Abstract
The present study evaluates the cytogenetic effects of both silver and gold nanoparticles on the root cells of Allium cepa. In this study, the root cells of Allium cepa were treated with both gold and silver nanoparticles of different concentrations (1 mg/L, 5 mg/L and 10 mg/L) along with control for 72 h. Experimental results revealed that after 72 h of exposure, a significant decrease in mitotic index (MI) from 68% (control) to 52.4% (1 mg/L), 47.3% (5 mg/L) and 41.4% (10 mg/L) for gold nanoparticles and 57.1% (1 mg/L), 53% (5 mg/l), 55.8% (10 mg/L) for silver nanoparticles. Through minute observation of the photograph, it was recorded that some specific chromosomal abnormalities such as stickiness of chromosome, chromosome breaks, nuclear notch, and clumped chromosome at different exposure conditions. Therefore, present results clearly suggest that Allium cepa root tip assay could be a viable path through which negative impact of both gold and silver nanoparticles can be demonstrated over a wide range of concentrations.
Keywords: Allium cepa, Gold nanoparticles, Silver nanoparticles, Mitotic index, Chromosomal aberrations
1. Introduction
Nanotechnology, is special branch of material science where bulk materials break down to small particles within the range of 1–100 nm in diameter by means of physical, chemical and biological methods. Nanotechnology is absolutely a new emerging field, and basically there are evidences of several negative effects on growth and development of plantlets [16] but due to its diverse application in various fields such as DNA sequencing, pharmaceuticals, cosmetics, agriculture, biomolecular detection and diagnostics [17], it has gained attention and synthesized and used extensively in various field. Research has shown both silver and gold nanoparticles can be synthesized easily has many applications which makes it easily accessible and quite popular among researchers. Recently a work has been done on synthesis of AgNps by using different types of fungi [18]. Previous research also highlighted on the synthesize the cysteinen capped AgNPs mediated by electrochemically active biofilm with enhanced activity and explore its antibacterial activity on Escherichia coli (E. coli) and Pseudomonas aeruginosa (P. aeruginosa) [19]. Gold nanoparticles also have attracted great interests in the fields of biological and medical applications in past few years [20]. There are various agents such as fungi, plant extract [23], different bacteria have been used for synthesis of AuNps. Synthesis of positively charged gold nanoparticles using a stainless-steel mesh is also reported by a researcher [21]. Few researchers also reported the synthesis of positively charged gold nanoparticles by an electrochemically active biofilm (EAB) [22]. Although nanoparticles are extremely toxic due to its shorter size compared to its bulk counter part [4]. Nanoparticles having small in size (<50 nm) can comfortably penetrate and pass into the lymphatic system and subsequently it reach to the vital organ and body tissues and exhibit their negative effect. On the other hand, potential toxicity of nanoparticles on terrestrial plant species is extremely limited [10]. Previous research highlighted that nanoparticles can generate reactive oxygen species in the plant body [5]. However, few previous research also demonstrated that nanoparticles can exhibit both positive and negative effect on higher plants [5], [7]. Some researchers discussed in their paper that nanosize SiO2 and TiO2 can enhanced nitrate reductase activity and reduced both germination and growth of soybean [8]. Another work indicated that MWCNTs (multiwall carbon nanotubes) at the concentration range of 10–40 mg/L dramatically enhanced the seed germination and growth of tomato plants [6]. Historically plants have been used as indicator organisms, in studies on mutagenesis in higher eukaryotes. Plant systems have a variety of well-defined genetic endpoints including alterations in ploidy, chromosomal aberrations, and sister chromatid exchanges. Among plant tests, Allium cepa is one of the most widely used. Allium cepa has been used for evaluating chromosomal aberrations since 1920s [3]. The Allium test is based on the chromosome study of the meristem cells of the apical root cells of A. cepa in order to determine the influences of genotoxic substances or aneugenic substances [24], [25]. Mitosis involves five phases, based on the physical state of the chromosomes and spindle. These phases are prophase, prometaphase, metaphase, anaphase, and telophase. Cytokinesis is the final physical cell division that follows telophase, and is therefore sometimes considered a sixth phase of mitosis. This is a short-term test, which can assess cytogenetic effects of nanoparticles suspended in a test solution. Keeping in mind the above fact present work is dedicated to judge the efficacy of silver and gold nanoparticles towards chromosomal aberrations of Allium cepa root under laboratory condition.
2. Materials and methods
2.1. Nanoparticles
Silver and gold nanoparticles were biosynthesized by plant extract and details of synthesis procedure was mentioned in our earlier report (Hajra and Mondal, 2016). The synthesized nanoparticles were further characterised by UV–Vis Spectrophotometer (Optizen POP), SEM-EDX (Scanning Electron Microscopy and Energy Dispersive Spectroscopy) (JEOL JSM-6390LV), TEM (Transmission electron microscopy) (JEOL JEM 1400 plus) and XRD (X-ray diffraction) (Bruker D8) for confirmation of its size, structure and nature.
