Abstract
Phytoremediation is considered as a novel environmental friendly technology, which uses plants to remove or immobilize heavy metals. The use of metal-resistant plant growth-promoting bacteria (PGPB) constitutes an important technology for enhancing biomass production as well as tolerance of the plants to heavy metals. In this study, we isolated twenty seven (NF1-NF27) chromium resistant bacteria. The bacteria were tested for heavy metals (Cr, Zn, Cu, Ni, Pb and Co) resistance, Cr(VI) reduction and PGPB characters (phosphate solubilization, production of IAA and siderophores). The results showed that the bacterial isolates resist to heavy metals and reduce Cr(VI), with varying capabilities. 37.14% of the isolates have the capacity of solubilizing phosphate, 28.57% are able to produce siderophores and all isolates have the ability to produce IAA. Isolate NF2 that showed high heavy metal resistance and plant growth promotion characteristics was identified by 16S rDNA sequence analysis as a strain of Cellulosimicrobium sp.. Pot culture experiments conducted under greenhouse conditions showed that this strain was able to promote plant growth of alfalfa in control and in heavy metals (Cr, Zn and Cu) spiked soils and increased metal uptake by the plants. Thus, the potential of Cellulosimicrobium sp. for both bioremediation and plant growth promotion has significance in the management of environmental pollution.
Keywords: Phytoremediation, Heavy metals, Bioaugmentation, Plant growth-promoting bacteria
1. Introduction
Even heavy metals are derived from parent rock and are found throughout the earth’s crust, anthropogenic activities are among the major environmental and human health problems. The soil pollution by toxic heavy metals has accelerated greatly by the use of heavy metals such as chromium, zinc, copper, cadmium and lead, in industrial activities such as tanning, mining, refining and manufacturing processes [1]. Heavy metals are used also in different fungicides and land chemical fertilizers, wastewater irrigation and sewage sludge causing heavy metal contamination of water resources and agricultural soils [2], [3]. Thus, remediation of heavy metals is necessary to protect the environment from their toxic effects [4]. Several efforts have been made to develop sustainable and environmental friendly technologies useful to extract and remove toxic heavy metals from water and soil.
Currently, conventional remediation methods of heavy metals contaminated soils are environmentally destructive and expensive [5]. Phytoremediation is recognized as a promising and cost-effective solution among the numerous methods used for the rehabilitation of contaminated sites. This technique is based on the use of plants to clean up and/or improve soil and water quality by inactivation or translocation of pollutants in different parts of the plant, without negative effects on the structure, fertility and the biological activity of the soil [6], [7]. Metal hyper accumulating plants have gained increased attention. However, phytoextraction efficacy of most metal hyperaccumulator plants is generally restricted by their low biomass and slow growth rate [8]. As an alternative, high biomass crops (e.g. maize, sunflower, etc.) and chelant-assisted phytoremediation were proposed to improve heavy metals solubilization and availability [9], [10], [11].
Isolation and application of microbial populations for remediation of heavy metal ions from the environment has attracted several researchers and metal-detoxifying plant growth promoting bacteria (PGPB) have been the object of particular attention [12], [13], [14]. Indeed, PGPB have potential for metals detoxification and for mitigation of plant’s stress in polluted environment. They enhance the growth of plants by various PGP traits e.g. phosphate solubilization, production of indole-3-acetic acid, siderophores, ammonia, hydrogen cyanide and nitrogen fixation [15].
Despite that a number of bacterial strains has been reported, there is a growing need to find novel microbial resources that can help improve growth and yield of plants in contaminated soils. Furthermore, the majority of the studies have involved single-metal resistant strains generally Cr. However, contaminated sites are generally subject to multi-contamination with heavy metals. Therefore, the present study was designed to isolate multi-heavy metals resistant bacteria and to examine their plant growth promoting (PGP) properties in order to select bacterial strains that could support plant growth and phytoremediation of heavy metals contaminated sites.
2. Material and methods
2.1. Isolation of heavy metals-resistant bacteria
The soil samples were collected from the rhizosphere of indigenous plants of a contaminated region in the Plain of Sais, Fez (Morocco). This area had been exposed to a large number of toxic industrial wastes. Indeed, the city of Fez is among the cities of Morocco in full urban and industrial expansion. Brick, plastic, tanning, cement and steel industry are among the most polluting industries in the region, with the production of large heavy metal amounts that pose a threat to the environment. Effluents produced daily by industries rich with heavy metals (Chromium, Lead, Zinc, Copper and Nickel) are simply dumped untreated into the Sebou River which is used for irrigation.
Soil sample suspensions were prepared by adding 1 g of rhizospheric soil to 100 mL distilled water. 100 µL suspension for each sample dilutions (10−1–10−8) were plated in Luria Broth (LB) agar (peptone 10 g, sodium chloride 10 g, yeast extract 5 g and agar 15 g in 1 L distilled water, pH 7) amended with 100 mg L−1 of Cr(VI) in the form of K2Cr2O7. Plates were incubated at 30 °C for 48 h. Colonies of different morphologies were then selected and streaked on separate agar plates amended with the same concentrations of K2Cr2O7.
The selected bacterial isolates were tested for their resistance against Cr (K2Cr2O7), Ni (NiCl2), Cu (CuSO4), Pb (ZnSO4), Co (CoSO4) and Zn (ZnSO4) by estimating the minimum inhibitory concentrations (MIC) for each bacterial isolate using dilution plate method. For this purpose, the bacterial isolates were submitted to concentrations ranging from 200 to 1000 mg L−1 for Cr and from 5 to 2000 mg L−1 for other metals. Heavy metals were filter sterilized and added separately to the agar medium.
The minimum inhibitory concentration (MIC) was defined as the lowest concentration at which no viable colony-forming units (CFU) were observed after 48 h of incubation at 30 °C [16].
2.2. Chromium reduction experiments
The reduction of chromium by the isolated bacteria was studied in 150 mL Erlenmeyer flask containing 50 mL of LB medium supplemented with chromium. 100 µL of 24 h bacterial cultures were added to the media and incubated at 30 °C with shaking (120 rpm). Two mL of the cultures were then centrifuged at 6000g for 10 min to remove the bacterial cells. Then the supernatant was used to measure the concentration of the non-reduced chromium Cr(VI) using colorimetric reagent S-diphenyl carbazide (DPC) [17]. Absorbance was measured at 500 nm. The cells culture was monitored by measuring optical density at 600 nm.
