Abstract
Breast cancer is the most common cancer in females, it accounts for one third of all malignancies affecting women. Appropriate biomarkers play significant role in predicting the prognosis and decide the specific therapy to each patient. In this study we aimed at evaluating the value of Ki-67 as a prognostic marker in breast cancer patients and to analyze the associations between Ki-67 and their clinicopathological parameters. This study included 92 patients with developed non metastatic breast cancer and 10 women had benign breast tumor served as controls. We measured the serum level by ELISA technique and tissue expression of Ki-67 by immunohistochemical technique. Our results showed that there were no statistically significant differences in serum Ki-67 levels between the two studied groups. As for Ki-67expression in breast cancer cells, the score increases with increase of tumor size, grade, premenopausal, Ki-67 expression in estrogen and progesterone receptor positive tumors showed lower values than estrogen and progesterone negative tumors, while higher Ki-67 expression was more frequently associated with HER2-positive. In conclusion; our study supports the finding that tissue Ki-67 expression may add prognostic information to that obtained from classical prognostic factors and can provide data of significant value to other important prognostic indicators such as pathological grading, and axillary lymph node involvement.
Keywords: Ki-67, Immunohistochemistry, Breast carcinoma, Tumor grade, Prognosis, Cell proliferation
1. Introduction
Breast cancer is the most common cancer in females, it accounts for one third of all malignancies affecting women, this cancer has high metastatic capacity leading to high mortality. Early detection of this disease leads to improvement of its outcome and increased survival rate [1]. The use of molecular biomarkers assures that breast cancer patients receive appropriate treatment. Biomarkers, such as estrogen receptor, progesterone receptor, HER2 play significant roles in predicting the prognosis and decide the specific therapy to each patient [2]. Although yet diagnoses and therapies are incomplete because many patients die of relapsed disease; thus, improved diagnosis using novel biomarkers is essential to improve diagnosis and treatment for breast cancer [3].
Treatment decisions are crucial step for breast cancer patients; proliferation marker Ki-67 is one of the most controversially discussed parameters. Ki-67 is a nuclear protein identified by Gerdes et al. in 1983, it was found in a Hodgkin lymphoma cell line [4]. It is associated with cellular proliferation, it is expressed in cell cycle phase S, G1, G2 and M phase in cell nucleus. Ki67 is detected by immunohistochemical technique [5]. Ki-67 expression differs throughout the cell cycle reaching peak during mitosis, it has a role in cell division and there is evidence of a function in ribosomal RNA synthesis [6]. The gene coding for Ki-67 (MKI67) is located on chromosome 10q25-ter. It was found that in normal breast tissue Ki-67 is expressed at low level in estrogen receptor negative cells [7]. The monoclonal antibody Ki-67 immunostaining can assess the growth of malignant cells; however; there is no accepted cut-off value for Ki-67 [8], [9], for this its use is limited in routine pathology [10], [11]. Nevertheless, in routine clinical work, Ki-67 is widely determined in breast cancer tissue and used as an additional factor for decision making on adjuvant treatment strategies [12].
A 2010 published review article concluded increasing evidence that Ki-67 is a valuable prognostic marker but as to its predictive role its applicability is limited [12]. No robust evidence was found that Ki-67 can serve as a tool to identify patients who will benefit from a specific chemotherapy or endocrine treatment
The aims of this work were to evaluate the value of serum and tissue expression of Ki-67 as a prognostic marker by ELISA and immunohistochemistry techniques in breast cancer patients, to analyze the associations between Ki-67 and other biomarkers in breast cancer patients and to assess the relationship of Ki-67 to histological grading.
2. Patients and methods
This study included 92 patients presented to the Outpatient Clinic in National Cancer Institute, Cairo University with primary breast carcinoma, 10 patients with benign breast tumor (pathologic diagnosis of fibroadenoma) served as positive control and 10 healthy women served as negative control, their age ranged from 33 to 62 years. Most of our cases (57) were initially diagnosed by fine needle aspiration cytology (FNAC), 22 cases by core biopsy and 13 cases by excision biopsy prior to surgery. Selected patients were subjected to breast surgery which was either radical or conservative surgery. All patients had undergone full clinical examination, routine laboratory investigations: complete blood count, liver and kidney function tests, chest X-ray, mammography, breast and abdominal ultrasonography and bone scan.