2.2. Test system and treatments
The gold and silver nanoparticles are diluted to three different concentrations i.e; 1 mg L−1, 5 mg L−1 and 10 mg L−1. Healthy onion bulbs were collected from the nearby vegetable market. Three healthy onion bulbs (12–15 g) were grown directly in the nanoparticles in cylindrical glass tubes in normal lighting condition at room temperature (20 °C) for 72 h along with control. The test suspension was replaced daily to maintain constant concentrations of suspensions of nanoparticles. When the roots reached to 2–3 cm they were cut and processed for slide preparation by following standard method [33]. Two replicates for each concentration were made. After that the dried roots were carefully shaved off in order to expose the fresh meristematic tissue. Then the roots of onion were grown in different medium containing both silver (1 mg L−1, 5 mg L−1 and 10 mg L−1) and gold (1 mg L−1, 5 mg L−1 and 10 mg L−1) nanoparticles along with control (double distilled water) during 72 h. After 72 h root tips were cut and fixed in ethanol and acetic acid mixture (3:1) for 24 h at 5 °C. Then the roots were dipped into 1 M HCl solution and were heated at 60 °C for 4–5 min followed by transferred to distilled water and kept for few minutes. Finally the root tips were crushed with 2% aceto orcein with flat end of metal rod and the cover slip was carefully lowered on the slide and the cover slip was sealed with clear finger nail polish. The prepared slides were ready for microscopic study.
2.3. Macroscopic examination
Macroscopic parameters were measured after 72 h of exposure. The roots were cut at their base and the number counted and the length along with the breadth were measured. The length and breadth of all roots per bulb was summarized and expressed as the total length and total breadth of the root system. The mean values for all parameters were calculated. Seven roots of each bulb were fixed in a freshly prepared mixture of absolute ethanol and glacial acetic acid (3:1 v/v) for 24 h at 4 °C [34].
2.4. Microscopic examination
Three bulbs were used for each concentration of which five root tips were used for each concentration to prepare slide for microscopic analysis. The slides of each treatment and control were prepared by following aceto orcein squash technique. The root tips were kept in 1 M HCl for about 4–5 min followed by staining with 2% aceto orcein. Staining was continued for about 10 min and then it was squashed. The cover slip was sealed with clear finger nail polish [34]. The slides were analysed with Olympus CH20imicroscope at 100X magnification. The mitotic index was calculated as the number of dividing cells per number of total observed cells [35]. Along with this portion of mitotic phases, the presence and frequency of chromosome aberrations (fragments, anaphase bridges etc.) and micronuclei were also determined. A minimum of 500 cells were counted for each slide.