2.3. Determination of PGP properties of heavy metals resistant bacteria
Isolates were tested for a number of important properties regarding PGP activities.
2.3.1. IAA production
IAA production was tested according to Bharadwaj et al. [18] method. Cells were cultured in 15 mL tubes containing 5 mL of Luria-Bertani Broth supplemented with 1 mg mL−1 tryptophan. The medium was incubated 5 days at 30 °C with shaking (200 rpm). After centrifugation at 10,000g for 10 min, 1 mL of supernatant was mixed with 2 mL of the Salkowski solution (1.2g FeCl3 6H2O in 100 mL of H2SO4 7.9 M). After incubation for 20 min at room temperature, the optical density was measured at 535 nm. The standard curve was made from serial dilutions of a solution of IAA 50 µg mL−1 in the LB medium.
2.3.2. Siderophores production
Siderophores secretion by bacterial isolates was detected using blue agar plates containing the dye Chrome azurol S (CAS) (Sigma–Aldrich) [19]. Siderophores excretion showed a change in color, from blue to orange halos around colonies.
2.3.3. Phosphate-solubilizing activity
The ability of the isolated bacteria to solubilize inorganic phosphate was studied by three successive subcultures on National Botanical Research Institute's phosphate growth (NBRIP) agar medium (10 g L−1 d-glucose, 5 g L−1 Ca3(PO4)2, 5 g L−1 MgCl2 6H2O, 0.25 g L−1 MgSO4·7H2O, 0.2 g L−1 KCl, 0.1 g L−1 (NH4)2SO4, 15 g L−1 agar, pH 7) [20]. From each young bacterial culture 10 μL was deposited on the medium. After 5 days incubation at 30 °C, the solubilization areas (halo area) and the diameters of colonies were measured. The following report was calculated to evaluate the degree of solubilization of tricalcium phosphate by different isolates [21]:
2.4. Identification and characterization of the selected bacterial isolates
A bacterial isolate that showed high metal resistance and interesting PGP traits was subjected to molecular identification. Bacterial DNA was extracted from cells using thermal shock protocol: in 1.5 mL microcentrifuge tube an isolated colony from a young LB-agar culture of the isolate was mixed with 50 μL of sterile distilled water. Then the tube was frozen at −20 °C for 30 min and heated at 95 °C for 3 min repeated from twice to thrice. After centrifugation at 7000g for 10 min, 2 μL of the supernatant was used in the reaction mix. Molecular identification approach involved the use of 16S rDNA analysis; due to the slow rates of evolution of this region between different species of bacteria. This gene encodes a 16S ribosomal RNA; it is commonly used suitable for phylogenetic studies. The rDNA 16S regions were amplified using primers fD1 (5′AGAGTT TGATCCTGGCTCAG3′) and RS16 (5′TACGGCTACCTT GTTACGACTT3′) [22]. The reaction mix contained 4 μL of dNTPs (1 mM), 4 μL of Taq buffer (5×), 0.2 μL of Taq polymerase (5 U/μL), 1.2 μL of MgCl2 (25 mM), 2 μL of fD1 (10 μM), 2 μL of RS16 (10 μM), 4.6 μL of pure H2O, and 2 μL of the DNA. The amplification protocol was under the following conditions: denaturation at 94 °C for 5 min, 35 cycles of denaturation at 94 °C for 30 s, primers annealing at 55 °C for 45 s, and primer extension at 72 °C for 90 s; final extension was performed at 72 °C for 10 min. The amplified PCR products were confirmed by gel electrophoresis on 1% agarose gel, and visualized under short-wavelength UV light stained with ethidium bromide. The sequence was initially analyzed by a similarity search using the BLAST function of GenBank at the National Center for Biotechnology Information (NCBI) electronic site (http://www.ncbi.nlm.nih.gov/) and the SeqMatch tool of the Ribosomal Database Project II (RDP, http://rdp.cme.msu.edu/) [23]. Using the PhyML program (www.phylogeny.fr) and some reference sequences from the GenBank, a phylogenetic tree was realised.
2.5. Effect on plant growth
Experiments were conducted to study the effect of the selected bacterial strain on Medicago sativa (Alfalfa) growth in the presence of Cr(VI), Zn and Cu. Alfalfa was used due to its fast growth and high biomass production features.
Alfalfa seeds were firstly sterilized in a solution of 70% ethanol (v/v) for 5 min, washed with sterile water and put into a solution of sodium hypochlorite (5% v/v) for 10 min. Then, they were washed with sterile water and germinated in plates with water-agar for 2 days. Finally, seedlings were transferred and sown in plastic pots (7 cm diameter and 10 cm high) containing non-spiked (control) or metal spiked soil (four replicate flasks per treatment containing three plants each). The soil used in this study was an agricultural alkaline (pH 7.8) clay loam soil from northern Morroco (Sais plaine). It was artificially contaminated with aqueous solutions of K2Cr2O7, CuSO4 or ZnCl2 to achieve final concentrations of 50, 250 and 500 mg kg−1, respectively.
For inoculation of the seedlings, bacterial strain was grown overnight in LB medium at 30 °C. Cells in the exponential phase were harvested by centrifugation at 6000 rpm for 20 min, washed twice with sterile saline solution (0.85% NaCl) and centrifuged again. Bacterial inoculum was prepared by resuspending pelleted cells in sterile saline solution to get an inoculum density of ca. 108 CFU mL−1. Seedlings were inoculated with 10 mL of a suspension of the bacterial cells. Ten milliliter of saline solution was also added to the control treatment pots (uninoculated). Pots were placed in a controlled growth room (16 h photoperiod, 28–30 °C temperature range) and watered daily.
Plants were harvested after 30 days, subdivided in roots and shoots and washed with deionized water, then fresh weight was measured.
2.6. Analysis of metals in plants
The washed root and shoot samples were dried at 70 °C for 24 h and grinded into fine powder. About 200 mg of powdered plant tissue was digested [24]. Total Cr, Cu and Zn content in the digest was determined by inductively coupled plasma atomic emission spectrometer (ICP-AES) (Jobin Yvon).