The criteria for selecting the patients were, (a) Presence of breast lump which was diagnosed as breast carcinoma (b) No systemic disease such as diabetes mellitus, hypertension, chronic inflammatory disease, liver, renal or heart failure, (c) No distant metastasis (d) No neoadjuvant therapy.
Ethical considerations; The study was conducted according to the Declaration of Helsinki and was approved by the Medical Research Ethical Committee - National research center, Cairo, Egypt (Approval No.14–031). Written informed consent was obtained from all participants before enrollment in the study.
2.1. Blood sampling
Fasting venous blood samples were collected from all participants. Within 30 min, the sera were separated by centrifugation at 3000 rpm for 10 min after a minimum time span of 30 min and serum were removed, aliquot, and stored at −80 °C until further processing.
2.2. Histopathological examination
The excised tumors and breast tissues were sent to the Pathology Department for final histopathological examination and diagnosis. Tumor tissues as well as benign breast tissues were preserved in formalin and embedded in paraffin wax. Sections were stained with routine hematoxylin-eosin staining and diagnosed according to the criteria of the World Health Organization and graded according to the modified Scarff-bloom and Richardson method.
Patient’s age, tumor type, size, grade, nodal status, estrogen receptor (ER) progesterone receptor (PR) and human epidermal growth factor 2 (Her2neu) status were reported in the histopathological reports.
2.3. Measurement of circulating Human Ki-67 level
Serum concentration of Ki-67 was measured using a commercially available immunoassay Quantikine kit purchased from (glory science co, USA) catalog number #:95075.
2.4. Tissue expression of Ki-67 in invasive breast carcinoma
The analysis of Ki-67 was done by immunohistochemical staining and the proportion of the malignant cells staining positive for the nuclear antigen Ki-67 was evaluated in a quantitative and visual way using light microscopes. The Ki-67-labeling index is the percentage of cells with Ki-67 positive nuclear immunostaining.
2.5. Statistical analysis
Data was collected in an EXCEL database (Microsoft Corporation, Redmond, WA, USA) and statistical calculations were performed with SPSS version 16. As statistical analysis revealed the data to have a skewed distribution, non-parametric tests were selected. Categorical and continuous variables were analyzed using chi square, Mann–Whitney U tests and Kruskal–Wallis tests. Continuous variables were modeled stratifying by median. All statistical analyses were based on two-tailed hypothesis tests where P < 0.05 was considered to indicate a statistically significant difference.
3. Result
In this study the clinical significance of serum and Ki-67 index as a potential marker in breast cancer among 92 breast cancer cases. Moreover, the relationships between the Ki-67 index and clinicopathological characteristic reflecting prognosis were studied.
Table 1 showed the relationship between serum level of Ki-67 and clinicopathological characteristic of breast cancer cases, while Table 2 showed serum Ki-67 concentrations in the healthy control, benign and malignant breast tumor groups; The median serum level of Ki-67 in the malignant group was 13.43 (11.78–16.32) ng/ml, which is lower than the median level of Ki-67 in benign breast disease group 14.46 (13.84–15.08). While the median serum levels of Ki-67 in healthy control was 3.92 (2.72–7.29) ng/ml. Overall, there was a statistically significant difference between healthy control group and both benign and malignant breast tumor groups (P ≤ 0.001). However, there were no statistically significant differences in serum Ki-67 (p = 0.264) levels between these groups (Fig. 1).
Table 1.
