Abstract
Micelles are highly attractive nano-drug delivery systems for targeted cancer therapy. While they have been demonstrated to significantly alleviate the side-effects of their cargo drugs, the therapy outcomes are usually suboptimal partially due to ineffective drug release and endosome entrapment. Stimulus-responsive nanoparticles have allowed controlled drug release in a smart fashion, and we want to use this concept to design novel micelles. Herein, we reported pH-sensitive paclitaxel (PTX)-loaded poly (ethylene glycol)-phenylhydrazone-dilaurate (PEG-BHyd-dC12) micelles (PEG-BHyd-dC12/PTX). The micelles were spherical, with an average particle size of ∼135 nm and a uniform size distribution. The pH-responsive properties of the micelles were certified by both colloidal stability and drug release profile, where the particle size was strikingly increased accompanied by faster drug release as pH decreased from 7.4 to 5.5. As a result, the micelles exhibited much stronger cytotoxicity than the pH-insensitive counterpart micelles against various types of cancer cells due to the hydrolysis of the building block polymers and subsequent rapid PTX release. Overall, these results demonstrate that the PEG-BHyd-dC12 micelle is a promising drug delivery system for cancer therapy.
Keywords: pH-sensitive, micelles, cancer, paclitaxel, endosomal escape
Introduction
With the development of nanotechnology, various materials such as polymers, lipid, and metals (oxides), have been widely applied to design drug delivery system, especially for cancer therapy (Farokhzad and Langer, 2009). Nanoparticles based on the above materials have been demonstrated to realize controlled drug release and effectively targeting drug delivery (Wilczewska et al., 2012). To this end, micelles composed of amphipathic copolymers have received wide attention owing to their attractive features, such as small and uniform size, tumor targeting ability via the enhanced permeability and retention (EPR) effect, high stability in aqueous solution and excellent biocompatibility (Felber et al., 2012; Liu J. et al., 2014; Wang et al., 2018).
However, albeit with the extensive research efforts, the clinical translations of micelles from bench to bedsides are rather limited, partially due to their suboptimal therapy outcomes caused by the inefficient drug release at the tumor site and the endosomal entrapment of micelles (Kanamala et al., 2016). Plain micelles exhibit relatively slow drug release rate, which may result in ineffective drug concentration inside targeted cells (Wu et al., 2013). To mitigate these issues, smarter micelles are desired to be equipped with endosomal escape and rapid drug release abilities, which could be able to provide sufficient drug concentration for effective killing of the tumor cells.
To achieve such goals, environmentally sensitive polymers that can respond to different stimuli to trigger drug release have been extensively investigated, such as light (Liu et al., 2012; Cao et al., 2013), temperature (Kim et al., 2010; Wang et al., 2014), ultrasound (Yin et al., 2013; Ahmed et al., 2015), magnetic field (Ao et al., 2014; Deng et al., 2015), pH (Liu Y. et al., 2014; Yuba et al., 2017), redox properties (Yin et al., 2015; Zhang et al., 2016), and enzyme activity (Rao and Khan, 2013; Harnoy et al., 2014). Among of them, the pH-sensitive polymeric micelle appears to be a highly appealing candidate due to the intrinsic differences between solid tumors and the surrounding normal tissues in terms of their relative acidity. The pH-sensitive polymer micelles were devised based on copolymers composed of hydrophobic and hydrophilic polymers linked via acid-liable bonds, including hydrazone (Mo et al., 2012), benzoic imine (Yuan et al., 2012), oxime (Liu B. et al., 2014), acetal (Li et al., 2016), ester (Gao et al., 2018) and orthoester (Tang et al., 2011). Hydrolysis of the acid-labile bonds leads to rapid drug release at an acidic pH.
Herein, we synthesized the amphiphilic polymer PEG-BHyd-dC12 via an acid-labile hydrazone bond and constructed pH-responsive micelles. The hydrophilic PEG segment on micelles surface affords high colloidal stability in vitro and long circulation time in vivo, while it is readily departed from micelles at the tumor site under acid conditions, which is beneficial for cellular uptake (Du et al., 2011). Paclitaxel (PTX), one of the most effective antitumor drugs, was encapsulated into micelles due to its hydrophobic nature, and released in a pH-responsive manner. For comparison, the pH-insensitive counterpart polymer of PEG-BAmi-dC12 was also synthesized for micelles preparation. The physicochemical characterization, colloidal stability, drug release, cellular uptake, and in vitro cytotoxicity of the micelles were evaluated.
Materials and Methods
Chemicals and Reagents
Paclitaxel (PTX), 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC), N-hydroxysulfosuccinimide (NHS), 4-dimethylaminopyridine (DMAP), lauroyl chloride, α-methoxy-x-amino-poly(ethylene glycol) (Mn = 2000) (MeO-PEG2000-NH2) were purchased from Shanghai Aladdin Reagent Co. Ltd. (Shanghai, China). mPEG-hydrazide (Mn = 2000) was from Seebio Biotech, Inc. (Shanghai, China), and 3,5-dihydroxybenzaldehyde was from Bide Pharmatech Ltd. (Shanghai, China). 3,5-Dihydroxybenzoic acid was obtained from Saen Chemical Technology Co. Ltd. (Shanghai, China). Potassium hydroxide (KOH), tetrahydrofuran (THF), dimethyl sulfoxide (DMSO), petroleum ether, ethyl acetate were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), coumarine (Cou-6) and 4′,6-diamidino-2-phenylindole (DAPI) were obtained from Sigma-Aldrich Co. (St. Louis, MO, United States). Lysotracker red was supplied from Beyotime Institute of Biotechnology (Jiangsu, China). Dulbecco’s modified Eagle’s medium (DMEM), RPMI 1640, penicillin, streptomycin, phosphate buffered saline (PBS), fetal bovine serum (FBS) were purchased from Gibco Life Technologies, Inc. (Carlsbad, CA, United States). Human lung cancer cells (A549), human breast cancer cells (MDA-MB-231), human ovarian cancer cells (A2780) were obtained from Xiangya cell center (Changsha, China). PTX-resistant human lung cancer cells (A549/T) was bought from Gefan Biotechnology Co., Ltd. (Shanghai, China).
Synthesis of the pH-Sensitive Copolymer PEG-BHyd-dC12
3,5-Dihydroxybenzaldehyde was dissolved in THF, followed by the addition of KOH. Lauroyl chloride was added dropwise into the above mixture and vigorously stirred for 6 h to yield 3,5-dilaurate benzaldehyde. The purified 3,5-dilaurate benzaldehyde and mPEG-hydrazide were dissolved in ethyl alcohol and stirred for 24 h. After purification, the final amphiphilic polymer PEG-BHyd-dC12 was obtained.
Synthesis of the pH-Insensitive Copolymer PEG-BAmi-dC12
First, lauroyl chloride was added dropwise to a mixture of 3,5-dihydroxybenzoic acid with KOH in anhydrous acetone at 0°C under stirring to obtain 3,5-dilaurate benzoic acid. Then, 3,5-dilaurate benzoic acid, EDC, DMAP and NHS were dissolved into DMSO and stirred at room temperature for 2 h, followed by the addition of MeO-PEG2000-NH2. The resulting solution was dialyzed and subsequently lyophilized to obtain PEG-BAmi-dC12.