2.5. Statistical analysis
All calculations were done using Minitab version17 software. The level of significance was accepted at p < 0.05. Cytogenecity was statistically analysed by Student’s t-test. The level of significance was accepted at p < 0.05.
3. Results
3.1. Synthesis and characterization of silver and gold nanoparticles
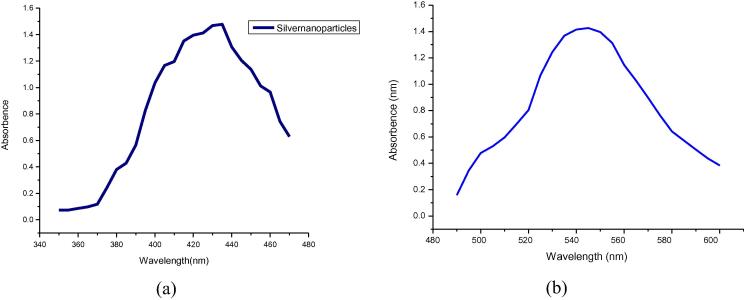
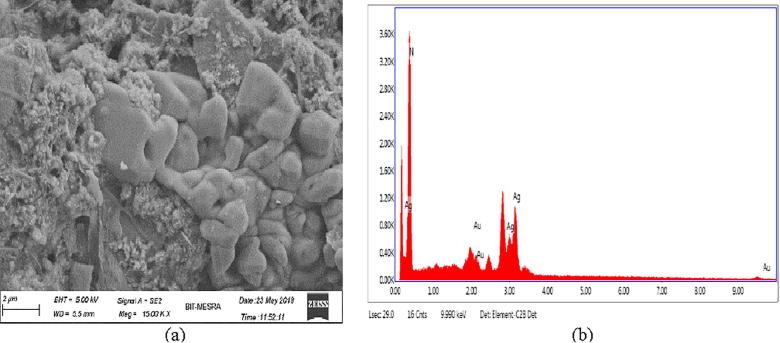
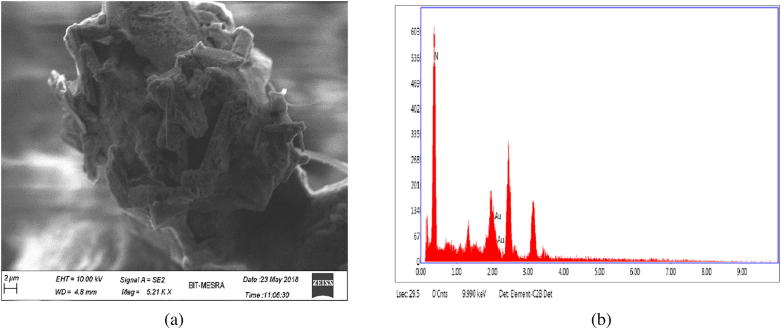
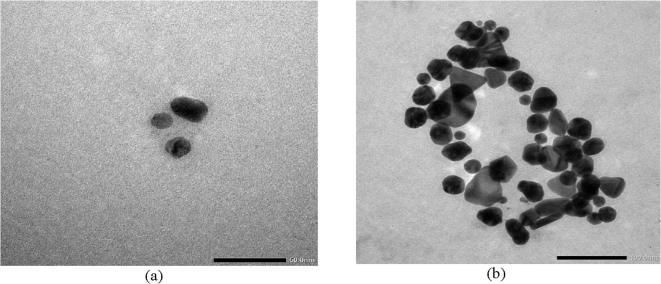
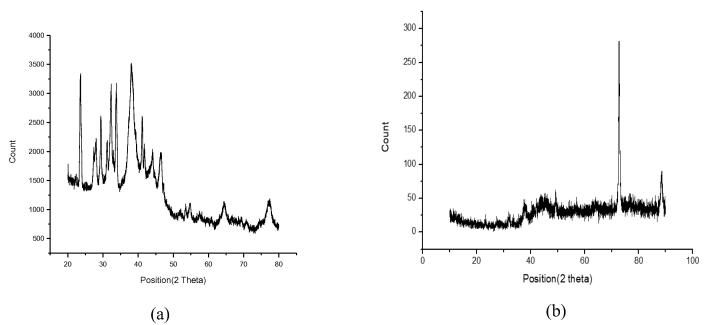
The sharp and distinct peaks of silver and gold nanoparticles were observed from UV–vis spectral signature at 440 nm and 550 nm respectively (Fig. 1). The surface morphology and existance of both silver and gold was assessed by SEM-EDX study (Figs. 2a and 2b). The exact size and shape of silver and gold nanoparticles were presented in Fig. 3. From the TEM it was observed that average size of silver and gold nanoparticles ranges between 25 and 40 nm and 17–24 nm, respectively. On the other hand, XRD study of both silver and gold highlighted the crystal structure of both the silver and gold nanoparticle (Fig. 4).
Fig. 1.
UV–Vis spectroscopy of (a) silver nanoparticle and (b) gold nanoparticle.
Fig. 2a.
(a) FESEM and (b) EDX of silver nanoparticle.
Fig. 2b.
(a) FESEM and (b) EDX of gold nanoparticle.
Fig. 3.
Transmission electron microscopy of (a) silver nanoparticle and (b) gold nanoparticle.
Fig. 4.
X-ray diffraction of (a) silver nanoparticle (b) gold nanoparticle.
3.2. Mitotic index
The effect of silver and gold nanoparticles on cell division and chromosomal behaviour of Allium cepa is investigated in this paper. Present study reveals there was no chromosomal aberration in control (Table 1). But silver and gold nanoparticles have significant effect on the occurrence of chromosomal aberrations in comparison with the control. From the experimental data it was seen that the mitotic index value for control was 68% and for gold nanoparticles it was 52.4%, 47.3% and 41.4% for 1 mg L−1, 5 mg L−1, 10 mg L−1 respectively (Table 1). It means in case of gold nanoparticles, the mitotic index decreased with increasing the concentration of the nanoparticles. But a reverse trend was observed in case of silver nanoparticles. The mitotic index value was 57.1% and 53% for 1 mg L−1 and 5 mg L−1 respectively but at 10 mg L−1 the value increases to 55.8% (Table 1).
Table 1.
Distribution of Allium cepa root tip cells treated with different concentrations of gold, silver nanoparticles.