Relation between KI-67 serum level and clinic-pathological characteristic of the 92 studied cases.
| Characteristics | Cases (n) | Ki-67 Median (IQR) (ng/ml) | P value | |
|---|---|---|---|---|
| Age | <50 years | 58 | 14.22 (11.88–19.93) | 0.628 |
| ≥50 years | 34 | 13.43 (11.77–15.70) | ||
| Histology | IDC | 78 | 13.43 (11.88–16.12) | 0.321 |
| ILC | 6 | 11.78 (11.78–14.05) | ||
| Mixed IDC &ILC | 6 | 19.21 (16.63–21.49) | ||
| Mixed IDC & IPC | 2 | 12.6 | ||
| Tumor size | pT1 | 10 | 12.81 (11.78–15.70) | 0.817 |
| pT2 | 60 | 13.53 (11.78–19.83) | ||
| pT3 | 16 | 13.02 (12.60–14.98) | ||
| pT4 | 6 | 14.26 (13.74–16.12) | ||
| Grade | 1 | 2 | 11.36 | 0.457 |
| 2 | 74 | 13.43 (11.78–16.74) | ||
| 3 | 16 | 13.84 (12.19–15.60) | ||
| Lymph nodes | pN0 | 44 | 13.02 (11.78–16.74) | 0.334 |
| pN1 | 2 | 11.98 | ||
| pN2 | 30 | 13.64 (11.78_14.26) | ||
| pN3 | 16 | 16.12 (13.02–19.21) | ||
| Estrogen receptor | −ve | 25 | 14.42 (11.98–17.53) | 0.608 |
| +ve | 67 | 12.19 (11.78–14.05) | ||
| Progesterone receptor | −ve | 28 | 13.82 (11.88–16.43) | 0.959 |
| +ve | 64 | 11.32 (11.05–15.67) | ||
| HER2neu | −ve | 61 | 12.43 (12.78–16.53) | 0.933 |
| +ve | 31 | 15.53 (11.98–16.53) | ||
IQR: inter-quartile range. HER2neu: human epidermal growth factor 2.
IDC: Invasive ductal carcinoma. ILC: Invasive lobular carcinoma.
IPC: Intracystic papillary carcinoma.
Table 2.
Serum Ki-67 level (ng/ml) in different studied groups.
 |
IQR: Inter-Quartile Range.
Fig. 1.
Comparison of serum Ki-67 concentrations in the two groups. Horizontal bars indicate the median values in each group.
Table 3 showed the relationship between Ki-67 expression in breast cancer tissue and the clinicopathological features of these patients. Regarding the size of tumor, nodal status and tumor grade; Ki-67 expression showed higher values as the tumor size increases, nodal affection increases and the grade advance. Regarding, Ki-67 expression in estrogen receptor positive tumors showed lower values than estrogen negative tumors. In our study, higher Ki-67 expression was more frequently associated with HER2-positive.
Table 3.
Relationship between Ki-67 expression in breast cancer tissues and the clinicopathological features of these patients (89 patients).
| Variables/(n) | KI-67 < 20 | KI-67 > 20 | p Value |
|---|---|---|---|
| Age/years | 0.0205* | ||
| ≤50 (56) | 19 | 37 | |
| >50 (33) | 14 | 19 | |
| Tumor grade | 0.042* | ||
| G1 (2) | 2 | 0 | |
| G2 (72) | 26 | 46 | |
| G3 (15) | 5 | 10 | |
| Tumor size | 0.013* | ||
| <2 cm (10) | 10 | 0 | |
| ≥2 cm (79) | 23 | 56 | |
| Lymph node | 0.056* | ||
| −ve (44) | 23 | 21 | |
| +ve (45) | 10 | 35 | |
| Estrogen receptors | 0.016* | ||
| −ve (25) | 12 | 13 | |
| +ve (64) | 21 | 43 | |
| Progesterone receptor | 0.0295 | ||
| −ve (28) | 13 | 15 | |
| +ve (61) | 20 | 41 | |
| HER2neu | 0.023* | ||
| −ve (60) | 24 | 36 | |
| +ve (29) | 9 | 20 |
HER2neu: human epidermal growth factor 2.
chi square test: P value <0.05 significant.