Characterization of Copolymers
The 1H-NMR spectra of PEG-BHyd-dC12 and PEG-BAmi-dC12 were recorded using a Bruker Avance 400 MHz NMR spectrometer (Varian, United States) with deuterated chloroform (CDCl3) as the solvent. The self-assembly behavior of polymers was investigated by the fluorescence probe technique (Xiong et al., 2017). First, 100 μL of pyrene in acetone (2.9 × 10−2mM) was evaporated to form a thin film on the flask bottom. Then, various concentrations of polymer solutions (from 0.1 μg/mL to 200 μg/mL) were added to the pyrene-coated vials and stored in the dark overnight. The fluorescence intensity ratio of I337/I334 in the emission spectra of pyrene was calculated and plotted against the logarithm of the polymer concentrations. The CMC value was obtained based on the fluorescence excitation spectra of the mixed solution.
Preparation of Micelles
PTX-loaded micelles were prepared by a thin-film hydration method. In brief, PEG-BHyd-dC12 or PEG-BAmi-dC12 (20.0 mg) and PTX (1 mg) were dissolved in dichloromethane (4 mL). The solution was evaporated under reduced pressure to form a uniform film. Deionized water (10 mL) was added and rotated for another 1 h. The obtained colloidal solution was then centrifuged at 3,000 rpm for 10 min and filtered through 0.45 μm pore size filter, followed by lyophilization. Blank micelles were prepared in a similar way in the absence of PTX.
Characterization of Micelles
The particle size, PDI, and zeta potential measurement were determined by dynamic light scattering (DLS) method using a Malvern Zeta Sizer Nano series (Nano ZS, Malvern Instruments, United Kingdom) at 25°C. The morphologies of the micelles were observed using transmission electron microscopy (TEM) (Titan G2-F20, FEI, United States).
The determination of PTX was carried out using a high-performance liquid chromatography (HPLC) system (LC-2010, Shimadzu, Tokyo, Japan). The chromatographic column was an ODS C18 (250 × 4.6 mm, 5 μm, Diamonsil, Beijing, China). The mobile phase consisted of mixtures of acetonitrile and water (55:45, v/v). The flow rate was 1 mL⋅min−1, and the detection wavelength was 227 nm. Micelles were centrifuged in an ultrafiltration tube (MWCO 10 kDa) at 5,000 rpm for 10 min and filtered through 0.22 μm filter to remove the unloaded PTX. PTX-loaded micelles were disrupted by methanol. The PTX loading content (LC) and encapsulated efficiency (EE) were calculated using the following formulae:
Colloidal Stability
Micelles were incubated with 10% FBS or 10 mM phosphate buffer solutions (pH 7.4, 6.5, and 5.5) at 37°C for 72 h, and the size was measured by DLS at different intervals.
In vitro Drug Release
The release study was assessed by the dialysis method. The release media was PBS solutions containing 0.5% Tween-80 with different pH values (5.5, 6.5, and 7.4). Typically, 2 mL of PTX-loaded micelles was placed in a dialysis bag (MWCO 3500) and dialyzed against 25 mL of buffer medium under mechanical shaking (100 rpm) at 37°C. At predetermined time intervals, 2 mL of release medium was withdrawn and replenished with an equal volume of fresh medium. The released PTX was detected by HPLC.
Cell Culture
A549 and A549/T cells were maintained in RPMI 1640 medium supplemented with 10% FBS, penicillin (50 U/mL) and streptomycin (50 U/mL) in a 5% CO2 atmosphere at 37°C. MDA-MB-231 and A2780 were maintained in DMEM medium supplemented with 10% FBS, penicillin (50 U/mL) and streptomycin (50 U/mL) in a 5% CO2 atmosphere at 37°C.
Intracellular Distribution
Cou-6 loaded micelles were constructed according to the above method, except the drug was replaced with Cou-6. A549 cells were seeded on glass coverslips in the 24-well plates at a density of 4 × 104 per well. After culturing for 24 h, Cou-6 loaded micelles ([Cou-6] = 200 ng/mL) were added and incubated for 1 h. Alternatively, the cells were incubated with Cou-6 loaded micelles for 1 h, then washed and cultured in fresh media for another 3 h. Then, the medium was replaced with 70 nM lysotracker red and incubated for another 1 h. Afterward, the cells were fixed with 4% formaldehyde for 20 min at room temperature and visualized using a CLSM (LSM 780, Carl Zeiss, Jena, German).
Cellular Uptake
A549 cells were seeded in 6-well plates with a density of 3 × 105 cells per well and incubated overnight, and then, the medium was replaced with Cou-6 loaded micelles at final Cou-6 concentration of 200 ng/mL. After 1 h or 4 h of incubation, the cells were harvested and quantified by flow cytometry (FACSVerse, BD, United States).
Cytotoxicity Assay
The cytotoxicity of micelles with or without an anticancer drug was determined by MTT assay. The cells were seeded in a 96-well plate at a density of 6,000 cells per well and maintained for 24 h. The medium was then replaced with the micelles and further incubated for 72 h. Then, 20 μL of MTT solution (5 mg/mL) was added to each well of the plate for another 4 h. Subsequently, 100 μL of DMSO was added to dissolve the formazan crystals, and the absorbance was measured at 570 nm by a microplate reader (ELX800, Bio-Tek, United States). The untreated cells were used as controls.
Hemolysis Tests
The hemocompatibility of micelles was evaluated by hemolysis assay (Yang et al., 2016). First, fresh rabbit blood was extracted from the heart of a rabbit. Subsequently, erythrocytes were obtained by centrifugation at 3,000 rpm for 15 min and washed with normal saline (NS). Serial dilutions of micelles were then added to the 2% erythrocytes (v/v) and incubated for 2 h at 37°C in a thermostatic water bath. Finally, the mixtures were centrifuged at 3,500 rpm for 15 min, and the supernatant of all samples was measured for UV absorbance (A) at 540 nm. NS and 0.5% Triton X-100 were regarded as the negative and positive controls, respectively. The hemolysis ratio was calculated as follows:
Statistical Analysis
The data were expressed as the mean ± standard deviation (SD). Statistical analysis was performed using a two-tailed Student’s t-test and analysis of variance (ANOVA) with the aid of SPSS 23.0 software. Differences were considered statistically significant when p-values were less than 0.05.
