Abstract
Marine environments are substantially untapped source for the isolation of bacteria with the capacity to produce various extracellular hydrolytic enzymes, which have important ecological roles and promising biotechnological applications. Hydrolases constitute a class of enzymes widely distributed in nature from bacteria to higher eukaryotes. Marine microbial communities are highly diverse and have evolved during extended evolutionary processes of physiological adaptations under the influence of a variety of ecological conditions and selection pressures. A number of marine hydrolases have been described, including amylases, lipases and proteases, which are being used extensively for biotechnological applications. The present study was carried out to isolate marine bacteria from continental slope sediments of the eastern Arabian Sea and explore their biotechnological potential. Among the 119 isolates screened, producers of amylases (15%), caseinases (40%), cellulases (40%), gelatinases (60%), lipases (26%), ligninases (33%), phytase (11%) and Malachite Green dye degraders (16%) were detected. Phylogenetic analysis based on 16S rRNA gene sequencing showed that predominant marine sediment bacteria possessing more than four enzymatic activities belonged to the phyla Firmicutes and Proteobacteria, was assigned to the genera Bacillus, Planococcus, Staphylococcus, Chryseomicrobium, Exiguobacterium and Halomonas. Biodegradation of the dye Malachite Green using the liquid decolorization assay showed that both the individual cultures (Bacillus vietnamensis, Planococcus maritimus and Bacillus pumilus) and their consortium were able to decolorize more than 70% of dye within 24 h of incubation. This is the first report on diversity and extracellular hydrolytic enzymatic activities and bioremediation properties of bacteria from continental slope sediment of eastern Arabian Sea.
Keywords: Arabian Sea, Continental slope, Marine bacteria, Extracellular hydrolytic enzymes, Malachite green dye degradation
1. Introduction
Marine environments are substantially untapped source for the isolation of bacteria with the capacity to produce various extracellular hydrolytic enzymes, which have important ecological roles and promising biotechnological applications. Since marine bacteria are adapted to extreme conditions like high salinity, low temperature and high pressure, the enzymes isolated from them have shown unique characteristics compared to the enzymes from terrestrial counterparts. Marine bacteria are capable of producing a wide spectrum of hydrolytic enzymes such as cellulase, amylase [1], protease [2], phytase [3], lipase and ligninase. Extracellular hydrolytic enzymes such as amylases, proteases, lipases, DNases, have quite diverse potential usages in different areas such as food industry, feed additive, biomedical sciences and chemical industries. Marine microbial enzymes also have potential to degrade large organic contaminants and hence showed bioremediation property. Because of the cost effectiveness and better activity, enzymes from microbial origin have gained much attention in industrial field. Such enzymes are thermostable, tolerant to a varied range of pH and other harsh conditions required in industrial applications. Novelty in their structure and characteristics has shown promising scope to the researchers in academia and industry [4]. However, there are very few reports on the application of marine microorganisms for isolation of enzymes.
Arabian Sea is one of the most productive regions of the world oceans and an area of intense scientific interest for the past several years [5]. The continental slope of Arabian Sea is largely unexplored, and may provide a rich source of the potential bacteria capable of producing extracellular hydrolytic enzymes. There are a number of studies reporting marine bacteria capable of producing various extracellular enzymes from different areas of Arabian Sea [6], [7], [8]. Yet, reports are scanty on marine bacteria isolated from sediments of the continental slope of the eastern Arabian Sea. Keeping in view the industrial importance of the extracellular enzymes, the present study was designed to study the diversity of culturable bacteria from various depths of the continental slope of the eastern Arabian Sea and evaluate their potential for producing extracellular hydrolytic enzymes.
2. Materials and methods
2.1. Isolation of bacteria
A total of 5 marine sediment samples were collected at various depths (250 m–1000 m) from the continental slope of eastern Arabian Sea (Table 1 and Fig. 1). The sediment samples were collected using a Van-Veen Grab. The samples were properly labeled and placed in sterile plastic bags. The sediment samples were transported to the laboratory and stored at 4 °C until microbial isolation. Sediment samples (1 g) were serially diluted and plated on to sterile nutrient agar prepared with sea water by spread plate technique. The plates were incubated at 37 °C for 5 days and checked periodically for the growth of bacteria. Bacterial counts were represented as CFU/g for each sediment sample. Colonies of different morphology were selected and were re-streaked on sterile agar plates to ensure purity. Pure cultures were maintained as glycerol stock at −80 °C. The bacterial isolates were stained by Gram’s reaction, visualized under oil immersion objective and the microscopic morphology was recorded.
