenhouse conditions
Data in Table 2 illustrated the efficacy of the supernatants of the transformed E. coli as soil drench on M. javanica reproduction. Obtained results showed that, the transformant T-CHI-NRC-6 induced the highly reduction in M. javanica J2s in soil, numbers of root galls, eggmassess, females and developmental stages as well as eggs/eggmass by 85.46%, 64.71%, 75.18%, 77.46%, 69.47% and 18.32% reduction, respectively as compared to control. The transformant CHI-NRC-4 induced 81.95%, 57.06%, 67.15%, 68.89%, 60.35% and 12.67% reduction in the aforementioned nematode parameters, respectively. The application of the wild type B. subtilis resulted in 70.61%, 25.29%, 20.93%, 58.41%, 29.47% and 9.57% reduction in the previous nematode parameters as compared to control. Eggplant growth parameters were improved by the transformant CHI-NRC-4 than with the T-CHI-NRC-6. The increase in shoot and root length, fresh and dry shoot weight and number of leaves were 38.73%, 56.07%, 48.61%, 61.54%, and 50% increase, respectively as compared to control as shown in (Table 3).
Table 2.
Effect of cultural supernatant of transformed chitinase -E. coli on reproduction of M. javanica associated with eggplant.
| Treatments | J2s in soil | % Red. | Galls | % Red. | Egg masses | % Red. | Females | % Red. | Dev. Stage | % Red. | Eggs/Egg mass | %Red. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B. subtilis | 293c | 70.61 | 127c | 25.29 | 96b | 29.93 | 131c | 58.41 | 201a | 29.47 | 463c | 9.57 |
| CHI-NRC-4 | 180d | 81.95 | 73d | 57.06 | 45c | 67.15 | 98d | 68.89 | 113b | 60.35 | 448c | 12.67 |
| T-CHI-NRC-6 | 145e | 85.46 | 60d | 64.71 | 34c | 75.18 | 71e | 77.46 | 87b | 69.47 | 419d | 18.32 |
| Control | 997a | – | 170a | – | 137a | – | 315a | – | 285a | – | 513a | – |
J2s: M. javanica second stage juveniles.
* Means not followed by the same letter are significantly different by Duncan‘s multiple range test (P ≤ 0.05).
Table 3.
Influence of cultural supernatant of transformed chitinase -E. coli on eggplant growth parameters.
| Treatments | Shoot length/cm | % Inc. | Shoot fresh weight/g | % Inc. | Shoot dry weight/g | % Inc. | Root length/cm | % Inc. | Leaves/plant | % Inc. |
|---|---|---|---|---|---|---|---|---|---|---|
| B. subtilis | 22.3a | 28.9 | 10.6a | 47.2 | 1.8a | 38.5 | 16.5a | 54.1 | 12a | 50.0 |
| CHI-NRC-4 | 24a | 38.7 | 10.7a | 48.6 | 2.1a | 61.5 | 16.7a | 56.1 | 12a | 50.0 |
| T-CHI-NRC-6 | 23.5a | 35.8 | 10.5a | 45.8 | 1.9a | 46.2 | 14.5a | 35.5 | 11ab | 37.5 |
| Control | 17.3b | – | 7.2b | – | 1.3b | – | 10.7b | – | 8c | – |
* Means not followed by the same letter are significantly different by Duncan‘s Multiple Range Test (P ≤ 0.05).
The present results are in agreement with the findings of [3], [2] who reported that, the nematicidal activity observed with the application of Bacillus strains is related with their proteolytic activity. A chitinase-producing bacterium, causes lysis of the eggshell of M. incognita, especially in the first-stage juvenile, and results in egg hatching inhibition and/or egg-kill. The lysis of the eggshell by chitinolytic activity plays a role in the control of root-knot nematode (M. incognita). Moreover Chitin played an important role in the formation of the egg shell and cuticle of C. elegans by acting as an important barrier to protect nematodes from infection by pathogenic microorganisms [16].
Farther more, Woo-Jin et al. [20] found that, after 7 days of incubation with bacteria Paenibacillus illinoisensis known as producing chitinase none of the eggs of M. incognita hatched. Mercer et al. [14] documented that the chitinase conflict with the egg hatching of M. hapla where the eggshells of root-knot nematode might be lysed with an aberrant change in egg shape and egg rupture.