Results and Discussion
Synthesis and Characterizations of Copolymers
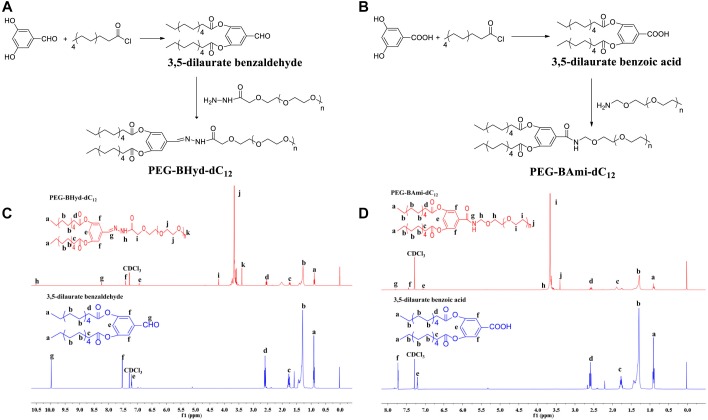
The synthesis schemes of PEG-BHyd-dC12 and PEG-BAmi-dC12 were illustrated in Figures 1A,B. To synthesize the PEG-BHyd-dC12 di-block amphiphilic polymer, the hydrophobic fragment of 3,5-dilaurate benzaldehyde was conjugated with the hydrophilic fragment of PEG through the linkage of hydrazone. The 3,5-dihydroxybenzaldehyde was first reacted with lauroyl chloride to form a 3,5-dilaurate benzaldehyde intermediate with a yield of 90%, and then the aldehyde group on 3,5-dilaurate benzaldehyde reacted with the hydrazine groups on mPEG-hydrazide to give PEG-BHyd-dC12 with a final yield was 69%. All of the synthetic compounds were characterized by 1H-NMR spectra (Figures 1C,D), which were in good agreement with their depicted structures as described in the following:
FIGURE 1.
Synthesis and characterization of PEG-BHyd-dC12 (A,C) and PEG-BAmi-dC12 (B,D). The synthesis of PEG-BHyd-dC12 (A) and PEG-BAmi-dC12 (B), H1-NMR spectrum of PEG-BHyd-dC12 (C), and PEG-BAmi-dC12 (D).
1H NMR of intermediate compound 3,5-dilaurate benzaldehyde: 1H NMR (400 MHz, CDCl3) δ (ppm) 0.91 (6H, t, -CH3), 1.21–1.45 (32H, m, -(CH2)n), 1.75 (4H, m, CO-βH), 2,59 (4H, t, CO-αH), 7.20 (1H, t, 4-ArH), 7.52 (2H, d, 2,6-ArH), 9.98 (1H, s, -CHO).
1H NMR of PEG-BHyd-dC12: 1H NMR (400 MHz, CDCl3) δ (ppm) 0.89 (6H, t, -CH3), 1.22–1.45 (32H, m, -(CH2)n), 1.74 (4H, m, CO-βH), 2,54 (4H, t, CO-αH), 3.39 (3H, s, -OCH3 from PEG), 3.50–3.84 ((-OCH2CH2-)n), 4.19 (2H, s, CO-αH, from PEG), 6.94 (1H, t, 4-ArH), 7.41 (2H, d, 2,6-ArH), 8.24 (1H, s, -NH), 10.5 (1H, s, -CH = N).
As for PEG-BHyd-dC12, the characteristic peaks at 3.5–3.84 ppm were from PEG, and the proton peak at 10.5 ppm indicated the formation of the hydrazone bond. In addition, the absence of proton peak of aldehyde (9.98 ppm) suggested that free 3,5-dilaurate benzaldehyde was removed in the purified PEG-BHyd-dC12.
1H NMR of intermediate compound 3,5-dilaurate benzoic acid: 1H NMR (400 MHz, CDCl3) δ (ppm) 0.90 (6H, t, -CH3), 1.21–1.44 (32H, m, -(CH2)n), 1.71 (4H, m, CO-βH), 2,61 (4H, t, CO-αH), 7.20 (1H, t, 4-ArH), 7.72 (2H, d, 2,6-ArH).
1H NMR of PEG-BAmi-dC12: 1H NMR (400 MHz, CDCl3) δ (ppm) 0.90 (6H, t, -CH3), 1.22–1.45 (32H, m, -(CH2)n), 1.75 (4H, m, CO-βH), 2,58 (4H, t, CO-αH), 3.40 (3H, s, -OCH3 from PEG), 3.50–3.84 ((-OCH2CH2-)n), 7.06 (1H, t, 4-ArH), 7.45 (2H, d, 2, 6-ArH), 7.79 (1H, d, -CONH).
The characteristic peaks of PEG (3.50–3.84 ppm) were obvious, and the peak of new amide bond can be seen at 7.79 ppm for PEG-BAmi-dC12.
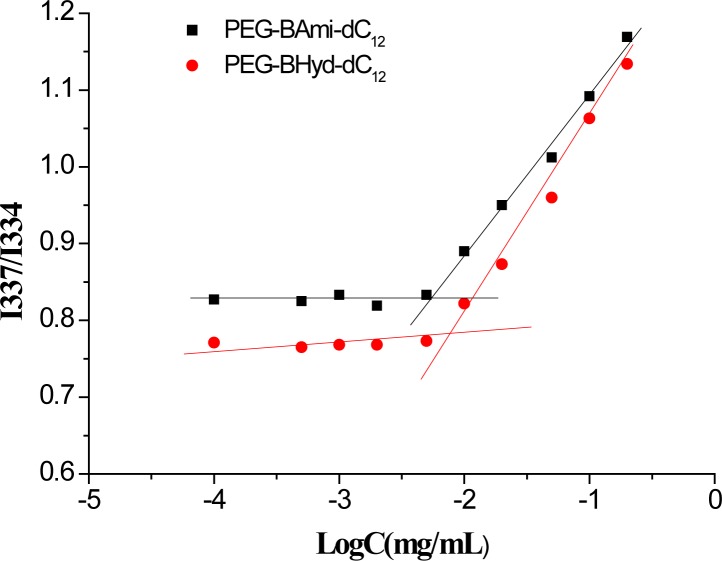
CMC Measurement
As amphiphilic materials, a key parameter for their applications as a nanocarrier is their CMC. Micelles can be formed at concentrations above the CMC. The CMC values of PEG-BHyd-dC12 and PEG-BAmi-dC12 were determined by a well-established method using pyrene as a fluorescence probe, resulting in 7.5 μg/mL for PEG-BHyd-dC12 and 5.6 μg/mL for PEG-BAmi-dC12 (Figure 2). These CMC values were within the typical concentration range for most polymeric micelle CMCs, which can be directly applied in vivo (Maysinger et al., 2007; Diezi et al., 2010; Owen et al., 2012). It is reasonable that these two polymers have comparable CMC values, as their structures are nearly identical; they only differed at the junction between the hydrophobic and hydrophilic blocks (one with a hydrazone bond and the other with an amide bond). Therefore, PEG-BAmi-dC12 is an excellent control to study the pH-responsive property of PEG-BHyd-dC12 for drug delivery.
FIGURE 2.
The CMC curve of PEG-BHyd-dC12 and PEG-BAmi-dC12.