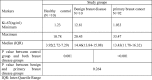
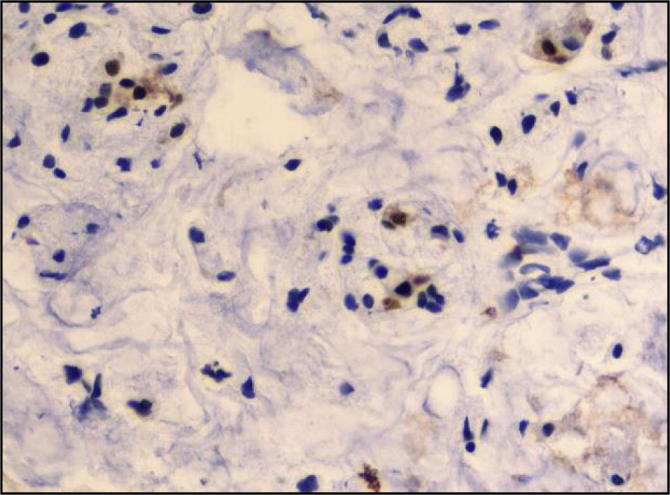
The distribution of the Ki-67 labeling index as measured by the percentage of positively stained cells is shown in Fig. 2, Fig. 3, Fig. 4, Fig. 5. Positive immunoperoxidase reaction in the Ki-67 antibody-stained sections was confined to the nuclei of carcinoma cells (Fig. 2) and was found to be expressed in 89/92 of the studied tissue samples. The proportion of Ki-67 positive cells in breast carcinomas varied from 0.8% to 70% (median, 7.5%). while 3 cases were completely negative for Ki-67 expression. Ki-67 scores counted by two independent pathologists (study Pathologists) were in good agreement. 33 cases had a value of 10–19% on the Ki-67 index (Fig. 3), 36 cases had value >20 and <50% (Fig. 4) and 20 cases had a value >50% (Fig. 5). The breast biopsy proved to be benign lesion by histopathology, there was no need to perform immunoreactivity to Ki-67 expression.
Fig. 2.
Nuclear immunohistochemical staining for Ki-67 in invasive breast carcinoma (Avidin-biotin-peroxidase complex (ABC) immunoperoxidase + Diaminobenzidine (DAB) chromogen × 400).
Fig. 3.

Invasive duct carcinoma, tumor cells showing expression of value of 10–19% on the Ki-67 index (ABC immunoperoxidase + DAB chromogen × 400).
Fig. 4.

Nuclear staining for Ki-67 with value >20 and <50% in a case of invasive duct carcinoma (ABC immunoperoxidase + DAB chromogen × 400).
Fig. 5.
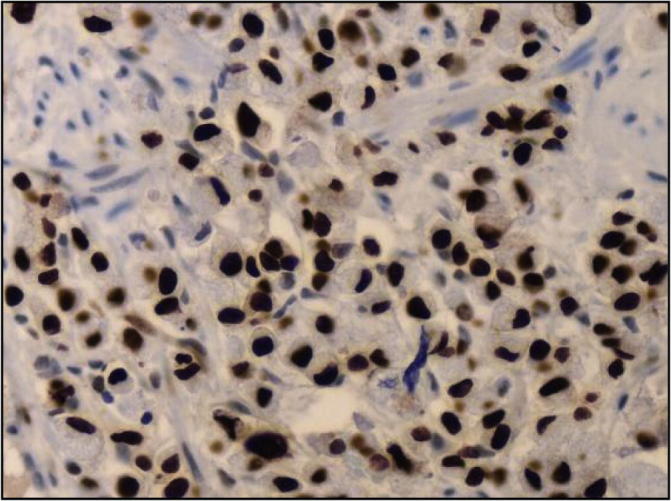
High score of nuclear staining (> 50%) for Ki-67 in a premenopausal female with invasive duct carcinoma (ABC immunoperoxidase + DAB chromogen × 400).
4. Discussion
Breast cancer is the most common female cancer worldwide and the second leading cause of cancer-related death, thus it is important to find good prognostic markers that can define patients who are at high risk of recurrence and choosing the suitable therapy for them [13]. Prognostic markers such tumor size, grade, age, histological type and estrogen receptor status influence the therapy decision. Cell proliferation is one of the most essential characteristics of cancer; thus, its measurement may provide useful information about disease status [14].