| No. of counted cells | Dividing cell | Prophase | Metaphase | Anaphase | Telophase | Mitotic index | Mean ± SD | |
|---|---|---|---|---|---|---|---|---|
| Control | ||||||||
| Replica1 | 500 | 340 | 317 | 12 | 6 | 5 | 68% | 68% ± 3 |
| Replica2 | 500 | 328 | 318 | 8 | 2 | 0 | 65% | |
| Replica3 | 500 | 355 | 341 | 11 | 2 | 1 | 71% | |
| Gold nano | ||||||||
| (1 mg/L) | ||||||||
| Replica1 | 500 | 272 | 260 | 8 | 3 | 1 | 54% | 52.4% ± 1.7 |
| Replica2 | 500 | 263 | 257 | 4 | 2 | 0 | 52.6% | |
| Replica3 | 500 | 264 | 253 | 7 | 3 | 1 | 50.6% | |
| (5 mg/L) | ||||||||
| Replica1 | 500 | 249 | 238 | 7 | 2 | 2 | 49.8% | 47.3% ± 2.19 |
| Replica2 | 500 | 233 | 221 | 7 | 4 | 1 | 46.6% | |
| Replica3 | 500 | 228 | 220 | 5 | 2 | 1 | 45.6% | |
| (10 mg/L) | ||||||||
| Replica1 | 500 | 213 | 206 | 4 | 2 | 1 | 42.6% | 41.4% ± 1.2 |
| Replica2 | 500 | 201 | 192 | 4 | 4 | 1 | 40.2% | |
| Replica3 | 500 | 207 | 201 | 3 | 3 | 0 | 41.4% | |
| Silver nano | ||||||||
| (1 mg/L) | ||||||||
| Replica1 | 500 | 291 | 280 | 7 | 4 | 0 | 58.2% | 57.1% ± 1.22 |
| Replica2 | 500 | 287 | 279 | 4 | 3 | 1 | 57.4% | |
| Replica3 | 500 | 279 | 268 | 5 | 4 | 2 | 55.8% | |
| (5 mg/L) | ||||||||
| Replica1 | 500 | 258 | 247 | 5 | 5 | 1 | 51.6% | 53% ± 1.4 |
| Replica2 | 500 | 265 | 251 | 7 | 5 | 2 | 53% | |
| Replica2 | 500 | 272 | 261 | 5 | 4 | 2 | 54.4% | |
| (10 mg/L) | ||||||||
| Replica1 | 500 | 285 | 277 | 4 | 3 | 1 | 57% | 55.8% ± 1.44 |
| Replica2 | 500 | 271 | 261 | 6 | 4 | 0 | 54.2% | |
| Replica3 | 500 | 281 | 272 | 5 | 4 | 0 | 56.2% | |
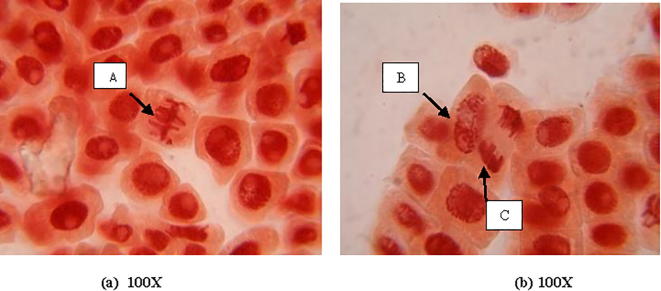
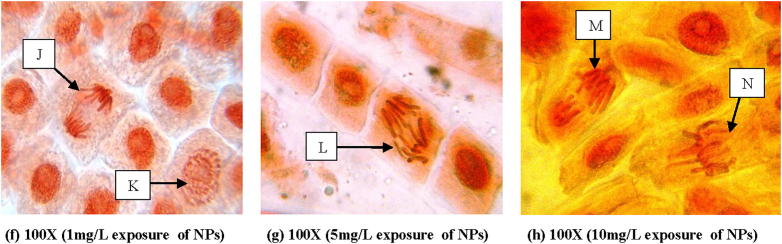
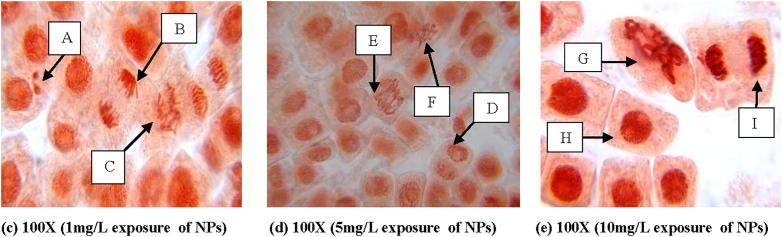
From Fig. 5 it is clear that there is no chromosomal aberration in control. At 10 mg L−1 concentration of gold, anaphase-telophase with fragment bridge, disturbed metaphase with unoriented chromosomes and at 5 mg L−1 of concentration anaphase with chromatin bridge were observed (Fig. 7). But, in case of silver, micronucleus at interphase was observed at 1 mg L−1 of concentration. Anaphase with broken chromosome bridge, vagrant chromosome in anaphase-telophase, disturbed metaphase with clumping chromosomes and lagging chromosomes were noticed at 5 mg L−1 and 10 mg L−1 concentration of silver nanoparticle respectively (Fig. 6).
Fig. 5.
Almost proper anaphase (A), prophase (B) and metaphase (C) showing no chromosomal aberration in control.
Fig. 7.
Gold nanoparticle induced chromosomal aberration in root tip cells of Allium cepa showing J – Chromosomal bridge, K – normal prophase, L – anaphase with chromatin bridge, M – anaphase-telophase with fragment bridge, N – disturbed metaphase with unoriented chromosomes at different exposure conditions of the nanoparticle.
Fig. 6.
Mitotic chromosomal aberrations in A. cepa root cells induced by silver nanoparticle showing A – micronucleus at interphase, B – Normal anaphase, C – chromosomal bridges, D – normal telophase, E – vagrant chromosome in anaphase-telophase, F – cell laggards, G – disturbed metaphase with clumping chromosomes, H – normal interphase cell, I – disturbed telophase at different exposure conditions of the nanoparticle.