Table 1.
Details of longitude and latitude of the sampling stations.
| No | Transect | Depth | Location |
|---|---|---|---|
| 1 | Trivandrum | 1000 | 08.32 N; 75.59 E |
| 2 | Trivandrum | 500 | 08.30 N; 76.16 E |
| 3 | Kollam | 400 | 09.00 N; 75.28 E |
| 4 | Kochi | 500 | 09.56 N; 75.32 E |
| 5 | Kannur | 1000 | 12.82 N; 73.86 E |
Fig. 1.
Map of the study area showing different sampling stations.
2.2. Screening for extracellular enzyme activities
The screening of extracellular hydrolytic enzymes producing bacteria was done by spot inoculation method on Basal Mineral Agar (BSA) medium [KH2PO4-0.1%, (NH4)2SO4-0.5%, MgSO4·7H2O-0.01%, NaCl-0.01%, and agar 2.0%] supplemented with specific substrates. Inoculated plates were incubated for 2–5 days and the enzyme activity was indicated by zone of clearance around the colony. Amylase activity was detected in BSA agar supplemented with 1% soluble starch followed by addition of 1% iodine solution after 2 days of incubation, allowing the visualization of clear halos around the colonies. The lipase activity was determined by the appearance of calcium salt crystals of the lauric acid around the colonies when bacteria were grown in BSA containing 1% Tween 80 (v/v). The cellulolytic activity of the isolates was evaluated by growing bacteria on 1% carboxymethylcellulose (CMC) agar plates. The plates were flooded with 0.1% congo red solution and incubated at room temperature for 30 min. The plates were washed twice with 5 M NaCl for 30 min. The presence of orange/colorless halo around the colony was indicative of cellulase activity. The activity of phytase was determined by growing bacteria on Sodium phytate containing medium followed by the appearance of clear zone around the colony. To determine proteolytic activity, skim milk was used as substrate while, gelatin was used as substrate to detect gelatinase activity. Detecting their ability to decolorize methylene blue, a synthetic lignin-like dye, assessed the ligninolytic potential of the bacterial strains. The bacterial isolates were tested for their capability to degrade Malachite Green, a recalcitrant triphenylmethane dye, by growing them in BSA media containing 0.01% (w/v) Malachite Green.
2.3. Malachite Green dye liquid decolorization assay
Decolorization experiments were conducted in 250 ml Erlenmeyer flask containing 100 ml of mineral broth (g/L) [ammonium sulfate 5 g, potassium dihydrogen phosphate 1 g, dipotassium hydrogen phosphate 2 g, magnesium sulfate 0.5 g, sodium chloride 0.1 g, manganese chloride 0.01 g, ferrous sulfate 0.01 g, sodium molybdate 0.01 g, at pH 7.2] supplemented with 100 mg/L Malachite Green dye. The decolorization efficiency of individual bacterial cultures (Planococcus maritimus, Bacillus vietnamensis and Bacillus pumilus) and equal amounts of each three cultures (5 ml of each; 108 cells/mL) as bacterial consortium was investigated by incubating the flasks at 37 °C on a shaker with 150 r.p.m up to 24 h. After incubation, 2 ml of culture filtrate from each was drawn and centrifuged at 10000 rpm for 15 min at 4 °C. After centrifugation, the supernatant was collected and the absorbance was measured at 620 nm using a spectrophotometer. The percentage decolorization was calculated using the following formula
Where
Initial absorbance-absorbance at 0 h of incubation.
Final absorbance-absorbance at incubation on time t.
2.4. Molecular identification of bacterial isolates
Bacteria isolated from sediments samples were identified by partial sequencing of 16S rDNA. The genomic DNA was extracted from bacterial strains using GenElute Bacterial Genomic DNA kit (Sigma-Aldrich) in accordance with the manufacturer’s instructions. The 16S rDNA gene was amplified from the extracted DNA using universal bacterial primers 27F (5′-AGAGTTTGA TCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′). The PCR amplification conditions were as follows; initial denaturation at 95 °C for 2 min followed by 30 cycles of 95 °C for 1 min, 58 °C for 30 s, 72 °C for 1 min and final extension at 72 °C for 7 min. The PCR products were sequenced by Sanger’s method (Scigenome pvt. Labs).