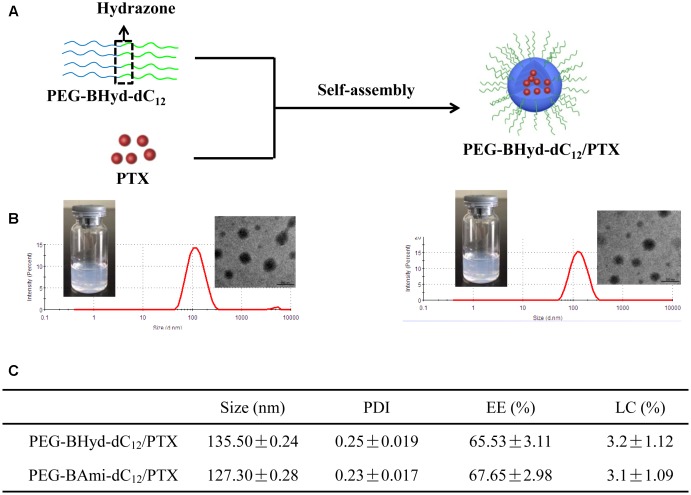
Preparation and Characterization of Micelles
From the above experiments, we have demonstrated that both PEG-BHyd-dC12 and PEG-BAmi-dC12 were able to self-assemble into micelles at very low concentrations, implying their applicability for the development of a nano-drug delivery system. We next used these polymers to prepare micelles, and the hydrophobic PTX was used a model to encapsulate into the hydrophobic core of the micelles (Figure 3A). The pH-sensitive micelles (PEG-BHyd-dC12/PTX) were prepared using a standard thin-film hydration method. After removing the organic solvents, the solution appeared to be semi-transparent with light-blue opalescence (Inset in Figure 3B, left), suggesting the successful preparation of nano-sized micelles. The particle size was approximately 135 nm as determined by DLS (Figure 3B, left); this size is suitable for passive accumulation in the tumor tissue through the EPR effect (Danhier et al., 2010). From TEM, the micelles were well dispersed with spherical morphology (Inset in Figure 3B, left). The LC efficiency of PEG-BHyd-dC12/PTX was 3% (Figure 3C), which was comparable to many other PTX-loading micelles reported previously, and was sufficient for subsequent in vitro/in vivo therapeutic applications (Lee et al., 2003; Zhu et al., 2010; Mei et al., 2015).
FIGURE 3.
Preparation and characterization of micelles. Schematic preparation (A), appearance, size distribution, and TEM images (B, left presents PEG-BHyd-dC12/PTX, right presents PEG-BAmi-dC12/PTX), characterization (C). Data were presented as mean ± standard deviation (SD; n = 3).
By using the same method, the pH-insensitive PEG-BAmi-dC12/PTX micelles were also prepared and characterized (Figure 3B, right; Figure 3C). Interestingly, these two types of micelles displayed quite similar properties in terms of appearance, particle size, morphology and drug loading efficiency. Therefore, a parallel comparison between these micelles can be made for their in vitro/in vivo biological performance, which can be rationalized by the pH-responsive bond linkage.
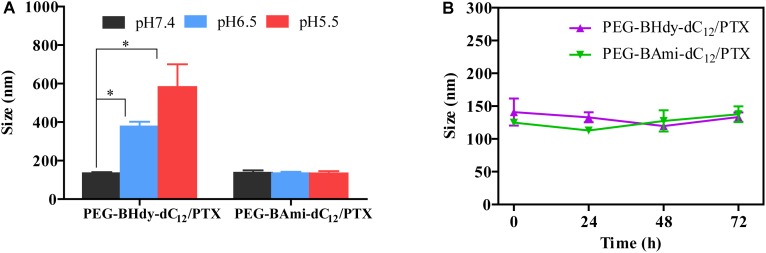
Colloidal Stability
The colloidal stability of the micelles was first studied under different buffer solutions. Interestingly, with pH decrease from 7.4 to 6.5 and 5.5, the particle size of PEG-BHyd-dC12/PTX markedly increased, while it remained unchanged for PEG-BAmi-dC12/PTX (Figure 4A). This can be rationalized by the pH-responsive property of the PEG-BHyd-dC12/PTX, which could swell and then collapse at lower pH (Li et al., 2016; Qiu et al., 2017). We also challenged the micelles with 10% FBS, and both types of micelles were quite stable even after 72 h incubation (Figure 4B). Therefore, the pH-sensitive micelles were stable in blood circulation and can rapidly collapse to release the payload under acidic conditions.
FIGURE 4.
Colloidal stability of micelles. Size change of PEG-BHyd-dC12/PTX micelles and PEG-BAmi-dC12/PTX micelles in phosphate buffers with different pH values (A) and 10% FBS (B) at 37°C for 72 h. The pHs were buffered by disodium hydrogen phosphate and sodium dihydrogen phosphate with total phosphate concentration of 10 mM. Data were shown as mean ± SD (n = 3). ∗p < 0.05.
In vitro Drug Release
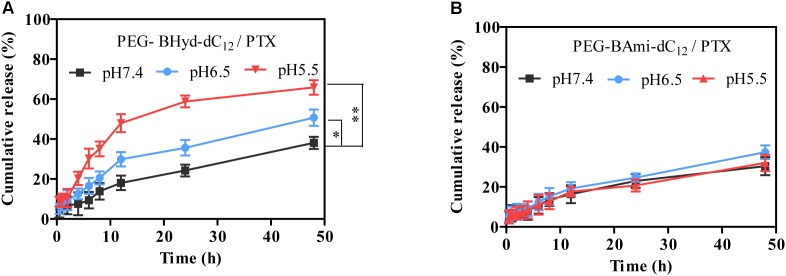
The release behavior of PTX from polymeric micelles was evaluated under various conditions at 37°C. Different buffer solutions were employed to simulate the micro-environment of the blood circulation (pH 7.4), tumor tissue (pH 6.5), and endosome (pH 5.5). We first studied the performance of pH-sensitive PEG-BHyd-dC12/PTX micelles. At pH 7.4, almost no PTX was released in the initial 4 h, which was followed by a sustained release phase with only 38% PTX release after 48 h (Figure 5A, black trace). Therefore, the micelles can stably encapsulate PTX for a long time, which is important for decreasing the side effects and increasing the drug accumulation in tumor sites. By lowering the pH to 6.5, a notable increase in drug release was observed at each time point (Figure 5A, blue trace). With further decrease of the pH to 5.5, the micelles showed an even higher rate of drug release (Figure 5A, red trace). After 48 h, the cumulative drug release was 50% and 65%, respectively, significantly higher than that at pH 7.4 (∼40%), indicating a good pH-responsive capability. This pH-responsive drug release profile can be ascribed to the hydrazone bond between the hydrophilic and hydrophobic chains of the polymer. As the pH decreases, the hydrazone bond tends to hydrolyse and thus the micelles collapse, resulting in burst drug release.
FIGURE 5.
Release profiles of PEG-BHyd-dC12/PTX micelles (A) and PEG-BAmi-dC12/PTX micelles (B) at different pHs at 37°C. The pHs were buffered by disodium hydrogen phosphate and sodium dihydrogen phosphate with total phosphate concentration of 10 mM. Data were shown as mean ± SD (n = 3). ∗p < 0.05, ∗∗p < 0.01.