The bioaccumulation factor (BAF) was calculated to estimate the metal uptake in different plant parts. It presents an index of the ability of a plant to accumulate a particular metal relative to its concentration in the medium [25].
2.7. Statistical analysis
Results from greenhouse experiments were expressed as mean ± SE. Data were submitted to ANOVA analysis for each data in order to determine significant differences between the means. A multiple range test at the 95% confidence level was performed using Tuckey’s method.
3. Results and discussion
3.1. Bacterial resistance to heavy metals
Isolating bacteria from metal-polluted sites is an advantage to have a bacterial flora adapted to the toxicity of heavy metals [26]. In this study, twenty-seven bacterial isolates were originally isolated for different morphological appearance of their colonies on LB agar medium amended with 100 mg L−1 of K2Cr2O7. All of bacteria were multiresistant to several heavy metals. Resistance of the selected twenty-seven bacterial isolates in terms of MIC (mg L−1) to metal concentrations is summarized in Table 1. All selected bacteria were found to resist to the tested heavy metals with varying capabilities ranging from 400 to 900 mg L−1 for Cr(VI), 400 to 2000 mg L−1 for Zn, 100 to 500 mg L−1 for Cu, 100 to 1000 mg L−1 for Ni, 100 to 2000 for pb and 400 to 1000 Co. Approximately 52% of isolates were able to grow at Cr(VI) concentrations >500, 74% of isolates resist to Zn concentrations >500 mg L−l; 48% of isolates resisted to Cu concentrations >300 mg L−l.
Table 1.
Tolerance to heavy metals (MIC (mg L−1)), time for total reduction of 100 mg L−1 Cr (VI) and PGP characteristics of bacterial isolates.
| MIC (mg L−1) |
Tot. red. of 100 mg L−1 Cr(VI) | PGP traits |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Strain | Cr(VI) | Zn | Cu | Ni | Pb | Co | Time (h) | PSI | IAA (μg mL−1) | Siderophores |
| NF1 | 600 | 500 | 400 | 500 | 1000 | 400 | 60 | – | 38.89 | + |
| NF2 | 800 | 1500 | 400 | 500 | 1500 | 400 | 48 | 2.66 | 88.53 | + |
| NF3 | 400 | 500 | 300 | 500 | 1500 | 500 | 60 | – | 56.83 | – |
| NF4 | 500 | 500 | 400 | 500 | 1500 | 400 | 72 | – | 32.26 | – |
| NF5 | 900 | 1000 | 400 | 500 | 1500 | 400 | 96 | – | 96.14 | + |
| NF6 | 900 | 1500 | 200 | 500 | 1500 | 1000 | 96 | 2.60 | 40.61 | – |
| NF7 | 400 | 500 | 300 | 500 | 1500 | 400 | 120 | – | 43.56 | + |
| NF8 | 600 | 1500 | 400 | 300 | 1500 | 400 | 96 | – | 12.51 | + |
| NF9 | 600 | 2000 | 400 | 500 | 1500 | 500 | 96 | – | 33.24 | + |
| NF10 | 700 | 1500 | 400 | 1000 | 1500 | 500 | 96 | 2.50 | 48.97 | – |
| NF11 | 400 | 1000 | 200 | 500 | 1500 | 500 | 120 | – | 74.26 | – |
| NF12 | 500 | 1500 | 400 | 500 | 1500 | 500 | 96 | – | 65.68 | – |
| NF13 | 500 | 1500 | 400 | 300 | 1500 | 1000 | 96 | 2.20 | 41.35 | – |
| NF14 | 600 | 500 | 300 | 500 | 1500 | 1000 | 96 | 2.45 | 39.63 | – |
| NF15 | 500 | 2000 | 300 | 500 | 1500 | 1000 | 72 | 3.33 | 36.68 | – |
| NF16 | 500 | 1000 | 300 | 500 | 1500 | 500 | 96 | 2.36 | 37.91 | – |
| NF17 | 500 | 1000 | 300 | 300 | 1500 | 500 | 96 | – | 43.07 | + |
| NF18 | 600 | 1000 | 300 | 300 | 2000 | 500 | 96 | – | 58.30 | + |
| NF19 | 700 | 500 | 400 | 500 | 1500 | 500 | 60 | – | 74.20 | + |
| NF20 | 700 | 1500 | 400 | 400 | 1500 | 500 | 72 | 2.50 | 43.32 | – |
| NF21 | 600 | 1500 | 300 | 400 | 1500 | 400 | 60 | 2.60 | 51.92 | – |
| NF22 | 400 | 1000 | 300 | 500 | 1500 | 500 | 96 | 2.66 | 43.56 | – |
| NF23 | 400 | 1000 | 400 | 1000 | 1500 | 500 | 120 | – | 74.28 | – |
| NF24 | 600 | 2000 | 300 | 400 | 1500 | 500 | 60 | 2.50 | 47.00 | – |
| NF25 | 500 | 1000 | 500 | 100 | 1500 | 500 | 144 | – | 53.14 | + |
| NF26 | 500 | 2000 | 300 | 500 | 1500 | 500 | 48 | 2.50 | 47.00 | – |
| NF27 | 700 | 400 | 100 | 500 | 1500 | 500 | 120 | 2.30 | 42.09 | – |
The high tolerance to metals could be attributed to the fact that these bacteria were isolated from contaminated soil with industrial effluents containing high levels of metals.
3.2. Bacterial Cr(VI) reduction
Chromium (VI) has been designated as a priority pollutant due to its carcinogenicity and mutagenicity. In contrast, the derivatives of Cr (III) are water insoluble at neutral pH, less toxic and mutagenic than Cr (VI). Therefore, reduction of Cr (VI) to Cr (III) is considered as a beneficial reaction in many chromium contaminated environments [27].
The results indicated that all the tested bacterial isolates showed varying potential to reduce the Cr (VI) in the liquid media. The bacterial isolates NF2 and NF26, performed total reduction of 100 mg L−1 of Cr (VI) in 48 h of incubation, 12 isolates have the ability to reduce it in 96 h while NF25 strain was able to achieve total reduction in 144 h.