In this study we evaluated the serum level and the immunohistochemical expression of Ki-67 in patients with breast cancer
There were no statistically significant differences in serum Ki-67 levels between the studied groups, up to our knowledge no researchers discuss its value in breast cancer prognosis. Ki-67 expression in breast cancer cells, the score increases with increase of tumor size, grade, premonopausal, Progesterone receptor (PR) negative, lymph node positive and estrogen negative tumors. These results were in agreement with Fausto et al. [15] who stated that high immunohistochemical expression Ki-67 level is associated with greater risk of recurrence (64 % increased risk) and that the proliferative marker Ki-67 has an independent prognostic value in terms of survival and relapse in patients with early-stage breast cancer (BC), and should be routinely assessed by pathologists.
In accordance with our results, other studies found that a higher Ki-67 index significantly correlated with increasing tumor size [16], [17], [18], +ve lymph nodes [16], [19]. A study included 194 women diagnosed as breast cancer reported significant correlation between Ki-67 and tumor size, tumor grade, PR, and lymph node status. another study reported that there was no correlation with ER positivity and tumor size [20]. A number of studies reported that among classical histopathological parameters, grading was strongly correlated to Ki-67-labeling indices [17], [21], [22]. Other studies showed that ER status has been largely identified as being inversely correlated with Ki-67, with the higher rates of ER positivity shown in the lowest proliferating tumors [16], [23], [24], [21].
Spyratos et al. [21] stated that cell proliferation is a major determinant of biological characteristic of breast cancer, they found that there is a strong correlation between the Ki-67 status and histological grade of cancer. Inwald et al. [17] declared that Ki-67 showed an association with the common histological parameters and there was close correlation between it and the tumor grade as these parameters has a close association with proliferation.
In our study, higher Ki-67 expression was more frequently associated with HER2-negative. This results was in agreement with study done by Stathopoulos et al., who reported HER2-negative had significantly higher Ki-67 values [24]. Contrary to our result, other studies founded that a higher Ki-67 index significantly correlated with HER2-positive, [16], [17], [20].
Although the prognostic role of Ki-67 is a matter of debate, several authors have also demonstrated the value of Ki-67 for predicting the benefit of adjuvant therapy in high-risk luminal B-like and node-negative patients [25], [26]. Penault-Llorca [27] showed that patients whose tumors had Ki-67 levels >20% benefit from the addition of docetaxel, thus it is useful for detecting therapy choices. A study reported that in univariate analyses, high Ki-67-labeling index was associated with vascular invasion [28]. Furthermore, other studies showed that vascular and lymphatic invasion associated with higher Ki-67 values [17], [29]. In a number of studies, Ki-67 has been divided based on ≥10% and <10% and other studies based on ≥20% and <20% such as our study, and even a few studies based on other numbers. For a good result about correlation between Ki-67 with other factors in BC, it needs that the researchers do the studies based on a constant and accurate number.
Nishimura et al. [28] demonstrated that high Ki-67 in primary tumors, irrespective of high or low Ki-67 in recurrent tumors, was significantly correlated with a lower survival rate, although, Ibrahim et al. [29] reported that patients with high Ki-67 in recurrent tumors showed significantly lower survival rates, irrespective of high or low Ki-67 in primary tumors. Also, in a meta-analysis involving 12,155 patients showed that Ki-67 positivity denotes a higher risk of recurrence and worse prognosis in early breast cancer patients [12]. Another study investigated the relationship between proliferation markers and patient survival confirmed that high Ki-67 is associated with worse survival rate [30].
These results were also in accordance with Nahed & Shiamaa 2016 [31], who found a high Ki-67 index (≥ 15%) was significantly correlated with adverse prognostic factors. High Ki-67 index (≥ 15%) was significantly correlated with ER−/PR−. High Ki-67 index (≥ 15%) is significantly correlated with high tumor grade.
5. Conclusion
Our study showed that serum Ki-67 levels had no clinical significant in the patients with cancer breast, also there is no significant between serum levels of Ki-67 in benign and malignant tumors.the results of present study support the finding that tissue Ki-67 expression may be considered a valuable potential biomarker and add a prognostic information to that obtained from classical prognostic factors such as pathological grading, tumor size, and lymph node involvement. our recommendation is to focus on standardization of Ki-67 assessment to avoid any contradictory results in Ki-67.