As a control, we also performed the drug release experiment with pH-insensitive PEG-BAmi-dC12/PTX micelles. In this case, slow and sustained drug release was seen under different conditions, and pH had little effect on the rate of drug release, giving a cumulative drug release of less than 40% after 48 h (Figure 5B). Considering the structural difference between PEG-BHyd-dC12/PTX and PEG-BAmi-dC12/PTX, these results further demonstrated critical role of the hydrazone bond for the pH-sensitive property of the PEG-BHyd-dC12/PTX micelles.
Intracellular Uptake Study
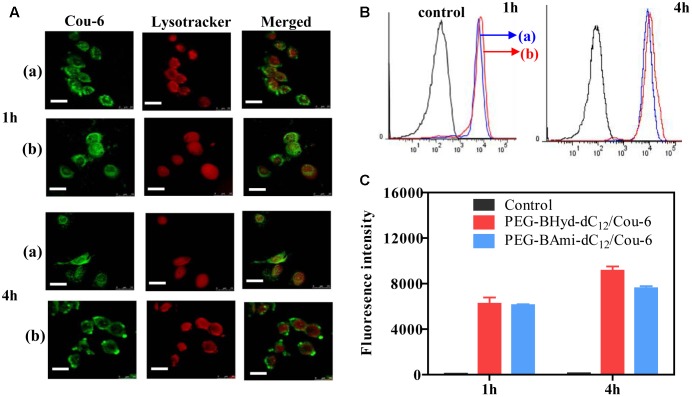
Having demonstrated the pH-responsive property of the PEG-BAmi-dC12/PTX micelles, we next studied the performance of the micelles inside cells. To conveniently track the micelles inside cells, Cou-6 (a hydrophobic green fluorophore) instead of PTX was encapsulated into micelles, and the acidic organelles (i.e., lysosomes and endosomes) were stained by Lysotracker red. A549 cancer cell line was used as a model since PTX has been widely used in clinic for lung cancer therapy (Singla et al., 2002). From confocal laser scanning microscopy (CLSM), substantial green fluorescence was observed for both types of micelles after 1 h incubation (Figure 6A), indicating a high level of cellular internalization. To visualize the co-localization of micelles and endo/lysosomes, we merged the green and red channels, and the emergence of orange spots indicated the localization of micelles in the endo/lysosomes. Both PEG-BHyd-dC12/Cou-6 and PEG-BAmi-dC12/Cou-6 micelles showed obvious spots after 1 h of incubation, consistent with the endocytosis pathway of the micelles (Zhang et al., 2017).
FIGURE 6.
Cellular uptake studies of PEG-BHyd-dC12/Cou-6 and PEG-BAmi-dC12/Cou-6 in A549 cells by using CLSM (A), flow cytometry (B), fluorescence intensities quantified from B (C). The (a) indicated PEG-BAmi-dC12/Cou-6 while the (b) represented PEG-BHyd-dC12/Cou-6. The scale bar is 25 μm.
We next studied the intracellular performance of the micelles. To do this, the cells were washed and cultured in fresh media so that further internalization of micelles was avoided. After 4 h incubation, the pH-insensitive micelles were still largely entrapped into the endo/lysosomes. In contrast, the orange spots of pH-sensitive micelles were weakened, and green color was evenly distributed throughout the cytoplasm, which showed minimal co-localization with the red fluorescence of the endo/lysosomes. The micelles detached from endosome due to hydrolysis of copolymer under acidic organelles, which facilitated efficient release of drug. Therefore, successful endo/lysosomal escape of pH-sensitive micelles was indicated. It is known that the successful escape of a nano-delivery system from the intracellular endosome/lysosome for drug release is a key issue in determining their therapeutic efficiency (Qiu et al., 2017). After cellular uptake, micelles were first entrapped into endosome/lysosome (Chou et al., 2011; Varkouhi et al., 2011). Once entering the endo/lysosomes, the pH-sensitive micelles were disassembled because of pH-triggered hydrolysis of the acid-labile chemical linkage, and the drug rapidly escaped from the endosome/lysosome, resulting in pH-triggered intracellular burst release (Fang et al., 2016).
To have a quantitative understanding, we next performed flow cytometry experiments to study the uptake of micelles by A549 cells (Figures 6B,C). After 1 h incubation, there was no difference in intensity between pH-sensitive and pH-insensitive micelles. Interestingly, after 4 h, the fluorescence from pH-responsive micelles was considerably higher than that of pH-insensitive micelles (Figure 6C), in agreement with a previous report (Qiu et al., 2017). While the pH-responsiveness of micelles has little effect on cell uptake process, the relative lower fluorescence for PEG-BAmi-dC12/PTX was likely due to the efflux of the micelles from cells to medium. As has been demonstrated, the endo/lysosome entrapped micelles can be pumped out by ATP-binding cassette protein B1 (ABCB1) transporter (Sakai-Kato et al., 2012). Since the pH-responsive micelles collapse faster in endo/lysosome, relatively less micelles were cleared from cells by this pump-out process, resulting in stronger fluorescence inside cells.
Cytotoxicity Assay
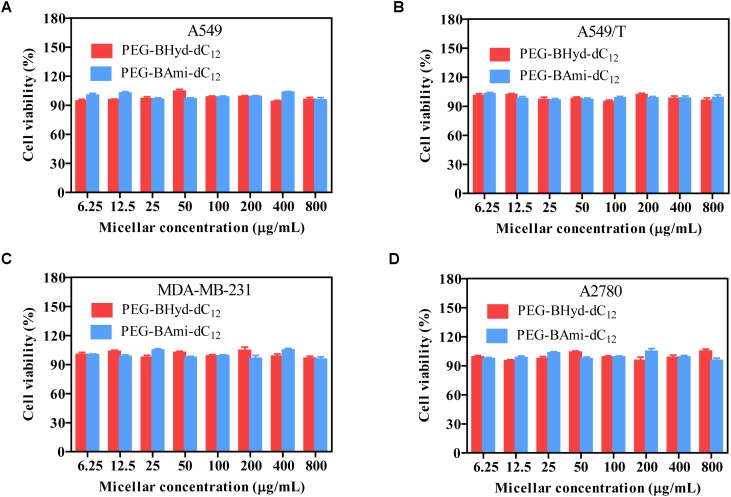
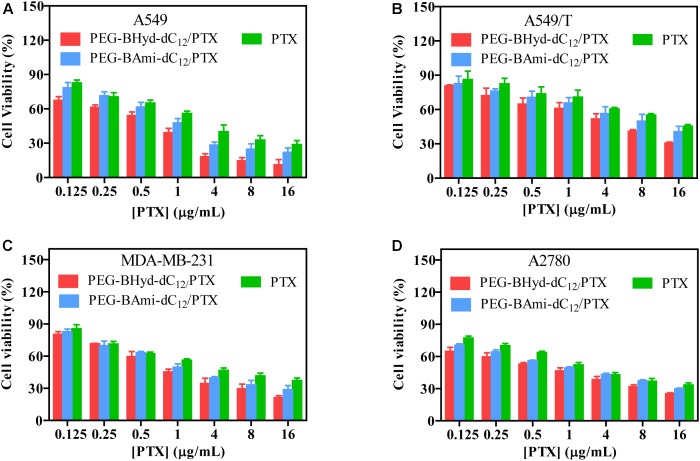
Cytotoxicity studies were performed by incubating micelles with different types of cells for 72 h, and cell viability was measured by MTT assay. The cytotoxicity of the polymers was tested by incubating the cells with blank micelles (without PTX loading), and all types of cells remained >90% viability with concentration up to 800 μg/mL, indicating high biocompatibility (Figure 7). As for A549, at the highest PTX concentration (16 μg/mL), the viabilities of cells incubated with PEG-BHyd-dC12/PTX, PEG-BAmi-dC12/PTX and free PTX dropped to 11%, 22%, and 28%, respectively, showing high toxicity to cancer cells (Figure 8A). The anti-cancer capability was quantified by measuring the half-maximal inhibitory concentration (IC50), which was in order of PEG-BHyd-dC12/PTX (0.57 μg/mL) < PEG-BAmi-dC12/PTX (1.1 μg/mL) < free PTX (1.87 μg/mL) (Table 1). Therefore, PEG-BHyd-dC12/PTX exhibited the highest activity, which was attributable to the pH-responsive property for rapid endo/lysosome drug escape to enhance the antitumor effect.