The ability to resist and reduce the hexavalent chromium by bacteria isolated from contaminated environments under field and laboratory conditions has been reported by several authors [28], [29], [30]. PGPB having the ability to reduce Cr(VI) have potential utilization for plant growth improvement as well as for Cr(VI) bioremediation [31].
Bacteria reduce Cr(VI) by chemical or enzymatic means. For example, chromate may act as terminal electron acceptor for gaining energy [32]. Reduction of Cr (VI) might be due to the chemical compounds like cysteine, sulphite, glutathione and thiosulfates might reduce Cr (VI) into Cr (III) [33]. The enzymatic activities of bacteria might also be one of the possible reasons for reduction of Cr (VI) by soluble and membrane-bound reductases that exist in several of aerobic, facultative and anaerobic bacteria [34].
3.3. PGP characteristics of the isolated bacteria
The importance of soil bacteria in heavy metal resistance and their ability to promote the host plant growth in a metal-contaminated environment make them the preferred choice for phytoremediation studies. Hence, plant growth-promoting characteristics such as the production of IAA, siderophores and solubilization of phosphate by the isolates were further investigated.
3.3.1. Production of indole acetic acid (IAA)
Results showed that all 27 isolates growing in medium added with tryptophan were able to produce IAA. Maximum IAA production were recorded in isolates NF5 (96.14 µg mL−1) and NF2(88.53) as compared to other isolates. The minimum amount of IAA production was recorded in isolate NF8 (12.24 µg mL−1). Several species of bacteria were reported to have the ability to produce IAA, an essential hormone in root development [35]. The bacterial IAA plays a very important role in improving the absorption of minerals and nutrients uptake therefore the enhancement of lateral and adventitious rooting leading, and inducing a bacterial proliferation on the roots by root exudation [4].
3.3.2. Production of siderophores
In this study, 37% of isolates were positive for siderophore production. Metal-resistant siderophore-producing bacteria greatly contributes to the survival and growth of plants particularly in contaminated soils because it plays an important role in reducing the metal toxicity and supplying the plant with iron [36].
3.3.3. Phosphate solubilization
Phosphorus is one of the major essential macronutrients for plant growth and development. However the concentration of soluble P in the soil is usually very low [37]. The use of phosphate solubilizing bacteria as inoculants can strongly increase simultaneously P uptake by the plant and crop yield [38].
Qualitative estimation of phosphorus solubilization was carried out in NBRIP agar medium. 48% of isolates were found to solubilise tricalcium phosphate, with the highest capability observed using isolates NF15 (PSI = 3.33), NF2 and NF22 (PSI = 2.66). Several Studies showed that PGP bacteria were responsible for solubilizing the insoluble P [39], [40], [41]. It was also reported that excretion of organic acids was one of the most important factors in phosphate solubilization [42].
3.4. Bacterial identification
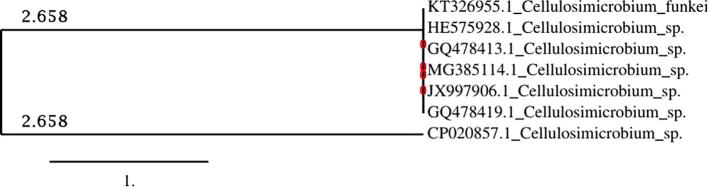
In this study, the bacterial isolate NF2 showed multiple PGP traits, such as the production of IAA and siderophores and phosphate solubilization activity. It has also high heavy metals resistance and Cr(VI) reduction ability. The 16S rRNA gene sequences were compared to NCBI database using BLAST analysis this bacterial isolate showed similarities of 100% to Cellulosimicrobium sp. (CP020857.1). The phylogenetic lineage of NF2 drawn from 16S rDNA sequence databases of some closely related members is presented in Fig. 1.
Fig. 1.
Phylogenetic tree derived from 16S rRNA gene sequence of Cellulosimicrobium sp. (CP020857.1), using the maximum likelihood method implemented in the PhyML program.
There is little work on the isolation and the characterization of Cellulosimicrobium as bacteria with potential for environmental management. Yet, species of this genus have recently been reported to possess significant potential as plant growth promoting bacteria and chromium bioremediation agents [43], [44], [45], [46].
3.5. Effect of bacterial inoculation on plant growth
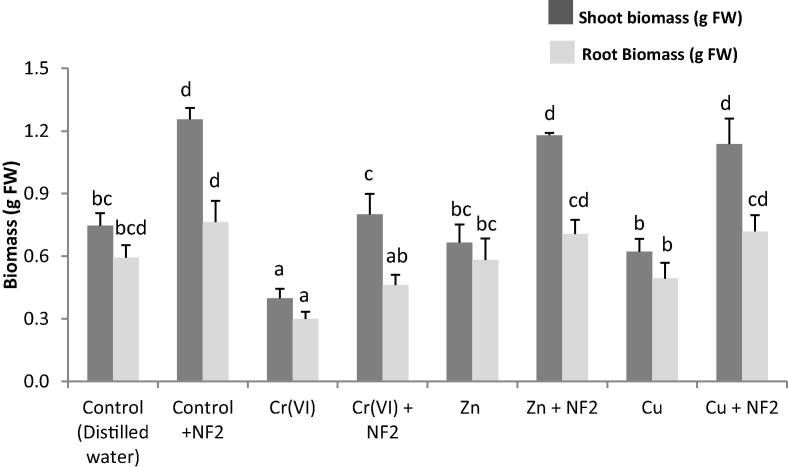
Based on PGP traits and metal resistance, the heavy metal-tolerant Cellulosimicrobium sp. was selected for pot culture experiments. Results show that this bacterium was able to improve plant growth by 68% and 28% for shoot and root control plants, respectively (Fig. 2). The detrimental effects of metals on plant growth were also reduced by bacterial inoculation, as revealed by the performance of the plants in Cr(VI)-, Zn- and Cu-spiked soils (Fig. 2). It showed greatly enhanced shoot biomass by 100%, 77%, and 82%, in the presence of Cr, Zn and Cu, respectively. Root biomass was improved by 54%, 21% and 46%, in the presence of Cr, Zn and Cu, respectively.
Fig. 2.