Sequence data were edited using BioEdit Sequence Alignment Editor (Version 7.2.6.1) and analyses were performed using BLAST (NCBI) search to find the closest with the closest relatives of strains. All the sequences of 16S rDNA were aligned using the multiple sequence alignment program CLUSTAL-X. Phylogenetic tree was constructed using MEGA software version 7 using Kimura-2 parameter model and neighbor joining method. All of the bacterial sequences were submitted to GenBank the under the accession numbers MF523634- MF523649, MF574279.
3. Results
3.1. Isolation and identification of marine sediment bacteria
In the present study, bacterial population isolated from marine sediments collected from 5 different locations of the continental slope of eastern Arabian Sea showed a range of 60–120 × 105 CFU/g. A total of 119 bacterial isolates showing different morphological characteristics were identified and designated as MSB1- MSB119.
3.2. Extracellular enzymatic activities of bacterial isolates
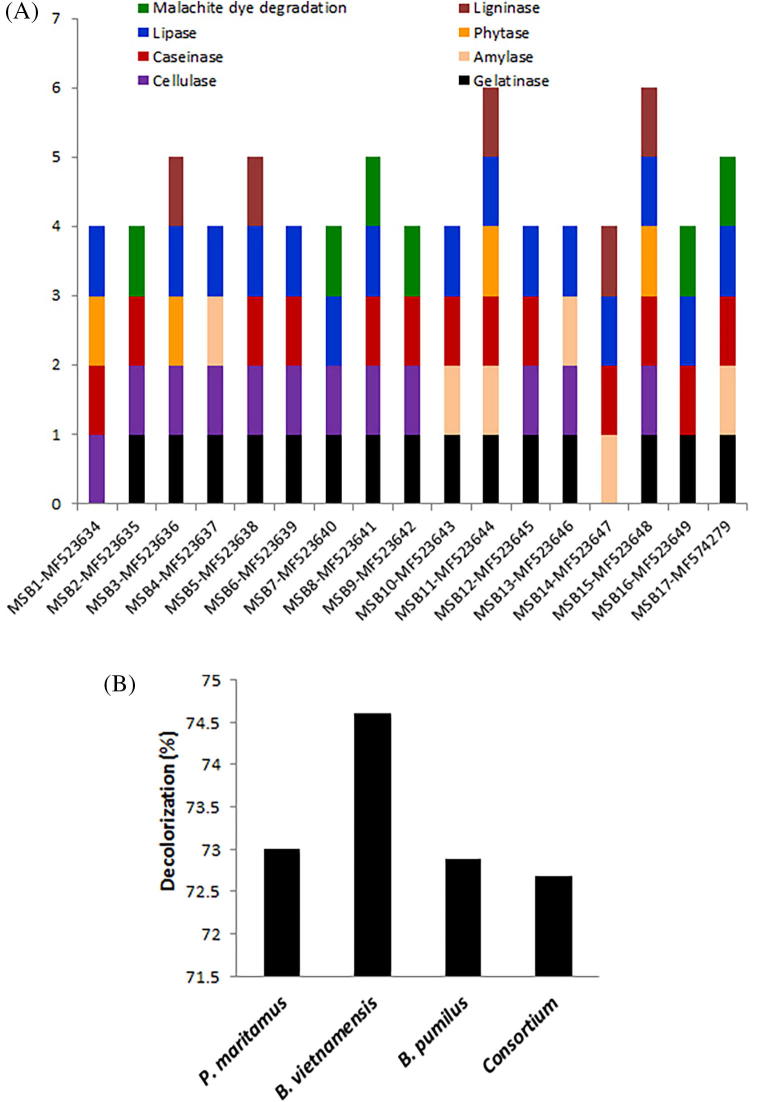
Out of 119 strains 60% of bacterial strains were able to produce gelatinase enzymes while 40% exhibited cellulolytic activity. In preliminary screening of lipase assay, 26% of bacterial strains were lipolytic on Tween 80 agar plates. Among the isolated bacteria, 15% were shown to produce amylase enzyme activities and 40% showed caseinase activity on skim milk agar plate. Out of 119 isolates screened, 14 strains showed positive activity for phytase and 40 strains showed ligninase activity. Out of 119 bacterial strains, 17 showed more than four enzymatic activities (Fig. 2A) and were selected for molecular identification. It is interesting to note that five enzyme activities were detected in MSB11 and MSB15 isolates. However, none of the isolates was able to produce all the tested activities.