FIGURE 7.
Cell viability of blank micelles after incubating with A549 (A), A549/T (B), MDA-MB-231 (C), and A2780 (D) cells for 72 h. Data were shown as mean ± SD (n = 4).
FIGURE 8.
Cell viability of PTX-loaded micelles and free PTX after incubating with A549 (A), A549/T (B), MDA-MB-231 (C), and A2780 (D) cells for 72 h. Data were shown as mean ± SD (n = 4).
Table 1.
IC50 value of the micelles and free PTX to A549, A549/T, MDA-MB-231, and A2780 cells for 72 h incubation (mean ± SD, n = 4).
| IC50 (μg/mL) |
||||
|---|---|---|---|---|
| A549 | A549/T | MDB-MA-231 | A2780 | |
| PTX | 1.87 ± 0.08 | 11.17 ± 1.15 | 2.99 ± 0.37 | 2.01 ± 0.04 |
| PEG-BHyd-dC12/PTX | 0.57 ± 0.16∗▲ | 3.04 ± 1.13∗▲ | 1.16 ± 0.06∗▲ | 0.75 ± 0.08∗∗▲ |
| PEG-BAmi-dC12/PTX | 1.10 ± 0.06# | 6.77 ± 0.30# | 1.64 ± 0.13# | 1.33 ± 0.13# |
PTX vs. PEG-BHyd-dC12/PTX, ∗p < 0.05, ∗∗p < 0.01; PTX vs. PEG-BAmi-dC12/PTX, #p < 0.05; PEG-BHyd-dC12/PTX vs. PEG-BAmi-dC12/PTX, ▲p < 0.05.
To test the generality, we further performed the anti-tumor assay by using MDA-MB-231 and A2780 cells, and analogous results were observed (Figures 8C,D). The PEG-BHyd-dC12/PTX displayed the best anti-cancer activity, followed by PEG-BAmi-dC12/PTX and then free PTX. Therefore, such micelles can be implemented for different types of cancer therapy. As one limitation of PTX for long-term cancer treatment is the acquired drug resistance by cancer cells (Yusuf et al., 2003), we also tested whether the nano-systems could reverse drug resistance by using PTX-resistant A549/T cells as a proof-of-concept. The cytotoxicity of PTX and micelles was also dose dependent (Figure 8B), while the overall IC50 value was much higher due to the drug resistance (Table 1). Notably, cytotoxicity of PEG-BHyd-dC12/PTX was 3.7-fold higher than that of free PTX, which may be useful to reverse drug resistance.
Hemolysis Assay
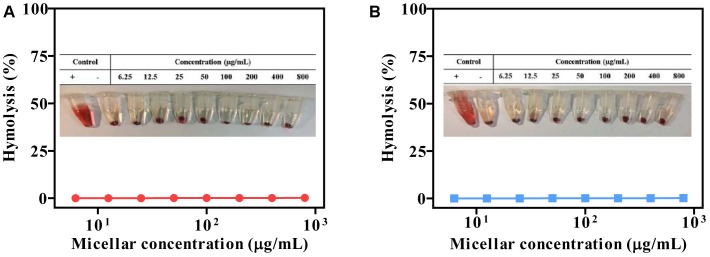
The biocompatibility of polymeric micelles is the prerequisite for biomedical application. We studied this property by using hemolysis assay. Typically, the micelles were incubated with erythrocytes, and the release of hemoglobin was measured to quantify the erythrocyte-damaging properties (Nogueira et al., 2013). The positive control of 0.5% Triton X-100 showed obvious hemolysis, as high as 100%, while the micelles produced less than 2% at different concentration (Figure 9). Therefore, the micelles were highly biocompatible and can be directly administrated by intravenous injection.
FIGURE 9.
Compatibility studies of micelles (pH 7.4). PEG-BHyd-dC12 (A) and PEG-BAmi-dC12 (B). “+” represents positive control by using 0.5% Triton X-100, and “–” represents negative control of non-treatment.
Conclusion
In this work, pH-sensitive PTX-loaded PEG-BHyd-dC12 micelles were constructed and characterized. These nanoparticles exhibited pH-dependent drug release profile and endosomal escape ability after intracellular delivery, and displayed enhanced anti-tumor activity compared with the pH-insensitive counterpart micelles and the free PTX. All of these results suggested that the PEG-BHyd-dC12 micelles-based drug delivery system is a promising drug carrier for targeted cancer treatment.
Author Contributions
YY performed the cell experiments and wrote the manuscript. ZW performed the synthesis and characterization. YP performed drug release. JD and WZ designed the experiments.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. Thanks to the supported of the Innovation-Driven Project of Central South University (Grant No. 20170030010004), National Natural Science Foundation of China (Grant Nos. 81573374 and 81502997), Key Laboratory Breeding Base of Hunan Oriented Fundamental and Applied Research in Innovative Pharmaceutics (Grant No. 2016TP1029), Hunan Engineering Research Center for Optimization of Drug Formulation and Early Clinical Evaluation (Grant No. 2015TP2005), and Director Fund of Jiangsu Key Laboratory of New Drug Research and Clinical Pharmacy (Grant No. ZR-XY201406).