Influence of Cellulosimicrobium sp. (NF2) inoculation on Medicago sativa plant growth in the absence (Control) and in the presence of heavy metals (Cr(VI), Zn and Cu). For the same parameter, values with different letters are significantly different (P < 0.05).
Several studies have also shown that metal-tolerant rhizobacteria were capable of stimulating plant growth in either the presence or absence of toxic concentrations of heavy metals like Cd [47], Ni [26], Pb [48] or Cr(VI) [32], [39], [40], [49]. Karthik et al. [44] showed the Cr(VI) tolerant and plant growth promotion ability of C. funkei strain under the Cr(VI) stress. In this study, cellulosimicrobium sp. was isolated from a multicontaminated site and showed an ability to increase plant growth under stress by Cr, Cu and Zn.
Plant growth promoting bacteria can increase the growth and development of the plants either indirectly by reducing the toxic effects of metals or directly by producing the phytohormones [50]. Indeed, PGP traits have been successfully implicated in promoting plant growth and concurrently mitigating the degree of toxicity or damage to plants exposed to stress generated by different heavy metals. Halstead et al. [51] have demonstrated that the high heavy metals concentration in soil affects the plant growth because it interferes with the uptake of nutrients such as phosphorus. This deficiency can be compensated by the phosphate-solubilization ability of bacteria that play a crucial role in enhancing the plants uptake of soil minerals such as P in metal-contaminated soils [52].
The phytohormone production by PGPB is shown to play a key role in plant–bacterial interactions and plant growth in contaminated soils by heavy metals [53]. In fact, many heavy metals resistant bacteria were capable of producing phytohormones such as auxin IAA even under stress conditions [54], [55]. Thus, the observed plant growth promotion under Pb stress after inoculation of plant with P. fluorescens was attributed to bacterial IAA production and excretion [56]. Madhaiyan et al. [57] reported the greater potential of the endophytic bacteria, Burkholderia sp. and Methylobacterium oryzae to enhance growth of Lycopersicon esculentum under Ni and Cd stress. This effect was assigned to the ability of the bacteria to lower the level of stress ethylene induced by Ni and Cd or to the possible contribution of the endophytes in reducing the phytotoxic effects of the metals through their capability of biosorption and bioaccumulation.
Production of siderophores may stimulate plant growth directly under iron limitation conditions [58], or indirectly by forming stable complexes with heavy metals such as Zn, Al, Cu and Pb and helping plants to alleviate the metal stresses [59]. Moreover, siderophores secreted by PGPB strains can decrease the formation of free radicals, so that it allows protecting microbial auxins from degradation and enabling them to enhance plant growth [60].
4. Effect of bacterial inoculation on metal uptake by plants
The total metal contents and the accumulation factor noticed in the shoots and the roots of alfalfa after 45 days of culture are shown in Table 2. The results show that the root tissues accumulated more metals than shoots in both inoculated and non-inoculated alfalfa plants. The inoculation of NF2 significantly increased the roots uptake of Cr, Zn and Cu by 43%, 37% and 53%, respectively. It also significantly increased shoots uptake of Zn and Cu (41% and 54%, respectively), while no significant difference was noticed in shoot chromium contents.
Table 2.
Effect of Cellulosimicrobium sp. (NF2) on Cr, Zn and Cu content (µg g−1) and bioaccumulation factor (BAF) of the shoots and roots of alfalfa grown on contaminated soils with Cr(VI), Zn and Cu, respectively. For each metal, values with different letters are significantly different (P < 0.05).
| Treatment | Metal analysed | Metal uptake (µg g−1 of dry weight) |
BAF |
||
|---|---|---|---|---|---|
| Roots | Shoots | Roots | Shoots | ||
| Cr(VI) | Cr | 15.03 a | 6.98 a | 0.301 | 0.140 |
| Cr(VI) + NF2 | Cr | 21.58b | 7.75 a | 0.432 | 0.155 |
| Zn | Zn | 312.05 a | 102.60 a | 0.624 | 0.205 |
| Zn + NF2 | Zn | 428.12b | 144.98b | 0.856 | 0.290 |
| Cu | Cu | 115.27 a | 39.07 a | 0.461 | 0.156 |
| Cu + NF2 | Cu | 177.44b | 60.00b | 0.710 | 0.240 |
Increased metal concentrations in plant tissues were reported in inoculated plants by many PGPB. For example, in Cu rich environment, Pantoea sp. inoculated alfalfa showed 15% increase in Cu accumulation and in Pb-Zn rich environment, Zn accumulation increased by 30.3% [61]. Bacillus cereus increased sequestration of heavy metals (Fe, Mn, Zn, and Cd) in Trifolium repens [62]. The bacteria Enterobacter sp. and Klebsiella sp. improved the accumulation of Cd, Pb and Zn by Brassica napus [63]. However, in some cases it has been reported that the inoculation with metal resistant bacteria decreased the uptake of metals by the plants and thereby increased plant biomass [64]. These effects were attributed to metals immobilization in the rhizosphere. This is particularly the case of chromium where most authors reported significantly lower Cr accumulation in bacterial inoculated plant due to the immobilization of Cr by bacteria through several ways, including adsorption, accumulation, reduction, secretion of cell surface associated polysaccharides and proteins [65], [66]. In the present study, it is noticeable that chromium uptake by the plant roots was significantly increased by NF2 inoculation probably through Cr (VI) reduction to Cr (III), which would have favored the immobilization of Cr in the rhizosphere and in the plant roots.
5. Conclusion
The isolated multi-metals resistant bacteria seemed to be efficient plant growth-promoting bacteria that could produce IAA, siderophores, solubilize the phosphate. Moreover, the ability of these bacteria to reduce Cr(VI) could lower Cr(VI) toxicity and support plant growth in Cr-contaminated soil. The selected PGPB, Cellulosimicrobium sp. NF2 was able to promote plant growth of alfalfa even under metal stress and increased metal uptake by the plants. It could therefore be a good choice for application in microbially assisted phytoremediation approaches for depollution of multi-metals contaminated soils.