3.3. Comparison of MI between silver and gold nanoparticles
The efficacy of silver and gold nanoparticles on the MI of Allium cepa was compared by applying statistical test (Table 3). From the result it has been found that mean of MI of AgNPs (1 mg L−1) is higher than the mean MI of AuNPs (1 mg L−1) and it is statistically significant (P < 0.03) (Table 3). Almost similar significant difference (P < 0.003) between AgNPs (5 mg L−1) and AuNPs (5 mg L−1) was also recorded (Table 4). But at higher concentration (10 mg L−1), MI of AgNP exhibited high trend of statistically significant (P < 0.001) than 10 mg L−1 of AuNPs (Table 5). Finally it may be concluded that AgNPs showed higher cell division at both lower (1 mg L−1) and higher (10 mg L−1) concentration than AuNPs.
Table 3.
Comparative status of MI exerted by 1 mg/L AuNPs and 1 mg/L AgNPs.
| Au NP 1 mg/L | Ag NP 1 mg/L | Mean | SD | t value | Significant level | ||
|---|---|---|---|---|---|---|---|
| 54 | 58.2 | Au NP | Ag NP | Au NP | Ag NP | ||
| 52.6 | 57.4 | 52.4 | 57.13 | 1.71 | 1.22 | 3.90 | P < 0.03 |
| 50.6 | 55.8 | ||||||
Table 4.
Comparative status of MI exerted by 5 mg/L AuNPs and 5 mg/L AgNPs.
| Au NP 5 mg/L | Ag NP 5 mg/L | Mean | SD | t value | Significant level | ||
|---|---|---|---|---|---|---|---|
| 49.8 | 51.6 | Au NP | Ag NP | Au NP | Ag NP | ||
| 46.6 | 53 | 53 | 47.33 | 2.19 | 1.40 | 3.77 | p < 0.033 |
| 45.6 | 54.4 | ||||||
Table 5.
Comparative status of MI exerted by 10 mg/L AuNPs and 10 mg/L AgNPs.
| Au NP 10 mg/L | Ag NP 10 mg/L | Mean | SD | t value | Significant level | ||
|---|---|---|---|---|---|---|---|
| 42.6 | 42.6 | Au NP | Ag NP | Au NP | Ag NP | ||
| 40.2 | 54.2 | 41.40 | 55.80 | 1.20 | 1.44 | 13.29 | p < 0.001 |
| 41.4 | 56.2 | ||||||
3.4. Comparison of chromosomal aberration between silver and gold nanoparticles
From the statistical results it was observed that gold (Au) nanoparticles of 10 mg L−1 showing highest chromosomal aberration in the roots of Allium cepa and it is significant at P < 0.05 level (Table 7). In comparison with AuNP, AgNP of 10 mg L−1 showed much lower chromosomal aberration with respect to control which is significant at P < 0.024 (Table 6). So, it can be conclude that AuNPs of higher (10 mg L−1) concentrations showed highest chromosomal aberration than AgNPs of 10 mg L−1.
Table 7.
Comparative status of chromosomal aberration of AgNPs with respect to control.
| Control | Ag 1 mg/L | Ag 5 mg/L | Ag 10 mg/L | t value of Ag1 vs conc. | t value of Ag5 vs conc. | t value of Ag10 vs conc. |
|---|---|---|---|---|---|---|
| 68 | 58.2 | 51.6 | 57 | 5.81 | 7.85 | 6.35 |
| p < 0.028 | p < 0.016 | p < 0.024 | ||||
| 65 | 57.4 | 53 | 54.2 | |||
| 71 | 55.8 | 54.4 | 56.2 |
Table 6.
Comparative status of chromosomal aberration of AuNPs with respect to control.
| Control | Au 1 mg/L | Au 5 mg/L | Au 10 mg/L | t value of Au1 vs conc. | t value of Au5 vs conc. | t value of Au10 vs conc. |
|---|---|---|---|---|---|---|
| 68 | 54 | 49.8 | 42.6 | 7.83 p < 0.004 |
9.63 P < 0.002 |
14.26 p < 0.005 |
| 65 | 52.6 | 46.6 | 40.2 | |||
| 71 | 50.6 | 45.6 | 41.4 |
3.5. Effect on length and breadth of Allium cepa root
Breadth is the measurement of width of the Allium cepa root which is measured by screw gauge and root length was measured by the centimeter scale. From Table 2 it was clear that the breadth of the roots of Allium cepa is highly affected by the nanoparticles. But in case of length there is no significant changes. Similar observation was recorded by other authors [11]. This may be due to low concentration of the nanoparticles which did not show any effects on the growth of root length as well as number of roots.
Table 2.