Fig. 2.
(A) Extracellular enzyme profile of 17 bacterial isolates (B) % decolorization of Malachite Green at 100 mg/L concentration. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Molecular identification of bacterial strains
Phylogenetic analysis of 17 bacterial isolates which showed more than four enzymatic activities were determined by sequencing 16S rDNA gene sequences (Fig. 3). The majority of the isolates was assigned to the phylum Firmicutes and included in the genera Bacillus (eight), Planococcus (two), Staphylococcus (two), Exiguobacterium (two) and Chryseomicrobium (one). Only two strains were belonged to Phylum Proteobacteria and included in the genus Halomonas. Only one strain in genus Halomonas showed 98% 16S rDNA similarity with known species, and might be a novel species. Only two strains belong to genus Staphylococcus and genus Bacillus showed 100% 16S rDNA similarity with known species.
Fig. 3.
Phylogenetic neighbour-joining tree based on 16S rRNA gene sequences, showing the diversity of marine sediment bacteria.
3.4. Decolorization of Malachite Green by marine bacteria
Of all the 119 strains, 16% isolates showed positive results for Malachite Green dye degradation by plate assay technique. The three bacterial strains (Planococcus maritimus, Bacillus vietnamensis and Bacillus pumilus) showing the highest decolorization activity in plate assay were further tested by liquid decolorization assay. The liquid decolorization assay showed that both the individual cultures and its consortium were able to decolorize more than 70% of Malachite Green dye within 24 h of incubation. Bacillus vietnamensis showed highest decolorization of Malachite Green (74.6%) followed by Planococcus maritimus (73%). The decolorization rate of the bacterial consortium (72.8%) was similar to that of individual culture of Bacillus pumilus (72.8%) (Fig. 2B).
4. Discussion
Microbial communities play a key role in degradation process of a large fraction of the organic matter in the ocean [9]. Observations of high degree of microbial diversity in the Arabian Sea [10], [11] led to the hypothesis that the eastern continental slope of Arabian Sea is an enormous source of microbial flora. Microbial diversity of the eastern Arabian Sea has been previously reported [8], [12], [13]. Our study reports the bacterial diversity from the sediments of eastern continental slope of Arabian Sea and their enzyme-producing potential. Furthermore, sediments from continental slope showed more bacterial diversity than the oxygen minimum zone of the eastern Arabian Sea as previously reported [7].
Bacteria from marine environment are well-known producers of extracellular hydrolytic enzymes [14]. With this background, the present study reports the extracellular enzyme profile of 119 bacterial strains including amylase, caseinase, cellulase, gelatinase, lipase, ligninase and phytase. They were also screened for Malachite Green dye degradation potential. All the 119 bacterial isolates produced at least one enzymatic activity, with gelatinase being the most frequently detected. Among the 119 bacterial isolates, 17 strains were able to produce more than four hydrolytic enzymes suggesting that almost 14% of the isolated bacterial strains possessed potent hydrolytic enzymes. The identification of these bacterial strains were done using 16S rDNA, a powerful tool for deducing phylogenetic and evolutionary relationships among bacteria.
Bacillus was found to be the dominant genera as previously reported [15] followed by Planococcus [16], Staphylococcus [17], Exiguobacterium, Halomonas and Chryseomicrobium [18] as observed in this investigation. Staphylococcus saprophyticus isolated in this study has been previously identified from marine environment [17]. One of the isolated Halomonas sp. is considered to be a novel species, due to it's relatively low sharing of <98% 16S rRNA gene sequence similarity to its closest type strain. In accordance with the present study, Arora et al. [18] have reported the isolation and molecular characterization of Chryseomicrobium imtechense from seawater of the Bay of Bengal. Bacteria that shared 99% 16S rDNA gene sequence identity differed in their bioactivity profiles may be considered to be different strains [19]. In this study also closely related strains showed different enzymatic profile. Planococcus maritimus (MSB2 and MSB16), Bacillus pumilus (MSB6 and MSB8) and Bacillus vietnamensis (MSB10 and MSB17) have shown different enzymatic activities.