Acknowledgments
The authors thankfully acknowledge the financial and scientific support of Microbial Biotechnology Laboratory and the “Centre Universitaire d’Interface” SMBA University, Fez, Morocco.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
References
- 1.Nriagu J.O. Toxic metal pollution in Africa. Sci Total Environ. 1992;121:1–37. doi: 10.1016/0048-9697(92)90304-b. [DOI] [PubMed] [Google Scholar]
- 2.Jadia C.D., Fulekar M.H. Phytoremediation of heavy metals: recent techniques. Afr J Biotechnol. 2009;8:921–928. [Google Scholar]
- 3.Akcil A., Erust C., Ozdemiroglu S., Fonti V., Beolchini F. A review of approaches and techniques used in aquatic contaminated sediments: metal removal and stabilization by chemical and biotechnological processes. J Clean Prod. 2015;86:24–26. [Google Scholar]
- 4.Glick B.R. Using soil bacteria to facilitate phytoremediation. Biotechnol Adv. 2010;28:367–374. doi: 10.1016/j.biotechadv.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Aboulroos S.A., Helal M.I.D., Kamel M.M. Remediation of Pb and Cd polluted soils using in situ immobilization and phytoextraction techniques. Soil Sediment Contam. 2006;15:199–215. [Google Scholar]
- 6.Ebbs S.D., Lasat M.M., Brady D.J., Cornish J., Gordon R., Kochian L.V. Phytoextraction of cadmium and zinc from a contaminated soil. J Environ Qual. 1997;26:1424–1430. [Google Scholar]
- 7.Salt D.E., Blaylock M., Kumar N.P., Dushenkov V., Ensley B.D., Chet I., Raskin I. Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Biotechnology (N. Y) 1995;13:468–474. doi: 10.1038/nbt0595-468. [DOI] [PubMed] [Google Scholar]
- 8.Lasat M.M. Phytoextraction of toxic metals: a review of biological mechanisms. J Environ Qual. 2002;31:109–120. [PubMed] [Google Scholar]
- 9.Baldantoni D., Bellino A., Cicatelli A., Castiglione S. Artificial mycorrhization does not influence the effects of iron availability on Fe, Zn, Cu, Pb and Cd accumulation in leaves of a heavy metal tolerant white poplar clone. Plant Biosyst. 2011;145:236–240. [Google Scholar]
- 10.Chen Y., Li X., Shen Z. Leaching and uptake of heavy metals by ten different species of plants during an EDTA-assisted phytoextraction process. Chemosphere. 2004;57:187–196. doi: 10.1016/j.chemosphere.2004.05.044. [DOI] [PubMed] [Google Scholar]
- 11.Grčman H., Vodnik D., Velikonja-Bolta Š., Leštan D. Ethylenediamine dissuccinate as a new chelate for environmentally safe enhanced: lead phytoextraction. J Environ Qual. 2003;32:500–506. doi: 10.2134/jeq2003.5000. [DOI] [PubMed] [Google Scholar]
- 12.Wenzel W.W. Rhizosphere processes and management in plant-assisted bioremediation (phytoremediation) of soils. Plant Soil. 2009;321:385–408. [Google Scholar]
- 13.Aafi N.E., Brhada F., Dary M., Maltouf A.F., Pajuelo E. Rhizostabilization of metals in soils using Lupinus luteus inoculated with the metal resistant rhizobacterium Serratia sp. MSMC 541. Int J Phytorem. 2012;14:261–274. doi: 10.1080/15226514.2011.604693. [DOI] [PubMed] [Google Scholar]
- 14.Yang Q., Tu S., Wang G., Liao X., Yan X. Effectiveness of applying arsenate reducing bacteria to enhance arsenic removal from polluted soils by Pteris vittata L. Int J Phytorem. 2012;14:89–99. doi: 10.1080/15226510903567471. [DOI] [PubMed] [Google Scholar]
- 15.Ahemad M., Khan M.S. Alleviation of fungicide-induced phytotoxicity in greengram [Vigna radiata (L.) Wilczek] using fungicide-tolerant and plant growth promoting Pseudomonas strain. Saudi J Biol Sci. 2012;19:451–459. doi: 10.1016/j.sjbs.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Washington J.A., Sutter V.L. Dilution tests procedures. In: Balows A., Hausler W.J., Truant J.P., Lennette E.H., editors. Manual of clinical microbiology. American Society for Microbiology; Washington D.C.: 1981. pp. 549–555. [Google Scholar]
- 17.Pattanapipitpaisal P., Brown N.L., Macaskie L. Chromate reduction and 16S rRNA identification of bacteria isolated from Cr(VI) contaminated site. Appl Microbiol Biotechnol. 2001;57:257–261. doi: 10.1007/s002530100758. [DOI] [PubMed] [Google Scholar]
- 18.Bharadwaj D.P., Lundquist P.O., Alström S. Arbuscular mycorrhizal fungal spore-associated bacteria affect mycorrhizal colonization, plant growth and potato pathogens. Soil Biol Biochem. 2008;40:2494–2501. [Google Scholar]
- 19.Schwyn B., Neilands J.B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 20.Nautiyal C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett. 1999;170:265–270. doi: 10.1111/j.1574-6968.1999.tb13383.x. [DOI] [PubMed] [Google Scholar]
- 21.Battini F., Cristani C., Giovannetti M., Agnolucci M. Multifunctionality and diversity of culturable bacterial communities strictly associated with spores of the plant beneficial symbiont Rhizophagus intraradices. Microbiol Res. 2016;183:68–79. doi: 10.1016/j.micres.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Weisburg D.J., Barns W.G., Pelletier S.M., Lane D.A. 16S Ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cole P., Wang J.R., Fish Q., Chai J.A., McGarrell B., Sun D.M., Brown Y., Alfaro C.J.M., Kuske A., Tiedje C.R. Ribosomal database project: data and tools for high throughput rRNA analysis. Nucl Acids Res. 2014;42:633–642. doi: 10.1093/nar/gkt1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies J.R., Fred T., Puryear D. Jeffrey, Newton Ronald J. Mycorrhizal fungi enhance accumulation and tolerance of chromium in sunflower (Helianthus annuus) J Plant Physiol. 2001;158:777–786. [Google Scholar]