4. Conclusions
The chitinase gene cloning technique is a promising biotechnological approach to improve the potentiality of bacterial strains to control plant parasitic nematode. Genetic manipulation by cloning the desired gene could result in new biocontrol strains with increased production of toxic compounds or lytic enzymes resulted in more suppression in nematode population and enhanced plant growth parameters. Chitinase, due to its high chitin-degrading activity, has been used as a bio-control agent against phyto-pathogenic bacteria, fungi, insects, and nematodes. The over expression of this gene using the gene cloning technique will therefore cause higher levels of the enzymes production on the root surface, which lead to faster and much effective interaction and neutralization of the invading nematode.
Acknowledgment
This work was funded by a grant code number 10120603 of In-House project, National Research Centre, Giza, Egypt.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
References
- 1.Abd-Elgawad M.M.M. Egyp J Agronematol. 2008;6:33–46. [Google Scholar]
- 2.Ann Y.C. Am J Exper Agric. 2013;3:794–805. [Google Scholar]
- 3.Cronin D., Moenne-Loccoz Y., Dunne C., Gara F.O. Eur J Plant Pathol. 1998;103:433–440. [Google Scholar]
- 4.Danismazoglu M., Ismail D., Sezen K., Ratoglu H., Nalacioglu R. Turk J Biol. 2014;31:1–16. [Google Scholar]
- 5.Dashti A.A., Jadaon M.M., Abdulsamad A.M., Dashti H.M. Kuwait Med J. 2009;41(2):117–122. [Google Scholar]
- 6.Dong X., Stothard P., Forsythe I.J., Wishart D.S. Nucl Acids Res. 2004;32:w660–w664. doi: 10.1093/nar/gkh410. (web server issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gortari M.C., Hours R.A. Mycol Prog. 2008;7:221–238. [Google Scholar]
- 8.Greco N., Brandonisio A., Elia F. Nemat Medit. 1992;20:13–16. [Google Scholar]
- 9.Hussain A.A., Abdel-Salam M.S., Abo-Ghalia H.H., Hegazy W.K., Hafez S.S. JGEB. 2017;15:77–85. doi: 10.1016/j.jgeb.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hussey R.S., Barker K.R. Plant Dis Rep. 1973;57:1025–1028. [Google Scholar]
- 11.Koenning S.R., Overstreet C., Noling J.W., Donald P.A., Becker J.O., Fortnum B.A. J Nemat. 2000;31:587–618. [PMC free article] [PubMed] [Google Scholar]
- 12.Kohli R.K., Batish D.R., Singh H.P., Arora V. J Punjab Acad Sci. 1999;1:127–131. [Google Scholar]
- 13.Lee Y.S., Kim K.Y. Biotech Biotech Equip. 2015;29(3):470–478. [Google Scholar]
- 14.Mercer C.F., Greenwood D.R., Grant J.L. Nematol. 1992;38:227–236. [Google Scholar]
- 15.Ploeg A.T. Plant Dis. 2002;86:505–508. doi: 10.1094/PDIS.2002.86.5.505. [DOI] [PubMed] [Google Scholar]
- 16.Radwan M.A., Farrag S.A.A., Abu-Elamayem M.M., Ahmed N.S. Biol Fert Soils. 2011;48:463–468. [Google Scholar]
- 17.Sambrook J., Russell D.W., editors. Molecular cloning: a laboratory manual. 3rd ed. Cold Spring Harbor Laboratory Press; N.Y.: 2001. [Google Scholar]
- 18.Tikhonov V.E., Lopez-Llorca L.V., Salinas J., Jansson H.B. Fungal Genet Biol. 2002;35:67–78. doi: 10.1006/fgbi.2001.1312. [DOI] [PubMed] [Google Scholar]
- 19.Tindall D. London: ELBS and Oxford University Press; 1978. p. 711.
- 20.Woo-Jin J., Soon-Ju J., Kyu-Nam A., Yu-Lan J., Ro-Dong P., Kil-Yong K. J Microbiol Biotechnol. 2002;12:865–871. [Google Scholar]