Macroscopic parameters (number of roots, average length of roots and average breadth of roots) after 72 h of exposure to nanoparticles.
| No. of roots (mean ± SD) | Root length (cm) (mean ± SD) | Root breadth (cm) (mean ± SD) | |
|---|---|---|---|
| Control | 6 ± 0.04 | 3.6 ± 0.01 | 1.1 ± 8.33 |
| Silver nanoparticle | |||
| 1 mg/L | 7 ± 0.1 | 4.23 ± 0.11 | 0.25 ± 0.06 |
| 5 mg/L | 10 ± 0.13 | 5.63 ± 0.13 | 0.28 ± 0.03 |
| 10 mg/L | 7 ± 0.01 | 0.53 ± 0.004 | 0.36 ± 0.021 |
| Gold nanoparticle | |||
| 1 mg/L | 3 ± 0.003 | 0.8 ± 0.001 | 0.24 ± 0.06 |
| 5 mg/L | 15 ± 0.11 | 3.05 ± 0.011 | 0.34 ± 0.006 |
| 10 mg/L | 6 ± 0.10 | 4.23 ± 0.53 | 0.34 ± 0.001 |
3.6. Comparative study of chromosomal aberration
Both the Tables 8 and 9 demonstrated the chromosomal aberration induced by silver and gold nanoparticles. All the mentioned nano induced aberration was demonstrated on Allium cepa except one (Vicia faba). On the other hand, gold nanoparticle based aberrations were also highlighted on A. Cepa. In both the cases sticky anaphase is the common observation. In the previous literatures [28], [29], [30], [31], [32], the findings were highlighted as sticky chromosomes, disturbed metaphase, micronuclei, decrease of MI index, bridge anaphase, laggard chromosome etc. are the common for both silver and gold nanoparticles in A. cepa. Present observations also support the previous findings.
Table 8.
Comparative study of chromosomal aberration on various plants induced by silver nanoparticle.
| Agent | Plant | Aberrations in chromosome | Reference |
|---|---|---|---|
| Silver nanoparticles | A. cepa | Sticky chromosomes in metaphase stage, disturbed metaphase, chromosomal break and laggard, Chromatin bridge | [7] |
| Chitosan-capped silver nanoparticles | A. cepa | Acentric fragment, double fragments, lagging chromosomes and micronuclei | [26] |
| Silver nanoparticales | Vicia faba | Chromatid and isochromatid types of gaps, breaks, and fragments, decrease in MI Index | [27] |
| Silver nanoparticales | Allium cepa and Nicotiana tabacum | Varying extent of DNA damage | [28] |
| Nano-silver | A. cepa | Disturbed metaphase, Cell showing metaphasic fragments, Anaphase showing multiple fragments | [29] |
| Silver nanoparticles | Root tips and flower buds of Allium cepa | Disturbed metaphase chromosomes, single and multiple bridge formation with fragmentation, anaphase chromosome protruded out, multipolar anaphase and sticky anaphase | [30] |
| Silver nanoparticle | A. cepa | Decrease in MI Index, lagging chromosomes, stickiness, anaphase with broken chromosome bridge | Present study |
Table 9.
Comparative study of chromosomal aberration on various plants induced by gold nanoparticle.
| Agent | Plant | Aberrations in chromosome | Reference |
|---|---|---|---|
| Gold nanoparticles | A. cepa | Clumped metaphase and sticky anaphase, formation of chromosomal bridge and laggard chromosome, anaphase bridge and chromosomal break | [31] |
| Gold nanorods | A. cepa | Diagonal anaphase, sticky chromosome, and chromosome bridge formation in anaphase, clumped chromosome, laggard chromosome, disturbed metaphase, chromosomal break, chromosomal bridge, sticky chromosome | [32] |
| Gold nanoparticles | A. cepa | Cell in anaphase showing laggards, Anaphase with broken chromosome bridge, disturbed metaphase with unoriented chromosomes | Present study |
4. Discussion
The biosynthesized silver and gold nanoparticles are stable in room temperature more than 180 days, that means both silver and gold salts were reduced and stabilized by the plant-origin biomolecules. The average size of both the nanoparticles ranges between 50 and 100 nm. Therefore, very high surface area and supossed to be lightly toxic for both plant and animal systems [41]. So far as nanoparticle toxicity is concerned, it mainly exhibited their toxicity in two different actions [42]: (i) toxicity due to complex nature of chemicals, and (ii) toxicity based on the size and shape of the nanoparticle. Varieties of plant species such as Vicia faba, Zea mays, Nicotiana tabacum, Allium cepa, Crepis capillaris, Hordeum vulgare etc. were extensively used towards assessment of environmental contamination [35]. However, use of onion (Allium cepa) root tip bioassay is a easy, reliable and simple test model which is easy to administer to check the genotoxic potential of environmental contaminants [36]. The cytotoxicity test through chromosomal aberrations using A. cepa test was popular since 1920 [37]. However, after that United Nations Environmental Programme (UNEP) [38] and the International Programme on Chemical Safety (IPCS) certified that A. cepa root tip assay is a vital chromosomal aberration test for in situ monitoring of environmental contaminants including various chemicals, nanoparticles, pharmaceuticals etc [39]. Recently another study highlighted that oxidative stress is an essential mechanism through which nanoparticles can exhibited toxicity in cells [40]. In this study both silver and gold nanoparticles exhibited cytotoxicity by decreasing the mitotic index in a dose dependent relationship [7]. Chromosomal aberration are the changes in structure of chromosomes. There are several factors such as DNA breaks, inhibition of DNA synthesis and replication of altered DNA which can induce structural chromosomal alterations [13]. Microscopic study revealed the presence of lagging chromosomes, stickiness, anaphase with broken chromosome bridge which in turn confirmed that chromosomal aberration has occurred. Here different kind of chrosomalaberration was observed with different kind of concentration of nanoparticles and salt. Both physiological and clastogenicaberrations like laggards, broken chromosome bridge, anaphase with multiple chromosome bridge were observed here. Quite similar observation was described by other researchers [7]. The decrease in MI index is probably due to the mitodepressive effects of both silver and gold nanoparticles i.e; it may interfere with the normal development of mitosis by preventing a large number of cells to entering the prophase, thus hampering the total cell cycle [2]. According to some researchers, various chromosomal abnormalities in metaphase and anaphase are due to the shifting of poles by depolymerization of spindle fibers. In the present study similar observations were noticed at 10 mg L−1 of gold and 5 mg L−1 of silver nanoparticles respectively [1]. Chromosome losses, delays, adherence, multipolarity and C-metaphases can be resulted by the action of aneugenic effects [12]. The formation of chromosomal bridge and fragmentation are attributed to chromosomal stickiness, which was followed by the failure of free anaphasic separation [1], [9]. Some researchers reported that in the endosperm cells of plants acentric chromosome fragments are pulled poleward at the time of phragmoplast formation by kinetochore independent process which may be one of the reason for anaphasic separation [14], [15]. Some authors also have reported that plants treated with gold nanorods were found to be develop oxidative stress which further results in the phytotoxicity towards plant cell [11].