- 25.Ghosh M., Singh S.P.A. Review on phytoremediation of heavy metals and utilization of it’s by products. Asian J Energy Environ. 2005;6:18. [Google Scholar]
- 26.Ma Y., Rajkumar M., Freitas H. Improvement of plant growth and nickel uptake by nickel resistant-plant-growth promoting bacteria. J Hazard Mater. 2009;166:1154–1161. doi: 10.1016/j.jhazmat.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 27.Mishra P.M., Naik G.K., Nayak A., Parida K.M. Facile synthesis of nano-structured magnetite in presence of natural surfactant for enhanced photocatalytic activity for water decomposition and Cr (VI) reduction. Chem Eng J. 2016;299:227–235. [Google Scholar]
- 28.Ilias M., Rafiqullah I.M., Debnath B.C., Bin Mannan K.S., Mozammel Hoq M. Isolation and characterization of chromium(VI)-reducing bacteria from tannery effluents. Indian J Microbiol. 2011;51:76–81. doi: 10.1007/s12088-011-0095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishra S., Doble M. Novel chromium tolerant microorganisms: isolation, characterization and their biosorption capacity. Ecotoxicol Environ Saf. 2008;71:874–879. doi: 10.1016/j.ecoenv.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 30.Joutey N. Tahri, Bahafid W., Sayel H., Nassef S., El Ghachtouli N., El Ghachtouli N. Leucobacter chromiireducens CRB2, a new strain with high Cr(VI) reduction potential isolated from tannery-contaminated soil (Fez, Morocco) Ann Microbiol. 2016;66:425–436. [Google Scholar]
- 31.Sayel H., Joutey N.T., Bahafid W., El Ghachtouli N. Chromium resistant bacteria: impact on plant growth in soil microcosm. Arch Environ Prot. 2014;40:81–89. [Google Scholar]
- 32.Wani R., Kodam K.M., Gawai K.R., Dhakephalkar P.K. Chromate reduction by Burkholderia cepacia MCMB-821, isolated from the pristine habitat of alkaline crater lake. Appl Microbiol Biotechnol. 2007;75:627–632. doi: 10.1007/s00253-007-0862-7. [DOI] [PubMed] [Google Scholar]
- 33.Donati E., Oliver C., Curutchet G. Reduction of chromium (VI) by the indirect action of Thiobacillus thioparus. Braz J Chem Eng. 2003;20:69–73. [Google Scholar]
- 34.Ramírez-Díaz M.I., Díaz-Pérez C., Vargas E., Riveros-Rosas H., Campos-García J., Cervantes C. Mechanisms of bacterial resistance to chromium compounds. Biometals. 2008;21:321–332. doi: 10.1007/s10534-007-9121-8. [DOI] [PubMed] [Google Scholar]
- 35.Lambrecht M., Okon Y., Vande Broek A., Vanderleyden J. Indole-3-acetic acid: a reciprocal signalling molecule in bacteria-plant interactions. Trends Microbiol. 2017;8:298–300. doi: 10.1016/s0966-842x(00)01732-7. [DOI] [PubMed] [Google Scholar]
- 36.Rajkumar M., Ae N., Prasad M.N.V., Freitas H. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 2010;28:142–149. doi: 10.1016/j.tibtech.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Zhu F., Qu L., Hong X., Sun X. Isolation and characterization of a phosphate-solubilizing halophilic bacterium Kushneria sp. ycwa18 from daqiao saltern on the coast of yellow sea of China. Evid Based Complement Alternat Med. 2011;2011:1–6. doi: 10.1155/2011/615032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodríguez H., Fraga R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv. 1999;17:319–339. doi: 10.1016/s0734-9750(99)00014-2. [DOI] [PubMed] [Google Scholar]
- 39.Hemambika B., Balasubramanian V., Kannan V.R., James R.A. Screening of chromium-resistant bacteria for plant growth-promoting activities. Soil Sediment Contam An Int J. 2013;22:717–736. [Google Scholar]
- 40.Rajkumar M., Nagendran R., Lee K.J., Lee W.H., Kim S.Z. Influence of plant growth promoting bacteria and Cr6+ on the growth of Indian mustard. Chemosphere. 2006;62:741–748. doi: 10.1016/j.chemosphere.2005.04.117. [DOI] [PubMed] [Google Scholar]
- 41.Chatterjee S., Sau G.B., Mukherjee S.K. Plant growth promotion by a hexavalent chromium reducing bacterial strain, Cellulosimicrobium cellulans KUCr3. World J Microbiol Biotechnol. 2009;25:1829–1836. [Google Scholar]
- 42.Hemambika B., Kannan V.R. Intrinsic characteristics of Cr(6+)-resistant bacteria isolated from an electroplating industry polluted soils for plant growth-promoting activities. Appl Biochem Biotechnol. 2012;167:1653–1667. doi: 10.1007/s12010-012-9606-y. [DOI] [PubMed] [Google Scholar]
- 43.Nabti E., Bensidhoum L., Tabli N., Dahel D., Weiss A., Rothballer M., Hartmann A. Growth stimulation of barley and biocontrol effect on plant pathogenic fungi by a Cellulosimicrobium sp. strain isolated from salt-affected rhizosphere soil in northwestern Algeria. Eur J Soil Biol. 2014;61:20–26. [Google Scholar]
- 44.Karthik C., Oves M., Thangabalu R., Sharma R., Santhosh S.B., Arulselvi P.I. Cellulosimicrobium funkei-like enhances the growth of Phaseolus vulgaris by modulating oxidative damage under Chromium (VI) toxicity. J Adv Res. 2016;7:839–850. doi: 10.1016/j.jare.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karthik C., Barathi S., Pugazhendhi A., Ramkumar V.S., Thi N.B.D., Arulselvi P.I. Evaluation of Cr (VI) reduction mechanism and removal by Cellulosimicrobium funkei strain AR8, a novel haloalkaliphilic bacterium. J Hazard Mater. 2017;333:42–53. doi: 10.1016/j.jhazmat.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 46.Bharagava R.N., Mishra S. Hexavalent chromium reduction potential of Cellulosimicrobium sp. isolated from common effluent treatment plant of tannery industries. Ecotoxicol Environ Saf. 2018;147:102–109. doi: 10.1016/j.ecoenv.2017.08.040. [DOI] [PubMed] [Google Scholar]