Acknowledgments
Acknowledgements
This work was financially supported by the National Research Centre, Cairo, Egypt.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Taneja P., Maglic D., Kai F. Classical and novel prognostic markers for breast cancer and their clinical signi cance. Clin Med Oncol. 2010;4:15–34. doi: 10.4137/cmo.s4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kittaneh M., Montero A.J., Glück S. Molecular pro ling for breast cancer: a com- prehensive review. Biomark Cancer. 2013;5:61–70. doi: 10.4137/BIC.S9455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerdes J., Schwab U., Lemke H., Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31(1):13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- 5.Gerdes J., Lemke H., Baisch H., Wacker H.H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133(4):1710–1715. [PubMed] [Google Scholar]
- 6.Scholzen T., Gerdes J. The Ki-67protein: from the known and the unknown. J Cell Physiol. 2000;182(3):311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Urruticoechea A., Smith I.E., Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol. 2005;23:7212–7220. doi: 10.1200/JCO.2005.07.501. [DOI] [PubMed] [Google Scholar]
- 8.Yerushalmi R., Woods R., Ravdin P.M., Hayes M.M., Gelmon K.A. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11:174–183. doi: 10.1016/S1470-2045(09)70262-1. [DOI] [PubMed] [Google Scholar]
- 9.Luporsi E., Andre ´ F., Spyratos F., Martin P.M., Jacquemier J., Penault-Llorca F. Ki-67: level of evidence and method- ological considerations for its role in the clinical management of breast cancer: analytical and critical review. Breast Cancer Res Treat. 2012;132(3):895–915. doi: 10.1007/s10549-011-1837-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gnant M, Harbeck N, Thomssen C, 2011. St. Gallen Summary of the Consensus Discussion. Breast Care (Basel) 6(2):136–141. [DOI] [PMC free article] [PubMed]
- 11.Untch M., Gerber B., Möbus V., Schneeweiss A., Thomssen C., Beckmann M.W. Zurich Consensus: Statement of German Experts on St. Gallen Conference 2011 on Primary Breast Cancer (Zurich 2011) Breast Care. 2011;6:144–152. [Google Scholar]
- 12.Yerushalmi R., Woods R., Ravdin P.M., Hayes M.M., Gelmon K.A. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11:174–183. doi: 10.1016/S1470-2045(09)70262-1. [DOI] [PubMed] [Google Scholar]
- 13.de Azambuja E., Cardoso F., de Castro G., Colozza M., Jr, Mano M.S., Durbecq V., Sotiriou C., Larsimont D., Piccart-Gebhart M.J., Paesmans M. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer. 2007;96(10):1504–1513. doi: 10.1038/sj.bjc.6603756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cianfrocca M., Goldstein L.J. Prognostic and predictive factors in early-stage breast cancer. Oncologist. 2004;9(6):606–616. doi: 10.1634/theoncologist.9-6-606. [DOI] [PubMed] [Google Scholar]
- 15.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Fausto P., Viale G., Cabiddu M., Barni S. Prognostic value of different cut-off levels of Ki-67 in breast cancer: a systematic review and meta-analysis of 64,196 patients. Breast Cancer Res Treat. 2015;153:477–491. doi: 10.1007/s10549-015-3559-0. [DOI] [PubMed] [Google Scholar]
- 17.Nishimura R, Osako T, Okumura Y, Hayashi M, Toyozumi Y, Arima N. Ki-67:2010. As a prognostic marker according to breast cancer subtype and a predictor of recurrence time in primary breast cancer. Exp Ther Med; 1:747–54. [DOI] [PMC free article] [PubMed]