5. Conclusion
Present study results reveal that both silver and gold nanoparticles shows negative effect on the roots of Allium cepa. Both the nanoparticles could penetrate into the root cell and cause significant changes in intracellular components, causing remarkable damage to the cell division. The mitotic index decreased from the control (68%) to that of 10 mg L−1 treated (41.4%) for gold nanoparticles and 5 mg L−1 treated (53%) for Silver nanoparticles. Moreover, the cell division was arrested, at metaphase stage for both the nanoparticles, showing lagging chromosomes, stickiness, and anaphase with broken chromosome bridge. Both silver and gold nanoparticles does not exhibited any variation in root length but remarkable changes was recorded in root diameter and number of roots for both types of nanoparticles. Therefore it can be concluded that nanoparticles supposed to be a potent hazardous component for the environment and entire ecological systems. More research should be done to unfold their overall fate, transport, end exposure pathways in the wider environment. Further study is going on to explore more about nanoparticles and their cytogenetic effects on plants.
Acknowledgements
The authors acknowledge their sincere thanks to the funding agency, DST Govt. of India F. No. SR/FST/ESI-141/2015(C), dated 26th May 2016 for providing necessary funds for conducting the present research. Authors also extending their sincere gratitude to all the faculty members and staff, Department of Environmental Science, The University of Burdwan, for their moral support and valuable suggestions for preparing this manuscript.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
References
- 1.Darlington C.D., Mcleish J. Nature. 1951;167:407–408. doi: 10.1038/167407a0. [DOI] [PubMed] [Google Scholar]
- 2.Elghamery A.A., Elnahas A.I., Mansour M.M. Cytologia. 2000;55:209–215. [Google Scholar]
- 3.Grant W.F. Mutat Res. 1982;99:273–291. doi: 10.1016/0165-1110(82)90046-x. [DOI] [PubMed] [Google Scholar]
- 4.Hajra A., Mondal N.K. IJSRES. 2015;3:0047–0061. [Google Scholar]
- 5.Hong F.S., Zhou J., Liu C., Yang F., Wu C., Zheng I., Yang P. Biol Trace Elem Res. 2005;105:269–279. doi: 10.1385/BTER:105:1-3:269. [DOI] [PubMed] [Google Scholar]
- 6.Khodakovskaya M., Dervishi E., Mahmood M., Xu Y., Li Z., Watanabe F., Biris A.S. ACS Nano. 2009;3:3221–3227. doi: 10.1021/nn900887m. [DOI] [PubMed] [Google Scholar]
- 7.Kumari M., Mukherjee A., Chandrasekaran N. Sci Total Environ. 2009;407:5243–5246. doi: 10.1016/j.scitotenv.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Lu C.M., Zhang C.Y., Wen J.Q., Wu G.R., Tao M.X. Soybean Sci. 2002;21:168–171. [Google Scholar]
- 9.Panda K.K., Achary V.M., Krishnaveni R., Padhi B.K., Sarangi S.N., Sahu S.N., Panda B.B. Toxicol In Vitro. 2011;25:1097–1105. doi: 10.1016/j.tiv.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 10.USEPA. Nanotechnology White Paper. Science Policy Council, USEPA, Washington, DC. EPA 100/B-07/001; February 2007. p. 1–119.