References
- Ahmed S. E., Martins A. M., Husseini G. A. (2015). The use of ultrasound to release chemotherapeutic drugs from micelles and liposomes. J. Drug Target. 23 16–42. 10.3109/1061186X.2014.954119 [DOI] [PubMed] [Google Scholar]
- Ao L., Wang B., Liu P., Huang L., Yue C., Gao D., et al. (2014). A folate- integrated magnetic polymer micelle for MRI and dual targeted drug delivery. Nanoscale 6 10710–10716. 10.1039/c4nr02484b [DOI] [PubMed] [Google Scholar]
- Cao J., Huang S., Chen Y., Li S., Li X., Deng D., et al. (2013). Near-infrared light-triggered micelles for fast controlled drug release in deep tissue. Biomaterials 34 6272–6283. 10.1016/j.biomaterials.2013.05.008 [DOI] [PubMed] [Google Scholar]
- Chou L. Y., Ming K., Chan W. C. (2011). Strategies for the intracellular delivery of nanoparticles. Chem. Soc. Rev. 40 233–245. 10.1039/c0cs00003e [DOI] [PubMed] [Google Scholar]
- Danhier F., Feron O., Préat V. (2010). To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 148 135–146. 10.1016/j.jconrel.2010.08.027 [DOI] [PubMed] [Google Scholar]
- Deng L., Ren J., Li J., Leng J., Qu Y., Lin C., et al. (2015). Magnetothermally responsive star-block copolymeric micelles for controlled drug delivery and enhanced thermo-chemotherapy. Nanoscale 7 9655–9663. 10.1039/c5nr00642b [DOI] [PubMed] [Google Scholar]
- Diezi T. A., Bae Y., Kwon G. S. (2010). Enhanced stability of PEG-block-poly(N-hexyl stearate l-aspartamide) micelles in the presence of serum proteins. Mol. Pharm. 7 1355–1360. 10.1021/mp100069p [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J. Z., Du X. J., Mao C. Q., Wang J. (2011). Tailor-made dual pH-sensitive polymer-doxorubicin nanoparticles for efficient anticancer drug delivery. J. Am. Chem. Soc. 133 17560–17563. 10.1021/ja207150n [DOI] [PubMed] [Google Scholar]
- Fang X. B., Zhang J. M., Xie X., Liu D., He C. W., Wan J. B., et al. (2016). pH-sensitive micelles based on acid-labile pluronic F68-curcumin conjugates for improved tumor intracellular drug delivery. Int. J. Pharm. 502 28–37. 10.1016/j.ijpharm.2016.01.029 [DOI] [PubMed] [Google Scholar]
- Farokhzad O. C., Langer R. (2009). Impact of nanotechnology on drug delivery. ACS Nano 3 16–20. 10.1021/nn900002m [DOI] [PubMed] [Google Scholar]
- Felber A. E., Dufresne M. H., Leroux J. C. (2012). pH-sensitive vesicles, polymeric micelles, and nanospheres prepared with polycarboxylates. Adv. Drug Deliv. Rev. 64 979–992. 10.1016/j.addr.2011.09.006 [DOI] [PubMed] [Google Scholar]
- Gao Y., Xiao Y., Liu S., Yu J. (2018). Camptothecin prodrug nanomicelle based on a boronate ester-linked diblock copolymer as the carrier of doxorubicin with enhanced cellular uptake. J. Biomater. Sci. Polym. Ed. 29 160–180. 10.1080/09205063.2017.1406632 [DOI] [PubMed] [Google Scholar]
- Harnoy A. J., Rosenbaum I., Tirosh E., Ebenstein Y., Shaharabani R., Beck R., et al. (2014). Enzyme-responsive amphiphilic PEG-dendron hybrids and their assembly into smart micellar nanocarriers. J. Am. Chem. Soc. 136 7531–7534. 10.1021/ja413036q [DOI] [PubMed] [Google Scholar]
- Kanamala M., Wilson W. R., Yang M., Palmer B. D., Wu Z. (2016). Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: a review. Biomaterials 85 152–167. 10.1016/j.biomaterials.2016.01.061 [DOI] [PubMed] [Google Scholar]
- Kim T. H., Chen Y., Mount C. W., Gombotz W. R., Li X., Pun S. H. (2010). Evaluation of temperature-sensitive, indocyanine green-encapsulating micelles for noninvasive near-infrared tumor imaging. Pharm. Res. 27 1900–1913. 10.1007/s11095-010-0190-y [DOI] [PubMed] [Google Scholar]
- Lee S. C., Kim C., Kwon I. C., Chung H., Jeong S. Y. (2003). Polymeric micelles of poly(2-ethyl-2-oxazoline)-block-poly(epsilon-caprolactone) copolymer as a carrier for paclitaxel. J. Control. Release 89 437–446. 10.1016/S0168-3659(03)00162-7 [DOI] [PubMed] [Google Scholar]
- Li M., Gao M., Fu Y., Chen C., Meng X., Fan A., et al. (2016). Acetal-linked polymeric prodrug micelles for enhanced curcumin delivery. Colloids Surf. B Biointerfaces 140 11–18. 10.1016/j.colsurfb.2015.12.025 [DOI] [PubMed] [Google Scholar]
- Liu B., Chen H., Li X., Zhao C., Liu Y., Zhu L., et al. (2014). pH-responsive flower-like micelles constructed via oxime linkage for anticancer drug delivery. RSC Adv. 4 48943–48951. 10.1039/c4ra08719d [DOI] [Google Scholar]
- Liu G., Chen C., Li D., Wang S., Ji J. (2012). Near-infrared light-sensitive micelles for enhanced intracellular drug delivery. J. Mater. Chem. 22 16865–16871. 10.1039/c2jm00045h [DOI] [Google Scholar]
- Liu J., Huang Y., Kumar A., Tan A., Jin S., Mozhi A., et al. (2014). pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol. Adv. 32 693–710. 10.1016/j.biotechadv.2013.11.009 [DOI] [PubMed] [Google Scholar]
- Liu Y., Feng L., Liu T., Zhang L., Yao Y., Yu D., et al. (2014). Multifunctional pH-sensitive polymeric nanoparticles for theranostics evaluated experimentally in cancer. Nanoscale 6 3231–3242. 10.1039/c3nr05647c [DOI] [PubMed] [Google Scholar]
- Maysinger D., Lovrić J., Eisenberg A., Savić R. (2007). Fate of micelles and quantum dots in cells. Eur. J. Pharm. Biopharm. 65 270–281. 10.1016/j.ejpb.2006.08.011 [DOI] [PubMed] [Google Scholar]
- Mei D., Lin Z., Fu J., He B., Gao W., Ma L., et al. (2015). The use of α-conotoxin ImI to actualize the targeted delivery of paclitaxel micelles to α7 nAChR-overexpressing breast cancer. Biomaterials 42 52–65. 10.1016/j.biomaterials.2014.11.044 [DOI] [PubMed] [Google Scholar]
- Mo G., Hu X., Liu S., Yue J., Wang R., Huang Y., et al. (2012). Influence of coupling bonds on the anti-tumor activity of polymer-pirarubicin conjugates. Eur. J. Pharm. Sci. 46 329–335. 10.1016/j.ejps.2012.02.013 [DOI] [PubMed] [Google Scholar]