- 18.Inwald E.C., Klinkhammer-Schalke M., Hofstädter F., Zeman F., Koller M., Gerstenhauer M. Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat. 2013;139:539–552. doi: 10.1007/s10549-013-2560-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.González-Sistal A., Sánchez A.B., Del Rio M.C., Arias J.I., Herranz M., Ruibal A. Association between tumor size and immunohistochemical expression of Ki-67, p53 and BCL2 in a node-negative breast cancer population selected from a breast cancer screening program. Anticancer Res. 2014;34:269–273. [PubMed] [Google Scholar]
- 20.Li F.Y., Wu S.G., Zhou J., Sun J.Y., Lin Q., Lin H.X. Prognostic value of Ki-67 in breast cancer patients with positive axillary lymph nodes: a retrospective cohort study. PLoS One. 2014;9:e87264. doi: 10.1371/journal.pone.0087264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haroon S., Hashmi A.A., Khurshid A., Kanpurwala M.A., Mujtuba S., Malik B. Ki67 index in breast cancer: correlation with other prognostic markers and potential in pakistani patients. Asian Pac J Cancer Prev. 2013;14:4353–4358. doi: 10.7314/apjcp.2013.14.7.4353. [DOI] [PubMed] [Google Scholar]
- 22.Spyratos F., Ferrero-Poüs M., Trassard M., Hacène K., Phillips E., Tubiana-Hulin M. Correlation between MIB-1 and other proliferation markers: clinical implications of the MIB-1 cutoff value. Cancer. 2002;94:2151–2159. doi: 10.1002/cncr.10458. [DOI] [PubMed] [Google Scholar]
- 23.Trihia H., Murray S., Price K., Gelber R.D., Golouh R., Goldhirsch A. Ki-67 expression in breast carcinoma: Its association with grading systems, clinical parameters, and other prognostic factors — A surrogate marker? Cancer. 2003;97:1321–1331. doi: 10.1002/cncr.11188. [DOI] [PubMed] [Google Scholar]
- 24.Kontzoglou K., Palla V., Karaolanis G., Karaiskos I., Alexiou I., Pateras I. Correlation between Ki67 and breast cancer prognosis. Oncology. 2013;84:219–225. doi: 10.1159/000346475. [DOI] [PubMed] [Google Scholar]
- 25.Stathopoulos G.P., Malamos N.A., Markopoulos C., Polychronis A., Armakolas A., Rigatos S. The role of Ki-67 in the proliferation and prognosis of breast cancer molecular classification subtypes. Anticancer Drugs. 2014;25:950–957. doi: 10.1097/CAD.0000000000000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polley M.Y., Leung S.C., Gao D. An international study to increase concordance in Ki67 scoring. Mod Pathol. 2015;28(6):778–786. doi: 10.1038/modpathol.2015.38. [DOI] [PubMed] [Google Scholar]
- 27.Criscitiello C., Disalvatore D., De Laurentiis M. High Ki- 67 score is indicative of a greater benefit from adjuvant chemotherapy when added to endocrine therapy in luminal B HER2 negative and node-positive breast cancer. Breast. 2014;23(1):69–75. doi: 10.1016/j.breast.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Penault-Llorca F., Andre ´ F., Sagan C. Ki67 expression and docetaxel efficacy in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27(17):2809–2815. doi: 10.1200/JCO.2008.18.2808. [DOI] [PubMed] [Google Scholar]
- 29.Nishimura R., Osako T., Nishiyama Y., Tashima R., Nakano M., Fujisue M. Prognostic significance of Ki-67 index value at the primary breast tumor in recurrent breast cancer. Mol Clin Oncol. 2014;2:1062–1068. doi: 10.3892/mco.2014.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibrahim T., Farolfi A., Scarpi E. Hormonal receptor, human epidermal growth factor receptor-2, and Ki67 discordance between primary breast cancer and paired metastases: clinical impact. Oncology. 2013;84:150–157. doi: 10.1159/000345795. [DOI] [PubMed] [Google Scholar]
- 31.Stuart-Harris R., Caldas C., Pinder S.E., Pharoah P. Proliferation markers and survival in early breast cancer: a systematic review and meta-analysis of 85 studies in 32,825 patients. Breast. 2008;17:323–334. doi: 10.1016/j.breast.2008.02.002. [DOI] [PubMed] [Google Scholar]