- 11.Wan Y., Li J., Ren H., Huang J., Yuan H. J Nanosci Nanotechno. 2014;14:6089–6094. doi: 10.1166/jnn.2014.8853. [DOI] [PubMed] [Google Scholar]
- 12.Leme D.M., Marin-Morales M.A. Mutat Res. 2009;682:71–81. doi: 10.1016/j.mrrev.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Albertini R.J., Anderson D., Douglas G.R., Hagmar L., Hemmink K., Merlo F., Natarajan A.T., Norppa H., Shuker D.E., Tice R., Waters M.D., Aitio A. Mutat Res. 2000;463:111–172. doi: 10.1016/s1383-5742(00)00049-1. [DOI] [PubMed] [Google Scholar]
- 14.Bajer A. Chromosoma. 1958;9:319–331. [PubMed] [Google Scholar]
- 15.Bajer A., Ostergen G. Hereditas. 1963;50:179–195. [Google Scholar]
- 16.Castiglione M.R., Giorgetti L., Geri C., Crempnini R. J Nanoparticle Res. 2011;13:2443–2449. [Google Scholar]
- 17.Sahana R., Daniel K., Sankar S.G., Archunan G., Vennison S.J., Sivakumar M. Green Chem Lett Rev. 2014;7:64–72. [Google Scholar]
- 18.Azhar U., Khan N.M., Khan M., Cho M.H., Khan M.M. Bioprocess Biosyst Eng. 2018;41:1–20. doi: 10.1007/s00449-017-1846-3. [DOI] [PubMed] [Google Scholar]
- 19.Khan M.M., Kalathil S., Lee J., Cho M.H. Bull Korean Chem Soc. 2012;33:8. [Google Scholar]
- 20.Linic S., Asiam U., Boerigter C., Morabito M. Nat Mater. 2015;14:567–576. doi: 10.1038/nmat4281. [DOI] [PubMed] [Google Scholar]
- 21.Han T.H., Khan M.M., Kalathil S., Lee J., Cho M.H. J Nanosci Nanotechnol. 2013;13:6140–6144. doi: 10.1166/jnn.2013.7660. [DOI] [PubMed] [Google Scholar]
- 22.Khan M.M., Lee J., Cho M.H. Int J Hydrogen Energy. 2013;38:5243–5250. [Google Scholar]
- 23.Ahmed S., Ikram S. Nano Res Appl. 2015;1 [Google Scholar]
- 24.Levan A. Hereditas. 1938;24:471–486. [Google Scholar]
- 25.Levan A. Hereditas. 1949;35:325–337. [Google Scholar]
- 26.Pesnya D.S. Caryologia: Int J Cytol Cytosyst Cytogen. 2013;66:275–281. [Google Scholar]
- 27.Patlolla A.K., Berry A., May L.B., Tchounwou P.B. Int J Environ Res Pub Health. 2012;9:1649–1662. doi: 10.3390/ijerph9051649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghosh M., Manivannan J., Sinhaa S., Chakraborty A., Mallick S.K., Bandyopadhyay M., Mukherjee A. Mutat Res. 2012;749:60–69. doi: 10.1016/j.mrgentox.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Babu K., Deepa M.A., Shankar S.G., Rai S. Int J Nanotechnol. 2008;2:2. [Google Scholar]
- 30.Saha N., Gupta S.D. J Hazard Mater. 2017;330:18–28. doi: 10.1016/j.jhazmat.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 31.Rajeshwari A., Suresh S., Chandrasekaran N., Mukherjee A. RSC Adv. 2016;6:24000–24009. [Google Scholar]
- 32.Rajeshwari A., Roy B., Chandrasekaran N., Mukherjee A. Plant Physiol Biochem. 2016;109:209–219. doi: 10.1016/j.plaphy.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Fiskesjo G. Allium test for screening chemicals; evaluation of cytologic parameters. In: Wang W., Gorsuch J.W., Hughes J.S., editors. Plants for environmental studies. CRC Lewis Publishers; Boca Raton, New York: 1997. [Google Scholar]
- 34.Klancˇnik K., Drobne D., Valant J., Koce J.D. Ecotox Environ Saf. 2011;74:85–92. doi: 10.1016/j.ecoenv.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Bakare A.A., Mosuro A.A., Osibanjo O. J Environ Biol. 2000;21:263–271. [PubMed] [Google Scholar]
- 36.Bakand S., Hayes A. Int J Mol Sci. 2016;17:929. doi: 10.3390/ijms17060929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satapathy R., Swamy P.A. Biol Sci. 2013;4:454–460. [Google Scholar]
- 38.Grant W.F. Mutat Res Fundam Mol Mech Mutagen. 1999;426:107–112. doi: 10.1016/s0027-5107(99)00050-0. [DOI] [PubMed] [Google Scholar]
- 39.Cabrera G., Rodriguez D. Mutat Res. 1999;426:211–214. doi: 10.1016/s0027-5107(99)00070-6. [DOI] [PubMed] [Google Scholar]
- 40.Mangalampalli B., Dumala N., Grover P. J Environ Sc. 2018;66:125–137. doi: 10.1016/j.jes.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 41.Jia G., Wang H., Yan L., Wang X., Pei R., Yan T., Zhao Y., Guo X. Environ Sci Technol. 2005;39:1378–1383. doi: 10.1021/es048729l. [DOI] [PubMed] [Google Scholar]
- 42.Brunner T.J., Wick P., Manser P., Spohn P., Grass R.N., Limbach L.K., Bruinink A., Stark W.J. Environ Sci Technol. 2006;40:4374–4381. doi: 10.1021/es052069i. [DOI] [PubMed] [Google Scholar]