- 47.Belimov A.A., Hontzeas N., Safronova V.I., Demchinskaya S.V., Piluzza G., Bullitta S., Glick B.R. Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.) Soil Biol Biochem. 2005;37:241–250. [Google Scholar]
- 48.Hasnain S., Yasmin S., Yasmin A. The effects of lead-resistant Pseudomonads on the growth of Triticum aestivum seedlings under lead stress. Environ Pollut. 1993;81:179–184. doi: 10.1016/0269-7491(93)90084-2. [DOI] [PubMed] [Google Scholar]
- 49.Maqbool Z., Asghar H.N., Shahzad T., Hussain S., Riaz M., Ali S., Maqsood M. Isolating, screening and applying chromium reducing bacteria to promote growth and yield of okra (Hibiscus esculentus L.) in chromium contaminated soils. Ecotoxicol Environ Saf. 2015;114:343–349. doi: 10.1016/j.ecoenv.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 50.Mallick I., Bhattacharyya C., Mukherji S., Dey D., Sarkar S.C., Mukhopadhyay U.K., Ghosh A. Effective rhizoinoculation and biofilm formation by arsenic immobilizing halophilic plant growth promoting bacteria (PGPB) isolated from mangrove rhizosphere: A step towards arsenic rhizoremediation. Sci Total Environ. 2018;610–611:1239–1250. doi: 10.1016/j.scitotenv.2017.07.234. [DOI] [PubMed] [Google Scholar]
- 51.Halstead R.L., Finn B.J., MacLean A.J. Extractability of nickel added to soils and its concentration in plants. Can J Soil Sci. 1969;49:335–342. [Google Scholar]
- 52.Zaidi S., Usmani S., Singh B.R., Musarrat J. Significance of Bacillus subtilis strain SJ-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere. 2006;64:991–997. doi: 10.1016/j.chemosphere.2005.12.057. [DOI] [PubMed] [Google Scholar]
- 53.Kuklinsky Sobral J., Araújo W.L., Mendes R., Geraldi I.O., Pizzirani Kleiner A.A., Azevedo J.L. Isolation and characterization of soybean associated bacteria and their potential for plant growth promotion. Environ Microbiol. 2004;6:1244–1251. doi: 10.1111/j.1462-2920.2004.00658.x. [DOI] [PubMed] [Google Scholar]
- 54.Dell’Amico E., Cavalca L., Andreoni V. Improvement of Brassica napus growth under cadmium stress by cadmium-resistant rhizobacteria. Soil Biol Biochem. 2008;40:74–84. [Google Scholar]
- 55.Chiboub M., Saadani O., Fatnassi I.C., Abdelkrim S., Abid G., Jebara M., Jebara S.H. Characterization of efficient plant-growth-promoting bacteria isolated from Sulla coronaria resistant to cadmium and to other heavy metals. C R Biol. 2016;339:391–398. doi: 10.1016/j.crvi.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 56.Sheng X.F., Xia J.J., Jiang C.Y., He L.Y., Qian M. Characterization of heavy metal-resistant endophytic bacteria from rape (Brassica napus) roots and their potential in promoting the growth and lead accumulation of rape. Environ Pollut. 2008;156:1164–1170. doi: 10.1016/j.envpol.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 57.Madhaiyan M., Poonguzhali S., Sa T. Metal tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.) Chemosphere. 2007;69:220–228. doi: 10.1016/j.chemosphere.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 58.Indiragandhi P., Anandham R., Madhaiyan M., Sa T.M. Characterization of plant growth-promoting traits of bacteria isolated from larval guts of diamondback moth Plutella xylostella (lepidoptera: plutellidae) Curr Microbiol. 2008;56:327–333. doi: 10.1007/s00284-007-9086-4. [DOI] [PubMed] [Google Scholar]
- 59.Neubauer U., Furrer G., Kayser A., Schulin R. Siderophores, NTA, and citrate: potential soil amendments to enhance heavy metal mobility in phytoremediation. Int J Phytoremediat. 2000;2:353–368. [Google Scholar]
- 60.Dimkpa C.O., Merten D., Svatoš A., Büchel G., Kothe E. Metal-induced oxidative stress impacting plant growth in contaminated soil is alleviated by microbial siderophores. Soil Biol Biochem. 2009;41:154–162. [Google Scholar]
- 61.Li S., Wang J., Gao N., Liu L., Chen Y. The effects of Pantoea sp. strain Y4-4 on alfalfa in the remediation of heavy-metal-contaminated soil, and auxiliary impacts of plant residues on the remediation of saline-alkali soils. Can J Microbiol. 2016;63:278–286. doi: 10.1139/cjm-2016-0369. [DOI] [PubMed] [Google Scholar]
- 62.Azcón R., del Carmen Perálvarez M., Roldán A., Barea J.M. Arbuscular mycorrhizal fungi, Bacillus cereus, and Candida parapsilosis from a multicontaminated soil alleviate metal toxicity in plants. Microb Ecol. 2010;59:668–677. doi: 10.1007/s00248-009-9618-5. [DOI] [PubMed] [Google Scholar]
- 63.Jing Y.X., Yan J.L., He H.D., Yang D.J., Xiao L., Zhong T., Li S.B. Characterization of bacteria in the rhizosphere soils of Polygonum pubescens and their potential in promoting growth and Cd, Pb, Zn uptake by Brassica napus. Int J Phytoremediat. 2014;16(4):321–333. doi: 10.1080/15226514.2013.773283. [DOI] [PubMed] [Google Scholar]
- 64.Madhaiyan M.S., Poonguzhali S., Sa T. Metal tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.) Chemosphere. 2007;69(2):220–228. doi: 10.1016/j.chemosphere.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 65.Oves M., Khan M.S., Zaidi A. Biosorption of heavy metals by Bacillus thuringiensis strain OSM29 originating from industrial effluent contaminated north Indian soil. Saudi J Biol Sci. 2013;20(2):121–129. doi: 10.1016/j.sjbs.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wani P.A., Khan M.S. Bacillus species enhance growth parameters of chickpea (Cicer arietinum L.) in chromium stressed soils. Food Chem Toxicol. 2010;48(11):3262–3267. doi: 10.1016/j.fct.2010.08.035. [DOI] [PubMed] [Google Scholar]