Cellulase producing Bacillus sp. was reported earlier [20]. Interestingly, it was found that B. aerius (MSB 5) and B. pumilus (MSB6) were highly potential with cellulase activity. B. pumilus (MSB6) showed highest amylase activity with maximum zone of clearance. B. aryabhattai (MSB1) showed efficient phytate degrading activity with a maximum zone of clearance around the colony, supporting the observations made by Pal Roy et al. [21]. Among Gram-negative bacterial isolates from hypersaline environment, most of the hydrolytic enzyme producers were belonged to the genus Halomonas [22]. There are reports that Halomonas sp. and Staphylococcus sp. are able to produce amylases, gelatinase, cellulase, and lipase [23]. Similarly, Halomonas and Staphylococcus genera were found to be potent extracellular hydrolytic enzyme producers. The caseinase and gelatinase activities of B. vietnamensis were previously reported [24]. In this study, amylase and ligninase enzymatic activities were also detected in B. vietnamensis, followed by caseinase and gelatinase activities. This study reported Exiguobacterium sp. as the producers of hydrolytic enzymes, in agreement with the study of Vishnivetskaya et al. [25], according to which most of the hydrolytic enzymes were identified from Exiguobacterium strains including esterases and alkaline proteases. Chryseomicrobium imtechense was found as the highest protease producer, in accordance with another study by Arora et al. [18].
Bioremediation by microbes for the degradation of xenobiotics seems a green solution to the problem of environmental pollution [26]. Malachite Green, a triphenylmethane dye is widely used in textile and paper industries. Because of their recalcitrant nature, the degradation of dyes is very difficult and has received considerable attention. Therefore, several studies were conducted to decolorize dyes from the environment using marine bacterial isolates [27]. B. pumilus and B. vietnamensis can effectively degrade Malachite Green dye supporting the similar observations made earlier where marine Bacillus species detected as effective tool for bioremediation of textile dyes [28]. In the present investigation, Planococcus, Staphylococcus and Chryseomicrobium sp. also significantly degrade Malachite Green dye incorporated in the culture medium. The result of biodegradation of Malachite Green dye using liquid culture assay showed that 70% of Malachite Green is very effectively decolorized by individual strains of B. vietnamensis, Planococcus maritimus and B. pumilus and within 24 h. Moreover, the consortium of the above three strains can be used for dye degradation.
5. Conclusion
The study suggested that the marine environment could be used as a promising source for bacteria with extracellular enzymatic activities. The study provides the possibility of using marine bacteria as a source of commercial enzymes to be applied in the industrial field. B. vietnamensis, P. maritimus and B. pumilus can be considered as promising bacterial strains for bioremediation of Malachite Green. Further, the metagenome will be isolated and analyzed from the sediment sample to find out the enzymatic potential of non-culturable bacteria as well to get a wider view of the biotechnological potential of marine bacteria.
5.1. Strengths and weaknesses of the article
The major strength of the article is the exotic nature of microorganisms itself. They are isolated from continental shelf sediments of a relatively under explored region of the Arabian Sea, depths ranging from 250 m to 1000 m. The pressure at these regions range from 40 to 100 times that of normal atmospheric pressure and the temperature is below 15 °C. This means the barophilic and psychrotrophic nature of the bacterial isolates mentioned in this paper may have very unique physiological capabilities, though we have not explored that at this point of time. However, we have plans to extend the work to detailed analysis. Bioremediation potential, especially dye removal efficiency, of selected isolates seems to be remarkable and could be exploited in bioremediation systems. The weakness of the paper is that the diversity we have reported is based on culture based techniques. However, metagenomic analyses could have revealed much higher diversity. Since our primary interest was on culturable organisms that could be exploited straight away in application mode we have looked at the culture based diversity. However, this is a first report on diversity and biotechnological potential of bacteria from the continental slope of eastern Arabian Sea.
Acknowledgments
Acknowledgement
The first author is grateful to Science and Engineering Research Board (SERB)-National Postdoctoral Fellowship grant (SERB PDF FILE NUMBER:PDF/2015/000554), Government of India, for financial support.
Conflict of interest
None declared.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
Contributor Information
Arakkaveettil Kabeer Farha, Email: https://orcid.org/0000-0001-7270-1461, farha.ak@gmail.com.
Abdulla Mohamed Hatha, Email: mohamedhatha@cusat.ac.in.