- Nogueira D. R., Tavano L., Mitjans M., Perez L., Infante M. R., Vinardell M. P. (2013). In vitro antitumor activity of methotrexate via pH-sensitive chitosan nanoparticles. Biomaterials 34 2758–2772. 10.1016/j.biomaterials.2013.01.005 [DOI] [PubMed] [Google Scholar]
- Owen S. C., Chan D. P., Shoichet M. S. (2012). Polymeric micelle stability. Nano Today 7 53–65. 10.1016/j.nantod.2012.01.002 [DOI] [Google Scholar]
- Qiu L., Zhu M., Gong K., Peng H., Ge L., Zhao L., et al. (2017). pH-triggered degradable polymeric micelles for targeted anti-tumor drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 78 912–922. 10.1016/j.msec.2017.04.137 [DOI] [PubMed] [Google Scholar]
- Rao J., Khan A. (2013). Enzyme sensitive synthetic polymer micelles based on the azobenzene motif. J. Am. Chem. Soc. 135 14056–14059. 10.1021/ja407514z [DOI] [PubMed] [Google Scholar]
- Sakai-Kato K., Ishikura K., Oshima Y., Tada M., Suzuki T., Ishii-Watabe A., et al. (2012). Evaluation of intracellular trafficking and clearance from HeLa cells of doxorubicin-bound block copolymers. Int. J. Pharm. 423 401–409. 10.1016/j.ijpharm.2011.12.022 [DOI] [PubMed] [Google Scholar]
- Singla A. K., Garg A., Aggarwal D. (2002). Paclitaxel and its formulations. Int. J. Pharm. 235 179–192. 10.1016/S0378-5173(01)00986-3 [DOI] [PubMed] [Google Scholar]
- Tang R., Ji W., Panus D., Palumbo R. N., Wang C. (2011). Block copolymer micelles with acid-labile ortho ester side-chains: synthesis, characterization, and enhanced drug delivery to human glioma cells. J. Control. Release 151 18–27. 10.1016/j.jconrel.2010.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varkouhi A. K., Scholte M., Storm G., Haisma H. J. (2011). Endosomal escape pathways for delivery of biologicals. J. Control. Release 151 220–228. 10.1016/j.jconrel.2010.11.004 [DOI] [PubMed] [Google Scholar]
- Wang X., Li S., Wan Z., Quan Z., Tan Q. (2014). Investigation of thermo-sensitive amphiphilic micelles as drug carriers for chemotherapy in cholangiocarcinoma in vitro and in vivo. Int. J. Pharm. 463 81–88. 10.1016/j.ijpharm.2013.12.046 [DOI] [PubMed] [Google Scholar]
- Wang Z., Deng X., Ding J., Zhou W., Zheng X., Tang G. (2018). Mechanisms of drug release in pH-sensitive micelles for tumour targeted drug delivery system: a review. Int. J. Pharm. 535 253–260. 10.1016/j.ijpharm.2017.11.003 [DOI] [PubMed] [Google Scholar]
- Wilczewska A. Z., Niemirowicz K., Markiewicz K. H., Car H. (2012). Nanoparticles as drug delivery systems. Pharmacol. Rep. 64 1020–1037. 10.1016/s1734-1140(12)70901-5 [DOI] [PubMed] [Google Scholar]
- Wu H., Zhu L., Torchilin V. P. (2013). pH-sensitive poly(histidine)- PEG/DSPE-PEG co-polymer micelles for cytosolic drug delivery. Biomaterials 34 1213–1222. 10.1016/j.biomaterials.2012.08.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong D., Zhang X., Peng S., Gu H., Zhang L. (2017). Smart pH-sensitive micelles based on redox degradable polymers as DOX/GNPs carriers for controlled drug release and CT imaging. Colloids Surf. B Biointerfaces 163 29–40. 10.1016/j.colsurfb.2017.12.008 [DOI] [PubMed] [Google Scholar]
- Yang M., Ding J., Zhang Y., Chang F., Wang J., Gao Z., et al. (2016). Activated macrophage-targeted dextran–methotrexate/folate conjugate prevents deterioration of collagen-induced arthritis in mice. J. Mater. Chem. B 4 2102–2113. 10.1039/c5tb02479j [DOI] [PubMed] [Google Scholar]
- Yin T., Wang L., Yin L., Zhou J., Huo M. (2015). Co-delivery of hydrophobic paclitaxel and hydrophilic AURKA specific siRNA by redox-sensitive micelles for effective treatment of breast cancer. Biomaterials 61 10–25. 10.1016/j.biomaterials.2015.05.022 [DOI] [PubMed] [Google Scholar]
- Yin T., Wang P., Li J., Zheng R., Zheng B., Cheng D., et al. (2013). Ultrasound-sensitive siRNA-loaded nanobubbles formed by hetero-assembly of polymeric micelles and liposomes and their therapeutic effect in gliomas. Biomaterials 34 4532–4543. 10.1016/j.biomaterials.2013.02.067 [DOI] [PubMed] [Google Scholar]
- Yuan Z., Que Z., Cheng S., Zhuo R., Li F. (2012). pH-triggered blooming of ‘nano-flowers’ for tumor intracellular drug delivery. Chem. Commun. 48 8129–8131. 10.1039/c2cc34225a [DOI] [PubMed] [Google Scholar]
- Yuba E., Yamaguchi A., Yoshizaki Y., Harada A., Kono K. (2017). Bioactive polysaccharide-based pH-sensitive polymers for cytoplasmic delivery of antigen and activation of antigen-specific immunity. Biomaterials 120 32–45. 10.1016/j.biomaterials.2016.12.021 [DOI] [PubMed] [Google Scholar]
- Yusuf R. Z., Duan Z., Lamendola D. E., Penson R. T., Seiden M. V. (2003). Paclitaxel resistance: molecular mechanisms and pharmacologic manipulation. Curr. Cancer Drug Targets 3 1–19. 10.2174/1568009033333754 [DOI] [PubMed] [Google Scholar]
- Zhang J., Zhao X., Chen Q., Yin X., Xin X., Li K., et al. (2017). Systematic evaluation of multifunctional paclitaxel-loaded polymeric mixed micelles as a potential anticancer remedy to overcome multidrug resistance. Acta Biomater. 50 381–395. 10.1016/j.actbio.2016.12.021 [DOI] [PubMed] [Google Scholar]
- Zhang P., Zhang H., He W., Zhao D., Song A., Luan Y. (2016). Disulfide-linked amphiphilic polymer-docetaxel conjugates assembled redox-sensitive micelles for efficient antitumor drug delivery. Biomacromolecules 17 1621–1632. 10.1021/acs.biomac.5b01758 [DOI] [PubMed] [Google Scholar]
- Zhu C., Jung S., Luo S., Meng F., Zhu X., Park T. G., et al. (2010). Co-delivery of siRNA and paclitaxel into cancer cells by biodegradable cationic micelles based on PDMAEMA-PCL-PDMAEMA triblock copolymers. Biomaterials 31 2408–2416. 10.1016/j.biomaterials.2009.11.077 [DOI] [PubMed] [Google Scholar]