References
- 1.Aygan A., Arikan B., Korkmaz H., Dinçer S. Braz J Microbiol. 2008;39:547–553. doi: 10.1590/S1517-838220080003000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fulzele R., DeSa E., Yadav A., Shouche Y. Braz J Microbiol. 2011;42:1364–1373. doi: 10.1590/S1517-838220110004000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uzair B., Ahmed N. J Basic Appl Sci. 2007;3:19–24. [Google Scholar]
- 4.Rao T.E., Imchen M., Kumavath R. Adv Food Nutr Res. 2017;80:149–163. doi: 10.1016/bs.afnr.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Smith S, Banse K, Cochran J, Codispoti L. In: US JGOFS planning report No.13, Woods Hole Oceanographic Institution, Woods Hole, Massachusetts; 1991. p. 164.
- 6.Tallur P.N., Sajjan D.B., Mulla S.I., Talwar M.P. 3 Biotech. 2016;6:28. doi: 10.1007/s13205-015-0332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Divya B., Soumya K.V., Nair S. Antonie Leeuwenhoek. 2010;98:9–18. doi: 10.1007/s10482-010-9423-7. [DOI] [PubMed] [Google Scholar]
- 8.Anas A., Nilayangod C., Jasmin C., Vinothkumar C.S. 3 Biotech. 2016;6:238. doi: 10.1007/s13205-016-0556-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belanger C., Desrosiers B., Lee B.K. Aquat Microb Ecol. 1997;13:187–196. [Google Scholar]
- 10.Boetius A., Ferdelman G., Timothy G., Karin L. Deep sea res part II: topical studies. Oceanography. 2000;47:2835–2875. [Google Scholar]
- 11.Augustine D., Jacob J.C., Remya K.D., Philip R. Int J Res Marine Sci. 2013;2:56–63. [Google Scholar]
- 12.Divya B., Parvathi A., LokaBharathi A. World J Microbiol Biotechnol. 2011;27:2821. [Google Scholar]
- 13.Sinimol S., Sarika A.R., Jayakumaran Nair A. 3 Biotech. 2016;6:1–7. doi: 10.1007/s13205-015-0318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fenice M., Gallo A.M., Juarez-Jimenez B., Gonzalez-Lopez J. Ann Microbiol. 2007;57:1–93. [Google Scholar]
- 15.Mondol M.A., Shin H.J., Islam M.T. Mar Drugs. 2013;11:2846–2872. doi: 10.3390/md11082846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joghee N.N., Gurunathan J. Carbohydr Res. 2014;383:76–81. doi: 10.1016/j.carres.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Li M., Huiru Z., Biting D., Yun J. BioTechnol Indian J. 2011;7:480–487. [Google Scholar]
- 18.Arora P.K., Chauhan A., Pant B., Korpole S. Int J Syst Evol Microbiol. 2011;61:1859–1864. doi: 10.1099/ijs.0.023184-0. [DOI] [PubMed] [Google Scholar]
- 19.Matobole R.M., van Zyl L.J., Parker-Nance S., Davies-Coleman M.T. Mar Drugs. 2017;15:47. doi: 10.3390/md15020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Priya I., Dhar M.K., Bajaj B.K., Koul S. Indian J. Microbiol. 2016;56:228–231. doi: 10.1007/s12088-016-0578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pal Roy M., Datta S., Ghosh S. Biotechnol Prog. 2017;33:633–641. doi: 10.1002/btpr.2452. [DOI] [PubMed] [Google Scholar]
- 22.De Lourdes Moreno M., Pérez D., García M.T., Mellado E. Life: Open Access J. 2013;3:38–51. doi: 10.3390/life3010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Rubaye M.T.S., Al-Musawi M.H.J., Fakhari J., Hosseini M. Biosci Biotech Res Asia. 2017;14 [Google Scholar]
- 24.Kim M., Nishiyama Y., Mura K., Tokue C. Biosci Biotechnol Biochem. 2004;68:1533–1540. doi: 10.1271/bbb.68.1533. [DOI] [PubMed] [Google Scholar]
- 25.Vishnivetskaya T.A., Kathariou S., Tiedje J.M. Extremophiles. 2009;13:541–555. doi: 10.1007/s00792-009-0243-5. [DOI] [PubMed] [Google Scholar]
- 26.Ali H., Ahmad W., Haq T. Afr J Biotechnol. 2009;8:1574–1576. [Google Scholar]
- 27.Raja P., Chellaram C., Jebasingh S.E.J., Maheshwari N., Chandrika M., Gladis C. Chem Pharm Res. 2013;5:146–151. [Google Scholar]
- 28.UmaMaheswari N., Sivagami S. Int J Pure Appl Biosci. 2016;4:123–128. [Google Scholar]